Abstract
Introduction
The purpose of this study was to investigate the clinical and radiographic outcomes at 2 years for patients who underwent an arthroscopic xenograft bone block procedure plus ASA for recurrent anteroinferior gleno-humeral instability.
Methods
This retrospective study was conducted on patients affected by chronic anteroinferior shoulder instability. The inclusion criteria were as follows: patients must be aged 18 years or older; have recurrent anteroinferior shoulder instability, a glenoid defect >10%, assessment by the Pico area measurement system, anterior capsular insufficiency, and an engaging Hill-Sachs lesion. The exclusion criteria were as follows: multidirectional instability, glenoid bone defect <10%, arthritis, and minimum follow-up less than 24 months. Clinical outcomes were evaluated according to Western Ontario Shoulder Instability Index (WOSI) and Rowe scale. Computed tomography (CT) results were evaluated to assess any signs of resorption or displacement of the xenograft at 24 months follow-up.
Results
Twenty patients that met all the inclusion criteria underwent arthroscopic xenograft bone block procedure and ASA. The mean preoperative Rowe score was 38.3 points, and it significantly improved (P < .001), increasing to 95.5 points. ROWE level at follow-up was excellent for 18 patients (90%), fair for 1 patient (5%), and poor for another patient (5%). The mean preoperative WOSI score was 1242 points, and it improved significantly (P <.0001), with a mean score of 120 points at follow-up. In all patients, the comparative study between CT scans performed postoperatively and at final follow-up did not reveal a volume reduction of the xenografts (P > .05) and absence areas affected by signs of resorption and breakage with 34.4% of postprocedural increase of the glenoid surface, were seen.
Conclusions
The combination of ASA and bone block procedure with a xenograft was effective in the glenoid reconstruction and restoration of shoulder stability. No radiographic evidence of graft resorption, graft displacement, or glenohumeral arthritis were observed at 24-month follow-up.
Level of Evidence
Level IV, therapeutic case series.
Introduction
The glenoid bone loss (GBL) threshold of 20% has given way to less acceptable amounts of bone loss; GBL >13% is now being used as a potential cutoff to recommend glenoid reconstruction.1, 2, 3, 4 This technique is typically performed by means of bone graft transfer5 or coracoid process transfer.6 The Latarjet technique has been increasingly used because the coracoid graft with coraco-brachialis muscle transfer on the glenoid neck could correct both the bone defect and the capsular deficiency.6 Bone graft techniques are associated with low risks of complications4 and are technically less demanding; however, they could be less effective in the presence of capsular deficiency or in patients who are affected by shoulder hyperlaxity.7,8 The autologous use of bone grafts from the iliac crest or distal clavicle can be either bicortical or tricortical with high osteogenic, osteoinductive, and osteoconductive properties, and can be done arthroscopically.9,10 Other solutions that avoid donor-site damage have included the use of refrigerated or fresh grafts from the bone bank.11 The critical aspect of allograft is the risk of bone resorption,9 as has been reported in recent studies.10,12 The use of equine grafts treated by a deantigenation enzymatic process has been reported to be safe and biomechanically reliable.13,14 Following these reports, we have chosen this type of biomaterial for creating a precontoured bone graft as an alternative to current autografts and allografts. The ASA technique, which consists of a tenodesis of the upper third of the subscapularis tendon on the glenoid neck as an augmentation of the Bankart repair, has a triple effect: address the stretched part of the subscapularis, amend capsular insufficiency, and restore the coracohumeral tension.8 The purpose was to investigate the clinical and radiographic outcomes at 2-year follow-up for patients who underwent an arthroscopic xenograft bone block procedure plus ASA for recurrent anteroinferior gleno-humeral instability. We hypothesized an absence of resorption of the xenograft on computed tomography (CT) scan and good clinical results.
Protocol Approval
This study was approved by the Institutional Review Board Ethics Committee of the local institutes (protocol no. 69/CE4-19 OSS 5 on March 2019 and protocol no. 103700/ASL 9 on May 2019).
