Abstract
Breast cancer is one of the leading causes of death in women worldwide. Recent studies have demonstrated that inflammation due to infections with microorganisms could play a role in breast cancer development. One of the known human pathogens, Borrelia burgdorferi, the causative agent of Lyme disease, has been shown to be present in various types of breast cancer and is associated with poor prognosis. We reported that B. burgdorferi can invade breast cancer cells and affect their tumorigenic phenotype. To better understand the genome-wide genetic changes caused by B. burgdorferi, we evaluated the microRNA (miRNA or miR) expression profiles of two triple-negative breast cancer cell lines and one non-tumorigenic mammary cell line before and after B. burgdorferi infection. Using a cancer-specific miRNA panel, four miRNAs (miR-206, 214-3p, 16-5p, and 20b-5p) were identified as potential markers for Borrelia-induced changes, and the results were confirmed by quantitative real-time reverse transcription (qRT-PCR). Among those miRNAs, miR-206 and 214 were the most significantly upregulated miRNAs. The cellular impact of miR-206 and 214 was evaluated using DIANA software to identify related molecular pathways and genes. Analyses showed that the cell cycle, checkpoints, DNA damage–repair, proto-oncogenes, and cancer-related signaling pathways are mostly affected by B. burgdorferi infection. Based on this information, we have identified potential miRNAs which could be further evaluated as biomarkers for tumorigenesis caused by pathogens in breast cancer cells.
Keywords: breast cancer, Borrelia burgdorferi, infection, microRNA, biomarkers
1. Introduction
Breast cancer is one of the leading causes of death in women, exhibiting a severe prognosis and high mortality rate [1]. Advancements in the stages of breast cancer make the prevention of the disease more complex; hence, detecting breast cancer at an earlier stage is essential for a better prognosis [2].
Breast cancer is a complex disease with multiple factors contributing to its development. For example, inherited mutations in genes such as BRCA1 and BRCA2 or exposure to toxic substances such as tobacco, smoke, and alcohol can increase a person’s risk of developing breast cancer [3,4,5]. The impact of other environmental factors such as lifestyle, diet, physical activity, hormonal influences, and exposure to radiation on breast cancer development is still being studied [4,5]. It is important to note that the development of breast cancer is influenced by a combination of these factors and further research is needed to fully understand the mechanisms that contribute to this disease.
Carcinogenesis can be initiated by a prolonged history of inflammation, which can be triggered by pathogenic infection, leading to a cascade of inflammatory events and a defective immune response [1,6]. To support this hypothesis, studies show that specific bacterial infections are significant risk factors for carcinomas of different organ systems including the breast, stomach, colon, and pancreas [7,8,9]. For example, a study comparing cancerous and normal ovarian samples showed that a higher percentage of samples in the ovarian cancer group tested positive for Chlamydia trachomatis infection in comparison to the control group [10,11]. Additionally, infection with Helicobacter pylori plays a crucial role in the development of gastric cancer because of the pathogen’s ability to disrupt the host’s mucosal homeostasis [9]. Further, multiple research groups have found that the advancement of colorectal cancer (CRC) is influenced by intestinal bacteria including H. pylori and the anaerobic bacteria Fusobacterium nucleatum [12,13,14,15]. Several studies present that F. nucleatum facilitates colorectal carcinoma by impacting the role of tumor-infiltrating lymphocytes and suppressing the action of natural killer (NK) cells, thus producing resistance to chemotherapy [14,15]. It was also revealed that an increased number of F. nucleatum in breast cancer cells enhances tumor progression and metastases by impairing the activity of T cells [14]. Another study explored the role of microbiomes in the progression of breast cancer and metastasis [16]. In this study, researchers identified a tumor, called the mouse mammary tumor virus-polyoma middle tumor antigen (MMTV-PyMT), that closely resembles human breast cancer in mice [16]. Researchers observed that breast tumor cells exhibited an oxygen microenvironment that significantly reduced anaerobes while increasing facultative anaerobes [16]. In light of this observation, it was concluded that PyMT spontaneous breast tumors contain significant quantities of live bacteria [16]. In addition, a substantial decrease in lung metastasis was observed following intratumor bacterial reduction by antibiotic treatment administered to eliminate tumor-resident microbiota [16]. These observations suggested that tumor-associated bacteria could assist in cancer spread and metastasis [16]. Additionally, it was shown that microorganisms are capable of inducing DNA damage in breast cancer tissues but not in normal breasts [17].
To further strengthen the above observation, findings from a recent microbiome study showed that several pathogenic bacteria were present in different types of breast cancer tissues [1]. One such microorganism was Borrelia burgdorferi, a spirochete that causes Lyme disease. The presence of B. burgdorferi in breast cancer tissues was also found to be associated with poor prognosis [1]. This information explains the need to further investigate the influential role of B. burgdorferi in the development of breast cancer.
Lyme disease is a vector-borne human disease that affects various body systems, causing musculoskeletal, neurological, and cardiac conditions [18]. B. burgdorferi manipulates the mammalian host cells after its entry by modifying its glycoproteins, which help it to invade and propagate in different body tissues [18,19]. It was reported that B. burgdorferi could invade human brain cells in vitro and induce an inflammatory process [19]. Further, it was also observed that by the mechanism of intracellular localization B. burgdorferi could stay hidden from the host’s immune system [19]. Our research group has recently found evidence that B. burgdorferi can invade the triple-negative MDA-MB-231 breast cancer cells at a significantly higher rate in comparison to normal mammary epithelial cells [20]. This study also showed that the in vitro invasion and migratory capability of breast cancer cells was significantly increased following B. burgdorferi infection [20]. Our current research focused on identifying the molecular changes that occur when B. burgdorferi infects breast tissues, and whether these changes could initiate or progress tumor development. In recent years, microRNAs (miRNA or miR) have been identified as novel cancer biomarkers that can play a vital role in the regulation of molecular mechanisms leading to tumorigenesis [21]. Therefore, the goal of this study was to identify changes in miRNA expression levels in B. burgdorferi-infected versus uninfected breast cancer cells to better understand the potential relationship between this infection and breast cancer development.
miRNAs are small non-coding RNAs that post-transcriptionally regulate gene expression [21]. miRNAs are involved in regulating many molecular pathways, and their dysregulation can lead to disease development, including various cancers [21]. Some miRNAs act as tumor suppressors and exhibit decreased expression in certain cancers, while other miRNAs are considered oncogenes and are expressed at higher levels in some cancer types [22]. miRNAs are also implicated in cancer-related processes such as inflammation and tumor progression [22,23]. Numerous studies have reported that the development of breast cancer involves the dysregulated expression of miRNAs [21]. Furthermore, previous research has reported that B. burgdorferi infection can affect the expression of miRNAs in host cells [24,25]. It is thus essential to understand the role of miRNAs in B. burgdorferi-infected breast carcinoma pathogenesis.
This study focused on analyzing the expression levels of several cancer-associated miRNAs in breast cancer cells following B. burgdorferi infection. These potential target miRNAs were selected based on results from an exploratory miRNA breast cancer array and a scientific literature review. A confirmatory study was carried out using quantitative real-time reverse transcription PCR (qRT-PCR), which is considered a standard method for gene expression measurement. This study also focused on analyzing the pathways regulated by our target miRNAs and their associated genes using DIANA software [26]. This research helps in better understanding the miRNAs that contribute to B. burgdorferi infection-induced responses and breast cancer development, as well as their associated pathways and genes.
2. Materials and Methods
2.1. Breast Cancer Array
A Qiagen human breast cancer miRCURY LNA miRNA Focus PCR panel (Qiagen, Hilden, Germany, Cat No: 339325) was utilized to identify target miRNAs that exhibit differential expression in response to B. burgdorferi infection along with a scientific literature review. The breast cancer miRNA panel is a ready-to-use 96-well PCR plate that can be used to quantitate the expression levels of 86 miRNAs specifically associated with breast cancer. This breast cancer array was analyzed by qRT-PCR following the manufacturer’s instructions. The cDNA samples used for this experiment were prepared using RNA extracted from uninfected and B. burgdorferi-infected MDA-MB-231 cells. The delta-delta Ct method was used to quantify any changes in miRNA expression, with SNORD49 acting as a reference gene.
