Key Points
Question
What is the association of overweight and obese body mass index (BMI) with posttreatment response, tumor recurrence, and survival outcomes among patients with head and neck cancer who underwent chemoradiotherapy?
Findings
In this cohort study involving 445 patients, both overweight and obese BMI were associated with complete metabolic response after chemoradiotherapy. Only overweight BMI was associated with improved overall survival, progression-free survival, and reduction in locoregional failure.
Meaning
This study suggests that overweight BMI is an independent factor favorably associated with complete metabolic response after chemoradiotherapy, survival, and locoregional failure.
Abstract
Importance
Combined modality therapy, such as chemoradiotherapy, often results in significant morbidity among patients with head and neck cancer. Although the role of body mass index (BMI) varies based on cancer subtypes, its association with treatment response, tumor recurrence, and survival outcomes among patients with head and neck cancer remains unclear.
Objective
To evaluate the role of BMI in treatment response, tumor recurrence, and survival outcomes among patients with head and neck cancer undergoing chemoradiotherapy.
Design, Setting, and Participants
This retrospective, observational, single-institution cohort study conducted at a comprehensive cancer center included 445 patients with nonmetastatic head and neck cancer who underwent chemoradiotherapy from January 1, 2005, to January 31, 2021.
Exposure
Normal vs overweight or obese BMI.
Main Outcomes and Measures
Metabolic response after chemoradiotherapy, locoregional failure (LRF), distant failure (DF), overall survival (OS), and progression-free survival (PFS), with Bonferroni correction used to adjust for multiple comparisons and P < .025 being considered statistically significant.
Results
A total of 445 patients (373 men [83.8%]; median age, 61 years [IQR, 55-66 years]; 107 [24.0%] with normal BMI, 179 [40.2%] with overweight BMI, and 159 [35.7%] with obese BMI) were included for analysis. Median follow-up was 48.1 months (IQR, 24.7-74.9 months). On Cox proportional hazards regression multivariable analysis, only overweight BMI was associated with improved OS (5-year OS, 71.5% vs 58.4%; adjusted hazard ratio [AHR], 0.59 [95% CI, 0.39-0.91]; P = .02) and PFS (5-year PFS, 68.3% vs 50.8%; AHR, 0.51 [95% CI, 0.34-0.75]; P < .001). On logistic multivariable analysis, overweight BMI (91.6% vs 73.8%; adjusted odds ratio [AOR], 0.86 [95% CI, 0.80-0.93]; P < .001) and obese BMI (90.6% vs 73.8%; AOR, 0.89 [95% CI, 0.81-0.96]; P = .005) were associated with complete metabolic response on follow-up positron emission tomography–computed tomography after treatments. On Fine-Gray multivariable analysis, overweight BMI was associated with reduction in LRF (5-year LRF, 7.0% vs 25.9%; AHR, 0.30 [95% CI, 0.12-0.71]; P = .01), but not DF (5-year DF, 17.4% vs 21.5%; AHR, 0.92 [95% CI, 0.47-1.77]; P = .79). Obese BMI was not associated with LRF (5-year LRF, 10.4% vs 25.9%; AHR, 0.63 [95% CI, 0.29-1.37]; P = .24) or DF (5-year DF, 15.0% vs 21.5%; AHR, 0.70 [95% CI, 0.35-1.38]; P = .30).
Conclusion
In this cohort study of patients with head and neck cancer, when compared with normal BMI, overweight BMI was an independent factor favorably associated with complete response after treatments, OS, PFS, and LRF. Further investigations are warranted to improve understanding on the role of BMI among patients with head and neck cancer.
This cohort study evaluates the role of body mass index (BMI) in treatment response, tumor recurrence, and survival outcomes among patients with head and neck cancer undergoing chemoradiotherapy.
Introduction
The prevalence of obesity is anticipated to increase, with nearly 1 in 2 adults having obesity by 2030.1 The prognostic role of body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) may vary based on cancer subtypes.2
Previous meta-analysis and prospective studies showed that both obesity and overweight were associated with worse all-cause mortality,3 cancer-related mortality,4 and the incidence of multiple types of cancer.5 However, while similar findings were noted for breast, ovarian, and colorectal cancer,6,7,8 obesity was a favorable prognostic factor for survival in lung cancer2,9,10 and renal cell carcinoma.2,11
A correlation between BMI and survival among patients with head and neck cancer was not observed in a meta-analysis.2 Combined modality therapies, such as chemoradiotherapy, often result in weight loss with muscle mass depletion, which is associated with poor prognosis.12,13,14,15 The role of BMI in this setting remains unclear. To address these knowledge gaps, we performed an observational cohort study to evaluate the association between BMI and survival outcomes.
