Abstract
Compared with men, women are disproportionately affected by alcohol, including greater risks of physiological damage, behavioral impairment, and relapse. One likely mechanism underlying the sexual disparity in this vulnerability is the fluctuation of ovarian hormones, particularly estradiol (E2), across phases of the menstrual cycle. Several pre-clinical and clinical studies have shown that higher E2 levels positively correlate with drinking, suggesting E2 may play a significant role in modulating drinking. Inhibitory control also modulates drinking; when it is reduced or compromised by alcohol, the drinker’s ability to stop the self-administration of alcohol could be impaired, leading to a binge episode. The present study aimed to examine the degree to which menstrual cycle phase can influence the disinhibiting effect of alcohol. Twenty-four healthy young-adult women participated in a within-subjects placebo-controlled study of the acute disinhibiting effect of 0.60 g/kg alcohol over the course of two test sessions. A cued go/no-go task measured the disinhibiting effects of alcohol and placebo beverages during the early follicular phase of the cycle when E2 levels were low and the late follicular phase (i.e., ovulation) when E2 was elevated. Results showed that the disinhibiting effect of alcohol increased nearly two-fold during the late follicular phase when E2 was elevated. These findings highlight the role of alcohol-induced disinhibition as a potential behavioral mechanism by which fluctuations in ovarian hormones as a function of the menstrual cycle contribute to increased risk for excessive alcohol usein women.
Keywords: alcohol, menstrual cycle, follicular phase, estradiol, disinhibition
Introduction
Alcohol use poses significant health problems, particularly when characterized by patterns of high intensity drinking (i.e., binges). In both sexes, alcohol use disorders are associated with comorbidities such as liver cirrhosis, cardiovascular disease, and cancers. However, compared with men, women are disproportionately affected by alcohol, including a greater risk of organ damage (Mumenthaler et al., 1999; Nolen-Hoeksema, 2004; Witt, 2007), and higher risk of neurotoxic effects as shown by preclinical studies (Hashimoto & Wiren, 2008; Satta et al., 2018; Wilhelm et al., 2016). Women also exhibit profiles of substance use that differ from men with more rapid acquisition, a greater incidence of relapse (Anker & Carroll, 2011; Becker et al., 2017; Hudson & Stamp, 2011), and a greater sensitivity to the several acute behaviorally-impairing effects of the drug (Miller et al., 2009; Weafer et al., 2010).
One likely mechanism underlying the sexual disparity in vulnerability to alcohol impairment is the fluctuation of ovarian hormones, in particular estradiol (E2), which is the predominant active form of estrogen in pre-menopausal women (Stillwell, 2016). Estradiol, as well as luteinizing hormone (LH), is elevated during the late follicular phase around ovulation (Stillwell, 2016). Despite their diverse mechanisms of action, E2 and alcohol share a couple of common neurotransmission targets, specifically glutamatergic, GABAergic, and dopaminergic receptors. GABAA receptors have long been known to mediate the CNS depressant effects of alcohol (Morrow et al., 1999; Morrow et al., 2001). Studies have shown that E2 reduces GABAergic transmission and elevates glutamate transmission via action on NMDA receptors (Barth et al., 2015; Kelly et al., 2003). E2 also modulates dopaminergic transmission in the mesolimbic pathway (Barth et al., 2015; Yoest et al., 2014), a region where alcohol is known to elevate dopamine levels, contributing to its rewarding properties (Di Chiara, 1997; Trantham-Davidson & Chandler, 2015).
Several pre-clinical and clinical studies have shown that higher E2 levels are associated with higher consumption of alcohol. Preclinical studies have reported associations between the estrous cycles of female rats and their levels of ad lib ethanol consumption (Lancaster et al., 1996; Roberts et al., 1998). For example, studies in freely-cycling rats observed increased ethanol consumption in conjunction with the onset of their estrous cycles, when E2 is elevated (e.g., Lancaster et al. 1996). Studies examining the effects of ovariectomization provide additional evidence for the link between E2 and ethanol consumption. Ovariectomization reduces alcohol consumption to levels observed in males (Ford et al., 2002), and when ovariectomized rats were injected with E2, alcohol consumption increased in a dose-dependent manner (Ford et al., 2002, 2004; Satta et al., 2018).
Clinical studies of women across their menstrual cycles also have shown that the late follicular phase is associated with increased drinking, suggesting that E2 and other ovarian hormones may play a significant role in modulating drinking (Erol et al., 2019; Martin et al., 1999; Muti et al., 1998). Furthermore, some clinical studies have observed a bidirectional relationship between drinking and E2 levels in women by showing that alcohol can also increase levels of E2 (Reichman et al., 1993). In addition to alcohol consumption, craving and subjective effects are reported to be higher during the follicular phase at the beginning of the menstrual cycle, a phase when estrogen (E2) is rising and progesterone (P4) is low (Evans & Foltin, 2010; Evans et al., 2002; Justice & de Wit, 1999, 2000; Sofuoglu et al., 1999; Terner & de Wit, 2006; Weinberger et al., 2015; White et al., 2002). Quit attempts are generally more successful during the second half of the menstrual cycle during the luteal phase when E2 declines rapidly and P4 is rising (Mazure et al., 2011; Perkins et al., 2000; Saladin et al., 2015).
Our own pilot data suggests that these menstrual cycle effects are due to fluctuations in estrogen and P4. Specifically, we demonstrated that follicular increases in E2 in the absence of elevated P4 (i.e., ovulatory E2) and a steeper slope of ovulatory E2 surges prospectively predicted a 3-fold increase in alcohol consumption the following day, an effect with potential clinical significance (Martel et al., 2017).
The relationship between menstrual cycle phase and increased alcohol consumption is generally assumed to reflect an E2-induced enhancement of the rewarding properties of alcohol, increasing the motivation to drink (Becker et al., 2017). Although traditional models of alcohol use disorders emphasize the rewarding effects in reinforcing its use, acute impairment of cognitive functions has also been implicated in its abuse potential. Alcohol is well-known for its acute disinhibiting effects on behavior. Laboratory studies of inhibitory control use stop-signal and cued go/no-go tasks, which are reaction time tasks used to model behavioral control as the ability to quickly activate a response to a go-signal and suddenly inhibit a response when a stop-signal occurs (Logan, 1994; Logan et al., 1984; Miller et al., 1991). Studies using these tasks have found that alcohol acutely impairs the ability to inhibit behavior (de Wit et al., 2000; Fillmore, 2012; Fillmore & Vogel-Sprott, 2000). The impairing effect of alcohol on the drinker’s inhibitory control also appears to contribute to the abuse potential. For instance, binge drinkers are more sensitive to the disinhibiting effects of the drug (Marczinski et al., 2007), and individual differences in sensitivity to the disinhibiting effects of alcohol predict ad lib consumption (Gan et al., 2014; Weafer & Fillmore, 2008). The ability to inhibit or terminate instigated and ongoing behaviors is likely integral to terminating a drinking episode. When inhibitory control is reduced or compromised by alcohol, the drinker’s ability to stop the self-administration of alcohol could be impaired. The acute impairment of inhibitory control likely occurs in conjunction with the drug’s rewarding effects that serve to positively reinforce continued consumption in the situation.
