Abstract
Watermelon (Citrullus lanatus) is consumed all over the world that contains a large number of seeds and rind, which is discarded. These by-products contain phytochemical compounds with great nutritional potential. This study aims to evaluate physicochemical properties and sensory values of watermelon rind candy. In this study in order to make the waste of watermelon a more sustainable and value-added food product, the watermelon rind was dried using an osmotic dehydration technique which comprises gradual impregnation of syrup (50 and 70% w/w – 1 to 5 h) before drying at 40 and 60 °C in 8 and 10 h. Various variables such as moisture content, chemical composition, water loss, solid gain, rehydration water, acidity, pH, antioxidant activity, antibacterial activity, residual toxins, phenolic and flavonoid contents during osmotic dehydration of watermelon were investigated. Results showed by rising temperatures, dehydration becomes more severe. Increasing the temperature in both osmotic samples in a concentrated solution (70%) and in osmotic samples with a dilute solution (50%) can enhance the mass transfer, water loss, solid absorption, as well as dehydration intensity. However, antioxidant activity, phenolic and flavonoid content significantly decreased after osmotic dehydration. TPC decreased from 35.83 mg/100 g to 27.45 mg/100 g and TFC of the watermelon rind (8.71 ± 0.01 mg/100 g) decreased to 2.63 ± 0.02 mg/100 g and also antioxidant activity after the osmotic process decreased from 61% to 40%. Also, osmotic dehydration had no significant impact on acidity and pH. The watermelon rind dehydrated sample (osmosis temperature: 40 °C, osmotic solution concentration: 70%, immersion duration: 5 h) was the best choice of panelists due to the highest score in the sensory evaluation including taste, texture, and overall acceptability. By determining the hardness of the watermelon rind candy and comparing it with the results of texture analysis of other dried products, it can be concluded that this product can be used as a healthy snack with longer shelf life properties.
Keywords: Watermelon rind candy, Osmotic dehydration, Hot air drying, Antimicrobial properties, Sensory analysis
1. Introduction
Watermelon (Citrullus lanatus), family of Cucurbitacea is known as a popular fruit crop that is originally from tropical and subtropical countries [1]. With a global production of almost 103 million tons in 2018, watermelon (Citrullus lanatus) is considered the second-largest fruit in the world by production [2]. The cultivated area of watermelon in Iran is around 132,786 ha, and its average yield is 26.8 tons per hectare, which ranks second in the world with a production of 3,568,134 tons in 2017 [3].
Watermelon can be taken for breakfast meal as an enjoyable appetizer or even snack by consumers [4]. It has the main macronutrients like protein, fat, carbohydrates, and also crude fiber and ash have been observed [5]. Moreover, watermelon is a considerable source of vitamins such as A, B vitamins (mainly B1 and B6), and minerals including K and Mg [6]. The therapeutic and pharmacological values of watermelon have been informed, which is associated with antioxidant components such as carotenoids and lycopene [[7], [8], [9], [10]]. Lycopene has been revealed to have a protective role against numerous types of cancer [11,12], as well it aids suppressing the inflammations that leads to different diseases including atherosclerosis, asthma, diabetes, arthritis, and cancers, especially, prostate cancer [13].
Watermelon consists of three major parts: the pulp (about 68% of the total weight), rind (approximately 30% of the total weight), and seed (about 2% of the total weight) [14,15]. The outer rind has a dark green color or pale green stripes, which can change to a yellowish-green color after ripening [16]. Watermelon rinds are typically consumed as pickles in the southern United States [17]. It also can be used in the formulation of jellies, fruit preserves, and conserves [18,19]. Although the watermelon rind is edible, many consumers avoid eating it because of its undesirable flavor. The inner rind consists of a high amount of citrulline, a non-essential amino acid, which has been conveyed to arginine (utilized in the urea cycle for the elimination of ammonia from the body) [20]. Some people suffer from an arginine deficiency, so the production of extracts or nutritional supplements containing watermelon rind has been considered to treat this specific type of deficiency [21]. This amino acid is capable of dilation of the arteries and improving blood circulation [22]. Environmental issues are raised because watermelon rind, which makes up around 30% of the entire fruit, is frequently thrown irresponsibly into the environment [23]. Due to the lack of understanding regarding potential conversion strategies of highly valuable components, the valorisation of this waste is limited [24,25].
According to Athmaselvi et al. [26], the watermelon rind contains approximately 95% water, which makes it vulnerable to deterioration. Therefore, it is vital to minimize the moisture content for the production of longer shelf-life by-products from watermelon rind [81]. Also, considering that the rind covers the about one-third total weight of this fruit and contains a significant amount of nutrients (protein, carbohydrate, fiber, and phenolic compounds [27,28], it is required to utilize watermelon rinds in the formulation of various food products. By using the drying process, the rind can become value-added and more sustainable food product and can be employed in order to prepare bakery products [27].
The purpose of using different drying techniques is to extend the storage time with minimal packaging and get better sustainability, reduce the volume and weight of the product, and increase the availability of various dried foods and medicinal plants in all seasons [29]. Hot air drying is one of the main drying methods in the food industry, as its relatively cheaper. The key issue with hot air drying is the long drying period, the change in sensory, nutritional, and physical properties of the product, as well as the alteration of texture, which causes surface hardening [30]. On the other hand, osmotic dehydration is a common drying process that removes the water from a lower concentration of solute to a greater concentration via a semipermeable membrane and leads to equilibrium between both sides of the membrane [31]. Osmotic dehydration is performed by placing the food product in a solution containing dissolved solids with higher osmotic pressure and less water activity than the product itself. In osmotic dehydration, the solutes used are mostly sugar syrup with fruit slices and salt (NaCl) or highly concentrated saltwater with vegetables. Among drying methods, osmotic dehydration is popular due to its minimal loss of color, flavor, aroma, and nutritional constituents [32].
The aim of this study is to investigate the potential of watermelon rind as a by-product of processing waste to prepare a healthy snack, and to determine changes in physicochemical properties during the preparation procedure.
2. Materials and methods
Sabzevar Jovin watermelon (Crimson Suite cultivar) was purchased from local fruit markets (Neishabour, Iran). Through preparing the sample, the thickness of the peel and the absence of any damage were considered.
2.1. Preparation of watermelon rind
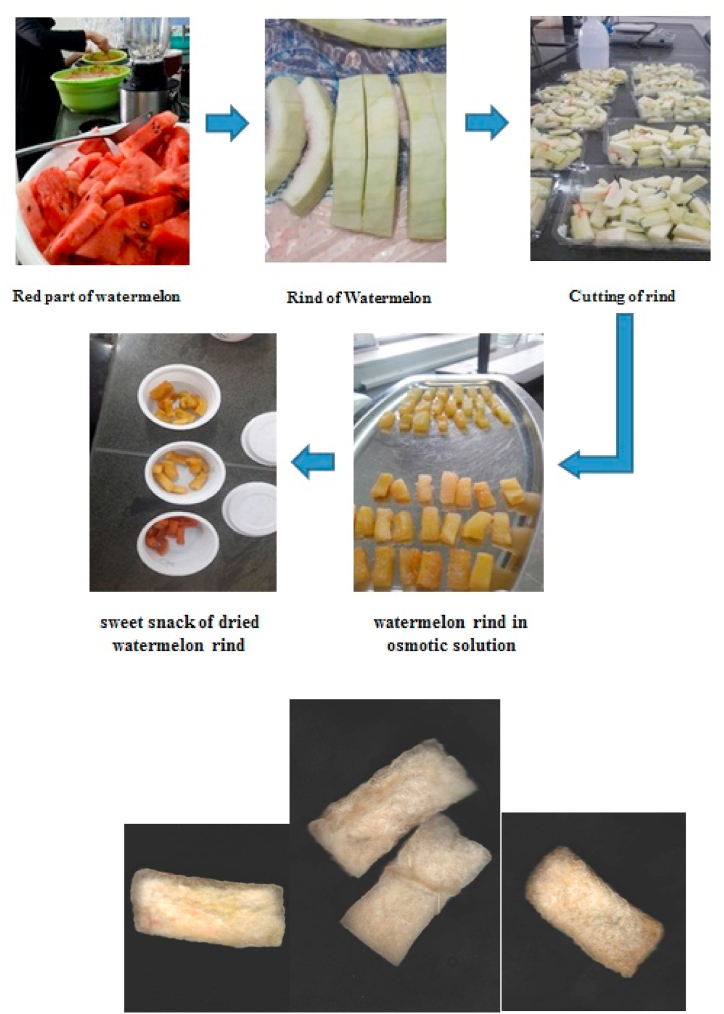
First, the red part of the watermelon was separated from the fruit and then the rinds were cut into small pieces with the help of a cutting device (mold). Then, the outer rind (green peel) was removed manually from the white part (Dimensions of the rinds after these steps: 3*1.5*0.7 cm3, approximately). In Scheme 1 the portions of rinds had been blanched by placing them in boiling water for 2 min to inactivate the enzymes. Cubes of watermelon rind randomly formed an experimental group and the parameters were measured in triplicate.
