Abstract
Cold physical plasma (CPP) technology is of high promise for various medical applications.
The interplay of specific components of physical plasma with living cells, tissues and organs on a structural and functional level is of paramount interest with the aim to induce therapeutic effects in a controlled and replicable fashion.
In contrast to other medical disciplines such as dermatology and oromaxillofacial surgery, research reports on CPP application in orthopaedics are scarce.
The present implementation of CPP in orthopaedics involves surface modifications of orthopaedic materials and biomaterials to optimize osseointegration. In addition, the influence of CPP on musculoskeletal cells and tissues is a focus of research, including possible adverse reactions and side effects. Its bactericidal aspects make CPP an attractive supplement to current treatment regimens in case of microbial inflammations such as periprosthetic joint infections. Attributed anticancerogenic and pro-apoptotic effects underline the clinical relevance of CPP as an additive in treating malignant bone lesions.
The present review outlines ongoing research in orthopaedics involving CPP; it distinguishes considerations for safe application and the need for more evidence-based research to facilitate robust clinical implementation.
Keywords: cold physical plasma, antimicrobial therapy, cancer therapy, plasma medicine, reactive oxygen species, skeletal regeneration, surface modification
Introduction
Plasma medicine has been investigated as a new branch for cold physical plasma (CPP) applications. Physical plasma can be defined as an aggregate state of matter with an overall quasineutral charge, comprised of different components such as motile electrons, ionized atoms and molecules, all interacting collectively due to long-range coulomb forces. The generation of physical plasma involves the ionization and excitation of a working gas such as argon, helium, oxygen or ambient air by energizing its atoms or molecules utilizing thermal, chemical, electrical or radiative sources. The characteristics of physical plasma vary greatly as a function of parameters such as gas type, energy source, surrounding pressure and electrode set-up that all influence its physiochemical traits.
Physical plasma medicine is focused on the interplay of specific plasma components with cells, tissues and organs on a structural and functional level. The consensus today is that the main therapeutic effects of CPP are mediated by reactive oxygen and nitrogen species (ROS/RNS), electric field generation and subsequent secondary reactions of these processes, placing the field of plasma medicine at the heart of redox biology (1, 2, 3). CPP is generated under atmospheric pressure conditions and temperatures at or below 40°C (near body temperature) and is of high promise for medical applications. Due to the relatively low temperatures CPP operates at, it is considered not to harm cells and tissues while being beneficial regarding cell proliferation, differentiation and migration, depending on the dose or concentration applied (4).
In the following, we briefly summarize the present medical applications of CPP in general and outline the possible approaches in orthopaedics. We discuss CPP antimicrobial aspects in the light of clinical challenges in orthopaedics, such as periprosthetic joint infections (PJIs). In addition, the utilization of CPP in the treatment of bone malignancies is discussed. Furthermore, we summarize possible surface modifications of orthopaedic materials and biomaterials by CPP and describe the influence of CPP on musculoskeletal cells and tissues, including associated adverse reactions and side effects.
Cold physical plasma: devices for experimental and clinical application
In medical settings, CPP is most commonly generated using plasma jet technologies that are of the dielectric barrier discharge (DBD) or non-DBD type (5). DBDs are characterized by the CPP discharge between an electrode insulated by a dielectric material and a ground electrode. To generate normal pressure CPP, the discharge gap lies within a range of 0.1–10 mm (6). Despite the existence of different DBD set-ups, floating electrode DBDs are primarily used for experimental and biomedical applications in vivo. Here, the second electrode is not grounded as it is represented by the organism treated (e.g. human) and is therefore called floating electrode (7). This enables excellent spatial and temporal precision in CPP irradiation of large treatment areas with high concentrations of generated plasma components within a small space. Both DBD and jet systems can utilize ambient air for plasma generation but can also be operated using noble gases. DBD treatment requires close proximity and a fixed distance to the target area, whereby flat and smooth surfaces receive an even plasma discharge distribution (6). Hence, in dermal pathologies, for instance, large-area chronic wounds benefit from larger DBDs, whereas small and cavity-rich injuries benefit from precision plasma jets.
An interesting aspect of plasma technology is its tunability by modifying the gas composition supplied to the source (8). In plasma jet devices, the working gas flows through an electric field for CPP ignition, and the resulting plasma effluent carries the reactive CPP particles to the treatment area (Fig. 1). Thus, CPP generation takes place at a greater distance and is being electrophysically independent of the target area, with only negligible currents passing through the treated body part (9). Nevertheless, this conclusion may not be generalized, as the generation of electric fields at the target has been demonstrated for various set-ups. The electric field profile is influenced by the electrophysical characteristics of the target and the treatment regime (10). Depending on the electrode configuration within these pen-like devices, dielectric free electrode jets, DBD jets, DBD-like jets and single-electrode jets can be distinguished (11, 12). The noble gases helium and argon in their pristine state or as mixtures with other gases such as nitrogen or oxygen are usually employed as working gases. Plasma jets can be used to treat surface areas as well as deeper tissue layers and visceral cavities (13).
Figure 1.
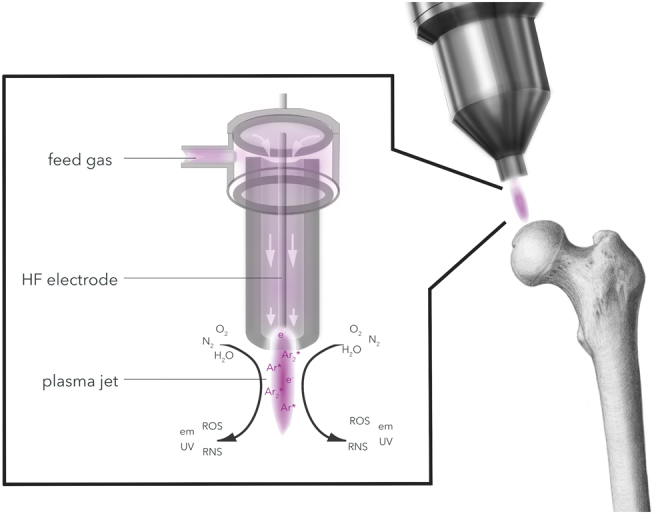
CPP is generated by adding energy to a neutral gas to yield a partially ionized gas that can be operated at or below body temperature (<40°C). In pen-like CPP devices, the transition of a neutral feeding gas (e.g. argon) through a strong electric field (HF electrode) results in the discharge of ions (Ar*) and electrons (e–). The interaction with ambient air species (O2, N2 and H2O) leads to the generation of bioreactive short-living oxygen species (ROS). Side products of this complex physiochemical process are low amounts of ultraviolet (UV) and electromagnetic (em) radiation.
Currently, a limited number of plasma devices are commercially available that have been CE-certified and recognized as medical devices for medical, not cosmetic, purposes through the EU Medical Devices Regulation (MDR 2017/745): kINPen MED plasma jet (neoplas med, Greifswald, Germany), SteriPlas plasma torch (ADTEC, Hunslow, UK), PlasmaDerm DBD (CINOGY Technologies, Duderstadt, Germany) and the battery-operated PlasmaCare DBD (terraplasma medical, Garching, Germany) (14). These CPP devices feature a comprehensive physical and biochemical characterization accompanied by safety analyses for in vivo applications (15). Despite the rather low number of commercially available equipment, a rising number of applications in clinical research are reported for CPP devices (16).
To characterize CPP devices in a more comprehensive and stringent fashion, the German DIN SPEC 91315 was established in 2014. The combination of biological, chemical and physical tests aims to standardize CPP device characterization while also creating minimal conditions of application safety (17). However, CPP device constructions and set-ups display great heterogeneity, especially in experimental practice, attributed to parameters like different gas flow rates, gas mixtures and discharge voltages, which ultimately hinders comparison of treatment regimens and observed biological effects (18). In addition to direct treatment by CPP, the use of plasma-treated liquids allows for so-called indirect treatments. Specifically, this means that a liquid is exposed to the plasma processes, allowing the entry of gas phase-generated ROS into the liquid, where the plentiful short-lived ROS/RNS react to a few relatively stable species that are in principle suitable for clinical application (19). In experimental research using plasma-treated liquids, primarily cell culture media or saline solutions were used, especially in the context of anticancer research (20). Due to a lack of consensus on the generation of these liquids at pharmaceutical quality, parameters such as sterility, ROS/RNS stability, storability, plasma source and management implementations hamper the translation of such experimental approaches to clinical settings, like musculoskeletal applications. In addition, clinical studies are not available. This is also true for direct CPP treatments (potentially, with specific adjustments for implementation) in orthopaedics and trauma surgery.
Cold physical plasma: an overview of applications in medicine
In medicine, the majority of clinical plasma applications are in the field of dermatology, especially to promote the healing of chronic wounds and ulcers (21). Here, the antimicrobial properties of CPP appear advantageous, as chronic wounds are a widespread problem, affecting about 2% of the population worldwide, and are caused by venous stasis, diabetes and peripheral arterial occlusive disease. Such ulcers usually present a medical challenge since proper healing is impaired in up to 15% of cases (22). In addition, bacterial superinfection can impair consolidation. First clinical trials applying CPP to chronic ulcers showed promising results by accelerating healing while simultaneously reducing the bacterial wound load (23). However, a recent randomized controlled clinical trial comparing plasma treatment against an antiseptic agent still showed superior responses in plasma-treated wound patients, suggesting plasma-mediated redox effects to be at least in part responsible for the clinical effects observed (21, 22). In addition to chronic wound healing, CPP was also shown to successfully stimulate healing in second- and third-degree burns, dog bites and probands with laser-ablated skin wounds (24, 25, 26).
Aside from the treatment of wounds, CPP has also proven to be successful for the treatment of split skin draft donor and recipient sites, in the context of surgical site infections (SSIs), in cardiac surgery, in the palliative treatment of head and neck cancer and as an additive treatment for metastatic colon cancer (27). The clinical relevance of CPP as an additive treatment option in cancer therapies was also demonstrated in a clinical study investigating CPP effects on squamous cell carcinoma of the oropharynx (28).
Due to the numerous properties of CPP, its application is also explored for other medical areas. These include but are not restricted to dentistry, general surgery, aesthetic medicine, the treatment of pigmentation disorders and ophthalmology (27). It therefore appears only consistent to explore the utilization of CPP in other medical disciplines. In orthopaedics, infection of wounds or implant sites is the main problem following surgical treatment. Here, CPP has great potential to be of use in clinical routine.
