Abstract
Introduction
Organ shortage, subsequent use of extended donor criteria organs and high-risk recipients needing redo-surgery are increasing the complexity of heart transplantation. Donor organ machine perfusion (MP) is an emerging technology allowing reduction of ischemia time as well as standardized evaluation of the organ. The aim of this study was to review the introduction of MP and analyze the results of heart transplantation after MP in our center.
Methods
In a retrospective single-center study, data from a prospectively collected database were analysed. From July 2018 to August 2021, fourteen hearts were retrieved and perfused using the Organ Care System (OCS), 12 hearts were transplanted. Criteria to use the OCS were based on donor/recipient characteristics. Primary objective was 30-day survival, secondary objectives were major cardiac adverse events, graft function, rejection episodes as well as overall survival in the follow-up and assessment of MP technical reliability.
Results
All patients survived the procedure and the postoperative 30-day interval. No MP related complications were noted. Graft ejection fraction beyond 14 days was ≥ 50% in all cases. Endomyocardial biopsy showed excellent results with no or mild rejection. Two donor hearts were rejected after OCS perfusion and evaluation.
Conclusion
Ex vivo normothermic MP during organ procurement is a safe and promising technique to expand the donor pool. Reduction of cold ischemic time while providing additional donor heart assessment and reconditioning options increased the number of acceptable donor hearts. Additional clinical trials are necessary to develop guidelines regarding the application of MP.
Keywords: Ex-vivo perfusion, Heart transplantation, Heart ischemic time, High-risk heart transplant recipients, Donor heart evaluation
1. Introduction
Despite of advances in the treatment of end-stage heart failure including long-term ventricular assist devices [1] and total artificial heart [2], [3], heart transplantation remains the gold standard warranting excellent long-term survival and quality of life. Mortality on the waiting list remains between 13 and 20% [4], [5]; moreover, due to the scarcity of organs, only the sickest of the recipients have a realistic chance of transplantation, as around 75% of heart recipients in Germany are transplanted under high urgent status [data from Eurotransplant statistics report library, report 2144P_Germany_heart]. Due to these adverse conditions, recipients are hospitalised and/or on MCS in up to 50% of the cases [6].
In Germany, due to transplant law restrictions, the number of donors declined to 10.7 per million. Additionally, donation after circulatory death (DCD) is still inhibited by law in Germany, allowing only donation after secured brain death. Available donors are older and therefore have pre-existent cardiovascular medical conditions [5], [7]. Limited supply with donor organs forces transplant teams to optimise organ evaluation and to broaden acceptance criteria. Nevertheless, offered donor heart grafts are declined in about 20–30% of the cases during the allocation process [5]. Main dilemma remains predicting allograft function from an extended criteria organ after a timespan of cold ischemic storage.
Cold Storage (CS) remains the recommended procedure for graft transportation since 50 years, to slow down biochemical reaction rates and decrease the rate of intracellular degradation of essential components necessary for organ viability. However, CS in itself is a major risk factor for primary graft dysfunction and postoperative mortality due to ischemic damage and subsequent reperfusion injuries of the graft in a time dependent manner [8], [9], [10]. Besides considerations regarding the cardioplegic/preservation solution, stable temperature levels within the range of 4–8 °C are deemed important, since lower and higher temperatures increase negative effects; moreover a hypothermic time span of 4–6 h should not be exceeded [11], [12], [13].
In contrast, in order to reduce negative effects of hypothermia, ex vivo warm graft machine perfusion (MP) of the heart in a beating, near-physiological state in a portable system is under investigation. A nutrient rich perfusion solution and improved oxygenation by the use of donor blood, should allow for a biochemically optimised environment. This method offers the unique possibility of assessing hemodynamics, biochemistry and structure of the beating heart up to the time of transplantation. MP offers real time quality control of donor hearts as well as providing the opportunity to extend graft preservation time. Moreover it allows for continuous assessment of heart grafts with extended criteria, which would not have been accepted under usual allocation and/or acceptance rules. The safety and effectiveness of portable, warm machine perfusion (PROCEED II) and the potential of expanding the donor pool (EXPAND) have been investigated and presented promising results [7], [14].
It was therefore the aim of this study to review our centre’s first experience with the introduction of warm MP, thereby analysing feasibility and outcome of heart transplantation using this new preservation technique.
2. Material and methods
In a retrospective single-center study, data from a prospectively collected database were analysed. From July 2018 to August 2021, fourteen hearts were retrieved and perfused using machine perfusion (Organ Care System (OCS), Transmedics), 12 hearts were transplanted. The study was approved by the local Ethics Committee (21–10062-BO) and conducted according to the declaration of Helsinki.
