Abstract
Plant species are a reservoir of natural compounds that can potentially be used to treat different diseases. Citrus medica Linn. belonging to the Rutaceae family, has been used for centuries in medicine for its antioxidant, anti-inflammatory, antimicrobial, antiviral, and antihyperglycemic properties. These activities are ascribable not only to the presence of health-promoting macronutrients and micronutrients, such as carbohydrates, minerals, amino acids, and vitamins, but also to specialized metabolites, such as flavonoids (apigenin, hesperetin, hesperidin, naringin, naringenin, rutin, quercetin, and diosmin), coumarins (citropten, scoparone, and bergapten), terpenes (limonene, γ-terpinene, limonin, and nomilin), and phenolic acids (p-coumaric acid, trans-ferulic acid, and chlorogenic acid). In recent years, particular attention has been focused on the antioxidant, anti-inflammatory, antimicrobial activity, antidiabetic, anticancer, and neuroprotective activity of C. medica. However, although many studies have reported this species’ chemical and biological properties, the literature has never been analyzed via a systematic approach. For this reason, using PubMed and Scopus as databases, we performed a systematic review of C. medica’s chemical composition and biological properties to inspire new research approaches and increase its curative application.
Keywords: Citrus medica Linn., phytochemical composition, biologic effects, systematic review
1. Introduction
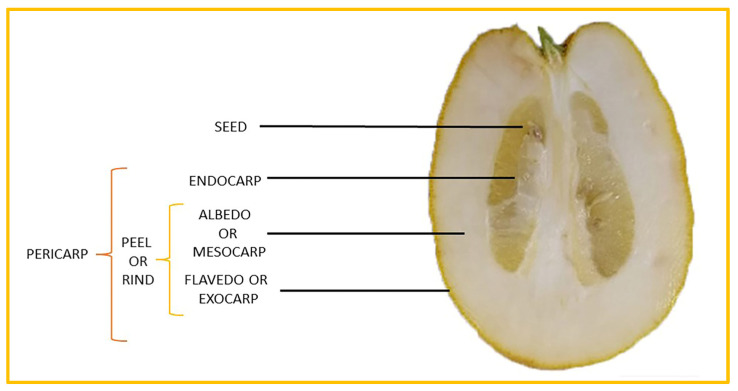
Citrus medica Linn., also called “cedar”, “citron”, “etrog”, “foshou”, and “fingered citron”, belonging to the Rutaceae family, is one of the three basic species of the genus Citrus, together with Citrus maxima Burm. (pomelo) and Citrus reticulata Blanco (mandarin). It is a short, medium-sized evergreen tree that reaches 4–8 m in height [1]. Its leaves are up to 20 cm long and its flowers grow in groups of three to twelve. The color of the fruit (size 20–30 cm) varies according to the state of maturation from green to yellow. Anatomically, the genus Citrus fruits are composed of exocarp, also called epicarp (flavedo or exterior peel), mesocarp (albedo), and endocarp (locule or segment membrane). Often, the albedo and flavedo are together referred to as the peel or rind. The exocarp or flavedo contains numerous essential oil (EO), glands, carotenoids, and chlorophyll. The mesocarp or white albedo portion of the peel contains cellulose, pectin, and hemicellulose, and it comprises 70% of the fruit, while the endocarp (the edible part of the fruit) and seeds constitute the minor part (Figure 1) [2].
Figure 1.
Horizontal cross-section of C. medica cultivar, Diamante Liscia, harvested in Italy.
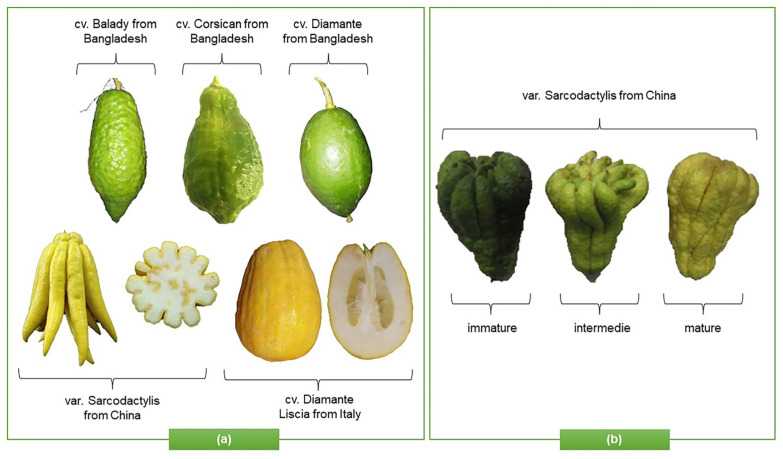
This species has an ancient origin. It was probably native to Asia Minor before arriving in Europe and, currently, it is widely cultivated in Italy, India, China, Indonesia, Australia, Brazil, and the USA. Furthermore, most citrus fruits prefer a temperate climate, with temperatures of 23–25 °C, and do not tolerate cold below 7–8 °C. “Diamante Liscia”, “Diamante Rugosa”, “Corsican”, “Badaly”, and “Maxima” are the best-known C. medica cultivars, while “Sarcodactylis” is the main Chinese variety (var.) (Figure 2a), with different morphological characteristics and phytochemical profiles that depend on the state of maturation (Figure 2b), genetic and agronomic factors, and the habitat [3].
Figure 2.
Representation of (a) morphological characteristics of some cultivars of C. medica from Italy, China, and Bangladesh (b) and in different states of maturation.
Usually, C. medica is consumed as a functional food, to prepare beverages, and for medicinal purposes [4]. Described by several botanists, such as Pliny and Theophrasty, due to its healing properties [5], C. medica is a rich source of bioactive compounds capable of preventing and treating various diseases.
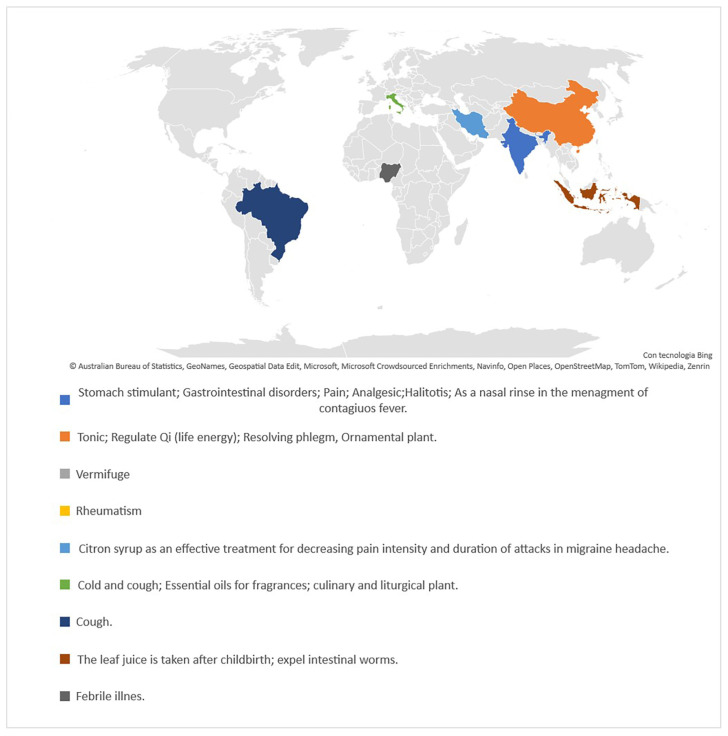
The species is widely used in Ayurvedic medicine for antioxidant, carminative, antibacterial, anticancer, and antiviral purposes, among others [6,7]. Recently Haridas et al. [8] suggested that the herbal formulation of C. medica and Zingiber officinalis Roscoe may have good potential for reducing the viral load of SARS-CoV-2 in the nasal passages. Additionally, citron oil is widely used in Persian folk medicine for musculoskeletal, gastrointestinal, and nervous ailments [9]. Furthermore, a juice-extract syrup also showed good activity against migraines [10]. Figure 3 represents the traditional uses in medicine of C. medica in different countries [11,12].
Figure 3.
Schematic representation of traditional uses in medicine of C. medica in different countries.
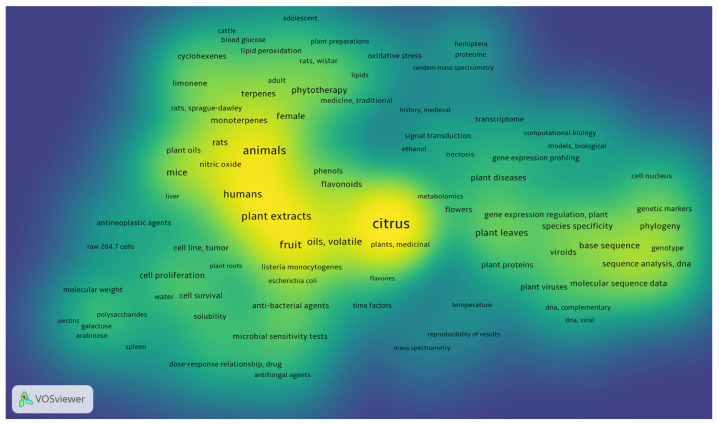
Due to the potential role of this plant in drug discovery, this systematic review presents a careful analysis of the studies regarding C. medica, with a particular focus on its chemical properties and biological activity. The density visualization (Figure 4) created with VOSviewer software, version 1.6.17 (© 2022, Centre for Science and Technology Studies, Leiden University, Leiden, The Netherlands) for Windows, is proposed to offer a quick visualization of the items that concern this systematic review. The image shows the density of the keywords that appear at least twice in the selected items.
Figure 4.
Density visualization of the main keywords in the articles analyzed.
2. Materials and Methods
2.1. Search Strategy
Based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, research analysis was performed from 1 July 2022 to 31 March 2023. The search was conducted using PubMed (http://www.ncbi.nlm.nih.gov/pubmed, accessed from 1 July 2022 to 31 March 2023) and Scopus (http://www.scopus.com, accessed from 1 July 2022 to 31 March 2023), using different keywords, including “Citrus medica” and other terms, as follows: “carotenoids”, “flavonoids”, “coumarins”, “terpenes”, “EO”, “polysaccharides”, “antioxidant activity”, “antimicrobial activity”, “antibacterial activity”, “anti-inflammatory activity”, “hyperglycaemic activity”, “hypoglycaemic activity”, “hypocholesterolemic activity”, “hypolipidemic activity”, “cytotoxic activity”, “analgesic activity”, “anticancer activity”, “antitumoral activity”, “anticholinesterase”. The research was confined to full-text and English publications only.
2.2. Study Selection
The study selection included English articles containing “Citrus medica” in the title or abstract accompanied by keywords. Articles that treated Citrus medica as Citrus bergamia were not included in this systematic review because they are two different plant species. The exclusion criteria were as follows: review articles, articles in languages other than English, book chapters, letters, conference papers, notes, manuscripts without full text available, short reports, and short surveys. Two investigators (V.C. and N.B.) screened the literature by analyzing titles, abstracts, and full texts. In case of disagreement, another reviewer was consulted (L.M.).
2.3. Data Extraction
All included articles were closely examined and information related to Citrus medica L.’s active metabolite extraction, phytochemical profile, and biological activity was extracted. For the biological activity, in vitro cell-free and cell-based experimentation was considered.
2.4. Methodological Quality Assessment
The methodological quality and the risk-of-bias assessment were carried out using a checklist adapted from Cochrane Handbook for Systematic Review of Interventions, appropriately adjusted for pre-clinical studies. Studies were analyzed based on criteria in Table 1.
Table 1.
| Checklist for Assessment of Risks of Bias in Pre-Clinical Studies |
|---|
| Are the hypothesis and objective of the study clearly described? |
| Are the main outcomes to be measured clearly described? |
| Are the main findings of the study clearly described? |
| Are the samples size calculations reported? |
| Are the animals randomly housed during the experiment? |
| Are the investigators blinded from knowledge which treatment used? |
| Are the outcome assessors blinded? |
| Is the dose/route of administration of Citrus medica L. properly reported? |
| Is the dose/route of administration of the drug in co-treatment properly reported? |
| Is the frequency of treatments adequately described? |
The studies that reported all the included parameters were considered of higher methodological quality. On the other hand, studies that lacked these criteria were considered at high risk of bias, while studies that did not completely fulfil the parameters were considered to have a medium risk of bias.
3. Results and Discussion
3.1. Study Characteristics
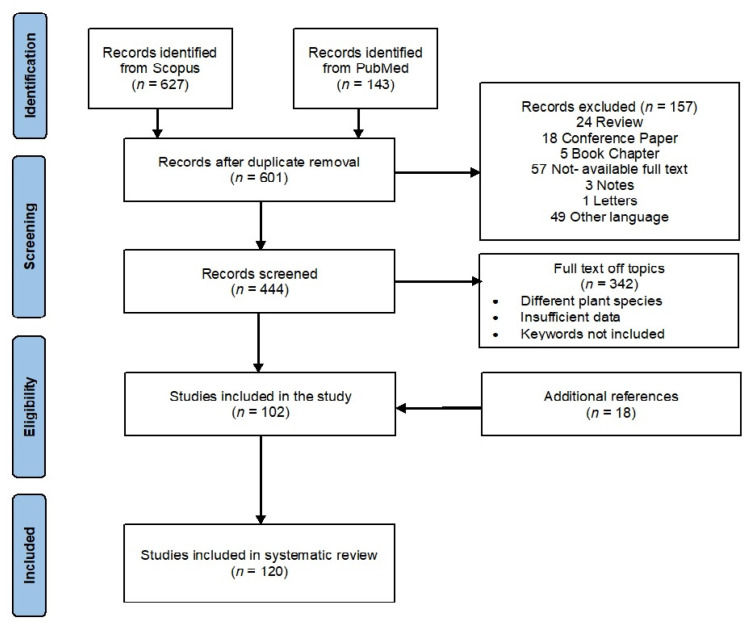
A preliminary survey of the literature led to the identification of 770 reports (627 from Scopus and 143 from PubMed). After checking for duplicates and articles that did not fit with the inclusion criteria, 499 results were removed, with 102 articles remaining. To these, 18 articles found in the bibliographies were added. Hence, the final reference list comprised 120 items (Figure 5).
Figure 5.
Flow diagram of the systematic review of the literature-search results based on PRISMA statement.
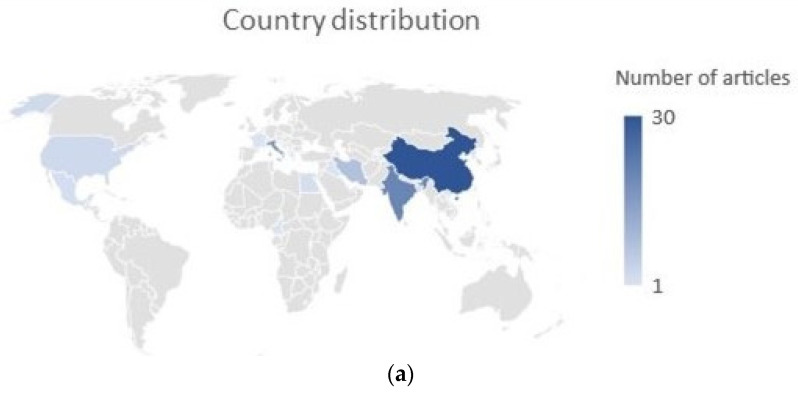
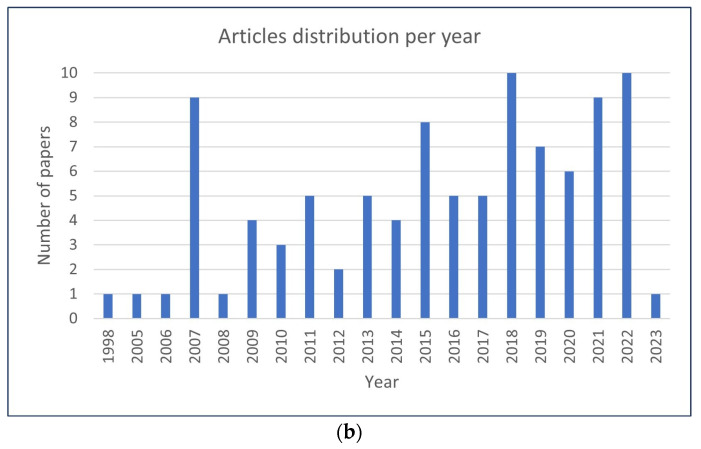
The selected papers originated in 17 countries; the country in which the greatest number of articles was published was China, followed by India and Italy (Figure 6a).
Figure 6.
(a) Representation of distribution of authors’ countries of origin; (b) distribution of the selected studies by year of publication.
3.2. Phytochemistry
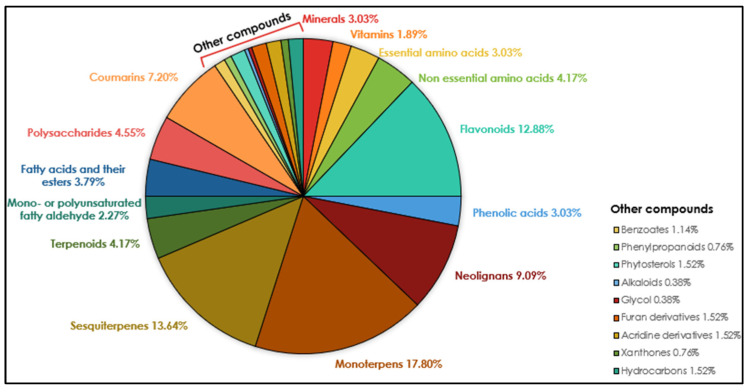
The phytochemicals identified in C. medica can be classified into nutrient compounds, such as vitamins, essential amino acids, non-essential amino acids, and minerals, and non-nutritive compounds, such as flavonoids, alkaloids, terpenes, and coumarins. The diagram (Figure 7) shows the metabolic profile of C. medica according to the classes of compounds found in the analyzed articles. This section closely analyzes the nutritional value and chemical composition of C. medica, including a screening of the extractive methods used.
Figure 7.
Metabolic profile of C. medica according to classes of compounds with percentage ranges in the plant. Values < 1.8% are grouped in other compounds.
3.2.1. Macronutrients and Micronutrients
Mahdi et al. [15] examined the nutritional composition of pulp and peel, and macronutrients, such as sugars, lipids, and proteins were determined; however, the significant contribution in terms of biological activity is due to the presence of micronutrients. The peel is richer in water-soluble vitamins than in pulp, especially in terms of vitamins B6, B1, and B2, with percentage contributions of 100 g of fresh weight (FW) to the Reference Daily Intake (C-RDI) of 779.11%, 304.69*, and 89.39%, respectively. Dadwal et al. [15] quantified the vitamin C in different parts of C. medica extracted by ultra-sonication and analyzed using UHPLC–QTOF–IMS with the following results: exocarp (7.95 ± 0.12 mg/100 g), mesocarp (3.05 ± 0.01 mg/100 g), endocarp (2.33 ± 0.02 mg/100 g), and seeds (3.11 ± 0.10 mg/100 g). Hasan et al. [16] analyzed the contents of vitamin C in citrus juice, finding 54 mg/100 g. Hence, it is possible to assert that juice represents the richest source of vitamin C. Furthermore, Dey et al. [3] investigated the kinetics degradation of vitamin C, indicating that temperatures above 40 °C caused the compound degradation. In addition to vitamin C, Citrus medica is also rich in Vitamin B, minerals (mainly present in the fruit peel and pulp), and non-essential amino acids. Table 2 reports all the nutrients found in C. medica.
Table 2.
Macronutrients, amino acids, minerals, and water-soluble vitamins identified in C. medica L.
