Abstract
OBJECTIVE
There are no commercially available hybrid closed-loop insulin delivery systems customized to achieve pregnancy-specific glucose targets in the U.S. This study aimed to evaluate the feasibility and performance of at-home use of a zone model predictive controller–based closed-loop insulin delivery system customized for pregnancies complicated by type 1 diabetes (CLC-P).
RESEARCH DESIGN AND METHODS
Pregnant women with type 1 diabetes using insulin pumps were enrolled in the second or early third trimester. After study sensor wear collecting run-in data on personal pump therapy and 2 days of supervised training, participants used CLC-P targeting 80–110 mg/dL during the day and 80–100 mg/dL overnight running on an unlocked smartphone at home. Meals and activities were unrestricted throughout the trial. The primary outcome was the continuous glucose monitoring percentage of time in the target range 63–140 mg/dL versus run-in.
RESULTS
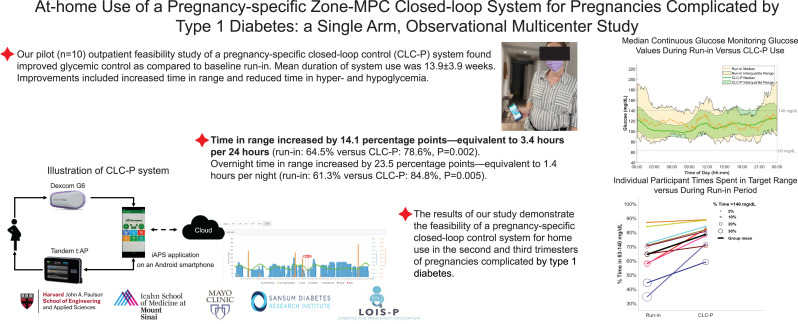
Ten participants (HbA1c 5.8 ± 0.6%) used the system from mean gestational age of 23.7 ± 3.5 weeks. Mean percentage time in range increased 14.1 percentage points, equivalent to 3.4 h per day, compared with run-in (run-in 64.5 ± 16.3% versus CLC-P 78.6 ± 9.2%; P = 0.002). During CLC-P use, there was significant decrease in both time over 140 mg/dL (P = 0.033) and the hypoglycemic ranges of less than 63 mg/dL and 54 mg/dL (P = 0.037 for both). Nine participants exceeded consensus goals of above 70% time in range during CLC-P use.
CONCLUSIONS
The results show that the extended use of CLC-P at home until delivery is feasible. Larger, randomized studies are needed to further evaluate system efficacy and pregnancy outcomes.
Graphical Abstract
Introduction
Pregnancies complicated by type 1 diabetes can be associated with significant maternal and fetal sequelae. Maternal hyperglycemia has been linked to complications including preeclampsia, medically indicated preterm delivery, labor abnormalities, need for cesarean delivery, and maternal birth trauma (1–4). Fetal and neonatal morbidity includes increased risk of congenital malformations, growth abnormalities (fetal growth restriction, small or large, for gestational age, fetus or neonate), oligohydramnios and polyhydramnios, stillbirth, birth trauma, neonatal hypoglycemia, hyperbilirubinemia, hypocalcemia, polycythemia, and neonatal intensive care admission (5–7). Maternal pregestational and gestational elevations in glycated hemoglobin, reduced time in glucose target range, maternal hyperglycemia, and episodes of severe maternal hypoglycemia have all been associated with poorer neonatal outcomes (6,8–10). The American Diabetes Association published consensus guidelines for the use of continuous glucose monitoring recommending that glycemic targets for pregnant women with type 1 diabetes achieve a glucose time in range goal of >70% between 63 and 140 mg/dL (3.5–7.8 mmol/L) (11). Despite increased adoption of continuous glucose monitoring (12), use of insulin pump therapy (13), and the use of more rapid insulin analogs (14), many pregnant women still struggle to achieve these targets and reduce glycemic variability to optimize pregnancy outcomes (15).
Hybrid closed-loop control systems have the potential to improve glycemic control during pregnancies complicated by type 1 diabetes; however, limited data are currently available for use of these systems during pregnancy (16–19). The two largest studies in this field from Stewart et al. (17,18) reported results for 16 women using closed-loop control compared with sensor-augmented pump use. These studies used an earlier noncommercial version of the CamAPS (CamDiab Ltd.), which is a closed-loop system that bears the Conformité Européenne mark (CE mark) for use in pregnant women with type 1 diabetes. In the U.S., there are currently no FDA-cleared closed-loop control systems for use during pregnancy, and none of the commercially available systems are customized to pregnancy-specific glucose targets. Our previously published data from a supervised 48-h hotel study demonstrated that glucose control using a zone model predictive controller (zone-MPC) specifically customized for pregnancy (CLC-P) is feasible (20). The results from this pilot study confirmed the algorithm tuning and indicated the need for further studies to evaluate the system for home use. Here we report the results of the first outpatient, extended duration study in the U.S. assessing the use of CLC-P during pregnancies complicated by type 1 diabetes.