There is also growing evidence that heightened E2 levels during the follicular phase weaken inhibitory pathways to compromise the ability to suppress pre-potent actions (Colzato et al., 2010). Indeed, studies assessing women’s levels of behavioral disinhibition and impulsivity at different points throughout their menstrual cycles report greater impulsivity and disinhibited behavior during the follicular phase when E2 is rising (Colzato et al., 2010; Diekhof, 2015; Kurath & Mata, 2018). Although the findings are not entirely consistent (Derntl et al., 2014; Op de Macks et al., 2016), they do suggest a putative link between elevated E2 increased disinhibition and impulsivity in women.
Taken together, the pre-clinical and clinical evidence for the involvement of menstrual cycle phase in drinking behavior and impulse control among women provides strong support for its role in the abuse potential of alcohol. Given evidence that elevated E2 can weaken inhibitory control in women, we tested the hypothesis that ovarian hormone levels during the late follicular phase can increase alcohol abuse potential specifically by increasing women’s sensitivity to the acute disinhibiting effect of alcohol. The study examined premenopausal adult women and compared their acute sensitivity to the disinhibiting effect of a controlled dose of 0.60 g/kg alcohol and placebo between two distinct phases of their menstrual cycle: early follicular phase when E2 levels are low, and the late follicular phase (i.e., ovulation) when E2 levels are the most elevated. We hypothesized that the acute disinhibiting effect of alcohol would be greater during the late follicular phase when E2 was high compared with the early phase when E2 is lowest.
Methods
Participants
Twenty-four adult premenopausal women participated in this study. All participants had to report consuming alcohol at least once per week, have regularly cycling periods, and no use of oral contraceptives or other hormone-based medication during the past three months. Volunteers completed questionnaires that provided demographic information, typical drinking habits, and physical and mental health status. Those with a self-reported psychiatric disorder, head trauma, or other central nervous system (CNS) injury were excluded from the study. Individuals with a clinician-diagnosed substance use disorder as determined using the Structured Clinical Interview for the DSM (SCID) were excluded as well. Those using any prescription psychoactive medication, or any medication contraindicated for alcohol use, were excluded. The sample excluded drinkers with a potential risk of alcohol use disorder as defined by an Alcohol Use Disorder Identification Test (AUDIT) score of 10 or higher. Less than 20% of volunteers failed to pass screening. Recruitment continued until we obtained 24 study-eligible subjects. Sample size was based on power calculations using pilot data.
Prior to test sessions recent use of amphetamine, barbiturates, benzodiazepines, cocaine, opiates, and tetrahydrocannabinol (THC) was assessed by means of urine analysis. Any participant who tested positive for any of these drugs was excluded from participation. Those who tested positive for THC were retained provided there was no THC use within the past two days prior to any test session (n= 4). No participants were breast-feeding or pregnant, as determined by self-report and urine human chorionic gonadotrophin (HCG) levels. Participants were recruited via notices posted on community bulletin boards and by social media advertisements. The University of Kentucky Medical Institutional Review Board approved the study (IRB Protocol 52637, Estradiol Effects on Behavioral and Reward Sensitivity to Alcohol Across the Menstrual Cycle). The study is registered as a phase one clinical trial in clinicaltrials.gov NCT04595682. All study volunteers provided informed consent prior to participation and received $340 for their participation.
Apparatus and Materials
Response Inhibition.
A cued go/no-go reaction time task was used to measure participants’ response inhibition to no-go targets and their reaction time to go targets (Fillmore & Weafer, 2004). The task required finger presses on a keyboard and measured the ability to inhibit prepotent behavioral response of executing a key press. Cues provided preliminary information regarding the type of target stimulus (i.e., go or no-go) that was likely to follow, and the cues had a high probability of signaling the correct target. Participants were instructed to press the forward slash (/) key on the keyboard as soon as a go (green) target appeared and to suppress the response when a no-go (blue) target was presented. The go cue conditions were of particular interest. Go cues generate response prepotency which speeds response time to go targets. However, subjects must overcome this response prepotency to inhibit the response if a no-go target is subsequently displayed. Response inhibition was measured by the proportion of no-go targets in which subjects failed to inhibit a response (p-inhibition failures) during the test. Poor inhibitory control was indicated by a higher proportion of inhibition failures (i.e., greater p-inhibition failure score). A test consisted of 250 trials with 700 ms inter-trial intervals and required 15 mins to complete. The task has been used in other research, has strong psychometrics, including reliability, and is highly sensitive to dose-dependent impairing effects of alcohol on drinkers’ inhibitory control (Fillmore & Weafer, 2013; Weafer & Fillmore, 2016). The task was operated by E-Prime experiment generation software (Psychology Software Tools, Pittsburgh, PA).
Subjective Effects.
Subjective effects were measured using visual analog scales (VAS). Participants rated the degree to which they experienced five subjective effects of alcohol: intoxication, stimulation, sedation, desire for more alcohol, and liking the effects. Participants indicated their response to each item using an electronic survey by sliding a vertical mark on a 100 mm line, with the left side, 0, indicating not at all and the right side, 100, indicating very much.
Timeline Follow Back (TLFB).
The TLFB (Sobell & Sobell, 1992) was used to assess daily patterns of alcohol use over the 4 weeks prior to the intake session. This measure used a structured calendar anchored with holidays and other notable dates to facilitate recall of past drinking episodes. For each day, participants estimated the number of standard drinks they consumed and the number of hours they spent drinking. Binge days were identified as days in which the reported alcohol use of a participant was estimated to yield a BAC of 80 mg/100 ml or higher based on a calculation using the participants’ body weight. The TLFB provided four measures of drinking habits: (a) drinking days (total number of days that alcohol was consumed), (b) drunk days (total number of days that participants reported feeling drunk), (c) binge days (total number of binge episodes), and (d) total drinks (total number of drinks consumed).
Blood Alcohol Concentrations.
Blood alcohol concentrations (BACs) were determined from breath samples measured by an Intoxilyzer, Model 400 (CMI, Inc., Owensboro, KY).
Ovulation Detection.
Ovulation was determined by detection kits (Wondfo Inc. Willowbrook, IL) for LH levels in the urine. A rise in LH triggers ovulation, and thus an increased concentration of LH in urine is typically indicative of eminent ovulation. Participants began completing ovulation tests on day 9 of their cycle (with day 0 being the first day of menstruation) and continued to complete one test per day within 30 mins of waking up until two consecutive positive tests were obtained.
Hormone Assessment.
Saliva samples were collected via passive drool by participants each morning 30 min after waking and subsequently frozen. Participants were instructed not to eat, drink, brush teeth, or smoke before saliva collection. No participant reported violation of this morning protocol in daily diaries. Salivary E2 (pg/mL), P4 (pg/mL), and LH (mcg/ml) were determined using enzyme immunoassay kits available through Salimetrics (State College, PA) and assayed through the University of Kentucky Clinical Center for Translational Science. For E2, the Salimetrics 17β-Estradiol immunoassay kit had a sensitivity of 0.1 pg/mL, and the sample precision (% coefficient of variation, CV) mean was 4.27%, inter-assay precision (% CV of controls between plates was 4.05%. For P4, the Salimetrics Progesterone immunoassay kits had a sensitivity of 5 pg/mL, the sample precision mean was 6.09%, and inter-assay precision was 6.68%. For LH, the Meso Scale Luteinizing Hormone Immunoassay kits had a sensitivity of 1.6 mcg/mL, the sample precision mean was 7.06%, and the inter-assay precision was 6.67%. These saliva-based assays demonstrate high and significant correlations of 0.8 (p < .01) with serum levels of E2 and P4, based on validity data provided by the kit manufacturer (Salimetrics, State College, PA). All participants showed peak P4 levels consistent with an ovulatory cycle (Howards et al., 2009).
