Abstract
Neuronal injury and apoptosis are important causes of the occurrence and development of many neurodegenerative diseases, such as cerebral ischemia, Alzheimer’s disease, and Parkinson’s disease. Although the detailed mechanism of some diseases is unknown, the loss of neurons in the brain is still the main pathological feature. By exerting the neuroprotective effects of drugs, it is of great significance to alleviate the symptoms and improve the prognosis of these diseases. Isoquinoline alkaloids are important active ingredients in many traditional Chinese medicines. These substances have a wide range of pharmacological effects and significant activity. Although some studies have suggested that isoquinoline alkaloids may have pharmacological activities for treating neurodegenerative diseases, there is currently a lack of a comprehensive summary regarding their mechanisms and characteristics in neuroprotection. This paper provides a comprehensive review of the active components found in isoquinoline alkaloids that have neuroprotective effects. It thoroughly explains the various mechanisms behind the neuroprotective effects of isoquinoline alkaloids and summarizes their common characteristics. This information can serve as a reference for further research on the neuroprotective effects of isoquinoline alkaloids.
Keywords: isoquinoline alkaloids, neuroprotection, nerve injury, mechanism
1. Introduction
Degenerative diseases of the nervous system have brought a significant medical and public health burden on people worldwide. With the acceleration of population aging, the prevalence and incidence of degenerative nervous system diseases have sharply increased in recent years [1]. Although the pathological mechanisms of cerebral ischemia, Alzheimer’s disease, and Parkinson’s disease differ, nerve cell injury and apoptosis are common pathological features [2]. After experiencing cerebral ischemia, the blood vessels and tissues in the brain may become damaged, leading to the destruction of the blood–brain barrier. Under the combined action of a series of cascade reactions, such as oxidative stress stimulation, the release of inflammatory factors, aggravation of autophagy, and mitochondrial dysfunction, a large number of nerve cells rapidly undergo apoptosis. This process directly leads to learning and cognitive dysfunction after cerebral ischemia [3]. The pathological mechanism of Alzheimer’s disease is still controversial, but studies have confirmed that progressive neurodegeneration is its main feature. Neuroinflammation and angiogenesis, neurogenesis, and neurological recovery dysfunction play an important role in the pathophysiology of AD [4]. The main cause of Parkinson’s disease is the degeneration and eventual loss of dopaminergic neurons in the substantia nigra. Dopamine replacement therapy has achieved remarkable results [5]. Therefore, drug research with neuroprotective effects, which can reduce nerve damage and promote neuronal repair and regeneration, is of great significance in alleviating the progression of such diseases and even curing them. However, the research progress of drugs with neuroprotective effects has been slow, and few such drugs have been marketed in recent years [6].
Isoquinoline alkaloids, including nuciferine, berberine, and tetrandrine, are the main active components extracted from traditional Chinese medicines, such as lotus leaf, Coptis chinensis Franch, and Stephania tetrandra S. Moore, respectively. Most of these traditional Chinese medicines have been used in clinical treatments for many years. The core functions are clearing heat and detoxifying, diuresis and detumescence, and promoting blood circulation and cooling the blood. Now, they have also been studied for their anti-inflammatory and anti-oxidant properties [7,8,9,10]. Upon observing their structures, we have found that they have a common structural basis and belong to isoquinoline alkaloids. By consulting the relevant literature on these alkaloids, it can be found that these compounds have a wide range of pharmacological effects and significant pharmacological activities, especially in improving nerve damage caused by neurodegenerative diseases. Studies have shown that the active ingredients of isoquinoline alkaloids can exert neuroprotective effects by inhibiting nerve injury inflammation, anti-oxidative damage, regulating autophagy, inhibiting intracellular calcium overload, and improving mitochondrial dysfunction, as well as promoting vascular endothelial cell proliferation, and neuronal repair and regeneration [11]. However, these alkaloids have not yet been used to treat neurodegenerative diseases, and the related research is not deep enough. Additionally, there is no review focused on a comprehensive summary of the neuroprotective effects of isoquinoline alkaloids.
Therefore, we used isoquinoline alkaloids, neuroprotection, nerve injury, and the names of some isoquinoline alkaloids, such as nuciferine, berberine, and tetrandrine, as well as some nerve injury mechanisms such as neuroinflammation, oxidative stress, and autophagy as keywords. After consulting a large amount of literature in PubMed and CNKI literature retrieval media, we have compiled a list of several representative isoquinoline alkaloids with neuroprotective effects, and comprehensively discuss their different mechanisms of neuroprotective effects and some common characteristics. It is hoped that this can provide a reference for further basic research on the neuroprotective effects of isoquinoline alkaloids.
2. Isoquinoline Alkaloids with Neuroprotective Effects
2.1. Nuciferine
Nuciferine is a natural product extracted from the traditional Chinese medicine, lotus leaf. Lotus leaf has the effect of dispelling dampness and decreasing phlegm, dispersing stasis, and lowering fever in the theory of traditional Chinese medicine. It is a traditional medicinal material for food therapy [12]. Nuciferine has been shown to have anti-inflammatory, antioxidant, anti-aging, hypolipidemic, and other pharmacological activities [13]. The physicochemical properties and metabolomics analysis of nuciferine showed that its absolute bioavailability is 69.56%. It has good fat solubility and high blood–brain barrier permeability, which are the bases for its treatment of central nervous system diseases [14]. At the same time, existing studies have also shown that nuciferine can produce neuroprotective effects by inhibiting neuroinflammation, reducing oxidative damage, and regulating autophagy [15].
2.2. Berberine
Berberine is a plant alkaloid extracted from Coptis chinensis Franch, which has a variety of pharmacological properties. Many studies have focused on lowering blood glucose, anti-obesity, and improving insulin resistance [16,17,18]. However, current studies have also shown that it has many pharmacological activities, such as anti-inflammatory, anticancer, cardiovascular, and central nervous system effects [19]. It is also a potential drug for the multitarget treatment of neurodegenerative diseases caused by metabolic disorders [20]. Usually, when neuronal damage is caused by neurometabolic diseases, multiple important organelles, including lysosomes, peroxisomes, and mitochondria, will undergo dysfunction, causing metabolic disorders and lead to a significant amount of neuronal apoptosis [21]. This feature of berberine provides a novel therapeutic idea for neuroprotection by improving metabolic disorders and optimizing energy metabolism.
2.3. Tetrandrine
Radix Stephanie Tetrandrine has been traditionally used as a medicine for dispelling wind and dampness, promoting diuresis, and reducing swelling. As the main alkaloid component of Radix Stephanie tetrandrae, tetrandrine has also received extensive attention in recent years [22]. Studies have demonstrated that this compound has a variety of neuroprotective pharmacological activities. The main neuroprotective mechanism involves regulating Ca2+ and K+ channels, maintaining intracellular calcium homeostasis, and reducing neuronal and glial cell damage caused by Ca2+ overload [23]. In addition, it can regulate central neurotransmitter transport and metabolism, inhibit neuroinflammation, improve vascular endothelial dysfunction, decrease oxidative stress, and regulate autophagy [24,25,26]. It has a highly comprehensive neuroprotective effect and is a potential neuroprotective agent.
2.4. Morphine
Morphine is a classic analgesic and sedative drug. At present, its clinical application is mainly to alleviate pain. Traditional studies have shown that morphine has a potent inhibitory effect on the central nervous system, and large doses of morphine can produce toxic effects on nerve cells [27]. However, we are cognizant that in recent years, many studies have shown that morphine can also exert neuroprotective effects through various mechanisms [28]. The main mechanisms include reducing intracellular Ca2+ overload, reducing cell oxidative damage, activating autophagy, and inhibiting intracellular toxic protein production to promote neuronal regeneration and differentiation [29,30,31,32]. At the same time, researchers have applied very low doses of morphine to very preterm infants, which has effectively improved the neurodevelopmental status of the infants [33]. This also reflects the neuroprotective potential of low-dose morphine. What’s more, studies have shown that morphine derivatives also have neuroprotective effects. For example, Hydrophone protects hippocampal CA1 neurons from ischemia-reperfusion injury by activating the mTOR signaling pathway, and methadone can improve cognitive dysfunction in drug withdrawal patients [34,35]. This suggests that derivatives of isoquinoline alkaloids may also have neuroprotective effects, but research in this area is more limited, and more related research is needed.
2.5. Tetrahydropalmatine
Tetrahydropalmatine is a key component of the traditional Chinese medicine Corydalis ambigua. In addition to the traditional analgesic, sedative, and hypnotic effects, many studies on the effects on the cardiovascular and central nervous systems have also made some progress [36,37]. Especially for neuroprotection, tetrahydropalmatine can not only inhibit the level of nerve injury inflammation, antioxidant damage, and regulate autophagy to reduce neuronal apoptosis, but also has a unique ability to promote vascular endothelial cell proliferation and neuronal repair and regeneration [38]. Angiogenesis plays an important role in wound healing, nerve defects, and nerve regeneration. Tetrahydropalmatine also has the potential to increase the expression of neurotrophic factors, promoting neurogenesis and enhancing the repair and regeneration abilities of neurons after injury [39].
The structures of several aforementioned alkaloids are shown in Figure 1. These structures all contain the same isoquinoline nucleus, which belongs to the active components of isoquinoline alkaloids. In addition to the above, a variety of isoquinoline alkaloids have neuroprotective effects, showing pharmacological activity in the treatment of neurodegenerative diseases [40]. In order to better demonstrate the neuroprotective effects and mechanisms of this type of compound, we selected about 10 representative isoquinoline alkaloids with the same isoquinoline nucleus structure and neuroprotective effect, as shown in Table 1.
Figure 1.
The chemical structure of isoquinoline alkaloids with neuroprotective effects. (a–e) These are the chemical structures of nuciferine, berberine, tetrandrine, morphine, and tetrahydropalmatine, respectively. They all have the same isoquinoline (benzopyridine) core structure.
Table 1.
