Abstract
Endoscopes are increasingly being used in middle ear surgery as an adjunct to or replacement for the operative microscope. The superior visualization of hidden areas and a minimally invasive transcanal approach to the pathology are some of the endoscope’s advantages. The aim of this review is to compare the surgical outcomes of a totally endoscopic transcanal approach with a conventional microscopic approach for type 1 tympanoplasty in patients with chronic otitis media (COM) in order to establish if endoscopic myringoplasty (EM) could be a better alternative to microscopic myringoplasty (MM). A literature review was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis recommendations. The selected articles were identified by searching PubMed Central, PubMed, MEDLINE and Embase databases for the relevant publications. Only studies where the same surgeon in the department performed both endoscopic and microscopic myringoplasty have been included in the review. The results suggest that with an endoscopic approach, minimally invasive myringoplasty can be achieved with a similar graft success rate and postoperative air–bone gap (ABG) improvement, a shorter operative time and less postoperative complications compared to a microscopic approach.
Keywords: endoscopic, microscopic, myringoplasty, chronic otitis media, review
1. Introduction
Chronic otitis media (COM) is a complex multifactorial inflammatory and infectious condition that is mainly characterized by middle ear mucosal inflammation with permanent tympanic membrane perforation and, in some cases, fixation or interruption of the ossicular chain.
The main objectives in treating chronic otitis media are to repair the tympanic membrane perforation, eradicate chronic infection and, if necessary, restore the integrity and mobility of the ossicular chain.
Microscopic myringoplasty (MM) has been the standard surgery for repairing perforated tympanic membranes since the 1950s, but since the late 1990s, endoscopic myringoplasty (EM) has been increasingly practiced.
The MM approach offers binocular vision along with an excellent stereoscopic surgical view and leaves both surgeons’ hands free, but it is limited by the straight-line vision that makes the visualization of the middle ear through the ear canal relatively difficult [1]. Therefore, conventional MM is originally performed using a postauricular incision, with or without drilling of the bony ear canal, in order to obtain adequate visualization and illumination [2]. A postauricular incision may produce surgical scarring, temporary loss of cutaneous sensation [3] and malposition of the ear.
The EM approach has several advantages when compared to the conventional postauricular MM: it avoids unnecessary incisions and soft tissue dissections, ensures easy access to hidden areas, eliminates the potential need for canalplasty, provides a shorter operative time and has lower complication rates [4,5,6,7].
In the last years, there have been several comparative studies published regarding the efficacy of the two operative approaches [2,5,8,9,10,11,12], but few systematic reviews regarding the comparison between endoscopic and microscopic tympanoplasty [13].
Therefore, we decided to perform a systematic review to compare the postoperative outcomes of the totally endoscopic transcanal approach with a conventional microscopic approach for myringoplasty in patients with COM in order to analyze if the endoscopic approach can provide at least the same results as the microscopic approach or even better outcomes in terms of graft success rates, hearing improvement, operative time and postoperative complications, and to establish if EM could be a better alternative to MM.
2. Materials and Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14]. No ethical approval was required as previously published studies were analyzed. The PICO framework was used to develop the specific research question:
Population: patients with chronic otitis media
Intervention: myringoplasty
Comparison: an endoscopic versus a microscopic approach
Outcome: graft success rate, air–bone gap (ABG) improvement, operative time, postoperative complications
2.1. Search Strategy
Two of the authors independently searched the PubMed Central, PubMed, MEDLINE and Embase databases for relevant publications on 8 March 2023. We searched for all available studies reporting comparisons between endoscopic type 1 tympanoplasty or myringoplasty and microscopic type 1 tympanoplasty or myringoplasty in patients with chronic otitis media. The following keywords were used for searching through the PubMed Central database: “((((endoscopic) AND microscopic)) AND (((myringoplasty) OR tympanoplasty) OR type 1 tympanoplasty)) AND ((chronic otitis media) OR COM)”.
2.2. Selection of Studies
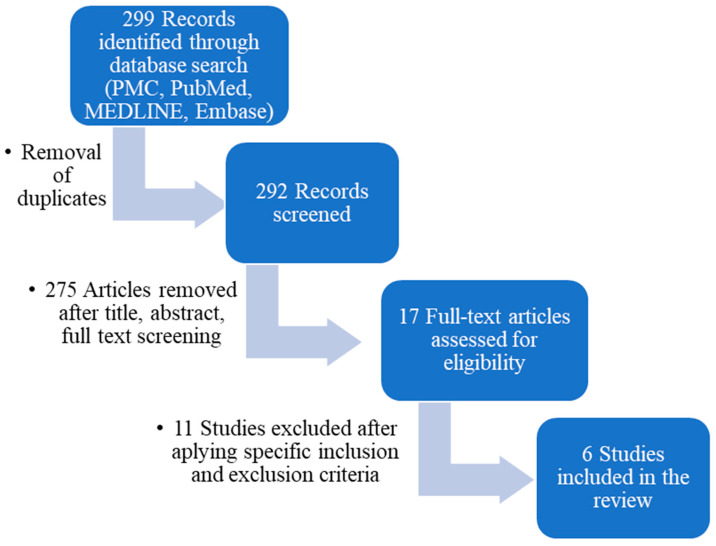
Only full-text English studies were selected, with no restriction regarding the date of publication. All duplicates were manually removed before the study titles and abstracts were screened. Lastly, the remaining studies had their full texts reviewed. The full texts of eligible articles were subsequently evaluated based on the inclusion and exclusion criteria (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram outlining the study design.