Research Design and Methods
Study Participants and Protocol
The study was approved by a central institutional review board (Mayo Foundation Institutional Review Board) and the Food and Drug Administration under an Investigational Device Exemption and was conducted in pregnant women with preexisting type 1 diabetes between May 2021 and October 2022 at three clinical centers in the U.S. All participants provided informed consent prior to enrollment. Data from all centers were collected and managed on the REDCap electronic data capturing tool hosted by Mayo Clinic (21).
Pregnant women aged 18–45 years with preexisting type 1 diabetes for more than 1 year, between 140/7 to 326/7 gestational weeks, already using an insulin pump, and with glycated hemoglobin of 9% or less with singleton pregnancy were enrolled. Participants were instructed to bolus insulin for all meals and snacks that contained ≥5 grams of carbohydrate unless treating hypoglycemia;participants used versions of either lispro or aspart insulin approved for use in the study pump during the trial. Those known to have cardiac disease, concurrent use of any noninsulin glucose-lowering agent other than metformin, any bleeding disorder, prior history of preterm premature rupture of membranes, significant hyperemesis interfering with carbohydrate intake, abnormal liver or renal function tests, or dermatological conditions that would preclude wearing a sensor or insulin infusion set were excluded from the study. Participants were enrolled in a phased manner. A data safety monitoring board consisting of three field experts separate from the study team initially followed study progress frequently and then every 3 months after safety requirements were met for the first three participants.
The study consisted of three phases: phase one was a run-in period of 1 to 2 weeks using the participant’s personal therapy and study-provided continuous glucose monitoring (Dexcom G6, Dexcom) (referred to as run-in); phase two was 2 days of system use in a supervised outpatient environment (three participants’ data from this study’s 2-day period are included in our previous publication [20]); phase three was CLC-P use at home until delivery (referred to as CLC-P). During CLC-P use, participants operated the study system at home with event-based remote monitoring including real-time alerts for prespecified glucose limits and connectivity issues sent to the participant’s study site team (Supplementary Material). Participants were required to perform blood glucose monitoring before meals, postprandial, and at bedtime during the first 2 weeks of home use per prespecified regulatory requirements (Supplementary Material). Each site conducted 24-, 48-, and 72-h phone check-in visits after dismissal to home setting to ensure safety and continued use of the study system, followed by weekly phone visits for the duration of system use for review of participants’ glucose control, insulin delivery, and monitoring for potential adverse events. Insulin delivery settings (basal rates, carbohydrate ratios, and correction factors) were adjusted weekly if clinically indicated or sooner based on glycemic control as pregnancy progressed. Biweekly pregnancy data were analyzed to monitor time in range and other continuous glucose monitoring metrics to assess safety in continuing participation in the study (Supplementary Material). According to prespecified regulatory requirements, study systems were discontinued before hospital admission and delivery, and participants were either transitioned safely to their home devices during delivery or maintained on intravenous insulin infusion based on clinical preferences.
Closed-loop System
The closed-loop system, interoperable artificial pancreas system (22), consisted of a Tandem insulin pump for research (t:AP insulin pump; Tandem Diabetes Care), a continuous glucose monitor, and a MPC–based algorithm customized for pregnancy (CLC-P). The system application resided in an unlocked Android phone (Google Pixel 3a). CLC-P was configured by total daily insulin delivery (manually entered at initiation and then updated if it changed by more than 10%) and physician-prescribed insulin pump treatment parameters (i.e., carbohydrate ratio, insulin sensitivity factor, and basal insulin profile) for personalization of insulin dosing decisions.
CLC-P was developed for use in pregnancies complicated by type 1 diabetes (23), and data on supervised use of the current algorithm have been previously reported (20). Every 5 min, the controller computes an optimal insulin microbolus to keep glucose in a target glucose zone of 80–110 mg/dL (4.4–6.1 mmol/L) during the day and 80–100 mg/dL (4.4–5.6 mmol/L) overnight (12:00 a.m. to 6:00 a.m.). The microbolus computations are subject to constraints, including a limit on the maximum insulin delivery allowed at a time step, which is a function of predefined limits and insulin on board.
