Abstract
Levonorgestrel intrauterine systems (LNG-IUSs) are complex drug-device combination products designed to release a hormonal contraceptive drug for up to 7 years. These drug delivery systems offers a great promise as a modern method of long-acting reversible contraceptives (LARCs) to improve women’s health. Unfortunately, there are some scientific challenges associated with the development of these products which are among the major reasons contributing to the availability of relatively few IUS products on the market. This review summarizes the formulation considerations (drug and excipient attributes), manufacturing methods, advances in characterization and in vitro drug release testing of IUSs, as well as factors influencing drug release from IUSs. A critical discussion on the major challenges to IUS product development is presented. Specifically, insights on bioequivalence evaluation, in vitro-in vivo correlation (IVIVC) establishment, and regulatory challenges are detailed. Lastly, methodological tools to overcome some of these hurdles to product development are proposed. The knowledge furnished through this review will be helpful towards obtaining better product understanding. Such understanding will facilitate the development of these complex drug products, as well as their regulatory approval process.
Keywords: Polydimethylsiloxane, Silicone, Levonorgestrel, Intrauterine device, Long-acting implants, Long-acting reversible contraceptives, Manufacturing, In vitro drug release, Drug-Device Combinations, Silica
Graphical Abstract

1. Introduction
The unmet clinical needs across several health conditions have led to the development of therapeutic products that combine drugs and devices which allow local administration, precise drug targeting and individualized patient therapy [1]. In recent years, drug-device combination products have introduced a new dynamic in the healthcare landscape providing more effective and novel treatment options to significantly advance patient care. Levonorgestrel intrauterine systems (LNG-IUSs) offer one such promising drug-device combination strategy. LNG-IUSs contain a hormonal contraceptive drug (LNG) in a polymer matrix around a vertical polyethylene stem that is covered with a polydimethylsiloxane (PDMS) membrane to regulate drug release in the uterine cavity [2]. IUSs, also known as long-acting reversible contraceptives (LARCs), are safe, extremely effective (with a typical-use first year pregnancy rate of 0.1% whereas all other methods have typical-use pregnancy rates of approximately 1% and above), easy to use (non-surgical), highly patient-compliant drug-device combination products that provide drug release for 3–7 years [3–6]. LNG-IUSs have been placed on the Essential Medicines List by the World Health organization (WHO) [7]. They are well accepted worldwide and have also shown important non-contraceptive benefits such as in the treatment of heavy menstrual bleeding [8]. Despite several advantages, there are a number of product development challenges associated with IUSs which have most likely contributed to the paucity of commercial IUS products and the lack of generic equivalents.
The design of IUSs is complex and involves the use of specialized manufacturing methods with numerous process variables. It is essential for pharmaceutical manufacturers to identify and understand the critical formulation and processing parameters to achieve robust product design and consistent performance. The design of drug-device combination products requires additional considerations to maintain the physical integrity of the device and prevent any adverse interactions between the drug and device components. The formulation excipients should be compatible with the materials used in the device, and all materials should be selected based on the manufacturing method. It is also important that suitable physicochemical characterization methods and physicomechanical tests are available to demonstrate that the product meets the manufacturing and performance requirements. To date, limited reports are available on the formulation and characterization of IUSs [9–11]. Two commercial IUS products, Mirena® and Liletta® have the same drug loading and although the daily drug release rates are fairly similar, by the end of five years of use the cumulative drug release percentages are different (60% for Mirena® and 49.8% for Liletta®) [2, 12, 13]. The excipients used in the formulation of IUSs (such as PDMS and silica) have been used in drug delivery for a long time. Nevertheless, the impact of excipient attributes on formulation and drug release of IUSs still remains unclear. Previous reports have elaborated that silicone polymers and additives are available in a wide range of chemistries which may impart different properties to the product [14–16]. IUSs are designed to achieve controlled release of the drug over several years and thus require high drug loading. Unanticipated changes and/or dose dumping during in vivo release may compromise product safety and efficacy. Therefore, it is imperative to understand and control the critical physicochemical attributes and in vitro release characteristics of these products. Furthermore, the extremely long-acting nature of these products and slow drug release rates pose challenges to the development of suitable in vitro release testing methods. Lately, researchers have developed real-time and accelerated in vitro release testing methods for LNG-IUSs which can be used for quality control and batch testing, formulation optimization, and may be applicable for bioequivalence testing [12, 17]. However, the suitability of these methods to reflect in vivo changes might still need to be addressed. In addition, drug release mechanisms from IUSs have not yet been fully understood. There are scientific hurdles for the establishment of IVIVCs such as the extremely long-acting nature and the local action of these drug products. With the increasing number of IUS products on market, the Food and Drug Administration (FDA) has recently issued some regulatory guidance to accelerate their generic drug product development. Despite these efforts, additional product-specific guidance is warranted to enable pharmaceutical manufacturers to uncover the complexities involved in these products and facilitate regulatory review.
The current manuscript offers a detailed description of the formulation components, manufacturing, characterization tools, in vitro drug release testing methods for IUSs and highlights the effect of formulation factors that may influence product performance. An in-depth discussion on the existing challenges to the development of these products is also provided. In addition, insights on possible strategies to overcome some of the critical challenges and future directions to guide research efforts to propel the development of long-acting IUSs are presented.
2. Evolution of contraceptive methods
The earliest practices of family planning until the late 19th and 20th centuries involved the withdrawal method and fertility awareness-based methods such as abstaining on fertile days [5, 18, 19]. Heavy metals such as copper, mercury and lead, and medicinal herbs such as pessaries of acacia and honey, silphium seeds (giant species of fennel), olive and cedar oils were traditionally used for birth control. These methods were not only less efficacious but some of them also resulted in toxicity and led to high mortality rates [19–21]. Mechanical contraceptives include the use of condoms, sponges, and vaginal diaphragms which are less effective, require proper insertion, and are associated with vaginal irritation and urinary tract infections. Surgical methods to induce permanent sterilization like vasectomy and hysterectomy are very invasive and cause a risk to patients’ lives [22].
In response to the problems of family planning, the FDA approved the use of the first hormonal oral contraceptive pill Enovid in 1960 [23]. However, the high doses of progestin and estrogen in the pill caused severe side-effects such as thromboembolism and stroke [24]. In 1960s, the Lippes loop entered into the market as the first traditional device for intrauterine insertion [25]. The Lippes loop was a non-medicated, flexible double S-shaped polyethylene loop. Later in 1970s, another device for intrauterine insertion included the Dalkon Shield which comprised of a plastic five-pronged crab-like shield [26]. However, these traditional devices were large in size, had design flaws, and led to serious injuries, infection, increased risk of pregnancy complications, and death [27]. Therefore, these devices were eventually banned by the FDA and withdrawn from the market. The introduction of fiberoptic technology in 1970s allowed safer and quicker surgical procedures such as tubular litigation which was a permanent sterilization method [28]. In 1988, Paragard, the first copper IUD became available in the US. The copper IUDs (Paragard TCu 380a, 32 mm × 36 mm) have a T-shaped frame wound with copper wire and have copper sleeves [29]. These IUDs were the first dosage forms for long-term contraception and were efficacious. However, they were later associated with irregular and heavy menstrual periods, increased likelihood of dysmenorrhea, abdominal pain and cramps, uterine perforations and bleeding [30]. Several safer options with progestin-based long-term hormonal contraceptives were made available in the 1990s with the approval of subdermal implants such as Norplant® (silicone rods of 2.4 mm diameter and 34 mm long providing contraceptive action for up to 5 years) and Depo-Provera 150 mg/mL (medroxyprogesterone acetate administered as a subcutaneous injection every 3 months) [31, 32]. In 1999, the FDA approved Plan B® pills (containing levonorgestrel) for emergency contraception [33]. Over the years, several combination oral contraceptive pills have been introduced in the market and the doses of estrogen/progestins have been progressively lowered to reduce the adverse events such as thromboembolism and stroke [34, 35].
The progestin revolution took off in the year 2000 with the FDA approval of the first hormonal intrauterine system Mirena® (52 mg LNG) which was indicated for the treatment of heavy menstrual bleeding and contraceptive purposes for up to 5 years initially [36]. The limitations of the traditional IUDs were overcome by LNG-IUSs. Instead of using copper or non-medicated elements, a hormonal contraceptive drug, LNG, was incorporated in the intrauterine device. LNG-IUSs are available as steroid reservoirs mounted on a T-shaped polyethylene frame (32 mm × 32 mm) [2]. Unlike the traditional IUDs, the hormonal IUSs offered a highly safe and efficacious method of long-term contraception without the heavy menstrual bleeding issues [37]. Two international multicenter clinical studies showed that the 5-year cumulative pregnancy rate for Mirena® was 0.5–1.1% whereas that of copper IUDs was 1.4–5.9% [38, 39]. Following the approval of the first hormonal IUS, other long-acting reversible methods of contraception such as vaginal rings (Nuvaring®, etonogestrel/ethinyl estradiol vaginal ring for 3 weeks), birth control transdermal patches (Xulane®, norelgestromin/ethinyl estradiol matrix-type transdermal system for 4 weeks), and single-rod implants (Nexplanon®, etonogestrel implant for 3 years) were developed and approved in the United States [40]. Recently, the FDA has approved Mirena® for pregnancy prevention for up to 7 years [36]. The use of LNG-IUSs has been rapidly increasing in the past two decades. Currently, three other LNG-IUSs: Liletta®, Skyla®, and Kyleena® are also available on the US market (Table 1). Fig. 1 depicts the timeline of the development of various contraceptive methods.
Table 1.
| Product name | Strength | Effective duration | In vivo drug release rate at the end of first year of use | In vivo drug release rate at the end of approved year of use | Applicant holder | Year of approval |
|---|---|---|---|---|---|---|
| Mirena® | 52 mg | 7 years* | 18.0 μg/ day | 8.0 μg/ day (7 years) | Bayer | 2000 |
| Liletta® | 52 mg | 6 years | 17.0 μg/ day | 8.6 μg/ day (6 years) | Allergan/ Medicines360 | 2015 |
| Kyleena® | 19.5 mg | 5 years | 9.8 μg / day | 7.4 μg/ day (5 years) | Bayer | 2016 |
| Skyla® | 13.5 mg | 3 years | 6.0 μg/ day | 5 μg/ day (3 years) | Bayer | 2013 |
The US FDA has extended the duration of use for Mirena® up to 8 years in 2022.
Fig. 1.

Evolution of various contraceptive methods.
3. Levonorgestrel intrauterine systems: modern day solutions to contraception
3.1. Description of LNG-IUSs
LNG-IUSs comprise of a cylindrical drug-polymer reservoir mounted around the vertical stem of a T-shaped polyethylene frame, and covered with an outer semi-opaque polymer membrane (Fig. 2A). The drug-polymer reservoir is a mixture of LNG and PDMS whereas the outer translucent membrane is primarily made of PDMS. The outer PDMS membrane controls the drug release rate from IUSs. This outer membrane has a smooth surface and does not cause abrasion in the uterine lining. The T-frame is composed of low-density polyethylene along with barium sulphate which makes it radio-opaque and capable of monitoring via ultrasound [2, 13]. The T-frame attaches the IUSs to the fundus and holds them in a vertical position in the uterine cavity. The two horizontal arms at the upper end of the T-frame are soft and flexible to facilitate insertion and avoid any irritation to the uterine tissues. The T-frame has a loop at the bottom end of the vertical stem and a blue polypropylene monofilament thread consisting of a copper-containing pigment as a colorant is attached to the loop [13].
Fig. 2.
