Abstract
Objectives:
To estimate the pooled proportion of extensively drug-resistant tuberculosis (XDR-TB) and pre-extensively drug-resistant tuberculosis (pre-XDR-TB) in patients with multidrug-resistant TB (MDR-TB).
Methods:
We systematically searched articles from electronic databases: MEDLINE (PubMed), ScienceDirect, and Google Scholar. We also searched gray literature from the different literature sources main outcome of the review was either XDR-TB or pre-XDR-TB in patients with MDR-TB. We used the random-effects model, considering the substantial heterogeneity among studies. Heterogeneity was assessed by subgroup analyses. STATA version 14 was used for analysis.
Results:
A total of 64 studies that reported on 12,711 patients with MDR-TB from 22 countries were retrieved. The pooled proportion of pre-XDR-TB was 26% (95% confidence interval [CI]: 22–31%), whereas XDR-TB in MDR-TB cases was 9% (95% CI: 7–11%) in patients treated for MDR-TB. The pooled proportion of resistance to fluoroquinolones was 27% (95% CI: 22–33%) and second-line injectable drugs was 11% (95% CI: 9–13%). Whereas the pooled resistance proportions to bedaquiline, clofazimine, delamanid, and linezolid were 5% (95% CI: 1–8%), 4% (95% CI: 0–10%), 5% (95% CI; 2–8%), and 4% (95% CI: 2–10%), respectively.
Conclusion:
The burden of pre-XDR-TB and XDR-TB in MDR-TB were considerable. The high burdens of pre-XDR-TB and XDR-TB in patients treated for MDR-TB suggests the need to strengthen TB programs and drug resistance surveillance.
Keywords: Pre-extensively drug-resistant tuberculosis, Extensively drug-resistant tuberculosis, Multidrug-resistant tuberculosis
Introduction
The rise of drug-resistant (DR) bacterial infections is becoming a major public health concern worldwide. It threatens global tuberculosis (TB) control programs and makes TB diagnosis and treatment challenging. In the past 20 years, DR-TB has spread across the world and continued to be a challenge to global TB control efforts [1]. A recent estimate indicated 465,000 incident cases of multidrug resistance/rifampicin (RIF) resistance (MDR/RR-TB) occurred worldwide [2]. In addition, an estimated 3.6% of new TB cases and 18% of previously treated TB cases have developed MDR-TB in 2021 [3]. Moreover, on average, 6.2% of XDR-TB was estimated in 2019 among patients treated for MDR-TB [2]. Prolonged duration required for the treatment, low cure rates, and the cost of drugs and toxicity make DR-TB treatment the most costly challenge [4].
Migration, housing conditions, poverty, and the emergence of other diseases, such as HIV and diabetes, are the factors fueled the burden of MDR/XDR-TB [5,6]. Furthermore, low laboratory diagnosis capabilities that delay DR-TB diagnosis and limited access to second-line MDR-TB treatment are associated with the transmission of resistant strains. Therefore, to stop the emergency of DR-TB strain, the best strategy is evidence-based diagnosis and treatment [7].
Before 2021, XDR-TB was defined as a disease caused by Mycobacterium tuberculosis with resistance to at least isoniazid (INH) and RIF (MDR-TB), with further resistance to any fluoroquinolones (FQs) and a second-line injectable drug (SLID) (kanamycin, amikacin, or capreomycin). Pre-XDR-TB is defined as TB with resistance to INH, RIF, and either an FQ or a second-line injectable agent but not both [4]. Based on new experimental and observational data, the World Health Organization (WHO) recently updated its guidelines, in which the late-generation FQs (levofloxacin and moxifloxacin) and WHO group A drugs (linezolid and bedaquiline) are recommended for the treatment of MDR-TB. In this guideline, XDR-TB is defined as an infection with MDR M. tuberculosis that is resistant to any FQs and at least one of the group A drugs. The most effective use of group A drugs to improve MDR-TB treatment requires appropriate drug susceptibility testing results [8].
The DR-TB treatment method has been updated in 2022. This document includes two new recommendations. The first regimen is the use of bedaquiline, pretomanid, linezolid, and moxifloxacin regimen for 6 months. This regimen is composed of bedaquiline, pretomanid, linezolid, and moxifloxacin and given to patients with MDR/RR-TB. However, patients with MDR/RR-TB with FQs additional resistance (pre-XDR-TB) should be treated for 9 months with all oral regimens. The consolidated guidelines includes the existing recommendations in the treatment regimens for INH-resistant TB with longer all oral regimens, monitoring of treatment response, timing of antiretroviral therapy in MDR/RR-TB for the patients infected with HIV, and the use of surgery for patients receiving MDR-TB treatment [8].
Several review studies have attempted to pool the proportion of MDR-TB cases. However, there are few review studies that attempted to estimate the pooled proportion of pre-XDR-TB and XDR-TB. Thus, we aimed to determine the pooled proportion of pre-XDR-TB and XDR-TB among patients diagnosed with MDR-TB from published primary studies.
Methods
Protocol registration
To prevent duplicates, the review study databases were searched for similar systematic reviews before this review commenced. The protocol of this systematic review and meta-analysis was registered in International Prospective Register of Systematic Reviews at the University of York database and obtained registration number PROSPERO ID: CRD42022343112.
Databases and search strategy
We followed the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines for reporting systematic reviews and meta-analyses [9,10]. We estimated the pooled proportion of pre-XDR-TB and XDR-TB in patients with MDR-TB for global occurrence. We conducted systematic searches of the following electronic databases: MEDLINE (PubMed), ScienceDirect, and Google Scholar, until July 20, 2022 for articles published in English, without limiting the year of publication. Studies that reported pre-XDR-TB and XDR-TB globally were included in the analysis. We used search terms: “(extensively drug-resistant tuberculosis OR XDR-TB) AND (pre-extensively drug-resistant tuberculosis OR Pre-XDR-TB) AND (drug-resistant tuberculosis OR DR-TB) AND (second-line drug resistance)” for the PubMed database search in both free text and medical subject heading.
Inclusion and exclusion criteria
We included cross-sectional studies that reported the proportion of either pre-XDR or XDR-TB among patients diagnosed with MDR-TB. However, we excluded studies that compared or validated the diagnostic methods for the detection of DR-TB and treatment outcomes. In addition, we excluded case studies, editorials, author comments, commentaries, general evaluations, and professional opinions to avoid duplicates.
Study selection
To identify potential studies, two authors (GD and BY) independently searched the electronic databases. Two reviewers (GD and DFG) independently screened the full-text papers to choose relevant articles based on the inclusion criteria. Differences between the two reviewers were resolved through discussion between the two authors (GD and DFG).
PICOS criteria
Participants: patients with MDR-TB with pre-XDR-TB and XDR-TB.
Intervention: not applicable.
Comparator: not applicable.
Outcome: pre-XDR-TB and XDR-TB among patients with MDR-TB.
Study design: observational studies.
Study setting: any setting in any country worldwide.
Definition of terms
Based on a previous 2021 definition, pre-XDR-TB and XDR-TB were defined as:
Pre-XDR-TB was defined as TB with resistance to INH and RIF and either an FQ or a second-line injectables.
XDR-TB referred to MDR-TB that is resistant to INH and rifampin plus any fluoroquinolone and at least one of the three SLIDs.
New TB case is defined as a newly registered episode of TB in a patient who has never been treated for TB or has taken anti-TB drugs for less than a month.
Previously treated TB case refers to a patient who has received anti-TB drugs in the past for a month or longer.
Data extraction
We extracted the data in a standard prepared Microsoft Excel sheet. Two authors (GD and BY) independently extracted the data from the selected primary studies. Data were extracted on the variables: first author name; year of publication; study period; study area (country); study design; a number of MDR-TB; a number of XDR-TB; a number of pre-XDR-TB; FLQ resistance; SLIDs resistance, new drugs resistance Bedaquiline (BDQ), Clofazimine (CFZ), Delamanid (DLM), and Linezolid (LZD), and previous treatment history. Discrepancies between the two authors’ on data records were resolved by consensus.
Risk of bias assessment and quality assessment
Two authors (GD and AA) evaluated the quality of the selected studies independently and in cases of inconsistencies a third reviewer (BY) was involved. We used Newcastle-Ottawa scale adapted for cross-sectional studies to assess the quality of the included studies. Newcastle-Ottawa scale rates the likelihood of bias in three domains of observational studies. These are the (1) selection of participants, (2) comparability, and (3) outcomes. For each numbered item in the selection and outcome categories, a study receives up to one point, and for comparability, a study may receive up to two points [11]. For low-, moderate-, and high-quality studies, the corresponding scores of 0–3, 4–6, and 7–9 were given, respectively. We used the I-squared statistic (I2) to assess the heterogeneity in the reported proportion. I2 ≥50% was used to indicate the presence of heterogeneity [12]. Moreover, a funnel plot was used to examine the possibility of publication bias.
Statistical analysis
We used the random-effects model to pool the proportion of pre-XDR-TB and XDR-TB and their 95% confidence interval (CI). The pooled proportion of pre-XDR-TB and XDR-TB in patients with MDR-TB was estimated using the “metaprop” command in STATA 14 (STATA Corporation, College Station, TX, USA). The estimates of pre-XDR-TB and XDR-TB pooled proportion were compared descriptively by the WHO regional categories and patient TB treatment history.
Results
Study selection
A total of 867 records were retrieved from the electronic and gray literature search and imported to EndNote reference manager. Of the total retrieved record, 389 remained after the duplicates were removed; Of 389 records, 298 were excluded by reviewing the title and abstract for population, intervention, and outcome difference with the current review. A total of 91 original articles were retrieved and fully articles were reviewed, and 27 were removed based on exclusion criteria. Finally, total of 64 articles were included in this review [5,13–75] (Figure 1).
Figure 1.
