Abstract
Background:
Mortality and morbidity from tuberculosis (TB) remain one of the most important public health issues. Although cell-mediated immunity is the main immune response against Mycobacterium tuberculosis (MTB), the role of B-cells during MTB infection and disease is unclear.
Methods:
Peripheral blood mononuclear cells (PBMC) were isolated from treatment naïve Pulmonary TB patients (TB, n = 16), latent TB-infected participants (LTBI, n = 17), and healthy controls (HC, n = 19). PBMCs were stained with various fluorescently labeled antibodies to define B-cell subsets using multicolor flow cytometry.
Results:
Atypical memory B cells (CD19+CD27−CD21−) and circulating marginal zone B-cells (CD19+CD27+CD21+IgM+IgD+CD23−) were significantly higher in active TB when compared to LTBI and HC. CD5+ regulatory B cells (Breg, CD19+CD24hiCD38hiCD5+) and resting B-cells (CD19+CD27+CD21+) in Active TB patients were significantly lower compared to HC and LTBI. Overall, there were no differences in B cell percentages (CD19+), naïve B cells (CD19+CD27−CD21+), Breg (CD19+CD24hiCD38hi), and activated memory B cells (CD19+CD27+CD21−) among the three study groups.
Conclusions:
These results indicated that multiple subsets of B cells were associated with TB infection and disease. It will be useful to examine these cell populations for their potential use as biomarkers for TB disease and LTBI.
Keywords: B cells, Immuno-phenotyping, Tuberculosis
1. Introduction
The communicable disease, tuberculosis (TB) is among the leading causes of death and disease in the world. Globally, approximately 10 million people developed TB in 2020. Africa accounted for 25% of all TB cases in WHO regions. According to WHO 2021 report, Ethiopia is among the 30 high-burden countries listed for TB and TB/HIV [1].
Infection with Mycobacterium tuberculosis in a human host can take one of three forms: latent infection, reactivation, or primary active infection [2]. For maximum protection against infection and disease progression, a better knowledge of the interactions and complementing effects of the different arms of the immune system against tuberculosis, as well as the way M. tuberculosis manipulates these responses, is critical [3–5].
Researchers studied B cells in tuberculosis, such as (CD19+) B cells, plasma cells (CD138+), regulatory B cells (Breg) (CD1d+CD5+, CD25+CD71+, CD24hiCD27+, and CD24hiCD38hi), circulating marginal zone (CMZ) (CD19+IgM+CD23−CD27+), and marker of memory based on the combined expression patterns of CD21 and CD27, or the combined expression of IgD and CD27 [6–8]. B cells can conceivably shape anti-tuberculosis immunity through a variety of means including direct effects of antibodies upon the pathogen, cytokine production, as well as influencing the intracellular killing mechanisms of leukocytes [5]. However, protection against intracellular pathogens is often generalized as exclusively T cell-mediated, with B cells and antibodies playing a limited role than they are perceived to play during extracellular infections [4,5,8]; therefore, most studies done on TB focused on T cells. Despite this, recent studies indicate that B cells are crucial in the fight against M. tuberculosis [3–5,9] although some studies reported conflicting results on the role of B cells in TB [6,8,10,11].
Given the paucity of studies on B cells in human TB in general and the lack of any such studies in Ethiopia, the goal of the present investigation was to assess frequencies of B cells and their many subsets using flow cytometric approaches in TB patients in Ethiopia.
2. Methods
2.1. Study population
A cross-sectional study was conducted from February 2018 to April 2018. Study participants were recruited from five public health centers, in Addis Ababa, Ethiopia. Newly diagnosed smear positive and rifampicin-sensitive pulmonary TB patients were recruited before the initiation of chemotherapy. As controls, apparently healthy participants were recruited from Voluntary Counseling and Testing (VCT) clinics of the same study sites as the TB patients. Individuals with LTBI and HC were classified based on QuantiFERON-TB Gold assay results. All participants were between the age of 18 and 75 and HIV-negative. Individuals treated with immunosuppressive drugs and those with chronic diseases such as diabetes were excluded from the study. Blood from all participants and sputum from TB patients was collected at the health center and transported to the Armauer Hansen Research Institute (AHRI) for laboratory investigations.