| Nutrient Compounds | Part of Plant | Quantitative | References |
|---|---|---|---|
| Minerals | |||
| Calcium (Ca) | peel, pulp | 107.39–195.91 mg/100 g FW | [17] |
| Copper (Cu) | peel, pulp | 0.061–0.45 mg/100 g FW | [17] |
| Iron (Fe) | peel, pulp | 0.82–2.92 mg/100 g FW | [17] |
| Magnesium (Mg) | peel, pulp | 5.86–16.29 mg/100 g FW | [17] |
| Manganese (Mn) | peel, pulp | 0.052–0.266 mg/100 g FW | [17] |
| Potassium (K) | peel, pulp | 126.04–263.27 mg/100 g FW | [17] |
| Sodium (Na) | peel, pulp | 6.74–27.92 mg/100 g FW | [17] |
| Zinc (Zn) | peel, pulp | 0.24–0.51 mg/100 g FW | [17] |
| Vitamins | |||
| Ascorbic acid (vitamin C) | peel, pulp, exocarp, mesocarp, endocarp, seeds | 0.23–2.39 mg/100 g FW | [17] |
| 2.33–7.95 mg/100 g DW | [15] | ||
| fructus | 11.61 ± 2.50 mg/100 g FW | [18] | |
| peel | - | [19] | |
| peel | - | [20] | |
| fructus | - | [21] | |
| juice | 18.49 ± 0.52 mg/100 g FW | [3] | |
| Niacin (vitamin B3) | peel, pulp | 0.05–0.63 mg/100 g FW | [17] |
| Pyridoxine (vitamin B6) | peel, pulp | 0.75–10.12 mg/100 g FW | [17] |
| Riboflavin (vitamin B2) | peel, pulp, exocarp, mesocarp, endocarp, seeds | 0.37–1.16 mg/100 g FW | [17] |
| 1.85–6.38 mg/100 g DW | [15] | ||
| Thiamin (vitamin B1) | peel, pulp, exocarp, endocarp | 1.32–3.65 mg/100 g FW | [17] |
| 0.18–0.40 mg/100 g DW | [15] | ||
| Essential amino acids | |||
| Histidine | peel, pulp | 7.68–38.04 mg/100 g FW | [17] |
| Isoleucine | peel, pulp | 16.14–81.95 mg/100 g FW | [17] |
| Leucine | peel, pulp | 30.05–126.24 mg/100 g FW | [17] |
| Lysine | peel, pulp | 27.37–94.46 mg/100 g FW | [17] |
| - | [22] | ||
| Methionine | peel, pulp | 1.63–11.53 mg/100 g FW | [17] |
| Phenylalanine | peel, pulp, exocarp, endocarp, mesocarp, seeds | 19.21–89.44 mg/100 g FW | [17] |
| - | [15] | ||
| Threonine | albedo, pulp | - | [22] |
| Valine | peel, pulp, albedo, pulp | 29.64–121.92 mg/100 g FW | [17] |
| - | [22] | ||
| Non-essential amino acids | |||
| Alanine | peel, pulp | 57.55–153.99 mg/100 g FW | [17] |
| albedo, pulp | [22] | ||
| Arginine | peel, pulp | 18.64–90.62 mg/100 g FW | [17] |
| Asparagine | peel, oil glands, albedo, pulp | - | [22] |
| Aspartic acid | peel, pulp | 232.86–637.32 mg/100 g FW | [17] |
| Cystine | peel, pulp | 1.76–1.82 mg/100 g FW | [17] |
| Glutamic acid | peel, pulp | 71.47–227.50 mg/100 g FW | [17] |
| Glycine | peel, pulp | 21.15–108.48 mg/100 g FW | [17] |
| Proline | peel, pulp | 55.22–150.18 mg/100 g FW | [17] |
| - | [15] | ||
| - | [22] | ||
| Serine | peel, pulp | 22.45–78.84 mg/100 g FW | [17] |
| Tryptophan | exocarp, endocarp, mesocarp | - | [17] |
| Tyrosine | peel, pulp | 12.51–53.74 mg/100 g FW | [17] |
| Macronutrients | |||
| Moisture content | peel, pulp | 81.78–86.03 g/100 g FW | [17] |
| Fat | peel, pulp | 0.39–0.56 g/100 g FW | [17] |
| Protein | peel, pulp | 0.80–2.99 g/100 g FW | [17] |
| Ash | peel, pulp | 0.44–1.23 g/100 g FW | [17] |
| Carbohydrates | peel, pulp | 9.19–16.60 g/100 g FW | [17] |
| Energy | peel, pulp | 53.74–73.06 g/100 g FW | [17] |
| Glucose | peel, pulp | 0.92–2.27 g/100 g FW | [17] |
| Fructose | peel, pulp | 1.60–2.95 g/100 g FW | [17] |
| Sucrose | peel, pulp | 0.27–1.03 g/100 g FW | [17] |
3.2.2. Polyphenols, Flavonoids, and Phenolic Acids
Flavonoids are a group of specialized metabolites with considerable health benefits, such as antiviral, antioxidant, antimicrobial, hypoglycaemic, and anti-inflammatory properties [10,23,24]. Malleshappa et al. [24] assessed the anti-inflammatory and nociceptive activity in ethanolic extract peels of some citrus fruits attributable to the high content of phenolic compounds. The flavonoids and polyphenols identified in C. medica can be classified into different structural categories: flavanones (naringin, narirutin, hesperidin, etc.), flavones (limocitrol 3-alpha-L-arabinopyranosyl-(1->3)-galactoside, scutellarein 4′-methyl ether 7-glucoside, vitexin, diosmin, etc.), polymethoxyflavones (nobiletin, tangeretin, 5-demethylnobiletin, etc.), anthocyanins (cyanidin 3-glucoside, cyanidin 3-(6′′-malonyl) glucoside, and peonidin 3-(6′′-malonyl) glucoside), flavonols (quercetin, rutin, and kaempferol, etc.), and phenolic acids, such as caffeic acid, chlorogenic acid, salicylic acid, gallic acid, benzoic acid, trans-cinnamic acid, p-coumaric acid, and trans-ferulic acid. These compounds are present in different percentages in all parts of C. medica, such as the fruits, flowers, leaves, roots, and stem barks. Dadwal et al. [15], after drying all the fruit parts and treating them with a hydroethanolic medium using UAE, detected flavonoids and other phenolic chemicals, using UHPLC–QTOF–MS, in the following order: exocarp > mesocarp > endocarp > seeds. Hesperidin was dominant in all the parts, with the highest concentration of 3307.25 mg/100 g in the exocarp extract, while naringin (295.15 mg/100 g), nobiletin (94.32 mg/100 g), and tangeretin (164.88 mg/100 g) were found in highest concentrations in the exocarp. This quantification was in agreement with the results presented by Adham [25], who demonstrated, through qualitative–quantitative analyses, that hesperidin is the dominant specialized metabolite in C. medica flavedo. Furthermore, a comparative study of flavedo extracts was performed by Taghvaeefard et al. [26], on two Iranian citron fruits: C. medica cv. macrocarpa (large citron) and cv. medica (small citron). The hesperidin content was 2.77 mg/g of dry weight of the fruit peel compared to 1.86 mg/g of dry weight of the flavedo from the small citron. In summary, the contents of flavone and flavonol in the small citron were twice those in the large citron obtained by macerating 200 mg of dried flavedo in methanol/acetic acid (85:15). The phytochemical profile does not depend only on the part of the plant analyzed, but also on the stage of maturation of the fruits. As reported by Menichini et al. [27], immature fruits showed a higher flavonoid contents than mature fruits. In addition to the aforementioned anti-inflammatory activity, C. medica’s antioxidant activity seems to be related to the amount of phenolic compounds. Specifically, a hydroalcoholic extract of C. medica cv Diamante demonstrated interesting antioxidant properties, probably due to the presence of high levels of hesperidin (224.3 ± 3.2 mg/kg of FW), hesperetin (203.8 ± 3.1 mg/kg of FW), rutin (156.5 ± 3.3 mg/kg of FW), quercetin (580.8 ± 3.1 mg/kg of FW), diosmin (372.53 ± 6.4 mg/kg of FW), and apigenin (941.0 ± 8.0 mg/kg of FW) [27]. The activity of these compounds has led to numerous studies on extraction from industrial by-products such as peel, seeds, and bagasse [28]. As a part of the recovery of industrial waste, the contents of flavonoids in citron seeds and their germinated shoots were compared: neohesperetin, didymin, naringenin, and hesperetin were significantly increased in the shoots after germination, with values of 14.63, 12.24, 10.51, and 20.01 mg/g DW, respectively, while the naringin and didymin were decreased compared to the citron seeds before germination [29]. Recent innovative procedures, such as microwave-assisted extraction, supercritical carbon dioxide, enzyme-assisted extraction, pulsed electric field, sub-critical water extraction, and solar-energy-assisted extraction have been proven to be good methods for the up-scaled application of the recovery of bioactive components present in low concentrations [30]. In this vein, Govindarajan et al. [31] investigated the optimum condition using a response surface methodology on the pectin yield from dried C. medica peel with the following parameters: microwave power of 480 W, irradiation time of 20 s, and dilution factor of 1:10 weight/volume (w/v). Recently, six new neolignans were identified and characterized by Ma et al. [32], compared to common extractions; in this case, the fruits (9.5 kg) of C. medica var. Sarcodactylis were air-dried, smashed, and extracted with 95% EtOH heating under reflux at 110 °C for 4 h with an electric heating jacket. The chemical properties of the polyphenols, flavonoids, and phenolic acids are reported in Table 3.
Table 3.
Flavonoids, phenolic acids, and neolignans identified in C. medica L.
| Compounds | Formula | Structure | Extraction Method | Chemical Analysis | Part of the Plant |
Quantitative | References |
|---|---|---|---|---|---|---|---|
| Apigenin | C15H12O5 |
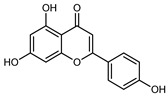
|
Maceration 70% EtOH | HPLC | flavedo | 62.80 mg/kg FW | [33] |
| Exhaustive maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 58.00–941.00 mg/kg FW | [27] | |||
| Maceration 100% EtOH | UPLC–DAD | peel and pulp | 24.26 ± 1.67 µg/g FW | [34] | |||
| Apigenin-6,8-di-C-glucoside | C27H30O15 |
|
UAE 50%MeOH | HPLC–Q/TOF–MS | fructus | - | [35] |
| Atalantoflavon | C21H18O4 |
|
Maceration Acetone |
COSY, NOESY, HMQC, HMBC, HR–ESI–MS | root bark, stem bark | - | [36] |
| Maceration MeOH | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] | |||
| Catechin | C15H14O6 |
|
UAE EtOH 80% | UHPLC–QTOF–IMS | mesocarp, endocarp, seeds | 5.14–57.87 mg/100 g DW | [15] |
| Maceration 100% EtOH | UPLC–DAD | flavedo, pulp | 4.34–68.78 µg/g FW | [34] | |||
| Dihydrokaem-pferide | C16H14O6 |
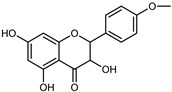
|
Maceration 70% MeOH | UV, MS, NMR | leaves | - | [38] |
| Dihydroquercetin | C15H12O7 |
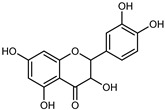
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, endocarp, seeds | - | [15] |
| Epicatechin | C15H14O6 |
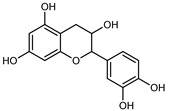
|
Maceration 100% EtOH | UPLC–DAD | flavedo, pulp | 9.85–105.10 µg/g FW | [34] |
| Eriocitrin (Eriodictyol-7-O-rutinoside) | C27H32O15 |
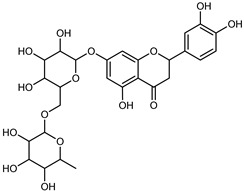
|
Maceration MeOH and 0.1% HCl | HPLC–PDA–MS | fructus | - | [39] |
| Herbacetin | C15H10O7 |
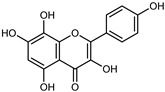
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, seeds | - | [15] |
| Hesperetin | C16H14O6 |
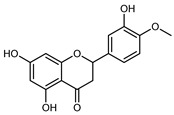
|
Dynamic maceration 70% EtOH | HPLC | flavedo | 0.39–1.82 mg/g DW | [26] |
| UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, endocarp, mesocarp, seeds | - | [15] | |||
| Maceration 70% EtOH | HPLC | flavedo | 50.4 mg/kg FW | [33] | |||
| Exhaustive maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 203.80 mg/kg FW | [27] | |||
| Hesperetin-7-O-rutinoside | C28H34O15 |
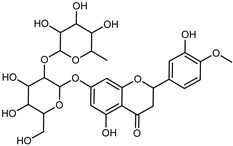
|
Maceration MeOH and 0.1% HCl | HPLC–PDA–MS | fructus | - | [39] |
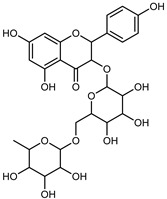
| Hesperidin | C28H34O15 |
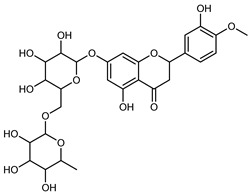
|
PLE MeOH | HPLC–DAD | fructus | 30.36 µg/mL | [40] |
| Dynamic maceration with 70% EtOH | HPLC | flavedo | 1.86–2.77 mg/g DW | [26] | |||
| UAE 80% EtOH | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 383.02–3307.25 mg/100 g DW | [15] | |||
| Exhaustive maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 9.00–224.30 mg/kg FW | [27] | |||
| UAE 50% MeOH | HPLC–Q/TOF–MS | fructus | 0.84–1.84 mg/g DW | [35] | |||
| Kaempferol 3-O-rutinoside | C27H32O15 |
|
Dynamic maceration 70% EtOH | HPLC | flavedo | - | [26] |
| Limocitrol 3-α-l-arabinopyranosyl-(1->3) -galactoside | C29H34O18 |
|
CPE 85% EtOH | UPLC–QTOF–MS/MS | fructus | - | [41] |
| Lonchocarpol A | C25H28O5 |
|
Maceration Acetone |
COSY, NOESY, HMQC, HMBC, HR–ESI–MS | root bark, stem bark | - | [36] |
| Naringenin 7-O-glucoside | C21H22O10 |
|
UAE 80% EtOH | UHPLC–QTOF–IMS | exocarp, mesocarp, seeds | - | [15] |
| Naringin | C27H32O14 |
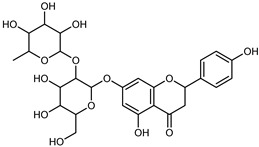
|
Exhaustive extraction 70% EtOH | HPLC | fructus | 556.00 mg/kg FW | [27] |
| UAE 80% EtOH | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 36.82–295.15 mg/100 g DW | [15] | |||
| UAE 80% EtOH | HPLC–QTOF–MS | fructus | 0.43–0.61 mg/g DW | [35] | |||
| Maceration 70% EtOH | HPLC | flavedo | 18.60 mg/kg FW | [33] | |||
| Neodiosmin (Diosmetin-7-O-neoheseridoside) | C28H32O15 |
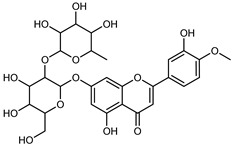
|
CPE 85% EtOH | UPLC–QTOF–MS/MS | fructus | - | [41] |
| Diosmin | Exhaustively maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 18.20–372.50 mg/kg FW | [27] | ||
| Neohesperidin (hesperetin-7-O-neohesperidoside) | C28H34O15 |
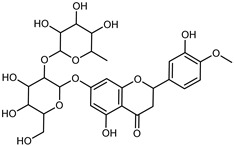
|
Maceration MeOH and 0.1% HCl | HPLC–PDA–MS | fructus | - | [39] |
| Nobiletin | C21H22O8 |
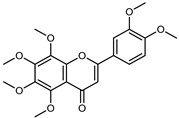
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 25.63–94.32 mg/100 g DW | [15] |
| Phloretin-3′, 5′-di-C-glucoside | C27H34O15 |
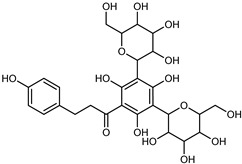
|
Maceration MeOH and 0.1%HCl | HPLC–PDA–MS | fructus | - | [39] |
| Quercetin | C15H10O7 |
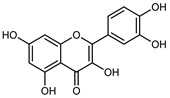
|
Soxhlet MeOH 65 °C | HPLC | fructus 2 | 0.025 mg/g DW | [42] |
| Maceration 70% EtOH | HPLC | flavedo | 18.20 mg/kg FW | [33] | |||
| Dynamic maceration 70% EtOH | HPLC | flavedo | 1.62–3.01 mg/g DW | [26] | |||
| Exhaustive maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 11.00–580.80 mg/kg FW | [27] | |||
| Rutin | C27H30O16 |
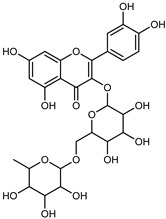
|
Maceration 100% EtOH | UPLC–DAD | flavedo and pulp | 19.39–115.47 µg/g FW | [34] |
| Dynamic maceration 70% EtOH | HPLC | flavedo | 0.20–0.42 mg/g DW | [26] | |||
| UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp | 74.08–328.82 mg/100 g DW | [15] | |||
| 70% MeOH | UV, MS, NMR | leaves | - | [38] | |||
| Sakuranetin | C16H14O5 |
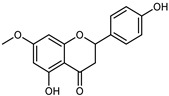
|
Maceration on cold 70% MeOH | UV, MS, NMR | leaves | - | [38] |
| Stachannin Scutellarein 4′-methyl ether 7-glucoside | C22H22011 |
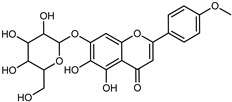
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, endocarp, seeds | - | [15] |
| Tangeritin | C20H20O7 |
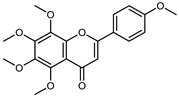
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 18.96–164.88 mg/100 g DW | [15] |
| Vitexin | C21H20010 |
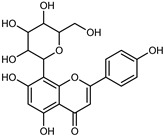
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, endocarp, seeds | - | [15] |
| Vitexin-2-rhamnoside | C27H30O14 |
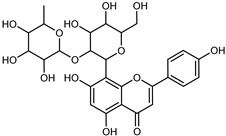
|
PLE MeOH | HPLC–DAD | fructus | - | [40] |
| 3,5,6-Trihydroxy-3′,4′,7-trimethoxyflavone | C18H16O8 |
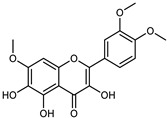
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | - | [15] |
| 5,7-Dihydroxy-3′, 4′, 5′-trimethoxyflavone | C18H16O7 |
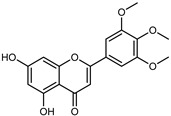
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, seeds | - | [15] |
| 5-Demethylnobiletin | C20H20O8 |
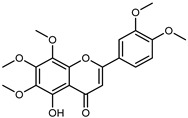
|
Dynamic maceration 70% EtOH | HPLC | flavedo | - | [26] |
| 6,8-di-C-glucosyldiosmetin | C28H32016 |
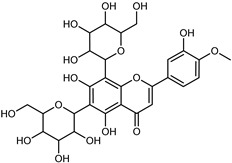
|
PLE MeOH | HPLC–DAD | fructus | 13.51 µg/mL | [40] |
| 7-O-Methyl-aromadendrin | C16H14O6 |
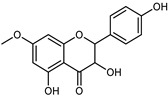
|
Maceration on cold 70% MeOH | UV, MS, NMR | leaves | - | [38] |
| Scoparin (Chrysoeriol 8-C-glucoside) |
C22H22O11 |
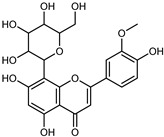
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Phenolic acids | |||||||
| Benzoic acid | C7H6O2 |
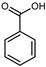
|
Soxhlet with MeOH | HPLC | fructus | 0.00103 mg/g DW | [42] |
| Caffeic acid | C9H8O4 |
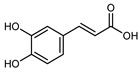
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 36.38–122.88 mg/100 g DW | [15] |
| 100% EtOH for 24 h | UPLC–DAD | flavedo, pulp | 6.97–7.11 µg/g FW | [34] | |||
| Chlorogenic acid | C16H18O9 |
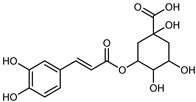
|
UAE EtOH 80% |
UHPLC–QTOF–IMS | mesocarp, endocarp, seeds | 66.66–109.85 mg/100 g DW | [15] |
| Gallic acid | C7H605 |
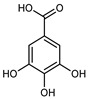
|
UAE EtOH 80% |
UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp | 13.51–26.36 mg/100 g DW | [15] |
| 100% EtOH | UPLC–DAD | flavedo, pulp | 16.84–39.02 µg/g FW | [34] | |||
| Soxhlet with MeOH | HPLC | fructus | 0.30 mg/g DW | [42] | |||
| p-Coumaric acid | C9H803 |
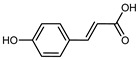
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 3.90–28.09 mg/100 g DW | [15] |
| Methyl-4-hydroxycinnamate | C10H10O3 |
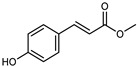
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Salicylic acid | C7H6O3 |
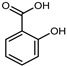
|
Soxhlet with MeOH | HPLC | fructus | 0.16 mg/g DW | [42] |
| trans-Cinnamic acid | C9H8O2 |
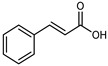
|
UAE EtOH 80% | UHPLC–QTOF–IMS | mesocarp, endocarp, seeds | 0.42–13.06 mg/100 g DW | [15] |
| trans-Ferulic acid | C10H10O4 |
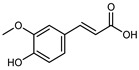
|
UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp. Endocarp, seeds | 19.85–96.79 mg/100 g DW | [15] |
| 100% EtOH | UPLC–DAD | flavedo and pulp | 106.36–295.97 µg/g FW | [34] | |||
| Dynamic maceration 70% EtOH | HPLC | flavedo | 0.21–1.08 mg/g DW | [26] | |||
| Neolignans | |||||||
| (7E)-1-Allyl alcohol-5,6-(11-isopropyl)-furanyl-3′,5′-dimethoxy-4′-glycerol-9′-isovalerate-3,4,7′,8′-benzodioxane neolignan | C33H40O11 |
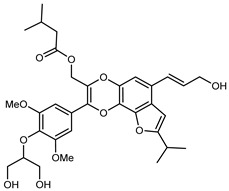
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| 2(7E,10′E,11E)-1-(9-Methoxyl)-propenyl-5-hydroxy-6-prenyl-8′-methylol-11′,16′-dihydroxy-15′,17′-dimethoxy-10′-phenylallyl alcohol-3,4,7′,8′-benzodioxane neolignan | C35H38O10 |
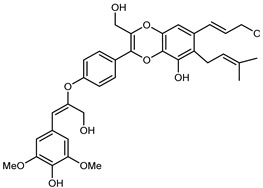
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7E,11E)-1-(9-Methoxyl)-propenyl-5-hydroxy-6-geranyl-16′-hydroxy-15′,17′-dimethoxyphenyl-8′,11′-dimethylol-benzofuranyl 3,4,7′,8′-benzodioxane neolignan | C39H44O11 |
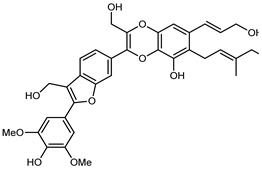
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| 1-(18,19-Dimethyl)-propanol-4-hydroxyl-5,6-(13-hydroxyl-12-methoxyl)-phenylethyl-7′-(4′-hydroxyl-5′-methoxy)-phenyl-9′-O-β-D glucopyranosyl-phenanthrofuran neolignan | C36H42O13 |
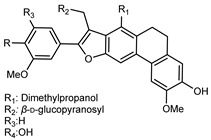
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| 1-(17-Furanyl)-ethyl-4-hydroxyl-5,6-(13-hydroxyl-12-methoxyl)- phenylethyl-7′-(3′,4′,5′-trimethoxy)-phenyl-9′-O-β-D-glucopyranosyl- phenanthrofuran neolignan | C39H42O14 |
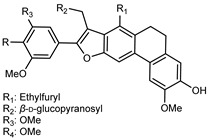
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| 1-(17Z)-Methyl-butanol-4-hydroxyl-5,6-(13-hydroxyl-12-methoxyl)- phenylethyl-7′-(4′-hydroxy-3′,5′-dimethoxy)-phenyl-9′-O-β-D-glucopyranosyl-phenanthrofuran neolignan | C37H42O14 |
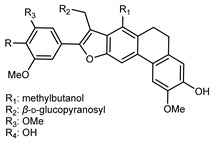
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (9′E)-4,5-(11,12-Dimethyl)- pyranyl-7′-(4′-hydroxy)- phenyl-4′-propenyl-8′-methylol-furanyl-6′-acetyl-1′,6-biphenyl-7-ketone | C32H28O6 |
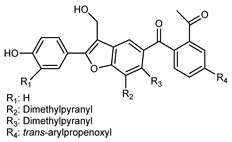
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (9E,9′E)-5-Isopentenyl-7′-(4′-hydroxy-5′-methoxy)-phenyl-4′-propenylketone-8′-methylol-furanyl-6′-acetyl-1′,6-biphenyl-7-ketone | C33H30O8 |
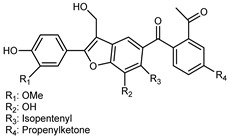
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7E,10E)-4,5′-Dihydroxy-5-isopentenol-6-(7,8-trans allyl)-alcohol7′-(4′-hydroxy-3′,5′-dimethoxyl)-phenyl-9′,9′-dimethylol-1′,7′- bineolignan | C34H34O11 |
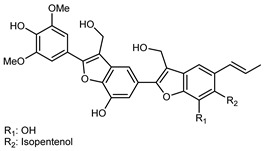
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7E)-5′-Hydroxy-4,5-(13,14-dimethyl)-pyranyl-6-allyl alcohol-7′-(4′-hydroxy-3′,5′-dimethoxyl)-phenyl-9′,9′-dimethylol-1′,7′-bineolignan | C34H32O10 |
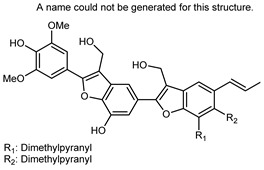
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7S,8R)-9′,3-Dimethoxyl isoamericanol | C20H22O7 |
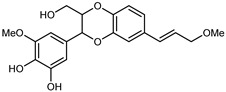
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7S,8R,7′S,8′R)-7,8–7′,8′-trans-7′,8′-Z-Sesquiverniciasin A | C27H25O9 |
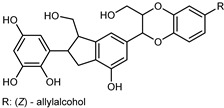
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7S,8R,7′S,8′R)-7,8–7′,8′-trans-7′,8′-E-Sesquiverniciasin A | C27H25O9 |
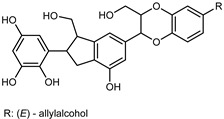
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Selamoellenin B | C21H24O7 |
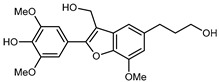
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Dendronbibisline A | C30H26O7 |
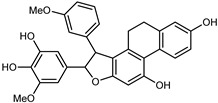
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Dendronbibisline B | C25H24O7 |
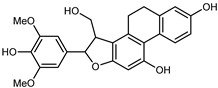
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Dendronbibisline C | C32H32O8 |
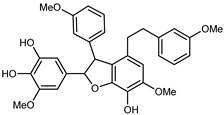
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Dendronbibisline D | C33H34O8 |
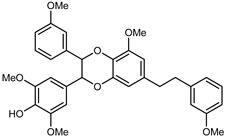
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Herpetiosol B | C30H34O9 |
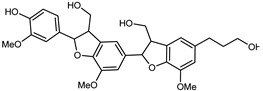
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Herpetosiols C | C31H34O9 |
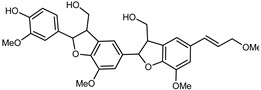
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Silychristin A | C25H22O10 |
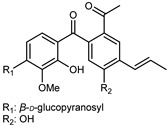
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Silychristin B | C25H22O10 |
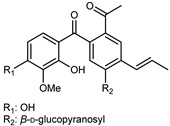
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7S,8R)-threo-1′-[3′-Hydroxy-7-(4-hydroxy-3- methoxyphenyl)-8-hydroxymethyl-7,8-dihydrobenzofuran]acryl-aldehyde | C19H18O6 |
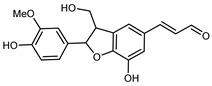
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (-)-(7R,8S,7′E)-4-Hydroxy-3,5,5′,9′-tetramethoxy-4′,7-epoxy-8,3′-neolign-7′-en-9-ol | C22H26O7 |
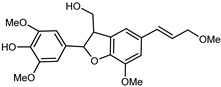
|
reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
3.2.3. Terpenes
Terpenes are a class of natural products formed by different isoprene units (C5H8) that determine structural classifications in monoterpenes, diterpenes, sesquiterpenes, triterpenes, and tetraterpenes. The EO, obtained primarily from the flavedo of C. medica, is rich in these specialized metabolites. In recent years, the attention focused on these molecules used as perfumes and for the preservation of foods has considerably increased, thanks to their antimicrobial activity against Saccharomyces cerevisiae [44]. Additionally, other studies have evaluated the anti-inflammatory and antioxidant activity associated with these molecules [28]. The composition of EO depends on several factors, such as the extraction method, different stages of fruit maturity [45], environmental factors, geographical location, and genetic variations. All these variables make the comparison between studies complex [46]. In fact, the peel oil of the C. medica var. Sarcodactylis profile reported by Jing et al., including limonene (41.8%), geranial (17.9%), neral (13.6%), citronellal (4.4%), and nerol (4.1%), was different from that reported by Venturini et al., using C. medica cv. Corsican [47]. However, in all these studies, limonene and γ-terpinene were the most abundant compounds identified in C. medica EO. Their quantity depends on the maturity stage of the fruit. In particular, the highest concentration of limonene (36.37%) was found in the immature stage, while the highest content of γ-terpinene (25.23%) was reported in the intermediated stage, but was reduced in mature fruits.