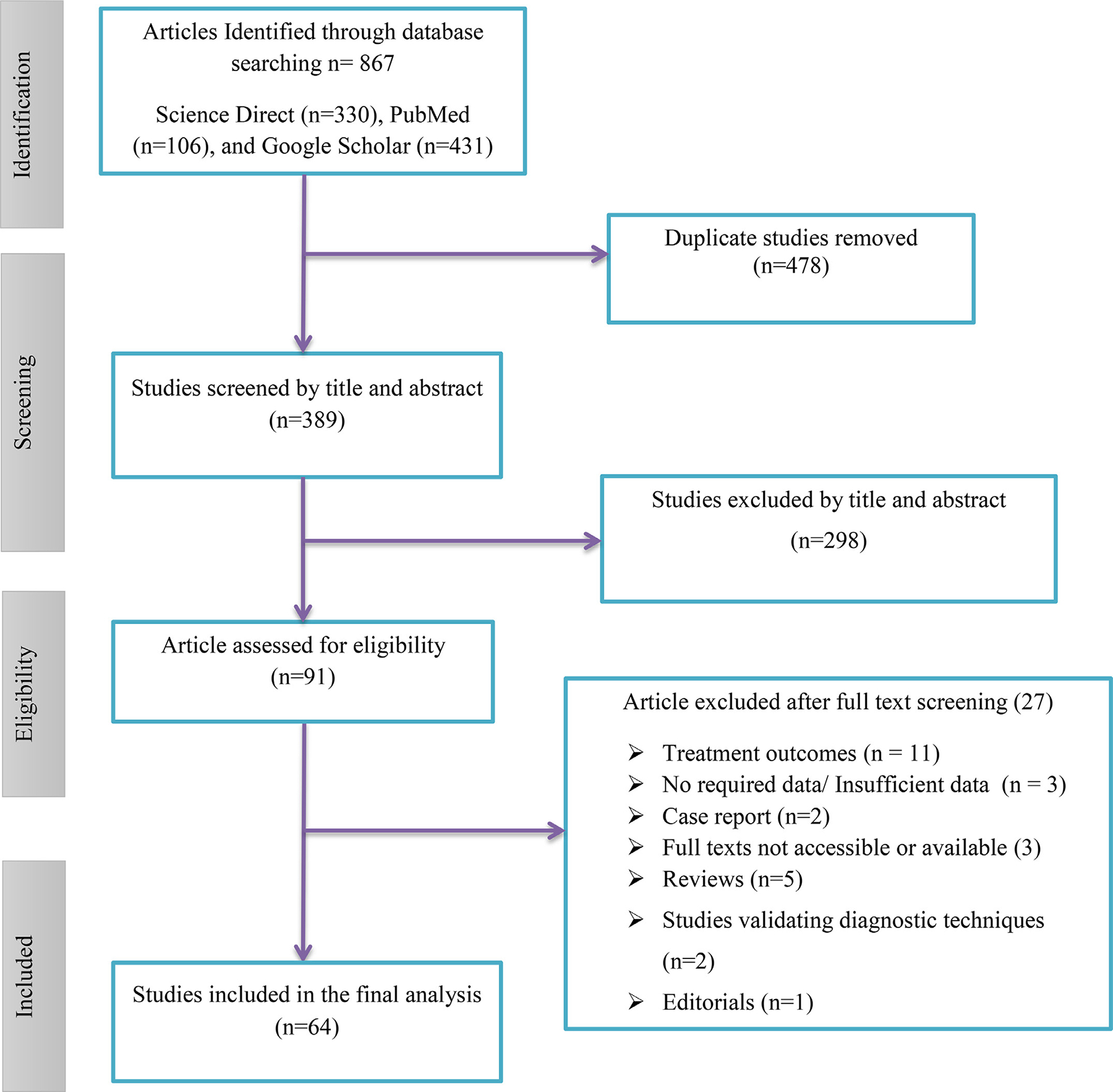
Flowchart describing the selection of studies for the systematic review and meta-analysis of extensively drug-resistant-TB and pre- extensively drug-resistant-TB TB in globally. TB, tuberculosis.
Characteristics of the studies included in the review
Detailed characteristics of included studies are depicted in Table 1. The included studies were reported from 22 countries across the WHO regions. A total of 13 studies were reported from in India [14,23,27,31,33,35,44,45,54,57–59,61] and 11 from China [13,20,29,53,66,67,69,70,72–74]. A total of 20 studies were reported from the Western Pacific [13,20,21,29,36–39,46,49,51,53,66,67,69–74] and 18 from South-East Asian regions [14,23,27,31–33,35,44,45,50,52,54, 57–59,61–63]. A total of 12 studies reported from African region [15,16,18,19,22,24,25,41,43,48,60,68]. The remaining 14 studies were reported from the Eastern Mediterranean, Americas, and European regions [5,17,26,28,30,34,40,42,47,55,56,64,65,75].
Table 1.
Characteristics of the individual studies on XDR-TB and pre-XDR-TB among DR-TB patients in globally included in the current systematic review and meta-analysis.
| First author, year | Study design | Country | WHO regions | Study period | MDR-TB | XDR-TB | Pre-XDR-TB | XDR-TB New | XDR-TB Previous treated | Pre- XDR-TB New | Pre- XDR-TB Previous treated | FQs resistance | SLIDs resistance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Adwani et al. [14] | cross-sectional | India | SEAR | 2014 | 227 | 11 | 127 | 11 | 0 | 127 | 0 | 127 | 19 |
| Agonafir et al. [15] | cross-sectional | Ethiopia | AFR | 2005–2006 | 46 | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 0 |
| Araujo et al. [75] | cross-sectional | Brazil | AMR | 2013–2019 | 33 | 2 | 3 | 1 | 1 | 1 | 2 | 5 | 2 |
| Elion Assiana et al. [16] | cross-sectional | Congo | AFR | 2018–2019 | 9 | 1 | 1 | NR | NR | NR | NR | 2 | 1 |
| Banerjee et al. [17] | cross-sectional | California | AMR | 1993–2006 | 424 | 18 | 77 | NR | NR | NR | NR | NR | NR |
| Bedru et al. [18] | cross-sectional | Ethiopia | AFR | 2017–2018 | 30 | 3 | 1 | 1 | 2 | 0 | 1 | NR | NR |
| Calver et al. [19] | cross-sectional | South Africa | AFR | 2003–2005 | 77 | 5 | 26 | NR | NR | NR | NR | NR | NR |
| Chen et al. [20] | cross-sectional | China | WPR | 2014 –2015 | 51 | 0 | 24 | 0 | 0 | 10 | 14 | 24 | 0 |
| Cheng et al. [21] | cross-sectional | Cambodia | WPR | 2012– 2017 | 118 | 3 | 16 | NR | NR | NR | NR | 15 | 5 |
| Dagne et al. [20] | cross-sectional | Ethiopia | AFR | 2019 | 99 | 1 | 8 | NR | NR | NR | NR | 7 | 3 |
| Dala et al. [21] | cross-sectional | India | SEAR | 2005–2013 | 340 | 33 | 193 | NR | NR | NR | NR | 179 | 41 |
| Daniel et al. [24] | cross-sectional | Nigeria | AFR | 2007–2011 | 50 | 0 | 10 | 0 | 10 | 0 | 10 | 8 | NR |
| Diriba et al. [25] | cross-sectional | Ethiopia | AFR | 2019 | 14 | 0 | 3 | 0 | 0 | 2 | 1 | 2 | 1 |
| Ennassiri et al. [26] | cross-sectional | Morocco | EMR | 2015 | 155 | 4 | 18 | NR | NR | NR | NR | 16 | 6 |
| Gadhav et al. [27] | cross-sectional | India | SEAR | 2019 | 700 | 23 | 143 | NR | NR | NR | NR | 106 | 58 |
| Gallo et al. [28] | cross-sectional | Brazil | AMR | 2011–2013 | 313 | 32 | 60 | 6 | 26 | 1 | 47 | 59 | 33 |
| He et al. [29] | cross-sectional | China | WPR | 2015 | 102 | 9 | 30 | NR | NR | NR | NR | 24 | 6 |
| Jabbar et al. [30] | cross-sectional | Pakistan | EMR | 2016–2017 | 62 | 5 | 0 | NR | NR | NR | NR | 5 | 5 |
| Jain et al. [31] | retrospective | India | SEAR | 2007–2009 | 130 | 11 | 55 | NR | NR | NR | NR | 36 | 19 |
| Jaksuwan et al. [32] | cross-sectional | Thailand | SEAR | 2005–2012 | 24 | 1 | 9 | NR | NR | NR | NR | NR | NR |
| James et al. [33] | cross-sectional | India | SEAR | 2003 –2007 | 103 | 45 | 0 | NR | NR | NR | NR | NR | NR |
| Javaid et al. [34] | cross-sectional | Pakistan | EMR | 2011–2012 | 132 | 2 | 65 | NR | NR | NR | NR | 67 | 5 |
| Kozinska et al. [5] | cross-sectional | Poland | EUR | 2000–2009 | 297 | 36 | 19 | NR | NR | NR | NR | NR | NR |
| Kumar et al. [35] | cross-sectional | India | SEAR | 2014–2016 | 173 | 3 | 33 | 1 | 2 | 5 | 28 | NR | NR |
| Kuo et al. [36] | cross-sectional | Taiwan | WPR | 2011–2015 | 63 | 4 | 0 | NR | NR | 0 | 0 | 4 | 4 |
| Lai et al. [37] | cross-sectional | Taiwan | WPR | 2000–2006 | 150 | 10 | 0 | 1 | 9 | NR | NR | 6 | 4 |
| Lee et al. [38] | cross-sectional | South Korea | WPR | 2011–2017 | 85 | 9 | 29 | NR | NR | NR | NR | 32 | 15 |
| Lee et al. [39] | cross-sectional | Korea | WPR | 2006–2013 | 145 | 55 | 0 | 27 | 28 | 0 | 0 | 43 | 12 |
| Macedo et al. [40] | cross-sectional | Portugal | EUR | 2008–2010 | 50 | 12 | 0 | NR | NR | NR | NR | NR | NR |
| Madukaji et al. [41] | cross-sectional | Nigeria | AFR | 2018–2019 | 101 | 12 | 16 | NR | NR | NR | NR | 5 | 12 |
| Matsui et al. [42] | cross-sectional | Brazil | AMR | 2016–2017 | 92 | 5 | 11 | 1 | 4 | 5 | 6 | NR | NR |
| Mbuh et al. [43] | cross-sectional | Cameroon | AFR | 2016–2017 | 75 | 1 | 2 | 0 | 1 | 0 | 2 | NR | NR |
| Misra et al. [44] | cohort study | India | SEAR | 2017–2019 | 62 | 48 | 11 | NR | NR | NR | NR | 48 | 11 |
| Mohan et al. [45] | cross-sectional | India | SEAR | 2012 | 87 | 3 | 0 | NR | NR | NR | NR | NR | NR |
| Mok et al. [46] | cross-sectional | Korea | WPR | 2010–2014 | 378 | 47 | 78 | 20 | 27 | 37 | 41 | 96 | 68 |
| Momen et al. [47] | cross-sectional | Morocco | EMR | 2015–2018 | 200 | 5 | 48 | 2 | 3 | 5 | 42 | 27 | 25 |
