Abstract
Background
Outpatients treated with oral anti-cancer drugs, including selective cyclin-dependent kinase 4/6 inhibitors (CDK4/6i), may benefit from a pharmacy practice setting adapted to support proper oral anti-cancer drug monitoring. This real-world study aimed to characterize patient-centered pharmacy practice aligned with American Society of Clinical Oncology (ASCO)/National Community Oncology Dispensing Association (NCODA) standards and to describe its impact on CDK4/6i treatment use.
Methods
This retrospective study included women with confirmed hormone receptor-positive/human epidermal growth factor 2 negative locally advanced or metastatic breast cancer treated with either palbociclib, abemaciclib or ribociclib combined with letrozole or fulvestrant. Pharmacists collected patient characteristics, clinical activities, and treatment patterns using data from the pharmacy chart. CDK4/6i treatment adherence rates were estimated based on medication claims data. Time-to-treatment discontinuation, a proxy for time-to-event, was assessed using the Kaplan-Meier estimate.
Results
Of the 195 patients assessed for eligibility, 65 were included in this study. The median observation duration was 13.6 months. An average of seven pharmaceutical care activities (range 2.8–21.7) per patient was documented for each treatment cycle. The mean proportion of days covered was 89.6%. The median time-to-treatment discontinuation was estimated at 44.2 months in patients treated with CDK4/6i + letrozole and 17.0 months in patients treated with CDK4/6i + fulvestrant. The average relative dose intensity was 85%, and the benefits of treatment were maintained regardless of the relative dose intensity levels.
Conclusion
A structured patient-centered pharmacy practice model integrating the ASCO/NCODA patient-centered standards and ongoing communication with patients and healthcare providers ensure timely refills, close monitoring, and allows patients to achieve high adherence and persistence rates comparable to those reported in clinical trials.
Keywords: Oncology pharmacy practice, breast cancer, CDK4/6 inhibitors, real-world evidence, quality improvement
Introduction
Breast cancer is the most common cancer and the second leading cause of cancer-related death in Canadian women, accounting for 25% of newly diagnosed cancers. While 5–10% of breast cancers are already metastatic at diagnosis, 20–30% of women with early stage breast cancer will eventually develop metastatic breast cancer (MBC).1,2 The majority of breast cancers (65–70%) are hormone receptor-positive (HR + ) and human epidermal growth factor receptor 2-negative (HER2−). Endocrine therapy, with or without a CDK4/6 inhibitor (CDK4/6i), is the mainstay for HR + /HER2− locally advanced inoperable or MBC, collectively referred to as advanced breast cancer (ABC). The addition of CDK4/6i to endocrine therapy has led to substantial progression-free survival (PFS) and overall survival (OS) in the first- and second-line ABC settings, making CDK4/6i therapy (palbociclib, ribociclib, or abemaciclib) plus standard endocrine therapy the treatment of choice in these contexts.3–8
The pharmacy delivery of oral anticancer drugs (OACDs), such as CDK4/6i, to outpatients represents a more convenient approach compared to intravenous chemotherapy, with only periodic visits to cancer care centers being necessary. However, patients must assume greater responsibility for their own care, which may lead to numerous challenges for clinicians including medication adherence, drug reimbursement, toxicity recognition and management, and suboptimal treatment outcomes.9,10
An independent pharmacy located in Montreal, Canada (Lariviere & Massicotte Pharmacy (thereafter referred to as LMP)), dedicated to the dispensing of specialty medicines for over 14 years, adapted its practice model to circumvent many of these challenges. The LMP structured and standardized its workflow to offer continuing support to patients and to ensure ongoing communication and collaboration with hospital teams as well as with community pharmacists that dispense patients’ general medication. These are in line with recent guidelines related to the dispensing and follow-up of patients treated with OACDs published collaboratively by the American Society of Clinical Oncology (ASCO) and the US National Community Oncology Dispensing Association (NCODA) (see Supplemental Table 1).11,12 In these patient-centered models, the practice aims to improve patient outcomes by minimizing administrative delays in patient treatment, promoting treatment adherence, preventing and managing adverse events, and empowering patients to become a partner in their own care. One can hypothesize that if the intensity of OACD follow-up in real-world practice relies on high practice standards, the resulting treatment outcomes should replicate those observed in clinical trials. 13
The objectives of this project were to characterize the practice processes at LMP for patients dispensed with CDK4/6i for the treatment of ABC, namely by measuring the frequency of pharmacists’ clinical activities based on ASCO/NCODA standards and analyzing the impact on patients’ use of CDK4/6i.
Methods
Study design and population
This is a retrospective pharmacy electronic chart review of patients with ABC who received an oral CDK4/6i, either in monotherapy or in combination with an aromatase inhibitor (e.g. letrozole or fulvestrant), at LMP between 16th March 2016 (approval of palbociclib in Canada), and 11 November 2020. At the time of protocol development, HR + /HER2− ABC was the only approved indication which reflected the clinical use of CDK4/6i. Women treated for HR + /HER2− ABC who initiated treatment with a CDK4/6i inhibitor with the first prescription filled at the LMP were eligible to participate in this study if they were 18 years or older and had not previously received a CDK4/6i. Patients had to have received at least three 28-day cycles of CDK4/6i and had not been dispensed with a CDK4/6i refill in another pharmacy during the study period. As suggested by a central IRB and the College of Pharmacy, the patients must have provided verbal consent for inclusion in the study.
