Abstract
Management of pancreaticoduodenal artery aneurysms (PDAAs) and gastroduodenal artery aneurysms (GDAAs) with concomitant celiac occlusion represents a challenging clinical scenario. Here, we describe a 62-year-old female with PDAA and GDAA complicated by celiac artery occlusion due to median arcuate ligament syndrome. We used a staged, minimally invasive approach consisting of: (1) a robotic median arcuate ligament release; (2) endovascular celiac artery stenting; and (3) visceral aneurysm coiling. The findings from this case report represent a novel treatment strategy for the management of PDAA/GDAA with celiac artery compression secondary to median arcuate ligament syndrome.
Keywords: Visceral artery aneurysm, Minimally invasive, Median arcuate ligament syndrome, Pancreaticoduodenal artery aneurysm, Gastroduodenal artery aneurysm
Pancreaticoduodenal artery aneurysms (PDAAs) and gastroduodenal artery aneurysms (GDAAs) account for just 3.5% of visceral artery aneurysms.1 These aneurysms are often associated with concomitant celiac artery stenosis or occlusion; the aneurysmal dilation thought to be secondary to chronic increased retrograde flow and turbulence through the pancreaticoduodenal arteries.2, 3, 4
The treatment of choice for PDAA and GDAA is coil embolization. However, in the setting of celiac occlusion, the pancreaticoduodenal artery (PDA) and gastroduodenal artery (GDA) may provide significant collateral network to the hepatic artery.5 Thus, coil embolization in patients with concomitant celiac occlusion may cause catastrophic hepatic ischemia and liver failure.6,7 In such cases, celiac revascularization should be considered and may be achieved with either endovascular or open techniques. Endovascular management with angioplasty and stenting of the celiac artery is a low-morbidity option; however, in patients with median arcuate ligament syndrome (MALS), durability is poor due to extrinsic compression.8 Open reconstruction options include direct reconstruction with an aorto-celiac bypass or an extra-anatomic approach, such as a right renal artery to hepatic artery bypass; however, many patients are not candidates for these major operations.9
Although management of PDAA and GDAA with concomitant celiac occlusion is challenging, treatment of PDAA and GDAA is essential due to their increased risk of rupture and associated morbidity and mortality.3,10,11 Herein, we present a novel, minimally invasive approach to this complex vascular pathology. The patient was adequately informed and consented to the publication of this case.
Case report
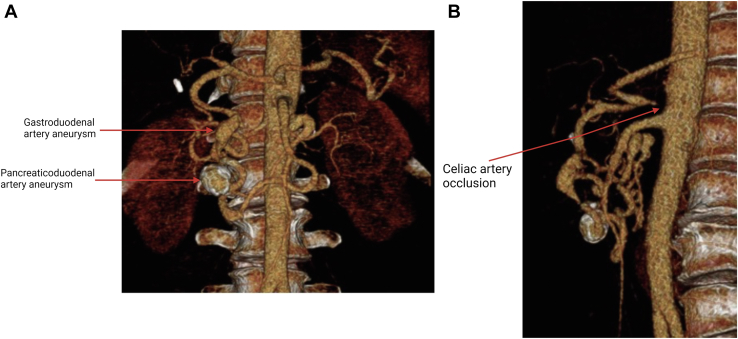
A 62-year-old female with past medical history hypertension, hyperlipidemia, and type 2 diabetes presented with a history of epigastric abdominal pain. She underwent a laparoscopic cholecystectomy in 2017 for presumed symptomatic cholelithiasis. She continued to have abdominal pain and subsequently underwent a magnetic resonance cholangiopancreatography to evaluate for choledocholithiasis, which demonstrated an incidental finding of PDAA and GDAA. The patient was referred to vascular surgery and a computed tomography angiography (CTA) was obtained, which demonstrated a 1.8-cm PDAA, a 1.9-cm GDAA, and celiac artery occlusion secondary to median arcuate ligament syndrome (Fig 1).
Fig 1.
Three-dimensional reconstruction of computed tomography angiography (CTA) images taken preoperatively. A, Anterior view showing pancreaticoduodenal artery aneurysm (PDAA) and gastroduodenal artery aneurysm (GDAA). B, Sagittal view demonstrating complete occlusion of the celiac artery.
We elected to manage the visceral artery aneurysms with coil embolization and to revascularize the celiac artery to ensure adequate hepatic perfusion. The patient was morbidly obese with a body mass index (BMI) of 40.8 kg/m2; hence, we felt she was not an optimal candidate for open celiac artery reconstruction. Therefore, we decided to employ a staged, minimally invasive approach. First, the patient underwent a robotic median arcuate ligament release. This was done to ensure durability of a subsequent endovascular revascularization of the celiac artery. Following the median arcuate release, the patient was admitted to the hospital. On postoperative day three during the index hospitalization, we took the patient to the operating room for the second stage of the procedure. We performed celiac artery angioplasty and stenting, followed by visceral artery aneurysm coil embolization. The right femoral artery was accessed using a micropuncture kit under ultrasound guidance. An aortogram in the lateral position was performed to mark the origin of the celiac artery. The celiac artery occlusion was crossed with an 0.014 wire and support catheter. Next, we pre-dilated the lesion with a 4-mm angioplasty balloon and deployed a 7- × 27-mm bare metal balloon-expandable stent (Boston Scientific Corporation, Marlborough, MA). Completion angiogram demonstrated excellent flow in the celiac artery. Next, the GDA was selected, and a visceral angiogram at this location showed both PDAA and GDAA. We embolized both aneurysm sacs with detachable coils ranging in diameter from 10 to 22 mm (Boston Scientific Corporation, Marlborough, MA). Completion angiogram showed cessation of flow in the aneurysm sacs. The patient reported resolution of her abdominal pain and no postoperative complications and was discharged on hospital admission day five.
