Summary
We characterized the role of structural variants, a largely unexplored type of genetic variation, in two non-Alzheimer’s dementias, namely Lewy body dementia (LBD) and frontotemporal dementia (FTD)/amyotrophic lateral sclerosis (ALS). To do this, we applied an advanced structural variant calling pipeline (GATK-SV) to short-read whole-genome sequence data from 5,213 European-ancestry cases and 4,132 controls. We discovered, replicated, and validated a deletion in TPCN1 as a novel risk locus for LBD and detected the known structural variants at the C9orf72 and MAPT loci as associated with FTD/ALS. We also identified rare pathogenic structural variants in both LBD and FTD/ALS. Finally, we assembled a catalog of structural variants that can be mined for new insights into the pathogenesis of these understudied forms of dementia.
Keywords: Lewy body dementia, frontotemporal dementia, amyotrophic lateral sclerosis, structural variant, genome-wide association study, resource, case-control study, non–Alzheimer's dementia
Graphical abstract
Highlights
-
•
Structural variants were called in the genomes of non-Alzheimer’s dementias
-
•
Discovery of TPCN1 as a novel risk locus for Lewy body dementia
-
•
Structural variants at C9orf72 and MAPT were present in frontotemporal dementia
-
•
Gene-set analyses identified likely pathogenic rare structural variants
This article from Kaivola, Chia, and Ding et al. describes the identification, characterization, and analysis of structural variants in genome data from patients with the non-Alzheimer’s dementias Lewy body dementia (LBD) and frontotemporal dementia (FTD)/amyotrophic lateral sclerosis (ALS).
Introduction
Structural variants are duplicated, deleted, inserted, inverted, or translocated DNA segments measuring at least 50 base pairs in length by current definition. This class of genomic variation is a major source of genetic diversity in the human genome that contributes to phenotypic heterogeneity.1,2 Not surprisingly, structural variants have been implicated in the pathogenesis of complex neurological disorders. For example, APP duplications,3,4 SNCA multiplications,5,6 and recombination events at the GBA locus7 are rare causes of Lewy body dementia (LBD), the second most common form of neurodegenerative dementia after Alzheimer’s disease. The discovery of repeat expansions in C9orf72 unified frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) into the same clinical spectrum.8,9,10 These findings highlight the pathogenic role of structural variants in disease and suggest that it may account for at least some of the unexplained heritability in age-related neurological conditions.
To date, identifying the structural variants underlying disease has relied on candidate-gene studies rather than unbiased genome-wide assessments. More recently, however, the study of structural variants has gained momentum due to the improved availability of whole-genome sequence datasets and modernized detection algorithms.11,12,13 These efforts are enhanced by the publication of structural variant catalogs, providing crucial insights into the frequency and heterogeneity of this understudied variant type in the human population.11,14,15,16
Here, we applied these advancements to systematically map genomic structural variants in large cohorts of patients diagnosed with non-Alzheimer’s dementias (LBD and FTD/ALS) and in unaffected controls.12,13 We exploited this resource to perform genome-wide association studies (GWASs) to identify common structural variants acting as risk modulators and identify rare, pathogenic structural variants in neurodegenerative disease genes. Our work discovered structural variants associated with risk of developing these fatal neurological conditions, confirming the critical role of this variant class in neurodegeneration. Building on our work, we compiled an interactive resource that can be investigated by other researchers for new insights into non-Alzheimer’s dementias.
Results
Structural variant identification in non-Alzheimer’s dementias
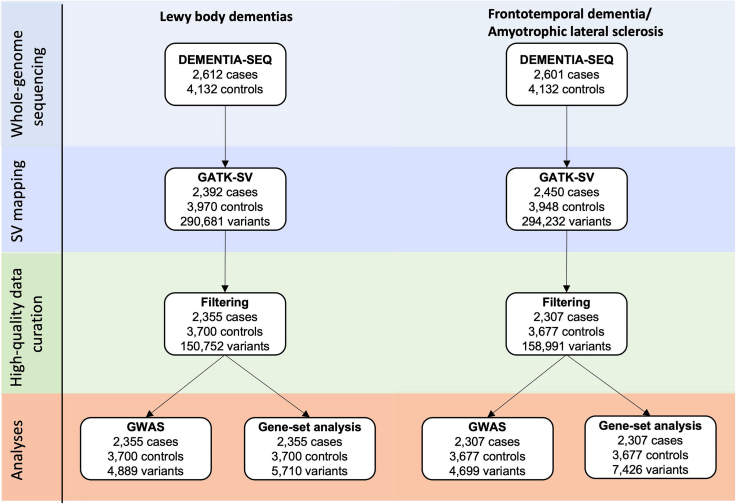
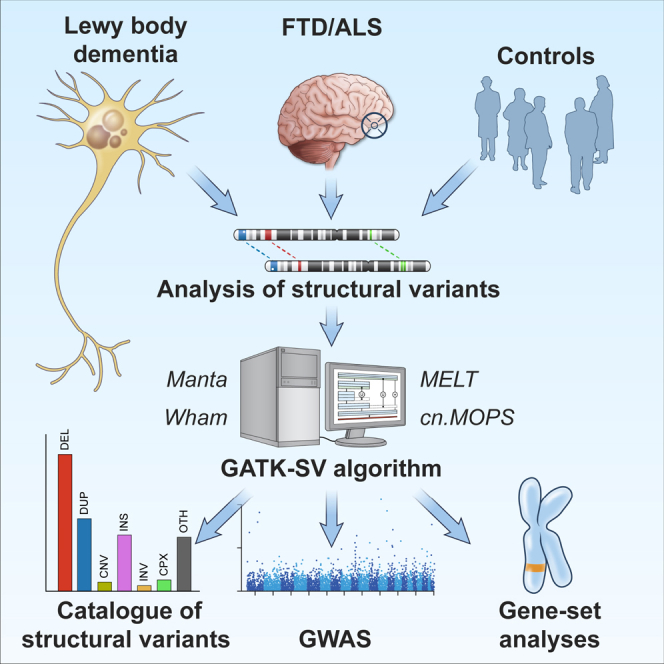
We mapped structural variants in whole-genome sequence data obtained from patients diagnosed with LBD (n = 2,612), FTD/ALS (n = 2,601), and unaffected individuals (n = 4,132) using the Genome Analysis Toolkit’s Structural Variant (GATK-SV) pipeline.11,12,13 This method improves the identification of structural variants in Illumina short-read whole-genome sequencing data by applying machine learning to combine information from five detection algorithms. Figure 1 summarizes the study workflow.
Figure 1.
Study flowchart
Schematic illustration of the analytical workflow. The same controls were used in the analysis of LBD and FTD/ALS.
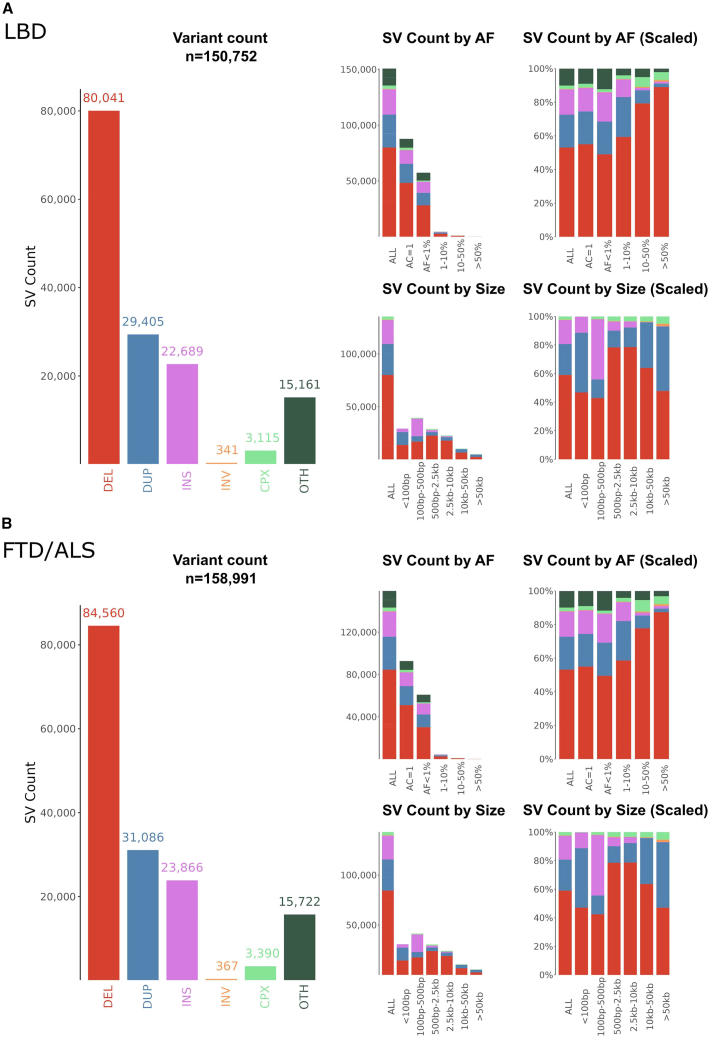
After applying stringent quality control and filtering steps, there were 150,752 structural variants in the 2,355 LBD cases and 3,700 controls available for the association analysis. Similarly, there were 158,991 structural variants in the 2,307 FTD/ALS cases and 3,677 controls. In the LBD case-control cohort, there were on average 895 autosomal structural variants per participant. In the FTD/ALS case-control cohort, there were 865 structural variants on average per participant (Figure 2 and Figures S1–S3 for descriptive statistics of filtered variants and Figures S4–S6 for summaries of unfiltered variants). The median structural variant length was 467 base pairs in the LBD case-control cohort and 479 base pairs in the FTD/ALS case-control cohort. As expected, the most common structural variants were deletions (53.1% of all structural variants in the LBD case-control cohort and 53.2% in the FTD/ALS case-control cohort) or duplications (19.5% and 19.6%). Further, most of the structural variants were rare (minor allele frequency [MAF] <1%; 96.3% of the observed structural variants in the LBD case-control cohort and 96.6% in the FTD/ALS cohort). The structural variant type, size, and allele frequency did not markedly differ between the cases and controls for LBD or FTD/ALS (Table S1).
Figure 2.
Descriptive statistics of the structural variants in the study cohorts
Descriptive statistics of high-quality subset of structural variants mapped by the GATK-SV pipeline in the (A) LBD case-control cohort and (B) FTD/ALS case-control cohort. AF, allele frequency; DEL, deletions; DUP, duplications; INS, insertions; INV, inversions; CPX, complex variant; OTH, other variant; SV, structural variant.