Furthermore, in the mature stage, the monoterpene-hydrocarbon content increased, but the amount of sesquiterpene hydrocarbons and total sesquiterpenes decreased [45]. According to Taghvaeefard et al., the main constituent of EO from the flavedo in C. medica var. macrocarpa was limonene (89.39%), while in C. medica var. medica, limonene (48.59%), linalool (22.98%), and linalyl acetate (8.21%) were the main components detected [26]. Regarding the extraction condition, a low yield represents a limiting factor for the recovery of EOs. Poiana et al. performed three different extraction techniques on C. medica cv. Diamante: the commonly used hydro-distillation of fresh and dried peel, supercritical carbon dioxide extraction (SCF–CO2), and solvent extraction using pentane. The contents of monoterpenes and limonene were higher with the hydro-distillation but decreased with the SCF–CO2, with which it was instead possible to observe an increase in sesquiterpenes. The reason for this is that mainly volatile molecules were extracted in the hydro distillates, while the high density of SCF–CO2 increased the solubility of the non-volatile compounds [48]. According to Bartolo et al., the best extraction method is the abrasion of rinds, except for limonene, which has a better yield with manual squeezing [49]. These techniques allow the extraction of a quantity of active metabolites that is greater than that of the oil obtained by simple maceration with hexane, which, as reported by Conforti et al., led to the identification of 45 compounds and an extraction yield of 0.13% [50]. In addition, Xing et al. obtained an extraction yield of 0.48% by using ultrasound-assisted hydro-distillation (UAHD) [51], while Wei et al. [52] demonstrated that under the optimal extraction parameters (microwave irradiation power, microwave irradiation time, and homogenization time) the essential oil yield (1.65% ± 0.05%) from solvent-free microwave extraction was 27.91% higher than that from hydro-distillation (HD) (1.29% ± 0.03%), which was probably due to the special heating mode of the microwave. Several studies also reported a comparison of yields obtained with hydro-distillation vs. steam HD. Jing et al. showed a low yield of oil obtained by the steam distillation of citrus peel (0.64 ± 0.07 g of oil/g) [47]. Wu et al. compared different fruit stages of C. medica, demonstrating that the EO-extraction yield ranged from 2.39 ± 0.08% w/w in the immature stage to 3.57 ± 0.12% w/w in the mature stage, which was higher than that reported by Peng et al. [53] (0.45%). The reasons for this difference could be the geographical origin and the different methods of extraction; in fact, in the first case, the EO was obtained by HD from fresh fruits grown in China, while in the second study, the oil was obtained by the steam-based hydro-distillation of dried fruits cultivated in Japan. Vitalini et al. [54] described different exocarp EO and hydrolate (HY) compositions. The volatile profile of the EO was characterized by limonene (66.9%) and γ-terpinene (20.0%) as the most abundant compounds, while α-terpineol (44.7%), and terpinen-4-ol (21.6%) were found in the HY extract. Furthermore, several minor monoterpene components, such as α-thujene (0.2%), α-pinene (0.6%), β-thujene (0.1%), β-myrcene (0.9%), β-pinene (0.8%), (+)-4-carene (0.2%), R-(+)-citronellal (0.1%), nerol acetate (0.2%), and geranyl acetate (0.1%), which were found in the EO, were missing in the hydrolate (HY). Instead, other compounds, such as β-terpinene (1.0%), linalol (5.7%), thymol (1.9%), and piperitenone (0.4%), were detected only in the HY. Furthermore, as with many other citrus fruits, C. medica is an important source of carotenoids, also called tetraterpenoids, which are made up of a carbon skeleton characterized by six isoprene units. Fanciullino et al. [55] analyzed the carotenoid contents of twenty-five citrus varieties and reported that in C. medica, β-cryptoxanthin was found without cis-violaxanthin, while in Citrus maxima, only cis-violaxanthin was found, with a lack of β-cryptoxanthin. The total carotenoid content in the extracts of Etrog citron and Diamante citron juices was identified by a comparison of their retention times and UV/vis spectra, showing 0.227 mg lycopene/L and 0.019 mg lycopene/L respectively. Other typical specialized compounds abundant in citrus fruits and the Rutaceae family are limonoids, which chemically constituted by variations in the structure of the furanolactone core. The most frequently present components in C. medica are limonin and nomilin, which are responsible for the bitter taste of the genus Citrus. Lim et al. [56] investigated the optimum conditions for the enzymatic hydrolysis of citron waste juice using the response surface methodology: the highest contents of limonin and nomilin were 3.49 mg/100 g (extraction conditions: pH 4.51, temperature 50.30 °C, time 48.34 min, and 0.21% yield) and 1.56 mg/100 g (extraction conditions: pH 4.59, temperature 50.08 °C, time 66.07 min, and 0.30%), respectively. The terpenes identified in C. medica are shown in Table 4. They are grouped into their respective categories based on the number of isoprene units: monterpenes, diterpenes, triterpenes, tetraterpenes, and polyterpenes.
Table 4.
Terpenes identified in different parts of Citrus medica L.
| Compounds | Formula | Structure | Extraction Method * | Analysis | Part of the Plant | Abundance | References |
|---|---|---|---|---|---|---|---|
| Monoterpenes | |||||||
| ɑ-Thujene | C10H16 |
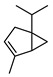
|
I | GC–MS | flavedo | 0.2% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.28–0.59% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.2–0.9% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | - | [57] | |||
| X | GC–MS | fructus | 1.20–1.29% | [53] | |||
| XI | GC–MS | fructus | 0.87% | [58] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| XV | GC–MS | exocarp, mesocarp | 0.4–0.5% | [54] | |||
| ɑ-Thujone | C10H16O |
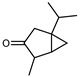
|
XIII | GC–MS | fresh fructus | 4.29–5.05% | [45] |
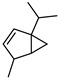
| Ꞵ-Thujene | C10H16 |
|
VIII | GC–MS–SPME | industrial essence | 0.78% | [57] |
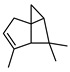
| ɑ-Pinene | C10H16 |
|
I | GC–MS | flavedo | 0.49% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.69–1.46% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.6–2.1% | [48] | |||
| X | GC–MS | fructus | 2.92–3.40% | [53] | |||
| XI | GC–MS | fructus | 1.99% | [58] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| XIII | GC–MS | fresh fructus | 6.38–7.73% | [45] | |||
| XV | GC–MS | exocarp, mesocarp | 1.4–1.6% | [54] | |||
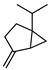
| Sabinene | C10H16 |
|
I | GC–MS | flavedo | 0.64% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.14–0.22% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| Camphene | C10H16 |
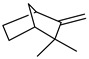
|
II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| V, VI, VII | GC–MS | flavedo | trace | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.04% | [57] | |||
| X | GC–MS | fructus | 0.02–0.03% | [53] | |||
| XIII | GC–MS | fresh fruit | 0.22–0.29% | [45] | |||
| cis-Sabinene hydrate | C10H18O |
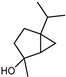
|
II, III, IV | HRGC–MS | flavedo | 0.04–0.06% | [49] |
| V, VI, VII | GC–MS | flavedo | trace | [48] | |||
| trans-Sabinene hydrate | C10H18O |
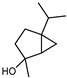
|
VIII | GC–MS–SPME | industrial essence | - | [57] |
| Ꞵ-Pinene | C10H16 |
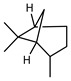
|
I | GC–MS | flavedo | 0.63% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.69–1.47% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 1.0–2.0% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 20.07% | [57] | |||
| X | GC–MS | fructus | 2.48–2.88% | [53] | |||
| XI | GC–MS | fructus | 2.02% | [58] | |||
| XII | HR–MAS–NMR | flavedo, oil glands | - | [22] | |||
| XIII | GC–MS | fresh fructus | 2.64–3.18% | [45] | |||
| XIV | GC–MS | exocarp, mesocarp | 2.4–2.5% | [54] | |||
| Myrcene | C10H16 |
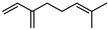
|
I | GC–MS | flavedo | 0.89 | [50] |
| II, III, IV | HRGC–MS | flavedo | 1.13–1.47% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.8–1.6% | [48] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| VIII | GC–MS–SPME | industrial essence | 2.24% | [57] | |||
| X | GC–MS | fructus | 1.64–1.76% | [53] | |||
| XI | GC–MS | fructus | 1.25% | [58] | |||
| Limonene | C10H16 |
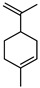
|
I | GC–MS | flavedo | 15.20% | [50] |
| II, III, IV | HRGC–MS | flavedo | 25.70–60.30 g/100 g DW | [49] | |||
| V, VI, VII | GC–MS | flavedo | 34.6–60.8% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 41.07% | [57] | |||
| X | GC–MS | fructus | 51.24–57.63% | [53] | |||
| XI | GC–MS | fructus | 52.44% | [58] | |||
| XII | HR–MAS–NMR | flavedo, oil glands, albedo | - | [22] | |||
| XIII | GC–MS | fresh fructus | 32.07–36.37% | [45] | |||
| XIV | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | - | [15] | |||
| XV | GC–MS | exocarp, mesocarp | 75.8–76.2% | [54] | |||
| Decane | C10H22 |
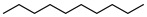
|
II, III, IV | HRGC–MS | flavedo | trace | [49] |
| Decanal | C10H20O |
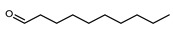
|
II, III, IV | HRGC–MS | flavedo | 0.04–0.07 | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.27% | [57] | |||
| Octyl acetate | C10H20O2 |
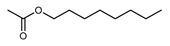
|
II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| VIII | GC–MS | industrial essence | 0.16% | [57] | |||
| Citronellol | C10H20O |
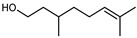
|
II, III, IV | HRGC–MS | flavedo | 0.03–0.11% | [49] |
| cis-Limonene oxide | C10H16O |
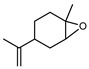
|
II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| VIII | GC–MS–SPME | industrial essence | - | [57] | |||
| trans-Limonene oxide | C10H16O |
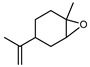
|
II, III, IV | HRGC–MS | flavedo | trace | [49] |
| VIII | GC–MS | industrial essence | 0.28% | [57] | |||
| trans-Carveol | C10H16O |
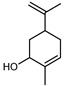
|
V, VI, VII | GC–MS | flavedo | 0.1% | [48] |
| Carveol | C10H16O |
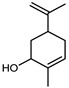
|
XV | GC–MS | mesocarp | 0.1% | [54] |
| Camphor | C10H16O |
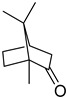
|
II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| Citronellal | C10H18O |
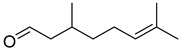
|
II, III, IV | HRGC–MS | flavedo | 0.04–0.06% | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1–0.2% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.27% | [57] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| XIII | GC–MS | fresh fructus | 0.11% | [45] | |||
| Borneol | C10H18O |
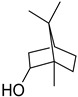
|
II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| (Z)-Ꞵ-Ocimene | C10H16 |
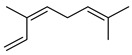
|
II, III, IV | HRGC–MS | flavedo | 0.75–1.19% | [49] |
| V, VI, VII | GC–MS | flavedo | 1.4–1.5% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | - | [57] | |||
| XI | GC–MS | fructus | 0.94% | [58] | |||
| (E)-Ꞵ-Ocimene | C10H16 |
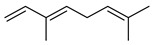
|
II, III, IV | HRGC–MS | flavedo | 1.10–1.74% | [49] |
| V, VI, VII | GC–MS | flavedo | 1.9–2.1% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.07% | [57] | |||
| XIII | GC–MS | fresh fructus | 0.55–0.99% | [45] | |||
| X | GC–MS | fructus | 0.23–0.93% | [53] | |||
| XI | GC–MS | fructus | 0.65% | [58] | |||
| XV | GC–MS | exocarp, mesocarp | 1.1–1.2% | [54] | |||
| Citral | C10H16O |
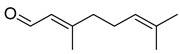
|
VIII | GC–MS–SPME | industrial essence | - | [57] |
| X | GC–MS | fructus | 1.96–2.34% | [53] | |||
| XII | HR–MAS–NMR | flavedo | - | [22] | |||
| XV | GC–MS | mesocarp | 0.1% | [54] | |||
| Octanal | C8H16O |
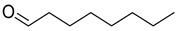
|
II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| V, VI, VII | GC–MS | flavedo | - | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.31% | [57] | |||
| ɑ-Phellandrene | C10H16 |
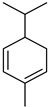
|
II, III, IV | HRGC–MS | flavedo | 0.04–0.05% | [49] |
| XV | GC–MS | mesocarp | trace | [54] | |||
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| X | GC–MS | fructus | 0.1% | [53] | |||
| δ-3-Carene | C10H16 |
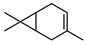
|
I | GC–MS | flavedo | 2.30% | [50] |
| II, III, IV | HRGC–MS | flavedo | trace | [49] | |||
| 3-Carene | C10H16 |
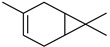
|
XIII | GC–MS | fresh fructus | 8.15–9.01% | [45] |
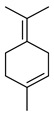
| 4-Carene | C10H16 |
|
VIII | GC–MS–SPME | industrial essence | 0.10% | [57] |
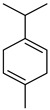
| γ-Terpinene | C10H16 |
|
I | GC–MS | flavedo | 10.27% | [50] |
| II, III, IV | HRGC–MS | flavedo | 21.19–23.44% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 22.1–24.6% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 8.35% | [57] | |||
| X | GC–MS | fructus | 27.01–33.71% | [53] | |||
| XI | GC–MS | fructus | 28.41% | [58] | |||
| XII | HR–MAS–NMR | flavedo, oil glands | - | [22] | |||
| XIII | GC–MS | fresh fructus | 22.44–25.23% | [45] | |||
| XV | GC–MS | exocarp, mesocarp | 15.0–16.5% | [54] | |||
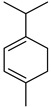
| ɑ-Terpinene | C10H16 |
|
II, III, IV | HRGC–MS | flavedo | 0.35–0.41% | [49] |
| V, VI, VII | GC–MS | flavedo | trace | [48] | |||
| X | GC–MS | fructus | 1.28% | [53] | |||
| XI | GC–MS | fructus | 0.81% | [58] | |||
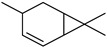
| Terpinolene | C10H16 |
|
I | GC–MS | flavedo | 0.91% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.87–1.00% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 1.0–1.2% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.33% | [57] | |||
| X | GC–MS | fructus | 1.25–1.54% | [53] | |||
| XIII | GC–MS | industrial essence | - | [45] | |||
| XV | GC–MS | exocarp, mesocarp | 0.2–0.6% | [54] | |||
| Linalool | C10H18O |
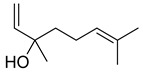
|
I | GC–MS | flavedo | 1.15% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.10–0.20 g/100 g DW | [47] | |||
| V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 1.73% | [57] | |||
| XIII | GC–MS | fresh fructus | 0.16–0.18% | [45] | |||
| Linalool oxide | C10H18O2 |
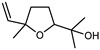
|
VIII | GC–MS–SPME | industrial essence | 0.28% | [57] |
| Allocimene | C10H16 |
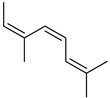
|
I | GC–MS | flavedo | 0.70% | [50] |
| Terpinen-4-ol | C10H18O |
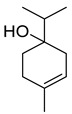
|
I | GC–MS | flavedo | 1.02% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.04–0.06% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1–0.2% | [48] | |||
| X | GC–MS | fructus | 0.34–0.51% | [53] | |||
| VIII | GC–MS–SPME | industrial essence | 0.31% | [57] | |||
| XIII | GC–MS | fresh fructus | 0.69–0.88% | [45] | |||
| ɑ-Terpineol | C10H18O |
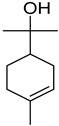
|
I | GC–MS | flavedo | 2.64% | [50] |
| V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.10% | [57] | |||
| X | GC–MS | fructus | 0.48–0.58% | [53] | |||
| XIII | GC–MS | fresh fruit | 1.17–1.61% | [45] | |||
| XV | GC–MS | exocarp, mesocarp | 0.1–0.4% | [54] | |||
| Nerol | C10H18O |
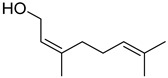
|
I | GC–MS | flavedo | 4.69% | [50] |
| V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] | |||
| XIII | GC–MS | fresh fructus | 0.9–1.53% | [45] | |||
| Neral | C10H16O |
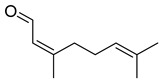
|
II, III, IV | HRGC–MS | flavedo | 1.20–9.40 g/100 g DW | [49] |
| V, VI, VII | GC–MS | flavedo | trace | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 2.49% | [57] | |||
| X | GC–MS | fructus | 0.45% | [53] | |||
| XII | HR–MAS–NMR | flavedo | - | [22] | |||
| XIII | GC–MS | fresh fructus | 1.04–1.60% | [45] | |||
| p-Cymen-8-ol | C10H14O |
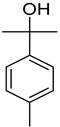
|
II, III, IV | HRGC–MS | flavedo | 0.01% | [47] |
| p-Cymene | C10H14 |
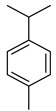
|
V, VI, VII | GC–MS | flavedo | 0.4–0.6% | [48] |
| XIV | GC–MS | exocarp, mesocarp | 0.2–0.7% | [54] | |||
| VIII | GC–MS–SPME | industrial essence | 5.92% | [57] | |||
| XIII | GC–MS | fresh fructus | 1.64–2.77% | [45] | |||
| Geraniol | C10H18O |
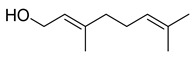
|
I | GC–MS | flavedo | 4.63% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.10–8.50 g/100 g DW | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1–0.7% | [48] | |||
| X | GC–MS | fructus | 0.55–0.58% | [53] | |||
| VIII | GC–MS-SPME | industrial essence | 0.27% | [57] | |||
| XIII | GC–MS | fresh fructus | 1.18–2.02% | [45] | |||
| Perillal | C10H14O |
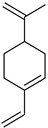
|
VIII | GC–MS–SPME | industrial essence | 0.10% | [57] |
| Cuminol | C10H14O |
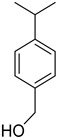
|
VIII | GC–MS–SPME | industrial essence | 0.03% | [57] |
| Carvacrol | C10H14O |
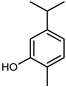
|
II, III, IV | HRGC–MS | flavedo | trace | [49] |
| Perilla aldehyde | C10H14O |
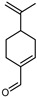
|
II, III, IV | HRGC–MS | flavedo | 0.01–0.02% | [49] |
| Sesquiterpenes | |||||||
| δ-Elemene | C15H24 |
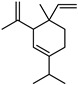
|
II, III, IV | HRGC–MS | flavedo | 0.06–0.16% | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| Ꞵ-Elemene | C15H24 |
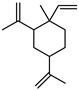
|
I | GC–MS | flavedo | 0.1% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.1% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| X | GC–MS | flavedo | 0.1% | [53] | |||
| Copaene | C15H24 |
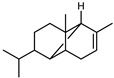
|
X | GC–MS | fructus | 0.02% | [53] |
| trans-Caryophyllene | C15H24 |
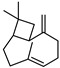
|
I | GC–MS | flavedo | 0.41% | [50] |
| ɑ-Bisabolol | C15H26O |
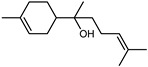
|
V, VI, VII | GC–MS | flavedo | 0.2% | [48] |
| ɑ-Bergamotene | C15H24 |
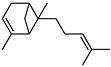
|
I | GC–MS | flavedo | 1.09% | [50] |
| XV | GC–MS | exocarp, mesocarp | 0.3–0.6% | [54] | |||
| X | GC–MS | fructus | 0.07% | [53] | |||
| V, VI, VII | GC–MS | flavedo | 0.2–1.7% | [48] | |||
| ɑ-Himachalene | C15H24 |
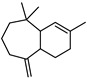
|
XV | GC–MS | exocarp, mesocarp | 0.1–0.6% | [54] |
| γ-Gurjunene | C15H24 |
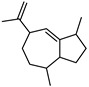
|
XV | GC–MS | mesocarp | trace | [54] |
| ɑ-Humulene | C15H24 |
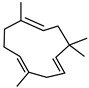
|
I | GC–MS | flavedo | 0.13% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.1% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| X | GC–MS | fructus | - | [53] | |||
| (Z)-β-Farnesene | C15H24 |
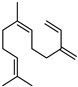
|
I | GC–MS | flavedo | 0.14% | [50] |
| II, III, IV | HRGC–MS | flavedo | trace | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| α-Bisabolene | C15H24 |
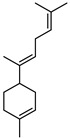
|
I | GC–MS | flavedo | 0.10% | [50] |
| IX | GC–MS | leaves | - | [59] | |||
| β-Bisabolene | C15H24 |
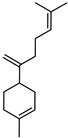
|
I | GC–MS | flavedo | 1.39% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.03–0.05% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.2–2.6% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.30% | [57] | |||
| Spathulenol | C15H24O |
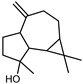
|
I | GC–MS | flavedo | 0.1% | [50] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| α-cis-Bergamotene | C15H24 |
