Abstract
Malaria is an infectious disease caused by Plasmodium spp. and it is mainly transmitted to humans by female mosquitoes of the genus Anopheles. Malaria is an important global public health problem due to its high rates of morbidity and mortality. At present, drug therapies and vector control with insecticides are respectively the most commonly used methods for the treatment and control of malaria. However, several studies have shown the resistance of Plasmodium to drugs that are recommended for the treatment of malaria. In view of this, it is necessary to carry out studies to discover new antimalarial molecules as lead compounds for the development of new medicines. In this sense, in the last few decades, animal venoms have attracted attention as a potential source for new antimalarial molecules. Therefore, the aim of this review was to summarize animal venom toxins with antimalarial activity found in the literature. From this research, 50 isolated substances, 4 venom fractions and 7 venom extracts from animals such as anurans, spiders, scorpions, snakes, and bees were identified. These toxins act as inhibitors at different key points in the biological cycle of Plasmodium and may be important in the context of the resistance of Plasmodium to currently available antimalarial drugs.
Keywords: malaria, Plasmodium, antimalarials, resistance to antimalarials, animal venom toxins
1. Introduction
Malaria remains a major public health problem [1]. In 2021, 247 million cases of malaria were registered worldwide, an increase from 245 million in 2020, with an estimated 619,000 deaths, the most vulnerable groups being children under 5 years of age, pregnant women and patients with HIV/AIDS. This increase was particularly evident in Africa (95%) [1]. Malaria is a potentially dangerous acute febrile infectious disease caused by Plasmodium spp. (species), which is transmitted to humans through the bite of an infected female Anopheles mosquito [2]. Currently, seven species are known to cause malaria in humans in different areas of the world (Table 1): Plasmodium falciparum, Plasmodium vivax, Plasmodium knowlesi, Plasmodium ovale, Plasmodium malariae, Plasmodium cynomolgi and Plasmodium simium.
P. falciparum malaria cases are most prevalent in the African region (mainly sub-Saharan Africa), Southeast Asia, the Eastern Mediterranean, and the Western Pacific region [1]. P. vivax is the dominant malaria species in much of Asia-Pacific, the Horn of Africa, and Central and South America, and caused 4.5 million cases worldwide in 2020 [3]. P. ovale is usually described as being limited to tropical Africa, the Middle East, Papua New Guinea, and Irian Jaya in Indonesia [4]. P. knowlesi has been reported in Malaysian Borneo; though cases have also been reported in Thailand, Myanmar, China, the Philippines, and Singapore [1]. P. malariae has been reported in Africa, the Southeastern Pacific and South America, P. simium in South America, and Plasmodium cynomolgi in Peninsular Malaysia, the Northern Sabah Kapit district in Sarawak and Malaysian Borneo (Table 1).
The containment of cases and progression towards the elimination of human malaria is related to adequate and timely treatment, in addition to vector control, mainly using insecticides [1,5]. Different antimalarials are currently available (Table 1 and Table 2) and are effective to some extent [1]. However, resistance to available antimalarials has been increasingly reported, and has become an important barrier to malaria elimination [1,6,7,8]. The discovery of new molecules or toxins may lead to medicines with greater antimalarial activity. As such, this review identifies and describes animal venom toxins with potential antimalarial activity.
2. Plasmodium Species Causing Human Malaria
The etiological agents of malaria are the protozoans of the genus Plasmodium, which are transmitted to the vertebrate host through the bite of infected Anopheles female mosquitoes [9]. More than 120 species of Plasmodium are known, but only 7 species of them are described as being capable of infecting humans, and P. malariae, P. vivax, and P. falciparum are the most common species [10,11]. P. falciparum is responsible for most of the severe cases and about 99% of malaria-associated deaths worldwide [1]. P. vixax is a predominant species in the Americas, causing 75% of malaria cases [1]; it can also cause severe cases, similarly to P. falciparum [12]. P. knowlesi, P. simium and P. cynomolgi are transmitted from primates to humans; however, the prevalence and clinical impact of these species are unclear, although the first species can cause severe manifestations [13,14]. P. malariae and P. ovale cause uncomplicated malaria, although they may sometimes be associated with other complications [13].
Female mosquitoes of the genus Anopheles are the vectors of Plasmodium spp. [1]. Anopheles are insects of great epidemiological importance [15]. Approximately 3500 mosquito species, grouped into 41 genera, are known. The genus Anopheles has a wide geographic distribution, and Antarctica is the only place that it is not found. This genus consists of about 430 species and, of these, only about 70 species are natural transmitters of malaria [15,16,17,18,19,20,21]. The Plasmodium-vector interaction is complex and there are factors (invasion of the intestinal cells of mosquitoes, ookinete escape, ookinete development time, vector immune response, among others, for example) that determine the specificity in the ecological relationship between vector-plasmodium and the geographical distribution of cases [22,23,24] (Table 1).
Table 1.
Distribution of Plasmodium species on the continents of the globe, their respective vectors and recommended treatments.
| Parasite | Vector | Location, Continent | Treatment | Ref. |
|---|---|---|---|---|
| P. vivax |
Anopheles albimanus
Anopheles albitarsis Anopheles aquasalis Anopheles darlingi Anopheles freeborni Anopheles marajoara Anopheles nuneztovaris Anopheles pseudopunctipennis Anopheles quadrimaculatus Anopheles cruzzi Anopheles bellator Anopheles brasiliensis Anopheles calderoni Anopheles triannulatus Anopheles neivai Anopheles deaneorum Anopheles oswaldoi Anopheles argyritarsis Anopheles dunhami |
Central and South America | CQ + PQ AS + PQ CQ + TQ |
[1,15,25,26,27,28,29] |
|
Anopheles annularis
Anopheles aconitus Anopheles subpictus |
South and Southeast Asia and Asia-Pacific | CQ AL DHA + PPQ AS + PY |
[1,15,28] | |
| Anopheles stephensi | Africa | CQ DHA + PPQ |
[1,15,26,27] | |
| P. falciparum |
Anopheles arabiensis
Anopheles funestus Anopheles gambae Anopheles stephensi Anopheles melas Anopheles merus Anopheles moucheti Anopheles nili |
Africa | AL AS + AQ AS + PY DHA + PPQ |
[1,15,20,30] |
|
Anopheles farauti
Anopheles Kiliensis Anopheles punctulatus Anopheles dirus Anopheles minimus Anopheles lesteri Anopheles sinensis Anopheles balabacensis Anopheles barbirostris |
Asia | AL AS + MQ AS + PY AS + SP AS + SP DHA + PPQ + PQ |
[1,15,30] | |
|
Anopheles atroparvus
Anopheles labranchiae Anopheles messeae Anopheles sacharovi Anopheles sergentii Anopheles superpictus |
Mediterranean | AL AS + SP DHA + PPQ |
[1,15,31] | |
|
Anopheles flavirostris
Anopheles koliensis Anopheles lesteri Anopheles leucosphyrus Anopheles maculatus Anopheles punctulatus Anopheles sinensis Anopheles sundaicus |
Western Pacific | AL AS + PY DHA + PPQ AS + MQ |
[1,15,30] | |
|
Anopheles darlingi
Anopheles deaneorum |
Central and South America | AL AS + MQ |
||
| P. malariae |
Anopheles stephensi, Anopheles gambiae |
Africa | CQ + PQ | [1,15,29,32,33,34] |
|
Anopheles freeborni
Anopheles dirus |
Southeastern Pacific | |||
| Anopheles darlingi | South America | |||
| P. ovale |
Anopheles gambiae
Anopheles funestus |
Africa | CQ + PQ and/or ART | [1,15,34] |
|
Anopheles flavirostris
Anopheles koliensis Anopheles lesteri |
Western Pacific | |||
| P. simium |
Anopheles nyssorhinchus
Anopheles Kerteszia |
South America | CQ + PQ | [1,11,15] |
| P. knowlesi |
Anopheles hackeri
Anopheles latens Anopheles sundaicus Anopheles dirus Anopheles hacker Anopheles cracens Anopheles introlatus |
Malaysia | CQ + PQ | [1,15] |
| P. cynomolgi | Anopheles balabacensis | Malaysia | CQ + PQ | [1,14,35] |
Abbreviations: CQ—chloroquine; PQ—primaquine; AS—artesunate; ART—artemisinin; MQ—mefloquine; AL—artemether-lumefantrine; AQ—amodiaquine; DHA—dihydroartemisinin; PPQ—piperaquine; PY—pyronaridine; SP—sulfadoxinepyrimethamine.
3. Plasmodium Life Cycle and Pathogenicity
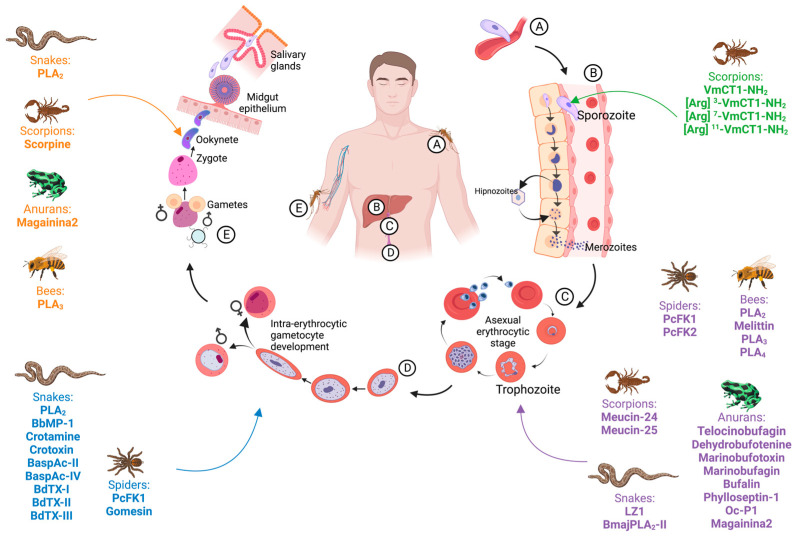
The life cycle of Plasmodium is heteroxenous and occurs in the mosquito vector and in vertebrates (e.g., humans) (Figure 1). The infection begins with the bite of a mosquito infected with Plasmodium sporozoites (Figure 1A) [36]. The sporozoites are inoculated during the blood meal (Figure 1A). They slip through the dermis and subsequently enter the blood circulation and migrate to the hepatic sinusoids to invade the hepatocytes [37] (Figure 1B). After the invasion, the sporozoites divide asexually by pre-erythrocytic schizogony and form pre-erythrocytic trophozoites that multiply, giving rise to tissue schizonts (Figure 1B) [25,37]. The duration of pre-erythrocytic schizogony varies according to the infectious species (8 to 27 days for P. vivax, 8 to 25 days for P. falciparum, 9 to 17 days for P. ovale, 15 to 30 days for P. malariae, 9 to 12 days for P. knowlesi and still unknown for P. simium and P. cynomolgi) [38]. At this stage, P. vivax and P. ovale form hypnozoites, which are latent forms of the parasite that are responsible for relapses of the disease months or years later [36,37].
Figure 1.
Plasmodium life cycle, showing the points at which toxins from animal venoms can act. Inoculation of sporozoites into the host’s epidermis (A); hepatic and pre-erythrocytic stage (B); erythrocytic stage (C); intraerythrocytic phase and gametocyte development (D); Anopheles spp. infection and parasite development in the mosquito midgut (E); male gamete (♂); female gamete (♀).
At the end of the first phase (Figure 1B), also called the exoerythrocytic or tissue stage, each infected hepatocyte releases thousands of exoerythrocytic merozoites. The number varies according to the species (about 2000 merozoites when the infection is by P. malariae; 10,000 when due to P. vivax; 40,000 when due to P. falciparum and 15,000 when due to P. ovale, though the quantity is still unknown for P. simium and P. cynomolgi) [39]. Merozoites released from hepatocytes invade red blood cells (Figure 1C), which initiates the erythrocytic phase. P. vivax preferentially invades young erythrocytes, P. falciparum invades erythrocytes in any evolutionary phase, while P. malariae invades old erythrocytes [39]. After invading the erythrocytes, merozoites divide asexually giving rise to ring forms, trophozoites, and young and mature schizonts [36]. During a period that varies from 48 to 72 h, the parasite develops inside the erythrocytes until it causes their rupture, thus releasing new merozoites that will invade new erythrocytes [39] (Figure 1C). The rupture and consequent release of merozoites into the bloodstream is clinically translated by the onset of the malarial paroxysm, which will be repeated at the end of the new cycle [39]. This cycle of invasion-multiplication-release-invasion is repeated [36]. After a period of asexual replication, some merozoites differentiate into male and female gametocytes (Figure 1D), which mature without cell division and become infectious to mosquitoes [39] (Figure 1E).
Mosquitoes become infected with Plasmodium during a blood meal from an infected host (Figure 1E). In the vector, the sexual reproduction (sporogony) of the malaria parasite occurs in the mosquito’s stomach after the differentiation of gametocytes into gametes and their fusion, with the formation of the zygote [39] (Figure 1E). The zygote transforms into a mobile form (ookinete) that transposes the peritrophic matrix and then migrates to the midgut wall of the insect and forms the oocyst, within which the sporozoites will develop [36,39] (Figure 1E). The sporozoites produced in the oocysts are released into the insect’s hemolymph and migrate to the salivary glands, from where they are transferred to the blood of the human host during the blood meal [39] (Figure 1A). The time required for completion of the sporogony cycle in insects varies depending on the Plasmodium species and the temperature, though it generally takes around 10 to 20 days [36].
During the life cycle of Plasmodium, it can invade the red blood cells to feed on hemoglobin. As it feeds, it ruptures the cells, releasing red blood cells and parasite debris, including malarial pigment (hemozoin) and glycophosphatidylinositol, called malarial toxin, thus causing the symptoms [40]. The more erythrocytes that are infected and rupture and release putative malarial toxins, the greater the pathogenesis or severity of malaria [41,42]. Putative malarial toxins activate peripheral blood mononuclear cells and stimulate the release of cytokines with a consequent systemic inflammatory response [41]. The balance between pro-inflammatory and anti-inflammatory cytokines, chemokines, growth factors, and effector systems determines the severity of the disease [41]. The pathogenicity of malaria also depends on the individual’s immunological characteristics, the genetic aspects of the parasite and host, previous exposure to infection, age, and nutritional, geographic and socioeconomic factors [43]. Clinical complications of malaria include severe anemia, acute renal failure, acute pulmonary edema, algid malaria, and cerebral malaria, and they can be avoided through early diagnosis and treatment [1,42].