Isoquinoline alkaloids with neuroprotective effects and their neuroprotective mechanisms.
| Alkaloid | Structure | Neuroprotective Mechanism | Reference |
|---|---|---|---|
| Papaverine |
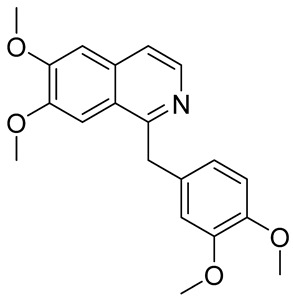
|
anti-inflammatory; anti-oxidation; anti-apoptosis; promote neurogenesis; inhibition of α-synuclein aggregation | [41,42,43,44,45,46] |
| Higenamine |
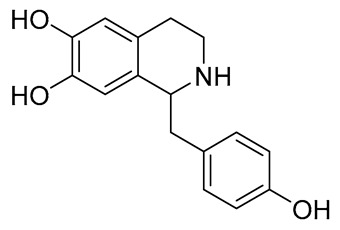
|
anti-inflammatory; anti-oxidation; anti-apoptosis; | [47,48,49] |
| Sinomenine |
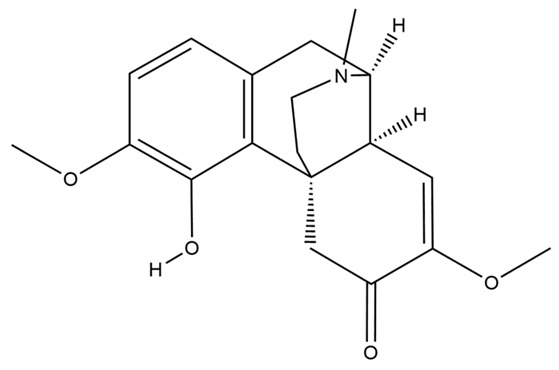
|
anti-inflammatory; anti-oxidation; regulating autophagy; anti-pyroptosis; anti-apoptosis; neuroimmune intervention; inhibition of Ca2+ overload |
[50,51,52,53,54,55,56,57,58] |
| Sanguinarine |
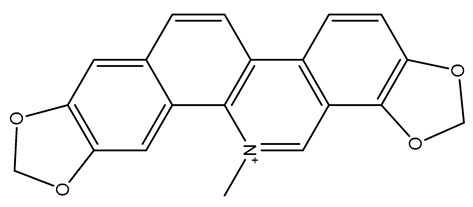
|
anti-inflammatory; anti-apoptosis; mitochondrial protection; inhibition of Ca2+ overload | [59,60,61,62] |
| Neferine |
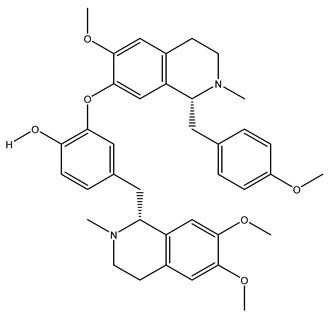
|
anti-inflammatory; anti-oxidation; anti-apoptosis; regulating autophagy; inhibition of Ca2+ overload; mitochondrial protection | [63,64,65,66,67,68,69,70] |
| Stepharine |
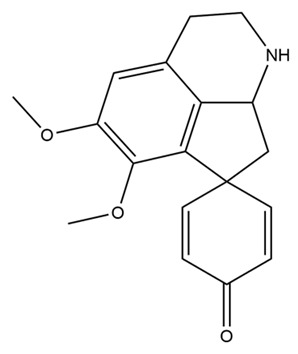
|
anti-inflammatory; anti-apoptosis; anti-oxidation | [71,72,73] |
| Dauricine |
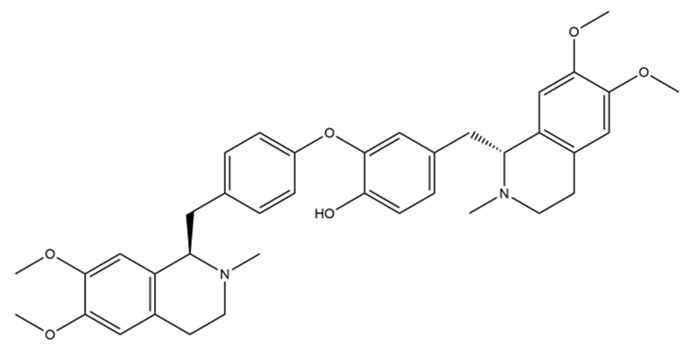
|
anti-inflammatory; anti-oxidation; anti-apoptosis; acceleration of Aβ protein degradation; inhibition of ferroptosis; enhance mitochondrial function |
[74,75,76,77,78] |
| Lycorine |
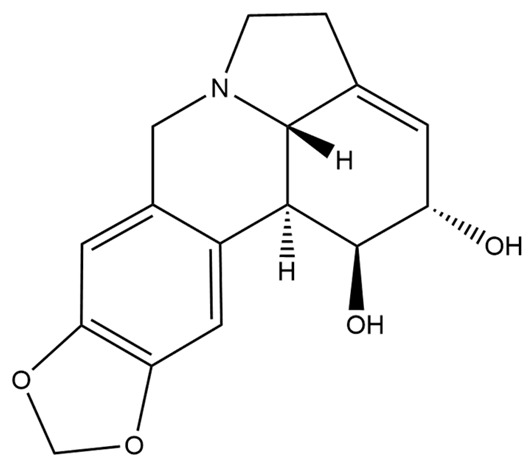
|
anti-oxidation; anti-apoptosis; | [79,80] |
| Piperine |
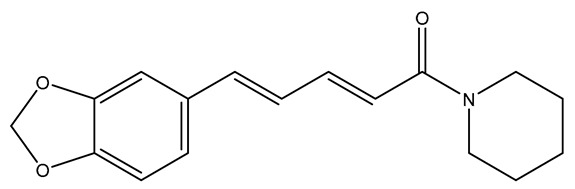
|
anti-inflammatory; anti-oxidation; anti-apoptosis; improve mitochondrial dysfunction; reduce the toxicity of excitatory amino acids; up-regulate nerve growth factor |
[81,82,83,84,85,86,87] |
| Jatrorrhizine |
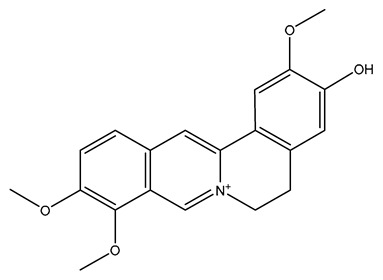
|
anti-inflammatory; anti-oxidation; anti-apoptosis; improve vascular endothelial dysfunction | [88,89,90] |
3. Neuroprotective Effect and Mechanism of Isoquinoline Alkaloids
The above studies have shown that a variety of isoquinoline alkaloids with a common structural basis, can exert neuroprotective effects based on a variety of different modes of action and have potential application value for the treatment of many neurodegenerative diseases. Recent reports have also revealed the intracellular signaling pathways and mechanisms that these alkaloids may target, thereby exerting their neuroprotective effects. In this section, we will briefly describe the mechanism of nerve injury, summarize the neuroprotective mechanisms and signaling pathways of the five common isoquinoline alkaloids mentioned in the previous section, and clarify their potential contribution to the treatment of neurodegenerative diseases.
3.1. Neuroprotection towards Inflammatory Injury
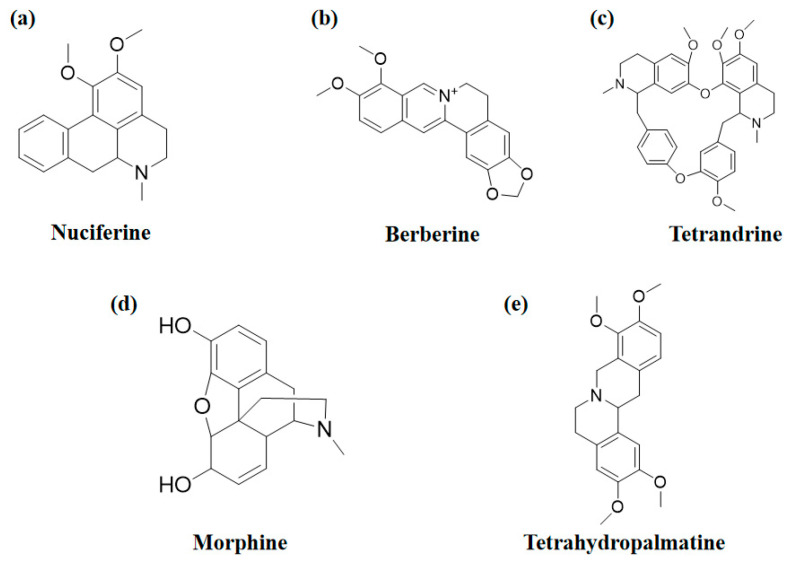
Almost all neurodegenerative diseases are accompanied by inflammation. When neurons are affected under the action of various intracellular mechanisms, abnormal protein metabolism and degeneration, organelle dysfunction, etc., will occur, resulting in a large amount of neuronal apoptosis [91]. Inflammation is usually regarded as a process of self-repair of the body, which helps to remove a large number of fragments caused by cell necrosis and death. Therefore, neuronal apoptosis will induce the activation of immune cells and the release of a large number of inflammatory factors. However, due to inflammation, brain tissue damage, blood–brain barrier damage, and edema, cerebrovascular dysfunction will worsen and induce further neuronal apoptosis [92]. Therefore, although inflammation is not the main pathological mechanism of most neurodegenerative diseases, it is a key event in these diseases. Reducing inflammatory damage is still of great significance for neuroprotection and disease treatment [93]. As shown in Figure 2, isoquinoline alkaloids have significant anti-inflammatory activity. It has been shown that tetrandrine can effectively inhibit the activation of NLRP3 inflammasome by inhibiting I/R-induced overexpression of inflammatory and apoptotic factors such as NLRP3, caspase-1, IL-1β, IL-18, and Sirt-1. Up-regulation of Sirt-3 further inhibited NLRP1 inflammasome activation and significantly reduced the neurological deficit, infarct volume, and brain water content in MCAO mice [94]. In addition, it can also down-regulate the expression of NSE, TNF-α, NF-κB, TRAF1, GADD34, p-PERK, IRE1α, CHOP, and p-JNK by regulating the IRE1α/JNK/CHOP signaling pathway, reducing the level of neuroinflammation and neuronal apoptosis in mice with traumatic brain injury (TBI) [95]. Studies have shown that tetrandrine can reverse the ectopic transcription of inflammation-related genes, including TNFα, IL-1β, IL-6, COX-2, iNOS, and p65, in 5XFAD mice (a transgenic model of AD), inhibiting the secretion of inflammatory cytokines TLR4, p65, iNOS, and COX-2 in microglia BV2 cells induced by Aβ1-42, and improving AD by reducing inflammation and neurotoxicity [96].