Antimicrobial cold physical plasma: towards orthopaedic indications
SSIs are serious complications in any surgical field and range among the leading causes of healthcare-associated infections worldwide. They represent a significant concern in terms of patient morbidity and mortality. Furthermore, SSIs constitute a financial burden to healthcare systems and negatively impact the patients’ quality of life.
In the presence of foreign body implants, such as joint replacements, the diagnostics and treatment of such infections is particularly difficult, and PJIs are known as a major complication after joint replacement. Periprosthetic infection rates range between 1.4% and 2.5% after primary joint arthroplasties (29) and up to 4% following revision arthroplasties (30). Current therapeutic strategies include debridement, antibiotic treatment and implant retention combined with an exchange of modular components for acute infections or removal of the infected prosthesis in either single-stage or two-stage revision for chronic situations (31). Although early diagnostics and treatment have improved over the last decade, PJIs are still considered one of the most unpredictable complications in need of an interdisciplinary therapeutic approach (32).
The known antimicrobial properties of CPP offer a novel approach to the high demand for enhancing antimicrobial strategies in orthopaedics and trauma surgery (33) (Fig. 2). Albeit the underlying mechanisms for the antimicrobial effects of CPP are still not fully understood, it is hypothesized that plasma discharges on the target area result in the accumulation of ROS/RNS along with immediate changes in cell membrane integrity, followed by leakage of intracellular microbial products (34). At equimolar concentrations, higher ROS levels are preferentially toxic to prokaryotes since eukaryotic cells generally have more elaborate antioxidant defence systems. In addition, ROS serve as signalling molecules and are part of physiological responses in cells in tissues (35).
Figure 2.
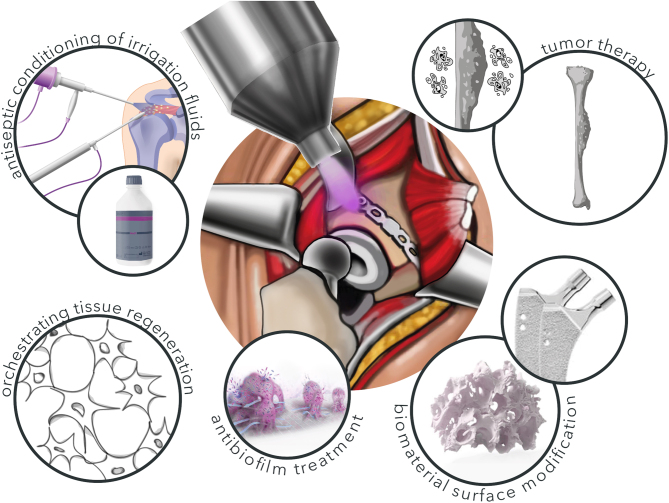
Possible CPP applications in musculoskeletal medicine. Due to modifications of treatment parameters, CPP can be utilized in several different clinical applications in musculoskeletal medicine. These include skeletal tumour therapy, biomaterial modification and cell regenerative approaches at lower treatment intensity. Besides this, CPP´s antiseptic properties are promising for the antibiofilm treatment in septic surgery and the conditioning of antiseptic irrigation fluids, e.g. in arthroscopy.
The antimicrobial plasma effects are of particular interest in the context of biofilm-mediated infections that are especially resistant to host defence and antimicrobial agents (36). Biofilms are aggregated communities of bacteria adherent to a living or non-living surface embedded within a matrix of self-produced or acquired extracellular polymeric substances (EPS), including polysaccharides, DNA, protein and lipids (37). While there is an increasing antimicrobial resistance crisis due to a lack of an effective antibiotic discovery pipeline (38), novel biofilm-specific strategies are desperately needed (30). Regarding the prevention of biofilms, previously mentioned methods of CPP-based surface modifications of implants have shown to minimize the adhesion as well as biofilm formation rate of bacteria without any negative impact regarding biocompatibility or osseointegration (39, 40).
With regard to its therapeutic potential, CPP was already proven in vitro to inactivate biofilms developed by different pathogens such as Enterococcus spp. (41), Staphylococcus epidermidis (42), Staphylococcus aureus (43), Klebsiella pneumoniae (44), Acinetobacter baumannii (45), Pseudomonas aeruginosa (46), Escherichia coli (42), Listeria monocytogenes (47), Salmonella typhimurium (47) and Candida albicans (48). Moreover, CPP was shown to facilitate the eradication of biofilms formed by multidrug-resistant bacteria in chronic wounds (49). The underlying mechanisms are yet to be fully understood. First studies suggest two distinct but parallel modes of action to mediate CPP anti-biofilm activity. First, the diffusion and penetration of plasma-derived ROS into the biofilm matrices cause damage to the bacterial cells. Second, a chemical break down of the EPS due to a physical etching effect leads to biofilm detachment from the solid substratum surface (43, 50). Unlike antibiotic therapy, CPP treatment of biofilms showed no development of resistance in bacterial strains so far (51). In general, CPP application shows a more efficient biofilm inactivation of gram-negative bacteria than gram-positive bacteria, correlating with the thicker cell wall of the latter (51). By contrast, a study investigating 194 clinical isolates of multidrug-resistant bacteria following in vitro plasma treatment found gram-positive bacteria to be more sensitive than gram-negative bacteria for a DBD device, while there was no difference between both populations when using a prototype of the plasma jet kINPen (52). Further research is necessary to determine the ideal plasma configurations for the clinical practicability of these reported effects.
Currently, the practical utilization of CPP is limited to topical applications. The intraoperative usage of CPP is technically possible, yet clinicians seem hesitant since standardized protocols for the sterile implementation of existing devices are currently not available.
As mentioned earlier, most clinical CPP application studies focus on healing chronic wounds (16). The combination of antimicrobial characteristics and the stimulation of tissue regeneration make CPP an attractive adjuvant for orthopaedic treatment concepts (11, 21). Wounds in orthopaedic and trauma surgery are commonly iatrogenic or acute. There are no studies investigating the direct application of CPP on postoperative sutured wounds. It has already been shown in an in vitro set-up that CPP treatment did not alter the mechanical properties of suture materials while showing promising results regarding the prevention and eradication of S. aureus and E. coli contamination (53). Further studies are necessary to determine the benefit of CPP applications to patients with traumatic wounds or wounds with healing disorders after sutures. Adapted DBD devices like the Plasmaderm dressing show promising first results in the context of such acute wound healing (54).
The antimicrobial properties of CPP imply the capability to be a cost-effective tool in future therapeutic regimes of orthopaedic and traumatology, especially in the case of PJI. Thus, CPP treatments have the potential to improve antimicrobial therapies in musculoskeletal surgery. Nevertheless, further clinical research is needed to tap the full therapeutic potential of CPP in case of musculoskeletal infections.
Cold physical plasma in bone cancer therapy
Osteosarcoma (OS) represents the most common primary malignancy of the skeleton, with an annual incidence of 4.4 per million in the general population (55). With an incidence of about 3 per million for each entity, Ewing’s sarcoma (ES) and chondrosarcoma (CS) are two more common malignant bone tumours usually diagnosed in childhood and adolescence.
Undesirable side effects and the lack of full effectiveness of conventional therapies require the development of new treatment approaches (56). As mentioned earlier, the introduction of CPP to oncology has shown promising results for a variety of tumour types in recent years (57). Till present, there are several potential mechanisms of plasma-mediated anticancer effects, albeit, given the heterogeneity of tumour types and genetics, an overall generalization seems not plausible (58). On the non-molecular level, the analysis of growth kinetics of different cancer cell lines revealed that the inhibition of cell proliferation is based on the direct reduction of initial cell numbers during CPP treatment and the long-term growth inhibition of remaining cells (59). In addition, CPP treatment is hypothesized to induce cancer cell apoptosis mediated by mitochondria, death receptors and the endoplasmic reticulum (60) as the result of an intracellular increase of ROS for which various cancers are known to be receptive (61). Another theory is that CPP induced singlet oxygen (1O2) formation that may contribute to the intracellular activation of apoptosis-associated signalling cascades (62), albeit the lifetimes of singlet oxygen in liquid or organic matrices are likely too short to be responsible for such an effect (63). Moreover, cells that are stressed or already harmed, for example, by chemotherapy, secrete several factors summarized as damage-associated molecular patterns (DAMPs). By interaction with defined receptors, DAMPs are known to result in sterile inflammation processes that subsequently evoke an immunomodulated anticancer response (64), which has been recently proven in vivo (65). In addition, evidence suggests that CPP can also restore cell sensitivity to chemotherapeutic agents, as demonstrated in breast cancer (66) and glioma cells (67). CPP also induces the suppression of integrin-dependent pathways involved in radio- and chemo-resistance in cancerogenic cells of different origins (68). Therefore, CPP exposure seems to be a promising approach for the additive treatment of OS, ES and CS, at least from what can be concluded from in vitro studies (69, 70, 71). As described for other cancer types, the mode of action elicited by CPP treatment of bone sarcoma cells is based on the disruption of cell membrane integrity and subsequent intracellular accumulation of ROS (69, 71, 72). In addition, it is reported that CPP induces changes in peroxiredoxin expression and the activation of p53 in OS cells (72). The growth-inhibiting effect of CPP in OS, ES and CS results from apoptosis, which is usually followed by cellular condensation and fragmentation (72). CPP exposure of OS cells also affects the gene expression of interleukins and inhibits angiogenic vascular endothelial growth factor (VEGF) (73). This indicates a possible influence of CPP on the tumour microenvironment. Other studies also showed that CPP application suppresses angiogenesis and thus may negatively influence tumour growth and metastasis (74). The CPP OS anticancer effects depend on plasma device parameters such as feed gas, gas flow rates and supply voltages, as well as on the distance from the liquid surface, exposure time and the type of cancer being treated (72).