4. Malaria Treatment
Antimalarial drugs can act by interrupting the multiplication of the parasite and, consequently, the inhibition of malarial infection by affecting different stages of the parasite throughout the cycle [44]. Antimalarial drugs target (a) the parasite asexual erythrocytic stages, (b) tissue schizonticides by targeting hypnozoites and gametocytocides, which destroys the sexual forms of the parasite in the bloodstream, thus preventing the transmission of malaria to the mosquito, and (c) the sporontocides, which prevents or inhibits the formation of oocysts and malaria sporozoites in the infected mosquito [45]. For the adequate treatment of malaria, the following is necessary: identification of the infectious species; identification of the susceptibility of the Plasmodium species to the drug; and the clinical status of the patient [46]. The antimalarials in current use are summarized in Table 1 and Table 2.
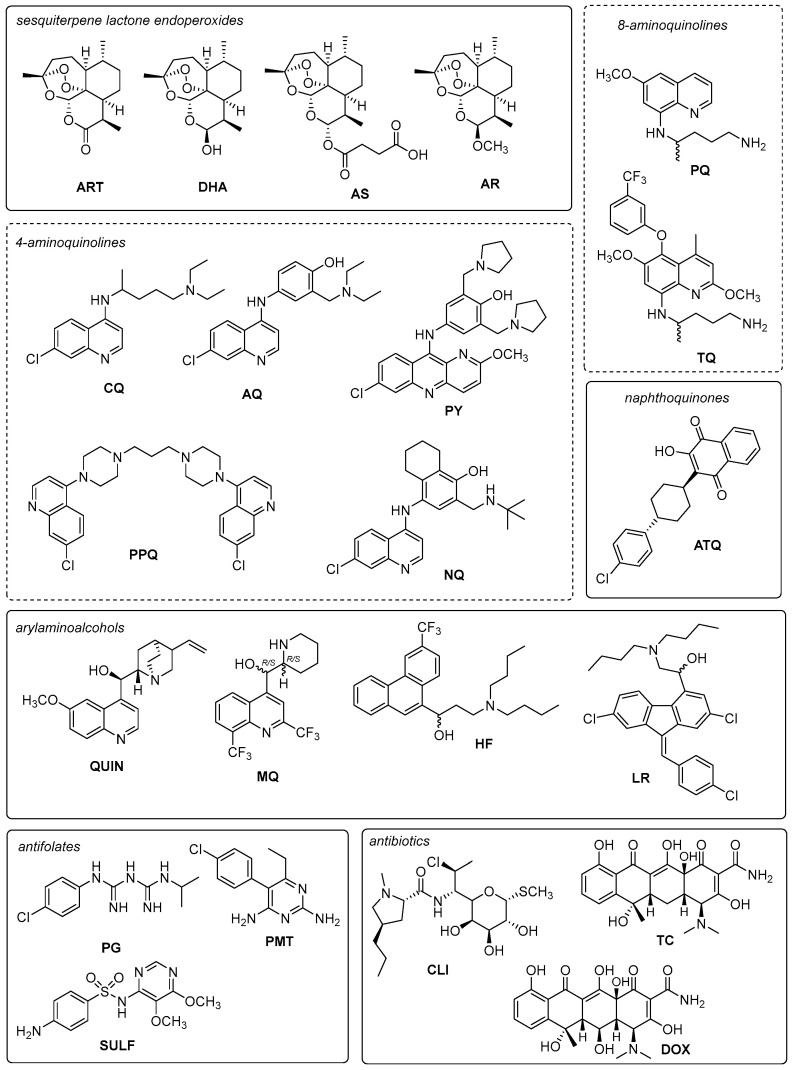
The available antimalarials are categorized into seven classes: (a) sesquiterpene lactone endoperoxides compounds: artemisinin (ART), dihydroartemisinin (DHA), artesunate (AS) and artemether (AR); (b) 4-aminoquinolines: chloroquine (CQ), amodiaquine (AQ), pyronaridine (PY), piperaquine (PPQ) and naphthoquine (NQ); (c) arylaminoalcohols: quinine (QUIN), mefloquine (MQ), halofantrine (HF) and lumefantrine (LR); (d) 8-aminoquinolines: primaquine (PQ) and tafenoquine (TQ); (e) antifolates: proguanil (PG), pyrimethamine (PMT) and sulfadoxine (SULF); f) naphthoquinones: atovaquone (ATQ) and (g) antibiotics: clindamycin (CLI), doxycycline (DOX) and tetracycline (TC) (Table 2, Figure 2). Their mechanisms of action are summarized in Table 2.
Figure 2.
Currently used antimalarial drugs.
However, in some regions, the first line of treatment is via artemisinin combination therapies (ACTs) + PQ/TQ due to CQ resistance [47,48,49,50]. Treatment for P. falciparum infections is performed by the combination of AR and LR or with MQ or QUIN, DOX and PQ [25]. For mixed infections caused by P. falciparum and P. vivax (or P. ovale), treatment should include a blood schizonticidal drug that is effective for P. falciparum, associated with PQ (tissue schizonticidal). If the mixed infection is P. falciparum and P. malariae, treatment should be directed towards P. falciparum only [25]. With regard to the treatment of severe and complicated malaria, the aim is to prevent the patient from dying, so doctors must follow a rigid treatment scheme that consists of modulating the dosage, and these schemes are already defined in the strategic plan for the treatment of malaria in each country. However, the WHO recommends the administration of injectable artesunate (intramuscular or intravenous), followed by an ACT-based treatment as soon as the patient can take oral medications. If injectable treatment is not possible, the patient should immediately be given artesunate intrarectally and transferred as soon as possible to a suitable site for full parenteral treatment [1,25] (Table 2).
Table 2.
Available drugs, their chemistry classes, origin, target species, points where it is active and mechanism of action.
| Class | Name | Origin of the Drug | Plasmodium spp. | Active against Stages | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|
| Sesquiterpene lactone endoperoxides | ART | Artemisia annua L. | P. falciparum and P. vivax | All | Protein metabolism | [50,51,52,53] |
| AS | Semi-synthetic derivative of artemisinin | P. vivax | All | [50,54,55,56] | ||
| AR | P. falciparum and P. vivax | All | [50,51,57] | |||
| DHA | P. falciparum and P. vivax | All | Not very well known. Probably protein metabolism | [46,51] | ||
| 4-aminoquinolines | CQ | Synthetic analogue of quinine | P. vivax | Blood stages (trophozoite and schizont) |
Digestion of hemoglobin | [50,54,55,56,57,58,59] |
| AQ | P. vivax | [50,55,60,61] | ||||
| PPQ | P. falciparum and P. vivax | Not very well known. Probably digestion of hemoglobin | [46,56,59,60] | |||
| PY | P. falciparum and P. vivax | [46,51,56,62] | ||||
| NQ | P. falciparum and P. vivax | [46,63] | ||||
| Arylaminoalcohols | QUIN | Cinchona calisaya L. | P. falciparum and P. vivax | Blood stages (trophozoite and gametocytes) |
Digestion of hemoglobin | [50,64] |
| MQ | Synthetic derivative of quinoline | P. falciparum and P. vivax | Blood stages (trophozoite, schizont and gametocytes) |
[50,52,56] | ||
| LR | Synthetic derivative of fluorene | P. falciparum and P. vivax | Not very well known. Probably digestion of hemoglobin | [50,64] | ||
| HF | Synthetic derivative of fluorene | P. falciparum | Digestion of hemoglobin | [65,66] | ||
| 8-Aminoquinolines | PQ | Synthetic, 8-aminoquinoline derivative | P. vivax | Forms quinoline-quinone metabolites that act as oxidants | [46,67] | |
| TQ | Synthetic analogue of primaquine | P. vivax and P. falciparum | Interferes with the polymerization of the heme group | [46,60] | ||
| Antifolates | PMT | Synthetic derivative of ethyl-pyrimidine | P. falciparum and P. vivax | Blood, liver (schizont) and mosquito (oocysts) stage | Inhibits dihydrofolate reductase enzyme and blocks plasmodium DNA synthesis | [46,68] |
| SULF | P. vivax | Blood and liver (schizont) stage | Inhibits parasite dihydropteroate synthetase | [46,69] | ||
| PG | Biguanide | P. falciparum and P. vivax | Blood (gametocyte) and liver (shizont) stages | Inhibits parasite dihydrofolate reductase | [46,50] | |
| Naphthoquinones | ATQ | Synthetic hydroxynaphthoquinone | P. falciparum and P. vivax | Inhibits electron transport in the mitochondria of parasites | [46,58] | |
| Antibiotics | CLI | Semisynthetic of lincomycin | P. falciparum | All blood stages | Inhibits protein synthesis in the Plasmodium apicoplast | [46,70] |
| DOX | Semi-synthetic of tetracycline | P. falciparum | [46,64,70] | |||
| TC | Semi-synthetic of chlortetracycline | P. falciparum | [50,70] |
Abbreviations: ART—artemisinin; DHA—dihydroartemisinin; AS—artesunate; AR—artemether; CQ—chloroquine; AQ—amodiaquine; PY—pyronaridine; PPQ—piperaquine; NQ—naphthoquine; QUIN—quinine; MQ—mefloquine; HF—halofantrine; LR—lumefantrine; PQ—prima-quine; TQ—tafenoquine; PG—proguanil; PMT—pyrimethamine; SULF—sulfadoxine; ATQ—atovaquone; CLI—clindamycin; DOX—doxycycline; TC—tetracycline.
Resistance to Antimalarials
Drug resistance to antimalarials threatens the control and elimination of malaria [71]. P. falciparum has developed resistance to all currently used antimalarials, but there is a variation in geographic distribution and degree of resistance (Table 3). The most resistant parasites are found in Southeast Asia (Table 3). Resistance is lowest in P. vivax, although resistance to CQ is found throughout Indonesia and Papua New Guinea [13]. CQ-resistance has spread much more slowly in P. vivax populations when compared to P. falciparum [48]. Possible causes include a small parasite load in P. vivax infections, early gametocytogenesis and transmission before resistant clones are selected in the host under drug pressure, and the very high genetic diversity in natural populations of P. vivax [48].
The resistance of Plasmodium spp. to antimalarial drugs has been mainly associated with genetic mechanisms (Table 3), thus several studies on molecular markers have identified and tracked genes expressed by the parasite, as well as key mutations [1]. Resistance to CQ is associated with the development of a transporter for CQ encoded by the CQ resistance transporter orthologue gene of P. vivax (pvcrt-o) or CQ resistance transporter gene of P. falciparum (pfcrt), which prevents its absorption and metabolization in the parasite’s food vacuoles (Table 3). The P. vivax multidrug resistance gene 1 (pvmdr1) and P. falciparum multidrug resistance gene 1 (pfmdr1) were also associated with resistance to CQ in P. vivax and P. falciparum, respectively (Table 3). Resistance to MQ was identified by the amplification of pvmdrl/pfmdr1 and over-expression of p-glycoprotein homologue 1 protein (pgh1) for P. vivax and P. falciparum, and the same strategy regarding HF and QUIN is used for P. falciparum (Table 3). In regard to resistance to AQ, P. vivax or P. falciparum reduce the affinity of binding of the competitive inhibitor to dihydrofolate reductase (dhfr), and P. falciparum uses the same strategy regarding PG and PMT (Table 3). In terms of resistance to ART, AS, AR, and LR, although not very well known, it has been associated with phenotypes expressed in trophozoite ring stages during the P. falciparum cycle and mutation of the Kelch protein 13 (pfk13) in specific sequences of the domain-containing protein 1 (btb-poz) and six kelch domains that somehow impede parasite protein metabolism (Table 3).
Table 3.
Plasmodium drug resistance, the location where documented and its resistance mechanism.
| Parasite | Drug Resistance | Location Where Plasmodium Resistance Has Been Documented | Resistance Mechanism | Ref. |
|---|---|---|---|---|
| P. vivax | CQ | Asia and Oceanian (Papua New Guinea and Indonesia), South America (Brazil) Africa (Madasgascar and South and Southeast Asia (India, Myanmar, Nepal, and Thailand) | Develops a transporter for chloroquine, encoded pvcrt-o Mutation of pvmdr1 and pvdhfr |
[72,73,74,75,76] |
| MQ | Southeast Asia (Western border of Thailand) and South America (Brazil) | Amplification of pvmdrl and over-expression of pghl | [75,77] | |
| AQ | Southeast Asia (Western border of Thailand) | Reduced dhfr affinity | [75,76] | |
| P. falciparum | ART | Southeast Asia and East Asia (Thailand, Vietnam, Myanmar, Laos, China), and Sub-Saharan Africa | Not well known Mutation of pfk13 in specific sequences of the BTB-POZ domains and six kelch domains (probably) |
[78,79] |
| AS | Southeast Asia (Western Cambodia) |
[78] | ||
| AR | Southeast Asia and Sub-Saharan Africa | [78,79] | ||
| LR | Southeast Asia and Sub-Saharan Africa | [77,78] | ||
| CQ | Southeast Asia (Western border of Thailand), Africa (Sub-Saharan Africa), South America (Brazil) | Develops a transporter for chloroquine, encoded pfcrt; Mutation of pfmdr1 and pfdhfr |
[75,80] | |
| AQ | Western border of Thailand | Reduced affinity for binding of the DHFR competitive inhibitor | [76] | |
| QUIN | Southeast Asia (Thailand, Thai Myanmar and Thai-Cambodian borders) | Amplification of pfmdrl and pghl overexpression | [76,81,82] | |
| PG | Southeast Asia (Thailand, Thai Myanmar and Thai-Cambodian borders) | Reduced affinity for binding of the dhfr competitive inhibitor | [76,81] | |
| MQ | Southeast Asia (Western border of Thailand, Thai-Myanmar and Thai-Cambodian borders) South America (Brazil) | Amplification of pfmdrl and pghl over-expression | [75,76,81,82,83,84] | |
| HF | Southeast Asia (Thailand) | Amplification of pfmdrl and pghl over-expression | [80,81] | |
| SULF | Southeast Asia (Western border of Thailand, Thai-Myanmar and Thai-Cambodian borders) |
dhps mutations | [75,76,81] | |
| PMT | Southeast Asia (Western border of Thailand, Thai-Myanmar and Thai-Cambodian borders) |
Reduced affinity for binding of the dhfr competitive inhibitor | [75,76,81] |
Abbreviations: CQ—chloroquine; MQ—mefloquine; AQ—amodiaquine; ART—artemisinin; AS—artesunate; AR—artemether; LR—lumefantrine; QUIN—quinine; PG—proguanil; HF—halofantrine; SULF—sulfadoxine; PMT—pyrimethamine; pvcrt-o—P. vivax chloroquine resistance transporter orthologue gene; pvdhfr—P. vivax dihydropteroate reductase; pvmde1—P. vivax multidrug resistance gene 1; pgh1—P-glycoprotein homologue 1 protein; DHFR—dihydrofolate reductase; pfk13—P. falciparum Kelch protein 13; pfcrt—P. falciparum chloroquine resistance transporter gene; pfmdr1—P. falciparum multidrug resistance gene 1; pfdhfr—P. falciparum dihydropteroate reductase; dhfr—competitive inhibitor to dihydro-folate reductase; dhps—deoxyhypusine synthase; BTB-POZ—domain-containing protein 1.