2.2. Mammalian Cell Culture
The normal mammary epithelial cell line MCF-10A (American Type Culture Collection, ATCC CRL-10317, Manassas, VA, USA) and the triple-negative breast cancer cell line MDA-MB-231 (ATCC HTB-26) were cultured using standard tissue culture conditions as previously reported (Gaur 2021). An additional triple-negative breast cancer cell line, HCC1806 (ATCC CRL-2335), was cultured and maintained in RPMI-1640 media (Sigma Aldrich, Louis, MO, USA) with 10% fetal bovine serum (FBS, Gemini Bio, West Sacramento, CA, USA) supplemented with 1% Penicillin–Streptomycin–Glutamine (PSG, ThermoFisher Scientific, Louis, MO, USA). Before the initiation of infection, cells were synchronized to the G0 phase of the cell cycle by incubating in serum-free media for 10 hours. The cells at G0 phase represent a quiescent phase in which cells are not actively dividing, thus allowing analysis of the initial events of the cell cycle [27]. In addition, synchronizing cells reduces the variability between cells, creating a more precise way of comparing experimental results and achieving increased reproducibility of experiments [27]. Following synchronization, a total of one million cells were infected with B. burgdorferi with a multiplicity of infection (MOI) of 60 as previously described [20].
2.3. Bacterial Culture
Low passage isolates (<6) of B. burgdorferi B31 strain (ATCC #35210) were utilized for infection following our previously published protocol (Gaur 2021). All experiments were conducted in three biological replicates (N = 3); four uninfected and four B. burgdorferi-infected cell samples from independent rounds of infection were collected and analyzed for each breast cell type studied (N = 12).
2.4. Total RNA Extraction
Total RNA from MCF-10A, MDA-MB-231, and HCC1806 cell samples (1 × 106 cells/sample) was extracted using a Qiagen miRNeasy Mini Kit (Qiagen Cat: 217004) following the manufacturer’s instructions. The extracted RNA was stored at −80 °C until further experimental application.
2.5. cDNA Synthesis/Quantitation
Single-stranded cDNA was synthesized using a miRCURY LNA RT Kit (Qiagen, 339340) following the manufacturer’s instructions. Each RT reaction required 200 ng of input RNA. cDNA was then quantitated using the Qubit 2.0 Fluorometer and Qubit ssDNA Assay Kit as recommended by the kit protocol (ThermoFisher Scientific, Q10212).
2.6. qPCR Analysis of miRNA
qPCR was used to analyze changes in the expression of target miRNAs between uninfected and infected breast cell samples. A miRCURY LNA SYBR Green PCR kit (Qiagen, 339345) and miRNA-specific primers (miRCURY LNA miRNA PCR Assays, Qiagen, 339306) were used to perform qPCR as per the manufacturer’s instructions. Each qPCR reaction was prepared using 60 ng of template cDNA. The expression of target miRNAs was measured in quadruplets for each of the three biological replicates (N = 12).
2.7. Calculating miRNA Expression Fold Changes and Statistical Analysis
miRNA expression fold changes were calculated by first obtaining the Cq (cycle of quantitation) values from each qPCR reaction. A total of 12 Cq values were obtained for each target miRNA, from which the three outliers based on the highest and lowest values were excluded from data analysis. Delta-delta Ct values and the expression fold change of each target miRNA between the uninfected and infected samples were calculated using the Microsoft Excel program (Microsoft, Redmont, WA, USA). Statistical analysis for the calculated expression fold changes was performed using a paired, two-tailed Student’s t-test in the Qiagen miRCURY LNA miRNA PCR data analysis tool (Qiagen). The data were normalized using the normalization method geNorm (pre-defined reference miRNA only). Using this method, data related to miRNA expression can be normalized based on reference miRNAs in a more accurate and reliable way. A stability measure of <1.5 was returned for the reference gene SNORD48, which was found to be the steadiest reference gene. The expression fold change of a miRNA with a p-value of <0.05 was considered statistically significant.
2.8. DIANA Analysis of Target miRNAs
The mirPath v.3 DIANA software and DIANA-TarBase v7.0 were used to identify cellular pathways and genes associated with the target miRNAs (https://dianalab.e-ce.uth.gr/html/mirpathv3/index.php?r=mirpath (accessed on 3 January 2023).
3. Results
The goal of this study was to identify any expression fold changes in cancer-associated miRNA molecules between B. burgdorferi-infected and uninfected triple-negative breast cancer cell lines MDA-MB-231 and HCC1806. miRNA expression level changes in response to infection were further compared between the two triple-negative breast cancer cell lines and the normal mammary epithelial cell line MCF10A. Along with a published literature review, we performed an exploratory study using a cancer-specific miRNA PCR panel to identify miRNAs with the greatest amount of differential expression in B. burgdorferi-infected breast cancer cells compared to the uninfected control [28,29,30,31]. These data led to the identification of four potential microRNAs for evaluation in our study using real-time PCR and pathway analysis.
3.1. Breast Cancer Array
In an early exploratory experiment, we used a qPCR array from Qiagen to identify miRNAs that are differentially expressed in B. burgdorferi-infected MDA-MB-231 cells compared to uninfected control cells (miRCURY LNA miRNA Focus PCR Panels www.qiagen.com (accessed on 10 December 2022). Results obtained from this array showed that the expression of 38 miRNAs was increased, while the expression of 48 miRNAs was decreased in B. burgdorferi-infected MDA-MB-231 cells (Supplementary Data). Further analyses showed that seven miRNAs were upregulated by >1.5-fold (Table 1), and ten miRNAs were downregulated by >1.5-fold (Table 2).
Table 1.
List of seven miRNAs observed to be upregulated in MDA-MB-231 breast cancer cells after infection with B. burgdorferi based on data from a Qiagen breast cancer microRNA panel.
| Upregulated miRNAs | |
|---|---|
| miRNA | Fold Change |
| miR-206 | 26.06 |
| miR-202-3p | 3.92 |
| miR-214-3p | 2.66 |
| miR-20b-5p | 2.11 |
| miR-146a-5p | 1.73 |
| miR-16-5p | 1.6 |
| miR-101-3p | 1.56 |
Table 2.
List of 10 miRNAs observed to be downregulated in MDA-MB-231 breast cancer cells after infection with B. burgdorferi based on data from a Qiagen breast cancer microRNA panel.
| Downregulated miRNAs | |
|---|---|
| miRNA | Fold Change |
| miR-22-3p | −1.55 |
| miR-423-5p | −1.55 |
| miR-133a-3p | −1.56 |
| miR-29b-3p | −1.56 |
| let-7b-5p | −1.63 |
| miR-155-5p | −1.66 |
| miR-200a-3p | −1.99 |
| miR-181a-5p | −2.14 |
| miR-145-5p | −2.16 |
| miR-143-3p | −2.4 |
3.2. Selection of Target miRNAs
Target miRNAs were selected based on results from the exploratory Qiagen breast cancer array panel and a review of published scientific literature. The breast cancer array data were used to identify four target miRNAs (miR-206, 214-3p, 16-5p, and 20b-5p) that exhibited the most differentiated Cq values between uninfected and B. burgdorferi-infected cells. All the miRNAs selected were found to be related to either breast cancer or reported to be dysregulated in association with B. burgdorferi infection (Table 3). To confirm that these four miRNAs were indeed dysregulated in B. burgdorferi-infected breast cancer cells compared to the uninfected control, our target miRNAs were further investigated by quantitative real-time PCR.
Table 3.
