Abstract
Background:
Early pregnancy loss (EPL), also referred to as miscarriage, is common, affecting approximately 1 million people in the United States annually. EPL can be treated with expectant management, medications, or surgical procedures - strategies that differ in patient experience, effectiveness, and cost. One of the medications used for EPL treatment, mifepristone, is uniquely regulated by the FDA.
Objectives:
To compare the cost-effectiveness from the healthcare sector perspective of medical management of EPL, using the standard of care medication regimen of mifepristone and misoprostol, to office uterine aspiration.
Study design:
We constructed a decision-analytic model to compare the cost effectiveness of EPL treatment with medical management to office uterine aspiration. Data on medical management came from the Pregnancy Failure Regimens (PreFaiR) randomized clinical trial and data on uterine aspiration came from the published literature. The analysis was from the healthcare sector perspective with a 30-day time horizon. Costs were in 2018 U.S. dollars. Effectiveness was measured in quality-adjust life years (QALYs) gained and the rate of complete gestational sac expulsion with no additional interventions. Our primary outcome was the incremental cost per QALY gained. Sensitivity analysis were performed to identify the key uncertainties.
Results:
Mean per-person costs were higher for uterine aspiration than for medical management ($828 [95% CI $789, 868] versus $661 [95% CI $556, $766], (p=0.004)). Uterine aspiration more frequently led to complete gestational sac expulsion (97.3% versus 83.8%, p=0.0001), however estimated QALYs were higher for medical management than uterine aspiration (0.082 [95% CI 0.8148, 0.08248] versus 0.079 [95% CI 0.0789, 0.0791], p<0.0001). Medical management dominated uterine aspiration, with lower costs and higher QALYs. The probability that medical management is cost-effective relative to uterine aspiration is 97.5% for all willingness-to-pay values ≥ $5,600/QALY. Sensitivity analysis did not identify any thresholds that would significantly change outcomes.
Conclusions:
Although office-based uterine aspiration more often results in treatment completion without further intervention, medical management with mifepristone pretreatment costs less and yielded similar QALYs, making it an attractive alternative. Our findings provide evidence that increasing access to mifepristone and eliminating unnecessary restrictions will improve early pregnancy care.
Keywords: health economics, mifepristone pretreatment, misoprostol, miscarriage, quality-adjusted life-years, uterine aspiration
Introduction
Early pregnancy loss (EPL), or miscarriage, is common, affecting over 1 million people in the United States annually.1 With an increased availability of highly sensitive pregnancy tests and early ultrasounds, many patients are diagnosed with an EPL prior to the onset of symptoms.2–4 Once diagnosed, pregnant individuals have three treatment routes available to them: expectant management (watching and waiting), surgical intervention (uterine aspiration in the office or operating room), or medical management (using medications to induce uterine contractions and expel tissue).5 These three options differ in effectiveness, patient experience, and cost.6–9
A 2018 multicenter randomized clinical trial of medical management of EPL demonstrated increased clinical effectiveness when the medication mifepristone was added as a pretreatment to the at the time standard regimen of misoprostol.10 Prior to mifepristone pretreatment, as many as 15–40% of patients opting for medical management with 800 mcg of vaginal misoprostol required either additional doses of medication or a uterine evacuation procedure to complete the process.6, 7 With improved medication management effectiveness, patient and clinician interest in expanding access to medication management has increased,11 and the COVID-19 pandemic has highlighted the importance of treatment options that minimize in-person clinic visits.12, 13 However, there are barriers to mifepristone access for EPL treatment. Mifepristone for EPL is off-label use of the medication approved to induce abortion, and mifepristone carries a U.S. Food and Drug Administration (FDA)-mandated Risk Evaluation and Mitigation Strategy (REMS) that requires both prescribing providers and dispensing pharmacies to be certified.14–16 Clinicians and payers may also perceive cost to be a barrier to the use of mifepristone.
