Abstract
Hydrogels based on various polymeric materials have been successfully developed in recent years for a variety of skin applications. Several studies have shown that hydrogels with regenerative, antibacterial, and antiinflammatory properties can provide faster and better healing outcomes, particularly in chronic diseases where the normal physiological healing process is significantly hampered. Various experimental tests are typically performed to assess these materials' ability to promote angiogenesis, re-epithelialization, and the production and maturation of new extracellular matrix. Immunohistochemistry is important in this context because it allows for the visualization of in situ target tissue factors involved in the various stages of wound healing using antibodies labelled with specific markers detectable with different microscopy techniques. This review provides an overview of the various immunohistochemical techniques that have been used in recent years to investigate the efficacy of various types of hydrogels in assisting skin healing processes. The large number of scientific articles published demonstrates immunohistochemistry's significant contribution to the development of engineered biomaterials suitable for treating skin injuries.
Key words: Immunohistochemistry, hydrogels, healing biomarkers, skin repair, skin regeneration
Introduction
Skin is the outer organ of vertebrates and, covering the whole body, represents the first line of contact with the environment. From the functional point of view, skin controls body temperature, detects sensation from external stimuli and acts as a protective barrier against physical, chemical and biological noxious agents, thus ensuring the maintenance of body homeostasis. Due to its protecting role, skin is vulnerable and its integrity can be easily compromised by several external factors causing injury, burns, microbial invasion, inflammation, premature aging and even cancer.1-3
In healthy individuals the process of cutaneous repair is very efficient. However, in chronic diseases such as diabetes, arteriosclerosis and vasculitis, the skin regenerative capability is significantly impaired; moreover, serious traumatic injuries (e.g., burns) hardly heal. In these cases, innovative solutions and therapeutic approaches are required to promote faster healing and restore skin functions. To this aim, different strategies have been proposed such as cytokine or stem cell therapy, autograft, allograft, xenotransplantation, and engineered skin substitutes.4,5
Among these approaches, skin substitutes are especially effective due to their easy availability, low risk of infection, reduction of scarring and reduced need for surgical procedures.6 Many materials in different forms have been investigated as skin dressing for tissue regeneration, such as films, patches, sponges, sheets, foams, nanofibres, hydrogels.7 Among them, hydrogels represent a very promising tools in the field of tissue engineering thanks to their unique three-dimensional (3D) structure, good permeability, low toxicity, easy preparation and application, suitability in absorbing wound exudates, and ability to provide a moist environment able to stimulate healing and patient compliance. Hydrogels have also been used for the 3D reconstruction in vitro of human skin for experimental purposes.8-10
Considering the increasing interest on hydrogels designed for skin repair and regeneration, this paper aims to present an overview of the most recent progresses in the development of high-performance hydrogels, highlighting the great contribution of immunohistochemistry (IHC) to the evaluation of the therapeutic potential of these innovative medical devices.
Application of hydrogels to skin wound healing and diseases
Through an accurate preparation and rational design, it is possible to obtain hydrogels endowed with specific functionalities useful for various skin applications. Hydrogels can be broadly classified according to either the material used (producing natural, synthetic or hybrid hydrogels) or the synthesis methods.
