Abstract
We developed recombinant variants of oncolytic vaccinia virus LIVP strain expressing interleukin-15 (IL-15) or its receptor subunit alpha (IL-15Rα) to stimulate IL-15-dependent immune cells. We evaluated their oncolytic activity either alone or in combination with each other in vitro and in vivo using the murine CT26 colon carcinoma and 4T1 breast carcinoma models. We demonstrated that the admixture of these recombinant variants could promote the generation of the IL-15/IL-15Rα complex. In vitro studies indicated that 4T1 breast cancer cells were more susceptible to the developed recombinant viruses. In vivo studies showed significant survival benefits and tumor regression in 4T1 breast cancer syngeneic mice that received a combination of LIVP-IL15-RFP with LIVP-IL15Ra-RFP. Histological analysis showed recruited lymphocytes at the tumor region, while no harmful effects to the liver or spleen of the animals were detected. Evaluating tumor-infiltrated lymphocytes represented profound activation of cytotoxic T cells and macrophages in mice receiving combination therapy. Thus, our experiments showed superior oncolytic effectiveness of simultaneous injection of LIVP-IL15-RFP and LIVP-IL15Ra-RFP in breast cancer-bearing mice. The combined therapy by these recombinant variants represents a potent and versatile approach for developing new immunotherapies for breast cancer.
Keywords: oncolytic virus, vaccinia virus, LIVP, interleukin-15, interleukin-15 receptor alpha, IL-15/IL-15Rα complex, breast adenocarcinoma, colon carcinoma
Graphical abstract

Baklaushev and colleagues developed vaccinia virus strains expressing IL-15 or its receptor IL-15Rα and evaluated in vivo. The results showed a significant increase in survival rate and tumor regression in 4T1 tumor-bearing mice that received combination therapy of VV-IL15 with VV-IL15-Ra associated with enhanced tumor-infiltrated lymphocytes.
Introduction
Oncolytic virus (OV) therapy is a promising novel approach to cancer treatment. The OVs act as immunostimulatory agents that selectively replicate and lyse tumor cells without harming the normal tissues. Previous studies showed that OVs could boost anti-cancer systemic immunity,1 and various gene engineering methods have been incorporated to enhance OVs’ therapeutic efficacy. For instance, T-VEC, the first FDA-approved OV for melanoma treatment, is an engineered oncolytic herpes simplex virus type 1 carrying human GM-CSF gene. Vaccinia viruses (VVs) are promising oncolytic agents for cancer therapy due to their high tumor tropism and the ability of selective tumor cell lysis as well as high safety profile.2,3 VVs have a large transgene-encoding capacity with efficient foreign gene expression. One appealing strategy to improve cancer viral therapy is to enhance the host’s antitumor immunity response via encoding transgene immunostimulatory molecules. Numerous recombinant VVs enhanced with immunogenic transgenes, such as interferons (IFNs), tumor necrosis factor (TNF), and a variety of interleukins (ILs), are currently being used in preclinical or clinical cancer therapy trials.4,5,6
Interleukin (IL)-15 is a pleiotropic cytokine that plays an essential role in developing, activating, and surviving T cells, natural killer (NK) cells, and NK-T cells.7 IL-15 receptor subunit alpha (IL-15Rα) is one of the primary receptors that stabilizes and enhances IL-15 bioactivity, and mediates its signaling pathway. It can bind to IL-15 independently from other subunits with high affinity, forming the IL-15/IL-15Rα complex on the surface of activated monocytes, and promoting T cell survival.8 In recent years, several studies testing oncolytic potential of IL-15 alone9,10,11,12,13 or in combination with its potential effectors were conducted; an oncolytic Western Reserve vaccinia virus encoding a fusion IL-15/IL-15Rα gene in combination with PD1 blockade caused remission of colon and ovarian cancer.14 Other OVs including measles and myxoma viruses have been engineered to express IL-15/IL-15Rα fusion protein, as well which represented promising results for melanoma treatment.15,16 Several phase I and II clinical trial reports verified IL-15 and IL-15Rα immune modulation in cancer patients.17
Vaccinia virus Lister strain from the Institute of Virus Preparation, Moscow, Russia (LIVP) is an attenuated variant reported to hold excellent oncolytic properties.18,19,20,21 Previously, it was demonstrated that this strain could treat highly immunosuppressive tumors such as triple-negative breast cancer (TNBC) models.22 Therefore, we utilized this strain for further modification and development of recombinant variants expressing murine IL-15 (LIVP-IL15-RFP) or murine IL-15Rα (LIVP-IL15Ra-RFP) with deletion of thymidine kinase (TK) to treat 4T1 murine breast cancer model, which represents a highly immunosuppressive tumor model, and CT26 murine colon carcinoma.23 Deletion of TK, a common strategy to increase tumor selectivity,24 was performed by inserting the reporter gene tagRFP (red fluorescent protein) into the TK locus. We hypothesized that combining LIVP-IL15-RFP with LIVP-IL15Ra-RFP can promote the formation of IL-15/IL-15Rα complex that produces a synergistic antitumor effect with a significant prolongation of survival compared to either therapy alone in 4T1 breast cancer models, correlating with an increased level of CD8+ tumor-infiltrated lymphocytes and cytokines that indirectly enhance activation of NK, B, and T cells.
Results
Construction of the recombinant viruses
The recombinant viruses were constructed by homologous recombination using tagRFP as the reporter gene for in vitro analysis (Figure 1A). Correctness of insertions was verified by the Sanger sequence of amplified TK gene from genomic DNA.
Figure 1.
Construction of recombinant LIVP strains expressing IL-15 and IL-15Rα by homologous recombination
(A) Scheme of genetic modification of TK gene. Microphotographs of BHK21 cells (B) and 4T1 cells (C) infected with LIVP-RFP recombinant strain (the same picture was obtained for LIVP-IL15-RFP and LIVP-IL15Ra-RFP; data are not shown). Red immunofluorescence merged with bright field microscopy. (D) Western blotting analysis in cell lysate/supernatant of infected cells by LIVP-IL15-RFP (left panel) normalized the protein concentration to β-actin (right panel). Description: Sprntnt Ctrl, Lysate Ctrl, a supernatant and a lysate of the control (non-infected) cells; IL-15 VV Inf. Suprntnt, IL-15 VV Inf. Lysate, a supernatant and a lysate of IL-15 VV or IL-15Ra infected cells, respectively.