Enrollment process
Potentially eligible patients were identified from the pharmacy database and divided into four equivalent subgroups, each of which was contacted by a different pharmacist by phone. A description of the research project was provided and consent was requested to use the information from the patient pharmacy records for research purposes. Consent was obtained between 11 November 2020 and 31 January 2021.
End points
The main objective was to describe the type and frequency of pharmacists’ clinical activities performed in our practice setting using the ASCO/NCODA pharmacy practice standards for benchmarking. Secondary objectives were to describe patients clinical outcomes such as treatment adherence rate as measured by the modified medication possession ratio (MPRm) and the proportion of days covered (PDC), the relative dose intensity (RDI) and the duration of treatment/time-to-treatment discontinuation (TTD). Finally, we also described time-to-treatment initiation (TTI). The underlying hypothesis that cannot be tested with this study design is that best practices in pharmacy will contribute to maximize treatment adherence, limit early discontinuation and ultimately improve treatment outcomes. 13
Data source and extraction
Once consent was provided, all study data were collected retrospectively from the available patient pharmacy records, including prescriptions, medical oncologist notes, and hospital pharmacy transfer plans to community pharmacies and pharmacy administrative files. Patient demographics, clinical and CDK4/6i information, and prescription dispensing histories were extracted and collected by 31 April 2021. The treatment pattern variables included dose modifications, dose interruptions, and the date of CDK4/6i discontinuation. All data were extracted by three experienced pharmacists who were trained on variable definitions and how to complete the electronic case report form (eCRF). The eCRF data were compiled and anonymized by the principal investigator prior to analysis.
Variable definitions and data analyses
LMP's structured approach was developed to allow fast, safe, and successful access to medication, as well as optimal patient outcomes. To appreciate the level of effort necessary to implement such processes, we evaluated the number of clinical activities required for dispensing CDK4/6i, including the number of laboratory test results verification, and the number of communications with patients, community pharmacists, other healthcare professionals and payer. Clinical activities and communications were only carried out by pharmacists. Administrative activities (e.g. follow-up with insurances to obtain prior authorization) could be done by either pharmacist or technicians. OACD, including CDK4/6i, require coordination between pharmacists, hospital medical teams, payers, and patient support groups offering financial assistance groups, which may delay patient receipt of the drug. TTI, defined as the time elapsed from prescription date to the treatment's start date, was used to reflect delays needed before patients could start their treatment. Time-to-treatment reimbursement by payer and time from reimbursement to treatment initiation was also measured to better understand how TTI was distributed. These analyses were mostly descriptive and no formal statistical hypotheses were tested.
Adherence measures were calculated and reported over the entire treatment period, ranging from treatment initiation to treatment discontinuation or data extraction, if the treatment was still ongoing. Prescription refill history was obtained using an electronic claims database system. Patients were considered to have continuous insurance eligibility. The PDC was calculated to account for early refills, which could lead to an overestimation of adherence using the MPRm method. These variables are defined as follows:
To evaluate the potential impact of dose modifications on outcomes, we calculated the RDI, which considers both drug interruptions and dose reduction over time. For the RDI calculation, the standard dose was set according to approved labels: palbociclib in combination with letrozole or fulvestrant (125 mg once daily for 21 days out of 28), abemaciclib in combination with either letrozole or fulvestrant (150 mg twice daily), and abemaciclib monotherapy (200 mg twice daily).
Statistical analyses were performed based on the estimated frequency distribution of data. The normality of the data was evaluated using the Kolmogorov–Smirnov test and QQ plot for visual evaluation of normal distribution for proper selection of the statistical test. The difference between the number of clinical activities during the first three cycles and the following did not follow a normal distribution. Therefore, a non-parametric paired Wilcoxon test was used to assess differences. The TTI and time-to-reimbursement variables were normally distributed. Student's t-tests were performed for these analyses. Duration of treatment/TTD measures, defined as the time elapsed from the medication's starting date to the treatment's discontinuation date, or death, were calculated using Kaplan-Meier estimates. Thus, TTD is a proxy for time to disease progression, death from any cause, or adverse events leading to CDK4/6i discontinuation. Patients who were still receiving CDK4/6i at the time of data collection were right-censored. Finally, TTD was stratified according to RDI levels (<85% and ≥85%) to obtain a better understanding of the link between treatment modifications and treatment persistence. Statistical and descriptive analyses were performed using GraphPad Prism version 5.0, with a significance level of 0.05.