Procedure
Individuals who responded to the advertisements contacted the laboratory and completed an online screening survey. Volunteers were told that the purpose of the experiment was to study the effects of ovarian hormones and alcohol on behavior. All sessions were conducted in the Human Behavioral Pharmacology Laboratory of the Department of Psychology and began between 10:00 a.m. and 6:00 p.m. Participants were tested individually by a research assistant. Before each test session, participants were instructed to fast for 4 hours and abstain from alcohol for 24 hours. At the beginning of each testing session, a zero BAC was verified for each participant. Urine samples were tested for the presence of drug metabolites in all participants (On Trak TesTstiks, Roche Diagnostics Corporation, Indianapolis, IN) and pregnancy (Mainline Confirms HGL, Mainline Technology, Ann Arbor, MI) before each session. All testing was conducted in a small room that consisted of a chair and a desk with a computer that operated the cued go/no-go task.
Intake Session.
Intake sessions were scheduled to coincide with the week that participants expected to start their cycle, occurring no more than 7 days prior to or 3 days following the onset of menstruation. During an initial intake session participants provided informed consent, completed questionnaires, and were familiarized with the lab environment and procedures and the cued go/no-go task. Women were provided with salivary test kits and instructed to provide a saliva sample each morning for the duration of a single menstrual cycle, during which the two alcohol test sessions were conducted. Participants were also provided with urine ovulation tests and instructed in their proper use. They were instructed to notify the lab via text or email the day their period began to schedule their test sessions.
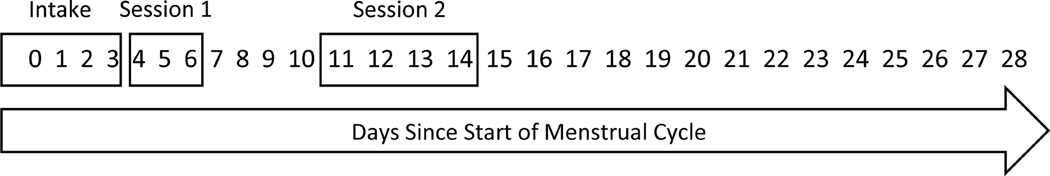
Women’s response to alcohol was tested on two separate days. Test sessions were scheduled based on the menstrual cycle (see Fig. 1), with day 0 being the start of menstruation. The first test session occurred on days 4–6 at the beginning of the follicular phase when E2 was low. The second test session occurred on days 11–14 during the late follicular phase coinciding with ovulation when E2 was high. This was confirmed with E2 assay results. The two test sessions were approximately one week apart.
Figure 1.
Timeline of session scheduling, with Day 0 being start of menstruation. Each session was scheduled within a set range of days. The intake session could occur up to seven days before onset of menstruation, which is not pictured.
Test Sessions.
The test sessions on each day were identical. During a session, inhibitory control and subjective effects were tested twice, first following 0.0 g/kg alcohol (a placebo) and second following an active alcohol dose of 0.60 g/kg abs. alcohol. The active dose produces an average peak BAC of 80-mg/100 ml approximately 60–75 min after drinking and was chosen on the basis of prior research that showed response inhibition is reliably impaired at this BAC (Abroms et al., 2003; Fillmore & Vogel-Sprott, 1999, 2000; Marczinski & Fillmore, 2003, 2005). The dose was administered as absolute alcohol divided equally into two drinks containing one part 95% abs. grain alcohol and three parts carbonated mix. Participants had six minutes to consume the entire beverage. This dosing procedure produces a mean rate of rise in BAC of 1.0 mg/100 ml/minute (Fillmore & Vogel-Sprott, 1998). The placebo followed the identical beverage administration protocol, and consisted of carbonated mix that was half the total volume of the 0.60 g/kg alcohol drink. This reduced volume enabled better absorption of the subsequent alcoholic dose. A small amount (3 ml) of alcohol was floated on the surface of the placebo beverage and was served in two glasses that had been sprayed with an alcohol mist that resembled condensation and provided a strong alcoholic scent as the beverages were consumed. Previous research has shown that individuals report this beverage contains alcohol (Fillmore et al., 1998).
Behavioral Testing.
At the outset of the session, women received the placebo dose. After 35 minutes, they completed the cued go/no-go task. Subjective effects were measured twice under placebo, 30 mins and 50 mins after consumption. Participants then received the alcohol dose (0.60 g/kg) and completed the cued go/no-go task 35 minutes following consumption. Subjective effects were measured five times following alcohol administration: 30, 50, 60, 90, and 120 mins. BAC was measured at regular 20–30 minute intervals throughout the session.
Criterion Measures and Data Analyses
Response Inhibition.
Failures of response inhibition on the cued go/no-go task were measured as the proportion of no-go targets in which a participant failed to inhibit a response. The measure of interest was the proportion of inhibition failure score in the go cue (i.e., prepotent) condition. This proportion was calculated based on the 25 trials in which no-go targets were preceded by go cues. Greater p-inhibition failures indicate poorer inhibitory control (i.e., disinhibition). Speed of responding to targets in the go cue condition was measured by the participant’s average RT (msec) for a test. Omission errors were also recorded when participants failed to respond to go targets. Omission errors were infrequent and occurred on no more than 2% of go target trials (~2 trials per test) in either dose condition. The task has been used in other research, has strong psychometrics, including reliability, and is highly sensitive to dose-dependent impairing effects of alcohol on drinkers’ inhibitory control (Fillmore & Weafer, 2013; Weafer & Fillmore, 2016).
Data Analyses of Cued Go No-go Measures.
Cycle phase and alcohol effects on p-inhibition failures and RT scores were analyzed using a 2 Phase (early vs. late follicular) x 2 Alcohol Dose (0.0 g/kg vs. 0.60 g/kg) repeated measures analysis of variance (ANOVA). Subjective effects were each analyzed by 2 Phase (early vs. late follicular) X 7 Time Interval repeated measures ANOVAs. The time intervals were: 30 and 50 mins post-placebo administration and 30, 50, 60, 90, 120 mins post-alcohol administration. BACs following the alcohol administration were analyzed by a 2 Phase (early vs. late follicular) X 5 time (30, 50, 60, 90, 120 min) repeated measures ANOVA.
Accountability.
We report how we determined our sample size, all data exclusions (if any), all manipulations, and all measures in the study. The study is registered as a phase one clinical trial in clinicaltrials.gov NCT04595682 and data will be available.
Results
Demographic and Drinking Habits
Table 1 reports participants’ mean demographic and drinking data. As seen in the table, participants reported drinking an average of 32 drinks during a 30-day period and reported drinking on an average of 12 days during the period. On average, participants reported feeling drunk on two occasions and engaging in one binge drinking episode. These reports are consistent with moderate alcohol consumption typical of the study population (Fillmore & Jude, 2011). The sample had a racial make-up of Caucasian (n = 16), African-American (n = 5), Hispanic (n = 1), and multiracial (n = 2).
Table 1.
Demographics and Drinking History
| Mean | SD | |
|---|---|---|
|
| ||
| Age | 24.04 | 2.48 |
| BMI | 23.98 | 3.28 |
| Education | 16.18 | 1.67 |
| TLFB Drinking Days | 11.50 | 4.53 |
| TLFB Drunk Days | 2.38 | 1.86 |
| TLFB Binge Days | 1.46 | 1.91 |
| TLFB Total Drinks | 31.50 | 17.93 |
Table 1. Composite demographic data for all participants (N=24). Age = years of age; Education = years of education (e.g., high school degree = 12 years); TLFB drinking days = total number of drinking days in the past 30 days; TLFB drunk days = total number of days in which the participant drank to a level that they felt drunk in the past 30 days; TLFB binge days = number of days that met binge drinking criteria in the past 30 days, defined as drinking to or in excess of 80 mg/100 ml; TLFB total drinks = TLFB total drinks consumed in the past 30 days.