2.2. QuantiFERON TB Gold Plus assay
The assay was performed according to the manufacturer’s instructions using the QFT-TB Gold Plus blood collection tubes composed of Nil, TB 1 antigen, TB 2 antigen, and Mitogen tubes and the QFT-TB Gold plus ELISA kit (Cellestis Limited, Carnegie, Victoria, Australia). Absorbance was read using a microplate reader with a 450 nm filter and with a 620 nm to 650 nm reference filter. The data was analyzed using the QFT-TB Gold plus Analysis Software [12].
2.3. PBMC isolation
Peripheral blood mononuclear cells (PBMC) were isolated from blood collected in heparinized tubes by density gradient centrifugation using Ficoll-Paque™PLUS solution (GE Healthcare). Briefly, whole blood was layered onto Leucosep tubes (BD) containing 15 ml of Ficoll and centrifuged for 20 min at 1700 RPM. The fluid above the separation membrane of the Leucosep tube which contains the PBMC was transferred into a new tube and first washed with phosphate-buffered saline (PBS) and then washed with FACS buffer (PBS, Heat-inactivated FBS (Fatal bovine serum) and Na2 EDTA) for 10 min at 1700 RPM.
2.4. Flow cytometry
Surface staining was done by incubating fresh PBMC with a cocktail of antibodies containing 5 μl CD19 PerCP (Clone 4G7, BD, Catalog No. 345778), 3 μl CD21 PE-CY7 (Clone B-ly4, BD, Catalog No. 561374), 20 μl CD27 FITC (Clone Ll28, BD, Catalog No. 340424), 3 μl CD23 BV421 (Clone M-L233, BD, Catalog No. 562707), 10 μl sIgD PE(Clone IA6–2, BD, Catalog No. 555779), 10 μl sIgM APC (Clone G20–127, BD, Catalog No. 551062) in panel one and 5 μl CD19 PerCP (Clone 4G7, BD, Catalog No. 345778), 3 μl CD21 PE-CY7 (Clone B-ly4, BD, Catalog No. 561374), 20 μl CD27 FITC (Clone Ll28, BD, Catalog No. 340424), 10 μl CD 5 PE(Clone Ll7F12, BD, Catalog No. 345782), 3 μl CD38 APC (Clone HB7, BD, Catalog No. 345807) and 3 μl CD24 BV421 (Clone ML5, BD, Catalog No. 562789) in panel two. Incubations were done for 20 min at room temperature in the dark. Then cells were washed and re-suspended in FACS (Fluorescence Activated Cell Sorting) buffer. Data were acquired on a BD FACSCanto II with Diva software and analyzed using FlowJo software (Version 9.6.2).
2.5. Gating strategy
The lymphocyte population was gated based on the forward scatter (FSC) and side scatter (SSC) position, followed by a singlets gate based on FSC height and area, then total B-cells were selected based on CD19 positivity. Memory B cell subsets were identified using CD27 and CD21 within a total B-cells gate. Switched and non-switched B cells were defined based on lack (IgM−IgD−) or presence (IgM+IgD+) of surface immunoglobulin, respectively. Marginal zone B cells were defined as non-switched CD27+CD19+CD21+CD23− B cells. Breg is classified by the combined expression of CD24 and CD38 with or without CD5. We used FMO (Fluorescence minus one) and unstained control to gate the target population.
2.6. Data quality assurance
Data quality was addressed by following standard operating procedures carefully for all laboratory tests. Reagents and PBMC were optimized for flow cytometry. The quality of the LJ medium was assured by sterility checking and inoculating of known M. tuberculosis isolate.
2.7. Data analysis and interpretation
Data was analyzed by JMP software. Differences in frequencies of B cell subsets across all groups were assessed using the non-parametric Kruskal-Wallis test, and comparisons of two groups were evaluated with the non-parametric Wilcoxon rank sum test. Data is presented as median (Interquartile range) and a P value of less than 0.05% was taken as a statistically significant difference.
2.8. Ethical consideration
Ethical approval was obtained from the Department of Medical Laboratory Sciences of Addis Ababa University, Addis Ababa City Administration Health Bureau, and Armauer Hansen Research Institute (AHRI)/All Africa Leprosy, Tuberculosis and Rehabilitation Training Center (ALERT) ethics review committee. All participants gave written informed consent before data and specimen collection.