The created recombinant viruses LIVP-IL15-RFP and LIVP-IL15Ra-RFP effectively infected both BHK-21 and 4T1 tumor cells in vitro (Figures 1B and 1C). Cell lysates and supernatant of infected BHK-21 cells with LIVP-IL15-RFP or LIVP-IL15Ra-RFP were harvested and analyzed for the presence of IL-15 and IL-15Rα by western blotting assay. IL-15 was detected both in lysates and supernatant (Figures 1D and S1), while IL-15Rα was only detected in the lysate (Figure S2). It is worthy to mention that IL-15Rα was seen in the cell lysate of the control (un-infected BHK-21 cells) and the lysate of infected cells since this receptor is naturally expressed by many cells and tissues.25,26 However, the relative intensity of immunoprecipitated proteins in infected cells was at least twice higher than the control (Figure S2).
VV recombinant strains efficiently propagate and lyse tumor cells in vitro
Before evaluating the therapeutic efficacy of the recombinant strains, we examined their capacity to replicate and lyse relevant murine tumor cell lines in vitro. Cytotoxicity of the viruses was evaluated at 24, 48, 72, 96, and 120 h following infection of 4T1, CT26, and BHK-21 cell cultures. Cytotoxicity begins 48 h post infection (Figure 2A). Results show that arming LIVP with IL-15 increases cytotoxicity in 4T1 and CT26 cell cultures. However, the combination of LIVP-IL15-RFP with LIVP-IL15Ra-RFP did not show any synergistic cytotoxic effect in vitro, and it appears LIVP-IL15Ra-RFP reduced the LIVP-IL15-RFP cytotoxicity to some extent.
Figure 2.
Cytotoxicity and replication characteristics of the developed strains
(A) Viability of BHK-21, 4T1, and CT26 cells through 120 h after infection (h.p.i) by MOI 1 of LIVP-RFP, LIVP-IL15-RFP, LIVP-IL15Rα-RFP, and combination of LIVP-IL15-RFP with LIVP-IL15Rα-RFP (LIVP-IL15-RFP + LIVP-IL15Rα-RFP). (B) Kinetics of recombinant viral strains in different cell lines (BHK-21,4T1, CT26). ANOVA was performed for statistical analysis, ∗p < 0.05 and ∗∗p < 0.01 indicate significance.
The rate of viral replication was determined by flow cytometry detecting red fluorescence emitted from infected tumor cell lines of 4T1, CT26, and BHK-21 with MOI 1 of LIVP-RFP, LIVP-IL15-RFP, LIVP-IL15Ra-RFP, and combination of LIVP-IL15-RFP with LIVP-IL15Ra-RFP. The results indicate that recombinant viruses can infect and replicate in the cancer cell culture of 4T1 more effectively than CT26 (Figure 2B).
Detection of IL-15/IL-15 receptor alpha complex in vitro
To determine the combination of the viruses LIVP-IL15-RFP with LIVP-IL15Ra-RFP can form the complex of the IL-15/IL-15Rα, both tumor model cell lines, CT26 and 4T1, were infected with virus combination at MOI 1. Cells infected with the parental virus LIVP-RFP served as control. The cell lysate was collected at indicated time points of 24, 48, and 72 h after infection, and the complex’s concentration was measured using an enzyme-linked immunosorbent assay (ELISA) specific to IL-15/IL-15Rα complex. The complex was detected in both cell lines, and its concentration increased over time (Figure S3). The difference in potential to form the complex between two cell lines was insignificant. In cells infected only with LIVP-IL15-RFP, the IL15/IL15Rα complex was also detected, since both cell lines naturally possess IL-15 receptors,25,26 but this amount was not considerable. As expected, cells infected with a non-cytokine-expressing virus (LIVP-RFP) had no detectable IL-15/IL-15Rα (Figure S3).
A combination of IL15-RFP and IL15Ra-RFP strains shows synergetic oncolytic effect in vivo
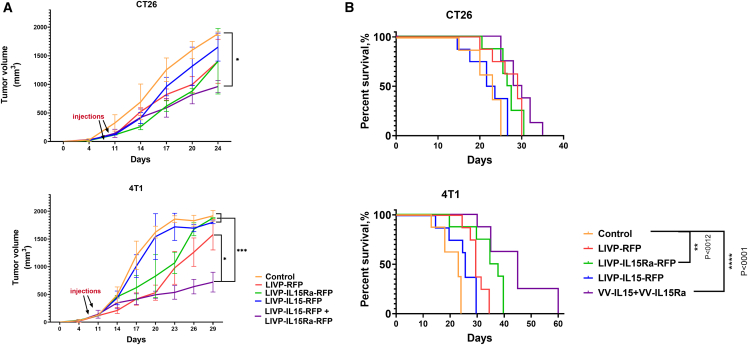
9 days following tumor implantation, while the tumor volume reached approximately 100 mm3, 108 PFU of purified strains in 100 μL of PBS was injected intratumorally two times into the mice bearing 4T1 breast tumors or CT26 colon carcinoma. Tumor volume was analyzed every other day, and survival rates were determined. Lower tumor volume was observed in all treated groups compared with the control group that received PBS. However, tumor progression was the weakest in 4T1 breast cancer-bearing mice that received a simultaneous injection of LIVP-IL15-RFP with LIVP-IL15Ra-RFP even though the dose of each component in the combined regime was twice lower compared with monotherapies (Figure 3A). This result indicates the superior treatment efficacy of combination therapy over monotherapy. Moreover, the survival rate was drastically prolonged in the group receiving combination therapy, consistent with tumor progression assessment (Figure 3B).
Figure 3.
