Abstract
Meningiomas are the most common extra-axial neoplasmof the central nervous system (CNS). There are a number of characteristic imaging features of meningiomas on magnetic resonance imaging (MRI) that allow an accurate diagnosis, however there are a number of atypical features that may be diagnostically challenging. Furthermore, a number of other neoplastic and non-neoplastic conditions may mimic meningiomas. This case highlights the importance of careful analysis of imaging findings and the need for consideration of all possible diagnoses, including rare or atypical presentations of common neoplasms such as meningiomas. Early detection and accurate diagnosis are crucial in determining the appropriate management and improving the outcomes for patients with intracranial tumors.
Keywords: Meningioma, Radiological features, Atypical aspect
Introduction
Meningiomas are a complex group of tumors that arise from the meninges, and they represent a significant proportion of intracranial tumors. These primary non-glial brain tumors are the most widely recognized extra-axial neoplasm, emerging from the arachnoid cap cells, and accounting for approximately 37.6% of all primary brain tumors [1,2]. Various risk factors are associated with meningiomas, including age, female sex, ethnic group, family history, genetic polymorphisms, and certain medical conditions such as Turner's syndrome, Werner's syndrome, neurofibromatosis 2, and familial cancer syndromes. Women are more likely to be affected than men, with an average age of diagnosis of 66 years [2], [3], [4].
Although meningiomas are usually considered benign, they can cause significant morbidity, presenting with a variety of non-specific, location-dependent symptoms that can lead to delays in diagnosis and management. Patients may experience new-onset headaches, seizures, focal neurological dysfunction, or cranial nerve palsies, which necessitate further diagnostic evaluation with neuroimaging [2]. Meningiomas are typically easily detectable on imaging studies such as CT and MRI, due to their characteristic appearance and location. They are most commonly found in the falcine, convexity, and parasagittal regions of the brain, but can occur in other areas as well [5,6]. Brain MRI shows a mass with high density, broadly attached to the dura. The mass displays an isointense to hypointense signal on T1-weighted images and an isointense to hyperintense signal on T2-weighted images. Following the administration of contrast, the mass exhibits a uniformly intense enhancement [5]. However, diagnosing meningiomas can be challenging, particularly when imaging reveals unusual findings that resemble other malignant or nonmalignant neoplasms. Therefore, a careful analysis of imaging findings is necessary to establish an accurate diagnosis, which can help with surgical planning [7].
In this article, we present a unique radiological feature of Meningothelial meningioma.
Case presentation
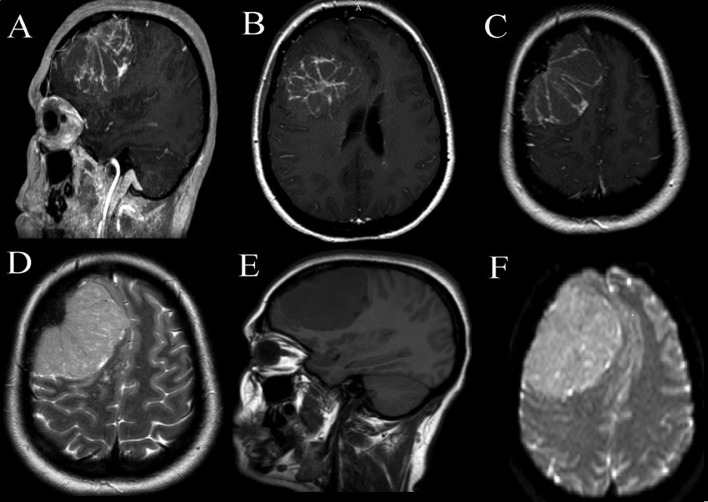
A 42-year-old right-handed female with a history of type 2 diabetes, she had progressive headache since 2 years. Neurological examination revealed frontal lobe syndrome with visual acuity: 02/10 in the right eye, and 01/10 in the left eye and bilateral papilledema. CT and MRI (Fig. 1) showed a right frontal convexity tumor with midline shift. Isoitense on T1WI, hyperintense on T2WI, with web-like enhancement after godalinium injection with no dural-tail. The ADC value was (0.69 × 10^− 3 mm2/s).
Fig. 1.