3. Results
3.1. Characteristics of study participants
We recruited smear-positive Tuberculosis patients before the initiation of anti-TB treatment and performed additional tests at AHRI, including a culture of sputum and the SD BIOLINE TB Ag MPT64 rapid test. The TB cases (N = 16) all had positive culture results and identified as M. tuberculosis complex by SD BIOLINE TB Ag MPT64 immunochromatographic assay. The LTBI group (N = 17) had positive interferon-gamma release assay (IGRA) using QuantiFERON (QFN) blood tests, whereas the healthy control group (N = 19) had negative results. There were more males than females in each group. The median age was 26, 29, and 26 years for TB, LTBI, and healthy controls (HC) cases, respectively. Body Mass Index (BMI) was significantly lower in active TB than in LTBI and HCs (p = 0.0007) (Table 1).
Table 1.
Characterization of study participants, in selected health centers, Addis Ababa, Ethiopia, 2018.
| Variables | TB (n = 16) | LTBI (n = 17) | HC (n = 19) | P-value |
|---|---|---|---|---|
|
| ||||
| Sex | 0.8727 | |||
| Male | 10 | 10 | 12 | |
| Female | 6 | 7 | 7 | |
| Age (Years) | ||||
| Median (IQR) | 26(21–35) | 29(26–33) | 26(22–28) | 0.2344 |
| BMI (kg/m2) | ||||
| Median (IQR) | 18.75(17.1–20.5) | 21.7(20–25.35) | 22(20–24) | 0.0007 |
TB, Tuberculosis; HC, Healthy control; LTBI, Latent tuberculosis infections; IQR, Interquartile Range; BMI, Body mass index.
3.2. Analysis of total B-cells frequencies during M.tuberculosis infection and disease
Total B-cells were defined as CD19+ cells within a lymphocyte population (Figs. 1 and 2). Hence, we assessed the differences in the total percentages of B-cells (CD19+) in PBMCs among active TB, LTBI, and HC. Differences in proportions of total B-cells (CD19+) were not statistically significant among the three groups (p = 0.2407) although frequencies of B-cells were lower in TB patients compared to the HC group (Table 2).
Fig. 1.

Typical example of gating strategy for memory B cells subsets (CD27+CD21−, CD27−CD21−, CD27+CD21+ and CD27−CD21+): (A) lymphocyte gate using forward scatter (FSC) and Side Scatter (SSC), (B) Gate on singlets based on FSC-height and area, (C) B-cells are identified using CD19, (D) Memory B cell subsets are identified using CD27 and CD21 within a total B-cell gate, (E) Class switched and non-class switched memory B cell gate on resting memory B-cell subsets, (F) Circulating Marginal zone B cells gate on non-class switched memory B cell.