Validation of structural variants using long-read sequencing
We compared structural variants mapped from short-read genome sequence data using the GATK-SV pipeline to long-read Nanopore sequencing data generated for 20 samples. This analysis was performed by applying Truvari bench,17 an algorithm that correlates structural variants based on type, size, and location. The validation rates of the raw data were consistent with prior observations and varied according to structural variant type, allele frequency, and quality.18,19,20 After the application of stringent quality filters, the highest concordance estimate was observed for deletions (mean validation rate = 84.3% and genotype concordance = 94.3%), whereas duplications had the lowest concordance rate (50.2% and 89.2%; Table S2). Common structural variants (MAF ≥ 1%) were more likely to be confirmed by long-read sequencing (n = 5,101 structural variants identified in the 20 participants; the validation rates were 84.7%, 52.5%, 61.6%, and 53.2% for deletions, duplications, insertions, and inversions, respectively). The validation rates for rare structural variants (MAF < 1%) were not as robust (n = 1,917 rare structural variants identified by Nanopore long-read sequencing in the 20 samples; the validation rates were 79.5%, 43.3%, 61.4%, and 0.0% for deletions, duplications, insertions, and inversions).
Genome-wide association studies of common structural variants in LBD and FTD/ALS
After quality control filtering, we performed GWASs on common (i.e., MAF ≥ 1%) high-quality variants separately for the LBD and FTD-ALS case-control cohorts. In total, 4,899 structural variants were tested for association with LBD, and 4,699 variants were tested for FTD/ALS.
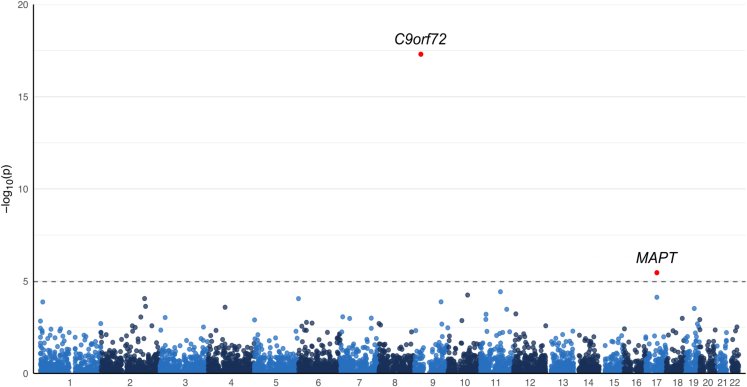
In the FTD/ALS structural variant GWAS, we identified two statistically significant signals (Table 1; Figure 3). The first structural variant was located on chromosome 9p21, corresponding to the C9orf72 hexanucleotide repeat expansion (p = 4.99 × 10−18, odds ratio [OR] = 14.47, 95% confidence interval [CI] = 7.90–26.49). The second association signal was due to the 673-kb complex, common structural variant in the MAPT locus on the long arm of chromosome 17 that is part of the H2 haplotype (p = 3.48 × 10−6, OR = 0.77, 95% CI = 0.68–0.86). Both loci are known risk loci for FTD/ALS,10,21 representing an internal validation of the GATK-SV pipeline.
Table 1.
Significantly associated structural variants in the LBD and FTD/ALS GWAS analyses
| Chr | Position | Gene | Type | Length (bp) | MAF cases | MAF controls | p value | OR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| LBD | 12 | 113,245,316 | TPCN1 | deletion | 309 | 0.075 | 0.052 | 9.10 × 10−6 | 1.43 (1.22–1.67) |
| FTD/ALS | 9 | 27,573,524 | C9orf72 | unresolveda | NA | 0.032 | 0.0018 | 4.99 × 10−18 | 14.47 (7.90–26.49) |
| FTD/ALS | 17 | 45,603,799 | MAPT | complex inversion | 673,211 | 0.17 | 0.19 | 3.48 × 10−6 | 0.77 (0.68–0.86) |
The Bonferroni threshold of significance was 1.02 × 10−5 (= 0.05/4,889 structural variants with an MAF ≥ 1%) for the LBD GWAS and 1.06 × 10−5 (=0.05/4,699) for the FTD/ALS GWAS. Chromosome positions are displayed according to reference genome build hg38. Chr, chromosome; LBD, Lewy body dementia; FTD, frontotemporal dementia; ALS, amyotrophic lateral sclerosis; bp, base pairs; MAF, minor allele frequency; OR, odds ratio; CI, confidence interval; NA, not applicable.
Confirmed to refer to the hexanucleotide repeat expansion in the C9orf72 gene.
Figure 3.
Genome-wide representation of the common structural variants in the FTD/ALS case-control cohort
Manhattan plot depicting the GWAS results (n = 2,307 FTD and 3,677 controls, and 4,699 structural variants with MAF ≥ 1%). The annotations show the break-end variant on chromosome 9, corresponding to the C9orf72 hexanucleotide repeat expansion, and a 673-kb complex inversion that is part of the MAPT H2 haplotype.
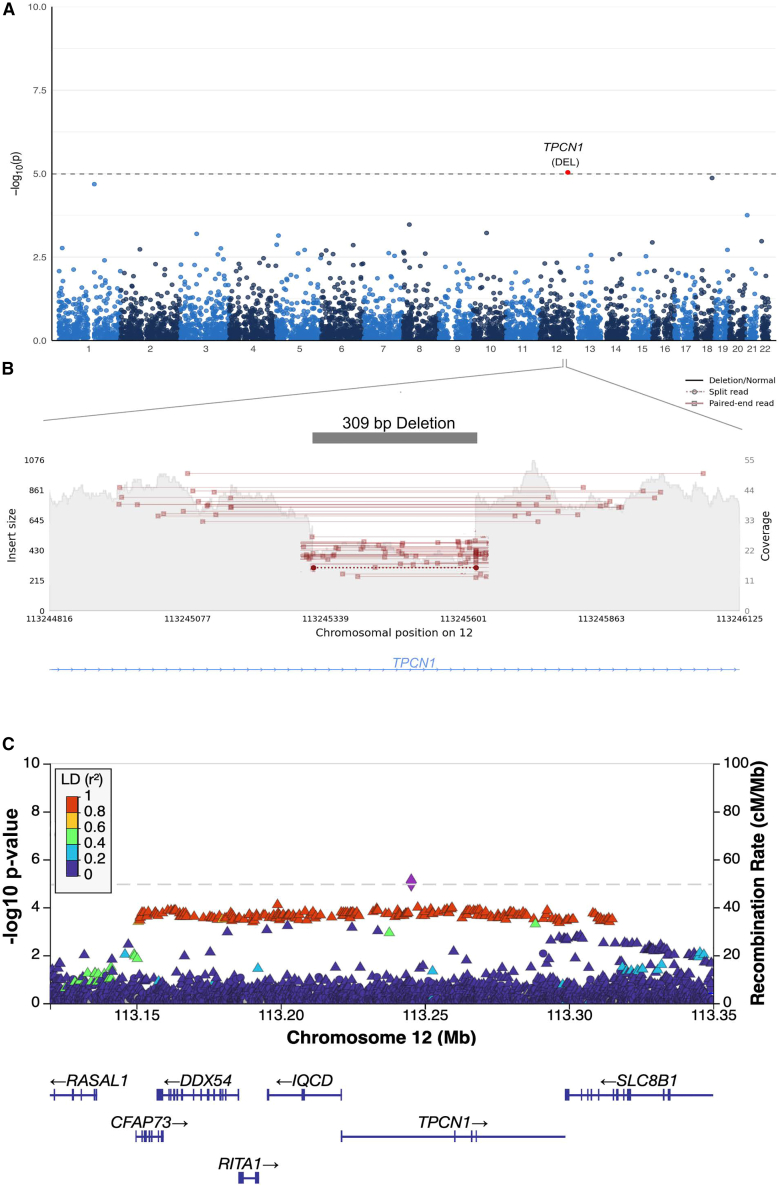
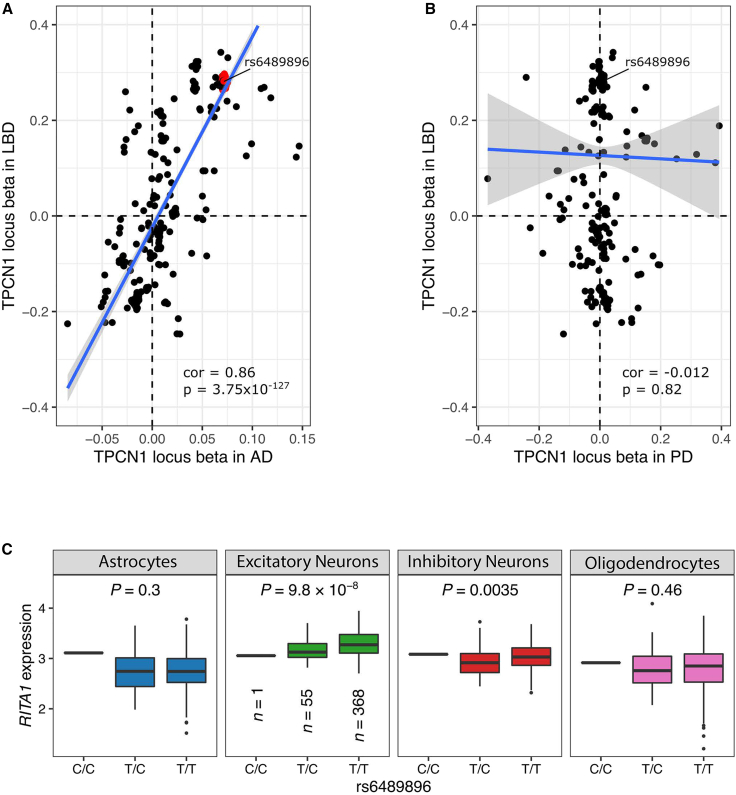
In the LBD structural variant GWAS, we identified a novel locus on chromosome 12q24.13 that reached Bonferroni-corrected statistical significance (p = 9.2 × 10−6, OR = 1.43, 95% CI = 1.22–1.67; Figure 4A). This association signal was driven by a 309-base-pair deletion that we validated using long-read sequencing (95.0% allele concordance rate; Table 1; Figure 4B). The deletion was located within intron 2 of TPCN1 (NM_017901.6; Figure 4C), a gene encoding the two-pore segment channel 1 that is highly expressed in neurons and glia. The same locus had previously been reported in an Alzheimer’s disease GWAS.22 To further explore this relationship, we generated a beta-beta plot of the TPCN1 locus to compare the association signals in LBD (obtained from our previously reported single-nucleotide variant GWAS12) with a published Alzheimer’s disease GWAS.22 This showed that the association signals at the TPCN1 locus were identical (Figure 5A). However, no such relationship was detected between the TPCN1 locus association in LBD versus a recent Parkinson’s disease GWAS23 (Figure 5B).
Figure 4.
Genome-wide representation of the common structural variants in LBD
For a Figure360 author presentation of this figure, see https://doi.org/10.1016/j.xgen.2023.100316.
(A) Manhattan plot depicting the GWAS results (n = 2,355 LBD cases and 3,700 controls; 4,889 structural variants with MAF > 1%).
(B) An illustration of the TPCN1 deletion in a heterozygous carrier. Paired-end reads that did not span the deletion have been omitted for clarity.
(C) The TPCN1 deletion (purple diamond) is the top association signal in the LBD analysis and shows strong linkage disequilibrium with nearby single-nucleotide variants.
Figure 5.
