Abstract
Purpose
Intra-articular injection is a well-established and increasingly used treatment for the patient with mild-to-moderate hip osteoarthritis. The objectives of this literature review and meta-analysis are to evaluate the effect of prior intra-articular injections on the risk of periprosthetic joint infection (PJI) in patients undergoing total hip arthroplasty (THA) and to try to identify which is the minimum waiting time between hip injection and replacement in order to reduce the risk of infection.
Methods
The database of PubMed, Embase, Google Scholar and Cochrane Library was systematically and independently searched, according to Preferred Reporting Items for Systematic Reviews and Meta–Analyses (PRISMA) guidelines. To assess the potential risk of bias and the applicability of the evidence found in the primary studies to the review, the Newcastle–Ottawa scale (NOS) was used. The statistical analysis was performed by using the software ’R’ version 4.2.2.
Results
The pooling of data revealed an increased risk of PJI in the injection group that was statistically significative (P = 0.0427). In the attempt to identify a ’safe time interval’ between the injection and the elective surgery, we conducted a further subgroup analysis: in the subgroup 0–3 months, we noted an increased risk of PJI after injection.
Conclusions
Intra-articular injection is a procedure that may increase the risk of developing periprosthetic infection. This risk is higher if the injection is performed less than 3 months before hip replacement.
Keywords: intra-articular injections, hyaluronic acid, corticosteroids, periprosthetic joint infections, hip infection, osteoarthritis, total hip arthroplasty
Introduction
Osteoarthritis (OA) is a degenerative disease that affects joints, especially hip and knee. Population aging leads to a progressive increase in the incidence of this disease. Half of the world's population aged 65 and older suffer from OA and 80% of people with symptomatic OA have limitations in movement, while 25% cannot perform their normal daily activities (1). The European Project on Osteoarthritis (EPOSA) has made it possible to obtain more accurate demographic data on the disease, involving six European countries, recording that the prevalence of OA is 30.4% (2).
Hip OA involves worsening groin pain and progressive reduction of joint excursion, leading to a significant deterioration in the quality of life and daily activities. The natural history of OA goes through various phases, more or less symptomatic, which last for years, at the end of which the only resolving treatment is the prosthetic replacement of the joint (3).
Nonoperative treatment for hip OA consists of a stepwise approach. This approach includes weight loss, activity modification, physiotherapy, oral analgesics including nonsteroidal anti-inflammatories, and intra-articular injections. Less invasive surgical options, such as arthroscopy, have very narrow indications in patients with OA (4).
Intra-articular injection is a well-accepted treatment for the patient with mild-to-moderate OA who has exhausted other noninvasive treatment options. Hip injection should always be performed under visualization of the joint by x-rays or ultrasound. It can also be performed with different types of medication: hyaluronic acid (HA), platelet-rich plasma (PRP), HA and PRP, corticosteroids (CS) and local anaesthetics (LA) (5, 6).
The infiltrative treatment may be ineffective in reducing pain and improving function (7). Therefore, it is necessary to propose a total hip arthroplasty (THA) to the patient. Nowadays, the correct timing between the last intra-articular hip injection and THA is unknown. In fact, this ‘safety’ time interval between the two procedures is essential to minimize the risk of periprosthetic joint infection (PJI) due to possible contamination during the previous hip injections.
The primary objectives of this literature review and meta-analysis are to evaluate the effect of prior intra-articular injections on the risk of PJI in patients undergoing THA and to try to identify a minimum waiting time interval between hip injection and replacement in order to reduce the risk of infection. The secondary objective is to evaluate the type of medication and their relative risk on the rate of PJI.
Materials and methods
This literature review and meta-analysis were performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta- Analyses) statement guidelines (8).
Search strategy and information sources
The database of PubMed, Embase, Google Scholar and Cochrane Library was systematically and independently searched until 15 December 2022 by two reviewers (MS and VC). The details of the literature search are reported in the Supplementary material section. Additional studies were eventually identified from the references of the retrieved papers.
Eligibility criteria
The inclusion criteria for the retrieved studies are as follows:
Population:patients older than 18 years undergoing THA.
Intervention:intra-articular injections of any drugs performed before the THA surgical procedure.
Comparison:a cohort of patients who did not undergo intra-articular injections prior to the THA surgical procedure.
Outcome: diagnosis of PJI (according by any definition used in the included studies).
Study type:all cohort studies, random and clinical controlled trials, prospective or retrospective.
The exclusion criteria adopted were
Absence of a control group;
Studies in which it was not possible to retrieve the incidence of PJI in each arm;
Studies not written in the English language;
Studies with duplicated data.
Study selection
Two reviewers (MS and VC) independently evaluated the studies for eligibility. Once the relevant studies were identified, their full text wasextracted and selected on the base of the inclusion and exclusion criteria. In case of disagreement, the senior authors (GS and GL) were sought to resolve the divergences.