The inclusion criteria consisted of: (1) patients with chronic suppurative otitis media with inactive disease and an intact ossicular chain; (2) studies comparing endoscopic with microscopic myringoplasty; (3) studies where the same surgeon carried out both endoscopic and microscopic myringoplasty; (4) studies providing outcome measures such as graft success rate, audiometric outcomes, or duration of surgery in both the endoscopic and microscopic groups.
The following exclusion criteria were applied: (1) studies including patients with cholesteatoma, ossicular chain disorders, adhesive/atelectatic otitis media, active/granulative COM; (2) studies performing simultaneous otologic procedures in addition to myringoplasty (e.g., ossicular chain reconstruction, mastoidectomy); (3) studies with non-available English full-text, duplicate publications, publications where original articles were inaccessible (e.g., only abstracts were available) and/or incomplete data were provided; (4) animal studies, in vitro studies; (5) review articles, case reports; (6) studies with only one approach assessed (only EM or only MM) or a mixture of the two (endoscope-assisted microscopic myringoplasty, microscopic-assisted endoscopic myringoplasty); and (7) subjects unrelated to the researched topic.
2.3. Data Extraction and Management
Two authors reviewed all the relevant studies and independently extracted the data; any discrepancies were resolved by consensus between the two authors.
For each selected article, the following information was noted in a template built for this study: the author, year of publication, period of the study, type of study, number of patients in total and in each comparative group, mean age of each comparative group, mean follow-up period in each group (Table 1), location and size of perforation, graft material, and graft technique in each comparative group (Table 2), graft success rate for each group, mean pre- and postoperative air–bone gap in each group, mean operative time for each approach (Table 3) and postoperative complications in each group.
Table 1.
Baseline characteristics.
| Author | Year of Publication | Study Period | Type of Study | Total Patients (n) | EM Patients (n) | MM Patients (n) | EM Age (Years) | MM Age (Years) | EM Follow-Up (Months) | MM Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Secaattin Gulsen, Adem Baltac [15] | 2019 | 2015–2018 | RNRC | 126 (149 ears) |
67 (78 ears) |
59 (71 ears) |
45.4 (15–61) | 54.8 (18–72) | 8.2 | 9.3 |
| Ahmad Daneshi et al. [16] | 2020 | 2014–2016 | RNRC | 130 | 75 | 55 | 39.85 (18–68) | 38.25 (16–77) | 12 | 12 |
| Qimei Yanget et al. [17] | 2022 | 2011–2016 | RNRC | 345 | 224 | 121 | 40.87 (18–67) | 38.56 (18–66) | 12.74 (6–48) | 14.08 (6–36) |
| Chin-Kuo Chen et al. [18] | 2022 | 2008–2018 | RNRC | 43 | 22 | 21 | 50.6 | 45.9 | 14 | 13.7 |
| Tzu-Yen Huang et al. [19] | 2018 | 2011–2014 | RNRC | 95 (100 ears) |
47 (50 ears) |
48 (50 ears) |
54.2 | 49.9 | 6 | 6 |
| A. C. Jyothi et al. [20] | 2017 | 2011–2013 | PRC | 120 | 60 | 60 | 28.5 | 31.4 | 12 | 12 |
RNRC = Retrospective non-randomized comparative; PRC = Prospective randomized comparative; EM = Endoscopic myringoplasty; MM = Microscopic myringoplasty.
Table 2.
Graft analysis.
| Author | EM Graft Material | MM Graft Material | EM Graft Technique | MM Graft Technique |
|---|---|---|---|---|
| Secaattin Gulsen, Adem Baltacı [15] | Tragal chondroperichondrial | Tragal chondroperichondrial | Underlay | Underlay (postaural) |
| Ahmad Daneshi et al. [16] | 0.5 mm thickness conchal chondroperichondrial | 0.5 mm thickness conchal chondroperichondrial | Underlay | Underlay (postaural) |
| Qimei Yanget et al. [17] | Tragal chondroperichondrial | Temporalis fascia | Underlay | Underlay (postaural) |
| Chin-Kuo Chen et al. [18] | Postconchal perichondrium | Temporalis fascia | Over-underlay | Over–underlay (postaural) |
| Tzu-Yen Huang et al. [19] | Tragal perichondrium | Temporalis fascia | Underlay | Underlay (postaural) |
| A. C. Jyothi et al. [20] | Temporalis fascia | Temporalis fascia | Underlay | Underlay (postaural) |
EM = Endoscopic myringoplasty; MM = Microscopic myringoplasty.