Genomic fine-mapping of the TPCN1 locus
(A) Scatterplot showing the beta regression coefficients of common variants (MAF ≥ 1%) at the TPCN1 locus in the Chia et al. LBD GWAS (y axis)12 and Bellenguez et al. Alzheimer’s disease GWAS (x axis).22
(B) Beta-beta plot comparing the TPCN1 locus variants in the Chia et al. LBD GWAS (y axis)12 with the Nalls et al. Parkinson’s disease GWAS results (excluding 23&Me data; x axis).23
(C) Single-nucleus RNA sequence analysis of the TPCN1 deletion-tagging SNP rs6489896 identified a significant cis-eQTL for RITA1 in excitatory neurons.
Replication of TPCN1 deletion in LBD
We identified the TPCN1 deletion in a replication cohort consisting of an additional 555 LBD cases and 274 control subjects. The MAF was 6.9% in these cases and 4.9% in the control samples, similar to what was observed in the discovery cohort. Furthermore, the TPCN1 deletion was significantly associated with LBD in this cohort (p = 0.024, OR = 2.46, 95% CI = 1.12–5.36), representing an independent replication of the novel risk locus. Meta-analysis of the discovery and replication cohorts showed the same direction of effect (p = 1.64 × 10−6, OR = 1.46, 95% CI = 1.25–1.70). The TPCN1 deletion associated with an increased risk of LBD was tagged by the single-nucleotide polymorphism rs6489896 (chr12:113,281,983:T>C; r2 = 0.95, D′ = 0.98), and a statistically significant association was also observed with this variant in the replication dataset (p = 0.024, OR = 2.46, 95% CI = 1.13–5.37).
Functional effect of the chromosome 12q24.13 deletion
In the Genotype-Tissue Expression (GTEx Analysis Research v8) database,24,25 rs6489896 was an expression quantitative trait locus (eQTL) for the RITA1 gene in the brain, with decreased expression levels in all of the evaluated brain regions (e.g., cortex p = 7.3 × 10−11, normalized effect size = −0.41). A single-nucleus, cell-type-level RNA sequencing dataset based on 424 brains of the Religious Orders Study/Memory and Aging Project (ROS/MAP) collection confirmed these findings26; only RITA1 gene expression in excitatory neurons had a statistically significant association with rs6489896 (p = 9.80 × 10−8, beta = −0.35; Figure 5C; Table S4). While these observations suggest that the deletion exerts a functional effect on the neighboring RITA1 gene, the location of the structural variant within intron 2 of TPCN1 means that we cannot exclude the possibility that the deletion is also influencing that gene. In that regard, it is interesting to note that rs6489896 was a splicing quantitative trait locus for TPCN1 in GTEx with the strongest association observed in subcutaneous adipose tissue (p = 9.80 × 10−40, normalized effect value −1.20). A similar effect was not observed in various brain regions, although this may reflect the small sample size limiting the power to detect such effects.
Identification of rare, disease-causing structural variants in LBD and FTD/ALS
Our cohort was not sufficiently powered to perform gene-burden analysis, as most of the genes did not have more than a single exonic structural variant. Nevertheless, to explore the role of rare structural variants in the pathogenesis of non-Alzheimer’s dementias, we examined their occurrence in 50 genes previously implicated in familial neurodegenerative diseases (see Table S5 for a list of the genes). Our analysis identified 83 and 81 exonic structural variants that mapped to these established neurodegenerative disease genes in the LBD and FTD/ALS cohorts, respectively (Table S6). Among these, we found a number of structural variants that are known disease-causing mutations. These included an SNCA duplication and an OPTN deletion, each detected in a single LBD case.
In the FTD/ALS case-control cohort, we identified a LRRK2 duplication in one case with a clinical diagnosis of non-fluent variant primary progressive aphasia. This form of dementia is most commonly a manifestation of a four-repeat tauopathy upon pathological evaluation.27 Mutations in LRRK2 have been identified as rare causes of four-repeat tauopathies,3,28 suggesting that this LRRK2 duplication could be disease causing. Additionally, we identified a heterozygous deletion of the promoter and the first two exons of CHCHD10 in one patient with FTD and motor neuron disease. Heterozygous missense mutations in CHCHD10, especially in exon 2, are a known cause of FTD/ALS,29,30,31 and a loss-of-function mechanism has been proposed.32 We also found a deletion of the last exon and 3′-untranslated region of FIG4 in one patient diagnosed with FTD. Heterozygous missense mutations in FIG4, including in exon 23, have been linked to FTD/ALS,33 and our case provides supportive evidence of their pathogenicity. Table 2 summarizes the clinical and pathological features of these cases.
Table 2.
Pathogenic, rare structural variants in the LBD and FTD/ALS case-control cohorts
| Chr | Position | Type | Length (bp) | MAF cases | MAF controls | Overlapping regions | Patient characteristic | |
|---|---|---|---|---|---|---|---|---|
| SNCA | 4 | 89,123,177 | duplication | 1,347,138 | 0.00021 | 0 | whole gene | pathologically diagnosed LBD |
| OPTN | 10 | 12,982,796 | deletion | 314,105 | 0.00021 | 0 | whole gene | pathologically diagnosed LBD |
| FIG4 | 6 | 109,822,689 | deletion | 8,753 | 0.00021 | 0 | exon 23, 3′ UTR | non-fluent variant PPA |
| LRRK2 | 12 | 40,073,033 | duplication | 327,105 | 0.00021 | 0 | whole gene | non-fluent variant PPA |
| CHCHD10 | 22 | 23,767,017 | deletion | 2,653 | 0.00021 | 0 | promoter, exons 1–2 | FTD (with motor neuron disease) |
Chromosome positions are displayed according to reference genome build hg38. PPA, primary progressive aphasia.
Resource for exploring structural variants in non-Alzheimer’s dementias
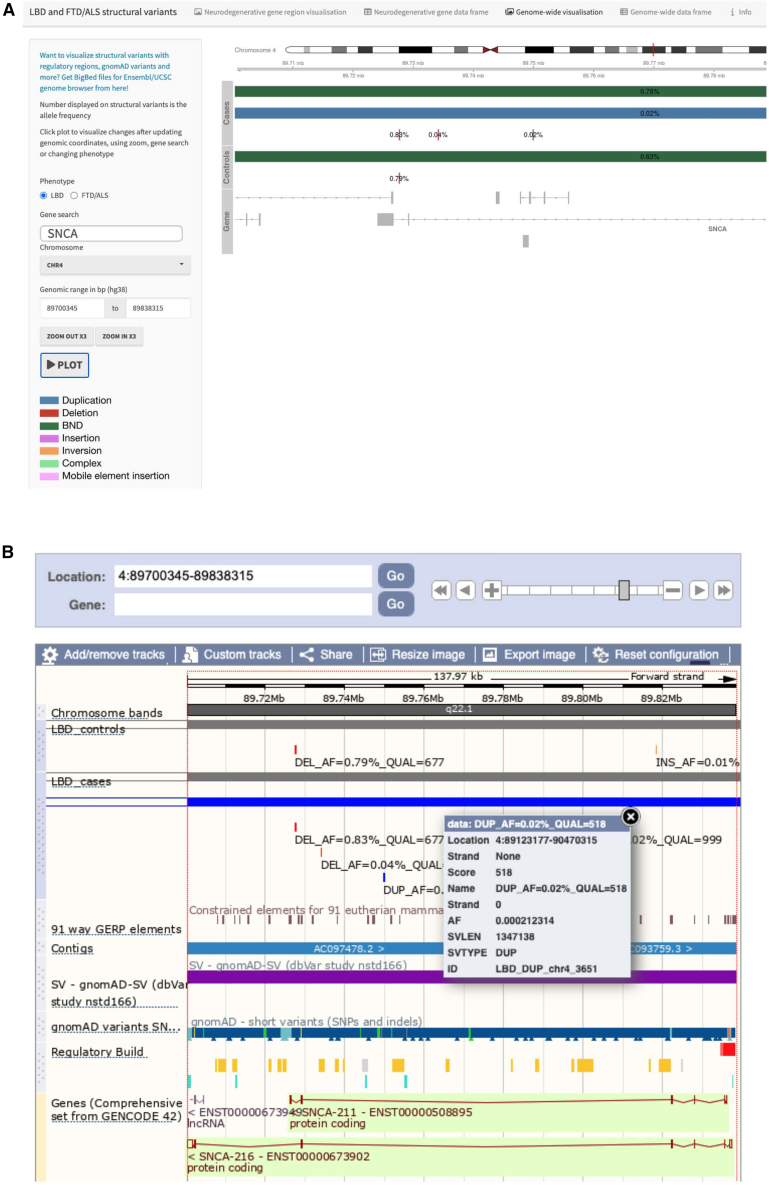
The structural variant dataset generated in this study is among the largest published to date in neurological disorders. As such, it represents a valuable resource that can be exploited for further research. We prioritized making our structural variant data easily accessible and user friendly. The raw sequence data are available in dbGaP (study accession phs001963.v1.p1), and details of the individual structural variants are provided in Tables S7 and S8. Moreover, we developed an online resource that enables researchers to explore the structural variant data. For example, the data can be filtered by several parameters, including location, gene name, structural variant type, overlapping genetic region, structural variant quality, case-control allele frequency, and combined annotation-dependent depletion (CADD) structural variant score (https://ndru-ndrs-lng-nih.shinyapps.io/non_ad_dementias_sv_app/; Figure 6A). The online resource also allows visualization of structural variants in a genomic context. Moreover, we created BigBed files of structural variants that can be viewed, e.g., in Ensembl and UCSC genome browsers as custom tracks (Figure 6B). This allows the structural variants to be explored with other available tracks, such as regulatory regions, known pathological variants, and gnomAD structural variants. We have made our programming code publicly available so that other researchers can apply it and modify it to match their needs (https://github.com/ruthchia/Structural_variant_analysis-LBD-FTD; https://doi.org/10.5281/zenodo.7796321).
Figure 6.
Interactive catalog of structural variants in non-Alzheimer’s dementias
(A) Screen shot of the online portal that lets the user filter structural variants and display genomic regions. For example, this image shows a large duplication encompassing the entire SNCA gene in an LBD case.
(B) Visualization of structural variants using BigBed files in the Ensembl genome browsers as custom tracks (https://www.ensembl.org).
Discussion
In this study, we investigated the role of structural variants in LBD and FTD/ALS, two forms of non-Alzheimer’s dementia. We applied a multi-algorithm pipeline to identify, characterize, and analyze structural variants and to create a resource.11 We conducted GWASs and neurodegenerative disease gene-set evaluations to elucidate the role of these variants in complex diseases. In this process, we identified a common structural variant in TPCN1 that modulates disease risk, a previously known pathogenic variant in C9orf72, a common complex variant at the MAPT locus, and likely pathogenic, rare variants in several other neurodegenerative disease genes.
In the FTD/ALS structural variant GWAS, we identified a break-end mutation at chromosome 9p21.2, corresponding to the C9orf72 hexanucleotide repeat expansion, and a 673-kb complex inversion related to the MAPT H2 haplotype. Both are structural variants known to be associated with FTD/ALS, demonstrating the ability of the analysis pipeline to capture this type of disease-associated genomic variation.