Methods
Our study was performed under a protocol approved by the Roswell Park Comprehensive Cancer Center institutional review board. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was reviewed, and our study follows the guideline. The study was conducted in accordance with the Declaration of Helsinki.16 A waiver of consent was obtained from the institutional review board of the Roswell Park Comprehensive Cancer Center due to the retrospective nature of the study making consent impractical and because contacting patients to obtain consent would pose a greater risk than the waiver.
Our retrospective database was queried for patients with head and neck cancer who underwent curative-intent definitive chemoradiotherapy at the Roswell Park Comprehensive Cancer Center between January 1, 2005, and January 31, 2021. Patients were excluded if they underwent surgery or radiotherapy alone, received a diagnosis of metastatic cancer, or had unknown BMI. Patients with low BMI (underweight, <18.5) were also excluded due to a small sample size (n <15).
Body mass index is stratified by normal weight (18.5-24.9), overweight (25.0-29.9), and obese (≥30). Other variables of interest were extracted, including age, self-reported gender, smoking history, Karnofsky performance status, race and ethnicity, number of comorbidities, primary disease site, cancer staging based on the American Joint Committee on Cancer Staging Manual, 7th edition,17 human papillomavirus (HPV) status, and chemotherapy. All missing values were coded as unknown for analysis. Other clinically pertinent variables were not captured in the database, such as treatment-related toxic effects. Race and ethnicity were self-reported, and this information was extracted from the electronic health record. Among patients who self-reported other racial and ethnic backgrounds, they included African American, American Indian or Alaska Native, Asian, Hispanic, and those who were unknown or declined to answer. Such categories were combined as a single group prior to performing our analyses because it would be challenging to show meaningful differences in outcomes due to their small subgroup sample sizes.
The primary end points of our study were overall survival (OS) and progression-free survival (PFS). These outcomes were defined as the time intervals from diagnosis to any death or last follow-up and from diagnosis to tumor progression or any death or last follow-up, respectively. Other end points included metabolic response of disease on positron emission tomography–computed tomography (PET-CT) after completing radiotherapy, locoregional failure (LRF), distant failure (DF), and treatment interruptions, defined as more than 56 days of the radiotherapy treatment course.18,19,20
Statistical Analysis
Comparison of baseline characteristics was performed using the Fisher exact test and the Mann-Whitney test as appropriate. Evaluation of survival outcomes was performed using the Kaplan-Meier method, log-rank tests, and Cox proportional hazards regression multivariable analyses. Logistic multivariable analysis was performed to identify variables associated with posttreatment responses and treatment interruptions. Fine-Gray multivariable analysis was performed to evaluate LRF and DF outcomes with death as a competing event. All multivariable analysis models were constructed using all patient and tumor variables as listed previously.
Propensity score matching was used to reduce selection bias. All baseline characteristics were considered for matching as deemed clinically pertinent. Matching was performed based on the nearest neighbor method in a 1:1 ratio with no replacements and a caliper distance of 0.2.21 Subgroup analyses were also performed to evaluate OS, PFS, LRF, and DF outcomes based on HPV status, which was assessed using p16 status among patients with oropharyngeal cancer.
Bonferroni correction was used to adjust for multiple comparison (normal vs overweight BMI and normal vs obese BMI). All P values were 2-sided, and P < .025 was deemed statistically significant. All statistical analyses were performed using R, version 4.2.1 (R Group for Statistical Computing).
Results
A total of 445 patients (373 men [83.8%]; median age, 61 years [IQR, 55-66 years]; 107 [24.0%] with normal BMI, 179 [40.2%] with overweight BMI, and 159 [35.7%] with obese BMI) met our criteria (Table). Most patients had a good Karnofsky performance status of 90 to 100 (339 [76.2%]) and underwent definitive chemoradiotherapy for oropharyngeal cancer (262 [58.9%]). There were 3 patients (0.7%) with treatment interruptions. Median follow-up was 48.1 months (IQR, 24.7-74.9 months).