Methods
A series of 20 patients (Table 1) treated for recurrent anteroinferior gleno-humeral instability with glenoid bone defects at 2 hospitals were retrospectively identified. The present study was approved by the Ethics Committee of the local institutes (protocol no. 69/CE4-19 OSS 5 on March 2019 and protocol no. 103700/ASL 9 on May 2019), and informed specific consent was obtained from all of the patients. The patients underwent operations in two hospitals by three different surgeons. The inclusion criteria were as follows: 1) age of 18 years or older; 2) recurrent antero-inferior shoulder instability; 3) glenoid defect >10%, as assessed by the Pico area measurement system in two-dimensional (2D)-CT; 4) anterior capsular insufficiency, as assessed during arthroscopic examination, and 5) an engaging Hill Sachs lesion. The exclusion criteria were as follows: 1) multidirectional instability, 2) a glenoid bone defect <10%, and 3) arthritis. Demographic data and preoperative clinical characteristics of the participants were collected: sex, age, shoulder operated, number of dislocations, GBL, and shoulder hyperlaxity. Clinical outcomes were evaluated using ROWE score, WOSI score, and measurements of external rotation in two positions with the arm adducted (external rotation 1 [ER1]) and with the arm at 90° of abduction (ER2) compared with contralateral shoulder. Hyperlaxity was evaluated, according to Neer and Coudane-Walch tests.7 Patients underwent surgery between March 2019 and September 2020 using all arthroscopic xenograft bone block technique fixed to the glenoid neck with double-button stabilization Bankart repair plus soft tissue augmentation with the upper third of the subscapularis tendon tenodesis technique (ASA). The indications were chronic anterior shoulder instability with a GBL >10%. The precontoured bone block xenograft was of equine origin and was cleaned through the Zymo-Teck process, an enzymatic deantigenation treatment that allows the complete removal of all immunogenic components13 without altering the biological and biomechanical properties of the treated graft (Bioteck, Turin, Italy).14, 15, 16 The preservation of the collagen component (type I bone collagen) allows the implanted material to physiologically respond to the action of the cellular elements involved in the regeneration process of the spongiosa of the glenoid neck, thus facilitating bone regeneration at the interface.17,18 We have chosen this kind of material due to the specific dense trabecular structure and high biomechanical resistance of the proximal epiphysis of the equine humerus. This precision-machined graft is composed of an anterior cortical part and a posterior spongy trabecular part that encounters the spongiosa of the glenoid neck.
Table 1.
Patient’s Demographics
| Number of participants | 20 |
| Sex (M/F) | 19/1 (95%) |
| Age | 31.0 ± 11.0 (25.8-36.2) |
| Follow-up (months) | 24 (20.0-31.7) |
| Shoulder operated (R/L) | 14/6 (70%) |
| Number of dislocations | 17.8 ± 22.6 (7.2-28.4) |
| Shoulder hyperlaxity (Y/N) | 14/6 (70%) |
| Glenoid bone loss (%) | 18.5 ± 4.7 (16.2-20.7) |
Number and (%) or means ± SD and (95% CI) of demographic and clinical characteristics of the study participants.
The dimensions of the xenograft are height 22 × 10 (maximum), length 10 (maximum) and thickness with two 3-mm holes for the passage of the fixation suture devices, which were preshaped to correspond to the 2 drill holes made by the posterior glenoid guide (Smith & Nephew Inc, Andover, MA), as described in the original study by Taverna.19
Surgical Technique
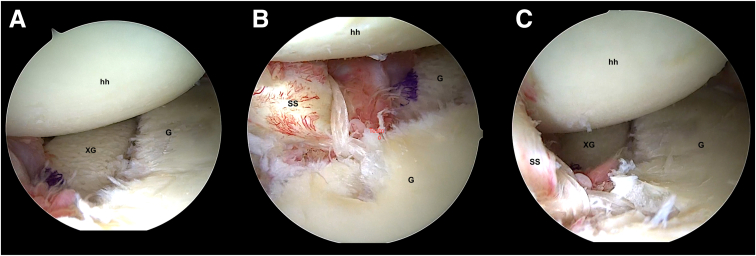
Patients, under general anesthesia, were placed in the lateral decubitus position for the arthroscopic procedure.19, 20, 21 Complete arthroscopic exploration was performed through standard portals, and all the lesions were identified. The anteroinferior labrum and capsule remnants were carefully detached from the neck of the glenoid cavity from the 2 o’clock to the 6 o'clock position in the case of the right shoulder and from the 11 o’clock to 6 o'clock position in the case of the left shoulder. The glenoid defect was debrided and freshened to leave it smooth and perpendicular to the articular glenoid surface. Two drill holes were made using the posterior glenoid guide (Smith & Nephew), according to the technique already described in the literature.19 After that, the xenograft was introduced into the joint through the rotator interval and was positioned in line with the defect. The correct position of the graft was checked from the anterosuperior portal (Fig 1A). The graft was fixed with 4 Endobuttons suture fixation device (Smith & Nephew). A suture tensioner device was used to secure the posterior round Endobuttons, tensioning it at 75 N. The procedure was then completed by repairing the anteroinferior capsule with 1 anchor, similar to part of a standard Bankart procedure, at the 5-o’clock position. After graft positioning and Bankart repair, the procedure was completed with ASA to allow the subscapularis tendon and the entire anteroinferior capsule to shift from inferior to superior to obtain better coverage of the graft and soft-tissue tightening (Fig 1, B and C). In all patients, a Bankart repair was performed.22 The ASA procedure was already described in previous publications.21,23,24
Fig 1.