2.1. Indication for MP
Criteria for using the MP over cold ischemic storage during retrieval were donor and recipient specific. Donor specific criteria were 1) estimated ischemic time > 4 h, 2) history of donor cardiac arrest, 3) inotrope therapy > 0.25 µg/kg/min, 4) left ventricular ejection fraction less than 45%, 5) donors older than 55 years, 6) coronary vessel disease or missing coronary angiography, 7) LV hypertrophy and 8) judgement of the retrieval surgeon. Recipient specific criterion was previous cardiac surgery, mainly left ventricular assist device. All patients gave written consent for orthotopic heart transplantation as well as MP preservation and assessment.
2.2. Retrieval and MP procedure
The MP is a portable perfusion and monitoring device used for perfusing the heart ex vivo with warm, oxygenated, nutrient-enriched donor blood while the heart is beating, thus imitating the in vivo, metabolically active state. The layout of the system has been described extensively [7], [14]. Shortly, the heart is retrieved under standard conditions with collection of 1.2–1.5 L of donor blood and installed in the system for further perfusion.
Warm oxygenated blood is pumped into the aorta, perfusing the coronary arteries while the deoxygenated blood of the coronary sinus flows in the right atrium and through the tricuspid valve (superior/inferior venae cava closed) in the right ventricle. It is then ejected into a pulmonary artery cannula and returned to the blood reservoir after passing through the oxygenator. Notably, a vent is inserted through the left atrial appendage and atrial/ventricular temporary pacing wires are inserted.
The heart is placed in the perfusion module and heart perfusion is commenced with a pump flow of 900 to 1200 mL/min aiming for a coronary flow between 650 and 850 mL/min.
Simultaneously the heart is being rewarmed to 34 °C. In case of ventricular or atrial arrhythmias the heart is initially shocked with 10 Joules and then in 5 Joules increments until sinus rhythm is established.
Venous and arterial blood gas samples are taken after commencing perfusion (starting concentration) and in hourly intervals up to end of MP (ending concentration) to quantify for lactate, electrolytes and haemoglobin using the CG4/CG8 cartridges in the IStat portable analyser (Abbott Point of Care Inc, Princeton, NJ). The criteria for organ acceptance, as formulated in the PROCEED II trial [14], were: stable or diminishing lactate levels, venous blood lactate levels lower than the arterial values, or lactate level less than 5 mmol/L at the end of the MP perfusion demonstrating lactate elimination by the donor heart, as well as stable coronary perfusion and good donor heart contractility.
Donor hearts fulfilling the above-mentioned criteria are finally accepted for transplantation. Before removal from the MP, donor hearts are perfused with 1 L of cold Bretschneider cardioplegia.
The total duration from donor cross-clamping to the connection on the OCS and from administration of cardioplegia on the MP to the release of the cross-clamp after implantation is the total time of cold ischemic storage. MP perfusion time is the time between the connection of the heart on the system and administration of cardioplegia before disconnecting it. The total preservation time is defined as the duration from donor cross-clamping and release of cross-clamping after transplantation.
2.3. Transplantation procedure
Hearts were transplanted using bicaval technique as a standard. Immunosuppression consisted of induction with rabbit antithymocyte globulin and maintenance with a combination of tacrolimus, mycophenolate mofetil and prednisone.
2.4. Data analysis
Thirty-day survival, major cardiac adverse events including left/right ventricular dysfunction and graft function, rejection episodes and overall survival were examined during follow-up. MP parameters such as coronary flow, aortic pressure, heart rate and arterial/venous lactate values were analysed as well as causes for final rejection of heart allograft after initial acceptance were assessed. Finally, potential troubleshooting with the device and/or the necessary logistics were evaluated using the written protocols of each MP procedure.
Data were analysed with SPSS version 27 (IBM SPSS Statistics, IBM Corp., Armonk, NY, USA) and presented as mean +/- standard deviation. Descriptive analysis and survival (Kaplan-Meier) analysis was performed.
3. Results
All transplanted patients (n = 12) survived the transplantation procedure and no patient was lost to follow-up.
3.1. Donor and recipient characteristics
Donors all met the criterias of brain death (DBD) and showed a median age of 54.3 years with equal distribution between male/female. Main cause of death was stroke or cerebrovascular event with 83.4%. One third of the donor offers were conducted in tertiary round as extended/center allocation. Two heart grafts were from donors after cardiopulmonary resuscitation. Details are given in Table 1.
Table 1.