Table. Baseline Characteristics.
| Characteristic | Before matching, No. (%) (N = 445) | After matching | |||||
|---|---|---|---|---|---|---|---|
| No. (%) | P value | No. (%) | P value | ||||
| Normal BMI (n = 81) | Overweight BMI (n = 81) | Normal BMI (n = 65) | Obese BMI (n = 65) | ||||
| BMI | |||||||
| Normal (18.5-24.9) | 107 (24.0) | 81 (100.0) | 0 | NA | 65 (100.0) | 0 | NA |
| Overweight (25.0-29.9) | 179 (40.2) | 0 | 81 (100.0) | 0 | 0 | ||
| Obese (≥30) | 159 (35.7) | 0 | 0 | 0 | 65 (100.0) | ||
| Gender | |||||||
| Man | 373 (83.8) | 67 (82.7) | 66 (81.5) | >.99 | 54 (83.1) | 55 (84.6) | >.99 |
| Woman | 72 (16.2) | 14 (17.3) | 15 (18.5) | 11 (16.9) | 10 (15.4) | ||
| Smoker | |||||||
| Never or former | 369 (82.9) | 64 (79.0) | 63 (77.8) | >.99 | 55 (84.6) | 51 (78.5) | .50 |
| Current | 76 (17.1) | 17 (21.0) | 18 (22.2) | 10 (15.4) | 14 (21.5) | ||
| Age, y | |||||||
| <65 | 320 (71.9) | 59 (72.8) | 61 (75.3) | .86 | 45 (69.2) | 50 (76.9) | .43 |
| ≥65 | 125 (28.1) | 22 (27.2) | 20 (24.7) | 20 (30.8) | 15 (23.1) | ||
| KPS | |||||||
| <90 | 106 (23.8) | 22 (27.2) | 22 (27.2) | >.99 | 16 (24.6) | 16 (24.6) | >.99 |
| 90-100 | 339 (76.2) | 59 (72.8) | 59 (72.8) | 49 (75.4) | 49 (75.4) | ||
| Race and ethnicity | |||||||
| White | 390 (87.6) | 72 (88.9) | 67 (82.7) | .37 | 55 (84.6) | 56 (86.2) | >.99 |
| Othera | 55 (12.4) | 9 (11.1) | 14 (17.3) | 10 (15.4) | 9 (13.8) | ||
| No. of comorbidities | |||||||
| 0 | 71 (16.0) | 12 (14.8) | 15 (18.5) | .78 | 11 (16.9) | 9 (13.8) | .88 |
| 1-3 | 272 (61.1) | 51 (63.0) | 47 (58.0) | 39 (60.0) | 42 (64.6) | ||
| >3 | 102 (22.9) | 18 (22.2) | 19 (23.5) | 15 (23.1) | 14 (21.5) | ||
| Primary disease site | |||||||
| Oropharynx | 262 (58.9) | 42 (51.9) | 45 (55.6) | .91 | 35 (53.8) | 38 (58.5) | .69 |
| Larynx | 102 (22.9) | 24 (29.6) | 23 (28.4) | 18 (27.7) | 19 (29.2) | ||
| Other | 81 (18.2) | 15 (18.5) | 13 (16.0) | 12 (18.5) | 8 (12.3) | ||
| T staging | |||||||
| 1-2 | 231 (51.9) | 30 (37.0) | 36 (44.4) | .42 | 27 (41.5) | 30 (46.2) | .72 |
| 3-4 | 214 (48.1) | 51 (63.0) | 45 (55.6) | 38 (58.5) | 35 (53.8) | ||
| N staging | |||||||
| 0 | 87 (19.6) | 18 (22.2) | 20 (24.7) | .81 | 15 (23.1) | 15 (23.1) | >.99 |
| 1 | 44 (9.9) | 13 (16.0) | 9 (11.1) | 6 (9.2) | 6 (9.2) | ||
| 2 | 278 (62.5) | 44 (54.3) | 47 (58.0) | 40 (61.5) | 39 (60.0) | ||
| 3 | 36 (8.1) | 6 (7.4) | 5 (6.2) | 4 (6.2) | 5 (7.7) | ||
| HPV | |||||||
| Negative | 76 (17.1) | 14 (17.3) | 14 (17.3) | >.99 | 12 (18.5) | 8 (12.3) | .68 |
| Positive | 225 (50.6) | 31 (38.3) | 32 (39.5) | 29 (44.6) | 31 (47.7) | ||
| Not available | 144 (32.4) | 36 (44.4) | 35 (43.2) | 24 (36.9) | 26 (40.0) | ||
| Chemotherapy | |||||||
| Cisplatin | 374 (84.0) | 70 (86.4) | 70 (86.4) | >.99 | 54 (83.1) | 56 (86.2) | .81 |
| Other | 71 (16.0) | 11 (13.6) | 11 (13.6) | 11 (16.9) | 9 (13.8) | ||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HPV, human papillomavirus; KPS, Karnofsky performance status; NA, not available.
Among patients who self-reported other racial and ethnic backgrounds, they included African American, American Indian or Alaska Native, Asian, Hispanic, and those who were unknown or declined to answer.
