Abstract
Introduction
PF-06439535 (bevacizumab-bvzr; Zirabev®) is a bevacizumab biosimilar. The stability profile and functional activity of PF-06439535 after dilution for intravenous infusion was evaluated following extended storage conditions.
Methods
PF-06439535 drug product was diluted in 0.9% sodium chloride to produce final concentrations of 1.4 and 16.5 mg/mL of PF-06439535, representing clinically relevant low and high doses for intravenous infusion. Three drug product lots and three infusion bag types (polyolefin, ethylene vinyl acetate, and polyvinyl chloride) were tested. To simulate the potential preparation and administration conditions encountered in a clinical setting, prepared drug solutions were initially stored at 25 ± 5°C for 24 ± 2 h, and then at 5 ± 3°C for up to 6 weeks. Extended storage was followed by storage at 25 ± 5°C for 24 ± 2 h before testing. Physicochemical and biological stability were evaluated according to visual characteristics and pH, protein concentration, particulate content, the proportions of molecular weight variants and charge variants, and relative potency. A wide range of analytical techniques optimized for PF-06439535 assessment were employed, such as size-exclusion chromatography, non-reducing sodium dodecyl sulfate capillary electrophoresis, cation-exchange chromatography, far-UV circular dichroism spectroscopy, differential scanning calorimetry, and an in vitro cell-based bioassay.
Results
For all concentrations, drug product lots, infusion bag types, and time points tested, there were no significant changes in protein concentration and no notable differences in visual characteristics (color, clarity, and visible particulates). The abundance of molecular weight variants and charge variants remained stable over the 6-week study period. There were no stability concerns with regard to sub-visible particles. There were no significant changes in primary, secondary, or tertiary structure. Finally, the in vitro relative potency of PF-06439535 was maintained throughout the study period.
Conclusions
The stability and biological activity of PF-06439535 was maintained after dilution and storage for up to 6 weeks at 2–8°C, demonstrating the integrity of diluted PF-06439535 under extended in-use conditions.
Keywords: Bevacizumab, biosimilar, extended in-use, stability
Introduction
Bevacizumab is a recombinant humanized monoclonal antibody that is approved in the United States and Europe as treatment for non-small cell lung cancer, metastatic colorectal cancer, metastatic renal cell cancer, and certain gynecological cancers.1–4 Additionally, bevacizumab is approved in the United States as treatment for glioblastoma multiforme and in Europe as treatment for metastatic breast cancer.1–4 Bevacizumab is directed against vascular endothelial growth factor1–4 and bevacizumab-based treatment regimens improve survival and response outcomes for patients with solid tumors.5,6
The availability of biosimilars may broaden access to biologic therapy and generate cost savings for healthcare systems. 7 The development of bevacizumab biosimilars has the potential to increase patient access to this treatment. Biosimilars are biologic drugs that are highly similar to a licensed biologic (i.e. originator or reference product [RP]), with no clinically meaningful differences in safety, purity, or potency from the RP.8–10 The US Food and Drug Administration (FDA), European Medicines Agency (EMA), and other regulatory agencies (e.g. the Pharmaceuticals and Medical Devices Agency of Japan, Health Canada, and the Therapeutic Goods Administration of Australia) require a rigorous analytical (structural and functional) assessment of the proposed biosimilar and the RP in order to support a determination of biosimilarity.8,9,11–13 US FDA and EMA regulatory pathways for biosimilar approval require head-to-head comparative assessments of the proposed biosimilar and the RP, including analytical, nonclinical, and clinical studies.8,9 These studies are performed in a step-wise manner, and regulatory approval is granted based on the totality of the evidence for biosimilarity that is accumulated over all stages of testing.8,9
PF-06439535 (bevacizumab-bvzr; Zirabev®; Pfizer Europe MA EIGG, Brussels, Belgium and Pfizer Inc, New York, NY,USA) is a bevacizumab biosimilar that was approved by the EMA and US FDA for use in all indications of the RP, except for in hepatocellular carcinoma (Avastin®; Genentech Inc, South San Francisco, CA, USA and Roche Registration GmbH, Grenzach-Wyhlen, Germany).3,4 Analytical and nonclinical toxicity studies demonstrated similarity of PF-06439535 to bevacizumab RPs sourced from the European Union (bevacizumab-EU) and from the US (bevacizumab-US).14–16 A clinical study, conducted in healthy volunteers, demonstrated similarity in pharmacokinetics, safety, and immunogenicity of PF-06439535 to bevacizumab-EU and bevacizumab-US. 17
Bevacizumab is given by intravenous (IV) infusion and must be diluted in 0.9% sodium chloride before administration. Once prepared, solutions of bevacizumab RP can be stored at 2–8°C for 30 days, and bevacizumab biosimilar PF-06439535 may be stored for 35 days plus an additional 48 h at 2–30°C, according to the EMA Summary of Product Characteristics.1,3 It is recommended that prepared dosing solutions are used immediately and “if not used immediately, in-use storage times and conditions are the responsibility of the user and would normally not be longer than 24 h at 2°C to 8°C, unless dilution has taken place in controlled and validated aseptic conditions”.1,3 However, in clinical practice, drug solutions are often prepared well in advance of their use to accommodate scheduling and logistics, and to provide flexibility. Therefore, product stability studies would be helpful to demonstrate that the physicochemical stability and activity of the biologic therapy is not compromised under extended in-use conditions.