List of target miRNAs with a focus on their associations with breast cancer and bacterial infection.
| miRNA | General Overview | Role in Breast Cancer/Infections |
|---|---|---|
| miR-206 | Oncogene |
|
| miR-214-3p | Oncogene | |
| miR-16-5p | Tumor suppressor | |
| miR-20b-5p | Oncogene |
3.3. Selection of a Reference Gene
A reliable reference gene that shows stable expression under both experimental and control conditions is necessary for data normalization and obtaining reliable expression fold change values [38,39]. Our study used a small nucleolar RNA, SNORD48, as a reference gene because of its undifferentiated expression and stability throughout the qRT-PCR experiments performed. Moreover, our scientific literature review identified SNORD48 as an ideal reference gene for analyzing the expression of miRNAs by qRT-PCR [39,40].
3.4. Validation of the Differential Expression of Four Selected miRNAs in B. burgdorferi-Infected Breast Cell Lines by qRT-PCR
Differential expressions of the four target miRNAs identified above were validated by comparing the expression levels of each miRNA between B. burgdorferi-infected and uninfected breast cells. Expression levels of each target miRNA were measured in uninfected and infected breast cancer cell lines HCC1806, MDA-MB-231, and normal breast cell line MCF10A using qRT-PCR. Differences in miRNA expression levels between the uninfected and infected samples are outlined in Table 4, Table 5 and Table 6 below.
Table 4.
Expression fold change of target miRNAs in B. burgdorferi-infected HCC1806 triple-negative breast cancer cells compared to uninfected control cells. qRT-PCR data were normalized using SNORD48 as a reference gene. Expression fold changes with a p-value of <0.05 were considered statistically significant.
| miRNA | Fold Change | p-Value |
|---|---|---|
| hsa-miR-206 | 4.92 | 1.34 × 10−3 |
| hsa-miR-214-3p | 2.22 | 2.66 × 10−3 |
| hsa-miR-16-5p | 0.94 | 3.89 × 10−1 |
| hsa-miR-20b-5p | 1.34 | 4.65 × 10−3 |
Table 5.
Expression fold changes of target miRNAs in B. burgdorferi-infected MDA-MB-231 triple-negative breast cancer cells compared to uninfected control cells. qRT-PCR data were normalized using SNORD48 as a reference gene. Expression fold changes with a p-value of <0.05 were considered statistically significant.
| miRNA | Fold Change | p-Value |
|---|---|---|
| miR-206 | 5.13 | 3.00 × 10−6 |
| miR-214-3p | 2.14 | 0.00 |
| miR-16-5p | 1.12 | 9.70 × 10−5 |
| miR-20b-5p | 1.3 | 2.16 × 10−2 |
Table 6.
Expression fold changes of target miRNAs in B. burgdorferi-infected MCF10A normal mammary epithelial cells compared to uninfected control cells. qRT-PCR data were normalized using SNORD48 as a reference gene. Expression fold changes with a p-value of <0.05 were considered statistically significant.
| miRNA | Fold Change | p-Value |
|---|---|---|
| miR-206 | 0.6 | 8.74 × 10−4 |
| miR-214-3p | 0.82 | 6.30 × 10−3 |
| miR-16-5p | 0.97 | 6.07 × 10−1 |
| miR-20b-5p | 0.85 | 8.44 × 10−3 |
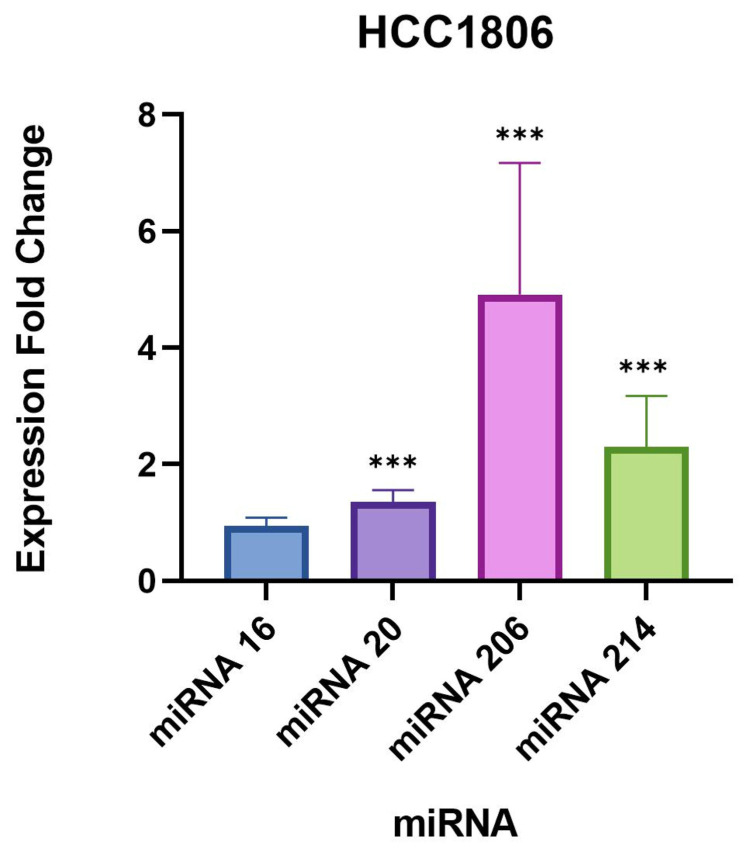
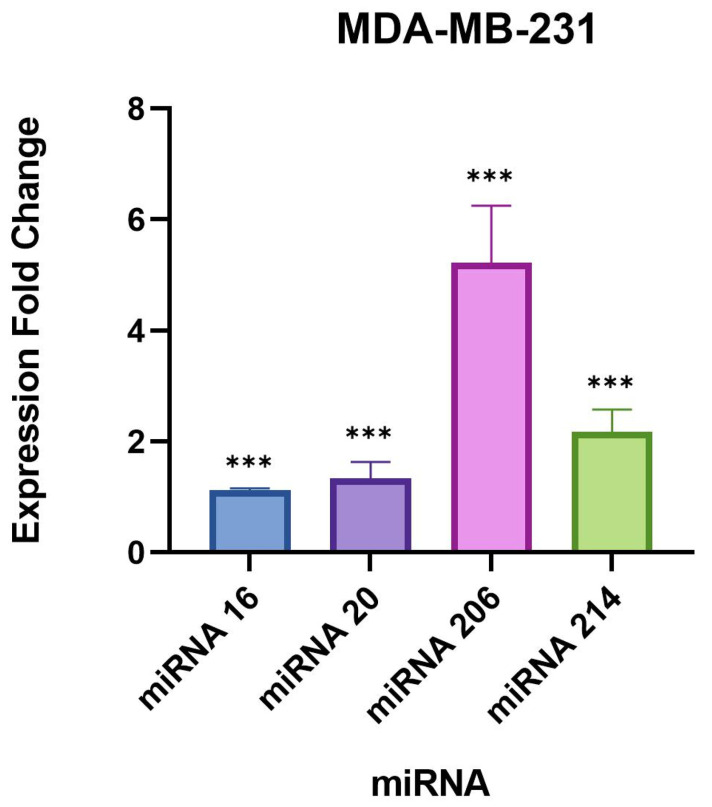
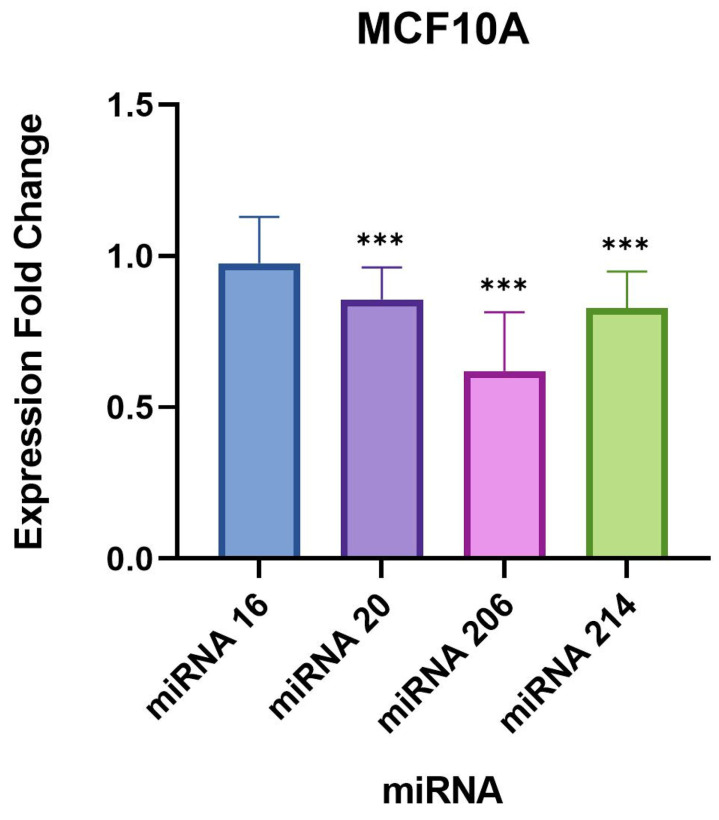
The largest difference in miRNA expression identified between uninfected and infected samples was observed for miR-206, which was significantly upregulated by approximately 5-fold in both triple-negative breast cancer cell lines (Table 4 and Table 5, Figure 1 and Figure 2). Interestingly, miR-206 was also slightly but significantly upregulated in the normal breast cell line MCF10A by 0.6-fold (Table 6, Figure 3). A very similar trend was found for miR-214-3p which was significantly upregulated in all three cell lines, such as miR-214-3p which was observed to be upregulated by approximately 2-fold in the MDA-MB-231 and HCC1806 cell samples, and 0.8-fold in the MCF10A cell samples (Table 4, Table 5 and Table 6, Figure 1, Figure 2 and Figure 3). A small but statistically significant change in the expression of miR-16-5p was observed only in the MDA-MB-231 cell line. A minor, statistically significant change in miR-20-5p expression was also found in all three cell lines, in the range of 0.8–1.3-fold (Table 4, Table 5 and Table 6, Figure 1, Figure 2 and Figure 3).
