Abstract
We used low and high molecular weight fluorescence tracers to investigate the entry of foreign solutes into the brain parenchyma and their exit from it by the glymphatic system, during experimentally induced depressive-like behavior in rats. The tail suspension test (TST), as an acute stressor, is known to induce such a type of behavior, considered to model the human major depressive disorder (MDD). Electroacupuncture (EAP) relieves both depressive-like behavior in rodents and the symptoms of MDD in humans. Here we report that 180 min after the intracisternal injection of the low molecular weight tracer Fluorescein-5-Isothiocianate Conjugated Dextran (FITC-d3), a 15-min duration TST tended to increase the control fluorescence in the brain of rats. Both EAP and sham EAP decreased the fluorescence of FITC-d3 in comparison with the TST, but not the control value. In addition, EAP and sham EAP counteracted the effects of TST. The high molecular weight tracer Ovalbumin Alexa Fluor 555 Conjugate (OA-45) failed to enter the brain parenchyma and accumulated at more superficial sites; however, EAP or sham EAP modified the distribution of fluorescence under TST application in a similar manner as that observed during the use of FITC-d3. It is concluded that EAP is possibly a valid treatment to slow down the entry of foreign solutes into the brain; in view of the comparable effects of EAP on FITC-d3 and OA-45 distribution, EAP seems to act before FITC-d3 passes the astroglial aquaporin-4 water channels, which are a critical constituent of the glymphatic system.
Keywords: Glymphatic system, Electroacupuncture, Tail suspension test, Depression-like behavior, Fluorescence tracers
Highlights
-
•
High and low molecular weight fluorescence tracers were used to investigate the glymphatic system of the rat.
-
•
The tail suspension test tended to induce an increase of fluorescence by the low molecular weight tracer.
-
•
Electroacupuncture decreased the gross fluorescence increase by both types of tracers.
-
•
Electroacupuncture interacted with the entry of both types of tracers into the brain parenchyma.
-
•
Electroacupuncture did not modulate the efficiency of the glymphathic system’s function.
1. Introduction
Major depressive disorder (MDD) is a serious and debilitating disorder characterized primarily by low mood and loss of interest that persists for at least 2 weeks (Malhi and Mann, 2018). Currently more than 280 million people suffer from depression worldwide and suicide is the fourth leading cause of death in 15–19-years old depressed patients (WHO, 2017; https://www.who.int/news-room/fact-sheets/detail/depression). Depression is also a major socio-economically disquieting problem recognized as the leading cause of disability. It is especially alarming that the incidence of depression is increasing year by year, and this is in teens faster than in adults (Miller and Campo, 2021). In children, this incidence has risen to 2.5%, while in adolescents it is about 5–10%, thereby equaling or even surmounting that observed in adults (see also above; Bylund and Reed, 2007).
Environmental stressors and genetic factors appear to initiate a plethora of morphologic and functional changes which eventually manifest themselves as depression (Jesulola et al., 2018). A battery of preferentially rodent tests have been used to model the symptoms of depression without exception based on the understanding that chronic stress is the most common risk factor of depression (Anderzhanova et al., 2017; Pryce and Fuchs, 2017. Although chronic stress models are considered to be more reliable to induce so called depressive-like behavior in rats/mice, acute stressful stimulation is easier to handle and is therefore frequently used (e.g. tail suspension test [TST], forced swim test, foot shock in combination with the measurement of sucrose consumption; Belovicova et al., 2017). In TST, used in this study, mice are suspended on their tails hanging freely in the air (for details see Section Materials and Methods); the animals try to escape, but after a certain time give up their futile trials and become immobile acquiring a state of “learned helplessness”. The time spent in learned helplessness is considered to be a measure of the depression-like behavioral state (see Zhao et al., 2022, and references within).
The relatively new concept of the glymphatic (glial lymphatic) system proposes that aquaporin channels, in the first instance belonging to the aquaporin-4 class (AQP4), localized on the end feet of astrocytes, are instrumental to transport nutrients and remove waste products and neurotoxins from the brain (Iliff et al., 2012). A series of subsequent studies have shown that the function of this system is greatly enhanced during sleep (Xie et al., 2013, Hladky and Barrand, 2019) and anesthesia (Hablitz et al., 2019), and is significantly impaired in aging (Kress et al., 2014), brain trauma (Piantino et al., 2019), Alzheimer's disease (Reeves et al., 2020), hypertension (Mortensen et al., 2019), stroke (Mestre et al., 2020a), and diabetes (Jiang et al., 2017). It was reported that chronic unpredictable mild stress (CUMS) inhibited the function of the glymphatic system (Xia et al., 2017, Liu et al., 2020) and prevented the metabolism of the exogenous, 42 amino acid-containing amyloid β protein (Aβ42) (Xia et al., 2017), which is thought to be involved in the pathogenesis of Alzheimer’s disease (Hardy and Selkoe, 2002, Hunter et al., 2018, Illes et al., 2019). Correspondingly, this form of stress increased the accumulation of endogenous and exogenous Aβ42 in the mouse brain (Xia et al., 2017). Long-term administration of polyunsaturated fatty acids (Liu et al., 2020) or fluoxetine treatment (Xia et al., 2017) reversed the deleterious modulation by CUMS of the glymphatic system.
Acupuncture refers to a family of procedures involving usually mechanical or electrical stimulation via solid metallic needles introduced through the skin at specific sites, termed acupoints (Johnson, 2006, Berman et al., 2010, Tang et al., 2016). In traditional acupuncture, needles are manipulated manually; electroacupuncture (EAP) means electrical stimulation via the needles having often stronger effects than manual manipulations. EAP has proved to be effective for the treatment of depression in both clinical (Zhao et al., 2019, Wang et al., 2016) and animal experimental settings (Guo et al., 2015, Yang et al., 2017). In this study, we aimed to clarify whether EAP or TST cause changes in the function of the glymphatic system, and whether EAP can improve the possibly harmful effect of TST. We found that stimulation of acupoint Zusanli (ST36) either with electric current or by simply introducing the acupuncture needle (sham EAP), is sufficient to slow down the distribution of foreign substances in the brain after their appearance in the cerebrospinal fluid (CSF).
2. Materials and methods
2.1. Animals
Groups of 6 male Sprague-Dawley rats (20–25 days old) were used in these experiments. Animals were maintained in standard laboratory conditions at 22–24 °C and in a natural light/dark cycle with free access to water and food.