Data extraction
Two reviewers (MS and VC) performed the data extraction independently. In case of disagreement, the senior authors (GS and GL) were sought to resolve the divergences. Data extracted from the eligible studies included first author names, year of publication, study design, study location (country), sample size, number of infected and non-infected patients in each cohort, mean age, the drugs injected, time from injection to surgery, the reference standard of PJI diagnosis and duration of follow-up.
Quality evaluation
Two reviewers (MS and VC) performed the data extraction independently. In case of disagreement, the senior author (GL) was sought to resolve the divergences. To assess the potential risk of bias and the applicability of the evidence found in the primary studies to the review, the Newcastle–Ottawa scale (NOS) was used (9).
Statistical analysis
The statistical analysis of this meta-analysis was performed by using the software ‘R’ version 4.2.2 (R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
A random-effects model was used to perform the meta-analysis, on the basis of the sparse nature of data and because we anticipated considerable between-study heterogeneity.
The meta-analytical methodology was
Mantel–Haenszel (MH) method for pooling data. MH method is specific for binary outcomes and was proposed for the common odds ratio in a stratified case–control study (10) and then extended to the risk ratio (RR) and risk difference as a measure of treatment effect for sparse data by Greenland and Robins (11).
The Paule–Mandel estimator was chosen for calculation of τ 2 (12).
We used Knapp–Hartung adjustments (13) to calculate the confidence interval (CI) around the pooled effect. Several studies (14, 15) showed that these adjustments can reduce the chance of false positives, especially when the number of studies is small.
Continuity correction of 0.5 in studies with zero cell frequencies was used only to calculate individual study results (16).
The pooled incidence of PJI was reported using RR with 95% CIs.
Forest plots were used to display the RR of PJI for each study, displaying heterogeneity statistics as well.
A L’Abbé plot (17) was also drawn to allow an easy and direct visualization of the summary measure of the risk of PJI and of the level of heterogeneity.
The heterogeneity was evaluated using Higgins and Thompson’s I 2 statistics (18), and Q-profile method was used for CI of τ 2 and τ (19). Prediction intervals were furthermore provided to better clarify the meaning of the heterogeneity measure reported (20).
The potential sources of heterogeneity were investigated as follows:
Checking for outliers and influential cases by performing an influence diagnostic (21). A Baujat plot was used to report the influence diagnostic (22). After we identified the influential studies, we performed and reported the results of a sensitivity analysis in which these studies are excluded.
Performing a sub-group analysis based on the timing of injection before THA, in an attempt to reduce the potential cause of heterogeneity.
Investigating the Publication Bias (PB) using Funnel Plots (when the P value was <0.05, the test for PB was considered statistically significant) and the Egger’s test (23).
Results
Search and selection process
The search strategies are reported in Supplementary Table 1 (see section on supplementary materials given at the end of this article) in the Supplementary material section, and the study flow chart is reported in Fig. 1. The process of literature search yielded 5538 articles, from which we removed 1500 records because they were duplicates and 3200 records for other reasons (e.g. other languages and not pertinent). After this first process, the remaining 838 papers were screened by evaluating titles and abstracts. At the end of the screening process, the full text of 30 articles was assessed for eligibility. Nineteen papers were excluded for several reasons (tenarticles reported cumulative data for hip and knee joints, three papers compared different numbers of injections and six articles did not have a control group). Finally, 11 papers were included in this systematic review (24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34). One study (28) was not included in the meta-analysis because, reporting no events for each arm, it did not provide any information for pooling data and was not indicative for the meta-analytic strategy.
Figure 1.
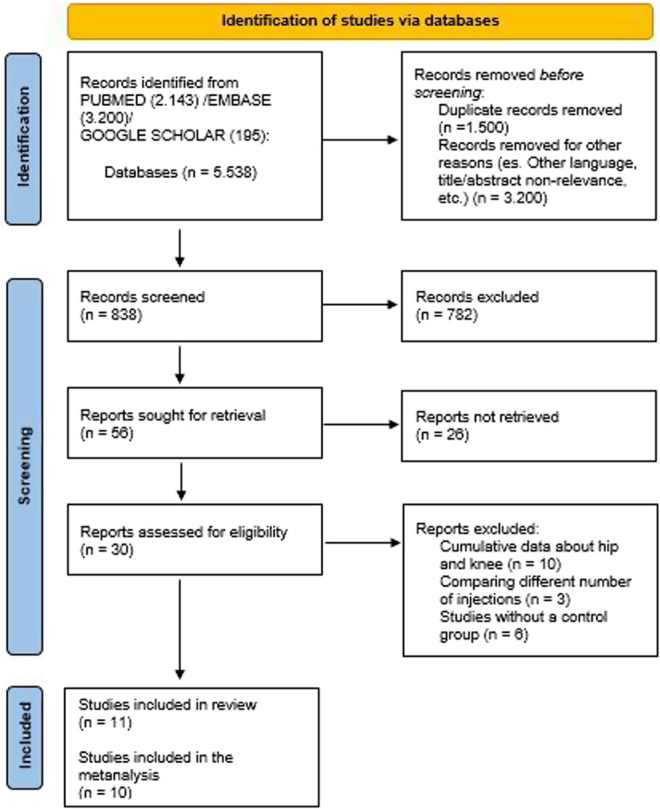
Study flowchart.