5. Antiplasmodial Toxins
In view of the morbidity and mortality rates associated with malaria, the high global distribution of malaria, the resistance of Plasmodium to the drugs used in the treatment, and the resistance of the vector (Anopheles spp.) to conventional insecticides, there is a need to seek alternatives for the development of new drugs for combating and controlling the transmission of malaria [85].
Animal venoms are a complex mixture that contains many proteins, enzymes, peptides, and small molecules [86,87]. Depending on the taxonomic group, the toxins present in venoms have different modes of action and can be used for defense against predators and pathogens in the environment [88,89,90]. It is known that animal venoms have a range of molecules with antimicrobial properties, thus making them an important resource for the investigation of compounds with antimalarial potential [90].
Over the years, several studies have been carried out to demonstrate the antiplasmodial effect of toxins from different taxonomic groups (Table 4). Snake toxins are much more commonly studied for this purpose; however, there are studies for toxins from arachnids, scorpions and bees [91,92,93,94]. Although studies with toxins are mostly carried out with venom from animals, there are records of antiplasmodial activity for the secretion of some frog species, whose venom inoculation system is passive [95]. Among the toxins that are active against Plasmodium, these are mainly peptides and enzymes [96,97]. Crude extracts and fractions of venom extracts from some animals have also been tested and have demonstrated antiplasmodial activity [98].
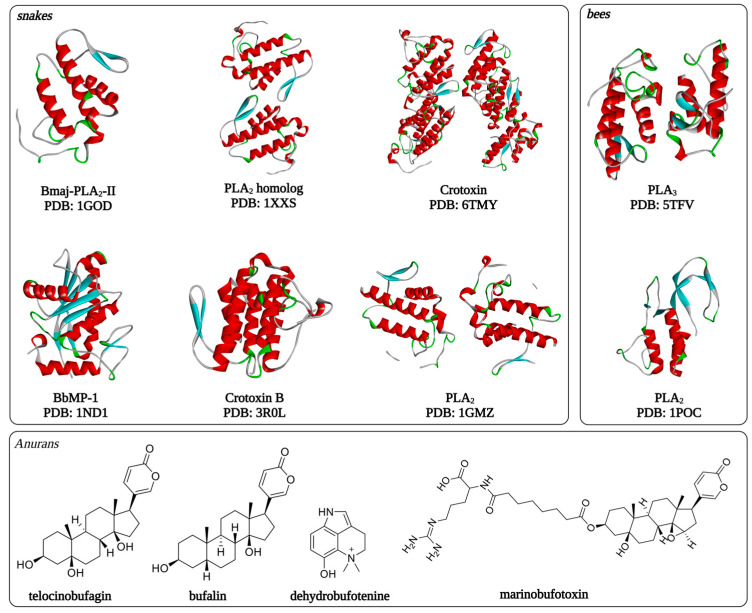
Table 4 summarizes the toxins from venomous animals that have shown activity against Plasmodium spp. Isolated substances and total extracts and fractions of extracts that have shown antiplasmodial activity in studies are evidenced. Figure 3 shows some of the three-dimensional structures of molecules isolated from animal venoms and which have antiplasmodial activity.
Figure 3.
Three-dimensional structures of animal venom toxins with antiplasmodial activity.
5.1. Snake Toxins against Plasmodium
Snakes are animals that belong to the class Reptilia and the order Squamata, and there are an estimated 3970 species around the world [99]. During foraging, some snake species capture their prey and kill it by constriction, but others have venom-inoculating structures [100]. The toxins present in snake venoms have a complex of substances with neurotoxic, cardiotoxic and inflammatory capacity [101], and are efficient in killing certain types of prey. Searches for bioactive substances in snake toxins are extensively carried out for the formulation of new drugs, with captopril being known as a successful case. It is a drug used to control blood pressure whose active ingredient was isolated from the venom of the snake Bothrops jararaca [102]. It is known that several species present toxins that are mainly derived from proteins that have antibacterial, antifungal, antiviral and antiparasitic characteristics [90].
5.1.1. Crude Snake Venoms
Extracts, fractions, enzymes, and some peptides found in snake toxins have antiplasmodial activity (Table 4). Terra et al. [103] showed that the crude venom extract of Micrurus spixii (Squamata, Elapidae) has great P. falciparum inhibitory power against its intraerythrocytic development (IC50 = 0.78 µg/mL). Hajialiani et al. [104] and Hajialiani et al. [105] tested Naja naja oxiana venom extracts against the parasites P. falciparum and P. berghei, respectively. In both studies, fraction 4, which was obtained from the crude extract of the venom of Naja naja oxiana, was used for anti-Plasmodium assays, and satisfactory inhibitory results were found (IC50 = 3.2 µg/mL) [104], as well as the interruption of parasitemia in 70%, 50% and 30% with the different concentrations of the fraction, 5, 2.5 and 1 mg/kg, respectively [105].
5.1.2. Peptides from Snake Venoms
The inhibitory activities of some peptides isolated from snake venoms were tested against Plasmodium spp. [106,107]. Maluf et al. [106] isolated a cationic polypeptide with 42 amino acid residues (YKQCHKKGGHCFPKEKICLPPSSDFGKMDCRWRWKCCKKGSG), called crotamine, from Crotalus durissus, and tested its activity against P. falciparum. This substance inhibited the development of P. falciparum, presenting an IC50 of 1.87 µg/mL. Similarly, Fang et al. [107] tested the LZ1 peptide (VKRWKKWWRKWKKWV-NH2 CAS #) derived from the cathelicidin (polypeptide) of Bungarus fasciatus (Squamata, Elapidae) against P. falciparum and P. berghei in in vitro and in vivo assays. In the in vitro antiplasmodial assay, strong suppression was observed for P. falciparum (IC50 = 3.045 µM); and, in the in vivo assay, it was possible to observe expressive antimalarial activity, with 39% (4 mg/kg), 35% (8 mg/kg) and 24% (12 mg/kg).
5.1.3. Phospholipase A2 from Snake Toxins
Phospholipase A2 (PLA2) are a superfamily of enzymes known to have the ability to catalyze the hydrolysis of fatty acids at the sn-2 position to produce free fatty acids and lysophospholipids. They are small molecules of between 14 and 38 kDa that have between 5 and 8 disulfide bridges. This superfamily comprises a number of proteins, which are classified into 15 groups and 5 types: secreted, cytosolic, Ca2+ independent, acetyl hydrolases and lysosomal [108]. PLA2s have been detected in venoms from many snakes of the Elapidae and Viperidae families, and are mostly present in toxins from species of the genera Bothrops and Crotalus (Table 4).
Of the total number of studies that provided evidence of the inhibitory activity of snake venoms against Plasmodium, 68% corresponded to PLA2 (Table 4). The first study to test the antiplasmodial activity of a PLA2 derived from snake toxins was carried out by Zieler et al. [109]. The authors noted that PLA2 isolated from the venom of the eastern diamondback rattlesnake (Crotalus adamanteus) inhibited Plasmodium gallinaceum oocyst formation in Aedes aegypti. The reduction in the rate and intensity of infection was 29% and 24%, respectively. Despite being a notable result, in this study neither the average inhibitory concentration nor the minimum lethal concentration of the molecule was evaluated. Guillaume et al. [110] evaluated seven PLA2s from groups IA, IB, IIA and III against in vitro intraerythrocytic development of P. falciparum. Anti-Plasmodium activity was tested for toxins from the vipers Agkistrodon halys and Vipera ammodytes, which both have PLA2s belonging to group IIA, and from the elapids Naja mossambica mossambica and Naja scutatus scutatus, which have PLA2s from group IA. All PLA2s, from both groups, inhibited Plasmodium development (IC50 = 0.023 nM for N. mossambica mossambica, 2.6 nM for N. scutatus scutatus, 0.823 nM for A. halys and 2.8 nM for V. ammodytes). Castilho et al. [96] tested Bothrops asper venom against P. falciparum using the whole venom, a catalytically active PLA2 (fraction V) and a PLA2 homologue (fraction VI) due to its enzymatic activity. Fraction V had an IC50 of 1.42 µg/mL while fraction VI had an IC50 of 22.89 µg/mL and the whole venom had an IC50 of 0.13 µg/mL, thus demonstrating high inhibitory power. Quintana et al. [111] tested two fractions with PLA2s from the crotoxin complex and PLA2 crotoxin B. Fractions 1 and 2 containing PLA2s from the crotoxin complex, as well as crotoxin B, inhibited the intraerythrocytic development of P. falciparum (IC50 = 0.17, 0.76 and 0.6 µg/mL, respectively). PLA2 (BmajPLA2-II) isolated from Bothrops marajoensis venom showed inhibition of P. falciparum development (IC50 = 6.41 μg/mL) [112]. PLA2s isolated from the venom of another species of the Bothrops genus were also evaluated against P. falciparum. PLA2s (BdTX-I and BdTX-II) and the BdTX-III analogue isolated from B. diporus also showed inhibitory characteristics (IC50 = 2.44, 0.0153 and 0.5913 µg/mL, respectively) [113].
Simões-Silva et al. [114] analyzed the venom of B. asper and isolated and characterized five new PLA2 isoforms, four of them acidic and one with the basic form, which were grouped into two groups: Asp49-PLA and Lys49-PLA2-like, respectively. Two PLA2s (BaspAc-II and BaspAc-IV) showed activity against P. falciparum (IC50 = 2.46 and 0.019 µg/mL, respectively). Furthermore, the mixture of the two PLA2s that were active against Plasmodium (BaspAc-II and BaspAc-IV) showed activity that was ten times greater than when tested individually, with a fractional inhibitory concentration (FIC) of 0.498 µg/mL, thus demonstrating a synergistic effect. Most of the tests used to evaluate the effect of PLA2s used CQ-resistant strains of Plasmodium. The positive and significant results regarding Plasmodium inhibition show that the molecule may be an important alternative for disease control [114].
5.2. Toxins from Anurans against Plasmodium
The class Amphibia is divided into 3 orders, namely Gymnophiona (caecilians), Caudata (salamanders and newts) and Anura (toads, frogs, and tree frogs), the latter being the most representative of the class with approximately 7500 described species [115,116]. Anurans have an integument that is devoid of hair, feathers, or scales for protection, and have developed strategies to avoid water loss and infections by pathogens in the environment [115]. They are highly predated by both vertebrates and invertebrates at all stages of development and, therefore, have several characteristics that reduce the risk of predation [117]. Anurans have a vast framework of substances in their skin and, due to their integument being unprotected, these substances act not only to control infections by pathogens, but are also used to protect against predators [88,118]. Several substances have been tested against microorganisms and have shown antimicrobial characteristics [88]; however, studies that investigate antiplasmodial activity in anuran venoms are still scarce.
5.2.1. Crude Anuran Venom
Antimicrobial assays involving crude extracts of anuran venoms are commonly performed [119]. Assays to assess antiplasmodial activities using crude extracts of anuran venoms have already been performed for bufonids [98,120]. The crude extract of Rhinella marina venom is active against P. falciparum (IC50 = 2.43 µg/mL) [120] and the venom of a bufonid (unspecified) showed good control of parasitemia against P. berghei [98]. The crude extracts tested showed inhibition of the development of trophozoites of both Plasmodium species [98,120].
5.2.2. Steroids from Anuran Venoms
The bufadienolide known as telocinobufagin is present in the secretion of the bufonids Rhinella marina and Rhaebo guttatus [95]. Bufadienolides are steroids that are commonly found in the secretion of bufonids, and are known to be associated with intoxication processes in domestic animals, as well as being involved in chemical defense events [121]. The steroid telocinobufagin, isolated from the secretions of both toad species, showed antiplasmodial activity in vitro for P. falciparum by interfering with the development of trophozoites (IC50 = 1280 µg/mL) [95]. Three other bufadienolides, marinobufotoxin, marinobufagin, and bufalin, were isolated from the secretion of R. marina and showed antiplasmodial activity in vitro for P. falciparum by interrupting the development of trophozoites (IC50 = 5.31, 3.89 and 3.44 µg/mL, respectively) [122].