Results
Patient baseline characteristics
A total of 195 adult patients had a prescription for CDK4/6i during the study period and were assessed for eligibility. Most patients were excluded because they passed away (n = 56), did not start treatment (n = 21), received only part of their treatment at the pharmacy (n = 19), had prior use of CDK4/6i (n = 8), or took <3 cycles of treatment (n = 4). As such, 87 patients were contacted, and 65 patients provided consent for data collection and were included in the study. Of these, 51 patients (78%) were treated with palbociclib, 14 (22%) with abemaciclib, and none received ribociclib.
The demographic and patient characteristics are described in Table 1. The median (range) age was 66 (39–94) years. Most patients were postmenopausal (94%) at the time of CDK4/6i initiation and had MBC (90%). CDK4/6i were generally combined with either letrozole (68%) or fulvestrant (29%), whereas two patients received abemaciclib as monotherapy. No CDK4/6i were combined with other AIs (exemestane or anastrozole) or tamoxifen in our practice setting. Most patients received CDK4/6i in the first-line setting (57%), and 20 patients (31%) received CDK4/6i as second-line treatment or beyond. The line of treatment was not available for eight patients (12%).
Table 1.
Patient and disease baseline characteristics at the time of CDK4/6 inhibitor treatment initiation.
| Characteristic | Patients (n = 65) |
|---|---|
| Age | |
| Median: year (range) | 66 (39–94) |
| <65 years: no. (%) | 30 (46) |
| ≥65 years: no. (%) | 35 (54) |
| Menopausal status a : no. (%) | |
| Pre- or perimenopause | 4 (6) |
| Post-menopause | 61 (94) |
| CDK4/6 inhibitor used: no. (%) | |
| Palbociclib | 51(78) |
| Abemaciclib | 14 (22) |
| Ribociclib | 0 (0) |
| Concurrent hormonal therapy: no. (%) | |
| Letrozole | 44 (68) |
| Fulvestrant | 19 (29) |
| None | 2 (3) |
| Line of treatment in ABC, MBC setting a : no. (%) | |
| First | 37 (57) |
| Second or more | 20 (31) |
| Unknown | 8 (12) |
| Tumor involvement a : no. (%) | |
| Metastatic | 59 (90) |
| Locally advanced | 3 (5) |
| Unknown | 3 (5) |
Abbreviations: ABC: advanced breast cancer; MBC: metastatic breast cancer; CDK4/6: cyclin-dependent kinase 4 and 6.
Menopausal status, line of treatment, and tumor involvement were either indicated on prescription, on patient support program enrollment form, self-reported, or inferred from pharmacologic profile.
At the time of data extraction, CDK4/6i treatment was still ongoing in 43 patients (66%), including 30 patients in the CDK4/6i + letrozole group, 11 patients in the CDK4/6i + fulvestrant group, and two patients on CDK4/6i monotherapy.
Clinical and administrative activities
Regarding the primary objective, the number of clinical interventions and activities required to dispense CDK4/6i during the observation period were evaluated and are presented in Table 2. There was an average of seven activities (range 2.8–21.7) per 28-day cycle. Communication with patients and laboratory result verification accounted for 70% of these activities, with an average of 3.1 (range 1.4–8) and 1.9 (range 0.8–4.2) activities per cycle, respectively. These activities were more frequent in the first three cycles, with an average of 11 per cycle (range 4.7–26.7), with a statistically significant difference (p < 0.0001) compared to cycles 4 and beyond.
Table 2.
Clinical and administrative activities related to patients treated with CDK4/6 inhibitors.
| Activity | Total | Cycles 1–3 | Cycles ≥ 4 | Cycles 1–3 vs Cycles ≥ 4 |
|---|---|---|---|---|
| Mean/cycle (range) | Mean/cycle (range) | Mean/cycle (range) | P value | |
| Total activities | 7.1 (2.8–21.7) | 11 (4.7–26.7) | 5.2 (1.5–17) | <0.0001 |
| Communications with patientsa | 3.1 (1.4–8) | 4.5 (2–10) | 2.4 (1–9) | <0.0001 |
| Verification of laboratory test results b | 1.9 (0.8–4.2) | 2.4 (1–5.7) | 1.6 (0.5–4.5) | <0.0001 |
| Communications with community pharmacists c | 0.3 (0–1.3) | 0.7 (0–3.3) | 0.2 (0–1) | <0.0001 |
| Communications with other healthcare professionalsc,d | 0.6 (0–3.7) | 1.1 (0–5.3) | 0.3 (0–2) | <0.0001 |
| Follow-up with payers e | 1.2 (0–8) | 2.2 (0.3–13) | 0.7 (0–2.5) | <0.0001 |
Activities with patients included any kind of communication for the corresponding period, including medication counselling, follow-ups with patients to assess or manage efficacy, toxicities, adherence to treatment, and calls from patients.
Laboratory test results verification refers to the number of times a pharmacist checked and documented laboratory test results for the corresponding period.
Communications with a healthcare professional included any form of direct or indirect communication (e.g. fax, email, phone call).
Other healthcare professional refers to any healthcare professional other than community pharmacist (e.g. medical oncologist, nurse, hospital pharmacist).