The figure shows the arthroscopic visualization during procedure. (A) Xenograft is placed over the anterior glenoid neck, at the same level of glenoid surface. hh, humeral head; G, glenoid; XG, xenograft. (B and C) In the final arthroscopic view (right shoulder) with patient in lateral decubitus position. Posterior portal (A). Anterosuperior portal (B). G, glenoid; hh, humeral head; SS, subscapularis tendon; XG, xenograft. The humeral head is shifted posteriorly by subscapularis tendon action.
The advancement of the subscapularis tendon over the graft, the closure of the anterior capsule, and the posterior shifting of the humeral head were assessed from the antero-superior portal. Following surgery, the patients wore a sling with the arm in adduction for 4 weeks, and active exercises of the elbow and hand were permitted. From the fourth week to the eighth week, physical therapy was prescribed for the recovery of full range of motion. Strengthening exercises started 10 weeks after surgery. Return to sports was not allowed until 6 months after surgery.
Computer Tomography and Clinical Assessment
All patients underwent postoperative 2D-CT within 2 days after surgery to assess correct graft positioning. Further 2D-CT examinations were conducted postoperatively at 24 months follow-up to assess any signs of resorption or displacement of the xenograft and were compared with that performed postoperatively. The mean xenograft volume (mm3) was used for the evaluation of bone resorption. The images were exported to Horos for reconstruction and analysis.25 The measurements of xenograft position were relative to the height of the glenoid from the anterior portion of the xenograft (medialization); similarly, the angles measured were formed by the glenoid plane and the anterior portion of xenografts (angulation). All the measurements were performed manually by two experienced radiologists (M.R. and F.D.). We arbitrarily consider measures <2 mm of medialization and angles <2° an optimal position of the xenograft (flush with the glenoid plane). We consider measures between 2 and 5 mm of medialization or angles between 2° and 5° as signs of a slight medialization/angulation and measures >5 mm or angles >5° as important signs of a medialization/angulation. Clinical outcomes were evaluated using the Western Ontario Shoulder Instability Index (WOSI) and the Rowe scale preoperatively at 24-month follow-up. Patients were asked to score their perceived degree of change on a 4-item anchor questionnaire to calculate the minimal clinically important difference (MCID) and patient-acceptable symptom state (PASS) of the WOSI. ER1 versus ER1 contralateral and ER2 versus ER2 contralateral were compared at follow-up.
Statistics
Statistical Package for the Social Sciences ver. 20.0 (SPSS Inc., Chicago, IL) was used for the statistical analysis. Number and percentage or mean and standard deviation (SD) of demographical data and preoperative clinical characteristics were calculated. Frequency, percent, valid percent, and cumulative percent of medialization or angulation placement of xenograft were calculated. Paired-sample t-test was used to assess differences between preoperative and postoperative values. Statistical significance was set at P < .05.
Results
Demographic data of the 20 participants in the study were reported in Table 1. Ninety-five percent of participants were male, and 70% of participants had both right shoulder operated and shoulder hyperlaxity. The following means ± SD were calculated: age (years), 31.0 ± 11.0; number of dislocations, 17.8 ± 22.6; glenoid bone loss (%) 18.5 ± 4.7. Clinical characteristics were reported in Table 4: ROWE score, 38.3 ±12.3; WOSI score, 1242 ± 107; ER1 (degrees), 76.8 ± 11.3 contralateral 86.3 ± 9.3 (P = .001); ER2 (degrees), 88.3 ± 5.2 contralateral 91.8 ± 9.3 (P = .001).
Table 4.