Scheme 1.
The steps of production of watermelon rind candy.
2.2. Antimicrobial activity
The antimicrobial activity was carried out by well diffusion technique against two microorganisms such as Escherichia coli and Staphylococcus aureus. The organisms were sub-cultured on Nutrient agar and stored in the refrigerator until further use. Briefly, approximately 150 μL of extract solution was poured into the well (of 8 mm in diameter) on Petri dishes with nutrient agar. The inoculated plates were put an incubator (37 °C for 24 h), then the inhibition area surrounding the wells were assessed.
2.3. Physicochemical properties
Some physical properties such as moisture [84], pH, ash [34], crude protein [35], crude fat and crude fiber [36] contents of dried watermelon rinds were analyzed according to standard AOAC methods [37].
2.4. Measurement of residual toxins
2.5. Measurement of heavy metals
The concentration of heavy metals in samples was measured by ICP- OES method [40]. Aluminum, Arsenic, Cadmium, Mercury, Chromium, Iron, Nickel, Lead, Zinc, and Copper were detected. The results obtained by the induced coupled plasma mass spectrometer were compared with the values in food standards and analyzed.
2.6. Preparation of osmotic solutions
Osmotic solutions prepared in this study include water and sucrose with weight concentrations of 50% and 70% sucrose. Temperatures (40 and 60 °C), and sucrose solution concentration (50 and 70 wt %), were considered as osmotic treatments. Watermelon rind pieces were placed in solutions in a ratio of 1:4 (watermelon rind: osmotic solution) for 1–5 h.
The parameters of water loss from watermelon rind (WL) and solids gain (SG) in the pre-treatment stage were measured using equations (1), (2)), respectively [41]:
| (1) |
| (2) |
M0 is the initial mass of samples before the osmotic process, M is the final mass after time t of the osmotic process, S is the dry mass of rinds after time t of the osmotic process, and S0 is the dry mass of fresh rinds.
2.7. Final drying of samples
The pretreated samples were dried by hot air at 60 °C for 8 and 10 h.
2.8. Measurement of water rehydration
To measure the Wr, first samples were dried (Wd). Then, they were immersed in water for 30 min at 50 °C. Afterward, the samples were taken out of water. Their surface water was completely dried and weighed by filter paper. Then the re-hydration capacity was calculated according to equation (3) [41].
| (3) |
2.9. Shrinkage measurement
The apparent volume of the samples (before processing and after drying) was measured by the solvent transfer technique [42]. First, initial volume of the sample was calculated from equation (4) and then, shrinkage percentage was calculated according to equation (5).
| (4) |
| (5) |
V0: initial volume of the sample.
M1: Sample weight (g)
M2: Pycnometer weight (g)
M3: Pycnometer weight of sample and toluene content (g)
: The density of toluene (g/cm3)
V: volume of the sample.
S: Shrinkage percentage.
2.10. Determination of color intensity
To probe the effect of different treatments on color changes of the samples, a computer vision system was applied. The images were captured using a flatbed scanner (HP ScanJet G4010, Hewlett Packard Co., CA, USA) with 600 dpi of resolution and the subsequent settings: highlight 190, shadows 40, and midtones 1 (scanning software HP Precisionscan Pro, Hewlett Packard Co.). The images were saved in JPEG format. To separate the true images from the background segmentation were performed. Then the images were converted into L*a*b* units [82]. In the L*a*b* space, the color perception is uniform, and therefore, the Euclidean distance between two colors is almost in agreement with the color difference perceived by the human eye. The net color difference (ΔE) was calculated with equation (6) [42].
| (6) |
Subscripts 1 and 2 are referred to as color components before and after treatments. Frying, respectively. In this study, the image analysis was managed using ImageJ software (National Institutes Health, Bethesda, Md, USA) version 1.43r.
2.11. Watermelon rind powder
The white layer of watermelon rind was cut into small portions and dried in front of a hot air oven at 60 °C for 24 h. The dehydrated rind was powdered in a mixer and sieved to a fine powder (100 mesh). Extraction of watermelon rind was carried out by use of an orbital shaker by adding 10 g watermelon rind powder in a flask containing 100 mL 80% aqueous ethanol at room temperature (25 ± 2 °C) at 200 rpm for 24 h and filtered through whatman's filter paper no.1. The extract was collected and kept at 4 °C.
2.12. Determination of total phenolic content (TPC)
Total phenolic content (TPC) was determined according to Folin–Ciocalteu method [43]. In this assay, 0.25 mL of the extract of watermelon rind powder and also the extract of watermelon rind candy were blended with 1.25 mL of Folin-Ciocalteu reagent and put to incubation at room temperature. After 5 min, 5 mL of sodium carbonate (20% w/v) was put into this mixture, and the solution was allowed to keep for 15 min at room temperature. Absorbance was recorded at 760 nm via UV–VIS spectrophotometer (JENWAY 7313, UK). TPC in the watermelon rind extract samples was reported in terms of gallic acid equivalents (mg GAE L−1).
2.13. Flavonoid content determination
Total flavonoid content was measured according to the aluminum chloride colorimetric approach. 1 ml of the extracted watermelon rind powder and also the extract of watermelon rind candy were blended with 3 mL of distilled water. Also, 0.1 mL of 5% NaNO2 was added to the above mixture. After 5 min, 0.1 mL of 10% AlCl3 solution was added and let to stand for 6 min. Then, 2 mL of 1 M NaOH was added; it was kept at ambient temperature for 30 min. The absorbance of samples was recorded at 510 nm with a UV–VIS spectrophotometer (JENWAY 7313, UK). The amount of flavonoid was calculated as quercetin equivalents from a calibration curve, which was plotted by making the quercetin solutions at concentrations ranging from 10 to 1000 mg mL−1 in ethanol.
2.14. Determination of antioxidant activities
Free radical scavenging activity {1,1-diphenyl-2-picryIhydrazyl radical (DPPH) test} of watermelon rind powder and watermelon rind candy were assessed according to the DPPH method [44]. Briefly, 100 μL of the extract solutions were mixed with 1 mL of 0.1 mM DPPH solution. The reaction mixtures were put in an incubator for 60 min in a dark place at ambient temperature; absorbance was recorded at 517 nm using a spectrophotometer. The DPPH radical scavenging activity was obtained using equation (7).
| (7) |
2.15. Texture analysis
Texture analysis of the samples was performed by a puncture test by a TA. XT plus Texture Analyzer (Stable Micro Systems, UK) coupled with a 30-kg load cell. For this test, the dried watermelon rind pieces were placed on the platform and a 2-mm probe was used to puncture them in the center. Five replications were completed for each sample and the average of obtained data were reported.
2.16. Sensory evaluation
The sensory was determined using a 9-point hedonic scale to evaluate sensory acceptability by panelists. Sensory factors of watermelon rind candy including color, smell, taste, texture, and total acceptability were evaluated [45].
Twenty employees from Neyshabur University of Medical Sciences were chosen for this study sensory assessment. The panellists' gender ratio was 40% female to 60% male. While the panelists' ages ranged from 21 to 30 years old, 31–40 years old, and over 50 years old.
After obtaining informed consent from the panelists, they had all received the recipes in advance and were all non-smokers. They rated the items from 1 to 9 based on their evaluations of taste, smell, color, texture, sweetness, and general attractiveness.
This experiment was approved by the Ethics Committee of Neyshabur University of Medical Sciences, Neyshabur, Iran (approval number: IR. NUMS.REC.1399.027).
2.17. Statistical analysis
Experimental data were analyzed using the analysis of SPSS statistical software (Chicago, IL, USA), and the values were reported as means ± standard deviation (SD). The significant differences among means were determined by the Duncan Multiple Range Test (DMRT) to express the significant differences among the mean values at the 0.05 level.
3. Results and discussion
3.1. Antibacterial activity
The antibacterial activity of watermelon rind extracts as indicated by zones of inhibition against Staphylococcus aureus (10.84 ± 0.19 mm), Bacillus cereus (11.38 ± 0.26), and E. coli (13.64 ± 0.21 mm) were determined. The larger zone of inhibition belongs to E. coli (>13 mm). Thus, the data exhibited that watermelon rind extract has higher antimicrobial efficacy on the growth of E. coli followed by B. cereus and S. aureus. Recently, Neglo et al. [46], reported that the rind had no inhibitory activity against Enterococcus faecalis, and S. typhi, E. coli. However, the antimicrobial activity of the rind has been observed against Staphylococcus aureus, Pseudomonas fluorescens, Bacillus subtilis, Micrococcus luteus Listeria innocua, Streptococcus thermophilus, Shigella sonnei, Salmonella enterica, and Klebsiella oxytoca microbes. The varying efficacies in the growth of various microorganisms could be due to phytochemical constituents such as flavonoids or phenolics. Also, alkaloids are identified as beneficial components of watermelon rind which can be responsible for an antimicrobial effect [5].