The comparative cost-effectiveness of medication management and in-office management has significant implications for clinical care and reproductive health policy. If medication management is preferred by many patients, decreases the need to access in-person clinical care during a pandemic, and is found to be cost-effective, clinicians and policy makers should increase efforts to improve mifepristone availability and reduce access burdens. Given the clinical efficacy of medical management of EPL using mifepristone pretreatment, and its proven cost-effectiveness compared to misoprostol-alone treatment for EPL,17 we built a decision-analytic model combining the Pregnancy Failure Regimens Trial (PreFaiR) data and data from the published literature to assess the cost-effectiveness of medical management with mifepristone-pretreatment followed by misoprostol (“medical management”) compared to an office-based uterine aspiration arm (“uterine aspiration”) for treatment of EPL.
Materials and methods
Trial Design and Participants
PreFaiR is a pragmatic comparative effectiveness trial conducted at three United States sites from May 1, 2014, to April 30, 2017, the details of which have been previously described.10 The trial randomized 300 women with anembryonic gestation or fetal demise before 12 completed gestational weeks with a closed cervical os to pretreatment with 200 mg mifepristone administered orally followed by 800 mcg misoprostol administered vaginally, or to 800 mcg misoprostol administered vaginally without pretreatment. One hundred forty-nine women were randomized to mifepristone-pretreatment, and data from 148 patients were evaluable for the medical management arm (mean [SD] age, 30.7 [6.3] years).10 Participants were scheduled to return at 24 to 96 hours after misoprostol use (“Day 3 Visit”) for assessment of treatment success. If the gestational sac was not expelled, participants were offered expectant management, a second dose of misoprostol, or office-based uterine aspiration. All participants were followed up for 30 days after randomization to verify pregnancy expulsion and to assess adverse effects.
We built a secondary analysis utilizing PreFaiR results10 to compare the healthcare sector perspective costs and health outcomes associated with medical management versus uterine aspiration. For the medical management arm, we used the mifepristone pretreatment-misoprostol arm results from the trial.10 The uterine aspiration arm was based on a demographically similar population, using the published literature to generate patient-level data.6–8, 10, 17 All uterine aspirations were assumed to be performed in the office setting with local anesthesia (without general anesthetic or sedative agents). As is standard practice and consistent with prior cost analyses, office uterine aspiration procedures were assumed to have been performed without ultrasound guidance.7,17, 18 This study has institutional review board approval from the University of Pennsylvania (Philadelphia, Pennsylvania), the University of California Davis (Davis, California) and the Albert Einstein College of Medicine (New York, New York).
Economic Evaluation Design
Incremental cost per quality-adjusted life year (QALY) gained and the incremental cost per complete gestational sac expulsion were calculated from the healthcare sector perspective to compare medical management and uterine aspiration for miscarriage management. This approach follows the recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine19, 20 and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) for health economic evaluations.21 An intent-to-treat (ITT) approach was used: all participants assigned to mifepristone pretreatment in the 2018 trial, regardless of treatment response, were included in the medical management arm. We used the 30-day follow-up period of the 2018 Schreiber et al trial10 as our time horizon. For the uterine aspiration arm, we modeled costs and outcomes for a demographically similar population choosing office uterine aspiration as their primary treatment approach.
Costs and Use of Resources
Healthcare sector perspective costs included, as recommended, costs incurred by payers and by patients for the therapies and for other EPL-related healthcare costs. We used a macro-costing approach: healthcare utilization data collected during the trial (medical management arm) or estimated from the literature (uterine aspiration arm) were combined with national average Medicare reimbursement rates or published prices to reflect costs to the healthcare sector for each EPL- or therapy-related clinical event (Supplemental Table 1). For generalizability, national average Medicare reimbursement rates were used rather than institution- or region-specific costs. The original study utilized detailed case report forms, collected at scheduled study visits or telephone calls at study days 3, 8, and 30, for information regarding resource use. Additionally, the number of EPL-related procedures performed, adverse clinical events (e.g. infections, additional office visits, emergency room visits, admissions), and other health care for EPL (e.g. medications) were collected from both case report forms and electronic medical record review during the original study. Unit cost estimates were applied to calculate the total costs for each participant. For medical management, costs include initial treatment with 200 mg mifepristone and 800 mcg misoprostol. For uterine aspiration, costs include an office-based procedure with local anesthesia, and estimated complication rates from the literature.6, 7 Results are expressed in 2018 US dollars.