| Namburete et al. [48] | cross-sectional | Mozambique | AFR | 2014–2015 | 25 | 0 | 6 | 0 | 0 | NR | NR | 6 | 0 |
| Nguyen et al. [49] | cross-sectional | Vietnamese | WPR | 2011 | 91 | 5 | 15 | 2 | 3 | 8 | 7 | 15 | 5 |
| Noor et al. [50] | cross-sectional | Bangladesh | SEAR | 2011–2012 | 59 | 2 | 9 | 0 | 2 | 0 | 9 | 7 | 2 |
| Park et al. [51] | retrospective | Korea | WPR | 2008 | 2,472 | 749 | 0 | 313 | 436 | 0 | 0 | NR | NR |
| Poudel et al. [52] | cross-sectional | Nepal | SEAR | 2007–2010 | 109 | 13 | 43 | NR | NR | NR | NR | NR | NR |
| Qi et al. [53] | cross-sectional | China | WPR | 2009–2011 | 249 | 31 | 77 | 10 | 21 | NR | NR | 89 | 41 |
| Ramachandran et al. [54] | cross-sectional | India | SEAR | 2005 | 216 | 7 | 0 | 0 | 7 | NR | NR | 52 | 10 |
| Riccardi et al. [55] | retrospective | Italy | EUR | 2000–2015 | 370 | 0 | 83 | 0 | 0 | NR | NR | NR | NR |
| Salvato et al. [56] | cross-sectional | Brazil | AMR | 2013–2014 | 87 | 4 | 8 | NR | NR | NR | NR | NR | NR |
| Sethi et al. [57] | cross-sectional | India | SEAR | 2018 | 687 | 59 | 265 | 6 | 53 | 103 | 192 | 295 | 70 |
| Sharma et al. [58] | retrospective | India | SEAR | 2003 | 211 | 5 | 25 | 5 | NR | NR | 21 | 14 | |
| Sharma et al. [59] | cross-sectional | India | SEAR | 2014–2016 | 49 | 1 | 9 | NR | NR | NR | NR | NR | NR |
| Shibabaw et al. [60] | cross-sectional | Ethiopia | AFR | 2016–2018 | 176 | 1 | 10 | 1 | 0 | 1 | 9 | NR | NR |
| Singhal et al. [61] | cross-sectional | India | SEAR | 2012–2013 | 87 | 10 | 43 | NR | NR | NR | NR | 41 | 16 |
| Tasnim et al. [62] | cross-sectional | Bangladesh | SEAR | 2016–2017 | 68 | 4 | 11 | 1 | 3 | 3 | 8 | 9 | 2 |
| Tuladhar et al. [63] | cross-sectional | Nepal | SEAR | 2015 | 57 | 1 | 29 | NR | NR | NR | NR | 21 | 8 |
| Ullah et al. [64] | retrospective | Pakistan | EMR | 2019–2020 | 180 | 8 | 62 | NR | NR | NR | NR | 62 | 8 |
| Vashakidze et al. [65] | cross-sectional | Georgia | EUR | 2005–2007 | 261 | 33 | 96 | 6 | 27 | NR | NR | 75 | 54 |
| Wang et al. [66] | cross-sectional | china | WPR | 2008–2012 | 206 | 41 | 90 | NR | NR | NR | NR | 90 | 35 |
| Wang et al. [67] | cross-sectional | china | WPR | 2020 | 391 | 28 | 94 | NR | NR | NR | NR | 68 | NR |
| Welekidan et al. [68] | cross-sectional | Ethiopia | AFR | 2018–2019 | 38 | 0 | 2 | NR | NR | NR | 2 | 2 | 0 |
| Xu et al. [69] | cross-sectional | China | WPR | 2015–2018 | 17 | 0 | 9 | NR | NR | 1 | 8 | 9 | NR |
| Yang et al. [70] | cross-sectional | China | WPR | 2008–2009 | 239 | 29 | 138 | 14 | 15 | 64 | 74 | 134 | 77 |
| Yang et al. [71] | cross-sectional | Korea | WPR | 2017 | 420 | 9 | 17 | NR | NR | NR | NR | NR | NR |
| Yao et al. [13] | cross-sectional | China | WPR | 2018–2019 | 425 | 29 | 282 | NR | NR | NR | NR | 311 | 171 |
| Yuan et al. [72] | cross-sectional | China | WPR | 2010–2011 | 77 | 16 | 26 | NR | NR | NR | NR | 16 | 13 |
| Yuan et al. [73] | cross-sectional | China | WPR | 2010–2011 | 159 | 13 | 0 | 3 | 10 | NR | NR | NR | NR |
| Zheng et al. [74] | cross-sectional | China | WPR | 2014–2016 | 88 | 9 | 44 | NR | NR | NR | NR | 34 | 11 |
AFR, African region; AMR, region of the Americas; DR-TB, drug-resistant tuberculosis; EMR, Eastern Mediterranean eegion; EUR, European region; FQs, fluoroquinolone; MDR-TB, multidrug-resistant tuberculosis; SEAR, South-East Asian region; SLID, second-line injectable drug; WPR, Western Pacific region; XDR-TB, extensively drug-resistant tuberculosis.
The data were extracted from 64 studies involving a total of 12,711 patients with MDR-TB who were treated from 2003 to 2020, with publication years ranging from 2008 to 2021. The sample size of MDR-TB in the included studies varied from nine [16] to 2472 [51]. Among the 64 studies, 53 reported pre-XDR cases, whereas 57 reported XDR-TB cases.
Pooled proportion of pre-XDR-TB
The pooled proportion of pre-XDR-TB among MDR-TB cases was 26% (95% CI: 22–31; I2 = 97.31%). China had the highest proportion of pre-XDR-TB (66%) [13] and Ethiopia the lowest (3%) [18]. In the Western Pacific, South-East Asian, Eastern Mediterranean, European, Americas, and African regions, the pooled proportions of pre-XDR-TB were 35% (95% CI: (95% CI: 24–41; I2 = 96.2%), 30% (95% CI: 15–45; I2 = 95.41%), 22% (95% CI: 5–39), 14% (95% CI: 10–19; I2 = 65.25%), and 12% (95% CI: 7–17; I2 = 79.68%), respectively (Figure 2).
Figure 2.
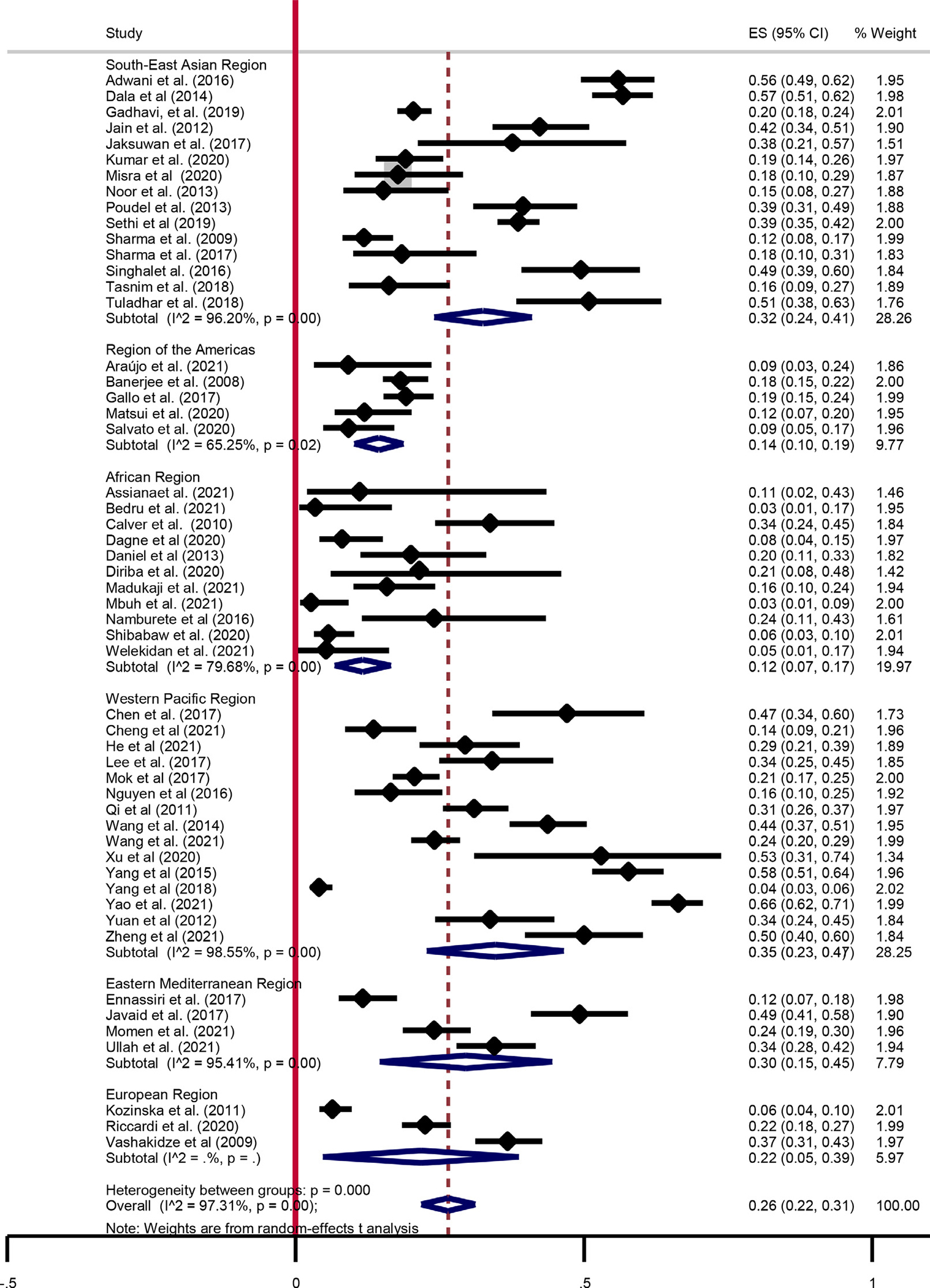
Summary of pooled estimates of pre-extensively drug-resistant-tuberculosis among multi drug-resistant-tuberculosis patients. CI, confidence interval; ES, effect size. New diagnosed cases Previously treated diagnosed cases.
In the current study, we also performed a subgroup analysis based on the treatment history of patients with MDR-TB (newly diagnosed and previously treated cases). In the newly diagnosed group, the data were extracted from 23 studies, with the sample sizes ranging from 14 [25] to 687 [57]. A study in China had the highest proportion of pre-XDR-TB (27%) [70], whereas Ethiopia and Cameroon had the lowest (1%) [43,60]. The pooled proportion of pre-XDR-TB among newly diagnosed MDR-TB cases was 9% (95% CI: 5–12; I2 = 96.32%). In the previously treated group, the data were extracted from 19 studies with sample sizes ranging from 14 [25] to 687 [57]. Similarly, the highest proportion of pre-XDR-TB (47%) was reported in China (69), whereas Ethiopia and Cameroon had the lowest (3%) [18,43]. The pooled proportion estimate of pre-XDR-TB proportion was 13% (95% CI: 8–18; I2 = 96.12%) (Figure 3).
Figure 3.