Figure 2.
Isoquinoline alkaloids exert neuroprotective effects by reducing inflammatory injury.
In addition to tetrandrine, berberine and nuciferine also have good anti-inflammatory activity. In addition to activating the PI3K-AKT signaling pathway and inhibiting the NF-kB signaling pathway to produce anti-inflammatory effects, berberine can also inhibit the production of TNF-α, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS), which helps to reduce neuroinflammation and prevent blood–brain barrier damage [97,98,99,100]. In addition to improving neuroinflammatory damage, berberine can also directly act on improving cognitive dysfunction in AD through a variety of other mechanisms. Nuciferine has been shown to inhibit the activation of NF-κB, reduce the release of pro-inflammatory mediators such as TNF-α, IL-1β, and PGE2, and inhibit the inflammatory response of BV2 cells induced by LPS. Further studies have shown that nuciferine can also activate PPAR-γ, inhibit neuroinflammatory damage caused by NF-kB, and exert neuroprotective effects [101].
3.2. Neuroprotection towards Oxidative Stress
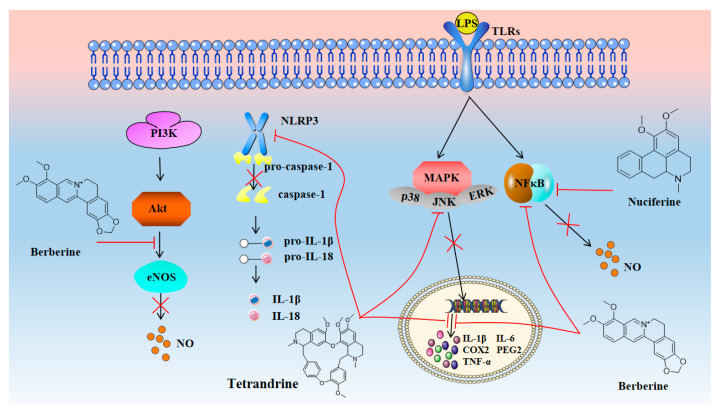
The relationship between free radical damage and inflammatory damage is inseparable, and there is a complex interaction between them [102]. Cells produce free radicals during normal metabolism, and the body contains antioxidants that neutralize these free radicals, sustaining a dynamic balance between antioxidants and free radicals. When pathological damage causes an excessive accumulation of free radicals, the balance is disrupted, and oxidative stress occurs. Inflammatory damage is one of the factors that can trigger oxidative stress [103]. However, oxidative stress can cause cell and tissue damage, which can activate a variety of transcription factors, leading to abnormal expression of genes involved in inflammatory pathways and promoting inflammatory responses. The cycle of the two further exacerbates the disease [104]. As shown in Figure 3, in addition to anti-inflammatory activity, isoquinoline alkaloids have significant antioxidant activity, and berberine can protect C17.2 neural stem cells from oxidative damage [105]. The main mechanism involves reducing the level of reactive oxygen species (ROS) in C17.2 cells through the (NRf1/2)-(NQO-1)-(HO-1) pathway while down-regulating the apoptosis factors caspase-3 and Bax, and up-regulating the anti-apoptotic factor Bcl2 to reduce apoptosis. In addition, berberine can also increase the viability of C17.2 cells by upregulating the expression of the extracellular signal-related kinase (ERK) and phosphorylated extracellular signal-related kinase (p-ERK), activating the WNT/β-Catenin signaling pathway, and increasing the expression levels of pre-neural factors ASCL1, NeuroG1, NeuroD2, and DCX. This further reduces oxidative damage to C17.2 neural stem cells and induces them to differentiate into neurons. Moreover, studies have shown that berberine exerts neuroprotective effects by activating antioxidant enzymes such as superoxide dismutase (SOD) and glutathione (GSH) to antagonize oxidative stress caused by chronic cerebral hypoperfusion [106].
Figure 3.
Isoquinoline alkaloids exert neuroprotective effects by reducing oxidative stress.
Another alkaloid that can reduce oxidative stress damage in nerve cells is nuciferine. CAT, SOD, and GSH-Px are well-known antioxidant enzymes because they have strong free radical scavenging effects in tissues and cells. Excessive levels of reactive oxygen species (ROS) in cells reduce the activity of these enzymes [107]. However, studies have shown that nuciferine can bring these enzyme activities in diabetic rats induced by alloxan to close-to-normal levels, thereby protecting nerve cells from oxidative damage [108]. It is a potential drug for the treatment of Alzheimer’s disease caused by diabetes by exerting neuroprotective effects. Similar to nuciferine, tetrahydropalmatine can also play an antioxidant role by increasing the levels of SOD, GSH, and CAT and reducing the level of MDA, effectively reducing the oxidative stress injury to nerve cells [109].
3.3. Neuroprotection towards Regulating Autophagy
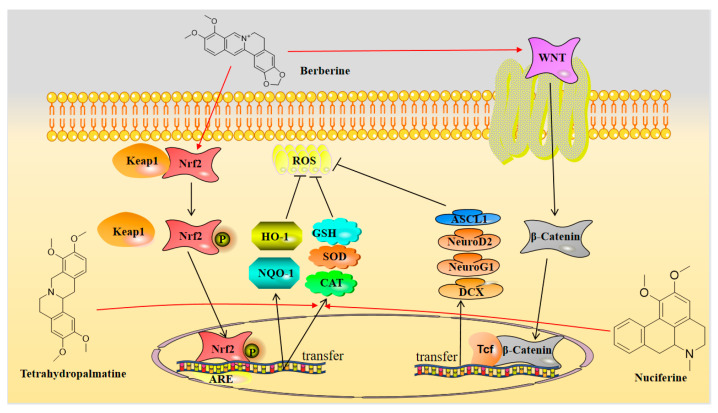
Autophagy is a cellular process of massive degradation of proteins and organelles in cells, which can lead to non-apoptotic programmed cell death called autophagic cell death [110]. The traditional theory holds that after neuronal damage leads to apoptosis, autophagy mediated by lysosomes, as the main organelles, will be induced and enhanced [111]. However, new studies have shown that although autophagy is associated with various cellular damage mechanisms, much apoptosis caused by enhanced autophagy will greatly weaken the autophagy function, leading to the aggravation of damage. This is a dynamic process in neuronal damage [112]. Therefore, the regulation of autophagy function is essential for the protection of neurons. As shown in Figure 4, in a mouse model of traumatic brain injury (TBI), the levels of malondialdehyde (MDA), glutathione (GSH), and glutathione peroxidase 4 (GPX4) in brain tissue were detected by enzyme-linked immunosorbent assay (ELISA). The levels of Beclin 1, light chain 3 (LC3) II/I, p62, GPX4, and ferritin heavy chain 1 (FTH1) were detected by Western blotting (WB) and immunofluorescence. It has been found that tetrandrine could reduce MDA content and increase the GSH content during the period after TBI. It can also reverse the changes in the expression levels of the above autophagy-related proteins after TBI and promote autophagy. Through antioxidative damage and regulation of autophagy, it effectively exerts neuroprotective effects and improves neurological function and reduces brain edema after TBI in mice [113].
Figure 4.
Isoquinoline alkaloids exert neuroprotective effects by regulating autophagy.
In addition, other studies have shown that tetrahydropalmatine can also play a role in autophagy regulation. Studies have found that the expression of Beclin-1 and LC3II/I increased after I/R injury in rats, while the expression of p62 decreased, which confirmed that autophagy was activated after I/R injury. Further studies have found that this is related to the inhibition of the PI3K/AKT/mTOR pathway. Tetrahydropalmatine can reactivate this pathway and reduce the level of autophagy [114]. Nuciferine also has an autophagy regulatory effect. Through the study of the rat pMCAO model, it was found that the levels of autophagy markers decreased, and the accumulation of autophagy substrates was reduced in the early stages of cerebral ischemia. This is related to the inhibition of the autophagy–lysosomal (ALP) pathway mediated by transcription factor EB (TFEB) [115]. In addition, other studies have shown that nuciferine can increase the expression of TFEB and activate the ALP pathway, making the above autophagy markers and autophagy substrates approach normal levels [116]. Although this study only showed that nuciferine regulates autophagy, we believe that regulating autophagy is also an important basis for nuciferine to exert neuroprotective effects.
3.4. Neuroprotection towards Calcium Overload
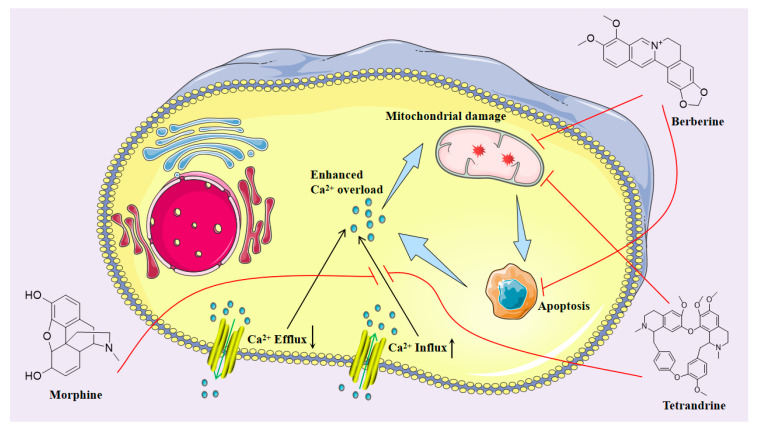
As an essential signaling molecule and cell function regulator, Ca2+ has made substantial progress in the study of function and mechanism [117]. It is also accepted that an imbalance in Ca2+ homeostasis is closely linked to the development of various human pathologies, including neurodegenerative diseases. Calcium homeostasis is closely related to neurodegeneration, neurotoxicity, neuroinflammation, autophagy, and mitochondrial function changes [118]. As shown in Figure 5, studies have confirmed that methamphetamine (MA) can affect the mitochondrial calcium ATPase responsible for pumping Ca2+ into the internal space of mitochondria for storage, directly produce neurotoxicity to cortical cells, and induce cell death with an increase in calcium load. A low dose of morphine can reduce calcium overload induced by MA in PC12 and U87 cells, and significantly improves cell viability by reducing cytotoxicity, caspase-3 activity, and intracellular calcium concentration. The neuroprotective effect of low-dose morphine may also be related to the reduction of inflammatory injury after calcium overload [119]. Berberine also inhibits intracellular calcium overload. Excitatory amino acid toxicity damage leads to the destruction of intracellular calcium balance. Calcium overload is a trigger for oligodendrocyte death. In vitro, the OGD/R ischemia model found that berberine can prevent intracellular calcium accumulation in a concentration-dependent manner, protecting OLN-93 oligodendrocytes from excitotoxicity-induced cell damage [120].