Effectiveness Outcome
The primary effectiveness outcome was the one-month QALY. QALYs were based on a modified utility score taken from the published EPL literature of 30-day trials, with successful medical management defined as 1, successful uterine aspiration as 0.95, and need for uterine aspiration or repeat dosage after failed medical or procedural treatment defined as 0.90. 7,17 QALYs were calculated from the utility scores, which were assumed to remain constant during the 30-day trial. 7,17 Other effectiveness outcomes included treatment success, defined for medical management as gestational sac expulsion with one dose of misoprostol at the first follow-up visit and for uterine aspiration as a successful procedure, with no additional interventions needed within 30 days after treatment. Any uterine aspirations performed for treatment failures were assumed to be 100% completed.
Statistical Analysis
Univariate cost and effectiveness measures were compared using unpaired t-tests to assess differences between medical management and uterine aspiration. The primary cost-effectiveness measure was the incremental cost-effectiveness ratio (ICER), defined as the difference in mean cost of medical management versus uterine aspiration, divided by the difference in mean QALYs of medical management versus uterine aspiration. A secondary ICER was calculated using treatment success as the effectiveness measure; the difference in mean cost was divided by the difference in treatment success with medical management versus uterine aspiration. Uncertainty in the estimated ICER was evaluated by generating pairs of differences in mean cost and mean QALYs from 5,000 replications of cost and QALY data and calculating 95% confidence intervals. The cost-effectiveness plane shows the differences between the medical management and uterine aspiration arms in mean cost on the y-axis and mean QALYs on the x-axis. The ICER was calculated for each replication and compared with a range of willingness-to-pay values (zero to $2,000,000 per QALY). Cost-effectiveness acceptability curves display the percentage of ICER replications that are cost effective below each willingness-to-pay value. An analogous approach was used for treatment success ICER.22–27
We conducted sensitivity analyses to identify the effect of key variables on cost-effectiveness results, including the cost of mifepristone for medical management, cost of uterine aspiration, frequency of treatment success, and utility score assumptions.
Statistical analyses were performed using Stata 14.2 (StataCorp, College Station, TX). P-values < 0.05 indicated significance; all analyses were two-sided.
Results
Resources and Costs
Estimated mean per-person costs were higher for uterine aspiration, $828 [95% CI, $789 to 868], than for medical management, $661 [95% CI, $556-$766], (p=0.004) (Table 1).
Table 1:
Costs and Outcomes of Early Pregnancy Loss Treatment with Medical Management versus Uterine Aspiration
| Mean Per-Person Costs | Medical Management (USD) | Uterine Aspiration (USD) | P-Value |
|---|---|---|---|
| Direct Costs - Formal Health Care Sector a | |||
| Treatment - mean (SD) | 519.34 (57.03) | 741.87 (0.0) | <0.0001 |
| Re-aspiration - mean (SD) | 41.71 (134.87) | 25.52 (130.37) | 0.223 |
| Repeat misoprostol - mean (SD) | 0.22 (0.80) | - | - |
| Unscheduled visitsb - mean (SD) | 14.13 (52.68) | 18.31 (59.21) | 0.468 |
| Other complicationsc - mean (SD) | 84.03 (625.48) | 33.41 (318.87) | 0.259 |
| Pain controld - mean (SD) | 1.31 (1.47) | 9.29 (0.0) | <0.0001 |
| Total healthcare perspective costse - mean (95% CI) | $660.75 (555.88–765.62) | $828.40 (789.12–867.68) | 0.004 |
| Effects | Medical Management | Uterine Aspiration | P-Value |
| Average QALYs per Person — mean (SD) | 0.082 (0.003) | 0.0790 (0.001) | <0.0001 |
| Completion rate after first treatment—mean (SD) | 83.8% (36.98) | 97.3% (16.22) | 0.0001 |
Medical management health care utilization based on data from Nagendra et al. 2020; uterine aspiration health care utilization based on data from Zhang et al. 2005, Rausch et al. 2012, and Cubo et al. 2019. Detail in Supplemental Table 1.