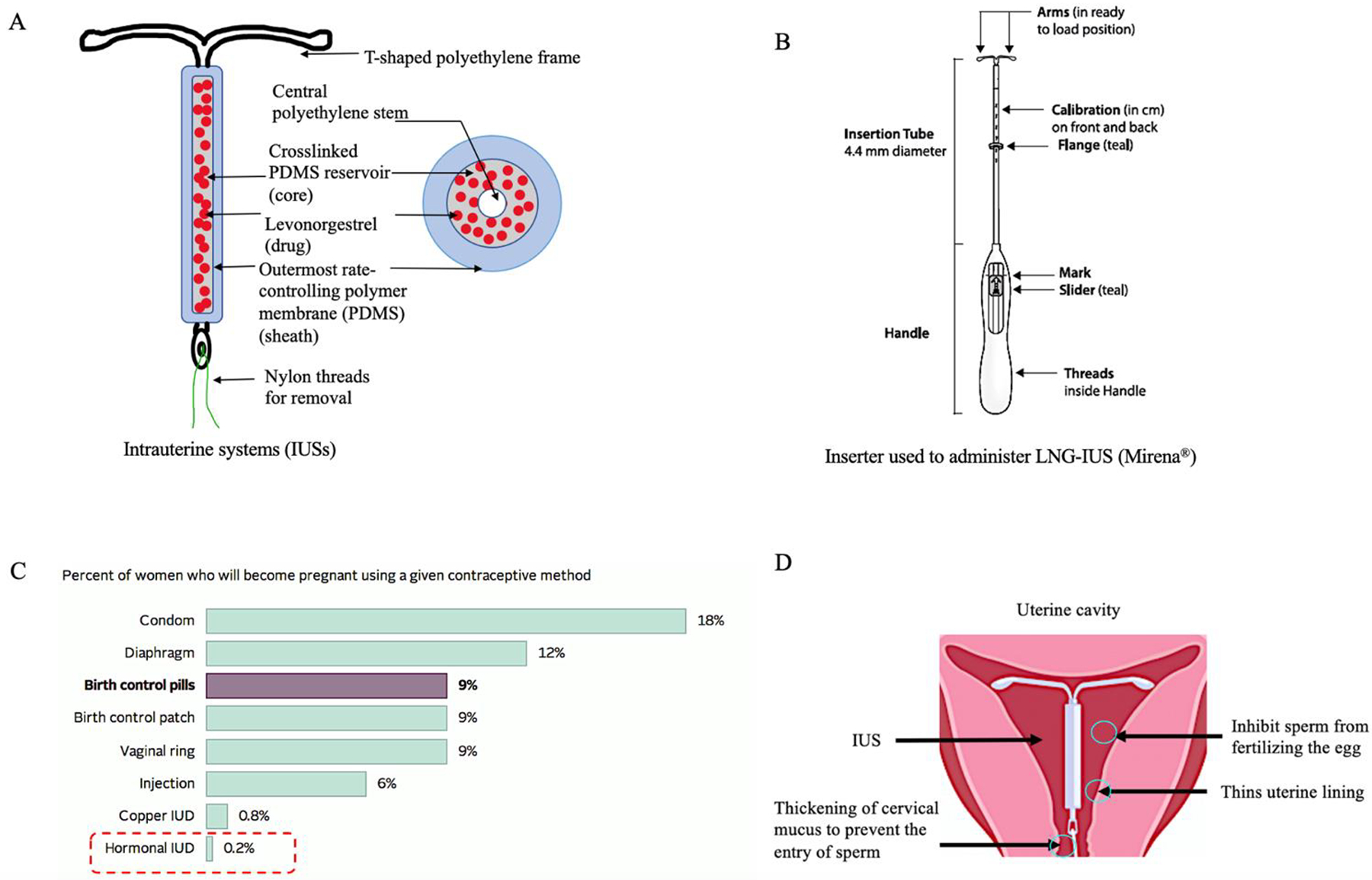
A) The structure of LNG-IUSs; B) inserter design for administration of IUSs in the uterine cavity; C) failure rates of different contraceptive methods; and D) mechanisms of pharmacological action of LNG-IUSs. [2, 5, 17, 36]
LNG-IUSs are packaged sterile with a single-use disposable inserter pre-loaded with the IUS product for intrauterine insertion. The inserter consists of a symmetric two-sided body and a slider that are integrated with a teal flange, an insertion tube and a plunger. The dimensions of the inserter may vary depending on the dimensions of different IUS products. A diagram of the inserter used for Mirena® is shown in Fig. 2B. LNG-IUSs are inserted using aseptic techniques by a trained medical practitioner. Usually, IUSs are inserted into the uterine cavity during the first seven days of the menstrual cycle. It is also considered safe to administer IUSs immediately after uncomplicated spontaneous or induced first-trimester and second-trimester abortions [2, 13, 41]. For postpartum insertion, it is recommended by the WHO to insert IUSs four weeks after childbirth to avoid the risk of perforation and expulsion [41]. LNG-IUSs are inserted to a uterine depth of 6–10 cm which is predetermined using ultrasound. Fundal positioning of IUSs is required to prevent its expulsion. Once the IUSs are administered using the inserter, the polypropylene monofilament threads are cut, leaving about 3 cm visible outside the cervix to monitor the position of IUSs after insertion and also to aid in IUS removal [2].
3.2. Advantages of LNG-IUSs and changing landscape of women’s healthcare
The introduction of LNG-IUSs has resulted in a drastic decline in the number of surgical irreversible contraceptive procedures being carried out, increased use of long-acting reversible contraceptives among women with a significant reduction in abortion and unintended pregnancy rates [42, 43]. Ultimately, this has improved the overall quality of life in women of different reproductive age groups. According to the Centers for Disease Prevention and Control (CDC), LNG-IUSs are the most methods of contraception with more than 99.5% efficacy [5]. LNG-IUSs have the lowest failure rates as compared to any other contraceptive method currently available on the market (Fig. 2C). The contraceptive efficacy heavily relies on patient compliance, and unlike oral pills, weekly or monthly injectables and transdermal patches, LNG-IUSs do not require patients to remember frequent administration of dosage regimens [44]. Moreover, the administration of IUSs is non-surgical (unlike the subdermal implants). Apart from its contraceptive action, the LNG-IUS product Mirena® provides additional advantages such as reduced menstrual blood loss and pain by suppressing endometrial proliferation [45, 46]. This offers improvements in hemoglobin and ferritin levels in the body benefitting in conditions like anemia [47, 48]. The use of LNG-IUSs in women in the reproductive age group of 15–44 years has increased from 2% in 2002 up to 14% in 2017 [49]. In a clinical study by Secura et al., it was demonstrated that 75% of women opted for a LARC method as their preferred choice. Out of which, 46% chose an IUS, 12% chose a copper-IUD, and 17% chose a subdermal implant [50]. In a study conducted on nulliparous women, administration of oral contraceptives and LNG-IUSs showed similar continuation rates for 1 year but the overall satisfaction rate was significantly higher with LNG-IUSs as compared to the oral contraceptives [51]. According to another report, the satisfaction rates after three years of LNG-IUS insertion were as high as 94% [52]. The rate of ectopic pregnancy with LNG-IUSs was 0.6/1000 women which is approximately 23% lower compared to the ectopic pregnancy rates in women using no contraceptive methods [46]. LNG-IUSs are well tolerated and its local action on the endometrium along with its low systemic side effects have led to its use in several other conditions such as benign menorrhagia, endometriosis, estrogen-induced endometrial hyperplasia, and uterine fibroids [52, 53].
3.3. Mechanisms of pharmacological action
The local mechanism through which LNG-IUSs induce a contraceptive action is not completely understood and it is believed to be brought about by multiple mechanisms (Fig. 2D). Direct intrauterine administration of LNG-IUSs results in high endometrial concentrations of 450–1500 ng/g of tissue weight [46]. LNG is rapidly absorbed by the capillary network underlying the basal endometrial mucosa and peak plasma concentrations of approximately 150–200 pg/mL were obtained after a few hours of administration [46]. The plasma concentrations of LNG gradually decline over several years of use. Local release of LNG induces changes in the endometrial morphology such as atrophy of endometrial glands, functional and morphological changes in stromal decidual lining, and inflammatory cell infiltration [54, 55]. Endometrial atrophy prevents the implantation of embryos in the uterus. The estrogen and progesterone receptors on the endometrial lining are downregulated and eventually endometrial activity is lost. The effect of LNG on endometrial atrophy is completely reversible on removal of the IUSs and no long-term adverse effects on the endometrium have been reported [56]. Physiological changes such as thickening of cervical mucus is observed which prevents the passage of sperm into the uterus. In addition to the mechanisms described above, LNG is also responsible for inhibition of sperm survival in the uterus [2]. Since LNG-IUSs act locally, the systemic adverse effects associated with steroidal contraceptives (such as elevated blood pressure, thromboembolism, and abdominal cramping) delivered via other routes can be avoided without compromising the efficacy.
4. Considerations in formulation development of IUSs
4.1. Polymer properties
PDMS is the major constituent in the reservoir and the outer membrane of IUSs. PDMS is a silicone polymer with repeating units of silicone and oxygen backbone with methyl substitution (Fig. 3A) [57]. Silicone polymers are transformed into a three-dimensional network through crosslinking by the formation of covalent bonds. This process is known as curing [14]. Crosslinking in PDMS occurs through one of the following reactions: a) addition curing; b) condensation curing; and c) peroxide curing. PDMS for addition curing is commercially available as two-part systems (supplied as Part A/Prepolymer A and Part B/Prepolymer B). Prepolymer A consists of mainly a silicone elastomer base while prepolymer B contains the crosslinking agent. Addition curing occurs through a reaction between vinyl groups of the elastomer base and hydride groups of the crosslinking agent in the presence of a platinum-based catalyst (Fig. 3B) [14]. This reaction usually takes place at high temperatures. Addition curing is commonly used for manufacturing pharmaceutical drug products since it does not yield any reaction by-products and provides good mechanical strength to the crosslinked polymer. Condensation curing can be carried out either using one-part or two-part PDMS polymers. Most condensation-based PDMS prepolymers are available as ready to use and do not require mixing. The crosslinking process begins as the product comes in contact with moisture in the atmosphere. These prepolymers contain acetoxy groups which get hydrolyzed by the ambient moisture to form silanols [14, 15, 58]. This allows further condensation to occur and adjacent PDMS chains become crosslinked. This reaction requires the presence of an organometallic catalyst such as tin and takes place at room temperature (known as room temperature vulcanization (RTV) process). Condensation curing is also used in some two-part systems in which reaction occurs between prepolymer A containing a hydroxy-terminated group and prepolymer B containing alkoxysilane (for example, tetra-n-propoxysilane) [14, 15, 58]. Condensation curing results in the formation of by-products such as acetic acid or alcohol which may react with drug or cause premature drug dissolution and lead to burst release. Crosslinked PDMS formed using condensation curing may result in slight shrinkage of the product and therefore this process may not be suitable for manufacturing drug products which require precise tolerances. Another method of forming crosslinked PDMS polymer is free radical chain polymerization with the use of organic peroxides [14]. This reaction is used for preparing high-consistency silicone products prepared through injection molding or extrusion. The presence of residual peroxides poses a major risk of toxicity and side-reactions with the drug leading to drug instability. Therefore, this method of crosslinking is not commonly used for the formulation of drug products.
Fig. 3.