Figure 5.
Isoquinoline alkaloids exert neuroprotective effects by alleviating inflammation and mitochondrial dysfunction after calcium overload.
3.5. Neuroprotection towards Mitochondrial Dysfunction
Energy metabolism is the foundation of cellular life activities. Mitochondria, known as the engine of human energy conversion, play a major role in cell life activities [121]. A variety of evidence has shown that energy metabolism disorders and neuronal damage caused by mitochondrial dysfunction are the pathological basis of various degenerative neurological diseases, including cerebral ischemia, Alzheimer’s disease, and Parkinson’s disease [122]. Weiyi Li et al. used several cell ischemic injury apoptosis models in their study, including a serum deprivation cell model, a glutamate-induced RGC-5 cell death model, and a hydrogen peroxide (H2O2)-induced RGC-5 cell death model, as well as an astrocyte-derived neuron-like RGC-5 cell model. It was found that tetrandrine treatment protected staurosporine-induced RGC-5 cells from serum deprivation-induced cell death, significantly increased the relative number of cells cultured with 1 mM H2O2, and significantly prevented 25 mM glutamic acid-induced cell death in a dose-dependent manner. It has a protective effect on a variety of cells. Further studies have shown that the protective effect of tetrandrine is related to the reduction of mitochondrial transmembrane potential (δψm), improvement of mitochondrial function, inhibition of caspase-3 activation induced by ischemia/reperfusion injury, and reduction of bcl-2 expression [123]. Morphine can also play a similar role in mitochondrial protection. The decrease in mitochondrial membrane potential means that the permeability of mitochondria increases after dysfunction, leading to the release of caspase and nuclease-activating protein. This is a major cause of apoptosis. Studies have shown that morphine can alleviate nicotine-induced mitochondrial dysfunction in PC12 cells and reduce caspase-3 release. As shown in Figure 5, this neuroprotective effect is also associated with a reduction in intracellular calcium levels [124]. There is a complex interaction between calcium overload, mitochondrial damage, and apoptosis [125].
In addition, studies on the zebrafish model of Parkinson’s disease, showed that berberine could easily penetrate the blood–brain barrier. Subcellular localization studies have shown that berberine rapidly and specifically accumulates in the mitochondria of PC12 cells, inhibited the accumulation of Pink1 protein and inhibited the overexpression of LC3 protein in 6-OHDA-injured cells. It is confirmed that mitochondria are the potential sites of berberine in the brain, and berberine may improve the nerve injury caused by Parkinson’s disease by regulating mitochondrial function [126].
3.6. Neuroprotective Effects of Promoting Vascular Endothelial Proliferation and Neuronal Regeneration
More and more studies have shown that isoquinoline alkaloids play a significant role in promoting the proliferation of vascular endothelial cells and the regeneration of neurons. By inducing the angiogenesis of endothelial cells and accelerating the recovery of damaged neuronal cell structure and function, it can rapidly improve memory and cognitive dysfunction caused by neuronal damage and improve the prognosis of ischemic stroke [127]. This is still of great significance for the treatment of neurodegenerative diseases by promoting the repair and regeneration of damaged neurons. Based on the clinical experience of traditional Chinese medicine, molecular docking was performed on tetrahydropalmatine and VEGFR2 to demonstrate their binding potential. Metabolomics analysis showed that it can increase the expression of VEGFR2, which is a trigger for angiogenesis and has the potential to promote angiogenesis and exert neuroprotective effects [128]. Studies have also shown that tetrandrine has a role in promoting angiogenesis. Vascular endothelial growth factor A (VEGF-A), which belongs to the same family as VEGFR2, plays an important role in angiogenesis. It has been observed that tetrandrine can increase the expression of VEGF-A mRNA in H9C2 cells. Tetrandrine treatment can increase the synthesis of new VEGF-A mRNA but has no impact on the stability of VEGF-A mRNA. This can enhance the angiogenesis activity of endothelial cells and improve blood flow recovery and capillary density after ischemic limb injury. This is associated with increased VEGF-A expression [129]. It is not only limited to the recovery of ischemic diseases but also of great significance for promoting neuronal repair and regeneration.
4. Summary and Outlook
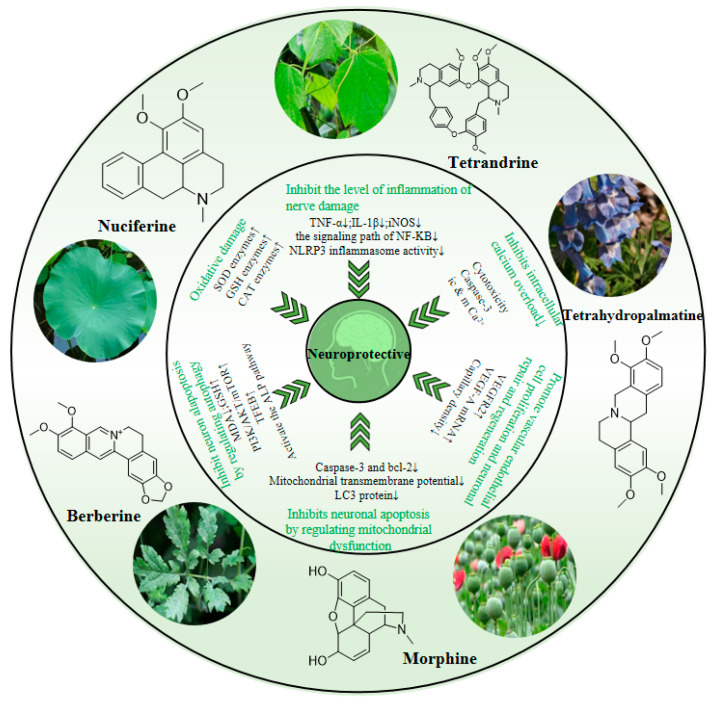
Finally, we comprehensively analyzed the neuroprotective mechanisms of isoquinoline alkaloids and the key targets of their regulation. As shown in Figure 6, we found that the same alkaloid component can exert direct or potential neuroprotective effects in many different ways. These mechanisms are closely related to each other, such as calcium overload, inflammation, and autophagy. There are both cascades and reciprocal causation. This indicates that isoquinoline alkaloids can exert neuroprotective effects through multiple links, multiple targets, and multiple pathways. Some existing studies have also confirmed our idea [130]. We further found that because these isoquinoline alkaloids have a common structural basis, they often reduce neuronal apoptosis through very similar mechanisms of action, with similar or even the same targets. For example, berberine, nuciferine, and tetrahydropalmatine, mentioned above, can increase SOD, GSH, and CAT in vivo to reduce oxidative damage. Tetrandrine and morphine can reduce the release of the apoptotic factor caspase-3. Both tetrahydropalmatine and tetrandrine can act on the same family of vascular endothelial growth factors (VEGF) to promote angiogenesis and neuronal regeneration. In the further analysis of the structural characteristics and pharmacological activities of these alkaloids, we found that both the five isoquinoline alkaloids we discussed in detail, and the dozens of isoquinoline alkaloids we listed in Table 1, have the following feature: the isoquinoline nucleus itself contains a benzene ring, most of these drugs are connected to multiple ether bonds on the benzene ring (most of them have three to four), and these ether bonds have the potential to form hydroxyl groups. These structures themselves directly contain a small number of hydroxyl groups, which means that their polarity is relatively low, which is conducive to their passage through the blood–brain barrier. If they pass through the blood–brain barrier, multiple ether bonds are converted into hydroxyl groups connected to the benzene ring, then these aromatic hydroxyl groups are very effective antioxidant structures. Based on this, we can even speculate that isoquinoline alkaloids may play a neuroprotective role mainly by exerting antioxidant effects combined with anti-inflammation, autophagy regulation, and inhibition of calcium overload. Of course, this conjecture has serious limitations, and more experimental verification is needed in order to provide a sufficient scientific foundation.
Figure 6.
Neuroprotective mechanism of isoquinoline alkaloids.
The neuroprotective isoquinoline alkaloids and their neuroprotective mechanisms are not limited to those mentioned above. These active effects are the fundamental pathological mechanisms shared by a variety of neurodegenerative diseases, indicating that isoquinoline alkaloids may not only be effective for a single neurodegenerative disease but also have therapeutic effects on a variety of neurodegenerative diseases. At the same time, we also found that they have strong similarities in the mechanism of action, signaling pathways, and target sites, and they share the same structural nucleus. Therefore, we have the following interesting thoughts: (1) The very similar mechanisms and targets of these alkaloids may be the basis for their combined use through different mechanisms to exert neuroprotective effects and synergistic effects in the treatment of neurodegenerative diseases. (2) The way these alkaloids exert neuroprotective effects is often multitarget and multi-channel. Whether or not their properties are better than the current clinical use of single-effect neuroprotective drugs, they may have greater potential for development as neuroprotective drugs. (3) They have the same isoquinoline core structure, which may help to study their structure–activity relationship. Perhaps we can extend the investigation to other components or derivatives with similar structures by studying the structure–activity relationship of one or several drugs.
However, a limiting factor for further research on isoquinoline alkaloids is the lack of basic research on the active components of these alkaloids. Although research on these alkaloids has gradually entered the field of vision of researchers, due to the many and complex mechanisms of these components, basic research on them still lacks systematic and comprehensive study. This undoubtedly brings difficulties for further research and clinical studies on isoquinoline alkaloids. Therefore, we believe that the study of these compounds should pay attention to their similarities and structural features. At the same time, we also believe that strengthening the study of these compounds is helpful in applying the advantages of multitarget and multi-pathway neuroprotective effects to the treatment of neurodegenerative diseases as soon as possible.