On Cox proportional hazards regression multivariable analysis (eTable 1 in Supplement 1), overweight BMI was associated with improved OS (5-year OS, 71.5% vs 58.4%; adjusted hazard ratio [AHR], 0.59 [95% CI, 0.39-0.91]; P = .02) and PFS (5-year PFS, 68.3% vs 50.8%; AHR, 0.51 [95% CI, 0.34-0.75]; P < .001). Obese BMI was not associated with either OS (AHR, 0.62 [95% CI, 0.39-0.98]; P = .04) or PFS (AHR, 0.66 [95% CI, 0.44-0.99]; P = .04). On logistic multivariable analysis (eTable 2 in Supplement 1), having overweight BMI (91.6% vs 73.8%; adjusted odds ratio [AOR], 0.86 [95% CI, 0.80-0.93]; P < .001) and obese BMI (90.6% vs 73.8%; AOR, 0.89 [95% CI, 0.81-0.96]; P = .005) were associated with complete metabolic response on follow-up PET-CT after treatments. Given the small number of treatment interruptions seen in our cohort, there was no association between BMI and treatment interruptions (overweight BMI: AOR, 1.00 [95% CI, 0.98-1.03]; P = .69; obese BMI: AOR, 0.99 [95% CI, 0.97-1.01]; P = .43).
On Fine-Gray multivariable analysis (eTable 3 in Supplement 1), overweight BMI was associated with a reduction in LRF (5-year LRF, 7.0% vs 25.9%; AHR, 0.30 [95% CI, 0.12-0.71]; P = .01) but not DF (5-year DF, 17.4% vs 21.5%; AHR, 0.92 [95% CI, 0.47-1.77]; P = .79). Obese BMI was not associated with LRF (5-year LRF, 10.4% vs 25.9%; AHR, 0.63 [95% CI, 0.29-1.37]; P = .24) or DF (5-year DF, 15.0% vs 21.5%; AHR, 0.70 [95% CI, 0.35-1.38]; P = .30).
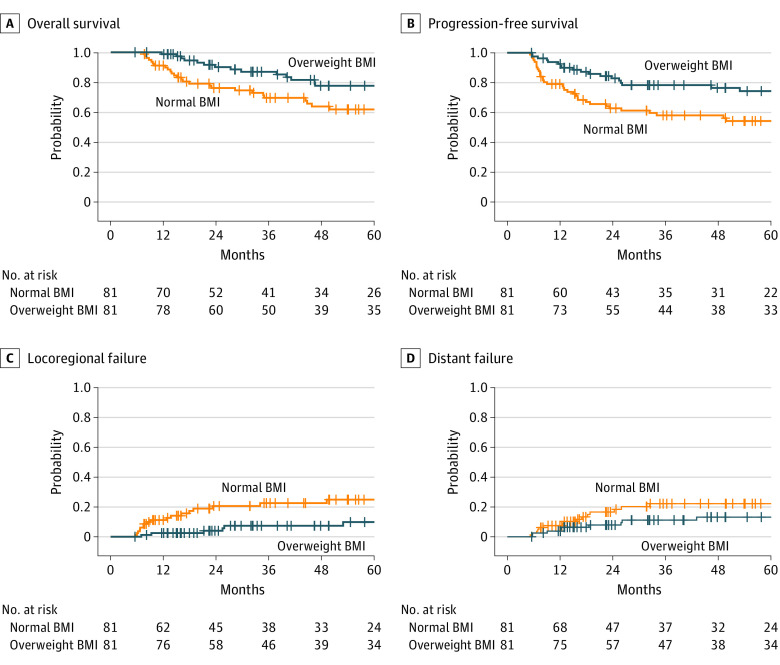
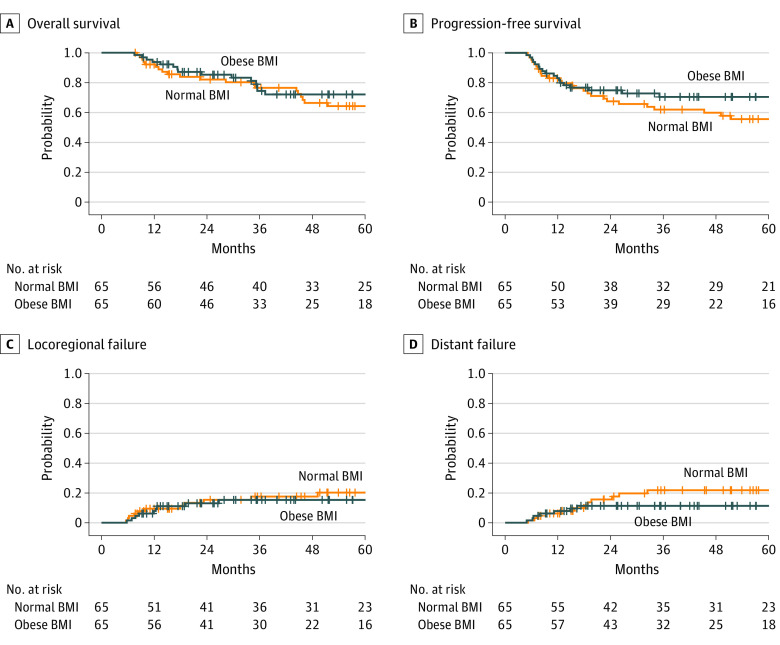
After propensity score matching, 81 matched pairs were identified for normal vs overweight BMI, and 65 matched pairs were identified for normal vs obese BMI. All baseline characteristics were well balanced (Table). Outcomes similar to the multivariable analysis were observed for overweight BMI (OS: AHR, 0.43 [95% CI, 0.24-0.77]; P = .004; PFS: AHR, 0.42 [95% CI, 0.25-0.71]; P = .001; LRF: AHR, 0.35 [95% CI, 0.15-0.82]; P = .02; DF: AHR, 0.66 [95% CI, 0.30-1.47]; P = .31) (Figure 1) and obese BMI (OS: AHR, 0.66 [95% CI, 0.36-1.22]; P = .18; PFS: AHR, 0.61 [95% CI, 0.35-1.08]; P = .09; LRF: AHR, 0.77 [95% CI, 0.33-1.78]; P = .53; DF: AHR, 0.59 [95% CI, 0.23-1.48]; P = .26) (Figure 2).
Figure 1. Kaplan-Meier and Cumulative Incidence Curves for Overall Survival, Progression-Free Survival, Locoregional Failure, and Distant Failure for Overweight vs Normal Body Mass Index (BMI) After Propensity Score Matching.
Figure 2. Kaplan-Meier and Cumulative Incidence Curves for Overall Survival, Progression-Free Survival, Locoregional Failure, and Distant Failure for Obese vs Normal Body Mass Index (BMI) After Propensity Score Matching.
On subgroup analysis, 76 patients with oropharyngeal cancer were identified for p16-negative, and 225 patients with oropharyngeal cancer were identified for p16-positive cases. When Cox and Fine-Gray multivariable analyses were repeated, the only statistically significant outcome was LRF for those with overweight BMI (AHR, 0.02 [95% CI, 0.001-0.29]; P = .005) (eTable 4 in Supplement 1).