The conditions under which extended in-use stability is evaluated are distinct from those used to assess shelf-life storage stability. In addition, the extended in-use stability of a biosimilar cannot be assumed to be identical to that of its RP as the biosimilar may use a different formulation, resulting in differences in stability from the RP. Furthermore, the physicochemical properties of a product would be better characterized using product-specific assays that have been specially selected and optimized by manufacturers during the course of a product's development, as compared with generic assays. Using a wide range of analytical techniques and an in vitro cell-based biological activity assay, all of which were optimized for bevacizumab assessment, we describe an extended in-use study that evaluated the stability profile and functional activity of PF-06439535 across multiple concentrations and time periods in infusion bags, representative of those encountered in a typical clinical settling.
Methods
Preparation
PF-06439535 drug product was injected into infusion bags containing 100 or 150 mL of 0.9% sodium chloride, using a sterile syringe, to produce final concentrations of 1.4 and 16.5 mg/mL of PF-06439535. Three drug product lots (Lot 1, Lot 2, and Lot 3) and three infusion bag types (polyolefin [PO], ethylene vinyl acetate [EVA], and polyvinyl chloride [PVC]) were tested. Thirty-six infusion bags (6 infusion bags per drug concentration per drug product lot) were prepared with PO infusion bags. Twenty-four additional infusion bags (6 infusion bags per drug concentration per infusion bag type) were prepared for drug product Lot 1 using EVA and PVC infusion bags. Additional PO infusion bags were prepared at 16.5 mg/mL to support the biophysical characterization and peptide mapping. Preparation of infusion bags was performed in a biosafety cabinet.
Study design
After infusion bag preparation, all dosing solutions were initially stored at 25 ± 5°C for 24 ± 2 h, and were then stored at 5 ± 3°C for 0, 1, 2, 4, or 6 weeks. At each time point, samples were removed from storage at 5 ± 3°C and maintained at 25 ± 5°C for an additional 24 ± 2 h before testing. Baseline samples (Week 0) were stored at 25 ± 5°C for a total of 48 h. Storage at room temperature before and after refrigerated storage simulated the time needed for drug preparation and administration in a clinical setting. At each time point, sample aliquots were removed from the infusion bag using a sterile syringe in a biosafety cabinet.
Visual characteristics and pH
The presence of visible particulates in solution was evaluated by visual inspection of samples (30 mL aliquot) against white and black panel backgrounds, with a light source of suitable intensity (2000–3750 lux). Sample clarity was determined by turbidimetry. Turbidity was measured using a HACH 2100AN turbidimeter, which was calibrated with formazin turbidity standards before use. Sample color was determined by visual examination. Samples were inspected against a white background and compared against a water reference and a brown color standard.
Samples were equilibrated to 22.5–25.4°C for at least 30 min and evaluated using a pH electrode and meter that was calibrated before use. Single pH measurements were taken for each sample.
Protein concentration
Protein concentration of PF-06439535 was determined using a SoloVPE variable path length instrument, with a Cary 50/60 UV spectrophotometer. Samples were equilibrated to room temperature and assayed neat. Each sample (100 μL) was read in triplicate using Quick Slope at 280 nm and scatter correction at 320 nm. Average protein concentration (mg/mL) for triplicate readings was reported.
Size-exclusion high-performance liquid chromatography (SE-HPLC)
Size-exclusion high-performance liquid chromatography (SE-HPLC) was used to assess the formation of high-molecular-mass species (HMMS) of PF-06439535. Samples were eluted isocratically (20 mM sodium phosphate, 400 mM sodium chloride, pH 6.0) on a Waters YMC-Pack Diol-200 column (8 × 300 mm) and UV detection was performed at a wavelength of 280 nm.
Non-reducing sodium dodecyl sulfate capillary gel electrophoresis (SDS-CGE)
Sodium dodecyl sulfate capillary gel electrophoresis (SDS-CGE) was used to assess intact antibody and antibody fragments. The assay was performed under non-reducing conditions, using the ProteomeLab IgG Purity/Heterogeneity Assay Kit or the ProteomeLab SDS-MW Analysis Kit. Electrophoretic separation was performed at 15 kV (normal polarity), and UV detection was carried out at 220 nm.
Peptide mapping
The 16.5 mg/mL samples were reduced using dithiothreitol. Samples were alkylated by adding iodoacetamide and incubation at room temperature for 30 min, and desalted using a P6 Bio-Gel® column. Digestion was performed by adding Lys-C/trypsin and incubating at 37°C for 60 min. Liquid chromatography–mass spectrometry (LC-MS) analysis was performed using a Thermo Orbitrap Fusion Lumos. Mass spectrometry analysis was performed using Biopharma Finder, v3.0.