Table 3.
Outcomes analysis.
| Outcome | Graft Success Rate | Pre-op. ABG (dB) | Post-op. ABG (dB) | Mean Operative Time (min) | ||||
|---|---|---|---|---|---|---|---|---|
| Authors | EM | MM | EM | MM | EM | MM | EM | MM |
| Secaattin Gulsen, Adem Baltacı [15] | 94.80% | 92.90% | 28.9 | 29.7 | 8.2 | 7.9 | 34.9 | 52.7 |
| Ahmad Daneshi et al. [16] | 97.30% | 96.40% | 25.2 | 24.9 | 15.7 | 14.4 | 76.7 | 161 |
| Qimei Yanget et al. [17] | 94.64% | 90.90% | 19.26 | 18.13 | 7.72 | 8.34 | 49.22 | 81.22 |
| Chin-Kuo Chen et al. [18] | 86.30% | 85.70% | 20.7 | 17.6 | 10.2 | 12.5 | 79.8 | 99.9 |
| Tzu-Yen Huang et al. [19] | 98% | 98% | 21.6 | 21.4 | 13.3 | 12.5 | 50.4 | 75.5 |
| A. C. Jyothi et al. [20] | 91.67% | 93.30% | 34.16 | 35.54 | 18 | 16 | 60 | 120 |
ABG = air–bone gap; EM = endoscopic myringoplasty; MM = microscopic myringoplasty.
3. Results
3.1. Results of the Literature Search
There were 299 articles identified in the databases. After the removal of duplicates, 292 studies remained. These studies were screened via the title, abstract and full-text, when needed, for relevance, leaving 17 studies for full-text review. Applying all the inclusion and exclusion criteria, there were another 11 studies excluded at this stage, leaving 6 eligible studies to be included in the present article [15,16,17,18,19,20].
The PRISMA flow diagram used to describe the flow of information throughout various phases of the systematic review is displayed in Figure 1.
3.2. Characteristics of the Included Studies
From the selected articles, five were retrospective non-randomized studies and one was a prospective randomized controlled trial.
The enrolled studies were conducted between 2008 and 2018. Four of the studies comprised over 100 patients each, and two of them had fewer than 100.
The total number of patients in the present review is 859 and the total number of ears analyzed is 887. Of these, 57.38% (n = 509) underwent EM and 42.62% (n = 378) MM.
The age of patients ranged from 15 to 77 years, with no significant difference in the mean age between the two comparative groups in each study.
Sex distribution. Overall, 47.26% (n = 406) of patients were male and 52.74% (n = 453) were female. In the EM groups, 50.5% (n = 250) of patients were male and 49.5% (n = 245) were female, whereas in the MM groups 42.86% (n = 156) of patients were male and 57.14% (n = 208) were female.
Perforation characteristics were assessed in four out of six studies with respect to its anatomic localization and size.
Regarding their anatomic localization, tympanic membrane perforations were grouped as central, marginal, anterior and posterior. In two studies, central perforations were more often described [16,18], whereas in one study, anterior perforations were more frequent, with no significant differences between the EM and MM groups [15]. With respect to the perforation size, they were classified as small, medium/moderate, large, subtotal or total, and the distribution of perforation size between the EM and MM groups was assessed. In one study [15], perforations of the tympanic membrane were classified as small (perforation of the tympanic membrane less than 25%), medium (between 25% and 75%) or large (more than 75%). The distribution of perforation size was similar between the EM and MM groups, with no statistically significant differences: 53.8% small, 29.5% medium and 16.7% large perforations in the EM group, and 54.9% small, 30.8% medium and 14.1% large perforations in the MM group. In the second study [16], the size of the perforation was assessed in the same way: small (<25%), moderate (25% to 75%) or large (>75%) of the surface of the tympanic membrane, although the distribution between the two groups was not described, but only general distribution, 18.5% small, 46.2% moderate and 35.4% large perforations. In the third study [17], all ears included in the study had subtotal perforation. Another study [18] included only large-sized perforations: 50–75% (large) or >75% (subtotal) of the pars tensa, with even distribution between the EM and MM groups: 72.7% large perforations and 27.3% subtotal perforations in the EM group and 76.2% large perforations and 23.8% subtotal perforations in the MM group.