3.2. Physicochemical properties of dried watermelon rind
Chemical properties, including the contents of carbohydrate, protein, moisture, ash, fiber, and fat in the dried watermelon rind, were determined to be 48.61 ± 0.02, 14.45 ± 0.11, 14.36 ± 0.03, 12.39 ± 0.05, 9.3 ± 0.12, 0.89 ± 0.04 (%). In the study of Al-Sayed and Ahmed, 2013, close results have been obtained that reveal watermelon rind powder contains a considerable amount of moisture, fat, protein, ash, and carbohydrates: 10.61%, 2.44%, 11.17% 13.09%, and 56% [47].
3.2.1. pH and acidity
pH and acidity were measured in samples with the minimum and maximum immersion period in an osmotic solution (immersion times of 1 and 5 h). According to Table 1, temperature, osmotic solution concentration, and immersion time had no significant effect on the pH and acidity index of osmotic samples.
Table 1.
Changes in acidity and pH.
| Code | pH | Acidity |
|---|---|---|
| a11 | 6.19 ± 0.03a | 0.0288 ± 0.02a |
| a15 | 6.34 ± 0.01a | 0.0131 ± 0.01a |
| a21 | 6.33 ± 0.01a | 0.032 ± 0.05a |
| a25 | 6.34 ± 0.01a | 0.0192 ± 0.02a |
| b11 | 6.29 ± 0.04a | 0.0192 ± 0.05a |
| b15 | 6.24 ± 0.03a | 0.0125 ± 0.04a |
| b21 | 6.12 ± 0.02a | 0.0122 ± 0.03a |
| b25 | 6.33 ± 0.03a | 0.0193 ± 0.03a |
(a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70).
3.3. Proximate composition
3.3.1. Moisture content
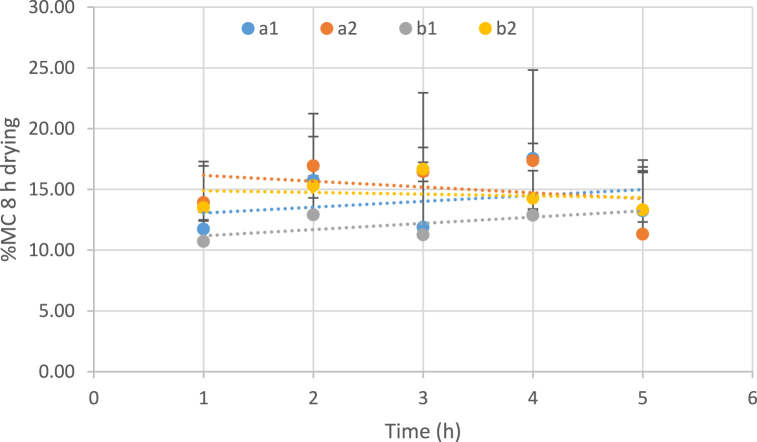
Drying is known as a necessary process in the development of dehydrated fruit snacks. The standard level of moisture in dehydrated fruit candy ranged from 12 to 21% [12]. Thus, the fruit candy can be stored for a long time and also be preserved from deterioration caused by different microorganisms. Fig. 1 shows the effect of temperature, the concentration of the osmotic solution, and immersion duration on the moisture content of the dried samples for 8 h.
Fig. 1.
Influence of temperatures, osmotic solution concentration and immersion time period on moisture content of the dried sample (8 h drying). (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70).
The effects of 50 and 70% concentrations of osmotic solution on the amount of moisture in dried products are different. Higher concentrations of osmotic solution (70%) cause a downward trend in the amount of moisture based on dry weight, and lower concentrations of osmotic solution (50%) cause an upward trend on this parameter. Osmotic drying at 50% concentration showed that increasing the temperature of the osmotic dehydration has a decreasing impact on the moisture content of the samples. However, in samples exposed to a 70% osmotic solution, temperature changes had almost no significant effect on the final moisture content (Fig. 1).
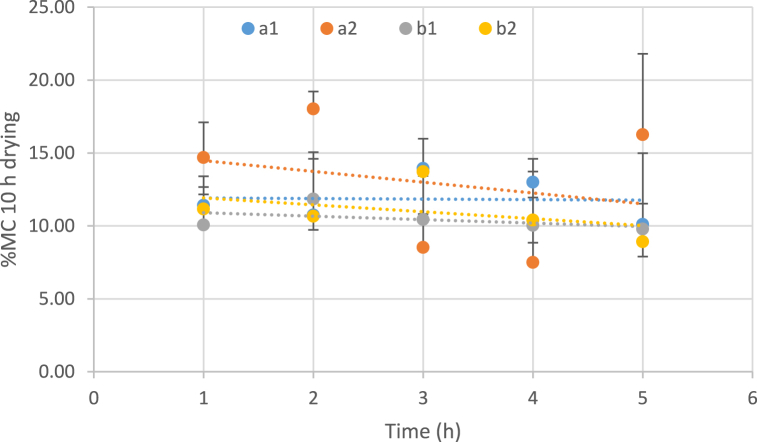
Hani Mohammad et al. (2015) indicated that the drying duration of dehydrated watermelon rind using the osmotic dehydration method (for 8, 14, and 20 h, at 50 °C) had a significant effect on decreasing the moisture content of the watermelon rind [45].
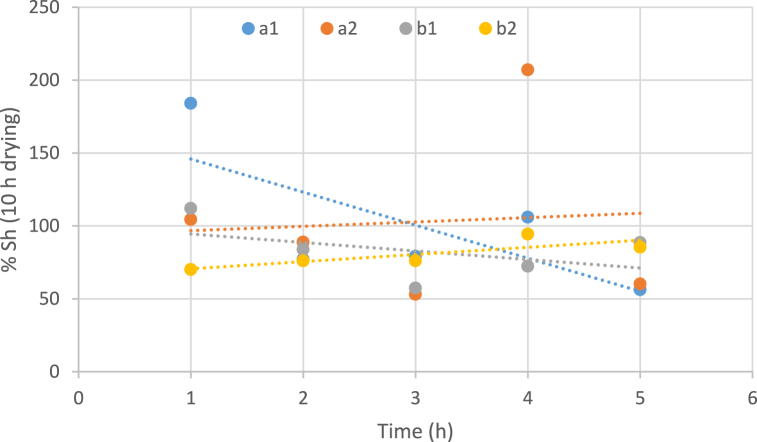
Increasing the immersion time of samples in osmotic solutions leads to a considerable reduction in the moisture content of the samples. According to this diagram, generally the higher temperature of the osmotic process causes a lower level of moisture content in the sweet snacks obtained from white watermelon (Fig. 2). Generally, the findings also indicated that the moisture content through the drying process dropped with time, which was in agreement with the nature of the drying features of different vegetables and fruits [48].
Fig. 2.
Influence of temperatures, concentration of osmotic solution and immersion duration on the moisture content (10 h drying). (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70).
3.3.2. Residual toxins
In watermelon skin samples, organochlorine and organophosphate toxins were assessed. The results of this test showed that the amount of none of the toxins was in the detectable range.
3.3.3. Heavy metals
The amounts of heavy metals (mg/l) were determined in the watermelon rind before dehydration. The heavy metals in the peel, including Ni > As > Pb > Hg > Cd were shown in Table 2 Heavy metals such as Cd, As, Hg, and Pb are highly toxic at high levels and biologically unnecessary even at low levels [49].
Table 2.
The heavy metal content in the raw sample of white watermelon rind.
| Metal | Quantity (mg/l) |
|---|---|
| Pb | 0.069> |
| Ni | 9.239 |
| As | 1.011 |
| Hg | <0.146 |
| Cd | <0.003 |
Among the identified metals, Ni showed the highest content (9.239 mg/l) and Cd was the lowest one in the watermelon rind. However, the concentration of Ni was lower than WHO (World Health Organization) safe limit (WHO limit in fruits = 10 ppm) [50].
3.3.4. Influence of temperature, concentration of the osmotic solution, and immersion duration on water loss
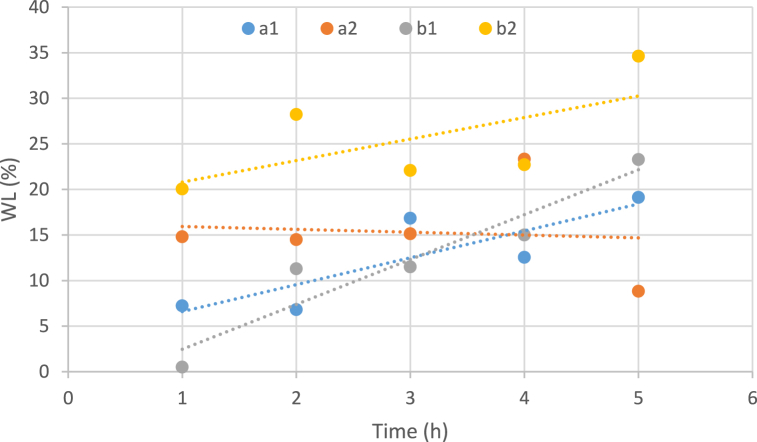
Rising temperatures increased the intensity of dehydration. The main reason behind this is the effect of increasing temperature on the permeability of the membrane and more water leaving the tissue into the osmotic solution. Increasing the temperature increases the osmotic potential and permeability of the sample membrane, due to the destructive effect on the cell wall of white watermelon rind samples. Regarding to the slope of the samples at 50% dilute concentration, a higher temperature (60 °C) has a stronger effect in relation to water loss (Fig. 3).