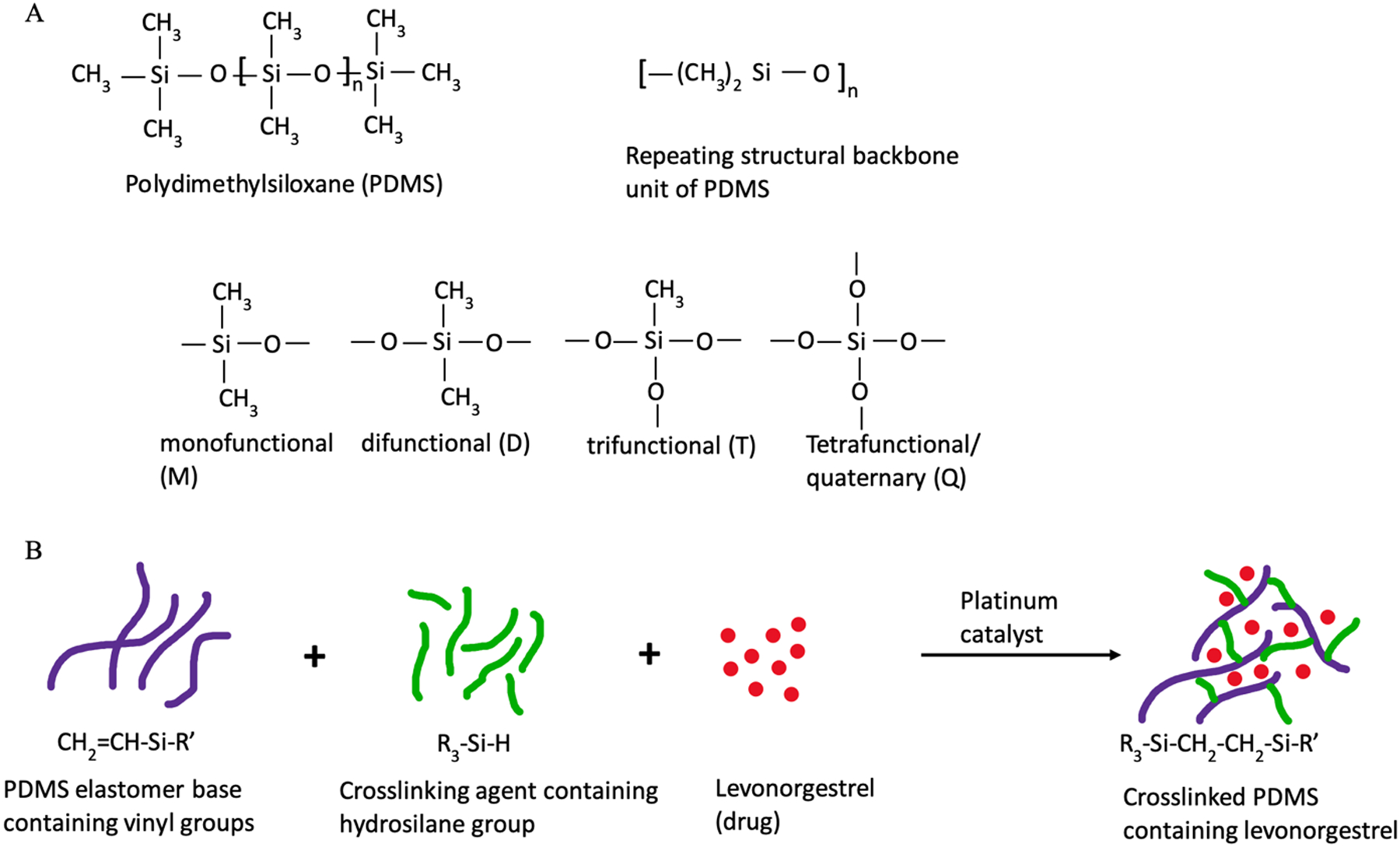
A) General chemical structure of PDMS and its various structural units; and B) addition reaction resulting in a crosslinked PDMS matrix containing the drug. [11, 17]
Crosslinking in PDMS is crucial to achieve the properties which makes it suitable for application in controlled drug delivery. PDMS crosslinking alters the surface reactivity, surface tension, thermal properties, tensile strength, and stability against moisture and oxidation. Fanse et al. showed that the degree of polymer crosslinking has a critical impact on the physicochemical and mechanical properties of LNG-IUSs [11]. High crosslinking density is essential to maintain good tensile strength, tear resistance, and elasticity to facilitate molding and demolding of IUSs during manufacturing, and to achieve the desired shape and configuration while mounting the cylindrical drug-polymer reservoirs on T-shaped polyethylene frame. These mechanical properties are also important to prevent mechanical abrasion of IUSs inside the uterine cavity during several years of its use.
In addition, the amount of non-crosslinked PDMS should be controlled in the formulations to prevent toxicity and achieve physiological inertness. The OSHA permissible exposure limits (TWA) for high viscosity silicone polymers is 6 mg/m3 and 80mg/m3/%SiO2 (silica) [59, 60]. Crosslinking imparts a hydrophobic polymer network capable of entrapping drugs and slowly releasing them over a prolonged time duration. Fanse et al. have shown that the crosslinking density should be controlled to achieve the desired drug release rates from LNG-IUSs [17].
PDMS can be synthesized in variable chain lengths to give polymers with different molecular weights and can be modified with different chemical groups on the surface to provide variable properties. The silicon and oxygen atoms in PDMS may be arranged into linear or cyclic structures and the end groups influence the properties of the polymer [61]. Typical endgroups are methyl, hydroxyl, and hydrogen. The methyl groups along the polymer chain can also be substituted by phenyl, fluoro, vinyl or ethyl functional groups which allows for tailoring the properties of the polymer [62]. For example, the presence of phenyl groups in low to medium concentrations reduces the crystallization tendency of PDMS and imparts higher elasticity even at very low temperatures [14, 62]. Trifluoropropyl-substituted chains increase the solubility parameter from approximately 15.5 MPa1/2 to 19.5 MPa1/2, which provides high solvent-resistance and reduces the swelling of PDMS in non-polar solvents [15, 62]. Aminopropyl and polyether side groups increase the water solubility whereas alkyl groups (methyl, ethyl or propyl) increase the hydrophobicity. Hydroxy-terminated endgroups have lower activation energy, lower degradation temperature, and higher reactivities as compared to the trimethylsiloxy-terminated endgroups. The presence of halogen-substituted PDMS increases the stability of the polymer [15, 62]. Cyclic siloxanes are used as intermediates to prepare high-molecular weight PDMS. Silicone copolymers with silicone as the hydrophobic part can also be prepared which gives excellent surfactant properties to the polymer. For instance, diethylsiloxane copolymers demonstrate greater hydrocarbon solubility than analogous dimethylsiloxane homopolymers [63].
4.2. Additives
Apart from PDMS, the prepolymers A and B also contain hydrolytic products of silica and catalysts (such as platinum, tin, rhodium) required for crosslinking. PDMS elastomers have extremely low tensile strength without any reinforcement which limits their applications for use as drug-devices. Therefore, additives such as resins, silica, and diatomaceous earth are commonly used as fillers to impart mechanical strength to the polymer [64]. Silicone resins are highly branched polymers. Often, silicone resins are reacted with other polymers to form PDMS-based copolymers which provide high hydrophobicity [15, 61]. Diatomaceous earth is obtained from natural sources and is used as a substitute for silica. It is a low density filler with relatively larger particle size than silica, and generally has a porous three-dimensional microstructure [65]. In a recent report, the use of diatomaceous earth as a filler in PDMS-based composites was shown to improve the tensile strength and elasticity, while reducing the weight loss and thermal degradation compared to the unfilled polymer [65]. Fumed silica, which comprises of aggregates of amorphous silica nanoparticles is one of the widely used reinforcing fillers. In a previous study, it was shown that the viscosity, storage modulus and tear resistance of PDMS-silica composites were directly dependent on the loading (% weight) of colloidal silica dispersions [66]. Differences in the size and morphology of filler particles as well as the surface functionality of the dispersion medium resulted in different interfacial interactions and polymer-filler interactions. It is believed that the surface polarity due to the hydroxyl groups on silica particles can form hydrogen bonding with PDMS and the large surface area of the silica nanoparticles (100–400 m2/g) encourages polymer adsorption onto the filler [14, 67]. Other additives used in LNG-IUSs may include pore-formers, complexing agents and permeability enhancers. The numerous polymeric architectures in PDMS and the various additives (Table 2) can be utilized to achieve the desired properties in LNG-IUSs.
Table 2.
Excipients (polymers and additives) that may be potentially used in the formulation of LNG-IUSs.
| Component | Formulation variables |
|---|---|
| Polymers | Polydimethylsiloxane-based (PDMS) (silicones) |
| Different attributes- molecular weight, viscosities, chain length, etc. | |
| Functional group substitutions | |
| Dimethyl-substituted | |
| Phenyl-substituted | |
| Fluoro-substituted | |
| Ethyl-substituted | |
| End-groups | |
| Alkyl (methyl, ethyl, or propyl) | |
| Aminopropyl | |
| Hydroxyl | |
| Additives | Silica |
| Diatomaceous earth | |
| Silicone resins | |
| Silicone oil |
4.3. Manufacturing methods for IUSs
Different manufacturing methods such as extrusion, compression, transfer molding, and injection molding are available to process silicone elastomers, which may be used in the preparation of implantable drug-devices such as LNG-IUSs. Suitable methods need to be selected for preparing the drug-polymer matrix (core) and the outer polymer membrane of IUSs (either the same method or different methods may be used). Most of the manufacturing methods require pre-mixing of the drug, polymer, and additives which can be carried out using a planetary mixer (for example, Flacktek® dual asymmetric centrifuge planetary speedmixer). The speedmixer is a bladeless mixer which rotates the sample clockwise at a specific angle around a central axis, while simultaneously counter-rotating the sample along its own axis [68]. Samples with a wide range of viscosities can be mixed with high reproducibility and throughput. Different sizes of sample holders can be used in the speedmixer which allows easy scale-up for bulk manufacturing. Alternatively, manual mixing may be used but it requires sufficient de-aeration of the mixture to remove air bubbles. A two-roll mill is another technique for mixing the drug, prepolymers, and the additives using counter-rotating horizontal rollers. Depending on the properties of the materials to be processed, the rollers may be heated electrically or cooled using a circulating water cooler [69].
Extrusion is a manufacturing process in which the mixture is added through a hopper into the die-cavity. The feed-rate can be controlled and the required amount of material can be fed into the die-cavity via the conveying screw (Fig. 4A). Screens of appropriate sizes can be used to eliminate foreign particles, homogenize the feed, and remove large air bubbles [16, 70]. The die-cavity can be designed to provide a suitable shape to the extrudate. The mixture can be cured in the die-cavity at high temperatures and finally extruded from the die. The viscosity of the material, pressure applied during the extrusion, and the die geometry (for example, dead volume or sharp corners in the die) are important parameters that need to be controlled [16, 70]. Bao et al developed an easy-to-use laboratory scale extrusion method using custom designed polycarbonate molds and a twin syringe (Fig. 4B) [9]. Briefly, the assembly composed of two 3-mL polypropylene syringes with the needle hub removed, a hollow cylindrical polycarbonate mold, a stainless steel metal rod, and two polycarbonate centering devices. The premixed drug-polymer mixture was transferred into the twin syringe assembly and extruded into the cylindrical polycarbonate mold through a plunger and cured at a high temperature. The cured drug-polymer reservoirs were demolded, further fabricated into a suitable length and mounted on a polyethylene frame.
Fig. 4.

Different methods that may be used for manufacturing of IUSs. A) extrusion method [16]; B) modified lab-scale extrusion using twin syringe method [9]; C) compression molding method [16]; and D) injection-molding method (single injection-molding and continuous overmolding method) [70, 73].
Compression molding is a method which is preferred for materials with high viscosity. The sample mixture is placed inside a cavity between a movable top mold and a fixed bottom mold (Fig. 4C). The molds and the cavity are heated as the moving arm of the top mold compresses the sample into the bottom mold to achieve the desired shape. The sample is then demolded from the ejector using either the mold releasing agents or via pressure ejection through a plunger [70]. The pressure applied on the sample, curing conditions, ejection pressure, and type of mold-releasing agents used can have critical impact on the quality of the product. The use of compression molding to design hollow cylindrical reservoirs of IUSs may be challenging (compared to solid cylinders, circular or rectangular molds) and thus this method may be less suitable for IUSs.
Injection molding is a widely used and efficient technique for processing large quantities of PDMS-based products with high accuracy. A schematic of the injection molding process is depicted in Fig. 4D [70, 71]. Two different tanks containing prepolymers A and B (premixed with drug) are pumped into a mixing chamber via metering units. The individual tanks containing the prepolymers can be either static or dynamic in which the material is constantly mixed and homogenized. The drug-polymer mixture is then fed into a dosing cylinder of the injection-molding machine. Specified quantities of the mixture are further injected into the mold via the conveyer screws in the dosing cylinder. The drug-polymer mixture is cured at an appropriate temperature and time in the mold and finally ejected using either a plunger, ejector pins, or mold-releasing agents. Controlling the injection force, injection speed, accurate dosing, mold temperatures, and eliminating air in the feed are very critical to achieve good reproducibility and product quality. In some cases, post-curing is performed at high temperatures for a short time to improve mechanical properties and eliminate residual volatile materials and by-products [16]. The injection molding method can be used to prepare the drug-polymer reservoir (core) as well as the outer polymer membrane. The outer polymer membrane can be molded separately and then coated onto the drug-polymer reservoir. Alternatively, injection overmolding can be carried out to mold the polymer membrane onto a previously cured drug-polymer reservoir. Premixed drug-polymer mixtures can also be fed into the injection molding machine and then extruded into a mold to form implants with the desired thickness.