Donor and Recipients characteristics. All values are given in mean, with range in brackets or percentage in parentheses, n = 12. BMI: Body mass index, LV: left ventricular, ICM: Ischemic cardiomyopathy, DCM: Dilative cardiomyopathy, LVAD: left ventricular assist device.
| Donor | |
|---|---|
| Age (years) | 54.3 [35–68] |
| Sex Female | 6 (50%) |
| Height (cm) | 172.1 [158–185] |
| Weight (kg) | 75.2 [52–100] |
| BMI (kg/m2) | 25.2 [19–31] |
| Cause of death | 10 (83.4%) |
| Stroke or cerebrovascular event Hypoxia/Anoxia |
1 (8.3%) |
| Trauma | 1 (8.3%) |
| Female donor to male recipient | None |
| Type of allocation | |
| Primary | 8 (66.7%) |
| Extended/Center Allocation | 4 (33.3%) |
| LV Ejection fraction (%) | 59.6 [55.0–70.0] |
| Donor Hemoglobin (g/dL) | 10.2 [7.6–13.4] |
| Cardiopulmonary resuscitation | 2 (16.7%) |
| Recipient | |
| Age (years) | 49.2 [19–66] |
| Sex Female | 7 (58.3%) |
| Height (cm) | 166.7 [152–182] |
| Weight (kg) | 72 [49–112] |
| BMI (kg/m2) | 25.8 [20.1–33.8] |
| Type of cardiomyopathy | |
| ICM | 5 (41.7%) |
| DCM | 4 (33.3%) |
| Other | 3 (25%) |
| On LVAD |
6 (50%) |
| High Urgent status | 5 (41.7%) |
| Pulmonary Hypertension | 5 (41.7%) |
| Diabetes | 1 (8.3%) |
Recipients were aged 49.2 years in median, with seven female and five male patients. Typically, most underlying disease was ischemic (5) and dilatative (4) cardiomyopathy. Other diagnoses included amyloidosis, non-compaction and hypertrophic non-obstructive cardiomyopathy. Half of all recipients were on LVAD therapy, and 5 were transplanted under High Urgent status (Table 1).
3.2. MP procedure and perioperative outcome data
Indication for use of machine perfusion was documented as previous cardiac surgery (6/12, 50%), donor age > 55 years (5/12, 42%), donor cardiac arrest (2/12, 17%), donor inotrope therapy (1/12, 8%), missing angiography (1/12, 8%) and reported donor heart ventricular hypertrophy (1/12, 8%), with multiple indications occurring in some cases.
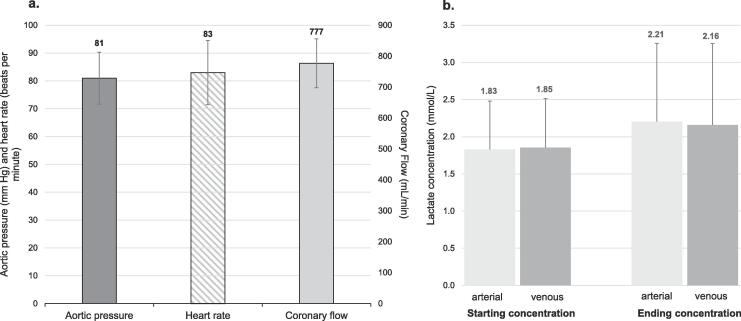
No technical issues occurred on any MP run. Hearts could be perfused in stable manner, reaching the targeted values of coronary flow and aortic pressure as shown in Fig. 1a. Lactate levels were taken from the arterial and venous lines regularly and reached 2.21 mmol/L and 2.16 mmol/L respectively, showing lactate consumption by the heart (Fig. 1b).
Fig. 1.
Data of MP. All values are mean ± standard deviation, total perfusion cases 12. A. Aortic pressure, heart rate and coronary flow. Aortic pressure and heart rate represented by the right y-axis, coronary flow by the left y-axis. B. Arterial/venous lactate concentration at start and end of perfusion.
MP perfusion time was 295.75 min in median, reaching up to 406 min, nonetheless leading to a cold/warm ischemia time of 82 ± 29 min. Heart allografts showed a median total out-of-body time of 377.75 min.
All patients survived the procedure, five of the patients (41.7%) received temporary extracorporal life support (ECLS) for 6.6 ± 4.2 days. Reasons for support were subjective ventricular dysfunction (assessed by the implanting surgeon) and/or cumulative inotrope dependency > 0.4 µg/kg/min. Liberal implementation of (ECLS) was favored as a strategy during the implementation of this new technique.
All patients could be weaned from ECLS, and could be transferred from Intensive Care Unit after 34.75 ± 31.2 days. No patient died within the 30-day period (Table 2).
Table 2.