Surgical approach. While for all subjects in the EM group the transcanal approach was utilized, those with MM were approached using the retroauricular technique. The surgeries in each study (both endoscopic and microscopic myringoplasties) were performed by the same surgeon.
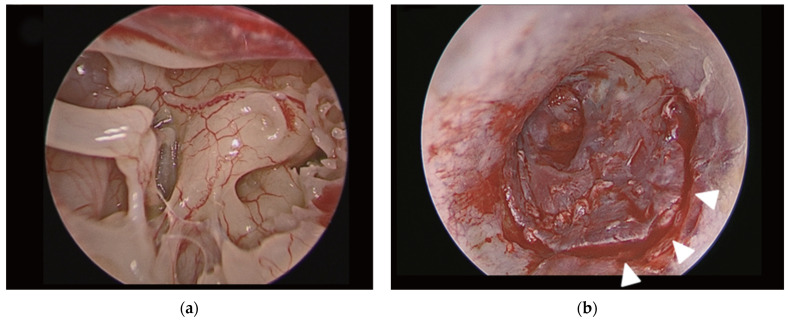
EM surgical technique (Figure 2). Perforation edges were freshened. Transcanal incisions were made. The tympanomeatal flap was elevated. The fibrous annulus was separated from the tympanic sulcus with the preservation of the chorda tympani nerve, and the middle ear space was reached. The mobility and integrity of the ossicular chain were checked by gentle palpation of the ossicles. The graft was positioned using an underlay technique. The tympanomeatal flap was placed in its original position and tightly supported with Gelfoam.
Figure 2.
(a) Endoscopic view of middle ear cavity during endoscopic myringoplasty; (b) view of transcanal incision (white arrow heads) in endoscopic myringoplasty. Final aspect of endoscopic myringoplasty. Adapted with the permission from Ref. [2]. Copyright © 2017 by Korean Society of Otorhinolaryngology-Head and Neck Surgery.
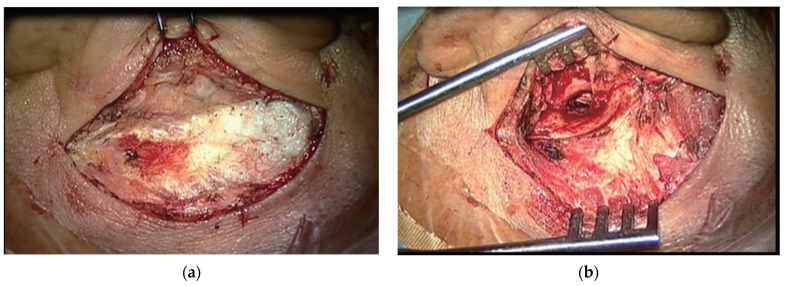
MM surgical technique (Figure 3). The perforation edges were freshened. A postauricular Wilde’s incision was performed. The tympanomeatal flap was elevated under the guidance of the surgical microscope. A canalplasty was practiced, when needed, by drilling the bony ear canal. The mobility and the integrity of the ossicular chain were checked. The graft was positioned with the underlay technique. The tympanomeatal flap was placed in its original position and supported with Gelfoam. The postaural wound was sutured and a compressive dressing was applied over it.
Figure 3.
(a) Postauricular incision for microscopic postauricular myringoplasty. (b) Microscopic view of operation field of myringoplasty. Adapted with the permission from Ref. [2]. Copyright © 2017 by Korean Society of Otorhinolaryngology-Head and Neck Surgery.
The graft material consisted of temporalis fascia, postconchal or tragal perichondrium, full-thickness tragal chondroperichondial graft, or a 0.5 mm-thickness conchal chondroperichondrial graft (Table 2).
The graft technique. An underlay graft technique was the preferred method in five studies, while one study used the over–underlay technique [18].
The follow-up period extended between 6 and 12 months.
Outcomes assessed. All studies included data regarding the following outcomes: graft success rate, hearing outcome in terms of pre- and postoperative air–bone gap and the mean operative time, in both groups. All the studies, except one, provided information about the postoperative complications [15,16,17,18,20].
3.3. Outcomes Analysis (Table 3)
3.3.1. Graft Success Rate
The graft success rate ranged between 86.30% [18] and 98% [19] in the EM group and between 85.70% [18] and 98% [19] in the MM group. There were no statistically significant differences found between the graft success rates in the EM group and the MM group in each study (Table 2).
3.3.2. Pre-Op and Post-Op ABG
In all studies, Pure Tone Audiometry (PTA) was performed pre- and postoperatively at frequencies of 500, 1000, 2000 and 4000 Hz to determine the Air Conduction (AC) thresholds, Bone Conduction (BC) thresholds and air–bone gap (ABG) values.
