Abstract
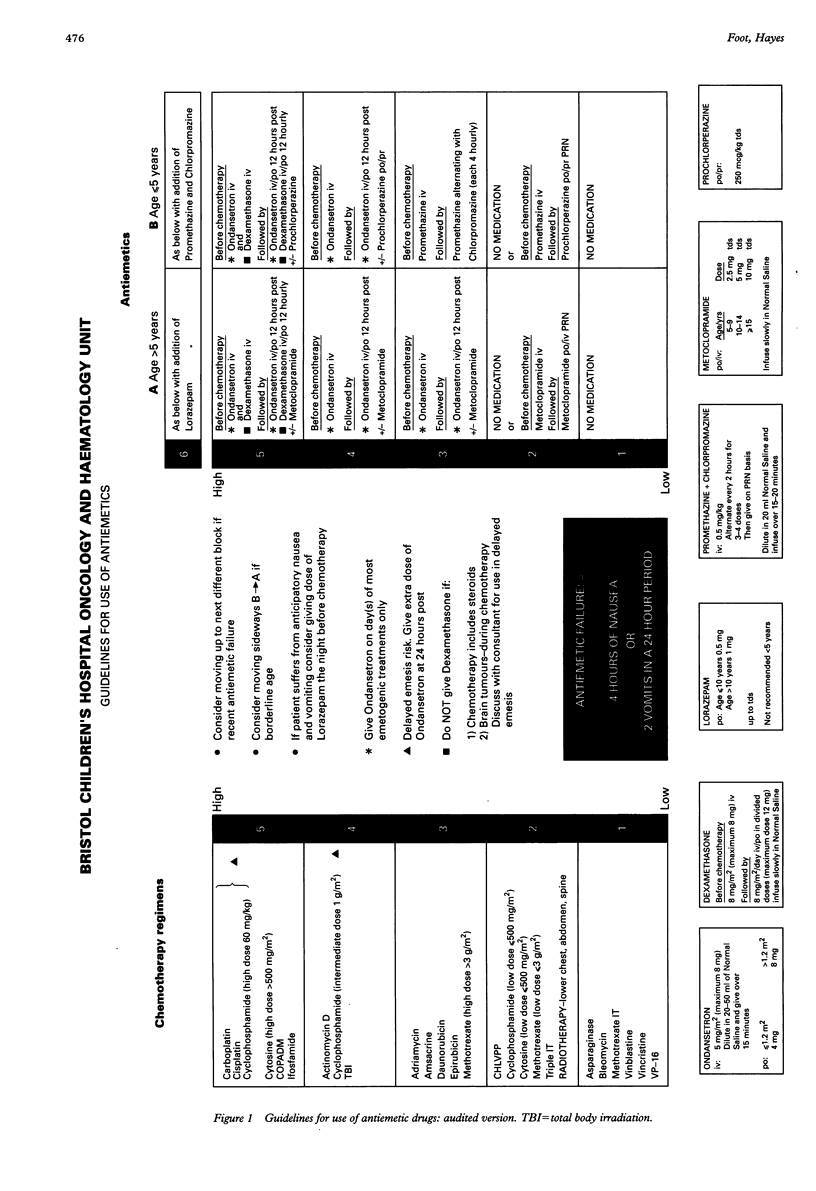
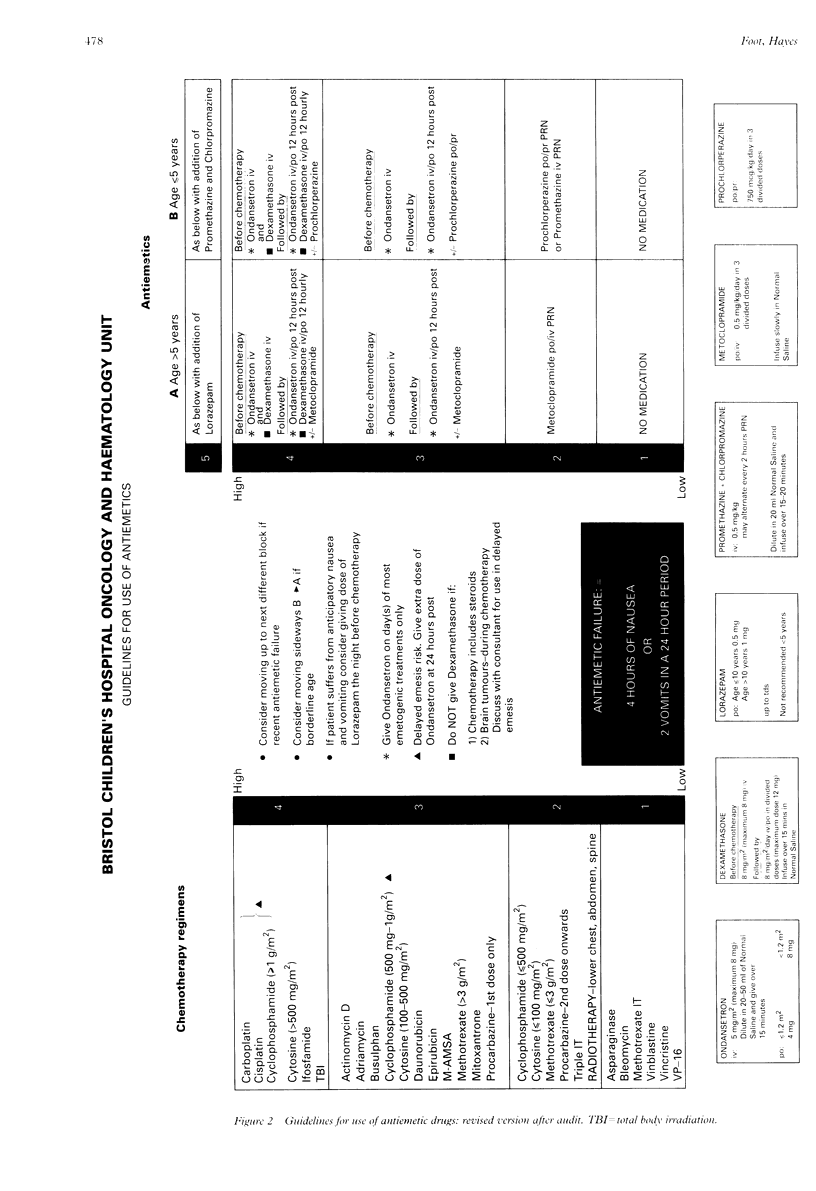
A system was devised to establish the optimum treatment for emesis for each individual child receiving cytotoxic treatment. Cytotoxic drugs were ranked on a scale (1-5), with antiemetic regimens correspondingly graded. An age division (< or = 5 years, > 5 years) was included. Cytotoxic treatment was given with co-administration of the parallel antiemetic regimen. Failure to control emesis required administration of a stronger regimen as defined in the guidelines. A prospective clinical audit was performed to monitor the efficacy and utility of the system using diary cards to record episodes of nausea or vomiting, or both, completed by the patient or a parent and the nursing staff. The following audit criteria were set: (a) 80% control with first courses of chemotherapy; (b) 85% control with subsequent courses of similar chemotherapy; and (c) 90% lack of anticipatory nausea. Sixty children (< 18 years) received emetogenic cytotoxic drugs from February-June 1993. The criteria were satisfied in two of three categories, with 82% control for first courses of chemotherapy, 83% control for subsequent courses of chemotherapy, and 90% lack of anticipatory nausea. The guidelines were workable and acceptable overall. Minor modifications have been made subsequent to the audit to improve their efficacy further.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. C., Gralla R., Reilly L., Kellick M., Young C. Metoclopramide: dose-related toxicity and preliminary antiemetic studies in children receiving cancer chemotherapy. J Clin Oncol. 1985 Aug;3(8):1136–1141. doi: 10.1200/JCO.1985.3.8.1136. [DOI] [PubMed] [Google Scholar]