Unscheduled visits were categorized as visits requiring a visit to a provider and a transvaginal ultrasound.
Other complications included visits to the office or emergency room related to the miscarriage, such as for pelvic inflammatory disease or need for a transfusion due to hemorrhage.
Pain control measures: All patients received a prescription to aid in pain management.
All costs reported in 2018 U.S. dollars ($).
Clinical Effectiveness and QALYs Outcomes
The effect of treatment completion was defined by complete gestational sac expulsion. With medical management, 83.8% of women had successful management after their initial treatment, compared to an estimated 97.3% of women with successful management with uterine aspiration (p=0.0001) (Table 1).
Estimated QALYs for uterine aspiration were 0.0790 [95% CI, 0.0789 to 0.0791], lower than for medical management 0.0820 [95% CI, 0.8148 to 0.08248] (p<0.0001) (Table 1).
Cost-effectiveness
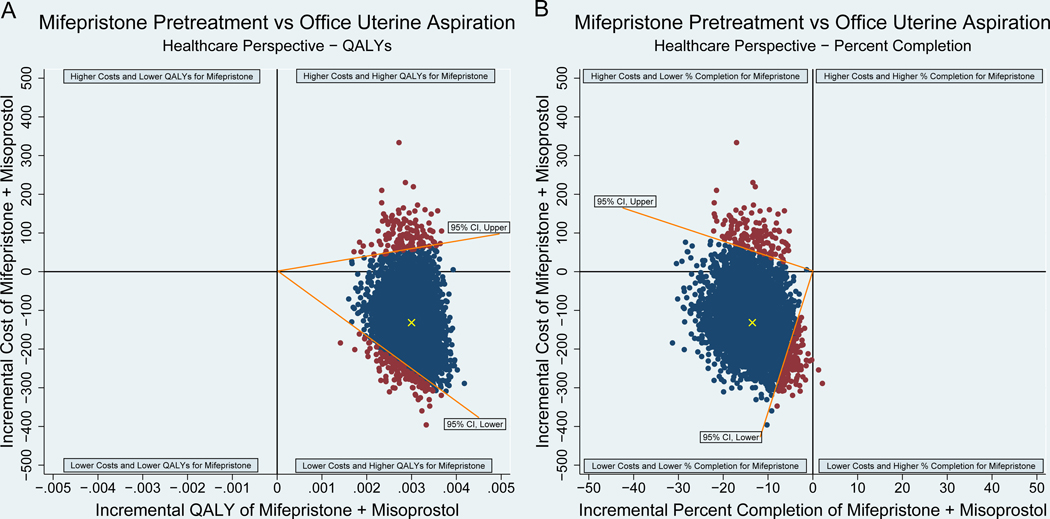
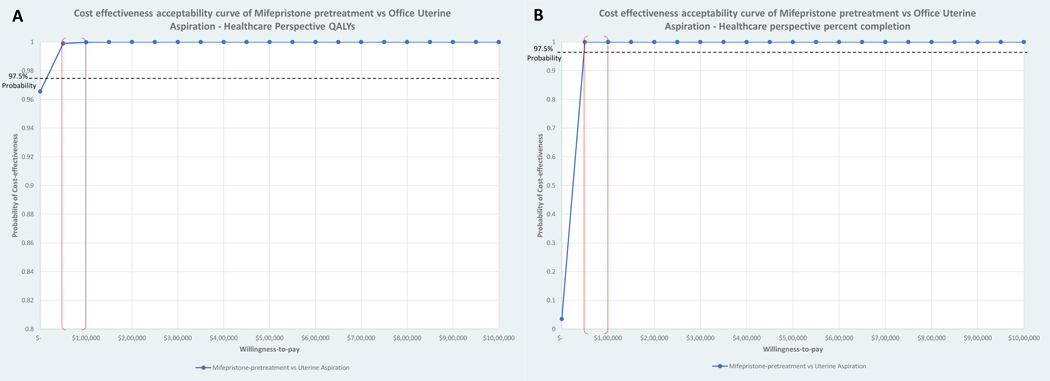
In comparing cost-effectiveness of medical management to uterine aspiration from the healthcare sector perspective, medical management was dominant, as costs were lower and QALYs were higher than for uterine aspiration. (Table 2, Figure 1) Cost-effectiveness acceptability curve analysis demonstrated that the probability that medical management is cost-effective relative to office uterine aspiration is 97.5% (corresponding to upper bound of 95% CI) for all willingness to pay values greater than $5,600 per QALY gained (Figure 2).