Discussion
To our knowledge, this is the largest study involving patients in the US treated with chemoradiotherapy for head and neck cancer that evaluated the role of BMI as a factor associated with survival, treatment response, and tumor recurrence outcomes. Overweight BMI and obese BMI were associated with complete metabolic response on follow-up PET-CT; however, only overweight BMI was an independent factor favorably associated with improved OS and PFS and a reduction in LRF. No association for OS and BMI was observed among HPV-positive patients.
Our finding of an association of overweight BMI with improved survival is consistent with a growing body of literature suggesting a higher BMI as a favorable prognostic factor.22,23,24,25,26,27,28,29 However, obese BMI was not associated with OS in our study. This finding is consistent with several reports,30,31,32 whereas other studies have reported survival benefits associated with obese BMI.22,23,24,25,28,29 Such discrepancies may be due to a nonlinear association between BMI and survival, with the highest survival seen in the overweight BMI range.33,34
To our knowledge, this is the first report for head and neck cancer to show that overweight BMI and obese BMI are associated with complete metabolic response on follow-up PET-CT. Our finding is consistent with another study suggesting a higher likelihood of pathologic complete response in rectal cancer among patients with obese BMI,35 whereas it is inconsistent with other studies suggesting that obese BMI is adversely associated with pathologic complete response in breast and rectal cancers.36,37,38,39
Reasons for this complex association may be multifactorial. Although obese BMI has been associated with worse postoperative complications,31,40 chronic inflammation for tumor development,41 and reduced antitumor immune response,42 several studies have suggested that obese BMI is a nutrient reserve to overcome toxic effects from combined modality therapies,33 which may be associated with improved LRF24 and DF.25,26 Such a complex interplay may explain the conflicting association between treatment-associated weight loss and survival for patients with head and neck cancer.15,27,43,44 This interplay may also explain the variations seen in markers for systematic inflammation,45 such as the neutrophil-lymphocyte ratio.46 Further complicating matters, studies have suggested that BMI alone may not be representative of one’s body fat composition and cachexia.47,48 Another quantitative measure correlated with BMI is skeletal muscle depletion measured based on CT imaging, which has been shown to be associated with worse survival24,49 and quality of life50 among patients with head and neck cancer.