5.2.3. Peptides from Anuran Venoms
Peptides are commonly a major component in anuran venoms and may be involved in communication [123] and defense events [124]. A large number of peptides present in the integument of anurans have an antimicrobial character and, for this reason, a series of studies have been carried out to test their activity against various microorganisms [125,126,127]. So far, only one peptide (phylloseptin-1), which was isolated from the tree frog Phyllomedusa azurea (Hylidae, Phyllomedusinae), has been tested for its activity against P. falciparum [128] and it showed the ability to inhibit the growth of trophozoites in in vivo experiments (MIC = 128 g/mL). Phylloseptin-1 is a peptide with 19 amino acid residues and presents an amidated C-terminal region (FLSLIPHAINAVSAIAKHN-NH2) [128]. Due to the high number of peptides already known and isolated from anuran venoms, it is suggested that more studies should be carried out to test their antiparasitic activity.
5.2.4. Alkaloids from Anuran Venoms
Anurans, for the most part, are not capable of synthesizing alkaloids in their bodies; therefore, this metabolite is obtained from the ingestion of ants, termites and mites [124] Like peptides, alkaloids are involved in chemical defense processes and, in tests, they have demonstrated antiviral [129], antibacterial and antifungal activity [126]. Five families of anurans have alkaloids in their secretions. Dendrobatidae presents the largest number of individuals known to have alkaloids in their toxins [86,118], the other families being Mantellidae, Eleutherodactylidae, Bufonidae, and Myobatrachidae. The alkaloid dehydrobufotenine, isolated from the secretion of the bufonid R. marina, was tested for antiplasmodial activity and showed great inhibitory activity against the development of P. falciparum trophozoites (IC50 = 19.11 µg/mL) [122].
5.3. Spider Venom Toxins against Plasmodium Species
Spiders belong to the class Arachnida, order Araneae, and there are approximately 50,000 species around the world [130]. Venom glands are present in most spiders, but they are absent in the family Uloboridae [131]. The glands are located either in the chelicerae or under the carapace [131]; however, the toxic potential of venoms varies according to the species, since it is used not only as a defense mechanism, but also in hunting events [132]. They are venomous animals that can cause harm to humans, which is why some species are considered a public health problem [133]. Their toxins have a range of substances that present bioactive properties, some of which are used in the pharmaceutical industry to produce drugs and even serums [134]. Some species have had their venoms tested and have demonstrated a broad spectrum of antimicrobial activities [135]; however, studies that evaluate their antiplasmodial potential are still scarce. The peptides psalmopeotoxin I (PcFK1) and psalmopeotoxin II (PcFK2) were isolated from the venom of the tarantula Psalmopeus cambridgei (Araneae, Theraphosidae) and were tested against Plasmodium sp. PcFK1 has 33 amino acid residues in its primary sequence (ACGILHDNCVYVPAQNPCCRGLQRYGKCLVQV), while PcFK2 has 28 amino acid residues (RCLPAGKTCVRGPMRVPCCGSCSQNKCT). Both peptides were tested in vitro against P. falciparum. It was observed that both PcFK1 and PcFK2 showed antiplasmodial activity by inhibiting the development of P. falciparum trophozoites (IC50 = 1.59 and 1.15 µg/mL, respectively) [136].
5.4. Scorpion Venom Toxins against Plasmodium Species
Like spiders, scorpions are arachnids and belong to the class Arachnida. They are animals that have an elongated body and a venom inoculating device (telson) at the tip of the tail [137]. Currently, approximately 2200 species are known [94,138]. Toxins from some scorpion species have been tested against P. falciparum, P. berghei and P. gallinaceum (Table 4). Scorpine, isolated from the venom of Pandinus imperator (Scorpionidae), was evaluated against P. berghei [97]. Scorpine is a peptide with 75 amino acid residues (GWINEEKIQKKIDERMGNTVLGRMAKAIVHKMAKNEFQCMANMDMLGNCEKHCQTSGEKGYCHGTKCKCGTPLSY), and presents inhibitory activity against gametocytes and ookinetes of P. berghei, with a minimum inhibitory concentration (MIC) of 50 μM (gametocytes) and 30 μM (ookinetes). Meucin-24, isolated from Mesobuthus eupeus (Buthidae), is a peptide that has 22 amino acid residues (GRGREFMSNLKEKLVKEKMKNS) in its primary structure and presents antiplasmodial activity for P. berghei and P. falciparum [139]. Meucin-24 can inhibit the development (40% inhibition) of both parasites at concentrations between 10 and 20 μM, while meucin-25, on the other hand, showed inhibitory activity of 50% at the same concentrations. VmCT1-NH2 and its analogues [Arg]3-VmCT1-NH2, [Arg]7-VmCT1-NH2 and [Arg]11-VmCT1-NH2 isolated from Vaejovis mexicanus (Vaejovidae) venom were tested for activity against P. gallinaceum. VmCT1-NH2, [Arg]3-VmCT1-NH2 and [Arg]7-VmCT1-NH2 showed inhibitory capacity in the sporozoite phase (IC50 = 0.49, 0.57 and 0.51 µg/mL, respectively) [140].
5.5. Bee Venom Toxins against Plasmodium Species
Bees are insects that belong to the order Hymenoptera and the superfamily Apoidea. They are eusocial and present an organization at a hierarchical level of castes. They are highly appreciated not only for their ecological importance (they are excellent pollinators of plants), but also for production of a highly appreciated wax [141]. They have a stinger and are considered venomous [142]. In the study of antiplasmodial substances, bee venom toxins have been analyzed and have shown promise, with the main activities being attributed to substances that originate from proteins (Table 4).
5.5.1. Peptides from Bee Venoms
Melittin and apamin were tested for their antiplasmodial activities. Melittin is a peptide that contains 26 amino acid residues in its primary structure (GIGAVLKVLTTGLPALISWIKRKRQQ) and is present in the venom of the bee Apis mellifera (Apidae) [94]. The first study to use synthetic melittin against malaria was carried out by Boman et al. [94] against P. falciparum and it was observed to inhibit the growth (in vitro) of the trophozoite and schizont forms at very low concentrations (MIC = 2 to 20 μM). Methyllin inhibited the intraerythrocytic growth of P. falciparum (IC50 = 10 µg/mL) [143], controlled parasitemia in vitro and in vivo of P. falciparum trophozoites at concentrations of 500, 250, and 125 μg/mL [144], and inhibited P. berghei ookinetes and gametocytes, in vitro, in Anopheles stephensi with a minimum inhibitory concentration of 25 μM [145]. Apamin reduces young P. falciparum trophozoites in vitro (MIC = 1 to 250 μg/mL). This peptide blocks potassium receptor channels and causes the parasite to become unviable [146].
5.5.2. Phospholipase A from Bee Venoms
Phospholipase A was more related to antiplasmodial activity in bee venoms. PLA5 presented in vitro activity against P. knowlesi, with a significant reduction in intraerythrocytic growth at concentrations of 2–4 μM [147]. PLA3 presented in vitro activity against P. falciparum and reduced the level of parasitemia due to it preventing the development of the schizont [148]. Other PLA3s acted to inhibit parasite development, especially by reducing oocyst development and being active against P. berghei and P. gallinaceum [149,150] and inhibiting intraerythrocytic growth of P. falciparum [110,151,152]. PLA4 reduced the development of P. falciparum schizonts and trophozoites in in vitro tests [153]. PLA2 inhibited the development of young trophozoites in cells infected with P. falciparum (IC50 = 1.1 × 106 μg/mL) [154].
Table 4.
Toxins from venomous animals that have shown activity against Plasmodium spp.
| Taxon | Family | Species | Chemical Class | Substance | Target Species | Development Stage | Magnitude of Activity | Model | Against | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μg/mL) | MIC (g/mL) | ||||||||||
| Anurans | Bufonidae | Rhinella marina | Bufadienolide | Telocinobufagin | P. f. | Trophozoites | 1.28 | ND | In vitro | CQ-resistant strain | [95] |
| Bufonidae | Rhaebo guttatus | Bufadienolide | Telocinobufagin | P. f. | Trophozoites | 1.28 | ND | In vitro | CQ-resistant strain | [95] | |
| Bufonidae | Rhinella marina | Alkaloid | Dehydrobufotenine | P. f. | Trophozoites | 19.11 | ND | In vitro | CQ-resistant strain | [122] | |
| Bufonidae | Rhinella marina | Bufadienolide | Marinobufotoxin | P. f. | Trophozoites | 5.31 | ND | In vitro | CQ-resistant strain | [122] | |
| Bufonidae | Rhinella marina | Bufadienolide | Marinobufagin | P. f. | Trophozoites | 3.89 | ND | In vitro | CQ-resistant strain | [122] | |
| Bufonidae | Rhinella marina | Bufadienolide | Bufalin | P. f. | Trophozoites | 3.44 | ND | In vitro | CQ-resistant strain | [122] | |
| Bufonidae | Not specified | Crude extract | Crude extract | P. berghei | ND | ND | ND | In vivo | Parasitemia | [98] | |
| Hylidae | Phyllomedusa azurea | Peptide | Phylloseptin-1 | P. f. | Trophozoites | ND | 128 | In vivo | Parasite growth in erythrocytes | [128] | |
| Leptodactylidae | Leptodactylus labyrinthicus | Peptide | Oc-P1 (ocellatins) | P. f. | Trophozoites | 26.71 | ND | In vitro | CQ-resistant strain | [155] | |
| Pipidae | Xenopus laevis | Peptide | Magainin2 | An. Gambiae | Zygotes, ookinetes, and merozoites | ND | 0.5–1 | In vitro | Parasite development in the mosquito | [156] | |
| Bufonidae | Rhinella marina | Crude extract | Crude extract | P. f. | Trophozoites | 2.43 | ND | In vitro | CQ-resistant strain | [120] | |
| Spiders | Theraphosidae | Psalmopoeus cambridgei | Peptide | Psalmopeotoxin I (PcFK1) | P. f. | Intraerythrocytic cycle | 116 | ND | In vitro | Artemisinin-resistant strain | [157] |
| Theraphosidae | Psalmopoeus cambridgei | Peptide | Psalmopeotoxin I (PcFK1) | P. f. | Trophozoites | 1.59 | ND | In vitro | Parasite growth in erythrocytes | [136] | |
| Theraphosidae | Psalmopoeus cambridgei | Peptide | Psalmopeotoxin II (PcFK2) | P. f. | Trophozoites | 1.15 | ND | In vitro | Parasite growth in erythrocytes | [136] | |
| Theraphosidae | Psalmopoeus cambridgei | Peptide | Psalmopeotoxin II’ (PcFK2’) | P. f. | Trophozoites | 9.2 | ND | In vitro | Parasite growth in erythrocytes | [158] | |
| Theraphosidae | Acanthoscurria gomesiana | Peptide | Gomesin | P. f. | Intraerythrocytic cycle | 75.8–86.6 | ND | In vitro | Parasite growth in erythrocytes | [159] | |
| Scorpions | Scorpionidae | Pandinus imperator | Peptide | Scorpine | P. berghei | Ookinetes and gametes | ND | 50 μM (fertilization); 3 μM (ookinete) | In vitro | Development of the para-site | [97] |
| Buthidae | Mesobuthus eupeus | Peptide | Meucin-24 | P. f./P. berghei | Inhibi-ting the erythrocyte development | ND | 40%;10 a 20 μM (inhibiting the development) | In vitro | Parasite growth in erythrocytes | [139] | |
| Buthidae | Mesobuthus eupeus | Peptide | Meucin-25 | P. f./P. berghei | Inhibiting the erythrocyte development | ND | 50%;10 a 20 μM (inhibiting the development) | In vitro | Parasite growth in erythrocytes | [139] | |
| Vaejovidae | Vaejovis mexicanus | Peptide | VmCT1-NH2 | P. gallinaceum | Sporozoites | 0.49 | ND | In vitro | Dead-cell staining | [140] | |
| Vaejovidae | Vaejovis mexicanus | Peptide | [Arg]3-VmCT1-NH2 | P. gallinaceum | Sporozoites | 0.57 | ND | In vitro | Dead-cell staining | [140] | |
| Vaejovidae | Vaejovis mexicanus | Peptide | [Arg]7-VmCT1-NH2 | P. gallinaceum | Sporozoites | 0.51 | ND | In vitro | Dead-cell staining | [140] | |
| Vaejovidae |
Vaejovis mexicanus
|
Peptide | [Arg]11-VmCT1-NH2 |
P. gallinaceum | Sporozoites | >1.6 | ND | In vitro | Dead-cell staining | [140] | |
| Snakes | Viperidae | Bothrops asper | Crude extract | Crude extract | P. f. | Intra-erythrocytic cycle | 0.13 | ND | In vitro | CQ-resistant strain | [96] |
| Viperidae | Bothrops asper | Enzyme | Fração V (Phospholipase A2) | P. f. | Intra-erythrocytic cycle | 1.42 | ND | In vitro | CQ-resistant strain | [96] | |
| Viperidae | Bothrops asper | Homologous | Fração VI (Homologo Phospholipase A2) | P. f. | Intra-erythrocytic cycle | 323.35 | ND | In vitro | CQ-resistant strain | [96] | |
| Elapidae | Bungarus fasciatus | Peptide | LZ1 | P. f. | Intra-erythrocytic cycle | 3.045 | ND | In vitro | CQ-resistant strain | [107] | |
| Elapidae | Bungarus fasciatus | Peptide | LZ1 | P. berghei | Intra-erythrocytic cycle | ND | 39% (4 mg/kg), 35% (8 mg/kg) e 24% (12 mg/kg) | In vivo | CQ-resistant strain | [107] | |
| Viperidae | Bothrops marajoensis | Enzyme | BmajPLA2-II | P. f. | Intra-erythrocytic cycle | 6.41 | ND | In vitro | CQ-resistant strain | [112] | |
| Viperidae | Agkistrodon halys | Enzyme | Phospholipase A2 (IIA) | P. f. | Intraerythrocytic development | 82.3 | ND | In vitro | Parasite growth in erythrocytes | [110] | |
| Elapidae | Naja mossambica mossambica | Enzyme | Phospholipase A2 (IA) | P. f. | Intraerythrocytic development | 0.023 | ND | In vitro | Parasite growth in erythrocytes | [110] | |