Virotherapy by the recombinant strains in vivo
(A) Subcutaneous CT26 colon carcinoma and 4T1 breast tumor progression in control treated with PBS and experimental groups treated with LIVP-RFP, LIVP-IL15-RFP, LIVP-IL15Rα-RFP, and a combination of LIVP-IL15-RFP+ LIVP-IL15Rα. ✝ represents the sacrifice of the animal. (B) The overall survival rate of BALB/c mice bearing CT26 colon carcinoma and 4T1 breast tumors treated by the recombinant viruses and the control group based on the Kaplan-Meier method. For statistical analysis, ANOVA and survival analysis were performed; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 indicate significance.
Recombinant strains modulate tumor microenvironment
To determine the effect of viruses on immune response in vivo, the immunocompetent BALB/c mice bearing subcutaneous 4T1 breast cancer tumors that had a better response to therapy rather than CT26 colon carcinoma models were used. 24 hours and 7 days after either monotherapy or combination therapy, tumor samples were collected, and tumor-infiltrated lymphocytes, monocytes, and leukocytes were analyzed. Analysis of T cells mirrored most tumor-infiltrating T cells in all treated mice were CD4+cells, although CD8+ cells were significantly elevated since 24 h after VV administration in the treated group by combination therapy (Figure S4). The level of F4/80+ cells 7 days after treatment in the combination therapy group was significantly higher than other groups, indicating treatment associated with a high level of macrophages. Level of CD45+ cells 7 days after treatment in all groups that received treatment except LIVP-RFP was significantly higher, representing a higher level of leukocyte infiltration (Figures 4 and S4).
Figure 4.
Recombinant strains alter immune system response
Analysis of tumor-infiltrated lymphocytes 7 days after complete treatment of 4T1 breast carcinoma-bearing mice (n = 3). The levels of F4/80+, CD45+, CD4 T+, and CD8+ T cells were measured. ANOVA was performed for statistical analysis; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 indicate significance.
Cytokine analysis of the serum and tumor tissue
The levels of various cytokines, including IFN -α, IFN-γ, IL-4, IL-6, IL-10, CCL2, CCL3, CCL4, CXCL9, CXCL10, TNF-α, vascular endothelial growth factor (VEGF), and GM-CSF from both sera and tumor samples from the 4T1 breast tumor-bearing mice of control and treated groups were measured 24 h and 7 days upon virus treatment completion. In serum samples from the group treated by a combination of LIVP-IL15-RFP with LIVP-IL15Ra, significantly increased levels of interferons (α and γ), GM-CSF, and TNF-α were observed (Figure 5), while in the tumor samples of the same group, elevated levels of CXCL9 and CXCL10 were noted (Figure 6). On the contrary, the group treated by LIVP-IL15-RFP had a surge of inflammatory cytokines of CCL2, VEGF, and IL-4. (Figures 5, 6, S5, and S6). Moreover, the presence of IL-15/IL-15Rα complex was measured in the same mice’s tumor-infiltrated fluids (TIFs) and sera. This complex was noticeably increased in TIFs samples of mice treated by a combination of LIVP-IL15-RFP with LIVP-IL15Ra-RFP (Figure 7). In serum samples, the IL-15/IL-15Rα complex was detected in both the combination therapy group and the group treated by LIVP-IL15-RFP alone (Figure S7).
Figure 5.
Cytokine analysis of the serum samples from the 4T1 tumor-bearing mice treated by LIVP-RFP, LIVP-IL15-RFP, LIVP-IL15Rα-RFP, and combination of LIVP-IL15-RFP+ LIVP-IL15Rα in comparison with the untreated mice (control group)
Statistical analysis was performed using a t test, with ∗p < 0.05 indicating significance. Designation: −1, 1 day after the last intratumoral VV injection; −7, 7 days after the last intratumoral VV injection.
Figure 6.
Cytokine analysis of the tumor samples from the 4T1 tumor-bearing mice treated by LIVP-RFP, LIVP-IL15-RFP, LIVP-IL15Rα-RFP, and combination of LIVP-IL15-RFP+ LIVP-IL15Rα in comparison with the untreated mice (control group)
Statistical analysis was performed using a t test, with ∗p < 0.05 indicating significance. Designation is the same as in Figure 5.
Figure 7.
ELISA of IL-15/IL-15Rα complex produced by viruses in tumor-infiltrated fluids of 4T1 breast cancer syngeneic mice in vivo
Designation: (−1), samples collected 24 h after complete treatment; (−7), samples collected 7 days after complete treatment. Statistical analysis was performed using a t test, with ∗p < 0.05 indicating significance.
Histology analysis
Histology analysis was done on 4T1 breast cancer models. Histology examination of the control samples showed a solid undifferentiated tumor with high cellularity formed by cells with polymorphic hyperchromic nuclei and peripheral infiltration of mononuclear cells and neutrophils (Figure 8, upper right panel). In tumor samples from mice treated by the recombinant oncolytic viruses, we found extensive confluent fields of necrosis as a homogeneous cell-free mass with fragments of karyorrhexis and many focal and confluent hemorrhages and a significant infiltration with immune cells (Figures 8, upper left panels, and S8). No signs of pathology were found on the liver sections of all animals (Figure 8, lower panels). At the same time, white pulp hypertrophy was observed in spleen sections of all animals with a tumor (Figure 8, middle panels), which indicated activation of the immune system in response to the neoplastic process.27
Figure 8.
Histological analysis of tumor and organs of the treated mice
Histology analysis of the liver, spleen, and the tumor treated by recombinant viruses expressing IL-15 or IL-15Rα and their combination compared with the parental virus (LIVP-RFP) and the control group.