In the LBD structural variant GWAS, we identified, validated, and replicated a significant association with a 309-base-pair deletion that overlaps intron 2 of the canonical transcript of TPCN1. TPCN1 encodes a voltage-dependent calcium channel that is expressed throughout the body34 and located at the endolysosomal membranes.35,36,37 Interestingly, TPCN1 has been reported to be essential for long-term potentiation in hippocampal neurons,38 and Tpcn1 knockout mice have been shown to have impaired memory and spatial learning.39 Furthermore, loss of presenilins decreases lysosomal calcium stores, which, in turn, alters TPCN1 levels by decreasing the functional dimer form.40
The potential role of the TPCN1 locus in dementia is supported by a recent GWAS in Alzheimer’s disease that reported a suggestive association between the intronic TPCN1 variant rs6489896 and Alzheimer’s disease.22 This rs6489896 variant tagged the 309-base-pair TPCN1 deletion in our data, and there was a near-perfect correlation between the haplotypes in the Alzheimer’s disease GWAS and our study (Figure 5A). These data indicate that the TPCN1 deletion is not specific to LBD. The suggestive association between TPCN1 and Alzheimer’s disease could be due to the well-established shared etiology between Alzheimer’s disease and LBD, or a subpopulation of LBD-variant Alzheimer’s disease patients. Interestingly, the TPCN1 locus did not correlate with Parkinson’s disease (Figure 5B). Furthermore, functional genomic evaluations based on bulk RNA sequencing and single-nuclear RNA sequencing data showed that the TPCN1 deletion-tagging rs6489896 variant strongly influences the expression of RITA1, a gene located centromeric to TPCN1 on the long arm of chromosome 12. RITA1 modulates Notch-signaling,41 although its exact functions remain elusive. Based on these preliminary data, we cannot rule out that other genes or a combination of genes within the TPCN1 locus may also influence disease risk.
We identified several rare structural variants of interest in our analysis of the neurodegenerative disease genes. SNCA duplications are an established rare cause of Parkinson’s disease and LBD,42 while OPTN deletions are a known cause of FTD43 and ALS,44 indicating that the observed structural variants are disease causing. Furthermore, optineurin, the protein encoded by OPTN, is deposited in Lewy bodies,45 the pathological hallmark of LBD, suggesting a molecular link between this pathogenic mutation and the LBD pathogenesis.
In conclusion, we used a multi-algorithm pipeline to create a resource of structural variants in LBD and FTD/ALS. We identified and validated common and rare structural variants driving disease risk in these neurological disorders and presented a resource that can be mined for further insights into this type of genomic variation. Our study highlighted the utility of using short-read whole-genome sequencing for structural variant discovery and demonstrated the value of studying this type of genomic variation to understand the underlying pathogenesis of non-Alzheimer’s dementias.
Limitations of the study
The main limitations of our study stem from the inherent difficulty of calling structural variants from short-read whole-genome sequencing data. As such, the validation rate of structural variants detected in short-read sequencing data is not ideal. To mitigate this problem, we used a multi-algorithm pipeline, GATK-SV,11 to create consensus structural variant calls and focused on a subset of high-quality structural variants in our analyses. Overall, the mean number of structural variant sites, the distribution of structural variant types, and the proportion of structural variant calls in Hardy-Weinberg equilibrium are within the expected limits. Moreover, we found good structural variant mapping precision and genotype concordance between short-read sequencing and long-read sequencing data (Table S2). These findings indicate that our structural variant calls are robust. However, there are no accepted filtering standards following the GATK-SV pipeline, and direct comparison with other studies employing different filters can be complex (Table S3). It is worth noting that breakpoint locations in structural variant calls are rarely exact. This issue also holds true when using a multi-algorithm pipeline that collapses sufficiently overlapping variants into a single structural variant. Therefore, structural variants where the functional impact is due to breakpoint location should be interpreted cautiously. Moreover, structural variants mapped to low-complexity repeat regions require validation since these regions are technically problematic to sequence, and the current human reference genome (GRCh38) can be incomplete at these loci. Another challenge when studying structural variants is the assessment of pathogenicity and unraveling the mechanisms by which they disrupt neuronal function. As previous data are scarce and the consequences of structural variants are not well understood, cell biology studies, especially those integrating other multi-omic data, are needed to fully understand the consequences of this mutation class.
Consortia
The members of The American Genome Center (TAGC) are Anthony R. Soltis, Coralie Viollet, Gauthaman Sukumar, Camille Alba, Nathaniel Lott, Elisa McGrath Martinez, Meila Tuck, Jatinder Singh, Dagmar Bacikova, Xijun Zhang, Daniel N. Hupalo, Adelani Adeleye, Matthew D. Wilkerson, Harvey B. Pollard, and Clifton L. Dalgard. The members of the International LBD Genomics Consortium are Sandra E. Black, Ziv Gan-Or, Julia Keith, Mario Masellis, Ekaterina Rogaeva, Alexis Brice, Suzanne Lesage, Georgia Xiromerisiou, Andrea Calvo, Antonio Canosa, Adriano Chio, Giancarlo Logroscino, Gabriele Mora, Reijko Krüger, Patrick May, Daniel Alcolea, Jordi Clarimon, Juan Fortea, Isabel Gonzalez-Aramburu, Jon Infante, Carmen Lage, Alberto Lleó, Pau Pastor, Pascual Sanchez-Juan, Francesca Brett, Dag Aarsland, Safa Al-Sarraj, Johannes Attems, Steve Gentleman, John A. Hardy, Angela K. Hodges, Seth Love, Ian G. McKeith, Christopher M. Morris, Huw R. Morris, Laura Palmer, Stuart Pickering-Brown, Mina Ryten, Alan J. Thomas, Claire Troakes, Marilyn S. Albert, Matthew J. Barrett, Thomas G. Beach, Lynn M. Bekris, David A. Bennett, Bradley F. Boeve, Clifton L. Dalgard, Ted M. Dawson, Dennis W. Dickson, Kelley Faber, Tanis Ferman, Luigi Ferrucci, Margaret E. Flanagan, Tatiana M. Foroud, Bernardino Ghetti, J. Raphael Gibbs, Alison Goate, David S. Goldstein, Neill R. Graff-Radford, Horacio Kaufmann, Walter A. Kukull, James B. Leverenz, Grisel Lopez, Qinwen Mao, Eliezer Masliah, Edwin Monuki, Kathy L. Newell, Jose-Alberto Palma, Matthew Perkins, Olga Pletnikova, Alan E. Renton, Susan M. Resnick, Liana S. Rosenthal, Owen A. Ross, Clemens R. Scherzer, Geidy E. Serrano, Vikram G. Shakkottai, Ellen Sidransky, Toshiko Tanaka, Nahid Tayebi, Eric Topol, Ali Torkamani, Juan C. Troncoso, Randy Woltjer, Zbigniew K. Wszolek, and Sonja W. Scholz. The members of the International ALS/FTD Consortium are Robert H. Baloh, Robert Bowser, Alexis Brice, James Broach, William Camu, Adriano Chiò, John Cooper-Knock, Carsten Drepper, Vivian E. Drory, Travis L. Dunckley, Eva Feldman, Pietro Fratta, Glenn Gerhard, Summer B. Gibson, Jonathan D. Glass, John A. Hardy, Matthew B. Harms, Terry D. Heiman-Patterson, Lilja Jansson, Janine Kirby, Justin Kwan, Hannu Laaksovirta, John E. Landers, Francesco Landi, Isabelle Le Ber, Serge Lumbroso, Daniel JL. MacGowan, Nicholas J. Maragakis, Kevin Mouzat, Liisa Myllykangas, Richard W. Orrell, Lyle W. Ostrow, Roger Pamphlett, Erik Pioro, Stefan M. Pulst, John M. Ravits, Wim Robberecht, Ekaterina Rogaeva, Jeffrey D. Rothstein, Michael Sendtner, Pamela J. Shaw, Katie C. Sidle, Zachary Simmons, Thor Stein, David J. Stone, Pentti J. Tienari, Bryan J. Traynor, Juan C. Troncoso, Miko Valori, Philip Van Damme, Vivianna M. Van Deerlin, Ludo Van Den Bosch, and Lorne Zinman. See Document S1 for consortium member affiliations.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human cerebral brain tissue and/or whole blood | A comprehensive list of study sites that provided biospecimens is listed in the supplemental information of this paper | N/A |
| Critical commercial assays | ||
| Neuro Consortium v1.1 genotyping array | Illumina | https://www.illumina.com/science/consortia/human-consortia/neuro-consortium.html |
| Nanobind Tissue Big DNA Kit | Circulomics | https://www.circulomics.com/ |
| NEBNext® Companion Module | New England Biolabs | E7180L |
| AMPure XP beads | Beckman Coulter | A63382 |
| Ligation Sequencing Kit | Oxford Nanopore technologies | SQK-LSK109 |
| Flow cell wash kit | Oxford Nanopore technologies | EXP-WSH004 |
| Deposited data | ||
| Human reference genome NCBI build 38, GRCh38 | Genome Reference Consortium | https://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/ |
| Gene region annotation data | Ensembl Biomart | https://www.ensembl.org/biomart/martview/ |
| Gene region annotation data | UCSC TableBrowser | https://genome.ucsc.edu/cgi-bin/hgTables |
| GeneHancer v.5.1.0 data | GeneCards | https://www.genecards.org/ |
| DEMENTIA-SEQ whole-genome sequencing data | dbGaP | https://www.ncbi.nlm.nih.gov/gap/Study Accession: phs001963.v2.p1 |
| Gene expression data | GTeX V8 | https://gtexportal.org/home/ |
| Structural variant data and programming code | This study |
https://github.com/ruthchia/Structural_variant_analysis-LBD-FTD https://doi.org/10.5281/zenodo.7796321 |
| Structural variants as BigBed files for custom tracks in Ensembl/UCSC genome browser | This study | https://github.com/ruthchia/Structural_variant_analysis-LBD-FTD |
| Online resource for structural variant exploration | This study | https://ndru-ndrs-lng-nih.shinyapps.io/non_ad_dementias_sv_app/ |
| Software and algorithms | ||
| GATK-SV structural variant calling pipeline (versions gatk-sv-v1_2020.6.7 to gatk-sv-0.13-beta.2021.3.15) | Collins R.L. et al.46 | https://github.com/broadinstitute/gatk-sv |
| Manta v.1.6.0 | Chen X. et al.47 | https://github.com/Illumina/manta |
| Guppy v.5.0.12 | Oxford Nanopore technologies | https://github.com/nanoporetech/megalodon |
| MinKNOW v.22.08.6 | Oxford Nanopore technologies | https://nanoporetech.com/ |
| MiniMap2 | Li H.48,49 | https://github.com/lh3/minimap2 |
| Sniffles2 | Sedlazeck F.J. et al.50 | https://github.com/fritzsedlazeck/Sniffles |
| PLINK v.1.9 and PLINK v.2.3 | Chang C.C. et al.51 | https://www.cog-genomics.org/plink/2.0/ |
| flashPCA v.2 | Abraham G. et al.52 | https://github.com/gabraham/flashpca |
| Samtools v.1.16.1 | Danecek P. et al.53 | https://samtools.github.io/bcftools/bcftools.html |
| Python 3.7. with packages pandas v. 1.3.5 and numpy v.1.21.6 | NA | www.python.org |
| Integrative Genomics Viewer | Robinson J.T. et al.54 | https://software.broadinstitute.org/software/igv/ |
| R v.4.1.3 with packages tidyverse v.1.3.2, stats v.4.1.3, data.table v.1.14.6, gmodels v.2.18.1.1, readxl v.1.4.1, Gviz v.1.38.4, AnnotationHub v.3.2.2, ggplot2 v.3.4.0, Seurat v.4, Matrix-eQTL v.2.3, shiny v.1.7.3, shinywidgets v.0.7.5, datamods v.1.4.0, shinythemes v.1.2.0, shinysky v.0.1.3 | R core team | https://www.r-project.org/ |
| Truvari v.3.5.0 | English A.C. et al.17 | https://github.com/ACEnglish/truvari |
| METAL 2020-05-05 | Willer C.J. et al.55 | http://csg.sph.umich.edu/abecasis/metal/ |
| CADD-SV v.1.1 | Kleinert P. et al.56 | https://cadd-sv.bihealth.org/ |
| CellRanger v.6.0.0 | 10x Genomics | https://support.10xgenomics.com/single-cell-gene-expression/software/overview/welcome |
| LocusZoom v.0.12 | Boughton A.P. et al.57 | http://locuszoom.org/ |
| Samplot June 18, 2021 | Belyeu J.R. et al.58 | https://github.com/ryanlayer/samplot |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Sonja W. Scholz (sonja.scholz@nih.gov).