Hormones
E2 varied as a function of cycle, with higher levels observed in the late follicular phase relative to the early follicular phase. LH followed a similar pattern to E2 during the follicular phase, with higher levels observed around ovulation. P4 increased from the early to late follicular phase, but with less consistency than E2 and LH.
Participants’ mean salivary hormone levels for E2 and P4 (pg/ml) and LH (mcg/ml) during early and late follicular phase were calculated as a 3-day average comprising the hormone level on the day of the laboratory session with levels on the day immediately prior to, and following, the laboratory session. Due to a coding error, data from two participants could not be analyzed. At early and late follicular phase, respective levels of E2 were 1.14 (SD = 0.51) and 1.45 (SD = 0.54). Respective levels of P4 were 81.6 (SD = 59.49) and 105.83 (SD = 41.88), and respective levels of LH were 20.20 (SD = 18.41) and 76.62 (SD = 104.05). Paired sample t tests comparing the late phase versus early phase sessions confirmed significantly higher late phase levels for E2, t(21) = −2.96, p = 0.007, and LH, t(21) = −2.94, p = 0.008, but not P4 (t = 0.063). The co-occurrence of ovulation with the late phase laboratory test session was confirmed using the urine ovulation tests, with 19 out of 24 participants demonstrating a positive ovulation test. Based on data from these 19 participants, the late phase test sessions occurred on average within 1.89 days of ovulation (SD = 1.94).
Blood Alcohol Concentration (BAC)
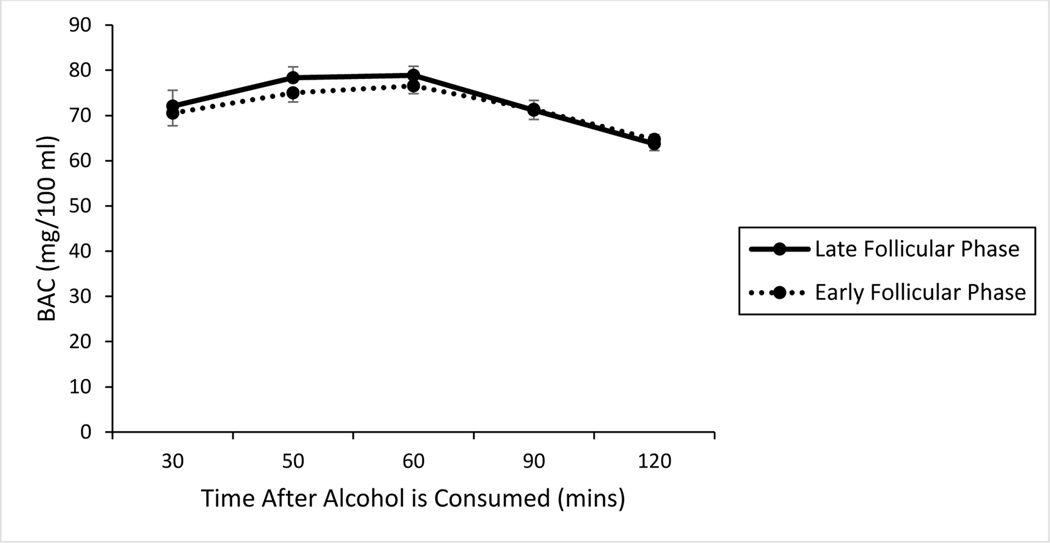
Fig. 2 shows a plot of BAC over time following alcohol administration. As seen in the figure, the mean BAC was nearly identical during tests occurring in the early and late follicular phase across the blood alcohol concentration curve. BAC peaked at 60 mins following consumption, with a mean value of 76.54 mg/100 ml (SD = 8.3) during the early follicular phase, and a mean value of 78.88 mg/100 ml (SD = 9.86) during the late follicular phase. Paired-samples t tests indicated no significant difference between the two test sessions at 30 min post alcohol administration when participants began the cued go/no-go task (p = 0.57), or in the peak BAC obtained during each session (p = 0.19). No detectable BACs were observed following the placebo administration.
Figure 2.
Mean BAC (mg/100 ml) following 0.60 g/kg alcohol from 30 to 120 min post-administration during the early and late follicular phase of the menstrual cycle. Vertical capped lines indicate standard error of the mean.
Disinhibition
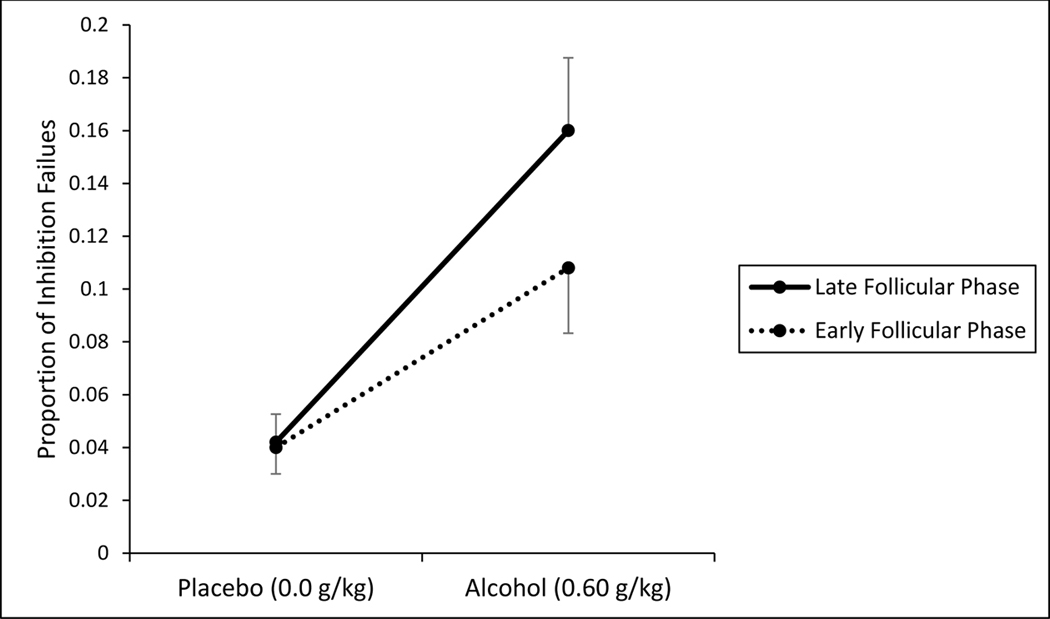
Fig. 3 shows the mean proportion of inhibition failures on the cued go/no-go task following placebo and alcohol administration during the early and late follicular phase. The figure shows a greater proportion of inhibitory failures following alcohol consumption relative to placebo. Indeed, a significant main effect of dose was observed, F (1, 23) = 22.97, p < 0.001, ηp2 = 0.50. A significant main effect of phase, F (1, 23) = 4.83, p = 0.038, ηp2 = 0.17, and a significant interaction between dose and phase were observed, F (1, 23) = 5.51, p = 0.028, ηp2 = 0.19. The figure illustrates the interaction. Inhibitory failures under alcohol were greater during the late versus early follicular phase, and this was confirmed by a simple effects comparison, t(23) = −43.054, p < 0.001. By contrast, inhibitory failures showed no difference between phases following placebo, t(23) = −0.166, p = 0.87. Fig. 3 shows the mean inhibition failures following placebo (i.e., in the sober state) at each phase to be nearly identical. Indeed, there was a significant correlation between participants’ inhibition failures on each placebo test, r = 0.522, p = 0.009, which demonstrates test-retest reliability of the measure.