Discussion
Breast cancer is the most prevalent life-threatening cancer causing the highest mortality among women worldwide. However, recent advances in cancer treatment have led to extraordinary progress noted in more efficient and less toxic treatments for breast cancer.28,29 OV therapy is a novel approach for treating cancer, as it can induce immune infiltration and direct cytolysis of tumor cells.30 OVs can also serve as a vector for local gene therapy by delivering several antitumor factors, such as cytokines, to enhance lymphocyte recruitment or tumor cell apoptosis.31 LIVP strain of VV is a promising agent for cancer immunotherapy21; the oncolytic virus GL-ONC1 which is under evaluation in phase I/II human clinical trials is based on this strain.32
Recently, IL-15 has emerged as a candidate immunomodulator for cancer therapy as it induces differentiation and proliferation of B, T, and NK cells. It also intensifies the cytolytic activity of CD8+ T cells. The complex of IL-15 with its high-affinity receptor subunit alpha (IL-15Rα), expressed on monocytes, macrophages, and antigen-presenting dendritic cells, demonstrates superior therapeutic advantages over IL-15 alone. This complex can drastically increase the half-life and bioavailability of IL-15.33,34 This phenomenon can be critical in the highly immunosuppressed tumor microenvironment (TME). In some previous studies, the IL-15/IL-15Rα complex was reported to cause significant tumor regression in melanoma and glioblastoma models associated with an enhanced level of CD8+T cells or dendritic cells in TME. At the same time, IL-15 alone could not do this.35,36 More recently, IL-15/IL-15Rα fusion protein has been developed, and engineered OVs expressing this multimeric complex have been introduced with favorable oncolytic properties on mice models.14,15,16 However, the advantage of this complex in clinical trials is not clear yet.37
Therefore, we engineered the oncolytic vaccinia virus LIVP expressing IL-15 and IL-15Rα to improve anti-cancer immune responses. All the variants have a deletion of TK to increase tumor selectivity. At first, we evaluated the capacity of developed variants to replicate and lyse tumor cells using murine 4T1 breast cancer and CT26 colon carcinoma models in vitro; all recombinant viruses had a higher level of replication and cytotoxicity in 4T1 cell culture than CT26. LIVP-IL15-RFP had an exceeding cytotoxicity and replication rate compared with other variants, and when mixed with LIVP-IL15Ra-RFP, the cytotoxicity was reduced. Then we investigated if combining LIVP-IL15-RFP with LIVP-IL15Ra-RFP can enhance the formation of IL-15/IL-15Rα complex. Both tumor models (4T1 and CT26) were infected by the LIVP-IL15-RFP/LIVP-IL15Ra-RFP admixture, and ELISA determined the presence of the complex in both cell lines. Furthermore, in the in vivo experiment, we observed the amount of this complex was drastically higher in the tumor-infiltrated fluid of the group that received a combination of the viruses compared with groups that received monotherapy.
The in vivo experiments were carried out on immune-competent BALB/c mice carrying murine CT26 colon carcinoma and 4T1 breast carcinoma; the combination therapy of the virus variant expressing IL-15 with the virus expressing IL-15-Rα significantly improved the therapeutic outcomes with prolonged survival and lower tumor progression rate compared with monotherapy using viruses expressing IL-15 or 1IL-15Rα alone, especially in the 4T1 breast cancer mouse models.
4T1 cell line shares major molecular features with human TNBC.38 TNBC lacks the expression of receptors for progesterone, estrogen, and human epidermal growth factor receptor 2 and is one of the most aggressive forms of breast cancer with the highest fatal rate.39 Previously, it was demonstrated that the vaccinia virus LIVP and MVA strains have superior potential to target this type of tumor.40,41
One of the main concerns with viral immunotherapy is its lethal or near-lethal toxicity, like cytokine release syndrome (CRS). Our data suggest an injection of LIVP-IL15-RFP alone can exacerbate CRS, as significant elevation of inflammatory cytokines such as IL-4, CCL2, and VEGF was observed in the treated group that only received LIVP-IL15-RFP. All three cytokines mentioned contribute to cancer progression and assist metastasis.42,43,44 In contrast, cytokine analysis of the group treated by combination therapy (LIVP-IL15-RFP + LIVP-IL15Ra-RFP) suggests that the presence of IL-15Rα can modulate the increase of inflammatory cytokines. Additionally, a higher level of cytokines such as GM-CSF, TNF-α, CXCL9, and CXCL10 was observed in this group. Several studies suggest that these cytokines play a critical role in tumor regression; however, understanding the tumor and cytokines interactions is a complex phenomenon, and it shall be noted that such interactions may also demonstrate paradoxical effects.45 GM-CSF is a hematopoietic cytokine with diverse effects on the immune system, including differentiation of myeloid cells, activation of T cells, maturation of dendritic cells, promotion of cell-mediated and humoral responses, as well as antitumor activity.46,47 Numerous studies demonstrated GM-CSF as a potent modulator for cancer treatment; the first approved oncolytic virus for melanoma treatment was an engineered herpes simplex virus talimogene laherparepvec (T-VEC) expressing GM-CSF.48 TNF-α has been widely implicated in cancer therapy due to its ability to inhibit cell proliferation and break vascularizing in TME.49
Under the generic name of tasonermin, it is used to remove soft tissue of sarcoma of the limbs.50 Another type of cytokines elevated during combination therapy was CXCLs (CXCL9 and 10); these cytokines have been reported to regulate immune cell differentiation, activation, and migration, leading to tumor regression.51
The histological observation indicated massive infiltration of immune cells and necrosis in tumor sections of animals treated by combination therapy. Consistently, analysis of tumor-infiltrated cells demonstrated the increase of total immune cells, T cells, and macrophages; the levels of CD45+, F4/80+ CD4+T, and CD8+T cells at TME of this treated group were drastically higher than the groups that received monotherapy. All of the effects observed in this study are indicative of the conversion of “cold” TME to “hot” TME with increased levels of tumor-suppressing cytotoxic immune cells, providing a solid rationale for combining OVs with the expression of various immune effectors and especially IL-15/IL-15Rα complex to affect TNBC.
Thus, co-therapy with oncolytic recombinant LIVP strains expressing IL-15 and IL-15Rα may be a facile approach for intratumoral delivery of the IL-15/IL-15Rα complex, enhancing the local antitumor immune response and can be considered as a promising platform for further clinical trials of oncolytic therapy of immunosuppressive TNBC.
Materials and methods
Cell culture
Rat2 TK−/− (rat fibroblasts deficient in TK gene expression) was provided by the Cell Proliferation laboratory, Engelhardt Institute of Molecular Biology (Moscow, Russia). BHK-21 (ATCC NO. CCL-10), 4T1 murine breast adenocarcinoma (ATCC NO. CRL-3406), CT26 murine colon carcinoma (ATCC NO. CRL-2638), and HEK293T (CRL-1573) cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All the cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (Glutamax supplemented/Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) and 100 μg/mL penicillin-streptomycin (PanEco, Russia). All cell cultures were incubated at 37°C in a 5% CO2 atmosphere. An Improved Neubauer hemocytometer was used for cell counting.