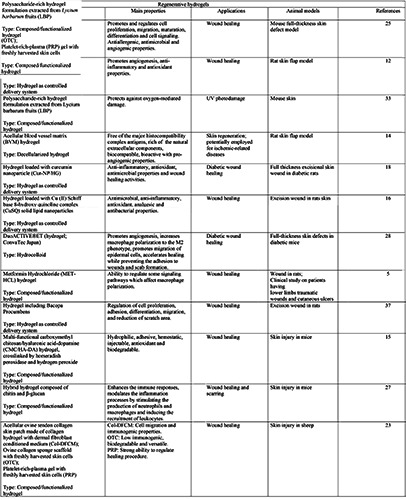
One of the most common applications is to support the regenerative process in different skin injuries such as chronic wounds, deep ulcers or burns, and in general skin loss. In the context of damaged skin, numerous biomimetic gels have been developed and modified to match the timescale for the distinct stages of acute wound healing (Table 1). These materials represent a promising dressing for skin treatment thank to their ability to act on a single or on various stages of the healing process. Since hydrogels act as temporary skin substitutes, they must be sterile, biocompatible, biodegradable/removable, non immunogenic, impermeable to exogenous bacteria, able to firmly cover the open wound thus guaranteeing a protective microenvironment.11 All these properties can be achieved by designing gels with specific physico-chemical characteristics or by adding sensitive agents. It is also possible to develop hydrogels able to respond to specific external physical stimuli. 11 For example, Zheng et al. developed an injectable pregel solution whose elastic modulus drastically outweighs the viscous modulus at 32°C, thus leading to phase transition from liquid to gel and providing a suitable cell survival environment.12 Moreover, hydrogels can be employed on the injured skin as space filling agents, 3D framework for cells, or delivery support for bioactive molecules.13 For example, some injectable hydrogels have shown a rapid adaptability to the irregularly shaped defects of the damaged skin, ensuring a complete cover with minimally invasive injection procedures.14,15 Other hydrogels were tailored to deliver bioactive molecules able to accelerate the healing process, angiogenesis, re-epithelialization as well as the production of extracellular matrix. The application of therapeutic substances incorporated into the hydrogel structure protects these compounds against the action of the local enzymes while ensuring their longterm efficacy by controlling their sustained release.12,13 Another possibility is the use of hydrogels containing nanoparticles 16-18 or microspheres19 as drug-delivery systems.
Also cell-loaded hydrogels may provide a therapeutic support in all stages of wound healing. For example, hydrogels enriched with human dermal fibroblasts and epidermal keratinocytes have been shown to accelerate the wound healing thanks to the ability of fibroblasts to secrete cytokines, growth factors and extracellular matrix components promoting the proliferation of the epidermal cells.20,21 Other studies proved the potential of undifferentiated stem cells to enhance the immunoregulation, release of the regenerative secretome, angiogenesis and cell recruitment in wound healing.22
A further type of healing-promoting procedure is based on hydrogels enriched with platelet-rich plasma (PRP).23 Indeed, PRP plays an essential role during the inflammatory phase by providing several cytokines involved in the regular healing process. 24
Finally, the polymers forming hydrogels can be also able to influence the different healing stages of injured skin by acting on cell proliferation, migration, maturation and differentiation, angiogenesis, cell signalling and inflammation.5,15,25-27
In chronic wounds caused by diseases such as diabetes, malignant tumours, infections, and vasculopathy, the physiological cascade of events occurring during the healing process is significantly compromised causing persistent infections, inflammatory response, loss of angiogenic potential, reduced extracellular matrix, and degradation of growth factors. To overcome these drawbacks, Kamar and collaborators developed a hydrogel loaded with curcumin nanoparticles that was able to improve the healing process in diabetic rats by promoting re-epithelialization, dermo-epidermal junction formation, dermis reorganization with significant increased of aquaporin-3 (AQP3), vascular endothelial growth factor (VEGF) expression and collagen deposition.18 Also hydrocolloid dressing proved to promote angiogenesis, reepithelialization and extracellular matrix remodelling in diabetic mice with full-thickness defects.28
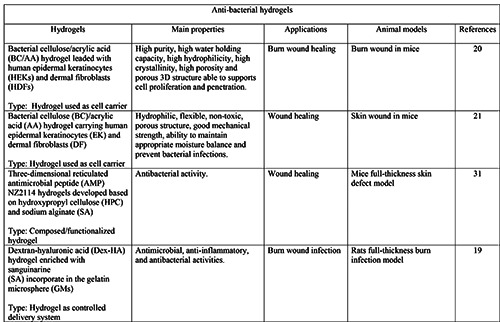
Moreover, in many chronic wounds the inflammatory response is impaired, leading to the development of important infections: here, the inability of immune cells to eliminate infection represents the main cause of persistent inflammation. To overcome these issues, several hydrogels made of or combined with antiseptic/antibacterial materials, and gels with immunoregulatory activity have been successfully formulated for wound healing and dermatological purposes (Tables 2 and 3).26,29-32 For example, Lacatusu et al. developed lipid nanostructured carriersbased hydrogels loaded with natural bioactive compounds able to improve the local antioxidant and anti-inflammatory activities in inflamed skin.17 Hydrogels also proved to be beneficial to burn wounds, with high-quality and efficient scar inhibition thanks to their water reserving, moist environment and antibacterial properties. 19,20 Finally, hydrogels also represent a spearhead in the cosmetics industry. For example, polysaccharide-rich hydrogels proved to successfully reduce the UV-induced photodamage in the skin of mice.33
Table 1.