Figure 1.
Expression fold changes of the target miRNAs in B. burgdorferi-infected HCC1806 triple-negative breast cancer cells compared to the uninfected HCC1806 cells. ***, p-value < 0.05.
Figure 2.
Expression fold changes of the target miRNAs in B. burgdorferi-infected MDA-MB-231 triple-negative breast cancer cells compared to the uninfected MDA-MB-231 cells. ***, p-value < 0.05.
Figure 3.
Expression fold changes of the target miRNAs in B. burgdorferi-infected MCF10A non-tumorigenic mammary epithelial cells compared to the uninfected MCF10A cells. ***, p-value < 0.05.
3.5. DIANA Analysis of Target miRNAs
The mirPath v.3 DIANA software was used to investigate the cellular pathways associated with each of the target miRNAs chosen in this study. Further assessment was performed using KEGG analysis and the Tarbase v7.0 database.
Multiple statistically significant pathways (p-value of <0.05) associated with each target miRNA were identified. Table 7 summarizes the pathways associated with each target miRNA and their corresponding p-values. The observed data show that the Gap junction pathway is most significantly affected by miR-206, followed by the cell cycle, mismatch repair, and the ECM (extracellular matrix) receptor interaction pathways. miR-214-3p most significantly affects the ECM–receptor interaction pathway but is also associated with the GAP junction, microRNAs in cancer, and additional cancer pathways. The cell cycle, p53 signaling pathway, and pathways in cancer were observed to have a significant association with miR-16-5p. For miR-20b-5p, the p53 signaling pathway and pathways in cancer were found to be statistically significant.
Table 7.
Cellular pathways associated with target miRNAs and their statistical significance (p-value < 0.05) obtained from DIANA analysis.
| miRNA | Pathway | p-Value |
|---|---|---|
| miR-206 | GAP junction | 6.48 × 10−6 |
| Cell cycle | 0.000197 | |
| Mismatch repair | 0.003945 | |
| ECM–receptor interaction | 0.03367 | |
| miR-214-3p | ECM–receptor interaction | 3.40 × 10−17 |
| GAP junction | 7.14 × 10−7 | |
| microRNAs in cancer | 0.004235964 | |
| Pathways in cancer | 0.036322571 | |
| miR-16-5p | Cell cycle | 3.38 × 10−6 |
| P53 signaling pathway | 0.000887863 | |
| Pathways in cancer | 0.001992202 | |
| miR-20b-5p | P53 signaling pathway | 0.0054649 |
| Pathways in cancer | 0.012188139 |
Next, shared pathways were identified using DIANA which was further analyzed to identify those associated with more than one of the four target miRNAs (Table 8). The GAP junction and ECM–receptor interaction pathways were found to be affected by both miR-206 and 214-3p. The cell cycle pathway has been associated with miR-206 and 16-5p. Finally, the p53 signaling pathway was observed to be affected by miR-16-5p and 20b-5p. Pathways in cancer were alternatively associated with three out of four target miRNAs, miR-214-3p, 16-5p, and 20b-5p. In summary, pathways in cancer are the only pathway affected by more than two of the four target miRNAs. The remaining two pathways, mismatch repair and microRNAs in cancer, are each influenced by only one target miRNA: miR-206 and 214-3p, respectively.
Table 8.
Representation of common pathways associated with target miRNAs identified using DIANA analysis. ✓: means Present.
| Pathway | miR-206 | miR-214-3p | miR-16-5p | miR-20b-5p |
|---|---|---|---|---|
| GAP junction | ✓ | ✓ | - | - |
| ECM–receptor interaction | ✓ | ✓ | - | - |
| Cell cycle | ✓ | - | ✓ | - |
| Pathways in cancer | - | ✓ | ✓ | ✓ |
| p53 signaling pathway | - | - | ✓ | ✓ |
| Mismatch repair | ✓ | - | - | - |
| MicroRNAs in cancer | - | ✓ | - | - |
| Total no of pathways | 4 | 4 | 3 | 2 |
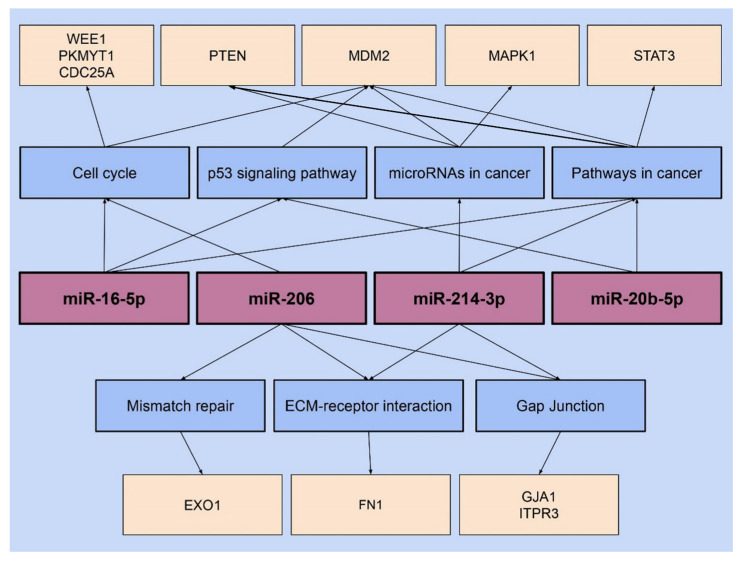
The pathways associated with each target miRNA were further analyzed to identify the specific genes affected by miRNA interaction; these genes are outlined in Figure 4. The ECM–receptor interaction pathway gene Fibronectin-1 protein (FN1) has been found to be affected by both miR-206 and 214. miR-206 and 214 also affect the Gap junction pathway genes Gap Junction Protein Alpha 1 (GJA1) and Inositol 1,4,5-trisphosphate receptor type 3 (ITPR3). miR-214 and 206 are each associated with additional genes related to other pathways. Two pathways associated with miR-214-3p, microRNAs in cancer and pathways in cancer, were both observed to involve the gene Phosphatase and Tensin homolog (PTEN). miRNA-214-3p is also associated with Mitogen-Activated Protein Kinase1 (MAPK1) in relation to the microRNAs in the cancer pathway. miR-206 has been associated with the Exonuclease 1 (EXO1) gene, which is related to the mismatch repair pathway. The cell cycle pathway-related genes WEE1 G2 Checkpoint Kinase (WEE1) and Protein Kinase Membrane Associated Tyrosine/Threonine 1 (PKMYT1) were both associated with miR-206. Different cell cycle-related genes including MDM2 (Murine Double Minute 2) and CDC25A (Cell Division Cycle 25A) were alternatively found to be affected by miR-16-5p. miR-16-5p and 20b-5p were found to be associated with STAT3 (Signal Transducer and Activator of Transcription 3), a gene related to pathways in cancer.