Figure 3.
Mean inhibition failures in response to go cue on cued go/no-go task under placebo and alcohol during the early and late follicular phase of the menstrual cycle. Vertical capped lines indicate standard error of the mean.
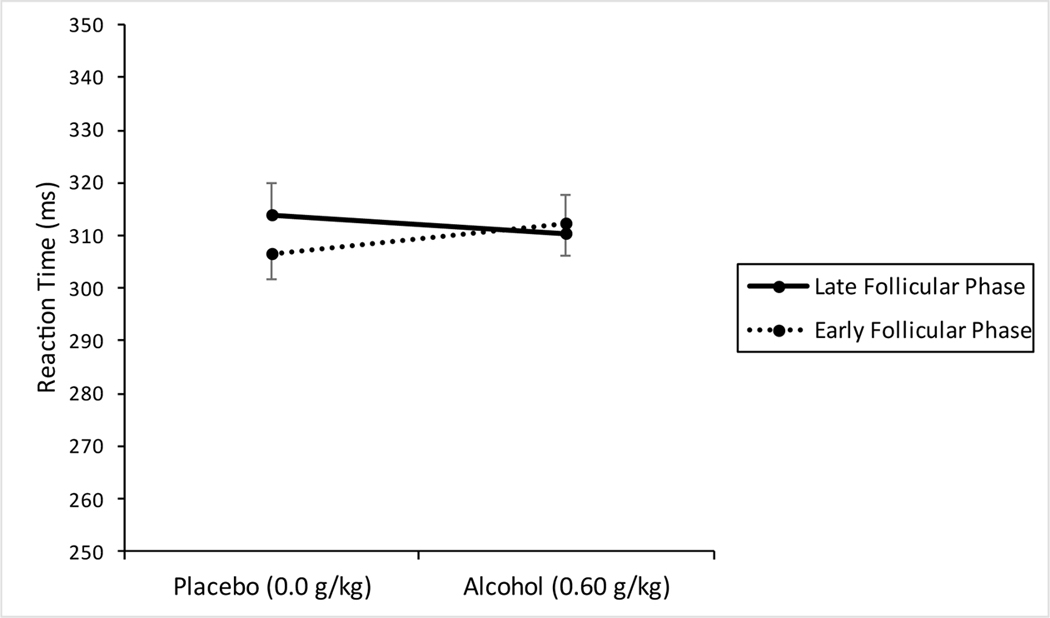
Fig. 4 shows the mean reaction time on the cued go/no-go task following placebo and alcohol during the early and late follicular phase. No significant main effects of dose, F (1, 23) = 0.14, p = 0.71, ηp2 = 0.01, phase, F (1, 23) = 1.15, p = 0.30, ηp2 = 0.05, or interaction were observed, F (1, 23) = 3.1, p = 0.092, ηp2 = 0.12.
Figure 4.
Mean reaction time (ms) in response to go cue on cued go/no-go task following placebo and 0.60 g/kg alcohol during the early and late follicular phase of the menstrual cycle. Vertical capped lines indicate standard error of the mean.
Subjective Effects
Table 2 shows the mean rating of the subjective effects (intoxication, desire, like, stimulation, sedation) throughout each session. The ratings generally followed a common pattern, with higher ratings following alcohol versus placebo. Ratings following alcohol tended to increase and then decline generally as a function of the rise and descent of participants’ BAC during the session. Four out of 528 ratings were lost due to a coding error; subjective effects values from these ratings were determined by averaging corresponding values from the two ratings completed before and after each lost rating. A significant main effect of time was observed for intoxication, liking, and simulation, ps < 0.001, but not for desire, p = 0.258, or sedation, p = 0.167. No significant main effect of phase or interaction between phase and time was observed for any item, ps > 0.212. Above-zero intoxication ratings at the 30 and 50 min tests following placebo indicated that participants expected they received some alcohol in the placebo beverage during each cycle phase. One-sample t tests confirmed the ratings were significantly greater than zero at each test (ps < 0.001).
Table 2.
Subjective Effects
| Phase | Placebo (0.0 g/kg) M (SD) |
Alcohol (0.60 g/kg) M (SD) |
||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 30 mins | 50 mins | 30 mins | 50 mins | 60 mins | 90 mins | 120 mins | ||
| Intoxication | Early | 29.63 (23.36) | 27.58 (23.14) | 74.42 (17.60) | 78.79 (14.45) | 75.71 (15.69) | 69.71 (16.65) | 57.42 (22.07) |
| Late | 25.67 (24.12) | 31.00 (25.56) | 74.75 (18.41) | 77.92 (15.96) | 74.17 (18.20) | 68.29 (17.70) | 58.92 (18.94) | |
|
|
||||||||
| Desire | Early | 31.42 (23.63) | 31.04 (22.62) | 33.42 (27.19) | 37.79 (31.02) | 35.29 (29.65) | 31.13 (26.46) | 29.75 (27.25) |
| Late | 37.29 (25.13) | 36.54 (25.93) | 35.63 (30.49) | 37.96 (27.64) | 39.54 (28.33) | 38.50 (30.67) | 25.00 (23.74) | |
|
|
||||||||
| Like | Early | 40.17 (23.38) | 36.50 (22.90) | 71.88 (23.90) | 57.73 (27.48) | 58.00 (24.55) | 57.50 (22.90) | 56.88 (18.20) |
| Late | 40.00 (23.41) | 42.63 (22.32) | 67.25 (19.44) | 64.96 (20.90) | 64.63 (19.22) | 58.00 (24.65) | 53.29 (25.17) | |
|
|
||||||||
| Stimulation | Early | 31.08 (23.30) | 29.38 (22.14) | 66.42 (18.24) | 66.42 (23.10) | 63.83 (25.64) | 58.96 (18.97) | 51.92 (20.14) |
| Late | 27.58 (23.81) | 28.88 (25.10) | 65.75 (21.71) | 60.13 (26.07) | 61.17 (25.76) | 56.17 (22.15) | 51.67 (22.23) | |
|
|
||||||||
| Sedation | Early | 34.75 (25.02) | 35.79 (25.99) | 43.71 (29.26) | 45.29 (30.67) | 45.00 (30.83) | 46.08 (30.26) | 40.58 (28.32) |
| Late | 39.00 (25.53) | 42.50 (27.26) | 43.13 (27.52) | 43.92 (31.45) | 40.88 (30.77) | 47.42 (24.67) | 44.21 (26.51) | |
Table 2. Composite subjective effects data for all participants (N=24). All ratings were given on a scale of 1–100. Each column indicates the number of minutes post-consumption that the rating was provided. Intoxication = participant’s subjective level of intoxication; Desire = participant’s desire to consume alcohol; Like = how much participant likes the effects she is feeling; Stimulation = participant’s subjective level of stimulation; Sedation = participant’s subjective level of sedation.