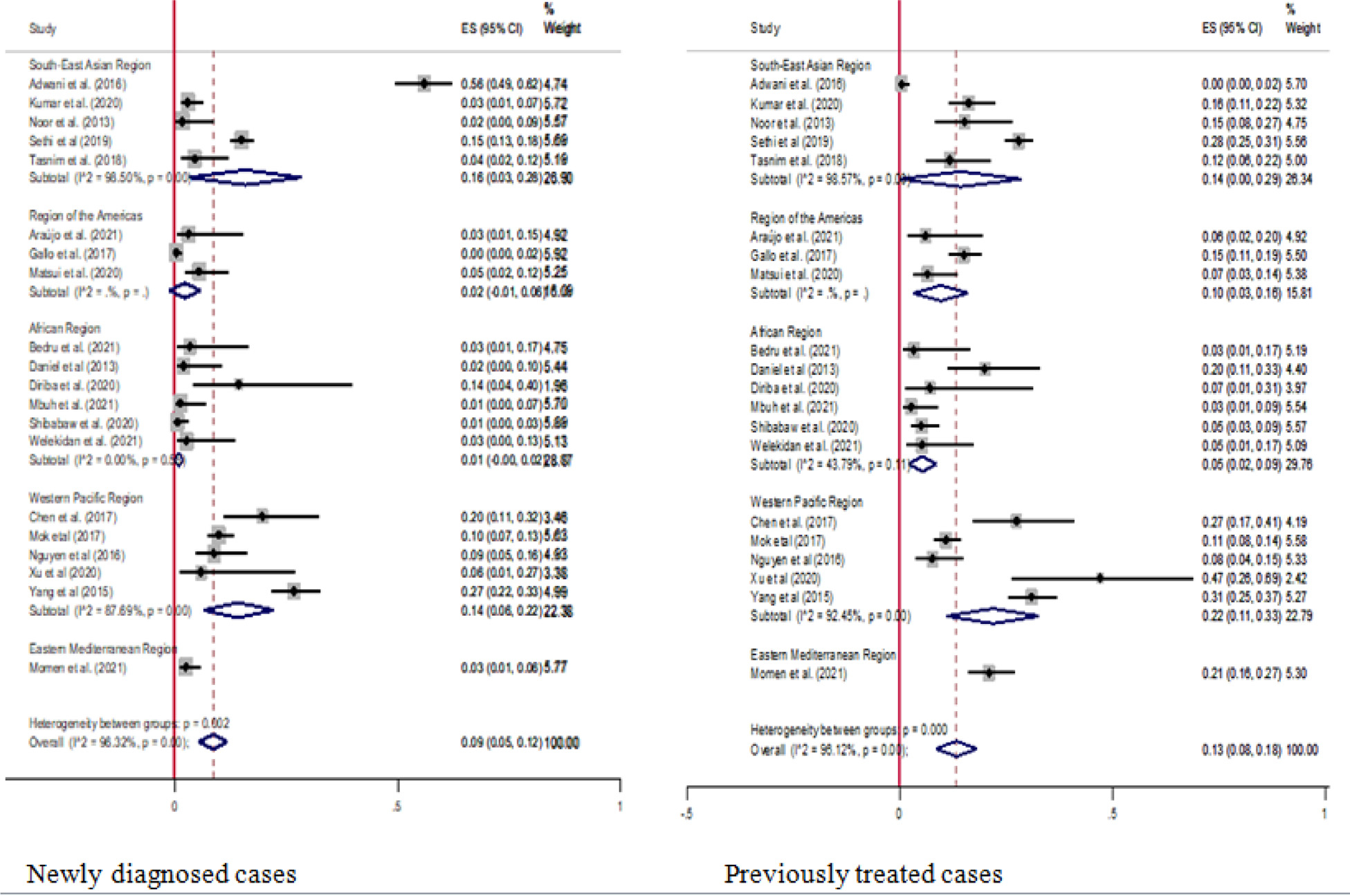
Pooled estimates of pre-extensively drug-resistant-tuberculosis among new and previous treated multi drug-resistant-tuberculosis patients. CI, confidence interval; ES, effect size.
Pooled proportion of XDR-TB
The proportion of XDR-TB was reported in all WHO regions. The estimated pooled proportion of XDR-TB among patients with MDR-TB was 9% (95% CI: 7–11; I2 = 95.98%). The highest proportion of XDR-TB was reported in India (77%) [44] and the lowest in Ethiopia [60] and Cameron (1%) [43]. The pooled proportions of XDR-TB in the Western Pacific, South-East Asian, Americas, African, and Eastern Mediterranean regions were 12% (95% CI: 7–17; I2 = 19.62%), 10% (95% CI: 6–13%; I2 = 94.54%), 6% (95% CI: 3–9; I2 = 57.54%), and 3% (95% CI: 1–5%; I2 = 65.68%), 3% (95% CI: 1–4; I2 = 19.62%), respectively (Figure 4).
Figure 4.
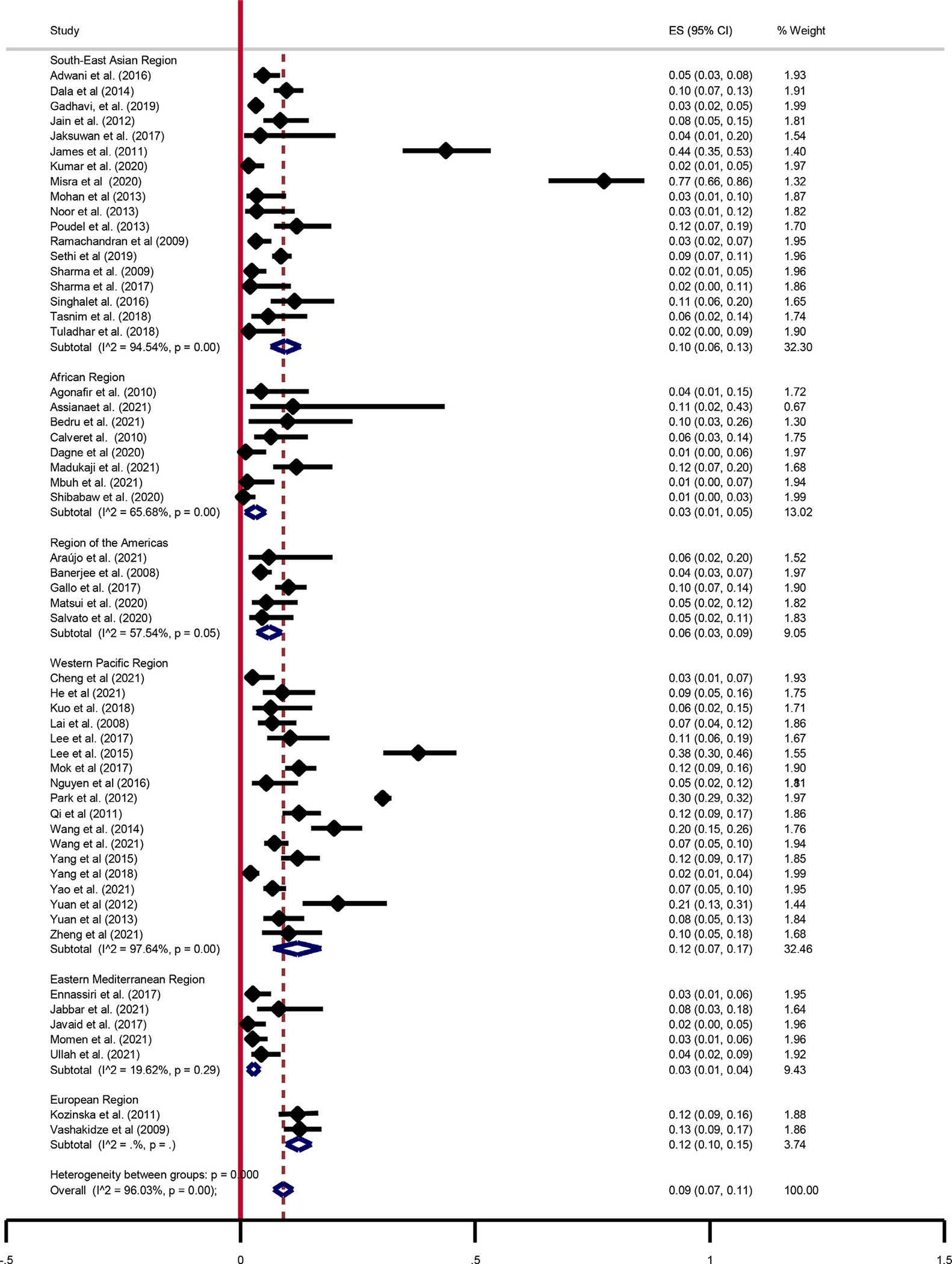
Pooled estimates of extensively drug-resistant-tuberculosis among multi drug-resistant-tuberculosis patients. CI, confidence interval; ES, effect size.
In the current study, we performed a subgroup analysis based on the treatment history of patients with MDR-TB (newly diagnosed and previously treated cases). In the newly diagnosed group, the data were extracted from 23 studies with a sample size ranges from nine [16] to 2472 [51]. Whereas the data was extracted from 25 studies, with sample sizes ranging from 33 [75] to 2472 [51], on previously treated patients. The pooled estimates of XDR-TB among newly diagnosed patients with MDR-TB were 3% (95% CI: 2–5; I2 = 93.58%) and 6% (95% CI: 4–8; I2 = 95.62%) among previously treated patients (Figure 5).
Figure 5.
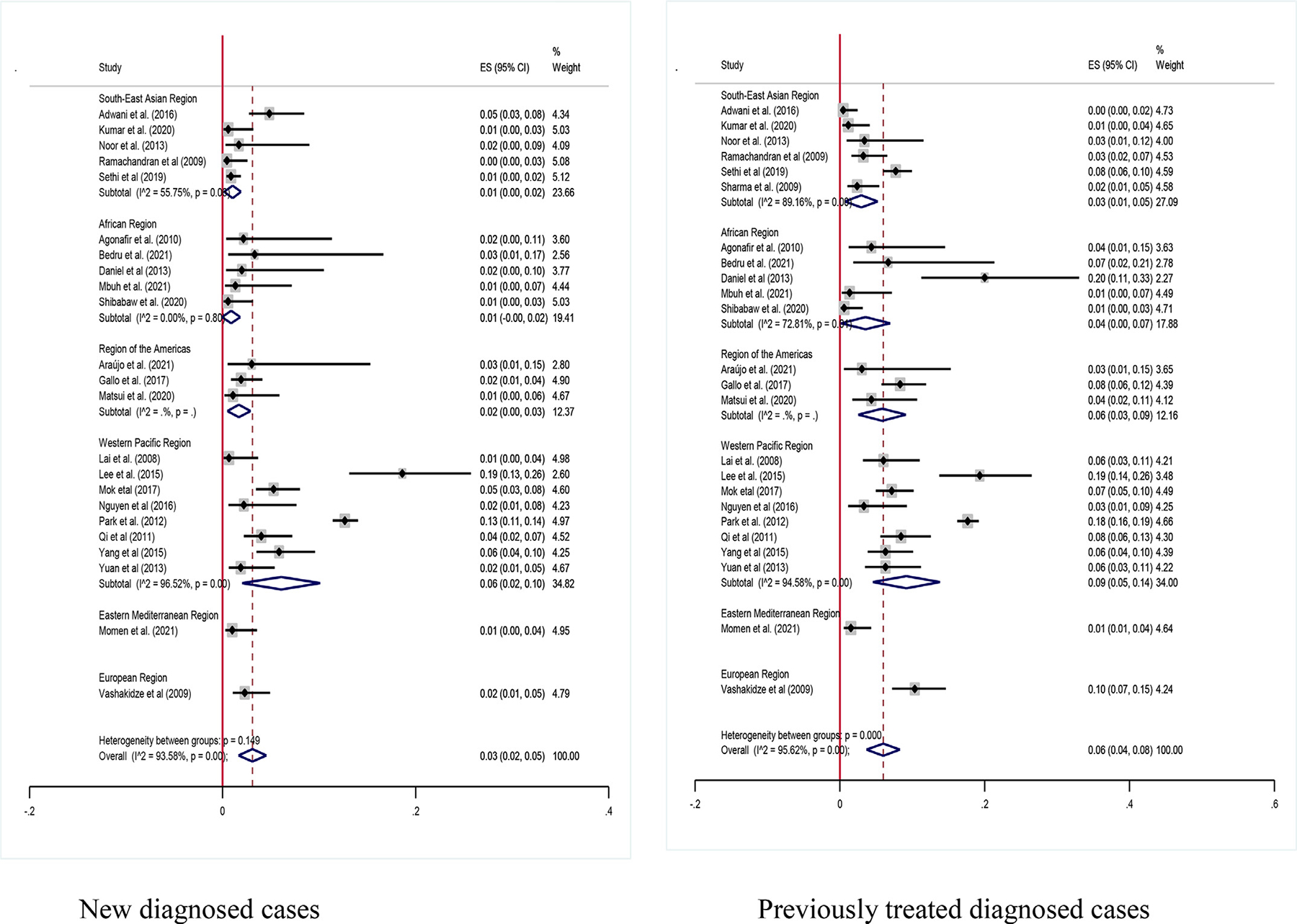
Pooled estimates of extensively drug-resistant-tuberculosis among new and previous treated multi drug-resistant-tuberculosis patients. CI, confidence interval; ES, effect size.