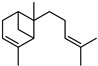
|
II, III, IV | HRGC–MS | flavedo | 0.02–0.03% | [49] |
| E-β-Caryophyllene | C15H24 |
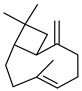
|
II, III, IV | HRGC–MS | flavedo | 0.10 g/100 g DW | [49] |
| VIII | GC–MS | industrial essence | 0.23% | [57] | |||
| IX | GC–MS | leaves | - | [59] | |||
| X | GC–MS | fructus | 0.06% | [53] | |||
| XIII | GC–MS | fresh fructus | 0.27–0.46% | [45] | |||
| XIV | UHPLC–QTOF–IMS | mesocarp | - | [15] | |||
| α-trans-Bergamotene | C15H24 |
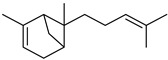
|
II, III, IV | HRGC–MS | flavedo | 0.29–0.45% | [49] |
| V, VI, VII | GC–MS | flavedo | 0.2–1.7% | [48] | |||
| IX | GC–MS | leaves | - | [59] | |||
| (E)-β-Farnesene | C15H24 |
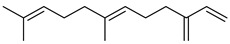
|
II, III, IV | HRGC–MS | flavedo | trace | [49] |
| XV | GC–MS | exocarp | 0.2% | [54] | |||
| IX | GC–MS | leaves | - | [59] | |||
| (Z)-β-Santalene | C15H24 |
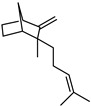
|
II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| Valencene | C15H24 |
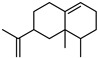
|
II, III, IV | HRGC–MS | flavedo | 0.03–0.07% | [49] |
| Bicyclogermacrene | C15H24 |
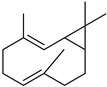
|
II, III, IV | HRGC–MS | flavedo | 0.03–0.04% | [49] |
| (Z)-α-Bisabolene | C15H24 |
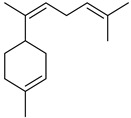
|
II, III, IV | HRGC–MS | flavedo | 0.03–0.05% | [49] |
| β-Cadinene | C15H24 |
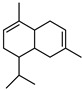
|
XIII | GC–MS | fresh fructus | 0.74–1.09% | [45] |
| α-Cedrene | C15H24 |
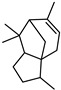
|
XIII | GC–MS | fresh fructus | 0.55–0.64% | [45] |
| (E,E)-α-Farnesene | C15H24 |
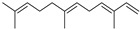
|
V, VI, VII | GC–MS | flavedo | trace | [48] |
| (Z)-α-Farnesene | C15H24 |
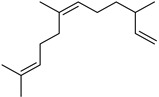
|
XV | GC–MS | exocarp | 0.6% | [54] |
| α-Farnesene | C15H24 |
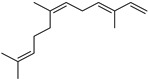
|
X | GC–MS | fructus | 0.1% | [53] |
| XV | GC–MS | mesocarp | 0.2% | [54] | |||
| (Z)-γ-Bisabolene | C15H24 |
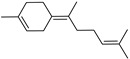
|
II, III, IV | HRGC–MS | flavedo | trace | [49] |
| Germacrene B | C15H24 |
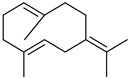
|
V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] |
| X | GC–MS | fructus | - | [53] | |||
| Gemacrene D | C15H24 |
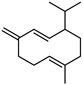
|
IX | GC–MS | leaves | - | [59] |
| X | GC–MS | fructus | 0.15–0.19% | [53] | |||
| Bicyclogermacrene | C15H24 |
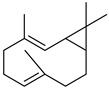
|
IX | GC–MS | leaves | - | [59] |
| X | GC–MS | fructus | 0.06% | [53] | |||
| (E)-Nerolidol | C15H26O |
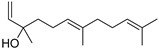
|
V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] |
| IX | GC–MS | leaves | - | [59] | |||
| Β-Bisabolene | C15H24 |
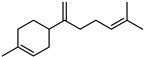
|
II, III, IV | HRGC–MS | flavedo | 0.40–0.67% | [49] |
| Farnesol | C15H26O |
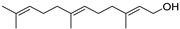
|
V, VI, VII | GC–MS | flavedo | trace | [48] |
| Farnesal | C15H24O |
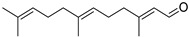
|
V, VI, VII | GC–MS | flavedo | trace | [48] |
| Triterpenoids (Limonoids) | |||||||
| Limonyl acetate | C28H34O9 |
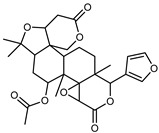
|
XIV | UHPLC–QTOF–IMS | exocarp, seeds | - | [15] |
| Limonin | C26H30O8 |
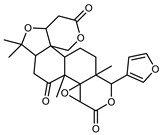
|
XVII | HPLC | citron waste | 3.08 mg/100 g DW | [56] |
| XVI | EI–MS, HR–EI–MS | fresh fructus | - | [43] | |||
| XIV | HPLC–Q/TOF–MS | fructus | 0.45–0.86 mg/g DW | [35] | |||
| XVIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] | |||
| Nomilin | C28H34O9 |
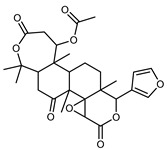
|
XVII | HPLC | citron waste | 0.87 mg/100 g DW | [56] |
| XVI | EI–MS, HR–EI–MS | fresh fructus | - | [43] | |||
| XIV | HPLC–Q/TOF–MS | fructus | 1.97–3.84 mg/g DW | [35] | |||
| Citrusin | C28H34O11 |
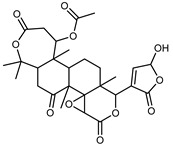
|
XVI | EI–MS, HR–EI–MS | fresh fructus | - | [43] |
| Obacunone | C26H30O7 |
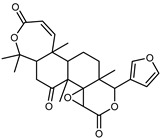
|
XVI | EI–MS, HR–EI–MS | fresh fructus | - | [43] |
| XIV | HPLC–Q/TOF–MS | fructus | 0.15–0.36 mg/g DW | [35] | |||
| Nomilinic acid | C28H36O10 |
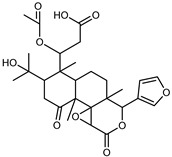
|
XIV | UHPLC–QTOF–IMS | exocarp, seeds | - | [15] |
| Terpenoids | |||||||
| Geranyl acetate | C12H20O2 |
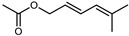
|
I | GC–MS | flavedo | 0.75% | [50] |
| Citronellyl acetate | C12H22O2 |
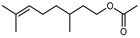
|
II, III, IV | HRGC–MS | flavedo | 0.10 g/100 g DW | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1–0.2% | [48] | |||
| Dihydrolinalyl acetate | C12H22O2 |
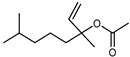
|
II, III, IV | HRGC–MS | flavedo | trace | [49] |
| β-lonone | C13H20O |
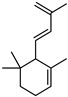
|
XIII | GC–MS | fresh fructus | 0.20–0.49% | [45] |
| Linalyl acetate | C12H20O2 |
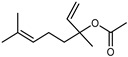
|
VIII | GC–MS | industrial essence | 1.82% | [57] |
* Essential oil extraction methods. I: Maceration of peel with n-hexane at room temperature; II: aspiration of the oil from the utricles present on the peel by means of a syringe with a thin needle; III: the rinds of the fruit were squeezed to cause the breaking of the utricles in order to release the oil, which was collected by extraction with hexane; IV: manual abrasion of the rind by means of a stainless-steel grater, followed by manual pressing and centrifugation of the water-oil emulsion; V: hydro-distillation; VI: soxhlet apparatus using pentane and ethanol as solvents; VII: SCF–CO2; VIII: alcoholic and industrial extraction method; IX: n-Hexanol was added to leaf powder; X: steam distillation; XI: distillation using a Clevenger-type apparatus; XII: the content of oil glands was obtained cutting the most superficial layer of flavedo to open oil glands; XIII: steam hydro-distillation; XIV: UAE; XV: exocarp and mesocarp were pulverized in liquid nitrogen with a chilled mortar and pestle, and then weighed and placed in MeOH. The mixtures were sonicated; XVI: MeOH under reflux; XVII: enzymatic treatment. XVIII: maceration in MeOH.
3.2.4. Coumarins
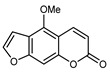
Coumarins are natural phytochemicals that are widely distributed in plants and are strongly related to numerous pharmacological activities. They belong to the lactone family, consist of a benzene ring fused to a α-pyrone ring, and can be classified into different subtypes. Several phytochemicals studies have shown the abundance of coumarins in citrus fruits; 5,7-dimethoxycumarin was found to be the most abundant coumarin (876.7 ± 4.7 µg/g) in a fruit extract (var. Sarcodactylis) using pressurized liquid extraction with methanol at 90 °C. This represents, together with hesperidin, a marker for the quality control of Citrus fruits [40]. Vitalini et al. also identified 5,7-dimethoxycumarin as the most abundant compound (50.6%), followed by 2-pyrone (23.4%), in a methanolic extract of the exocarp of a variety of C. medica from Switzerland. Furan derivatives were the main class of compounds detected by GC–MS, of which 5-hydroxymethylfurfural was the main exponent, with relative percentages of 14.7% and 24.8% in the exocarp extract and in the mesocarp extract, respectively. In addition, 2-Furanmethanol (3.9% for the exocarp extract; 6.7% for the mesocarp extract) and furaneol (3.1% for the exocarp extract; 3.6% for the mesocarp extract) were present in both extracts. Furthermore, 2-pyrone (33.1%) and 2,3-butanediol (23.7%) were the main component non-furanoic derivatives present in the mesocarp [54]. Coumarins were also detected in the root bark of C. medica. Wang et al. identified two coumarins, xanthyletin and xanthoxyletin, by micellar electrokinetic capillary chromatography; their amounts (1.6 mg/g and 0.7 mg/g, respectively) were lower than those found in other Citrus species, such as C. reticulata (3.6 mg/g and 1.5 mg/g, respectively) [60]. In Citrus fruits, other coumarins have also been identified in lower amounts or in traces, such as 7-hydroxycoumarin, 6,7-dimethoxycoumarin, and bergapten [40]. All the other coumarins found in C. medica are listed in Table 5.
Table 5.
Coumarins identified in various parts of C. medica L.
| Compounds | Formula | Structure | Extraction Method | Method Analyses | Part of the Plant | Quantitative | References |
|---|---|---|---|---|---|---|---|
| Oxypeucedanin hydrate | C16H16O6 |
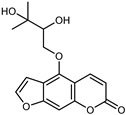
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | 2.03–21.30 g/100 g DW | [43] |
| Scoparone (6,7-dimethoxycoumarin) |
C11H10O4 |
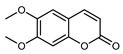
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| PLE MeOH | HPLCDAD | fructus | 38.79 µg/mL | [40] | |||
| UAE | HPLC–Q/TOF–MS | fructus | - | [35] | |||
| Skimmin | C15H16O8 |
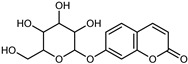
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Haploperoside A | C22H28O13 |
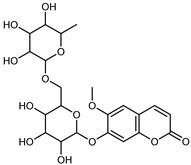
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Leptodactylone | C11H10O5 |
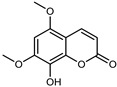
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Herniarin (7-methoycoumarin) | C10H8O3 |
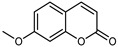
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Isomeranzin | C15H16O4 |
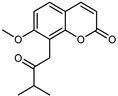
|
UAE | HPLC–Q/TOF–MS | fructus | - | [35] |
| Scopoletin | C10H8O4 |
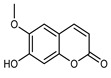
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| PLE MeOH | HPLC–DAD | fructus | 53.56 µg/mL | [40] | |||
| Isoscopoletin | C10H8O4 |
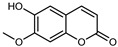
|
PLE MeOH | HPLC–DAD | fructus | 63.06 µg/mL | [40] |
| Umbelliferone (7-hydroxycoumarin) | C9H6O3 |
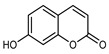
|
MeOH under reflux | EI-MS, HR–EI–MS | fresh fruit | - | [43] |
| PLE MeOH | HPLC–DAD | fructus | 40.23 µg/mL | [40] | |||
| Nordentatin | C19H20O4 |
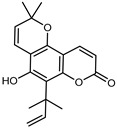
|
MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Maceration in acetone | COSY, NOESY, HMQC, HMBC, HR–ESI–MS | root bark, stem bark | - | [36] | |||
| 2-pyrone | C5H4O2 |
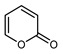
|
Maceration and UAE MeOH | GC–MS | exocarp, mesocarp | 23.4–33.1% | [54] |
| Citropten (5,7-dimethoxycoumarin) | C11H10O4 |
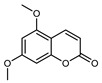
|
PLE MeOH | HPLC–DAD | fructus | 106.47 µg/mL | [40] |
| MeOH under reflux | HR–EI–MS1 | fresh fruits | 0.16–0.45 mg/g DW | [43] | |||
| Maceration of peel with n-hexane at room temperature | GC–MS | fructus | 12.64% | [50] | |||
| UAE | HPLC–Q/TOF–MS | fructus | 0.18–0.45 mg/g DW | [35] | |||
| Bergapten | C12H8O4 |
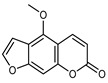
|
PLE MeOH | HPLC–DAD | fructus | 35.07 µg/mL | [40] |
| UAE | HPLC–Q/TOF–MS | fructus | - | [35] | |||
| Maceration MeOH | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] | |||
| Citrumedin-B | C24H28O4 |
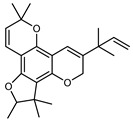
|
Acetone at room temperature | COSY, NOESY, HMQC, HMBC, HR–ESI–MS | root bark, stem bark | - | [36] |
| Xanthyletin | C14H12O3 |
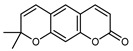
|
PLE MeOH | HPLC–DAD | fructus | - | [40] |
| UAE with CHCl3 | MEKC (micellar electrokinetic capillary chromatography) | root bark | - | [60] | |||
| Xanthoxyletin | C15H14O4 |
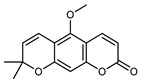
|
UAE with CHCl3 | MEKC (micellar electrokinetic capillary chromatography) | root bark | - | [60] |
| 5,8-dimethoxhypsoralene | C12H8O4 |
|
Maceration MeOH | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
3.3. Other Compounds
Other compounds were identified in C. medica, and they are classified in the following Table 6. In addition, several authors isolated and characterized new polysaccharides (listed in Table 7), which may be endowed with potential bioactivities.
Table 6.
Other compounds identified in C. medica L.
| Compounds | Formula | Structure | * Extraction Method | Method Analyses | Part of the Plant | Abundance | References |
|---|---|---|---|---|---|---|---|
| Alkaloids | |||||||
| 1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid | C12H12N2O2 |
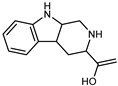
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Acridine derivatives | |||||||
| Medicacridone | C20H21NO4 |
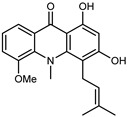
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Citracridone-I | C20H19NO5 |
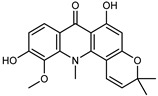
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| citracridone-III | C19H17NO5 |
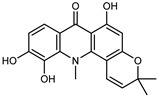
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| 5-hydroxynoracronycine 3 | C19H17NO4 |
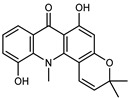
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Xanthones | |||||||
| Medicaxanthone | C51H75O8 |
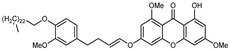
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Lichenxanthone | C15H12O6 |
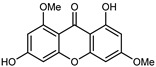
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Glycol | |||||||
| 2,3-Butanediol | C4H10O2 |
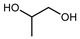
|
X | GC–MS | exocarp, mesocarp | 23.7% | [54] |
| Furan derivatives | |||||||
| Furfural | C5H4O2 |
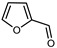
|
X | GC–MS | exocarp, mesocarp | 3.9% | [54] |
| 2(3H)-Furanone, 5-methyl | C8H12O2 |
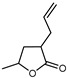
|
X | GC–MS | exocarp, mesocarp | 0.9% | [54] |
| 5- 5-Hydroxymethylfurfural | C6H6O3 |
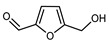
|
X | GC–MS | exocarp, mesocarp | 1.9% | [54] |
| Hydrocarbons | |||||||
| 1,3-Cyclopentadiene | C5H6 |
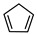
|
XIII | GC–MS | fresh fructus | 1.75–2.36% | [45] |
| Benzene | C6H6 |
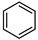
|
XI | GC–MS | fructus | 1.67% | [58] |
| Eicosane | C20H42 |
|
I | GC–MS | flavedo | 0.10% | [50] |
| Nonacosane | C29H60 |

|
I | GC–MS | flavedo | 0.10% | [50] |
| Mono or polyunsaturated aldehyde | |||||||
| Undecanal | C11H22O |
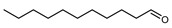
|
V, VI, VII | GC–MS | flavedo | 0.1–0.2% | [48] |
| II, III, IV | HRGC–MS | flavedo | 0.03–0.06% | [49] | |||
| Dodecanal | C12H24O |

|
II, III, IV | HRGC–MS | flavedo | 0.02–0.03% | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| 9,17-octadecadienal | C18H32O |
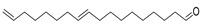
|
I | GC–MS | flavedo | 9.29% | [50] |
| 16-Octadecenal | C18H34O |

|
I | GC–MS | flavedo | 0.10% | [50] |
| Nonanal | C9H18O |
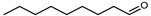
|
II, III, IV | HRGC–MS | flavedo | 0.04–0.07% | [49] |
| Tetradecanal | C14H28O |

|
V, VI, VII | GC–MS | flavedo | 0.1% | [48] |
| Pentadecanal | C15H30O |

|
V, VI, VII | GC–MS | flavedo | 0.1% | [48] |
| Phenylpropanoids | |||||||
| Coniferin | C16H22O8 |
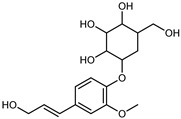
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Syringin | C17H24O9 |
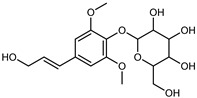
|
IX | EI–MS, HR–EI-MS | fresh fruit | - | [43] |
| Phytosterols | |||||||
| Lupeol | C26H32O7 |
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Stigmasterol | C29H48O |
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Ꞵ-Sitosterol | C29H50O |
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Fatty acids and their esters | |||||||
| Lauric acid | C12H24O2 |
|
I | GC–MS | flavedo | 0.11% | [50] |
| Myristic acid | C14H28O2 |
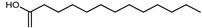
|
I | GC–MS | flavedo | 0.23% | [50] |
| Palmitic acid | C16H32O2 |
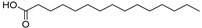
|
I | GC–MS | flavedo | 5.17% | [50] |
| Hexadecanal | C16H32O |

|
XIII | GC–MS | mesocarp | 1.6% | [54] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| Pentadecanoic acid methyl ester | C16H32O2 |
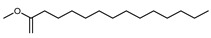
|
I | GC–MS | flavedo | 0.22% | [50] |
| Palmitoleic acid | C17H32O2 |
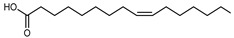
|
I | GC–MS | flavedo | 0.19% | [50] |
| Heptadecanoic acid | C17H34O2 |
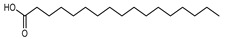
|
I | GC–MS | flavedo | 0.20% | [50] |
| Stearic acid | C18H36O2 |
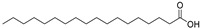
|
I | GC–MS | flavedo | 0.18% | [50] |
| 16-Octadecenal | C18H34O |

|
I | GC–MS | flavedo | 0.10% | [50] |
| Linoleic acid, methyl ester | C19H34O2 |
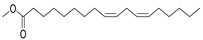
|
I | GC–MS | flavedo | 0.19% | [50] |
| Linolenic acid, methyl ester | C19H32O2 |
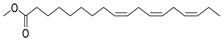
|
I | GC–MS | flavedo | 0.41%0.30% | [50] |
| Stearic acid, methyl ester | C19H38O2 |
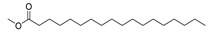
|
I | GC–MS | flavedo | 0.30% | [50] |
| Benzoates | |||||||
| Methyl vanillate methyl ester |
C9H10O4 |
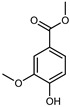
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Methyl benzoate | C8H8O2 |
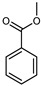
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Methyl paraben | C8H8O3 |
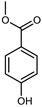
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
* Extraction method. I: Maceration of peel with n-hexane at room temperature; II: aspiration of the oil from the utricles present on the peel by means of a syringe with a thin needle; III: the rinds of the fruit were squeezed to cause the breaking of the utricles in order to release the oil, which was collected by extraction with hexane; IV: manual abrasion of the rind by means of a stainless steel grater, followed by manual pressing and centrifugation of the water–oil emulsion; V: hydro-distillation; VI: soxhlet apparatus using pentane and ethanol as solvents; VII: SCF–CO2; VIII: maceration in MeOH; IX: MeOH under reflux; X: maceration and UAE in MeOH; XI: distillation using a Clevenger-type apparatus; XII: steam-based hydro-distillation; XIII: exocarp and mesocarp were pulverized in liquid nitrogen with a chilled mortar and pestle, and then weighed and placed in MeOH. The mixtures were sonicated.
Table 7.
New polysaccharides isolated from C. medica L.
| Compounds | Molecular Weight | Extraction Method | Method Analyses | Part of the Plant | Abundance | References |
|---|---|---|---|---|---|---|
| Polysaccharides | ||||||
| CMSPB80-1 | 103 kDa | Alkali extraction | HPGPC, FT–IR, methylation analysis, GC–MS, NMR | fructus | - | [61] |
| CMSPW90-1 | 18.8 kDa | Hot water | HPGPC, FT–IR, methylation analysis, GC–MS, NMR | pulp | - | [62] |
| CMSPW90-M1 | 75.4 kDa | Hot water | HPGPC, FT–IR, methylation analysis, GC–MS, NMR | pulp | - | [62] |
| CMSPA90-1 | 17.6 kDa | Acid extraction | HPGPC, FT–IR, methylation analysis, GC–MS, NMR | fructus | 97.77% ± 1.4% (w/w) DW | [63] |
| FCp-1, FCp-2, FCp-3, and FCp-4 | 113.9, 32.6, 140.3, and 177.1 kDa respectively |
Hot water | acid hydrolysis, methylation, IR, GC–MS, and NMR | fructus | - | [64] |
| CM-1 and CM-2 | 21.520 kDa, 22.303 kDa respectively |
Hot water | Monosaccharide composition, linkage, and NMR | fructus | - | [65] |
| K-CLMP | 3.76 × 103 kDa | Hot water | methylation analysis, NMR | fructus | 5.81% | [66] |
| Crude polysaccharides (FCPs) | - | Hot water | FT–IR | fructus | 3.19 ± 0.10% | [67] |
3.4. Biological Activity
After establishing the large presence of bioactive compounds, it is necessary to investigate the capacities of specific compounds or extracts obtained by different extraction methods and different parts of C. medica to achieve a defined biological effect. Antioxidant and antimicrobial activities have been widely studied, particularly analgesic, anti-inflammatory, and hypoglycemic activities. All these activities are reported in the Table 8.
Table 8.