Perioperative outcome and Follow-up data. All values are given in mean, with range in brackets or percentage in parentheses, n = 12. OCS: Organ care system, ICU: Intensive care unit, MCS: mechanical circulatory support, EMB: Endomyocardial biopsy (after guidelines of the International Society of Heart and Lung Transplantation).
| Perioperative data | |
|---|---|
| OCS Perfusion (min) | 295.75 [221–406] |
| Out of body time (min) | 377.75 [255–466] |
| Cold/warm ischemia (min) | 82 [47–147] |
| Ventricular dysfunction/inotrope dependency requiring MCS | 5 (41.7%) |
| Time on MCS (days) | 6.6 [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] |
| Graft failure | 0 (0%) |
| ICU (days) | 34.75 [6–112] |
| 30d mortality | 0 (0%) |
| Follow-up data | |
| Ejection fraction (%) | 57.25 [50–64] |
| EMB results (worst) | |
| 2R | 1 (8%) |
| 1R | 3 (25%) |
| 0R | 8 (67%) |
| Overall mortality | 3 (25%) |
| survival days | 50, 234, 329 |
| Cause of death | |
| Cardiovascular | 0 |
| sepsis/abdominal ischemia | 2 (16%) |
| sepsis/respiratory | 1 (8%) |
3.3. Long term follow up
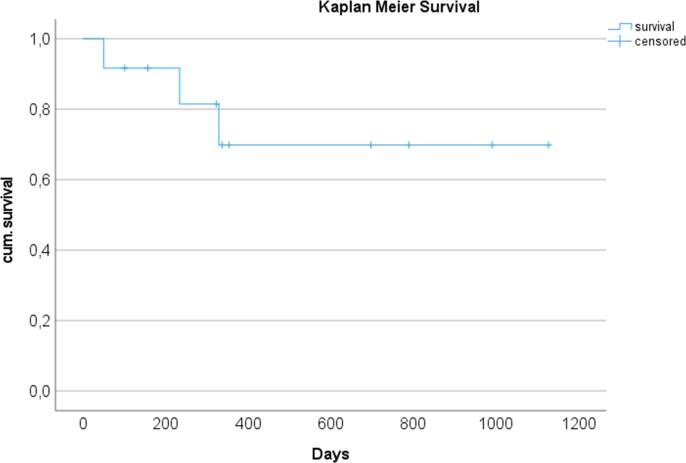
The overall survival as shown in Fig. 2 was 75% over 3 years with the longest survivor having reached 1320 days (status on 01.03.2022) and is ongoing.
Fig. 2.
Kaplan-Meier plot of 3 year survival after heart transplantation (n = 12).
No patient presented cardiac-related complications. Two patients developed abdominal ischemia and consecutive sepsis on day 50 and 234, respectively, the first patient without having been discharged and under treatment in the cardiology department, the other after discharge and readmission with abdominal symptoms. The third patient was admitted from home after developing a severe respiratory infection with consecutive sepsis and died after 329 days. The other 9 patients are alive and in stable health condition.
During follow-up, regular echocardiographic examinations and endomyocardial biopsy showed good cardiac function with left ventricular ejection fraction of 57% in the mean and no severe rejection, with only one episode of 2R in one patient which was successfully treated with corticosteroids. Up to date, 67% of the recipients showed 0R and 25% 1R (after guidelines of the International Society of Heart and Lung Transplantation).The follow-up data are shown in Table 2.
3.4. Rejected heart allografts after MP perfusion
Two donor hearts were rejected after MP perfusion.
Case 1
The offer was accepted for a high urgent 46 year old male patient on LVAD. MP was planned due abnormal electrocardiography, missing coronary angiography and estimated time of ischemia > 4 h. On-site inspection revealed calcification in the left anterior descending coronary artery by digital touch, however retrieval and further evaluation was decided because of good function by visual assessment. MP perfusion showed no substantial abnormalities, lactate levels staying stable (1.59 to 1.92 mmol/L in arterial and from 1.52 to 1.9 mmol/L in venous samples). Back at our centre angiography on MP was performed, revealing coronary artery disease with multiple stenosis of the left anterior descending coronary artery. The implanting surgeon finally rejected the heart considering the coronary disease.
Case 2
The offer was accepted for a high urgent 66 year old male patient with amyloidosis. MP retrieval was planned due to age > 55 years, inotropic therapy > 0.25 µg/kg/min norepinephrine and echocardiography with suspected left ventricular hypertrophy and aortic valve insufficiency grade I. Initial start of MP showed a 45 min episode of ventricular fibrillation not responding to multiple (25 documented) defibrillation attempts; after 45 min the heart converted finally after defibrillation in sinus rhythm and was overpaced with 90 bpm. During the whole perfusion time the aortic pressure could not be stabilised, showing mean pressure of 62 mmHG. Lactate levels increased steadily from 1.59 to 4.2 mmol/L in arterial and from 1.52 to 3.9 mmol/L in venous samples. Left ventricle venting showed significant flow of blood, indicating a higher aortic valve insufficiency. Moreover, palpatory feeling indicated a relevant hypertrophy of the septum. The implanting surgeon finally rejected the heart considering the above mentioned irregularities.