Generation of recombinant viruses
LIVP strain with deletion of TK and expression of tagRFP reporter gene under the control of 7.5k promoter (LIVP-RFP) was constructed previously. Total RNA was extracted from the mouse heart and mammary glands to get correspondent mRNA of IL-15 and IL-15Rα, respectively (CleanRNA Standart kit, Evrogen, Russia). Subsequently, complementary DNA was constructed by a SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific, USA) according to the manufacturer’s instruction. Fragments were amplified by PCR using specific primers for mouse IL-15 or IL-15Rα (Table S1) using Q5 High Fidelity polymerase (NEB, USA). Then they were inserted by sticky ends ligation into the recombination plasmid previously developed at the Cell Proliferation laboratory EIMB RAS. Transfection for recombination was performed using Lipofectamine 3000 (Thermo Fisher Scientific, USA) in HEK293T cells, followed by infection with wild-type LIVP. TK-deleted recombinant variants were selected on Rat-2 TK− cells (deficient in the TK expression) treated with 2-bromodeoxyuridine after infection at MOI 1 of the virus variants gained after transfection. All transgenes were controlled by 7.5k promoter and IL-15 or IL-15Rα-RFP co-expression cassettes linked by 2A self-processing peptide (Figure 1A). Insertions were confirmed by sequencing genomic DNA. Also, western blotting was used to verify the presence of IL-15 and IL-15Rα in infected cells. Expression of tagRFP in infected cells was confirmed using fluorescent microscopy. All the recombinant viruses were purified by the sucrose gradient method for in vitro and in vivo experiments.52
Detection of mouse IL-15 and IL-15Rα produced by recombinant viruses in infected cells
Western blot analysis was performed to verify IL-15 and IL-15Ra expression by the viruses. A confluent monolayer of BHK-21 cells in a six-well plate was infected at MOI 1 of LIVP-IL15-RFP or LIVP-IL15Ra-RFP. Mock-infected cells were used as a negative control. The cells were lysed using RIPA buffer, then subjected to 12% SDS-PAGE with further transfer onto a membrane (PVDF membrane, Millipore, USA) and stained with anti-IL-15 (cat.no# MAB2471, R&D Systems, China) and anti-IL-15Ra (cat.no. # sc-374023, Santa Cruz, USA) antibodies at 1:2,000 dilution in 2% fat-free milk. The secondary antibody was a monoclonal anti-mouse IgGκ BP-HRP conjugated (sc-516102, Santa Cruz Biotechnology, USA), used at a 1:2,500 dilution. Blots were visualized by ChemiDoc System (Bio-Rad, USA).
Viral titer and cytotoxicity estimation
In this study, BHK-21 cells were used for viral propagation and titer estimation. In a 96-well plate, 1 × 104 cells were seeded per well. Upon forming a monolayer, the nascent medium was discarded and replaced with a medium with decreased FBS content (2%). Cells were infected with 10-fold serial dilutions of strains LIVP-RFP, LIVP-IL15-RFP, and LIVP-IL15Ra-RFP and then incubated for 48 h. TCID50 (50% tissue culture infective dose) was assessed by the Reed-Muench method.53 In addition, a cytotoxicity test was performed using the MTT assay to evaluate the recombinant virus’s capability to kill tumor cells. 4T1, CT26, and BHK-21 (reference) cell lines were seeded at 1 × 104 cells per well in 96-well plates, followed by infection with the MOI 1 of the viruses (LIVP-RFP, LIVP-IL15-RFP, LIVP-IL15Ra-RFP, and mixture of LIVP-IL15-RFP and LIVP- IL15Ra-RFP). Cell viability was determined by MTT assay at indicated time points of 24, 48, and 72 h after infection.54
Assessment of viral replication in vitro by flow cytometry
As the RFP expression rate correlates with the viral replication efficacy in infected cells, flow cytometry was used to assess the kinetics of viral replication. 4T1, CT26, and BHK-21 cells were seeded at 1 × 105 cells per well in 12-well plates and then infected with LIVP-RFP, LIVP-IL15-RFP, LIVP-IL15Ra-RFP, and a combination of LIVP-IL15-RFP and LIVP- IL15Ra-RFP at MOI 1. Cells were harvested at 24 and 48 h post infection for analysis. Samples were analyzed (10,000 events per sample) by detection of red fluorescence using a BD LSR Fortessa cytofluorimeter (Beckman Dickinson, USA), the FACS Diva (Beckman Dickinson, USA), and Flowing Software 2.0 (Turku Bioscience Center, Finland).
Enzyme-linked immunosorbent assay
To determine the ability of LIVP-IL15-RFP/LIVP-IL15Ra-RFP mixture to direct formation of the IL-15/IL-15Rα complex, 105 of 4T1 and CT26 cells were seeded in a 12-well plate and infected at MOI 1 of the viruses. 24, 48, and 72 h after infection, supernatant and lysate were collected. The presence and concentration of the complex were assessed by ELISA using a murine IL-15/IL-15R complex ELISA kit per the manufacturer’s recommendations (#BMS6023, Invitrogen, USA).
The IL-15/IL-15Rα complex level in sera and TIFs of treated 4T1 mice models were measured 1 and 7 days after treatment (n = 3 per each time point) using the same ELISA kit.