Biological activities of C. medica L.
| Test/Model | Concentration/Dosage Tested/Results | Part of the Plant | Extraction Method | Reference |
|---|---|---|---|---|
| Antioxidant activity | ||||
| ABTS | RSA 87.94% 0.2 mg/mL | fructus | CPE | [41] |
| DPPH | RSA 89.86% at 0.8 mg/mL | |||
| ORAC | 928.64 µmol TE/g | |||
| DPPH | 112.18 μg ascorbic acid/ mL | fructus | Soxhlet | [42] |
| NO | 112.18 μg ascorbic acid/ mL | |||
| TPC | 177.50 ± 4.95 mgGAE/g | |||
| TFC | 165.52 ± 0.65 mgQUE/g | |||
| TPC | 227.45 ± 1.04 mg GAE/100 g FW | peel | MAE | [17] |
| 88.76 ± 1.38 mg GAE/100 g FW | pulp | |||
| DPPH | 22.79 ± 0.12 IC50 μg GAE/mL | peel | ||
| 22.79 ± 0.12 IC50 μg GAE/mL | pulp | |||
| ABTS | 214.81 ± 1.45 mg TE/100 g FW | peel | ||
| 71.53 ± 0.84 mg TE/100 g FW | pulp | |||
| DPPH | EC50 827.26 µg/mL | peel | Maceration | [20] |
| TPC | 66.36 μg GAE/mg | peel | ||
| 51.21 μg GAE/mg | pulp | |||
| TFC | 40.17μg cathecol equivalent/mg | peel | ||
| 37.9μg cathecol equivalent/mg | pulp | |||
| DPPH | 0.80 ± 0.07 (IC50 mg/mL) | peel | Maceration | [33] |
| ABTS | 3.48 ± 1.0 (IC50 mg/mL) | |||
| BCB | 0.23 ± 0.002 (IC50 mg/mL) | |||
| FRAP | 3.9 ± 0.5 P (µm Fe (II)/g) | |||
| DPPH | 147 ± 1.23 IC50 µg/mL | peel | Maceration | [50] |
| BCB | 3 ± 0.05 IC50 µg/mL at 30 min | |||
| Bovine brain peroxidation assay | 2472 ± 4.19 IC50 µg/mL | |||
| DPPH | 72.00 ± 0.82% scavenging activity | juice | Maceration | [3] |
| TPC | 309.08 ± 3.06 mgGAE/g | |||
| DPPH | EC50 102.9 µg/mL | leaves | Maceration | [38] |
| TPC | 398.0 ± 3.2 mg/100 g FW | flowers | Exhaustive maceration | [27] |
| 401.6 ± 5.1 mg/100 g FW | leaves | |||
| 181.3 ± 3.1 mg/100 g FW | immature mesocarp | |||
| 262.6 ± 3.7 mg/100 g FW | immature endocarp | |||
| 123.1 ± 6.5 mg/100 g FW | mature mesocarp | |||
| 109.4 ± 2.9 mg/100 g FW | mature endocarp | |||
| TFC | 266.9 ± 7.2 mg QUE/100 g FW | flowers | ||
| 97.5 ± 2.8 mg QUE/100 g FW | leaves | |||
| 95.7 ± 3.2 mg QUE/100 g FW | immature mesocarp | |||
| 64.9 ± 3.2 mg QUE/100 g FW | immature endocarp | |||
| 43.1 ± 1.2 mg QUE/100 g FW | mature mesocarp | |||
| 37.5 ± 1.6 mg QUE/100 g FW | mature endocarp | |||
| DPPH | 425.0 ± 2.95 µg Ascorbic acid/mL | flowers | ||
| 502.0 ± 3.01 µg Ascorbic acid/mL | leaves | |||
| 382.0 ± 2.45 µg Ascorbic acid/mL | immature mesocarp | |||
| >1000 µg Ascorbic acid/mL | immature endocarp | |||
| >1000 µg Ascorbic acid/mL | mature mesocarp | |||
| >1000 µg Ascorbic acid/mL | mature endocarp | |||
| BCB | 2.8 ± 0.002 µg/mL at 30 min | flowers | ||
| >100 µg/mL at 30 min | leaves | |||
| 3.7 ± 0.007 µg/mL at 30 min | immature mesocarp | |||
| 4.1 ± 0.009 µg/mL at 30 min | immature endocarp | |||
| 36.6 ± 0.075 µg/mL at 30 min | mature mesocarp | |||
| 3.5 ± 0.008 µg/mL at 30 min | mature endocarp | |||
| TPC | 227.45 mg GAE/100 g FW | peel | Maceration 70% MeOH | [26] |
| 88.76 mg GAE/100 g FW | pulp | |||
| DPPH | IC50 22.79 μg GAE/ml | peel | ||
| IC50 54.74 μg GAE/mL | pulp | |||
| TPC | 2.52 ± 0.07 mg GAE/g | exocarp | UAE | [54] |
| 1.74 ± 0.02 mg GAE/g | mesocarp | |||
| TFC | 2.20 ± 0.26 mg QE/g | exocarp | ||
| 1.50 ± 0.06 mg QE/g | mesocarp | |||
| ABTS | 55.8 ± 5.4% RSA | exocarp | ||
| 52.0 ± 0.4% RSA | mesocarp | |||
| 54.1 ± 0.2% RSA | EO | Hydro-distillation | ||
| 3.1 ± 0.2% RSA | Hy | |||
| DPPH | 55.7 ± 1.20% RSA | exocarp | UAE | |
| 46.7 ± 0.82% RSA | mesocarp | |||
| 26.4 ± 0.74% RSA | EO | Hydro-distillation | ||
| 2.5 ± 0.3% RSA | Hy | |||
| DPPH | 77.2% RSA | EO | Hydro-distillation | [46] |
| TPC | 2.74 ± 1.12 mg GAE/g | fructus | UAE | [35] |
| TFC | 2.41 ± 2.03 mg QUE/g | fructus | ||
| DPPH | 1.48 ± 1.82 TE mM/g | fructus | ||
| ABTS | 0.92 ± 2.08 TE mM/g | fructus | ||
| FRAP | 0.38 ± 1.98 FeII mM/g | fructus | ||
| TPC | 31.60 ± 0.35 mg GAE/g | fructus | Maceration and UAE | [15] |
| TFC | 15.38 ± 0.02 mg RE/g | |||
| DPPH | EC50 78.00% μg/mL | |||
| DPPH | 47.45% (3.2 mg/mL) | fructus | Maceration in 95% ethanol and 0.3 mol/L of NaOH solution overnight | [61] |
| ABTS | 49.58% (3.2 mg/mL) | |||
| DPPH | 90.24% at 1.0 mg/mL | fructus | CPE | [41] |
| ORAC | 928.64 µmol TE/g | |||
| Hydroxyl RSA | 81.5% at 0.8 mg/mL | fructus | Digestion | [45] |
| Superoxide anion radical scavenging activity | 7.7 to 73.5% at 0.05 to 0.8 mg/mL | |||
| TPC | 25.8 ± 2.8 mg GAE/g of DW | by-products | Maceration 96% EtOH | [68] |
| DPPH | 43.8 ± 0.3% | |||
| Antimicrobial, antiviral and antifungal activity | ||||
| MTT | 95% inhibition at 0.5 µg/µL on Madin Darby canine kidney (MDCK) cell line with Avian influenza A virus (H5N1 | EO | Hydro-distillation | [69] |
| Agar diffusion assay | MIC: C. albicans 3 µg/mL, B. subtilis 25 µg/mL, K. pneumonia 25 µg/mL | fructus | Hydro-distillation | [41] |
| Inibition zone (mm): B. subtilis 13, B. cereus 21, S. aureus 12, K. pneumonia 15, C. albicans 27, A. niger 11 | leaves | |||
| Plaque reduction assay | 50% at 0.504 µg/µl | fructus | ||
| 95% at 0.5 µg/µl | leaves | |||
| Plate count analysis | Saccharomyces cerevisiae: 3 min at 500 ppm | fructus | Hydro-distillation | [44] |
| Plate count analysis | Bacteria survival: E. coli (600 ppm) 1 log decrease at day 3, S. Enteritidis (600 ppm) 3 log decrease at day 3, L. monocytogenes (600 ppm) 4 log decrease at day 3 | fructus | Hydro-distillation | [70] |
| Disc diffusion test | Inibition zone (mm): mold growth on bread from 8.54 ± 1.27 mm to 15.26 ± 2.16 mm | flower and fructus | Hydro-distillation | [71] |
| Inibition zone (mm): mold growth on bread > 90 mm | leaves | Hydro-distillation | ||
| Agar diffusion assay | MIC (µL/mL): Lactobacillus curvatus, Weissella viridescens, Leuconostoc mesenteroides, Enterococcus faecium, Lactobacillus reuteri, Lactobacillus dextrinicus, Lactobacillus sakei, and Pediococcus dextrinicus from 7.33 ± 0.57 to 9.00 ± 0.00 | fructus | Hydro-distillation | [72] |
| Agar diffusion assay | MIC (mg/mL): Gram-positive from 0.625 to 1.25; Gram-negative bacteria 2.5 | fructus | Hydro-distillation | [73] |
| Plate count analysis | MIC (mg/mL): Gluconobacter cerinus, Dekkera bruxellensis, Candida zemplinina, Hanseniaspora uvarum, Pichia guilliermondii, and Zygosaccharomyces bailii from 530 to 4240 | fructus | Hydro-distillation | [74] |
| Oxford cup method | MIC (mg/mL): Fusarium oxysporum 9.38, Fusarium solani 12.05, and Cylindrocarpon destructans 8.44 | fructus | Hydro-distillation | [75] |
| Plate count analysis | Yersinia enterocolitica O9, Proteus spp., Klebsiella pneumoniae, and E. coli: not effective | aerial parts | Hydro-distillation | [76] |
| Agar diffusion assay | MIC (mg/L): Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Listeria monocytogenes, Salmonella Enteritidis, Salmonella Typhimurium, Pseudomonas fragi, Saccharomyces cerevisiae, and Aspergillus niger < 2000 | fructus | Hydro-distillation | [77] |
| Agar diffusion assay | MIC (v/v%): Escherichia coli, Pseudomonas Aeruginosa, Salmonella paratyphi B, Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis, Candida albicans, and Aspergillus flavus from 1 and 4% v/v | peel | Hydro-distillation | [46] |
| Biofilm formation | Inhibition of biofilm formation (%): Staphylococcus aureus 100% at 0.75 mg/mL | fructus | Ultrasonic/microwave assisted hydro-distillation | [78] |
| Inhibition of biofilm formation (%): Staphylococcus aureus 83l% at 0.75 mg/mL | Maceration | |||
| Agar diffusion assay | Gold fingered citron MIC (mg/mL): Bacillus subtilis, Streptococcus pneumoniae, Enterococcus faecalis, Escherichia coli, and Staphylococcus aureus from 0.00 to 10.82 ± 02 | fructus | ultrasonic | [79] |
| Cantonese fingered citron MIC (mg/mL): Bacillus subtilis, Streptococcus pneumoniae, Enterococcus faecalis, Escherichia coli, and Staphylococcus aureus from 0.00 to 9.81 ± 0.20 | ||||
| Sichuan fingered citron MIC (mg/mL): Bacillus subtilis, Streptococcus pneumoniae, Enterococcus faecalis, Escherichia coli, and Staphylococcus aureus from 0.00 to 10.83 ± 0.24 | ||||
| Agar diffusion assay | Sulphur nanoparticles MIC (µg/mL): Listeria monocytogenes, Salmonella typhi, Chromobacterium violaceum, Fusarium oxysporum, and Aspergillus flavus from 250 ± 1.21 to 700 ± 1.88 | leaves | Hydro-distillation | [80] |
| Aluminium oxide nanoparticles MIC (µg/mL): Listeria monocytogenes, Salmonella typhi, Chromobacterium violaceum, Fusarium oxysporum, and Aspergillus flavus from 150 ± 2.77 to 1000 ± 1.1 | ||||
| Tetrazolium microplate Assay | Nanoemulsions MIC (μL/mL): Escherichia coli, Bacillus subtilis, and Staphylococcus aureus from 0.16 to >2.5 | commercial EO | Commercial EO | [81] |
| Mycelial growth assay | Nanoemulsions mycelial growth inhibition (%): Penicillium citrinum and Aspergillus niger from 3.6 ± 0.6 to 27.0 ± 1.1 | |||
| Agar diffusion assay | ZnO nanoparticles inhibition zone (mm): Streptomyces sannanesis, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella enterica, Candida albicans, and Aspergillus niger from 22 to 25 | peel | Maceration | [82] |
| Agar diffusion assay | Ethyl acetate and ethanolic extract MIC (mg/mL): Staphylococcus auricularis not active, Streptococcus mitis not active, Streptococcus pneumoniae not active, Klebsiella pneumoniae, and Escherichia coli from 12.5 to 25. | peel | Reflex extraction | [83] |
| MIC (mg/mL): Staphylococcus auricularis, Streptococcus mitis, Streptococcus pneumoniae, and Klebsiella pneumoniae from 1.5625 to 6.25; Escherichia coli not active | juice | Hand squeezing | ||
| Agar diffusion assay | Zone of inhibition (mm): Bacillus subtilis, Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris, Aspergillus flavus, A. niger, and Candida albicans from 0 to 23 | root, leaf, bark, peel, pulp | Maceration | [84] |
| juice | Hand squeezing | |||
| Agar diffusion assay | Bacillus subtilis, Staphylococcus aureus, and Escherichia coli; Klebsiella pneumonia not active | juice | Hand squeezing | [85] |
| Pour plate | (At 2.0 mg/mL) MIC: P. aeruginosa 82.8%, S. aureus 100% | fructus | Maceration | [21] |
| Microtiter-plate | E. coli, L. monocytegenes, P. carotovorum, Ps. aeruginosa, and S. aureus MIC (mg/mL): 7–10 | peel | Maceration | [34] |
| pulp | ||||
| Adherence and invasion in HeLA cells | Adherence (50.8 to 91%), invasion (85.1 to 94.8%) in C. jejuni strain | by-products | Maceration | [68] |
| Motility | Inhibition C. jejuni: 35–50% | peles, seeds, bagasse | Maceration | [86] |
| Biofilm formation | Inhibition C. jejuni: 60–75%, | |||
| Disk diffusion | E. coli, K. pneumoniae, P. aeruginosa, Propionibacterium acnes, Salmonella typhi, and fungi Fusarium culmorum, F. oxysporum, and F. graminearum; zone of inhibition:10–30 mm | fructus | Squeezing | [87] |
| Crystal violet staining | MIC = 1.25% (v/v) |
fructus | Carbon-quantum-dots synthesis | [88] |
| Cytotoxic activity | ||||
| MTT | Growth inhibition 56.5 ± 3.6% 50 µg/mL | peel | Hydro-distillation | [89] |
| Growth inhibition 30.2 ± 2.2% and 73.3 ± 2.6% cell death at 25 and 50 μg/mL | [90] | |||
| EC50 1.24 ± 0.42 EO EC50 2.97 ± 0.07 Hy | fructus | [54] | ||
| EC50 1.76 ± 0.32 | exocarp | Maceration and UAE | [54] | |
| 50% growth inhibition at 7.80 and 9.50 μmol/L | peel | Semisynthesis | [91] | |
| IC50 from 60.5 to 80.0 μM | bark | Maceration | [37] | |
| Anti-inflammatory and analgesic activity | ||||
| NO | 56% after 12 h, 83% after 24 h at 0.063 mg/mL | fructus | Hydro-distillation | [77] |
| IC50 17.0 mg/mL | peel | [92] | ||
| 10.87–82.77% at 500 mg (60–300 min) | [24] | |||
| 250–500 mg at 30–120 min | ||||
| Clinical study | > than placebo in reduction in headache-attack intensity | juice | Syrup | [10] |
| Hypoglycemic activity | ||||
| α-amylase | IC50 625 ± 8.53 µg/mL | peel | Maceration | [50] |
| >1000 IC50 µg/mL | flowers | [27] | ||
| 438.5 ± 5.2 IC50 µg/mL | leaves | |||
| 702.2 ± 5.7 IC50 µg/mL | immature mesocarp | |||
| 702.2 ± 5.7 IC50 µg/mL | immature endocarp | |||
| 707.4 ± 5.6 IC50 µg/mL | mature mesocarp | |||
| 426.0 ± 4.4 IC50 µg/mL | mature endocarp | |||
| α -glucosidase | >1000 IC50 µg/mL | flowers | ||
| 777.8 ± 5.4 IC50 µg/mL | leaves | |||
| 539.7 ± 6.4 IC50 µg/mL | immature mesocarp | |||
| 472.9 ± 4.7 IC50 µg/mL | immature endocarp | |||
| 633.1 ± 3.4 IC50 µg/mL | mature mesocarp | |||
| 574.1 ± 5.8 IC50 µg/mL | mature endocarp | |||
| Plasma glucose level | Glucose (mg/dL): from 213 (60 min) to 155 (120 min) | epicarp | Hydro-distillation | [53] |
| Glucose (mg/dL): from 228 (60 min) to 216(120 min) | pulp | |||
| From glucose level (mg/dL) 106.8 ± 5.87 to 105.2 ± 8.35 (after 1 month) at 200 mg/kg/day; from glucose level (mg/dL) 109.3 ± 5.04 to 87.4 ± 6.30 (after 1 month) at 400 mg/kg/day | leaves | Maceration | [38] | |
3.4.1. Antioxidant Activity
Antioxidants are compounds that are able to neutralize free radicals, which can damage the body’s cells [93]. The C. medica has significant antioxidant properties due to the presence of an excellent antioxidant, ascorbic acid, in addition to known phenolic compounds, flavonoids, carotenoids, and heteropolysaccharides, which contribute to antioxidant capacity in citrus fruits. Several studies have evaluated the antioxidant activities of different parts of C. medica in combination with the total contents of phenols and flavonoids, widely known as molecules with marked antioxidant activity [36,94,95,96]. In fact, Luo et al. [41] investigated the major flavonoids of finger citron, prepared by continuous phase-transition extraction (CPE), purified with AB-8 macroporous resins and then identified by UHPLC–QTOF–MS, showing good radical-scavenging activity on an in vitro test. The scavenging capacities of DPPH and ABTS were investigated from 0.1 mg/mL to 1 mg/mL using ascorbic acid as the standard. At the concentration of 1.0 mg/mL, the DPPH radical scavenging was 90.24%, and no significant differences were found from that of 0.8 mg/mL, which was 89.86%, compared with the 94.74% inhibition of ascorbic acid. At a low concentration (0.2 mg/mL), the purified extract showed a scavenging capacity of ABTS radicals equal to 87.94% compared with the ascorbic acid. Considering the wide use of the fruit in the diet, the result demonstrated with the ORAC (oxygen radical absorbance capacity) test, of 928.64 μmol TE (Trolox equivalent)/g, is of significant interest. Furthermore, Mondal et al. [42] reported a radical-scavenging potency of C. medica fruit methanol extract (IC50 112.18 μg/mL) using ascorbic acid as the standard (IC50 25.53 μg/mL). On the other hand, the IC50 values calculated for the C. medica-fruit methanol extract and ascorbic acid for the nitric oxide (NO) radical-scavenging assay were 117.38 μg/mL and 45.23 μg/mL, respectively. As reported by Mahdi et al. [15], Foshou (C. medica var. Sarcodactylis) peel showed significantly higher antioxidant properties and nutritional contents than the pulp. The aqueous extracts of Foshou peel and pulp contained 227.45 mg GAE (gallic acid equivalent)/100 g FW and 88.76 mg GAE/100 g FW of total phenolic compounds, with DPPH scavenging-activity levels of IC50 22.79 μg GAE/mL and 54.74 μg GAE/mL, respectively. The antioxidant capacities of the Foshou peel and pulp was 214.81 mg TE/100 g FW and 71.53 mg TE/100 g FW, respectively. Pallavi et al. [97] also compared the antioxidant activities of peel and pulp extracts from five varieties of C. medica: citron, sour orange, lemon, pomelo, and orange. The citron peel and pulp demonstrated the highest phenolic contents (66.36 μg GAE/mg for the peel and 51.21 μg GAE/mg for the pulp) and flavonoid contents (40.17 μg catechol/mg for the peel and 37.9 μg catechol/mg for the pulp) compared to the other varieties. Similar results were obtained for the total antioxidant content (140.17 µg ascorbic acid equivalent/mg for the peel and 116.11 µg ascorbic acid equivalent/mg for pulp) and the DPPH peel and pulp radical scavenging (EC50 (half maximal effective concentration) 827.26 µg/mL and 4089.64 µg/mL, respectively); however, the orange pulp was the least active (EC50 3628.44 µg/mL), followed by the citron pulp (EC50 4089.64 µg/mL).
The antioxidant activity of peel was extensively studied. As reported by Menichini et al. [33], hydroalcoholic peel extract inhibited both DPPH and ABTS radicals with IC50 values of 0.80 ± 0.07 and 3.48 ± 1.0 mg/mL, respectively, compared with ascorbic acid (0.002 ± 0.01 and 0.009 ± 0.0003). In the β-carotene-bleaching test, the peel extract reduced the β-carotene discoloration, exhibiting good activity (IC50 0.23 ± 0.002 mg/mL) with propyl gallate, with IC50 0.001 ± 0.0001 mg/mL. Conforti et al. also reported the activity of a peel n-hexane extract, showing an IC50 of 147 ± 1.23 µg/mL against DPPH radicals with ascorbic acid as the positive control (2.00 ± 0.03 IC50 µg/mL) and an antioxidant activity of IC50 3.00 ± 0.05 µg/mL, as evaluated by a β-carotene bleaching assay at 30 min, with propyl gallate as positive control (IC50 1 ± 0.04 µg/mL). The authors reported different results, probably due to the different extraction used.
The antioxidant activities of juice, leaves, and flowers has not been extensively studied but is equally important. Dey et al. analyzed the antioxidant power of 0.1 mL of concentrated juice from three cultivars, Diamante, Balady, and Corsican, with the following test data: 72.00 ± 0.82% for DPPH radical-scavenging activity and 309.08 ± 3.06 mg GAE/g for TPC [3]. Furthermore, the methanol-extract leaves showed antioxidant activity (EC50 102.9 µg/mL), when compared to the ascorbic acid (EC50 49.28 µg/mL) [38].
The antioxidant capacity depends on the species, cultivar, stage of maturation, pedoclimatic conditions, and other agronomic factors. Wu et al. [45] reported the antioxidant activity of EO obtained from C. medica fruits in different maturation stages. A stronger DPPH radical inhibition was reported for the EO of the fruit in the immature stage, of 78.4 ± 2.6%, compared to that in the mature stage, of 63.8 ± 2.1%. Furthermore, the EO obtained from the immature stage showed greater reducing power by converting the Fe3+/ferricyanide complex into the ferrous form than those collected during the mature stage. The differences between these results may have been due to the different chemical compositions, since even if the activity is principally attributed to the most abundant compounds, the antagonistic effect of a compound present in smaller amounts must be considered. Such results can also be affected by changes in humidity and temperature during different seasons. To support this, Menichini et al. [27] demonstrated that the highest total contents of phenols and flavonoids were in immature fruits, although the greatest content was found in flowers (398.0 ± 3.2 and 266.9 ± 7.2 mg/100 g, respectively), followed by leaves (401.6 ± 5.1 and 97.5 ± 2.8 mg/100 g respectively). Despite the greater content present in the flowers, the best DPPH scavenging activity was demonstrated by the mesocarp of immature fruits (IC50 of 382.0 µg/mL), followed by flower and leaf extracts, with IC50 values of 425.0 and 502.0 µg/mL, respectively. By contrast, the flowers showed the highest inhibition of linoleic acid oxidation (IC50 value of 2.8 µg/mL) after 30 min of incubation. Indeed, Taghvaeefard et al. [26] compared the antioxidant activities of flavedos from two Iranian citron fruits, proving that the antioxidant activity was higher in the large citron, cv. macrocarpa (IC50 170.142.5 mL/L), than in the small citron, cv. medica (IC50 280.125 mL/L). Although the total contents of phenols were comparable, and the content of flavonoids in the small citron was twice that in the large citron, this confirmed the different compound contents in the different Citrus varieties and the synergistic activities of some compounds. These differences could be attributed to the different chemical compositions of the same Citrus species in different regions. Many studies have also investigated the antioxidant activity of EO. Vitalini et al. reported good radical-scavenging activity against the ABTS of EO and methanol exocarp and mesocarp extracts of 54.1 ± 0.2%, 55.8 ± 5.4%, and 52.0 ± 0.4%, respectively. The methanolic exocarp and mesocarp extracts also showed good activity towards the stable DPPH radical, reporting a percentage of inhibition of 55.7 ± 1.20% for the exocarp extract and of 46.7 ± 0.82% for that of the mesocarp. These activities can be associated with the presence of compounds such as flavonoids and polyphenols. In fact, the authors reported a total exocarp-extract polyphenol content of 2.52 ± 0.07 mg GAE/g in the fruit part compared to 1.74 ± 0.02 mg GAE/g in the fruit part of the mesocarp, along with a total flavonoid content of 2.20 ± 0.26 mg QE/g in the fruit part of the exocarp versus 1.50 ± 0.06 mg QE/g in the fruit part of the mesocarp extract [54].