The amount of bolus insulin required before meals is calculated based on the user-entered carbohydrate amount, prescribed carbohydrate ratio, insulin sensitivity factor, and glucose level at that time. Meal boluses were automatically reduced by 20% if the premeal glucose was below 70 mg/dL (3.9 mmol/L). To mitigate the increased risk of postprandial hyperglycemia in pregnancy, correction boluses were automatically administered with the meal bolus to bring the glucose to 90 mg/dL (5 mmol/L) when the premeal glucose was above 100 mg/dL (5.6 mmol/L). The controller was designed to intensify insulin delivery when the blood glucose values were trending upward while in the 120–180 mg/dL (6.7–10 mmol/L) range.
For enhanced protection against hypoglycemia, the system has a safety layer—health monitoring system—designed to work in parallel with the controller. The system warns the user through audiovisual advisory alarms and informs the research team via text messages for impending hypoglycemia. These alarms were triggered when glucose was predicted to fall below 65 mg/dL (3.6 mmol/L) in the next 15 min (Supplementary Material) (24).
Study End Points
The primary efficacy end point was the percentage of time within the pregnancy-specific target glucose range of 63–140 mg/dL (3.5–7.8 mmol/L), as assessed by continuous glucose monitoring during CLC-P use compared with run-in. Secondary efficacy end points included overnight and 2-h postprandial time in range, as well as mean glucose and glycemic variability. Safety end points included frequency and duration of mild, moderate, or severe hypoglycemia. Mild and moderate hypoglycemia were defined as below thresholds of 63 or 54 mg/dL (3.5 or 3.0 mmol/L), respectively. Severe hypoglycemia was defined as requiring active third-party treatment. Hypoglycemic events were defined as time <54 mg/dL for 15 consecutive minutes followed by time >70 mg/dL for 15 consecutive minutes. Additional safety end points included percent time in hyperglycemia above predefined thresholds of 140, 180, and 250 mg/dL (7.8, 10, and 13.9 mmol/L). Hyperglycemia events were classified as diabetic ketoacidosis if the following criteria were present: symptoms such as polyuria, polydipsia, nausea, or vomiting; serum ketones >1.5 mmol/L or large/moderate urine ketones; either arterial blood pH <7.30 or venous pH <7.24 or serum bicarbonate <15; and treatment provided in a health care facility. Usability outcomes included active time in closed-loop, continuous glucose monitoring use time, device issues, and total daily insulin delivery along with basal and bolus insulin delivery.
The clinical study also reviewed any serious adverse events, planned and unplanned outside interventions, and unanticipated adverse device effects as well as maternal, fetal, and neonatal outcomes (Supplementary Material).
Data Analysis
Analyses were conducted on an intention-to-treat basis. Each participant’s glucose control during run-in served as their own baseline reference, and the outcomes from CLC-P use were compared against the reference period. For continuous outcomes, a paired t test was used if the normality assumption was met and a Wilcoxon signed-rank test was used otherwise. A Shapiro-Wilk test was used for testing the normality assumption. Hypoglycemic events were analyzed with Poisson regression adjusted for random participant effect. All P values were two-tailed, and the results are interpreted based on the statistical significance threshold of 0.05.
Postprandial 2-h glucose time in range was calculated based on the continuous glucose monitoring data collected within 2 h of meal boluses only when no other meal was reported within this period. Both postprandial time in range and the percentage of time in closed loop were computed exclusively for CLC-P use.
Results
Between May 2021 and May 2022, 10 participants (mean age 32.6 ± 4.3 years, diabetes duration of 16.6 ± 7.8 years, glycated hemoglobin 5.8 ± 0.6%) were enrolled in the study and completed all three study phases. Participant characteristics are summarized in Table 1. The average duration of CLC-P use was 14 ± 4 weeks per participant. Across participants, continuous glucose monitoring use time and active time in closed loop were 98.5 ± 0.6% and 94.1 ± 4.2%, respectively.
Table 1.