Fig. 2.
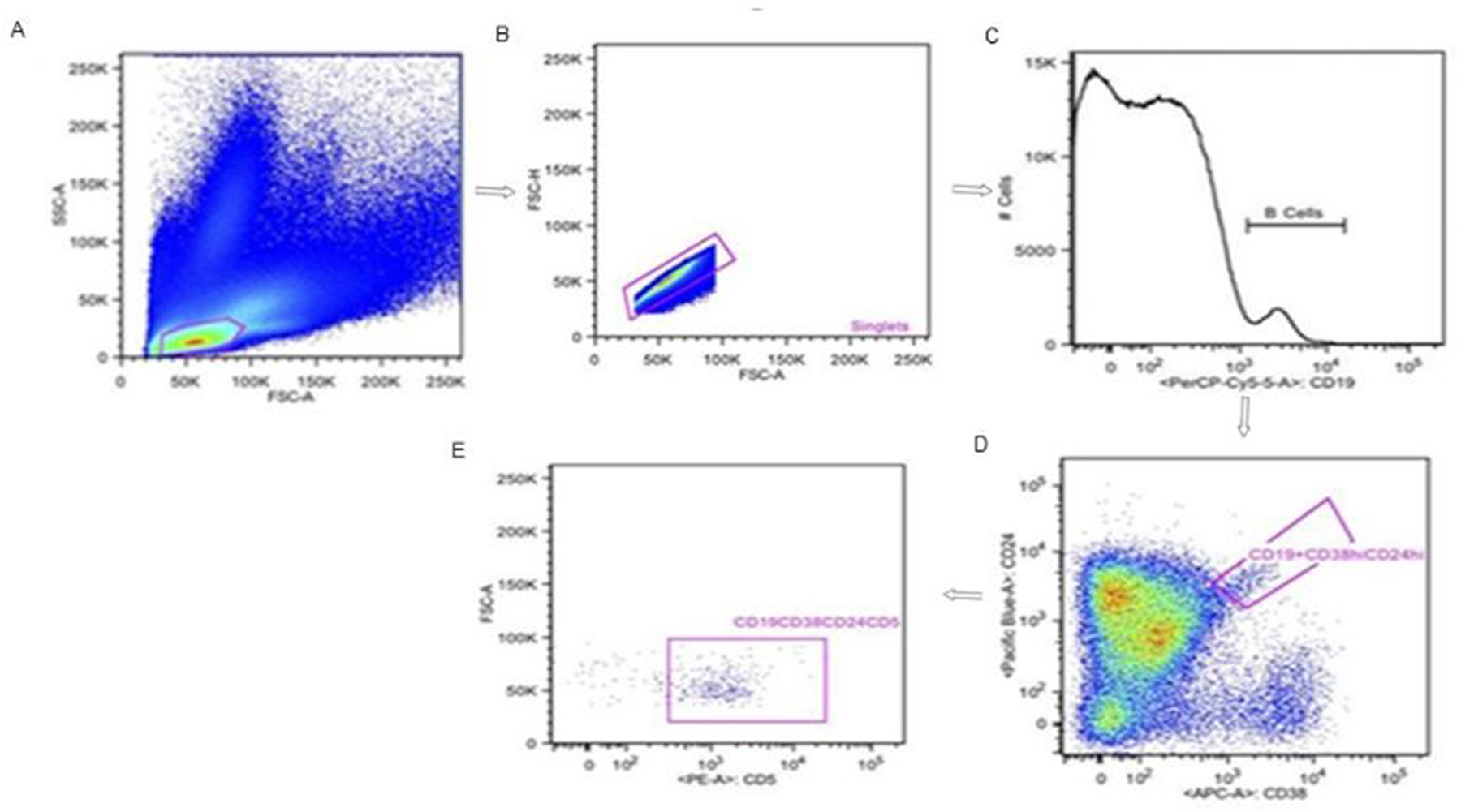
Typical example of gating strategy for regulatory B cell:(A) Lymphocyte gate using FSC and SSC, (B) Gate on singlet based on FSC-height and area, (C) B-cells are identified using CD19, (D) Regulatory B cell subsets are identified using CD24 and CD38 within a total B cell gate, (E) Alternative subset of regulatory B cells gate on CD24 and CD 38(CD19+CD24hiCD38hiCD5+).
Table 2.
Percentage of B cells subsets [Median (IQR)] in the study participants, Addis Ababa, Ethiopia, 2018.
| B cells subsets | HCs | LTBI | TB | Kruskal-wallis test P-value | P-values of Wilcoxon rank sum test |
||
|---|---|---|---|---|---|---|---|
| HCs vs. LTBI | HCs vs. TB | LTBI vs. TB | |||||
|
| |||||||
| Total B cells % | 3(1.6–5.3) | 1.9(1.0–3.3) | 1.5(0.8–5.2) | 0.2407 | 0.0962 | 0.0913 | 0.6656 |
| Naïve B cells % | 70.7(64–78) | 64.7(56.6–71.9) | 60.9(45–74.7) | 0.1156 | 0.1095 | 0.0661 | 0.5167 |
| aMBCs % | 11.5 (6.4–16.2) | 10.5(8.5–15.3) | 20.8(13.6–34.3) | 0.0024 | 0.7393 | 0.0036 | 0.0017 |
| AMBCs % | 5.3 (3.8–7.3) | 6.9(4.8–11.3) | 9.9(4.1–16.7) | 0.1134 | 0.1406 | 0.0591 | 0.428 |
| RMBCs % | 10.9 (8.3–14) | 15(11.2–19.5) | 3.2(2.2–5.3) | <.0001 | 0.0573 | <0.001 | <0.0001 |
| CSMBCs % | 62.7 (54.9–68.7) | 71.4(65.6–76.3) | 70.4(66.3–84.4) | 0.0065 | 0.0041 | 0.0118 | 0.4937 |
| NCSMBCs % | 27.6 (21.4–35) | 19.5(14.1–23.2) | 12.9(6.1–25.6) | 0.0011 | 0.0027 | <0.0021 | 0.1213 |
| CMZ % | 75.9(63.6–87.1) | 84.6(70.55–88.8) | 94.1(87.5–96.95) | 0.0015 | 0.2165 | 0.0012 | 0.0059 |
| Breg % | 13(7.0–17.8) | 7.64(3.6–14.2) | 7.47(3.7–10.5) | 0.1635 | 0.0842 | 0.1405 | 0.7458 |
| CD5+ Breg % | 49.3(41.367.6) | 47.9(41.3–58.2) | 34.5(25–42.5) | 0.0043 | 0.5792 | 0.0044 | 0.0042 |
IQR: Interquartile Range; HC: Healthy Control; LTBI: latent TB infection; TB: tuberculosis; aMBCs: atypical memory B cells; AMBCs: Activated memory B cells; RMBCs: Resting memory B cells; CSMBCs: Class switched memory B cells; NCSMBCs: Non-class switched memory B cells; CMZ: Circulating marginal zone; Breg: Regulatory B cells.