On the other hand, the highest antioxidant activity of the EO from C. medica var. macrocarpa Risso was measured by Ghani et al. at the over-ripe stage (76.08% ± 0.51% radical-scavenging activity), which was probably due to specific compounds of a different variety [98]. In agreement with Guo et al., the EO of Citrus medica cv. Sarcodactylis showed the highest antioxidant activities, of 77.2%, in a DPPH assay [47].
Overall, antioxidant bioactive compounds are often confirmed by in vitro assays. However, they are characterized by poor absorption through biological barriers. To enhance their beneficial effects and to overcome this limitation, Zhao et al. [35] developed Ca–alginate microbeads with the polysaccharide-filler-controlled delivery of phenolic compounds. A C. medica-fruit extract was analyzed for the total phenolic (31.60 ± 0.35 mg GAE/g) and flavonoid (15.38 ± 0.02 mg RE (rutin equivalent)/g) contents. In addition, phenolic compounds entrapped in microbeads were identified using UHPLC–DAD–QTOF–IMS after in vitro digestion. The quantification results showed that the alginate extract and pectin filler (APE) had the highest amounts of phenolics, particularly hesperidin (264.11 mg/100 g), tangeretin (29.67 mg/100 g), and nobiletin (127.14 mg/100 g) [99]. Recently, Peng et al. [61] extracted a crude polysaccharide from the residues of C. medica var. Sarcodactylis (CMSPB80-1). The CMSPB80-1 exhibited the highest DPPH radical-scavenging rate, of 47.45% (3.2 mg/mL), while the highest ABTS radical-scavenging rate was 49.58% (3.2 mg/mL), within a concentration range of stable free radicals from 0.05 to 3.2 mg/mL. Similarly, Luo et al. [66] isolated from fresh fruits of C. medica var. Sarcodactyilis a new type of galactorhamnan, named K-CMLP (Citrus medica polysaccharide), consisting of rhamnose, galactose, and glucose, which exhibited free-radical scavenging (IC50 2.5520 mg/mL) that was lower than those other extracts obtained from whole fruit, according to a DPPH test,. Previously, Wu et al. [67], showed an increase in O2•− scavenging ability in citron-fruit polysaccharides, from 7.7% to 73.5%, when the concentration increased from 0.05 mg/mL to 0.80 mg/mL. Additionally, Wu et al. [100] studied three drying methods, freeze drying, hot-air drying, and vacuum drying, to enhance the physicochemical and antioxidant properties of finger-citron polysaccharides. The results showed that the maximum yield (88.7% radical-scavenging activity) was obtained by freeze-drying, suggesting that it may be possible to obtain a novel polysaccharide with strong radical-scavenging capacity through the optimization of freeze-drying parameters. These studies demonstrate that antioxidant activity is linked not only to phenolic compounds and their derivatives, but also to heteropolysaccharide molecules, and represents a reuse of waste resulting from fruit processing. On this basis et al. [68] investigated the total phenolic content of Citrus-aurantium-L.-, C.-limon-, and C.-medica-by-product extracts, reporting values of 92.0 ± 4.8, 41.7 ± 13.1, and 25.8 ± 2.8 mg GAE/g of DW, respectively. They also evaluated the total flavonoid contents (TFCs) of citrus-by-product extracts, reporting the highest content for C. aurantium (161.09 ± 0.2 mg QE (quercetin equivalent)/g), followed by C. limon and C. medica (63.97 ± 0.3 and 29.29 ± 5.6 mg QE/g, respectively). In addition, the antioxidant capacities of citrus by-product extracts were investigated: the highest DPPH radical-scavenging activity was detected for the by-product of the extract of C. aurantium (90.1 ± 0.6%), followed by those of the extracts of C. limon (44.6 ± 1.2%) and C. medica (43.8 ± 0.3%), according to the total-phenolic- and flavonoid-content results.
The ability of C. medica to act on intracellular ROS concentrations was investigated in human epidermal keratinocytes (HaCaT) stimulated by H2O2. The extract obtained from the whole fruit significantly decreased the intracellular ROS compared to the positive control, probably acting on the endogenous antioxidant defenses. In fact, as shown by the authors, the superoxide dismutase (SOD) and catalase (CAT) enzymes were upregulated by the extract [101].
3.4.2. Antibacterial, Antiviral, and Antifungal Activity
Bacteria may develop resistance to conventional drugs; therefore, there is an increasing need to find new antimicrobial agents. Medicinal plants are rich sources of bioactive compounds which also display antimicrobial activities [102]. Among the many biological properties of C. medica, its antimicrobial properties are undoubtedly the most heavily investigated, probably due to the strong presence of phytochemicals, such as citral, linalool, and limonene [103,104,105], which are endowed with antimicrobial effects. Most of the relevant studies concern citron’s antibacterial activities, but some researchers have also focused their attention on the antiviral properties of this species. For example, C. medica var. Sarcodactylis EO was screened for its antiviral activity by El Hawary et al. [69]. The investigation was carried out by incubating the Madin Darby canine kidney (MDCK) cell line with Avian influenza A virus (H5N1). At a concentration of 0.5 µg/µL, the EO from leaves showed 95% inhibition, while the inhibition by the fruit EO was lower (50%). According to previous studies on the antiviral activity against H5N1, limonene, which is present in high quantities in the citron EO, seems to be one of the main factors responsible for this activity [104]. Many researchers investigated the ability of C. medica EO to reduce food spoilage. Belletti et al. [44] investigated the effect of citron EO, citral, and (E)-2-hexenal on the spoilage of noncarbonated beverages inoculated with different amounts of a S. cerevisiae strain combined with a mild heat treatment (55 °C). They found that the citron EO had the highest capacity to prevent yeast growth in terms of time, which was probably due to the presence of other molecules with synergistic actions, such as β-pinene and limonene. The authors investigated the antimicrobial potential of Salmonella enteritidis, Escherichia coli, and Listeria monocytogenes in fruit-based salads packaged in plastic containers. Regarding noncarbonated beverages, the citron EO showed the best results, avoiding the undesirable cytotoxicity of the citral, since the shelf life of the fruit-based salads was doubled [70]. Wu et al. [71] demonstrated, in a disc-diffusion test, that the fungal-inhibitory activity of EO depends on the contents of the compounds. Compared to the flower and fruit EOs, the leaf EO is richer in oxygenated monoterpenes, to which the antifungal activity can be attributed. The former (inhibition zone ranging from 8.54 ± 1.27 mm to 15.26 ± 2.16 mm) showed a smaller inhibition zone than the latter (inhibition zone > 90.00 mm) against mold growth on Chinese steamed bread. The effect of the EO obtained from C. medica fresh finger fruits on lactic acid bacteria isolated from vacuum-packed cooked and cured sausages was assessed by Khorsandi et al. The results were encouraging because the EO was able to act against the bacteria by reducing the spoilage of the food [72]. The effects of finger-citron EO on food-borne bacteria (E. coli, St. aureus, Bacillus subtilis, and Micrococcus luteus) were investigated by Li et al. [73]. They demonstrated a stronger effect of the extract on Gram-positive (minimum inhibitory concentrations (MIC) ranging from 0.625 to 1.25 mg/mL) than on Gram-negative bacteria (MIC 2.5 mg/mL). The C. medica EO can also be applied in the wine industry; Mitropoulou et al. [74] demonstrated that the spoilage and microbial growth of wine (inoculated with Gluconobacter cerinus, Oenococcus oeni, Pediococcus pentosaceus, Dekkera bruxellensis, Candida zemplinina, Hanseniaspora uvarum, Pichia guilliermondii, or Zygosaccharomyces bailii) was considerably delayed after treatment with EO (18 days, compared to the 9 days used for the control). The MIC of the C. medica EO on Panax notoginseng Burkill root fungi (Fusarium oxysporum, Fusarium solani, and Cylindrocarpon destructans) were 9.38 mg/mL, 12.05 mg/mL, and 8.44 mg/mL, respectively; these values were not significantly higher than those of the positive control, hymexazol (MIC values 0.12 mg/mL, 0.16 mg/mL and 0.18 mg/mL) [75]. The EO from the aerial parts of C. medica was not effective on Yersinia enterocolitica O9, Proteus spp., Klebsiella pneumoniae, or E. coli, as demonstrated by Al-mariri and Safi [76]. Mitropoulou et al. [77] analyzed the EO phytochemical composition, and limonene was the main constituent identified by SPME GC/MS (88%) and GC/MS (64%). The antimicrobial effects of limonene and those of the whole phytocomplex on several bacteria and fungi were compared: the MIC values were significantly (p < 0.05) lower for the C. medica EO (<2000 mg/L) than for the limonene (>5000 mg/L), probably due to the synergistic effects of other components. The EO from C. medica var. Sarcodactylis Swingle, cultivated in China, showed strong antimicrobial activities against the bacteria and fungi tested (MIC ranging from 1 and 4% v/v), probably due to the strong presence of linalool, which showed lower MIC (0.125–0.5% v/v) compared to the other compounds identified [46]. Zang et al. [78] compared the effect of the extraction technique on the antimicrobial activity of the EO from C. medica var. Sarcodactyilis. They found an increased inhibition of biofilm formation from S. aureus in the ultrasonic/microwave-assisted hydro-distillation and hydro-distillation extraction (100% of inhibition at 0.75 mg/mL) compared to the solvent extraction (83% at the same concentration). This difference was attributed by the authors to the high presence of volatile components in the hydrodistilled extracts.
Three kinds of fingered citron (Citrus medica L. var. Sarcodactylis Swingle) EO showed different antimicrobial activities: the Gold, Cantonese, and Sichuan fingered citron EOs showed the strongest antibacterial activity on S. aureus, E. faecalis, and E. coli, respectively [79]. The Eos were nano-functionalized by inclusion in sulphur- and aluminum-oxide nanoparticles, showing the highest inhibitory-activity levels on Salmonella typhi (21 mm) and F. oxysporum, respectively. A good increase in growth inhibition was observed for EO in combination with antibiotics [80]. The nanoemulsion of the EO from C. medica L. var. Sarcodactylis was developed by Li et al., who demonstrated the increased antibacterial activity of an extract against E. coli, B. sublitis, and S. aureus. The same was not observed with fungi, whose growth was reduced more by the free EO than the nanoemulsion [81].
Citron-peel extracts obtained with different solvents (acetone, DMSO, methanol, petroleum ether) were tested for their antibacterial capabilities on several pathogenic microorganisms, which were either fungi or bacteria. Overall, the solvent that made it possible to obtain the extract with the strongest antimicrobial activity was the DMSO, since a larger inhibition zone than that of the control (Gentamycin for bacteria and Ketoconazole for fungi) was observed [105]. Ethyl acetate and ethanol 80% peel extracts exhibited larger inhibition zones (10 mm and 22 mm, respectively) at 100 mg/mL compared to juice extracts, against which the bacterium showed complete resistance [83]. A peel extract of the C. medica variety grown in the Kumaun region in Uttarakhand, India was tested for its antimicrobial capacity against P. aeruginosa without any results, while the pulp and juice extracts from the same variety were active against this bacterium. The researchers also tested root, leaf, and bark extracts on several bacterial strains. The extracts that showed the strongest activities were the root and juice extracts with, 19-nn and 17-mm inhibition zones, respectively, which even higher than that of the standard drug (chloramphenicol, 14 mm) [84]. In contrast, Sharma et al. stated that a C. medica-juice extract had no effect on the growth of any of the bacteria they tested (B. subtilis, S. aureus, Es. Coli, and K. pneumoniae), while it was active against fungi (Aspergillus niger and C. albicans) [85]. In 2015, Shende et al. synthetized copper nanoparticles containing the juice of C. medica collected in Amravati, Maharashtra, and India. They demonstrated that the encapsulation of the extract in nanoparticles strongly increased the inhibitory activity of the C. medica against the tested bacteria, E. coli, K. pneumoniae, P. aeruginosa, Propionibacterium acnes, and Salmonella typhi, and fungi, Fusarium culmorum, F. oxysporum, and F. graminearum [87]. Castillo et al. assessed the antimicrobial activity of Campylobacter jejuni from C. medica by-products (peel, seeds, and bagasse). The extract reduced the swarm motility (35–40%) and biofilm formation (60–75%). Quorum sensing was performed by measuring the Autoinducer-2 activity, which was reduced by about 90% by the extract [86]. The study continued with the analysis of the effects of C. medica by-products on C. jejuni adherence (which was from reduced by 50.8% to 91%) and invasion (reduced by 85.1% to 94.8%) to human tumor cells (HeLa), while the gene expression of adhesion (cadF) and invasion (ciaB) molecules was significantly (p ≤ 0.05) reduced [68]. Peel and pulp extracts of C. medica cv. medica and C. medica cv. Salò were found to exert antibacterial activities against E. coli, L. monocytogenes, P. aeruginosa, S. aureus, and Pectobacterium carotovorum (due to the high contents of phenolic acids and flavonoids), with a strong capacity to inhibit biofilm formation, especially on L. monocytogenes [34]. Keerthana et al. demonstrated that the use of nanoparticles could be a good strategy to enhance the antimicrobial activity of an extract. In fact, the inclusion of C. medica-peel extract in ZnO nanoparticles increased the sensitivity of the bacterial strains, with the largest inhibition zone against S. sannanesis (25 mm), followed by B. subtilis (24 mm), Pseudomonas aeruginosa (23 mm), and Salmonella enterica (22 mm) [82]. Researchers attributed the strong bactericidal capacity of ZnO nanoparticles to their production of reactive oxygen species (ROS) in water suspensions [106]. Nanomaterials were obtained from C. medica by Selvaraju et al. Specifically, starting with the fruit extract, they obtained carbon quantum dots (CQDs) and tested their capacity to act on the pathogen P. aeruginosa. As shown by the crystal violet staining assay, the CQDs inhibited the growth of the bacteria with a MIC of 1.25% (v/v) [88]. A cyclic peptide previously isolated from the fruit peel of C. medica var. Sarcodactylis Swingle was synthetized by Dahiya and Kumar [91]. They evaluated the antimicrobial activity of the new peptide against the Gram-positive bacteria B. subtilis and Staphylococcus aureus, as well as the Gram-negative bacteria P. aeruginosa and E. coli, in comparison to the standard drug, ciprofloxacin. The peptide was active only against P. aeruginosa, showing a similar MIC value to the ciprofloxacin (6 μg/mL), with an inhibition zone 28 mm in diameter, compared to the 25 mm displayed by the reference drug. In addition, the peptide was also active against Candida albicans, with a 22-mm-diameter inhibition zone, compared to the 20 mm displayed by the griseofulvin. Chromatographic fractionation and the enrichment of phenolic components from C. medica var. Sarcodactylis fruit strongly increased the antibacterial and antibiofilm capacity of the species against S. aureus, with a 100% inhibition of biofilm formation at 2.0 mg/mL [21]. A C. medica var. Sarcodactylis exocarp ethanol extract showed stronger inhibition against Bacillus cereus (MIC 2.5 mg/mL) than against E. coli (MIC 10 mg/mL). The higher content of coumarin may explain the stronger antimicrobial activity of the exocarp extract [7].
3.4.3. Cytotoxic Activity
As stated by the World Health Organization, together with cardiovascular diseases, cancer is an important cause of mortality worldwide, which, according to recent trends, could even rise above heart diseases as the leading cause of death [107]. Despite the great therapeutic advances in traditional cancer therapies, they feature several disadvantages, such as systemic toxicity, drug resistance, and side effects [108]. Natural products have been found to possess antitumor and tumor-preventive properties. Citrus species, including C. medica, are traditionally used for anticancer applications [109].
Nair et al. used Dalton’s lymphoma ascites (DLA) cells to evaluate the toxicity of C. medica peel-oil and peel-water extracts. The peel-oil extract induced 30.2 ± 2.2% and 73.3 ± 2.6% cell death at 25 µg/mL and 50 µg/mL, respectively, while the C. medica peel-water extract was less toxic, showing 56.5 ± 3.6% inhibition at 50 µg/mL [90].
The cytotoxic effects of the EOs extracted from two different cultivars of C. medica (cv. ‘liscia’ and cv. ‘rugosa’) grown in the Campania region, Italy, and limonene, as the major component (67.2% for C. medica cv. liscia and 62.8% for C. medica cv. rugosa), was evaluated on a human neuroblastoma cell line (SH-SY5Y). The limonene and C. medica cv. ‘rugosa’ EO showed an IC50 > 2000 µg/mL, while the C. medica cv. ‘liscia’ EO showed an IC50 of 718.2 µg/mL [110]. An exocarp ethanol extract from C. medica var. Sarcodactylis was more toxic (EC50 1.76 mg/mL) than a mesocarp extract (EC50 not attained) in a HL60 leukemia cell line [54]. This might be explained by the strong presence of coumarins, which have been reported to have good anticancer activity [111].
Dahiya and Kumar [91] synthesized a cyclic peptide, sarcodactylamide, previously isolated from the fruit peel of C. medica var. Sarcodactylis Swingle. The synthesis was carried out by coupling two tetrapeptide units (Boc–Leu–Pro–Trp–Leu–OMe and Boc–Ile–Ala–Ala–Gly–OMe) after the deprotection of carboxyl and amino terminals and the cyclization of the linear octapeptide segment. The authors proved that the new peptide reduced the proliferation of DLA and Ehrlich’s ascites carcinoma (EAC) cell lines, which were previously injected into the peritoneal cavities of healthy albino mice. A 50% growth inhibition was obtained at 7.80 μmol/L and 9.50 μmol/L, respectively, for the DLA and EAC cells, which was lower than that of the positive control, 5-fluorouracil (37.36 and 90.55 μmol/L).
A new prenylated acridone alkaloid, medicacridone, and a new ferulate xanthone, medicaxanthone, were identified for the first time in the methanol extract of a C. medica bark collected in Cameroon. The newly isolated compounds and the already known compounds, citracridone, 5-hydroxynoracronycine, citracridone-III, lichenxanthone, lichenxanthone, and atalantoflavone, were tested for their cytotoxic activity against the human prostate adenocarcinoma cell line PC-3, showing weak activity (IC50 from 60.5 to 80.0 μM) compared to that of the positive control, Doxorubicin (IC50 0.9 μM) [37].
3.4.4. Anti-Inflammatory and Analgesic Activity
The citron C. medica is rich in flavonoids, such as hesperidin, naringin, and apigenin, which possess strong anti-inflammatory activities [112]. The effect of the C. medica EO on nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated macrophages was tested by Mitropoulou et al. [77], who obtained rates of inhibition of NO production of 56% after 12 h and of 83% after 24 h at 0.063 mg/mL of EO. No significant inhibition of NO production was observed at the lowest concentration tested (50% after 12 h, and 80% after 24 h at 0.018 mg/mL). The EO obtained from the C. medica cv. Diamante peel, was instead able to reduce NO production in LPS-stimulated macrophages and possessed anti-inflammatory activity, with an IC50 value of 17.0 mg/mL, compared to indomethacin, which was used as a positive control (IC50 of 53.0 mg/mL) [92]. Flower and leaf extracts of the same cultivar showed an inhibitory effect on LPS-induced NO production in a macrophage RAW 264.7 cell line in a dose-dependent manner, although the IC50 levels were notably high (525.0 mg/mL and 574.0 mg/mL, respectively) [27]. The EO from fingered citron (C. medica L. var. Sarcodactylis) was able to reduce the production of the inflammatory cytokines tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6) in LPS-activated macrophages (RAW 264.7 cells) at 40% to 80% at the highest concentration (0.02%). The extract prevented the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-kB) activation by inhibiting the nuclear factor of kappa light polypeptide gene enhancer in B-cell inhibitor, alpha (IkB-α) phosphorylation. Furthermore, the levels of phosphorylated mitogen-activated protein kinases (MAPKs: c-Jun NH2-terminal kinase, JNK, and extracellular-signal-regulated kinase, ERK) were significantly decreased after the pre-treatment of the LPS-stimulated cells [27]. Similar activity was shown by the fruit extract in HaCat cells stimulated by LPS [101]. A C. medica. var. Sarcodactylis Swingle fruit was included in a formulation of ten herbs, which was tested against systemic lupus erythematosus (SLE). The authors found that the formulation can inhibit the membrane-bound B-cell activating factor (mBAFF)-induced upregulation of BAFF and its receptor, BAFF-R, Bcl-2, interleukin 10 (IL-10), and NF-κB, in YAC-1 cells (isolated rat peripheral-blood lymphocytes); thus, it can be administrated in combination with glucocorticoids in order to reduce toxicity and to improve efficacy [100]. An in vivo application of the anti-inflammatory activity of a peel extract of C. medica was carried out by Sood et al. [113]. Wistar rats received an ethyl-acetate extract of C. medica peel (400 mg/kg) via oral administration, which caused an approximately twofold reduction in the volume of carrageenan-induced paw edema after 12 h. Subsequently, the ability of the C. medica peel extract to reduce paw edema was also investigated by Malleshappa et al. [24] by using ethanol as an extraction solvent. After 5 h, the ethanolic peel extract reduced the paw edema by 82.77 ± 0.88%, in a manner that was comparable to that of the standard utilized (indomethacin), which caused a reduction in volume of 88.62 ±1.16%. Therefore, although the results are not comparable, as they are expressed differently, both extracts showed anti-edematogenic activities in preclinical studies.
One of the common features of inflammatory responses is the development of pain [114]. Ethanol [24] and ethyl acetate [83] extracts of C. medica peel were also investigated for their potential analgesic effects using the hot-plate test. This method measures the response to an acute nociceptive stimulus by placing an animal on a heated surface. Both extracts showed significant analgesic activity against hyperalgesia induced by thermal stimuli. The oral administration of the ethanolic extract (400 mg/Kg) led an extension of the rats’ reaction times compared with the control (carrageenan-treated) group, as well as the ethyl-acetate extract (500 mg/Kg). No significant differences were observed compared to the diclofenac sodium (12.5 mg/Kg) control group. Regarding the carrageenan-induced paw-edema test, it was not possible to compare the results due to their different forms of expression.