Baseline characteristics of the 10 study participants
| Characteristic | Value |
|---|---|
| Age, years | 32.6 ± 4.3 |
| Ethnicity: not Hispanic or Latino, no. (%) | 10 (100) |
| Race: White, no. (%) | 10 (100) |
| Duration of diabetes, years | 16.6 ± 7.8 |
| Weight, kg | 75.7 ± 17.2 |
| BMI* | 27.2 ± 4.9 |
| Glycated hemoglobin, % | 5.8 ± 0.6 |
| Gestational age at enrollment, weeks | 22.0 ± 3.4 |
| First pregnancy, no. (%)† | 4 (40) |
| Pump type, no. (%) | |
| Sensor-augmented pump | 6 (60) |
| Predictive low-glucose suspend | 3 (30) |
| Hybrid closed loop | 1 (10) |
| Duration of pump use, no. (%) | |
| 6 months to 1 year | 1 (10) |
| 1–5 years | 3 (30) |
| 5–10 years | 1 (1) |
| >10 years | 5 (50) |
| CGM user at enrollment, no. (%) | 10 (100) |
| Duration of CGM use, no. (%) | |
| 3–6 months | 1 (10) |
| 1–5 years | 7 (70) |
| 5–10 years | 1 (10) |
| >10 years | 1 (10) |
| Metformin use | 0 (0) |
| Average total daily insulin during run-in, units | 41.7 ± 15 |
| Average total daily insulin during run-in, units/kg | 0.55 ± 0.18 |
Data are mean ± SD unless otherwise indicated. CGM, continuous glucose monitoring.
BMI is the weight in kilograms divided by the square of the height in meters.
See Supplementary Material for details of participants’ obstetric history.
Glucose Control Outcomes
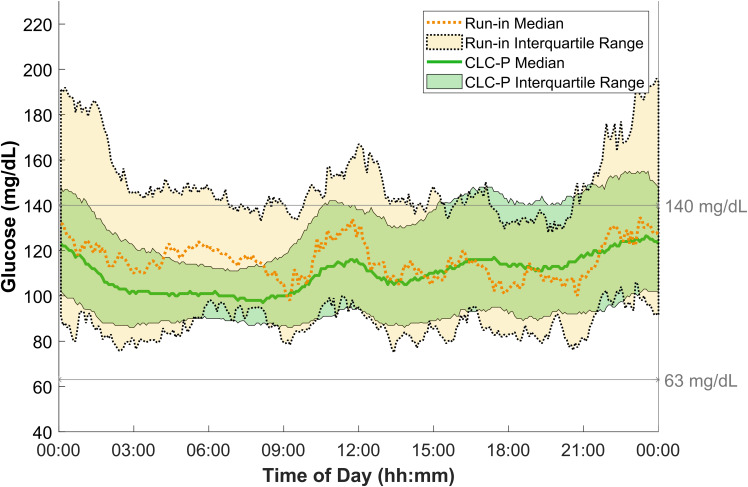
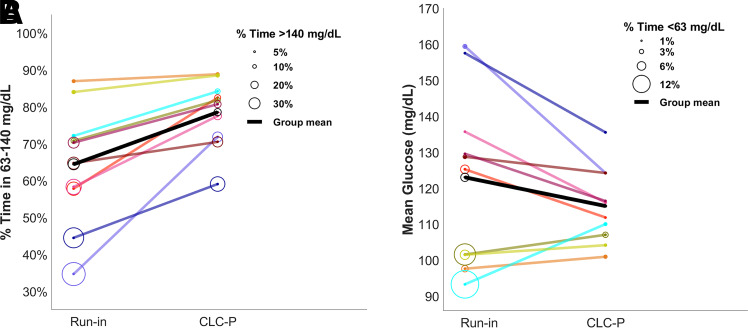
Compared with the participants’ run-in, average time in range was significantly higher during CLC-P use for both 24-h (run-in 64.5% versus CLC-P 78.6%; P = 0.002) and overnight (run-in 61.3% versus CLC-P 84.8%; P = 0.005). Time in range increased by 14.1 percentage points—equivalent to 3.4 h per 24 h. Overnight time in range increased by 23.5 percentage points—equivalent to 1.4 h per night. Mean glucose was 123 mg/dL (6.8 mmol/L) during run-in and 115 mg/dL (6.4 mmol/L) during CLC-P use (P = 0.139). These outcomes were accompanied by lower time above 140 mg/dL (7.8 mmol/L) (run-in 29.8% versus CLC-P 19.7%; P = 0.033), lower time below 63 mg/dL (3.5 mmol/L) (run-in 3.7% versus CLC-P 1.57%; P = 0.037), and lower time below 54 mg/dL (3 mmol/L) (run-in 1.0% versus CLC-P 0.4%; P = 0.037) during CLC-P use. The number of hypoglycemic events per week also decreased significantly on CLC-P (run-in 4.0 versus CLC-P 0.7; P < 0.001). Detailed results are shown in Table 2, and Fig. 1 shows the 24-h median profiles. Nine participants achieved the recommended threshold of 70% for time in range during CLC-P use, and all participants had a higher time in range compared with run-in (Fig. 2). Postprandial 2-h time in range was 73.4 ± 11.0% per participant. A breakdown of these outcomes for each individual participant and other outcomes are detailed in Supplementary Material.