Measures of Clinical and Radiographic Outcome at 24 Months After Surgery
| P∗ | |||
|---|---|---|---|
| Means ± SD | 95% CI | ||
| After surgery | 1503 ± 12.9 | 1497-1509 | .102 |
| 24 months follow-up | 1493 ± 15.2 | 1486-1500 | |
| Difference | 10.2 ± 107.2 | −40.0-60.0 | |
| Changes of outcomes after 24 months from surgery | |||
|---|---|---|---|
| Preoperative | Follow up | P∗ | |
| ROWE | 38.3 ± 12.3 (32.5-44.0) | 95.5 ± 9.4 (91.1-99.9) | <.001 |
| WOSI | 1242 ± 107 (1192-1292) | 120 ± 181 (35-205) | <.001 |
| External rotations of the operated and contralateral shoulders at 24 months follow-up | ||||
|---|---|---|---|---|
| ER | ER Contralateral | Differences | P∗ | |
| ER1 vs ER1 contralateral | 76.8 ± 11.3 (71.5-82.0) | 86.3 ± 9.3 (81.9-90.6) | 9.5 ± 6.3 (6.6-12.4) | <.001 |
| ER2 vs ER2 contralateral | 88.3 ± 5.2 (85.8-90.7) | 91.8 ± 6.5 (88.7-94.8) | 3.5 ± 3.3 (1.9-4.8) | .017 |
Values are presented as means ± SD and 95% CI of xenograft volume (mm3).
P of Student’s t-test.CI, confidence interval; ER, external rotation; SD, standard deviation.
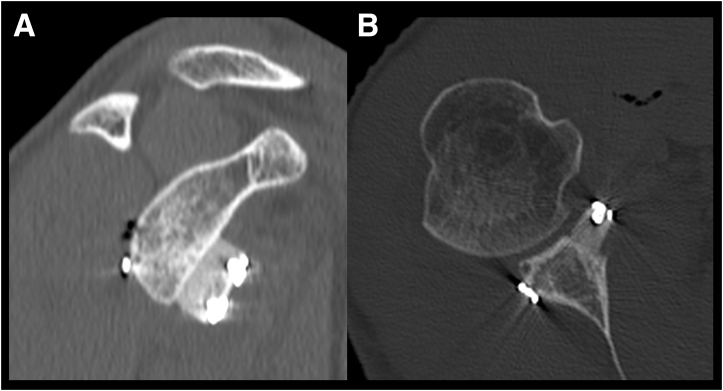
The operative mean time was 70 minutes (minimum 55, maximum 85). No intraoperative graft rupture was observed during mechanical compression with the specific tensioner. The postoperative 2D-CT studies revealed appropriate placement of the xenografts (flush with glenoid plane) and the correct entry location of the glenoid tunnels (Fig 2).
Fig 2.
(A and B) Sagittal and axial views at computed tomography scan postoperative control that shows a correct positioning of the xenograft.
The positioning of xenograft remained optimal in 75% of the 20 patients 24 months later (Table 2), whereas only 1 participant presented an angulation more than 5° at 24-month follow-up.
Table 2.
Medialization of Xenograft Placement After Surgery and at 24-Month Follow-Up
| Placement | Frequency | Percent | Valid Percent |
Cumulative Percent |
|---|---|---|---|---|
| After surgery: | ||||
| Optimum position | 15 | 75.0 | 75.0 | 75.0 |
| Slight medialization | 5 | 25.0 | 25.0 | 100.0 |
| Total | 20 | 100.0 | 100.0 | |
| 24 months follow-up: | ||||
| Optimum position | 15 | 75.0 | 75.0 | 75.0 |
| Slight medialization | 5 | 25.0 | 25.0 | 100.0 |
| Total | 20 | 100.0 | 100.0 | |
Values are provided regarding medialization of xenograft placement after surgery and at 24-month follow-up. Optimum position of xenograft: <2 mm. Slight medialization: between 2 mm and 5 mm; medialization: >5 mm.
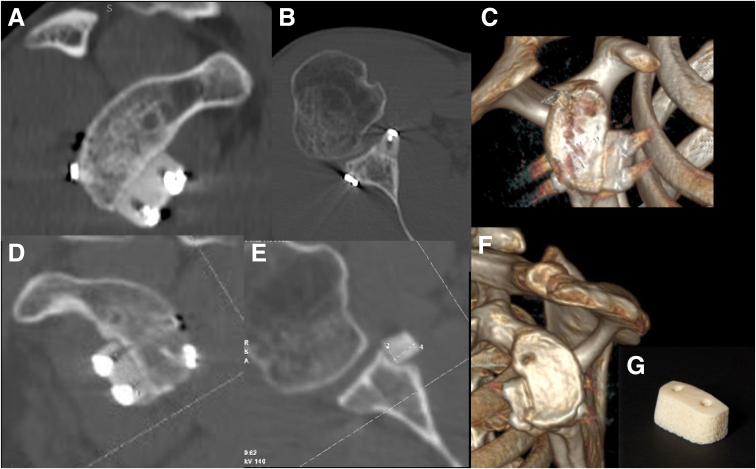
In all patients, the comparative study between 2D-CT scans performed at follow-up did not reveal a volume reduction of the xenografts and any areas affected by signs of resorption and breakage at 24-month follow-up (Fig 3, A-C).