Materials availability
This study did not generate new unique reagents. Short-read whole-genome sequencing data have been deposited in dbGaP (accession code phs001963.v2.p1).
Experimental model and subject details
Patient and control cohorts
A total of 2,612 LBD cases, 2,601 FTD/ALS cases, and 4,132 unaffected controls were included in the study. These cohorts have been described elsewhere.12,13 Briefly, LBD patients were diagnosed with pathologically definite (69.05% of the cohort) or clinically probable disease (30.95%) according to the McKeith and Emre consensus criteria.59,60 These consensus criteria guide optimal methods to establish the clinical and pathological diagnosis of LBD, including diagnostic biomarkers. The FTD/ALS cohort included 1,377 patients diagnosed with FTD spectrum disorders, including the known subtypes of behavioral variant FTD, primary progressive aphasia, and progressive supranuclear palsy, and 1,065 patients diagnosed with ALS. Patients with FTD were diagnosed according to the Neary criteria61 or the Movement Disorders Society criteria for progressive supranuclear palsy.62 These criteria define core measures and several supportive and exclusion criteria for establishing a diagnosis of FTD. Patients with ALS were diagnosed according to the revised El Escorial criteria.63 These criteria classify patients according to the level of diagnostic certainty and have been shown to be specific to the diagnosis of ALS.
The control participants included convenience control genomes obtained from the Wellderly cohort (n = 1,202 healthy, aged European-ancestry individuals recruited in the United States)64 and the Accelerating Medicine Partnership – Parkinson’s Disease Initiative (n = 1,016; www.amp-pd.org). The control cohorts were selected based on a lack of evidence of cognitive decline in their clinical history and the absence of neurological deficits on neurological examination. Pathologically confirmed control individuals (n = 605) had no evidence of notable neurodegenerative disease on histopathological examination. The appropriate institutional review boards of the participating institutions approved the study (study identification numbers 03-AG-N329 and NCT02014246), and informed consent was obtained from all subjects or their surrogate decision-makers according to the Declaration of Helsinki.
The LBD replication cohort consisted of 667 independent cases with a clinical or neuropathological diagnosis of LBD and 274 neuropathologically unaffected control subjects. All samples were of European ancestry. The demographic characteristics of the study cohorts are summarized in Table S9.
Method details
Short-read whole-genome sequencing
The genomes were generated using PCR-free library preparations followed by 150 base-pair, paired-end sequencing on an Illumina HiSeq X Ten sequencing platform (version 2.5 chemistry, Illumina), as described elsewhere.12,13 The average sequencing read-depth was 35x, and the mean coverage per genome was 36.3 (95% CI = 29.3–43.3).
Structural variant mapping
We used the Broad Institute’s GATK-SV pipeline (in cohort mode) following default settings for structural variant discovery and downstream filtering.46,65 This pipeline leverages five algorithms to call structural variants: (1) Manta,47 (2) the Mobile Element Locator Tool (MELT),66 (3) WHole-genome Alignment Metrics (WHAM),67 (4) the Mixture Of PoissonS model for CNV detection (cn.MOPS),68 and (5) GATK gCNV.69 The LBD cases (n = 2,612) and the control subjects (n = 4,132) were called together, and the FTD/ALS cases (n = 2,601) and the same control subjects (n = 4,132) were independently called together using the GATK-SV pipeline. This approach was chosen to ensure accurate identification of rare structural variants in the case cohorts.
Sample quality control and sample batching
We excluded 239 samples from the LBD case-control cohort (representing 3.5% of the total cohort) and 259 samples (3.9%) from the FTD/ALS case-control cohort for failing at least one of the following quality control metrics: median sequencing coverage in 100 base-pair bins, dosage bias score δ, autosomal ploidy spread, Z score of outlier 1 megabase bins, chimera rate, pairwise alignment rate, read length, library contamination, ambiguous sex genotypes, and discordance between inferred and reported sex. We kept samples with non-canonical sex chromosome configurations in their batches and removed all non-autosomal structural variant calls.
Of note, the LBD and FTD cohorts used the same initial set of control samples. However, there was a difference of 23 control samples after the GATK-SV pipeline was applied due to the initial quality control steps in the pipeline. The thresholds for sample exclusion are not fixed. Instead, they are based on values derived from the analyzed samples, including metrics for ancestry and coverage. This filtering approach led to minor differences in which control samples were excluded when they were called separately with the LBD cases and FTD cases.
Following quality control, we subdivided the filtered samples into 16 batches. We ranked and binned the samples based on sex, median 100 base-pair binned coverage, and dosage bias scores so that samples of corresponding quality were batched together.
Structural variant evidence collection
The structural variant evidence of individual samples collected in this step included: raw structural variant/copy number variant calls and raw evidence-binned read counts, split reads, discordant read-pairs, and single nucleotide polymorphism B-allele frequency from five different structural variant algorithms: Manta (v1.4),47 MELT (v2.2.0),66 Wham (v1.7),67 cn.MOPS (v1.20.1),68 and GATK-gCNV.69 One control sample in the LBD case-control cohort and two FTD/ALS cases had a high number of improper pairs in MELT and were excluded. We then aggregated the structural variant calls from the five algorithms and standardized them to meet the specifications required for the structural variant discovery pipeline.
Structural variant discovery
Structural variant discovery consisted of first clustering structural variants across all batches for each structural variant calling algorithm. Then, low-quality variants and samples with outlying variant numbers were removed. The filtered variants were combined for each variant calling algorithm across batches and genotypes. After assigning variant probability scores, all probable variants were re-genotyped and all variants from all variant calling algorithms across all batches were combined and complex variants were resolved.
Downstream filtering
Initial GATK-SV filtering steps included: (1) minGQ filtering with a 1% false discovery rate threshold, (2) FilterOutlierSamples, (3) BatchEffect, and (4) FilterCleanupQualRecalibration. In total, we filtered out 160 outliers in the LBD cohort and 76 in the FTD/ALS cohort. The final call set included 290,681 structural variants in the LBD cohort (n = 2,392 cases and n = 3,970 controls) and 294,232 variants in the FTD/ALS cohort (n = 2,450 cases and n = 3,948 controls).
Data curation
To create a high-quality subset of structural variants and samples for subsequent genetic analyses, we further filtered structural variants after the GATK-SV pipeline. We included structural variants with “PASS”, “MULTIALLELIC” or “UNRESOLVED” filter. We set genotypes with a genotype quality (GQ) < 300 to missing and included variants with a genotyping rate >95% and Hardy-Weinberg equilibrium p value > 1x10−6 (mid-p adjustment). We excluded individuals with missing information and individuals who had failed previous single-nucleotide variant/indel calling.12 This resulted in 2,355 LBD cases and 3,700 controls with 150,752 structural variants, and 2,307 FTD/ALS cases and 3,677 controls with 158,991 structural variants. These samples and variants underwent principal component analysis based on single-nucleotide polymorphism data.12 For GWASes, we additionally filtered out structural variants with MAF <1% in cases, QUAL <500, variants within 100 kb of telomeres, variants within 2.5 Mb of centromeres, and variants within the variable, diversity, joining recombinant regions.
Validation of structural variants using nanopore long-read sequencing
We performed long-read Nanopore genome sequencing in 20 samples that had also been sequenced on the short-read Illumina HiSeq X10 platform. High molecular weight DNA extraction and long-read sequencing were performed on an Oxford Nanopore PromethION platform, as per the manufacturer’s instructions. The tissue and library preparation steps followed a protocol described elsewhere: https://www.protocols.io/view/processing-human-frontal-cortex-brain-tissue-for-p-kxygxzmmov8j/v1. Briefly, the Nanobind Tissue Big DNA Kit (Circulomics) was used to extract DNA from cerebellar tissue on a KingFisher Apex instrument (Thermo Fisher Scientific, USA). The DNA was sheared to a target size of 30 kb on a Megaruptor 3 instrument (Diagenode, BE) and quantified using a Tapestation 4200 (Agilent Technologies). Next, the NEBNext Companion Module (New England Biolabs, USA) was used for DNA and end-repair, followed by AMPure XP bead purification (Beckman Coulter) and adaptor ligation (Ligation Sequencing Kit; Oxford Nanopore Technologies). PromethION R.9.4.1 flow cells were primed and loaded with 400 ng of the DNA libraries. One sample was loaded on each flow cell and flushed using the wash kit (Oxford Nanopore Technologies). Libraries were reloaded every 24 h to maximize the data output, with a total run time of 72 h. We performed base calling using Guppy (v.5.0.12; Oxford Nanopore Technologies) and MinKNOW (version 22.08.6; Oxford Nanopore Technologies) on a PromethION compute device. The reads were assembled to the reference genome (GRCh38) using MiniMap248,49 and structural variants were called with sniffles2.70
We used the T. bench algorithm to study the structural variant validation rate per sample and per structural variant type with different MAF thresholds (all, <1%, ≥1%).17 A structural variant was validated if (1) the structural variant type matched, (2) breakpoint distance was within 200 base pairs, and (3) the reciprocal size overlap was >70% between the short-read and long-read structural variant calls.
We validated the top GWAS hits and the structural variants of interest in the neurodegenerative diseases genes using long-read sequencing (Nanopore), and genotyping array-based CNV calling by visualization of B-allele frequencies and log-R-ratio (Illumina, Neuro Consortium array v1.1). If DNA was exhausted in sequencing, we validated structural variant calls visually using Integrative Genomics Viewer.54 Validation data are presented in the Supplementary Materials.