Extrusion and injection molding may be the most suitable techniques to prepare the outer polymer membrane either via batch processing or continuous processing. Other methods such as spray-coating and dipping method may also be used to coat the polymer membrane on the reservoirs [72]. However, it is very challenging to achieve a uniform thickness of the polymer membrane and batch-to-batch consistency via these methods. Another technique commonly employed for coating the outer polymer membrane is the use of a solvent swelling method. In this method, the polymer membrane prepared using one of the above methods or a prefabricated membrane is allowed to swell in a nonpolar solvent. The swollen membrane is removed from the solvent and the excess solvent on the surface is wiped. Finally, this swollen membrane is covered on the drug-polymer reservoir and vacuum dried to remove the solvent. According to one of the methods described in a patent, the prefabricated tubing can also be swollen mechanically with the use of a pressurized gas [72].
5. Characterization of IUSs
Several spectral, thermal, and mechanical techniques for characterization of PDMS-based LNG-IUSs have been reported in the literature. A suitable method for evaluating the degree of crosslinking in IUSs is essential to ensure complete curing at the specified conditions, assess the impact of formulation components on processability and to determine any deviations in the manufacturing process. Two different methods to determine the extent of polymer crosslinking in LNG-IUSs have been reported [11, 17]. The first method determines the degree of PDMS crosslinking (expressed as percentage weight of the formulation) based on the total soluble gel fraction of LNG-IUSs using solvent extraction. The second method determines crosslinking density (expressed in moles/cm3) based on the polymer swelling properties using the Flory-Rehner theory.
FTIR and NMR are widely available tools for detecting structural variations arising due to PDMS with different substitutions and end groups [11, 15, 58, 62]. The silicones in LNG-IUSs show strong absorption bands at 1260 cm−1, 1000–1100 cm−1 and 790 cm−1 in the mid-IR range. The characteristic FTIR peak at 912 cm−1 is attributed to the -Si-H (hydrosilane) groups in the crosslinking agent and can provide useful information on the crosslinking density of the product. Solution-state 1H NMR shows signals at approximately 4.7 ppm corresponding to the hydrosilane groups and multiplets between 5.7 to 6.3 ppm corresponding to vinyl protons in the PDMS elastomer base. Structural details about the type of substituents on the silicone backbone such as phenyl, methyl, vinyl may be obtained using 13C NMR. The structure of silicone polymers is also assessable using 29Si solid state NMR [15, 17]. However, the low natural abundance of 29Si (4.7%) leads to decreased sensitivity and long accumulation time for measurements.
Gas chromatography coupled with mass spectrometry (GC-MS) can be used to detect PDMS oligomers and other volatile substances in IUSs [58]. Gel permeation chromatography (GPC) (also known as size exclusion chromatography) is a commonly employed technique to obtain information on molecular weight and distribution. Atomic absorption spectroscopy and X-ray fluorescence allow quantification of silicone content in the formulations [62]. However, these techniques will be applicable for accurate quantification if standards can be prepared in the same matrix as the sample. Surface tension measurement is a useful technique to determine the relative hydrophobicity of PDMS incorporated with different additives [62]. Changes in the contact angle arising from differences in surface free energies can provide an indication of solvent penetration and drug diffusion through the polymer.
Variations in material attributes, filler content, and processing conditions may impact the thermal characteristics and stability of the formulation. Thermal events such as melting and crystallization of drug and polymer, residual solvent, weight loss, and degradation can be investigated using DSC and TGA. DSC can be typically used at low temperatures for monitoring polymer melting (at approximately −42°C) and crystallization (at approximately −70°C). DSC can also be utilized to monitor the exothermic enthalpy changes associated with the PDMS crosslinking reaction in IUSs [11, 15, 62]. On the other hand, TGA is used for monitoring polymer degradation profiles at high temperatures (above 300°C). However, TGA does not give information on the nature of the volatilized species and this requires the use of advanced hyphenated techniques such as GC-MS.
Characterization of mechanical strength of IUSs is an inevitable aspect in the evaluation of product performance. Currently, there are no standardized methods available for characterization of the mechanical properties of IUSs. A recent report has described the use of: a durometer for hardness testing of the drug-polymer reservoirs and the outer polymer membranes, a texture analyzer for determining the elasticity and tensile strength, and a dynamic mechanical analyzer for analyzing the moduli properties and glass transition in LNG-IUSs [11]. Such techniques can be used for product evaluation and quality control purposes during formulation development and screening.
6. Drug release testing of IUSs
According to the existing literature reports, in vitro drug release testing of IUSs have been carried out using water shaker baths [9, 12, 74]. The formulations are typically placed in tightly capped Pyrex® glass bottles containing release media and are kept in a water shaker bath at 37°C with a rotation speed of 100 rpm. Two small steel clips may be clamped on the polyethylene frame of the IUSs to ensure that they sink at the bottom of the glass bottles. Selection of a suitable release medium for intrauterine delivery is challenging due to: (i) variable pH in the intrauterine cavity (ranging from pH 8 in the fallopian tubes, 6.5–7 in the uterus, and gradually becoming acidic towards the vaginal cavity); (ii) physiological changes due to age and metabolism resulting in compositional changes in the uterine fluids; and (iii) varying physiological conditions throughout the menstrual cycle and with disease states [75]. Normal saline (0.9% w/v sodium chloride) is used as the release media for real-time in vitro drug release testing of IUSs [9, 12]. The high amount of Na+, H+ and Cl− ions in the uterine cavity (which maintains the ionic balance and homeostasis) may explain the use of saline as a release medium. The volume of release medium is dependent on the drug loading in IUSs and ensuring that sink conditions are maintained throughout the test. Since drug release from IUSs is extremely slow (over several years), accelerated in vitro release tests have been reported [12, 17]. For optimization of the in vitro release method, the impact of surfactants, organic solvents, and temperature on drug release from IUSs was investigated [12]. Surfactants had a relatively lower effect on accelerating drug release from LNG-IUSs. The drug release rates depicted a temperature-dependent release and obeyed the Arrhenius law. Use of organic solvents greatly increased the release rates of LNG-IUSs and followed the rank order: tert-butanol > isopropyl alcohol > ethanol [12]. Bao et al. utilized 20% v/v tert-butanol in phosphate buffered saline (PBS) with 0.25% w/v of SDS as the release media for accelerated testing of Q1/Q2 equivalent LNG-IUSs with different drug loading and product design parameters [10, 12]. Fanse et al. reported the use of 45% v/v tert-butanol in PBS for accelerated testing of non-Q1/Q2 equivalent LNG-IUSs with different crosslinking densities [17]. All of the above mentioned real-time and accelerated in vitro release testing methods demonstrated good discriminatory ability and reproducibility, and can be used for batch testing and quality control of IUSs during product development.
6.1. Factors affecting drug release from IUSs
To achieve long-term release (3–7 years) in LNG-IUSs, a large amount of drug is incorporated into these systems. Consequently, unanticipated changes in in vivo drug release and/or dose dumping may occur which may lead to adverse consequences in terms of product safety and efficacy. Therefore, it is essential to understand the physicochemical properties affecting drug release from long-acting LNG-IUSs.
6.1.1. Critical drug attributes
The drug particles in LNG-IUSs are in the solid-state and critical drug attributes such as particle size, morphology, and crystallinity may affect the drug release. In a previous report, it was shown that drug release from LNG-IUSs (for 12 months) with a small drug particle size (approximately 12 μm) had a slightly higher release rate (p-value = 0.21) than LNG-IUSs with a larger drug particle size (approximately 31 μm) [10]. A different report revealed that drug particle size did not have any significant impact on the release rates from LNG-IUSs [17]. This could probably be due to the relatively smaller difference in the drug particle sizes used (approximately 10 μm and 5 μm). Since daily drug release rates of LNG-IUSs are extremely low and the results were reported for only up to 4 months, differences in drug release might be observed on continued testing for longer duration. The morphology of drug particles, extent of crystallinity, and/or the presence of polymorphic forms may also influence the drug release rates. The release rate is also dependent on the amount of drug present in the reservoirs of IUSs. Different drug loading can be achieved in IUSs to obtain different target daily drug release rates. The daily drug release rate (%) of LNG-IUSs is inversely proportional to the drug loading [9]. This means that IUSs with lower drug loading will show faster release rates and will sustain the drug release for a shorter time duration.
6.1.2. Excipients
PDMS is the release-controlling excipient present in the drug-polymer reservoir as well as the outer polymer membrane of LNG-IUSs. Modifications in the physicochemical properties of the formulations induced by the substitution of surface organofunctional groups or different endgroups in PDMS (for example, hydroxyl, hydrogen, or vinyl) may modulate the hydrophobicity of the polymer and consequently the drug release from IUSs. Introduction of trifluoropropyl groups on PDMS has been shown to reduce the permeability of lipophilic steroidal drugs through the PDMS matrix [14, 72]. This is believed to occur due to suppression in the swelling behavior of PDMS due to the presence of the fluoropropyl groups. Different drug release rates can be achieved by substitution or grafting of poly(alkylene oxide) groups to the polysiloxane groups of PDMS. It was shown in a study that the relative permeability of LNG and 17-β-estradiol in the PDMS matrix increased proportionally to the concentration of polyethylene oxide added to the formulations [76]. In another study, addition of polyethylene glycol and propylene glycol increased the drug release rates from indomethacin-PDMS transdermal patches which was attributed to higher pore formation and subsequent water absorption [77]. The fillers added to improve the structural integrity of the matrix may also have an impact on drug release from IUSs. The presence of silica also increases the moisture content in the polymer which may affect the initial burst release of the drug [14, 58]. The matrix and/or the outer polymer membrane may also consist of complex forming agents such as cyclodextrins in small quantities to adjust the initial burst release of the drug [72]. PDMS with differences in molecular weight, viscosities, and chain functionality (mono-, di-, tri-, or quaternary-functional units) lead to differences in the hydrophobicity of the matrix, and show different polymer-solvent interaction parameters as well as swelling behavior in various organic solvents [15]. This may result in different diffusion coefficients of the drug through the polymer matrix. Furthermore, the prepolymer mixing ratio is an important material attribute of LNG-IUSs. Since the formation of the polymer matrix depends on crosslinking between the prepolymers A and B, it is critical to mix these prepolymers in a suitable stochiometric ratio to achieve the highest crosslinking density. The vendors suggest a mixing ratio for PDMS polymers, however, the recommended conditions may not necessarily provide the highest crosslinking density in the final formulations. Fanse et al. showed the impact of prepolymer ratios on the crosslinking density of LNG-IUSs. It was observed that the crosslinking density influences the formulation properties such as porosity, polymer crystallinity, solid-state characteristics, and hydrophobicity which subsequently affect drug release from LNG-IUSs [17].
6.1.3. Processing variables
Variations in the manufacturing method for LNG-IUSs may have a significant impact on the formulation and drug release properties. Depending on the curing technique (addition curing or condensation curing), different by-products may be generated which can affect drug solubilization, permeation, and/or degradation. Processing conditions such as curing temperature and time are very critical to ensure complete polymer crosslinking and to avoid overcuring of the polymer, degradation, and potential drug-polymer interactions. Therefore, optimizing the curing conditions is essential to ensure product quality and avoid irregular in vitro and in vivo drug release. As discussed previously, viscosity of the drug-polymer mixture and the pressure applied during extrusion and/or compression may have an impact on the microstructure of LNG-IUSs which can impact their drug release characteristics. The viscosity of some silicone polymers may increase during storage due to a particular polymer-additive orientation which can be reversed with time [70]. This may affect the manufacturability of IUSs and ultimately their drug release characteristics. However, this may not be a concern for processing methods using automated extrusion and injection molding. This is because the conveyer screw causes mixing of the prepolymers and a subsequent reduction in the viscosity, enabling the material to flow better and provide accurate dosing to the mold with every batch. Ultimately, this will ensure reproducibility in both in vitro and in vivo release behavior. Therefore, selection of the manufacturing method and control of processing variables is pivotal in ensuring good performance of LNG-IUSs.