Follow-ups with insurance included any communication done in order to verify whether a CDK4/6 inhibitor was covered or not, and any communication with patient support programs to verify and coordinate financial assistance, if applicable.
Time-to-treatment initiation
The TTI and time-to-reimbursement were evaluated and are presented in Table 3. A mean TTI of 18.5 days (range 1–73) was observed in patients treated with CDK4/6i. A statistically significant difference is noted in the delays before initiating treatment advantaging public insurance compared to private insurance (12.2 vs 29.3 days; p = 0.0061).
Table 3.
Time-to-treatment initiation and reimbursement for CDK4/6 inhibitors.
| Total | Public payer | Private payer | Public vs Private | |
|---|---|---|---|---|
| Characteristic | (n = 41) a | (n = 26) | (n = 15) | P value |
| Time-to-treatment initiation b , days | ||||
| Median (IQR) | 14.0 (8–20) | 13.5 (8–16.25) | 21.0 (9–55) | 0.0061 |
| Mean (range) | 18.5 (1–73) | 12.2 (1–23) | 29.3 (3–73) | |
| Time-to-reimbursement c , days | ||||
| Median (IQR) | 10.0 (4–15) | 9.0 (3.8–13) | 16.0 (4–51) | 0.0016 |
| Mean (range) | 14.5 (0–70) | 8.3 (0–21) | 25.1 (1–70) | |
| Time from reimbursement to initiation, days | ||||
| Median (IQR) | 3.0 (2–5) | 3.0 (2–6) | 4.0 (2–5) | 0.9493 |
| Mean (range) | 4.0 (0–12) | 3.9 (0–11) | 4.1 (1–12) |
Abbreviations: IQR: interquartile range.
24 patients initiated their treatment through a compassionate use program and were thus excluded for these analyses.
Time-to-treatment initiation was defined as the period between the date of the prescription and the start of the treatment.
Time-to-reimbursement was calculated from the date of the prescription until the drug was approved for reimbursement.
Treatment adherence and clinical outcomes
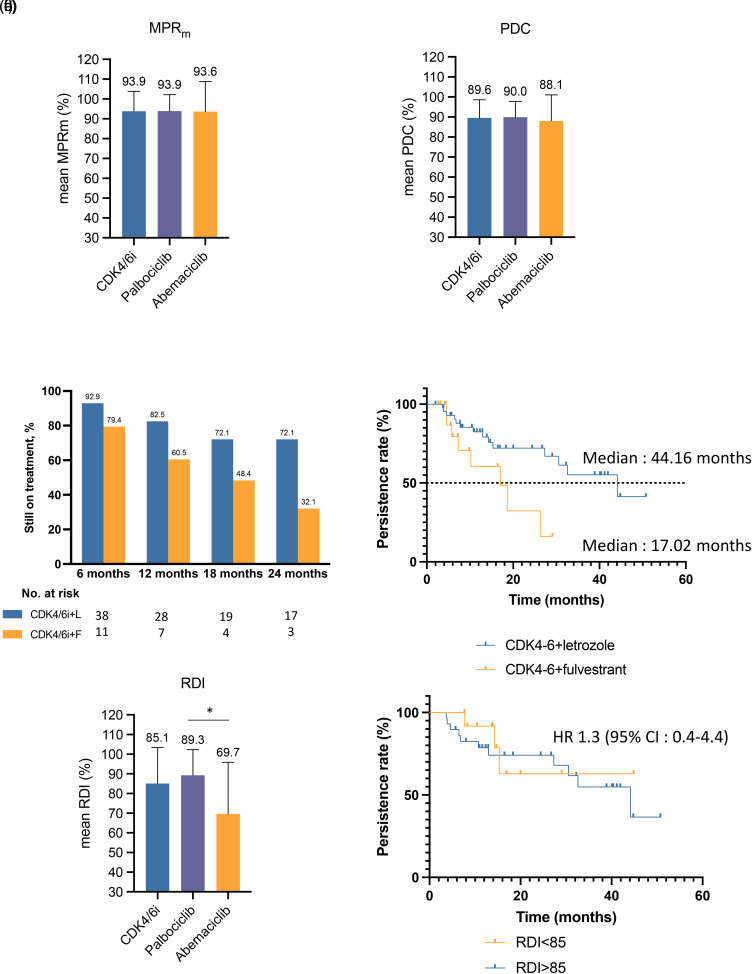
Throughout the study period, patients received an average of 17 cycles of CDK4/6i (ranging from 3–52 cycles). The average MPRm and PDC values were 93.9% and 89.6%, respectively (Figure 1(a) and (b)). Notably, adherence measures were similar for palbociclib- and abemaciclib-treated patients.
Figure 1.