4. Discussion
In this single center retrospective study, we demonstrated that the introduction and use of donor heart machine perfusion was feasible and safe. Short term and intermediate term results are excellent and no early or midterm death due to cardiac causes occurred. In addition, by thorough evaluation during donor organ perfusion, 2 borderline hearts were extracted from the transplant procedure due to contraindications uncovered by organ perfusion.
4.1. Introduction of the technique
After theoretical training of the whole team including perfusionists and after surgical training on procurement and perfusion of pig hearts, the DSO (German Organ procurement organisation) was involved for transport and further logistics. In order to obtain the necessary donor blood for MP, donor management protocol was adapted to include a request of hemoglobine > 10 mg/dl at procurement time and explantation teams informed other organ teams at arrival in order to manage retrieval in general agreement.
4.2. Outcome
In this collective, survival was 92% after 3 months and 75% after 3 years. This is in accordance with international survival data showing a survival of 90% and 80% after 3 months and 3 years respectively [6], when taking in account the small collective and higher risk when using extended criteria allografts. Additionally, survival in Germany show lower rates when considering the german quality report 2020 with 73% survival after 3 years [15]. In summary, outcomes of heart transplantation after MP are comparable to standard transplantation procedure with CS.
4.3. Ischemia time and graft function
Despite CS the anaerobic metabolism continues and myocardial injury occurs time-dependent, resulting in a doubled risk of early graft failure comparing 3 to 6 h of CS [16]. Furthermore, ischemia/reperfusion injury after CS might lead to damage such as cardiac allograft vasculopathy (CAV) or myocardial scar formation with increased mortality [17], [18]. Current practice calls for an ischemic time of less than 4 h, however this is often exceeded due to transport or complex redo cases.
In our cohort total ischemia time was 82 min in the mean, demonstrating that very short ischemic times can be achieved with MP. The broad range of 47 – 147 min can be explained by different practices, reperfusing after anastomosis of left atrium/ aorta or reperfusing after the complete procedure. Considering a mean out of body time of 377 min, these results emphasize the possibilities of mobile MP, in which donor hearts were perfused during transport and up to implantation for 295.75 min in the mean, overcoming long range transportation and recipient preparation surgery in redo cases. These findings are in accordance with reports from others using MP [14], [19], [20], one center even reporting extension of MP up to almost 16 h, still with a reduced cold ischemic time of 68 min [21].
Ischemic times in our case series were considerable lower compared to international published data using CS, probably reducing primary graft dysfunction (PGD). This might be a reason why use of MP resulted in excellent short term posttransplant outcomes and a low rate of primary graft dysfunction. In our case series cardiac function and 30 d survival was comparable to results from Chan et al. [20]. They found a trend towards higher rates of freedom from CAV using MP compared to CS. However, rate and type of rejection was not different. In our series, biopsies showed no or only minor rejection of the graft during follow-up, with only 1 rejection type 2R occurring in one patient, which after treatment with corticosteroids, caused no further complications. In another study Sato et al. [22] found no difference in intima thickening one year after heart transplantation after MP compared to a control group, with no signs of CAV or development of non-fatal major cardiac events being recognised.
In our case series 60% of the patients had previous surgery and 50% were on mechanical assist. We found MP to allow for precise planning of surgery and for comfort to work without extreme time pressure for example in redo cases. Similar experiences were reported from Kaliyev et al. in MCS recipients [19].
The use of mechanical support with prolonged venoarterial ECLS in 5 patients (41.7%) is to be seen as a strategic change in more liberal use of mechanical support in order to minimize postoperative heart allograft stress and inotropic support and therefore should not be considered as a postoperative complication in this study.
4.4. MP in organ retrieval
In 2020, 1344 potential organ donors were registered in Germany, resulting in 913 procurements. Out of these only 400 hearts were relayed to the allocation process, leading to 320 transplanted heart grafts [5]. These numbers underline the potential of standardized evaluation of all donor organs.
Evaluation of the donor heart during a standard procurement is usually uneventful. However, when the donor is instable, procurement might be initiated without time for the evaluation of an organ, that is most dependent of acute management i.e. during blood loss, variations in electrolytes or blood pressure. These scenarios followed by CS forces the surgeon to make decisions relying what he get in the “black (ice-) box”.
In contrast, MP enables standardized monitoring of aortic pressure, coronary blood flow, oxygen saturation and arterial/venous lactate concentration in consecutive, even online measurements under „laboratory” conditions. Especially the consumption/production of lactate deducted from the difference of arterial and venous concentration can serve as useful surrogate parameter of cell damage [14]. Additionally, MP allows for visual assessment and for angiography in order to assess coronary anatomy and pathology during perfusion when deemed necessary [23]. In our series we had one unstable donor, one missing coronary angiography and 7 estimated ischemic times exceeding 4 h when taking in account transportation and preparation time of redo cases.