Fig 3.
The figure shows the computed tomography scans with sagittal (A), axial (B) views and 3D reconstruction (C) at 2-year follow-up and at 4-year follow-up (D-F). Absence of graft resorption can be noted. In axial view, there is a slight angulation of the xenograft, although it is still in good position (D). A precontoured xenograft is used for the procedure (G).
The mean surface area was 86.4% (84.6-88.1), and it increased after graft implantation to 122.3% (118-125.9). At follow-up, the mean surface area decreased to 116.1% (112.6-118.4). No signs of secondary osteoarthritis were noted (Fig 3, D-F).
ROWE and WOSI scores presented a significant improvement (P < .001) at 24-month follow-up, passing from 38.3 and 1242 points to 95.5 and 120 points, respectively. ROWE level at follow-up was excellent. Operative mean time was 70 minutes (minimum 55, maximum 85). Clinical outcomes were excellent for 18 patients (90%), fair for 1 patient (5%), and poor for another patient (5%) (Table 3).
Table 3.
Angulation of Xenograft After Surgery and at 24-Month Follow-Up
| Score | Frequency | Percent | Valid Percent |
Cumulative Percent |
|---|---|---|---|---|
| After surgery: | ||||
| Optimum position | 15 | 75.0 | 75.0 | 75.0 |
| Slight angulation | 5 | 25.0 | 25.0 | 100.0 |
| Total | 20 | 100.0 | 100.0 | |
| 24 Months follow-up: | ||||
| Optimum position | 15 | 75.0 | 75.0 | 75.0 |
| Slight angulation | 4 | 20.0 | 20.0 | 95.0 |
| Angulation | 1 | 5.0 | 5.0 | 100.0 |
| Total | 20 | 100.0 | 100.0 | |
Optimum position of Xenograft <2°: slight medialization between 2° and 5°; angulation of xenograft > 5°.
At final follow-up, the mean deficit of external rotation was 9.5° with the arm at the side of the trunk (ER1 position), and the mean deficit was 3.5° with the arm in 90° of abduction (ER2 position) (Table 4).
We could not calculate the MCID and PASS of the WOSI score by the anchor-based method questionnaire because only one patient was present in the “no change” group. From the analysis of CT scan measurements, we did not highlight complete fusion between the graft and the scapular neck, no recurrent dislocation, no deep infection disease transmission and recurrent dislocation occurred.
Discussion
The most important finding of this study was that the implanted equine xenograft15,16,26 did not show any resorption sign on CT scan at 2-year follow-up. Furthermore, the association of ASA yielded excellent results in 18 of the 20 patients of the present series. Nowadays, it is well known that GBL >13% in the anterior glenoid rim always requires glenoid surgical reconstruction to avoid early failure of simple soft-tissue repair.1,12
The optimal grafting procedure to address the GBL is still controversial. There is considerable concern about the bone remodeling process of coracoid grafts.27 Free bone grafts provide a more anatomical surgical repair and less complications compared to the Latarjet procedure28, 29, 30 but are less indicated in the presence of a concomitant anterior capsular deficiency. Furthermore, the effectiveness of soft tissue augmentation to a free bone block procedure has been already described.31 On the contrary, a high resorption rate of the allograft has been described, and there is a concern regarding donor morbidity of the autograft and length of operating time. In our series, intraoperative or early postoperative complications were not observed, including hematomas, disease transmission, or neurovascular injuries.
The average time of the operation was 70 minutes (minimum 55 minutes, maximum 85). There were no adverse biological reactions to the graft. The literature on the use of xenografts for restoring articular defects is almost poor.