6.1.4. Outer membrane
The outer polymer membrane presents a diffusion barrier to the drug and therefore the thickness of the membrane significantly affects drug release. Drug release rates decrease as the thickness of the outer membrane increases [10]. LNG-IUSs with a drug-polymer matrix in the core and an outer rate-controlling polymer membrane are a combination of a reservoir and a monolithic matrix system for controlled drug release. However, in the absence of the outer membrane, LNG-IUSs behave as monolithic matrix systems. Drug release from LNG-IUSs without an outer membrane were reported to follow zero-order kinetics [12]. This could be due to the high drug loading (50% w/w drug loading) and the excess drug particles present in the solid-state which acted as a constant source of drug diffusion providing zero-order release. On the other hand, LNG-IUSs follow a first order model in the presence of the outer polymer membrane indicating that these systems behave as reservoirs with daily drug release rates decreasing exponentially with time [12, 17]. As discussed previously, the outer membrane is composed of PDMS and other additives. The different attributes of PDMS (such as molecular weight, endgroup substitution, and degree of crosslinking) as well as the additive properties (such as the type, purity, and concentration) used in the preparation of the outer membrane may significantly influence the drug release characteristics. In a previous report, drug release from LNG-IUSs with outer polymer membranes obtained from different sources was investigated [10]. The membranes with similar dimensions obtained from various sources showed significant differences in their structural, thermal, and mechanical characteristics as determined via FTIR, DSC, TGA and texture analyzer. Usually, polymer membranes with high mechanical strength may be expected to show low drug release rates. Interestingly, it was observed that drug release from the LNG-IUSs was inversely correlated to the mechanical strength of the outer polymer membranes [10]. In a later report, a direct correlation between the polymer crosslinking density and the tensile strength, moduli properties, and porosity in LNG-IUSs was established [17]. The outer polymer membranes with a greater mechanical strength may have a higher crosslinking density and therefore a higher porosity resulting in faster drug release rates. Variation in the properties of the outer polymer membrane allow modulation of the drug release from LNG-IUSs.
6.1.5. Design parameters and drug-device configuration
Drug-device combination products such as LNG-IUSs require additional considerations in terms of product design parameters which influence the rate of delivery. Critical factors include: the length of drug-polymer reservoirs, the thickness of reservoirs, the dimensions of the outer polymer membrane, the shape and the size of the polyethylene frame, the arrangement of the reservoirs and the outer membrane on the polyethylene frame, as well as interactions of the device constituents with the drug. These factors not only control the drug release from the IUSs but are also important for easy administration and maintaining product integrity throughout their lifecycle. Previous reports demonstrating the impact of product design parameters on drug release from LNG-IUSs showed that non-hollow reservoirs had the slowest drug release rates while the release rates increased as the internal diameter of the reservoir increased [10]. Similarly, LNG-IUSs with longer reservoirs demonstrated fast drug release. It was concluded through this study that the dimensional differences in IUSs resulted in different surface areas and a linear correlation was established between the surface area of the reservoirs and the release rates of LNG-IUSs. A comparison of the release profiles of LNG-IUSs prepared using one-segment reservoirs (single drug-polymer reservoir, 12.5 mm in length) and two-segment reservoirs (two drug-polymer reservoirs, both ends of each segment were open and each segment was 6.25 mm in length separated on the polyethylene frame) was conducted [10]. The release rate from LNG-IUSs with two-segment reservoirs was significantly higher than the one-segment reservoirs. This was due to the higher surface area and direct exposure of the reservoirs to the release media (due to both ends being open). The configuration of the IUSs is another approach to obtain the desired drug release characteristics.
6.2. Drug release mechanisms
The intricate design and the complex matrix of excipients in LNG-IUSs leads to complicated drug release mechanisms which are not yet fully understood. However, there are a few reports in the literature that contribute to an improved understanding of the release mechanisms from LNG-IUSs. Modeling of LNG-IUS release kinetics was previously performed and explained using a two-phase model (zero-order plus Higuchi) [12]. The structure of LNG-IUS is a combination of a monolithic matrix and a reservoir system (Fig. 5A). Due to the high drug loading, the system contains both saturated drug solution and excess solid drug particles and thus acts as a reservoir with a constant activity source. Initially, drug diffusion occurs from the surface of the monolithic drug-polymer reservoir through the outer polymer membrane. With the progression of time, as the drug is depleted from the surface, diffusion occurs from the core of the polymer matrix to the interface of the reservoir and outer polymer membrane (Phase 1) (Fig. 5B). This is further followed by drug diffusion through the outer polymer membrane (Phase 2). Equation 1 explains the release kinetics of LNG-IUSs [78].
Fig. 5.

Depiction of LNG-IUS device structure combining the monolithic and reservoir systems; and B) concentration profile in LNG-IUS. At time t, a drug depleting layer (Phase 2) of thickness x has formed immediately adjacent to the outer membrane of thickness λ. (The drug reservoir PDMS matrix is designated as Phase 1 and the outer PDMS membrane is Phase 2.) [12]
| (Equation 1) |
Mt = cumulative drug released at time t
dMt/dt = steady-state drug release rate at time t
A = total surface area
D1= diffusion coefficient of drug in phase 1
D2 = diffusion coefficient of drug in phase 2
λ = thickness of outer polymer membrane
K = partition coefficient of drug
x = thickness of drug-depleted layer at the interface of monolithic reservoir and outer polymer membrane
C1(s) = solubility of drug in the matrix
Since a saturation state is maintained initially within the drug-polymer reservoir at the surface of the outer membrane, the thickness of the drug-depleted layer (x) is very small and the diffusion coefficient of drug in the matrix is high. In this case, xD2 << K λ D1 and the release rate follows zero-order kinetics until approximately 30% release (constant release). After approximately 30% release, there is a significant increase in the thickness of x that drug diffusion from the inner core of the polymer matrix to the outer membrane becomes the rate-limiting step. At this point, xD2 >> K λ D1 and equation 1 reduces to the Higuchi equation indicating matrix-controlled diffusion [12].
Another report has detailed the effects of polymer crosslinking on the drug release mechanisms from LNG-IUSs [17]. Interestingly, high drug release rates were obtained from the formulations with high crosslinking densities. It was shown that PDMS forms a solid-state porous branched network at high crosslinking densities facilitating fast solvent uptake and rapid drug release (Fig. 6). Additionally, these formulations have low polymer crystallinity and high hydrophobicity and therefore the drug is more soluble in the polymer resulting in its rapid diffusion through the polymer matrix. The crosslinking density of the polymer has a significant impact on the microstructure of LNG-IUSs. Non-crosslinked polymer in formulations with low crosslinking density exist in the gel state which coats the drug particles thereby restricting access to the solvent molecules (Fig. 6). Moreover, the non-crosslinked polymer is believed to exist as aggregated dangling chain units which provide a tortuous path for drug diffusion leading to slower drug release rates [17].
Fig. 6.

Influence of polymer crosslinking on drug release mechanisms from LNG-IUSs. [17]
7. Challenges to the development of IUSs
7.1. Selection of suitable excipients
The excipients in IUSs are complex mixtures of polymers and additives. Besides the PDMS elastomer base and the crosslinking agent in the prepolymers A and B respectively, the following excipients add to the complexity of the matrix: silicone hydrolysis products, a platinum-based catalyst, inhibitors, and fillers in various concentrations. The wide range of variability in the excipient attributes in IUSs warrants the understanding of their impact on in-process as well as finished product characteristics. To date, there have been no previous studies investigating the effects of different chemical substitution, end group, molecular weight or viscosity of the PDMS polymer, as well as the type and concentration of the fillers on drug release from IUSs. In addition, the use of various excipients may impart different mechanical strength to the formulations which is critical in the design of drug-device combination products such as IUSs. For the successful development of IUSs with desired characteristics, challenges exist not only in material selection for the drug-polymer reservoir (core) but also for the rate-controlling outer polymer membrane. Variability in the physicochemical properties of excipients may compromise the quality and performance attributes of the product. Since IUSs are long-acting complex drug products, gaining knowledge and understanding of the excipients will accelerate the development of these products.
7.2. Manufacturing challenges
Despite several processing techniques (as discussed in section 4.3), currently, there is only one report in the literature describing the manufacturing of LNG-IUSs [9]. This may be due to the requirement of sophisticated instrumentation for manufacturing IUSs and the associated challenges as compared to conventional formulations. One of the major difficulties in manufacturing these drug-devices is the choice of mold materials and achieving precise device shape. The molds used to prepare IUSs should be carefully designed to avoid any sharp edges or corners which may damage the surface. There may be interactions between the mold material and the drug or polymer at high temperatures which may negatively impact the curing process as well as lead to difficulties in demolding [9]. A commonly observed problem with the use of platinum-based addition curing systems is the poisoning of catalysts which negatively affects the curing process. Sulfur-containing materials, organotin-based compounds, and some amine-containing substances may react with the catalyst and prevent the complete curing of the formulations [79]. Several PDMS-based formulations use lubricants such as silicone oil for efficient mixing, better flow properties, and easy ejection from the mold. However, there have been no previous studies demonstrating the impact of silicone oil concentrations on drug release from IUSs. The amount of lubricants used in IUSs may affect their mechanical strength, processing conditions, and release behavior and thus the concentration of excipients in the formulations should be controlled.
Mixing of silicone prepolymers and additives with the drug is an additional challenge, especially for IUSs with very high drug loading. Inadvertent changes in the prepolymer ratios may occur during manual mixing or due to inaccurate dosing of the prepolymers using automated pumps during injection molding. Additionally, the mixing method may also have an impact on the final product consistency and drug release. Therefore, a suitable mixing method should be selected based on the polymer viscosity and batch size. A previous report showed that LNG-IUSs prepared using planetary mixing had higher drug release rates than those prepared using manual mixing [17]. This was attributed to the high porosity of the formulations mixed using planetary mixers. In addition, the heat generated during planetary mixing may lead to higher drug solubility in the polymer and consequently faster drug diffusion. The incorporation of air bubbles during the mixing, extrusion, compression, or injection molding processes should be controlled. Excess air bubbles in the formulation may be an issue for product content uniformity and complete filling in the mold cavities. To avoid this, adequate venting should be ensured during manufacturing [70]. For processing methods using twin-screw extruders, the material may accumulate in the dead space of the mixing barrel and vulcanize with time. Manufacturing techniques such as injection molding use stainless steel screens to reduce contamination and provide better dimensional control. However, this may also result in generation of backpressure which needs to be controlled [16, 70].
Since IUSs are drug-device combination products, their manufacturing requires consideration of additional aspects of device assembly and configuration. Appropriate precautions must be taken to maintain product integrity while mounting the drug-polymer reservoirs on the polyethylene frame. The overmolding technique via the injection molding method to coat the outer polymer membrane on drug-polymer reservoirs offers high throughput, accuracy and product consistency. However, this technique requires high precision and is expensive. On the other hand, conventional methods such as spray-coating and dipping may encounter challenges in terms of achieving a uniform thickness of the polymer membrane.
7.3. Challenges in performance testing
Despite recent advances in the development of characterization tools for LNG-IUSs, the limited sensitivity of several characterization techniques to detect minor variations at low concentrations can be a challenge. In addition, the complex composition of the excipients in IUSs may pose additional difficulties in quantification of the product variations. Some of the techniques (e.g., NMR and GC) require extraction of the sample in a strong nonpolar solvent. These methods often encounter limitations such as low extraction recovery yield, interference arising from excipients and/or drug-excipient interactions.