In regard to performance of MP, perfusion pressure and metabolic parameters were stable during the perfusion time. Coronary flow and aortic pressure are important values and need to be maintained in a target range (mean aortic pressure between 60 mm Hg and 90 mm Hg, and coronary blood flow between 650 mL/min and 850 mL/min). Compared to data presented in the PROCEED II trial [14], our haemodynamic as well as metabolic values in MP donor hearts were comparable, showing consumption of lactate and values less than 5 mmol/L in the perfused hearts. Furthermore, reconditioning measures such as adapting the perfusion pressures, when applicable, were applied. The EXPAND trial has already reported the use of hearts with extended donor criteria (EDC), transplanting 75 of 93 identified EDC hearts successfully with excellent results and leading to an utilization rate of 81% [7], Garcia et al reporting 86,7% in a study using machine perfusion in heart transplantation with an adverse donor/recipient profile [24]. Our results show a comparable utilization rate of 86% directly after introduction and confirm the possibilities of MP. Furthermore, MP can be used to resuscitate and assess hearts in the setting of donation after circulatory death (DCD) in the countries allowing this type of donation, leading to excellent results and utilization rates as demonstrated experimentally [25] as well as clinically [26], increasing the donor pool with comparable results to donation after brain death (DBD). This is particularly interesting since DCD donors are expected to become a major source of heart donation, accounting for one third of the heart transplant activity in some experienced centers [26].
Further clinical and experimental studies should be performed to assess additional evaluation parameters, since ex vivo perfusion allows for sampling during the whole procedure. Biomarkers in the perfusate, further cardiac imaging procedures and even biopsies may lead to a more sophisticated method of assessment of the perfused graft with predictive data quality [27], [28]. Kaliyev et al. reported of better myocardial protection using blood cardioplegia instead of Custodiol cardioplegia during explantation [29]. Finally, therapeutic possibilities have to be explored in order to progress from organ reconditioning to organ repair [30].
4.4.1. Discarded organs
No organ was declined or discarded because of technical issues. Two donor hearts after MP were declined for further transplantation. We consider these cases as an expression of a new dimension of decision-making. The continuous metabolic and functional assessment of the donor heart together with short ischemic times broadens confidence when finally accepting an organ and allows, on the other hand to uncover pathological findings in donor hearts that would have been otherwise accepted by present standards. Therefore, evaluation under standardised conditions up to final acceptance by the implanting surgeon such as in MP might be of great advantage.
5. Strengths and limitations
Our study is strong for thorough data collection and completeness of data. In addition a protocol for prospective data collection was used. No patient was lost to follow up and all patients will be followed in the future for further reports.
However several limitations of this study have to be considered, when interpreting the results. First the retrospective single center design that describes an institutional experience. Second the results may be influenced by center-specific protocols, techniques and geographic variables during organ retrieval in different hospitals. Thirdly. patients were inhomogeneous due to different indications and age groups.
5.1. Conclusion
Ex vivo normothermic MP during organ procurement is a safe and promising technique allowing for good outcomes after heart transplantation. Control and Reduction of cold ischemic time while providing standardized donor heart assessment and potential reconditioning shall increase the number of acceptable donor hearts. Clear guidelines regarding the application of MP have to be developed in order to sift out donor hearts that benefit from MP.
This retrospective study did not receive any grants or funding and was approved by the local Ethics Committee (21-10062-BO).
CRediT authorship contribution statement
Nikolaus Pizanis: Conceptualization, Investigation, Data curation, Methodology, Formal analysis, Project administration, Validation, Supervision, Writing – original draft, Writing – review & editing. Alexandros Merkourios Dimitriou: Conceptualization, Investigation, Formal analysis, Software, Writing – original draft. Achim Koch: Conceptualization, Investigation, Data curation, Methodology, Writing – review & editing. Peter Luedike: Methodology, Data curation, Writing – review & editing. Maria Papathanasiou: Investigation, Data curation, Writing – review & editing. Tienush Rassaf: Writing – review & editing. Arjang Ruhparwar: Methodology, Validation, Writing – review & editing. Bastian Schmack: Investigation, Data curation, Writing – review & editing. Alexander Weymann: Data curation, Writing – review & editing. Katja Bettina Ferenz: Writing – review & editing. Markus Kamler: Conceptualization, Methodology, Validation, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank the following colleagues for their participation in organ retrieval, machine perfusion management and cardiac transplantation assistance: George Ayoub, Omar Abou Issa, Christos Ilias and Angenita Munneke. Additionally, we would like to thank Klaus Kreikemeier for the help in the data acquisition. The authors received no funding for this study. For publication purpose only, we acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
References
- 1.Kirklin J.K., Pagani F.D., Kormos R.L., et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J. Heart Lung Transplant. 2017;36(10):1080–1086. doi: 10.1016/j.healun.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Kirsch M.E., Nguyen A., Mastroianni C., et al. SynCardia temporary total artificial heart as bridge to transplantation: current results at la pitié hospital. Ann. Thorac. Surg. 2013;95(5):1640–1646. doi: 10.1016/j.athoracsur.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen A., Pellerin M., Perrault L.P., et al. Experience with the SynCardia total artificial heart in a Canadian centre. Can. J. Surg. 2017;60(6):375–379. doi: 10.1503/cjs.003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eurotransplant annual report 2020. Available from: <https://www.eurotransplant.org/statistics/annual-report/>.