Fig. 3.
Influence of temperature variables, concentration of osmotic solution and immersion duration on water loss (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70).
Also, cross-examination of temperature and dilute concentration showed that increasing the temperature in both osmotic samples in concentrated solution (70%) and in osmotic samples in dilute solution (50%) increases the dehydration intensity.
Generally, the temperature and sucrose concentration parameters both exerted a noticeable effect (P ≤ 0.05) on the water loss response. Increasing the concentration of the osmotic solution increases the osmotic pressure between the sample and the osmotic solution. Therefore, the osmotic action increases, and the water loss of the sample increases. Increasing the concentration of the osmotic solution, separately, has a considerable increasing effect on the dehydration intensity of the sample tissue. On the other hand, the effect of increasing sucrose concentration on water loss response was greater than that of increasing temperatures. Higher solution concentrations led to an enhancement in the osmotic pressure gradients and greater water loss occurred [51]. Furthermore, the osmotic drying process with a longer period will change the absorption features of the cell wall and cause more WL [52].
Studies on drying strawberry leaves with osmotic dehydration by Kalbasi et al. have shown that increasing the immersion time, calcium chloride and the amount of fructose syrup relative to sucrose and the treatment temperature of strawberry samples increase the amount of water leaving the tissue [53].
According to Uddin et al. (2004) study results presented that immersion period and sucrose concentration were the most effective factors in causing water loss in osmotically dehydrated carrots. It has been observed that vacuum osmotic dehydration was strongly effective in improving mass transfer over the osmotic dehydration of variety of fruits, such as cranberries [40], mangos [54], and papayas [55].
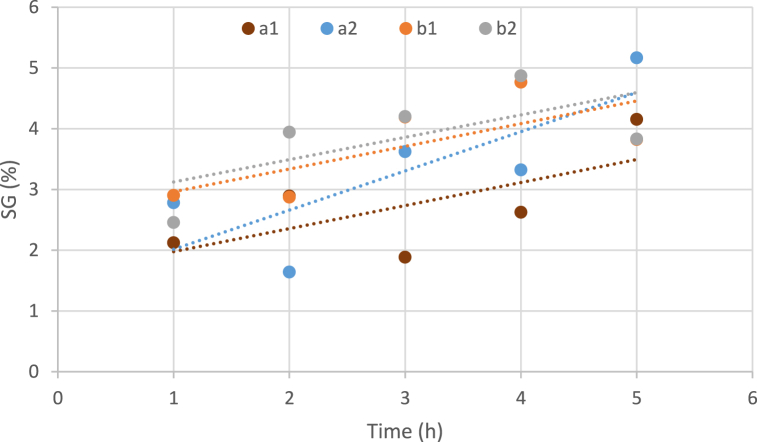
3.3.5. Influence of temperature, the concentration of osmotic solution, and immersion duration on solid gain
Fig. 4 shows the effects of different temperatures and sucrose concentrations of the solution on the SG caused by osmotic dehydration of the watermelon rind slices. The increase in the concentration of osmotic solution caused an increase in the absorption of solids, which is due to increased osmotic pressure and swelling of watermelon white rind cell membranes. The osmotic solution and drying temperature significantly affected the solid gain. The osmotic solution and drying temperature significantly affected the samples. Osmotic dehydration is one of the major pre-treatments utilized before the drying process that eliminates water from cellular solids [56,57]. A mutual study of temperature and concentration of osmotic solution indicates that at a concentration of 70% in osmotic samples, the increase in temperature shows no significant change in the absorption of solids, and in both graphs, this trend is upward. Pavcov et al. (2009) and Kaltsa et al. (2011) obtained similar results on apricot and pomegranate samples, which were due to the long exposure of the samples to the osmotic solution [58,59]. Additionally, Hung chia et al. (2018) prepared watermelon rind via vacuum osmotic dehydration and atmospheric pressure. Water loss and solid gain of watermelon rind increased by enhancing syrup concentration, temperature, and immersion period for both vacuum osmotic dehydration and atmospheric pressure, however vacuum osmotic dehydration significantly enhanced the mass transfer rate (11). The results of current research by Stavropoulou et al. (2022) that evaluated the effect of osmotic dehydration on the qualitative properties of button mushroom (Agaricus bisporus) which dried in hot air”, displayed that by increasing the concentration of NaCl solution from 5% to 10%, the amount of dehydration and solids uptake enhanced considerably. Also, demonstrated that the osmotic pretreatment can significantly influence mass transfer and improve the quality [60] properties of button mushrooms before traditional freezing. Regarding synergistic impacts, the interactions between osmotic concentrations and temperature were realized to have a significant positive effect on SG.
Fig. 4.
Influence of temperature variables, concentration of osmotic solution and immersion duration on solid gain (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70).
In osmotic samples with a dilute solution (50%), the temperature rising causes an increase in dry matter. But in this case, the slope of the two graphs and the amount and intensity of this increase are almost equal. Similar results in the increase of WL and SG have been also reported in research on green figs [61] and banana slices [62].
The results of Dermesonlouoglou et al. [63], Lazou et al. [64], Gabriela et al. [48], Amiryousefi and Mohebbi [65] researches on the evaluation of SG and WL factors during the osmotic dehydration process of pumpkin, tomato, mango and potato products, confirm the results of this paper.
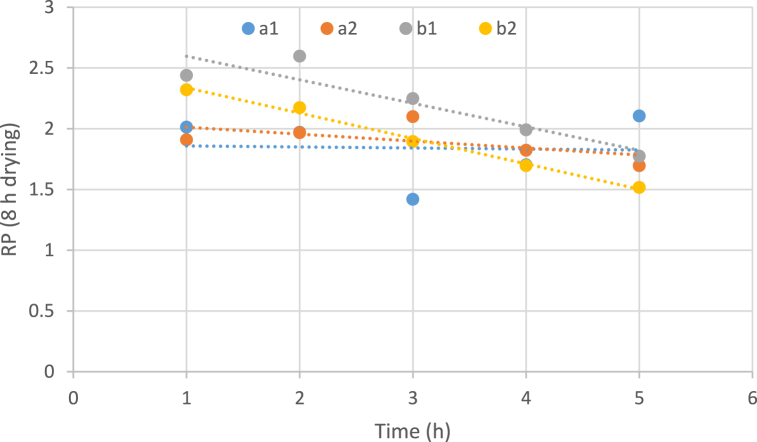
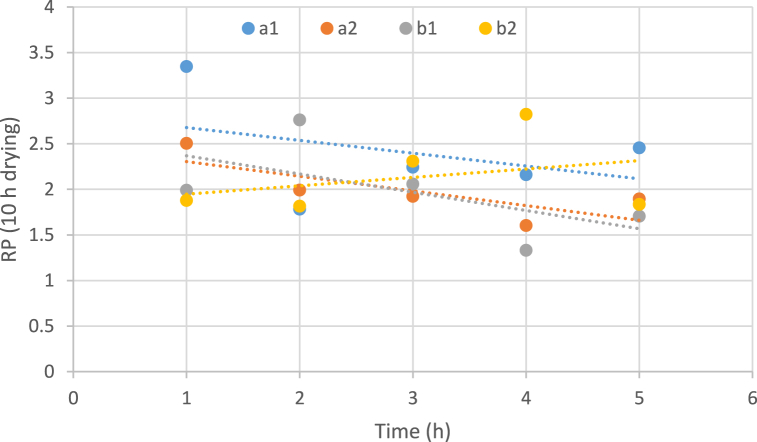
3.3.6. The effect of temperature, the concentration of osmotic solution, and immersion duration on water rehydration
In Fig. 5, Fig. 6 increasing the immersion time of the sample in an osmotic solution reduces the rehydration capacity of water in all samples. At higher temperatures (60 °C), this downward trend occurs with a greater slope. Although the samples had more water rehydration capacity in the early hours at this particular temperature (60 °C) than at low temperatures, in the last hours of condensation, the water rehydration capacity of the samples significantly decreased. Similar results in the case of rehydration of osmotically pretreated and dehydrated carrots were also revealed by Rastogi et al. (2004). In a study conducted by Shekar & Javadi. (2019). ultrasound-assisted osmotic dehydration pretreatment led to a decrease in rehydration ratio of the dried apple slices. The main reason for this decrease is the filling of the empty pores of the sample tissue with an osmotic solvent [66,67].
Fig. 5.
Impact of temperature, concentration of osmotic solution and immersion duration on water rehydration of dried samples (8 h drying). (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70).