Hydrogels recently developed for regenerative purpose.
Immunohistochemistry for hydrogel development
To provide information on the effects of hydrogels designed for skin applications, different techniques are commonly used. Among these, IHC is the method of choice for the localization and semi-quantification of protein expression in tissue and cell components of the skin.
In injured skin, wound healing is the common physiological process governed by a sequence of phases corresponding to inflammation, proliferation and remodelling. In normal healing, all these stages are characterized by the activities of multiple cell types and messenger factors able to restore the homeostasis and proper tissue/organ function.34,35 These mechanisms are impaired in traumatic or chronic injuries and, in this case, hydrogels may be a helpful tool to restore the normal wound healing process. The immunohistochemical detection of wound healing biomarkers is essential to assess the suitability of hydrogels for skin repair and regeneration. Most studies have been focused on factors involved in promoting fibroblast proliferation, extracellular matrix remodelling and angiogenesis, including cell growth factors among which transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), vascular endothelial cell growth factor (VEGF), hepatocyte growth factor (HGF), and interleukins such as IL-6 and IL-22 (Figure 1). All the studies considered in this review applied IHC at light microscopy, sometimes using fluorescent markers21,23 but more frequently using enzyme-based revealing systems,12,14-16,18,25,27,28,31,33 probably because of the intrinsic autofluorescence of the skin that prevents the visualization or leads to underestimation of the signal arising from the fluorophore bound to the antibody.36
There are various examples of application of IHC to detect markers of the wound healing phases. The expression of tumour necrosis factor (TNF-α), a pro-inflammatory cytokine released by platelets during the inflammatory stage, was investigated by Abou El-ezz et al. in formalin-fixed paraffin-embedded (FFPE) rat skin sections by using secondary horseradish-peroxidase (HRP)-labelled antibodies and diaminobenzidine tetrahydrochloride (DAB) to develop the colour. The immunopositivity for TNF-α was digitally quantified as the percentage of labelled area in the wounds. IHC staining revealed that hydrogels loaded with the Cu (II) Schiff base 8-hydroxy quinoline complex were able to decrease inflammation; moreover, using the same detection and quantification methods, the authors found an increase of VEGF, that is known to promote angiogenesis and improve vascularization. 16 Similarly, IHC was used in other investigations to evaluate the levels of VEGF in healthy and diabetic rat models of wound treated with Apelin-13-loaded chitosan (CH)/s-sodiumglycerophosphate (s-GP) gel or hydrogel loaded with curcumin nanoparticles (Cur-NP/HG).12,18 In particular, the rat flap zones were subjected to IHC staining using streptavidinperoxidase/ DAB method and the images, collected with an inverted microscope, were used to measure the integral absorbance of the VEGF level.12 Similarly, Kamar et al. incubated FFPE rat skin sections with an anti-VEGF primary antibody and biotinylated secondary antibody, and the area of VEGF-positive staining was measured.18 IHC staining of CD34 and AQP3 was performed by the same authors with similar samples and techniques to evaluate the cell differentiation and maturation occurring during the proliferative phase of the healing process.12,18 In addition, the level of CD34 expression was measured by Zheng et al. through the integral absorbance as previously reported for the VEGF.12
Table 2.
Hydrogels recently developed for antibacterial purpose.
Epidermal maturation has also been investigated in sections of mouse and sheep skin, by evaluating the immunolabelling for cytokeratin 14 (CK-14,, a marker of the proliferating basal keratinocytes), and for involucrin and CK10 (two markers linked to the final maturation stage of the epidermal cells): fluorescent secondary antibodies were here used, and images taken by confocal fluorescence microscopy.21,23 The re-epithelialization process was also studied by Kang et al. on mouse skin through the immunodetection of CK14 and CK15 using an HRP-conjugated secondary antibody colour-developed with DAB.27
Another essential process that occurs during the proliferative phase is the formation of new blood vessels: this process can be easily monitored by IHC, by detecting angiogenic markers such as FGF, TGF-β, the hypoxia-inducible factor (HIF), VEGF and CD31: a semi-quantitative assessment of the angiogenetic process was obtained by measuring the integral absorbance and mean optical density of the immunostaining and by counting the new blood vessels per square millimetre area as an estimate of the microvessel formation.14,16,25,31
Biomarkers related to the remodelling phase of wound healing have been also widely studied throught IHC to set up hydrogels for skin applications. This phase is mainly characterized by wound contraction and collagen deposition. In the granulation tissue, fibroblasts are activated to become myofibroblasts, which possess contractile ability mediated by stress fibres made of alpha-smooth muscle actin (α-SMA). Thus α-SMA is a suitable marker used in several works to evaluate hydrogel ability to enhance cutaneous wound healing. Many authors applied secondary antibodies conjugated with Alexa Fluor probes, or with streptavidin-biotin complex followed by chromogenic detection with DAB, in FFPE mouse skin samples.15,21,25
Table 3.