2.2. Electroacupuncture
Rats were fixed to a wooden block by two Velcro brand hooks and loop fasteners as well as an additional tape before EAP. EAP was delivered to the Zusanli acupoint (ST36). An electrical current of 1 mA and a frequency of 100 Hz was applied for 30 min, by an “acupoint nerve electrostimulator” (HANS-200; Nanjing Jisheng Medical Technology Co., Jiangsu, China). EAP was delivered through stainless steel needles (2.5 cm long, 0.25 mm diameter; Hwato-Med. Co.; Jiangsu, China) introduced 4–6 mm deep below the skin. Both low frequency (4–15 Hz; Yu et al., 2006; Yu et al., 2023) and high frequency (100 Hz; Wu et al., 2015; Xu et al., 2020) electrical stimulation are able to reverse depressive-like behavior; we used the latter variant of EAP in the present experiments.
One of the needles was inserted to the Zusanli acupoint (ST36), and the other one was inserted to a non-acupoint located somewhat distal from ST36 toward the tail and opposite to the knee joint (Torres-Rosas et al., 2014). This non-acupoint can be found over the semitendinous muscle near the tail base and is neither referred in the acupoint map of rodents nor close to any major nerve. In the sham group of animals, the needles were again positioned at ST36 and the non-acupoint, but now without electrical stimulation. A second control condition was used as well by introducing one of the needles to the non-acupuncture point near the tail base and the other needle besides it; electrical stimulation was delivered as usual. This latter procedure did not alter the fluorescence intensity of the tracers in comparison with the respective control values at the 180-min time-point (not shown). Our results agree with the previous finding that EAP delivered to this non-acupoint (and the simultaneous immobilization of the animals) failed to exert a behavioral effect e.g. on pain sensation (Zhang et al., 2020).
2.3. Tail suspension test (TST)
Because of the young age and low weight of the rats, the TST did not cause any damage to their tails. The animals were suspended on a 55-cm high laboratory rack by adhesive tape about 1 cm from the tail tip. The approximate distance between the animal's nose and the operating floor was 20–25 cm. The animals were separated from each other by baffles to prevent mutual interference during the suspension time. We increased the short and more usual duration of the TST in the second half of the experiments from 5 to 15 min, in order to obtain a still stronger learned helplessness/depression-like reaction, assuming that the resulting change in the function of the glymphatic system will become more pronounced. TST was performed only a single time in each animal and at roughly the same time of the day (10:00 a.m. to 12:00 a.m.).
It has to be taken into consideration that immobilization of the animals also causes a so-called restraint stress (Xu et al., 2017), which will contribute to the extent of depression-like behavior. Although both immobilization and TST induce depression-like state, the direction of changes in fluorescence intensity of the tracers are just the opposite. Moreover, restraint stress is equally present following EAP+TST as well as sham EAP+TST and therefore the demonstrated mutual interrelationship between the changes in fluorescence intensity by TST and EAP should be in fact due to the acupuncture procedure.
2.4. Measurement of the intracerebral distribution of fluorescent markers
Cisterna magna injections were performed 24 h after either experimental manipulation. Rats were anesthetized via intraperitoneal application of pentobarbital sodium (40 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) and then fixed in a stereotaxic frame; the atlanto-occipital membrane was surgically exposed followed by a durotomy, and a 30 GA injection needle was inserted into the cisterna magna. Two different fluorescent tracers were used, namely one of a small molecular weight (Fluorescein Isothiocyanate-d3; FITC-d3; 3 kDa) and another one of a large molecular weight (Ovalbumin Alexa Fluor 555 Conjugate; OA-45; 45 kDa). Both tracers were purchased from Invitrogen, Carlsbad, CA, USA. 1% solutions of the fluorescent tracers were mixed in a 1:1 ratio, and then infused at a rate of 1 µl/min for 10 min with a micro-syringe pump (RWD Life Science, San Diego, CA, USA) into the cisterna magna according to Kress et al. (2014). Rats were sacrificed 0.5, 3, and 6 h after the beginning of infusion and were transcardially perfused with saline and 4% paraformaldehyde. The brains were removed and postfixed overnight at 4 °C. Coronal sections (100 µm thick) of the whole brain were cut by a vibratome (VT1200S; Leica Biosystem, Muttenz, Switzerland). For each animal, 6 slices (bregma +1.5 mm∼−3.5 mm) were selected. Images were prepared by a Slideviewer (VS200; Olympus, Tokyo, Japan) and the program ImageJ (NIH, Bethesda, MD, USA) was used to calculate the mean fluorescence intensity of tracers in brain slices.
In experiments, when the interaction of TST and EAP or sham EAP was measured, a recovery of the fluorescence intensity of either tracer was observed at the 180-min time-points (see Figs. 1–4B). Although we did not determine at these time-points the counteraction of the TST immobility duration by EAP, it is noteworthy that in previous experiments this was already noted (Zhang and Chen, 2022).
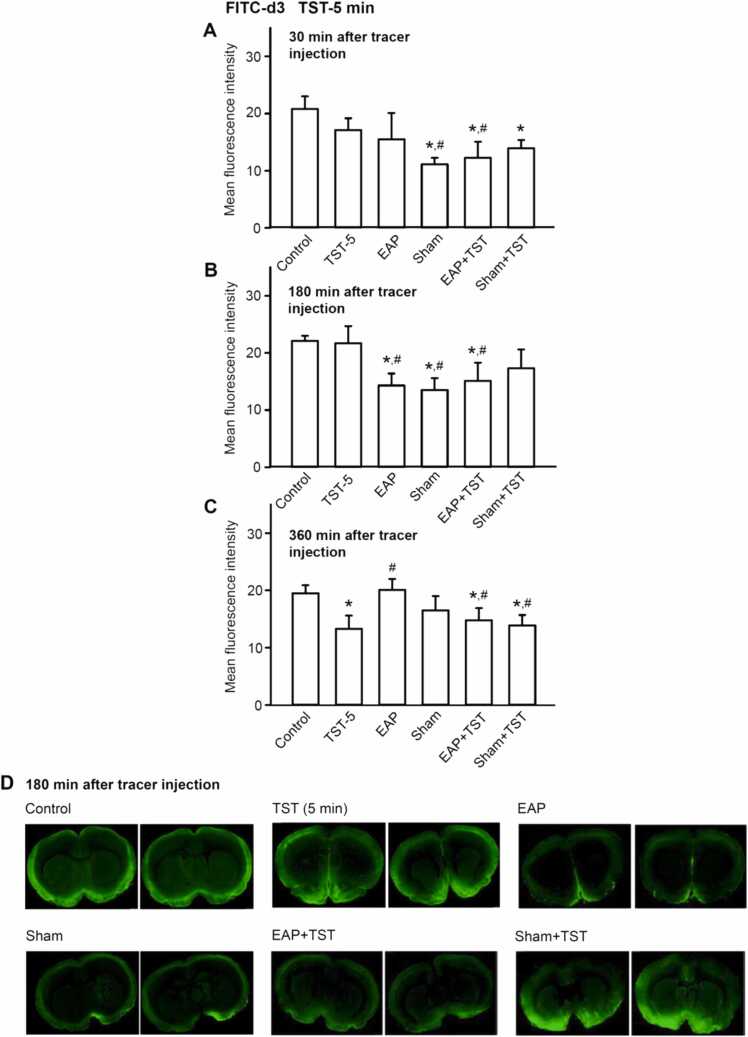
Fig. 1.