Included studies characteristics
The details of the studies included in this systematic review are reported in Table 1. All papers were published from 2005 to 2021. Three studies were conducted in Europe (26, 27, 32), three in Canada (24, 28, 29) and the remaining five studies in USA (25, 30, 31, 33, 34). All studies were retrospective, six adopted a matched cohort design (24, 25, 26, 27, 28, 33) and the remaining were case–control studies. The studies reported outcomes for CS injections in six cases (24, 25, 26, 27, 28, 33), for HA in one case (32) and for CS or HA in the remaining four papers. The sample sizes were variable in the included studies, varying from 40 to 168 537 cases. There was no uniformity in diagnostic criteria adopted for the PJI diagnosis, and in several studies, the definition of PJI was not reported at all (25, 26, 29, 31, 33, 34). The follow-up in the studies is variable, spanning from 3 months to 71 months. The time intercurred between the last articular injection and the elective surgery is, similarly, widely variable, ranging from 2 weeks to a maximum of 42.9 months.
Table 1.
Characteristics of the included studies.
| Study | P/R | Study design | Sample size | Drug injected | Diagnostic criteria of PJI | Time from injection to surgery | Follow-up | NOS score | |
|---|---|---|---|---|---|---|---|---|---|
| Study group | Control group | ||||||||
| Kaspar & de Beer (24) | R | MC | 40 | 40 | CS | Revision caused by PJI | 0.5–42.9 mo | 29.8 mo | 8 |
| McIntosh et al. (25) | R | MC | 224 | 224 | CS | Not reported | Mean: 112 days | 2.7 years | 6 |
| Sreekumar et al. (26) | R | MC | 68 | 136 | CS | Not reported | Median: 11 mo | 1 year | 7 |
| Meermans et al. (27) | R | MC | 175 | 175 | CS | Identified by local clinical signs | <12 mo | 71 mo | 8 |
| Croft & Rockwood (28) | R | MC | 48 | 48 | CS | Revision caused by PJI | Mean: 5.9 mo | 10.45 mo | 7 |
| Ravi et al. (29) | R | CC | 1691 | 35 413 | CS or HA | Not reported | < 12 mo | 2 years | 8 |
| Schairer et al. (30) | R | CC | 5421 | 168 537 | CS or HA | Revision caused by PJI | <12 mo | 1 year | 8 |
| Werner et al. (31) | R | CC | 3368 | 31 229 | CS or HA | Not reported | <12 mo | 6 mo | 6 |
| Colen et al. (32) | R | CC | 118 | 495 | HA | MIS definition | <6 mo | 52 mo | 6 |
| Forlenza et al. (33) | R | MC | 29 058 | 29 058 | CS | Not reported | <6 mo | 6 mo | 8 |
| Tang et al. (34) | R | CC | 342 | 2998 | CS or HA | Not reported | 12.4 ± 11 mo | 3 mo | 7 |