Pre-op ABG values ranged from 20.7 dB [18] to 34.16 dB [20] in the EM group and from 17.6 dB [18] to 35.54 dB [20] in the MM group. On the other hand, post-op ABG values ranged from 7.72 dB [17] to 18 dB [20] in the EM group and from 7.9 dB [15] to 16 dB [20] in the MM group. One study found the difference between hearing improvement in the EM group and the MM group being statistically significant at 1 month follow-up in favor of the EM group (p = 0.063 [15]), but not at 12 months, and one study found the difference between ABG improvement in the EM group and the MM group being statistically significant at 12 months follow-up in favor of the MM group (p = 0.0001 [20]).
3.3.3. Mean Operative Time
All studies reported operation time data. The EM group had a shorter mean operative time than the MM group; this finding was consistent in all studies and statistically significant in five of six studies (p < 0.05 [15]; p < 0.001 [16]; p < 0.0001 [17]; p = 0.006 [18]; p < 0.0001 [19]). The mean operative time in the EM group varied between 34.9 [15] and 79.8 min [18] and between 52.7 [15] and 161 min [16] in the MM group.
3.3.4. Postoperative Complications
Four of the six studies [15,16,17,20] investigated postoperative complications that appeared in each group. The reported postoperative complications in the MM groups included: numbness around the ear [15,17], tinnitus [17], wet ear/granulation tissue [16,17,20], abnormal taste/dysgeusia [15,17], postauricular hematoma, wound infection, otitis externa, asymmetry of the auricle and wound dehiscence [15]. For the EM groups, the reported postoperative complications included: tinnitus [17], wet ear/granulation tissue [20], abnormal taste/dysgeusia [15,17] and otitis externa [15].
Postoperative pain was assessed in four studies [16,17,18,20]; one of them used the WILDA’s pain assessment guide [20], two studies used the visual analog scaling (VAS) method (0 for no pain and 10 for the worst pain imaginable) [16,18] and one study only mentioned the percentage of patients that experienced ear pain postoperatively, irrespective of its intensity [17]. In the first case, the pain score was found to be 5 in the MM group as compared to 4 in the EM group [20]. In the studies using the VAS method, the pain scale scores did not differ significantly between the groups in one study [18], while postoperative pain was found to be significantly lower in patients who underwent endoscopic surgery (p < 0.001) in the other one [16].
3.4. Subgroup Analysis
3.4.1. Graft Material
Graft material varied between groups and between studies, consisting of either temporalis fascia, postconchal or tragal perichondrium, full-thickness tragal chondroperichondial graft or a 0.5 mm-thickness conchal chondroperichondrial graft. Temporalis muscle fascia tended to be used more frequently in the MM group [17,18,19,20], as the approach was postaural and the graft place was close to the incision site. Tragal/conchal perichondrium as well as tragal/conchal chondroperichondial grafts were used in the EM group [15,16,17,18], the last being also used in the MM group in two studies [15,16].
Graft Success Rate
Groups with temporalis fascia grafts had a graft success rate varying from 85.70% [18] to 98% [20], with both the lowest and highest graft success rate values belonging to the MM group.
The graft success rate in groups with tragal chondroperichondrial grafts was between 92.90% in the MM group and 94.80% in the EM group [15], while for conchal chondroperichondrial grafts, success rates varied between 96.40% in the MM group and 97.30% in the EM group [16].
Postconchal perichondrium had a graft success rate of 86.30% [18] and tragal perichondrium of 98% [19], both being surgically placed using an endoscopic approach.
Post-Op AGB
For the temporalis muscle fascia graft, a post-op ABG of 8.34 to 18 dB was noted, with smaller post-op ABGs for MM groups [17,18,19,20].
For tragal chondroperichondrial grafts, the smallest post-op ABG was in an EM group (7.72 dB) [17], but similar to the MM group from another study (7.9 dB) [15].
Conchal chondroperichondrial grafts had a comparable mean post-op ABG between the EM and MM groups from the same study: 15.7 dB and 14.4 dB [16].
The smallest mean post-op ABG in the present review was achieved using a tragal chondroperichondrial graft (7.72 dB [17]) and the highest using temporalis fascia (18 dB [20]). Another study revealed very good results regarding post-op ABG using temporalis fascia grafts, of 8.34 dB [17]. Further studies are needed to compare the outcomes of myringoplasty in correlation with the graft material used.
Mean Operative Time
The shortest operative times were noted in both the EM and MM groups that used tragal chondroperichondrial grafts, 34.9 and 52.7 min [15], while the longest mean operative times were needed for myringoplasties that used temporalis fascia as graft material: 120 min, 99.9 min, 81.22 min and 75.5 min [17,18,19,20]; but also for 0.5 mm-thickness conchal chondroperichondrial graft, having the longest mean operative time in MM groups: 161 min [16] and almost the longest mean operative time in EM groups: 76.7 min [16].
Nonhomogenous data from the studies on postoperative complications did not allow us to conduct a subgroup analysis for this specific outcome.