Structural variant evaluation in the LBD replication cohort
We used the Manta algorithm to detect the structural variants within the TPCN1 locus on chromosome 12q24.13 in the 667 LBD cases and the 274 controls making up the replication cohort.47 This analysis used default settings and focused on the region defined by the TPCN1 deletion plus 1,000 flanking base-pairs on either side. Result files were merged with bcftools,53 and missing genotypes were set to reference homozygotes. We used flashPCA (v.2)52 based on single nucleotide polymorphism data to calculate the principal components. We then used the ‘step()’ function, as implemented in the R (v.4.0.3) ‘stats’ package (https://www.R-project.org/), to select the appropriate covariates from age, sex, and principal components 1–10 to include in the association model. Next, we excluded related samples and samples with missing covariates. This left 555 LBD cases and 274 controls available for the association analysis. Logistic regression on LBD status and deletion genotype, adjusted for age and principal components 1–5, was performed using PLINK2.51 The age of 144 samples was expressed as a 10-year interval (e.g., 60–69 years), and the middle point of age (e.g., 65) was used for these subjects. We performed a meta-analysis of the discovery and replication cohorts using the inverse-variant weighted method implemented in METAL.55
Neurodegenerative disease gene analysis
We extracted structural variants that were located within 50 neurodegenerative disease genes (+/− 1Mb, Table S5). To annotate structural variants that overlapped these genes, we retrieved the Ensembl canonical transcript gene regions, exon ranges, and gene strand information using the UCSC Table Browser (GRCh38, GENCODE V38).71 We defined promoter regions as the 1kb flanking region upstream of the transcription start site on the transcribed strand. We used PLINK2 (v.2.3)51 to calculate structural variant allele frequencies in the cases and controls. To annotate structural variants, we used CADD-SV (v.1.1) with default settings to calculate scores for duplications, deletions, and insertions (including mobile element insertions).56
To help explore structural variants within neurodegenerative disease gene regions, we created an interactive app using the R packages shiny, shinywidgets, and datamods. Prerendered images for the interactive app were created using the Gviz package.72
Quantification and statistical analysis
Genome-wide association studies
We used flashPCA (v.2)52 to calculate the principal components for the LBD case-control and the FTD/ALS case-control cohorts. We then used the ‘step()’ function, as implemented in the R (v.4.0.3) ‘stats’ package (https://www.R-project.org/), to select the appropriate covariates for each association analysis. In the LBD GWAS, we adjusted for age, sex, and principal components 1, 3, 4, 7, and 8. In the FTD/ALS GWAS, we adjusted for age, sex, and principal components 1, 2, 3, and 7. We used logistic Firth hybrid regression implemented in PLINK251 to perform the genome-wide association analyses. The Bonferroni threshold of significance was 1.02x10−5 (=0.05/4,889 structural variants with a MAF ≥1%) for the LBD GWAS and 1.06x10−5 (= 0.05/4,699) for the FTD/ALS GWAS.
Brain expression analysis
We examined the expression of TPCN1 and neighboring genes in a single-nucleus RNA-seq dataset from the Religious Orders Study/Memory and Aging Project (ROS/MAP) cohort.26 These data were prepared from 424 dorsolateral prefrontal cortexes of individuals of advanced age using the 10x Genomics Single Cell 3′ kit, as described elsewhere.26 Sequencing reads were processed, and the unique molecule identifier (UMI) count matrix was generated using Cell Ranger software (v.6.0.0, 10x Genomics). The classification of the cell types was performed by clustering cells by gene expression using the R package Seurat (v.4).73 The “pseudobulk” gene expression matrix was constructed by aggregating UMI counts of the same cell type of the same donor and normalizing them to the log2 counts per million reads mapped (CPM) values. Genotyping was performed by whole-genome sequencing followed by GATK processing. Mapping of cis-eQTL was performed using Matrix-eQTL (v.2.3) for single nucleotide polymorphisms within 1Mb of the transcription start sites.74
Acknowledgments
The authors are grateful to all patients, their family members, and caregivers, as well as all healthy participants, for making this study possible. This research was supported in part by the Intramural Research Program of the US National Institutes of Health (National Institute on Aging, National Institute of Neurological Disorders and Stroke; project numbers 1ZIAAG000935 [PI B.J.T.], 1ZIANS003154 [PI S.W.S.]). K.K. was supported by grants from The Päivikki and Sakari Sohlberg Foundation and the Finnish Parkinson Foundation. We are grateful to the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona, for the provision of human biological materials. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026, National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 and P30 AG072980, Arizona Alzheimer’s Disease Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901, and 1001 to the Arizona Parkinson’s Disease Consortium), and the Michael J. Fox Foundation for Parkinson’s Research. The study used tissue samples and data from the Johns Hopkins Morris K. Udall Center of Excellence for Parkinson’s Disease Research (NIH P50 NS38377). We thank the members of the Laboratory of Neurogenetics (NIH) for their collegial support and technical assistance. We thank members of the North American Brain Expression Consortium (NABEC) for providing samples derived from brain tissue. Brain tissue for the NABEC cohort was obtained from the Baltimore Longitudinal Study on Aging at the Johns Hopkins School of Medicine, the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, the Banner Sun Health Research Institute Brain and Body Donation Program, and the University of Kentucky Alzheimer’s Disease Center Brain Bank. We thank the UK Brain Expression Consortium (UKBEC) and the Northwestern University Brain Bank for providing DNA or tissue samples. The ROS/MAP study was supported by the National Institute on Aging (RF1 AG057473, U01 AG061356). This work utilized the computational resources of the NIH HPC Biowulf cluster USA (http://hpc.nih.gov). A complete list of acknowledgments is given in the supplemental information.
Author contributions
Conceptualization, S.W.S., B.J.T., K.B., and J.R.G.; methodology, S.W.S., B.J.T., C.L.D., R.C., K.B., M.R., A.R., and S.S.; software, J.D., J.R.G., R.L.C., H.T., M.B., X.Z., and K.K.; formal analysis, K.K., R.C., J.D., R.L.C., J.R.G., V.M., M.F., D.A.B., and P.L.D.; investigation, K.K., M.R., R.C., C.L.D., R.L.W., R.L.C., A.R., S.S., K.B., P.A.J., L.M., T.M.D., L.S.R., M.S.A., O.P., J.C.T., M.M., J.K., S.E.B., L.F., S.M.R., The American Genome Center; resources, International LBD Genomics Consortium, International ALS/FTD Consortium, E.T., A.T., T.M.F., J.E.L., L.M., S.D., C.M., L.M., S.D., A.C., G.E.S., T.G.B., P.T., T.F., B.G., N.R.G.-R., B.F.B., Z.K.W., D.W.D., A.C., D.A.B., P.L.D., O.A.R., C.L.D., J.R.G., B.J.T., and S.W.S.; data curation, K.K., R.C., J.D., and M.R.; writing – original draft, K.K.; writing – review & editing, all authors; visualization, K.K., J.D., and R.C.; supervision, O.A.R., C.L.D., J.R.G., S.W.S., and B.J.T.; funding acquisition, S.W.S. and B.J.T.
Declaration of interests
S.W.S. serves on the Scientific Advisory Council of the Lewy Body Dementia Association and the Multiple System Atrophy Coalition. S.W.S. and B.J.T. receive research support from Cerevel Therapeutics. B.J.T. holds patents on the clinical testing and therapeutic implications of the C9orf72 repeat expansion. H.R.M. is employed by the University College London. In the last 12 months, he reports paid consultancy from Roche and Amylyx; lecture fees/honoraria from BMJ, Kyowa Kirin, and Movement Disorders Society; and research grants from Parkinson’s UK, Cure Parkinson’s Trust, PSP Association, Medical Research Council, and the Michael J Fox Foundation. H.R.M. is a co-applicant on a patent application related to C9orf72 “method for diagnosing a neurodegenerative disease” (PCT/GB2012/052140).
Published: May 4, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xgen.2023.100316.
Contributor Information
Sonja W. Scholz, Email: sonja.scholz@nih.gov.
The American Genome Center:
Anthony R. Soltis, Coralie Viollet, Gauthaman Sukumar, Camille Alba, Nathaniel Lott, Elisa McGrath Martinez, Meila Tuck, Jatinder Singh, Dagmar Bacikova, Xijun Zhang, Daniel N. Hupalo, Adelani Adeleye, Matthew D. Wilkerson, Harvey B. Pollard, and Clifton L. Dalgard
International LBD Genomics Consortium:
Sandra E. Black, Ziv Gan-Or, Julia Keith, Mario Masellis, Ekaterina Rogaeva, Alexis Brice, Suzanne Lesage, Georgia Xiromerisiou, Andrea Calvo, Antonio Canosa, Adriano Chio, Giancarlo Logroscino, Gabriele Mora, Reijko Krüger, Patrick May, Daniel Alcolea, Jordi Clarimon, Juan Fortea, Isabel Gonzalez-Aramburu, Jon Infante, Carmen Lage, Alberto Lleó, Pau Pastor, Pascual Sanchez-Juan, Francesca Brett, Dag Aarsland, Safa Al-Sarraj, Johannes Attems, Steve Gentleman, John A. Hardy, Angela K. Hodges, Seth Love, Ian G. McKeith, Christopher M. Morris, Huw R. Morris, Laura Palmer, Stuart Pickering-Brown, Mina Ryten, Alan J. Thomas, Claire Troakes, Marilyn S. Albert, Matthew J. Barrett, Thomas G. Beach, Lynn M. Bekris, David A. Bennett, Bradley F. Boeve, Clifton L. Dalgard, Ted M. Dawson, Dennis W. Dickson, Kelley Faber, Tanis Ferman, Luigi Ferrucci, Margaret E. Flanagan, Tatiana M. Foroud, Bernardino Ghetti, J. Raphael Gibbs, Alison Goate, David S. Goldstein, Neill R. Graff-Radford, Horacio Kaufmann, Walter A. Kukull, James B. Leverenz, Grisel Lopez, Qinwen Mao, Eliezer Masliah, Edwin Monuki, Kathy L. Newell, Jose-Alberto Palma, Matthew Perkins, Olga Pletnikova, Alan E. Renton, Susan M. Resnick, Liana S. Rosenthal, Owen A. Ross, Clemens R. Scherzer, Geidy E. Serrano, Vikram G. Shakkottai, Ellen Sidransky, Toshiko Tanaka, Nahid Tayebi, Eric Topol, Ali Torkamani, Juan C. Troncoso, Randy Woltjer, Zbigniew K. Wszolek, and Sonja W. Scholz
International ALS/FTD Consortium:
Robert H. Baloh, Robert Bowser, Alexis Brice, James Broach, William Camu, Adriano Chiò, John Cooper-Knock, Carsten Drepper, Vivian E. Drory, Travis L. Dunckley, Eva Feldman, Pietro Fratta, Glenn Gerhard, Summer B. Gibson, Jonathan D. Glass, John A. Hardy, Matthew B. Harms, Terry D. Heiman-Patterson, Lilja Jansson, Janine Kirby, Justin Kwan, Hannu Laaksovirta, John E. Landers, Francesco Landi, Isabelle Le Ber, Serge Lumbroso, Daniel J.L. MacGowan, Nicholas J. Maragakis, Kevin Mouzat, Liisa Myllykangas, Richard W. Orrell, Lyle W. Ostrow, Roger Pamphlett, Erik Pioro, Stefan M. Pulst, John M. Ravits, Wim Robberecht, Ekaterina Rogaeva, Jeffrey D. Rothstein, Michael Sendtner, Pamela J. Shaw, Katie C. Sidle, Zachary Simmons, Thor Stein, David J. Stone, Pentti J. Tienari, Bryan J. Traynor, Juan C. Troncoso, Miko Valori, Philip Van Damme, Vivianna M. Van Deerlin, Ludo Van Den Bosch, and Lorne Zinman
Supplemental information
The table lists the structural variants that were discovered in the study cohorts in 50 genes previously implicated in monogenic forms of neurodegeneration. BAF, B allele frequency; BND, break-end variant; CPX, complex variant; DEL, deletion; DUP, duplication; INS, insertion; INV, inversion; ME, mobile element (Alu, LINE1, SVA); OTH, other variant; PE, paired-end read; RD, read depth; SR, split read. Relates to Table 2.