Finally, we also hope that this review will enable more researchers to focus on the neuroprotective effects of isoquinoline alkaloids and provide some ideas for conducting more in-depth research in this field.
Author Contributions
Conceptualization, Q.L. and J.L.; methodology, J.L.; software, Y.W. (Yarong Wu); validation, Y.Y., B.X. and Y.W. (Yuhao Wu); formal analysis, S.D.; investigation, Y.W. (Yarong Wu); resources, Q.L.; data curation, S.D. and Y.Y.; writing—original draft preparation, J.L.; writing—review and editing, Y.Y., B.X. and Y.W. (Yuhao Wu); visualization, B.X. and Y.W. (Yuhao Wu); supervision, Q.L. and J.L.; project administration, Q.L. and J.L.; funding acquisition, Q.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This paper is supported by the Key Laboratory of Neuropsychiatric Drug Research of Zhejiang Province (2019E10021); the Medical and Health Science and Technology Project of Zhejiang Province (2021KY136); the Natural Science Foundation of Zhejiang province (LY21H280010); and the Zhejiang Province Health High-level Talent Training Project (WJW2022007).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bondy S.C. Anthropogenic pollutants may increase the incidence of neurodegenerative disease in an aging population. Toxicology. 2016;341–343:41–46. doi: 10.1016/j.tox.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Takanashi M., Hattori N. Neurodegenerative diseases. Nihon. Rinsho. 2012;70:94–98. [PubMed] [Google Scholar]
- 3.Jurcau A., Simion A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021;23:14. doi: 10.3390/ijms23010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boese A.C., Hamblin M.H., Lee J.P. Neural stem cell therapy for neurovascular injury in Alzheimer’s disease. Exp. Neurol. 2020;324:113112. doi: 10.1016/j.expneurol.2019.113112. [DOI] [PubMed] [Google Scholar]
- 5.Latif S., Jahangeer M., Maknoon Razia D., Ashiq M., Ghaffar A., Akram M., El Allam A., Bouyahya A., Garipova L., Shariati M.A., et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta. 2021;522:114–126. doi: 10.1016/j.cca.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Kins S., Schäfer K.H., Endres K. Drug development for neurodegenerative diseases. Biol. Chem. 2021;403:1. doi: 10.1515/hsz-2021-0413. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q., Su C.P., Zhang H.M., Ren Y.L., Wang W., Guo S.Z. Anti-inflammatory mechanism of heat-clearing and detoxifying Chinese herbs. Zhongguo Zhong Yao Za Zhi. 2018;43:3787–3794. doi: 10.1016/j.jacc.2018.08.101. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Wang L., Lou G., Zeng H.-R., Hu J., Huang Q., Peng W., Yang X.-B. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 2019;57:193–225. doi: 10.1080/13880209.2019.1577466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi Y., Ni S., Heng X., Qu S., Ge P., Zhao X., Yao Z., Guo R., Yang N., Zhang Q., et al. Uncovering the Potential Mechanisms of Coptis chinensis Franch. for Serious Mental Illness by Network Pharmacology and Pharmacology-Based Analysis. Drug Des. Dev. Ther. 2022;16:325–342. doi: 10.2147/DDDT.S342028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Qi D., Gao Y., Liang C., Zhang Y., Ma Z., Liu Y., Peng H., Zhang Y., Qin H., et al. History of uses, phytochemistry, pharmacological activities, quality control and toxicity of the root of Stephania tetrandra S. Moore: A review. J. Ethnopharmacol. 2020;260:112995. doi: 10.1016/j.jep.2020.112995. [DOI] [PubMed] [Google Scholar]
- 11.Wąsik A., Antkiewicz-Michaluk L. The mechanism of neuroprotective action of natural compounds. Pharmacol. Rep. 2017;69:851–860. doi: 10.1016/j.pharep.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H., Han L., Shi W., Fang X., Hong Y., Cao Y. Research Advances in Lotus Leaf as Chinese Dietary Herbal Medicine. Am. J. Chin. Med. 2022;50:1423–1445. doi: 10.1142/S0192415X22500616. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y., Lu J., Sun L., Lyu X., Chang X.Y., Mi X., Hu M.G., Wu C., Chen X. Akkermansia muciniphila: A potential novel mechanism of nuciferine to improve hyperlipidemia. Biomed. Pharmacother. 2021;133:111014. doi: 10.1016/j.biopha.2020.111014. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Wu X., Mi Y., Zhang B., Gu S., Liu G., Li X. PLGA nanoparticles for the oral delivery of nuciferine: Preparation, physicochemical characterization and in vitro/in vivo studies. Drug Deliv. 2017;24:443–451. doi: 10.1080/10717544.2016.1261381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C., Duan F., Xie Y., Wan Q., Liu H., Gong J., Huang L., Song Z. Nuciferine attenuates acute ischemic stroke in a rat model: A metabolomic approach for the mechanistic study. Mol. Omics. 2022;18:765–778. doi: 10.1039/D2MO00158F. [DOI] [PubMed] [Google Scholar]
- 16.Sun R., Kong B., Yang N., Cao B., Feng D., Yu X., Ge C., Feng S., Fei F., Huang J., et al. The Hypoglycemic Effect of Berberine and Berberrubine Involves Modulation of Intestinal Farnesoid X Receptor Signaling Pathway and Inhibition of Hepatic Gluconeogenesis. Drug Metab. Dispos. 2021;49:276–286. doi: 10.1124/dmd.120.000215. [DOI] [PubMed] [Google Scholar]
- 17.Imenshahidi M., Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res. 2019;33:504–523. doi: 10.1002/ptr.6252. [DOI] [PubMed] [Google Scholar]
- 18.Wang S., Xu Z., Cai B., Chen Q. Berberine as a Potential Multi-Target Agent for Metabolic Diseases: A Review of Investigations for Berberine. Endocr. Metab. Immune Disord.-Drug Targets. 2021;21:971–979. doi: 10.2174/1871530320666200910105612. [DOI] [PubMed] [Google Scholar]
- 19.Baska A., Leis K., Gałązka P. Berberine in the Treatment of Diabetes Mellitus: A Review. Endocr. Metab. Immune Disord.-Drug Targets. 2021;21:1379–1386. doi: 10.2174/1568026620666201022144405. [DOI] [PubMed] [Google Scholar]
- 20.Finkbeiner S. The Autophagy Lysosomal Pathway and Neurodegeneration. Cold Spring Harb. Perspect. Biol. 2020;12:a033993. doi: 10.1101/cshperspect.a033993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habtemariam S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res. 2020;155:104722. doi: 10.1016/j.phrs.2020.104722. [DOI] [PubMed] [Google Scholar]
- 22.Shang W., Zhang J., Song H., Zhu S., Zhang A., Hua Y., Han S., Fu Y. Mechanism of Tetrandrine Against Endometrial Cancer Based on Network Pharmacology. Drug Des. Dev. Ther. 2021;15:2907–2919. doi: 10.2147/DDDT.S307670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen J.Y., Zhang J., Chen S., Chen Y., Zhang Y., Ma Z.Y., Zhang F., Xie W.M., Fan Y.F., Duan J.S., et al. Endothelium-derived hydrogen sulfide acts as a hyperpolarizing factor and exerts neuroprotective effects via activation of large-conductance Ca2+-activated K+ channels. Br. J. Pharmacol. 2021;178:4155–4175. doi: 10.1111/bph.15607. [DOI] [PubMed] [Google Scholar]
- 24.Wang H., Chen L., Wang S., Tian X., Zhang L., Li H., Li C., Xue Y., Wang Q., Fang L., et al. Tetrandrine promotes angiogenesis via transcriptional regulation of VEGF-A. Vascul. Pharmacol. 2021;141:106920. doi: 10.1016/j.vph.2021.106920. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Shi M., Liu L., Wang J., Zhu M., Chen H. Tetrandrine Inhibits Skeletal Muscle Differentiation by Blocking Autophagic Flux. Int. J. Mol. Sci. 2022;23:8148. doi: 10.3390/ijms23158148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J., Yu P., Dai F., Jiang H., Ma Z. Tetrandrine reduces oxidative stress, apoptosis, and extracellular matrix degradation and improves intervertebral disc degeneration by inducing autophagy. Bioengineered. 2022;13:3944–3957. doi: 10.1080/21655979.2022.2031396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhaleh H., Azadbakht M., Bidmeshki Pour A. Low concentrations of morphine enhanced the neuroglia-like differentiation. Bratisl. Lek. Listy. 2020;121:271–277. doi: 10.4149/BLL_2020_041. [DOI] [PubMed] [Google Scholar]
- 28.Hussain G., Rasul A., Anwar H., Aziz N., Razzaq A., Wei W., Ali M., Li J., Li X. Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018;14:341–357. doi: 10.7150/ijbs.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magdy S., Gamal M., Samir N.F., Rashed L. IκB kinase inhibition remodeled connexins, pannexin-1, and excitatory amino-acid transporters expressions to promote neuroprotection of galantamine and morphine. J. Cell Physiol. 2021;236:7516–7532. doi: 10.1002/jcp.30387. [DOI] [PubMed] [Google Scholar]
- 30.Amini K., Zhaleh H., Tahvilian R., Farnia V. Low concentration of morphine protects against cell death, oxidative stress and calcium accumulation by nicotine in PC12 cells. Bratisl. Lek. Listy. 2019;120:256–262. doi: 10.4149/BLL_2019_042. [DOI] [PubMed] [Google Scholar]