| Elapidae | Naja scutatus scutatus | Enzyme | Phospholipase A2 (IA) | P. f. | Intraerythrocytic development | 2.6 | ND | In vitro | Parasite growth in erythrocytes | [110] | |
| Viperidae | Vipera ammodytes | Enzyme | Phospholipase A2 (IIA) | P. f. | intraerythrocytic development | 2.8 | ND | In vitro | Parasite growth in erythrocytes | [110] | |
| Elapidae | Naja naja oxiana | Extract | Fraction 4 | P. f. | Intraerythrocytic development | 0.368 | ND | In vitro | Parasite growth in erythrocytes | [104] | |
| Elapidae | Naja naja oxiana | Extract | Fraction 4 | P. berghei | Intraerythrocytic development | ND | 70%(5 mg/kg); 50%(2.5 mg/kg); 30%(1 mg/kg) | In vivo | Parasite growth in erythrocytes | [105] | |
| Viperidae | Bothrops brazili | Metalloproteinase | BbMP-1 | P. f. | Intra-erythrocytic development | 3.2 | ND | In vitro | Parasite growth in erythrocytes | [160] | |
| Viperidae | Crotalus durissus | Peptide | Crotamine | P. f. | Intra-erythrocytic development | 1.87 | ND | In vitro | CQ-resistant strain | [106] | |
| Viperidae | Crotalus durissus cumanensis | Enzyma (Fraction 1) | Crotoxin (Phospholipase A2) | P. f. | Intra-erythrocytic development | 0.17 | ND | In vitro | CQ-resistant strain | [111] | |
| Viperidae | Crotalus durissus cumanensis | Enzyme (Fraction 2) | Crotoxin (Phospholipase A2) | P. f. | Intra-erythrocytic development | 0.76 | ND | In vitro | CQ-resistant strain | [111] | |
| Viperidae | Crotalus durissus cumanensis | Enzyme | Crotoxin B (Phospolipase A2) | P. f. | Intra-erythrocytic development | 0.6 | ND | In vitro | CQ-resistant strain | [111] | |
| Viperidae | Crotalus durissus cumanensis | Crude extract | Crude extract | P. f. | Intra-erythrocytic development | 0.17 | ND | In vitro | CQ-resistant strain | [111] | |
| Viperidae | Bothrops asper | Enzyme | BaspAc-II | P. f. | Intra-erythrocytic development | 2.46 | ND | In vitro | CQ-resistant strain | [114] | |
| Viperidae | Bothrops asper | Enzyme | BaspAc-IV | P. f. | Intra-erythrocytic development | 0.019 | ND | In vitro | CQ-resistant strain | [114] | |
| Elapidae | Micrurus spixii | Crude extract | Crude extract | P. f. | Intra-erythrocytic development | ≤0.78 | ND | In vitro | CQ-resistant strain | [103] | |
| Viperidae | Bothrops diporus | Enzyme | BdTX-I (Phospholipase A2) | P. f. | Intra-erythrocytic development | 2.44 | ND | In vitro | CQ-resistant strain | [113] | |
| Viperidae | Bothrops diporus | Enzyme | BdTX-II (Phospholipase A2) | P. f. | Intra-erythrocytic development | 0.0153 | ND | In vitro | CQ-resistant strain | [113] | |
| Viperidae | Bothrops diporus | Enzyme | BdTX-III (Phospholipase A2) Homologo | P. f. | Intra-erythrocytic development | 0.59 | ND | In vitro | CQ-resistant strain | [113] | |
| Viperidae | Crotalus adamanteus | Enzyme | Phospholipase A2 | P. gallinaceum | Oocyst formation | ND | ND | In vitro | ND | [109] | |
| Bees | Not applicable | Not applicable | Enzyme | Phospholipase A2 | P. f. | Young trophozoites | 1.1 × 10−6 | ND | In vitro | Intra-erythrocytic growth | [154] |
| Not applicable | Not applicable | Enzyme | Phospholipase A3 | P. f. | Tropho-zoites | 1.69 × 10−5 | ND | In vitro | Intraerythrocytic growth | [110] | |
| Not applicable | Not applicable | Enzyme | Phospholipase A2 | P. f. | Mature trophozoites | ND | ND | In vitro | Intraerythrocytic growth | [155] | |
| Apidae | Apis mellifera | Enzyme | Phospholipase A3 | P. berghei | Oocysts | ND | ND | In vitro | Development of the parasite | [149] | |
| Not applicable | Not described | Enzyme | Phospholipase A3 | P. gallinaceum | Oocysts | ND | ND | In vitro | Development of the parasite | [150] | |
| Apidae | Apis mellifera | Peptide | Melittin | P. f. | Not specified | 10 | ND | In vitro | Intraerythrocytic growth | [143] | |
| Not applicable | Not applicable | Enzyme | Phospholipase A3 | P. f. | Not specified | ND | ND | In vitro | Intraerythrocytic growth | [151] | |
| Not applicable | Not applicable | Enzyme | Phospholipase A3 | P. f. | Schizonts | ND | ND | In vitro | Parasitemia | [148] | |
| Not applicable | Not applicable | Enzyme | Phospholipase A4 | P. f. | Trophozoites and schizonts | ND | ND | In vitro | Parasitemia | [153] | |
| Not applicable | Not applicable | Peptide | Melittin | P. f. | Trophozoites and schizonts | ND | ND | In vitro | Development of the parasite | [94] | |
| Not applicable | Not applicable | Peptide | Melittin | P. f. | Trophozoites | ND | ND | In vitro and in vivo | Parasitemia | [144] | |
| Not applicable | Not applicable | Enzyme | Phospholipase A5 | P. knowlesi | Trophozoites | ND | ND | In vitro | Intraerythrocytic growth | [147] | |
Abbreviations: IC50—Half-maximal inhibitory concentration; MIC—Minimum inhibitory concentration; Not determined (ND); NMR 1H—Hydrogen-1 nuclear magnetic resonance; NMR 13C—Carbon-13 nuclear magnetic resonance; F-moc—Fluorenylmethoxycarbonyl protecting group; HPLC—High-performance liquid chromatography; MALDI-TOF—Matrix-assisted laser desorption/ionization-time of flight; TOF MS—Time-of-flight mass spectrometry; RP-HPLC—Reversed-phase HPLC; SDS-PAGE—Sodium dodecyl sulfate-polyacrylamide gel electrophoresis; Ref.—References; P. f.—Plasmodium falciparum; P. v.—Plasmodium vivax; CQ—chloroquine.
6. Conclusions and Future Perspectives
Drug therapies and vector control via insecticides are respectively the most used methods for the treatment and control of malaria; however, several studies have shown resistance of some Plasmodium species to the drugs that are recommended for their treatment. In view of this, it is necessary to carry out studies to discover new antimalarial molecules as lead compounds for the development of medicines. As such, in the last few decades, animal venoms have attracted attention for being a potential source for new antimalarial molecules.
In this review, we evidenced 50 substances, 4 fractions and 7 toxins extracted from the venoms of animals. Anurans, snakes, spiders, scorpions and bees have been studied, and all of them have shown immeasurable antimalarial activities against Plasmodium spp., acting in distinct phases of its biological cycle and with its consequent inhibition. However, it is emphasized that more studies should be carried out in order to unravel the mechanism of action of the toxins in the inhibition of Plasmodium spp., as they represent a major milestone in the face of the resistance to current antimalarial drugs.
Author Contributions
Onceptualization, H.H.F.K., G.C.d.M. and W.M.M.; methodology, A.A.X.A. and A.M.R.; software, M.B.P.; validation, M.A.S. and I.S.d.O.; formal analysis, D.C.B.-d.-S. and H.H.F.K.; investigation, Z.M.S. and A.L.B.; resources, G.C.d.M. and W.M.M.; data curation, M.B.P. and I.S.d.O.; writing—original draft preparation, Z.M.S. and A.L.B.; writing—review and editing, Z.M.S.; visualization, D.C.B.-d.-S.; supervision, H.H.F.K. and G.C.d.M.; project administration, H.H.F.K. and W.M.M.; funding acquisition, W.M.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Key Contribution
Animal venom toxins can inhibit the growth of Plasmodium spp. and are promising substances in biotechnological and medical fields.
Funding Statement
This research was financed by Fundação de Amparo à Pesquisa do Estado do Amazonas—FAPEAM—RESOLUÇÃO N. 005/2022, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—Finance code 001), Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) (Pró-Estado Program-#002/2008, #007/2018, and #005/2019), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) via scholarships provided to I.O. (No. 2020/13176-3 and No. 2022/08964-8). We also would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the productivity grants provided to W.M.M. (No. 309207/2020-7), H.H.F.K. (No. 305942/2020-4), G.C.M. (No. 315156/2021-0), M.P. (No. 307184/2020-0) and D.C.B.d.-S. (bolsista visitante nacional II da Fapeam). The author H.H.F.K. also acknowledges FAPEAM for the PRODOC project (Call 003/2022).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO World Malaria Report 2022. World Health Organization. [(accessed on 18 January 2023)]. Available online: https://www.who.int/publications/i/item/9789240064898.
- 2.Meibalan E., Marti M. Biology of Malaria Transmission. Cold Spring Harb. Perspect. Med. 2017;7:a025452. doi: 10.1101/cshperspect.a025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatesan P. The future of malaria control in light of RTS, S. Lancet Microbe. 2022;3:e251. doi: 10.1016/S2666-5247(22)00070-2. [DOI] [PubMed] [Google Scholar]
- 4.Win T.T., Jalloh A., Tantular I.S., Tsuboi T., Ferreira M.U., Kimura M., Kawamoto F. Molecular Analysis of Plasmodium ovale Variants. Emerg. Infect. Dis. 2004;10:1235–1240. doi: 10.3201/eid1007.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemingway J. The role of vector control in stopping the transmission of malaria: Threats and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130431. doi: 10.1098/rstb.2013.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doenças no Portal Fiocruz Malária. [(accessed on 3 April 2023)]. Available online: https://portal.fiocruz.br/doenca/malaria.
- 7.Instituto Oswaldo Cruz Avanços e Perspectivas Para a Eliminação da Malária. 2022. [(accessed on 3 April 2023)]. Available online: https://www.ioc.fiocruz.br.
- 8.PAHO. Pan American Health Organization Plan of Action for Malaria Elimination 2021–2025. [(accessed on 3 April 2023)]. Available online: https://iris.paho.org/handle/10665.2/56616.
- 9.Bensch S., Hellgren O.H., Pérez-Tris J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- 10.Phillips M.A., Burrows J.N., Manyando C., van Huijsduijnen R.H., Van Voorhis W.C., Wells T.N.C. Malaria. Nat. Rev. Dis. Primers. 2017;3:17050. doi: 10.1038/nrdp.2017.50. [DOI] [PubMed] [Google Scholar]
- 11.Brasil P., Zalis M.G., De Pina-Costa A., Siqueira A.M., Bianco C., Jr., Silva S., Areas A.L.L., Pelajo-Machado M., De Alvarenga D.A.M., da Silva Santelli A.C.F., et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: A molecular epidemiological investigation. Lancet Glob. Health. 2017;5:1038–1046. doi: 10.1016/S2214-109X(17)30333-9. [DOI] [PubMed] [Google Scholar]
- 12.Filho A.C.d.A., De Lacerda M.V.G., Okoshi K., Okoshi K., Okoshi M.P.A. Malária e o Endotélio Vascular. Arq. Bras. Cardiol. 2014;103:165–169. doi: 10.5935/abc.20140088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varo R., Chaccour C., Bassat Q. Apdate on malaria. Med. Clin. 2020;155:395–402. doi: 10.1016/j.medcli.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Ta T.H., Hisam S., Lanza M., Jiram A.I., Ismael N., Rubio J.M. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar. J. 2014;13:68. doi: 10.1186/1475-2875-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinka M.E., Bangs M.J., Manguin S., Rubio-Palis Y., Chareonviriyaphap T., Coetzee M., Mbogo C.M., Hemingway J., Patil A.P., Temperley W.H., et al. A global map of dominant malaria vectors. Parasites Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consoli R., Oliveira R.L. Principais Mosquitos de Importância Sanitária no Brasil. Fiocruz; Rio de Janeiro, Brazil: 1998. [(accessed on 18 January 2023)]. pp. 1–224. Available online: http://books.scielo.org. [Google Scholar]
- 17.Lozovei A.L. Culicidae (Mosquitos) In: Marcondes C.B., editor. Entomologia Médica e Veterinária. 2nd ed. Atheneu; São Paulo, Brazil: 2011. pp. 107–174. [Google Scholar]
- 18.Hay S.I., Sinka M.E., Okara R.M., Kabaria C.W., Mbithi P.M., Tago C.C., Benz D., Gething P.W., Howes R.E., Patil A.P., et al. Developing global maps of the dominant Anopheles vectors of human malaria. PLoS Med. 2010;7:e1000209. doi: 10.1371/journal.pmed.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Economic Forum Guidelines for Employer-Based Malária Control Programmes. 2006. [(accessed on 22 March 2023)]. Available online: http://www.weforum.org/pdf/Malária.pdf.
- 20.Sinka M.E., Bangs M.J., Manguin S., Coetzee M., Mbogo C.M., Hemingway J., Patil A.P., Temperley W.H., Gething P.W., Kabaria C.W., et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic precis. Parasit. Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faran M.E., Linthicum K.J. A Handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae) Mosq. Syst. 1981;13:1–81. [Google Scholar]
- 22.Sinden R.E. A cell biologist’s view of host cell recognition and invasion by malarial parasites. Trans. R. Soc. Trop. Med. Hyg. 1985;79:598–605. doi: 10.1016/0035-9203(85)90165-8. [DOI] [PubMed] [Google Scholar]
- 23.Smith R.C., Jacobs-Lorena M. Plasmodium-Mosquito Interactions: A Tale of Roadblocks and Detours. Adv. Insect Phys. 2010;39:119–149. doi: 10.1016/B978-0-12-381387-9.00004-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A., Edwards M.J., Jacobs-Lorena M. The journey of the malaria parasite in the mosquito: Hopes for the new century. Parasitol. Today. 2000;16:196–201. doi: 10.1016/S0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 25.Brasil. Ministério da Saúde Guia Prático de Tratamento da Malária No Brasil. [(accessed on 2 March 2023)]; Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_pratico_malaria.pdf.