Table 2.
Continuous glucose monitoring outcomes for run-in versus CLC-P use
| Variable | Run-in§ | CLC-P | Absolute difference (95% CI) | P value† |
|---|---|---|---|---|
| Primary outcome | ||||
| Time in 63–140 mg/dL, % | 64.5 ± 16.3 | 78.6 ± 9.2 | 14.1 (6.6 to 21.7) | 0.002 |
| Secondary outcomes | ||||
| Overnight time in 63–140 mg/dL, %‡ | 61.3 ± 20.7 | 84.8 ± 7.7 | 23.5 (9.0 to 37.9) | 0.005 |
| Postprandial time in 63–140 mg/dL, %* | NA | 73.4 ± 11.0 | NA | NA |
| Time <63 mg/dL, % [IQR]†† | 3.7 [1.5 to 6.4] | 1.6 [1.4 to 2.1] | −2.8 (−8.3 to −0.3) | 0.037 |
| Time <54 mg/dL, % [IQR]†† | 1.0 [0.3 to 2.2] | 0.4 [0.3 to 0.4] | −0.9 (−3.7 to −0.02) | 0.037 |
| Time >140 mg/dL, % | 29.8 ± 19.5 | 19.7 ± 9.5 | −10.1 (−19.2 to −1.0) | 0.033 |
| Time >180 mg/dL, % [IQR]†† | 7.2 [4.0 to 13.6] | 3.4 [3.0 to 8.0] | −5.3 (−13.6 to −1.2) | 0.002 |
| Time >250 mg/dL, % [IQR]†† | 0.3 [0.0 to 1.9] | 0.2 [0.1 to 0.6] | NA‡‡ | NA‡‡ |
| Mean glucose, mg/dL | 123.1 ± 24.1 | 115.1 ± 10.6 | −8.0 (−19.1 to 3.1) | 0.139 |
| Hypoglycemic events per week** | 4.0 ± 4.7 | 0.7 ± 0.6 | −5.4 (−7.7 to −3.7) | <0.001 |
Data are means ± SD unless otherwise indicated. To convert values for glucose to millimoles per liter, multiply by 0.05551. IQR, interquartile range; NA, not applicable.
The run-in week is defined as the last 7 days before the day participants switched to CLC-P. Mean gestational age at start of CLC-P was 23.7 ± 3.5 weeks and ended at a mean of 37.9 ± 1.1 weeks. One participant’s run-in was calculated based on their last 8 days of data instead because they had 1 day missing within the last 7 days before switching to CLC-P.
P values were calculated only for the outcomes that had been prespecified in the statistical plan.
Overnight is defined as 12:00 a.m. to 6:00 a.m.
Outcomes are calculated only for the CLC-P use period. Participants followed their regular treatment during run-in, including how they reported their carbohydrate intake. Thus, postprandial outcome is calculated only for the CLC-P use period, where participants were instructed to input all their carbohydrate intakes.
Hypoglycemic events are defined as time <54 mg/dL for 15 consecutive minutes followed by time >70 mg/dL for 15 consecutive minutes. This outcome is modeled with Poisson regression, and the model is adjusted for the participant effect (random effect).
Differences had skewed distributions, and thus these outcomes were analyzed via Wilcoxon signed rank test, while all others were analyzed via paired t test.
Most of the values were approximately zero, leading to not computable P values and CIs.
Figure 1.
Median continuous glucose monitoring glucose values during run-in versus CLC-P use. Comparison of glucose levels based on continuous glucose monitoring data between CLC-P (solid lines indicating median, and green shading indicating interquartile range) and run-in (dashed lines indicating median, and yellow shading indicating interquartile range). To convert values for glucose to millimoles per liter, multiply by 0.05551.
Figure 2.
Individual participant times spent in target range and above target range, mean glucose, and time spent below target range. A: Percent time spent in the target range 63–140 mg/dL (line) and above the target range (circle size). B: Mean continuous glucose monitoring–measured glucose (line) and percent time spent below the target range (circle size). Each participant’s data are depicted with a separate color, and individual results are provided in Supplementary Material. The same color is used for the same participants throughout the panels. To convert values for glucose to millimoles per liter, multiply by 0.05551.