3.4.5. Other Activities
Few studies have reported the hypoglycemic activity of C. medica and its potential application in diabetes treatment. The enzymes α-amylase and α-glucosidase are implicated in the reduction in post-prandial hyperglycemia by retarding the adsorption of glucose [115], and the use of inhibitors of both enzymes represents a therapeutic strategy. Natural compounds possess a promising ability to regulate hyperglycemia via the downregulation of α-amylase and α-glucosidase enzymes, with fewer side effects than conventional drugs [116]. As stated in many studies, the C. medica isolated flavonoids apigenin and hesperetin could be the molecules responsible for its hypoglycemic activity [117], in addition to terpenoids, whose access to enzymatic sites may be facilitated by their lipophilicity [118]. The flowers, leaves, and fruits (endocarp and mesocarp) of C. medica cv Diamante at two maturation stages were investigated for their potential hypoglycemic effects by Menichini et al. [27]. All the extracts showed weaker inhibition of amylase and glucosidase enzymes than the control (acarbose: IC50 50.0 ± 0.9 and 35.5 ± 1.2 µg/mL, respectively). The flowers inhibited enzyme activity, with IC50 > 1000 μg/mL, while the leaves were more active on the α-amylase (IC50 438.5 ± 5.2 μg/mL) than on the α-glucosidase (IC50 777.5 ± 5.4 μg/mL). The maturation stages affected the endocarp activity on the carbohydrate-hydrolyzing enzymes: the α-amylase was inhibited more by the mature fruits (IC50 426.0 ± 4.4 μg/mL) than by the immature extract (IC50 844.5 ± 3.6 μg/mL), while the opposite was observed for the α-glucosidase (IC50 574.1 ± 5.8 μg/mL and IC50 472.9 ± 4.7 μg/mL, respectively). The mesocarp extract showed higher IC50 values, which were indicative of lower inhibitory activity against both enzymes; no difference in α-amylase inhibition was observed, while, regarding the α-glucosidase, the immature fruit showed stronger activity. Peng et al. [53] suggested the use of C. medica var. Sarcodactylis fruit extracts in type 2 diabetes mellitus, reporting an insulin-secretagogue effect. In a preclinical study, C. medica cv Diamante hydroalcoholic peel extract significantly (p < 0.05) decreased the serum glucose level (187.8 ± 20.6 mg/dL, at 600 mg/kg) compared to that in the control group (282.9 ± 60.1 mg/dL) [33]. The 70% aqueous methanol extract from C. medica var. Etrog leaves decreased serum levels of glucose in a dose-dependent manner, with 105.2 ± 8.35 mg/dL and 87.4 ± 6.30 mg/dL at 200 and 400 mg/kg, respectively, compared to the standard Gliclazide (110.8 ± 7.24 mg/dL) and diabetic control groups (172.3 ± 82.09 mg/dL) [38]. Conforti et al. [50] demonstrated that C. medica cv. Diamante peel extract was able to inhibit the α-amylase enzyme (IC50 value of 625 µg/mL), which was significantly different from the control utilized (acarbose, IC50 of 50 ± 0.58 µg/mL). The same extract was found to inhibit the acetylcholinesterase activity with IC50 values of 621 ± 7.83 µg/mL, which was ascribed by the authors to the presence of monoterpenes. Cholinesterase inhibition was also investigated by Tundis et al. [92]. The effects of EO obtained by hydro-distillation, cold-pressing, and supercritical carbon-dioxide extraction on acetylcholinesterase and butyrylcholinesterase were examined: hydro-distillation showed the strongest inhibitory activity against both enzymes (IC50 values of 171.3 mg/mL and 154.6 mg/mL respectively). However, the IC50 of the standard molecule utilized was much higher (physostigmine IC50 values of 0.2 ± 0.004 2.4 ± 0.02 mg/mL). These activities are also attributable to the presence of flavonoids; Dehghan et al. [119] investigated the interactions of hesperidin, diosmin, rutin, and naringin with protein targets of Alzheimer’s, Parkinson’s, and Huntington’s diseases using computational drug-design methods. Further studies are required to investigate the pharmacokinetic profiles of these compounds to increase their absorption in brain tissue; their use could represent a strategy against neurodegenerative diseases if co-administered with conventional drugs to reduce their potential toxic effects. Furthermore, C. medica is also an important source of polysaccharides, which can have many biological properties. The biological activity of polysaccharides seems to be related to their water-solubility; moreover, the presence of arabinose, mannose, glucose, or galactose might be linked to its immunomodulatory activity [120]. This is the case with the new heteropolysaccharide, CMSPB80-1, isolated by Peng et al. [61], which showed an immunoregulatory activity by increasing the production of NO by RAW264.7 macrophages and splenocyte proliferation. The proliferation of splenocytes is also enhanced by the sulfate derivative (CMSPW90-M1) of the heteropolysaccharide CMSPW90-1, which, in addition, was able to increase the phagocytosis of RAW264.7 cells [62]. The same activity has been observed for the heteropolysaccharide, CMSPA90-1, isolated by Gao et al. [63]. Furthermore, FCp-3 is a water-soluble polysaccharide identified in C. medica fruit by He et al., who demonstrated how this polysaccharide increased the proliferation of both splenocytes and thymocytes, potentially suggesting a potential immunomodulatory activity [64]. A new type of arabinoxylan (CM-1) and a new type of galactoarabinan (CM-2) were isolated for the first time from a whole fruit and exhibited antiproliferative activities against cancer-cell lines and immunostimulatory properties by increasing the secretion of pro-inflammatory cytokines, such as TNF-α and IL-6 [65]. By contrast, the galactorhamnan polysaccharide, K-CMLP (mainly composed of rhamnose and galactose), exerted anti-inflammatory effects by diminishing the production of TNF-α and IL-6 [66].
4. Conclusions
Over the last decades, the use of plant-derived extracts has received increased attention due to concerns over the possible adverse health effects caused by the use of conventional medicine. This review summarizes the main chemical properties and biological activities, examining new research approaches to share the knowledge on the therapeutic and nutraceutical properties of C. medica. The limitations on the introduction of C. medica into medical practice in relation to the activities screened in this review are due to the scarcity of preclinical and clinical studies. Additional in vivo experiments are certainly required to better investigate the effects of this species on the entire organism. As highlighted in our research, the biological properties of C. medica can be ascribed to the presence of specific molecules, such as polyphenols, alkaloids, coumarins, and terpenes, and to macronutrients and micronutrients, such as carbohydrates, minerals, vitamins, and amino acids, which are endowed with properties that are beneficial for health.
In conclusion, based on the present literature review, it is possible to assert that C. medica can be considered an excellent candidate for treating various pathologies, mainly related to inflammation, oxidative stress, and microbial infection. However, new studies are needed to maximize C. medica’s potential for human health.
Abbreviations
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| APE | Alginate extract and pectin filler |
| COSY | Correlation spectroscopy |
| CPE | Continuous phase-transition extraction |
| C-RDI | Contribution reference daily intake |
| DLA | Dalton’s lymphoma ascites |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DW | Dry weight |
| EC50 | Half maximal effective concentration |
| EAC | Ehrlich’s ascites carcinoma |
| EtOH | Ethanol |
| EI-MS | Electron ionization–mass spectrometry |
| EO | Essential oil |
| ERK | Extracellular signal-regulated kinase |
| FW | FW |
| GAE | Gallic acid equivalent |
| GC–MS | Gas chromatography–mass spectrometry |
| GC–MS–SPME | Gas chromatography–mass spectrometry–solid-phase microextraction |
| HeLa | Henrietta Lacks |
| HL60 | Human leukemia cell line |
| HMQC | Heteronuclear multiple quantum coherence |
| HMBC | Heteronuclear multiple bond coherence |
| HPGPC | High-performance gel-permeation chromatography |
| HPLC | High-performance liquid chromatography |
| HPLC–PDA–MS | High-performance liquid chromatography–photodiode array–mass spectrometry |
| HPLC–QTOF–MS | High-performance liquid chromatography–quadruple time of flight–mass spectrometry |
| HRGC–MS | High-resolution gas chromatography–mass spectrometry |
| HR–ESI–MS | High-resolution–electrospray ionization–mass spectrometry |
| HR–EI–MS | High-resolution–electron ionization–mass spectrometry |
| HR–MAS–NMR | High-resolution–magic angle sinning–nuclear magnetic resonance |
| HY | Hydrolat |
| IC50 | Half-maximal inhibitory concentration |
| IkB-α: | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| IL-6 | Interleukin 6 |
| IL-1β | Interleukin 1β |
| IL-10 | Interleukin 10 |
| JNK | c-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| MAPKs | Mitogen-activated protein kinase |
| MeOH | Methanol |
| MIC | Minimum inhibitory concentration |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NOESY | Nuclear Overhauser effect spectroscopy |
| PC-3 | Human prostate adenocarcinoma cell line |
| PLE | Pressure liquid extraction |
| RAW 264.7 | Macrophage cell line |
| RSA | Radical-scavenging activity |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SCF–CO2 | Super-critical fluid–carbon bioxide |
| SH-SY5Y | Human neuroblastoma cell line |
| TFC | Total flavonoid content |
| TNF-α | Tumor necrosis factor alpha |
| TPC | Total phenolic content |
| UV | Ultraviolet |
| UAHD | Hydro-distillation ultrasound-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| UHPLC–QTOF–IMS | Ultra-performance liquid chromatography–quadruple time of flight–mass spectrometry |
| Var. | Variety |
Author Contributions
V.C. and N.B.: conceptualization. N.B., V.C., I.F., L.L., M.P., D.R., C.M., L.M. and N.T.T.: investigation, data curation. V.C. and N.B.: writing—original draft preparation, writing—review and editing and visualization. N.B., V.C., I.F., L.L., M.P., D.R., C.M., L.M. and N.T.T.: addressing of PRISMA guidelines and validation. L.M. and N.T.T.: structural-similarity analysis. L.M.: resources, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. All authors have read and agreed to the published version of the manuscript.
Funding Statement
This research was supported by Regione Basilicata; Project PAPRIKANET—Plant Active Products: Research, Innovation and Knowledge Advancements Through an International Network CUP: C39J20001740002 program 2014IT16RFOP022; Italian Ministry of the Economic Development, “Fondo per la Crescita Sostenibile—Sportello “Agrifood” PON I&C 2014–2020”, Project FULLNESS n. F/200099/01–03/X45; Project SPIA—Valorization of by-products from the agro-food chain CUP: G49J19001350004; and DGR n. 527/2019 “PO FESR BASILICATA 2014-2020—Axis I—Research, Innovation Action and Technological Development—Action 1B.1.2.1- Avviso Pubblico per il sostegno alla creazione e sviluppo dei Cluster Tecnologici della Regione Basilicata e alla realizzazione di progetti di Ricerca e Sviluppo”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ramadugu C., Keremane M.L., Hu X., Karp D., Federici C.T., Kahn T., Roose M.L., Lee R.F. Genetic analysis of citron (Citrus medica L.) using simple sequence repeats and single nucleotide polymorphisms. Sci. Hortic. 2015;195:124–137. doi: 10.1016/j.scienta.2015.09.004. [DOI] [Google Scholar]
- 2.Hayat K. Citrus molecular phylogeny antioxidant properties and medicinal uses. Nova. Sci. 2014;3:235. [Google Scholar]
- 3.Dey P., Hoque M., Islam M., Monalisa K. Kinetics of antioxidants degradation and quality changes of citron (Citrus medica) fruit juice concentrate during storage. J. Microbiol. Biotechnol. Food Sci. 2021;2021:230–234. [Google Scholar]
- 4.Pieroni A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J. Ethnopharmacol. 2000;70:235–273. doi: 10.1016/S0378-8741(99)00207-X. [DOI] [PubMed] [Google Scholar]
- 5.Secundus C.P. Naturalis Historiae, Libri XXXVII. Vol. 6 Nabu Press; Charleston, SC, USA: 1865. Lipsiae, in Aedibus B. G. Teubneri. [Google Scholar]
- 6.Pieroni A., Quave C.L., Villanelli M.L., Mangino P., Sabbatini G., Santini L., Boccetti T., Profili M., Ciccioli T., Rampa L.G. Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. J. Ethnopharmacol. 2004;91:331–344. doi: 10.1016/j.jep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Sahoo C.R., Sahoo J., Mahapatra M., Lenka D., Sahu P.K., Dehury B., Padhy R.N., Paidesetty S.K. Coumarin derivatives as promising antibacterial agent (s) Arab. J. Chem. 2021;14:102922. doi: 10.1016/j.arabjc.2020.102922. [DOI] [Google Scholar]
- 8.Haridas M., Sasidhar V., Nath P., Abhithaj J., Sabu A., Rammanohar P. Compounds of Citrus medica and Zingiber officinale for COVID-19 inhibition: In silico evidence for cues from Ayurveda. Future J. Pharm. Sci. 2021;7:13. doi: 10.1186/s43094-020-00171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarshenas M.M., Mahmoodian R., Moein M. Colorimetric determination of flavonoids in Citrus medica peel traditional medicinal oil. Int. J. Pharmacogn. Phytochem. Res. 2015;7:696–700. [Google Scholar]
- 10.Jafarpour M., Yousefi G., Hamedi A., Shariat A., Salehi A., Heydari M. Effect of a traditional syrup from Citrus medica L. fruit juice on migraine headache: A randomized double blind placebo controlled clinical trial. J. Ethnopharmacol. 2016;179:170–176. doi: 10.1016/j.jep.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Kalariya M., Prajapati R., Chavda J. Pharmacological potential of Citrus medica: A review. Pharma Sci. Monit. 2019;10:66–81. [Google Scholar]
- 12.Karp D., Hu X. The citron (Citrus medica L.) in China. Hortic. Rev. 2018;45:143–196. [Google Scholar]
- 13.Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., Thomas J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10:14651858. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponticelli M., Lela L., Moles M., Mangieri C., Bisaccia D., Faraone I., Falabella R., Milella L. The healing bitterness of Gentiana lutea L., phytochemistry and biological activities: A systematic review. Phytochemistry. 2022;206:113518. doi: 10.1016/j.phytochem.2022.113518. [DOI] [PubMed] [Google Scholar]
- 15.Dadwal V., Joshi R., Gupta M. A comparative metabolomic investigation in fruit sections of Citrus medica L. and Citrus maxima L. detecting potential bioactive metabolites using UHPLC-QTOF-IMS. Food Res. Int. 2022;157:111486. doi: 10.1016/j.foodres.2022.111486. [DOI] [PubMed] [Google Scholar]
- 16.Hasan M.M., Roy P., Alam M., Hoque M.M., Zzaman W. Antimicrobial activity of peels and physicochemical properties of juice prepared from indigenous citrus fruits of Sylhet region, Bangladesh. Heliyon. 2022;8:e09948. doi: 10.1016/j.heliyon.2022.e09948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahdi A.A., Al-Ansi W., Ahmed M.I., Xiaoyun C., Mohammed J.K., Sulieman A.A., Mushtaq B.S., Harimana Y., Wang H. Microwave assisted extraction of the bioactive compounds from peel/pulp of Citrus medica L. var. sarcodactylis swingle along with its nutritional profiling. J. Food Meas. Charact. 2020;14:283–292. doi: 10.1007/s11694-019-00290-6. [DOI] [Google Scholar]
- 18.Khomdram S., Barthakur S., Devi G.S. Biochemical and molecular analysis of wild endemic fruits of the Manipur region of India. Int. J. Fruit Sci. 2014;14:253–266. doi: 10.1080/15538362.2013.818483. [DOI] [Google Scholar]
- 19.Madhavi N., Jyothi B. Colorimetric determination of vitamin C in fresh and dilute fruit juices and effect of thermal exposure on concentration at various stages. Int. J. Pharma Bio. Sci. 2016;7:197–211. [Google Scholar]
- 20.Pallavi M., Ck R., Krishna V., Parveen S. Quantitative phytochemical analysis and antioxidant activities of some citrus fruits of South India. Asian J. Pharm. Clin. Res. 2017;10:198–205. [Google Scholar]
- 21.Wang E., Li Y., Maguy B.L., Lou Z., Wang H., Zhao W., Chen X. Separation and enrichment of phenolics improved the antibiofilm and antibacterial activity of the fractions from Citrus medica L. var. sarcodactylis in vitro and in tofu. Food Chem. 2019;294:533–538. doi: 10.1016/j.foodchem.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 22.Mucci A., Parenti F., Righi V., Schenetti L. Citron and lemon under the lens of HR-MAS NMR spectroscopy. Food Chem. 2013;141:3167–3176. doi: 10.1016/j.foodchem.2013.05.151. [DOI] [PubMed] [Google Scholar]
- 23.Bellavite P., Donzelli A. Hesperidin and SARS-CoV-2: New light on the healthy function of citrus fruits. Antioxidants. 2020;9:742. doi: 10.3390/antiox9080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malleshappa P., Kumaran R.C., Venkatarangaiah K., Parveen S. Peels of citrus fruits: A potential source of anti-inflammatory and anti-nociceptive agents. Pharmacogn. J. 2018;10:s172–s178. doi: 10.5530/pj.2018.6s.30. [DOI] [Google Scholar]
- 25.Adham A.N. Comparative antimicrobrial activity of peel and juice extract of citrus fruits growing in Kurdistan/Iraq. Am. J. Microbiol. Re. 2015;5:155–159. [Google Scholar]
- 26.Taghvaeefard N., Ghani A., Hosseinifarahi M. Comparative study of phytochemical profile and antioxidant activity of flavedo from two Iranian citron fruit (Citrus medica L.) J. Food Meas. Charact. 2021;15:2821–2830. doi: 10.1007/s11694-021-00859-0. [DOI] [Google Scholar]
- 27.Menichini F., Loizzo M.R., Bonesi M., Conforti F., De Luca D., Statti G.A., de Cindio B., Menichini F., Tundis R. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food Chem. Toxicol. 2011;49:1549–1555. doi: 10.1016/j.fct.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 28.Adham A.N. Qualitative and quantitative estimation of hesperidin in peel and juice of citrus fruits by RP-HPLC method growing in Kurdistan region/Iraq. Int. J. Pharm. Sci. Rev. Res. 2015;33:220–224. [Google Scholar]
- 29.Choi I.-W., Choi S.-Y., Ji J.-R. Flavonoids and functional properties of germinated citron (Citrus junos Sieb. ex TANAKA) shoots. Food Sci. Biotechnol. 2009;18:1224–1229. [Google Scholar]
- 30.Tahir T., Ashfaq M., Shahzad M.I., Bukhari S.T. Recent Approaches in the Extraction of Citrus Metabolites. Curr. Biotechnol. 2019;8:85–95. [Google Scholar]
- 31.Govindarajan D.K., Viswalingam N., Meganathan Y., Devaraj B.S., Sivamani S., Sivarajasekar N. Response surface methodology optimization for extraction of pectin from waste rinds of Citrus medica. AIP Conf. Proc. 2022;2446:020013. [Google Scholar]
- 32.Ma Q.-G., Wei R.-R., Yang M., Huang X.-Y., Wang F., Dong J.-H., Sang Z.-P. Isolation and characterization of neolignan derivatives with hepatoprotective and neuroprotective activities from the fruits of Citrus medica L. var. Sarcodactylis Swingle. Bioorg. Chem. 2021;107:104622. doi: 10.1016/j.bioorg.2020.104622. [DOI] [PubMed] [Google Scholar]
- 33.Menichini F., Tundis R., Loizzo M.R., Bonesi M., D’Angelo D., Lombardi P., Mastellone V. Citrus medica L. cv Diamante (Rutaceae) peel extract improves glycaemic status of Zucker diabetic fatty (ZDF) rats and protects against oxidative stress. J. Enzyme Inhib. 2016;31:1270–1276. doi: 10.3109/14756366.2015.1115400. [DOI] [PubMed] [Google Scholar]
- 34.Fratianni F., Cozzolino A., De Feo V., Coppola R., Ombra M.N., Nazzaro F. Polyphenols, antioxidant, antibacterial, and biofilm inhibitory activities of peel and pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò cultivated in southern Italy. Molecules. 2019;24:4577. doi: 10.3390/molecules24244577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao P., Duan L., Guo L., Dou L.-L., Dong X., Zhou P., Li P., Liu E.-H. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 2015;173:54–60. doi: 10.1016/j.foodchem.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Chan Y.-Y., Li C.-H., Shen Y.-C., Wu T.-S. Anti-inflammatory principles from the stem and root barks of Citrus medica. Chem. Pharm. Bull. 2010;58:61–65. doi: 10.1248/cpb.58.61. [DOI] [PubMed] [Google Scholar]
- 37.Fomani M., Happi E.N., Francois-Hugues P., Lallemand M.-C., Waffo A.F.K., Wansi J.D. A prenylated acridone alkaloid and ferulate xanthone from barks of Citrus medica (Rutaceae) ZNB. 2015;70:71–75. doi: 10.1515/znb-2014-0179. [DOI] [Google Scholar]
- 38.Hetta M., El-Alfy T., Yassin N., Abdel-Rahman R., Kadry E. Phytochemical and antihyperglycemic studies on Citrus medica L. leaves (Etrog) growing in Egypt. Int. J. Pharmacogn. Phytochem. Res. 2013;5:271–277. [Google Scholar]
- 39.Roowi S., Crozier A. Flavonoids in tropical citrus species. J. Agric. Food Chem. 2011;59:12217–12225. doi: 10.1021/jf203022f. [DOI] [PubMed] [Google Scholar]
- 40.Chu J., Li S.-L., Yin Z.-Q., Ye W.-C., Zhang Q.-W. Simultaneous quantification of coumarins, flavonoids and limonoids in Fructus Citri Sarcodactylis by high performance liquid chromatography coupled with diode array detector. J. Pharm. Biomed. Anal. 2012;66:170–175. doi: 10.1016/j.jpba.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 41.Luo X., Wang J., Chen H., Zhou A., Song M., Zhong Q., Chen H., Cao Y. Identification of flavoanoids from finger citron and evaluation on their antioxidative and antiaging activities. Front. Nutr. 2020;207:584900. doi: 10.3389/fnut.2020.584900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mondal M., Saha S., Sarkar C., Hossen M.S., Hossain M.S., Khalipha A.B.R., Islam M.F., Wahed T.B., Islam M.T., Rauf A. Role of Citrus medica L. Fruits Extract in Combatting the Hematological and Hepatic Toxic Effects of Carbofuran. Chem. Res. Toxicol. 2021;34:1890–1902. doi: 10.1021/acs.chemrestox.1c00166. [DOI] [PubMed] [Google Scholar]