Although it has been demonstrated in animal models that direct topical CPP treatment in vivo can reduce tumour growth (75, 76), osseous tumours are mostly inaccessible to direct local CPP treatment. Similar to applications that aim to treat biofilms, indirect CPP treatment through plasma-treated liquids is particularly interesting. Plasma-treated liquids induce apoptosis of sarcoma cells (70), but the effectiveness observed is lower when compared to direct CPP treatment. It was shown that plasma-treated liquids induce mitochondrial network aberrations, disruption of endoplasmic-mitochondrial calcium homeostasis, autophagy and cell death in various OS cell lines (77, 78). Furthermore, activation of resistance pathways against oxidative stress and inhibition of relevant kinases such as 5' adenosine monophosphate-activated protein kinase or signal transducer and activator of transcription 3 in OS cells were demonstrated in vitro following CPP exposure (79). Further, in vitro studies also suggested selective cytotoxicity of indirect CPP treatment in OS cells over mesenchymal stromal cells (MSCs) (79) and other less malignant cells (70). As mentioned earlier, it is assumed that the selectivity of CPP on cancerous over non-cancerous cells is based on the higher sensitivity of malignant cells to oxidants (80). However, this assumption does not regard the different responses of sarcoma cell subpopulations to ROS and RNS. The only study investigating the effects of CPP on actual tumours of murine organotypic OS ex vivo cultures demonstrated reduced tumour cell viability following treatment (81).
In summary, CPP treatment of skeletal sarcomas results in significant anti-oncogenic effects by inhibiting cell growth which is mediated by the impairment of cell membrane functionality and induction of apoptosis. Therefore, CPP applications could be a promising addition to existing therapies (Fig. 2). Nevertheless, the treatment of skeletal sarcomas using CPP needs to be further characterized, especially in vivo.
Cold physical plasma-induced surface modifications of orthopaedic materials
A promising application of CPP in orthopaedics is the functionalization of implant surfaces (39). The main alterations induced by CPP or thermal physical plasma treatment of implant materials are a modified surface roughness (82), i.e. an increased surface area, altered chemical speciation by oxidation of the implant material surface (83) and additive coatings (40). Major goals of physical plasma-induced alterations of orthopaedic implant materials are to enhance osseointegration to minimize the risk of implant loosening (84) and to inhibit bacterial biofilm formation, ultimately reducing the incidence of peri-implant infections (33) (Fig. 2).
Generally, thermal plasma spraying is a well-established manufacturing process and the main plasma-based method for additive surface modifications of orthopaedic implant materials. Implant materials modified by CPP-assisted coating deposition methods are not yet in clinical use but have gained popularity in implant material research in recent years. A major advantage of CPP-based additive manufacturing processes is that they allow for the coating of orthopaedic and dental materials with thermally instable materials, pharmaceuticals and even biomolecules (40, 85). Plasma material surface activation can be considered as the yielding process step since plasma activation leads to higher binding affinity of the respective coating material. The process steps of plasma activation and coating are carried out either sequentially or simultaneously. Biological effects induced by CPP-assisted coating are attributed to the applied bioactive molecules or to a synergistic effect of coating and plasma modification of the surface (86, 87). Biological effects mediated by CPP-assisted coating with biomimetic molecules have mainly been demonstrated in vitro. They include increased functionality and differentiation potential of primary MSCs and mesenchymal cell lines on various implant materials. Human bone marrow-derived MSCs (hBM-MSCs), in particular, are the focus of therapies addressing bone regeneration due to easy accessibility during standard orthopaedic interventions and their high affinity to differentiate into the osteoblastic lineage. Therefore, their interaction with implant materials is of paramount interest (88).
CPP-assisted coating with Arg–Gly–Asp (RGD) peptide sequences on titanium alloys and polylactide acid (PLA) was shown to result in increased adhesion and proliferation of MSCs (87) and human OS cells (MG-63) (89). CPP-assisted coatings of titanium–aluminium–vanadium (TiAlV) alloys with human collagen I also showed promising in vitro results since CPP-assisted coating was superior to coating by absorption, as demonstrated by subsequent analyses of MSC adhesion, proliferation and osteogenic differentiation (90). In vivo experiments demonstrated that CPP-assisted coating with bovine collagen I leads to improved fixation of titanium screws in bone, indicated by an increase of necessary removal force (91). Enhanced chondrogenic differentiation of MSCs was demonstrated after CPP-assisted coating of nanofibrous poly(ε-caprolactone) (PCL) scaffolds with chondroitin sulphate (92). Moreover, CPP-assisted coating of PCL nanofibers with gelatin led to increased cell adhesion and proliferation of MSCs (93). The possibility of coating with thermally instable materials also opens up new promising opportunities for coating with antimicrobial substances. By applying CPP, titanium was successfully coated with catechol/quinone groups to immobilize dispersin B that is known to possess antibiofilm properties (94). Moreover, guanidine coating of ultrahigh-molecular-weight polyethylene (UHMWPE) assisted by CPP showed antimicrobial activity against E. coli and S. aureus (95).
Another strategy for modifying implant surfaces, particularly suggested in experimental dentistry, is direct surface treatment with CPP without additive surface modification (39, 40). Direct treatment with CPP aims to improve biocompatibility, promote early wound healing and enhance osseointegration through increased osteoblast (OB) function and antimicrobial modification in terms of reduced potential for biofilm formation. Preclinical studies focusing on in vitro biocompatibility of CPP-modified implant materials are mostly performed by culturing musculoskeletal cells on the respective material. In vitro studies demonstrated improved biocompatibility of titanium and zirconium surfaces of implants by CPP treatment.
It has been shown that cellular proliferation, adhesion, spreading and osteogenic differentiation can be positively influenced by CPP treatment of implant surfaces, while these effects are predominantly attributed to plasma-enhanced wettability (82, 87, 96). Helium-based CPP treatment of non-metallic biomaterials such as PLA scaffolds also increased the adhesion and proliferation of MSCs and OBs (97). Argon-based CPP treatment of various synthetic bone grafts showed increased protein absorption and cell adhesion of murine OBs (98). An important step for biocompatibility assessment is the evaluation of possible inflammatory effects induced by CPP-based surface modification since the innate immune system plays an important role in the early phases of foreign body response (99). The numerous in vitro studies on CPP-modified biocompatibility of mesenchymal cells consequently led to in vivo studies showing promising results. Improved osseointegration was demonstrated by increased bone-to-implant contact and bone area fraction occupancy at the radius diaphysis after treatment of pristine and calcium phosphate-coated titanium with argon-based CPP in a canine model (83, 100). Histomorphometric analyses also revealed increased bone-to-implant contact and an enhanced proportion of newly formed bone adjacent to zirconium–titanium implants treated with argon-based CPP in canine mandibles (101). Comparable results were obtained following the implantation of argon-based CPP-treated dental titanium implants in the frontal bone of miniature pigs (102). Treatment of titanium with an argon/oxygen-based CPP showed both osteoinductive effects in vitro and increased osseointegration in the rat jaw compared to untreated titanium (103). The aforementioned in vivo studies did not report any adverse peri-implant side effects. A further goal of treating orthopaedic implant materials with CPP is the improvement of tribological properties. It was shown that UHMWPE reinforced with multi-walled carbon nanotubes exhibited optimized tribological properties when treated with argon-based CPP (104).
In addition to improved mechanical properties and osseointegration following CPP treatment, material modification to impart antimicrobial or antibiofilm properties to metallic biomaterials represents another important approach. Anti-biofilm surface modifications by direct CPP treatment have been suggested, particularly in experimental and pre-clinical studies. Most studies focusing on the antimicrobial effects of CPP have a dental background, i.e. aiming to reduce the adherence of oral bacteria or to eliminate oral biofilms on titanium and zirconia surfaces (82, 105, 106). However, several studies have also focussed on the elimination of bacteria relevant for orthopaedic surgery and PJIs. It was reported that air-based CPP could inactivate E. coli and S. aureus generated biofilms on materials commonly used in orthopaedic surgery, such as stainless steel, UHMWPE and TiAlV alloys (107). Treatment of titanium surfaces with a clinical CPP device was superior to treatment with a clinical erbium-doped yttrium–aluminium–granat laser device regarding antibiofilm activity (108). Furthermore, the combination of CPP treatment and mechanical cleaning is a promising approach for the decontamination of colonized implant surfaces (109).
Generally, CPP-assisted coating with bioactive molecules is a promising approach for biomaterial research and should be pursued further. The perspective to generate functionalized implants through CPP, especially the coating with biomolecules, will not only open up new avenues but also become an integral part of orthopaedic biomaterial research.
Cold physical plasma – a promising tool in skeletal regeneration
Since CPP is considered safe in general and largely biocompatible, a rising number of studies investigate its potential in skeletal regeneration. Bone healing and bone formation during remodelling are characterized by a continuous process of destruction, resorption and reformation of extracellular matrix mediated by MSCs, OBs and osteoclasts.
First studies investigating the influence of CPP on bone marrow, periodontal MSCs and adipose-derived MSCs (AD-MSCs) show stimulating effects regarding proliferation and differentiation, therefore indicating CPP as promising in the context of therapies aiming to enhance bone regeneration (110, 111, 112). The in vitro treatment of AD-MSCs with a helium-driven CPP device resulted in a slightly higher proliferation rate with no DNA-damaging effects evident when compared to controls (110). Similarly, helium CPP treatment of hBM-MSCs nearly doubled their proliferation rate, leading to an increased expression of surface markers associated with pluripotency and stemness, thereby underlining CPP´s potential regarding therapeutic approaches in regenerative medicine (113). However, similar to already mentioned applications, these effects appear to be dependent on exposure time, experimental set-up and treatment conditions, as demonstrated, for example, in periodontal ligament MSCs. Therefore, successful therapeutic application requires careful consideration and fine-tuning of these parameters (111, 114).
CPP treatment of MSCs favours the induction of an osteogenic phenotype. It increases cellular ALP activity levels (111) and leads to an upregulation of ß-catenin, runt-related transcription factor 2 and bone morphogenetic protein 2 expression (112) that are all markers for early osteogenic differentiation. Furthermore, CPP treatment increases the deposition of mineralized extracellular matrix (112). The CPP-induced osteogenic effects appear to be independent of the distance between the device and cells, as demonstrated by Eggers et al. utilizing a DBD plasma device, although these results are questionable in the case of plasma jets (115). This underlines the feasibility of CPP devices for clinical applications (116). Analyses of key apoptotic markers such as p53, apoptotic protease-activating factor 1 and caspases 3 and 9 in OB-like cells had been shown to be downregulated after CPP treatment in vitro. Additionally, CPP treatment leads to changes in cell morphology characteristic for increased cell migration. It was therefore concluded that CPP exhibits beneficial properties to support hard tissue repair (117).