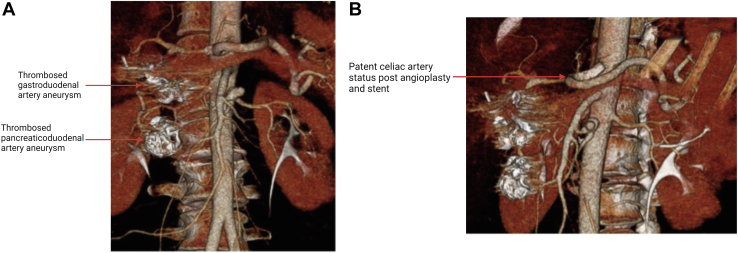
We obtained an ultrasound at 1 month postoperatively, which showed patent celiac artery with a velocity consistent with no remaining stricture. A CTA at 6 months showed patent celiac artery, and the GDA and PDA aneurysms were thrombosed (Fig 2). The patient will have continued surveillance with a duplex ultrasound at 12 months and annually thereafter.12
Fig 2.
Postoperative computed tomography angiography (CTA). A, Anterior view showing thrombosed pancreaticoduodenal artery aneurysm (PDAA) and gastroduodenal artery aneurysm (GDAA). B, Sagittal view demonstrating patent celiac artery after stenting.
Discussion
GDAA and PDAA represent a rare subset of visceral artery aneurysms with an elevated risk of rupture, independent of size.13 Recent clinical practice guidelines for visceral artery aneurysms from the Society for Vascular Surgery recommend intervention for GDAA and PDAA in patients with acceptable operative risk regardless of aneurysm size, given the high risk of rupture. The primary treatment of option for aneurysms of the pancreaticoduodenal arcade is coil embolization.7
Often, these aneurysms are associated with celiac artery stenosis or occlusion, due to atherosclerosis or MALS.2 In fact, impaired flow in the celiac axis has a causal relationship with the development of PDAA and GDAA; celiac stenosis/occlusion causes increased turbulent and retrograde flow through the superior mesenteric artery (SMA) and pancreaticoduodenal arcade, resulting in aneurysmal dilation.14,15 Concomitant celiac artery stenosis/occlusion complicates the surgical management of PDAA and GDAA. To date, there are no consensus guidelines regarding celiac artery reconstruction in this context. Coil embolization of PDAA and GDAA interrupts collateral networks between the SMA and celiac artery, which can lead to hepatic ischemia and subsequent liver failure; however, this risk appears to be low. In a single-institution retrospective review of 20 patients with PDAA and GDAA, the majority of patients had a concomitant celiac artery lesion and underwent endovascular coiling without celiac artery revascularization.16 At a median of 6 months, there were no occurrences of hepatic ischemia; therefore, the authors concluded that celiac revascularization may not be necessary. In our case, the patient had tandem aneurysms in the pancreaticoduodenal arcade, which necessitated coil embolization of the GDA and the superior PDA. We surmised that disruption of collateral flow in both the GDA and superior PDA would place our patient at increased risk for postoperative hepatic ischemia; therefore, we elected to pursue a celiac artery revascularization.
Traditional treatment options for celiac revascularization include endovascular stenting or open reconstruction. Endovascular stenting of the celiac artery is a minimally invasive option. However, in patients with MALS, extrinsic compression by the median arcuate ligament decreases durability.17 Surgical reconstruction provides a more durable option; however, not all patients are candidates for open abdominal surgery, which confers increased morbidity and mortality. In this case, we utilized a novel minimally invasive approach with a robotic median arcuate ligament release followed by endovascular stenting of the celiac artery with embolization of the visceral aneurysms. Release of the median arcuate ligament along with celiac artery stenting decreases the risk of hepatic ischemia by restoring celiac flow and may reduce the risk of aneurysmal recurrence given repair of the underlying pathophysiology. Utilizing minimally invasive techniques allowed for a relatively short recovery as well as an overall decrease in risk to the patient.
In conclusion, there is limited data available regarding the treatment of PDAA and GDAA with concomitant celiac artery stenosis. Here we report a case of MALS with PDAA and GDAA treated with a staged, minimally invasive approach. Although there is no consensus for the treatment of PDAA and GDAA with concomitant celiac stenosis, this staged, minimally invasive approach may provide a safe and technically feasible treatment option for patients with PDAA and GDAA secondary to MALS.