In our study, BMI was not associated with survival outcomes among HPV-positive patients, consistent with prior studies.32,51 Although a few other studies have suggested that a higher BMI is associated with improved survival,41 they also included patients with an underweight BMI as a reference group, which was previously shown to be associated with worse survival outcomes.33,34 Although a lack of association between BMI, HPV, and survival in our study may be due to smaller subgroup sample sizes, interaction among these variables warrants further investigation. For example, despite adipose tissue–promoting pathways, including PI3K-PTEN-Akt-mTOR and Ras-Raf-MAPK associated with HPV-associated head and neck cancers,52 patients with a high BMI were more likely to have greater treatment-related weight loss44,53 associated with changes in tumor microenvironment and inflammation that may potentiate treatments.54
Limitations
Our retrospective study has inherent limitations. In our study, BMI was analyzed as a categorical variable with 3 different strata (normal, overweight, and obese) instead of as a continuous variable. The association between BMI and survival outcomes has been previously shown to be complex and nonlinear,33,34 and there may be more clinically pertinent, model-derived BMI cutoffs associated with clinical outcomes. Our BMI variable was also collected at a single time point, and our analysis did not include dynamic changes in BMI prior to the diagnosis of head and neck cancer, during chemoradiotherapy, or after the completion of all treatments. Such changes may be more clinically pertinent in prognosticating clinical outcomes than a single measure of BMI. In addition, only 40% to 45% of patients with overweight BMI and obese BMI were matched, suggesting that our matched cohort may not be representative of our overall cohort. However, our findings from the matched cohorts were consistent with those from the overall cohort. Other clinical outcomes, such as toxicity profiles, were unavailable for analysis. Furthermore, our findings may not be generalizable for other patient cohorts who underwent surgery, induction systemic therapy, or radiotherapy alone.
Conclusions
Our cohort study suggests that overweight BMI is an independent, favorable factor associated with complete response after treatments, OS, PFS, and LRF. Further investigations are warranted to improve our understanding on the role of BMI among patients with head and neck cancer.
eTable 1. Cox Multivariable Analysis for Overall and Progression-Free Survival
eTable 2. Logistic Multivariable Analysis for Post-treatment Response
eTable 3. Fine-Gray Multivariable Analysis for Locoregional and Distant Failure
eTable 4. Cox and Fine-Gray Multivariable Analysis for Survival and Tumor Recurrence Outcomes Stratified by p16 Status
Data Sharing Statement
References
- 1.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 2.Petrelli F, Cortellini A, Indini A, et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e213520. doi: 10.1001/jamanetworkopen.2021.3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. doi: 10.1136/bmj.i2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625-1638. doi: 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 5.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901-1914. doi: 10.1093/annonc/mdu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2012;5(7):901-910. doi: 10.1158/1940-6207.CAPR-12-0048 [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Liu J, Wang X, Li M, Gan Y, Tang Y. Association of obesity and overweight with overall survival in colorectal cancer patients: a meta-analysis of 29 studies. Cancer Causes Control. 2014;25(11):1489-1502. doi: 10.1007/s10552-014-0450-y [DOI] [PubMed] [Google Scholar]
- 9.Li S, Wang Z, Huang J, et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the “obesity paradox” really exist? Eur J Cardiothorac Surg. 2017;51(5):817-828. [DOI] [PubMed] [Google Scholar]
- 10.Shen N, Fu P, Cui B, Bu CY, Bi JW. Associations between body mass index and the risk of mortality from lung cancer: a dose-response PRISMA-compliant meta-analysis of prospective cohort studies. Medicine (Baltimore). 2017;96(34):e7721. doi: 10.1097/MD.0000000000007721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagheri M, Speakman JR, Shemirani F, Djafarian K. Renal cell carcinoma survival and body mass index: a dose-response meta-analysis reveals another potential paradox within a paradox. Int J Obes (Lond). 2016;40(12):1817-1822. doi: 10.1038/ijo.2016.171 [DOI] [PubMed] [Google Scholar]