- 43.Chan Y.-Y., Hwang T.-L., Kuo P.-C., Hung H.-Y., Wu T.-S. Constituents of the Fruits of Citrus medica L. var. sarcodactylis and the Effect of 6, 7-Dimethoxy-coumarin on Superoxide Anion Formation and Elastase Release. Molecules. 2017;22:1454. doi: 10.3390/molecules22091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belletti N., Kamdem S.S., Patrignani F., Lanciotti R., Covelli A., Gardini F. Antimicrobial activity of aroma compounds against Saccharomyces cerevisiae and improvement of microbiological stability of soft drinks as assessed by logistic regression. Appl. Environ. Microbiol. 2007;73:5580–5586. doi: 10.1128/AEM.00351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Z., Li H., Yang Y., Zhan Y., Tu D. Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind. Crops Prod. 2013;46:311–316. doi: 10.1016/j.indcrop.2013.02.015. [DOI] [Google Scholar]
- 46.Guo J.-J., Gao Z.-P., Xia J.-L., Ritenour M.A., Li G.-Y., Shan Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT. 2018;97:825–839. doi: 10.1016/j.lwt.2018.07.060. [DOI] [Google Scholar]
- 47.Jing L., Lei Z., Zhang G., Pilon A.C., Huhman D.V., Xie R., Xi W., Zhou Z., Sumner L.W. Metabolite profiles of essential oils in citrus peels and their taxonomic implications. Metabolomics. 2015;11:952–963. doi: 10.1007/s11306-014-0751-x. [DOI] [Google Scholar]
- 48.Poiana M., Sicari V., Mincione B. A comparison between the chemical composition of the oil, solvent extract and supercritical carbon dioxide extract of Citrus medica cv. Diamante. J. Essent. Oil Res. 1998;10:145–152. doi: 10.1080/10412905.1998.9700866. [DOI] [Google Scholar]
- 49.Gabriele B., Fazio A., Dugo P., Costa R., Mondello L. Essential oil composition of Citrus medica L. Cv. Diamante (Diamante citron) determined after using different extraction methods. J. Sep. Sci. 2009;32:99–108. doi: 10.1002/jssc.200800404. [DOI] [PubMed] [Google Scholar]
- 50.Conforti F., Statti G.A., Tundis R., Loizzo M.R., Menichini F. In vitro activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer’s disease. Phytother. Res. 2007;21:427–433. doi: 10.1002/ptr.2077. [DOI] [PubMed] [Google Scholar]
- 51.Xing C., Qin C., Li X., Zhang F., Linhardt R.J., Sun P., Zhang A. Chemical composition and biological activities of essential oil isolated by HS-SPME and UAHD from fruits of bergamot. LWT. 2019;104:38–44. doi: 10.1016/j.lwt.2019.01.020. [DOI] [Google Scholar]
- 52.Wei L., Yu X., Li H., Zhu M., Pu D., Lu Q., Bao Y., Zu Y. Optimization of solvent-free microwave extraction of essential oil from the fresh peel of Citrus medica L. var. arcodactylis Swingle by response surface methodology, chemical composition and activity characterization. Sci. Hortic. 2023;309:111663. doi: 10.1016/j.scienta.2022.111663. [DOI] [Google Scholar]
- 53.Peng C.-H., Ker Y.-B., Weng C.-F., Peng C.-C., Huang C.-N., Lin L.-Y., Peng R.Y. Insulin secretagogue bioactivity of finger citron fruit (Citrus medica L. var. Sarcodactylis Hort, Rutaceae) J. Agric. Food Chem. 2009;57:8812–8819. doi: 10.1021/jf902143x. [DOI] [PubMed] [Google Scholar]
- 54.Vitalini S., Iriti M., Ovidi E., Laghezza Masci V., Tiezzi A., Garzoli S. Detection of Volatiles by HS-SPME-GC/MS and Biological Effect Evaluation of Buddha’s Hand Fruit. Molecules. 2022;27:1666. doi: 10.3390/molecules27051666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fanciullino A.-L., Dhuique-Mayer C., Luro F., Casanova J., Morillon R., Ollitrault P. Carotenoid diversity in cultivated citrus is highly influenced by genetic factors. J. Agric. Food Chem. 2006;54:4397–4406. doi: 10.1021/jf0526644. [DOI] [PubMed] [Google Scholar]
- 56.Lim J.Y., Yoon H.-S., Kim K.-Y., Kim K.-S., Noh J.G., Song I.G. Optimum conditions for the enzymatic hydrolysis of citron waste juice using response surface methodology (RSM) Food Sci. Biotechnol. 2010;19:1135–1142. doi: 10.1007/s10068-010-0162-3. [DOI] [Google Scholar]
- 57.Belletti N., Ndagijimana M., Sisto C., Guerzoni M.E., Lanciotti R., Gardini F. Evaluation of the antimicrobial activity of citrus essences on Saccharomyces cerevisiae. J. Agric. Food Chem. 2004;52:6932–6938. doi: 10.1021/jf049444v. [DOI] [PubMed] [Google Scholar]
- 58.Kim K.-N., Ko Y.-J., Yang H.-M., Ham Y.-M., Roh S.W., Jeon Y.-J., Ahn G., Kang M.-C., Yoon W.-J., Kim D. Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-κB signaling pathways in LPS-activated RAW 264.7 cells. Food Chem. Toxicol. 2013;57:126–131. doi: 10.1016/j.fct.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Gancel A.L., Ollitrault P., Froelicher Y., Tomi F., Jacquemond C., Luro F., Brillouet J.M. Citrus somatic allotetraploid hybrids exhibit a differential reduction of leaf sesquiterpenoid biosynthesis compared with their parents. Flavour. Fragr. J. 2005;20:626–632. doi: 10.1002/ffj.1512. [DOI] [Google Scholar]
- 60.Wang S.F., Ju Y., Chen X.G., De Hu Z. Separation and determination of coumarins in the root bark of three citrus plants by micellar electrokinetic capillary chromatography. Planta Med. 2003;69:483–486. doi: 10.1055/s-2003-39697. [DOI] [PubMed] [Google Scholar]
- 61.Peng B., Yang J., Huang W., Peng D., Bi S., Song L., Wen Y., Zhu J., Chen Y., Yu R. Structural characterization and immunoregulatory activity of a novel heteropolysaccharide from bergamot (Citrus medica L. var. sarcodactylis) by alkali extraction. Ind. Crops Prod. 2019;140:111617. doi: 10.1016/j.indcrop.2019.111617. [DOI] [Google Scholar]
- 62.Peng B., Luo Y., Hu X., Song L., Yang J., Zhu J., Wen Y., Yu R. Isolation, structural characterization, and immunostimulatory activity of a new water-soluble polysaccharide and its sulfated derivative from Citrus medica L. var. sarcodactylis. Int. J. Biol. Macromol. 2019;123:500–511. doi: 10.1016/j.ijbiomac.2018.11.113. [DOI] [PubMed] [Google Scholar]
- 63.Gao Y., Peng B., Xu Y., Yang J.-n., Song L.-y., Bi S.-x., Chen Y., Zhu J.-h., Wen Y., Yu R.-m. Structural characterization and immunoregulatory activity of a new polysaccharide from Citrus medica L. var. sarcodactylis. RSC Adv. 2019;9:6603–6612. doi: 10.1039/C8RA10664A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He Z., Liang F., Zhang Y., Pan Y. Water-soluble polysaccharides from finger citron fruits (Citrus medica L. var. sarcodactylis) Carbohydr. Res. 2014;388:100–104. doi: 10.1016/j.carres.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 65.Chang X., Shen C.-Y., Jiang J.-G. Structural characterization of novel arabinoxylan and galactoarabinan from citron with potential antitumor and immunostimulatory activities. Carbohydr. Polym. 2021;269:118331. doi: 10.1016/j.carbpol.2021.118331. [DOI] [PubMed] [Google Scholar]
- 66.Luo B., Lv J., Li K., Liao P., Chen P. Structural Characterization and Anti-inflammatory Activity of a Galactorhamnan Polysaccharide From Citrus medica L. var. sarcodactylis. Front. Nutr. 2022;9:916976. doi: 10.3389/fnut.2022.916976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Z., Li H., Tu D., Yang Y., Zhan Y. Extraction optimization, preliminary characterization, and in vitro antioxidant activities of crude polysaccharides from finger citron. Ind. Crops Prod. 2013;44:145–151. doi: 10.1016/j.indcrop.2012.11.008. [DOI] [Google Scholar]
- 68.Castillo S., Dávila-Aviña J., Heredia N., Garcia S. Antioxidant activity and influence of Citrus byproduct extracts on adherence and invasion of Campylobacter jejuni and on the relative expression of cadF and ciaB. Food Sci. Biotechnol. 2017;26:453–459. doi: 10.1007/s10068-017-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El Hawary S.S.E., Hashim F.A., Ibrahim N.A., Matloub A.A., ElSayed A.M., Farid M.A., Kutkat O., El-Abd E.A., Ali M., Shata H.M. A Comparative study of Chemical Compositions, Antimicrobial and Antiviral Activities of Essential Oils for Citrus medica var. sarcodactylis Swingle Fruits and Leaves along with Limonia acidissima L. Leaves. Egypt J. Chem. 2022;65:2–4. doi: 10.21608/ejchem.2021.108413.4958. [DOI] [Google Scholar]
- 70.Belletti N., Lanciotti R., Patrignani F., Gardini F. Antimicrobial efficacy of citron essential oil on spoilage and pathogenic microorganisms in fruit-based salads. J. Food Sci. 2008;73:M331–M338. doi: 10.1111/j.1750-3841.2008.00866.x. [DOI] [PubMed] [Google Scholar]
- 71.Wu K., Jin R., Bao X., Yu G., Yi F. Potential roles of essential oils from the flower, fruit and leaf of Citrus medica L. var. sarcodactylis in preventing spoilage of Chinese steamed bread. Food Biosci. 2021;43:101271. doi: 10.1016/j.fbio.2021.101271. [DOI] [Google Scholar]
- 72.Khorsandi A., Ziaee E., Shad E., Razmjooei M., Eskandari M.H., Aminlari M. Antibacterial effect of essential oils against spoilage bacteria from vacuum-packed cooked cured sausages. J. Food Prot. 2018;81:1386–1393. doi: 10.4315/0362-028X.JFP-17-474. [DOI] [PubMed] [Google Scholar]
- 73.Li Z.-H., Cai M., Liu Y.-S., Sun P.-L., Luo S.-L. Antibacterial activity and mechanisms of essential oil from Citrus medica L. var. sarcodactylis. Molecules. 2019;24:1577. doi: 10.3390/molecules24081577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitropoulou G., Nikolaou A., Santarmaki V., Sgouros G., Kourkoutas Y. Citrus medica and Cinnamomum zeylanicum essential oils as potential biopreservatives against spoilage in low alcohol wine products. Foods. 2020;9:577. doi: 10.3390/foods9050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen W.F., Wong L.L., Zhang X., Zhou F.X., Xia F., Tang L.P., Li X. New 4, 5-seco-20 (10→5)-abeo-Abietane Diterpenoids with Anti-Inflammatory Activity from Isodon lophanthoides var. graciliflorus (Benth.) H. Hara. Chem. Biodivers. 2019;16:e1900206. doi: 10.1002/cbdv.201900206. [DOI] [PubMed] [Google Scholar]
- 76.Al-Mariri A., Safi M. In vitro antibacterial activity of several plant extracts and oils against some gram-negative bacteria. Iran. J. Med. Sci. 2014;39:36. [PMC free article] [PubMed] [Google Scholar]
- 77.Mitropoulou G., Fitsiou E., Spyridopoulou K., Tiptiri-Kourpeti A., Bardouki H., Vamvakias M., Panas P., Chlichlia K., Pappa A., Kourkoutas Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT. 2017;84:344–352. doi: 10.1016/j.lwt.2017.05.036. [DOI] [Google Scholar]
- 78.Zhang H., Lou Z., Chen X., Cui Y., Wang H., Kou X., Ma C. Effect of simultaneous ultrasonic and microwave assisted hydrodistillation on the yield, composition, antibacterial and antibiofilm activity of essential oils from Citrus medica L. var. sarcodactylis. J. Food Eng. 2019;244:126–135. doi: 10.1016/j.jfoodeng.2018.09.014. [DOI] [Google Scholar]
- 79.Wang F., You H., Guo Y., Wei Y., Xia P., Yang Z., Ren M., Guo H., Han R., Yang D. Essential oils from three kinds of fingered citrons and their antibacterial activities. Ind. Crops Prod. 2020;147:112172. doi: 10.1016/j.indcrop.2020.112172. [DOI] [Google Scholar]
- 80.Suryavanshi P., Pandit R., Gade A., Derita M., Zachino S., Rai M. Colletotrichum sp.-mediated synthesis of sulphur and aluminium oxide nanoparticles and its in vitro activity against selected food-borne pathogens. LWT. 2017;81:188–194. doi: 10.1016/j.lwt.2017.03.038. [DOI] [Google Scholar]
- 81.Li Z.-H., Cai M., Liu Y.-s., Sun P.-L. Development of finger citron (Citrus medica L. var. sarcodactylis) essential oil loaded nanoemulsion and its antimicrobial activity. Food Control. 2018;94:317–323. doi: 10.1016/j.foodcont.2018.07.009. [DOI] [Google Scholar]
- 82.Keerthana P., Vijayakumar S., Vidhya E., Punitha V., Nilavukkarasi M., Praseetha P. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti-infection and growth promotion in plants. Ind. Crops Prod. 2021;170:113762. [Google Scholar]
- 83.Adham A.N. Phytochemical analysis and evaluation antibacterial activity of Citrus medica peel and juice growing in Kurdistan/Iraq. J. Appl. Pharm. Sci. 2015;5:136–141. doi: 10.7324/JAPS.2015.501023. [DOI] [Google Scholar]
- 84.Sah A.N., Juyal V., Melkani A.B. Antimicrobial activity of six different parts of the plant Citrus medica Linn. Pharmacogn. J. 2011;3:80–83. doi: 10.5530/pj.2011.21.15. [DOI] [Google Scholar]
- 85.Sharma R., Verma S., Rana S., Rana A. Rapid screening and quantification of major organic acids in citrus fruits and their bioactivity studies. J. Food Sci. Technol. 2018;55:1339–1349. doi: 10.1007/s13197-018-3045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castillo S., Heredia N., Arechiga-Carvajal E., García S. Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol. 2014;28:106–122. doi: 10.1080/08905436.2014.895947. [DOI] [Google Scholar]
- 87.Shende S., Ingle A.P., Gade A., Rai M. Green synthesis of copper nanoparticles by Citrus medica Linn.(Idilimbu) juice and its antimicrobial activity. World J. Microbiol. Biotechnol. 2015;31:865–873. doi: 10.1007/s11274-015-1840-3. [DOI] [PubMed] [Google Scholar]
- 88.Selvaraju N., Ganesh P.S., Palrasu V., Venugopal G., Mariappan V. Evaluation of Antimicrobial and Antibiofilm Activity of Citrus medica Fruit Juice Based Carbon Dots against Pseudomonas aeruginosa. ACS Omega. 2022;7:36227–36234. doi: 10.1021/acsomega.2c03465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nair A., Kurup Sr R., Nair A.S., Baby S. Citrus peels prevent cancer. Phytomedicine. 2018;50:231–237. doi: 10.1016/j.phymed.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Ajikumaran N.S., Rajani Kurup S., Akhila S., Sabulal B. Citrus peels prevent cancer. Phytomedicine. 2018;50:231–237. doi: 10.1016/j.phymed.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 91.Dahiya R., Kumar A. Synthetic and biological studies on a cyclopolypeptide of plant origin. J. Zhejiang Univ. Sci. B. 2008;9:391–400. doi: 10.1631/jzus.B0720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tundis R., Calabria U., Bonesi M., Calabria U., Calabria U. Chemical composition and bioactivity of Citrus medica L. cv. Diamante essential oil obtained by hydrodistillation. Nat. Prod. Res. 2011;25:789–799. doi: 10.1080/14786410902900085. [DOI] [PubMed] [Google Scholar]
- 93.Singh B., Singh J.P., Kaur A., Singh N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020;132:109114. doi: 10.1016/j.foodres.2020.109114. [DOI] [PubMed] [Google Scholar]
- 94.Chen W., Zheng J., Li Y., Guo W. Effects of high temperature on photosynthesis, chlorophyll fluorescence, chloroplast ultrastructure, and antioxidant activities in fingered citron. Russ. J. Plant Physiol. 2012;59:732–740. doi: 10.1134/S1021443712060040. [DOI] [Google Scholar]
- 95.Ebrahimi Y., Hasanvand A., Valibeik A., Ebrahimi F., Abbaszadeh S. Natural antioxidants and medicinal plants effective on hyperlipidemia. Res. J. Pharm. Technol. 2019;12:1457–1462. doi: 10.5958/0974-360X.2019.00242.7. [DOI] [Google Scholar]
- 96.Jayaprakasha G., Patil B.S. In vitro evaluation of the antioxidant activities in fruit extracts from citron and blood orange. Food Chem. 2007;101:410–418. doi: 10.1016/j.foodchem.2005.12.038. [DOI] [Google Scholar]
- 97.Pallavi M., Ramesh C., Siddesha J., Krishna V., Kavitha G., Nethravathi A., Parveen S., KM A.K. Anti-hyperlipidemic effects of citrus fruit peel extracts against high fat diet-induced hyperlipidemia in rats. Int. J. Res. Pharm. Sci. 2021;12:2226–2232. doi: 10.26452/ijrps.v12i3.4837. [DOI] [Google Scholar]
- 98.Ghani A., Taghvaeefard N., Hosseinifarahi M., Dakhlaoui S., Msaada K. Essential oil composition and antioxidant activity of citron fruit (Citrus medica var. macrocarpa Risso.) peel as relation to ripening stages. Int. J. Environ. Health Res. 2022:1–11. doi: 10.1080/09603123.2022.2084514. [DOI] [PubMed] [Google Scholar]
- 99.Dadwal V., Joshi R., Gupta M. Formulation, characterization and in vitro digestion of polysaccharide reinforced Ca-alginate microbeads encapsulating Citrus medica L. phenolics. LTW. 2021;152:112290. doi: 10.1016/j.lwt.2021.112290. [DOI] [Google Scholar]
- 100.Wu Z. Effect of different drying methods on chemical composition and bioactivity of finger citron polysaccharides. Int. J. Biol. Macromol. 2015;76:218–223. doi: 10.1016/j.ijbiomac.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 101.Mengsa H., Kun X., Pei L., Jun L. Five Rutaceae family ethanol extracts alleviate H2O2 and LPS-induced inflammation via NF-κB and JAK-STAT3 pathway in HaCaT cells. Chin. J. Nat. Med. 2022;20:937–947. doi: 10.1016/S1875-5364(22)60217-6. [DOI] [PubMed] [Google Scholar]
- 102.Hanafy S.M., Abd El-Shafea Y.M., Saleh W.D., Fathy H.M. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J. Genet. Eng. Biotechnol. 2021;19:80. doi: 10.1186/s43141-021-00151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herman A., Tambor K., Herman A. Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 2016;72:165–172. doi: 10.1007/s00284-015-0933-4. [DOI] [PubMed] [Google Scholar]
- 104.Nagy M.M., Al-Mahdy D.A., Abd El Aziz O.M., Kandil A.M., Tantawy M.A., El Alfy T.S. Chemical composition and antiviral activity of essential oils from Citrus reshni hort. ex Tanaka (Cleopatra mandarin) cultivated in Egypt. J. Essent. Oil-Bear. Plants. 2018;21:264–272. doi: 10.1080/0972060X.2018.1436986. [DOI] [Google Scholar]
- 105.Punitha V., Vijayakumar S., Nilavukkarasi M., Vidhya E., Praseetha P. Fruit peels that unlock curative potential: Determination of biomedical application and bioactive compounds. S. Afr. J. Bot. 2022;150:1051–1060. doi: 10.1016/j.sajb.2022.09.022. [DOI] [Google Scholar]
- 106.Thi T.U.D., Nguyen T.T., Thi Y.D., Thi K.H.T., Phan B.T., Pham K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020;10:23899–23907. doi: 10.1039/d0ra04926c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bray F., Laversanne M., Weiderpass E., Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 108.Nyrop K.A., Damone E.M., Deal A.M., Wheeler S.B., Charlot M., Reeve B.B., Basch E., Shachar S.S., Carey L.A., Reeder-Hayes K.E., et al. Patient-reported treatment toxicity and adverse events in Black and White women receiving chemotherapy for early breast cancer. Breast Cancer Res. Treat. 2022;191:409–422. doi: 10.1007/s10549-021-06439-6. [DOI] [PubMed] [Google Scholar]
- 109.Cirmi S., Maugeri A., Ferlazzo N., Gangemi S., Calapai G., Schumacher U., Navarra M. Anticancer potential of citrus juices and their extracts: A systematic review of both preclinical and clinical studies. Front. Pharmacol. 2017;8:420. doi: 10.3389/fphar.2017.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aliberti L., Caputo L., De Feo V., De Martino L., Nazzaro F., Souza L.F. Chemical composition and in vitro antimicrobial, cytotoxic, and central nervous system activities of the essential oils of Citrus medica L. cv.‘Liscia’and C. medica cv.‘Rugosa’cultivated in Southern Italy. Molecules. 2016;21:1244. doi: 10.3390/molecules21091244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rawat A., Reddy A.V.B. Recent advances on anticancer activity of coumarin derivatives. Eur. J. Med. Chem. 2022;5:100038. doi: 10.1016/j.ejmcr.2022.100038. [DOI] [Google Scholar]
- 112.Xiao S., Liu W., Bi J., Liu S., Zhao H., Gong N., Xing D., Gao H., Gong M. Anti-inflammatory effect of hesperidin enhances chondrogenesis of human mesenchymal stem cells for cartilage tissue repair. J. Inflamm. 2018;15:14. doi: 10.1186/s12950-018-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sood S., Bansal S., Muthuraman A., Gill N., Bali M. Therapeutic potential of Citrus medica L. peel extract in carrageenan induced inflammatory pain in rat. J. Med. Plant Res. 2009;3:123–133. doi: 10.3923/rjmp.2009.123.133. [DOI] [Google Scholar]
- 114.Marouf B.H., Hussain S.A., Ali Z.S., Ahmmad R.S. Resveratrol supplementation reduces pain and inflammation in knee osteoarthritis patients treated with meloxicam: A randomized placebo-controlled study. J. Med. Food. 2018;21:1253–1259. doi: 10.1089/jmf.2017.4176. [DOI] [PubMed] [Google Scholar]
- 115.Awosika T.O., Aluko R.E. Inhibition of the in vitro activities of α-amylase, α-glucosidase and pancreatic lipase by yellow field pea (Pisum sativum L.) protein hydrolysates. Int. J. Food Sci. Technol. 2019;54:2021–2034. doi: 10.1111/ijfs.14087. [DOI] [Google Scholar]
- 116.Tolmie M., Bester M.J., Apostolides Z. Inhibition of α-glucosidase and α-amylase by herbal compounds for the treatment of type 2 diabetes: A validation of in silico reverse docking with in vitro enzyme assays. J. Diabetes. 2021;13:779–791. doi: 10.1111/1753-0407.13163. [DOI] [PubMed] [Google Scholar]
- 117.Abdou H.M., Hamaad F.A., Ali E.Y., Ghoneum M.H. Antidiabetic efficacy of Trifolium alexandrinum extracts hesperetin and quercetin in ameliorating carbohydrate metabolism and activating IR and AMPK signaling in the pancreatic tissues of diabetic rats. Biomed. Pharmacother. 2022;149:112838. doi: 10.1016/j.biopha.2022.112838. [DOI] [PubMed] [Google Scholar]
- 118.Wahyuono R.A., Hesse J., Hipler U.-C., Elsner P., Böhm V. In vitro lipophilic antioxidant capacity, antidiabetic and antibacterial activity of citrus fruits extracts from Aceh, Indonesia. Antioxidants. 2017;6:11. doi: 10.3390/antiox6010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dehghan M., Fathinejad F., Farzaei M.H., Barzegari E. In silico unraveling of molecular anti-neurodegenerative profile of Citrus medica flavonoids against novel pharmaceutical targets. Chem. Pap. 2022;77:595–610. doi: 10.1007/s11696-022-02496-3. [DOI] [Google Scholar]
- 120.Figueiredo R.T., Bittencourt V.C.B., Lopes L.C.L., Sassaki G., Barreto-Bergter E. Toll-like receptors (TLR2 and TLR4) recognize polysaccharides of Pseudallescheria boydii cell wall. Carbohydr. Res. 2012;356:260–264. doi: 10.1016/j.carres.2012.02.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.