Pooled proportion estimates of FQs, SLID, and new drugs (BDQ, CFZ, DLM, and LZD)
In this study, we estimated the pooled proportion of resistance to FQs, SLIDs, and new drugs among patients with MDR-TB. The highest proportion of FQs resistance was 77% [44], whereas the lowest proportion was 4% [15,37]. Furthermore, the highest proportion of SLIDs resistance was 40% [13], whereas the lowest proportion was 3% [50,62]. The overall pooled proportion of FQs resistance among MDR-TB cases were 27% (95% CI: 22–33; I2 = 97.53%) and 11% (95% CI: 9–13; I2 = 91.31%) SLIDs resistance (Figure 6).
Figure 6.
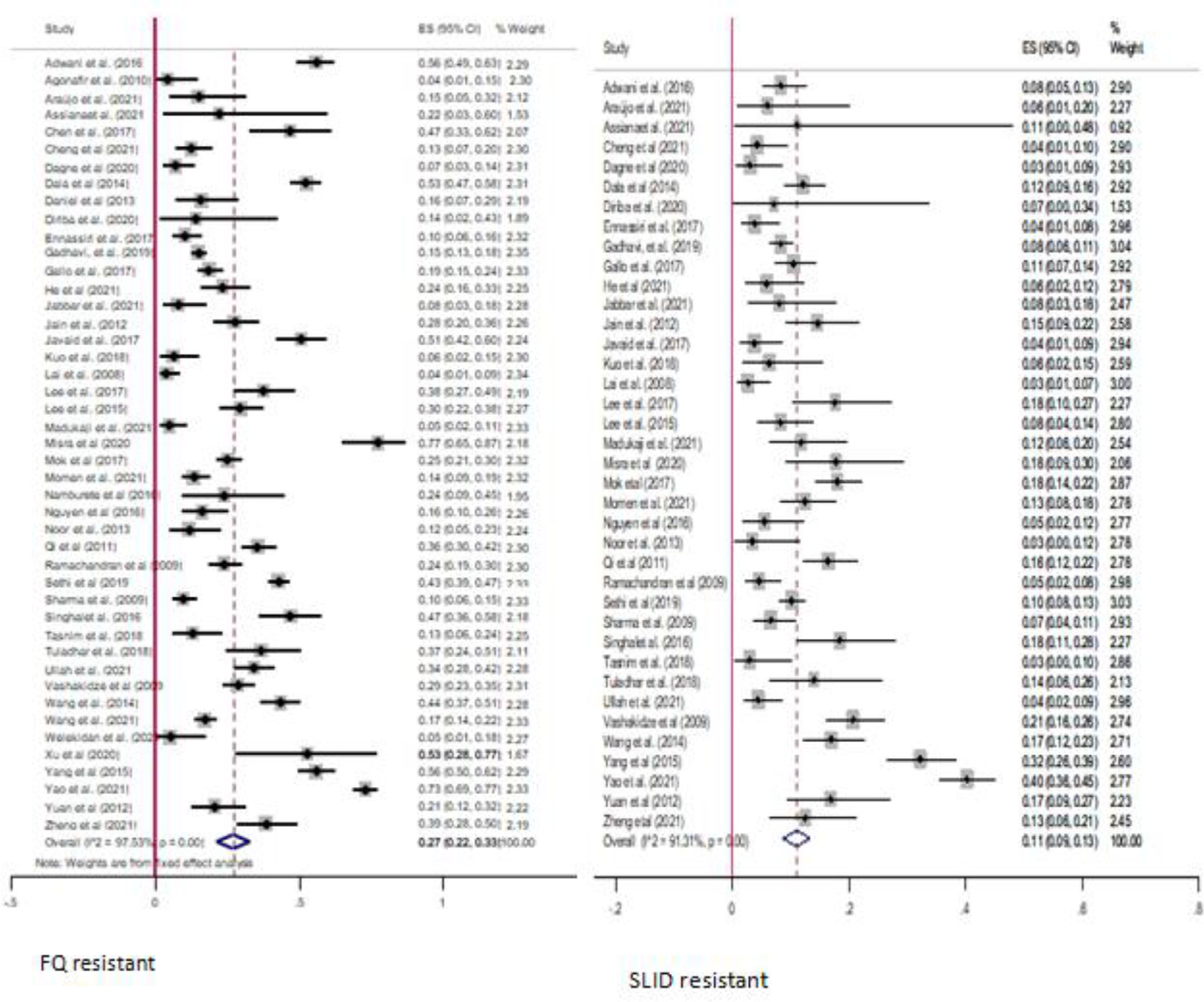
Summary of pooled estimates of FQs resistance and SLIDs resistance among multi drug-resistant-tuberculosis patients. CI, confidence interval; ES, effect size; FQs, fluoroquinolone; SLID, second-line injectable drug.
In this study, we performed a subgroup analysis to estimate the pooled new drug resistance among patients with MDR-TB. The pooled proportion of new drugs resistance was estimated from five studies for BDQ and LZD, four studies for DLM, and three studies for CFZ [13,29,67,71,74]. The sample size of the included studies ranged from 88 [74] to 425 [13]. The pooled proportion of resistance to new drugs among patients with MDR-TB was 5% (95% CI: 1–8; I2 = 90.84%) for BDQ, 4% (95% CI: 0–10; I2 = 84.27%) for CFZ, 5% (95% CI: 2–8; I2 = 80.80%) for DLM, and 4% (95% CI: 2–10; I2 = 67.39%) for LZD (Figure 7).
Figure 7.
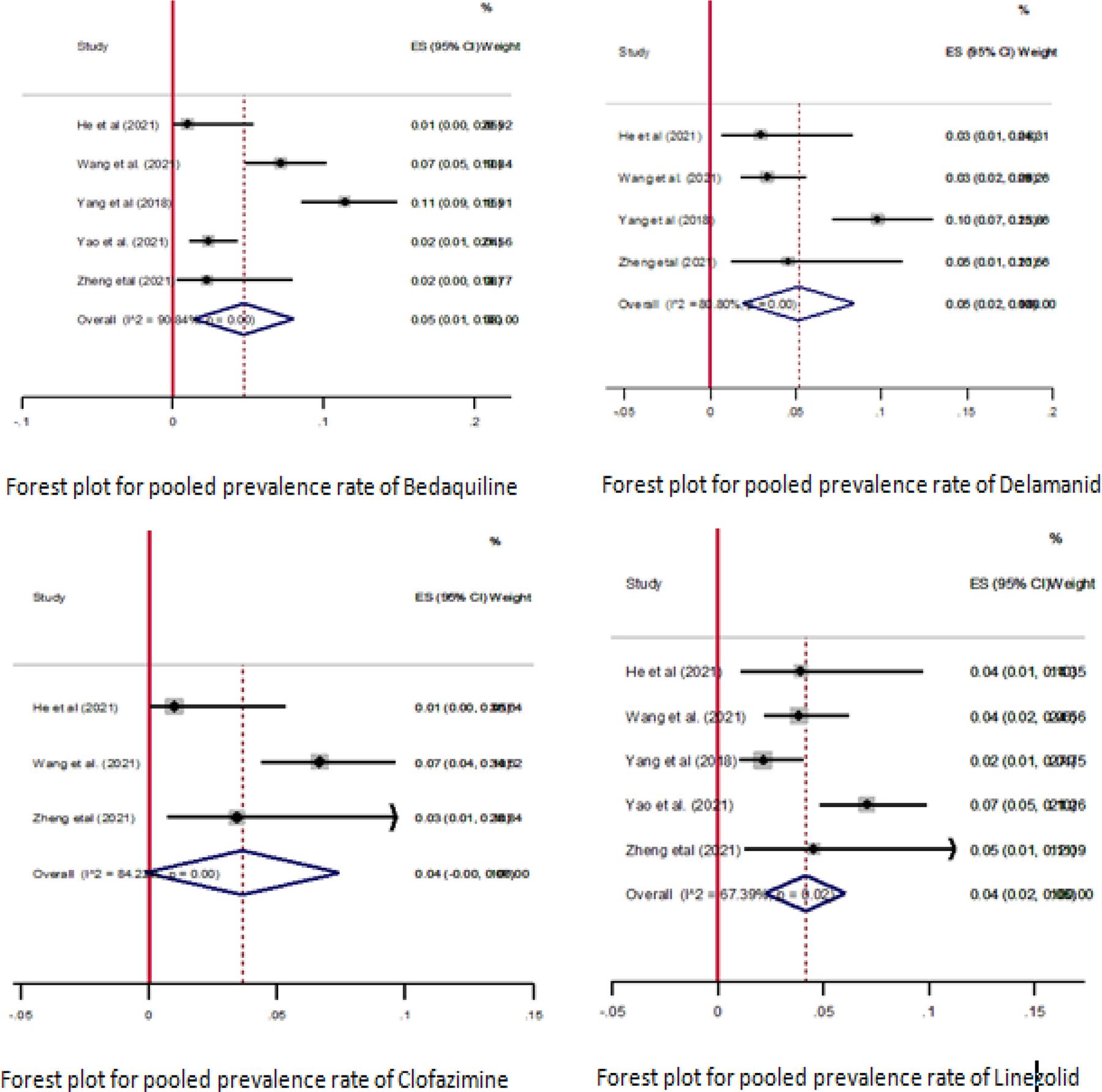
Summary of the pooled prevalence of new drug resistance among multi drug-resistant-tuberculosis patients. CI, confidence interval; ES, effect size.
Publication bias
We assessed the publication bias using funnel plots with the effect size and their standard errors. Visual inspection showed that the presence of publication bias was observed for the majority of the estimation of pre-XDR-TB, with fewer studies clustered at the tip of the funnel and the others distributed to the right and left corners of the funnel. The funnel plot for XDR-TB patients was relatively symmetrical, with only few studies visible in the right corners (Figure 8).
Figure 8.