In 1988, Krödel and Melzer reported the radiological and clinical results of 46 patients who received a Kiel bovine bone xenograft using the Eden-Lange technique at a medium-term follow-up.32 The graft was inserted in a scapular neck groove. Radiological bony consolidation was found in 80% of patients, whereas bone resorption occurred in 14%.33 However, this bone was of bovine origin and was cleaned and treated with heat using a different process. In contrast, the xenograft (Fig 3G) of equine origin with high biomechanical resistance due to dense trabecular structure of the proximal epiphysis of the equine humerus we used, underwent a particular enzyme process consisting of hydrogen peroxide treatment and e-beam irradiation.13 This process resulted in effective and safe virus clearance and antigen inactivation while preserving the type I collagen structures, which were useful for the activation of endogenous growth factors, which is responsible for osteointegration and bone remodeling processes.17,18 The CT scans performed at 24 months of follow-up did not reveal any significant volume reduction of the xenografts, and no graft areas affected by signs of resorption were observed. In all patients, the grafts were well positioned, and angulation was reported in only 1 case. No breakage of the graft was seen. Furthermore, the mean increased glenoid surface area was 116.1% at follow-up and, in relation to a completely intact glenoid surface area (100%), glenoid surface augmentation leads to subsequent anterior bony effective support of the humeral head.33
In addition, the CT at 2-year follow-up highlighted either the stability or the absence of resorption of the graft. We performed in 2 of the 20 patients a subsequent control at CT at 4 years (Fig 3F). Therefore, it can be assumed that even if complete bone integration of the xenograft with the scapular neck did not occur, the stability of the graft could have been provided by periosteal penetration and fibrous integration between xenograft, periosteum, and anterior soft tissues. In other words, we can see that the xenograft used for glenoid reconstruction has a similar effect as a bio-prosthesis34 when compared to traditional bone grafts. No radiological signs of osteoarthritis were noted at the average follow-up. Moreover, in our series, we observed a significant improvement in functional and subjective outcomes, except for two patients.
A highly significant improvement of ROWE and WOSI scores was registered. ROWE level at follow-up was excellent for most of participants (90%).
At final follow-up, the external rotation on the operated shoulder was not compromised. From this study, it can be assumed that such excellent results observed in our critical group of patients are due to both the enlargement of the glenoid surface and to the concomitant effect of the ASA technique in restoring anterior capsular deficiency.8
In traumatic anterior instability, the humeral head comes out anteriorly and inferiorly; the rotator interval always separates as a part of the dislocation. As the interval tears and stretches, force is applied to the subscapularis upper border, which can become lax as it drops inferiorly.8 In addition, the coracohumeral ligament has 2 bands, 1 of which is attached to the subscapularis tendon that may be torn or stretched with anterior shoulder instability. In most cases of Bankart repair, even with complete repair, there may be residual capsule stretching and capsular deficiency left.8 Our deduction from the critical evaluation of the results is that this surgical technique could be a valid option in the treatment of chronic shoulder instability in patients affected by large GBL and capsular insufficiency.
Limitations
This study has several limitations. First, the inclusion criteria that were used to obtain a pure case group resulted in a limited study size. The interobserver reproducibility of the different radiographic measurements was not assessed; consequently, bias may have occurred in the positioning of the bone landmarks. The shape and volume of the precontour graft are an arbitrary standard used for all types of glenoid defects; therefore, it lacks the anatomical morphological precision to be specific for any kind of patient. None of the patients were evaluated using MRI to study the entity and quality of scar tissue surrounding the graft and its relationship with the subscapularis muscle, the scapular neck bone, and the joint capsule. and the upper third of the subscapularis tendon. Finally, there was no control group for the stabilization technique used in this study.