Assessment of therapeutic efficacy
All animals used in this study were authorized by the Engelhardt Institute of Molecular Biology, Moscow, Russia. Female 6-week-old BALB/c mice were used to establish 4T1 breast cancer or CT26 colon carcinoma models; 1×106 of 4T1 or CT26 cells were injected subcutaneously into the mice’s right flanks. The mice were housed under controlled conditions, with free access to food and water. Five groups of animals were used (n = 13 for each therapy): LIVP-RFP, LIVP-IL15-RFP, LIVP-IL15Ra-RFP, the combination of LIVP-IL15-RFP with LIVP- IL15Ra-RFP, and control group. While tumors reached approximately 100 mm3 in volume, 1 × 108 PFU (plaque-forming units) of the viruses (for combination therapy 5 × 107 of each virus) in 100 μL of PBS was injected intratumorally twice with an interval of 2 days to all mice in each corresponding group (9th and 11th days), and the control group received PBS. The tumor volume was measured by calipers every other day from the first day of tumor formation until tumors of all animals in the control group gained the maximum tumor volume indicated by the animal care guidelines. Tumor growth and survival curves were determined accordingly.
Analysis of tumor-infiltrating lymphocytes
4T1 breast cancer syngeneic mice treated with viruses or PBS were sacrificed 24 h and 7 days after virus injection (n = 3 for each time point), and their tumors were collected in RPMI-1640 containing 2% FBS and 1 mg/ml collagenase IV (Sigma, USA). After 30 min of incubation at 37°C, samples were homogenized in PBS containing 2% FBS and 1 mM EDTA. After obtaining single-cell suspension, 107 cells were incubated with Fc Block (anti-mouse CD16/CD32; 101302) and then stained with antibodies: PE anti-mouse CD4 (100512), Pacific Blue anti-mouse CD8a (100725), FITC anti-mouse CD45 (103108), and PE anti-mouse F4/80 (123110) antibodies (all from Biolegend, USA). The stained cells were acquired on BD LSR Fortessa cytofluorimeter (Beckman Dickinson, USA), and data were analyzed using Flowing Software 2.0 (Turku Bioscience Center, Finland).
Assessment of released cytokines
1 day and 7 days after the therapy, sera and tumor samples without necrosis were collected from three mice from each group to analyze the level of released cytokines during the treatment. 0.25 g of tumor tissue was homogenized and processed for this experiment. LEGENDplex MU Cytokine Release Syndrome Panel (13-plex) w/FP kit (BioLegend, USA) was used to determine the level of the interferon (IFN)-α, IFN-γ, interleukin (IL)-4, IL-6, IL-10, chemokine C-C motif ligand 2 (CCL2 or MCP-1), CCL3 (or MIP-1α), CCL4 (or MIP-1β), chemokine C-X-C motif ligand 9 (CXCL9 or MIG), CXCL10 (or IP-10), TNF-α, VEGF, and granulocyte-macrophage colony-stimulating factor (GM-CSF).
Histological study
At the end of the experiment, the 4T1 breast cancer syngeneic mice were sacrificed by an overdose of propofol administered intraperitoneally. The histological study was performed on three mice from each group. Following apnea, the animal's thorax and mediastinum were dissected, the right atrium was opened for blood removal, the left ventricle was punctured, and the animals were perfused with 100 mL of 10% buffered formalin solution (Thermo Fisher Scientific, USA). Samples from tumors, spleen, and liver of treated and control mice were harvested and post-fixed in the same solution at 4°С overnight, followed by paraffin embedding. 5-μm-thick paraffin sections were prepared and stained using standard hematoxylin and eosin staining.55
Statistics
All data were checked for normality and represented as mean ± SD. Statistical analysis was performed using ANOVA analysis. Differences were considered significant by ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. All graphs and statistical analysis were performed by GraphPad Prism version 8.0.2 (GraphPad Software, USA).
Data availability
The data analyzed in this research are available from the corresponding author upon request.
Acknowledgments
The local Ethical Committee of the Engelhardt Institute of Molecular Biology, Moscow, Russia approved the animal study. (Protocol No. 1 from April 22, 2022). In vivo experiment was conducted per directive 2010/63/EU on protecting animals used for scientific purposes by the European Parliament and the Council of European Union dated September 22, 2010. Mice were housed under standard conditions with access to water and food ad libitum. All efforts were made to minimize the pain of animals and keep the number of mice required for the experiment at an absolute minimum. The development of oncolytic viruses was supported by the Russian Science Foundation (grant #20-75-10157). In vivo experiments were carried out with the support of the Russian Science Foundation (grant # 22-64-00057). All the molecular studies were supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2019-1660). The authors are grateful to Dr. Katya A. Chernavina and Senior staff Innocka Isaeva for their help with the histological investigation and Dr. Natalie Kushnir for her kind help with the manuscript editing.
Author contributions
Conceptualization, Y.S., A.L., and P.C.; methodology, V.B., Y.S., and A.L.; formal analysis, Y.S. and A.L.; investigation, Y.S., P.V., V.K., G.Y., A.S., A.L., D.K., K.Z., and M.V.; histology F.Z. and V.B.; resources, V.B., S.K, and A.L.; writing—original draft preparation, Y.S.; writing—review and editing, Y.S., P.C. A.L., and V.B.; visualization, Y.S., V.B., and A.L.; supervision, P.C., V.B., and A.L.; project administration, A.T, A.L., and P.C.; funding acquisition, A.L, G.Y., and P.C. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
The authors report no conflicts of interest in this work.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omto.2023.05.002.