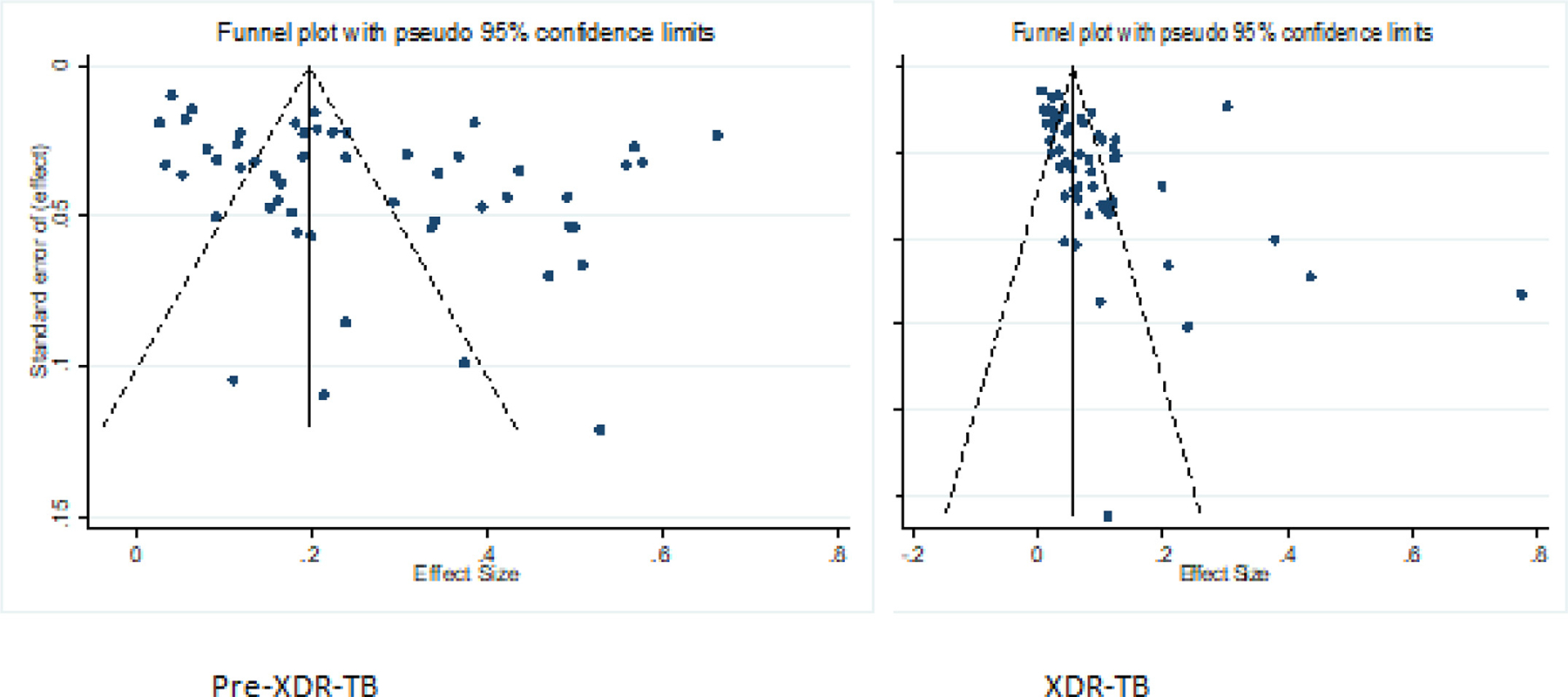
Funnel plots analyzing publication bias among studies evaluated for pre-XDR-TB and XDR-TB. XDR-TB, extensively drug-resistant-tuberculosis
Discussion
This systematic review and meta-analysis estimated the pooled proportion of pre-XDR and XDR-TB among patients diagnosed with MDR-TB from the study reported worldwide. The pooled proportions of XDR-TB among new patients with MDR-TB were 3% and 6% in previously treated patients. The pooled proportions of pre-XDR-TB among new patients with MDR-TB were 9% and 13% among previously treated patients. The overall pooled proportion of pre-XDR was 26%, whereas the proportion of XDR-TB was 9% among patients diagnosed with MDR-TB. The pooled proportion of FQs resistance was 27% and the proportion of SLIDs resistance was 11%. A considerable proportion of resistance to new drugs BDQ (5%), CFZ (4%), DLM (5%), and LZD (4%) were also reported worldwide.
In the current review, the pooled proportion of XDR-TB was 9%. This is relatively higher than the proportion reported by the WHO global TB report in 2019, in which the proportion of XDR-TB was 6.2% [4]. This substantial difference could be due to the fact that the current meta-analysis was based on the findings from published clinical studies that reported data from diverse patient populations in various settings. The data, therefore, effectively entails regional influences and different epidemiological factors contribute to drug resistance and do not involve selective sampling of patients. Moreover, the proportion reported in the current review might reflect the status of suspected isolates referred for resistance testing rather than the might actual prevalence that estimated from representative participates. In contrast, the proportion given by WHO is based on the estimation from the TB program report, which could lead to underestimation, whereas the current review is based on the primary studies reported by independent researchers worldwide, which could be more representative. The results of the current review findings were relatively similar to the 2018 WHO global TB report, in which the proportion of XDR-TB was 8.5% [76].
The proportion of XDR-TB among newly diagnosed patients with MDR-TB was 3% and 6% in previously treated patients. The combined proportion of pre-XDR-TB patients among the newly diagnosed patients with MDR-TB was 9% and 13% in the previously treated patients. The WHO estimate showed that 25,038 cases of pre-XDR-TB or XDR-TB were detected worldwide in 2022 [3]. However, there is limited information on the burden of pre-XDR-TB and XDR-TB among MDR-TB cases based on their previous treatment history.
The findings of the current study showed that more than a quarter of patients with MDR-TB had pre-XDR-TB with the majority were resistant to FQs. The pooled proportion of pre-XDR-TB in the current review is higher than the WHO estimate of 2021 [77]. The study results show that the proportion of pre-XDR-TB is higher and strains remains a major global public health concern in the area of antimicrobial resistance.
Based on the subgroup analysis, there are differences in the proportion of pre-XDR and XDR-TB in the WHO-defined regions of the world. The Western Pacific and South-East Asian regions have the highest rates of pre-XDR-TB and XDR-TB proportion. These regions should primarily examine the major risk factors for the high rates of DR-TB and intensify their efforts to address factors associated with high prevalence of DR-TB. The Beijing family is highly prevalent in these two regions and could be among the factors associated with the high proportion of DR-TB in the region [61]. The higher proportion of pre-XDR and XDR-TB might be due to the considerable variation in the coverage of high MDR/RR-TB burden countries and the high burden of the Beijing family.
The current review determined the proportion of FQs resistance cases. The pooled proportion of FQs resistance among MDR-TB cases was 27%. This finding is higher than the estimate of WHO in 2019, in which the proportion of FQs was 20.8% [4]. This difference is most likely due to the fact that majority of the included publications being from countries with high proportion of DR-TB. In addition, the possible reasons behind the high proportion of FQs are access and indiscriminate use of some of the commonly available FQ antibiotics for the treatment of various infection diseases [78]. Furthermore, the pooled proportion of SLID resistance among patients with MDR-TB was found to be 11%. The proportion of FQs resistance was equal to resistance to SLID proportion. This might be due to the fact that injectable drugs are less frequently used than FQs to treat common infections.
WHO has updated the MDR-TB treatment recommendations, in which injectable drugs are replaced by new drugs (BDQ, CFZ, DLM, and LZD). The update is required because the SLIDs are associated with an increase in deaths, treatment failures, relapses, and severe side effects, including permanent hearing loss [79]. Despite the limited evidence on new drugs, five published studies were included in the current review. In the current review, the proportion of resistance to new drugs (BDQ, CFZ, DLM, and LZD) among patients with MDR-TB was considerable. The occurrence of drug resistance among these four new anti-TB drugs was highlighted by the relatively higher proportion of resistance to BDQ and DLM. The introduction of new drugs may represent a new era in the care of patients with DR-TB by minimizing the toxicity associated with injectable drugs, reducing the spread of disease, reducing mortality rates, and improving successful treatment outcomes [31]. However, our findings revealed that 4–5% of patients with MDR-TB developed resistance to new drugs. Our findings imply that appropriate strategies are required to reduce resistance acquired during treatment.
Our review has several strengths. We used a random-effects model to address the problem of heterogeneity on the effect sizes between the included studies. In addition, we conducted a subgroup analysis using previous TB treatment history to determine the potential sources of heterogeneity. Although we cannot exclude the risk of publication bias, we used a sensitive search strategy and included a large number of studies. Moreover, we included a large number of studies that published from different parts of the world, which increases the generalizability of our findings. The current review study has some limitations. We included the studies that were published in English only, which could induce publication bias. In addition, the majority of the included studies were reported from the Western Pacific and South-East Asian regions, which could have overestimated the proportion of pre-XDR-TB and XDR-TB in this region and might have induced variation in the coverage of high MDR/RR-TB burden among the countries. Moreover, we did not evaluate the effect of HIV and other factors that could have predicted the proportion of pre-XDR-TB and XDR-TB due to the lack of data on potential predictors from the included studies. Despite these limitations listed previously, the current study results would not be affected by these limitations.
Conclusion
The current review study showed the presence of a higher proportion of pre-XDR-TB and XDR-TB than the WHO estimates. The highest proportions of pre-XDR-TB and XDR-TB were observed in the Western Pacific and South-East Asian regions. A considerable proportion of resistance to new drugs was also observed. Programmatic interventions are required to reduce the occurrence of pre-XDR-TB and XDR-TB. Countries should implement robust passive or active surveillance of DR-TB to understand the current burden of resistance to second-line and newly introduced drugs.
Supplementary Material
Acknowledgments
The authors would like to acknowledge those responsible for the primary studies included in this study. We also acknowledge the Ethiopian Public Health Institute for nonfinancial support, including access to internet searching.
Funding
The authors did not receive specific funding for this work.
Abbreviations:
- AFR
African region
- AMR
Region of the Americas
- BDQ
Bedaquiline
- CFZ
Clofazimine
- CI
Confidence interval
- DLM
Delamanid
- DR-TB
Drug-resistant tuberculosis
- EMR
Eastern Mediterranean reegion
- ES
Effect size
- EUR
European region
- FQs
Fluoroquinolone
- INH
Isoniazid
- LZD
Linezolid
- MDR-TB
Multidrug-resistant tuberculosis
- pre-XDR-TB
pre-extensively drug-resistant tuberculosis
- RIF
Rifampicin
- SEAR
South-East Asian region
- SLID
Second-line injectable drug
- TB
Tuberculosis
- WHO
World Health Organization
- WPR
Western Pacific region
- XDR-TB
Extensively drug-resistant tuberculosis
Footnotes
Declaration of competing interests
The authors have no competing interests to declare.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.04.392.
Availability of data and materials
All relevant data are available from the corresponding author upon request.