- 5.German Organ Procurement Organization (Deutsche Stiftung für Organspende, DSO) annual report 2020. Available from: <https://dso.de/organspende/statistiken-berichte/jahresbericht>.
- 6.Khush K.K., Cherikh W.S., Chambers D.C., et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J. Heart Lung Transplant. 2019;38(10):1056–1066. doi: 10.1016/j.healun.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jawitz O.K., Devore A.D., Patel C.B., Bryner B.S., Schroder J.N. EXPANDing the donor pool: quantifying the potential impact of a portable organ-care system for expanded criteria heart donation. J. Card. Fail. 2021;27(12):1462–1465. doi: 10.1016/j.cardfail.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Reich H.J., Kobashigawa J.A., Aintablian T., Ramzy D., Kittleson M.M., Esmailian F. Effects of older donor age and cold ischemic time on long-term outcomes of heart transplantation. Tex Heart Inst. J. 2018;45(1):17–22. doi: 10.14503/THIJ-16-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicoara A., Ruffin D., Cooter M., et al. Primary graft dysfunction after heart transplantation: incidence, trends, and associated risk factors. Am. J. Transplant. 2018;18(6):1461–1470. doi: 10.1111/ajt.14588. [DOI] [PubMed] [Google Scholar]
- 10.Singh S.S.A., Dalzell J.R., Berry C., Al-attar N. Primary graft dysfunction after heart transplantation: a thorn amongst the roses. Heart Fail. Rev. 2019;24(5):805–820. doi: 10.1007/s10741-019-09794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minasian S.M., Galagudza M.M., Dmitriev Y.V., Karpov A.A., Vlasov T.D. Preservation of the donor heart: from basic science to clinical studies. Interact. Cardiovasc. Thorac. Surg. 2015;20(4):510–519. doi: 10.1093/icvts/ivu432. [DOI] [PubMed] [Google Scholar]
- 12.Bernard M., Cartoux C., Caus T., Sciaky M., Cozzone P.J. The influence of temperature on metabolic and cellular protection of the heart during long-term ischemia: a study using P-31 magnetic resonance spectroscopy and biochemical analyses. Cryobiology. 1998;37(4):309–317. doi: 10.1006/cryo.1998.2126. [DOI] [PubMed] [Google Scholar]
- 13.Jahania M.S., Sanchez J.A., Narayan P., Lasley R.D., Mentzer R.M., Jr. Heart preservation for transplantation: principles and strategies. Ann Thorac Surg. 1999;68(5):1983–1987. doi: 10.1016/s0003-4975(99)01028-0. [DOI] [PubMed] [Google Scholar]
- 14.Ardehali A., Esmailian F., Deng M., et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet. 2015;385(9987):2577–2584. doi: 10.1016/S0140-6736(15)60261-6. [DOI] [PubMed] [Google Scholar]
- 15.IQTIG Qualitaetsreport 2020. Available from: <https://iqtig.org/downloads/berichte/2019/IQTIG_Qualitaetsreport-2020_2021-02-11.pdf>.