Maternal and Neonatal Events and Outcomes
Maternal events were notable for an episode of ketosis with hyperglycemia in two participants at 342/7 and 366/7 weeks’ gestation with peak ketone meter measurement elevations of 2.0 and 1.4 mmol/L, respectively. Both were due to infusion set occlusions, which were managed in the outpatient setting (Supplementary Material). Two women developed coronavirus disease 2019 with mild symptoms. There were no severe hypoglycemic events or admissions for ketoacidosis.
One woman developed gestational hypertension, and another had exacerbation of prepregnancy hypertension. None of the women developed preeclampsia, eclampsia, polyhydramnios, or oligohydramnios. All participants elected to continue system use until delivery, except for one participant who preferred to transition to her personal devices 9 days early because of a concern about difficulty transitioning to her personal devices while in active labor. The mean gestational age at delivery was 37.8 ± 0.9 weeks. No infants were born before 37 weeks’ gestational age. Three participants had scheduled cesarean deliveries (two repeat, one for malpresentation), two had emergent cesarean deliveries (one for gestational hypertension in setting of malpresentation and one for a failed contraction stress test), and five had vaginal deliveries. No deliveries were complicated by shoulder dystocia.
Median birth weight was 3,515 g (range 2,880 to 3,941 g), with three infants large for gestational age and one infant small for gestational age (25). There were no episodes of neonatal hypoglycemia requiring IV dextrose. One infant delivered at 394/7 weeks of gestation was admitted to the neonatal intensive care unit for 2 days for observation and received antibiotic therapy after an event of mild respiratory distress and a dusky appearance attributed to the newborn swallowing amniotic fluid (identified by chest X-ray as reported in the discharge documentation); the infant and mother were discharged to home on day 2 after antibiotics were completed. No other complications were reported, and no infants had congenital malformations, based on newborn chart record assessment.
Conclusions
Our pilot outpatient feasibility study reports the first pregnancy-specific closed-loop control system outcomes during extended at-home use in the U.S. During system use, participants had improved glycemic control as compared with their baseline from run-in (at mean gestational age of 22.7 weeks [range 15.7–26.9]), including improved time in range and reduced time in hyperglycemia and hypoglycemia, demonstrating that it is feasible to aim for and to achieve pregnancy-specific targets safely in a home setting in the U.S. using a customized hybrid closed-loop system. Glucose control improved or remained in target time in range on the CLC-P system for each individual participant, and all but one participant’s mean time in range were above the recommended goals for pregnancy. Significant improvement over participants’ personal therapy suggests CLC-P is effective in achieving glycemic control for the pregnancy-specific recommendation without an increase in hypoglycemia. Mean glucose was lower for most participants, except for those with a high percentage of hypoglycemia at enrollment. It is important to note that one participant was using a commercial hybrid closed-loop system off-label, and three were using an insulin pump with a predictive low-glucose suspend feature for their standard therapy during run-in. No formal user experiences were collected in our study; participants did, however, elect to continue experimental system use until approaching delivery irrespective of enrollment time in range (TIR) or insulin pump regimen. Currently, there are limited published data reporting outcomes of closed-loop systems in pregnancy. In 2016, in the first outpatient, open-label, randomized, crossover study comparing a hybrid closed-loop research system with sensor-augmented pump therapy worn overnight for 4 weeks, Stewart et al. (17) found that system users spent significantly more time in target range (74.7% versus 59.5%) and had lower overnight mean glucose levels (119 versus 133 mg/dL [6.6 versus 7.4 mmol/dL]). In a separate report by the same group comparing day-and-night system use to sensor-augmented pump therapy during pregnancy, participants had a comparable percentage of time in range (62.3% versus 60.1%), mean glucose values, and proportions of time spent above 140 mg/dL (7.8 mmol/L) (18). Our study was small in number, with a different design that cannot be directly compared with the above studies; nonetheless, our findings are encouraging and show a clinically and statistically significant increase in time in range compared with run-in.