- 31.Wang B., Su C.J., Liu T.T., Zhou Y., Feng Y., Huang Y., Liu X., Wang Z.H., Chen L.H., Luo W.F., et al. The Neuroprotection of Low-Dose Morphine in Cellular and Animal Models of Parkinson’s Disease Through Ameliorating Endoplasmic Reticulum (ER) Stress and Activating Autophagy. Front. Mol. Neurosci. 2018;11:120. doi: 10.3389/fnmol.2018.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X.Y., Li J.F., Li T.Z., Pan C.X., Xue F.S., Wang G.Y. Morphine pretreatment protects against cerebral ischemic injury via a cPKCγ-mediated anti-apoptosis pathway. Exp. Ther. Med. 2021;22:1016. doi: 10.3892/etm.2021.10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luzzati M., Coviello C., De Veye H.S., Dudink J., Lammertink F., Dani C., Koopmans C., Benders M., Tataranno M.L. Morphine exposure and neurodevelopmental outcome in infants born extremely preterm. Dev. Med. Child Neurol. 2023 doi: 10.1111/dmcn.15510. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Xie W., Xie W., Kang Z., Jiang C., Liu N. Hydromorphone protects CA1 neurons by activating mTOR pathway. Neurosci. Lett. 2018;687:49–54. doi: 10.1016/j.neulet.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S., Iudicello J.E., Shi C., Letendre S., Knight A., Li J., Riggs P.K., Franklin DR J.r., Duarte N., Jin H., et al. Absence of neurocognitive impairment in a large Chinese sample of HCV-infected injection drug users receiving methadone treatment. Drug Alcohol Depend. 2014;137:29–35. doi: 10.1016/j.drugalcdep.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z.Y., Zhao W.R., Shi W.T., Xiao Y., Ma Z.L., Xue J.G., Zhang L.Q., Ye Q., Chen X.L., Tang J.Y. Endothelial-Dependent and Independent Vascular Relaxation Effect of Tetrahydropalmatine on Rat Aorta. Front. Pharmacol. 2019;10:336. doi: 10.3389/fphar.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Q., Meng X., Wang S. A Comprehensive Review on the Chemical Properties, Plant Sources, Pharmacological Activities, Pharmacokinetic and Toxicological Characteristics of Tetrahydropalmatine. Front. Pharmacol. 2022;13:890078. doi: 10.3389/fphar.2022.890078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C.L., Huang Q.L., Zhu Q., He J., Chen J., Zhang F., Cao Z.Y. Alkaloids from Corydalis decumbens modulate neuronal excitability. Bioorg. Chem. 2020;99:103795. doi: 10.1016/j.bioorg.2020.103795. [DOI] [PubMed] [Google Scholar]
- 39.Liu L., Liu M., Zhao W., Zhao Y.L., Wang Y. Levo-tetrahydropalmatine: A new potential medication for methamphetamine addiction and neurotoxicity. Exp. Neurol. 2021;344:113809. doi: 10.1016/j.expneurol.2021.113809. [DOI] [PubMed] [Google Scholar]
- 40.Cahlíková L., Vrabec R., Pidaný F., Peřinová R., Maafi N., Mamun A.A., Ritomská A., Wijaya V., Blunden G. Recent Progress on Biological Activity of Amaryllidaceae and Further Isoquinoline Alkaloids in Connection with Alzheimer’s Disease. Molecules. 2021;26:5240. doi: 10.3390/molecules26175240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saglam E., Zırh S., Aktas C.C., Muftuoglu S.F., Bilginer B. Papaverine provides neuroprotection by suppressing neuroinflammation and apoptosis in the traumatic brain injury via RAGE- NF-B pathway. J. Neuroimmunol. 2021;352:577476. doi: 10.1016/j.jneuroim.2021.577476. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y.Y., Park J.S., Leem Y.H., Park J.E., Kim D.Y., Choi Y.H., Park E.M., Kang J.L., Kim H.S. The phosphodiesterase 10 inhibitor papaverine exerts anti-inflammatory and neuroprotective effects via the PKA signaling pathway in neuroinflammation and Parkinson’s disease mouse models. J. Neuroinflammation. 2019;16:246. doi: 10.1186/s12974-019-1649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leem Y.H., Park J.S., Park J.E., Kim D.Y., Kim H.S. Papaverine Exerts Neuroprotective Effect by Inhibiting NLRP3 Inflammasome Activation in an MPTP-Induced Microglial Priming Mouse Model Challenged with LPS. Biomol. Ther. 2021;29:295–302. doi: 10.4062/biomolther.2021.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luhach K., Kulkarni G.T., Singh V.P., Sharma B. Attenuation of neurobehavioural abnormalities by papaverine in prenatal valproic acid rat model of ASD. Eur. J. Pharmacol. 2021;890:173663. doi: 10.1016/j.ejphar.2020.173663. [DOI] [PubMed] [Google Scholar]
- 45.Guan S., Liu Q., Gu H., Zhang Y.Y., Wei P.L., Qi Y.F., Liu J., Wang Z. Pluripotent anti-inflammatory immunomodulatory effects of papaverine against cerebral ischemic-reperfusion injury. J. Pharmacol. Sci. 2020;144:69–75. doi: 10.1016/j.jphs.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Leem Y.H., Park J.S., Park J.E., Kim D.Y., Kang J.L., Kim H.S. Papaverine inhibits α-synuclein aggregation by modulating neuroinflammation and matrix metalloproteinase-3 expression in the subacute MPTP/P mouse model of Parkinson’s disease. Biomed. Pharmacother. 2020;130:110576. doi: 10.1016/j.biopha.2020.110576. [DOI] [PubMed] [Google Scholar]
- 47.Yang X., Du W., Zhang Y., Wang H., He M. Neuroprotective Effects of Higenamine Against the Alzheimer’s Disease Via Amelioration of Cognitive Impairment, Aβ Burden, Apoptosis and Regulation of Akt/GSK3β Signaling Pathway. Dose Response. 2020;18:1559325820972205. doi: 10.1177/1559325820972205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu M.P., Zhang Y.S., Zhou Q.M., Xiong J., Dong Y.R., Yan C. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT signaling pathway. Pharmacol. Res. 2016;104:115–123. doi: 10.1016/j.phrs.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S., Chu S., Ai Q., Zhang Z., Gao Y., Lin M., Liu Y., Hu Y., Li X., Peng Y., et al. Anti-inflammatory effects of higenamine (Hig) on LPS-activated mouse microglia (BV2) through NF-κB and Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2020;85:106629. doi: 10.1016/j.intimp.2020.106629. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y., Wang H., Li L., Li X., Wang Q., Ding H., Wang X., Ye Z., Wu L., Zhang X., et al. Sinomenine Provides Neuroprotection in Model of Traumatic Brain Injury via the Nrf2-ARE Pathway. Front. Neurosci. 2016;10:580. doi: 10.3389/fnins.2016.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Q., Zhou W., Wang Y., Kou F., Lyu C., Wei H. Metabolic mechanism and anti-inflammation effects of sinomenine and its major metabolites N-demethylsinomenine and sinomenine-N-oxide. Life Sci. 2020;261:118433. doi: 10.1016/j.lfs.2020.118433. [DOI] [PubMed] [Google Scholar]
- 52.Bao X., He Y., Huang L., Li H., Li Q., Huang Y. Sinomenine exerts a neuroprotective effect on PD mouse model through inhibiting PI3K/AKT/mTOR pathway to enhance autophagy. Int. J. Neurosci. 2022:1–9. doi: 10.1080/00207454.2022.2100780. [DOI] [PubMed] [Google Scholar]
- 53.Gao B., Wu Y., Yang Y.J., Li W.Z., Dong K., Zhou J., Yin Y.Y., Huang D.K., Wu W.N. Sinomenine exerts anticonvulsant profile and neuroprotective activity in pentylenetetrazole kindled rats: Involvement of inhibition of NLRP1 inflammasome. J. Neuroinflammation. 2018;15:152. doi: 10.1186/s12974-018-1199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Z., Qiu N., Ou Y., Wei Q., Tang W., Zheng M., Xing Y., Li J.J., Ling Y., Li J., et al. N-Demethylsinomenine, an active metabolite of sinomenine, attenuates chronic neuropathic and inflammatory pain in mice. Sci. Rep. 2021;11:9300. doi: 10.1038/s41598-021-88521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramazi S., Fahanik-Babaei J., Mohamadi-Zarch S.M., Tashakori-Miyanroudi M., Nourabadi D., Nazari-Serenjeh M., Roghani M. Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: Involvement of oxidative stress, inflammation and pyroptosis. J. Chem. Neuroanat. 2020;108:101800. doi: 10.1016/j.jchemneu.2020.101800. [DOI] [PubMed] [Google Scholar]
- 56.Singh D., Agrawal A., Singal C.M.S., Pandey H.S., Seth P., Sharma S.K. Sinomenine inhibits amyloid beta-induced astrocyte activation and protects neurons against indirect toxicity. Mol. Brain. 2020;13:30. doi: 10.1186/s13041-020-00569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai W.D., Wang S., You W.T., Chen S.J., Wen J.J., Yuan C.R., Zheng M.J., Jin Y., Yu J., Wen C.P. Sinomenine regulates immune cell subsets: Potential neuro-immune intervene for precise treatment of chronic pain. Front. Cell Dev. Biol. 2022;10:1041006. doi: 10.3389/fcell.2022.1041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu W.N., Wu P.F., Chen X.L., Zhang Z., Gu J., Yang Y.J., Xiong Q.J., Ni L., Wang F., Chen J.G. Sinomenine protects against ischaemic brain injury: Involvement of co-inhibition of acid-sensing ion channel 1a and L-type calcium channels. Br. J. Pharmacol. 2011;164:1445–1459. doi: 10.1111/j.1476-5381.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q., Dai P., Bao H., Liang P., Wang W., Xing A., Sun J. Anti-inflammatory and neuroprotective effects of sanguinarine following cerebral ischemia in rats. Exp. Ther. Med. 2017;13:263–268. doi: 10.3892/etm.2016.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S.Y., Jin M.L., Kim Y.H., Kim C.M., Lee S.J., Park G. Involvement of heme oxygenase-1 in neuroprotection by sanguinarine against glutamate-triggered apoptosis in HT22 neuronal cells. Environ. Toxicol. Pharmacol. 2014;38:701–710. doi: 10.1016/j.etap.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 61.Yu C., Li P., Wang Y.X., Zhang K.G., Zheng Z.C., Liang L.S. Sanguinarine Attenuates Neuropathic Pain by Inhibiting P38 MAPK Activated Neuroinflammation in Rat Model. Drug Des. Devel. Ther. 2020;14:4725–4733. doi: 10.2147/DDDT.S276424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li P., Wang Y.X., Yang G., Zheng Z.C., Yu C. Sanguinarine Attenuates Neuropathic Pain in a Rat Model of Chronic Constriction Injury. Biomed. Res. Int. 2021;2021:3689829. doi: 10.1155/2021/3689829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin T.Y., Hung C.Y., Chiu K.M., Lee M.Y., Lu C.W., Wang S.J. Neferine, an Alkaloid from Lotus Seed Embryos, Exerts Antiseizure and Neuroprotective Effects in a Kainic Acid-Induced Seizure Model in Rats. Int. J. Mol. Sci. 2022;23:4130. doi: 10.3390/ijms23084130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sengking J., Oka C., Yawoot N., Tocharus J., Chaichompoo W., Suksamrarn A., Tocharus C. Protective Effect of Neferine in Permanent Cerebral Ischemic Rats via Anti-Oxidative and Anti-Apoptotic Mechanisms. Neurotox. Res. 2022;40:1348–1359. doi: 10.1007/s12640-022-00568-6. [DOI] [PubMed] [Google Scholar]