Discussion
This study aimed to determine the influence of menstrual cycle phase on sensitivity to the acute disinhibiting effect of alcohol in young women. Results showed that heightened sensitivity to the acute disinhibiting effect of alcohol was associated with the late follicular phase, which salivary data confirmed was characterized by elevated E2 and LH. The effect of cycle phase on disinhibition was specific to the intoxicated state, as disinhibition following placebo did not differ as a function of cycle phase. The difference in alcohol sensitivity was marked. Women displayed a 1.5-fold increase in the disinhibiting effect of alcohol during the late follicular phase versus the early follicular phase. The disinhibiting effects of alcohol cannot be explained by any speed/accuracy tradeoff in task performance as neither alcohol nor cycle phase had any effect on women’s reaction time during task performance. Furthermore, the difference in sensitivity to the disinhibiting effect of alcohol between early and late follicular phase cannot be explained by differences in the BACs at those times as BACs did not differ significantly as a function of cycle phase. Testing at the late follicular phase of the menstrual cycle was designed to coincide with ovulation. Although the timing of ovulation can be somewhat unpredictable, our hormone analyses from participants’ salivary samples confirmed that our scheduling protocol for testing during late follicular phase was successful at targeting ovulation. On average, sessions were scheduled within two days of ovulation.
In the sober state (i.e., following placebo) women’s level of inhibitory control was remarkably consistent across cycle phase. Indeed, following placebo, women’s cued go/no-go task performance showed nearly identical mean levels of inhibitory control between early and late follicular phase. This stability in sober-state inhibitory control was also indicated by evidence for its test-retest reliability across the early and late follicular phase. Regarding the subjective effects of alcohol, women reported higher ratings of effects in response to alcohol versus placebo that generally increased and declined as a function of the rising and descending BAC during the test session. However, the results showed no differences in the intensity of the subjective effects of alcohol between early and late follicular phase. Although the fixed administration order of placebo followed by alcohol is a limitation, the consistency of the behavioral and subjective responses to placebo at each cycle phase indicates no observation of a learning or a practice effect suggesting little potential for any order of testing effects.
To our knowledge, this is the first study to systematically test the effect of menstrual cycle phase and ovarian hormone levels on the acute behavioral disinhibiting effect of a controlled dose of alcohol in women. The study provides important new information on the potential role of cycle phase and possibly E2 to sensitize women to acute behavioral effects of alcohol during a critical phase of the menstrual cycle. Other researchers have speculated that women are at a greater likelihood of engaging in impulsive behavior, especially after drinking, when E2 is elevated (i.e., during ovulation) (Colzato et al., 2010; Diekhof, 2015; Kurath & Mata, 2018). One such impulsive behavior is binge drinking. It has been argued that alcohol-induced impairment of inhibitory control from an initial drink or two might reduce the ability to terminate continued drinking behavior in the situation, thus resulting in excessive, binge drinking (Fillmore, 2003, 2012). Research from our lab supports this account by showing that drinkers who display greater impairment of inhibitory control from an initial dose of alcohol also consume more alcohol as indicated by self-reported drinking patterns and when given ad lib access to alcohol in the laboratory (Weafer & Fillmore, 2008). To the extent that initial disinhibiting effects of alcohol can promote binge drinking, the current evidence would suggest that risk of such binge drinking in women might be greater during the late follicular phase when E2 is elevated, close to and during ovulation. Such risks also might be exacerbated in women with higher trait impulsivity, or by environments that provoke impulsive actions.
In addition to promoting binge use, a phase-induced elevation in sensitivity to the disinhibiting effect of alcohol might be relevant to women who are seeking to abstain or limit their alcohol consumption. All participants in the present study were social drinkers, due to both a desire for generalizability and ethical concerns surrounding the inclusion of heavy drinkers. However, based on the present data, women with alcohol use disorder could be at a higher risk for relapsing or violating their consumption limits during the late follicular phase when E2 is elevated, which could undermine treatment efficacy. Adding to the potential concern is that women might be unaware of any elevated risk during this time of their cycle. This is supported by our findings that the intensity of women’s perceived effects of alcohol, as measured by self-report scales in our study, did not increase from early to late follicular phase. This suggests that the participants might not have perceived the effects of alcohol to be any more intense during ovulation despite evidence of their greater sensitivity to its disinhibiting effect at this time.
The findings from this study are consistent with those of pre-clinical studies which found evidence for increased drinking when E2 is high by utilizing estrous cycle tracking and estradiol replacement in ovariectomized female rats (Lancaster et al., 1996; Roberts et al., 1998). The majority of these studies have focused on the relationship between E2 levels and the rewarding effects of alcohol, mediated through DA, GABA, and glutamate. Indeed, elevated reward has long been the suspected mechanism promoting increased drinking. The present study suggests a different mechanism underlying this relationship between estradiol and elevated drinking, impaired impulse control, by demonstrating that during the late follicular phase when E2 is elevated, sensitivity to disinhibition is elevated as well. However, it should be noted that the ovarian hormones P4 and LH vary as a function of cycle as well, and more research into their effects on alcohol consumption and disinhibition is needed. Further evidence is also needed to understand the neural basis for the cycle phase differences in alcohol-induced sensitivity to disinhibition. Some neuroimaging studies have linked activation in the anterior cingulate and DLPFC to disinhibition (Tekin & Cummings, 2002; van Holst et al., 2012), making these regions an appropriate starting point for investigating the neural bases by which ovarian hormones, such as a E2, might modulate the effects of alcohol on the inhibitory control of behavior.
Some limitations should be considered, such as the use of a single active dose. This dose and administration procedure has been shown to reliably yield BACs of 80 mg/100 ml in women of this age range (Abroms et al., 2003; Fillmore & Vogel-Sprott, 1999). Despite its relevance as a legal limit BAC for DUI per se laws in most of the United States, it is also important to test for cycle phase effects at lower doses. Our studies have demonstrated acute disinhibiting effects of alcohol at BACs as low as 50 mg/100 ml (Marczinski & Fillmore, 2003). The possibility that cycle phase, specifically the late follicular phase, can further lower the BAC threshold for impaired impulse control awaits to be examined. The findings are also limited to a single domain of impulsivity, inhibition of a pre-potent action. Impulsivity is a broad behavioral construct, commonly described as multifaceted. Inhibitory control as measured in the current study is only one aspect of this broad construct. Our measure of inhibitory control was focused on the individual’s ability to briefly prevent the initiation of an instigated action. Equally important to impulsivity is the likelihood of initiating an action despite potential costs to doing so. Delay discounting tasks have long been used to assess this other dimension of impulsivity, particularly in drug-using populations (Petry, 2001; Reynolds, 2006), and our findings should be considered within the greater context of general impulsivity.
An additional limitation of this study concerns its generalizability. This study was conducted in women with menstrual cycles unaltered by contraceptives. About one-quarter (26.1%) of reproductive-age women use hormonal contraceptives (Daniels & Abma, 2018), and the results are not generalizable to that population. However, the statistic might be an overestimation as it represents the percentage of women in the US who use oral contraceptives or long-acting reversible contraceptives such as an intrauterine device (IUD). Although the majority of therapeutics in these categories are hormonal, there are exceptions such as the copper IUD. On the other hand, this statistic represents women aged 15–49 years. The percentage of women using hormonal contraceptives likely varies by age and might be higher in younger adult women. Regardless, changes in sensitivity to the disinhibiting effects of alcohol could be quite different under hormonal contraception. The need for studies in women who use contraceptives is important as it represents a sizable portion of the population.
The focus of this study was limited to disinhibition, which is primarily relevant after a drinking episode has begun. However, the decision to begin drinking is known to be influenced by the appetitive properties of alcohol, and by cues that signal the availability of alcohol. We did not find evidence for heightened rewarding effects of alcohol as a function of cycle phase using self-reports of subjective effects. However, other measures of rewarding effects, such as attentional bias or cue reactivity to alcohol, could assess how the incentive salience of the drug might be influenced by cycle phase.