Besides bone formation, osteoclastic bone resorption is of particular importance in bone homeostasis (118). Osteoclastogenesis is driven by the receptor interaction of receptor activator of nuclear factor κB (RANK) and its’ ligand (RANKL) (119). Indeed, it is known that ROS are involved in osteoclastogenesis and also act as intracellular messengers in RANK/RANKL signalling (120). In a rodent model of periodontitis, it was described that a 2 min CPP treatment of alveolar bone leads to a decrease in RANKL and an increase in expression of the osteoclastogenesis inhibitor osteoprotegerin (OPG) thereby influencing the balance between bone formation and degradation (121). Shimatani et al. analysed CPP-mediated bone regeneration of large ulna defects on the foreleg of rabbits. Using a helium-driven plasma jet, the direct CPP treatment for 10 min significantly increased new bone formation within the defects compared to untreated control animals. Therefore, the authors suggested that CPP treatment may enhance bone healing capacity and reduces the rate of non-unions (122).
In summary, the current literature on in vitro data underlines CPPs’ potential as a powerful novel tool to aid skeletal regeneration. Yet, till now, the number of applications in animal models and clinical trials is still scarce. Consequently, further studies are essential to comprehensively investigate the underlying mechanism of CPP-enhanced bone formation to evaluate potential clinical application in orthopaedic and trauma surgery.
Adverse effects and side effects related to cold physical plasma
ROS are widely recognized for their dual role as they can be either harmful or beneficial in living systems which strongly depends on dosage and route as well as chronicity of exposure (123). Bone remodelling relies on a close equilibrium between bone formation and resorption that can be influenced by ROS (124). At physiologically low concentrations, ROS mediate intracellular signalling pathways that stimulate cell proliferation, osteogenic differentiation (125) and osteoclastic formation, thus influencing bone remodelling (126). High concentrations of ROS, however, were demonstrated to elicit detrimental effects in many diseases, including bone pathologies (121, 127). High ROS concentrations negatively affect bone formation by promoting osteoclastogenesis (128) while inhibiting osteoblastogenesis (129), thereby shifting the balance in tissue homeostasis towards bone resorption. It was shown that high concentrations of hydrogen peroxide could negatively affect osteogenic differentiation and viability in the M3C3-T1 osteoblastic cell line (130). The cytotoxic side effects of ROS seem contradictory to studies underlining CPP biocompatibility and safety in rodent models and humans (131, 132). However, it should be noted that CPP applications are often single events, lasting only for a few seconds or minutes, with most ROS being generated quickly reacting with biomolecules thereafter. Accordingly, single CPP treatment of oral mucosa in mice for up to 1 min showed only transient and mild inflammatory reactions accompanied by focal superficial ulcerations and necrosis, which had been completely regenerated and re-epithelialized after seven days (133). Long-term risk assessment of monthly repeated oral CPP treatments performed in mice over 1 year (murine equivalent to 60 human years) was well tolerated, and no carcinogenesis was evident in a total of 450 mice included in the study. With regard to invasive lesions generated experimentally by repeated exposure to the carcinogen DBP, kINPen plasma treatment even reduced the number of animals with invasive tumours, suggesting potential protection from malignancy formation (134).
Similar results were reported for applications in humans. CCP exposure of laser-induced skin lesions revealed no cancerous effects in an 5-year follow-up while favourably affecting proliferation and wound healing (26). This underlines not only the short-term but also the long-term safety of direct tissue treatment with plasma jet technologies. Two clinical trials report on CPP treatment of patients suffering from advanced squamous cell carcinoma of the head and neck region. Only mild and temporary reactions of malaise, exhaustion and dry mouth-like symptoms were observed for the treatment of different dermatological conditions with impaired wound healing and superinfection. Therefore, plasma jet applications can be considered safe in the case of direct tissue contact that is not prone to drying effects induced by the feed gas (135).
Yet, CPP also has the potential to damage and kill cells as a function of exposure time and working gas, which can be employed for disinfection or sterilization procedures. As mentioned earlier, anticancer therapies focus on CPP-mediated induction of cellular apoptosis that can also affect healthy cells (136). CPP effectively induces the DNA-damage response as frequently seen by γH2A.X phosphorylation across several cell types and plasma sources, but this is a consequence of oxidative stress signalling and apoptosis rather than direct DNA damage (137). A variety of studies using protocols qualified by the Organization for Economic Co-operation and Development (OECD) have failed to find increased genotoxicity, such as micronucleus formation, following CPP treatment (138, 139). Nevertheless, defining CPP dosing to correlate tissue effects is mandatory in plasma medicine (140). For regenerative in vitro approaches, biocompatible CPP doses were described to range from a few seconds/cm2 to 2 min/cm2 depending on single or repetitive application (114, 138, 141). Within this range, no mutagenic effects are evident, as shown for keratinocytes in vitro (138). Applied to primary human BM-MSCs for 30 s/cm2, CPP treatment was shown to be biocompatible while conserving BM-MSC functionality (114). Clearly, the biocompatibility of CPP treatment regimens is often determined by temporal duration. This definition does not consider specific exposure regimes and gas compositions influencing the complex ROS/RNS-mediated tissue interactions (142, 143). Consequently, Cheng et al. introduced the concept of equivalent total oxidation potentials (ETOPs) to reliably define an applied CPP dose and its biological effects.
In the case of orthopaedic relevant cell types, the treatment of BM-MSCs with plasma-treated liquids was shown to be more capable compared to direct CPP exposure in vitro (144). Moreover, helium gas mixtures were advantageous in treating MSCs in vitro. Such studies on modifying CPP´s treatment regimens indicate that gas mixture, dosimetry, exposure time and administration procedure need pre-considerations to achieve the treatment goals that include maintaining overall tissue integrity (114, 145).
Taken together, there is no solid and experimentally robust evidence, e.g. based on OECD-accredited experimental protocols and cell lines, that suggests genotoxic effects of gas plasma treatment. Nevertheless, the deposition of ROS induces oxidative stress, which can trigger inflammation and DNA-damage responses as part of routine biological reflexes to supraphysiological ROS levels. Indeed, such risk profiling, including long-term in vivo studies, yet need to be performed in orthopaedic applications to qualify plasma technology also for those indications in the long term. Such considerations must be supported by in vitro studies that utilize relevant musculoskeletal cell sources to facilitate the proper translation of CPP-based technologies into routine clinical applications in orthopaedic and trauma surgery.
Discussion and future perspective
Considering the array of requirements for treatment regimens, it is unlikely that a single solution will suit all indications mentioned in the field of orthopaedics. Even though the development or modification of a CPP device could be beneficiary, it will be decisive to adhere to established standards to ensure comparability of future research. Regarding orthopaedic and traumatology necessities, a plasma device with the capability to treat the intramedullary cavity could draw special interest. Moreover, an indirect application via plasma-treated liquids could be a useful tool in arthroscopic as well as in septic revision surgery. Despite promising results in a variety of orthopaedic indications ranging from implant material modification over tissue regeneration up to bone cancer and antimicrobial therapies, CPP applications are not commonly implicated as a treatment option in orthopaedics and traumatology yet. The German Association of Oral and Maxillofacial Surgery published a guideline for the therapeutic use of CPP (S2k-Level, AWMF Registry No. 007–107, February 2022). However, other bodies such as the German Society for Orthopaedics and Trauma Surgery did not contribute to the development of the guideline. Therefore, well-designed translational studies are needed to further corroborate and also validate the positive effects of CPP treatment reported in vitro. With the overall aim to introduce CPP therapies in orthopaedics, linking profound understanding of the underlying mechanisms of CPP with tissue viability, cytotoxicity and possible adverse effects could additionally improve the therapeutic potential. For instance, due to promising preclinical data on the CPP-based surface modification of implant materials that lead to enhanced bone ingrowth in a reproducible manner, this approach appears qualified to undergo the next step in the translational workflow. Nevertheless, pending questions regarding side effects like possible changes in implant longevity or corrosion resistance and the release of metal ions and nanoparticles need to be answered. Also, in relation to skeletal regeneration, additional fundamental research is needed to solidify CPP as a sound therapeutic option. PJIs are still one of the most serious complications of modern arthroplasty. Thus, innovative therapies are needed urgently. Considering CPP´s broad antimicrobial properties, irrigation with plasma-treated liquids may be implemented in treatment protocols of PJIs, if further studies validate a standardized and safe utilization of CPP.
Conclusion
Taken together, CPP has proven to be a beneficial and cost-effective treatment option for various applications and shows great potential in musculoskeletal medicine. While well established in dermatology and oral and maxillofacial surgery, further research is necessary to explore the potential feasibility of CPP in orthopaedic and trauma surgery.
ICMJE conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The authors acknowledge the support for the Article Processing Charge from the DFG (German Research Foundation, 393148499) and the Open Access Publication Fund of the University of Greifswald. J.S. receives the Domagk Master Class (DMC) scholarship funded by the University Medicine Greifswald.