Brain MRI shows a right frontal convexity tumor with midline shift with web-like enhancement after godalinium injection with no dural-tail. And the ADC value was (0.69 × 10^− 3 mm2/s).
The patient underwent for surgery, using the right frontal approach, the bone flap and the dura was infiltrated. The tumor was identified as a grayish mass with moderate vascularity and soft consistency, and resection was performed with piecemeal fashion technique. Gross total resection was achieved.
The patient had an uneventful recovery and remained symptom-free at follow-up visits, and postoperative imaging confirmed complete removal.
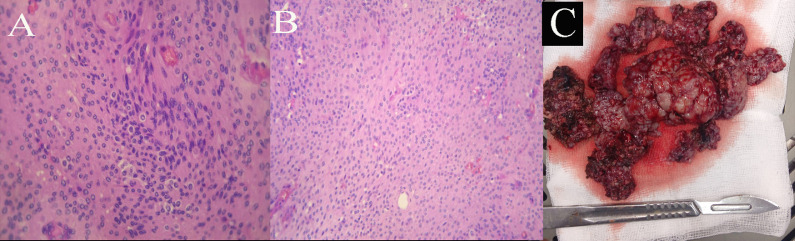
The pathological examination (Fig. 2) found grade 1 meningothelial meningioma.
Fig. 2.
(A, B) pathological examination shows grade 1 meningothelial meningioma. (C) preoperative image shows peacemeal resection.
Discussion
Meningiomas are primary brain tumors that usually arise from the meninges and are the most common form of such tumors. While most meningiomas are slow growing and benign, some can be malignant and aggressive [2]. These tumors are diagnosed more often in women than in men, with a female-to-male ratio of about 3:2. Although they can occur in people of any age, meningiomas are most commonly diagnosed in people over the age of 65 [2].
Clinical symptoms of meningiomas can vary depending on the tumor's location, size, and grade. Some meningiomas may not cause symptoms, especially if they are small and located in a noncritical area of the brain. However, as the tumor grows, it can put pressure on surrounding brain tissue, which can lead to a variety of symptoms, including headaches, seizures, weakness or numbness, vision or hearing problems, personality changes, memory loss, and balance or coordination problems [2].
Computed tomography (CT) is often the first imaging modality used to evaluate suspected meningiomas. Meningiomas typically present as a sharply circumscribed lobular mass with a broad-based dural attachment. On unenhanced CT, they appear as extra-axial masses that are homogeneous and hyperdense. Following contrast administration, they demonstrate homogeneous enhancement, which aids in their identification [5,8].
In addition, meningiomas are frequently associated with intratumoral calcification, which may appear as a speckled hyperdense appearance on CT. Bony changes associated with meningiomas can also be observed on CT imaging, including hyperostosis and osteolysis. Hyperostosis, the most commonly observed bony finding, manifests as bony thickening on CT and is seen in up to 25-49% of meningiomas, with the convexity and sphenoid wing being the most common locations. Hyperostosis may be reactive or associated with osseous tumor invasion, and strong enhancement within hyperostotic bone makes tumor invasion more likely [9,10].
Magnetic resonance imaging (MRI) is considered the preferred imaging method to distinguish meningiomas from other brain tumors, due to the characteristic appearance they display. This appearance can vary, depending on factors such as tumor size, location, and histological subtype [11,12]. Typically, meningiomas show up as a well-defined, enhancing mass on T1-weighted images, with or without surrounding edema. The degree of enhancement can vary from mild to intense, and it is usually homogeneous, although some tumors may have heterogeneous enhancement. In some cases, the enhancing portion of the tumor may be eccentric or located in a peripheral location, which is suggestive of a meningioma with a high grade of malignancy. On T2-weighted images, meningiomas are typically hyperintense compared to the surrounding brain tissue, with well-defined borders. However, the signal intensity may be variable, depending on the tumor's histology, and some tumors may have areas of low signal intensity, which are suggestive of calcifications or hemorrhages [5,8,13,14].