Adherence and duration of treatment. (a) Mean modified medication possession ratio (MPRm) and (b) mean proportion of days covered (PDC) are shown in patients treated with either CDK4/6i, palbociclib, and abemaciclib combined with letrozole or fulvestrant. (c) Landmark persistence rates are shown in patients treated with either CDK4/6i combined with letrozole or fulvestrant. Rates were estimated using Kaplan-Meier analyses. (d) Persistence rates as assessed by the investigators; the median time-to-treatment discontinuation was 44.16 months among 44 patients in the CDK4/6i + letrozole group and 17.02 months among 19 patients in the CDK4/6 + fulvestrant group. (e) Relative dose intensity is shown for patients treated with either CDK4/6i, palbociclib, and abemaciclib *p = 0.023. (f) Persistence rates of patients stratified according to their RDI level. Patients achieving and RDI of 85% or higher had similar time-to-treatment discontinuation than patients have an RDI below 85% (HR: 1.3 [95% CI :0.4–4.4]).
Abbreviations: MPRm: modified medication possession ratio; PDC: proportion of days covered; CDK4/6i: cyclin-dependent kinase 4 and 6 inhibitors; L: letrozole; F: fulvestrant; RDI: relative dose intensity; HR: hazard ratio; CI: confidence interval.
The treatment interruptions and dose reductions are shown in Table 4. After a mean (range) follow-up duration of 17.3 months (range 3.2–50.8), 41 patients (63%) had at least one treatment interruption, and the majority of them were in the first three cycles of treatment. The treatment interruption rates were similar for palbociclib (63%) and abemaciclib (64%). In the first three cycles of treatment, 16% of patients treated with palbociclib had at least one dose reduction compared to 43% of patients treated with abemaciclib. During the study period, 33% and 71% of patients had a dose reduction with palbociclib and abemaciclib, respectively.
Table 4.
Treatment modifications of CDK4/6 inhibitors.
| Treatment modification | CDK4/6i (n = 65) | Palbociclib (n = 51) | Abemaciclib (n = 14) |
|---|---|---|---|
| Interruptions a : no. (%) | |||
| Cycles 1–3 | 32 (49) | 24 (47) | 8 (57) |
| Total | 41 (63) | 32 (63) | 9 (64) |
| Reductions: no. (%) | |||
| Cycles 1–3 | 14 (22) | 8 (16) | 6 (43) |
| Total | 27 (42) | 17 (33) | 10 (71) |
| Duration of follow-up b | |||
| median (IQR), months | 13.6 (7.0–26.6) | 13.9 (6.4–29.0) | 12.4 (7.3–16.5) |
| mean (range), months | 17.3 (3.2–50.8) | 18.5 (3.2–50.8) | 13.2 (3.8–40.3) |
Abbreviations : IQR: interquartile range.
Interruptions were defined as delays >24 h of the planned start date of a new treatment cycle.
Duration of follow-up was defined as the time between the treatment initiation date and the treatment discontinuation date or date of last contact, whichever came first.
At the time of data collection, CDK4/6i treatment was discontinued in 22 patients (34%): 14 patients in the CDK4/6i + letrozole group and 8 patients in the CDK4/6i + fulvestrant group. In the CDK4/6i + letrozole subgroup, the 6-month, 12-month, and 24-month persistence rates were 92.9%, 82.5%, and 72.1%, respectively. Patients receiving CDK4/6i + fulvestrant had 6-month, 12-month, and 24-month persistence rates of 79.4%, 60.5%, and 32.1%, respectively (Figure 1(c)). The median TTD was estimated at 44.2 months among patients treated with CDK4/6i + letrozole and 17 months among patients treated with CDK4/6i + fulvestrant (Figure 1(d)).
The mean RDI was 89.3% for patients treated with palbociclib and 69.7% for patients treated with abemaciclib, with an average RDI of 85.1% for both CDK4/6i combined (Figure 1(e)). The TTD was described in relation to the RDI level in patients treated with CDK4/6i + letrozole (Figure 1(f)). No statistically significant differences in TTD were observed between patients with RDI <85% and those with RDI ≥85% (Figure 1(f)). This analysis was not performed for the CDK4/6i + fulvestrant subgroup because the sample size was insufficient.
Discussion
In this study, we analyzed the impact of a patient-centered practice model with a proactive and systematic follow-up structure aligned with ASCO/NCODA standards in a CDK4/6i population treated for ABC in a community pharmacy dedicated to specialized treatment, including OACDs. 11 This model translated into an average of seven clinical and administrative activities for each 28-day CDK4/6i treatment cycle. While the first three cycles required more intensive and statistically different involvement from our team, the following cycles still required an important effort (11.0 vs 5.2; p < 0.0001). Direct communication with patients and verification of laboratory test results were the most common activities performed (70%). Direct patient communications include systematic initial teaching, 7-day follow-up call after the treatment initiation, and monthly adherence call are done systematically for every patient. Other communications included follow-ups related to adherence, adverse events, adjustment of antiemetic medication, planning of next refill and/or laboratory test recommendations (Supplemental Figure 1). The number of communications reflects the high needs of patients treated with CDK4/6i as they have numerous questions, preoccupations, side effects, and reimbursement issues that need attention to allow safe and effective treatment continuity. To our knowledge, this is the first study to comprehensively show the clinical and administrative activity requirements for implementing ASCO/NCODA standards.