References
- 1.Obradović BM Ivković SS & Kuraica MM. Spectroscopic measurement of electric field in dielectric barrier discharge in helium. Applied Physics Letters 200892 191501. ( 10.1063/1.2927477) [DOI] [Google Scholar]
- 2.Babaeva NY & Kushner MJ. Intracellular electric fields produced by dielectric barrier discharge treatment of skin. Journal of Physics. Part D 201043 185206. ( 10.1088/0022-3727/43/18/185206) [DOI] [Google Scholar]
- 3.Vijayarangan V Delalande A Dozias S Pouvesle J-M Pichon C & Robert E. Cold atmospheric plasma parameters investigation for efficient drug delivery in HeLa cells. IEEE Transactions on Radiation and Plasma Medical Sciences 20172109–115. ( 10.1109/TRPMS.2017.2759322) [DOI] [Google Scholar]
- 4.Tan F Fang Y Zhu L & Al-Rubeai M. Controlling stem cell fate using cold atmospheric plasma. Stem Cell Research and Therapy 202011 368. ( 10.1186/s13287-020-01886-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weltmann KD Kindel E von Woedtke T Hähnel M Stieber M & Brandenburg R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure and Applied Chemistry 2010821223–1237. ( 10.1351/PAC-CON-09-10-35) [DOI] [Google Scholar]
- 6.Brandenburg R. Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Science and Technology 201726. ( 10.1088/1361-6595/aa6426) [DOI] [Google Scholar]
- 7.Fridman G Shereshevsky A Jost MM Brooks AD Fridman A Gutsol A Vasilets V & Friedman G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chemistry and Plasma Processing 200727163–176. ( 10.1007/s11090-007-9048-4) [DOI] [Google Scholar]
- 8.Matthes R Bekeschus S Bender C Koban I Hübner NO & Kramer A. Pilot-study on the influence of carrier gas and plasma application (open resp. delimited) modifications on physical plasma and its antimicrobial effect against Pseudomonas aeruginosa and Staphylococcus aureus. GMS Krankenhaushygiene Interdisziplinär 20127Doc02. ( 10.3205/dgkh000186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter J Brandenburg R & Weltmann K. Atmospheric pressure plasma jets: an overview of devices and new directions. Plasma Sources Science and Technology 201524 064001. ( 10.1088/0963-0252/24/6/064001) [DOI] [Google Scholar]
- 10.Stancampiano A Chung T-H Dozias S Pouvesle J-M Mir LM & Robert E. Mimicking of human body electrical characteristic for easier translation of plasma biomedical studies to clinical applications. IEEE Transactions on Radiation and Plasma Medical Sciences 20194335–342. ( 10.1109/TRPMS.2019.2936667) [DOI] [Google Scholar]
- 11.von Woedtke T Emmert S Metelmann H-R Rupf S & Weltmann K-D. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Physics of Plasmas 202027 070601. ( 10.1063/5.0008093) [DOI] [Google Scholar]
- 12.Lu X Laroussi M & Puech V. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Science and Technology 201221 034005. ( 10.1088/0963-0252/21/3/034005) [DOI] [Google Scholar]
- 13.Decauchy H Pavy A Camus M Fouassier L & Dufour T. Cold plasma endoscopy applied to biliary ducts: feasibility risk assessment on human-like and porcine models for the treatment of cholangiocarcinoma. Journal of Physics. Part D 202255 455401. ( 10.1088/1361-6463/ac8c4d) [DOI] [Google Scholar]
- 14.Bekeschus S Schmidt A Weltmann K-D & von Woedtke T. The plasma jet kINPen – A powerful tool for wound healing. Clinical Plasma Medicine 2016419–28. ( 10.1016/j.cpme.2016.01.001) [DOI] [Google Scholar]
- 15.Mann MS Tiede R Gavenis K Daeschlein G Bussiahn R Weltmann K-D Emmert S Woedtke Tv & Ahmed R. Introduction to DIN-specification 91315 based on the characterization of the plasma jet kINPen® MED. Clinical Plasma Medicine 2016435–45. ( 10.1016/j.cpme.2016.06.001) [DOI] [Google Scholar]
- 16.Braný D Dvorská D Halašová E & Škovierová H. Cold atmospheric plasma: a powerful tool for modern medicine. International Journal of Molecular Sciences 202021 2932. ( 10.3390/ijms21082932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SPEC D.91315 General Requirements for Plasma Sources in Medicine. Berlin: Beuth-Verlag; 2014. [Google Scholar]
- 18.Lin L & Keidar M. A map of control for cold atmospheric plasma jets: from physical mechanisms to optimizations. Applied Physics Reviews 20218 011306. ( 10.1063/5.0022534) [DOI] [Google Scholar]
- 19.Freund E & Bekeschus S. Gas plasma-oxidized liquids for cancer treatment: preclinical relevance, immuno-oncology, and clinical obstacles. IEEE Transactions on Radiation and Plasma Medical Sciences 20215761–774. ( 10.1109/TRPMS.2020.3029982) [DOI] [Google Scholar]
- 20.Tanaka H Bekeschus S Yan DY Hori M Keidar M & Laroussi M. Plasma-treated solutions (PTS) in cancer therapy. Cancers 202113. ( 10.3390/cancers13071737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekeschus S von Woedtke T Emmert S & Schmidt A. Medical gas plasma-stimulated wound healing: evidence and mechanisms. Redox Biology 202146 102116. ( 10.1016/j.redox.2021.102116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scotton MF Miot HA & Abbade LPF. Factors that influence healing of chronic venous leg ulcers: a retrospective cohort. Anais Brasileiros de Dermatologia 201489414–422. ( 10.1590/abd1806-4841.20142687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isbary G, Heinlin J, Shimizu T, Zimmermann JL, Morfill G, Schmidt HU, Monetti R, Steffes B, Bunk W, Li Y, et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. British Journal of Dermatology 2012167404–410. ( 10.1111/j.1365-2133.2012.10923.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betancourt-Angeles M Pena-Eguiluz R Lopez-Callejas R Dominguez-Cadena NA Mercado-Cabrera A Munoz-Infante J Rodríguez-Méndez BG Valencia-Alvarado R & Moreno-Tapia JA. Treatment in the healing of burns with a cold plasma source. International Journal of Burns and Trauma 20177142–146. [PMC free article] [PubMed] [Google Scholar]
- 25.Winter S Meyer-Lindenberg A Wolf G Reese S & Nolff MC. In vitro evaluation of the decontamination effect of cold atmospheric argon plasma on selected bacteria frequently encountered in small animal bite injuries. Journal of Microbiological Methods 2020169 105728. ( 10.1016/j.mimet.2019.105728) [DOI] [PubMed] [Google Scholar]
- 26.Metelmann H-R, Vu TT, Do HT, Le TNB, Hoang THA, Phi TTT, Luong TML, Doan VT, Nguyen TTH, Nguyen THM, et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: a clinical long term observation. Clinical Plasma Medicine 2013130–35. ( 10.1016/j.cpme.2012.12.001) [DOI] [Google Scholar]
- 27.Metelmann H-R Von Woedtke T & Weltmann K-D. Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application. Berlin: Springer; 2018. [Google Scholar]
- 28.Metelmann H-R, Seebauer C, Miller V, Fridman A, Bauer G, Graves DB, Pouvesle J, Rutkowski R, Schuster M, Bekeschus S, et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clinical Plasma Medicine 201896–13. ( 10.1016/j.cpme.2017.09.001) [DOI] [Google Scholar]
- 29.Marang-van de Mheen PJ Bragan Turner E Liew S Mutalima N Tran T Rasmussen S Nelissen RGHH & Gordon A. Variation in Prosthetic Joint Infection and treatment strategies during 4.5 years of follow-up after primary joint arthroplasty using administrative data of 41397 patients across Australian, European and United States hospitals. BMC Musculoskeletal Disorders 201718 207. ( 10.1186/s12891-017-1569-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izakovicova P Borens O & Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Reviews 20194482–494. ( 10.1302/2058-5241.4.180092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuiper JW Willink RT Moojen DJ van den Bekerom MP & Colen S. Treatment of acute periprosthetic infections with prosthesis retention: review of current concepts. World Journal of Orthopedics 20145667–676. ( 10.5312/wjo.v5.i5.667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karczewski D Winkler T Renz N Trampuz A Lieb E Perka C & Müller M. A standardized interdisciplinary algorithm for the treatment of prosthetic joint infections. Bone and Joint Journal 2019101–B132–139. ( 10.1302/0301-620X.101B2.BJJ-2018-1056.R1) [DOI] [PubMed] [Google Scholar]
- 33.Benčina M Resnik M Starič P & Junkar I. Use of plasma technologies for antibacterial surface properties of metals. Molecules 202126. ( 10.3390/molecules26051418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pompl R, Jamitzky F, Shimizu T, Steffes B, Bunk W, Schmidt H, Georgi M, Ramrath K, Stolz W, Stark RW, et al. The effect of low-temperature plasma on bacteria as observed by repeated AFM imaging. New Journal of Physics 200911115023–115034. ( 10.1088/1367-2630/11/11/115023) [DOI] [Google Scholar]
- 35.Sies H Belousov VV Chandel NS Davies MJ Jones DP Mann GE Murphy MP Yamamoto M & Winterbourn C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nature Reviews. Molecular Cell Biology 202223499–515. ( 10.1038/s41580-022-00456-z) [DOI] [PubMed] [Google Scholar]
- 36.Zimmerli W & Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunology and Medical Microbiology 201265158–168. ( 10.1111/j.1574-695X.2012.00938.x) [DOI] [PubMed] [Google Scholar]
- 37.Hall-Stoodley L Stoodley P Kathju S Høiby N Moser C Costerton JW Moter A & Bjarnsholt T. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunology and Medical Microbiology 201265127–145. ( 10.1111/j.1574-695X.2012.00968.x) [DOI] [PubMed] [Google Scholar]
- 38.Lewis K. The science of antibiotic discovery. Cell 202018129–45. ( 10.1016/j.cell.2020.02.056) [DOI] [PubMed] [Google Scholar]
- 39.Hui WL Perrotti V Iaculli F Piattelli A & Quaranta A. The emerging role of cold atmospheric plasma in implantology: a review of the literature. Nanomaterials (Basel) 202010. ( 10.3390/nano10081505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan F Fang Y Zhu L & Al-Rubeai M. Cold atmospheric plasma as an interface biotechnology for enhancing surgical implants. Critical Reviews in Biotechnology 202141425–440. ( 10.1080/07388551.2020.1853671) [DOI] [PubMed] [Google Scholar]