Our data set has expected limitations due to our focus on evaluating the feasibility of such a system in a vulnerable and understudied patient population, including a small sample size and without use during the first trimester because of regulatory requirements (completion of organogenesis before experimental system use). In addition, there was no randomized control group; instead, each participant’s 1-week unsupervised run-in period was used as their control. Our safety plan included close monitoring of participants, frequent contacts with the participants by the study team for dose titrations or any other needed education or interventions, and rigorous oversight because of regulatory requirements, and it may have contributed to the study outcomes. Based on differences in study design and the small number of participants evaluated, we are not able to directly compare our findings to the results of the few publications currently in this area. Nonetheless, CLC-P use enabled 9 out of 10 participants to achieve consensus recommendations for time in pregnancy range. Strengths of this study include its multicenter design, inclusion of participants struggling with high time above range or high time below range, the system’s use through a wide range of gestational ages, and no restrictions on daily life activities. Our data, at present, are the longest duration and first in the U.S. study of a pregnancy-specific system at home.
The results of our study confirm the feasibility of pregnancy-specific closed-loop control for home use and demonstrate the effectiveness of glycemic control for the pregnancy-specific requirements without severe adverse events or an increase in hypoglycemia. Future studies with larger sample sizes, use during early pregnancy and during delivery, and formal participant satisfaction surveys are needed to explore the generalizability of the results.
Article Information
Acknowledgments. The authors acknowledge Dr. Lois Jovanovič, a pioneer in the field of diabetes and pregnancy, for inspiring the team to pursue the design and clinical evaluation of the pregnancy-specific closed-loop system in the home setting.
Funding. Financial support for this study was provided by the National Institutes of Health (R01DK120358). Product support was provided by Dexcom, Inc. (AP-2020-014). Additional financial support was provided by Helmsley Charitable Trust (grant no. G-2204-05196). REDCap data management was supported by Research Computing Facility grant UL1TR002377.
Duality of Interest. Y.C.K. has received research and product support paid to his institution from Tandem Diabetes Care, Roche Diabetes, and Dexcom, Inc. C.J.L. has received research support and supplies from Insulet Corporation, Abbott Diabetes, Tandem Diabetes Care, and Dexcom, Inc. paid to her institution and has received consulting fees from Dexcom, Inc. and Eli Lilly and Company. B.O. is currently an employee of Insulet Corporation. Work performed on this study was part of her academic appointment at Harvard University and is independent of her employment with Insulet Corporation. K.C. has received research support paid to her institution from Insulet Corporation, Abbott, Medtronic Diabetes, and Eli Lilly and Company; has received research support and supplies from Dexcom, Inc.; and has received consulting fees from Dexcom, Inc. and Abbott Diabetes. G.O., C.M.L., and S.O. have received research supplies and support paid to their institution from Tandem Diabetes Care, Insulet Corporation, Abbott Diabetes, and Dexcom, Inc. W.K.K. has received research funding from AstraZeneca, Roche, and Biogen. J.E.P. is an employee and shareholder of Tandem Diabetes Care. Participation on this project was performed while he was an employee of Sansum Diabetes Research Institute. F.J.D. reports equity and licensed intellectual property (IP), and is a member of the Scientific Advisory Board of Mode AGC. E.D. has received personal fees from Roche and Eli Lilly and Company; holds patents on artificial pancreas technology; has received product support from Insulet Corporation, Tandem Diabetes Care, Roche, and Dexcom, Inc.; and is currently an employee and shareholder of Eli Lilly and Company. The work presented in this article was performed as part of his academic appointment and is independent of his employment with Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.J.L., Y.C.K., K.C., G.O., J.E.P., and E.D. developed the concept and protocol design of the study. C.J.L., Y.C.K., B.O., K.C., G.O., R.J.K., C.M.L., M.M.C., D.D., S.M.-S., S.O., C.R., S.R., J.E.P., and E.D. conducted the study. B.O., I.Z., and W.K.K. performed statistical analysis of study data. C.J.L., Y.C.K., B.O., K.C., G.O., R.J.K., C.M.L., M.M.C., S.O., and S.R. interpreted qualitative study data. C.J.L., Y.C.K., and B.O. wrote the first draft of this manuscript. M.C.T., S.D., and F.J.D., in addition to all of the authors listed, edited, reviewed, and approved the final version of this manuscript. E.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Portions of this study’s results were presented at the Advanced Technologies and Treatments for Diabetes (ATTD) 2023 International Diabetes Congress (Berlin, Germany), 23–26 February 2023.
Footnotes
Clinical trial reg. no. NCT04492566, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.22709128.
C.J.L., Y.C.K., and B.O. contributed equally to this work.
A complete list of members of the LOIS-P Diabetes and Pregnancy Consortium can be found in the supplementary material online.