Conclusions
The combination of ASA and bone block procedure with a xenograft was effective in the glenoid reconstruction and restoration of shoulder stability. No radiographic evidence of graft resorption, graft displacement, or glenohumeral arthritis were observed at the 24-month follow-up.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: M.M. reports consulting fees from Arthrex. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Lemmex D., Cárdenas G., Ricks M., Woodmass J., Chelli M., Boileau P. Arthroscopic management of anterior glenoid bone loss. JBJS Rev. 2020;8:e0049. doi: 10.2106/JBJS.RVW.19.00049. [DOI] [PubMed] [Google Scholar]
- 2.Bigliani LU, Newton PM, Steinmann SP, Connor PM, Mcllveen SJ. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med 26:41-45. [DOI] [PubMed]
- 3.Shaha J.S., Cook J.B., Song D.J., et al. Redefining “critical” bone loss in shoulder instability: Functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43:1719–1725. doi: 10.1177/0363546515578250. [DOI] [PubMed] [Google Scholar]
- 4.Burkhart S.S., De Beer J.F. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: Significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16:677–694. doi: 10.1053/jars.2000.17715. [DOI] [PubMed] [Google Scholar]
- 5.Auffarth A., Kralinger F., Resch H. Anatomical glenoid reconstruction via a J-bone graft for recurrent posttraumatic anterior shoulder dislocation. Oper Orthop Traumatol. 2011:23453–23461. doi: 10.1007/s00064-011-0055-5. [DOI] [PubMed] [Google Scholar]
- 6.Boylan M.R., Strauss E.J., Jazrawi L.M., Virk M.S. The Latarjet-Patte procedure: Past, present, and future. Bull Hosp Jt Dis (2013) 2022;80:80–87. [PubMed] [Google Scholar]
- 7.Ropars M., Fournier A., Campillo B., et al. Clinical assessment of external rotation for the diagnosis of anterior shoulder hyperlaxity. Orthop Traumatol Surg Res. 2010;96(8 Suppl):S84–S87. doi: 10.1016/j.otsr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Maiotti M., Russo R., Zanini A., et al. Bankart repair with subscapularis augmentation in athletes with shoulder hyperlaxity. Arthroscopy. 2021;37:2055–2062. doi: 10.1016/j.arthro.2021.01.062. [DOI] [PubMed] [Google Scholar]
- 9.Russo R., Maiotti M., Cozzolino A., et al. Arthroscopic iliac crest bone allograft combined with subscapularis upper-third tenodesis shows a low recurrence rate in the treatment of recurrent anterior shoulder instability associated with critical bone loss. Arthroscopy. 2021;37:824–833. doi: 10.1016/j.arthro.2020.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Russo R., Maiotti M., Taverna E., Rao C. Arthroscopic bone graft procedure combined with arthroscopic subscapularis augmentation for recurrent anterior instability with glenoid bone defect. Arthrosc Tech. 2018;7:e623–e632. doi: 10.1016/j.eats.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong I., John R., Ma J., Coady C.M. Arthroscopic anatomic glenoid reconstruction using distal tibial allograft for recurrent anterior shoulder instability: Clinical and radiographic outcomes. Am J Sports Med. 2020;48:3316–3321. doi: 10.1177/0363546520960119. [DOI] [PubMed] [Google Scholar]
- 12.Degen R.M., Camp C.L., Werner B.C., Dines D.M., Dines J.S. Trends in bone-block augmentation among recently trained orthopaedic surgeons treating anterior shoulder instability. J Bone Joint Surg Am. 2016;98:e56. doi: 10.2106/JBJS.15.01478. [DOI] [PubMed] [Google Scholar]
- 13.Cusinato R., Pacenti M., Martello T., Fattori P., Morroni M., Palù G. Effectiveness of hydrogen peroxide and electron-beam irradiation treatment for removal and inactivation of viruses in equine-derived xenografts. J Virol Methods. 2016;232:39–46. doi: 10.1016/j.jviromet.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Santini S., Barbera P., Modena M., Schiavon R., Bonato M. Equine-derived bone substitutes in orthopedics and traumatology: authors’ experience. Minerva Chir. 2011;66:63–72. [PubMed] [Google Scholar]
- 15.Perrotti V., Nicholls B.M., Horton M.A., Piattelli A. Human osteoclast formation and activity on a xenogenous bone mineral. J Biomed Mater Res A. 2009;90:238–246. doi: 10.1002/jbm.a.32079. [DOI] [PubMed] [Google Scholar]
- 16.Di Stefano D.A., Zaniol T., Cinci L., Pieri L. Chemical, clinical and histomorphometric comparison between equine bone manufactured through enzymatic antigen-elimination and bovine bone made non-antigenic using a high-temperature process in post-extractive socket grafting. A comparative retrospective clinical study. Dent J (Basel) 2019;7:70. doi: 10.3390/dj7030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gungormus M. The effect on osteogenesis of type I collagen applied to experimental bone defects. Dent Traumatol. 2004;20:334–337. doi: 10.1111/j.1600-9657.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu G., Hu Y.Y., Zhao J.N., Wu S.J., Xiong Z., Lu R. Effect of type I collagen on the adhesion, proliferation, and osteoblastic gene expression of bone marrow-derived mesenchymal stem cells. Chin J Traumatol. 2004;7:358–362. [PubMed] [Google Scholar]