The table lists the structural variants in the study cohorts that were included in the analyses. BAF, B allele frequency; BND, break-end variant; CPX, complex variant; DEL, deletion; DUP, duplication; INS, insertion; INV, inversion; ME, mobile element (Alu, LINE1, SVA); OTH, other variant; PE, paired-end read; RD, read depth; SR, split read. Relates to Figure 2
The table lists the unfiltered structural variants in the LBD and FTD/ALS study cohorts. BAF, B allele frequency; BND, break-end variant; CPX, complex variant; DEL, deletion; DUP, duplication; INS, insertion; INV, inversion; ME, mobile element (Alu, LINE1, SVA); OTH, other variant; PE, paired-end read; RD, read depth; SR, split read. Relates to STAR Methods
Data and code availability
All original code and genome browser data tracks on structural variants have been deposited in GitHub: https://github.com/ruthchia/Structural_variant_analysis-LBD-FTD and Zenodo: https://doi.org/10.5281/zenodo.7796321. The GATK-SV pipeline used to map structural variants is available from GitHub: https://github.com/broadinstitute/gatk-sv. The structural variant resource app is available at https://ndru-ndrs-lng-nih.shinyapps.io/non_ad_dementias_sv_app/. Annotated structural variant calls in neurodegenerative disease genes are summarized in Table S6.
References
- 1.Ho S.S., Urban A.E., Mills R.E. Structural variation in the sequencing era. Nat. Rev. Genet. 2020;21:171–189. doi: 10.1038/s41576-019-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeler D.A., Srinivasan M., Egholm M., Shen Y., Chen L., McGuire A., He W., Chen Y.J., Makhijani V., Roth G.T., et al. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872–876. doi: 10.1038/nature06884. [DOI] [PubMed] [Google Scholar]
- 3.Blauwendraat C., Pletnikova O., Geiger J.T., Murphy N.A., Abramzon Y., Rudow G., Mamais A., Sabir M.S., Crain B., Ahmed S., et al. Genetic analysis of neurodegenerative diseases in a pathology cohort. Neurobiol. Aging. 2019;76 doi: 10.1016/j.neurobiolaging.2018.11.007. 214.e1–214214.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyant-Marechal I., Berger E., Laquerrière A., Rovelet-Lecrux A., Viennet G., Frebourg T., Rumbach L., Campion D., Hannequin D. Intrafamilial diversity of phenotype associated with app duplication. Neurology. 2008;71:1925–1926. doi: 10.1212/01.wnl.0000339400.64213.56. [DOI] [PubMed] [Google Scholar]
- 5.Farrer M., Kachergus J., Forno L., Lincoln S., Wang D.S., Hulihan M., Maraganore D., Gwinn-Hardy K., Wszolek Z., Dickson D., Langston J.W. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann. Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 6.Obi T., Nishioka K., Ross O.A., Terada T., Yamazaki K., Sugiura A., Takanashi M., Mizoguchi K., Mori H., Mizuno Y., Hattori N. Clinicopathologic study of a SNCA gene duplication patient with Parkinson disease and dementia. Neurology. 2008;70:238–241. doi: 10.1212/01.wnl.0000299387.59159.db. [DOI] [PubMed] [Google Scholar]
- 7.Toffoli M., Chen X., Sedlazeck F.J., Lee C.Y., Mullin S., Higgins A., Koletsi S., Garcia-Segura M.E., Sammler E., Scholz S.W., et al. Comprehensive short and long read sequencing analysis for the Gaucher and Parkinson's disease-associated GBA gene. Commun. Biol. 2022;5:670. doi: 10.1038/s42003-022-03610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majounie E., Renton A.E., Mok K., Dopper E.G.P., Waite A., Rollinson S., Chiò A., Restagno G., Nicolaou N., Simon-Sanchez J., et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–330. doi: 10.1016/S1474-4422(12)70043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L., et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abel H.J., Larson D.E., Regier A.A., Chiang C., Das I., Kanchi K.L., Layer R.M., Neale B.M., Salerno W.J., Reeves C., et al. Mapping and characterization of structural variation in 17,795 human genomes. Nature. 2020;583:83–89. doi: 10.1038/s41586-020-2371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chia R., Sabir M.S., Bandres-Ciga S., Saez-Atienzar S., Reynolds R.H., Gustavsson E., Walton R.L., Ahmed S., Viollet C., Ding J., et al. Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat. Genet. 2021;53:294–303. doi: 10.1038/s41588-021-00785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewan R., Chia R., Ding J., Hickman R.A., Stein T.D., Abramzon Y., Ahmed S., Sabir M.S., Portley M.K., Tucci A., et al. Pathogenic huntingtin repeat expansions in patients with frontotemporal dementia and amyotrophic lateral sclerosis. Neuron. 2021;109:448–460.e4. doi: 10.1016/j.neuron.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakobsson M., Scholz S.W., Scheet P., Gibbs J.R., VanLiere J.M., Fung H.C., Szpiech Z.A., Degnan J.H., Wang K., Guerreiro R., et al. Genotype, haplotype and copy-number variation in worldwide human populations. Nature. 2008;451:998–1003. doi: 10.1038/nature06742. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Roberts N.D., Wala J.A., Shapira O., Schumacher S.E., Kumar K., Khurana E., Waszak S., Korbel J.O., Haber J.E., et al. Patterns of somatic structural variation in human cancer genomes. Nature. 2020;578:112–121. doi: 10.1038/s41586-019-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudmant P.H., Rausch T., Gardner E.J., Handsaker R.E., Abyzov A., Huddleston J., Zhang Y., Ye K., Jun G., Fritz M.H.Y., et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. doi: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.English A.C., Menon V.K., Gibbs R.A., Metcalf G.A., Sedlazeck F.J. Truvari: refined structural variant comparison preserves allelic diversity. bioRxiv. 2022 doi: 10.1101/2022.02.21.481353. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrska-Bishop M., Evani U.S., Zhao X., Basile A.O., Abel H.J., Regier A.A., Corvelo A., Clarke W.E., Musunuri R., Nagulapalli K., et al. High-coverage whole-genome sequencing of the expanded 1000 Genomes Project cohort including 602 trios. Cell. 2022;185:3426–3440.e19. doi: 10.1016/j.cell.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vialle R.A., de Paiva Lopes K., Bennett D.A., Crary J.F., Raj T. Integrating whole-genome sequencing with multi-omic data reveals the impact of structural variants on gene regulation in the human brain. Nat. Neurosci. 2022;25:504–514. doi: 10.1038/s41593-022-01031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billingsley K.J., Ding J., Jerez P.A., Illarianova A., Grenn F.P., Makarious M.B., Moore A., Vitale D., Reed X., Hernandez D., et al. Genome-wide analysis of structural variants in Parkinson’s disease using short-read sequencing data. 2022. BioRxiv. [DOI]
- 21.Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 22.Bellenguez C., Küçükali F., Jansen I.E., Kleineidam L., Moreno-Grau S., Amin N., Naj A.C., Campos-Martin R., Grenier-Boley B., Andrade V., et al. New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat. Genet. 2022;54:412–436. doi: 10.1038/s41588-022-01024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nalls M.A., Blauwendraat C., Vallerga C.L., Heilbron K., Bandres-Ciga S., Chang D., Tan M., Kia D.A., Noyce A.J., Xue A., et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18:1091–1102. doi: 10.1016/S1474-4422(19)30320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GTEx Consortium The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–1330. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eraslan G., Drokhlyansky E., Anand S., Fiskin E., Subramanian A., Slyper M., Wang J., Van Wittenberghe N., Rouhana J.M., Waldman J., et al. Single-nucleus cross-tissue molecular reference maps toward understanding disease gene function. Science. 2022;376:eabl4290. doi: 10.1126/science.abl4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita M., Gao Z., Zeng L., McCabe C., White C.C., Ng B., Green G.S., Rozenblatt-Rosen O., Phillips D., Amir-Zilberstein L., et al. Cell-subtype specific effects of genetic variation in the aging and Alzheimer cortex. bioRxiv. 2022 doi: 10.1101/2022.11.07.515446. [DOI] [PubMed] [Google Scholar]
- 27.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat. Rev. Neurol. 2010;6:88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajput A., Dickson D.W., Robinson C.A., Ross O.A., Dächsel J.C., Lincoln S.J., Cobb S.A., Rajput M.L., Farrer M.J. Parkinsonism, Lrrk2 G2019S, and tau neuropathology. Neurology. 2006;67:1506–1508. doi: 10.1212/01.wnl.0000240220.33950.0c. [DOI] [PubMed] [Google Scholar]
- 29.Bannwarth S., Ait-El-Mkadem S., Chaussenot A., Genin E.C., Lacas-Gervais S., Fragaki K., Berg-Alonso L., Kageyama Y., Serre V., Moore D.G., et al. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain. 2014;137:2329–2345. doi: 10.1093/brain/awu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dols-Icardo O., Nebot I., Gorostidi A., Ortega-Cubero S., Hernández I., Rojas-García R., García-Redondo A., Povedano M., Lladó A., Álvarez V., et al. Analysis of the CHCHD10 gene in patients with frontotemporal dementia and amyotrophic lateral sclerosis from Spain. Brain. 2015;138:e400. doi: 10.1093/brain/awv175. [DOI] [PubMed] [Google Scholar]
- 31.Müller K., Andersen P.M., Hübers A., Marroquin N., Volk A.E., Danzer K.M., Meitinger T., Ludolph A.C., Strom T.M., Weishaupt J.H. Two novel mutations in conserved codons indicate that CHCHD10 is a gene associated with motor neuron disease. Brain. 2014;137:e309. doi: 10.1093/brain/awu227. [DOI] [PubMed] [Google Scholar]
- 32.Woo J.A.A., Liu T., Trotter C., Fang C.C., De Narvaez E., LePochat P., Maslar D., Bukhari A., Zhao X., Deonarine A., et al. Loss of function CHCHD10 mutations in cytoplasmic TDP-43 accumulation and synaptic integrity. Nat. Commun. 2017;8:15558. doi: 10.1038/ncomms15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow C.Y., Landers J.E., Bergren S.K., Sapp P.C., Grant A.E., Jones J.M., Everett L., Lenk G.M., McKenna-Yasek D.M., Weisman L.S., et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 2009;84:85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishibashi K., Suzuki M., Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem. Biophys. Res. Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- 35.Cang C., Bekele B., Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol. 2014;10:463–469. doi: 10.1038/nchembio.1522. [DOI] [PubMed] [Google Scholar]