Meningiomas can present with different characteristics depending on the histological subtype, and the meningothelial variant is the most common one. After surgical resection, this subtype has a low recurrence rate. Imaging studies, including nonenhanced CT, T1-weighted MRI, T2-weighted MRI, and contrast-enhanced CT or MRI, usually show a hyperdense mass with a broad-based dural attachment, isointense to hypointense signal on T1WI, isointense to hyperintense signal on T2WI, and homogeneous intense enhancement following contrast administration. Another hallmark feature is an enhancing dural tail [5].
In our case, the patient initially presented with imaging findings suggestive of a meningioma. The histopathological analysis confirmed the diagnosis of meningioma, and further examination revealed it to be a meningothelial variant. The unique pattern of enhancement seen on imaging was atypical for this subtype.
The apparent diffusion coefficient (ADC) values of meningiomas can be variable on diffusion-weighted imaging (DWI), regardless of the tumor grade. Higher grades of meningiomas tend to have lower ADC values, but low ADC values can also be seen in grade I meningiomas. Magnetic resonance spectroscopy (MRS) can detect elevated choline and alanine levels, as well as diminished N-acetylaspartate (NAA) levels in meningiomas. Elevated alanine levels are relatively specific for meningiomas, but they can be difficult to identify. Other studies have also reported elevated choline and alanine levels in meningiomas, as well as reduced NAA levels. Perfusion imaging generally reveals high relative cerebral blood flow (rCBF) and relative cerebral blood volume (rCBV), although rCBV quantitation can be confounded by substantial gadolinium leakage with the dynamic susceptibility contrast (DSC) technique. These imaging techniques can be useful for the diagnosis and characterization of meningiomas, as well as for planning treatment strategies [5,[15], [16], [17]].
The grading of meningiomas is a crucial prognostic factor that guides treatment decisions and follow-up protocols. In the past, meningiomas were classified into various subtypes with different malignancy grades. However, the current approach regards meningiomas as a single tumor type with 15 subtypes, and the grading system has shifted towards a within-tumor grading regardless of subtype. The higher-risk subtypes, such as chordoid and clear cell meningiomas, are now assigned as grade 2 due to their higher likelihood of recurrence. Brain-invasive meningiomas are also generally associated with a higher risk of recurrence and are now classified as an atypical meningioma CNS WHO grade 2 in the WHO 2016 classification. While rhabdoid and papillary meningiomas may exhibit more aggressive behavior, classified as grade 3 meningioma [8,[18], [19], [20], [21]].
Meningiomas are treated on a case-by-case basis and may involve a range of options such as observation, surgical resection, radiotherapy, and in rare cases, chemotherapy. Advances in neurosurgery, neuroimaging, and neuroanesthesia have contributed to better long-term outcomes and overall survival rates for patients. Observation is a common approach for patients with incidentally detected small and asymptomatic meningiomas, with regular MRI scans to monitor for any changes. Surgical resection is typically the first choice for symptomatic meningiomas or larger tumors that are expected to cause symptoms soon. The extent of resection is a critical factor in recurrence rates, and complete removal is the primary goal. Radiotherapy is used as the primary treatment for unresectable meningiomas and as an adjuvant therapy after surgery in cases of WHO grade II and III meningiomas. For patients with recurrent or progressive meningiomas that no longer respond to surgery or radiotherapy, salvage systemic therapy may be used, although its efficacy is limited [22].
Conclusion
Meningioma are benign brain tumors, Brain MRI is preferred imagine method, it typically shows extra axial tumors with homogenous enhancement. Early detection and accurate diagnosis are crucial in determining the appropriate management.
Patient consent
Written informed consent for the publication of this case report was obtained from the patient.