Hydrogels recently developed for anti-inflammatory purpose.
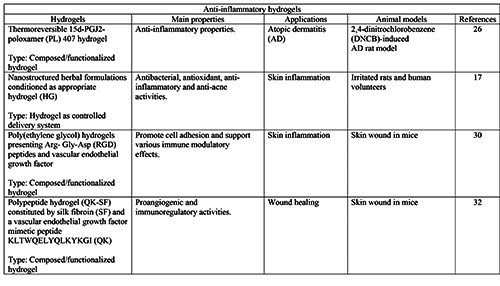
Figure 1.
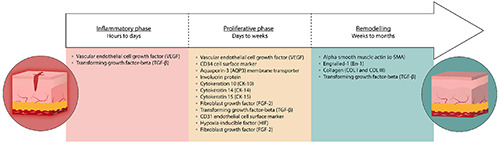
Factors involved in the different phases of cutaneous wound healing commonly investigated by IHC.
Another protein marker that was used for studying the effects of hydrogels on wound healing and scarring27 is engrailed-1 (En- 1), which is expressed by fibroblasts and leads to an over-deposition of the extracellular matrix and promotes the formation of scars in skin wounds.
An alternative target easily identifiable by IHC is collagen. Collagen type I represents the main structural element of the dermis that gradually replace collagen III, the major component of the granulation tissue. The immunolabelling for collagen I and collagen III was performed on FFPE rat or mouse skin samples using fluorescent (Alexa Fluor)21 or enzymatic (HRP/DAB or DAB) revealing systems28,33,37 in order to evaluate the ability of various kind of hydrogels to promote matrix remodelling during the regenerative process. In addition, Neves et al. demonstrated that polysaccharide-rich hydrogel formulation combined with photobiomodulation provides a concomitantly increase in the levels of collagen I, III and FGF-2, thus demonstrating that these combined treatments are effective in repairing the UV-induced photodamage in the skin of hairless mice.33
Another biomarker widely studied is TGF-β that is involved in different processes of the normal tissue repair mechanisms, including inflammation, angiogenesis, fibroblast proliferation, collagen synthesis and deposition as well as matrix remodelling. An overexpression of TGF-β1 was observed by Tawfeek et al. in wound treated with metformin hydrochloride hydrogel in skin samples, thus demonstrating the effective topical healing activity of this hydrogel type:5 in this work, a semi-quantitative analysis of the immunopositivity was performed according to the Remmele immunoreactive score.38
Most of the markers mentioned above have also been used to evaluate the regenerative potential of hydrogels in the case of skin damages caused by burns or inflammatory states.20,26 For example Napimoga et al. examined the efficacy of the topical 15d-PGJ2-poloxamer 407 hydrogel in an rat model of atopic dermatitis: a significant reduction of the immunopositivity positivity for the transcription factor RORϒt and TNF-α was observed after the topical treatment, suggesting that this hydrogel was able to improve atopic dermatitis through a suppression of the immune response.26
Concluding remarks
This review summarizes the recent advances in the development of hydrogels as medical devices for skin repair and regeneration. The research on hydrogels has greatly increased in the last years because of their several advantages compared to the classical therapeutic approaches; in particular, the hydrogels can be loaded with cells or bioactive compounds having antimicrobial, anti-inflammatory, angiogenic or proliferative properties, thus providing an active contribution to the wound healing.
Notably, IHC proved to be a key technique to test the efficacy of hydrogels. In fact, to assess the actual ability of hydrogels to improve the healing process, different biomarkers specifically expressed in the different healing phases need to be detected in skin sample and IHC proved to be a versatile, relatively simple and affordable technique for their visualization in situ. Thus, the classical immunohistochemical techniques have become indispensable for the development of engineered biomaterials in the leading-edge research field of regenerative medicine.