Delaying the initiation of treatment for ABC can negatively impact the treatment outcomes and is a major source of anxiety and distress for patients.14–18 Apart from patients that started their CDK4/6i through a compassionate use program, our study shows that a mean TTI of 18.5 days (or median of 14 days) was needed. In a Canadian study, the median time to start first-line treatment with a CDK4/6i after the metastatic diagnosis was 1.4 months. 19 In a more general setting, other studies showed that the median number of days from OACD prescription to patient receipt of the drug was 7–12 days, showing that obtaining OACD, such as CDK4/6i, is complex and prone to unwanted delays.20–24 In our study, this delay was mostly accounted for by the time for payers to approve reimbursements (mean 14.5 days, median 10 days). Comparatively to regular medications which are usually covered from the start without an expiry date and for which a single call to payers would be unexpected, CDK4/6i require prior authorizations and follow-ups with insurance to validate that drug coverage is granted. To minimize delays before starting treatment and to limit undue drug interruptions throughout the treatment, communications and follow-ups with payers were frequent in our practice setting, particularly in the first few cycles. Without such efforts, a longer TTI and a lower drug adherence could be expected. Payers handle prescriptions differently, which could account for variability for time to insurance approval, ranging from 1 to 70 days. Even if we could not verify whether the prior authorization form was sent to public/private payers at the same time the prescription was written, this delay appears to be longer than expected as other studies have showed that PA for an OACD could be obtained as quickly as 1 day.24,25 Processing time appears to be treatment- and localization-dependent. Still, earlier intervention and new health policies are needed to reduce processing time by payers to allow rapid CDK4/6i coverage. Nonetheless, once reimbursement was granted, patients were able to initiate treatment within four calendar days on average.
These clinical activities demonstrate that patients taking OACDs, especially CDK4/6i, have high healthcare needs, including patient education, drug interaction and side effect management, drug coverage, and follow-ups. These activities are allowing proper quality of care and safe administration of OACDs in the outpatient setting and follow the ASCO/NCODA standards. 11 To implement such standards and accompany patients throughout their treatment, the community pharmacies need to adapt their ratio of pharmacists to patients. Structured and systematic clinical approaches could translate into better adherence rates and clinical outcomes, including higher adherence rates (e.g. MPRm, PDC, RDI) and persistence rates (e.g. TTD).
A significant number of patients struggle to adhere to OACDs treatment as prescribed. 26 Poor adherence to OACDs can impede treatment efficacy and decrease response rates.23,26 A study showed that patients filling their oncolytic prescriptions at a specialized pharmacy in the USA had a 15% higher adherence rate and a lower prescription abandonment rate compared to traditional pharmacies. 27 In the present study, we showed that patients followed at a pharmacy dedicated to OACDs reached a high level of adherence with an average PDC and MPR of over 90% throughout the study period, which is higher than a previously published study reporting a 6-month PDC of 81% in patients on CDK4/6i in combination with hormonal therapy dispensed in a specialized pharmacy. 28
Most dose modifications have been implemented to manage toxicities. During the study period, an average of 63% of patients required at least one dose interruption, which is in the range of 52–83% previously reported in CDK4/6i clinical trials.3,4,29 The percentage of dose reduction of 33% that we observed among patients treated with palbociclib was similar to the dose reduction rates reported in PALOMA-2 (36%) and PALOMA-3 (34%).3,4 This frequency also appears to be somewhat comparable to other real-world evidence (RWE) studies of patients treated with palbociclib that reported dose reduction ranging between 14.3% and 57%.30,31 On the other hand, the percentage of patients on abemaciclib that required at least one dose reduction was higher in this study (71%) than what was previously reported in MONARCH-3 and -2 clinical trials (43–47%).5,6 This could be attributable to the small population of patients taking this molecule in our study.
The overall impact of dose modifications was evaluated using RDI, which considers both drug interruptions and dose reductions over time. Its relation with TTD was also assessed. The overall RDI of patients treated with CDK4/6i was 85%, which is similar to those reported in the corresponding highly structured clinical trials, which ranged between 85% and 87%.29,32 As expected, the higher dose reduction rate in abemaciclib-treated patients led to a lower RDI than that in palbociclib-treated patients (68.7% vs 89.3%; p = 0.023). Assuming that patients continuing therapy benefit from it and those who discontinue have an unfavorable benefit-risk ratio, TTD is another interesting treatment outcome to evaluate. The median TTD of patients included in this study, either on palbociclib or abemaciclib plus letrozole as first-line treatment, was 44.2 months. These results supplant the reported duration of treatment in PALOMA-2 and MONARCH-3, which were of 19.8 and 15.3 months, respectively.3,32,33 For patients with a more advanced disease, the studies PALOMA-3 and MONARCH-2 showed a median PFS of 9.5 months and 16.2 months, respectively, for treatment with palbociclib or abemaciclib with fulvestrant.4,6 In PALOMA-3, the reported duration of treatment was 10.8 months. In the present study, the median TTD of women receiving either palbociclib or abemaciclib and fulvestrant as a second-line treatment was 17.02 months. The durations of treatment reported are also higher than those previously reported in clinical trials or other RWE studies. 34
Various factors could potentially explain, at least partly, these differences. Since the ethics required the consent of patients, a selection bias could have been introduced, as only alive patients could give their consent to participate in this study. The retrospective nature of the study, based on pharmacy electronic health records, might have introduced some missing data or under-reported characteristics. Finally, all study patients were receiving treatment at the LMP, and these patients may not be representative of the overall ABC population. Despite these limitations, our results suggest that successfully adapting our activities as per ASCO/NCODA standards to better meet specific OACD-related patient needs may translate into improved treatment outcomes.