Fig. 6.
Impact of temperature, concentration of osmotic solution and immersion duration on water rehydration of dried samples (10 h drying). (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70).
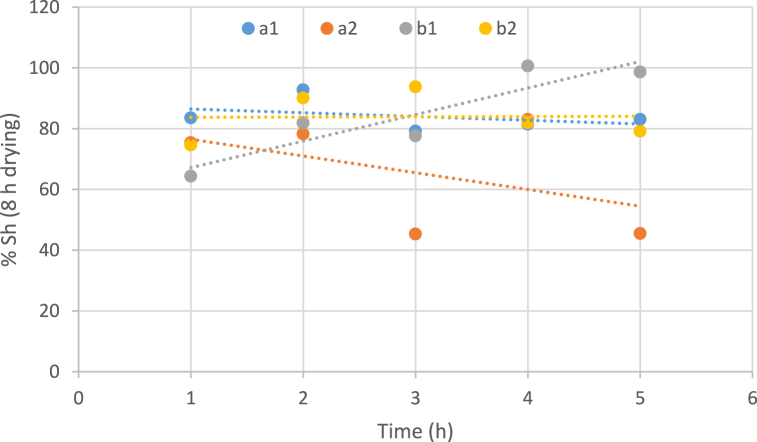
3.3.7. The effect of temperature, the concentration of osmotic solution and immersion duration on shrinkage
According to Fig. 7, Fig. 8, the effect of temperature variables, concentration of the osmotic solution, and immersion period on shrinkage has been determined. It seems that the changes in these variables do not have a significant effect on the process of shrinkage. In most of the samples, the variables change ineffectively or cause a decrease in shrinkage, especially at a temperature of 40 °C.
Fig. 7.
Impact of temperature, concentration of osmotic solution and immersion duration on shrinkage of dried samples (8 h drying). (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70).
Fig. 8.
Impact of temperature, concentration of osmotic solution and immersion duration on shrinkage of dried samples (10 h drying). (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70).
But unlike the obtained results of other samples, in the graph of the sample which was exposed to a solution with a lower concentration and a higher temperature (concentration 50% and temperature 60 °C) during the osmosis process and then in a hot air dryer for 8 h, a significant increase in the percentage of shrinkage was observed from the beginning to the end of the osmosis process (Fig. 7). Shrinkage causes water absorption more difficult and at a slower rate. By comparing the shrinkage diagram of this specific sample with the WL diagram, it showed that the highest intensity of dehydration was reported for this sample, too. Therefore, the high intensity of dehydration can be a good reason for increased shrinkage.
The osmosis process does not have a significant impact on the amount of shrinkage of dried samples of white watermelon skin; however, the amount of reabsorption of water in these samples was lower than that in the samples without pretreatment, which is due to the absorption of solids from the osmotic solution by the tissue of the sample during the osmotic process. The shrinkage of dried fruits could be associated with glass transition and water status [68]. Similar behavior has been observed by Maskan M. (2001) on the drying of kiwis through microwave and hot air drying [69]. However, Pavkov et al., 2021 demonstrated that during the hot air drying process, osmotic dehydration lowered the effective diffusivity of water. Also, the shrinking of apricot halves during the hot air drying process was reduced as a result [70].
3.3.8. Determination of color intensity
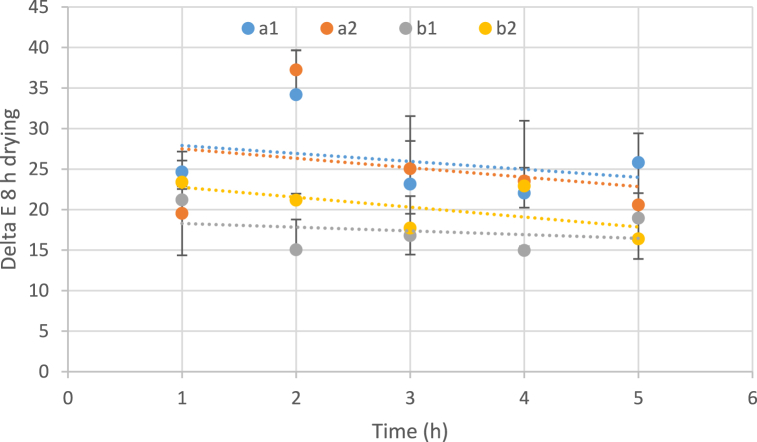
The color intensity of the dehydrated watermelon rind was determined by image processing based on the L*a*b* color system. The results showed that increasing the temperature during drying had no significant effect on the color intensity of the samples. However, higher osmotic concentrations showed a considerable increase in the L* value. Also, the L* value for dehydrated watermelon rind samples increased significantly after the osmotic process [81]. The b* values for dehydrated watermelon rinds were slightly increased with increasing temperature. The samples were exposed to a hot air dryer for 8 h, color changes are less at early hours; therefore, the samples that had been exposed to the osmosis process for a long time showed a reduction in color intensity (Fig. 9). The color changes of samples can be due to non-enzymatic browning reactions, as well as the development of brown pigments [69,71,72]. Kresic et al. (2004) reported that condensation of reducing sugars with pigments and amino acid transformation cause non-enyzmatic browning, which is responsible for the darkening of fruit tissue over the drying process [73]. In addition, obtained data showed that all samples that have been exposed to hot air drying for a longer time (10 h) are accompanied by an increase in color. It can be concluded that the increase in the time of the thermal process led to signs of burning along with an increase in color.
Fig. 9.
Influence of temperatures, concentration of osmotic solution and immersion duration on the colour intensity (8 h drying). (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70)
The impact of osmotic dehydration pre-treatments in sucrose solutions on dried Kosia pear slices were evaluated by Taşova et al., 2022, which showed the least color change was identified in control samples. They retained their color values better than treated samples [74].
3.3.9. Total phenolic, flavonoid compounds and antioxidant activity of watermelon rind powder, and its content during the osmotic process
The health-promoting phytochemicals including total phenolic content (TPC), total flavonoid compounds, and antioxidant activities in watermelon rind candy during the osmosis process were determined, and the obtained results are shown in Table 3. Measurements of osmotically dehydrated watermelon rind were carried out at two different temperatures (40 and 60 °C) and sucrose concentrations (50 and 70%) after immersion times of 1 and 5 h.
Table 3.
Total phenolic, flavonoid compounds and antioxidant activity of dried watermelon rind during the osmosis process after 10 h drying.
| Code | TPC (mg GAE/100 g) | Flavonoid (mg QUE/100 g) | Antioxidant activity (%) |
|---|---|---|---|
| a15 | 35.83 ± 0.13c | 26.60 ± 0.12d | 61 ± 0.05d |
| a25 | 27.45 ± 0.04b | 18.82 ± 0.06b | 49 ± 0.01b |
| b15 | 30.25 ± 0.09a | 23.45 ± 0.01c | 54 ± 0.12c |
| b25 | 22.94 ± 0.11a | 16.94 ± 0.13a | 40 ± 0.16a |
(a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70, immersion times of 1 and 5 h).
The phenolic compounds of plant-based products have been related to antioxidant potential [75]. From the obtained data, TPC in watermelon rind powder was found at 209.85 mg/100 g and the total flavonoid was 260.95 mg/100 g. Also, the antioxidant activity of watermelon rind powder was evaluated at about 72%. It can be observed from Table 3 that TPC in watermelon rind candy with different osmotic solution concentrations decreased from 35.83 mg/100 g at 50% concentration to 27.45 mg/100 g at 70% concentration of osmotic solution at 40 °C, and from 30.25 mg/100 g at 50% concentration to 22.94 mg/100 g at 70% concentration at 60 °C, respectively. Also, the total flavonoid content decreased from 26.60 to 18.82 mg/100 g at 50% concentration (at 40 °C), and from 23.45 to 16.94 mg/100 g at 70% concentration (at 60 °C). Flavonoid content was evaluated by Johnson et al. [76] and results displayed that the flavonoid content of the watermelon rind (8.71 ± 0.01 mg/100 g) decreased by drying to 2.63 ± 0.02 mg/100 g.
The antioxidantactivity was also assessed via the DPPH assays. The DPPH test has been commonly used for antioxidant activity assessment because this method is sensitive enough to identify bioactive components even at low concentrations [77]. The DPPH radical scavenging potential of raw watermelon rind was reported at 34.48 ± 1.63% and the phenolic content was found at 0.026 ± 0.006 mgGAE/g [46]. In the present study, antioxidant activity after the osmotic process decreased significantly, from 61% to 40% at 50% concentration (40 °C), and from 54% to 40% at 70% concentration (60 °C). Among different concentrations, 50% at 40 °C exhibited the strongest antioxidant activity (Table 3). These results revealed that higher phenolic contents exhibited higher scavenging activity. However, slight decreases in the TPC, TFC, and DPPH assays may be attributed to high temperatures during the drying process. On the other hand, increasing the concentration of the osmotic solutions leads to a significant decrease in the TPC and flavonoid content as well as the antioxidant activity of the snacks. Additionally, different concentrations and temperatures have been revealed to influence the amount of bioactive compounds which exert antioxidant properties. Generally, reduction in phenolic content has been associated with heat treatments which lead to the solubility change or the chemical reactivity of polyphenols [76,78]. The watermelon rind is a good source of natural antioxidants and the results of this study showed that a considerable amount of antioxidants can be preserved after the osmotic and drying process.