- 16.Lund L.H., Edwards L.B., Kucheryavaya A.Y., Benden C., Dipchand A.I., Goldfarb S., Levvey B.J., Meiser B., Rossano J.W., Yusen R.D., Stehlik J. The registry of the international society for heart and lung transplantation: thirty-second official adult heart transplantation report–2015; Focus theme: early graft failure. J. Heart Lung Transplant. 2015;34(10):1244–1254. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Yamani M.H., Haji S.A., Starling R.C., Tuzcu E.M., Ratliff N.B., Cook D.J., et al. Myocardial ischemic-fibrotic injury after human heart transplantation is associated with increased progression of vasculopathy, decreased cellular rejection and poor long-term outcome. J. Am. Coll. Cardiol. 2002;39(6):970–977. doi: 10.1016/s0735-1097(02)01714-x. [DOI] [PubMed] [Google Scholar]
- 18.Butler C.R., Kim D.H., Chow K., Toma M., Thompson R., Mengel M., et al. Cardiovascular MRI predicts 5-year adverse clinical outcome in heart trans-plant recipients. Am. J. Transplant. 2014;14(9):2055–2061. doi: 10.1111/ajt.12811. [DOI] [PubMed] [Google Scholar]
- 19.Kaliyev R., Lesbekov T., Bekbossynov S., Nurmykhametova Z., Bekbossynova M., Novikova S., Medressova A., Smagulov N., Faizov L., Samalavicius R., Pya Y. Heart transplantation of patients with ventricular assist devices: impact of normothermic ex-vivo preservation using organ care system compared with cold storage. J. Cardiothorac. Surg. 2020;15:323. doi: 10.1186/s13019-020-01367-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan J.L., Kobashigawa J.A., Reich H.J., Ramzy D., Thottam M.M., Yu Z., Aintablian T.L., Liou F., Patel J.K., Kittleson M.M., Czer L.S., Trento A., Esmailian F. Intermediate outcomes with ex-vivo allograft perfusion for heart transplantation. J. Heart Lung Transplant. 2017;36(3):258–263. doi: 10.1016/j.healun.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Kaliyev R., Bekbossynov S., Nurmykhametova Z. Sixteen-hour ex vivo donor heart perfusion during long-distance transportation for heart transplantation. Artif. Organs. 2019;43(3):319–320. doi: 10.1111/aor.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato T., Azarbal B., Cheng R., Esmailian F., Patel J., Kittleson M., Czer L., Thottam M., Levine R., Dimbil S., Olymbios M., Anzai T., Hamilton M.A., Khayal T., Kobashigawa J.A. Does ex vivo perfusion lead to more or less intimal thickening in the first-year post-heart transplantation? Clin. Transplant. 2019;33(8):e13648. doi: 10.1111/ctr.13648. [DOI] [PubMed] [Google Scholar]
- 23.Ghodsizad A., Bordel V., Ungerer M., Karck M., Bekeredjian R., Ruhparwar A. Ex vivo coronary angiography of a donor heart in the organ care system. HeartSurg Forum. 2012;15(3):E161–E163. doi: 10.1532/HSF98.20111146. [DOI] [PubMed] [Google Scholar]
- 24.García Sáez D., Zych B., Sabashnikov A., Bowles C.T., De Robertis F., Mohite P.N., Popov A.F., Maunz O., Patil N.P., Weymann A., Pitt T., McBrearty L., Pates B., Hards R., Amrani M., Bahrami T., Banner N.R., Simon A.R. Evaluation of the organ care system in heart transplantation with an adverse donor/recipient profile. Ann. Thorac. Surg. 2014;98(6):2099–2105. doi: 10.1016/j.athoracsur.2014.06.098. [DOI] [PubMed] [Google Scholar]
- 25.García Sáez D., Elbetanony A., Lezberg P., Hassanein A., Bowles C.T., Popov A.F., Zych B., Sabashnikov A., Mohite P., Simon A.R. Ex vivo heart perfusion after cardiocirculatory death; a porcine model. J. Surg. Res. 2015;195(1):311–314. doi: 10.1016/j.jss.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 26.Scheuer S.E., Jansz P.C., Macdonald P.S. Heart transplantation following donation after circulatory death: expanding the donor pool. J. Heart Lung Transplant. 2021;40(9):882–889. doi: 10.1016/j.healun.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Bona M., Wyss R.K., Arnold M., Méndez-Carmona N., Sanz M.N., Günsch D., Barile L., Carrel T.P., Longnus S.L. Cardiac graft assessment in the era of machine perfusion: current and future biomarkers. J. Am. Heart Assoc. 2021;10(4):e018966. doi: 10.1161/JAHA.120.018966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beuth J., Falter F., Pinto Ribeiro R.V., Badiwala M., Meineri M. New strategies to expand and optimize heart donor pool: ex vivo heart perfusion and donation after circulatory death: a review of current research and future trends. Anesth. Analg. 2019;128(3):406–413. doi: 10.1213/ANE.0000000000003919. [DOI] [PubMed] [Google Scholar]
- 29.Kaliyev R., Lesbekov T., Bekbossynov S., Bekbossynova M., Nurmykhametova Z., Novikova S., Smagulov N., Medressova A., Faizov L., Ashyrov Z., la Fleur P., Samalavicius R., Pya Y. Comparison of Custodiol vs warm blood cardioplegia and conditioning of donor hearts during transportation with the organ care system. J. Card. Surg. 2019;34(10):969–975. doi: 10.1111/jocs.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resch T., Cardini B., Oberhuber R., Weissenbacher A., Dumfarth J., Krapf C., Boesmueller C., Oefner D., Grimm M., Schneeberger S. Transplanting marginal organs in the era of modern machine perfusion and advanced organ monitoring. Front. Immunol. 2020;11:631. doi: 10.3389/fimmu.2020.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]