Methionine oxidation in the Fc domain
A proteolytic mapping method was used to monitor the oxidized and non-oxidized forms of a methionine containing peptide fragment from the Fc domain of PF-06439535. Enzymatic digestion of PF-06439535 was performed using Lys-C. The resulting peptide fragments were separated by reversed-phase high-performance liquid chromatography using a Zorbax 300SB column (4.6 × 250 mm, 5-micron). Samples were injected onto the column, and the separated peptide fragments were detected using UV absorbance at 214 nm.
Cation-exchange chromatography
Cation-exchange chromatography (CEX) was used to separate protein variants based on charge. CEX separation was performed using a Dionex ProPac WCX-10 cation-exchange column (4 × 250 mm, 10-micron). UV detection was performed at 214 nm.
Sub-visible particle counts by light obscuration
Particle counting was performed by light obscuration using an HIAC/Royco Model 9703 or 9703+ Particle Size Analyzer. A total of four successive measurements were performed on each 1 mL of sample. The first measurement was discarded, and the average number of particles ≥10 μm and ≥25 μm in size was counted across the final three measurements in each container.
Biological activity
The relative potency of PF-06439535 was determined with an in vitro cell-based, growth-inhibition assay. Cells were incubated with PF-06439535 and diluted recombinant human vascular endothelial growth factor. Luminescence from each well in the 96-well plate was measured after adding Cell Titer-Glo® and using a SPECTRAmax M5 microplate reader. Dose-dependent sigmoidal curve response plots were generated and fitted to a 4-parameter log model. The assay was performed using three assay plates.
Far-UV circular dichroism spectroscopy
The degree and type of secondary structure present in samples of PF-06439535 was evaluated by far-UV circular dichroism (CD) spectroscopy as orthogonal assessment of structural integrity. CD spectra were measured using a Chirascan V100 Circular Dichroism spectrometer, and recorded from 190 to 260 nm. Samples were measured using a 0.5-mm path-length quartz cell that was maintained at 25 ± 5°C. For each sample, the average of three separate scans was calculated, and spectra were baseline corrected.
Differential scanning calorimetry
The thermal stability of PF-06439535 was evaluated by differential scanning calorimetry (DSC) as additional orthogonal assessment of structural integrity. DSC measurements were performed using a MicroCal PEAQ-DSC. Samples were volumetrically diluted and scanned from 45°C to 95°C. Scans were recorded at a rate of 100°C/h. The resulting thermograms were baseline subtracted and plotted.
Results
Visual characteristics and pH
There were no significant changes in color or clarity from the initial to final time point for any samples. All samples and every timepoint were essentially free from visible particulates (Supplementary table 1). At all concentrations and time points tested, the pH of PF-06439535 dosing solutions remained consistent (data not shown).
Protein concentration
For both concentrations of PF-06439535 dosing solutions and across all infusion bag types and drug product lots, there was no significant change from baseline in the measured protein concentration, and there were no trends in changes of protein concentration throughout the study period (Table 1).
Table 1.
Protein concentration (mg/mL) of PF-06439535 during extended storage.
| Storage time (weeks) | PO infusion bags | EVA infusion bags | PVC infusion bags | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.4 mg/mL | 16.5 mg/mL | 1.4 mg/mL | 16.5 mg/mL | 1.4 mg/mL | 16.5 mg/mL | |||||
| Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 1 | Lot 1 | Lot 1 | |
| Concentration (mg/mL) | Concentration (mg/mL) | Concentration (mg/mL) | Concentration (mg/mL) | |||||||
| 0 | 1.4 | 1.5 | 1.5 | 16.6 | 16.5 | 16.5 | 1.4 | 16.4 | 1.3 | 15.1 |
| 1 | 1.5 | 1.5 | 1.5 | 16.4 | 16.5 | 16.6 | 1.4 | 16.5 | 1.2 | 15.2 |
| 2 | 1.5 | 1.5 | 1.5 | 16.4 | 16.5 | 16.5 | 1.4 | 16.5 | 1.3 | 15.1 |
| 4 | 1.4 | 1.3 | 1.3 | 16.5 | 16.5 | 16.5 | 1.4 | 16.5 | 1.2 | 15.2 |
| 6 | 1.4 | 1.3 | 1.4 | 16.4 | 16.5 | 16.5 | 1.4 | 16.6 | 1.3 | 15.1 |
EVA: ethylene vinyl acetate; PO: polyolefin; PVC: polyvinyl chloride.
Size-exclusion chromatography
The major peak in each chromatogram (Supplementary Figure 1) corresponds to the monomeric form of PF-06439535, which comprised ≥97% of all detected peaks across all time points, concentrations, infusion bag types, and drug product lots tested (Table 2). The minor peaks in the chromatogram correspond to HMMS, which constituted ≤1.9% of all detected peaks and remained relatively stable throughout the study (Table 2).
Table 2.