3.3. Distribution of memory B-cell subsets in active TB, LTBI, and HC
In this study memory B-cells subsets were detected in PBMCs based on the combined expression patterns of CD27 and CD21 within a total B-cell population gate. Naive B-cells were identified as CD21+CD27−; resting B-cells as CD21+CD27+; atypical memory B-cells as CD21−CD27-and activated B-cells as CD21−CD27+. Likewise, class-switched, and non-class-switched memory B cell subsets were defined as IgM− IgD− and IgM+ IgD+, respectively (Table 3).
Table 3.
Definitions of B cells subsets.
| Parameter | Subset |
|---|---|
|
| |
| CD19+ | B cell |
| CD19+CD27−CD21+ | Naïve B cell |
| CD19+CD27−CD21− | Atypical Memory B cell |
| CD19+CD27+CD21− | Activated Memory B cell |
| CD19+CD27+CD21+ | Resting Memory B cells |
| CD19+CD27+CD21+sIgM+sIgD+ | Non-class switched Memory B cell |
| CD19+CD27+CD21+ sIgM−sIgD− | Class switched Memory B cell |
| CD19+CD27+CD21+sIgM+sIgD+CD23− | Circulating Marginal Zone |
| CD19+CD24hiCD38hi | Regulatory B cells |
| CD19+CD24hiCD38hiCD5+ | CD5+ Regulatory B cells |
Frequencies of naïve and activated memory B-cells did not differ among the evaluated study participants. (Table 2). Active TB groups showed a significant decrease in resting memory B cells percentage compared to both HCs (p = 0.001) and LTBI (p=<0.0001) groups. In active TB cases, however, atypical memory B cells increased compared to LTBI and HCs, (p = 0.0017 and p = 0.0036, respectively) (Table 2).
The relative fraction of B-cells with class-switched memory phenotype was higher in active TB compared to HC (p = 0.0118) (Table 2). In contrast, significantly decreased proportions of non-class switched memory B cells were observed in the TB group compared to the HC group (p = 0.0021). The percentage of class-switched memory B-cells (IgM− IgD−) in LTBI cases was significantly higher (p = 0.0041) than in HC whereas Non-class switched memory B cells (IgM+ IgD+) were significantly lower (p = 0.0027) (Table 2).
Additionally, the percentage of CMZ B cells CD19+CD27+CD21+sIgM+sIgD+CD23− within non-class switched memory B cells was higher in the TB group compared to the HCs (p = 0.0012) and LTBI (p = 0.0059). There was no statistically significant difference observed between LTBI and HC (Table 2).
3.4. The proportion of regulatory B-cells (Breg) subsets among study participants
Regulatory B cells in TB have been characterized as CD24hiCD38hi and CD1d+CD5+. Accordingly, we evaluated the frequency of Bregs in PBMCs between active TB, LTBI, and HC.
The proportions of Breg (CD19+CD24hiCD38hi) were similar among the three groups. We also assessed the alternative CD5 expressing subset (CD19+CD24hiCD38hiCD5+). Significantly lower Bregs among active TB patients were observed compared to LTBI (p = 0.0042) and HCs (p = 0.0044). There was no between HCs and LTBI (Table 2).