Figure 4.
miRNA pathways and the related genes of each target miRNA through DIANA analysis.
4. Discussion
Previous research has demonstrated that the dysregulation of miRNA expression plays a significant role in breast cancer development [21,28,29,30,31]. Other reports have indicated that B. burgdorferi infection can affect the expression of certain miRNAs in host cells [25]. Therefore, we aimed to understand the impact of B. burgdorferi infection on the expression and function of miRNAs in breast cancer. In this study, miRNAs of interest were first identified from an exploratory breast cancer array and extensive literature reviews [21,28,29,30,31]. The expression of the target miRNAs was then further validated by qRT-PCR.
We found a very significant expression change of two miRNAs, miR-206 and 214-3p, in B. burgdorferi-infected triple-negative breast cancer cell lines. Studies have previously demonstrated an upregulated expression of these miRNAs in breast cancer cells [28,41], especially miR-206 which was found to be associated with poor prognosis in breast cancer patients [28]. Another study found that miR-206 facilitates the invasion, migration, and proliferation of breast cancer cells by suppressing the expression of the G-protein-coupled receptor neurokinin-1 (NK1R-FL) [41]. Moreover, the proliferation of breast cancer cells was significantly reduced with the downregulation of miR-206 [41]. These previous studies indicate that miR-206 likely plays a role in inducing a tumorigenic response.
Another important effect of miR-206 is the regulation of matrix metalloproteinase 9 (MMP9) expression, a protein that acts in tissue reassembly by activating cytokines and chemokines that degenerate the extracellular matrix [32]. In a mycobacterial infection study, miR-206 was found to regulate the expression of MMP9 by influencing tissue inhibitors of metalloproteinases such as TIMP3 [33]. Another study identified an upregulated expression of miR-206 in the failing hearts of mice and cardiac fibroblast cells and was found to be associated with reduced TIMP 3 activity [42]. Previously, our research group detected a significant increase in the expression of MMP9 in B. burgdorferi-infected MDA-MB-231 cells compared to the uninfected control group [20]. In contrast, the normal mammary epithelial cell line, MCF10A, did not show any change in MMP9 expression after infection with B. burgdorferi [20]. This finding demonstrated that the migratory and invasive characteristics of breast cancer cells were influenced by the level of tissue remodeling markers following infection with B. burgdorferi [20].
miR-214-3p also exhibited significant upregulation in our study which is supported by previous studies that have found miR-214-3p upregulation to be associated with the progression of triple-negative breast cancer [29,43]. One study observed that miR-214 promotes breast cancer progression by impairing the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signal transduction (PI3K/Akt/mTOR) pathway [29]. This pathway is one of the most important cell signaling pathways as it plays an essential role in regulating cell proliferation, apoptosis, and differentiation [29]. Another study indicated that miR-214 was significantly upregulated in sporadic breast cancer patients compared to the hereditary form [43]. Furthermore, triple-negative breast cancer patients with a higher expression of miR-214 exhibited a poorer prognosis than patients with a lower expression of miR-214 [43]. In yet another study, miR-214 was among the miRNAs found to be differentially expressed in primary astrocytes after infection with B. burgdorferi [35]. Moreover, a research study observed that triple-negative breast cancer cells’ viability, migration, and invasion capacity were higher with miR-214-3p upregulation and that inhibiting this miRNA suppressed these effects [44]. Furthermore, miR-214-3p was found to target ST6GAL1 by negative feedback regulation [44]. ST6GAL1 is a sialyltransferase whose transformation relates to the growth, invasion, and metastasis of the tumor [44,45]. It was identified that knocking down ST6GAL1 repressed triple-negative breast cancer cells’ viability, migration, and invasion abilities, which were restored by lowering the expression of miR-214-3p, indicating the association of this miRNA in breast cancer development [44].
In our study, the remaining targets, miR-16-5p and 20b-5p, did not demonstrate drastic expression changes compared to miR-206 and 214-3p, but were still statistically significant in the evaluated breast cell lines. A recent study published in 2021 concluded that the overexpression of miR-16-5p in breast cancer tissues resulted in cell cycle arrest and inhibited breast cancer cell proliferation [30]. An upregulation of ANLN (Anillin actin-binding protein) was identified in breast carcinoma and was a target of miR-16-5p in breast cancer cells [30]. ANLN is involved in cell division and is upregulated in various cancers such as pancreatic and cervical cancer [46,47]. Furthermore, it was discovered that miR-16-5p suppressed ANLN expression contributing to the inhibition of breast cancer cells, similar to the overexpression of miR-16-5p [30]. Additionally, it was reported that infection with Helicobacter pylori (H. pylori) induces an inflammatory process by the persistent colonization of the gastric mucosa, which could ultimately progress to more severe diseases such as gastric carcinoma [36,48]. Research also demonstrated that infection of the gastric epithelial cells resulted in an upregulation of miR-16, suggesting the role of bacterial infection in the dysregulation of this miRNA [48]. miR-20b-5p, evaluated in our study, is also implicated in playing a critical role in the development of breast cancer [31,37]. One study observed that miR-20b-5p significantly promoted breast cancer cell migration and proliferation capacity [31]. Researchers also demonstrated that upregulation of miR-20b-5p in breast cancer stem cells (BCSCs) inhibited their apoptosis and facilitated proliferation [31]. Further, an in vivo experiment involving xenograft tumors of breast cancer cells indicated that miR-20b-5p overexpression led to their significant enlargement [31]. Another study revealed the overexpression of miR-20b in breast cancer cells inhibited a critical tumor suppressor gene, PTEN, by binding to its 3′-UTR [37]. Additionally, the upregulation of miR-20b significantly promoted the proliferation and colony formation of breast cancer cells, whereas its downregulation reduced the growth of breast cancer cells [37]. PTEN downregulation activates the critical oncogenic PI3K/Akt signaling pathway, hence regulating cell proliferation, migration, cell cycle, and apoptosis during the development of cancer [37,49].
Our study further analyzed the pathways and genes associated with each target miRNA using the mirPath v.3 DIANA software. Multiple pathways were found to be associated with each miRNA studied, and each of these pathways involved multiple genes regulated by the corresponding miRNA. For example, miR-206 and 214-5p were found to influence two pathways, ECM–receptor interaction and Gap junction. The ECM–receptor interaction pathway involves communication between cells and the extracellular matrix, a process that assists in the development of cell–matrix interactions and ECM remodeling [50]. Mutation in this pathway can result in the development of a tumor, augment the migration of tumor cells, and initiate metastatic progression by redesigning the ECM in distant organs [50]. Relevantly, one research study has demonstrated a direct association between ECM remodeling and breast cancer progression [51]. The study identified advanced tumor growth caused by increased collagen-I deposition in mouse mammary epithelial cells, along with aggressive tumor progression and invasive lung metastasis [51]. In both miR-206 and 214-5p, the genes FN1 and GJA1 were found to be associated with the ECM–receptor interaction and Gap junction pathway, respectively [26].