3.4.2. Graft Technique
All studies included in the review used the underlay graft technique, except one study that used the over–underlay technique [18]. Looking at this specific study’s outcomes in comparison with the other five studies included in the review, the following observations can be made: it has the lowest graft success rates in both the EM and MM groups, the highest mean operative time in EM groups, but satisfactory post-op ABGs in both groups. However, the results cannot be generalized because this study consisted of a small number of patients, collected over a long period of time, including patients from the beginning of the endoscopic era. Further studies are needed to elucidate which graft technique has better outcomes.
4. Discussion
The present review comprised a relatively large number of patients and it revealed a comparable graft success rate between endoscopic and microscopic approaches. The graft success rate via the EM ranged from 86.30% to 98%, which is consistent with that of the MM. There were no statistically significant differences found between graft success rates in the EM group and the MM group in each study.
Age is a factor previously associated with the graft success rate and it might influence the results [21]. In the present review, studies that had similar age distribution between the two groups (EM and MM) were included.
Previous studies suggested that the comparable graft success rate is more likely associated with the grafting technique than surgical approach [22]. In this review, all authors, except one, used the underlay graft technique. Additional studies comparing the different grafting techniques might clarify this aspect.
The graft material could also be considered a variable that might influence postoperative outcomes. Conceptually, one might anticipate significant conductive hearing loss in cartilage myringoplasty, especially in the lower tones, with a tympanic membrane that is rigid and thick. However, in several studies and meta-analyses, the subgroup of full-thickness cartilage grafts revealed a slight, but significantly superior, hearing outcome than the temporalis fascia graft group [23]. Moreover, Gerber et al. demonstrated that cartilage does not impede sound transmission [24]. Four meta-analyses [25,26,27,28] and one systemic review [29] showed no difference regarding audiometric results between the cartilage and temporalis fascia grafts. More research is needed in this direction as well. The hearing improvements of EM and MM were comparable, which supported previous studies, suggesting that an endoscopic approach can be a good alternative to microscopic technique for patients with COM that require myringoplasty. In our review, the EM was superior to the MM in terms of operation time and postoperative complications. The mean operative time in EM groups was significantly shorter than that in MM groups. Suturing the postauricular incision in the MM group might extend the operation time. Moreover, postoperative complications seemed to be more likely to appear in the MM group, and they were mostly related to the postaural incision. On the other hand, sometimes, bleeding may be an inconvenience and a time-consuming factor during the endoscopic approach, that could even lead, in some cases, to a conversion to the microscopic approach. Yet, we found the shortest average operative time in EM groups, which is consistent with relevant studies in the literature, and no study included in our review reported incomplete endoscopic surgery with a microscopic conversion due to bleeding. The management of bleeding in endoscopic ear surgery is feasible through widely available hemostatic agents such as the injection of diluted epinephrine, cottonoids soaked with epinephrine (1:1000), mono- or bipolar cautery, washing with hydrogen peroxide, and self-suctioning instruments, and even the highest bleeding scores could be managed in an exclusively endoscopic technique in a study conducted by Anschuetz L. et al. [30]
Looking beyond myringoplasty, the place of the endoscopic approach in middle ear surgery is yet to be established. Further research is needed to make it clear if an endoscopic approach could possibly be superior to a microscopic approach in solving various technical difficulties in more complex procedures than myringoplasty, such as cholesteatoma surgery or congenital anomalies [6,7,31].
Limitations of the Study
There are some limitations to our study that should be addressed in future studies. First, a lack of randomized controlled studies. The retrospective nature of all but one study included in our review is problematic in terms of controlling selection and allocation bias. For instance, in one study, it was suggested to the patients included in the EM group to use this specific approach in their cases in order to decrease postoperative pain [15]. Second, the risk factors that could influence surgical outcomes, such as age and size or site of the tympanic membrane perforation, were inconsistent among some of the included studies. Deviated outcomes are theoretically possible due to these uncontrolled factors. Finally, further larger cohort studies, ideally based on randomized controlled trials, are necessary to support our current interpretations.
5. Conclusions
The endoscopic approach allows the surgeon to perform a minimally invasive transcanal myringoplasty, avoiding surgical scarring, a temporary loss of cutaneous sensation and malposition of the ear, with comparable results to conventional MM in terms of the graft success rates and ABG gain, with a shorter operative time and fewer postoperative complications.
Since EM is an efficient minimally invasive technique, it should always be considered when performing myringoplasty in patients with COM.
Author Contributions
I.S. had equal contribution with C.D.S. in designing the research, data acquisition, analysis and interpretation of data, and wrote the manuscript. V.Z. and R.H. performed the experiments, data acquisition, analysis and interpretation of data and manuscript drafting. A.M.V., I.S. and C.D.S. contributed to statistical analysis, critical revision of the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Institute of Phonoaudiology and Functional ENT Surgery.