- 65.Baskaran R., Poornima P., Huang C.Y., Padma V.V. Neferine prevents NF-κB translocation and protects muscle cells from oxidative stress and apoptosis induced by hypoxia. Biofactors. 2016;42:407–417. doi: 10.1002/biof.1286. [DOI] [PubMed] [Google Scholar]
- 66.Chen J., Tang M., Liu M., Jiang Y., Liu B., Liu S. Neferine and lianzixin extracts have protective effects on undifferentiated caffeine-damaged PC12 cells. BMC Complement. Med. Ther. 2020;20:76. doi: 10.1186/s12906-020-2872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sengking J., Oka C., Wicha P., Yawoot N., Tocharus J., Chaichompoo W., Suksamrarn A., Tocharus C. Neferine Protects Against Brain Damage in Permanent Cerebral Ischemic Rat Associated with Autophagy Suppression and AMPK/mTOR Regulation. Mol. Neurobiol. 2021;58:6304–6315. doi: 10.1007/s12035-021-02554-z. [DOI] [PubMed] [Google Scholar]
- 68.Wong V.K., Wu A.G., Wang J.R., Liu L., Law B.Y. Neferine attenuates the protein level and toxicity of mutant huntingtin in PC-12 cells via induction of autophagy. Molecules. 2015;20:3496–3514. doi: 10.3390/molecules20033496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong Y., He S., Huang K., Liang M. Neferine suppresses vascular endothelial inflammation by inhibiting the NF-κB signaling pathway. Arch. Biochem. Biophys. 2020;696:108595. doi: 10.1016/j.abb.2020.108595. [DOI] [PubMed] [Google Scholar]
- 70.Wu C., Chen J., Yang R., Duan F., Li S., Chen X. Mitochondrial protective effect of neferine through the modulation of nuclear factor erythroid 2-related factor 2 signalling in ischaemic stroke. Br. J. Pharmacol. 2019;176:400–415. doi: 10.1111/bph.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hao T., Yang Y., Li N., Mi Y., Zhang G., Song J., Liang Y., Xiao J., Zhou D., He D. Inflammatory mechanism of cerebral ischemia-reperfusion injury with treatment of stepharine in rats. Phytomedicine. 2020;79:153353. doi: 10.1016/j.phymed.2020.153353. [DOI] [PubMed] [Google Scholar]
- 72.Xiao J., Wang Y., Yang Y., Liu J., Chen G., Lin B., Hou Y., Li N. Natural potential neuroinflammatory inhibitors from Stephania epigaea H.S. Lo. Bioorganic Chem. 2020;107:104597. doi: 10.1016/j.bioorg.2020.104597. [DOI] [PubMed] [Google Scholar]
- 73.Al-Amin M.Y., Lahiry A., Ferdous R., Hasan M.K., Kader M.A., Alam A.K., Saud Z.A., Sadik M.G. Stephania japonica Ameliorates Scopolamine-Induced Memory Impairment in Mice through Inhibition of Acetylcholinesterase and Oxidative Stress. Adv. Pharmacol. Pharm. Sci. 2022;2022:8305271. doi: 10.1155/2022/8305271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L., Pu Z., Li M., Wang K., Deng L., Chen W. Antioxidative and antiapoptosis: Neuroprotective effects of dauricine in Alzheimer’s disease models. Life Sci. 2020;243:117237. doi: 10.1016/j.lfs.2019.117237. [DOI] [PubMed] [Google Scholar]
- 75.Pu Z., Ma S., Wang L., Shang L., Luo Y., Chen W. Amyloid-beta Degradation and Neuroprotection of Dauricine Mediated by Unfolded Protein Response in a Caenorhabditis elegans Model of Alzheimer’s disease. Neuroscience. 2018;392:25–37. doi: 10.1016/j.neuroscience.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 76.Wang K., Wang L., Chen L., Peng C., Luo B., Mo J., Chen W. Intranasal administration of dauricine loaded on graphene oxide: Multi-target therapy for Alzheimer’s disease. Drug Deliv. 2021;28:580–593. doi: 10.1080/10717544.2021.1895909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li M., Liu G., Wang K., Wang L., Fu X., Lim L.Y., Chen W., Mo J. Metal ion-responsive nanocarrier derived from phosphonated calix [4]arenes for delivering dauricine specifically to sites of brain injury in a mouse model of intracerebral hemorrhage. J. Nanobiotechnol. 2020;18:61. doi: 10.1186/s12951-020-00616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen C., Liu P., Wang J., Yu H., Zhang Z., Liu J., Chen X., Zhu F., Yang X. Dauricine Attenuates Spatial Memory Impairment and Alzheimer-Like Pathologies by Enhancing Mitochondrial Function in a Mouse Model of Alzheimer’s Disease. Front. Cell Dev. Biol. 2021;8:624339. doi: 10.3389/fcell.2020.624339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Omoruyi S.I., Ibrakaw A.S., Ekpo O.E., Boatwright J.S., Cupido C.N., Hussein A.A. Neuroprotective Activities of Crossyne flava Bulbs and Amaryllidaceae Alkaloids: Implications for Parkinson’s Disease. Molecules. 2021;26:3990. doi: 10.3390/molecules26133990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cortes N., Castañeda C., Osorio E.H., Cardona-Gomez G.P., Osorio E. Amaryllidaceae alkaloids as agents with protective effects against oxidative neural cell injury. Life Sci. 2018;203:54–65. doi: 10.1016/j.lfs.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 81.Hua S., Liu J., Zhang Y., Li J., Zhang X., Dong L., Zhao Y., Fu X. Piperine as a neuroprotective functional component in rats with cerebral ischemic injury. Food Sci. Nutr. 2019;7:3443–3451. doi: 10.1002/fsn3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adefegha S.A., Oboh G., Okeke B.M. Comparative effects of berberine and piperine on the neuroprotective potential of neostigmine. J. Complement. Integr. Med. 2021;18:491–497. doi: 10.1515/jcim-2020-0055. [DOI] [PubMed] [Google Scholar]
- 83.Kumar P., Singh S., Jamwal S. Neuroprotective potential of quercetin in combination with piperine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Neural Regen. Res. 2017;12:1137–1144. doi: 10.4103/1673-5374.211194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaushik P., Ali M., Salman M., Tabassum H., Parvez S. Harnessing the mitochondrial integrity for neuroprotection: Therapeutic role of piperine against experimental ischemic stroke. Neurochem. Int. 2021;149:105138. doi: 10.1016/j.neuint.2021.105138. [DOI] [PubMed] [Google Scholar]
- 85.Hsieh T.Y., Chang Y., Wang S.J. Piperine Provides Neuroprotection against Kainic Acid-Induced Neurotoxicity via Maintaining NGF Signalling Pathway. Molecules. 2022;27:2638. doi: 10.3390/molecules27092638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh S., Kumar P. Neuroprotective potential of curcumin in combination with piperine against 6-hydroxy dopamine induced motor deficit and neurochemical alterations in rats. Inflammopharmacology. 2017;25:69–79. doi: 10.1007/s10787-016-0297-9. [DOI] [PubMed] [Google Scholar]
- 87.Salman M., Tabassum H., Parvez S. Piperine mitigates behavioral impairments and provides neuroprotection against 3-nitropropinoic acid-induced Huntington disease-like symptoms. Nutr. Neurosci. 2022;25:100–109. doi: 10.1080/1028415X.2020.1721645. [DOI] [PubMed] [Google Scholar]
- 88.Duan W., Chen X. Jatrorrhizine can improve nerve cell injury induced by Aβ 25-35, acting through miR-223-3p/HDAC4 axis. Am. J. Transl. Res. 2021;13:4644–4655. [PMC free article] [PubMed] [Google Scholar]
- 89.Luo T., Shen X.Y., Li S., Ouyang T., Mai Q.A., Wang H.Q. The Protective Effect of Jatrorrhizine Against Oxidative Stress in Primary Rat Cortical Neurons. CNS Neurol. Disord. Drug Targets. 2017;16:617–623. doi: 10.2174/1871527315666160711101210. [DOI] [PubMed] [Google Scholar]
- 90.Wu G., Mu T., Zhang L., Chen X. Jatrorrhizine Hydrochloride alleviates tert-butyl hydroperoxide-induced endothelial cell injury through its anti-inflammatory activity and PPAR-γ activation. Cell Mol. Biol. 2020;66:125–129. doi: 10.14715/cmb/2020.66.2.20. [DOI] [PubMed] [Google Scholar]
- 91.Ramírez Hernández E., Alanis Olvera B., Carmona González D., Guerrero Marín O., Pantoja Mercado D., Valencia Gil L., Hernández-Zimbrón L.F., Sánchez Salgado J.L., Limón I.D., Zenteno E. Neuroinflammation and galectins: A key relationship in neurodegenerative diseases. Glycoconj. J. 2022;39:685–699. doi: 10.1007/s10719-022-10064-w. [DOI] [PubMed] [Google Scholar]
- 92.Yang C., Hawkins K.E., Doré S., Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019;316:C135–C153. doi: 10.1152/ajpcell.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muthuraju S., Zakaria R., Karuppan M.K.M., Al-Rahbi B. The Role of Neuroinflammation in Cellular Damage in Neurodegenerative Diseases. Biomed. Res. Int. 2020;2020:9231452. doi: 10.1155/2020/9231452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J., Guo M., Ma R., Wu M., Zhang Y. Tetrandrine alleviates cerebral ischemia/reperfusion injury by suppressing NLRP3 inflammasome activation via Sirt-1. PeerJ. 2020;8:e9042. doi: 10.7717/peerj.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu H., He S., Li C., Wang J., Zou Q., Liao Y., Chen R. Tetrandrine alleviates inflammation and neuron apoptosis in experimental traumatic brain injury by regulating the IRE1α/JNK/CHOP signal pathway. Brain Behav. 2022;12:e2786. doi: 10.1002/brb3.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ren D., Fu Y., Wang L., Liu J., Zhong X., Yuan J., Jiang C., Wang H., Li Z. Tetrandrine ameliorated Alzheimer’s disease through suppressing microglial inflammatory activation and neurotoxicity in the 5XFAD mouse. Phytomedicine. 2021;90:153627. doi: 10.1016/j.phymed.2021.153627. [DOI] [PubMed] [Google Scholar]