CC, case–control study; CS, corticosteroids; HA, hyaluronic acid; MC, matched cohort study; MIS, Musculoskeletal Infection Society; mo, months; NOS: Newcastle–Ottawa scale; P, prospective; PJI, peri-prosthetic joint infection; R, retrospective.
The quality analyses of the included studies reported the following Newcastle–Ottawa score: 8 points for five studies (24, 27, 29, 30, 33); 7 points for three studies (26, 28, 34); and 6 points for three studies (25, 31, 32).
Meta-analysis
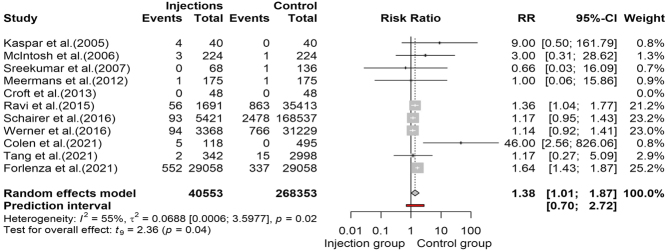
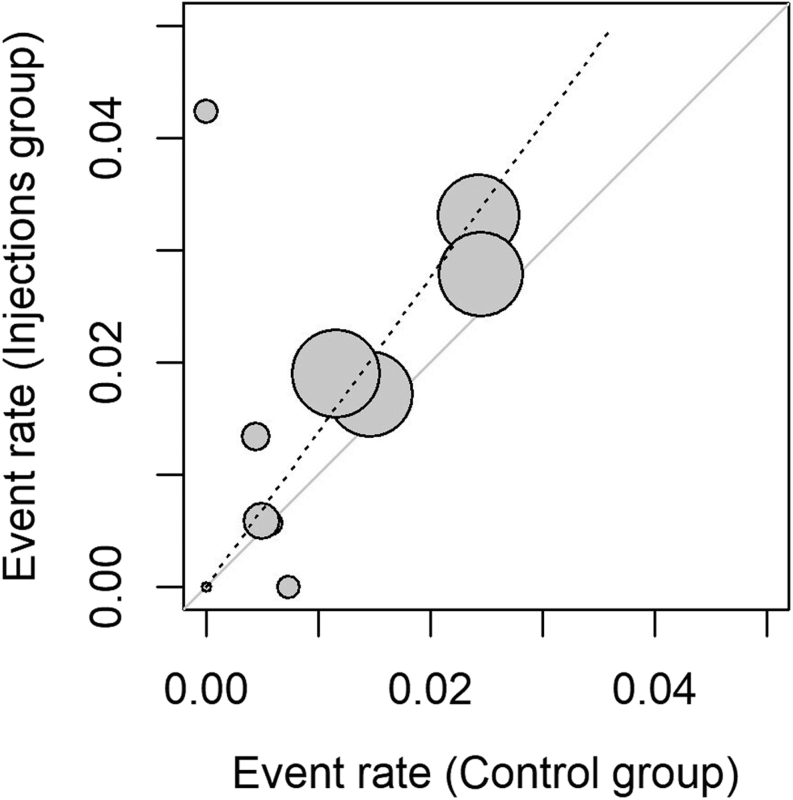
This meta-analytic process combined ten studies (24, 25, 26, 27, 29, 30, 31, 32, 33, 34), including 308 810 observations and 5272 events (PJI after articular injection). The pooling of data revealed an increased risk of PJI in the injection group (RR: 1.38, 95% CI: 1.01, 2.72) that was statistically significative (t = 2.36, P = 0.0427). The forest plot and the l’Abbé plot for the meta-analysis including all the studies are presented in Figs. 2 and 3, respectively. The prediction interval ranged from 0.7 to 2.72, indicating that negative risk in the injection group cannot be ruled out for future studies.
Figure 2.
Forest plot reproducing meta-analysis of all included studies.
Figure 3.
L’Abbé plot.
Study heterogeneity and sensitivity analysis
The between-study heterogeneity variance was estimated at τ 2 = 0.07 (95% CI: 0.01, 5.59), with an I 2 value of 55% (95% CI: 8.3%, 77.9%), indicating the presence of a moderate heterogeneity. The Baujat plot presented in Fig. 4 summarizes the influence diagnostic performed on our meta-analysis. In particular, we identified two influential studies, one having a large impact of heterogeneity but a small weight on the effect size (32) and one heavily influenced both heterogeneity and pooled effect (33). A sensitivity analysis was subsequently made by excluding the two influential cases, obtaining still a significant increase in PJI RR in the injection group, although smaller than the previous results (RR: 1.2; 95% CI: 1.08, 1.35; t = 3.87; P = 0.0061). Interestingly, in the latter analysis, the heterogeneity was annulled (τ 2 = 0, 95% CI: 0; 0.9; I 2 = 0%, 95% CI: 0%, 67.6%; Q = 3.81, df = 7, P = 0.8019) (Table 2).
Figure 4.
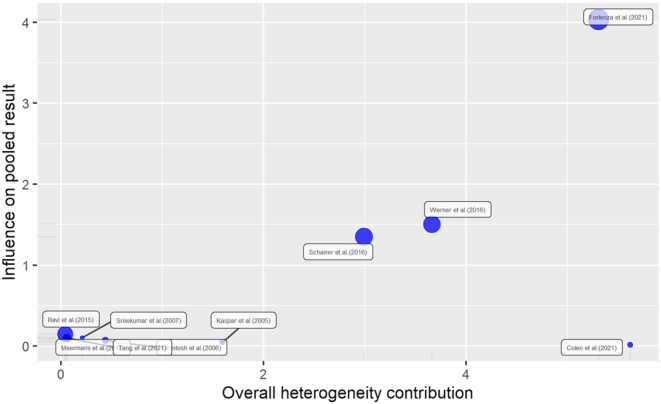
The Baujat plot for all studies.
Table 2.
Sensitivity analysis with the exclusion of the influential studies.