The Gap junction pathway, regulated by miR-206 and 214-5p, consists of intercellular channels that lead to the direct transmission of ions and small molecules between the cytosolic compartments of adjacent cells [52]. The upregulated expression of the FN1 gene has been correlated with the development of breast cancer and poor prognosis [53]. It has also been demonstrated that, when upregulated, miR-206 targets and leads to the irregular expression of the FN1 gene in Hirschsprung disease patients [54]. Moreover, B. burgdorferi targets the FN1 protein by expressing BBK32, a fibronectin-binding protein [55]. An upregulated expression of the GJA1 gene has been demonstrated to play a significant role in the vesicle-mediated transport of the transcellular location of Borrelia bavariensis and resulting in infection of the human brain microvascular endothelial cells [56]. This report, therefore, contributes to a better understanding of the resulting molecular changes caused by the dysregulation of miR-206 and 214-5p related to pathways and the associated genes [53,55,56]. Further, our data support the hypothesis that changes in miRNA expression can be a contributing factor to the development of breast cancer after B. burgdorferi infection although more research should investigate this connection further.
In addition, miR-206 is associated with two other pathways, cell cycle, and mismatch repair, suggesting it may play a role in maintaining cellular homeostasis [26]. According to the DIANA analysis, the cell cycle is influenced by genes WEE1 and PKMYT1 which play a vital role in recognizing and repairing DNA damage [26,57]. Both these genes exhibit an upregulated expression in breast cancer cells and act as oncogenes, assisting in further growth and carcinoma progression [57]. It has been reported that as malignant transformation is induced, WEE1 upregulation in cancer cells might promote tumorigenesis by maintaining the levels of genomic instability [57]. The DIANA analysis further shows that the mismatch repair pathway, regulated by miR-206, involves the EXO1 gene, which regulates the mechanisms of DNA damage checkpoints and repair [58]. The EXO1 gene was investigated in a study using MDA-MB-231 breast cancer cells where an increase in gene expression was observed compared to the normal mammary epithelial cell line, MCF10A [58]. This report suggests that the elevated expression of this EXO1 gene is associated with breast cancer development and poor prognosis by affecting the mismatch repair pathway of the cells [58]. The role of miR-206 may be a contributing factor in carcinogenesis, impaired cellular function, and DNA damage through its relation to the cell cycle and mismatch repair pathway [26].
DIANA analysis of miR-214-3p shows that this miRNA can influence two other pathways, microRNAs in cancer and pathways in cancer. Both these pathways involve the PTEN and MAPK1 genes [26]. PTEN mostly acts as a tumor suppressor, and it has been observed that loss of PTEN expression is associated with breast cancer progression [59]. Further, a research study has shown that in breast cancer cells, the upregulated expression of the MAPK pathway is associated with reduced PTEN expression [59]. Through DIANA analysis, miR-214-3p appears to influence another gene, the MDM2, which is involved in cancer pathways [26]. There is evidence that the members of the MDM family play a crucial role in regulating the fate of cell development [60]. This function of MDM is achieved by the negative control of an essential tumor suppressor gene, p53 [60]. In addition, research has concluded that the overexpression of the MDM2 gene is related to the augmentation of breast cancer [60]. Hence, the role of miR-214-3p in these pathways obtained by DIANA analysis describes its potential effect on breast cancer development [26]. In this study, a higher expression of miR-206 and 214-3p in the B. burgdorferi-infected triple-negative breast cancer cells was confirmed which provided strong evidence that these miRNAs may play a crucial role in the spirochetal infection of mammalian epithelial cells.
The DIANA analysis for miR-16-5p showed that it affects three pathways, one of which is the cell cycle pathway associated with the genes MDM2 and CDC25A [26]. The CDC25A gene regulates the G1/S and G2/M checkpoints of the cell cycle, and its dysregulation has been implemented in the development of triple-negative breast cancer [61]. Thus, miR-16-5p might be regulating genes involved in cell cycle pathways. Further DIANA analysis identified that miR-16-5p and 20b-5p share two other pathways. Both miRNAs were found to be associated with pathways in cancer and the p53 signaling pathway coded by the STAT3 and MDM2 genes [26]. STAT3 regulates the progress of breast cancer and the proliferation of cells by targeting oncogenes [62]. As mentioned earlier, miR-16-5p and 20b-5p were associated with the p53 signaling pathway, which involves the p53 tumor suppressor gene, whose dysregulation led to the growth of more aggressive breast cancer and poor prognosis [63].
In our DIANA analysis, it was further seen that out of the four target miRNAs, the MDM2 gene was associated with three miRNAs, miR-214-3p, 16-5p, and 20b-5p [26]. MDM2 is found to act as an oncoprotein mainly by degrading the tumor suppressor gene p53 via E3 ubiquitin ligase action [60,64]. The association of our target miRNAs with MDM2 has been a significant observation as this gene is found to be dysregulated in several cancers [60,64]. In addition, the pathway in cancer, as seen in miR-16-5p and 20b-5p, was also observed in miR-214-3p, making it the only pathway that is observed to be influenced by three miRNAs [26]. Hence, by analyzing the miRNA pathways using the DIANA, it was established that most of these target miRNAs influence cellular mechanism pathways associated with oncogenes, suggesting a possible role in the development and progression of breast cancer cells and B. burgdorferi infection.
5. Conclusions
In summary, this research study demonstrated that B. burgdorferi infection can cause the dysregulation of several miRNAs as observed in normal and tumorigenic breast cancer cell lines. The most statistically significant upregulations were observed for miR-206 and 214-3p in B. burgdorferi-infected breast cell lines compared to the uninfected breast cells. However, miRNAs can target hundreds of genes, thus, even the slight elevation of their expression can severely impact gene regulation. miRNA pathway analysis using DIANA also emphasized their potential role in regulating multiple oncogenes and augmenting the development of breast cancer. In addition, these miRNAs could be eventually targeted as a tool for therapeutic and diagnostic purposes for the analysis of breast cancer progression and management. Consequently, the miRNA molecules and their pathways with associated genes should be further evaluated to understand their role in breast cancer and the influence of B. burgdorferi infection in a more extensive form.
Acknowledgments
This work was supported by donations from the Pink Clover Foundation and National Philanthropic Trust. The supported organizations had no role in study design, data collection, and analysis, the decision to publish, or the preparation of the manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11061475/s1, Supplementary Data.