- 41.Theinkom F Singer L Cieplik F Cantzler S Weilemann H Cantzler M Hiller KA Maisch T & Zimmermann JL. Antibacterial efficacy of cold atmospheric plasma against Enterococcus faecalis planktonic cultures and biofilms in vitro. PLoS One 201914 e0223925. ( 10.1371/journal.pone.0223925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modic M Kovač J Nicholls JR Kos Š Serša G Cvelbar U & Walsh JL. Targeted plasma functionalization of titanium inhibits polymicrobial biofilm recolonization and stimulates cell function. Applied Surface Science 20194871176–1188. ( 10.1016/j.apsusc.2019.05.153) [DOI] [Google Scholar]
- 43.Traba C & Liang JF. Susceptibility of Staphylococcus aureus biofilms to reactive discharge gases. Biofouling 201127763–772. ( 10.1080/08927014.2011.602188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MJ Kwon JS Jiang HB Choi EH Park G & Kim KM. The antibacterial effect of non-thermal atmospheric pressure plasma treatment of titanium surfaces according to the bacterial wall structure. Scientific Reports 201991938. ( 10.1038/s41598-019-39414-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flynn PB Graham WG & Gilmore BF. Acinetobacter baumannii biofilm biomass mediates tolerance to cold plasma. Letters in Applied Microbiology 201968344–349. ( 10.1111/lam.13122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soler-Arango J Figoli C Muraca G Bosch A & Brelles-Mariño G. The Pseudomonas aeruginosa biofilm matrix and cells are drastically impacted by gas discharge plasma treatment: a comprehensive model explaining plasma-mediated biofilm eradication. PLoS One 201914 e0216817. ( 10.1371/journal.pone.0216817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Govaert M Smet C Vergauwen L Ećimović B Walsh JL Baka M & Van Impe J. Influence of plasma characteristics on the efficacy of cold atmospheric plasma (CAP) for inactivation of Listeria monocytogenes and Salmonella Typhimurium biofilms. Innovative Food Science and Emerging Technologies 201952376–386. ( 10.1016/j.ifset.2019.01.013) [DOI] [Google Scholar]
- 48.Maisch T Shimizu T Isbary G Heinlin J Karrer S Klämpfl TG Li YF Morfill G & Zimmermann JL. Contact-free inactivation of Candida albicans biofilms by cold atmospheric air plasma. Applied and Environmental Microbiology 2012784242–4247. ( 10.1128/AEM.07235-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maho T, Binois R, Brulé-Morabito F, Demasure M, Douat C, Dozias S, Escot Bocanegra P, Goard I, Hocqueloux L, Le Helloco C, et al. Anti-bacterial action of plasma multi-jets in the context of chronic wound healing. Applied Sciences 202111 9598. ( 10.3390/app11209598) [DOI] [Google Scholar]
- 50.Khosravi S Jafari S Zamani H & Nilkar M. Inactivation of Staphylococcus aureus and Escherichia coli biofilms by air-based atmospheric-pressure DBD plasma. Applied Biochemistry and Biotechnology 20211933641–3650. ( 10.1007/s12010-021-03636-3) [DOI] [PubMed] [Google Scholar]
- 51.Scholtz V Vaňková E Kašparová P Premanath R Karunasagar I & Julák J. Non-thermal plasma treatment of ESKAPE pathogens: a review. Frontiers in Microbiology 202112 737635. ( 10.3389/fmicb.2021.737635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daeschlein G, Napp M, von Podewils S, Lutze S, Emmert S, Lange A, Klare I, Haase H, Gümbel D, von Woedtke T, et al. In vitro susceptibility of multidrug resistant skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD). Plasma Processes and Polymers 201411175–183. ( 10.1002/ppap.201300070) [DOI] [Google Scholar]
- 53.Ercan UK İbiş F Dikyol C Horzum N Karaman O Yıldırım Ç Çukur E & Demirci EA. Prevention of bacterial colonization on non-thermal atmospheric plasma treated surgical sutures for control and prevention of surgical site infections. PLoS One 201813 e0202703. ( 10.1371/journal.pone.0202703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Welzen A Hoch M Wahl P Weber F Rode S Tietze JK Boeckmann L Emmert S & Thiem A. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacology and Physiology 202134328–336. ( 10.1159/000517524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savage SA & Mirabello L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma 20112011 548151. ( 10.1155/2011/548151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isakoff MS Bielack SS Meltzer P & Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. Journal of Clinical Oncology 2015333029–3035. ( 10.1200/JCO.2014.59.4895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keidar M Yan D & Sherman JH. Cold Plasma Cancer Therapy. San Rafael, CA: Morgan & Claypool Publishers; 2019. [Google Scholar]
- 58.Bekeschus S, Liebelt G, Menz J, Berner J, Sagwal SK, Wende K, Weltmann KD, Boeckmann L, von Woedtke T, Metelmann HR, et al. Tumor cell metabolism correlates with resistance to gas plasma treatment: the evaluation of three dogmas. Free Radical Biology and Medicine 202116712–28. ( 10.1016/j.freeradbiomed.2021.02.035) [DOI] [PubMed] [Google Scholar]
- 59.Haralambiev L Bandyophadyay A Suchy B Weiss M Kramer A Bekeschus S Ekkernkamp A Mustea A Kaderali L & Stope MB. Determination of immediate vs. kinetic growth retardation in physically plasma-treated cells by experimental and modelling data. Anticancer Research 2020403743–3749. ( 10.21873/anticanres.14363) [DOI] [PubMed] [Google Scholar]
- 60.Redza-Dutordoir M & Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta 201618632977–2992. ( 10.1016/j.bbamcr.2016.09.012) [DOI] [PubMed] [Google Scholar]
- 61.Trachootham D Alexandre J & Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Reviews. Drug Discovery 20098579–591. ( 10.1038/nrd2803) [DOI] [PubMed] [Google Scholar]
- 62.Bauer G. The antitumor effect of singlet oxygen. Anticancer Research 2016365649–5663. ( 10.21873/anticanres.11148) [DOI] [PubMed] [Google Scholar]
- 63.Wende K von Woedtke T Weltmann KD & Bekeschus S. Chemistry and biochemistry of cold physical plasma derived reactive species in liquids. Biological Chemistry 201840019–38. ( 10.1515/hsz-2018-0242) [DOI] [PubMed] [Google Scholar]
- 64.Krysko DV Garg AD Kaczmarek A Krysko O Agostinis P & Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nature Reviews. Cancer 201212860–875. ( 10.1038/nrc3380) [DOI] [PubMed] [Google Scholar]
- 65.Bekeschus S Clemen R Nießner F Sagwal SK Freund E & Schmidt A. Medical gas plasma jet technology targets murine melanoma in an immunogenic fashion. Advanced Science 20207 1903438. ( 10.1002/advs.201903438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S Lee H Jeong D Ham J Park S Choi EH & Kim SJ. Cold atmospheric plasma restores tamoxifen sensitivity in resistant MCF-7 breast cancer cell. Free Radical Biology and Medicine 2017110280–290. ( 10.1016/j.freeradbiomed.2017.06.017) [DOI] [PubMed] [Google Scholar]
- 67.Koritzer J, Boxhammer V, Schafer A, Shimizu T, Klampfl TG, Li YF, Welz C, Schwenk-Zieger S, Morfill GE, Zimmermann JL, et al. Restoration of sensitivity in chemo-resistant glioma cells by cold atmospheric plasma. PLoS One 20138 e64498. ( 10.1371/journal.pone.0064498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eke I & Cordes N. Focal adhesion signaling and therapy resistance in cancer. Seminars in Cancer Biology 20153165–75. ( 10.1016/j.semcancer.2014.07.009) [DOI] [PubMed] [Google Scholar]
- 69.Haralambiev L Nitsch A Einenkel R Muzzio DO Gelbrich N Burchardt M Zygmunt M Ekkernkamp A Stope MB & Gümbel D. The effect of cold atmospheric plasma on the membrane permeability of human osteosarcoma cells. Anticancer Research 202040841–846. ( 10.21873/anticanres.14016) [DOI] [PubMed] [Google Scholar]
- 70.Canal C Fontelo R Hamouda I Guillem-Marti J Cvelbar U & Ginebra MP. Plasma-induced selectivity in bone cancer cells death. Free Radical Biology and Medicine 201711072–80. ( 10.1016/j.freeradbiomed.2017.05.023) [DOI] [PubMed] [Google Scholar]
- 71.Guembel D, Gelbrich N, Weiss M, Napp M, Daeschlein G, Sckell A, Ender SA, Kramer A, Burchardt M, Ekkernkamp A, et al. New treatment options for osteosarcoma–inactivation of osteosarcoma cells by cold atmospheric plasma. Anticancer Research 2016365915–5922. ( 10.21873/anticanres.11178) [DOI] [PubMed] [Google Scholar]
- 72.Haralambiev L Wien L Gelbrich N Lange J Bakir S Kramer A Burchardt M Ekkernkamp A Gümbel D & Stope MB. Cold atmospheric plasma inhibits the growth of osteosarcoma cells by inducing apoptosis, independent of the device used. Oncology Letters 202019283–290. ( 10.3892/ol.2019.11115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haralambiev L Wien L Gelbrich N Kramer A Mustea A Burchardt M Ekkernkamp A Stope MB & Gümbel D. Effects of cold atmospheric plasma on the expression of chemokines, growth factors, TNF superfamily members, interleukins, and cytokines in human osteosarcoma cells. Anticancer Research 201939151–157. ( 10.21873/anticanres.13091) [DOI] [PubMed] [Google Scholar]