Supplemental information
References
- 1.Ylösmäki E., Cerullo V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020;65:25–36. doi: 10.1016/j.copbio.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Thorne S.H. Next-generation oncolytic vaccinia vectors. Methods Mol. Biol. 2012;797:205–215. doi: 10.1007/978-1-61779-340-0_14. [DOI] [PubMed] [Google Scholar]
- 3.Thorne S.H., Hwang T.H., Kirn D.H. Vaccinia virus and oncolytic virotherapy of cancer. Curr. Opin. Mol. Ther. 2005;7:359–365. [PubMed] [Google Scholar]
- 4.Whilding L.M., Archibald K.M., Kulbe H., Balkwill F.R., Öberg D., McNeish I.A. Vaccinia virus induces programmed necrosis in ovarian cancer cells. Mol. Ther. 2013;21:2074–2086. doi: 10.1038/mt.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. USA. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J., Xiao Z., Zhao Q., Li M., Wu X., Zhang L., Hu W., Cho C.H. Anti-cancer therapy with TNFα and IFNγ: a comprehensive review. Cell Prolif. 2018;51 doi: 10.1111/cpr.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhode P.R., Egan J.O., Xu W., Hong H., Webb G.M., Chen X., Liu B., Zhu X., Wen J., You L., et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol. Res. 2016;4:49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois S., Mariner J., Waldmann T.A., Tagaya Y. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 9.van Rikxoort M., Michaelis M., Wolschek M., Muster T., Egorov A., Seipelt J., Doerr H.W., Cinatl J., Jr. Oncolytic effects of a novel influenza A virus expressing interleukin-15 from the NS reading frame. PLoS One. 2012;7:e36506. doi: 10.1371/journal.pone.0036506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephenson K.B., Barra N.G., Davies E., Ashkar A.A., Lichty B.D. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012;19:238–246. doi: 10.1038/cgt.2011.81. [DOI] [PubMed] [Google Scholar]
- 11.Niu Z., Bai F., Sun T., Tian H., Yu D., Yin J., Li S., Li T., Cao H., Yu Q., et al. Recombinant newcastle disease virus expressing IL15 demonstrates promising antitumor efficiency in melanoma model. Technol. Cancer Res. Treat. 2015;14:607–615. doi: 10.7785/tcrt.2012.500414. [DOI] [PubMed] [Google Scholar]
- 12.Yan Y., Li S., Jia T., Du X., Xu Y., Zhao Y., Li L., Liang K., Liang W., Sun H., Li R. Combined therapy with CTL cells and oncolytic adenovirus expressing IL-15-induced enhanced antitumor activity. Tumour Biol. 2015;36:4535–4543. doi: 10.1007/s13277-015-3098-7. [DOI] [PubMed] [Google Scholar]
- 13.Nishio N., Dotti G. Oncolytic virus expressing RANTES and IL-15 enhances function of CAR-modified T cells in solid tumors. Oncoimmunology. 2015;4 doi: 10.4161/21505594.2014.988098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowalsky S.J., Liu Z., Feist M., Berkey S.E., Ma C., Ravindranathan R., Dai E., Roy E.J., Guo Z.S., Bartlett D.L. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol. Ther. 2018;26:2476–2486. doi: 10.1016/j.ymthe.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backhaus P.S., Veinalde R., Hartmann L., Dunder J.E., Jeworowski L.M., Albert J., Hoyler B., Poth T., Jager D., Ungerechts G., Engeland C.E. Immunological effects and viral gene expression determine the efficacy of oncolytic measles vaccines encoding IL-12 or IL-15 agonists. Viruses. 2019;11 doi: 10.3390/v11100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tosic V., Thomas D.L., Kranz D.M., Liu J., McFadden G., Shisler J.L., MacNeill A.L., Roy E.J. Myxoma virus expressing a fusion protein of interleukin-15 (IL15) and IL15 receptor alpha has enhanced antitumor activity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger C., Berger M., Hackman R.C., Gough M., Elliott C., Jensen M.C., Riddell S.R. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yakubitskiy S.N., Kolosova I.V., Maksyutov R.A., Shchelkunov S.N. Attenuation of vaccinia virus. Acta Nat. 2015;7:113–121. [PMC free article] [PubMed] [Google Scholar]
- 19.Shvalov A.N., Sivolobova G.F., Kuligina E.V., Kochneva G.V. Complete genome sequence of vaccinia virus strain L-IVP. Genome Announc. 2016;4 doi: 10.1128/genomeA.00372-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentschev I., Adelfinger M., Josupeit R., Rudolph S., Ehrig K., Donat U., Weibel S., Chen N.G., Yu Y.A., Zhang Q., et al. Preclinical evaluation of oncolytic vaccinia virus for therapy of canine soft tissue sarcoma. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shakiba Y., Vorobyev P.O., Naumenko V.A., Kochetkov D.V., Zajtseva K.V., Valikhov M.P., Yusubalieva G.M., Gumennaya Y.D., Emelyanov E.A., Semkina A.S., et al. Oncolytic efficacy of a recombinant vaccinia virus strain expressing bacterial flagellin in solid tumor models. Viruses. 2023;15:828. doi: 10.3390/v15040828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gholami S., Marano A., Chen N.G., Aguilar R.J., Frentzen A., Chen C.H., Lou E., Fujisawa S., Eveno C., Belin L., et al. A novel vaccinia virus with dual oncolytic and anti-angiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res. Treat. 2014;148:489–499. doi: 10.1007/s10549-014-3180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y., Huang X., Liu X., He Y., Hu Z., Xu W., Cao G., He W. Remodels the immunosuppressive tumor microenvironment by combination of Bacillus calmette-guerin and anti-PD-L1 in an orthotopic triple-negative breast cancer mouse model. Onco. Targets Ther. 2021;14:2247–2258. doi: 10.2147/OTT.S294129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd C.M., Hruby D.E. Construction of recombinant vaccinia virus: cloning into the thymidine kinase locus. Methods Mol. Biol. 2004;269:31–40. doi: 10.1385/1-59259-789-0:031. [DOI] [PubMed] [Google Scholar]
- 25.Steel J.C., Waldmann T.A., Morris J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budagian V., Bulanova E., Paus R., Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Xiao L.S., Hu C.Y., Cui H., Li R.N., Hong C., Li Q.M., Huang C.Y., Dong Z.Y., Zhu H.B., Liu L. Splenomegaly in predicting the survival of patients with advanced primary liver cancer treated with immune checkpoint inhibitors. Cancer Med. 2022;11:4880–4888. doi: 10.1002/cam4.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waks A.G., Winer E.P. Breast cancer treatment: a review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 29.El Masri J., Phadke S. Breast cancer epidemiology and contemporary breast cancer care: a review of the literature and clinical applications. Clin. Obstet. Gynecol. 2022;65:461–481. doi: 10.1097/GRF.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 30.Guse K., Cerullo V., Hemminki A. Oncolytic vaccinia virus for the treatment of cancer. Expert Opin. Biol. Ther. 2011;11:595–608. doi: 10.1517/14712598.2011.558838. [DOI] [PubMed] [Google Scholar]