Table 2:
Incremental Cost-effectiveness Ratio of Treatment of Early Pregnancy Loss with Medical Management versus Uterine Aspiration, by Measure of Health Outcome
| Health Outcome: Quality-adjusted life-years | Health Outcome: Completion after first treatment | ||
|---|---|---|---|
| Difference in mean costs per person | −$167.65 | Difference in mean costs per person | −$167.65 |
| Difference in mean QALYs per person | 0.0030 | Difference in completion rate after first treatment | −13.5% |
| ICER |
Medical management dominant
ICER (−55,883.33) 95% CI (−$99,683.07 – $5,531.71) per QALY |
ICER | $12.42 per one percentage point in completion rate 95% CI (−$1.25 – $45.64) |
Figure 1:
Incremental Cost-Effectiveness Ratio (ICER) Scatter Plot for Mifepristone Pretreatment versus Office Uterine Aspiration, by Measure of Health Outcome
Caption: Authors’ analysis. Scatterplots of points representing pairs of mean differences in cost and mean differences in QALYs (A) and percent completion (B) for mifepristone pretreatment versus office uterine aspiration from 5000 bootstrapped replications with replacement. The healthcare sector perspective in cost per QALY gained is in panel A and the healthcare perspective in cost per percent completion gained is in panel B. The difference in mean cost is on the y-axis and difference in mean QALY (A) or percent completion (B) is on the x-axis. Points that lie above the horizontal axis represent replications in which mifepristone pretreatment costs more than office uterine aspiration, while points below indicate replications in which office uterine aspiration costs more than mifepristone pretreatment. Points to the right of the vertical axis represent replications in which mifepristone pretreatment was more effective than office uterine aspiration, while points on the left indicate replications in which office uterine aspiration was more effective than mifepristone pretreatment. Estimates in panel A fell in the lower right quadrant, this shows mifepristone pretreatment as “dominant,” with lower mean costs and higher mean QALYs than office uterine aspiration. In panel B estimates fell in the lower left quadrant, this shows mifepristone pretreatment has lower cost and lower percent completion rates than office uterine aspiration. The blue points are within the 95% confidence interval (CI), the red points are outside the 95% CI, and the yellow points indicate the ICER point estimates.
Figure 2:
Cost-Effectiveness Acceptability Curve for Mifepristone Pretreatment versus Uterine Aspiration, by Measure of Health Outcome
Caption: Authors’ analysis. Cost-effectiveness acceptability curves (CEAC) for mifepristone pretreatment versus uterine aspiration. The 5,000 bootstrapped ICER replications with replacement were used to derive cost-effectiveness acceptability frontiers, which plot the probability of the optimal strategy being cost-effective across a range of values of willingness-to-pay per QALY gained (A) or per percent completion gained (B). These probabilities were graphed to create cost effectiveness acceptability curves for mifepristone pretreatment versus uterine aspiration. The common maximum willingness-to-pay per QALY gained threshold of $100,000 is indicated by the red line in panel A. Mifepristone pretreatment had a 97.5% probability of being cost-effective (corresponding to upper bound of 95% CI) compared with office uterine aspiration at a willingness-to-pay per QALY gained threshold of $5,600 and at $46 per one percentage point in completion rate gained.