Public Health Significance:
The findings of this study demonstrate that around ovulation, women exhibit increased disinhibition while intoxicated. Greater disinhibition can impair the drinker’s ability to terminate a drinking episode, leading to a binge. Thus, women may be at a greater risk for excessive alcohol use during the late follicular phase of the menstrual cycle around ovulation.
Footnotes
The authors declare no conflict of interest
References
- Abroms BD, Fillmore MT, & Marczinski CA (2003). Alcohol-induced impairment of behavioral control: effects on the alteration and suppression of prepotent responses. J Stud Alcohol, 64(5), 687–695. 10.15288/jsa.2003.64.687 [DOI] [PubMed] [Google Scholar]
- Anker JJ, & Carroll ME (2011). Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci, 8, 73–96. 10.1007/7854_2010_93 [DOI] [PubMed] [Google Scholar]
- Barth C, Villringer A, & Sacher J.(2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci, 9, 37. 10.3389/fnins.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, & Reed BG (2017). Sex differences, gender and addiction. J Neurosci Res, 95(1–2), 136–147. 10.1002/jnr.23963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Hertsig G, van den Wildenberg WP, & Hommel B.(2010). Estrogen modulates inhibitory control in healthy human females: evidence from the stop-signal paradigm. Neuroscience, 167(3), 709–715. 10.1016/j.neuroscience.2010.02.029 [DOI] [PubMed] [Google Scholar]
- Daniels K, & Abma JC (2018). Current Contraceptive Status Among Women Aged 15–49: United States, 2015 – 2017. Centers for Disease Control and Prevention. [Google Scholar]
- de Wit H, Crean J, & Richards JB (2000). Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci, 114(4), 830–837. 10.1037//0735-7044.114.4.830 [DOI] [PubMed] [Google Scholar]
- Derntl B, Pintzinger N, Kryspin-Exner I, & Schopf V.(2014). The impact of sex hormone concentrations on decision-making in females and males. Front Neurosci, 8, 352. 10.3389/fnins.2014.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G.(1997). Alcohol and dopamine. Alcohol Health Res World, 21(2), 108–114. https://www.ncbi.nlm.nih.gov/pubmed/15704345 [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK (2015). Be quick about it. Endogenous estradiol level, menstrual cycle phase and trait impulsiveness predict impulsive choice in the context of reward acquisition. Horm Behav, 74, 186–193. 10.1016/j.yhbeh.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Erol A, Ho AM, Winham SJ, & Karpyak VM (2019). Sex hormones in alcohol consumption: a systematic review of evidence. Addict Biol, 24(2), 157–169. 10.1111/adb.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, & Foltin RW (2010). Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav, 58(1), 13–21. 10.1016/j.yhbeh.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, & Foltin RW (2002). The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl), 159(4), 397–406. 10.1007/s00213-001-0944-7 [DOI] [PubMed] [Google Scholar]
- Fillmore MT (2003). Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev, 2(3), 179–197. 10.1177/1534582303257007 [DOI] [PubMed] [Google Scholar]
- Fillmore MT (2012). Drug abuse and behavioral disinhibition. In Verster PCJC, Brady K, and Galanter M.(Ed.), Drug Abuse and Addiction in Mental Illness: Causes, Consequences and Treatment (pp. 25–34). Springer Publishing. [Google Scholar]
- Fillmore MT, Carscadden JL, & Vogel-Sprott M.(1998). Alcohol, cognitive impairment and expectancies. Journal of Studies on Alcohol, 59(2), 174–179. 10.15288/jsa.1998.59.174 [DOI] [PubMed] [Google Scholar]
- Fillmore MT, & Jude R.(2011). Defining “binge” drinking as five drinks per occasion or drinking to a .08% BAC: which is more sensitive to risk? Am J Addict, 20(5), 468–475. 10.1111/j.1521-0391.2011.00156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, & Vogel-Sprott M.(1998). Behavioral impairment under alcohol: cognitive and pharmacokinetic factors. Alcohol Clin Exp Res, 22(7), 1476–1482. https://www.ncbi.nlm.nih.gov/pubmed/9802531 [PubMed] [Google Scholar]
- Fillmore MT, & Vogel-Sprott M.(1999). An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharmacol, 7(1), 49–55. 10.1037//1064-1297.7.1.49 [DOI] [PubMed] [Google Scholar]
- Fillmore MT, & Vogel-Sprott M.(2000). Response inhibition under alcohol: effects of cognitive and motivational conflict. J Stud Alcohol, 61(2), 239–246. 10.15288/jsa.2000.61.239 [DOI] [PubMed] [Google Scholar]
- Fillmore MT, & Weafer J.(2004). Alcohol impairment of behavior in men and women. Addiction, 99(10), 1237–1246. 10.1111/j.1360-0443.2004.00805.x [DOI] [PubMed] [Google Scholar]
- Fillmore MT, & Weafer J.(2013). Behavioral inhibition and addiction. In Wit J. M. H. d. (Ed.), The Wiley-Blackwell handbook of addiction psychopharmacology (pp. 135–164). Wiley Blackwell. [Google Scholar]
- Ford MM, Eldridge JC, & Samson HH (2002). Ethanol consumption in the female Long-Evans rat: a modulatory role of estradiol. Alcohol, 26(2), 103–113. 10.1016/s0741-8329(01)00203-8 [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, & Samson HH (2004). Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clin Exp Res, 28(1), 20–28. 10.1097/01.ALC.0000108647.62718.5A [DOI] [PubMed] [Google Scholar]
- Gan G, Guevara A, Marxen M, Neumann M, Junger E, Kobiella A, Mennigen E, Pilhatsch M, Schwarz D, Zimmermann US, & Smolka MN (2014). Alcohol-induced impairment of inhibitory control is linked to attenuated brain responses in right fronto-temporal cortex. Biol Psychiatry, 76(9), 698–707. 10.1016/j.biopsych.2013.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto JG, & Wiren KM (2008). Neurotoxic consequences of chronic alcohol withdrawal: expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology, 33(5), 1084–1096. 10.1038/sj.npp.1301494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, & Hovey KM (2009). Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol, 169(1), 105–112. 10.1093/aje/kwn287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A, & Stamp JA (2011). Ovarian hormones and propensity to drug relapse: a review. Neurosci Biobehav Rev, 35(3), 427–436. 10.1016/j.neubiorev.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Justice AJ, & de Wit H.(1999). Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl), 145(1), 67–75. 10.1007/s002130051033 [DOI] [PubMed] [Google Scholar]
- Justice AJ, & De Wit H.(2000). Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav, 66(3), 509–515. 10.1016/s0091-3057(00)00218-5 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, & Ronnekleiv OK (2003). Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci, 1007, 6–16. 10.1196/annals.1286.001 [DOI] [PubMed] [Google Scholar]
- Kurath J, & Mata R.(2018). Individual differences in risk taking and endogeneous levels of testosterone, estradiol, and cortisol: A systematic literature search and three independent meta-analyses. Neurosci Biobehav Rev, 90, 428–446. 10.1016/j.neubiorev.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, & Wren SB (1996). Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res, 20(6), 1043–1049. 10.1111/j.1530-0277.1996.tb01945.x [DOI] [PubMed] [Google Scholar]
- Logan GD (1994). On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In Carr DDTH (Ed.), Inhibitory processes in attention, memory, and language (pp. 189–239). Academic Press. [Google Scholar]