| Analysis | RR | 95% CI | P value | 95% PI | I2 | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| Main analysis | 1.38 | 1.01–1.87 | 0.0427 | 0.70–2.72 | 55% | 8.3–77.9% | 0.018 |
| Influential cases removed* | 1.20 | 1.08–1.35 | 0.0061 | 1.03–1.41 | 0% | 0–67.6% | 0.8019 |
Subgroup analysis
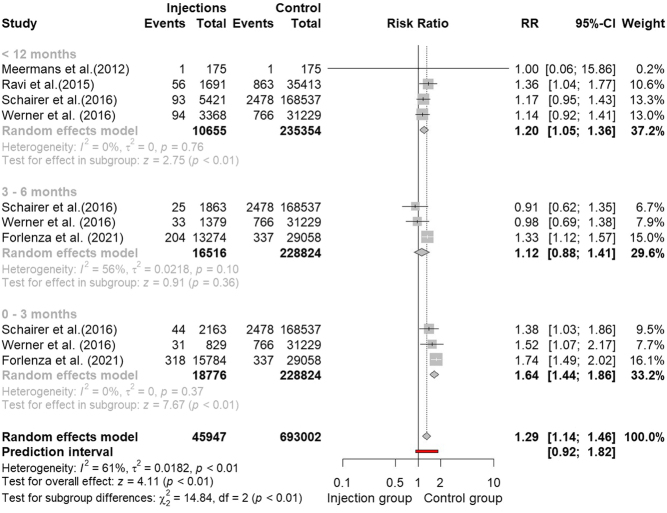
In the attempt to identify a ‘safe time interval’ between the injection and the elective surgery, we conducted a further subgroup analysis of the papers reporting data within 12 months of interval from injection to THA procedure and in which it was possible to subdivide the time interval into three subgroups: 0–3 months, 3–6 months and 6–12 months (27, 29, 30, 31, 33). No scientific paper reported data after 12 months, and in any case, it is believed that after 1 year, there can no longer be cause–effect between the two parameters. In the subgroup 0–3 months, we noted an increased risk of PJI after injection (RR: 1.64, 95% CI: 1.44, 1.86, P < 0.01; I 2 = 0%, P = 0.37). No significative increased risk was reported for the subgroup 3–6 months (RR: 1.12, 95% CI: 0.88, 1.41, P = 0.36; I 2 = 56%, P = 0.10), while a significative increase in the risk of PJI after injection was identified in the <12 months group (RR: 1.20, 95% CI: 1.05, 1.36, P < 0.01; I 2 = 0%, P = 0.76) (Fig. 5).
Figure 5.
Forest plot of subgroup analysis based on timing of injection before surgery.
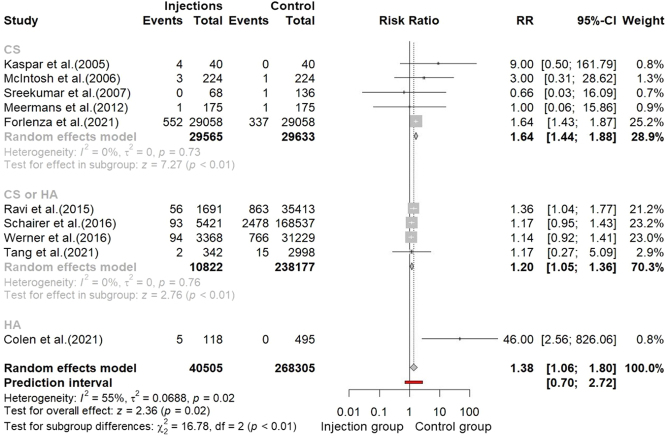
A further subgroup analysis was performed in the attempt to investigate if the type of drug injected was able to influence the overall risk of PJI. In five studies, only CS was used as a drug for injections (24, 25, 26, 27, 33). For this subgroup, the pooling of data revealed a significative increased relative risk of PJI (RR: 1.64, 95% CI: 1.44, 1.88, P < 0.01; I 2 = 0%, P = 0.73). In four studies, both CS and HA were used for intra-articular injections (29, 30, 31, 34). The pooled results for this group were RR: 1.20, 95% CI: 1.05, 1.36, P < 0.01; I 2 = 0%, P = 0.76. Only one study reported results for HA use only (32) (Fig. 6).
Figure 6.
Forest plot of subgroup analysis based on type of injection.
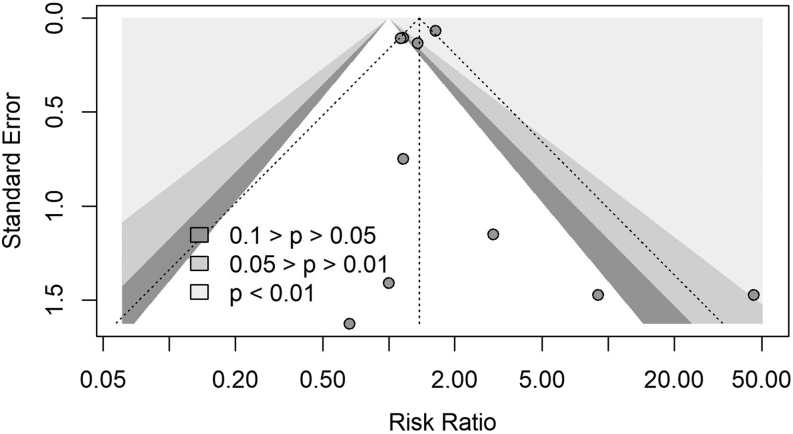
Publication bias
No publication bias was evidenced: Eggers' test does not indicate the presence of funnel plot asymmetry (intercept: 0.338; 95% CI: −0.98, −1.66; t = 0.503; P = 0.63) (Fig. 7).
Figure 7.
Funnel plot for identification of publication bias.
Discussion
The main finding of this meta-analysis is that intra-articular injection is a procedure that may increase the risk of developing periprosthetic infection. This risk is higher if the injection is performed less than 3 months before hip replacement.