- 12.Hua X, Liu S, Liao JF, et al. When the loss costs too much: a systematic review and meta-analysis of sarcopenia in head and neck cancer. Front Oncol. 2020;9:1561. doi: 10.3389/fonc.2019.01561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson W, Alexander N, Schipper M, Fig L, Feng F, Jolly S. Characterization of changes in total body composition for patients with head and neck cancer undergoing chemoradiotherapy using dual-energy x-ray absorptiometry. Head Neck. 2014;36(9):1356-1362. [DOI] [PubMed] [Google Scholar]
- 14.Jager-Wittenaar H, Dijkstra PU, Vissink A, et al. Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head Neck. 2011;33(6):863-870. doi: 10.1002/hed.21546 [DOI] [PubMed] [Google Scholar]
- 15.Langius JA, Bakker S, Rietveld DH, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer. 2013;109(5):1093-1099. doi: 10.1038/bjc.2013.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. Springer; 2010. [Google Scholar]
- 18.McCloskey SA, Jaggernauth W, Rigual NR, et al. Radiation treatment interruptions greater than one week and low hemoglobin levels (12 g/dL) are predictors of local regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2009;32(6):587-591. doi: 10.1097/COC.0b013e3181967dd0 [DOI] [PubMed] [Google Scholar]
- 19.Platek ME, McCloskey SA, Cruz M, et al. Quantification of the effect of treatment duration on local-regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Head Neck. 2013;35(5):684-688. doi: 10.1002/hed.23024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rades D, Stoehr M, Kazic N, et al. Locally advanced stage IV squamous cell carcinoma of the head and neck: impact of pre-radiotherapy hemoglobin level and interruptions during radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(4):1108-1114. doi: 10.1016/j.ijrobp.2007.07.2380 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arthur AE, Peterson KE, Rozek LS, et al. ; UM Head and Neck SPORE Program . Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. Am J Clin Nutr. 2013;97(2):360-368. doi: 10.3945/ajcn.112.044859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaudet MM, Patel AV, Sun J, et al. Prospective studies of body mass index with head and neck cancer incidence and mortality. Cancer Epidemiol Biomarkers Prev. 2012;21(3):497-503. doi: 10.1158/1055-9965.EPI-11-0935 [DOI] [PubMed] [Google Scholar]
- 24.Grossberg AJ, Chamchod S, Fuller CD, et al. Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol. 2016;2(6):782-789. doi: 10.1001/jamaoncol.2015.6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hicks DF, Bakst R, Doucette J, et al. Impact of obesity on outcomes for patients with head and neck cancer. Oral Oncol. 2018;83:11-17. doi: 10.1016/j.oraloncology.2018.05.027 [DOI] [PubMed] [Google Scholar]
- 26.Hollander Dd, Kampman E, van Herpen CM. Pretreatment body mass index and head and neck cancer outcome: a review of the literature. Crit Rev Oncol Hematol. 2015;96(2):328-338. doi: 10.1016/j.critrevonc.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 27.Karnell LH, Sperry SM, Anderson CM, Pagedar NA. Influence of body composition on survival in patients with head and neck cancer. Head Neck. 2016;38(suppl 1):E261-E267. doi: 10.1002/hed.23983 [DOI] [PubMed] [Google Scholar]
- 28.McRackan TR, Watkins JM, Herrin AE, et al. Effect of body mass index on chemoradiation outcomes in head and neck cancer. Laryngoscope. 2008;118(7):1180-1185. doi: 10.1097/MLG.0b013e31816fca5c [DOI] [PubMed] [Google Scholar]
- 29.Pai PC, Chuang CC, Tseng CK, et al. Impact of pretreatment body mass index on patients with head-and-neck cancer treated with radiation. Int J Radiat Oncol Biol Phys. 2012;83(1):e93-e100. doi: 10.1016/j.ijrobp.2011.11.071 [DOI] [PubMed] [Google Scholar]
- 30.Iyengar NM, Kochhar A, Morris PG, et al. Impact of obesity on the survival of patients with early-stage squamous cell carcinoma of the oral tongue. Cancer. 2014;120(7):983-991. doi: 10.1002/cncr.28532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Sun L, Sun L. Influence of body mass index on survival and prognosis in squamous cell carcinoma of head and neck. Cancer Manag Res. 2020;12:3203-3210. doi: 10.2147/CMAR.S249775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan X, Nelson HH, Langevin SM, et al. Obesity and head and neck cancer risk and survival by human papillomavirus serology. Cancer Causes Control. 2015;26(1):111-119. doi: 10.1007/s10552-014-0490-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56. doi: 10.1007/s11912-016-0539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H, Ma SJ, Farrugia M, et al. Machine learning incorporating host factors for predicting survival in head and neck squamous cell carcinoma patients. Cancers (Basel). 2021;13(18):4559. doi: 10.3390/cancers13184559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SY, Kim CH, Kim YJ, Kwak HD, Ju JK, Kim HR. Obesity as an independent predictive factor for pathologic complete response after neoadjuvant chemoradiation in rectal cancer. Ann Surg Treat Res. 2019;96(3):116-122. doi: 10.4174/astr.2019.96.3.