Mean fluorescence intensity of coronal brain slices of rats indicating the appearance of the low molecular weight tracer Fluorescein-5-Isothiocyanate-Conjugated Dextran (FITC-d3; 3 kDa) at the level of bregma + 1.5 mm ∼ − 3.5 mm. The tracer was infused via the cisterna magna into the CSF and rats were killed 30, 180, and 360 min afterwards. Electroacupuncture (EAP) was delivered for 30 min at a frequency of 100 Hz (1 mA) to the left Zusanli (ST36) acupoint via insertion of a stainless steel needle. The sham acupuncture procedure meant that such a needle was inserted for 30 min to the Zusanli acupoint without electrical stimulation. The tail suspension test (TST) was applied for 5 min, immediately after EAP or sham EAP. Then, the mean fluorescence derived from these 6 slices and the mean fluorescence derived from the other 6 slices of additional rats under each treatment were averaged and plotted in A-C. Means±S.E.M. from 6 animals. *P < 0.05; statistically significant difference from the respective control values. #P < 0.05; statistically significant difference from the respective TST values. One-way ANOVA followed by the Holm-Sidak test was used in all cases. Two brain slices are shown out of the 6 slices prepared from each brain (D).
Fig. 4.
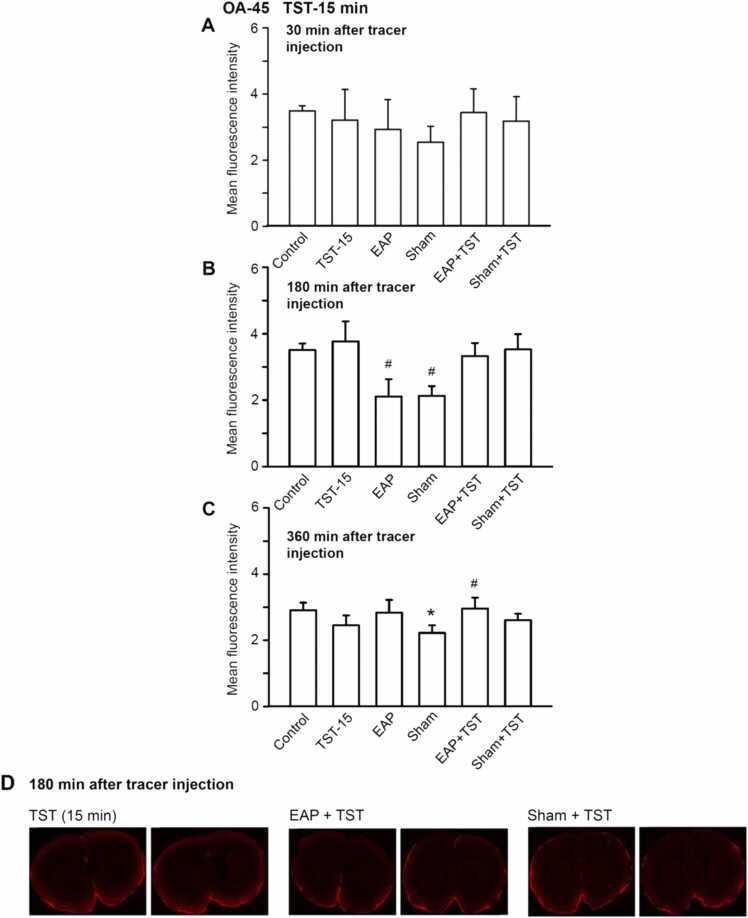
Fluorescence intensity of coronal brain slices of rats indicating the appearance of the low molecular weight tracer OA-45 (45 kDa) at the level of bregma + 1.5 mm ∼ − 3.5 mm. All procedures were as described in the Legend to Fig. 1, except the duration of TST, which was increased from 5 to 15 min. Means±S.E.M. from 6 animals. *P < 0.05; statistically significant difference from the control value. #P < 0.05; statistically significant difference from the respective TST values. One-way ANOVA followed by the Holm-Sidak (A, C) or Tukey test (B) were used as adequate. Two brain slices are shown out of the 6 slices prepared from each brain for the indicated treatments (D).
2.5. Data analysis
All data were expressed as means ± S.E.M. of 6 animals, calculated each from the mean of their 6 slices, respectively. SigmaPlot 14.0 was used for plotting the data and for statistical evaluations. Multiple comparisons between data were performed by one-way ANOVA followed by the parametric Holm-Sidak test or the non-parametric Tukey test, as defined by the Shapiro-Wilk normality test. The post-hoc test was run only if F or H achieved P < 0.05. A probability level of 0.05 or less was considered throughout to accept statistical significance.
3. Results
As mentioned in the Methods Section, we applied two fluorescence tracers, one of a low molecular weight (FITC-d3; 3 kDa) and the other one of a high molecular weight (OA-45; 45 kDa) into the cisterna magna to measure the entry, distribution and clearance of these tracers into, within and from, respectively, the brain parenchyma. Our reason to use 3 and 45 kDa tracers was that the only routes between the perivascular spaces and the wider brain interstitium are through ∼20 nm clefts (Mathiisen et al., 2010) between overlapping astroglial endfeet. The lower molecular weight tracer readily passes these gaps, while the higher molecular weight tracer cannot do that (Yang et al., 2013).