Informed Consent Statement
Informed consent was obtained from all subjects during each study included in the review.
Data Availability Statement
Data are available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Shakya D., Kc A., Nepal A. A Comparative Study of Endoscopic versus Microscopic Cartilage Type I Tympanoplasty. Int. Arch. Otorhinolaryngol. 2020;24:e80–e85. doi: 10.1055/s-0039-1693139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi N., Noh Y., Park W., Lee J.J., Yook S., Choi J.E., Chung W.-H., Cho Y.-S., Hong S.H., Moon I.J. Comparison of endoscopic tympanoplasty to microscopic tympanoplasty. Clin. Exp. Otorhinolaryngol. 2017;10:44–49. doi: 10.21053/ceo.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang H.S., Ahn S.K., Jeon S.Y., Hur D.G., Kim J.P., Park J.J., Kim D.W., Woo S.H. Sensation recovery of auricle following chronic ear surgery by retroauricular incision. Eur. Arch. Otorhinolaryngol. 2012;269:101–106. doi: 10.1007/s00405-011-1638-3. [DOI] [PubMed] [Google Scholar]
- 4.Kozin E.D., Gulati S., Kaplan A.B., Lehmann A.E., Remenschneider A.K., Alyson Kaplan B.A., Landegger L.D., Cohen M.S., Lee D.J. Systematic Review of Endoscopic Middle Ear Surgery Outcomes. Laryngoscope. 2015;125:1205–1214. doi: 10.1002/lary.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S., Cho H.-H. Transcanal Endoscopic Tympanoplasty for Pediatric Patients Under 15 Years of Age with Chronic Otitis Media. Clin. Exp. Otorhinolaryngol. 2020;13:41–46. doi: 10.21053/ceo.2019.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badr-el-Dine M. Value of ear endoscopy in cholesteatoma surgery. Otol. Neurotol. 2002;23:631–635. doi: 10.1097/00129492-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Thomassin J.M., Korchia D., Doris J.M. Endoscopic-guided otosurgery in the prevention of residual cholesteatomas. Laryngoscope. 1993;103:939–943. doi: 10.1288/00005537-199308000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Zakir I., Ahmad A.N., Pasha H.A., Aqil S., Akhtar S. Comparison of Endoscopic versus Microscopic Tympanoplasty. Iran J. Otorhinolaryngol. 2022;34:139. doi: 10.22038/IJORL.2022.61234.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahawerawat K., Kasemsiri P. Comparison of the clinical outcome of endoscopic push-through myringoplasty and microscopic overlay myringoplasty: Matching co-variated designs. BMC Surg. 2022;22:44. doi: 10.1186/s12893-022-01504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pal R., Surana P. Comparative Study between Microscopic and Endoscopic Tympanoplasty Type I. Indian J. Otolaryngol. Head Neck Surg. 2019;71((Suppl. S2)):1467–1473. doi: 10.1007/s12070-018-1555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Wang W., Xu K., Hu M., Ma Y., Lin P. Comparison of clinical outcome between endoscopic and postauricular incision microscopic type-1 tympanoplasty. Acta Otolaryngol. 2021;141:29–33. doi: 10.1080/00016489.2020.1821091. [DOI] [PubMed] [Google Scholar]
- 12.Cheng X., Wu S., Wang W. Efficacy of Otomicroscopy Combined with Otoendoscopy Double-Lens Technology-Assisted Tympanic Membrane Repair on Elderly Patients with Chronic Suppurative Otitis Media. Evid. Based Complement. Altern. Med. 2021;2021:5164907. doi: 10.1155/2021/5164907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Lee S.-Y., Lee D.Y., Seo Y., Kim Y.H. Can Endoscopic Tympanoplasty Be a Good Alternative to Microscopic Tympanoplasty? A Systematic Review and Meta-Analysis. Clin. Exp. Otorhinolaryngol. 2019;12:145–155. doi: 10.21053/ceo.2018.01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulsen S., Baltacı A. Comparison of endoscopic transcanal and microscopic approach in Type 1 tympanoplasty. J. Otorhinolaryngol. 2021;87:157–163. doi: 10.1016/j.bjorl.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daneshi A., Daneshvar A., Asghari A., Farhadi M., Mohebbi S., Mohseni M., Yazdani N., Mohammadi S., Hosseinzadeh F. Endoscopic versus Microscopic Cartilage Myringoplasty in Chronic Otitis Media. Iran J. Otorhinolaryngol. 2020;32:263–269. doi: 10.22038/ijorl.2020.44015.