References
- [1].Mirzayev F, Viney K, Linh NN, Gonzalez-Angulo L, Gegia M, Jaramillo E, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J 2021;57. doi: 10.1183/13993003.03300-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization. Global Tubeclosis report 2020. [Google Scholar]
- [3].World Health Organization Global Tubeclosis report. Geneva: World Health Organization; 2022. [Google Scholar]
- [4].World Health Organization Global tuberculosis report. Geneva: World Health Organization; 2019. [Google Scholar]
- [5].Kozińska M, Brzostek A, Krawiecka D, Rybczyńska M, Zwolska Z, Augustynowicz-Kopeć E. MDR, pre-XDR and XDR drug-resistant tuberculosis in Poland in 2000–2009. Pneumonol Alergol Pol 2011;79:278–87. doi: 10.5603/ARM.27646. [DOI] [PubMed] [Google Scholar]
- [6].Dunachie S, Chamnan P. The double burden of diabetes and global infection in low and middle-income countries. Trans R Soc Trop Med Hyg 2019;113:56–64. doi: 10.1093/trstmh/try124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shenoi S, Friedland G. Extensively drug-resistant tuberculosis: a new face to an old pathogen. Annu Rev Med 2009;60:307–20. doi: 10.1146/annurev.med.60. 053107.103955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Health Organization World. Consolidated guidelines on tuberculosis Drug-resistant tuberculosis treatment 2022 update. Geneva: World Health Organization; 2022. [PubMed] [Google Scholar]
- [9].Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med 2013;10:e1001419. doi: 10.1371/journal.pmed.1001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, Altman DG, Group PRISMA. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014;14:45. doi: 10.1186/147122881445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- [13].Yao C, Guo H, Li Q, Zhang X, Shang Y, Li T, et al. Prevalence of extensively drug-resistant tuberculosis in a Chinese multidrug-resistant TB cohort after redefinition. Antimicrob Resist Infect Control 2021;10:126. doi: 10.1186/s13756-021-009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adwani S, Desai UD, Joshi JM. Prevalence of pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) at a Tertiary Care Center in Mumbai. J Krishna Inst Med Sci Univ 2016;5:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Agonafir M, Lemma E, Wolde-Meskel D, Goshu S, Santhanam A, Girmachew F, et al. Phenotypic and genotypic analysis of multidrug-resistant tuberculosis in Ethiopia. Int J Tuberc Lung Dis 2010;14:1259–65. [PubMed] [Google Scholar]
- [16].Elion Assiana DO, Abdul JBPA, Linguissi LSG, Epola M, Vouvoungui JC, Mabiala A, et al. Epidemiological profile of multidrug-resistant and extensively drug-resistant Mycobacterium Tubrculosis among Congolese patients. Ann Clin Microbiol Antimicrob 2021;20:84. doi: 10.1186/s12941-021-00488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Banerjee R, Allen J, Westenhouse J, Oh P, Elms W, Desmond E, et al. Extensively drug-resistant tuberculosis in california, 1993–2006. Clin Infect Dis 2008;47:450–7. doi: 10.1086/590009. [DOI] [PubMed] [Google Scholar]
- [18].Bedru H, Fikru M, Niguse W, Jemal A, Getinet G, Gobena A, et al. Drug resistance pattern of M. tuberculosis complex in Oromia region of Ethiopia. Infect Drug Resist 2021;14:1679–89. doi: 10.2147/IDR.S294559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Calver AD, Falmer AA, Murray M, Strauss OJ, Streicher EM, Hanekom M, et al. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence. South Africa. Emerg Infect Dis 2010;16:264–71. doi: 10.3201/eid1602.090968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen J, Peng P, Du Y, Ren Y, Chen L, Rao Y, et al. Early detection of multidrug- and pre-extensively drug-resistant tuberculosis from smear-positive sputum by direct sequencing. BMC Infect Dis 2017;17:300. doi: 10.1186/s12879-017-24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng S, Hide M, Pheng SH, Kerléguer A, Delvallez G, Sam S, et al. Resistance to second-line anti-TB drugs in Cambodia: a phenotypic and genetic study. Infect Drug Resist 2021;14:1089–104. doi: 10.2147/IDR.S289907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dagne B, Desta K, Fekade R, Amare M, Tadesse M, Diriba G, et al. The Epidemiology of first and second-line drug-resistance Mycobacterium tuberculosis complex common species: evidence from selected TB treatment initiating centers in Ethiopia. PLoS One 2021;16:e0245687. doi: 10.1371/journal.pone.0245687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dalal A, Pawaskar A, Das M, Desai R, Prabhudesai P, Chhajed P, et al. Resistance patterns among multidrug-resistant tuberculosis patients in greater metropolitan Mumbai: trends over time. PLoS One 2015;10:e0116798. doi: 10.1371/journal.pone.0116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Daniel O, Osman E, Oladimeji O, Dairo OG. Pre-extensive drug resistant tuberculosis (pre-XDR-TB) among MDR-TB patents in Nigeria. Glob Adv Res J Microbiol 2013;2:22–5. [Google Scholar]
- [25].Diriba G, Kebede A, Tola HH, Yenew B, Moga S, Addise D, Alemu A, Mohammed Z, Getahun M, Fantahun M, Tadesse M, Dagne B, Amare M, Assefa G, Abera D, Desta K. Molecular characterization and drug resistance patterns of Mycobacterium tuberculosis complex in extrapulmonary tuberculosis patients in Addis Ababa, Ethiopia. PLoS One 2020;15:e0243493. doi: 10.1371/journal.pone.0243493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ennassiri W, Jaouhari S, Cherki W, Charof R, Filali-Maltouf A, Lahlou O. Extensively drug-resistant tuberculosis (XDR-TB) in Morocco. J Glob Antimicrob Resist 2017;11:75–80. doi: 10.1016/j.jgar.2017.07.002. [DOI] [PubMed] [Google Scholar]
- [27].Gadhavi H, Goyal A, Aring B, Mullan S. Detection of pre-extensively drug resistance (PRE-XDR TB) and extensively drug resistance (XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) patient by line probe assay. Int J Curr Microbiol Appl Sci 2019;8:1012–16. doi: 10.20546/ijcmas.2019.810.118. [DOI] [Google Scholar]
- [28].Gallo JF, Pinhata JMW, Simonsen V, Galesi VMN, Ferrazoli L, Oliveira RS. Prevalence, associated factors, outcomes and transmission of extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in Sao Paulo, Brazil: a cross-sectional study. Clin Microbiol Infect 2018;24:889–95. doi: 10.1016/j.cmi.2017.11.015. [DOI] [PubMed] [Google Scholar]
- [29].He W, Liu C, Liu D, Ma A, Song Y, He P, et al. Prevalence of Mycobacterium tuberculosis resistant to bedaquiline and delamanid in China. J Glob Antimicrob Resist 2021;26:241–8. doi: 10.1016/j.jgar.2021.06.007. [DOI] [PubMed] [Google Scholar]
- [30].Jabbar A, Khan TA, Rehman H, Khan AS, Ahmad S, Khan SN. Burden of Drug resistant tuberculosis in newly diagnosed tuberculosis patients of Khyber Pakhtunkhwa, Pakistan. J Pak Med Assoc 2021;71:912–15. doi: 10.47391/JPMA.08-926. [DOI] [PubMed] [Google Scholar]
- [31].Jain A, Dixit P, Prasad R. Pre-XDR & XDR in MDR and ofloxacin and kanamycin resistance in non-MDR Mycobacterium tuberculosis isolates. Tuberculosis (Edinb) 2012;92:404–6. doi: 10.1016/j.tube.2012.05.010. [DOI] [PubMed] [Google Scholar]
- [32].Jaksuwan R, Tharavichikul P, Patumanond J, Chuchottaworn C, Chanwong S, Smithtikarn S, et al. Genotypic distribution of multidrug-resistant and extensively drug-resistant tuberculosis in northern Thailand. Infect Drug Resist 2017;10:167–74. doi: 10.2147/IDR.S130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].James P, Gupta R, Christopher DJ, Thankagunam B, Veeraraghavan B. MDR- and XDR-TB among suspected drug-resistant TB patients in a tertiary care hospital in India. Clin Respir J 2011;5:19–25. doi: 10.1111/j.1752-699X.2009.00184.x. [DOI] [PubMed] [Google Scholar]
- [34].Javaid A, Hasan R, Zafar A, Chaudry MA, Qayyum S, Qadeer E, et al. Pattern of first- and second-line drug resistance among pulmonary tuberculosis retreatment cases in Pakistan. Int J Tuberc Lung Dis 2017;21:303–8. doi: 10.5588/ijtld.16.0444. [DOI] [PubMed] [Google Scholar]
- [35].Senthil Kumar RS. Prevalence of pre-extensively drug-resistant tuberculosis and extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in South Tamil Nadu. Int J Sci Stud 2020;8:96–9. [Google Scholar]
- [36].Kuo CY, Wang WH, Huang CH, Chen YH, Lu PL. Resistance to first- and second-line antituberculosis drugs in Southern Taiwan: implications for empirical treatment. J Microbiol Immunol Infect 2018;51:88–93. doi: 10.1016/j.jmii.2017.05.008. [DOI] [PubMed] [Google Scholar]
- [37].Lai CC, Tan CK, Huang YT, Chou CH, Hung CC, Yang PC, et al. Extensively drug-resistant Mycobacterium tuberculosis during a trend of decreasing drug resistance from 2000 through 2006 at a Medical Center in Taiwan. Clin Infect Dis 2008;47:e57–63. doi: 10.1086/591702. [DOI] [PubMed] [Google Scholar]
- [38].Lee YS, Lee BY, Jo KW, Shim TS. Performance of the GenoType MTBDRsl assay for the detection second-line anti-tuberculosis drug resistance. J Infect Chemother 2017;23:820–5. doi: 10.1016/j.jiac.2017.08.010. [DOI] [PubMed] [Google Scholar]
- [39].Lee HY, Lee J, Lee YS, Kim MY, Lee HK, Lee YM, et al. Drug-resistance pattern of Mycobacterium tuberculosis strains from patients with pulmonary and extrapulmonary tuberculosis during 2006 to 2013 in a Korean tertiary medical center. Korean J Intern Med 2015;30:325–34. doi: 10.3904/kjim.2015.30.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Macedo R, Antunes AF, Villar M, Portugal I. Multidrug and extensively drug-resistant tuberculosis in Lisbon and Vale do Tejo, Portugal, from 2008 to 2010. Int J Mycobacteriol 2012;1:131–6. doi: 10.1016/j.ijmyco.2012.07.001. [DOI] [PubMed] [Google Scholar]