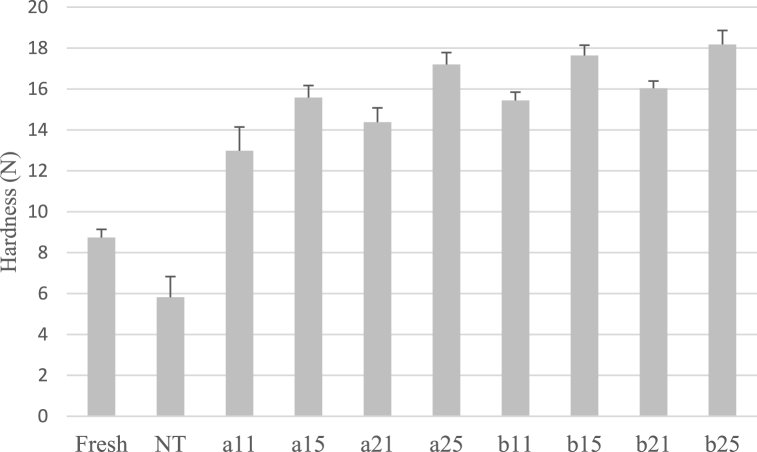
3.4. Texture analysis
Tenderness shows the amount of force that is needed to press the sample. Thus, the higher amount of this value indicates a quite firmer texture [79]. The obtained results show that the hardness was significantly (p < 0.05) influenced by temperature, osmotic duration time, and concentration of the osmotic solution (Fig. 10). Among all samples, the sample of b25 (dried at 60 °C and 70% concentration) demonstrated a significant (p < 0.05) the highest hardness (18.18 N). On the other hand, increasing the immersion time, temperature, and sugar concentration of the samples noticeably enhances the value of hardness in all samples. It is associated with the change in the cell membrane of tissue structure over-drying [79]. Moreover, osmotic dehydration can enhance the textural quality of dried fruits and lessen structural collapse during further drying. Rahaman et al. (2019) noted an increase in the chewiness of the osmotic dehydration-pretreated plum, which may be attributed to sucrose adhering to the fruit's surface [80].
Fig. 10.
Impact of temperature, concentration of osmotic solution and immersion duration on tenderness of dried samples (NT: non-treated dried watermelon rind, (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70, immersion times of 1 and 5 h).
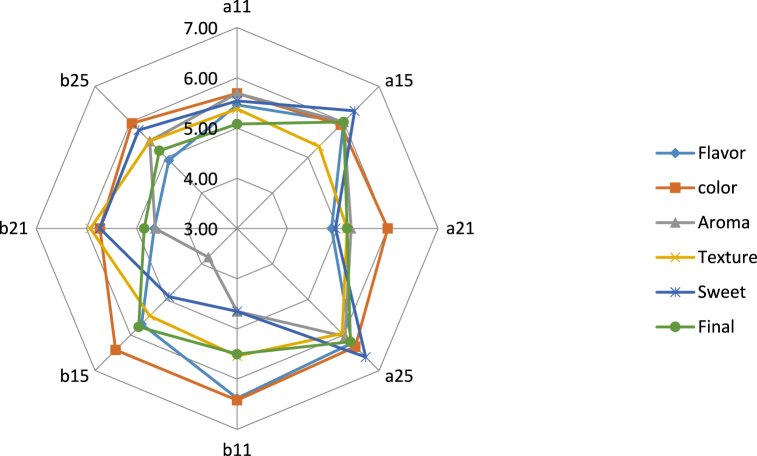
3.5. Sensory evaluation results
The results of the sensory evaluation analysis are illustrated in Fig. 11. Analysis of variance has been performed for color, flavor, sweetness, aroma, texture, and overall acceptability and the findings revealed that there is a significant difference (P < 0.05) among the samples. The results indicated that there were no significant differences in color and texture scores among all the samples. For the sweet attribute, there were significant differences in the sample dried at 60 °C, at 50% concentration, for 1 h and the sample dried at 40 °C, at 70%, for 5 h. For final acceptance, the sample (osmosis temperature: 40 °C, osmotic solution concentration: 70%, immersion duration: 3 h) received the highest score (6.5) compared to the sample that dried at 70% and 60 °C for 1 h (4.85). In addition, the sample (osmosis temperature: 40 °C, osmotic solution concentration: 70%, immersion duration: 3 h) is the most preferred sample in terms of texture taste parameters and general acceptance, and the sample (osmosis temperature: 40 °C, osmotic solution concentration: 70%, immersion duration: 5 h) was selected as the best sample in terms of odor, texture, and sweetness parameters. A study by Hani Mohammad et al. reported that osmotically dehydrated watermelon rind candy (dried for 14 h, at 50 °C) was the most pleasant sample for the panelists due to its high score for the sensory evaluations such as taste, texture and, overall acceptability [45].
Fig. 11.
Sensory evaluation of watermelon rind candy (a: temperature 40 °C, b: temperature 60 °C, 1: Sugar concentration 50 and 2: Sugar concentration 70, immersion times of 1 and 5 h).
In this study, we could investigate other likely effective variables, including various temperatures, concentration of osmotic solution and immersion time. Therefore, it is suggested to check these variables by other researchers. In addition, the other possible conversion strategies of food waste can be noted in future investigations. Also, osmotic pretreatment coupled with various techniques such as ultrasound, high hydrostatic pressure, and so on can be investigated in the future.
4. Conclusion
Watermelon white rind, which was once considered agricultural waste or a waste of processing units, can now be introduced as a pragmatic food product after processing. The osmotic dehydration technique is an effective method in turning the watermelon rind waste into the more sustainable product with lower environmental concerns. In this research, watermelon white rind, which is known as watermelon by-product was processed by the osmotic dehydration method and then dried by hot air to obtain candy using watermelon waste processing to prepare a tasty and healthy snack. During this process, the impact of different parameters of the osmotic process (osmotic process temperature, osmotic solution concentration, osmotic process time and hot air drying time) on the characteristics of watermelon white rind was investigated. Increasing the temperature of the osmotic process increases the rate of dehydration. Increases in the osmotic solution concentration and immersion duration cause an increase in the absorption of solids. The results of this study showed that the osmosis process had a slight effect on the wrinkling rate of dried watermelon white rinds.
Author contribution statement
Alieh Rezagholizade-shirvan: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Samira Shokri: Analyzed and interpreted the data; Wrote the paper.
Seyede Mahsa Dadpour: Analyzed and interpreted the data; Wrote the paper.