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Cosimo S, Porcu L, Agbor-Tarh D, et al. Effect of body mass index on response to neo-adjuvant therapy in HER2-positive breast cancer: an exploratory analysis of the NeoALTTO trial. Breast Cancer Res. 2020;22(1):115. doi: 10.1186/s13058-020-01356-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karatas F, Erdem GU, Sahin S, et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2017;32:237-244. doi: 10.1016/j.breast.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 38.Park IJ, You YN, Skibber JM, et al. Oncologic and functional hazards of obesity among patients with locally advanced rectal cancer following neoadjuvant chemoradiation therapy. Am J Clin Oncol. 2017;40(3):277-282. doi: 10.1097/COC.0000000000000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Zhang S, Yee D, et al. Impact of body mass index on pathological complete response following neoadjuvant chemotherapy in operable breast cancer: a meta-analysis. Breast Cancer. 2021;28(3):618-629. doi: 10.1007/s12282-020-01194-w [DOI] [PubMed] [Google Scholar]
- 40.Deneuve S, Tan HK, Eghiaian A, Temam S. Management and outcome of head and neck squamous cell carcinomas in obese patients. Oral Oncol. 2011;47(7):631-635. doi: 10.1016/j.oraloncology.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 41.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421-449. doi: 10.1146/annurev-pathol-012615-044359 [DOI] [PubMed] [Google Scholar]
- 42.Ringel AE, Drijvers JM, Baker GJ, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183(7):1848-1866. doi: 10.1016/j.cell.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghadjar P, Hayoz S, Zimmermann F, et al. ; Swiss Group for Clinical Cancer Research (SAKK) . Impact of weight loss on survival after chemoradiation for locally advanced head and neck cancer: secondary results of a randomized phase III trial (SAKK 10/94). Radiat Oncol. 2015;10:21. doi: 10.1186/s13014-014-0319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han HR, Hermann GM, Ma SJ, et al. Matched pair analysis to evaluate weight loss during radiation therapy for head and neck cancer as a prognostic factor for survival. Ann Transl Med. 2021;9(10):914. doi: 10.21037/atm-20-4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murad LD, Silva TQ, Schilithz AOC, Monteiro MC, Murad LB, Fialho E. Body mass index alters the predictive value of the neutrophil-to-lymphocyte ratio and systemic inflammation response index in laryngeal squamous cell carcinoma patients. Nutr Cancer. 2022;74(4):1261-1269. doi: 10.1080/01635581.2021.1952447 [DOI] [PubMed] [Google Scholar]
- 46.Ma SJ, Yu H, Khan M, et al. Evaluation of optimal threshold of neutrophil-lymphocyte ratio and its association with survival outcomes among patients with head and neck cancer. JAMA Netw Open. 2022;5(4):e227567. doi: 10.1001/jamanetworkopen.2022.7567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539-1547. doi: 10.1200/JCO.2012.45.2722 [DOI] [PubMed] [Google Scholar]
- 48.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629-635. doi: 10.1016/S1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 49.Shaver AL, Platek ME, Singh AK, et al. Effect of musculature on mortality, a retrospective cohort study. BMC Cancer. 2022;22(1):688. doi: 10.1186/s12885-022-09751-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaver AL, Noyes K, Ochs-Balcom HM, et al. A retrospective cohort study of myosteatosis and quality of life in head and neck cancer patients. Cancers (Basel). 2021;13(17):4283. doi: 10.3390/cancers13174283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anantharaman D, Billot A, Waterboer T, et al. Predictors of oropharyngeal cancer survival in Europe. Oral Oncol. 2018;81:89-94. doi: 10.1016/j.oraloncology.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 52.Chung CH, Guthrie VB, Masica DL, et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann Oncol. 2015;26(6):1216-1223. doi: 10.1093/annonc/mdv109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nourissat A, Bairati I, Samson E, et al. Predictors of weight loss during radiotherapy in patients with stage I or II head and neck cancer. Cancer. 2010;116(9):2275-2283. doi: 10.1002/cncr.25041 [DOI] [PubMed] [Google Scholar]
- 54.O’Flanagan CH, Smith LA, McDonell SB, Hursting SD. When less may be more: calorie restriction and response to cancer therapy. BMC Med. 2017;15(1):106. doi: 10.1186/s12916-017-0873-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Cox Multivariable Analysis for Overall and Progression-Free Survival
eTable 2. Logistic Multivariable Analysis for Post-treatment Response
eTable 3. Fine-Gray Multivariable Analysis for Locoregional and Distant Failure
eTable 4. Cox and Fine-Gray Multivariable Analysis for Survival and Tumor Recurrence Outcomes Stratified by p16 Status
Data Sharing Statement