Author Contributions
Conceptualization, A.D., M.M. and E.S.; methodology, A.D. and M.M.; validation, V.A.K., J.A.V. and E.S.; formal analysis, A.D. and M.M.; investigation, A.D. and M.M.; resources, E.S.; data curation, A.D., M.M. and J.A.V.; writing—original draft preparation, A.D. and E.S.; writing—review and editing, A.D., M.M., V.A.K., J.A.V. and E.S.; visualization, A.D. and J.A.V.; supervision, E.S.; project administration, E.S.; funding acquisition, E.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available at a reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Banerjee S., Tian T., Wei Z., Shih N., Feldman M.D., Peck K.N., DeMichele A.M., Alwine J.C., Robertson E.S. Distinct microbial signatures associated with different breast cancer types. Front. Microbiol. 2018;9:951. doi: 10.3389/fmicb.2018.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Y.S., Zhao Z., Yang Z.N., Xu F., Lu H.J., Zhu Z.Y., Shi W., Jiang J., Yao P.P., Zhu H.P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017;13:1387–1397. doi: 10.7150/ijbs.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peto J., Collins N., Barfoot R., Seal S., Warren W., Rahman N., Easton D.F., Evans C., Deacon J., Stratton M.R. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J. Natl. Cancer Inst. 1999;91:943–949. doi: 10.1093/jnci/91.11.943. [DOI] [PubMed] [Google Scholar]
- 4.Dieterich M., Stubert J., Reimer T., Erickson N., Berling A. Influence of lifestyle factors on breast cancer risk. Breast Care. 2014;9:407–414. doi: 10.1159/000369571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiatt R.A., Brody J.G. Environmental Determinants of Breast Cancer. Annu. Rev. Public Health. 2018;39:113–133. doi: 10.1146/annurev-publhealth-040617-014101. [DOI] [PubMed] [Google Scholar]
- 6.Garrett W.S. Cancer and the microbiota. Science. 2015;348:80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azevedo M.M., Pina-Vaz C., Baltazar F. Microbes and Cancer: Friends or Faux? Int. J. Mol. Sci. 2020;21:3115. doi: 10.3390/ijms21093115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muhammad J.S., Zaidi S.F., Sugiyama T. Epidemiological ins and outs of Helicobacter pylori: A review. J. Pak. Med. Assoc. 2012;62:955–959. [PubMed] [Google Scholar]
- 9.Camilo V., Sugiyama T., Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2017;22((Suppl. S1)):e12405. doi: 10.1111/hel.12405. [DOI] [PubMed] [Google Scholar]
- 10.Xie X., Yang M., Ding Y., Chen J. Microbial infection, inflammation, and epithelial ovarian cancer. Oncol. Lett. 2017;14:1911–1919. doi: 10.3892/ol.2017.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanmughapriya S., Senthilkumar G., Vinodhini K., Das B.C., Vasanthi N., Natarajaseenivasan K. Viral and bacterial etiologies of epithelial ovarian cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:2311–2317. doi: 10.1007/s10096-012-1570-5. [DOI] [PubMed] [Google Scholar]
- 12.Xu S., Yin W., Zhang Y., Lv Q., Yang Y., He J. Foes or Friends? Bacteria enriched in the tumor microenvironment of colorectal cancer. Cancers. 2020;12:372. doi: 10.3390/cancers12020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur J.C., Gharaibeh R.Z., Mühlbauer M., Perez-Chanona E., Uronis J.M., McCafferty J., Fodor A.A., Jobin C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat. Commun. 2014;5:4724. doi: 10.1038/ncomms5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parhi L., Alon-Maimon T., Sol A., Nejman D., Shhadeh A., Fainsod-Levi T., Yajuk O., Isaacson B., Abed J., Maalouf N., et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020;11:3259. doi: 10.1038/s41467-020-16967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu T., Guo F., Yu Y., Sun T., Ma D., Han J., Qian Y., Kryczek I., Sun D., Nagarsheth N., et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170:548–563.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu A., Yao B., Dong T., Chen Y., Yao J., Liu Y., Li H., Bai H., Liu X., Zhang Y., et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185:1356–1372.e26. doi: 10.1016/j.cell.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Urbaniak C., Gloor G.B., Brackstone M., Scott L., Tangney M., Reid G. The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 2016;82:5039–5048. doi: 10.1128/AEM.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dattwyler R.J., Luft B.J. Overview of the clinical manifestations of Borrelia burgdorferi infection. Can. J. Infect. Dis. 1991;2:61–63. doi: 10.1155/1991/902928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livengood J.A., Gilmore R.D., Jr. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 2006;8:2832–2840. doi: 10.1016/j.micinf.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Gaur G., Sawant J.Y., Chavan A.S., Khatri V.A., Liu Y.-H., Zhang M., Sapi E. Effect of invasion of Borrelia burgdorferi in normal and neoplastic mammary epithelial cells. Antibiotics. 2021;10:1295. doi: 10.3390/antibiotics10111295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan B.M., Robles A.I., Harris C.C. Genetic variation in microRNA networks: The implications for cancer research. Nat. Rev. Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q., Yang Z., Shi Y., Fan D. MiRNAs in human cancers: The diagnostic and therapeutic implications. Curr. Pharm. Des. 2014;20:5336–5347. doi: 10.2174/1381612820666140128204914. [DOI] [PubMed] [Google Scholar]
- 23.Hayes J., Peruzzi P.P., Lawler S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Lochhead R.B., Ma Y., Zachary J.F., Baltimore D., Zhao J.L., Weis J.H., O’Connell R.M., Weis J.J. MicroRNA-146a provides feedback regulation of Lyme arthritis but not carditis during infection with Borrelia burgdorferi. PLoS Pathog. 2014;10:e1004212. doi: 10.1371/journal.ppat.1004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lochhead R.B., Strle K., Kim N.D., Kohler M.J., Arvikar S.L., Aversa J.M., Steere A.C. MicroRNA expression shows inflammatory dysregulation and tumor like proliferative responses in joints of patients with postinfectious Lyme arthritis. Arthritis Rheumatol. 2017;69:1100–1110. doi: 10.1002/art.40039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlachos I.S., Zagganas K., Paraskevopoulou M.D., Georgakilas G., Karagkouni D., Vergoulis T., Dalamagas T., Hatzigeorgiou A.G. DIANA-miRPath v3.0, deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43:W460–W466. doi: 10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligasová A., Koberna K. Strengths and Weaknesses of Cell Synchronization Protocols Based on Inhibition of DNA Synthesis. Int. J. Mol. Sci. 2021;22:10759. doi: 10.3390/ijms221910759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quan Y., Huang X., Quan X. Expression of miRNA-206 and miRNA-145 in breast cancer and correlation with prognosis. Oncol. Lett. 2018;16:6638–6642. doi: 10.3892/ol.2018.9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Zhao Z., Li S., Dong L., Li Y., Mao Y., Liang Y., Tao Y., Ma J. Inhibition of miR 214 attenuates the migration and invasion of triple-negative Breast Cancer cells. Mol. Med. Rep. 2019;19:4035–4042. doi: 10.3892/mmr.2019.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z., Hu S., Li X., Liu Z., Han D., Wang Y., Wei L., Zhang G., Wang X. MiR-16-5p suppresses breast cancer proliferation by targeting ANLN. BMC Cancer. 2021;21:1188. doi: 10.1186/s12885-021-08914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia L., Li F., Qiu J., Feng Z., Xu Z., Chen Z., Sun J. Oncogenic miR-20b-5p contributes to malignant behaviors of breast cancer stem cells by bidirectionally regulating CCND1 and E2F1. BMC Cancer. 2020;20:949. doi: 10.1186/s12885-020-07395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng L., Xiong Y., Liu J., Yang X., Wang L., Zhang S., Liu M., Wang D. MMP-9-Related microRNAs as Prognostic Markers for Hemorrhagic Transformation in Cardioembolic Stroke Patients. Front. Neurol. 2019;10:945. doi: 10.3389/fneur.2019.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu X., Zeng L., Liu Z., Ke X., Lei L., Li G. MicroRNA-206 regulates the secretion of inflammatory cytokines and MMP9 expression by targeting TIMP3 in Mycobacterium tuberculosis-infected THP-1 human macrophages. Biochem. Biophys. Res. Commun. 2016;477:167–173. doi: 10.1016/j.bbrc.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Wright K., de Silva K., Plain K.M., Purdie A.C., Blair T.A., Duggin I.G., Britton W.J., Oehlers S.H. Mycobacterial infection-induced miR-206 inhibits protective neutrophil recruitment via the CXCL12/CXCR4 signaling Chang axis. PLoS Pathog. 2021;17:e1009186. doi: 10.1371/journal.ppat.1009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casselli T., Qureshi H., Peterson E., Perley D., Blake E., Jokinen B., Abbas A., Nechaev S., Watt J.A., Dhasarathy A., et al. MicroRNA and mRNA transcriptome profiling in primary human astrocytes infected with Borrelia burgdorferi. PLoS ONE. 2017;12:e0170961. doi: 10.1371/journal.pone.0170961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui L., Markou A., Stratton C.W., Lianidou E. Diagnosis and Assessment of Microbial Infections with Host and Microbial MicroRNA Profiles. Adv. Tech. Diagn. Microbiol. 2018:563–597. doi: 10.1007/978-3-319-95111-9_23. [DOI] [Google Scholar]
- 37.Zhou W., Shi G., Zhang Q., Wu Q., Li B., Zhang Z. MicroRNA-20b promotes cell growth of breast cancer cells partly via targeting phosphatase and tensin homologue (PTEN) Cell Biosci. 2014;4:62. doi: 10.1186/2045-3701-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong L., Lee K., Russell I., Chen C. Endogenous Controls for Real-Time Quantitation of miRNA Using TaqMan® MicroRNA Assays. Applied Biosystems Application Note, Publication 127AP11-01. 2007. [(accessed on 12 December 2022)]. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/Application-Notes/cms_044972.pdf.