Size-exclusion high-performance liquid chromatography analysis of PF-06439535 during extended storage.
| PO infusion bags | EVA infusion bags | PVC infusion bags | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.4 mg/mL | 16.5 mg/mL | 1.4 mg/mL | 16.5 mg/mL | 1.4 14;mg/mL | 16.5 mg/mL | ||||||
| Storage time (weeks) | Species (%) | Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 1 | Lot 1 | Lot 1 |
| 0 | Monomer | 98.4 | 98.6 | 98.5 | 98.3 | 98.5 | 98.5 | 98.4 | 98.3 | 98.2 | 98.3 |
| HMMS | 1.4 | 1.2 | 1.3 | 1.5 | 1.3 | 1.3 | 1.4 | 1.5 | 1.5 | 1.5 | |
| 1 | Monomer | 98.4 | 98.6 | 98.6 | 98.2 | 98.4 | 98.5 | 98.4 | 98.2 | 98.3 | 98.2 |
| HMMS | 1.4 | 1.2 | 1.2 | 1.6 | 1.4 | 1.3 | 1.4 | 1.6 | 1.4 | 1.6 | |
| 2 | Monomer | 97.5 | 98.3 | 98.5 | 98.5 | 98.7 | 98.7 | 98.5 | 98.5 | 98.4 | 98.5 |
| HMMS | 1.2 | 1.1 | 1.1 | 1.3 | 1.1 | 1.1 | 1.2 | 1.3 | 1.3 | 1.3 | |
| 4 | Monomer | 98.1 | 98.2 | 98.4 | 98.0 | 98.3 | 98.3 | 98.0 | 98.0 | 98.1 | 98.1 |
| HMMS | 1.6 | 1.6 | 1.4 | 1.8 | 1.5 | 1.5 | 1.8 | 1.8 | 1.7 | 1.7 | |
| 6 | Monomer | 98.2 | 98.4 | 98.4 | 98.0 | 98.2 | 98.3 | 98.2 | 97.9 | 98.0 | 98.0 |
| HMMS | 1.6 | 1.4 | 1.4 | 1.8 | 1.6 | 1.5 | 1.6 | 1.9 | 1.8 | 1.8 | |
EVA: ethylene vinyl acetate; HMMS: high-molecular-mass species; PO: polyolefin; PVC: polyvinyl chloride.
Non-reducing SDS capillary gel electrophoresis
The proportions of intact antibody and antibody fragments were quantified by non-reducing SDS-CGE (Supplementary Figure 2). Abundance of intact antibody was ≥93% for all time points, concentrations, infusion bag types, and drug product lots analyzed, with little change from baseline throughout the duration of the study (Table 3). The low concentration samples appeared to have slightly lower intact IgG and slightly higher levels of fragmentation than the high concentration samples (Table 3). However, none of the samples showed a trend in terms of the increase and/or decrease in the abundance of either species (Table 3).
Table 3.
Non-reducing SDS capillary gel electrophoresis of PF-06439535 during extended storage.
| PO infusion bags | EVA infusion bags | PVC infusion bags | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.4 mg/mL | 16.5 mg/mL | 1.4 mg/mL | 16.5 mg/mL | 1.4 14;mg/mL | 16.5 mg/mL | ||||||
| Storage time (weeks) | Species (%) | Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 1 | Lot 1 | Lot 1 |
| 0 | Intact IgG | 94.5 | 94.4 | 93.9 | 96.4 | 96.4 | 96.5 | 94.3 | 96.4 | 94.9 | 96.6 |
| Fragments | 5.2 | 5.3 | 5.8 | 3.3 | 3.6 | 3.5 | 5.4 | 3.4 | 5.1 | 3.4 | |
| 1 | Intact IgG | 94.9 | 94.5 | 94.7 | 96.0 | 96.3 | 96.3 | 95.1 | 96.5 | 94.8 | 96.6 |
| Fragments | 4.8 | 5.5 | 5.3 | 3.6 | 3.7 | 3.7 | 4.9 | 3.2 | 5.2 | 3.1 | |
| 2 | Intact IgG | 95.2 | 94.7 | 94.8 | 96.5 | 96.5 | 96.0 | 94.9 | 96.5 | 95.0 | 96.9 |
| Fragments | 4.8 | 5.3 | 5.2 | 3.1 | 3.5 | 3.7 | 5.1 | 3.2 | 5.0 | 3.1 | |
| 4 | Intact IgG | 95.0 | 94.0 | 94.4 | 96.1 | 96.1 | 96.3 | 94.9 | 96.2 | 95.2 | 96.2 |
| Fragments | 5.0 | 5.8 | 5.4 | 3.6 | 3.7 | 3.7 | 5.1 | 3.5 | 4.8 | 3.6 | |
| 6 | Intact IgG | 94.4 | 94.6 | 93.8 | 95.9 | 96.8 | 96.4 | 94.4 | 96.0 | 94.7 | 96.6 |
| Fragments | 5.3 | 5.4 | 6.2 | 3.5 | 2.9 | 3.6 | 5.3 | 3.7 | 4.9 | 3.1 | |
EVA: ethylene vinyl acetate; IgG: immunoglobulin G; PO: polyolefin; PVC: polyvinyl chloride.