Cost-effectiveness by treatment success of medical management to uterine aspiration was also evaluated. Costs for medical management were lower than uterine aspiration but uterine aspiration had higher treatment success, resulting in an ICER of $12.42 per one percentage point in completion rate gained. (Table 2, Figure 1) Cost effectiveness acceptability curve analysis demonstrated the probability that medical management is cost effective relative to uterine aspiration is 97.5% for all willingness to pay values greater than $46.00 per one percentage point in completion rate gained (Figure 2).
Sensitivity Analyses
Threshold analysis demonstrated the effort of key variables on mifepristone-pretreatment cost-effectiveness. With a decrease in cost of an in-office uterine aspiration procedure from $475 to $11 , or an increase in cost of mifepristone from $54 to $518 per dose, medical management would remain cost-effective at the generally accepted maximum willingness to pay of approximately $100,000/QALY.28 With a decrease of percentage completion rate for medical management from 83.8% to 28.4%, medical management would remain cost-effective at $100,000/QALY. With a decrease in utility score from 1.0 to 0.9335 for successful medical management, an increase in utility score from 0.95 to 0.9999 for successful in office uterine aspiration, or a decrease in utility score from 0.9 to 0.46 for retreatment after failed medical or procedural treatment, mifepristone pretreatment would remain cost effective at $100,00/QALY.
Comment
Principal findings
Our study demonstrates that from the healthcare perspective, medical management with mifepristone pretreatment followed by misoprostol was cost-effective when compared to office uterine aspiration for EPL treatment, with higher effectiveness (QALYs) and lower costs. Our analysis demonstrates that the ICER for medical management is well below the maximum willingness-to-pay threshold of approximately $100,000 per QALY gained.28
Results in the context of what is known
The improved efficacy of mifepristone pretreatment over misoprostol alone has changed the standard of care for medical management of EPL.17,5 Prior research comparing the cost-effectiveness of medical management and uterine aspiration for EPL management were based on medical management protocols using misoprostol alone.7 Our analysis provides additional information for patients seeking EPL treatment, clinicians incorporating the full range of EPL care into their practices, healthcare payers, and policy makers.
Clinical implications
The COVID-19 pandemic has highlighted the importance of increasing access to EPL treatment options that reduce the need for in-person clinical visits. As the pandemic has impacted access to hospital services, the importance of an effective treatment option that can be offered through telemedicine has become essential.24, 25 This study contributes to a growing body of literature showing medication management with mifepristone and misoprostol for EPL to be safe, effective, and cost-effective. Unfortunately, clinicians are hindered in prescribing mifepristone due to unnecessary restrictions, limiting the widespread use of this treatment strategy.14, 15, 29 We have shown that cost is not a barrier.
The FDA initially designated mifepristone as a medication requiring REMS following its approval in September 2000 for medical termination of intrauterine pregnancy. The REMS regulations for mifepristone initially required providers to be certified to prescribe mifepristone, mifepristone to be dispensed in a clinic or hospital setting, and prescribers to obtain a signed patient agreement form prior to dispensing the medication.15 These restrictions have prevented many patients from accessing mifepristone and are particularly burdensome for Black and underinsured patients, who are more likely to seek treatment in emergency care settings.30 In April 2021, the FDA announced their intention to “exercise enforcement discretion” during the COVID-19 public health emergency, and in December 2021 the FDA modified the REMS by removing the requirement that mifepristone be dispensed only in certain healthcare settings, specifically clinics, medical offices, and hospitals (referred to as the “in-person dispensing requirement”) and adding a requirement that pharmacies that dispense the drug be certified.15 How these changes will impact patients who wish to use mifepristone “off-label” for miscarriage management remains to be seen, but evidence of cost-effectiveness and the recent reduction in regulatory barriers may result in improved care and access for patients suffering from the most common complication in pregnancy.