- [41].Madukaji L, Okohu I, Usman S, Oyedum U, Enagi A, Usman A, et al. Early detection of pre-XDR TB with line probe assay in a high TB burden country. Afr Health Sci 2021;21:968–74. doi: 10.4314/ahs.v21i3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Matsui T, Pinhata JMW, Rabello MCDS, Brandão AP, Ferrazoli L, Leão SC, et al. Frequency of first and second-line drug resistance-associated mutations among resistant Mycobacterium tuberculosis clinical isolates from Sao Paulo, Brazil. Mem Inst Oswaldo Cruz 2020;115:e200055. doi: 10.1590/0074-02760200055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mbuh TP, Wandji A, Keugni L, Mboh S, Ane-Anyangwe I, Mbacham WF, et al. Predictors of drug-resistant tuberculosis among high-risk population diagnosed under national program conditions in the littoral region, Cameroon. BioMed Res Int 2021;2021:8817442. doi: 10.1155/2021/8817442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Misra R, Kesarwani V, Nath A. Assessment of burden of drug-resistant tuberculosis at a tertiary care centre in northern India: a prospective single centre cohort study. BMJ Open 2021;11:e044096. doi: 10.1136/bmjopen2020-044096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mohan K, Rawall S, Pawar UM, Sadani M, Nagad P, Nene A, et al. Drug resistance patterns in 111 cases of drug-resistant tuberculosis spine. Eur Spine J 2013;22:647–52. doi: 10.1007/s00586012-2154x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mok JH, Kang BH, Lee T, Lee HK, Jang HJ, Cho YJ, et al. Additional drug resistance patterns among multidrug-resistant tuberculosis patients in Korea: implications for regimen design. J Korean Med Sci 2017;32:636–41. doi: 10.3346/jkms.2017.32.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Momen G, Aainouss A, Lamaammal A, Chettioui F, Blaghen M, Messoudi M, et al. Molecular characterization of mutations associated with resistance to second line drugs in Mycobacterium tuberculosis patients from Casablanca. Morocco. Rev Inst Med Trop Sao Paulo 2021;63:e19. doi: 10.1590/S1678-9946202163019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Namburete EI, Tivane I, Lisboa M, Passeri M, Pocente R, Ferro JJ, et al. Drug-resistant tuberculosis in Central Mozambique: the role of a rapid genotypic susceptibility testing. BMC Infect Dis 2016;16:423. doi: 10.1186/s12879-016-1766x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nguyen HB, Nguyen NV, Tran HT, Nguyen HV, Bui QT. Prevalence of resistance to second-line tuberculosis drug among multidrug-resistant tuberculosis patients in Viet Nam. Western Pac Surveill Resp J 2016;7:35–40. doi: 10.5365/WPSAR.2016.7.2.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Noor R, Akhter S, Rahman F, Munshi SK, Kamal SM, Feroz F. Frequency of extensively drug-resistant tuberculosis (XDR-TB) among re-treatment cases in NIDCH, Dhaka, Bangladesh. J Infect Chemother 2013;19:243–8. doi: 10.1007/s10156-012-04908. [DOI] [PubMed] [Google Scholar]
- [51].Park YS, Hong SJ, Boo YK, Hwang ES, Kim HJ, Cho SH, et al. The national status of tuberculosis using nationwide medical records survey of patients with tuberculosis in Korea. Tuberc Respir Dis (Seoul) 2012;73:48–55. doi: 10.4046/trd.2012.73.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Poudel A, Maharjan B, Nakajima C, Fukushima Y, Pandey BD, Beneke A, et al. Characterization of extensively drug-resistant Mycobacterium tuberculosis in Nepal. Tuberculosis (Edinb) 2013;93:84–8. doi: 10.1016/j.tube.2012.10.007. [DOI] [PubMed] [Google Scholar]
- [53].Qi YC, Ma MJ, Li DJ, Chen MJ, Lu QB, Li XJ, et al. Multidrug-resistant and extensively drug-resistant tuberculosis in multi-ethnic region, Xinjiang Uygur Autonomous Region, China. PLoS One 2012;7:e32103. doi: 10.1371/journal.pone.0032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ramachandran R, Nalini S, Chandrasekar R, Dave PV, Sanghvi AS, Wares F, et al. Surveillance of drug-resistant tuberculosis in the state of Gujarat, India. Int J Tuberc Lung Dis 2009;13:1154–60. [PubMed] [Google Scholar]
- [55].Riccardi N, Pontarelli A, Alagna R, Saderi L, Ferrarese M, Castellotti P, et al. Epidemiology and treatment outcome of MDR and pre-XDR TB in international migrants at two reference centers in the North of Italy: a cross-sectional study coordinated by Stop TB Italia Onlus. Public Health 2020;180:17–21. doi: 10.1016/j.puhe.2019.10.022. [DOI] [PubMed] [Google Scholar]
- [56].Salvato RS, Costa ERD, Reis AJ, Schiefelbein SH, Halon ML, Barcellos RB, et al. First insights into circulating XDR and pre-XDR Mycobacterium tuberculosis in Southern Brazil. Infect Genet Evol 2020;78:104127. doi: 10.1016/j.meegid.2019.104127. [DOI] [PubMed] [Google Scholar]
- [57].Sethi S, Agarwal P, Khaneja R, Kumar N, Kumar N, Chandna J, et al. Second-line drug resistance characterization in Mycobacterium tuberculosis by genotype MTBDRsl assay. J Epidemiol Glob Health 2020;10:42–5. doi: 10.2991/jegh.k.191215.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sharma SK, George N, Kadhiravan T, Saha PK, Mishra HK, Hanif M. Prevalence of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: a retrospective hospital-based study. Indian J Med Res 2009;130:392–5. [PubMed] [Google Scholar]
- [59].Sharma SK, Chaubey J, Singh BK, Sharma R, Mittal A, Sharma A. Drug resistance patterns among extra-pulmonary tuberculosis cases in a tertiary care centre in North India. Int J Tuberc Lung Dis 2017;21:1112–17. doi: 10.5588/ijtld.16.0939. [DOI] [PubMed] [Google Scholar]
- [60].Shibabaw A, Gelaw B, Gebreyes W, Robinson R, Wang SH, Tessema B. The burden of pre-extensively and extensively drug-resistant tuberculosis among MDR-TB patients in the Amhara region, Ethiopia. PLoS One 2020;15:e0229040. doi: 10.1371/journal.pone.0229040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Singhal P, Dixit P, Singh P, Jaiswal I, Singh M, Jain A. A study on pre-XDR & XDR tuberculosis & their prevalent genotypes in clinical isolates of Mycobacterium tuberculosis in north India. Indian J Med Res 2016;143:341–7. doi: 10.4103/0971-5916.182625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tasnim T, Tarafder S, Alam FM, Sattar H, Mostofa Kamal SM. Pre-extensively drug resistant tuberculosis (pre-XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) patients in Bangladesh. J Tuberc Res 2018;06:199–206. doi: 10.4236/jtr.2018.63018. [DOI] [Google Scholar]
- [63].Tuladhar P, Khadka DK, Banjara MR, Tuladhar R. Second line drugs resistant mycobacterium tuberculosis in multi-drug resistant tuberculosis patients2. J Inst Sci Technol 2018;22:168–74. doi: 10.3126/jist.v22i2.19609. [DOI] [Google Scholar]
- [64].Ullah B, Saboor R, Iqbal MZ, Bhatti AA, Rehman AU, Akram M. Frequency of pre-extensively drug resistant tuberculosis and extensively drug resistant tuberculosis. Pak J Med Health Sci 2021;15:959–61. doi: 10.53350/pjmhs21155959. [DOI] [Google Scholar]
- [65].Vashakidze L, Salakaia A, Shubladze N, Cynamon M, Barbakadze K, Kikvidze M, et al. Prevalence and risk factors for drug resistance among hospitalized tuberculosis patients in Georgia. Int J Tuberc Lung Dis 2009;13:1148–53. [PMC free article] [PubMed] [Google Scholar]
- [66].Wang H, Zhang X, Luo T, Li X, Tian P, Xu Y, et al. Prediction of XDR/pre-XDR tuberculosis by genetic mutations among MDR cases from a hospital in Shandong. China. Tuberculosis (Edinb) 2014;94:277–81. doi: 10.1016/j.tube.2014.03.005. [DOI] [PubMed] [Google Scholar]
- [67].Wang G, Jiang G, Jing W, Zong Z, Yu X, Chen S, et al. Prevalence and molecular characterizations of seven additional drug resistance among multidrug-resistant tuberculosis in China: a subsequent study of a national survey. J Infect 2021;82:371–7. doi: 10.1016/j.jinf.2021.02.004. [DOI] [PubMed] [Google Scholar]
- [68].Welekidan LN, Skjerve E, Dejene TA, Gebremichael MW, Brynildsrud O, Tønjum T, et al. Frequency and patterns of first- and second-line drug resistance-conferring mutations in Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients in a cross-sectional study in Tigray Region. Ethiopia. J Glob Antimicrob Resist 2021;24:6–13. doi: 10.1016/j.jgar.2020.11.017. [DOI] [PubMed] [Google Scholar]
- [69].Xu Y, Li Q, Zhu M, Wu X, Wang D, Luo J, et al. The epidemiological characteristics and profile of drug-resistant tuberculosis among children with tuberculosis in Sichuan, China, 2015–2018: a retrospective study. Med (Baltim) 2020;99:e22608. doi: 10.1097/MD.0000000000022608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yang X, Yuan Y, Pang Y, Wang B, Bai Y, Wang Y, et al. The burden of MDR/XDR tuberculosis in coastal plains population of China. PLoS One 2015;10:e0117361. doi: 10.1371/journal.pone.0117361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yang JS, Kim KJ, Choi H, Lee SH. Delamanid, bedaquiline, and linezolid minimum inhibitory concentration distributions and resistance-related gene mutations in multidrug-resistant and extensively drug-resistant tuberculosis in Korea. Ann Lab Med 2018;38:563–8. doi: 10.3343/alm.2018.38.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yuan X, Zhang T, Kawakami K, Zhu J, Li H, Lei J, et al. Molecular characterization of multidrug- and extensively drug-resistant Mycobacterium tuberculosis strains in Jiangxi. China. J Clin Microbiol 2012;50:2404–13. doi: 10.1128/JCM.06860-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yuan X, Zhang T, Kawakami K, Zhu J, Zhen W, Li W, et al. Genotyping and clinical characteristics of multidrug and extensively drug-resistant tuberculosis in a tertiary care tuberculosis hospital in China. BMC Infect Dis 2013;13:315. doi: 10.1186/1471-2334-13-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zheng H, He W, Jiao W, Xia H, Sun L, Wang S, et al. Molecular characterization of multidrug-resistant tuberculosis against levofloxacin, moxifloxacin, bedaquiline, linezolid, clofazimine, and delamanid in southwest of China. BMC Infect Dis 2021;21:330. doi: 10.1186/s12879-021-06024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Araújoa LG, Gracia MT, Zaccariottoc TR, Morettia ML, Levy CE, Resende MR. Clinical outcomes and molecular characterization of drug-resistant tuberculosis in pre- and extensively drug-resistant disease based on line probe assays. Braz J Infect Dis 2021;25:101544. doi: 10.1016/j.bjid.2021.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].World Health Organization Global tuberculosis report. Geneva: World Health Organization; 2018. [Google Scholar]
- [77].World Health Organization. WHO announces updated definitions of extensively drug-resistant tuberculosis, Geneva: World Health Organization; 2021. [Google Scholar]
- [78].Heidary M, Shirani M, Moradi M, Goudarzi M, Pouriran R, Rezaeian T, et al. Tuberculosis challenges: resistance, co-infection, diagnosis, and treatment. Eur J Microbiol Immunol (Bp) 2022;12:1–17. doi: 10.1556/1886.2021.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Global Fund Advocates Network Making The Switch: Saving more lives with optimal treatment for drug-resistant TB. Paris: Medecins Sans Frontieres; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available from the corresponding author upon request.