References
- 1.Wong R, Geyer S, Weninger W, Guimberteau JC, Wong JK. The dynamic anatomy and patterning of skin. Exp Dermatol 2016;25:92-8. [DOI] [PubMed] [Google Scholar]
- 2.Dąbrowska AK, Spano F, Derler S, Adlhart C, Spencer ND, Rossi RM. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res Technol 2018;24:165-74. [DOI] [PubMed] [Google Scholar]
- 3.Lee H-J, Kim M. Skin barrier function and the microbiome. Int J Mol Sci 2022;23:13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santema TB, Poyck PPC, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev 2016;2:CD011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tawfeek HM, Abou-Taleb DAE, Badary DM, Ibrahim M, Abdellatif AAH. Pharmaceutical, clinical, and immunohistochemical studies of metformin hydrochloride topical hydrogel for wound healing application. Arch Dermatol Res 2020;312:113-21. [DOI] [PubMed] [Google Scholar]
- 6.Varkey M, Ding J, Tredget EE. Advances in skin substitutes - Potential of tissue engineered skin for facilitating anti-fibrotic healing. J Funct Biomater 2015;6:547-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamoun EA, Kenawy ERS, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVAbased hydrogel dressings. J Adv Res 2017;8:217-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carton F, Malatesta M. In vitro models of biological barriers for nanomedical research. Int J Mol Sci 2022;23:8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carton F, Malatesta M. Assessing the interactions between nanoparticles and biological barriers in vitro: A new challenge for microscopy techniques in nanomedicine. Eur J Histochem 2022;66:3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan SH, Chua DAC, Tang JRJ, Bonnard C, Leavesley D, Liang K. Design of hydrogel-based scaffolds for in vitro three-dimensional human skin model reconstruction. Acta Biomater 2022;153:13-37. [DOI] [PubMed] [Google Scholar]
- 11.Hao R, Cui Z, Zhang X, Tian M, Zhang L, Rao F, et al. Rational design and preparation of functional hydrogels for skin wound healing. Front Chem 2022;9:839055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng W, Wang J, Xie L, Xie H, Chen C, Zhang C, et al. An injectable thermosensitive hydrogel for sustained release of apelin-13 to enhance flap survival in rat random skin flap. J Mater Sci Mater Med 2019;30:106. [DOI] [PubMed] [Google Scholar]
- 13.Zagorska-Dziok M, Sobczak M. Hydrogel-based active substance release systems for cosmetology and dermatology application: A review. Pharmaceutics 2020;12:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu W, Xu P, Feng B, Lu Y, Bai J, Zhang J, et al. A hydrogel derived from acellular blood vessel extracellular matrix to promote angiogenesis. J Biomater Appl 2019;33:1301-13. [DOI] [PubMed] [Google Scholar]
- 15.Cui L, Li J, Guan S, Zhang K, Zhang K, Li J. Injectable multifunctional CMC/HA-DA hydrogel for repairing skin injury. Mater Today Bio 2022;14:100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abou El-ezz D, Abdel-Rahman LH, Al-Farhan BS, Mostafa DA, Ayad EG, Basha MT, et al. Enhanced in vivo wound healing efficacy of a novel hydrogel loaded with copper (II) Schiff base quinoline complex (CuSQ) solid lipid nanoparticles. Pharmaceuticals 2022;15:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacatusu I, Istrati D, Bordei N, Popescu M, Seciu AM, Panteli LM, et al. Synergism of plant extract and vegetable oils-based lipid nanocarriers: Emerging trends in development of advanced cosmetic prototype products. Mater Sci Eng C 2020;108:110412. [DOI] [PubMed] [Google Scholar]
- 18.Kamar SS, Abdel-Kader DH, Rashed LA. Beneficial effect of curcumin nanoparticles-hydrogel on excisional skin wound healing in type-I diabetic rat: Histological and immunohistochemical studies. Ann Anat 2019;222:94-102. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Q, Jiang M, Liu Q, Yan S, Feng L, Lan Y, et al. Enhanced healing activity of burn wound infection by a dextran-HA hydrogel enriched with sanguinarine. Biomater Sci 2018;6:2472-86. [DOI] [PubMed] [Google Scholar]