Currently, all literature reports use water shaker baths for in vitro release testing of LNG-IUSs. It is challenging to develop a compendial in vitro release testing method due to the extremely long-acting nature of IUSs and the very low daily drug release rates [2, 13, 80, 81]. A previous study reported the use of USP apparatus II for release testing of Q1/Q2 equivalent LNG-IUSs [10]. Higher drug release was observed using the USP apparatus II compared to the water shaker bath method. The use of USP apparatus IV for accelerated in vitro drug release testing of LNG-IUSs with organic solvent-resistant polyether ether ketone (PEEK) cells has been investigated (Fanse et al unpublished data [82]). Organic solvent leakage and mechanical challenges for ultra-long release testing of such products with extremely low drug release rates are the major concerns when using the compendial methods. Due to all of the above mentioned reasons, the development of compendial in vitro release testing methods for ultra-long-acting implants such as LNG-IUSs is difficult. The use of a suitable accelerated release method reduces the testing time considerably (up to 2–4 months compared to 3–7 years). Most of the reports in the literature utilize the full release profiles of LNG-IUSs via accelerated release testing to understand the drug release mechanisms. However, it is essential to establish a correlation between the accelerated and real-time in vitro release methods to demonstrate the suitability of a given method. This is particularly important for polymers such as PDMS which have different affinity and permeability towards hydroalcoholic and aqueous media.
7.4. IVIVC
In vitro- in vivo correlation (IVIVC) is defined by the FDA as “a predictive mathematical model describing the relationship between an in vitro property of a dosage form (rate or extent of drug release) and a relevant in vivo response (amount of drug absorbed or plasma drug concentrations)” [83]. The four levels of IVIVC included in the FDA guidance are: (i) Level A which establishes a point-to-point correlation between in vitro drug release and the fraction of drug absorbed in vivo; (ii) level B correlates the in vitro mean dissolution time with either the in vivo mean residence time, mean dissolution time or mean absorption time; (iii) level C represents a single point correlation between an in vitro dissolution parameter (such as the time required for 50% drug dissolution T50) and a pharmacokinetic parameter (such as Cmax, AUC, or Tmax); and (iv) multiple Level C which establishes a correlation between one or more pharmacokinetic parameters with at least three in vitro release time points (to include the initial, middle, and late-stage of the release profile). A Level A IVIVC is desirable from a regulatory perspective. Not only does IVIVC provide a predictive tool that can eliminate the need for clinical bioequivalence studies, but it also provides a better understanding of the formulation, helps in interpreting batch-to-batch variations and optimizing the formulation development process [84]. Compared to oral dosage forms, establishment of IVIVCs for complex parenteral drug products is challenging mainly due to their complex characteristics and lack of standardized, compendial in vitro release testing methods [85, 86]. To date, there are no reports in the literature describing the establishment of IVIVCs for LNG-IUSs. Given the advantages and need of IVIVCs in product development, it is worth discussing the challenges in IVIVC development for IUS products.
The first challenge is the development of biorelevant in vitro dissolution methods for IUSs that are capable of mimicking the dynamic and complex physiological conditions. In vitro drug release testing methods have been developed for LNG-IUSs which are discriminatory, robust, and capable of reflecting the differences in product properties [10]. These methods may be successfully utilized to control product quality during formulation development and optimization, as well as for batch release testing. However, these methods have not yet been validated for their use in the development of IVIVCs. Moreover, the drug release from these products is extremely long which makes IVIVC development difficult. Furthermore, an incomplete understanding of release mechanisms and product variables affecting drug release from IUSs presents additional challenges. One of the critical steps in developing IVIVCs is to understand and obtain in vivo drug input for correlation with the in vitro release [87]. A major challenge in the IVIVC development of IUSs is their locally-acting nature. The drug release rates of LNG-IUSs do not correlate with the in vivo plasma levels. Such non-linear response to drug input limits the deconvolution and model building for IVIVCs. Another vital aspect that needs to be considered for IVIVC development is the availability of suitable analytical methods to accurately quantify the extremely low concentrations of LNG in the plasma and/or endometrium. Drug concentrations in LNG-IUSs have been determined using HPLC and UPLC chromatographic methods [12, 17]. More sensitive techniques such as LC-MS and radioimmunoassay may be utilized to determine picogram levels of drug.
The presence of the mucosal lining of the uterus acts as a permeability barrier for endometrial drug absorption from IUSs [88]. Intersubject variability in in vivo performance of IUSs presents a further challenge. Factors such as expression of drug transporters and metabolizing enzymes, tissue permeability, local microbiome, immune cells, hormonal milieu, blood supply, and protein binding are well-known important contributors to drug disposition and pharmacokinetics [89]. Notably, all of these factors vary throughout the menstrual cycle as well as with age and disease conditions [75, 90]. Therefore, this may lead to different drug exposures and high intersubject variability. For instance, the thickness of the uterine tissue has been shown to change with differences in estrogen and progesterone levels in the body, the presence of inflammatory conditions and infections, and the menstrual cycle [89]. Uterine contractions and the blood generated during the menstrual cycle may dilute or expel the locally-delivered drugs [91]. Efflux transporters such as P-gp (ABCB1) and BCRP (ABCG2) and enzymes such as cytochrome P450 and 17-β-dehydrogenases which are highly expressed in the uterus may also contribute to intersubject variability [89].
Overall, due to all the challenges discussed above, the development of IVIVCs for IUSs is extremely difficult.
7.5. Regulatory aspects
As defined in the section 503(g) of the Federal Food, Drug, and Cosmetic Act (FD&C) and 21 CFR part 3, a product consisting of two or more different types of regulated medical components (drug, device, and/or biological product) is called a combination product [92]. Accordingly, a product can be classified as a drug/device, drug/biologic, biologic/device, or drug/device/biologic depending on its constituents. Since IUSs comprise of drug formulations mounted on T-frames along with inserters to administer these products packaged together, they are classified as drug-device combination products. The drug is required to achieve contraceptive efficacy, and the T-frame has the function of delivering the product via an inserter as well as to hold it inside the uterine cavity. In recent years, the FDA has published some draft guidance that is useful for the development and approval of drug-device combination products such as the classification of combination products, cGMP requirements, postmarketing safety, and approach for bridging in new NDAs for drug-devices [93–95]. In January 2022, the FDA issued a guidance discussing the principles and approaches to determine premarket submission pathways for combination products [96].
The regulatory requirements for combination products arise from the statutory and regulatory requirements applicable to the individual constituents (for example, drug and device individually) [96]. Additionally, these products are subject to special regulatory requirements to address the overlap and distinctions applicable to the respective constituents of the combination products [97]. The primary jurisdiction for regulation of combination products depends on the primary mode of action (PMOA) of the product i.e., if the PMOA is attributable to the drug, device, or biologic constituent [96]. A Premarket Approval Application (PMA), De Novo classification, or 510(k) pathways may be applicable for device-led combination products whereas an New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) is typically the marketing authorization pathway for a drug-led combination product.
The FDA also classifies long-acting IUSs as complex drug products. Per the FDA guidelines, a drug product containing either a complex active ingredient, polymer or peptide; has a complex route of delivery; has a complex dosage form; or is a drug-device combination is deemed to be a complex drug product [98]. IUSs have complex mixtures of excipients, complicated manufacturing methods involving in situ crosslinking of the polymer, and an intricate matrix-reservoir design which is critical in determining the drug release rates from IUSs. Demonstrating Q1/Q2 sameness and establishing therapeutic equivalence of these complex products can be taxing. The FDA has released a draft guidance on demonstrating equivalence of generic complex drug substances and formulations to promote generic drug development of complex products [99, 100]. However, the efforts to streamline these regulatory processes are still ongoing.
Drug manufacturers have to follow a long and challenging path to bring such a complex combination product to the market. The overall cost and time for the development may also be high. Furthermore, with new regulations and relatively less experience in industry for development of such products, manufacturers may face additional challenges in the aspects of: product evaluation, selection of testing methods, and setting specifications [97, 101, 102]. Considering that the dynamic regulatory landscape of complex combination products is a big challenge for pharmaceutical companies, it is essential to be fully informed on the latest regulations and understand the distinctions arising from them [101, 103]. Efforts to develop detailed guidance on requirements for combination products and product-specific guidance for IUSs by the regulatory agencies will be highly beneficial.
8. Future outlook
IUSs offer great promise as a contraceptive method. With increasing awareness in the public, the myths and misconceptions associated with traditional IUDs are diminishing as observed by the steadily growing use of LNG-IUSs. Consequently, this has resulted in a rising demand for LNG-IUSs over the past few years [49, 104]. However, the limited number of IUS products, their high cost, and the consequent limited availability to low-income countries are some of the existing barriers [105, 106]. Therefore, generic drug development to introduce more affordable IUS products may increase the use of this highly beneficial contraceptive method.
Manufacturing of IUSs can be improved through in-depth knowledge about the process controls and variables, as well as developing a design space for IUS products. Future efforts can be made in designing new manufacturing methods with potential for continuous manufacturing of these products to reduce batch variations and ensure high product consistency. Recently, the application of 3D printing technology for the development of implantable medical products and biomaterials has received great interest [107, 108]. The use of this technology for the development of IUS products may be evaluated in the future. Selection of suitable excipients is crucial to support the adoption of such advanced manufacturing technologies. Further, the excipient characteristics should be elucidated to establish correlations between the physicochemical properties, mechanical properties, and drug release from IUSs. This will ultimately lead to obtaining a better understanding of the release mechanisms from IUSs.
Since the complex nature of IUSs limits the development of conventional IVIVC analysis, non-traditional approaches need to be followed. The FDA has recently issued a draft guidance suggesting a combination of two studies: in vitro release testing and in vivo/ex vivo study to demonstrate bioequivalence in generic LNG-IUS products [109]. The formulations must follow a similar in vitro dissolution profile and the in utero drug release at different time points should be similar. Residual amounts of LNG following product administration and its subsequent removal at different time points should be measured [109]. Advanced imaging techniques may be utilized to elucidate drug distribution and release behavior. Mathematical modeling and simulation tools may be helpful in explaining drug release mechanisms and correlating in vitro release with in vivo performance.
Development of intrauterine products consisting of other hormonal drugs or with a combination of multiple drugs may be considered for future development. For example, IUSs with a combination of estrogen and progesterone-based hormonal drugs may be developed. Anti-retroviral drugs may be incorporated for patients with HIV infection. LNG-IUSs containing antimicrobial drugs may be developed for contraceptive purposes while providing a simultaneous treatment option for microbial infections in the upper reproductive tract. This will also usher the way for pharmaceutical companies to reengineer these drug products with 505b(2) potential. Considering the patient-benefits offered by IUSs as well as the revenue potential of these novel dosage forms for the manufacturers, more efforts should be undertaken to surmount the challenges and improve product access.
9. Conclusions
This review summarizes the role of IUSs in the changing landscape of women’s healthcare, assesses the current state of research, highlights the product development challenges, and future opportunities. Selection of excipients, manufacturing challenges, and development of appropriate in vitro release testing methods are deemed as some of the key obstacles for IUS product development. Establishment of conventional IVIVCs seems impractical for locally-acting complex products such as IUSs and thus alternative approaches to prove pharmaceutical equivalence need to be adapted. Drug-device combination products such as IUSs require special considerations for the selection of performance tests (e.g., crosslinking density, mechanical properties, and in vitro drug release testing), as well as specification setting. Detailed understanding of the excipient properties, the formulation and characterization aspects, the factors affecting drug release from IUSs, and the release mechanisms is very critical. Besides all of the above mentioned elements, increasing clarity of regulatory aspects and efforts to develop product-specific guidance by the FDA will be pivotal for the availability of these products on the market. Given the scarcity of previous literature reports on IUSs, this comprehensive review will help in the advancement of knowledge and serve as a ground for future research on long-acting IUSs as well as other PDMS-based complex drug products.
Acknowledgments
The authors acknowledge the support from the U.S. Food and Drug Administration grant (1U01FD005443-01) and Pfizer Distinguished Chair Technology Funds.