4. Discussion
B lymphocytes are identified by the expression of CD19 surface antigen, which is present at an early stage of bone marrow development and persists throughout the maturation process [13]. Different subsets of B cells can be distinguished in peripheral blood and other tissues. These are classified according to lineage and differentiation markers and include nave B-cells, immature B-cells, plasma cells, and memory B-cells. Memory B-cells can be further divided based on their expression of CD21 and CD27 or IgD and CD27. Class-switched human B cells are IgM−IgD− (double-negative) and non-class-switched human B cells are IgM+IgD+ (double-positive) [6,13,14]. B-cells have a regulatory phenotype as well, where they produce cytokines such as IL10 and TGF-alpha. Several phenotypes of B-cells with regulatory functions have been termed Bregs, which include, CD19+CD24hiCD38hi B-cells as well as CD5+ Breg cells [6,8].
Human B-cells have been understudied compared to the large number of studies assessing T-cell contributions in the control of M. tuberculosis infection. Studies of B-cells in TB suggest that these cells may contribute to disease pathology other than producing antibodies. Nevertheless, data are often contradictory [6–11]. Therefore, we studied B-cell phenotypes in patients with active TB, with LTBI, and in HC. We identified B-cells subsets based on ten markers.
Percentage and the total number of CD19+ B cells in previous studies of active untreated TB patients have been reported to be decreased relative to healthy controls in some [6,10] with other reports showing higher levels [11]. In our study, we observed decreased levels of B cells. Whether the frequency is low because of reduced total numbers in the body, or due to sequestration from the blood into diseased tissues is not clear. Variability in the dynamics of B cell tissue distribution, generation, and death may underly some of the discrepancies seen in the literature.
Similarly, levels of memory B-cells subsets were assessed in our study. We observed that active TB patients had higher frequencies of class-switched (IgM−IgD−) memory B lymphocytes, but not naïve B cells (CD21+CD27−) in their circulation than healthy controls. Class switching is associated with B cell memory, leading to more efficient clearance of infections than primary antibody responses [15]. Moreover, resting memory (CD21+CD27+) B-cell frequency was significantly decreased in active TB patients when compared to LTBI and HCs. In this investigation, proportions of activated memory B cells (CD21−CD27+) in active TB patients were higher than LTBI; consistent with findings in a previous study about active tuberculosis [6]; however, these differences did not achieve statistical significance in both studies. Furthermore, the study has shown that HIV-1 infection leads to increased numbers of activated memory B cells [16].
In the present study Atypical memory (CD21−CD27−) B cells were also investigated. Atypical memory B cells have been found in healthy individuals at low numbers, suggesting in disease settings these cells are an expansion of normally occurring cells. However, atypical memory B cells do not actively secrete antibodies or differentiate into antibody-secreting cells (ASCs) and also, exhibit reduced B cell receptor (BCR)-mediated signaling. Accordingly, they exhibit markedly impaired BCR-triggered Ca2+ mobilization, proliferation, and cytokine production [17,18]. In line with our report, phenotypic studies revealed that CD21−CD27− atypical memory B cells were similarly increased in the peripheral blood of patients with active TB than LTBI and HC, with levels normalizing after therapy [6]. Additionally, chronic infections such as hepatitis C virus [19], HIV-1 [16], and malaria [18,20] have been associated with atypical memory B cells. In contrast, decreased numbers of CD10−CD21−CD27− atypical memory B cells were found in patients with untreated erythema nodosum leprosum (ENL) reactions [15].
Marginal zone (MZ) B cells reside at the interface between the circulation and the white pulp of the spleen. They provide a first line of defense against infections by blood-borne viruses and encapsulated bacteria by rapidly producing IgM and class-switched IgG antibodies [21]. In peripheral blood, circulating marginal zone B cells have been described as IgM + IgD + CD27+ [22,23] and CD19+IgM+CD23−CD27+ [7]. In this study the number of circulating marginal zone B-cells (CD19+CD27+CD21+IgM+IgD+CD23−) in active TB cases was at a higher percentage than in LTBI; the contrary is reported by Du Plessis WJ et al. [7].