Peptide mapping
Total ion chromatograms from LC-MS analysis at baseline and at 6 weeks were highly similar, demonstrating that there was no change in the primary structure of PF-06439535 (Figure 1). The high degree of similarity between the chromatograms also indicates that there was no significant post-translational modification (oxidation or deamidation) of PF-06439535 throughout the 6-week study period.
Figure 1.
Peptide mapping of PF-06439535 (16.5 mg/mL) in PO infusion bags at (a) 0 and (b) 6 weeks. PO: polyolefin.
Methionine oxidation in the Fc domain
For all concentrations, infusion bag types, and drug product lots tested, there was no significant change in the degree of oxidation observed for the methionine residue (258) in the Fc domain of PF-06439535, and the abundance of the oxidized fragment (≤4.5%) remained low throughout the study period (Table 4). The data show that there was little methionine oxidation of PF-06439535 in this extended in-use storage study, consistent with its long-term drug product stability profile.
Table 4.
Methionine oxidation in PF-06439535 during extended storage.
| PO infusion bags | EVA infusion bags | PVC infusion bags | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.4 mg/mL | 16.5 mg/mL | 1.4 mg/mL | 16.5 mg/mL | 1.4 14;mg/mL | 16.5 mg/mL | |||||
| Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 1 | Lot 1 | Lot 1 | |
| Storage time (weeks) | Percentage (%) of methionine oxidation | Percentage (%) of methionine oxidation | Percentage (%) of methionine oxidation |
Percentage (%) of methionine oxidation |
||||||
| 0 | 3.0 | 3.3 | 3.1 | 3.1 | 3.4 | 3.2 | 3.6 | 3.1 | 3.6 | 3.2 |
| 1 | 2.9 | 3.1 | 3.0 | 3.0 | 3.4 | 3.1 | 3.7 | 3.2 | 3.2 | 3.0 |
| 2 | 3.1 | 3.4 | 3.2 | 3.4 | 3.4 | 3.4 | 4.2 | 3.4 | 3.4 | 3.2 |
| 4 | 3.1 | 3.2 | 3.3 | 3.2 | 3.4 | 3.3 | 4.4 | 3.3 | 3.3 | 3.2 |
| 6 | 3.1 | 3.3 | 3.3 | 3.2 | 3.5 | 3.3 | 4.5 | 3.6 | 3.6 | 3.3 |
EVA: ethylene vinyl acetate; PO: polyolefin; PVC: polyvinyl chloride.
Cation-exchange chromatography
The major peak in the chromatogram corresponds to the main PF-06439535 charge species, which constituted >50% of all detected peaks for all concentrations, drug product lots, infusion bag types, and time points tested (Supplementary Figure 3). The peaks eluting before and after the main peak constituted the acidic and basic variants, respectively. There was no significant change in the abundance of the main species or of the acidic or basic variants throughout the duration of the study (Figure 2).
Figure 2.
Cation-exchange analysis of PF-06439535 (PO infusion bags) (a) 1.4 mg/mL and (b) 16.5 mg/mL. AUC: area under the curve; PO: polyolefin.
Sub-visible particle counting
The sub-visible particle results were highly variable (Table 5), which was expected for samples of diluted dosing solutions. There were no trends (increases or decreases) in particle formation over time and no trends associated with a particular concentration or infusion bag type.
Table 5.
Sub-visible particle quantitation of PF-06439535 samples during extended storage.
| PO infusion bags | EVA infusion bags | PVC infusion bags | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.4 mg/mL | 16.5 mg/mL | 1.4 mg/mL | 16.5 mg/mL | 1.4 mg/mL | 16.5 mg/mL | ||||||
| Storage time (weeks) | Particles/mL | Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 1 | Lot 1 | Lot 1 |
| 0 | Particles ≥10 µm | 13 | 17 | 28 | 14 | 10 | 13 | 18 | 25 | 14 | 17 |
| Particles ≥25 µm | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 1 | Particles ≥10 µm | 39 | 110 | 64 | 23 | 8 | 10 | 14 | 50 | 38 | 9 |
| Particles ≥25 µm | 3 | 5 | 2 | 1 | 1 | 1 | 0 | 1 | 3 | 2 | |
| 2 | Particles ≥10 µm | 10 | 18 | 16 | 10 | 20 | 5 | 36 | 39 | 15 | 3 |
| Particles ≥25 µm | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 4 | Particles ≥10 µm | 64 | 13 | 12 | 13 | 13 | 13 | 245 | 49 | 23 | 6 |
| Particles ≥25 µm | 3 | 1 | 1 | 0 | 0 | 0 | 6 | 0 | 1 | 1 | |
| 6 | Particles ≥10 µm | 10 | 13 | 17 | 13 | 10 | 8 | 36 | 25 | 16 | 7 |
| Particles ≥25 µm | 0 | 1 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | |
EVA: ethylene vinyl acetate; PO: polyolefin; PVC: polyvinyl chloride.