- 19.Taverna E., D’Ambrosi R., Perfetti C., Garavaglia G. Arthroscopic bone graft procedure for anterior inferior glenohumeral instability. Arthrosc Tech. 2014;3:e653–e660. doi: 10.1016/j.eats.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Eichinger J.K., Hartshorn T., Zhou H., Matzkin E.G., Warner J.P. A comparison of the lateral decubitus and beach-chair positions for shoulder surgery: Advantages and complications. J Am Acad Orthop Surg. 2015;23:18–28. doi: 10.5435/JAAOS-23-01-18. [DOI] [PubMed] [Google Scholar]
- 21.Maiotti M., Massoni C. Arthroscopic augmentation with subscapularis tendon in anterior shoulder instability with capsulolabral deficiency. Arthrosc Tech. 2013;2:e303–e310. doi: 10.1016/j.eats.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegl U.J., Smith S.D., Todd J.N., Coatney G.A., Wijdicks C.A., Millett P.J. Biomechanical comparison of arthroscopic single- and double-row repair techniques for acute bony Bankart lesions. Am J Sports Med. 2014;42:1939–1946. doi: 10.1177/0363546514532782. [DOI] [PubMed] [Google Scholar]
- 23.Maiotti M., Russo R., Zanini A., Schröter S., Massoni C., Bianchedi D. Arthroscopic Bankart repair and subscapularis augmentation: an alternative technique treating anterior shoulder instability with bone loss. J Shoulder Elbow Surg. 2016;25:898–906. doi: 10.1016/j.jse.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Maiotti M., Massoni C., Russo R., Schroter S., Zanini A., Bianchedi D. Arthroscopic subscapularis augmentation of Bankart repair in chronic anterior shoulder instability with bone loss less than 25% and capsular deficiency: Clinical multicenter study. Arthroscopy. 2017;33:902–909. doi: 10.1016/j.arthro.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Paglia F., Caporlingua A., Armocida D., Rizzo F., Santoro A., D’angelo L. Preoperative 3D volume reconstruction of the posterior wall of the sphenoid sinus with Horos: A free, simple and reliable tool in endoscopic endonasal trans-sphenoidal surgery. Neurocirugia (English Edition) 2022;33:219–226. doi: 10.1016/j.neucie.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Artese L., Piattelli A., Di Stefano D.A., et al. Sinus lift with autologous bone alone or in addition to equine bone: An immunohistochemical study in man. Implant Dent. 2011;20:383–388. doi: 10.1097/ID.0b013e3182310b3d. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y.M., Jiang C.Y., Lu Y., Li F.L., Wu G. Coracoid bone graft resorption after Latarjet procedure is underestimated: a new classification system and a clinical review with computed tomography evaluation. J Shoulder Elbow Surg. 2015;24:1782–1788. doi: 10.1016/j.jse.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 28.Russo R., Maiotti M., Taverna E. Arthroscopic bone graft procedure combined with arthroscopic subscapularis augmentation (ASA) for recurrent anterior instability with glenoid bone defect: a cadaver study. J Exp Orthop. 2018;5:5. doi: 10.1186/s40634-018-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank R.M., Gregory B., O’Brien M., et al. Ninety-day complications following the Latarjet procedure. J Shoulder Elbow Surg. 2019;28:88–94. doi: 10.1016/j.jse.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Griesser M.J., Harris J.D., McCoy B.W., et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: A systematic review. J Shoulder Elbow Surg. 2013;22:286–292. doi: 10.1016/j.jse.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Callegari J.J., McGarry M., Crook L., et al. The addition of remplissage to free bone block restores translation and stiffness compared to bone block alone or Latarjet in a bipolar bone loss model. Arthroscopy. 2022;38:2609–2617. doi: 10.1016/j.arthro.2022.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Melzer C., Manz P., Krödel A., Stürz H. Operative therapy for recurrent shoulder dislocation with special regard to long-term clinical and radiological results using M. Lange technique. Arch Orthop Trauma Surg. 1989;108:107–111. doi: 10.1007/BF00932166. [DOI] [PubMed] [Google Scholar]
- 33.Moroder P., Blocher M., Auffarth A., et al. Clinical and computed tomography-radiologic outcome after bony glenoid augmentation in recurrent anterior shoulder instability without significant glenoid bone loss. J Shoulder Elbow Surg. 2014;23:420–426. doi: 10.1016/j.jse.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 34.Manji R.A., Lee W., Cooper D.K.C. Xenograft bioprosthetic heart valves: Past, present and future. Int J Surg. 2015;23:280–284. doi: 10.1016/j.ijsu.2015.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.