A consensus set of cell surface markers to specifically identify Breg has not been defined. Since numerous cell surface markers have been linked to Breg, a combination of these markers has been proposed as a method of better identifying Breg populations [24,25]. Therefore, in our study, we assessed CD19+CD24hiCD38hiBreg cells and an alternative CD19+CD24hiCD38hiCD5+ Breg marker combination, the latter of which are a main source of IL-10 that contribute to Breg suppressive function [6]. A higher level of IL-10 has been found in sputum and bronchoalveolar lavage fluid (BALF) [26]. Consistent with a previous report by Joosten and colleagues [6], we found in our study of human PBMC that CD19+CD24hiCD38hi Breg was not significantly different among the three study groups. However, in our study, the CD5+ subset was significantly lower in active TB patients when compared to LTBI and HCs. Contrary to our finding, Zhang M et al. reported significantly higher frequencies of CD19+CD5+CD1d+ B cells with stronger suppressive activity in TB patients than in healthy donors [8].
A limitation of our study was that we did not assess TB cases following treatment. However, we identified multiple subsets of B cells associated with TB infection and disease from a high TB burden country.
5. Conclusion
We observed significant differences in total B cells and B cell subsets in TB patients compared with healthy controls or those with latent TB, consistent with the involvement of B cells in the immuno-pathogenesis of TB. Based on changes in the profiles of B-cells during Mycobacterium tuberculosis infection and disease, these markers may be useful as biomarkers for TB disease and LTBI identification. These findings need to be confirmed and translated into clinically useful tests through large-scale longitudinal studies.
Acknowledgments
We would like to thank the study volunteers for their participation. The scientific advice and technical assistance from Emawayish Andarge, Azeb Tarekegn, Tsehayinesh Lemma, Tigist Beyene, and Ruth Solomon were very much appreciated.
Funding sources
This study was mainly supported by Armauer Hansen Research Institute (AHRI) and partly by Addis Ababa University (AAU).
Abbreviations
- AHRI
Armauer Hansen Research Institute
- ASCs
antibody-secreting cells
- BALF
Bronchoalveolar lavage fluid
- BCR
B cell receptor
- Bregs
Regulatory B cells
- CMZ
Circulating Marginal Zone
- DCs
Dendritic cells
- EDTA Na2
Ethylenediaminetetraacetic acid disodium
- ELISA
Enzyme-linked Immuno-Sorbent Assay
- FACS
Fluorescence Activated Cell Sorting
- FBS
Fetal bovine serum
- FMO
Fluorescence minus one
- FSC
Forward scatter
- HCs
Healthy controls
- HIV
Human Immunodeficiency Virus
- IGRA
Interferon gamma release assay
- LJ
Lowenstein Jensen Media
- LTBI
Latent tuberculosis infection
- MTB
Mycobacterium tuberculosis
- PBMC
Peripheral blood mononuclear cell
- PBS:
Phosphate buffered saline
- PTB
Pulmonary tuberculosis
- QFN:
QuantiFERON
- sIg:
Surface immunoglobulin
- SSC
Side scatter
- TB
Tuberculosis
- TGF:
Transforming growth factor
- VCT
Voluntary Counseling and testing
- WHO
World Health Organization
Footnotes
Declaration of competing interest
The authors declare that they have no competing interests.
References
- [1].World Health Organization. Global tuberculosis report. Geneva, Switzerland: WHO; 2021. [Google Scholar]
- [2].Sutherland JS, Lalor MK, Black GF, Ambrose LR, Loxton AG, Chegou NN, et al. Analysis of host responses to Mycobacterium tuberculosis antigens in a multi-site study of subjects with different TB and HIV infection states in sub-Saharan Africa. PLoS One 2013;8(9):pone.0074080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Achkar JM, Chan J, Casadevall A. B cells and Antibodies in the Defense against Mycobacterium tuberculosis infection. Immunol Rev 2015. March;264(1):167–81. 10.1111/imr.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kozakiewicz L, Phuah J, Flynn J, Chan J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv Exp Med Biol 2013;783:225–50. 10.1007/978-1-4614-6111-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur J Immunol 2009;39(3):676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Joosten SA, van Meijgaarden KE, del Nonno F, Baiocchini A, Petrone L, Vanini V, et al. Patients with tuberculosis have a dysfunctional circulating B-cell compartment, which normalizes following successful treatment. PLoS Pathog 2016;12(6). 