Circular dichroism spectroscopy
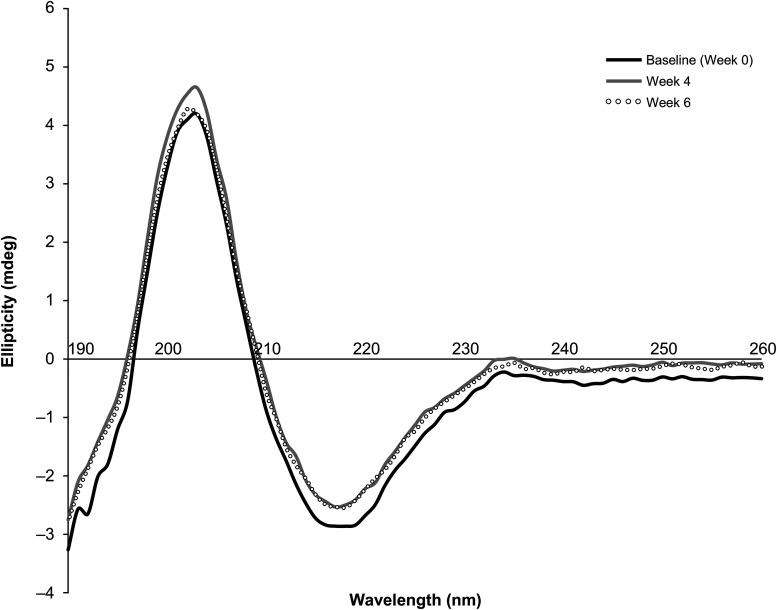
CD spectra of PF-06439535 at the 16.5 mg/mL concentration were recorded at 0 (baseline), 4, and 6 weeks (Figure 3). The CD spectra from all three time points overlay closely (Figure 3), demonstrating that there was no change in the secondary structure of PF-06439535 throughout the duration of the study.
Figure 3.
Far-ultraviolet circular dichroism spectroscopy of PF-06439535 (16.5 mg/mL) in PO infusion bags at 0 (black), 4 (gray), and 6 (open black circles) weeks. PO: polyolefin.
Differential scanning calorimetry
DSC thermograms of PF-06439535 at the 16.5 mg/mL concentration were recorded at 0 (baseline), 4, and 6 weeks (Figure 4). The thermograms from all three time points overlay closely (Figure 4), demonstrating that there was no change in the thermal stability of PF-06439535 throughout the duration of the study. The data are consistent with structural information obtained from primary biochemical and functional assessments, and other biophysical assays.
Figure 4.
Differential scanning calorimetry of PF-06439535 (16.5 mg/mL) in PO infusion bags at baseline 0 (black), 4 (gray), and 6 (open black circles) weeks. Cp: heat capacity; PO: polyolefin.
Biological activity
As assessed using a cell-based growth-inhibition assay, the in vitro relative potency of PF-06439535 was maintained throughout the study period (Table 6).
Table 6.
Relative in vitro (cell-based assay) potency of PF-06439535 during extended storage.
| Storage time (weeks) | PO infusion bags | EVA infusion bags | PVC infusion bags | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.4 mg/mL | 16.5 mg/mL | 1.4 mg/mL | 16.5 mg/mL | 1.4 mg/mL | 16.5 mg/mL | |||||
| Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 2 | Lot 3 | Lot 1 | Lot 1 | Lot 1 | Lot 1 | |
| Relative Potency (%) | Relative Potency (%) | Relative Potency (%) | Relative Potency (%) | |||||||
| 6 | 98 | 103 | 99 | 105 | 101 | 99 | 103 | 100 | 92 | 102 |
EVA: ethylene vinyl acetate; PO: polyolefin; PVC: polyvinyl chloride.
Discussion
This study was conducted to evaluate the stability and relative potency of PF-06439535 under extended in-use conditions, representative of those encountered in a typical clinical setting. Drug solutions were diluted to a range of concentrations (1.4 and 16.5 mg/mL) and stored for 24 h at 25 ± 5°C before being stored in infusion bags for an extended period of up to 6 weeks at 2–8°C. Extended storage was then followed by storage at 25 ± 5°C for 24 h. These concentrations were selected to cover potential doses (i.e. clinically relevant high and low doses) that could be administered to patients, and these storage conditions were intended to reflect the potential preparation and administration conditions encountered in a clinical setting.
Drug stability was assessed using a wide range of analy-tical techniques and an in vitro cell-based biological activity assay capable of detecting degradation products and variants which could affect product integrity or potency. The stability indicating assays in this study were selected based on their ability to detect the primary degradation pathways investigated during product development. For all concentrations, drug product lots, infusion bag types, and time points tested, there was no significant change in protein concentration, demonstrating minimal degradation and/or loss due to adsorption over the 6-week study period. There were also no notable differences in visual characteristics (color, clarity, and visible particulates) for any of the samples and no trends (increases or decreases) in particle formation over time, indicating that there are no stability concerns around sub-visible or visible particles. The relative abundance of HMMS and monomer did not change significantly throughout the study, as demonstrated by SEC analysis. This finding demonstrated structural integrity of the intact bevacizumab monoclonal antibody in a storage condition typically found during extended in-use and was further supported by orthogonal methods, such as non-reducing SDS-CGE, which demonstrated that there was a low abundance of fragments.