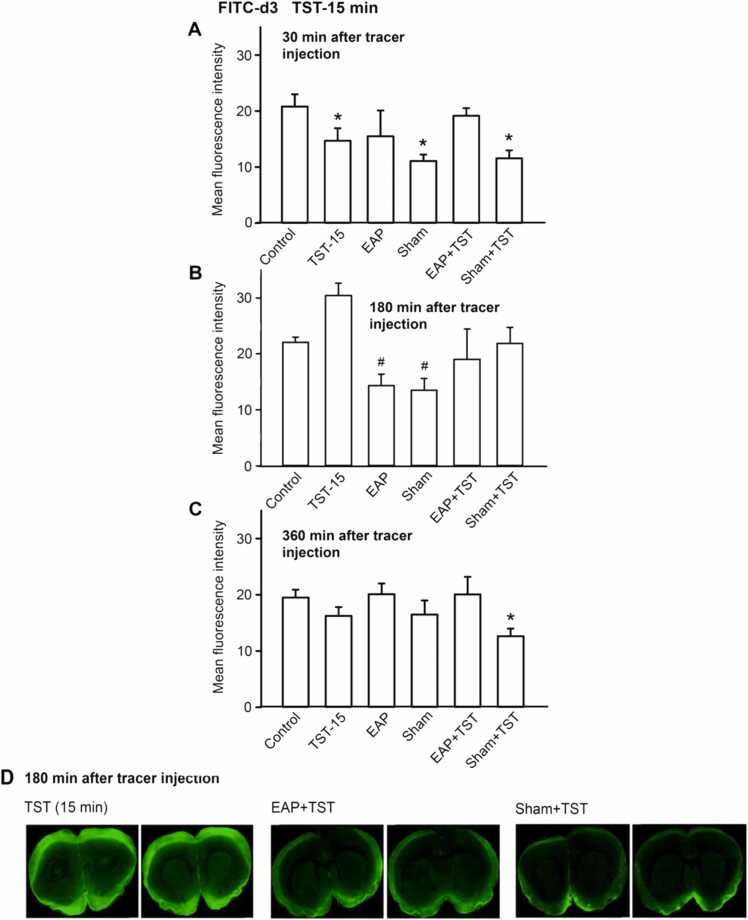
Demonstrative snapshots shown in Figs. 1–4D show that the intracisternal application of both tracers to rats caused a preferential increase of fluorescence intensity in the cortical and subcortical areas. We did not aim at discriminating between fluorescence changes in different areas of the brain, but rather determined the overall fluorescence. We noticed by measuring the fluorescence of FITC-d3 in the brain parenchyma, that both 30 and 360 min after its intracisternal application, rather inconsistent effects were observed (Fig. 1 A, C and Fig. 2 A, C). However, 180 min after FITC-d3 injection, both EAP and sham EAP uniformly decreased the fluorescence in comparison with the value measured under 5-min TST but also under control conditions (Fig. 1B). This means that EAP/sham EAP reduced the distribution of the small molecular foreign body in the brain, exerting a beneficial effect. When the duration of TST was increased from 5 to 15 min, a trend to an enhancement of fluorescence intensity was observed, although this change did not reach the level of statistical significance. EAP and sham EAP caused now a prominent decrease, which existed only in comparison with the TST, but not the control value (Fig. 2B). In addition, both EAP and sham EAP counteracted the effects of TST.
Fig. 2.
Fluorescence intensity of coronal brain slices of rats indicating the appearance of the low molecular weight tracer FITC-d3 (3 kDa) at the level of bregma + 1.5 mm ∼ − 3.5 mm. All procedures were as described in the Legend to Fig. 1, except the duration of TST, which was increased from 5 to 15 min. Means±S.E.M. from 6 animals. *P < 0.05; statistically significant difference from the respective control values. P < 0.05; statistically significant difference from the respective TST values. One-way ANOVA followed by the Holm-Sidak (A, C) or Tukey test (B) were used as adequate. Two brain slices are shown out of the 6 slices prepared from each brain for the indicated treatments (D).
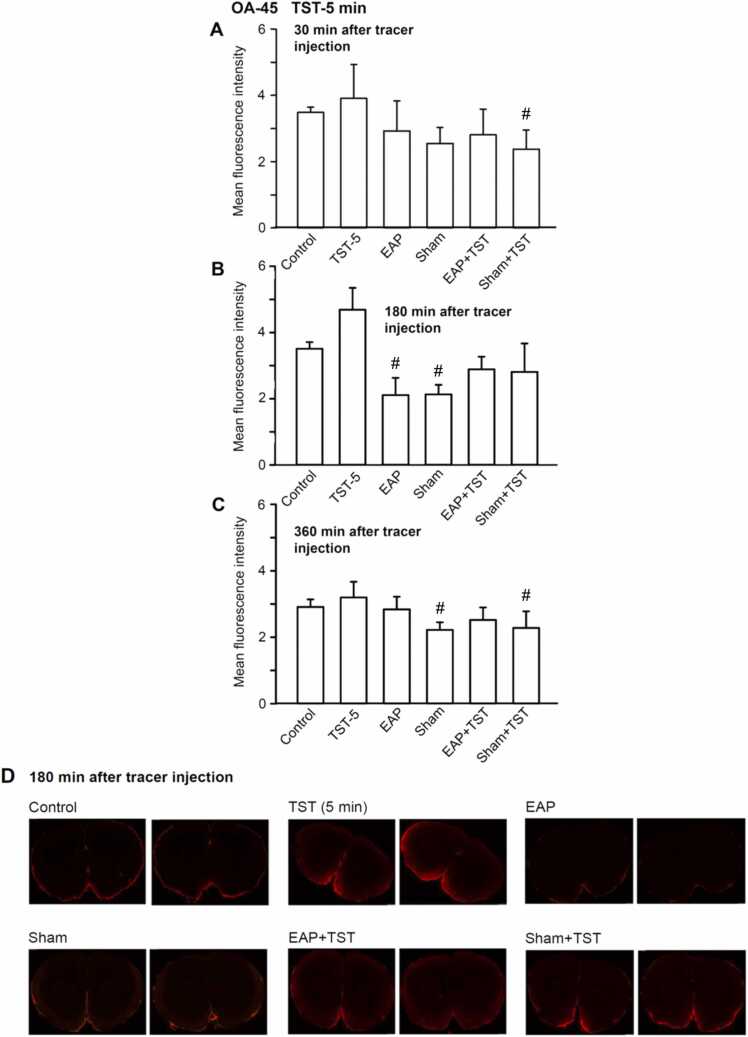
In the following experiments, we used instead of FITC-3d, the high molecular weight tracer OA-45. The fluorescence intensity measured with this tracer was about one fifth of that measured with FITC-d3, indicating that OA-45 entered the brain in a much lower quantity than its low molecular weight counterpart (Fig. 3A-D). While apparently intracisternally infused FITC-d3 moved into the brain parenchyma and exchanged with the brain interstitium, OA-45 became trapped in the perivascular space and could not move freely into and through the brain. Thus, this tracer failed to enter the glymphatic system, that permits CSF to exchange with the brain interstitial fluid.