Abbreviations
- LNG
levonorgestrel
- IUS
intrauterine system
- LARC
long-acting reversible contraceptives
- IVIVC
in vitro-in vivo correlation
- PDMS
polydimethylsiloxane
- WHO
World Health Organization
- FDA
Food and Drug Administration
- IUD
intrauterine device
- CDC
Center for Disease Prevention and Control
- Q1/Q2 equivalent
qualitatively and quantitatively equivalent
- RTV
room-temperature vulcanization
- FTIR
Fourier-transformed infrared spectroscopy
- NMR
nuclear magnetic resonance
- GC-MS
gas chromatography-mass spectrometry
- GPC
gel permeation chromatography
- DSC
differential scanning calorimetry
- TGA
thermogravimetric analysis
- HPLC
high performance liquid chromatography
- UPLC
ultra performance liquid chromatography
- PMOA
primary mode of action
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bayarri L Drug-device combination products: regulatory landscape and market growth. Drugs Today (Barc). 2015;51(8):505–13. doi: 10.1358/dot.2015.51.8.2376223. [DOI] [PubMed] [Google Scholar]
- 2.Mirena FDA label https://wwwaccessdatafdagov/drugsatfda_docs/label/2021/021225s042lblpdf. (Accessed 28 March 2022).
- 3.Hormonal IUS knowledgebase https://wwwmayoclinicorg/tests-procedures/mirena/about/pac-20391354. (Accessed 11 March 2022)
- 4.Rademacher KH, Sripipatana T, Pfitzer A, Mackay A, Thurston S, Jackson A, et al. A Global Learning Agenda for the Levonorgestrel Intrauterine System (LNG IUS): Addressing Challenges and Opportunities to Increase Access. Global Health: Science and Practice. 2018;6(4):635. doi: 10.9745/GHSP-D-18-00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. https://wwwcdcgov/reproductivehealth/contraception/indexhtm. (Accessed 5 April 2022).
- 6.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(WHO) WHO. 19th WHO Model List of Essential Medicines. https://wwwwhoint/medicines/publications/essentialmedicines/EML2015_8-May-15pdf?ua=1. (Accessed 24 March 2022)
- 8.Hubacher D The Levonorgestrel Intrauterine System: Reasons to Expand Access to the Public Sector of Africa. Global Health: Science and Practice. 2015;3(4):532. doi: 10.9745/GHSP-D-15-00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao Q, Gu B, Price CF, Zou Y, Wang Y, Kozak D, et al. Manufacturing and characterization of long-acting levonorgestrel intrauterine systems. International Journal of Pharmaceutics. 2018;550(1):447–54. doi: 10.1016/j.ijpharm.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao Q, Zou Y, Wang Y, Choi S, Burgess DJ. Impact of product design parameters on in vitro release from intrauterine systems. International Journal of Pharmaceutics. 2020;578:119135. doi: 10.1016/j.ijpharm.2020.119135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanse S, Bao Q, Zou Y, Wang Y, Burgess DJ. Effect of crosslinking on the physicochemical properties of polydimethylsiloxane-based levonorgestrel intrauterine systems. International Journal of Pharmaceutics. 2021;609:121192. doi: 10.1016/j.ijpharm.2021.121192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao Q, Zou Y, Wang Y, Kozak D, Choi S, Burgess DJ. Drug release testing of long-acting intrauterine systems. Journal of Controlled Release. 2019;316:349–58. doi: 10.1016/j.jconrel.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liletta FDA label. https://wwwaccessdatafdagov/drugsatfda_docs/label/2019/206229s008lblpdf. (Accessed 28 March 2022).
- 14.Colas A, Rudolf L, Curtis J. Silicone Biomaterials : History and Chemistry & Medical Applications of Silicones. An Introduction to Materials in Medicine. Biomaterials Science 2nd edition, Elsevier; 2004. [Google Scholar]
- 15.Kuo A. Polymer Data Handbook. Polymer Data Handbook. Oxford University Press; 1999. [Google Scholar]
- 16.Momentive. Silicone elastomers product guide. https://wwwpolymershapescom/wp-content/uploads/2020/04/Polymershapes_Momentive_Brochure-Silicone-Elastomerspdf. (Accessed 14 April 2022).
- 17.Fanse S, Bao Q, Zou Y, Wang Y, Burgess DJ. Impact of polymer crosslinking on release mechanisms from long-acting levonorgestrel intrauterine systems. International Journal of Pharmaceutics. 2022;612:121383. doi: 10.1016/j.ijpharm.2021.121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerrero AM. Age-old methods of contraception. Initiatives Popul. 1977;3(1):20–5. [PubMed] [Google Scholar]
- 19.Ancient birth control methods. https://flohealth/menstrual-cycle/sex/birth-control/ancient-birth-control-methods. (Accessed 4 May 2022).
- 20.Mbabajende V Historical survey of modern reversible contraceptive methods. Imbonezamuryango. 1986(5):14–7. [PubMed] [Google Scholar]
- 21.Bhatt N, Deshpande M. A Critical Review and Scientific Prospective on Contraceptive Therapeutics from Ayurveda and Allied Ancient Knowledge. Frontiers in Pharmacology. 2021;12. doi: 10.3389/fphar.2021.629591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quarini CA. History of contraception. Women’s Health Medicine. 2005;2(5):28–30. doi: 10.1383/wohm.2005.2.5.28. [DOI] [Google Scholar]
- 23.Buttar A, Seward S. “Enovid: The First Hormonal Birth Control Pill”. Embryo Project Encyclopedia. 2009. (Accessed 4 May 2022). [Google Scholar]
- 24.Junod SW. https://www.fda.gov/media/110456/download. US Food and Drug Administration. 1998. (Accessed 3 May 2022).
- 25.Peipert JF. Lippes loop and the first IUDs: lessons from a bygone era. American Journal of Obstetrics & Gynecology. 2018;219(2):127–8. doi: 10.1016/j.ajog.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Hubacher D The Checkered History and Bright Future of Intrauterine Contraception In the United States. Perspectives on Sexual and Reproductive Health. 2002;34(2):98–103. [PubMed] [Google Scholar]
- 27.Horwitz R. “The Dalkon Shield”. Embryo Project Encyclopedia. 2018. (Accessed 29 April 2022). [Google Scholar]
- 28.Jennifer D Sterilization as Cyborg Performance: Reproductive Freedom and the Regulation of Sterilization. Frontiers: A Journal of Women Studies. 2014;35(1):107–31. doi: 10.5250/fronjwomestud.35.1.0107. [DOI] [Google Scholar]
- 29.Paragard FDA label. https://wwwaccessdatafdagov/drugsatfda_docs/label/2019/018680s069s070lblpdf. (Accessed 11 March 2022).
- 30.Trieman K, Liskin L, Kols A, Rinehart W. Johns Hopkins School of Public Health, Population Information Program. Baltimore, MD: 1995. IUDs-an update. Population Reports, Series B. (6) [Google Scholar]
- 31.Haupt A. Norplant implant now a step closer to U.S. use. Popul Today. 1989;17(7–8):4. [PubMed] [Google Scholar]
- 32.Green W. The FDA, contraceptive marketing approval and products liability litigation: Depo-Provera and the risk of osteoporosis. Food Drug Law J. 2013;68(2):115–35. [PubMed] [Google Scholar]
- 33.Weismiller DG. Emergency contraception. Am Fam Physician. 2004;70(4):707–14. [PubMed] [Google Scholar]
- 34.Christin-Maitre S History of oral contraceptive drugs and their use worldwide. Best Practice & Research Clinical Endocrinology & Metabolism. 2013;27(1):3–12. doi: 10.1016/j.beem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Leblanc ES, Laws A. Benefits and risks of third-generation oral contraceptives. J Gen Intern Med. 1999;14(10):625–32. doi: 10.1046/j.1525-1497.1999.08108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirena Prescribing Information https://wwwmirena-uscom. (Accessed 17 March 2022).
- 37.Yoost J. Understanding benefits and addressing misperceptions and barriers to intrauterine device access among populations in the United States. Patient Prefer Adherence. 2014;8:947–57. doi: 10.2147/PPA.S45710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sivin I, el Mahgoub S, McCarthy T, Mishell DR Jr., Shoupe D, Alvarez F, et al. Long-term contraception with the levonorgestrel 20 mcg/day (LNg 20) and the copper T 380Ag intrauterine devices: a five-year randomized study. Contraception. 1990;42(4):361–78. doi: 10.1016/0010-7824(90)90046-x. [DOI] [PubMed] [Google Scholar]
- 39.Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49(1):56–72. doi: 10.1016/0010-7824(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 40.Britton LE, Alspaugh A, Greene MZ, McLemore MR. CE: An Evidence-Based Update on Contraception. Am J Nurs. 2020;120(2):22–33. doi: 10.1097/01.NAJ.0000654304.29632.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beatty M, Blumenthal P. The levonorgestrel-releasing intrauterine system: Safety, efficacy, and patient acceptability Therapeutics and Clinical Risk Management 2009;5:561–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peipert JF, Madden T, Allsworth JE, Secura GM. Preventing unintended pregnancies by providing no-cost contraception. Obstet Gynecol. 2012;120(6):1291–7. doi: 10.1097/aog.0b013e318273eb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pohjoranta E, Suhonen S, Gissler M, Ikonen P, Mentula M, Heikinheimo O. Early provision of intrauterine contraception as part of abortion care-5-year results of a randomised controlled trial. Hum Reprod. 2020;35(4):796–804. doi: 10.1093/humrep/deaa031. [DOI] [PubMed] [Google Scholar]
- 44.Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969–80. doi: 10.2165/11591290-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sitruk-Ware R, Nath A, Mishell DR Jr. Contraception technology: past, present and future. Contraception. 2013;87(3):319–30. doi: 10.1016/j.contraception.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sitruk-Ware R, Inki P. The levonorgestrel intrauterine system: long-term contraception and therapeutic effects. Womens Health (Lond). 2005;1(2):171–82. doi: 10.2217/17455057.1.2.171. [DOI] [PubMed] [Google Scholar]
- 47.Lowe RF, Prata N. Hemoglobin and serum ferritin levels in women using copper-releasing or levonorgestrel-releasing intrauterine devices: a systematic review. Contraception. 2013;87(4):486–96. doi: 10.1016/j.contraception.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 48.Ontario. HQ. Levonorgestrel-Releasing Intrauterine System (52 mg) for Idiopathic Heavy Menstrual Bleeding: A Health Technology Assessment. Ont Health Technol Assess Ser. 2016;16(18):1–119. [PMC free article] [PubMed] [Google Scholar]
- 49.Intrauterine Devices (IUDs): Access for Women in the US. https://wwwkfforg/womens-health-policy/fact-sheet/intrauterine-devices-iuds-access-for-women-in-the-u-s/. 2020. (Accessed 24 April 2022).
- 50.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203(2):115.e1–7. doi: 10.1016/j.ajog.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suhonen S, Haukkamaa M, Jakobsson T, Rauramo I. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception. 2004;69(5):407–12. doi: 10.1016/j.contraception.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Attia AM, Ibrahim MM, Abou-Setta AM. Role of the levonorgestrel intrauterine system in effective contraception. Patient Prefer Adherence. 2013;7:777–85. doi: 10.2147/PPA.S36948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gemzell-Danielsson K, Kubba A, Caetano C, Faustmann T, Lukkari-Lax E, Heikinheimo O. Thirty years of mirena: A story of innovation and change in women’s healthcare. Acta Obstet Gynecol Scand. 2021;100(4):614–8. doi: 10.1111/aogs.14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips V, Graham CT, Manek S, McCluggage WG. The effects of the levonorgestrel intrauterine system (Mirena coil) on endometrial morphology. J Clin Pathol. 2003;56(4):305–7. doi: 10.1136/jcp.56.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taraborrelli S. Physiology, production and action of progesterone. Acta obstetricia et gynecologica Scandinavica. 2015;94. doi: 10.1111/aogs.12771. [DOI] [PubMed] [Google Scholar]
- 56.Dinehart E, Lathi RB, Aghajanova L. Levonorgestrel IUD: is there a long-lasting effect on return to fertility? J Assist Reprod Genet. 2020;37(1):45–52. doi: 10.1007/s10815-019-01624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pieńkowska K. Review of Current Pharmaceutical Applications of Polysiloxanes (Silicones). Handbook of Polymers for Pharmaceutical Technologies. Scrivener Publishing LLC; 2015. p. 363–81. [Google Scholar]
- 58.Colas ARL, Corning D. Silicones in Pharmaceutical Applications. Dow Corning healthcare Industries; 2004. [Google Scholar]
- 59.Linear Polydimethylsiloxanes. https://wwwecetocorg/wp-content/uploads/2014/08/JACC-026pdf. (Accessed 27 March 2022).