From the Eastern Vascular Society
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Barrionuevo P., Malas M.B., Nejim B., Haddad A., Morrow A., Ponce O., et al. A systematic review and meta-analysis of the management of visceral artery aneurysms. J Vasc Surg. 2019;70:1694–1699. doi: 10.1016/j.jvs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 2.Sutton D., Lawton G. Coeliac stenosis or occlusion with aneurysm of the collateral supply. Clin Radiol. 1973;24:49–53. doi: 10.1016/s0009-9260(73)80114-x. [DOI] [PubMed] [Google Scholar]
- 3.Moore E., Matthews M.R., Minion D.J., Quick R., Schwarcz T.H., Loh F.K., et al. Surgical management of peripancreatic arterial aneurysms. J Vasc Surg. 2004;40:247–253. doi: 10.1016/j.jvs.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 4.Vandy F.C., Sell K.A., Eliason J.L., Coleman D.M., Rectenwald J.E., Stanley J.C. Pancreaticoduodenal and gastroduodenal artery aneurysms associated with celiac artery occlusive disease. Ann Vasc Surg. 2017;41:32–40. doi: 10.1016/j.avsg.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 5.van Petersen A.S., Kolkman J.J., Meerwaldt R., Huisman A.B., van der Palen J., Zeebregts C.J., et al. Mesenteric stenosis, collaterals, and compensatory blood flow. J Vasc Surg. 2014;60:111–119. doi: 10.1016/j.jvs.2014.01.063. 119 e1-2. [DOI] [PubMed] [Google Scholar]
- 6.Katsura M., Gushimiyagi M., Takara H., Mototake H. True aneurysm of the pancreaticoduodenal arteries: a single institution experience. J Gastrointest Surg. 2010;14:1409–1413. doi: 10.1007/s11605-010-1257-0. [DOI] [PubMed] [Google Scholar]
- 7.Chaer R.A., Abularrage C.J., Coleman D.M., Eslami M.H., Kashyap V.S., Rockman C., et al. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J Vasc Surg. 2020;72:3S–39S. doi: 10.1016/j.jvs.2020.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi Y., Morikage N., Samura M., Harada T., Yamashita O., Suehiro K., et al. Treatment options for celiac stenosis and pancreaticoduodenal artery aneurysms. Ann Vasc Surg. 2017;41:e21–e23. doi: 10.1016/j.avsg.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 9.Bowens N.M., Woo E.Y., Fairman R.M. Reno-hepatic artery bypass for an inferior pancreaticoduodenal artery aneurysm with associated celiac occlusion. J Vasc Surg. 2011;53:1696–1698. doi: 10.1016/j.jvs.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Stone W.M., Abbas M.A., Gloviczki P., Fowl R.J., Cherry K.J. Celiac arterial aneurysms: a critical reappraisal of a rare entity. Arch Surg. 2002;137:670–674. doi: 10.1001/archsurg.137.6.670. [DOI] [PubMed] [Google Scholar]
- 11.Shukla A.J., Eid R., Fish L., Avgerinos E., Marone L., Makaroun M., et al. Contemporary outcomes of intact and ruptured visceral artery aneurysms. J Vasc Surg. 2015;61:1442–1447. doi: 10.1016/j.jvs.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Zierler R.E., Jordan W.D., Lal B.K., Mussa F., Leers S., Fulton J., et al. The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures. J Vasc Surg. 2018;68:256–284. doi: 10.1016/j.jvs.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 13.de Perrot M., Berney T., Deleaval J., Buhler L., Mentha G., Morel P. Management of true aneurysms of the pancreaticoduodenal arteries. Ann Surg. 1999;229:416–420. doi: 10.1097/00000658-199903000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong M.B., Stadtlander K.S., Grove M.K. Pancreaticoduodenal artery aneurysm associated with median arcuate ligament syndrome. Ann Vasc Surg. 2014;28:741.e1–741.e5. doi: 10.1016/j.avsg.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Murata S., Tajima H., Fukunaga T., Abe Y., Niggemann P., Onozawa S., et al. Management of pancreaticoduodenal artery aneurysms: results of superselective transcatheter embolization. AJR Am J Roentgenol. 2006;187:W290–W298. doi: 10.2214/AJR.04.1726. [DOI] [PubMed] [Google Scholar]
- 16.Boll J.M., Sharp K.W., Garrard C.L., Naslund T.C., Curci J.A., Valentine R.J. Does management of true aneurysms of peripancreatic arteries require repair of associated celiac artery stenosis? J Am Coll Surg. 2017;224:199–203. doi: 10.1016/j.jamcollsurg.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Delis K.T., Gloviczki P., Altuwaijri M., McKusick M.A. Median arcuate ligament syndrome: open celiac artery reconstruction and ligament division after endovascular failure. J Vasc Surg. 2007;46:799–802. doi: 10.1016/j.jvs.2007.05.049. [DOI] [PubMed] [Google Scholar]