Strengths and limitations
Our study utilized economic data collected prospectively from a pragmatic randomized clinical trial for the medical management arm. However, our analysis has some limitations. Our study utilized 2018 National Medicare reimbursement rates to calculate healthcare costs to improve generalization, but actual costs for healthcare and reimbursement rates may vary by region and payer. The cost of mifepristone was included in healthcare costs but may also vary by region and year purchased. The use of a uterine aspiration group modeled with data from the published literature necessitated different sources of healthcare utilization data between the comparison groups. Our sensitivity analyses were performed with these limitations in mind in order to demonstrate thresholds at which mifepristone-pretreatment would no longer be cost-effective.
Additionally, further studies must be done to evaluate the QALYs for early pregnancy loss and other obstetric and gynecologic procedures to establish standard measures that can be used across our field. We made assumptions based on limited literature regarding the assignment of utility preference score values for successful medical management, failed medical management, successful uterine aspiration, and failed uterine aspiration. We conducted sensitivity analyses to further determine the smallest difference at which medical management remained cost-effective.
Our study examined only the healthcare perspective when comparing these two effective EPL treatment options. There is a need to assess the societal sector perspective of EPL management to conduct more comprehensive cost-effectiveness analyses and better identify the complete costs associated with treatment.
Conclusion
Medical management of EPL with mifepristone pretreatment and misoprostol is cost effective compared to office uterine aspiration, with similar QALYs and lower costs, making it a high-value care alternative. Increasing access to mifepristone and eliminating unnecessary restrictions will improve early pregnancy care.
Supplementary Material
AJOG at a Glance:
A. Why was the study conducted?
This study compares the cost-effectiveness of medical management of early pregnancy loss using mifepristone and misoprostol to office uterine aspiration.
B. What are the key findings?
Medical management with mifepristone and misoprostol dominated office uterine aspiration from the healthcare sector perspective because costs were lower and quality-adjusted life-years were similar.
C. What does this study add to what is already known?
Given the cost-effectiveness of medical management of early pregnancy loss with mifepristone and misoprostol, increasing access to mifepristone and eliminating unnecessary restrictions will substantially improve early pregnancy care.
Acknowledgements:
We thank the members of the PreFaiR trial team and the study participants for their dedication
Primary Funding Source:
Supported by the National Institute of Child Health and Human Development of the National Institutes of Health (Eunice Kennedy Shriver award number R01-HD0719-20 [to Dr. Schreiber] and Women’s Reproductive Health Research award number K12-HD001265-20 [to Dr. Sonalkar]), and a Society of Family Planning Research Fund Midcareer Mentor Award (Schreiber). The funding sources were not involved in study design; collection, analysis, and interpretation of data; writing of the report; or in the decision to submit the article for publication.
Footnotes
Trial Registration: www.clinicaltrials.gov, registration #: NCT02012491
Disclosures: The authors report no conflicts of interest.
Condensation: Medical management of early pregnancy loss is cost-effective compared to office uterine aspiration, with lower mean costs and similar quality-adjusted life-years.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep 2012;60:1–21. [PubMed] [Google Scholar]
- 2.Sapra KJ, Joseph KS, Galea S, Bates LM, Buck Louis GM, Ananth CV. Signs and Symptoms of Early Pregnancy Loss. Reprod Sci 2017. Apr; 24(4): 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD. Incidence of early loss of pregnancy. N Engl J Med 1988;319:189–194. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril 2003; 79(3):577–84. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. Early pregnancy loss. ACOG Practice Bulletin No. 200. Obstet Gynecol 2018;132(5):e197–e207 [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Gilles JM, Barnhart K, Creinin MD, Westhoff C, Frederick MM; National Institute of Child Health Human Development (NICHD) Management of Early Pregnancy Failure Trial. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353(8):761–769. doi: 10.1056/NEJMoa044064 [DOI] [PubMed] [Google Scholar]