- 39.Masè M., Grasso M., Avogaro L., D’Amato E., Tessarolo F., Graffigna A., Denti M.A., Ravelli F. Selection of reference genes is critical for miRNA expression analysis in human cardiac tissue. A focus on atrial fibrillation. Sci. Rep. 2017;7:41127. doi: 10.1038/srep41127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bignotti E., Calza S., Tassi R.A., Zanotti L., Bandiera E., Sartori E., Odicino F.E., Ravaggi A., Todeschini P., Romani C. Identification of stably expressed reference small non-coding RNAs for microRNA quantification in high-grade serous ovarian carcinoma tissues. J. Cell. Mol. Med. 2016;20:2341–2348. doi: 10.1111/jcmm.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., Wang M., Tong Y., Liu X., Zhang L., Dong D., Shao J., Zhou Y. MiR-206 Promotes Cancer Progression by Targeting Full-Length Neurokinin-1 Receptor in Breast Cancer. Technol. Cancer Res. Treat. 2019;18:1533033819875168. doi: 10.1177/1533033819875168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Limana F., Esposito G., D’Arcangelo D., Di Carlo A., Romani S., Melillo G., Mangoni A., Bertolami C., Pompilio G., Germani A., et al. HMGB1 attenuates cardiac remodeling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLoS ONE. 2011;6:e19845. doi: 10.1371/journal.pone.0019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalniete D., Nakazawa-Miklaševiča M., Štrumfa I., Āboliņš A., Irmejs A., Gardovskis J., Miklaševičs E. High expression of miR-214 is associated with a worse disease-specific survival of the triple-negative breast cancer patients. Hered. Cancer Clin. Pract. 2015;13:7. doi: 10.1186/s13053-015-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao Y., Zhao Z., Ma J., Dong L., Liang Y., Li S., Mao Y., Li Y., Zhang Y. MiR-214-3p regulates the viability, invasion, migration and EMT of TNBC cells by targeting ST6GAL1. Cytotechnology. 2019;71:1155–1165. doi: 10.1007/s10616-019-00352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S., Lu J., Xu Z., Zou X., Sun X., Xu Y., Shan A., Lu J., Yan X., Cui Y., et al. Differential expression of ST6GAL1 in the tumor progression of colorectal cancer. Biochem. Biophys. Res. Commun. 2017;486:1090–1096. doi: 10.1016/j.bbrc.2017.03.167. [DOI] [PubMed] [Google Scholar]
- 46.Wang A., Dai H., Gong Y., Zhang C., Shu J., Luo Y., Jiang Y., Liu W., Bie P. ANLN-induced EZH2 upregulation promotes pancreatic cancer progression by mediating miR-218-5p/LASP1 signaling axis. J. Exp. Clin. Cancer Res. 2019;38:347. doi: 10.1186/s13046-019-1340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia L., Su X., Shen J., Meng Q., Yan J., Zhang C., Chen Y., Wang H., Xu M. ANLN functions as a key candidate gene in cervical cancer as determined by integrated bioinformatic analysis. Cancer Manag. Res. 2018;10:663–670. doi: 10.2147/CMAR.S162813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao B., Liu Z., Li B.S., Tang B., Li W., Guo G., Shi Y., Wang F., Wu Y., Tong W.D., et al. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J. Infect. Dis. 2009;200:916–925. doi: 10.1086/605443. [DOI] [PubMed] [Google Scholar]
- 49.Chalhoub N., Baker S.J. PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkler J., Abisoye-Ogunniyan A., Metcalf K.J., Werb Z. Concepts of extracellular matrix remodeling in tumor progression and metastasis. Nat. Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Provenzano P.P., Inman D.R., Eliceiri K.W., Knittel J.G., Yan L., Rueden C.T., White J.G., Keely P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodenough D.A., Paul D.L. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X.X., Luo J.H., Wu L.Q. FN1 overexpression is correlated with unfavorable prognosis and immune infiltrates in breast cancer. Front. Genet. 2022;13:913659. doi: 10.3389/fgene.2022.913659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunadi B., Budi N.Y.P., Kalim A.S., Santiko W., Musthofa F.D., Iskandar K., Makhmudi A. Aberrant expressions of miRNA-206 target, FN1, in multifactorial Hirschsprung disease. Orphanet J. Rare Dis. 2019;14:5. doi: 10.1186/s13023-018-0973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raibaud S., Schwarz-Linek U., Kim J.H., Jenkins H.T., Baines E.R., Gurusiddappa S., Höök M., Potts J.R. Borrelia burgdorferi binds fibronectin through a tandem beta-zipper, a common mechanism of fibronectin binding in staphylococci, streptococci, and spirochetes. J. Biol. Chem. 2005;280(19):18803–18809. doi: 10.1074/jbc.M501731200. [DOI] [PubMed] [Google Scholar]
- 56.Tkáčová Z., Bhide K., Mochnáčová E., Petroušková P., Hruškovicová J., Kulkarni A., Bhide M. Comprehensive mapping of the cell response to Borrelia bavariensis in the brain microvascular endothelial cells in vitro using RNA-Seq. Front. Microbiol. 2021;12:760627. doi: 10.3389/fmicb.2021.760627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghelli Luserna di Rorà A., Cerchione C., Martinelli G., Simonetti G. A WEE1 family business: Regulation of mitosis, cancer progression, and therapeutic target. J. Hematol. Oncol. 2020;13:126. doi: 10.1186/s13045-020-00959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J., Zhang J. Elevated EXO1 expression is associated with breast carcinogenesis and poor prognosis. Ann. Transl. Med. 2021;9:135. doi: 10.21037/atm-20-7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebbesen S.H., Scaltriti M., Bialucha C.U., Morse N., Kastenhuber E.R., Wen H.Y., Dow L.E., Baselga J., Lowe S.W. Pten loss promotes MAPK pathway dependency in HER2/neu breast carcinomas. Proc. Natl. Acad. Sci. USA. 2016;113:3030–3035. doi: 10.1073/pnas.1523693113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haupt S., Vijayakumaran R., Miranda P.J., Burgess A., Lim E., Haupt Y. The role of MDM2 and MDM4 in breast cancer development and prevention. J. Mol. Cell. Biol. 2017;9:53–61. doi: 10.1093/jmcb/mjx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zacksenhaus E., Liu J.C., Granieri L., Vorobieva I., Wang D.Y., Ghanbari-Azarnier R., Li H., Ali A., Chung P.E.D., Ju Y.J., et al. CDC25 as a common therapeutic target for triple-negative breast cancer—The challenges ahead. Mol. Cell. Oncol. 2018;5:e1481814. doi: 10.1080/23723556.2018.1481814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma J.H., Qin L., Li X. Role of STAT3 signaling pathway in breast cancer. Cell. Commun. Signal. 2020;18:33. doi: 10.1186/s12964-020-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasco M., Shami S., Crook T. The p53 pathway in breast cancer. Breast Cancer Res. 2002;4:70–76. doi: 10.1186/bcr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mendoza M., Mandani G., Momand J. The MDM2 gene family. Biomol. Concepts. 2014;5:9–19. doi: 10.1515/bmc-2013-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available at a reasonable request from the corresponding author.