Mohamad Reza Amiryousefi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully acknowledge the support of the Neyshabur University of Medical Sciences for funding this project (project number 98-01-120). The authors would like to thank all those who helped us during this project especially Ms. Faezeh Mokhtari Mobarake and Ms. Maryam Eskandari.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e17300.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Huerta-Reyes M., Tavera-Hernández R., Alvarado-Sansininea J.J., Jiménez-Estrada M. Selected species of the cucurbitaceae family used in Mexico for the treatment of diabetes mellitus. Molecules. 2022;27:3440. doi: 10.3390/molecules27113440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.F.C.A . 2019. o.h.w.f.o.f.e.d.Q.v.a.o.M. [Google Scholar]
- 3.FAO. Food and Agriculture Organization of the United Nations, Rome FAOSTAT: core production data. 2014. http://faostat.fao.org/site/340/default.aspx
- 4.Gladvin G., Sudhaakr G., Swathi V., Santhisri K. Mineral and vitamin compositions contents in watermelon peel (Rind) International Journal of Current Microbiology and Applied Sciences. 2017;5:129–133. [Google Scholar]
- 5.Tabiri B., Agbenorhevi J.K., Wireko-Manu F.D., Ompouma E.I. Watermelon seeds as food: nutrient composition, phytochemicals and antioxidant activity. Int. J. Nutr. Food Sci. 2016;5:139–144. [Google Scholar]
- 6.Vinhas A.S., Silva C.S., Matos C., Moutinho C., Ferreira da Vinha A. Valorization of watermelon fruit (Citrullus lanatus) byproducts: phytochemical and biofunctional properties with emphasis on recent trends and advances. World Journal of Advance Healthcare Research. 2021;5:302–309. [Google Scholar]
- 7.Burton-Freeman B., Freeman M., Zhang X., Sandhu A., Edirisinghe I. Watermelon and L-citrulline in cardio-metabolic health: review of the evidence 2000–2020. Curr. Atherosclerosis Rep. 2021;23:1–24. doi: 10.1007/s11883-021-00978-5. [DOI] [PubMed] [Google Scholar]
- 8.Dokhani N., Nazer M., Skokri S., Darvishi M. Determination and evaluating the antioxidant properties of ziziphus nummularia (burm. F.) wight & arn., crataegus pontica K. Koch and scrophularia striata boiss. Egypt. J. Vet. Sci. 2022;53:423–429. [Google Scholar]
- 9.Falahi E., Delshadian Z., Ahmadvand H., Shokri Jokar S. Head space volatile constituents and antioxidant properties of five traditional Iranian wild edible plants grown in west of Iran. AIMS Agriculture and Food. 2019;4(4):1034–1053. doi: 10.3934/agrfood.2019.4.1034. [DOI] [Google Scholar]
- 10.Bahmani M., Shokri S., Nosrati Akhtar Z., Abbaszadeh S., Manouchehri A. The effect of pomegranate seed oil on human health, especially epidemiology of polycystic ovary syndrome; a systematic review. JBRA Assist Reprod. 2022 doi: 10.5935/1518-0557.20210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhoshani N.M., Al-Johani N.S., Alkeraishan N., Alarifi S., Alkahtani S. Effect of lycopene as an adjuvant therapy with 5-florouracil in human colon cancer. Saudi J. Biol. Sci. 2022;29 doi: 10.1016/j.sjbs.2022.103392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hani M.N.F., Zahidah W.W.N., Saniah K., Irwani H.M. Effects of drying on the physical characteristics of dehydrated watermelon rind candies. J. Trop. Agric. Food Sci. 2014;42:115–123. [Google Scholar]
- 13.Ilic D., Forbes K.M., Hassed C. 2011. Lycopene for the Prevention of Prostate Cancer, Cochrane Database of Systematic Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamuz S., Munekata P.E., Gullón B., Rocchetti G., Montesano D., Lorenzo J.M. Citrullus lanatus as source of bioactive components: an up-to-date review. Trends Food Sci. Technol. 2021;111:208–222. [Google Scholar]
- 15.Sewald M., DeVries J. 2015. Food product shelf life. [Google Scholar]
- 16.Liu D., Yang H., Yuan Y., Zhu H., Zhang M., Wei X., Sun D., Wang X., Yang S., Yang L. Comparative transcriptome analysis provides insights into yellow rind formation and preliminary mapping of the Clyr (Yellow Rind) gene in watermelon. Front. Plant Sci. 2020;11:192. doi: 10.3389/fpls.2020.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandel H.L., Bkovitchs N., Korman S.H. Elevated plasma citrulline and arginine due to consumption of watermelon (Citrullus vulgaris) Ber. Dtsch. Chem. Ges. 2005;28:467–472. doi: 10.1007/s10545-005-0467-1. [DOI] [PubMed] [Google Scholar]
- 18.Milala M., Luther A., Burah B. Nutritional comparison of processed and unprocessed Citrillus lanatus (watermelon) seeds for possible use in feed formulation. American Journal of Food and Nutrition. 2018;6:33–36. [Google Scholar]
- 19.Dane F., Liu J. Diversity and origin of cultivated and citron type watermelon (Citrullus lanatus) Genet. Resour. Crop Evol. 2007;54:1255–1265. [Google Scholar]
- 20.Collins J.K., Wu G., Perkins-Veazie P., Spears K., Claypool P.L., Baker R.A., Clevidence B.A. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition. 2007;23:261–266. doi: 10.1016/j.nut.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Janabi M., Rad A., Esteri S. Investigating the effect of different levels of pectin on tissue profile, color and sensory evaluation of watermelon marmalade. Journal of Innovation in Food Science and Technology. 2017;9 [Google Scholar]
- 22.Oseni O.A., Okoye V.I. Studies of phytochemical and antioxidant properties of the fruit of watermelon (Citrullus lanatus). (Thunb.) J. Pharmaceut. Biomed. Sci. 2013;27:508–514. [Google Scholar]
- 23.Hartati I., Subekti E. Microwave assisted extraction of watermelon rind pectin. Int. J. ChemTech Res. 2015;8:163–170. [Google Scholar]
- 24.Dranca F., Oroian M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018;113:327–350. doi: 10.1016/j.foodres.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 25.Gomez B., Gullon B., Remoroza C., Schols H.A., Parajo J.C., Alonso J.L. Purification, characterization, and prebiotic properties of pectic oligosaccharides from orange peel wastes. J. Agric. Food Chem. 2014;62:9769–9782. doi: 10.1021/jf503475b. [DOI] [PubMed] [Google Scholar]
- 26.Athmaselvi K., Alagusundaram K., Kavitha C., Arumuganathan T. Impact of pretreatment on colour and texture of watermelon rind. Int. Agrophys. 2012;26 [Google Scholar]
- 27.Hoque M.M., Iqbal A. Drying of watermelon rind and development of cakes from rind powder. International journal of novel research in life sciences. 2015;2:14–21. [Google Scholar]
- 28.Naknaen P., Itthisoponkul T., Sondee A., Angsombat N. Utilization of watermelon rind waste as a potential source of dietary fiber to improve health promoting properties and reduce glycemic index for cookie making. Food Sci. Biotechnol. 2016;25:415–424. doi: 10.1007/s10068-016-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma G., Prasad S. Optimization of process parameters for microwave drying of garlic cloves. J. Food Eng. 2006;75:441–446. [Google Scholar]
- 30.Liu Y., Zeng Y., Wang Q., Sun C., Xi H. Drying characteristics, microstructure, glass transition temperature, and quality of ultrasound‐strengthened hot air drying on pear slices. J. Food Process. Preserv. 2019;43 [Google Scholar]
- 31.Akbarian M., Ghasemkhani N., Moayedi F. Osmotic dehydration of fruits in food industrial: a review. Int. J. Biosci. 2014;4:42–57. [Google Scholar]
- 32.Yadav A.K., Singh S.V. Osmotic dehydration of fruits and vegetables: a review. J. Food Sci. Technol. 2014;51:1654–1673. doi: 10.1007/s13197-012-0659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Institute of Standards . 2008. Industrial Research of Iran Cereals, Pulses and by products - Determination of Ash Yeild by Incineration. [Google Scholar]
- 35.Institute of Standards and Industrial Research of Iran . 2014. Cereals and Pulses – Determination of the Nitrogen Content and Calculation of the Crude Protein Content – Kjeldahl Method. [Google Scholar]
- 36.Institute of Standards and Industrial Research of Iran . 2009. Agricultural Food Products – Determination of Crude Fibre Contents – General Method. [Google Scholar]
- 37.Institute of Standards and Industrial Research of Iran Cereals . 2007. Fruit Juices – Test Methods. [Google Scholar]
- 38.Chandra S., Mahindrakar A.N., Shinde L.P. Gas chromatography- mass spectrometry determination of pesticide residue in fruits. Int. J. ChemTech Res. 2014;6(1):124–130. [Google Scholar]
- 39.Shokrzadeh M., Karami M., Jafari Valoujaei M., Zamani Renani A. Measuring diazinon residue in thampson orange. Journal of Mazandaran University of Medical Sciences. 2013;23:91–99. [Google Scholar]
- 40.Wray D., Ramaswamy H.S. Microwave‐osmotic/microwave‐vacuum drying of whole cranberries: comparison with other methods. J. Food Sci. 2015;80:E2792–E2802. doi: 10.1111/1750-3841.13132. [DOI] [PubMed] [Google Scholar]
- 41.Shahidi F., maleki M. Evaluation of increase in turnip phenolic compounds in osmotic solution containing sour tea extract and evaluation of its drying air with hot air. J. Food Sci. Technol. 2018;88(16):231–251. [Google Scholar]