Finally, patients treated with either CDK4/6i combined with letrozole and having an RDI <85% had a similar TTD compared to patients with an RDI ≥ 85%, thus supporting dose modifications as a proper means of managing toxicities without affecting treatment outcomes. This corroborates a previous study showing similar results with ribociclib, where RDI level was not associated with treatment efficacy. 35 To our knowledge, this is the first study showing how RDI level for patients treated with palbociclib or abemaclib and letrozole is related to TTD. Similar results could be expected in combination with fulvestrant in second-line settings, however this analysis could not be performed for the CDK4/6i + fulvestrant subgroup because the sample size was insufficient. Notably, studies have reported that the PFS benefit of abemaciclib and palbociclib in combination with either letrozole or fulvestrant was not affected by dose reductions.36–38
Conclusion
This RWE study used CDK4/6i as an archetype of the ever-growing OACD class to describe how patient adherence and clinical outcomes can be optimized through an evolved pharmacy workflow integrating ASCO/NCODA standards of practice. We recognize that implementing such a structured approach integrating clinical pharmacy practice standards and adapting workflow may represent a considerable level of effort, but it could contribute positively to patient adherence and persistence. Similar pharmaceutical care evaluations should be performed regularly, as they drive continuous improvement. Further studies are needed to demonstrate prospectively the impact of ASCO/NCODA standards of practice on patient outcomes.
Supplemental Material
Supplemental material, sj-pptx-1-opp-10.1177_10781552221102884 for Cyclin-dependent kinase 4/6 inhibitor treatment use in women treated for advanced breast cancer: Integrating ASCO/NCODA patient-centered standards in a community pharmacy by Alexandre Marineau, Catherine St-Pierre, Roxanne Lessard-Hurtubise, Marie-Ève David, Jean-Philippe Adam and Isabelle Chabot in Journal of Oncology Pharmacy Practice
Acknowledgments
The authors would like to thank Johanne Sylvain and Sarwat Tobia for their involvement in obtaining patient consent. They also thank Lucie Blais (Faculty of Pharmacy, University of Montreal) for her input into the study protocol and Michael Reff, Executive Director of NCODA, for his comments on the manuscript.
Footnotes
Author contributions: AM, CSP, JPA, and IC developed the study protocol, reviewed, and interpreted the results. AM conducted the analyses and wrote the manuscript draft. AM, CSP, JPA, and IC revised the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: A first draft of the protocol was supported by an unrestricted educational grant from Novartis. The finalization of the protocol, data collection, analysis and manuscript development were supported by an unrestricted educational grant by Pfizer.
ORCID iDs: Alexandre Marineau https://orcid.org/0000-0003-0163-8391
Jean-Philippe Adam https://orcid.org/0000-0002-7938-0944
Supplemental material: Supplemental material for this article is available online.
References
- 1.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005; 10:20−29. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers 2019; 5(1): 66. [DOI] [PubMed] [Google Scholar]
- 3.Finn RS, Martin M, Rugo HS. et al. : palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 4.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2–negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17: 425–4439. [DOI] [PubMed] [Google Scholar]
- 5.Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638–3646. [DOI] [PubMed] [Google Scholar]
- 6.Sledge GW, Jr., Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR + /HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875–2884. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 8.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018; 36: 2465−2472. [DOI] [PubMed] [Google Scholar]
- 9.Barillet M, Prevost V, Joly F, et al. Oral antineoplastic agents: how do we care about adherence? Br J Clin Pharmacol 2015; 80: 1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raborn ML, Pelletier EM, Smith DB, et al. Patient out-of-pocket payments for oral oncolytics: results from a 2009 US claims data analysis. J Oncol Pract 2012; 8: 9s–15s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillmon MS, Kennedy EB, Anderson MK, et al. Patient-Centered standards for medically integrated dispensing: ASCO/NCODA standards. J Clin Oncol 2020; 38: 633–644. [DOI] [PubMed] [Google Scholar]
- 12.Dillmon MS, Kennedy EB, Reff M. Patient-Centered medically integrated dispensing: ASCO/NCODA standards summary. JCO Oncol Pract 2020; 16: 344–347. [DOI] [PubMed] [Google Scholar]
- 13.Arthurs G, Simpson J, Brown A, et al. The effectiveness of therapeutic patient education on adherence to oral anti-cancer medicines in adult cancer patients in ambulatory care settings: a systematic review. JBI Database System Rev Implement Rep 2015; 13: 244–292. [DOI] [PubMed] [Google Scholar]