- 26.Auburn S., Getachew S., Pearson R.D., Amato R., Miotto O., Trimarsanto H., Zhu S.J., Rumaseb A., Marfurt J., Noviyanti R., et al. Genomic Analysis of Plasmodium vivax in Southern Ethiopia Reveals Selective Pressures in Multiple Parasite Mechanisms. J. Infect. Dis. 2019;220:1738–1749. doi: 10.1093/infdis/jiz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tadesse F.G., Ashine T., Teka H., Esayas E., Messenger L.A., Chali W., Meerstein-Kessel L., Walker T., Behaksra S.W., Lanke K., et al. Anopheles stephensi Mosquitoes as Vectors of Plasmodium vivax and falciparum, Horn of Africa, 2019. Emerg. Infect. Dis. 2021;27:603–607. doi: 10.3201/eid2702.200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buyon L.E., Elsworth B., Duraisingh M.T. The molecular basis of antimalarial drug resistance in Plasmodium vivax. Int. J. Parasitol. Drugs Drug. Resist. 2021;16:23–37. doi: 10.1016/j.ijpddr.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Governo do Estado do São Paulo Secretaria de Estado da Saúde. SUCEN—Superintendencia de Controle de Endemias. [(accessed on 22 March 2023)]; Available online: https://saude.sp.gov.br/sucen-superintendencia-de-controle-de-endemias/programas/malaria/vetores.
- 30.Goodarzi E., Beiranvand R., Darvishi I., Naghibzadeh-Tehami A., Bechashk S.M., Naemi H., Khazaei Z. Geographical distribution of falciparum malaria in the world and its relationship with the human development index (HDI): Countries based on the WHO report in 2017. J. Public Health. 2020;30:655–664. doi: 10.1007/s10389-020-01336-6. [DOI] [Google Scholar]
- 31.Hertig E. Distribution of Anopheles vectors and potential malaria transmission stability in Europe and the Mediterranean area under future climate change. Parasites Vectors. 2019;12:18. doi: 10.1186/s13071-018-3278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC Malaria. Centers for Disease Control and Prevention. [(accessed on 8 March 2023)]; Available online: https://www.cdc.gov/dpdx/malaria/index.html.
- 33.FIOCRUZ Fundação Oswaldo Cruz—Rondônia. Plasmodium. [(accessed on 11 March 2023)]. Available online: https://www.rondonia.fiocruz.br/pivem/plasmodium/
- 34.Hawadak J., Nana R.R.D., Singh V. Global trend of Plasmodium malariae and Plasmodium ovale spp. malaria infections in the last two decades (2000–2020): A systematic review and meta-analysis. Parasites Vectors. 2021;14:297. doi: 10.1186/s13071-021-04797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bykersma A. The New Zoonotic Malaria: Plasmodium cynolmolgi. Trop. Med. Infect. Dis. 2021;6:46. doi: 10.3390/tropicalmed6020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nogueira F., Do Rosário V.E. Métodos para avaliação da atividade antimalárica nas diferentes fases do ciclo de vida do Plasmodium. Rev. Pan-Amaz. Saúde. 2010;1:2176–6215. doi: 10.5123/S2176-62232010000300015. [DOI] [Google Scholar]
- 37.Prudêncio M., Rodrigues A., Mota M.M. The silent path to thousands of merozoites: The Plasmodium liver stage. Nat. Rev. Microbiol. 2006;11:849–856. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- 38.Ng L.C., Ooi E.E., Lee C.C., Lee P.J., Tan N., Pei S.W., Tu T.M., Loh J.P., Leo Y.S. Naturally Acquired Human Plasmodium knowlesi Infection, Singapore. Emerg. Infect. Dis. 2008;14:814–816. doi: 10.3201/eid1405.070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ministério da Saúde Manual de Diagnóstico Laboratorial da Malária. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica e Diretoria Técnica de Gestão. Brasília: Ministério da Saúde. [(accessed on 10 March 2023)];2005 Available online: https://www.gov.br/saude/pt-br.
- 40.Schofield L., Hackett F. Signal Transduction in Host Cells by a Glycosylphosphatidyllnositol Toxin of Malaria Parasites. J. Exp. Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langhorne J., Ndungu F.M., Sponaas A.-M., Marsh K. Immunity to malaria: More questions than answers. Nat. Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 42.Mawson A.R. The pathogenesis of malaria: A new perspective. Pathog. Glob. Health. 2013;107:122–129. doi: 10.1179/2047773213Y.0000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gazzinelli R.T., Kalantari P., Fitzgerald K.A., Golenbock D.T. Innate sensing of malaria parasites. Nat. Rev. Immunol. 2014;14:744–757. doi: 10.1038/nri3742. [DOI] [PubMed] [Google Scholar]
- 44.Ministério da Saúde Guia Prático de Tratamento da Malaria No Brasil. [(accessed on 10 March 2023)];2005 Available online: https://bvsms.saude.gov.br/bvs.
- 45.Alam A., Goyal M., Iqbal M.S., Pal C., Bindu S., Maity P., Bandyopadhyay U. Novel antimalarial drug targets: Hope for new antimalarial drugs. Expert Rev. Clin. Pharmacol. 2009;2:469–489. doi: 10.1586/ecp.09.28. [DOI] [PubMed] [Google Scholar]
- 46.Fármacos antimaláricos. [(accessed on 11 March 2023)]. Available online: https://www.lecturio.com/pt/concepts/farmacos-antimalaricos/
- 47.Thriemer K., Ley B., von Seidlein L. Towards the elimination of Plasmodium vivax malaria: Implementing the radical cure. PLoS Med. 2021;18:e1003494. doi: 10.1371/journal.pmed.1003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price R.N., von Seidlein L., Valecha N., Nosten F., Baird J.K., White N.J. Global extent of chloroquine-resistant Plasmodium vivax: A systematic review and meta-analysis. Lancet Infect. Dis. 2014;14:982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO World Malaria Report 2019. World Health Organization. [(accessed on 11 March 2023)]. Available online: https://www.who.int/publications/i/item/9789241565721.
- 50.FUNASA, Ministério da Saúde Fundação Nacional de Saúde. Manual de Terapeutica da Malária. [(accessed on 11 March 2023)]; Available online: https://bvsms.saude.gov.br/bvs/publicacoes/funasa/manu_terapeutica_malaria.pdf.
- 51.Brice B.K., William Y., Lacina O., Félix Y., Hugues A., Léonardo B., André M., Joseph D. In vitro susceptibility of Plasmodium falciparum isolates from Abidjan, Côte d’Ivoire, to artemisinin, chloroquine, dihydroartemisinin and pyronaridine. Tanzan. J. Health Res. 2010;12:73–79. doi: 10.4314/thrb.v12i1.56364. [DOI] [PubMed] [Google Scholar]
- 52.Kerschbaumer G., Wernsdorfer G., Wiedermann U., Congpouong K., Sirichaisinthop J., Wernsdorfer W.H. Synergism between mefloquine and artemisinin and its enhancement by retinol in Plasmodium falciparum in vitro. Wien. Klin. Wochenschr. 2010;3:57–60. doi: 10.1007/s00508-010-1439-5. [DOI] [PubMed] [Google Scholar]
- 53.Pendey A.V., Tekwani B.L., Singh R.L., Chauhan V.S. Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite. J. Biol. Chem. 1999;274:19383–19388. doi: 10.1074/jbc.274.27.19383. [DOI] [PubMed] [Google Scholar]
- 54.Aguiar A.C.C., Pereira D.B., Amaral N.S., De Marco L., Krettli A.U. Plasmodium vivax and Plasmodium falciparum ex vivo susceptibility to antimalarials and gene characterization in Rondônia, West Amazon, Brazil. Malar. J. 2014;13:73. doi: 10.1186/1475-2875-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernández D., Segura C., Arboleda M., Garavito G., Blair S., Pabón A. In Vitro Susceptibility of Plasmodium vivax to Antimalarials in Colombia. Antimicrob. Agents Chemother. 2014;58:6354–6359. doi: 10.1128/AAC.03191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng W., Zhao H., Zhao W., Yang Q., Li X., Li X., Duan M., Wang X., Li C., Xiang Z., et al. Molecular Surveillance and Ex Vivo Drug Susceptibilities of Plasmodium vivax Isolates from the China–Myanmar Border. Front. Cell. Infect. Microbiol. 2021;11:738075. doi: 10.3389/fcimb.2021.738075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao W., Li X., Yang Q., Zhou L., Duan M., Pan M., Qin Y., Li X., Wang X., Zeng W., et al. In vitro susceptibility profile of Plasmodium falciparum clinical isolates from Ghana to antimalarial drugs and polymorphisms in resistance markers. Front. Cell. Infect. Microbiol. 2022;12:1015957. doi: 10.3389/fcimb.2022.1015957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treiber M., Wernsdorfer G., Wiedermann U., Congpuong K., Sirichaisinthop J., Wernsdorfer W.H. Sensitivity of Plasmodium vivax to chloroquine, mefloquine, artemisinin and atovaquone in north-western Thailand. Wien. Klin. Wochenschr. 2011;123:20–25. doi: 10.1007/s00508-011-0044-6. [DOI] [PubMed] [Google Scholar]
- 59.Marfurt J., Wirjanata G., Prayoga P., Chalfein F., Leonardo L., Sebayang B.F., Apriyanti D., Sihombing M.A.E.M., Trianty L., Suwanarusk R., et al. Longitudinal ex vivo and molecular trends of chloroquine and piperaquine activity against Plasmodium falciparum and P. vivax before and after introduction of artemisinin-based combination therapy in Papua, Indonesia. Int. J. Parasitol. Drugs Drug. Resist. 2021;17:46–56. doi: 10.1016/j.ijpddr.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell B.M., Udomsangpetch R., Rieckmann K.H., Kotecka B.M., Coleman R.E., Sattabongkot J. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 2003;47:170–173. doi: 10.1128/AAC.47.1.170-173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasugian A.R., Tjitra E., Ratcliff A., Siswantoro H., Kenangalem E., Wuwung R.M., Purba H.L., Piera K.A., Chalfien F., Marfurt J., et al. In vivo and in vitro efficacy of amodiaquine monotherapy for treatment of infection by chloroquine-resistant Plasmodium vivax. Antimicrob. Agents Chemother. 2009;53:1094–1099. doi: 10.1128/AAC.01511-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price R.N., Marfurt J., Chalfein F., Kenangalem E., Piera K.A., Tjitra E., Anstey N.M., Russell B. In vitro activity of pyronaridine against multidrug-resistant Plasmodium falciparum and Plasmodium vivax. Antimicrob. Agents Chemother. 2010;54:5146–5150. doi: 10.1128/AAC.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wirjanata G., Sebayang B.F., Chalfein F., Payoga P., Handayuni I., Trianty L., Kenangalem E., Noviyanti R., Campo B., Poespoprodjo J.R., et al. Potent Ex. Vivo Activity of Naphthoquine and Methylene Blue against Drug-Resistant Clinical Isolates of Plasmodium falciparum and Plasmodium vivax. Antimicrob. Agents Chemother. 2015;59:6117–6124. doi: 10.1128/AAC.00874-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agarwal P., Anvikar A.R., Pillai C.R., Srivastava K. In vitro susceptibility of Indian Plasmodium falciparum isolates to different antimalarial drugs & antibiotics. Indian J. Med. Res. 2017;146:622–628. doi: 10.4103/ijmr.IJMR_1688_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Randrianarivelojosia M., Ratsimbasoa A., Randrianasolo L., Randrianarijaona A., Jambou R. In-vitro sensitivity of Plasmodium falciparum to chloroquine, halofantrine, mefloquine and quinine in Madagascar. East. Afr. Med. J. 2002;79:237–241. doi: 10.4314/eamj.v79i5.8860. [DOI] [PubMed] [Google Scholar]
- 66.Yavo W., Bla K.B., Djaman A.J., Assi S.B., Basco L.K., Mazabraud A., Koné M. In vitro susceptibility of Plasmodium falciparum to monodesethylamodiaquine, quinine, mefloquine and halofantrine in Abidjan (Côte d’Ivoire) Afr. Health Sci. 2010;10:111–116. [PMC free article] [PubMed] [Google Scholar]
- 67.Dembélé L., Franetich J.F., Soulard V., Amanzougaghene N., Tajeri S., Bousema T., van Gemert G.J., Le Grand R., Dereuddre-Bosquet N., Baird J.K., et al. Chloroquine Potentiates Primaquine Activity against Active and Latent Hepatic Plasmodia Ex. Vivo: Potentials and Pitfalls. Antimicrob. Agents Chemother. 2020;65:1416–1420. doi: 10.1128/AAC.01416-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mbaye A., Gaye A., Dieye B., Ndiaye Y.D., Bei A.K., Affara M., Deme A.B., Yade M.S., Diongue K., Ndiaye I.M., et al. Ex vivo susceptibility and genotyping of Plasmodium falciparum isolates from Pikine, Senegal. Malar. J. 2017;16:250. doi: 10.1186/s12936-017-1897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baird J.K. Resistance to therapies for infection by Plasmodium vivax. Clin. Microbiol. Rev. 2009;22:508–534. doi: 10.1128/CMR.00008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Held J., Zanger P., Issifou S., Kremsner P.G., Mordmüller B. In vitro activity of tigecycline in Plasmodium falciparum culture-adapted strains and clinical isolates from Gabon. Int. J. Antimicrob. Agents. 2010;35:587–589. doi: 10.1016/j.ijantimicag.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Menard D., Dondorp A. Antimalarial Drug Resistance: A Threat to Malaria Elimination. Cold Spring Harb. Perspect. Med. 2017;7:a025619. doi: 10.1101/cshperspect.a025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rieckmann K.H., Davis D.R., Hutton D.C. Plasmodium vivax resistance to chloroquine? Lancet. 1989;2:1183–1184. doi: 10.1016/s0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- 73.Gama B.E., De Oliveira N.K.A., De Souza J.M., Daniel-Ribeiro C.T., Ferreira-da-Cruz M.d.F. Characterisation of pvmdr1 and pvdhfr genes associated with chemoresistance in Brazilian Plasmodium vivax isolates. Mem. Inst. Oswaldo Cruz. 2009;104:1009–1011. doi: 10.1590/S0074-02762009000700012. [DOI] [PubMed] [Google Scholar]