- 20.Mohamad N, Loh EYX, Fauzi MB, Ng MH, Mohd Amin MCI. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv Transl Res 2019;9:444-52. [DOI] [PubMed] [Google Scholar]
- 21.Loh EYX, Mohamad N, Fauzi MB, Ng MH, Ng SF, Mohd Amin MCI. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci Rep 2018;8:2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan F, Saha S, Hanjaya-Putra D. Biomimetic hydrogels to promote wound healing. Front Bioeng Biotechnol 2021;9:718377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramaniam T, Shaiful Hadi N, Sulaiman S, Fauzi MB, Hj Idrus RB, Chowdhury SR, et al. Comparison of three different skin substitutes in promoting wound healing in an ovine model. Burns 2022;48:1198-208. [DOI] [PubMed] [Google Scholar]
- 24.Gad SB, Hafez MH, El-Sayed YS. Platelet-rich plasma and/or sildenafil topical applications accelerate and better repair wound healing in rats through regulation of proinflammatory cytokines and collagen/TGF-β1 pathway. Environ Sci Pollut Res Int 2020;27:40757-68. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Cao X, Jiang H, Che X, Xu X, Ma B, et al. SIKVAVmodified chitosan hydrogel as a skin substitutes for wound closure in mice. Molecules 2018;23:2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napimoga MH, Clemente-Napimoga JT, Machabanski NM, Juliani MEA, Acras PHBC, Macedo CG, et al. The 15d‑PGJ2 hydrogel ameliorates atopic dermatitis through suppression of the immune response. Mol Med Rep 2019;19:4536-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang X, Lei J, Yang C, Zhang P, Li X, Zheng S, et al. A hybrid hydrogel composed of chitin and β-glucan for the effective management of wound healing and scarring. Biomater Sci 2022;10:6024-36. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi T, Ito M, Yamaguchi S, Watanabe S, Honda M, Imahashi T, et al. Hydrocolloid dressing improves wound healing by increasing M2 macrophage polarization in mice with diabetes. Nagoya J Med Sci 2020;82:487-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng Y, Li S, Tang H, Tao X, Fan Y, Huang Y. Medical applications of hydrogels in skin infections: A review. Infect Drug Resist 2023;16:391-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Yague MA, Hymel LA, Olingy CE, McClain C, Ogle ME, Garcia JR, et al. Analyzing immune response to engineered hydrogels by hierarchical clustering of inflammatory cell subsets. Sci Adv 2022;8:eabd8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y, Yang N, Teng D, Mao R, Hao Y, Ma X, et al. Antibacterial peptide NZ2114-loaded hydrogel accelerates Staphylococcus aureus-infected wound healing. Appl Microbiol Biotechnol 2022;106:3639-56. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Wang L, Guo C, Qiu M, Cheng L, Chen K, et al. Vascularized polypeptide hydrogel modulates macrophage polarization for wound healing. Acta Biomaterialia 2023;155:218-34. [DOI] [PubMed] [Google Scholar]
- 33.Neves LMG, Parizotto NA, Tim CR, Floriano EM, Lopez RFV, Venancio T, et al. Polysaccharide-rich hydrogel formulation combined with photobiomodulation repairs UVinduced photodamage in mice skin. Wound Repair Regen 2020;28:645-55. [DOI] [PubMed] [Google Scholar]
- 34.Wallace HA, Basehore BM, Zito PM. Wound healing phases. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 35.Muire PJ, Thompson MA, Christy RJ, Natesan S. Advances in immunomodulation and immune engineering approaches to improve healing of extremity wounds. Int J Mol Sci 2022;23:4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappellozza E, Boschi F, Sguizzato M, Esposito E, Cortesi R, Malatesta M, et al. A spectrofluorometric analysis to evaluate transcutaneous biodistribution of fluorescent nanoparticulate gel formulations. Eur J Histochem 2022;66:3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Cuazitl A, Gomez-Garcia MDC, Hidalgo-Alegria O, Flores OM, Nunez-Gastelum JA, Martinez ESM, et al. Characterization of polyphenolic compounds from bacopa procumbens and their effects on wound-healing process. Molecules 2022;27:6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue].[Article in German]. Pathologe 1987;8:138-40. [PubMed] [Google Scholar]