Hip osteoarthritis: conservative or not conservative treatments
The demand for hip prosthetic replacement is constantly increasing. There are several well-recognized risk factors for the development of PJI, including previous invasive procedures and exposure to contaminants. PJIis one of the most serious complications of joint replacement surgery and all the most important precautions to avoid it must be implemented. A meta-analysis estimates the rate of surgical site infection to be 2.5% and the rate of deep PJI to be 0.9% after THA (35).
Patients who undergo hip replacement have often suffered from hip OA for years and have certainly resorted to alternative therapies before surgical treatment, and among these there are intra-articular injections.
Recently, having undergone infiltrative treatments before surgery, it has been recognized as an important risk factor for the development of PJI (33).
Hip infiltrative treatment and risk of periprosthetic infection: evidences from the literature
Our meta-analysis provided cumulative evidence from 11 studies, including 308 906 observations, demonstrating a statistically significative 38% increased risk for PJI for patients who have a prior history of intra-articular injections. Our analysis, however, was deeply influenced by two studies (32, 33) that had a great impact on heterogeneity and pooled effect. The results obtained performing the sensitivity analysis, by removing the two influential studies, appear to be more realistic, providing a 20% of increased risk of PJI after intra-articular injection (RR: 1.20; 95% CI: 1.08, 1.35; t = 3.87; P = 0.0061) Table 2. Although reduced, the latter pooled risk is still statistically significant and in line with more recent meta-analyses (Nie et al. reported an RR of 1.24; 95% CI: 1.11–1.38; P = 0.002 (36); Albanese et al. reported an OR = 1.17; 95% CI: 1.01, 1.36; P = 0.04 (37)).
Recently, several authors have suggested that the timing of injections before surgery could significantly influence the risk of developing PJI (33, 38), but at the moment there are no guidelines on the correct timing between hip injection and prosthetic replacement and few data are reported in the literature, as recently highlighted by Li H. et al. (39). Available data arise from retrospective studies which are often underpowered, with widely disparate results (25, 40). On the other hand, the conclusion of all studies is that the risk increases the shorter the time interval between intra-articular injection and THA. Therefore, one of the purposes of this meta-analysis is to clarify the minimum time that must elapse between the last hip injection and the prosthetic replacement, so as to minimize the risk of PJI. Our analysis of the pooled data demonstrated that within 3 months from injections, the risk of developing PJI appears to be 64% increased (RR: 1.64; 95% CI: 1.44, 1.86; P < 0.01; I 2 = 0%; P = 0.37). No definitive results were found for the interval time 6–12 months, in which the pooled effect had not reached the statistical significance (P = 0.36). At 12 months, the RR returned to be in line with the general risk found for the cohort of patients who received articular injections at any time (RR: 1.20; 95% CI: 1.05, 1.36; P < 0.01; I 2 = 0%; P = 0.76). This result needs to be carefully considered in order to reduce the risk of PJI. Although the number of available studies is still too small to accurately define the real extent of the risk, a minimum interval of 3 months is advisable between the two procedures. Albanese et al. confirm the same result limited to the use of CS in their recently published meta-analysis (37).
The role of corticosteroids
One of the suggested pathogenetic mechanisms in developing PJI after intra-articular injections is the prolonged immunosuppressive effect of CS agents (38). In order to investigate whether the use of CS actually increases the infectious risk compared to HA, we performed a subgroup analysis based on the type of drug used for the articular injection. The available data, however, was limited. Only six studies clearly differentiated the cohort of patient who received the cohort on the basis of the drug used, in five cases CS was used (24, 25, 26, 27, 33) and in only one study HA was used (32)). Four studies did not discriminate between CS and HA (29, 30, 31, 34). None of the included studies reported results for PRP or bone marrow-derived mesenchymal stem cells (BMDC). Although the available studies did not allow to draw definitive conclusions, we found that CS articular injection significantly increases the risk of PJI with respect to the general risk (64% vs 38%). Further investigations, including other agents like PRP or BMDC, are needed, and this could be the subject of a subsequent literature review.
Similarly, it would be interesting to evaluate whether and how repeated hip injections lead to a higher risk of infection than a single one. The study by Chambers et al. suggests that repeated injections are associated with a statistically higher risk of infection (41).
Limits of our meta-analysis
Our meta-analysis has several limitations. The included studies are all retrospective, and there is heterogeneity, albeit moderate. Furthermore, almost all the studies included have foreseen the use of CS. We cannot definitively demonstrate whether this could have influenced the results. Finally, not all studies included used the Musculo Skeletal infection Society (MSIS) criteria for the diagnosis of PJI. On the other hand, there are many strengths of this meta-analysis: a high number of patients including big datasets by national registries, low publication bias and a high-quality score of the studies according to NOS.
Conclusions
Intra-articular injection is a procedure that may increase the risk of developing PJI if performed less than 3 months before THA. For this reason, it is essential that the orthopaedic surgeon investigates whether the patient has been injected in the previous 3 months before scheduling surgery. The patient that requests infiltrative treatment of the hip joint must be informed of this risk as well as of all possible complications.