- 42.Amiryousefi M.R., Mohebbi M., Khodaiyan F. Applying an intelligent model and sensitivity analysis to inspect mass transfer kinetics, shrinkage and crust color changes of deep-fat fried ostrich meat cubes. Meat Sci. 2014;96:172–178. doi: 10.1016/j.meatsci.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Rezagholizade-shirvan A., Najafi M.F., Behmadi H., Masrournia M. Preparation of nano-composites based on curcumin/chitosan-PVA-alginate to improve stability, antioxidant, antibacterial and anticancer activity of curcumin. Inorg. Chem. Commun. 2022;145 [Google Scholar]
- 44.Rezagholizade-shirvan A., Masrournia M., Fathi Najafi M., Behmadi H. Synthesis and characterization of nanoparticles based on chitosan-biopolymers systems as nanocarrier agents for curcumin: study on pharmaceutical and environmental applications. Polym. Bull. 2023;80:1495–1517. [Google Scholar]
- 45.Muhamad N.F.H., Zainon W.N.Z.W., Kormin S., Ali M.S. Processing of watermelon rind dehydrated candy. Int. J. Sci. Eng. 2015;8:6–9. [Google Scholar]
- 46.Neglo D., Tettey C.O., Essuman E.K., Kortei N.K., Boakye A.A., Hunkpe G., Amarh F., Kwashie P., Devi W.S. Comparative antioxidant and antimicrobial activities of the peels, rind, pulp and seeds of watermelon (Citrullus lanatus) fruit. Scientific African. 2021;11 [Google Scholar]
- 47.Al-Sayed H.M., Ahmed A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. (Cairo) 2013;58:83–95. [Google Scholar]
- 48.Gabriela A., Pompeu T., Paulo C., Carneiro F., Hilary C. Osmotic dehydration of mango: effects of temperature and process time. Int. Sugar J. 2004;12:70–71. [Google Scholar]
- 49.Mehrgan M.S., Shekarabi S.P.H., Hassanzadeh B., Alhosseini S.H.S. Seasonal variations of Cadmium and lead concentrations in water, sediments, and tissues of fish in mellat artificial lake, Iran. Journal of Human, Environment, and Health Promotion. 2019;5:177. [Google Scholar]
- 50.Yaqub G., Khan A., Zishan Ahmad M., Irshad U. Determination of concentration of heavy metals in fruits, vegetables, groundwater, and soil samples of the cement industry and nearby communities and assessment of associated health risks. J. Food Qual. 2021:2021. [Google Scholar]
- 51.Azoubel P.M., Murr F.E.X. Mass transfer kinetics of osmotic dehydration of cherry tomato. J. Food Eng. 2004;61:291–295. [Google Scholar]
- 52.Zhang P., Zhou L., Bi J., Liu X., Lyu J., Chen Q., Wu X. Drying kinetics and quality attributes of peach cylinders as affected by osmotic pretreatments and infrared radiation drying. Int. J. Food Eng. 2017;13 [Google Scholar]
- 53.Kalbasi A.H.F. Investigation of effective factors in the speed of osmotic drying process of Lebanese yellow apple. Iran. J. Agric. Sci. 2000 April 20;(2):31. [Google Scholar]
- 54.Lin X., Luo C., Chen Y. Effects of vacuum impregnation with sucrose solution on mango tissue. J. Food Sci. 2016;81:E1412–E1418. doi: 10.1111/1750-3841.13309. [DOI] [PubMed] [Google Scholar]
- 55.Moreno J., Bugueño G., Velasco V., Petzold G., Tabilo‐Munizaga G. Osmotic dehydration and vacuum impregnation on physicochemical properties of Chilean papaya (Carica candamarcensis) J. Food Sci. 2004;69:FEP102–FEP106. [Google Scholar]
- 56.Silveira E.T., Rahman M.S., Buckle K.A. Osmotic dehydration of pineapple: kinetics and product quality. Food Res. Int. 1996;29:227–233. [Google Scholar]
- 57.Bashir N., Sood M., Bandral J. Food preservation by osmotic dehydration-A Review. Chemical Science Review and Letters. 2020;9:337–341. [Google Scholar]
- 58.Pavkov I., Babić L., Babić M., Radojčin M. Osmotic drying of apricot (Prunus armeniaca) in sucrose solution. Agric. Conspectus Sci. 2009;74:253–257. [Google Scholar]
- 59.Kaltsa O., Yanniotis S., Polissiou M., Mandala I. Stability, physical properties and acceptance of salad dressings containing saffron (Crocus sativus) or pomegranate juice powder as affected by high shear (HS) and ultrasonication (US) process. LWT. 2018;97:404–413. [Google Scholar]
- 60.Stavropoulou N.A., Pavlidis V.-A., Giannakourou M.C. Optimization of osmotic dehydration of white mushrooms by response surface methodology for shelf-life extension and quality improvement of frozen end-products. Foods. 2022;11:2354. doi: 10.3390/foods11152354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Mello R.E., Corrêa J.L.G., Lopes F.J., de Souza A.U., da Silva K.C.R. Kinetics of the pulsed vacuum osmotic dehydration of green fig (Ficus carica L.) Heat Mass Tran. 2019;55:1685–1691. [Google Scholar]
- 62.Tiroutchelvame D., Maran J.P., Pragalyaashree M. Response surface analysis and optimization of osmotic dehydration of Musa acuminata slices. J. Microbiol. Biotechnol. Food Sci. 2021;2021:1016–1020. [Google Scholar]
- 63.Dermesonlouoglou E., Paraskevopoulou E., Andreou V., Taoukis P. Osmotic dehydration for the production of novel pumpkin cut products of enhanced nutritional value and sustainability. Appl. Sci. 2020;10:6225. [Google Scholar]
- 64.Lazou A.Ε., Dermesonlouoglou E.K., Giannakourou M.C. Modeling and evaluation of the osmotic pretreatment of tomatoes (S. lycopersicum) with alternative sweeteners for the production of candied products. Food Bioprocess Technol. 2020;13:948–961. [Google Scholar]
- 65.Amiryousefi M.R., Mohebbi M. Neural network approach for modeling the mass transfer of potato slices during osmotic dehydration using genetic algorithm. Afr. J. Agric. Res. 2010;5:70–77. [Google Scholar]
- 66.Rastogi N., Nayak C., Raghavarao K. Influence of osmotic pre-treatments on rehydration characteristics of carrots. J. Food Eng. 2004;65:287–292. [Google Scholar]
- 67.Shekar F., Javadi A. The effect of ultrasound-assisted osmotic dehydration pretreatment on the convective drying of apple slices (var. Golab) Journal of Food Biosciences and Technology. 2019;9:83–94. [Google Scholar]
- 68.Li X., Bi J., Chen Q., Jin X., Wu X., Zhou M. Texture improvement and deformation inhibition of hot air-dried apple cubes via osmotic pretreatment coupled with instant control pressure drop (DIC) LWT. 2019;101:351–359. [Google Scholar]
- 69.Maskan M. Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. J. Food Eng. 2001;48:177–182. [Google Scholar]
- 70.Pavkov I., Radojčin M., Stamenković Z., Kešelj K., Tylewicz U., Sipos P., Ponjičan O., Sedlar A. Effects of osmotic dehydration on the hot air drying of apricot halves: drying kinetics, mass transfer, and shrinkage. Processes. 2021;9:202. [Google Scholar]
- 71.Diamante L., Durand M., Savage G.P., Vanhanen L.P. 2010. Effect of Temperature on the Drying Characteristics, Colour and Ascorbic Acid Content of Green and Gold Kiwifruits. [Google Scholar]
- 72.Correa D.A., Morales J.J., Martelo R.J. 2018. Kinetics of Mass Transfer during Melon Osmotic Dehydration. [Google Scholar]
- 73.Krešić G., Lelas V. Effects of processing on nutritional composition and quality evaluation of candied celeriac. Sadhana. 2004;29:1–12. [Google Scholar]
- 74.Taşova M., Polatci H., Gökdoğan O. Effect of osmotic dehydration pre‐treatments on physicochemical and energy parameters of Kosia (Nashi) pear slices dried in a convective oven. J. Food Process. Preserv. 2022;46 [Google Scholar]
- 75.Generalić Mekinić I., Skroza D., Ljubenkov I., Katalinić V., Šimat V. Antioxidant and antimicrobial potential of phenolic metabolites from traditionally used Mediterranean herbs and spices. Foods. 2019;8:579. doi: 10.3390/foods8110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson J., Iwang E., Hemen J., Odey M., Efiong E., Eteng O. Evaluation of anti-nutrient contents of watermelon Citrullus lanatus. Ann. Biol. Res. 2012;3:5145–5150. [Google Scholar]
- 77.Sreedhar V., Nath L.R., Gopal N.M., Nath M.S. In-vitro antioxidant activity and free radical scavenging potential of roots of Vitex trifoliata. Res. J. Pharmaceut. Biol. Chem. Sci. 2010;1:1036–1044. [Google Scholar]
- 78.Arfaoui L. Dietary plant polyphenols: effects of food processing on their content and bioavailability. Molecules. 2021;26:2959. doi: 10.3390/molecules26102959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X., Bi J., Jin X., Li X., Zhao Y., Song Y. Effect of pectin osmosis or degradation on the water migration and texture properties of apple cube dried by instant controlled pressure drop drying (DIC) LWT. 2020;125 [Google Scholar]
- 80.Rahaman A., Zeng X.-A., Kumari A., Rafiq M., Siddeeg A., Manzoor M.F., Baloch Z., Ahmed Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104643. [DOI] [PubMed] [Google Scholar]
- 81.Rezagholizade-shirvan A., Kalantarmahdavi M., Amiryousefi M.R. Evaluation of the effect of basil seed gum, Tragacanth gum, pectin, and coating formulation with corn flour on oil absorption and sensory properties of watermelon rind chips. Heliyon. 2023;9(6):e16976. doi: 10.1016/j.heliyon.2023.e16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amiryousefi M.R., Mohebbi M., Tehranifar A. Pomegranate seed clustering by machine vision. Food Sci. Nutr. 2018;6:18–26. doi: 10.1002/fsn3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amiryousefi M.R., Mohebbi M., Khodaiyan F., Ahsaee M.G. MOO of DFF of ostrich meat plates. J. Food Process. Preserv. 2014;38:1472–1479. doi: 10.1111/jfpp.12106. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.