- 74.Rungsihirunrat K., Muhamad P., Chaijaroenkul W., Kuesap J., Na-Bangchang K. Plasmodium vivax Drug Resistance Genes; Pvmdr1 and Pvcrt-o Polymorphisms in Relation to Chloroquine Sensitivity from a Malaria Endemic Area of Thailand. Korean J. Parasitol. 2015;53:43–49. doi: 10.3347/kjp.2015.53.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chotivanich K., Udomsangpetch R., Chierakul W., Newton P.N., Ruangveerayuth R., Pukrittayakamee S., Looareesuwan S., White N.J. In vitro efficacy of antimalarial drugs against Plasmodium vivax on the western border of Thailand. Am. J. Trop. Med. Hyg. 2004;70:395–397. doi: 10.4269/ajtmh.2004.70.395. [DOI] [PubMed] [Google Scholar]
- 76.Fidock D.A., Nomura T., Talley A.K., Cooper R.A., Dzekunov S.M., Ferdig M.T., Ursos L.M., Sidhu A.B., Naude B., Deitsch K.W., et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell. 2000;6:861–871. doi: 10.1016/S1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alecrim M.d.G., Alecrim W., Macêdo V. Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev. Soc. Bras. Med. Trop. 1999;32:67–68. doi: 10.1590/S0037-86821999000100013. [DOI] [PubMed] [Google Scholar]
- 78.Noronha E., Alecrim M.d.G., Romero G.A.S., Macêdo V. Resistência à mefloquina do tipo RIII em crianças com malária falciparum em Manaus, AM, Brasil. Rev. Soc. Bras. Med. Trop. 2000;32:201–205. doi: 10.1590/S0037-86822000000200008. [DOI] [PubMed] [Google Scholar]
- 79.Couto A.A., Calvosa V.S., Lima J.E., Souza J.M. Evolução da resistencia in vitro do Plasmodium falciparum a antimaláricos em área de prospecção de ouro no Estado do Amapá, entre 1983 e 1990. Rev. Soc. Bras. Med. Trop. 1993;26:215–220. doi: 10.1590/S0037-86821993000400003. [DOI] [PubMed] [Google Scholar]
- 80.Cowman A.F. Mechanisms of drug resistance in malaria. Aust. N. Z. J. Med. 1995;25:837–844. doi: 10.1111/j.1445-5994.1995.tb02889.x. [DOI] [PubMed] [Google Scholar]
- 81.Reed M.B., Saliba K.J., Caruana S.R., Kirk K., Cowman A.F. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 82.Fairhurst R.M., Dondorp A.M. Artemisinin-Resistant Plasmodium falciparum Malaria. Microbiol. Spectr. 2016;4:1–16. doi: 10.1128/microbiolspec.EI10-0013-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takala-Harrison S., Clark T.G., Jacob C.G., Cummings M.P., Miotto O., Dondorp A.M., Fakuda M.M., Nosten F., Noedl H., Imwong M., et al. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc. Natl. Acad. Sci. USA. 2013;110:240–245. doi: 10.1073/pnas.1211205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silva-Pinto A., Domingos J., Cardoso M., Reis A., Benavente E.D., Caldas J.P., Conceição C., Toscano C., Baptista-Fernandes T., Clark T.G., et al. Artemether-lumefantrine treatment failure of uncomplicated Plasmodium falciparum malaria in travellers coming from Angola and Mozambique. Int. J. Infect. Dis. 2021;110:151–154. doi: 10.1016/j.ijid.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farooq U., Mahajan R.C. Drug resistance in malaria. J. Vector Borne Dis. 2004;41:45–53. [PubMed] [Google Scholar]
- 86.Utkin Y.N. Animal venom studies: Current benefits and future developments. World J. Biol. Chem. 2015;6:28–33. doi: 10.4331/wjbc.v6.i2.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arbuckle K. Toxinology. Springer; Dordrecht, The Netherlands: 2017. Evolutionary Context of Venom in Animals; pp. 3–31. [DOI] [Google Scholar]
- 88.Daly L.E., Kirke P.N., Molloy A., Weir D.G., Scott J.M. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274:1698–1702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- 89.Schendel V., Rash L.D., Jenner R.A., Undheim E.A.B. The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins. 2019;11:666. doi: 10.3390/toxins11110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oguiura N., Sanches L., Duarte P.V., Sulca-López M.A., Machini M.T. Past, Present, and Future of Naturally Occurring Antimicrobials Related to Snake Venoms. Animals. 2023;13:744. doi: 10.3390/ani13040744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abdullahi Z.U., Musa S.S., He D., Bello U.M. Antiprotozoal Effect of Snake Venoms and Their Fractions: A Systematic Review. Pathogens. 2021;10:1632. doi: 10.3390/pathogens10121632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gopalakrishnakone P., Corzop G., De Lima M.E., Diego-García E. Toxinology. Springer; Dordrecht, The Netherlands: 2015. Spider Venoms; pp. 1–467. [Google Scholar]
- 93.Ahmadi S., Knerr J.M., Argemi L., Bordon K.C.F., Pucca M.B., Cerni F.A., Arantes E.C., Çaliskan F., Laustsen A.H. Scorpion Venom: Detriments and Benefits. Biomedicines. 2020;8:118. doi: 10.3390/biomedicines8050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bomam H.G., Wade D., Bomam I.A., Wahlin B., Merrifield R.B. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989;259:103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- 95.Banfi F.F., Guedes K.d.S., Andrighetti C.R., Aguiar A.C., Debiase B.W., Noronha J.d.C., Rodrigues D.d.J., Júnior G.M.V., Sanchez B.A.M. Antiplasmodial and Cytotoxic Activities of Toad Venoms from Southern Amazon, Brazil. Korean J. Parasitol. 2016;54:415–421. doi: 10.3347/kjp.2016.54.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castillo J.C.Q., Vargas L.J., Segura C., Gutiérrez J.M., Pérez J.C.A. In vitro Antiplasmodial Activity of Phospholipases A2 and a Phospholipase Homologue Isolated from the Venom of the Snake Bothrops asper. Toxins. 2012;4:1500–1516. doi: 10.3390/toxins4121500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conde R., Zamudio F.Z., Rodríguez M.H., Possani L.D. Scorpine, an anti-malaria and anti-bacterial agent puri¢ed from scorpion venom. FEBS Lett. 2000;471:165–168. doi: 10.1016/S0014-5793(00)01384-3. [DOI] [PubMed] [Google Scholar]
- 98.Ayim J.O., Pam V.A., Uzoigwe N.R., Omalu I.C.J., Ombugadu A., Ameh S.F., Anyebe G.E., Abe E.M., Tanko N.S., Ahmed H.O., et al. The Antiplasmodial Activities of Bufonidae (Toad) Venom Crude Extract on Plasmodium Berghei in Swiss Albino Mice. Open Access Biog. Sci. Res. 2021;8:1–15. doi: 10.46718/JBGSR.2021.08.000190. [DOI] [Google Scholar]
- 99.The Reptile Database 2022. [(accessed on 14 March 2023)]. Available online: http://www.reptile-database.org/
- 100.De Fraga R., Magnusson W.E., Abrahão C.R., Sanaiotti T., Lima A.P. Habitat Selection by Bothrops atrox (Serpentes: Viperidae) in Central Amazonia, Brazil. Copeia. 2013;4:684–690. doi: 10.1643/CE-11-098. [DOI] [Google Scholar]
- 101.Ferraz C.R., Arrahman A., Xie C., Casewell N.R., Lewis R.J., Kool J., Cardoso F.C. Multifunctional Toxins in Snake Venoms and Therapeutic Implications: From Pain to Hemorrhage and Necrosis. Front. Ecol. Evol. 2019;7:218. doi: 10.3389/fevo.2019.00218. [DOI] [Google Scholar]
- 102.Oliveira A.L., Viegas M.F., Da Silva S.L., Soares A.M., Ramos M.J., Fernandes P.A. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 2022;6:451–469. doi: 10.1038/s41570-022-00393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Terra A.L.C., Morreira-Dill L.S., Simões-Silva R., Monteiro J.R.N., Cavalcante W.L.G., Gallacci M., Barros N.B., Nicolete R., Teles C.L.G., Medeiros P.S.M., et al. Biological characterization of the Amazon coral Micrurus spixii snake venom: Isolation of a new neurotoxic phospholipase A2. Toxicon. 2015;103:1–11. doi: 10.1016/j.toxicon.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 104.Hajialiani F., Elmi T., Mohamadi M., Sadeghi S., Shahbazzadeh D., Ghaffarifar F., Dalimi A., Arjmand M., Tabatabaie F., Zamani Z. Analysis of the active fraction of Iranian Naja naja oxiana snake venom on the metabolite profiles of the malaria parasite by 1 HNMR in vitro. Iran. J. Basic Med. Sci. 2020;23:534–543. doi: 10.22038/IJBMS.2020.39386.9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hajialiani F., Sadeghi S., Shahbazzadeh D., Tabatabaie F., Zamani Z. Assessing Anti-malaria Effect of Naja Naja Oxiana Snake Venom by Real-time Polymerase Chain Reaction Method. Complement. Med. J. 2021;11:68–81. doi: 10.32598/cmja.11.1.1049.1. [DOI] [Google Scholar]
- 106.Maluf S.E.C., Mas C.D., Oliveira E.B., Melo P.M., Carmona A.K., Gazarini M.L., Hayashi M.A.F. Inhibition of malaria parasite Plasmodium falciparum development by crotamine, a cell penetrating peptide from the snake venom. Peptides. 2016;78:11–16. doi: 10.1016/j.peptides.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 107.Fang Y., He X., Zhang P., Chen C., Mwangi J., Xu C., Mo G., Lai R., Zhang Z. In vitro and In vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin. Toxins. 2019;11:379. doi: 10.3390/toxins11070379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parthasarathy S., Steinbrecher U.P., Barnett J., Witztum J.L., Steinberg D. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc. Natl. Acad. Sci. USA. 1985;82:3000–3004. doi: 10.1073/pnas.82.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zieler H., Keister D.B., Dvorak J.A., Ribeiro J.M.C. A snake venom phospholipase A2 blocks malaria parasite development in the mosquito midgut by inhibiting ookinete association with the midgut surface. J. Exp. Biol. 2001;204:4157–4167. doi: 10.1242/jeb.204.23.4157. [DOI] [PubMed] [Google Scholar]
- 110.Guillaume C., Deregnaucourt C., Clavey V., Schével J. Anti-Plasmodium properties of group IA, IB, IIA and III secreted phospholipases A2 are serum-dependent. Toxicon. 2004;43:311–318. doi: 10.1016/j.toxicon.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 111.Quintana J.C., Chacón A.M., Vargas L., Segura C., Guitérrez J.M., Alarcón J.C. Antiplasmodial effect of the venom of Crotalus durissus cumanensis, crotoxin complex and Crotoxin B. Acta Trop. 2012;124:126–132. doi: 10.1016/j.actatropica.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 112.Grabner A.N., Alfonso J., Kayano A.M., Moreira-Dill L., Dos Santos A.P.d.A., Caldeira C.A.S., Sobrinho J.C., Gómez A., Grabner F.P., Cardoso F.F., et al. BmajPLA2-II, a basic Lys49-phospholipase A2 homologue from Bothrops marajoensis snake venom with parasiticidal potential. Int. J. Biol. Macromol. 2017;102:571–581. doi: 10.1016/j.ijbiomac.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 113.Vitorino K.A., Alfonso J.J., Gómez A.F., Santos A.P.A., Antunes Y.R., Caldeira C.A.d.S., Gómez C.V., Teles C.B.G., Soares A.M., Calderon L.A. Antimalarial activity of basic phospholipases A2 isolated from Paraguayan Bothrops diporus venom against Plasmodium falciparum. Toxicon. 2020;8:100056. doi: 10.1016/j.toxcx.2020.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simões-Silva R., Alfonso J.J., Gómez A.F., Sobrinho J.C., Kayano A.M., De Medeiros D.S.S., Teles C.B.G., Quintero A., Fuly A.L., Gómez C.V., et al. Synergism of in vitro plasmodicidal activity of phospholipase A2 isoforms isolated from panamanian Bothrops asper venom. Chem. Biol. Interact. 2021;346:109581. doi: 10.1016/j.cbi.2021.109581. [DOI] [PubMed] [Google Scholar]
- 115.Pough F.H., Janis C.M., Heiser J.B. Avida dos Vertebrados. 4ª Ed. São Pauilo. 1-718: Atheneu. [(accessed on 14 March 2023)]. Available online: http://www.avesmarinhas.com.br/A%20Vida%20dos%20Vertebrados.pdf.