- 31.Zeh H.J., Bartlett D.L. Development of a replication-selective, oncolytic poxvirus for the treatment of human cancers. Cancer Gene Ther. 2002;9:1001–1012. doi: 10.1038/sj.cgt.7700549. [DOI] [PubMed] [Google Scholar]
- 32.Lauer U., Zimmermann M., Sturm J., Koppenhoefer U., Bitzer M., Malek N.P., Glatzle J., Koenigsrainer A., Moehle R., Fend F., et al. Phase I/II clinical trial of a genetically modified and oncolytic vaccinia virus GL-ONC1 in patients with unresactable, chemotherapy-resistant peritoneal carcinomatosis. J. Clin. Oncol. 2013;31:3098. doi: 10.1200/jco.2013.31.15_suppl.3098. [DOI] [Google Scholar]
- 33.Van den Bergh J.M., Lion E., Van Tendeloo V.F., Smits E.L. IL-15 receptor alpha as the magic wand to boost the success of IL-15 antitumor therapies: the upswing of IL-15 transpresentation. Pharmacol. Ther. 2017;170:73–79. doi: 10.1016/j.pharmthera.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Stoklasek T.A., Schluns K.S., Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epardaud M., Elpek K.G., Rubinstein M.P., Yonekura A.R., Bellemare-Pelletier A., Bronson R., Hamerman J.A., Goldrath A.W., Turley S.J. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 36.Gaston D.C., Odom C.I., Li L., Markert J.M., Roth J.C., Cassady K.A., Whitley R.J., Parker J.N. Production of bioactive soluble interleukin-15 in complex with interleukin-15 receptor alpha from a conditionally-replicating oncolytic HSV-1. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isvoranu G., Surcel M., Munteanu A.N., Bratu O.G., Ionita-Radu F., Neagu M.T., Chiritoiu-Butnaru M. Therapeutic potential of interleukin-15 in cancer (review) Exp. Ther. Med. 2021;22:675. doi: 10.3892/etm.2021.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrörs B., Boegel S., Albrecht C., Bukur T., Bukur V., Holtsträter C., Ritzel C., Manninen K., Tadmor A.D., Vormehr M., et al. Multi-omics characterization of the 4T1 murine mammary gland tumor model. Front. Oncol. 2020;10:1195. doi: 10.3389/fonc.2020.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z., Qiu Y., Lu W., Jiang Y., Wang J. Immunotherapeutic interventions of triple negative breast cancer. J. Transl. Med. 2018;16:147. doi: 10.1186/s12967-018-1514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kochneva G., Sivolobova G., Tkacheva A., Grazhdantseva A., Troitskaya O., Nushtaeva A., Tkachenko A., Kuligina E., Richter V., Koval O. Engineering of double recombinant vaccinia virus with enhanced oncolytic potential for solid tumor virotherapy. Oncotarget. 2016;7:74171–74188. doi: 10.18632/oncotarget.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greiner S., Humrich J.Y., Thuman P., Sauter B., Schuler G., Jenne L. The highly attenuated vaccinia virus strain modified virus Ankara induces apoptosis in melanoma cells and allows bystander dendritic cells to generate a potent anti-tumoral immunity. Clin. Exp. Immunol. 2006;146:344–353. doi: 10.1111/j.1365-2249.2006.03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z., Chen L., Qin Z. Paradoxical roles of IL-4 in tumor immunity. Cell. Mol. Immunol. 2009;6:415–422. doi: 10.1038/cmi.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin J., Lin J., Xu A., Lou J., Qian C., Li X., Wang Y., Yu W., Tao H. CCL2: an important mediator between tumor cells and host cells in tumor microenvironment. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.722916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin K., Shen Y., He K., Xu Z., Li G., Teng L. Aflibercept (VEGF trap): one more double-edged sword of anti-VEGF therapy for cancer? Clin. Transl. Oncol. 2010;12:526–532. doi: 10.1007/s12094-010-0550-4. [DOI] [PubMed] [Google Scholar]
- 45.Chulpanova D.S., Kitaeva K.V., Green A.R., Rizvanov A.A., Solovyeva V.V. Molecular aspects and future perspectives of cytokine-based anti-cancer immunotherapy. Front. Cell Dev. Biol. 2020;8:402. doi: 10.3389/fcell.2020.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conlon K.C., Miljkovic M.D., Waldmann T.A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res. 2019;39:6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan W.L., Shen K.Y., Tien C.Y., Chen Y.A., Liu S.J. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy. 2017;9:347–360. doi: 10.2217/imt-2016-0141. [DOI] [PubMed] [Google Scholar]
- 48.Russell S.J., Peng K.W. Oncolytic virotherapy: a contest between apples and oranges. Mol. Ther. 2017;25:1107–1116. doi: 10.1016/j.ymthe.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Horssen R., Ten Hagen T.L.M., Eggermont A.M.M. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 50.Trnka J., Špaček M., Šírová V., Mitáš P., Hodková G., Kubinyi J., Špunda R., Lindner J. [Hyperthermic isolated limb perfusion combined with Tasonermin - a perfusion leakage monitoring technique] Klin. Onkol. 2016;29:375–379. doi: 10.14735/amko2016375. [DOI] [PubMed] [Google Scholar]
- 51.Tokunaga R., Zhang W., Naseem M., Puccini A., Berger M.D., Soni S., McSkane M., Baba H., Lenz H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - a target for novel cancer therapy. Cancer Treat. Rev. 2018;63:40–47. doi: 10.1016/j.ctrv.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cotter C.A., Earl P.L., Wyatt L.S., Moss B. Preparation of cell cultures and vaccinia virus stocks. Curr. Protoc. Protein Sci. 2017;89:5.12.1–5.12.18. doi: 10.1002/cpps.34. [DOI] [PubMed] [Google Scholar]
- 53.Ramakrishnan M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016;5:85–86. doi: 10.5501/wjv.v5.i2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan D.M. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol. Biol. 1998;79:179–183. doi: 10.1385/0-89603-448-8:179. [DOI] [PubMed] [Google Scholar]
- 55.Feldman A.T., Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014;1180:31–43. doi: 10.1007/978-1-4939-1050-2_3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this research are available from the corresponding author upon request.