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q., Wang B., Zhang J., Liu H., Xu M., Zhang W. Comparison of endoscopic and microscopic tympanoplasty in patients with chronic otitis media. Eur. Arch. Otorhinolaryngol. 2022;279:4801–4807. doi: 10.1007/s00405-022-07273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C.-K., Hsu H.-C., Wang M. Endoscopic tympanoplasty with post-conchal perichondrium in repairing large-sized eardrum perforations. Eur. Arch. Otorhinolaryngol. 2022;279:5667–5674. doi: 10.1007/s00405-022-07476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang T.-Y., Ho K.-Y., Wang L.-F., Chien C.-Y., Wang H.-M. A Comparative Study of Endoscopic and Microscopic Approach Type 1 Tympanoplasty for Simple Chronic Otitis Media. J. Int. Adv. Otol. 2016;12:28–31. doi: 10.5152/iao.2015.1011. [DOI] [PubMed] [Google Scholar]
- 20.Jyothi A.C., Shrikrishna B.H., Kulkarni N.H., Kumar A. Endoscopic Myringoplasty versus Microscopic Myringoplasty in Tubotympanic CSOM: A Comparative Study of 120 Cases. Indian J. Otolaryngol. Head Neck Surg. 2017;69:357–362. doi: 10.1007/s12070-017-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng C.C., Lai M.T., Wu C.C., Yuan S.P., Ding Y.F. Endoscopic transcanal myringoplasty for anterior perforations of the tympanic membrane. JAMA Otolaryngol. Head Neck Surg. 2016;142:1088–1093. doi: 10.1001/jamaoto.2016.2114. [DOI] [PubMed] [Google Scholar]
- 22.Tseng C.C., Lai M.T., Wu C.C., Yuan S.P., Ding Y.F. Comparison of the efficacy of endoscopic tympanoplasty and microscopic tympanoplasty: A systematic review and meta-analysis. Laryngoscope. 2017;127:1890–1896. doi: 10.1002/lary.26379. [DOI] [PubMed] [Google Scholar]
- 23.Yang T., Wu X., Peng X., Zhang Y., Xie S., Sun H. Comparison of cartilage graft and fascia in type 1 tympanoplasty: Systematic review andmeta-analysis. Acta Otolaryngol. 2016;136:1085–1090. doi: 10.1080/00016489.2016.1195013. [DOI] [PubMed] [Google Scholar]
- 24.Gerber M.J., Mason J.C., Lambert P.R. Hearing results after primary cartilage tympanoplasty. Laryngoscope. 2000;110:1994–1999. doi: 10.1097/00005537-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Iacovou E., Vlastarakos P.V., Papacharalampous G., Kyrodimos E., Nikolopoulos T.P. Is cartilage better than temporalis muscle fascia in type I tympanoplasty? Implications for current surgical practice. Eur. Arch. Otorhinolaryngol. 2013;270:2803–2813. doi: 10.1007/s00405-012-2329-4. [DOI] [PubMed] [Google Scholar]
- 26.Jalali M.M., Motasaddi M., Kouhi A., Dabiri S., Robabeh Soleimani R. Comparison of cartilage with temporalis fascia tympanoplasty: A meta-analysis of comparative studies. Laryngoscope. 2017;127:2139–2148. doi: 10.1002/lary.26451. [DOI] [PubMed] [Google Scholar]
- 27.Jeffery C.C., Shillington C., Andrews C., Ho A. The palisade cartilage tympanoplasty technique: A systematic review and metaanalysis. Otolaryngol. Head Neck Surg. 2017;46:48–51. doi: 10.1186/s40463-017-0225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyons S.A., Su T., Vissers L.E., Peters J.P., Smit A.L., Grolman W. Fascia compared to one-piece composite cartilage-perichondrium grafting for tympanoplasty. Laryngoscope. 2016;126:1662–1670. doi: 10.1002/lary.25772. [DOI] [PubMed] [Google Scholar]
- 29.Mohamad S., Khan I., Hussain S.M. Is cartilage tympanoplasty more effective than fascia tympanoplasty? A systematic review. Otol. Neurotol. 2012;33:699–705. doi: 10.1097/MAO.0b013e318254fbc2. [DOI] [PubMed] [Google Scholar]
- 30.Anschuetz L., Bonali M., Guarino P., Fabbri F.B., Alicandri-Ciufelli M., Villari D., Caversaccio M., Presutti L. Management of Bleeding in Exclusive Endoscopic Ear Surgery: Pilot Clinical Experience. Otolaryngol. Head Neck Surg. 2017;157:700–706. doi: 10.1177/0194599817726982. [DOI] [PubMed] [Google Scholar]
- 31.Gheorghe D.C., Epure V., Oprea D., Zamfir-Chiru-Anton A. Persistent Stapedial Artery, Oval Window Atresia and Congenital Stapes Agenesis—Case Report. Medicina. 2023;59:461. doi: 10.3390/medicina59030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request to the corresponding author.