Supplementary Material
ICMJE conflict of interest statement
Luigi Zagra is an associate editor on the editorial board of EFORT Open Reviews. Luigi Zagra was not involved in the review or editorial process for this paper on which he is listed as an author. The other authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported..
Funding
This work did not receive any specific grant from any funding agency in thepublic, commercial, or not-for-profit sector.
Author contribution statement
MS, VC and FD collected and analysed data; GS, LZ and GL revised the manuscript.
References
- 1.Woolf AD & Pfleger B. Burden of major musculoskeletal conditions. Bulletin of the World Health Organization 200381646–656. [PMC free article] [PubMed] [Google Scholar]
- 2.Castell MV, van der Pas S, Otero A, Siviero P, Dennison E, Denkinger M, Pedersen N, Sanchez-Martinez M, Queipo R, van Schoor N, et al. Osteoarthritis and frailty in elderly individuals across six European countries: results from the European Project on OSteoArthritis (EPOSA). BMC Musculoskeletal Disorders 201516 359. ( 10.1186/s12891-015-0807-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y Wei X Zhou J & Wei L. The age-related changes in cartilage and osteoarthritis. BioMed Research International 20132013916530. ( 10.1155/2013/916530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaishya R Pariyo GB Agarwal AK & Vijay V. Non-operative management of osteoarthritis of the knee joint. Journal of Clinical Orthopaedics and Trauma 20167170–176. ( 10.1016/j.jcot.2016.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennell KL Hunter DJ & Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Current Rheumatology Reports 201719 24. ( 10.1007/s11926-017-0652-x) [DOI] [PubMed] [Google Scholar]
- 6.Gazendam A Ekhtiari S Bozzo A Phillips M & Bhandari M. Intra-articular saline injection is as effective as corticosteroids, platelet-rich plasma and hyaluronic acid for hip osteoarthritis pain: a systematic review and network meta-analysis of randomised controlled trials. British Journal of Sports Medicine 202155256–261. ( 10.1136/bjsports-2020-102179) [DOI] [PubMed] [Google Scholar]
- 7.Wu B Li YM & Liu YC. Efficacy of intra-articular hyaluronic acid injections in hip osteoarthritis: a meta-analysis of randomized controlled trials. Oncotarget 2017886865–86876. ( 10.18632/oncotarget.20995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021372 n71. ( 10.1136/bmj.n71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells G Shea B O’Connell D Peterson J Welch V Losos M & Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (date last accessed 21 December 2022) 2021. [Google Scholar]
- 10.Mantel N & Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 195922719–748. [PubMed] [Google Scholar]
- 11.Greenland S & Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 19854155–68. ( 10.2307/2530643) [DOI] [PubMed] [Google Scholar]
- 12.Paule RC & Mandel J. Consensus values and weighting factors. Journal of Research of the National Bureau of Standards 198287 377–385. ( 10.6028/jres.087.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapp G & Hartung J. Improved tests for a random effects meta-regression with a single covariate. Statistics in Medicine 2003222693–2710. ( 10.1002/sim.1482) [DOI] [PubMed] [Google Scholar]
- 14.IntHout J Ioannidis JP & Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Medical Research Methodology 201414 25. ( 10.1186/1471-2288-14-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langan D Higgins JPT Jackson D Bowden J Veroniki AA Kontopantelis E Viechtbauer W & Simmonds M. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Research Synthesis Methods 20191083–98. ( 10.1002/jrsm.1316) [DOI] [PubMed] [Google Scholar]
- 16.Gart JJ & Zweifel JR. On the bias of various estimators of the logit and its variance with application to quantal bioassay. Biometrika 196754181–187. ( 10.1093/biomet/54.1-2.181) [DOI] [PubMed] [Google Scholar]
- 17.L’Abbé KA Detsky AS & O’Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine 1987107224–233. ( 10.7326/0003-4819-107-2-224) [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT & Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002211539–1558. ( 10.1002/sim.1186) [DOI] [PubMed] [Google Scholar]
- 19.Viechtbauer W. Confidence intervals for the amount of heterogeneity in meta-analysis. Statistics in Medicine 20072637–52. ( 10.1002/sim.2514) [DOI] [PubMed] [Google Scholar]
- 20.IntHout J Ioannidis JPA Rovers MM & Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 20166 e010247. ( 10.1136/bmjopen-2015-010247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viechtbauer W & Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Research Synthesis Methods 20101112–125. ( 10.1002/jrsm.11) [DOI] [PubMed] [Google Scholar]
- 22.Baujat B Mahé C Pignon JP & Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Statistics in Medicine 2002212641–2652. ( 10.1002/sim.1221) [DOI] [PubMed] [Google Scholar]