- 97.Rezaee R., Monemi A., SadeghiBonjar M.A., Hashemzaei M. Berberine Alleviates Paclitaxel-Induced Neuropathy. J. Pharmacopunct. 2019;22:90–94. doi: 10.3831/KPI.2019.22.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shang X.F., Yang C.J., Morris-Natschke S.L., Li J.C., Yin X.D., Liu Y.Q., Guo X., Peng J.W., Goto M., Zhang J.Y. Biologically active isoquinoline alkaloids covering 2014–2018. Med. Res. Rev. 2020;40:2212–2289. doi: 10.1002/med.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kong Y.R., Tay K.C., Su Y.X., Wong C.K., Tan W.N., Khaw K.Y. Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. Molecules. 2021;26:728. doi: 10.3390/molecules26030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aski M.L., Rezvani M.E., Khaksari M., Hafizi Z., Pirmoradi Z., Niknazar S., Mehrjerdi F.Z. Neuroprotective effect of berberine chloride on cognitive impairment and hippocampal damage in experimental model of vascular dementia. Iran. J. Basic Med. Sci. 2018;21:53–58. doi: 10.22038/IJBMS.2017.23195.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song C., Cao J., Lei Y., Chi H., Kong P., Chen G., Yu T., Li J., Kumar P.R., Xia J. Nuciferine prevents bone loss by disrupting multinucleated osteoclast formation and promoting type H vessel formation. FASEB J. 2020;34:4798–4811. doi: 10.1096/fj.201902551R. [DOI] [PubMed] [Google Scholar]
- 102.Breijyeh Z., Karaman R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules. 2020;25:5789. doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Di Liberto V., Mudò G. Role of Bioactive Molecules on Neuroprotection, Oxidative Stress, and Neuroinflammation Modulation. Int. J. Mol. Sci. 2022;23:15925. doi: 10.3390/ijms232415925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ibarrola J., Lu Q., Zennaro M.C., Jaffe I.Z. Mechanism by Which Inflammation and Oxidative Stress Induce Mineralocorticoid Receptor Gene Expression in Aging Vascular Smooth Muscle Cells. Hypertension. 2023;80:111–124. doi: 10.1161/HYPERTENSIONAHA.122.19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shou J.W., Cheung C.K., Gao J., Shi W.W., Shaw P.C. Berberine Protects C17.2 Neural Stem Cells From Oxidative Damage Followed by Inducing Neuronal Differentiation. Front. Cell Neurosci. 2019;13:395. doi: 10.3389/fncel.2019.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun K., Luo Z.L., Hu C., Gong T.L., Tang G.H., Wu S.P. Protective effect and immune mechanism of berberine on cerebral ischemia/reperfusion injury in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2020;36:656–661. doi: 10.12047/j.cjap.6001.2020.137. [DOI] [PubMed] [Google Scholar]
- 107.Almalki D.A., Alghamdi S.A. Hepatorenal protective effects of some plant extracts on experimental diabetes in male rats. Int. J. Pharmacol. 2019;15:238–247. [Google Scholar]
- 108.Khan S., Khan H.U., Khan F.A., Shah A., Wadood A., Ahmad S., Almehmadi M., Alsaiari A.A., Shah F.U., Kamran N. Anti-Alzheimer and Antioxidant Effects of Nelumbo nucifera L. Alkaloids, Nuciferine and Norcoclaurine in Alloxan-Induced Diabetic Albino Rats. Pharmaceuticals. 2022;15:1205. doi: 10.3390/ph15101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qu Z., Zhang J., Yang H., Huo L., Gao J., Chen H., Gao W. Protective effect of tetrahydropalmatine against d-galactose induced memory impairment in rat. Physiol. Behav. 2016;154:114–125. doi: 10.1016/j.physbeh.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y., Levine B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015;22:367–376. doi: 10.1038/cdd.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buratta S., Tancini B., Sagini K., Delo F., Chiaradia E., Urbanelli L., Emiliani C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020;21:2576. doi: 10.3390/ijms21072576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Denton D., Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26:605–616. doi: 10.1038/s41418-018-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu H., He S., Wang J., Li C., Liao Y., Zou Q., Chen R. Tetrandrine Ameliorates Traumatic Brain Injury by Regulating Autophagy to Reduce Ferroptosis. Neurochem. Res. 2022;47:1574–1587. doi: 10.1007/s11064-022-03553-9. [DOI] [PubMed] [Google Scholar]
- 114.Wen H., Zhang H., Wang W., Li Y. Tetrahydropalmatine protects against acute lung injury induced by limb ischemia/reperfusion through restoring PI3K/AKT/mTOR-mediated autophagy in rats. Pulm. Pharmacol. Ther. 2020;64:101947. doi: 10.1016/j.pupt.2020.101947. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y., Xue X., Zhang H., Che X., Luo J., Wang P., Xu J., Xing Z., Yuan L., Liu Y., et al. Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia. Autophagy. 2019;15:493–509. doi: 10.1080/15548627.2018.1531196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Du X., Di Malta C., Fang Z., Shen T., Niu X., Chen M., Jin B., Yu H., Lei L., Gao W., et al. Nuciferine protects against high-fat diet-induced hepatic steatosis and insulin resistance via activating TFEB-mediated autophagy-lysosomal pathway. Acta Pharm. Sin B. 2022;12:2869–2886. doi: 10.1016/j.apsb.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paez P.M., Lyons D.A. Calcium Signaling in the Oligodendrocyte Lineage: Regulators and Consequences. Annu. Rev. Neurosci. 2020;43:163–186. doi: 10.1146/annurev-neuro-100719-093305. [DOI] [PubMed] [Google Scholar]
- 118.Calvo-Rodriguez M., Hernando-Pérez E., López-Vázquez S., Núñez J., Villalobos C., Núñez L. Remodeling of Intracellular Ca2+ Homeostasis in Rat Hippocampal Neurons Aged In Vitro. Int. J. Mol. Sci. 2020;21:1549. doi: 10.3390/ijms21041549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu D.D., Hoeven R., Rong R., Cho W.C. Rhynchophylline Protects Cultured Rat Neurons against Methamphetamine Cytotoxicity. Evid.-Based Complement. Altern. Med. 2012;2012:636091. doi: 10.1155/2012/636091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang H., Liu C., Mei X., Cao Y., Guo Z., Yuan Y., Zhao Z., Song C., Guo Y., Shen Z. Berberine attenuated pro-inflammatory factors and protect against neuronal damage via triggering oligodendrocyte autophagy in spinal cord injury. Oncotarget. 2017;8:98312–98321. doi: 10.18632/oncotarget.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu S.B., Pekkurnaz G. Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis. J. Mol. Biol. 2018;430:3922–3941. doi: 10.1016/j.jmb.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao X.Y., Lu M.H., Yuan D.J., Xu D.E., Yao P.P., Ji W.L., Chen H., Liu W.L., Yan C.X., Xia Y.Y., et al. Mitochondrial Dysfunction in Neural Injury. Front. Neurosci. 2019;13:30. doi: 10.3389/fnins.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li W., Yang C., Lu J., Huang P., Barnstable C.J., Zhang C., Zhang S.S. Tetrandrine protects mouse retinal ganglion cells from ischemic injury. Drug Des. Devel. Ther. 2014;8:327–339. doi: 10.2147/DDDT.S55407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kasala S., Briyal S., Prazad P., Ranjan A.K., Stefanov G., Donovan R., Gulati A. Exposure to Morphine and Caffeine Induces Apoptosis and Mitochondrial Dysfunction in a Neonatal Rat Brain. Front. Pediatr. 2020;8:593. doi: 10.3389/fped.2020.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Biczo G., Vegh E.T., Shalbueva N., Mareninova O.A., Elperin J., Lotshaw E., Gretler S., Lugea A., Malla S.R., Dawson D., et al. Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models. Gastroenterology. 2018;154:689–703. doi: 10.1053/j.gastro.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang L., Sheng W., Tan Z., Ren Q., Wang R., Stoika R., Liu X., Liu K., Shang X., Jin M. Treatment of Parkinson’s disease in Zebrafish model with a berberine derivative capable of crossing blood brain barrier, targeting mitochondria, and convenient for bioimaging experiments. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2021;249:109151. doi: 10.1016/j.cbpc.2021.109151. [DOI] [PubMed] [Google Scholar]
- 127.Wang T., Fang X., Yin Z.S. Endothelial progenitor cell-conditioned medium promotes angiogenesis and is neuroprotective after spinal cord injury. Neural Regen. Res. 2018;13:887–895. doi: 10.4103/1673-5374.232484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cui H., Yang X., Wang Z., Li G., Li L., Huo S., Zhang B., He R., Chen K., Xu B., et al. Tetrahydropalmatine triggers angiogenesis via regulation of arginine biosynthesis. Pharmacol. Res. 2021;163:105242. doi: 10.1016/j.phrs.2020.105242. [DOI] [PubMed] [Google Scholar]
- 129.Hao H.F., Liu L.M., Pan C.S., Wang C.S., Gao Y.S., Fan J.Y., Han J.Y. Rhynchophylline Ameliorates Endothelial Dysfunction via Src-PI3K/Akt-eNOS Cascade in the Cultured Intrarenal Arteries of Spontaneous Hypertensive Rats. Front. Physiol. 2017;8:928. doi: 10.3389/fphys.2017.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Plazas E., Avila M.M.C., Muñoz D.R., Cuca S L.E. Natural isoquinoline alkaloids: Pharmacological features and multi-target potential for complex diseases. Pharmacol. Res. 2022;177:106126. doi: 10.1016/j.phrs.2022.106126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.