- 60.OSHA Reproductive Hazards. https://wwwoshagov/reproductive-hazards/hazards. (Accessed 15 April 2022).
- 61.Noll W, Glenz O, Gerhard H. Chemistry and technology of silicones. New York: Academic Press Inc., London; 1968. [Google Scholar]
- 62.Andriot M, DeGroot JV, Meeks R, Gerlach e, Jungk M, Wolf AT, et al. Silicones in Industrial Applications. Dow Corning Europe, Dow Corning Healthcare Industries. https://citeseerxistpsuedu/viewdoc/download?doi=10115291841&rep=rep1&type=pdf. (Accessed 24 March 2022). [Google Scholar]
- 63.Arkles B Reactive Silicones: Forging New Polymer Links. Gelest Inc., PA; 2013. [Google Scholar]
- 64.Lassen C, Hansen CL, Mikkelsen S, Maag J. Siloxanes - Consumption, Toxicity and Alternatives. Danish Ministry of the Envirnonment; https://www2mstdk/udgiv/publications/2005/87-7614-756-8/pdf/87-7614-757-6pdf. 2005. [Google Scholar]
- 65.Olewnik-Kruszkowska E, Brzozowska W, Adamczyk A, Gierszewska M, Wojtczak I, Sprynskyy M. Effect of Diatomaceous Biosilica and Talc on the Properties of Dielectric Elastomer Based Composites. Energies. 2020;13(21). doi: 10.3390/en13215828. [DOI] [Google Scholar]
- 66.Sumi D, Dhanabalan A, Thimmappa BHS, Krishnamurthy S. Effect of colloidal silica dispersions on the properties of PDMS‐colloidal silica composites. Journal of applied polymer science. 2012;125(S1):E515–E22. doi: 10.1002/app.36226. [DOI] [Google Scholar]
- 67.Noll W. Chapter 4 - Organosilanes with Organofunctional Groups. In: Noll W, editor. Chemistry and Technology of Silicones. Academic Press; 1968. p. 124–89. [Google Scholar]
- 68.Flacktek. https://speedmixercom/the-technology/. (Accessed 19 April 2022).
- 69.Drobny JG. 4 - Processing Methods Applicable to Thermoplastic Elastomers. In: Drobny JG, editor. Handbook of Thermoplastic Elastomers (Second Edition). Oxford: William Andrew Publishing; 2014. p. 33–173. [Google Scholar]
- 70.Wacker. Solid and liquid silicone rubber- material and processing guidelines. https://wwwwackercom/h/medias/6709-ENpdf. (Accessed 13 April 2022).
- 71.Arburg. Silicone injection moulding. https://wwwarburgcom/fileadmin/redaktion/mediathek/prospekte/arburg_silicone_526416_en_gb/#. (Accessed 14 April 2022).
- 72.Duesterberg B, Ahola M, Pihlaja J, Lyytikäinen H, Jukarainen H, Kleemola S, et al. Intrauterine delivery system for contraception. United States Patent, Bayer Oy. 2013. [Google Scholar]
- 73.Promed Pharma. Pharmaceutical Combination products. https://promedmoldingcom/promed-pharma/. (Accessed 23 April 2022).
- 74.Tommi M, Juha A, Harri J, Matti L, Jarkko R. Drug delivery device, especially for delivery of levonorgestrel. WIPO International Patent Application, Leiras Oy. 2001. [Google Scholar]
- 75.Ng KYB, Mingels R, Morgan H, Macklon N, Cheong Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: a systematic review. Human Reproduction Update. 2017;24(1):15–34. doi: 10.1093/humupd/dmx028. [DOI] [PubMed] [Google Scholar]
- 76.Jukareinen H, Markkulla K, Ala-Sorvari J, Lehtinen M, Ruohonen J. Membrane or matrix for controlling the permeation rate of drugs. US Patent application, Schering Oy. 2002. [Google Scholar]
- 77.Mikolaszek B, Kazlauske J, Larsson A, Sznitowska M. Controlled Drug Release by the Pore Structure in Polydimethylsiloxane Transdermal Patches. Polymers. 2020;12(7). doi: 10.3390/polym12071520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chien YW. Controlled release of biologically active agents. Journal of Pharmaceutical Sciences 1987; 77 (4): 371. doi: 10.1002/jps.2600770422. [DOI] [Google Scholar]
- 79.Nusil Product Guide. Avantor sciences. https://www.avantorsciences.com/pages/en/drug-delivery. (Accessed 18 April 2022).
- 80.Skyla FDA label. https://wwwaccessdatafdagov/drugsatfda_docs/label/2017/203159s007lblpdf. (Accessed 13 March 2022).
- 81.Kyleena FDA label. https://wwwaccessdatafdagov/drugsatfda_docs/label/2016/208224s000lblpdf. (Accessed 13 March 2022).
- 82.Suraj F, Quanying B, Diane B. Development of accelerated in vitro drug release testing methods for long-acting levonorgestrel intrauterine systems. (unpublished).
- 83.Rockville M. FDA Guidance for Industry: Extended release oral dosage forms: Development, evaluation and application of in vitro/in vivo correlation. 1997. BP2. [DOI] [PubMed]
- 84.Nuventra. ‘What is IVIVC?’. https://wwwnuventracom/resources/blog/what-is-ivivc/. 2019. (Accessed 23 April 2022).
- 85.Shen J, Burgess DJ. In vitro–in vivo correlation for complex non-oral drug products: Where do we stand? Journal of Controlled Release. 2015;219:644–51. doi: 10.1016/j.jconrel.2015.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burgess DJ, Crommelin DJA, Hussain AS, Chen M-L. Assuring quality and performance of sustained and controlled release parenterals. European Journal of Pharmaceutical Sciences. 2004;21(5):679–90. doi: 10.1016/j.ejps.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 87.Zhou D, Qiu Y. Dissolution and in vitro-in vivo correlation: understanding Biopharmaceutics properties for pharmaceutical product development and manufacturing II. Journal of Validation Technology 2010. p. 57. [Google Scholar]
- 88.Yarbrough V, Winkle S, Herbst-Kralovetz M. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Human Reproduction Update. 2015; 21(3): 353–77. doi: 10.1093/humupd/dmu065. [DOI] [PubMed] [Google Scholar]
- 89.Patel SK, Valicherla GR, Micklo AC, Rohan LC. Drug delivery strategies for management of women’s health issues in the upper genital tract. Advanced Drug Delivery Reviews. 2021;177:113955. doi: 10.1016/j.addr.2021.113955. [DOI] [PubMed] [Google Scholar]
- 90.Piccinato CA, Neme RM, Torres N, Silvério R, Pazzini VB, Rosa ESJC, et al. Is cytochrome P450 3A4 regulated by menstrual cycle hormones in control endometrium and endometriosis? Mol Cell Biochem. 2017;427(1–2):81–9. doi: 10.1007/s11010-016-2899-3. [DOI] [PubMed] [Google Scholar]
- 91.Schnyer AN, Jensen JT, Edelman A, Han L. Do menstrual cups increase risk of IUD expulsion? A survey of self-reported IUD and menstrual hygiene product use in the United States. The European Journal of Contraception & Reproductive Health Care. 2019;24(5):368–72. doi: 10.1080/13625187.2019.1643836. [DOI] [PubMed] [Google Scholar]
- 92.FDA. Combination Products Guide. https://wwwfdagov/combination-products/about-combination-products/combination-product-definition-combination-product-types. 2018. (Accessed 25 April 2022).
- 93.FDA. Current Good Manufacturing Practice Requirements for Combination Products. https://wwwfdagov/regulatory-information/search-fda-guidance-documents/current-good-manufacturing-practice-requirements-combination-products. 2017. (Accessed 25 April 2022).
- 94.FDA. Classification of Products as Drugs and Devices and Additional Product Classification Issues. https://wwwfdagov/regulatory-information/search-fda-guidance-documents/classification-products-drugs-and-devices-and-additional-product-classification-issues. 2017. (Accessed 25 April 2022).
- 95.FDA. Bridging for Drug-Device and Biologic-Device Combination Products. https://wwwfdagov/regulatory-information/search-fda-guidance-documents/bridging-drug-device-and-biologic-device-combination-products. 2019. (Accessed 25 April 2022).
- 96.FDA. Principles of Premarket Pathways for Combination Products. https://wwwfdagov/regulatory-information/search-fda-guidance-documents/principles-premarket-pathways-combination-products. 2022. (Accessed 28 April 2022).
- 97.Lewis AL. 11 - Methods for the characterisation and evaluation of drug-device combination products. In: Boutrand J-P, editor. Biocompatibility and Performance of Medical Devices. Woodhead Publishing; 2012. p. 233–68. [Google Scholar]
- 98.FDA. Introduction to Complex Products and FDA Considerations. https://wwwfdagov/media/108937/download. 2017.
- 99.Lionberger RA. FDA critical path initiatives: opportunities for generic drug development. AAPS J. 2008;10(1):103–9. doi: 10.1208/s12248-008-9010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.FDA. Formal Meetings Between FDA and ANDA Applicants of Complex Products Under GDUFA. https://wwwfdagov/media/107626/download. 2020. (Accessed 2 May 2022).
- 101.Liu J, Chiesl T, Kamberi M. Test Method Selection and Specification Setting in Stability Testing for Combination Medical Device Products. Journal of GXP Compliance. 2018. [Google Scholar]
- 102.Klein K, Borchard G, Shah VP, Flühmann B, McNeil SE, de Vlieger JSB. A pragmatic regulatory approach for complex generics through the U.S. FDA 505(j) or 505(b)(2) approval pathways. Ann N Y Acad Sci. 2021;1502(1):5–13. doi: 10.1111/nyas.14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tian J, Song X, Wang Y, Cheng M, Lu S, Xu W, et al. Regulatory perspectives of combination products. Bioactive Materials. 2022;10:492–503. doi: 10.1016/j.bioactmat.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gemzell-Danielsson K, Kubba A, Caetano C, Faustmann T, Lukkari-Lax E, Heikinheimo O. More than just contraception: the impact of the levonorgestrel-releasing intrauterine system on public health over 30 years. BMJ Sexual & Reproductive Health. 2021;47(3):228. doi: 10.1136/bmjsrh-2020-200962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rademacher KH, Solomon M, Brett T, Bratt JH, Pascual C, Njunguru J, et al. Expanding Access to a New, More Affordable Levonorgestrel Intrauterine System in Kenya: Service Delivery Costs Compared With Other Contraceptive Methods and Perspectives of Key Opinion Leaders. Glob Health Sci Pract. 2016;4 Suppl 2(Suppl 2):S83–S93. doi: 10.9745/GHSP-D-15-00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adedini SA, Omisakin OA, Somefun OD. Trends, patterns and determinants of long-acting reversible methods of contraception among women in sub-Saharan Africa. PLOS ONE. 2019;14(6):e0217574. doi: 10.1371/journal.pone.0217574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Domsta V, Seidlitz A. 3D-Printing of Drug-Eluting Implants: An Overview of the Current Developments Described in the Literature. Molecules. 2021;26(13). doi: 10.3390/molecules26134066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Z, Yang Y. Application of 3D Printing in Implantable Medical Devices. BioMed Research International. 2021;2021:6653967. doi: 10.1155/2021/6653967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.FDA Draft Guidance on Levonorgestrel. https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_021225.pdf. 2020. (Accessed 3 May 2022).