Results from LC-MS analysis, CD spectroscopy, and DSC analysis of PF-06439535 at 16.5 mg/mL were highly similar at all time points assessed. These findings indicate that there was no significant change in primary structure, and no changes in secondary or tertiary structure content. Furthermore, there was no significant change in oxidation state and in the abundance of charge variants, as demonstrated by CEX analysis. The in vitro relative potency of PF-06439535 was maintained throughout the study period, as demonstrated by the cell growth-inhibition assay, demonstrating that biological function of PF-06439535 is preserved and adding evidence of molecular structure stability that is seen in biochemical and biophysical assays.
To date, one study has evaluated the stability of a bevacizumab biosimilar (SB8) after dilution for IV infusion. SB8 was prepared at different concentrations (1.4 and 16.5 mg/mL) before being stored for 45 days at 5 ± 3°C and then for 3 days at 30 ± 2°C. 18 Results from the Park et al. study were similar to our findings for PF-06439535. For SB8, the visual characteristics, pH, protein concentrations, and abundance of sub-visible particles and molecular weight species remained stable over time. 18 In addition, the biological function of SB8 was maintained over the course of the study. 18 Stability studies under extended in-use conditions have been performed for other biologics, such as trastuzumab, rituximab, and infliximab.19–24 However, these studies varied in the analytical techniques and testing conditions used.
A strength of this study is the use of validated product-specific assays. Extended stability studies performed with generic, non-product-specific assays may not be sensitive enough to detect small amounts of protein degradation during extended in-use conditions. In contrast, the methods described here are robust and have been shown to be highly sensitive to small changes in protein quality. Combined with a deep knowledge of PF-06439535 degradation pathways, which have been investigated thoroughly during product development, the assays described in this paper allow us to ensure the physicochemical stability of PF-06439535 and that the biosimilar is safe and effective during extended in-use conditions. Additionally, the assays performed were chosen to align with the testing protocols suggested in the National Health Service Standard Protocol for deriving an assessment of stability. 25
A limitation of the current study is that it did not evaluate microbiological growth potential under the conditions tested, which is an important factor to consider when setting in-use periods in clinical practice. In the clinic, in-use hold times would not typically exceed 24 h unless dilution was performed under controlled and validated aseptic conditions. In order to reduce the risk of microbial contamination, products should be prepared and in-use periods should be determined based on local pharmacy regulations and guidance along with the in-use periods specified in the local PF-06439535 package insert.
In conclusion, a comprehensive set of analytical tech-niques and an in vitro cell-based biological activity assay demonstrated that the stability and biological activity of PF-06439535 is maintained after dilution and storage for an extended period of up to 6 weeks at 2–8°C. These findings demonstrate the integrity of diluted PF-06439535 under extended in-use conditions.
Supplemental Material
Supplemental material, sj-pdf-1-opp-10.1177_10781552221088020 for Physicochemical stability of PF-06439535 (bevacizumab-bvzr; Zirabev®), a bevacizumab biosimilar, under extended in-use conditions by Sarah Weiser, Chris Burns and Edward R. Zartler in Journal of Oncology Pharmacy Practice
Acknowledgements
This study was funded by Pfizer. Medical writing support was provided by Elyse Smith, PhD, of Engage Scientific Solutions and was funded by Pfizer. The authors thank Angel Bair, Rebecca Ingram, and Chandra Webb, of Pfizer, and Chee-Keng Ng, formerly of Pfizer, for their expertise and advice about the study design and conduct and for helpful discussions during development of the manuscript; Rui Cao and Sean Shen, of Pfizer, for the generation of mass spectrometric data; Smita Soni and Natalya Topilina, of Pfizer, for the generation of biophysical data; Ashley Farber, Cassie Sleger, and Samantha Stecha, of PPD Laboratories, for the generation of biochemical data; and Benjamin Grunder, Parag Kohle, and Sumedha Mitra, of Pfizer, for helpful discussions during development of the manuscript.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SW and CB are employees of and own stock or options in Pfizer. ERZ was an employee of and held stock or options in Pfizer at the time of the study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Pfizer.
ORCID iD: Sarah Weiser https://orcid.org/0000-0002-7899-678X
Supplemental material: Supplemental material is available for this article online.
References
- 1.European Medicines Agency. Avastin (bevacizumab) summary of product characteristics, https://www.ema.europa.eu/en/medicines/human/EPAR/avastin (2018, accessed 6 December 2018).
- 2.Genentech Inc. Avastin (bevacizumab) US prescribing information, https://www.gene.com/download/pdf/avastin_prescribing.pdf (2018, accessed 6 December 2018).
- 3.European Medicines Agency. Zirabev (bevacizumab) summary of opinion (initial authorisation), https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-zirabev_en.pdf (2019, accessed 21 March 2019).
- 4.Pfizer Inc. Zirabev (bevacizumab-bvzr) US prescribing information, https://labeling.pfizer.com/ShowLabeling.aspx?id=11860 (2020, accessed 30 November 2020).