Fig. 3.
Mean fluorescence intensity of coronal brain slices of rats indicating the appearance of the high molecular weight tracer Ovalbumin Alexa Fluor 555 Conjugate (OA-45; 45 kDa) at the level of bregma + 1.5 mm ∼ − 3.5 mm. The tail suspension test (TST) was applied for 5 min; all other procedures were as described in the Legend to Fig. 1. Means±S.E.M. from 6 animals. #P < 0.05; statistically significant difference from the respective TST values. One-way ANOVA followed by the Holm-Sidak (A, C) or Tukey test (B) were used as adequate. Two brain slices are shown out of the 6 slices prepared from each brain for the indicated treatments (D).
30 and 360 min after the intracisternal application of OA-45 there was no major and consequent difference between the effect of any of the treatments from the control fluorescence (3 A, C; 4 A, C). However, at the 180-min time-point already a 5 min duration TST tended to increase the fluorescence intensity (Fig. 3B; see also Fig. 2B for FITC-d3); in comparison with this value, both EAP and sham EAP caused manifest depression. EAP plus TST, or sham EAP plus TST induced again less decrease of fluorescence intensity than EAP or sham EAP alone, indicating antagonism of the TST effect by either of these procedures.
An increase of the duration of TST from 5 to 15 min left the decrease of the fluorescence changes of OA-45, 180 min after tracer application, unchanged (Fig. 4B; compare with Fig. 3B.
4. Discussion
It is well known that the CSF functions as a sink for brain extracellular solutes, but it was for a considerable time unclear how solutes from the brain interstitium move from the parenchyma to the CSF. In the meantime, several lines of evidence suggest that bulk flow drainage via the glymphatic (glial lymphatic) system, dependent on astroglial water channels, constitutes paravascular CSF pathways (Iliff et al., 2012, Kress et al., 2014). In consequence, meningeal lymphatic vessels represent a major efflux route for the CSF and its solutes (Mestre et al., 2020b). The passage of solutes into the brain parenchyma and out of it is believed to occur via the following routes: (1) The entry of subarachnoidal CSF into the brain along paravascular spaces is driven by the pulsation of cerebral arteries and arterioles and (2) the corresponding efflux pathway consists of perivenous spaces, highly dependent on arousal state and AQP4 expression in astrocytic end-feet terminating in the blood-brain barrier (BBB). The first evidence for the existence of a glymphatic system was supplied by the intracisternal injection of low and high molecular weight fluorescent tracers and measurement of their appearance in the brain by fluorescent microscopy (Iliff et al., 2012, Kress et al., 2014, Yang et al., 2017). We adapted this approach of the group around Maiken Nedergaard by using the low molecular tracer FITC-d3 (3 kDa) and its high molecular counterpart OA-45 (45 kDa).
Interstitial solutes, such as Aβ, were previously thought to be cleared from the brain primarily by transport through the BBB (Zlokovic, 2008, Abbott et al., 2010). BBB transport and glymphatic clearance have the same purpose in removing interstitial solutes such as Aβ from the brain, and they seem to be complementary in this respect (Kress et al., 2014). Our results suggest that OA-45 mainly accumulated in the perivascular space, in accordance with its failure to enter the brain tissue through the BBB/paraarterial CSF inflow channels. The BBB and the glymphatic system are not only functionally complementary, but they are also affected by some common factors, such as arterial pulsation, aging, inflammation and sleep disturbances, which all lead to BBB disruption and glymphatic system dysfunction (Harding et al., 2017, Iliff et al., 2013, Guo et al., 2015, Verheggen et al., 2018).
In view of the discovery of the glymphatic system and its significance for the maintenance of homeostatic conditions in the CNS, we hypothesized that the distribution of foreign solutes under pathological conditions might be improved by EAP. As already mentioned in the Introduction, EAP has been proved to be effective for the treatment of both MDD and depression-like symptoms in rodents (Zhao et al., 2019, Guo et al., 2015, Yang et al., 2017). EAP is an important therapeutic alternative to anti-depressant drugs, due to its advantages of less side effects, easy operation and definite, although modest, curative effect (Smith et al., 2018). There are different hypotheses on its mechanism of action to treat MDD. Presently, acupuncture is mainly believed to prevent synaptic plasticity dysfunctions (Han et al., 2018, She et al., 2015, Zhang et al., 2019, Yue et al., 2018, Guo et al., 2014), inflammation (Han et al., 2018, Duan et al., 2016, Mo et al., 2014, Cai et al., 2019), and gene expression (Duan et al., 2014, Zheng et al., 2019). Thus there are a number of tissue functions whose beneficial modulation by EAP may improve depressive conditions.
In contrast to CUMS induced by chronic stressors, we used TST as an acute stressor to initiate depressive-like behavior, and found, according to expectations, that the effect of TST was relieved by EAP or sham EAP. In accordance with the efficiency of sham EAP, large multicenter clinical trials conducted in Germany and the United States consistently revealed that the true and sham acupuncture do not differ in their effectiveness in decreasing pain levels across multiple chronic pain disorders (Colquhoun and Novella, 2013, Madsen et al., 2009).
A number of animal studies show that EAP can regulate the permeability of the BBB. It was e.g. reported that EAP pretreatment significantly attenuated the ischemia-reperfusion-induced increase in BBB permeability and brain edema, possibly by alleviating the degradation of claudin and inhibiting the expression of p-caveolin-1 in endothelial cells (Zou et al., 2015). Another group of authors found that EAP preconditioning reduced ischemic brain injury by inhibiting BBB disruption and brain edema via the modulation of NADPH oxidase 4 (NOX4), AQP4 expression and ROS production (Jung et al., 2016). Additionally, a time-dependent increase in the BBB permeability was noted following an 8 min EAP stimulation of the acupoints Baihui (GV20) and Shuigou (GV26) in rats (Zhang et al., 2018). Another study reported that EAP treatment may counteract cognitive impairment by improving the clearance of accumulated Aβ in the brain by promoting the function of the glymphatic system (Liang et al., 2021).