- 116.American Museum of Natural History. Anphibian Species of the World 6.1, na Online Reference. [(accessed on 14 March 2023)]. Available online: https://amphibiansoftheworld.amnh.org/
- 117.Toledo L.F., Sazuima I., Haddad C.F.B. Behavioural defences of anurans: An overview. Ethol. Ecol. Evol. 2011;23:1–25. doi: 10.1080/03949370.2010.534321. [DOI] [Google Scholar]
- 118.Saporito R.A., Spande T., Garraffo M.A., Donnelly M.A. Arthropod Alkaloids in Poison Frogs: A Review of the ‘Dietary Hypothesis’. Heterocycles. 2009;79:277–297. doi: 10.3987/REV-08-SR(D)11. [DOI] [Google Scholar]
- 119.Rodrigues C., Ibáñez R., Rollins-Smith L.A., Gutiérrez M., Durant-Archibold A.A. Antimicrobial Secretions of Toads (Anura, Bufonidae): Bioactive Extracts and Isolated Compounds against Human Pathogens. Antibiotcs. 2020;9:843. doi: 10.3390/antibiotics9120843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Medeiros D.S.S., Rego T.B., Dos Santos A.P.d.A., Pontes A.S., Moreira-Dill L.S., Matos N.B., Zuliani J.P., Stábeli R.G., Teles C.B.G., Soares A.M., et al. Biochemical and Biological Profile of Parotoid Secretion of the Amazonian Rhinella marina (Anura: Bufonidae) BioMed Res. Int. 2019;2019:2492315. doi: 10.1155/2019/4209743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Da Silva P.R.F., Jardim E.C., França L.R., Lemes A.G., Silva P.C. Tratamento de cães envenenados experimentalmente por bufadienolídeos substâncias encontradas na secreção de glândulas paratoides dos sapos do gênero Bufu. [(accessed on 18 January 2023)];Pesqui. Agropecuária Trop. 1978 1:114–120. Available online: http://repositorio.bc.ufg.br/handle/ri/11859. [Google Scholar]
- 122.Benfi F.F., Krombauer C., Da Fonseca A.L., Nunes R.R., Andrade S.N., De Rezende M.A., Chaves M.H., Filho E.d.S.M., Taranto A.G., Rodrigues D.d.J., et al. Dehydrobufotenin extracted from the Amazonian toad Rhinella marina (Anura: Bufonidae) as a prototype molecule for the development of antiplasmodial drugs. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021;27:1–16. doi: 10.1590/1678-9199-JVATITD-2020-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Menke M., Peram P.S., Starnberger I., Hödl W., Vences M., Schulz S. Frogolide—An Unprecedented Sesquiterpene Macrolactone from Scent Glands of African Frogs. Eur. J. Org. Chem. 2018;2018:2651–2656. doi: 10.1002/ejoc.201800199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saporito R.A., Donnelly M.A., Spande T.F., Garraffo H.M. A review of chemical ecology in poison frogs. Chemoecology. 2012;22:159–168. doi: 10.1007/s00049-011-0088-0. [DOI] [Google Scholar]
- 125.Nascimento A.C.C., Fontes W., Sebben A., Castro M.S. Antimicrobial Peptides from Anurans Skin Secrestions. Protein Pept. Lett. 2003;10:227–238. doi: 10.2174/0929866033478933. [DOI] [PubMed] [Google Scholar]
- 126.Mendes V.A., Barbaro K.C., Sciani J.M., Vassão R.C., Pimenta D.C., Jered C., Antoniazzi M.M. The cutaneous secretion of the casque-headed tree frog Corythomantis greeningi: Biochemical characterization and some biological effects. Toxicon. 2016;122:133–141. doi: 10.1016/j.toxicon.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 127.Nogueira T.A.C., Kaefer I.L., Sartim M.A., Pucca M.B., Sachett J., Barros A.L., Júnior M.B.A., Baía-da-Silva D.C., Bernarde P.S., Koolen H.H.F., et al. The Amazonian kambô frog Phyllomedusa bicolor (Amphibia: Phyllomedusidae): Current knowledge on biology, phylogeography, toxinology, ethnopharmacology and medical aspects. Front. Pharmacol. 2022;13:997318. doi: 10.3389/fphar.2022.997318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kuckelhaus S.A.S., Leite J.R.S.A., Muniz-Junqueira M.I., Sampaio R.N., Bloch C., Jr., Tosta E. Antiplasmodial and antileishmanial activities of phylloseptin-1, an antimicrobial peptide from the skin secretion of Phyllomedusa azurea (Amphibia) Exp. Parasitol. 2009;123:11–16. doi: 10.1016/j.exppara.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 129.Neto R.d.S.C., Vigerelli H., Jared C., Antoniazzi M.M., Chaves L.B., Da Silva A.d.C.R., De Melo R.L., Sciani J.M., Pimenta D.C. Synergic effects between ocellatin-F1 and bufotenine on the inhibition of BHK-21 cellular infection by the rabies vírus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21:1–8. doi: 10.1186/s40409-015-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jager P., Arnedo M.A., Azevedo G.H.F., Baehr B., Bonaldo A.B., Haddad C.R., Harms D., Hormiga G., Labarque F.M., Muster C., et al. Twenty years, eight legs, one concept: Describing spider biodiversity in Zootaxa (Arachnida: Araneae) Zootaxa. 2021;4979:131–146. doi: 10.11646/zootaxa.4979.1.14. [DOI] [PubMed] [Google Scholar]
- 131.Britannica. Venom. [(accessed on 26 April 2023)]. Available online: https://www.britannica.com/animal/spider-arachnid/Venom.
- 132.Menin M., Rodrigues D.d.J., De Azevedo C.S. Predation on amphibians by spiders (Arachnida, Araneae) in the Neotropical region. Phyllomedusa. 2005;4:39–47. doi: 10.11606/issn.2316-9079.v4i1p39-47. [DOI] [Google Scholar]
- 133.Buts W.C. Envenomation by the Brown Recluse Spider (Aranae, Scytodidae) and Related Species. A Public Health Problem in the United States. Clin. Toxicol. 1971;4:515–524. doi: 10.3109/15563657108990974. [DOI] [PubMed] [Google Scholar]
- 134.Chaves-Moreira D., Matsubara F.H., Schemczssen-Graeff Z., De Bona E., Heidemann V.R., Guerra-Duarte C., Gremski L.H., Cháves-Olórtegui C., Seniff-Ribeiro A., Chaim O.M., et al. Brown Spider (Loxosceles) Venom Toxins as Potential Biotools for the Development of Novel Therapeutics. Toxins. 2019;11:355. doi: 10.3390/toxins11060355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corzo G., Escoubas P. Pharmacologically active spider peptide toxins. Cell. Mol. Life Sci. 2003;60:2009–2426. doi: 10.1007/s00018-003-3108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Choi S.-J., Parent R., Guillaume C., Deregnaucourt C., Delabre C., Ojcius D.M., Montagne J.-J., Célérier M.-L., Phelipot A., Amiche M., et al. Isolation and characterization of Psalmopeotoxin I and II: Two novel antimalarial peptides from the venom of the tarantula Psalmopoeus cambridgei. FEBS Lett. 2004;572:109–117. doi: 10.1016/j.febslet.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 137.Seyedian R., Jalali A., Babaee M.M., Pipelzadeh M.H., Rezaee S. A biodistribution study of Hemiscorpius lepturus scorpion venom and available polyclonal antivenom in rats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012;18:375–383. doi: 10.1590/S1678-91992012000400005. [DOI] [Google Scholar]
- 138.Szilagyi-Zecchim V.J., Fernandes A.L., Castagna C.L., Voltolini J.C. Abundance of scorpions Tityus serrulatus and Tityus bahiensis associated with climate in urban area (Scorpiones, Buthidae) Indian Soc. Arachnol. 2012;1:15–23. [Google Scholar]
- 139.Gao B., Xu J., Rodriguez M.d.C., Lanz-Mendoza H., Hernández-Rivas R., Du W., Zhu S. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie. 2010;92:350–359. doi: 10.1016/j.biochi.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 140.Pedron C.N., Silva A.F., Torres M.D.T., De Oliveira C.S., Andrade G.P., Cerchiaro G., Pinhal M.A.S., De la Fuente-Nunez C., Oliveira V.X., Jr. Net charge tuning modulates the antiplasmodial and anticancer properties of peptides derived from scorpion venom. J. Pept. Sci. 2021;27:e3296. doi: 10.1002/psc.3296. [DOI] [PubMed] [Google Scholar]
- 141.Gonçalves P.S., De Araújo W.S. Diversity of Eusocial Bees in Natural and Anthropized Areas of a Tropical Dry Forest in the Parque da Sapucaia (Montes Claros, Minas Gerais, Brazil) Sociobiology. 2021;68:125–136. doi: 10.13102/sociobiology.v68i1.5305. [DOI] [Google Scholar]
- 142.Bava R., Castagna F., Musella V., Lupia C., Palma E., Britti D. Therapeutic Use of Bee Venom and Potential Applications in Veterinary Medicine. Vet. Sci. 2023;10:119. doi: 10.3390/vetsci10020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pretzel J., Mohring F., Rahlfs S., Becker K. Yellow Biotechnology I. Advances in Biochemical Engineering/Biotechnology. Springer; Berlin/Heidelberg, Germany: 2013. Antiparasitic peptides; pp. 157–192. [DOI] [PubMed] [Google Scholar]
- 144.Favuzza P., Guffart E., Tamborrini M., Scherer B., Dreyer A.M., Rufer A.C., Erny J., Hoernshemeyer J., Thoma R., Schmid G., et al. Structure of the malaria vaccine candidate antigen CyRPA and its complex with a parasite invasion inhibitory antibody. eLife. 2017;6:e20383. doi: 10.7554/eLife.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carter V., Underhill A., Baber I., Sylla L., Baby M., Large-Thiery I., Zettor A., Bourgouin C., Langel U., Faye I., et al. Killer Bee Molecules: Antimicrobial Peptides as Effector Molecules to Target Sporogonic Stages of Plasmodium. PLoS Pathog. 2013;9:e1003790. doi: 10.1371/journal.ppat.1003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Waller K.L., Kim K., McDonald T.V. Plasmodium falciparum: Growth response to potassium channel blocking compounds. Exp. Parasitol. 2008;120:280–285. doi: 10.1016/j.exppara.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 147.Van der Shaft P.H., Beaumelle B., Vial H., Roelofsen B., Op den Kamp J.A.F., Van Deenen L.L.M. Phospholipid organization in monkey erythrocytes upon Plasmodium knowlesi infection. BBA. 1987;901:1–14. doi: 10.1016/0005-2736(87)90250-1. [DOI] [PubMed] [Google Scholar]
- 148.Joshi P., Gupta C.M. Abnormal membrane phospholipid organization in Plasmodium fakiparum-infected human erythrocytes. Br. J. Haematol. 1988;68:255–259. doi: 10.1111/j.1365-2141.1988.tb06198.x. [DOI] [PubMed] [Google Scholar]
- 149.Morreira L.A., Ito J., Ghosh A., Devenport M., Zielers H., Abraham E.G., Crisanti A., Nolan T., Catteruccia F., Jacobs-Lorena M. Bee Venom Phospholipase Inhibits Malaria Parasite Development in Transgenic Mosquitoes. J. Biol. Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- 150.Rodrigues F.G., Santos M.N., De Carvalho T.X.T., Rocha B.C., Riehle A., Pimenta P.F.P., Abraham E.G., Jacobs-Lorena M., De Brito C.F.A., Moreira L.A. Blackwell Publishing Ltd Expression of a mutated phospholipase A2 in transgenic Aedes fluviatilis mosquitoes impacts Plasmodium gallinaceum development. Isect Mol. Biol. 2008;17:175–183. doi: 10.1111/j.1365-2583.2008.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Naik R.S., Krishnegowda G., Ockenhouse C.F., Gowda D.C. Naturally Elicited Antibodies to Glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum Require Intact GPI Structures for Binding and Are Directed Primarily against the Conserved Glycan Moiety. Infect. Immun. 2006;74:1412–1415. doi: 10.1128/IAI.74.2.1412-1415.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guillaume C., Calzada C., Lagarde M., Schrével J., Deregnaucourt C. Interplay between lipoproteins and bee venom phospholipase A2 in relation to their anti-Plasmodium toxicity. J. Lipid Res. 2006;47:1493–1506. doi: 10.1194/jlr.M600111-JLR200. [DOI] [PubMed] [Google Scholar]
- 153.Maguire P.A., Prudhomme J., Sherman I.W. Alterations in erythrocyte membrane phospholipid organization due to the intracellular growth of the human malaria parasite, Plasmodium falciparum. Parasitology. 1991;102:179–186. doi: 10.1017/S0031182000062466. [DOI] [PubMed] [Google Scholar]
- 154.Deregnaucourt C., Schrével J. Bee Venom Phospholipase A2 Induces Stage-specific Growth Arrest of the Intraerythrocytic Plasmodium falciparum via Modifications of Human Serum Components. J. Biol. Chem. 2000;275:39973–39980. doi: 10.1074/jbc.M006712200. [DOI] [PubMed] [Google Scholar]
- 155.Barbosa G.A. Síntese química e avaliação das propriedades antibacterianas e antiparasitárias de análogos de peptídeos antimicrobianos de anuros. Tese de doutorado. Em Biologia Molecular. Universidade de Brasília. 2015. [(accessed on 15 March 2023)]. Available online: https://repositorio.unb.br/handle/10482/20415.
- 156.Gwadz R.W., Kaslow D., Lee J.-Y., Maloy W.L., Zasloff M., Miller L.H. Effects of Magainins and Cecropins on the Sporogonic Development of Malaria Parasites in Mosquitoes. Infect. Immun. 1989;57:2628–2633. doi: 10.1128/iai.57.9.2628-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bastianelly G., Bouillon A., Nguyen C., Crublet E., Pêtres S., Gorgette O., Le-Nguyen D., Barale J.-C., Nilges M. Computational Reverse-Engineering of a Spider-Venom Derived Peptide Active Against Plasmodium falciparum SUB1. PLoS ONE. 2011;6:e21812. doi: 10.1371/journal.pone.0021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kamolkijkarn P., Prasertdee T., Netirojjanakul C., Sarnpitak P., Ruchirawat S., Deechongkit S. Synthesis, biophysical, and biological studies of wild-type and mutant psalmopeotoxins—Anti-malarial cysteine knot peptides from Psalmopoeus cambridgei. Peptides. 2010;31:533–540. doi: 10.1016/j.peptides.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 159.Moreira C.K., Rodrigues F.G., Ghosh A., Varotti F.d.P., Miranda A., Daffre S., Jacobs-Lorena M., Moreira L.A. Effect of the antimicrobial peptide gomesin against different life stages of Plasmodium spp. Exp. Parasitol. 2007;116:346–353. doi: 10.1016/j.exppara.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kayano A.M., Simões-Silva R., Medeiros P.S.M., Maltarollo V.G., Horio K.M., Oliveira E., Albericio F., Da Silva S.L., Aguiar A.C.C., Krettli A.U. BbMP-1, a new metalloproteinase isolated from Bothrops brazili snake venom with in vitro antiplasmodial properties. Toxicon. 2015;106:30–41. doi: 10.1016/j.toxicon.2015.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.