- Grunberg S. M., Hesketh P. J. Control of chemotherapy-induced emesis. N Engl J Med. 1993 Dec 9;329(24):1790–1796. doi: 10.1056/NEJM199312093292408. [DOI] [PubMed] [Google Scholar]
- Hewitt M., Cornish J., Pamphilon D., Oakhill A. Effective emetic control during conditioning of children for bone marrow transplantation using ondansetron, a 5-HT3 antagonist. Bone Marrow Transplant. 1991 Jun;7(6):431–433. [PubMed] [Google Scholar]
- Mehta P., Gross S., Graham-Pole J., Gardner R. Methylprednisolone for chemotherapy-induced emesis: a double-blind randomized trial in children. J Pediatr. 1986 May;108(5 Pt 1):774–776. doi: 10.1016/s0022-3476(86)81066-6. [DOI] [PubMed] [Google Scholar]
- Pinkerton C. R., Williams D., Wootton C., Meller S. T., McElwain T. J. 5-HT3 antagonist ondansetron--an effective outpatient antiemetic in cancer treatment. Arch Dis Child. 1990 Aug;65(8):822–825. doi: 10.1136/adc.65.8.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Newlands E. S., Rustin G. J., Begent R. H., Howells N., McQuade B., Bagshawe K. D. Comparison of ondansetron and ondansetron plus dexamethasone as antiemetic prophylaxis during cisplatin-containing chemotherapy. Lancet. 1991 Aug 24;338(8765):487–490. doi: 10.1016/0140-6736(91)90555-4. [DOI] [PubMed] [Google Scholar]
- Terrin B. N., McWilliams N. B., Maurer H. M. Side effects of metoclopramide as an antiemetic in childhood cancer chemotherapy. J Pediatr. 1984 Jan;104(1):138–140. doi: 10.1016/s0022-3476(84)80613-7. [DOI] [PubMed] [Google Scholar]
- van Hoff J., Hockenberry-Eaton M. J., Patterson K., Hutter J. J. A survey of antiemetic use in children with cancer. Am J Dis Child. 1991 Jul;145(7):773–778. [PubMed] [Google Scholar]