We found that EAP and sham EAP both reduced the fluorescence intensity in the rat brain, signaling the decreased presence of low and high molecular weight tracers after their intracisternal infusion. Because a selective effect on the low molecular weight tracer distribution would suggest an interaction with the glymphatic pathway responsible for the clearance of interstitial solutes, it can be concluded that the site of action of EAP and sham EAP is located before the astroglial aquaporin-4 water channels. Nonetheless our results supply evidence for the fact that stimulation of ST36 either with electrical current or mechanically by simple contact with the acupuncture needle (sham EAP) is sufficient to slow down the distribution of foreign substances in the brain after their appearance in the CSF. We assume, but did not test in the present study, that stressful procedures other than TST, such as forced swimming or foot shock (measured by sucrose consumption), might also induce effects similar to those caused by TST.
Funding
Our work was supported by grants from (‘The Project First-Class Disciplines Development’; CZYHW1901) of the Chengdu University of Traditional Chinese Medicine to Y.T. and P.I. in order to build up the “International Joint Research Centre on Purinergic Signalling of Sichuan Province”, grants of the State Administration of Foreign Experts Affairs to support the stays of P.I. and P.R. in Chengdu (G2022036004), and Sichuan Science and Technology Program (2019YFH0108, 2022YFH0006).
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Ethical Statement
All experimental procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Chengdu University of Traditional Chinese Medicine (protocol code, AM1362, 9 March 2020).
CRediT authorship contribution statement
Conceptualization, J.R.H., P.R., P.I., Y.T.; Methodology and Investigation, J.R.H., B.L., H.H.Y.; Data curing, J.R.H., B.L., H.H.Y.; Writing, original draft preparation, J.R.H., P.I., Y.T.; Review and editing, P.R., H.H.Y.; Funding acquisition, P.I., Y.T.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgements
None.
Contributor Information
Yong Tang, Email: tangyong@cdutcm.edu.cn.
Peter Illes, Email: peter.illes@medizin.uni-leipzig.de.
References
- Abbott N.J., Patabendige A.A.K., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Anderzhanova E., Kirmeier T., Wotjak C.T. Animal models in psychiatric research: the RDoC system as a new framework for endophenotype-oriented translational neuroscience. Neurobiol. Stress. 2017;7:47–56. doi: 10.1016/j.ynstr.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belovicova K., Bogi E., Csatlosova K., Dubovicky M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017;10:40–43. doi: 10.1515/intox-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman B.M., Langevin H.M., Witt C.M., Dubner R. Acupuncture for chronic low back pain. N. Engl. J. Med. 2010;363:454–461. doi: 10.1056/NEJMct0806114. [DOI] [PubMed] [Google Scholar]
- Bylund D.B., Reed A.L. Childhood and adolescent depression: why do children and adults respond differently to antidepressant drugs? Neurochem. Int. 2007;51:246–253. doi: 10.1016/j.neuint.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Ma W., Wang G.-T., Li Y.-J., Shen W.-D. Antidepressant, anti-inflammatory, and antioxidant effects of electroacupuncture through sonic hedgehog-signaling pathway in a rat model of poststroke depression. Neuropsychiatr. Dis. Treat. 2019;15:1403–1411. doi: 10.2147/NDT.S205033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Novella S.P. Acupuncture is theatrical placebo. Anesth. Analg. 2013;116:1360–1363. doi: 10.1213/ANE.0b013e31828f2d5e. [DOI] [PubMed] [Google Scholar]
- Duan D., Tu Y., Yang X., Liu P. Electroacupuncture restores 5-HT system deficit in chronic mild stress-induced depressed rats. Evid. -Based Complement. Altern. Med.: eCAM. 2016;2016 doi: 10.1155/2016/7950635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D., Yang X., Ya T., Chen L. Hippocampal gene expression in a rat model of depression after electroacupuncture at the Baihui and Yintang acupoints. Neural Regen. Res. 2014;9:76–83. doi: 10.4103/1673-5374.125333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Guo Z., Yang X., Sun L., Wang S., Yingge A., He X., Ya T. The Alterations of IL-1Beta, IL-6, and TGF-beta levels in hippocampal CA3 Region of chronic restraint stress rats after electroacupuncture (EA) pretreatment. Evid. -Based Complement. Altern. Med.: eCAM. 2014;2014 doi: 10.1155/2014/369158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Tu Y., Guo T.-W., Wu Y.-C., Yang X.-Q., Sun L., Yang X.-J., Zhang W.-Y., Wang Y., Zhang X.-H. Electroacupuncture pretreatment exhibits anti-depressive effects by regulating hippocampal proteomics in rats with chronic restraint stress. Neural Regen. Res. 2015;10:1298–1304. doi: 10.4103/1673-5374.162764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz L.M., Vinitsky H.S., Sun Q., Stæger F.F., Sigurdsson B., Mortensen K.N., Lilius T.O., Nedergaard M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019;5:eaav5447. doi: 10.1126/sciadv.aav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Wu H., Yin P., Chen Z., Cao X., Duan Y., Xu J., Lao L., Xu S. Electroacupuncture restores hippocampal synaptic plasticity via modulation of 5-HT receptors in a rat model of depression. Brain Res. Bull. 2018;139:256–262. doi: 10.1016/j.brainresbull.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Harding A., Robinson S., Crean S., Singhrao S.K. Can better management of periodontal disease delay the onset and progression of Alzheimer's disease? J. Alzheimer'S. Dis.: JAD. 2017;58:337–348. doi: 10.3233/JAD-170046. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Sci. (N. Y., N. Y. ) 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hladky S.B., Barrand M.A. Metabolite clearance during wakefulness and sleep. Handb. Exp. Pharmacol. 2019;253:385–423. doi: 10.1007/164_2017_37. [DOI] [PubMed] [Google Scholar]
- Hunter S., Smailagic N., Brayne C. Aβ and the dementia syndrome: Simple versus complex perspectives. Eur. J. Clin. Investig. 2018;48 doi: 10.1111/eci.13025. [DOI] [PubMed] [Google Scholar]
- Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., Nagelhus E.A., Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff J.J., Wang M., Zeppenfeld D.M., Venkataraman A., Plog B.A., Liao Y., Deane R., Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci.: Off. J. Soc. Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P., Rubini P., Huang L., Tang Y. The P2X7 receptor: a new therapeutic target in Alzheimer's disease. Expert Opin. Ther. Targets. 2019;23:165–176. doi: 10.1080/14728222.2019.1575811. [DOI] [PubMed] [Google Scholar]
- Jesulola E., Micalos P., Baguley I.J. Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model - are we there yet? Behav. brain Res. 2018;341:79–90. doi: 10.1016/j.bbr.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Zhang L., Ding G., Davoodi-Bojd E., Li Q., Li L., Sadry N., Nedergaard M., Chopp M., Zhang Z. Impairment of the glymphatic system after diabetes. J. Cereb. Blood Flow. Metab.: Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2017;37:1326–1337. doi: 10.1177/0271678X16654702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.I. The clinical effectiveness of acupuncture for pain relief--you can be certain of uncertainty. Acupunct. Med.: J. Br. Med. Acupunct. Soc. 2006;24:71–79. doi: 10.1136/aim.24.2.71. [DOI] [PubMed] [Google Scholar]
- Jung Y.S., Lee S.-W., Park J.H., Seo H.B., Choi B.T., Shin H.K. Electroacupuncture preconditioning reduces ROS generation with NOX4 down-regulation and ameliorates blood-brain barrier disruption after ischemic stroke. J. Biomed. Sci. 2016;23:32. doi: 10.1186/s12929-016-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress B.T., Iliff J.J., Xia M., Wang M., Wei H.S., Zeppenfeld D., Xie L., Kang H., Xu Q., Liew J.A., Plog B.A., Ding F., Deane R., Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P.-Z., Li L., Zhang Y.-N., Shen Y., Zhang L.-L., Zhou J., Wang Z.-J., Wang S., Yang S. Electroacupuncture improves clearance of amyloid-β through the glymphatic system in the SAMP8 mouse model of Alzheimer's disease. Neural Plast. 2021;2021 doi: 10.1155/2021/9960304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Hao J., Yao E., Cao J., Zheng X., Di Yao, Zhang C., Li J., Pan D., Luo X., Wang M., Wang W. Polyunsaturated fatty acid supplement alleviates depression-incident cognitive dysfunction by protecting the cerebrovascular and glymphatic systems. Brain, Behav., Immun. 2020;89:357–370. doi: 10.1016/j.bbi.2020.07.022. [DOI] [PubMed] [Google Scholar]
- Madsen M.V., Gøtzsche P.C., Hróbjartsson A. Acupuncture treatment for pain: systematic review of randomised clinical trials with acupuncture, placebo acupuncture, and no acupuncture groups. BMJ (Clin. Res. Ed. ) 2009;338:a3115. doi: 10.1136/bmj.a3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi G.S., Mann J.J. Depression. Lancet (Lond., Engl. ) 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- Mathiisen T.M., Lehre K.P., Danbolt N.C., Ottersen O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- Mestre H., Du T., Sweeney A.M., Liu G., Samson A.J., Peng W., Mortensen K.N., Stæger F.F., Bork P.A.R., Bashford L., Toro E.R., Tithof J., Kelley D.H., Thomas J.H., Hjorth P.G., Martens E.A., Mehta R.I., Solis O., Blinder P., Kleinfeld D., Hirase H., Mori Y., Nedergaard M. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Sci. (N. Y., N. Y. ) 2020:367. doi: 10.1126/science.aax7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre H., Mori Y., Nedergaard M. The brain's glymphatic system: current controversies. Trends Neurosci. 2020;43:458–466. doi: 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L., Campo J.V. Depression in adolescents. N. Engl. J. Med. 2021;385:445–449. doi: 10.1056/NEJMra2033475. [DOI] [PubMed] [Google Scholar]
- Mo Y., Yao H., Song H., Wang X., Chen W., Abulizi J., Xu A., Tang Y., Han X., Li Z. Alteration of behavioral changes and hippocampus galanin expression in chronic unpredictable mild stress-induced depression rats and effect of electroacupuncture treatment. Evid. -Based Complement. Altern. Med.: eCAM. 2014;2014 doi: 10.1155/2014/179796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen K.N., Sanggaard S., Mestre H., Lee H., Kostrikov S., Xavier A.L.R., Gjedde A., Benveniste H., Nedergaard M. Impaired glymphatic transport in spontaneously hypertensive rats. J. Neurosci.: Off. J. Soc. Neurosci. 2019;39:6365–6377. doi: 10.1523/JNEUROSCI.1974-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantino J., Lim M.M., Newgard C.D., Iliff J. Linking traumatic brain injury, sleep disruption and post-traumatic headache: a potential role for glymphatic pathway dysfunction. Curr. Pain. Headache Rep. 2019;23:62. doi: 10.1007/s11916-019-0799-4. [DOI] [PubMed] [Google Scholar]
- Pryce C.R., Fuchs E. Chronic psychosocial stressors in adulthood: studies in mice, rats and tree shrews. Neurobiol. Stress. 2017;6:94–103. doi: 10.1016/j.ynstr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves B.C., Karimy J.K., Kundishora A.J., Mestre H., Cerci H.M., Matouk C., Alper S.L., Lundgaard I., Nedergaard M., Kahle K.T. Glymphatic system impairment in alzheimer's disease and idiopathic normal pressure hydrocephalus. Trends Mol. Med. 2020;26:285–295. doi: 10.1016/j.molmed.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She Y., Xu J., Duan Y., Su N., Sun Y., Cao X., Lao L., Zhang R., Xu S. Possible antidepressant effects and mechanism of electroacupuncture in behaviors and hippocampal synaptic plasticity in a depression rat model. Brain Res. 2015;1629:291–297. doi: 10.1016/j.brainres.2015.10.033. [DOI] [PubMed] [Google Scholar]
- Smith C.A., Armour M., Lee M.S., Wang L.-Q., Hay P.J. Acupuncture for depression. Cochrane Database Syst. Rev. 2018;3 doi: 10.1002/14651858.CD004046.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Yin H.-Y., Rubini P., Illes P. Acupuncture-induced analgesia: a neurobiological basis in purinergic signaling. Neurosci.: a Rev. J. bringing Neurobiol., Neurol. Psychiatry. 2016;22:563–578. doi: 10.1177/1073858416654453. [DOI] [PubMed] [Google Scholar]