- 7.Rausch M, Lorch S, Chung K, Frederick M, Zhang J, Barnhart K. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97(2):355–360. doi: 10.1016/j.fertnstert.2011.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cubo AM, Soto ZM, Haro-Pérez A, Hernández Hernández ME, Doyague MJ, Sayagués JM. Medical versus surgical treatment of first trimester spontaneous abortion: A cost-minimization analysis. PLoS ONE. 2019;14(1): e0210449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber CA, Chavez V, Whittaker PG, Ratcliffe SJ, Easley E, Barg FK. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128(6):1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber CA, Creinin MD, Atrio J, Sonalkar S, Ratcliffe SJ, Barnhart KT. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378(23):2161–2170. doi: 10.1056/NEJMoa1715726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deFiebre G, Srinivasulu S, Maldonado L, Romero D, Prine L, Rubin SE. Barriers and Enablers to Family Physicians’ Provision of Early Pregnancy Loss Management in the United States. Women’s Health Issues. 2021; 31(1):57–64. [DOI] [PubMed] [Google Scholar]
- 12.Kohn JE, Snow JL, Simons HR, Seymour JW, Thompson TA, Grossman D. Medication abortion provided through telemedicine in four U.S. states. Obstet Gynecol. 2019;134(2):343–350. doi: 10.1097/AOG.0000000000003357. [DOI] [PubMed] [Google Scholar]
- 13.Ehrenreich K, Kaller S, Raifman S, Grossman D. Women’s experiences using telemedicine to attend abortion information visits in Utah: A qualitative study. Womens Health Issues. 2019;29(5):407–413. doi: 10.1016/j.whi.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Srinivasulu S, Yavari R, Burbaker L, Riker L, Prine L, Rubin SE. US clinicians’ perspectives on how mifepristone regulations affect access to medication abortion and early pregnancy loss care in primary care. Contraception. 2021;104:92–97. [DOI] [PubMed] [Google Scholar]
- 15.Food US and Administration Drug. Mifeprex risk evaluation and mitigation strategy (REMS) program. December 16, 2021. Accessed December 21, 2021. Available from: https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm [Google Scholar]
- 16.Danco. For Health Professionals Accessed March through May 2020. Available from: https://www.earlyoptionpill.com [Google Scholar]
- 17.Nagendra D, Koelper N, Loza-Avalos SE, Sonalkar S, Chen M, Atrio J, et al. Cost-effectiveness of mifepristone pre-treatment for the medical management of nonviable early pregnancy. JAMA Netw Open. 2020;3(3):e201594. doi: 10.1001/jamanetworkopen.2020.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalton VK, Harris L, Weisman CS, Guire K, Lebovic D. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet & Gynecol. 2006; 108:103–10. [DOI] [PubMed] [Google Scholar]
- 19.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 20.Sanders GD, Maciejewski ML, Basu A. Overview of cost-effectiveness analysis. JAMA. 2019;321(14):1400–1401. doi: 10.1001/jama.2019.1265 [DOI] [PubMed] [Google Scholar]
- 21.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 22.Efron B, Tibshirani R. An Introduction to the Bootstrap. Chapman & Hall; 1994. [Google Scholar]
- 23.Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000;19(23):3219–3236. doi: [DOI] [PubMed] [Google Scholar]
- 24.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. 2nd ed. Oxford University Press; 2013. [Google Scholar]
- 25.Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II: an ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–172. doi: 10.1016/j.jval.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 26.Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves: facts, fallacies and frequently asked questions. Health Econ. 2004;13(5):405–415. [DOI] [PubMed] [Google Scholar]
- 27.Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187:106–108. doi: 10.1192/bjp.187.2.106 [DOI] [PubMed] [Google Scholar]
- 28.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness — the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. . doi: . [DOI] [PubMed] [Google Scholar]
- 29.Thompson A, Singh D, Ghorashi AR, Donovan MK, Ma J, Rikelman J. The disproportionate burdens of the mifepristone REMS. Contraception. 2021;104(1):16–19. doi: 10/1016/j.contraception.2021.05.001 [DOI] [PubMed] [Google Scholar]
- 30.Flynn AN, Shorter JM, Roe AH, Sonalkar S, Schreiber CA. The Burden of the Risk Evaluation and Mitigation Strategy (REMS) on providers and patients experiencing early pregnancy loss: A commentary. Contraception. 2021; 104:29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.