Footnotes
Acknowledgments: This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Competing Interests: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
References
- 1.De Robles P, Fiest KM, Frolkis AD, Pringsheim T, Atta C, St. Germaine-Smith C, et al. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro Oncol. 2015;17(6):776–783. doi: 10.1093/neuonc/nou283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:V1–100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnan V, Mittal MK, Sinha M, Thukral BB. Imaging spectrum of meningiomas: a review of uncommon imaging appearances and their histopathological and prognostic significance. Polish J Radiol. 2019;84:e630–e653. doi: 10.5114/pjr.2019.92421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hortobágyi T, Bencze J, Varkoly G, Kouhsari MC, Klekner Á. Meningioma recurrence. Open Med. 2016;11(1):168–173. doi: 10.1515/med-2016-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buetow MP, Buetow PC, Smirniotopoulos JG. Typical, atypical, and misleading features in meningioma. Radiographics. 1991;11(6):1087–1106. doi: 10.1148/radiographics.11.6.1749851. [DOI] [PubMed] [Google Scholar]
- 6.Tokgoz N, Oner YA, Kaymaz M, Ucar M, Yilmaz G, Tali TE. Primary intraosseous meningioma: CT and MRI appearance. Am J Neuroradiol. 2005;26(8):2053–2056. [PMC free article] [PubMed] [Google Scholar]
- 7.Gangadhar K, Santhosh D, Fatterpekar G. Imaging Features of Intracranial Meningiomas with Histopathological Correlation: A Relook into Old Disease. Nepal J Radiol. 2013;3(1):14–32. [Google Scholar]
- 8.O'Leary S, Adams WM, Parrish RW, Mukonoweshuro W. Atypical imaging appearances of intracranial meningiomas. Clin Radiol. 2007;62(1):10–17. doi: 10.1016/j.crad.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Sheporaitis LA, Osborn AG, Smirniotopoulos JG, Clunie DA, Howieson J, Agostino AND. Intercranial meningioma. Am J Neuroradiol. 1992:29–37. [PMC free article] [PubMed] [Google Scholar]
- 10.Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107(5):905–912. doi: 10.3171/JNS-07/11/0905. [DOI] [PubMed] [Google Scholar]
- 11.Ihwan A, Rafika R, Cangara MH, Sjukur KJ, Faruk M. Correlation between radiological images and histopathological type of meningioma: a cohort study. Ethiop J Health Sci. 2022;32(3):597–604. doi: 10.4314/ejhs.v32i3.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhat AR, Wani MA, Kirmani AR, Ramzan AU. Histological-subtypes and anatomical location correlated in meningeal brain tumors (meningiomas) J Neurosci Rural Pract. 2014;5(3):244–249. doi: 10.4103/0976-3147.133568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams LC, Böker SM, Bender YY, Fallenberg EM, Wagner M, Buchert R, et al. Assessment of intracranial meningioma-associated calcifications using susceptibility-weighted MRI. J Magn Reson Imaging. 2017;46(4):1177–1186. doi: 10.1002/jmri.25614. [DOI] [PubMed] [Google Scholar]
- 14.Tamrazi B, Shiroishi MS, Liu CSJ. Advanced imaging of intracranial meningiomas. Neurosurg Clin N Am. 2016;27(2):137–143. doi: 10.1016/j.nec.2015.11.004. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald L. Mary seacole and claims of evidence-based practice and global influence. Nurs Open. 2016;3(1):5–18. doi: 10.1002/nop2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Yang Y, Xie Z, Zuo W, Jiang H, Zhao X, et al. Decreased Poly(ADP-Ribose) polymerase 1 expression attenuates glucose oxidase-induced damage in rat cochlear marginal strial cells. Mol Neurobiol. 2016;53(9):5971–5984. doi: 10.1007/s12035-015-9469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakyemez B, Yildirim N, Erdoǧan C, Kocaeli H, Korfali E, Parlak M. Meningiomas with conventional MRI findings resembling intraaxial tumors: can perfusion-weighted MRI be helpful in differentiation? Neuroradiology. 2006;48(10):695–702. doi: 10.1007/s00234-006-0115-y. [DOI] [PubMed] [Google Scholar]
- 18.Baumgarten P, Gessler F, Schittenhelm J, Skardelly M, Tews DS, Senft C, et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol. 2016;132(3):479–481. doi: 10.1007/s00401-016-1598-1. [DOI] [PubMed] [Google Scholar]
- 19.Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. 2022;128(1):47–58. doi: 10.1002/cncr.33918. [DOI] [PubMed] [Google Scholar]
- 20.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-Oncology. 2021;23(June):1821–1834. doi: 10.1093/neuonc/noab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Zhao W, Hou Y, Wen C, Wang J, Wu P, et al. An overview of managements in meningiomas. Front Oncol. 2020;10(August):1–12. doi: 10.3389/fonc.2020.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]