- Torres-Rosas R., Yehia G., Pena G., Mishra P., Thompson-Bonilla M.R., Moreno-Eutimio M.A., Arriaga-Pizano L.A., Isibasi A., Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med. 2014;20:291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheggen I.C.M., Van Boxtel M.P.J., Verhey F.R.J., Jansen J.F.A., Backes W.H. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci. Biobehav. Rev. 2018;90:26–33. doi: 10.1016/j.neubiorev.2018.03.028. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang Z., Liu J., Chen J., Liu X., Nie G., Byun J.-S., Liang Y., Park J., Huang R., Liu M., Liu B., Kong J. Repeated acupuncture treatments modulate amygdala resting state functional connectivity of depressive patients. NeuroImag. Clin. 2016;12:746–752. doi: 10.1016/j.nicl.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.Y., Jiang Y.L., He X.F., Zhao X.Y., Shao X.M., Du J.Y., Fang J.Q. Effects of electroacupuncture with dominant frequency at SP 6 and ST 36 based on meridian theory on pain-depression dyad in rats. Evid. -Based Complement. Altern. Med. 2015 doi: 10.1155/2015/732845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Yang L., Sun G., Qi S., Li B. Mechanism of depression as a risk factor in the development of Alzheimer's disease: the function of AQP4 and the glymphatic system. Psychopharmacology. 2017;234:365–379. doi: 10.1007/s00213-016-4473-9. [DOI] [PubMed] [Google Scholar]
- Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O'Donnell J., Christensen D.J., Nicholson C., Iliff J.J., Takano T., Deane R., Nedergaard M. Sleep drives metabolite clearance from the adult brain. Sci. (N. Y., N. Y. ) 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Wang K., Lu C., Dong L., Chen Y., Wang Q., Shi Z., Yang Y., Chen S., Liu X. Effects of the chronic restraint stress induced depression on reward-related learning in rats. Behav. brain Res. 2017;321:185–192. doi: 10.1016/j.bbr.2016.12.045. [DOI] [PubMed] [Google Scholar]
- Xu, X., Zheng, P., Zhao, H., Song, B., Wang, F., 2020, Effect of electroacupuncture at GV20 on sleep deprivation-induced depression-like behavior in mice. 481813. [DOI] [PMC free article] [PubMed]
- Yang L., Kress B.T., Weber H.J., Thiyagarajan M., Wang B., Deane R., Benveniste H., Iliff J.J., Nedergaard M. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med. 2013;11:107. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Guo Z., Lu J., Zhao B., Fei Y., Li J., Jiang H., Sun L., Wang Y., Sun Y., Bao T. The role of MAPK and dopaminergic synapse signaling pathways in antidepressant effect of electroacupuncture pretreatment in chronic restraint stress rats. Evid. -Based Complement. Altern. Med.: eCAM. 2017;2017 doi: 10.1155/2017/2357653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Li X.Y., Cao X.D., Wu G.C. Sucrose preference is restored by electro-acupuncture combined with chlorimipramine in the depression-model rat. Acupunct. Electrother. Res. 2006;31:223–232. doi: 10.3727/036012906815844201. [DOI] [PubMed] [Google Scholar]
- Yu L., Wang Y., Zhang H., Li M., Chen G., Hao J., Xie M. Involvement of purinergic P2Y1R in antidepressant-like effects of electroacupuncture treatment on social isolation stress mice. Purinergic Signal. 2023;19:55–68. doi: 10.1007/s11302-021-09827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue N., Li B., Yang L., Han Q.-Q., Huang H.-J., Wang Y.-L., Wang J., Yu R., Wu G.-C., Liu Q., Yu J. Electro-acupuncture alleviates chronic unpredictable stress-induced depressive- and anxiety-like behavior and hippocampal neuroinflammation in rat model of depression. Front. Mol. Neurosci. 2018;11:149. doi: 10.3389/fnmol.2018.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.M., Chen Z.Y. Electroacupuncture alleviates depression-like behaviors via a neural mechanism involving activation of Nucleus Accumbens Shell. World J. Biol. Psychiatry. 2022 doi: 10.1080/15622975.2022.2155993. [DOI] [PubMed] [Google Scholar]
- Zhang J., Lin X., Zhou H., Chen Y., Xiao S., Jiao J., Zhao Y., Di Z. Electroacupuncture: a new approach to open the blood-brain barrier in rats recovering from middle cerebral artery occlusion. Acupunct. Med.: J. Br. Med. Acupunct. Soc. 2018;36:377–385. doi: 10.1136/acupmed-2017-011496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhang Z., Zhang J., Zhong Z., Yao Z., Qu S., Huang Y. Electroacupuncture improves antidepressant effects in CUMS rats by protecting hippocampal synapse and mitochondrion: an ultrastructural and iTRAQ proteomic study. Evid. -Based Complement. Altern. Med.: eCAM. 2019;2019 doi: 10.1155/2019/3424698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Huang L., Kozlov S.A., Rubini P., Tang Y., Illes P. Acupuncture alleviates acid- and purine-induced pain in rodents. Br. J. Pharmacol. 2020;177:77–92. doi: 10.1111/bph.14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li Z., Wang Y., Ma X., Wang X., Wang X., Liu J., Huang Y., Zhang J., Li L., Hu X., Jiang J., Qu S., Chai Q., Song M., Yang X., Bao T., Fei Y. Manual or electroacupuncture as an add-on therapy to SSRIs for depression: a randomized controlled trial. J. Psychiatr. Res. 2019;114:24–33. doi: 10.1016/j.jpsychires.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Zhao Y.F., Ren W.J., Zhang Y., He J.R., Yin H.Y., Liao Y., Rubini P., Deussig J.M., Verkhratsky A., Yuan Z.Q., Illes P., Tang Y. High, in contrast to low levels of acute stress induce depressive-like behavior by involving astrocytic, in addition to microgial P2×7 receptors in the rodent hippocampus. Int. J. Mol. Sci. 2022;23:1904. doi: 10.3390/ijms23031904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., He J., Guo L., Yao L., Zheng X., Yang Z., Xia Y., Wu X., Su Y., Xu N., Chen Y. Transcriptome analysis on maternal separation rats with depression-related manifestations ameliorated by electroacupuncture. Front. Neurosci. 2019;13:314. doi: 10.3389/fnins.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zou R., Wu Z., Cui S. Electroacupuncture pretreatment attenuates blood‑brain barrier disruption following cerebral ischemia/reperfusion. Mol. Med. Rep. 2015;12:2027–2034. doi: 10.3892/mmr.2015.3672. [DOI] [PubMed] [Google Scholar]