- 5.Amit L, Ben-Aharon I, Vidal L, et al. The impact of Bevacizumab (Avastin) on survival in metastatic solid tumors--a meta-analysis and systematic review. PloS One 2013; 8: e51780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roviello G, Bachelot T, Hudis CA, et al. The role of bevacizumab in solid tumours: a literature based meta-analysis of randomised trials. Eur J Cancer 2017; 75: 245–258. [DOI] [PubMed] [Google Scholar]
- 7.IMS Institute for Healthcare Informatics. Delivering on the potential of biosimilar medicines, https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/delivering-on-the-potential-of-biosimilar-medicines.pdf (2016, accessed 6 December 2018).
- 8.Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf (2014, accessed 6 December 2018).
- 9.US Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf (2015, accessed 6 December 2018).
- 10.World Health Organization. Guidelines on evaluation of Similar Biotherapeutic Products (SBPs), http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf (2009, accessed 6 December 2018).
- 11.Health Canada. Guidance document: information and submission requirements for biosimilar biologic drugs, https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/brgtherap/applic-demande/guides/seb-pbu/seb-pbu-2016-eng.pdf (2016, accessed 2 April 2019).
- 12.Ministry of Health and Labour and Welfare. Guideline for the quality, safety, and efficacy assurance of follow-on biologics, https://www.pmda.go.jp/files/000153851.pdf (2009, accessed 2 April 2019).
- 13.Australian Government: Department of Health (Therapeutic Goods Administration). Biosimilar medicines regulation, Version 2.2, https://www.tga.gov.au/sites/default/files/biosimilar-medicines-regulation.pdf (2018, accessed 2 April 2020).
- 14.Peraza MA, Rule KE, Shiue MHI, et al. Nonclinical assessments of the potential biosimilar PF-06439535 and bevacizumab. Regul Toxicol Pharmacol 2018; 95: 236–243. [DOI] [PubMed] [Google Scholar]
- 15.Grunder B, Costigan L, Johnson K, et al. Characterization and similarity assessment of bevacizumab and a proposed biosimilar. American Association of Pharmaceutical Scientists (AAPS) Annual Meeting and Exhibition, San Diego, CA, 3–5 November 2014. [Google Scholar]
- 16.Rule K, Peraza M, Shiue M, et al. Nonclinical development of PF-06439535, a potential biosimilar to bevacizumab. IASLC 16th World Conference on Lung Cancer (WCLC), Denver, CO, 6–9 September 2015. [Google Scholar]
- 17.Knight B, Rassam D, Liao S, et al. A phase I pharmacokinetics study comparing PF-06439535 (a potential biosimilar) with bevacizumab in healthy male volunteers. Cancer Chemother Pharmacol 2016; 77: 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park D, Kim J, Yun J, et al. Evaluation of the physico-chemical and biological stability of SB8 (Aybintio), a proposed biosimilar to bevacizumab, under ambient and in-use conditions. Adv Ther 2020; 37: 4308–4324. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Lee JW, Kang HY, et al. In-use physicochemical and biological stability of the trastuzumab biosimilar CT-P6 upon preparation for intravenous infusion. BioDrugs 2018; 32: 619–625. [DOI] [PubMed] [Google Scholar]
- 20.Lamanna WC, Heller K, Schneider D, et al. The in-use stability of the rituximab biosimilar Rixathon(R)/Riximyo(R) upon preparation for intravenous infusion. J Oncol Pharm Pract 2019; 25: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pabari RM, Ryan B, Ahmad W, et al. Physical and structural stability of the monoclonal antibody, trastuzumab (Herceptin(R)), intravenous solutions. Curr Pharm Biotechnol 2013; 14: 220–225. [DOI] [PubMed] [Google Scholar]
- 22.Paul M, Vieillard V, Da Silva Lemos R, et al. Long-term physico-chemical stability of diluted trastuzumab. Int J Pharm 2013; 448: 101–104. [DOI] [PubMed] [Google Scholar]
- 23.Tokhadze N, Chennell P, Le Basle Y, et al. Stability of infliximab solutions in different temperature and dilution conditions. J Pharm Biomed Anal 2018; 150: 386–395. [DOI] [PubMed] [Google Scholar]
- 24.Young BL, Khan MA, Chapman TJ, et al. Evaluation of the physicochemical and functional stability of diluted REMSIMA(R) upon extended storage--A study compliant with NHS (UK) guidance. Int J Pharm 2015; 496: 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NHS Pharmaceutical Research and Development Working Group. Standard protocol for deriving and assessment of stability, part 2: aseptic preparations (biopharmaceuticals), edition 3, April 2017 (Yellow Cover), https://www.sps.nhs.uk/articles/https-www-sps-nhs-uk-wp-content-uploads-2020-08-stability-part-2-biopharmaceuticals-v4-aug-2020-pdf/ (2017, accessed 30 November 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-opp-10.1177_10781552221088020 for Physicochemical stability of PF-06439535 (bevacizumab-bvzr; Zirabev®), a bevacizumab biosimilar, under extended in-use conditions by Sarah Weiser, Chris Burns and Edward R. Zartler in Journal of Oncology Pharmacy Practice