- Logan GD, Cowan WB, & Davis KA (1984). On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform, 10(2), 276–291. 10.1037//0096-1523.10.2.276 [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, & Fillmore MT (2007). Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Behav, 21(3), 346–354. 10.1037/0893-164X.21.3.346 [DOI] [PubMed] [Google Scholar]
- Marczinski CA, & Fillmore MT (2003). Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Exp Clin Psychopharmacol, 11(1), 110–117. 10.1037//1064-1297.11.1.110 [DOI] [PubMed] [Google Scholar]
- Marczinski CA, & Fillmore MT (2005). Compensating for alcohol-induced impairment of control: effects on inhibition and activation of behavior. Psychopharmacology (Berl), 181(2), 337–346. 10.1007/s00213-005-2269-4 [DOI] [PubMed] [Google Scholar]
- Martel MM, Eisenlohr-Moul T, & Roberts B.(2017). Interactive effects of ovarian steroid hormones on alcohol use and binge drinking across the menstrual cycle. J Abnorm Psychol, 126(8), 1104–1113. 10.1037/abn0000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Mainous AG 3rd, Curry T, & Martin D.(1999). Alcohol use in adolescent females: correlates with estradiol and testosterone. Am J Addict, 8(1), 9–14. 10.1080/105504999306036 [DOI] [PubMed] [Google Scholar]
- Mazure CM, Toll B, McKee SA, Wu R, & O’Malley SS (2011). Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trial of bupropion. Drug Alcohol Depend, 114(1), 68–72. 10.1016/j.drugalcdep.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Schaffer R, & Hackley SA (1991). Effects of preliminary information in a Go versus No-go task. Acta Psychol (Amst), 76(3), 241–292. 10.1016/0001-6918(91)90022-r [DOI] [PubMed] [Google Scholar]
- Miller MA, Weafer J, & Fillmore MT (2009). Gender differences in alcohol impairment of simulated driving performance and driving-related skills. Alcohol Alcohol, 44(6), 586–593. 10.1093/alcalc/agp051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, & Grant KA (1999). Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res, 23(12), 1933–1940. 10.1111/j.1530-0277.1999.tb04094.x [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, & Matthews DB (2001). The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev, 37(1–3), 98–109. 10.1016/s0165-0173(01)00127-8 [DOI] [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, O’Hara R, & Yesavage JA (1999). Gender differences in moderate drinking effects. Alcohol Res Health, 23(1), 55–64. https://www.ncbi.nlm.nih.gov/pubmed/10890798 [PMC free article] [PubMed] [Google Scholar]
- Muti P, Trevisan M, Micheli A, Krogh V, Bolelli G, Sciajno R, Schunemann HJ, & Berrino F.(1998). Alcohol consumption and total estradiol in premenopausal women. Cancer Epidemiol Biomarkers Prev, 7(3), 189–193. https://www.ncbi.nlm.nih.gov/pubmed/9521430 [PubMed] [Google Scholar]
- Nolen-Hoeksema S.(2004). Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev, 24(8), 981–1010. 10.1016/j.cpr.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Op de Macks ZA, Bunge SA, Bell ON, Wilbrecht L, Kriegsfeld LJ, Kayser AS, & Dahl RE (2016). Risky decision-making in adolescent girls: The role of pubertal hormones and reward circuitry. Psychoneuroendocrinology, 74, 77–91. 10.1016/j.psyneuen.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Levine M, Marcus M, Shiffman S, D’Amico D, Miller A, Keins A, Ashcom J, & Broge M.(2000). Tobacco withdrawal in women and menstrual cycle phase. J Consult Clin Psychol, 68(1), 176–180. 10.1037/0022-006X.68.1.176 [DOI] [PubMed] [Google Scholar]
- Petry NM (2001). Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl), 154(3), 243–250. 10.1007/s002130000638 [DOI] [PubMed] [Google Scholar]
- Psychology Software Tools, Inc. (2017). E-Prime: Documentation Article. Retrieved from https://support.pstnet.com.
- Reichman ME, Judd JT, Longcope C, Schatzkin A, Clevidence BA, Nair PP, Campbell WS, & Taylor PR (1993). Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst, 85(9), 722–727. 10.1093/jnci/85.9.722 [DOI] [PubMed] [Google Scholar]
- Reynolds B.(2006). A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol, 17(8), 651–667. 10.1097/FBP.0b013e3280115f99 [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, & Koob GF (1998). Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res, 22(7), 1564–1569. https://www.ncbi.nlm.nih.gov/pubmed/9802543 [PubMed] [Google Scholar]
- Saladin ME, McClure EA, Baker NL, Carpenter MJ, Ramakrishnan V, Hartwell KJ, & Gray KM (2015). Increasing progesterone levels are associated with smoking abstinence among free-cycling women smokers who receive brief pharmacotherapy. Nicotine Tob Res, 17(4), 398–406. 10.1093/ntr/ntu262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta R, Hilderbrand ER, & Lasek AW (2018). Ovarian Hormones Contribute to High Levels of Binge-Like Drinking by Female Mice. Alcohol Clin Exp Res, 42(2), 286–294. 10.1111/acer.13571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back. In Measuring alcohol consumption (pp. 41–72). Humana Press. [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, & Hatsukami DK (1999). Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol, 7(3), 274–283. 10.1037//1064-1297.7.3.274 [DOI] [PubMed] [Google Scholar]
- Stillwell W.(2016). Bioactive Lipids. In An Introduction to Biological Membranes (pp. 453–478). Elsevier. [Google Scholar]
- Tekin S, & Cummings JL (2002). Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res, 53(2), 647–654. 10.1016/s0022-3999(02)00428-2 [DOI] [PubMed] [Google Scholar]
- Terner JM, & de Wit H.(2006). Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend, 84(1), 1–13. 10.1016/j.drugalcdep.2005.12.007 [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, & Chandler LJ (2015). Alcohol-induced alterations in dopamine modulation of prefrontal activity. Alcohol, 49(8), 773–779. 10.1016/j.alcohol.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst RJ, van Holstein M, van den Brink W, Veltman DJ, & Goudriaan AE (2012). Response inhibition during cue reactivity in problem gamblers: an fMRI study. PLoS One, 7(3), e30909. 10.1371/journal.pone.0030909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, & Fillmore MT (2008). Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology (Berl), 201(3), 315–324. 10.1007/s00213-008-1284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, & Fillmore MT (2016). Low dose alcohol effects on measures of impulsive behavior. Current Addiction Reports, 3(1), 75–84. [Google Scholar]
- Weafer J, Miller MA, & Fillmore MT (2010). Response conflict as an environmental determinant of gender differences in sensitivity to alcohol impairment. Curr Drug Abuse Rev, 3(3), 147–155. 10.2174/1874473711003030147 [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Smith PH, Allen SS, Cosgrove KP, Saladin ME, Gray KM, Mazure CM, Wetherington CL, & McKee SA (2015). Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res, 17(4), 407–421. 10.1093/ntr/ntu249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJ, & de Wit H.(2002). Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav, 73(4), 729–741. 10.1016/s0091-3057(02)00818-3 [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Andrew MR, & Wiren KM (2016). Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain Pathol, 26(4), 433–451. 10.1111/bpa.12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED (2007). Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol, 29(1), 81–95. 10.1016/j.ntt.2006.10.013 [DOI] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, & Becker JB (2014). Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem, 14(2), 83–89. 10.2174/1871524914666141226103135 [DOI] [PMC free article] [PubMed] [Google Scholar]