References
- 1. Holmes VA, Young IS, Patterson CC, et al.; Diabetes and Pre-eclampsia Intervention Trial Study Group . Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes Care 2011;34:1683–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maresh MJ, Holmes VA, Patterson CC, et al.; Diabetes and Pre-eclampsia Intervention Trial Study Group . Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care 2015;38:34–42 [DOI] [PubMed] [Google Scholar]
- 3. Cohen O, Keidar N, Simchen M, Weisz B, Dolitsky M, Sivan E. Macrosomia in well controlled CSII treated type I diabetic pregnancy. Gynecol Endocrinol 2008;24:611–613 [DOI] [PubMed] [Google Scholar]
- 4. Ekbom P, Damm P, Feldt-Rasmussen B, Feldt-Rasmussen U, Jensen DM, Mathiesen ER. Elevated third-trimester haemoglobin A 1c predicts preterm delivery in type 1 diabetes. J Diabetes Complications 2008;22:297–302 [DOI] [PubMed] [Google Scholar]
- 5. Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care 2009;32:2005–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evers IM, de Valk HW, Visser GH. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ 2004;328:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy HR, Bell R, Cartwright C, et al. Improved pregnancy outcomes in women with type 1 and type 2 diabetes but substantial clinic-to-clinic variations: a prospective nationwide study. Diabetologia 2017;60:1668–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inkster ME, Fahey TP, Donnan PT, Leese GP, Mires GJ, Murphy DJ. Poor glycated haemoglobin control and adverse pregnancy outcomes in type 1 and type 2 diabetes mellitus: systematic review of observational studies. BMC Pregnancy Childbirth 2006;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feig DS, Donovan LE, Corcoy R, et al.; CONCEPTT Collaborative Group . Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, Damm P, Mathiesen ER. Hypoglycaemia during pregnancy in women with type 1 diabetes. Diabet Med 2012;29:558–566 [DOI] [PubMed] [Google Scholar]
- 11. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levy CJ, Foster NC, DuBose SN, et al. Changes in device uptake and glycemic control among pregnant women with type 1 diabetes: data from the T1D exchange. J Diabetes Sci Technol 2021;15:1297–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nosova EV, O’Malley G, Dassau E, Levy CJ. Leveraging technology for the treatment of type 1 diabetes in pregnancy: a review of past, current, and future therapeutic tools. J Diabetes 2020;12:714–732 [DOI] [PubMed] [Google Scholar]
- 14. Toledano Y, Hadar E, Hod M. Pharmacotherapy for hyperglycemia in pregnancy - the new insulins. Diabetes Res Clin Pract 2018;145:59–66 [DOI] [PubMed] [Google Scholar]
- 15. O’Malley G, Ozaslan B, Levy CJ, et al. Longitudinal observation of insulin use and glucose sensor metrics in pregnant women with type 1 diabetes using continuous glucose monitors and insulin pumps: the LOIS-P study. Diabetes Technol Ther 2021;23:807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy HR, Elleri D, Allen JM, et al. Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care 2011;34:406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stewart ZA, Wilinska ME, Hartnell S, et al. Closed-loop insulin delivery during pregnancy in women with type 1 diabetes. N Engl J Med 2016;375:644–654 [DOI] [PubMed] [Google Scholar]
- 18. Stewart ZA, Wilinska ME, Hartnell S, et al. Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Diabetes Care 2018;41:1391–1399 [DOI] [PubMed] [Google Scholar]
- 19. Polsky S, Akturk HK. Case series of a hybrid closed-loop system used in pregnancies in clinical practice. Diabetes Metab Res Rev 2020;36:e3248. [DOI] [PubMed] [Google Scholar]
- 20. Ozaslan B, Levy CJ, Kudva YC, et al. Feasibility of closed-loop insulin delivery with a pregnancy-specific zone model predictive control algorithm. Diabetes Technol Ther 2022;24:471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deshpande S, Pinsker JE, Zavitsanou S, et al. Design and clinical evaluation of the Interoperable Artificial Pancreas System (iAPS) smartphone app: interoperable components with modular design for progressive artificial pancreas research and development. Diabetes Technol Ther 2019;21:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozaslan B, Deshpande S, Doyle FJ III, Dassau E. Zone–MPC automated insulin delivery algorithm tuned for pregnancy complicated by type 1 diabetes. Front Endocrinol 2021;12:768639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harvey RA, Dassau E, Zisser H, Seborg DE, Jovanovič L, Doyle FJ III. Design of the health monitoring system for the artificial pancreas: low glucose prediction module. J Diabetes Sci Technol 2012;6:1345–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr 2003;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]