10.1371/journal.ppat.1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].du Plessis WJ, Keyser A, Walzl G, Loxton AG. Phenotypic analysis of peripheral B cell populations during Mycobacterium tuberculosis infection and disease. J Inflamm 2016;13:23. 10.1186/s12950-016-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang M, Zheng X, Zhang J, Zhu Y, Zhu X, Liu H, et al. CD19+CD1d+CD5+ B cell frequencies are increased in patients with tuberculosis and suppress Th17 responses. Cell Immunol 2012;274:89–97. 10.1016/j.cellimm.2012.01.007. [DOI] [PubMed] [Google Scholar]
- [9].Raja A Immunology of tuberculosis. Indian J Med Res 2004;120(4):213–32. [PubMed] [Google Scholar]
- [10].Hernandez J, Velazquez C, Valenzuela O, Robles-Zepeda R, Ruiz-Bustos E, Navarro M, et al. Low number of peripheral blood B lymphocytes in patients with pulmonary tuberculosis. Immunol Invest 2010;39(3):197–205. 10.3109/08820130903586346. [DOI] [PubMed] [Google Scholar]
- [11].Wu YE, Zhang SW, Peng WG, Li KS, Li K, Jiang JK, et al. Changes in lymphocyte subsets in the peripheral blood of patients with active pulmonary tuberculosis. J Int Med Res 2009;37(6):1742–9. 10.1177/147323000903700610. [DOI] [PubMed] [Google Scholar]
- [12].QuantiFERON®-TB Gold Plus (QFT®-Plus) ELISA Package Insert. QFTPlus_ELISA_R04_022016.
- [13].Veneri D, Ortolani R, Franchini M, Tridente G, Pizzolo G, Vella A. Expression of CD27 and CD23 on peripheral blood B lymphocytes in humans of different ages. Blood Transfus 2009;7(1):29–34. 10.2450/2008.0007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaminski DA, Wei C, Qian Y, Rosenberg AF, Sanz I. Advances in human B cell phenotypic profiling. Front Immunol 2012;3:302. 10.3389/fimmu.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Negera E, Walker SL, Bekele Y, Dockrell HM, Lockwood DN. Increased activated memory B-cells in the peripheral blood of patients with erythema nodosum leprosum reactions. PLoS Neglected Trop Dis 2017;11(12):e0006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Amu S, Lavy-Shahaf G, Cagigi A, Hejdeman B, Nozza S, Lopalco L, et al. Frequency and phenotype of B cell subpopulations in young and aged HIV-1 infected patients receiving ART. Retrovirology 2014;11:76. 10.1186/s12977-014-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Karnell JL, Kumar V, Wang J, Wang S, Voynova E, Ettinger R. Role of CD11c (+) T-bet(+) B cells in human health and disease. Cell Immunol 2017;321:40–5. 10.1016/j.cellimm.2017.05.008. [DOI] [PubMed] [Google Scholar]
- [18].Portugal S, Tipton CM, Sohn H, Kone Y, Wang J, Li S, et al. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife 2015;8:4. 10.7554/eLife.07218.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Charles ED, Brunetti C, Marukian S, Ritola KD, Talal AH, Marks K, et al. Clonal B cells in patients with hepatitis C virus-associated mixed cryoglobulinemia contain an expanded anergic CD21low B-cell subset. Blood 2011;117(20):5425–37. 10.1182/blood-2010-10-312942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol 2009;183(3):2176–82. 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 2013;13(2):118–32. 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley M, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a pre-diversified immunoglobulin repertoire. Blood 2014;104(12):3647–54. 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Scheeren FA, Nagasawa N, Weijer K, Cupedo T, Kirberg J, Legrand N, et al. T cell – independent development and induction of somatic hypermutation in human IgM + IgD + CD27 + B cells. J Exp Med 2018;205(9):2033–42. 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dai YC, Zhong J, Xu JF. Regulatory B cells in infectious disease (Review). Mol Med Rep 2017;16(1):3–10. 10.3892/mmr.2017.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jiao Y, Wang X, Zhang T, Sun L, Wang R, Li W, et al. Regulatory B cells correlate with HIV disease progression. Microbiol Immunol 2014;58(8):449–55. 10.1111/1348-0421.12171. [DOI] [PubMed] [Google Scholar]
- [26].Almeida AS, Lago PM, Boechat N, Huard RC, Lazzarini LCO, Santos AR, et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol 2009;183:718–31. 10.4049/jimmunol.0801212. [DOI] [PubMed] [Google Scholar]