- 14.Konieczny M, Cipora E, Roczniak W, et al. Impact of time to initiation of treatment on the quality of life of women with breast cancer. Int J Environ Res Public Health 2020; 17(22): 8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open 2020; 3(12): e2030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khorana AA, Tullio K, Elson P, et al. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PLoS One 2019; 14(3): e0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung SY, Sereika SM, Linkov F, et al. The effect of delays in treatment for breast cancer metastasis on survival. Breast Cancer Res Treat 2011; 130: 953–964. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin JM, Anderson RT, Ferketich AK, et al. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol 2012; 30: 4493–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaro CP, Batra A, Lupichuk S. First-Line treatment with a cyclin-dependent kinase 4/6 inhibitor plus an aromatase inhibitor for metastatic breast cancer in Alberta. Curr Oncol 2021; 28: 2270–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtenstein MR, Beauchemin MP, Raghunathan RR, et al. Association between insurance plan, prior authorization, and time to receipt of oral anticancer drugs. J Clin Oncol 2021; 39: 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neil D, Accordino MK, Wright JD, et al. Delay in receipt of newly prescribed oral anticancer drugs. J Clin Oncol 2019; 37: 6541–6541. [Google Scholar]
- 22.Wang AA, Tapia C, Bhanji Y, et al. Barriers to receipt of novel oral oncolytics: a single-institution quality improvement investigation. J Oncol Pharm Pract 2020; 26: 279–285. [DOI] [PubMed] [Google Scholar]
- 23.Geynisman DM, Wickersham KE. Adherence to targeted oral anticancer medications. Discov Med 2013; 15: 231–241. [PMC free article] [PubMed] [Google Scholar]
- 24.Niccolai JL, Roman DL, Julius JM, et al. Potential obstacles in the acquisition of oral anticancer medications. J Oncol Pract 2017; 13: e29–e36. [DOI] [PubMed] [Google Scholar]
- 25.Geynisman DM, Meeker CR, Doyle JL, et al. Provider and patient burdens of obtaining oral anticancer medications. Am J Manag Care 2018; 24: e128–e133. [PMC free article] [PubMed] [Google Scholar]
- 26.Greer JA, Amoyal N, Nisotel L, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist 2016; 21: 354–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokes M, Reyes C, Xia Y, et al. Impact of pharmacy channel on adherence to oral oncolytics. BMC Health Serv Res 2017; 17(1): 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husinka L, Koerner PH, Miller RT, et al. Review of cyclin-dependent kinase 4/6 inhibitors in the treatment of advanced or metastatic breast cancer. J Drug Assess 2020; 10: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR + /HER2- advanced breast cancer. J Natl Cancer Inst 2019; 111: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mycock K, Zhan L, Taylor-Stokes G, et al. Real-World palbociclib use in HR + /HER2- advanced breast cancer in Canada: the IRIS study. Curr Oncol 2021; 28: 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkie J, Schickli MA, Berger MJ, et al. Progression-Free survival for real-world use of palbociclib in hormone receptor-positive metastatic breast cancer. Clin Breast Cancer 2020; 20: 33–40. [DOI] [PubMed] [Google Scholar]
- 32.Johnston S, Martin M, Di Leo A, et al. MONARCH 3 Final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019; 5(5):. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eli Lilly Canada Inc.: Product Monograph – Verzenio. https://pi.lilly.com/ca/verzenio-ca-pm.pdf. p.14 Published April 4, 2019. Updated January 10, 2022 Accessed March 1, 2022.
- 34.Harbeck N, Bartlett M, Spurden D, et al. CDK4/6 Inhibitors in HR + /HER2- advanced/metastatic breast cancer: a systematic literature review of real-world evidence studies. Future Oncol 2021; 17: 2107–2122. [DOI] [PubMed] [Google Scholar]
- 35.Burris HA, Chan A, Bardia A, et al. Safety and impact of dose reductions on efficacy in the randomised MONALEESA-2. − 3, and − 7 trials in hormone receptor-positive, HER2-negative advanced breast cancer. Br J Cancer 2021; 125: 679−686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rugo HS, Huober J, Garcia-Saenz JA, et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2–negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist 2021; 26: e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng J, Yu Y, Durairaj C, et al. Impact of dose reduction on efficacy: implications of exposure-response analysis of palbociclib. Target Oncol 2021; 16: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ismail RK, van Breeschoten J, Wouters M, et al. Palbociclib dose reductions and the effect on clinical outcomes in patients with advanced breast cancer. Breast 2021; 60: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pptx-1-opp-10.1177_10781552221102884 for Cyclin-dependent kinase 4/6 inhibitor treatment use in women treated for advanced breast cancer: Integrating ASCO/NCODA patient-centered standards in a community pharmacy by Alexandre Marineau, Catherine St-Pierre, Roxanne Lessard-Hurtubise, Marie-Ève David, Jean-Philippe Adam and Isabelle Chabot in Journal of Oncology Pharmacy Practice