- 23.Egger M Davey Smith G Schneider M & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997315629–634. ( 10.1136/bmj.315.7109.629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaspar S de V & de Beer J. Infection in hip arthroplasty after previous injection of steroid. Journal of Bone and Joint Surgery. British Volume 200587-B454–457. ( 10.1302/0301-620X.87B4.15546) [DOI] [PubMed] [Google Scholar]
- 25.McIntosh AL Hanssen AD Wenger DE & Osmon DR. Recent intraarticular steroid injection may increase infection rates in primary THA. Clinical Orthopaedics and Related Research 200645150–54. ( 10.1097/01.blo.0000229318.51254.79) [DOI] [PubMed] [Google Scholar]
- 26.Sreekumar R Venkiteswaran R & Raut V. Infection in primary hip arthroplasty after previous steroid infiltration. International Orthopaedics 200731125–128. ( 10.1007/s00264-006-0152-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meermans G Corten K & Simon JP. Is the infection rate in primary THA increased after steroid injection? Clinical Orthopaedics and Related Research 20124703213–3219. ( 10.1007/s11999-012-2390-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croft S & Rockwood P. Risk of intraarticular steroid injection before total hip arthroplasty. Current Orthopaedic Practice 201324185–188. ( 10.1097/BCO.0b013e3182847788) [DOI] [Google Scholar]
- 29.Ravi B Escott BG Wasserstein D Croxford R Hollands S Paterson JM Kreder HJ & Hawker GA. Intraarticular hip injection and early revision surgery following total hip arthroplasty: a retrospective cohort study. Arthritis and Rheumatology 201567162–168. ( 10.1002/art.38886) [DOI] [PubMed] [Google Scholar]
- 30.Schairer WW Nwachukwu BU Mayman DJ Lyman S & Jerabek SA. Preoperative hip injections increase the rate of periprosthetic infection after total hip arthroplasty. Journal of Arthroplasty 201631(Supplement) 166–169.e1. ( 10.1016/j.arth.2016.04.008) [DOI] [PubMed] [Google Scholar]
- 31.Werner BC Cancienne JM & Browne JA. The timing of total hip arthroplasty after intraarticular hip injection affects postoperative infection risk. Journal of Arthroplasty 201631820–823. ( 10.1016/j.arth.2015.08.032) [DOI] [PubMed] [Google Scholar]
- 32.Colen S Hoorntje A Maeckelbergh L van Diemen M Dalemans A van den Bekerom MPJ & Mulier M. Intra-articular hyaluronic acid injections less than 6 months before total hip arthroplasty: is it safe? A retrospective cohort study in 565 patients. Journal of Arthroplasty 2021361003–1008. ( 10.1016/j.arth.2020.09.024) [DOI] [PubMed] [Google Scholar]
- 33.Forlenza EM Burnett RA Korrapati A Yang J Forsythe B & Della Valle CJ. Preoperative corticosteroid injections demonstrate a temporal and dose-dependent relationship with the rate of postoperative infection following total hip arthroplasty. Journal of Arthroplasty 2021362033–2037.e1. ( 10.1016/j.arth.2021.01.076) [DOI] [PubMed] [Google Scholar]
- 34.Tang A Almetwali O Zak SG Bernstein JA Schwarzkopf R & Aggarwal VK. Do preoperative intra-articular corticosteroid and hyaluronic acid injections affect time to total joint arthroplasty? Journal of Clinical Orthopaedics and Trauma 20211649–57. ( 10.1016/j.jcot.2020.12.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindeque B Hartman Z Noshchenko A & Cruse M. Infection after primary total hip arthroplasty. Orthopedics 201437257–265. ( 10.3928/01477447-20140401-08) [DOI] [PubMed] [Google Scholar]
- 36.Nie F & Li W. Impact of prior intra-articular injections on the risk of prosthetic joint infection following total joint arthroplasty: a systematic review and meta-analysis. Frontiers in Surgery 20218 737529. ( 10.3389/fsurg.2021.737529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albanese J Feltri P Boffa A Werner BC Traina F & Filardo G. Infection risk increases after total hip arthroplasty within 3 months following intra-articular corticosteroid injection. A meta-analysis on knee and hip arthroplasty. Journal of Arthroplasty 2022. ( 10.1016/j.arth.2022.12.038) [DOI] [PubMed] [Google Scholar]
- 38.Charalambous CP Prodromidis AD & Kwaees TA. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. Journal of Arthroplasty 2014292175–2180. ( 10.1016/j.arth.2014.07.013) [DOI] [PubMed] [Google Scholar]
- 39.Li H Xing D Ke Y & Lin J. Safety of intra-articular steroid injections prior to arthroplasty: best evidence selection and risk of bias considerations. International Journal of Rheumatic Diseases 201821982–991. ( 10.1111/1756-185X.13314) [DOI] [PubMed] [Google Scholar]
- 40.Chitre AR Fehily MJ & Bamford DJ. Total hip replacement after intra-articular injection of local anaesthetic and steroid. Journal of Bone and Joint Surgery. British Volume 200789166–168. ( 10.1302/0301-620X.89B2.18428) [DOI] [PubMed] [Google Scholar]
- 41.Chambers AW Lacy KW Liow MHL Manalo JPM Freiberg AA & Kwon YM. Multiple hip intra-articular steroid injections increase risk of periprosthetic joint infection compared with single injections. Journal of Arthroplasty 2017321980–1983. ( 10.1016/j.arth.2017.01.030) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a