- 74.Haralambiev L Neuffer O Nitsch A Kross NC Bekeschus S Hinz P Mustea A Ekkernkamp A Gümbel D & Stope MB. Inhibition of angiogenesis by treatment with cold atmospheric plasma as a promising therapeutic approach in oncology. International Journal of Molecular Sciences 202021 7098. ( 10.3390/ijms21197098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Binenbaum Y Ben-David G Gil Z Slutsker YZ Ryzhkov MA Felsteiner J Krasik YE & Cohen JT. Cold atmospheric plasma, created at the tip of an elongated flexible capillary using low electric current, can slow the progression of melanoma. PLoS One 201712 e0169457. ( 10.1371/journal.pone.0169457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirpour S Piroozmand S Soleimani N Jalali Faharani N Ghomi H Fotovat Eskandari H Sharifi AM Mirpour S Eftekhari M & Nikkhah M. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Scientific Reports 20166 29048. ( 10.1038/srep29048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ito T Ando T Suzuki-Karasaki M Tokunaga T Yoshida Y Ochiai T Tokuhashi Y & Suzuki-Karasaki Y. Cold PSM, but not TRAIL, triggers autophagic cell death: a therapeutic advantage of PSM over TRAIL. International Journal of Oncology 201853503–514. ( 10.3892/ijo.2018.4413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tokunaga T Ando T Suzuki-Karasaki M Ito T Onoe-Takahashi A Ochiai T Soma M & Suzuki-Karasaki Y. Plasma-stimulated medium kills TRAIL-resistant human malignant cells by promoting caspase-independent cell death via membrane potential and calcium dynamics modulation. International Journal of Oncology 201852697–708. ( 10.3892/ijo.2018.4251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tornin J Mateu-Sanz M Rodríguez A Labay C Rodríguez R & Canal C. Pyruvate plays a main role in the antitumoral selectivity of cold atmospheric plasma in osteosarcoma. Scientific Reports 20199 10681. ( 10.1038/s41598-019-47128-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yusupov M Van der Paal J Neyts EC & Bogaerts A. Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes. Biochimica et Biophysica Acta. General Subjects 20171861839–847. ( 10.1016/j.bbagen.2017.01.030) [DOI] [PubMed] [Google Scholar]
- 81.Mateu-Sanz M Tornín J Brulin B Khlyustova A Ginebra M-P Layrolle P & Canal C. Cold plasma-treated ringer’s saline: a weapon to target osteosarcoma. Cancers 202012 227. ( 10.3390/cancers12010227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y Guo J Zhou X Liu Z Wang C Wang K Zhang J & Wang Z. A novel cold atmospheric pressure air plasma jet for peri-implantitis treatment: an in vitro study. Dental Materials Journal 201837157–166. ( 10.4012/dmj.2017-030) [DOI] [PubMed] [Google Scholar]
- 83.Coelho PG Giro G Teixeira HS Marin C Witek L Thompson VP Tovar N & Silva NR. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. Journal of Biomedical Materials Research. Part A 20121001901–1906. ( 10.1002/jbm.a.34127) [DOI] [PubMed] [Google Scholar]
- 84.Zhang BG Myers DE Wallace GG Brandt M & Choong PF. Bioactive coatings for orthopaedic implants—recent trends in development of implant coatings. International Journal of Molecular Sciences 20141511878–11921. ( 10.3390/ijms150711878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshinari M Matsuzaka K & Inoue T. Surface modification by cold-plasma technique for dental implants—bio-functionalization with binding pharmaceuticals. Japanese Dental Science Review 20114789–101. ( 10.1016/j.jdsr.2011.03.001) [DOI] [Google Scholar]
- 86.Permyakova ES Kiryukhantsev-Korneev PV Gudz KY Konopatsky AS Polčak J Zhitnyak IY Gloushankova NA Shtansky DV & Manakhov AM. Comparison of different approaches to surface functionalization of biodegradable polycaprolactone scaffolds. Nanomaterials 20199 1769. ( 10.3390/nano9121769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karaman O Kelebek S Demirci EA Ibis F Ulu M & Ercan UK. Synergistic effect of cold plasma treatment and RGD peptide coating on cell proliferation over titanium surfaces. Tissue Engineering and Regenerative Medicine 20181513–24. ( 10.1007/s13770-017-0087-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oryan A Kamali A Moshiri A & Baghaban Eslaminejad M. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells, Tissues, Organs 201720459–83. ( 10.1159/000469704) [DOI] [PubMed] [Google Scholar]
- 89.Mörke C, Rebl H, Finke B, Dubs M, Nestler P, Airoudj A, Roucoules V, Schnabelrauch M, Körtge A, Anselme K, et al. Abrogated cell contact guidance on amino-functionalized microgrooves. ACS Applied Materials and Interfaces 2017910461–10471. ( 10.1021/acsami.6b16430) [DOI] [PubMed] [Google Scholar]
- 90.Tan F & Al-Rubeai M. Customizable implant-specific and tissue-specific extracellular matrix protein coatings fabricated using atmospheric plasma. Frontiers in Bioengineering and Biotechnology 20197247. ( 10.3389/fbioe.2019.00247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O'Neill L Twomey B Tan F O'Donoghue J & Hunt JA. Collagen coating of titanium implants using nonthermal plasma. Plasma Medicine 20211163–79. ( 10.1615/PlasmaMed.2021039685) [DOI] [Google Scholar]
- 92.Meghdadi M Pezeshki-Modaress M Irani S Atyabi SM & Zandi M. Chondroitin sulfate immobilized PCL nanofibers enhance chondrogenic differentiation of mesenchymal stem cells. International Journal of Biological Macromolecules 2019136616–624. ( 10.1016/j.ijbiomac.2019.06.061) [DOI] [PubMed] [Google Scholar]
- 93.Meghdadi M Atyabi SM Pezeshki-Modaress M Irani S Noormohammadi Z & Zandi M. Cold atmospheric plasma as a promising approach for gelatin immobilization on poly (ε-caprolactone) electrospun scaffolds. Progress in Biomaterials 2019865–75. ( 10.1007/s40204-019-0111-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Czuba U Quintana R De Pauw-Gillet MC Bourguignon M Moreno-Couranjou M Alexandre M Detrembleur C & Choquet P. Atmospheric plasma deposition of methacrylate layers containing catechol/quinone groups: an alternative to polydopamine bioconjugation for biomedical applications. Advanced Healthcare Materials 20187e1701059. ( 10.1002/adhm.201701059) [DOI] [PubMed] [Google Scholar]
- 95.Yim JH Fleischman MS Rodriguez-Santiago V Piehler LT Williams AA Leadore JL & Pappas DD. Development of antimicrobial coatings by atmospheric pressure plasma using a guanidine-based precursor. ACS Applied Materials and Interfaces 2013511836–11843. ( 10.1021/am403503a) [DOI] [PubMed] [Google Scholar]
- 96.Duske K Koban I Kindel E Schroder K Nebe B Holtfreter B Jablonowski L Weltmann KD & Kocher T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. Journal of Clinical Periodontology 201239400–407. ( 10.1111/j.1600-051X.2012.01853.x) [DOI] [PubMed] [Google Scholar]
- 97.Wang M Favi P Cheng X Golshan NH Ziemer KS Keidar M & Webster TJ. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomaterialia 201646256–265. ( 10.1016/j.actbio.2016.09.030) [DOI] [PubMed] [Google Scholar]
- 98.Canullo L Genova T Naenni N Nakajima Y Masuda K & Mussano F. Plasma of argon enhances the adhesion of murine osteoblasts on different graft materials. Annals of Anatomy 2018218265–270. ( 10.1016/j.aanat.2018.03.005) [DOI] [PubMed] [Google Scholar]
- 99.Anderson JM Rodriguez A & Chang DT. Foreign body reaction to biomaterials. Seminars in Immunology 20082086–100. ( 10.1016/j.smim.2007.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giro G Tovar N Witek L Marin C Silva NR Bonfante EA & Coelho PG. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. Journal of Biomedical Materials Research. Part A 201310198–103. ( 10.1002/jbm.a.34304) [DOI] [PubMed] [Google Scholar]
- 101.Canullo L Tallarico M Botticelli D Alccayhuaman KAA Martins Neto EC & Xavier SP. Hard and soft tissue changes around implants activated using plasma of argon: a histomorphometric study in dog. Clinical Oral Implants Research 201829389–395. ( 10.1111/clr.13134) [DOI] [PubMed] [Google Scholar]
- 102.Naujokat H Harder S Schulz LY Wiltfang J Florke C & Acil Y. Surface conditioning with cold argon plasma and its effect on the osseointegration of dental implants in miniature pigs. Journal of Cranio-Maxillo-Facial Surgery 201947484–490. ( 10.1016/j.jcms.2018.12.011) [DOI] [PubMed] [Google Scholar]
- 103.Zheng Z Ao X Xie P Wu J Dong Y Yu D Wang J Zhu Z Xu HHK & Chen W. Effects of novel non-thermal atmospheric plasma treatment of titanium on physical and biological improvements and in vivo osseointegration in rats. Scientific Reports 20201010637. ( 10.1038/s41598-020-67678-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Naresh Kumar N Yap SL Bt Samsudin FND Khan MZ & Pattela Srinivasa RS. Effect of argon plasma treatment on tribological properties of UHMWPE/MWCNT nanocomposites. Polymers 20168 295. ( 10.3390/polym8080295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rupf S, Idlibi AN, Marrawi FA, Hannig M, Schubert A, von Mueller L, Spitzer W, Holtmann H, Lehmann A, Rueppell A, et al. Removing biofilms from microstructured titanium ex vivo: a novel approach using atmospheric plasma technology. PLoS One 20116 e25893. ( 10.1371/journal.pone.0025893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Preissner S Wirtz HC Tietz AK Abu-Sirhan S Herbst SR Hartwig S Pierdzioch P Schmidt-Westhausen AM Dommisch H & Hertel M. Bactericidal efficacy of tissue tolerable plasma on microrough titanium dental implants: an in-vitro-study. Journal of Biophotonics 20169637–644. ( 10.1002/jbio.201500189) [DOI] [PubMed] [Google Scholar]
- 107.Ibis F Oflaz H & Ercan UK. Biofilm inactivation and prevention on common implant material surfaces by nonthermal DBD plasma treatment. Plasma Medicine 2016633–45. ( 10.1615/PlasmaMed.2016015846) [DOI] [Google Scholar]
- 108.Ulu M Pekbagriyanik T Ibis F Enhos S & Ercan UK. Antibiofilm efficacies of cold plasma and Er: YAG laser on Staphylococcus aureus biofilm on titanium for nonsurgical treatment of periimplantitis. Nigerian Journal of Clinical Practice 201821758–765. ( 10.4103/njcp.njcp_261_17) [DOI] [PubMed] [Google Scholar]
- 109.Duske K Jablonowski L Koban I Matthes R Holtfreter B Sckell A Nebe JB von Woedtke T Weltmann KD & Kocher T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 201552327–334. ( 10.1016/j.biomaterials.2015.02.035) [DOI] [PubMed] [Google Scholar]