- 36.She J., Guo J., Chen Q., Zeng W., Jiang Y., Bai X.C. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature. 2018;556:130–134. doi: 10.1038/nature26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X., Zhang X., Dong X.P., Samie M., Li X., Cheng X., Goschka A., Shen D., Zhou Y., Harlow J., et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster W.J., Taylor H.B.C., Padamsey Z., Jeans A.F., Galione A., Emptage N.J. Hippocampal mGluR1-dependent long-term potentiation requires NAADP-mediated acidic store Ca(2+) signaling. Sci. Signal. 2018;11:eaat9093. doi: 10.1126/scisignal.aat9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallmann R.T., Klugbauer N. Genetic inactivation of two-pore channel 1 impairs spatial learning and memory. Behav. Genet. 2020;50:401–410. doi: 10.1007/s10519-020-10011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neely Kayala K.M., Dickinson G.D., Minassian A., Walls K.C., Green K.N., Laferla F.M. Presenilin-null cells have altered two-pore calcium channel expression and lysosomal calcium: implications for lysosomal function. Brain Res. 2012;1489:8–16. doi: 10.1016/j.brainres.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wacker S.A., Alvarado C., von Wichert G., Knippschild U., Wiedenmann J., Clauss K., Nienhaus G.U., Hameister H., Baumann B., Borggrefe T., et al. RITA, a novel modulator of Notch signalling, acts via nuclear export of RBP-J. EMBO J. 2011;30:43–56. doi: 10.1038/emboj.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs J., Nilsson C., Kachergus J., Munz M., Larsson E.M., Schüle B., Langston J.W., Middleton F.A., Ross O.A., Hulihan M., et al. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology. 2007;68:916–922. doi: 10.1212/01.wnl.0000254458.17630.c5. [DOI] [PubMed] [Google Scholar]
- 43.Pottier C., Bieniek K.F., Finch N., van de Vorst M., Baker M., Perkersen R., Brown P., Ravenscroft T., van Blitterswijk M., Nicholson A.M., et al. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol. 2015;130:77–92. doi: 10.1007/s00401-015-1436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iida A., Hosono N., Sano M., Kamei T., Oshima S., Tokuda T., Nakajima M., Kubo M., Nakamura Y., Ikegawa S. Novel deletion mutations of OPTN in amyotrophic lateral sclerosis in Japanese. Neurobiol. Aging. 2012;33:1843.e19–1843.e24. doi: 10.1016/j.neurobiolaging.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 45.Osawa T., Mizuno Y., Fujita Y., Takatama M., Nakazato Y., Okamoto K. Optineurin in neurodegenerative diseases. Neuropathology. 2011;31:569–574. doi: 10.1111/j.1440-1789.2011.01199.x. [DOI] [PubMed] [Google Scholar]
- 46.Collins R.L., Brand H., Karczewski K.J., Zhao X., Alföldi J., Francioli L.C., Khera A.V., Lowther C., Gauthier L.D., Wang H., et al. A structural variation reference for medical and population genetics. Nature. 2020;581:444–451. doi: 10.1038/s41586-020-2287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X., Schulz-Trieglaff O., Shaw R., Barnes B., Schlesinger F., Källberg M., Cox A.J., Kruglyak S., Saunders C.T. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32:1220–1222. doi: 10.1093/bioinformatics/btv710. [DOI] [PubMed] [Google Scholar]
- 48.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H. New strategies to improve minimap2 alignment accuracy. Bioinformatics. 2021;37:4572–4574. doi: 10.1093/bioinformatics/btab705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sedlazeck F.J., Rescheneder P., Smolka M., Fang H., Nattestad M., von Haeseler A., Schatz M.C. Accurate detection of complex structural variations using single-molecule sequencing. Nat. Methods. 2018;15:461–468. doi: 10.1038/s41592-018-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang C.C., Chow C.C., Tellier L.C., Vattikuti S., Purcell S.M., Lee J.J. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abraham G., Qiu Y., Inouye M. FlashPCA2: principal component analysis of Biobank-scale genotype datasets. Bioinformatics. 2017;33:2776–2778. doi: 10.1093/bioinformatics/btx299. [DOI] [PubMed] [Google Scholar]
- 53.Danecek P., Bonfield J.K., Liddle J., Marshall J., Ohan V., Pollard M.O., Whitwham A., Keane T., McCarthy S.A., Davies R.M., Li H. Twelve years of SAMtools and BCFtools. GigaScience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willer C.J., Li Y., Abecasis G.R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kleinert P., Kircher M. A framework to score the effects of structural variants in health and disease. Genome Res. 2022;32:766–777. doi: 10.1101/gr.275995.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boughton A.P., Welch R.P., Flickinger M., VandeHaar P., Taliun D., Abecasis G.R., Boehnke M. LocusZoom.js: interactive and embeddable visualization of genetic association study results. Bioinformatics. 2021;37:3017–3018. doi: 10.1093/bioinformatics/btab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belyeu J.R., Chowdhury M., Brown J., Pedersen B.S., Cormier M.J., Quinlan A.R., Layer R.M. Samplot: a platform for structural variant visual validation and automated filtering. Genome Biol. 2021;22:161. doi: 10.1186/s13059-021-02380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emre M., Aarsland D., Brown R., Burn D.J., Duyckaerts C., Mizuno Y., Broe G.A., Cummings J., Dickson D.W., Gauthier S., et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov. Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 60.McKeith I.G., Boeve B.F., Dickson D.W., Halliday G., Taylor J.P., Weintraub D., Aarsland D., Galvin J., Attems J., Ballard C.G., et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S., Freedman M., Kertesz A., Robert P.H., Albert M., et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 62.Höglinger G.U., Respondek G., Stamelou M., Kurz C., Josephs K.A., Lang A.E., Mollenhauer B., Müller U., Nilsson C., Whitwell J.L., et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov. Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brooks B.R., Miller R.G., Swash M., Munsat T.L., World Federation of Neurology Research Group on Motor Neuron Diseases El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 64.Erikson G.A., Bodian D.L., Rueda M., Molparia B., Scott E.R., Scott-Van Zeeland A.A., Topol S.E., Wineinger N.E., Niederhuber J.E., Topol E.J., Torkamani A. Whole-genome sequencing of a healthy aging cohort. Cell. 2016;165:1002–1011. doi: 10.1016/j.cell.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werling D.M., Brand H., An J.Y., Stone M.R., Zhu L., Glessner J.T., Collins R.L., Dong S., Layer R.M., Markenscoff-Papadimitriou E., et al. An analytical framework for whole-genome sequence association studies and its implications for autism spectrum disorder. Nat. Genet. 2018;50:727–736. doi: 10.1038/s41588-018-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gardner E.J., Lam V.K., Harris D.N., Chuang N.T., Scott E.C., Pittard W.S., Mills R.E., Devine S.E., 1000 Genomes Project Consortium the mobile element locator tool (MELT): population-scale mobile element discovery and biology. Genome Res. 2017;27:1916–1929. doi: 10.1101/gr.218032.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kronenberg Z.N., Osborne E.J., Cone K.R., Kennedy B.J., Domyan E.T., Shapiro M.D., Elde N.C., Yandell M. Wham: identifying structural variants of biological consequence. PLoS Comput. Biol. 2015;11:e1004572. doi: 10.1371/journal.pcbi.1004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klambauer G., Schwarzbauer K., Mayr A., Clevert D.A., Mitterecker A., Bodenhofer U., Hochreiter S. cn.MOPS: mixture of Poissons for discovering copy number variations in next-generation sequencing data with a low false discovery rate. Nucleic Acids Res. 2012;40:e69. doi: 10.1093/nar/gks003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van der Auwera G.A., O’Connor B.D. O’Reilly Media; 2020. Genomics in the Cloud: Using Docker, GATK, and WDL in Terra. [Google Scholar]
- 70.Smolka M., Paulin L.F., Grochowski C.M., Mahmoud M., Behera S., Gandhi M., Hong K., Pehlivan D., Scholz S.W., Carvalho C.M.B., et al. Comprehensive structural variant detection: from mosaic to population-level. bioRxiv. 2022 doi: 10.1101/2022.04.04.487055. Preprint at. [DOI] [Google Scholar]
- 71.Karolchik D., Hinrichs A.S., Furey T.S., Roskin K.M., Sugnet C.W., Haussler D., Kent W.J. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004;32:D493–D496. doi: 10.1093/nar/gkh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hahne F., Ivanek R. Visualizing genomic data using Gviz and bioconductor. Methods Mol. Biol. 2016;1418:335–351. doi: 10.1007/978-1-4939-3578-9_16. [DOI] [PubMed] [Google Scholar]
- 73.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., 3rd, Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shabalin A.A. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–1358. doi: 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The table lists the structural variants that were discovered in the study cohorts in 50 genes previously implicated in monogenic forms of neurodegeneration. BAF, B allele frequency; BND, break-end variant; CPX, complex variant; DEL, deletion; DUP, duplication; INS, insertion; INV, inversion; ME, mobile element (Alu, LINE1, SVA); OTH, other variant; PE, paired-end read; RD, read depth; SR, split read. Relates to Table 2.
The table lists the structural variants in the study cohorts that were included in the analyses. BAF, B allele frequency; BND, break-end variant; CPX, complex variant; DEL, deletion; DUP, duplication; INS, insertion; INV, inversion; ME, mobile element (Alu, LINE1, SVA); OTH, other variant; PE, paired-end read; RD, read depth; SR, split read. Relates to Figure 2
The table lists the unfiltered structural variants in the LBD and FTD/ALS study cohorts. BAF, B allele frequency; BND, break-end variant; CPX, complex variant; DEL, deletion; DUP, duplication; INS, insertion; INV, inversion; ME, mobile element (Alu, LINE1, SVA); OTH, other variant; PE, paired-end read; RD, read depth; SR, split read. Relates to STAR Methods
Data Availability Statement
All original code and genome browser data tracks on structural variants have been deposited in GitHub: https://github.com/ruthchia/Structural_variant_analysis-LBD-FTD and Zenodo: https://doi.org/10.5281/zenodo.7796321. The GATK-SV pipeline used to map structural variants is available from GitHub: https://github.com/broadinstitute/gatk-sv. The structural variant resource app is available at https://ndru-ndrs-lng-nih.shinyapps.io/non_ad_dementias_sv_app/. Annotated structural variant calls in neurodegenerative disease genes are summarized in Table S6.