- 110.Park J Lee H Lee HJ Kim GC Kim DY Han S & Song K. Non-thermal atmospheric pressure plasma efficiently promotes the proliferation of adipose tissue-derived stem cells by activating NO-response pathways. Scientific Reports 20166 39298. ( 10.1038/srep39298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miletić M Mojsilović S Đorđević IO Maletić D Puač N Lazović S Malović G Milenković P Lj Petrović Z & Bugarski D. Effects of non-thermal atmospheric plasma on human periodontal ligament mesenchymal stem cells. Journal of Physics. Part D 201346 345401. ( 10.1088/0022-3727/46/34/345401) [DOI] [Google Scholar]
- 112.Choi BB Choi JH Kang TH Lee SJ & Kim GC. Enhancement of osteoblast differentiation using no-ozone cold plasma on human periodontal ligament cells. Biomedicines 20219 1542. ( 10.3390/biomedicines9111542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park J Lee H Lee HJ Kim GC Kim SS Han S & Song K. Non-thermal atmospheric pressure plasma is an excellent tool to activate proliferation in various mesoderm-derived human adult stem cells. Free Radical Biology and Medicine 2019134374–384. ( 10.1016/j.freeradbiomed.2019.01.032) [DOI] [PubMed] [Google Scholar]
- 114.Fischer M Schoon J Freund E Miebach L Weltmann KD Bekeschus S & Wassilew GI. Biocompatible gas plasma treatment affects secretion profiles but not osteogenic differentiation in patient-derived mesenchymal stromal cells. International Journal of Molecular Sciences 202223. ( 10.3390/ijms23042038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miebach L Freund E Clemen R Weltmann KD Metelmann HR von Woedtke T Gerling T Wende K & Bekeschus S. Conductivity augments ROS and RNS delivery and tumor toxicity of an argon plasma jet. Free Radical Biology and Medicine 2022180210–219. ( 10.1016/j.freeradbiomed.2022.01.014) [DOI] [PubMed] [Google Scholar]
- 116.Eggers B Wagenheim AM Jung S Kleinheinz J Nokhbehsaim M Kramer FJ & Sielker S. Effect of cold atmospheric plasma (CAP) on osteogenic differentiation potential of human osteoblasts. International Journal of Molecular Sciences 202223 2503. ( 10.3390/ijms23052503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eggers B Marciniak J Memmert S Wagner G Deschner J Kramer FJ & Nokhbehsaim M. Influences of cold atmospheric plasma on apoptosis related molecules in osteoblast-like cells in vitro. Head and Face Medicine 202117 37. ( 10.1186/s13005-021-00287-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Charles JF & Aliprantis AO. Osteoclasts: more than ‘bone eaters’. Trends in Molecular Medicine 201420449–459. ( 10.1016/j.molmed.2014.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S-i, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of the National Academy of Sciences of the United States of America 1998953597–3602. ( 10.1073/pnas.95.7.3597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee NK Choi YG Baik JY Han SY Jeong DW Bae YS Kim N & Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005106852–859. ( 10.1182/blood-2004-09-3662) [DOI] [PubMed] [Google Scholar]
- 121.Zhang Y, Xiong Y, Xie P, Ao X, Zheng Z, Dong X, Li H, Yu Q, Zhu Z, Chen M, et al. Non-thermal plasma reduces periodontitis-induced alveolar bone loss in rats. Biochemical and Biophysical Research Communications 20185032040–2046. ( 10.1016/j.bbrc.2018.07.154) [DOI] [PubMed] [Google Scholar]
- 122.Shimatani A Toyoda H Orita K Hirakawa Y Aoki K Oh JS Shirafuji T & Nakamura H. In vivo study on the healing of bone defect treated with non-thermal atmospheric pressure gas discharge plasma. PLoS One 202116 e0255861. ( 10.1371/journal.pone.0255861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Filaire E & Toumi H. Reactive oxygen species and exercise on bone metabolism: friend or enemy? Joint Bone Spine 201279341–346. ( 10.1016/j.jbspin.2012.03.007) [DOI] [PubMed] [Google Scholar]
- 124.Hameister R Kaur C Dheen ST Lohmann CH & Singh G. Reactive oxygen/nitrogen species (ROS/RNS) and oxidative stress in arthroplasty. Journal of Biomedical Materials Research. Part B, Applied Biomaterials 20201082073–2087. ( 10.1002/jbm.b.34546) [DOI] [PubMed] [Google Scholar]
- 125.Arakaki N Yamashita A Niimi S & Yamazaki T. Involvement of reactive oxygen species in osteoblastic differentiation of MC3T3-E1 cells accompanied by mitochondrial morphological dynamics. Biomedical Research 201334161–166. ( 10.2220/biomedres.34.161) [DOI] [PubMed] [Google Scholar]
- 126.Banfi G Iorio EL & Corsi MM. Oxidative stress, free radicals and bone remodeling. Clinical Chemistry and Laboratory Medicine 2008461550–1555. ( 10.1515/CCLM.2008.302) [DOI] [PubMed] [Google Scholar]
- 127.Alfadda AA & Sallam RM. Reactive oxygen species in health and disease. Journal of Biomedicine and Biotechnology 20122012936486. ( 10.1155/2012/936486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bai XC Lu D Liu AL Zhang ZM Li XM Zou ZP Zeng WS Cheng BL & Luo SQ. Reactive oxygen species stimulates receptor activator of NF-κB ligand expression in osteoblast. Journal of Biological Chemistry 200528017497–17506. ( 10.1074/jbc.M409332200) [DOI] [PubMed] [Google Scholar]
- 129.Arai M Shibata Y Pugdee K Abiko Y & Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life 20075927–33. ( 10.1080/15216540601156188) [DOI] [PubMed] [Google Scholar]
- 130.Fatokun AA Stone TW & Smith RA. Responses of differentiated MC3T3-E1 osteoblast-like cells to reactive oxygen species. European Journal of Pharmacology 200858735–41. ( 10.1016/j.ejphar.2008.03.024) [DOI] [PubMed] [Google Scholar]
- 131.Lademann JM, Richter H, Alborova A, Humme D, Patzelt A, Kramer A, Weltmann KD, Hartmann B, Ottomann C, Fluhr JW, et al. Risk assessment of the application of a plasma jet in dermatology. Journal of Biomedical Optics 200914 054025. ( 10.1117/1.3247156) [DOI] [PubMed] [Google Scholar]
- 132.Daeschlein G, Scholz S, Ahmed R, Majumdar A, von Woedtke T, Haase H, Niggemeier M, Kindel E, Brandenburg R, Weltmann KD, et al. Cold plasma is well-tolerated and does not disturb skin barrier or reduce skin moisture. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 201210509–515. ( 10.1111/j.1610-0387.2012.07857.x) [DOI] [PubMed] [Google Scholar]
- 133.Jablonowski L, Kocher T, Schindler A, Müller K, Dombrowski F, von Woedtke T, Arnold T, Lehmann A, Rupf S, Evert M, et al. Side effects by oral application of atmospheric pressure plasma on the mucosa in mice. PLoS One 201914 e0215099. ( 10.1371/journal.pone.0215099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Evert K, Kocher T, Schindler A, Müller M, Müller K, Pink C, Holtfreter B, Schmidt A, Dombrowski F, Schubert A, et al. Repeated exposure of the oral mucosa over 12 months with cold plasma is not carcinogenic in mice. Scientific Reports 202111 20672. ( 10.1038/s41598-021-99924-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Emmert S, Brehmer F, Hänßle H, Helmke A, Mertens N, Ahmed R, Simon D, Wandke D, Maus-Friedrichs W, Däschlein G, et al. Atmospheric pressure plasma in dermatology: ulcus treatment and much more. Clinical Plasma Medicine 2013124–29. ( 10.1016/j.cpme.2012.11.002) [DOI] [Google Scholar]
- 136.Sakudo A Yagyu Y & Onodera T. Disinfection and sterilization using plasma technology: fundamentals and future perspectives for biological applications. International Journal of Molecular Sciences 201920 5216. ( 10.3390/ijms20205216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bekeschus S Schütz CS Nießner F Wende K Weltmann K-D Gelbrich N von Woedtke T Schmidt A & Stope MB. Elevated H2AX phosphorylation observed with kINPen plasma treatment is not caused by ROS-mediated DNA damage but is the consequence of apoptosis. Oxidative Medicine and Cellular Longevity 201920191–15. ( 10.1155/2019/8535163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maisch T Bosserhoff AK Unger P Heider J Shimizu T Zimmermann JL Morfill GE Landthaler M & Karrer S. Investigation of toxicity and mutagenicity of cold atmospheric argon plasma. Environmental and Molecular Mutagenesis 201758172–177. ( 10.1002/em.22086) [DOI] [PubMed] [Google Scholar]
- 139.Bekeschus S Schmidt A Kramer A Metelmann HR Adler F von Woedtke T Niessner F Weltmann KD & Wende K. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environmental and Molecular Mutagenesis 201859268–277. ( 10.1002/em.22172) [DOI] [PubMed] [Google Scholar]
- 140.Weltmann K & Von Woedtke T. Plasma medicine—current state of research and medical application. Plasma Physics and Controlled Fusion 201759 014031. ( 10.1088/0741-3335/59/1/014031) [DOI] [Google Scholar]
- 141.Przekora A, Audemar M, Pawlat J, Canal C, Thomann JS, Labay C, Wojcik M, Kwiatkowski M, Terebun P, Ginalska G, et al. Positive effect of cold atmospheric nitrogen plasma on the behavior of mesenchymal stem cells cultured on a bone scaffold containing iron oxide-loaded silica nanoparticles catalyst. International Journal of Molecular Sciences 202021 4738. ( 10.3390/ijms21134738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheng H Luo J Song K Zhao F Liu D Nie L & Lu X. On the dose of plasma medicine: plasma-activated medium (PAM) and its effect on cell viability. Physics of Plasmas 202229 063506. ( 10.1063/5.0089357) [DOI] [Google Scholar]
- 143.Cheng H Xu J Li X Liu D & Lu X. On the dose of plasma medicine: equivalent total oxidation potential (ETOP). Physics of Plasmas 202027 063514. ( 10.1063/5.0008881) [DOI] [Google Scholar]
- 144.Hajizadeh K Hajisharifi K & Mehdian H. Morphological risk assessment of cold atmospheric plasma-based therapy: bone marrow mesenchymal stem cells in treatment zone proximity. Journal of Physics. Part D 201952 495203. ( 10.1088/1361-6463/ab3f65) [DOI] [Google Scholar]
- 145.Bourdens M Jeanson Y Taurand M Juin N Carrière A Clément F Casteilla L Bulteau AL & Planat-Bénard V. Short exposure to cold atmospheric plasma induces senescence in human skin fibroblasts and adipose mesenchymal stromal cells. Scientific Reports 201998671. ( 10.1038/s41598-019-45191-2) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a