Abstract
Objectives:
To determine the time interval between mifepristone and misoprostol administration associated with the most efficacious early pregnancy loss (EPL) management.
Study Design:
We performed a secondary analysis of a randomized trial. Participants with EPL were instructed to take 200 mg oral mifepristone followed by 800 mcg vaginal misoprostol 24 hours later. The primary outcome was gestational sac expulsion at the first follow-up visit (1–4 days after misoprostol use) after a single dose of misoprostol and no additional intervention within 30 days after treatment. Despite specification of drug timing, participants used the medication over a range of time. We graphed sliding average estimates of success and assessed the proportion of treatment successes over time to define timing interval cohorts for analysis. We used multivariable generalized linear regression to assess the association between time interval and success.
Results:
Of 139 eligible participants, 70 (50.4%) self-administered misoprostol before 24 hours, and 69 (49.6%) at or after 24 hours. We defined the following time intervals: 0 to 6 hours (n = 22); 7 to 20 hours (n = 29); and 21 to 48 hours (n = 88). Success occurred in 96.6% of the 7- to 20-hour cohort compared to 54.6% and 87.5% of the cohorts self-administering misoprostol earlier or later, respectively. When adjusting for race, gestational age, diagnosis, bleeding at presentation, insurance status, and enrollment site, participants administering misoprostol between 0 and 6 hours (adjusted risk ratio 0.58, 95% CI 0.40–0.85) and 21 to 48 hours (adjusted risk ratio 0.91, 95% CI 0.72–0.99) had a lower risk of success when compared to participants administering 7 to 20 hours after mifepristone.
Conclusions:
These data suggest that medical management of EPL has the highest likelihood of success when misoprostol is self-administered 7 to 20 hours after mifepristone.
Implications:
These preliminary data suggest that patients have the highest likelihood of success when misoprostol is taken between 7 and 20 hours after mifepristone. In contrast with medical abortion, simultaneous medication administration may not be as effective as delayed. Future research is needed to confirm the optimal medication time interval.
Keywords: Early pregnancy loss, Medical management, Mifepristone, Miscarriage
1. Introduction
Early pregnancy loss (EPL) occurs in 15% to 20% of clinically recognized pregnancies [1]. Medical management of EPL is a safe alternative to expectant or surgical management, and pretreatment with 200 mg of oral mifepristone followed by 800 mcg of vaginal misoprostol has become the standard of care [2–4].
Mifepristone is a competitive progesterone receptor antagonist, increasing uterine contractility and sensitizing the myometrium to prostaglandin [5]. After oral administration, mifepristone is rapidly absorbed, with peak serum concentration occurring within 60 to 90 minutes of administration, and slowly metabolized, with a half-life between 25 and 30 hours [6, 7]. Given this pharmacokinetic profile, medical abortion investigators have explored shortening the interval between mifepristone and misoprostol and have demonstrated that vaginal administration of misoprostol 6 to 8 hours after mifepristone is as effective as vaginal administration 24 hours after mifepristone [8]. Some studies of medical abortion suggest that mifepristone and vaginal misoprostol may be administered simultaneously [9, 10], however, meta-analyses have shown that the time interval of > 24 hours may actually be most efficacious [11]. Additionally, the literature supports a 24-hour interval between mifepristone and misoprostol for EPL management [3, 12]. Alternative time intervals have been less well studied for EPL than for induced abortion.
During the conduct of a comparative effectiveness trial of mifepristone pretreatment vs. misoprostol alone for EPL management, we noted that many participants did not adhere strictly to the time interval between medications that was indicated by the protocol. Given this observed variability in timing intervals, we sought to preliminarily elucidate the time interval between mifepristone and misoprostol that is associated with the highest treatment success. We hypothesized that, similar to medical abortion, mifepristone and misoprostol could be administered at time intervals shorter than 24 hours without compromising treatment success.
2. Material and methods
We performed a secondary analysis of data from a randomized clinical trial of medical management of EPL, the Comparative Effectiveness of Pregnancy Failure Management Regimens trial [3]. In the primary clinical trial, 300 women diagnosed with EPL from multiple centers who desired medical management were randomized to misoprostol-alone (misoprostol 800 mcg vaginally) or mifepristone-pretreatment (mifepristone 200 mg orally followed by misoprostol 800 mcg vaginally 24 hours later). Women were eligible if they were 18 years or older and diagnosed with a nonviable intrauterine pregnancy between 5 and 12 completed weeks gestation. The primary outcome of the study was treatment success, defined by complete expulsion of the gestational sac on ultrasound evaluation within 1 to 4 days after a single dose of misoprostol and no additional intervention within 30 days after treatment. The current report evaluated data from the mifepristone-pretreatment arm only. Study staff specified that participants should administer misoprostol 24 hours after mifepristone administration and wrote this planned time as a reminder on the participants daily diary card. Despite this protocol, we observed a range of medication time intervals. Staff recorded mifepristone administration time, and participants self-recorded misoprostol administration time through a daily written diary, allowing us to compute the time interval between the two.
We used a Locally Weighted Scatterplot Smoothing (LOWESS) graph to visualize a sliding interval average of the proportion of treatment success over the range of medication use timing intervals [13]. We then defined time interval cohorts for analysis based on our LOWESS graph. Three time intervals were defined from visual inspection of the graph and were driven by the distribution of treatment failures to ensure that at least one failure was present in every interval. Subsequent analysis sought to identify whether subject level factors were associated with the observed success.
Associations between time interval and treatment success were assessed after adjustment for identified covariates using a multivariable generalized linear regression assuming log link with a robust Poisson error structure. We performed bivariate analyses of treatment success by participant characteristics (race, gravidity, parity, age, gestational age, diagnosis, bleeding at time of presentation, insurance status, and enrollment site) and used a backward stepwise selection process for variables with p < 0.20 to obtain our final set of model covariates. Variables with p < 0.05 along with other important clinically important confounders such as gestational age, diagnosis, and bleeding at time of presentation were included in the final model [14, 15]. All data were analyzed using Stata 14.2 (College Station, TX).
3. Results
Of the 149 participants in the mifepristone-pretreatment arm, 148 had medication timing data evaluable for analysis. The majority of participants took misoprostol within 48 hours from mifepristone ingestion, and we chose to limit our analysis to this group (N = 139). Of these participants, 70 (50.4%) self-administered misoprostol before 24 hours, and 69 (49.6%) at or after 24 hours. Using LOWESS graphing, we assessed the proportion of treatment success according to different medication time interval ranges and grouped participants in the following cohorts for analysis based on variability in success rates over time: 0 to 6 hours (n = 22); 7 to 20 hours (n = 29); and 21 to 48 hours (n = 88; Fig. 1). Participants in these timing groups did not differ statistically in age, gravidity, parity, or gestational age. Participants who self-identified as Black/African-American, were without private insurance, and enrolled at the University of Pennsylvania were more likely to administer misoprostol prior to 21 hours (p < 0.01, p < 0.01, and p < 0.01, respectively; Table 1). Gestational age, diagnosis and bleeding had a trend to-ward differences between timing cohorts and these clinical differences are notable even if not statistically significant.
Fig. 1.
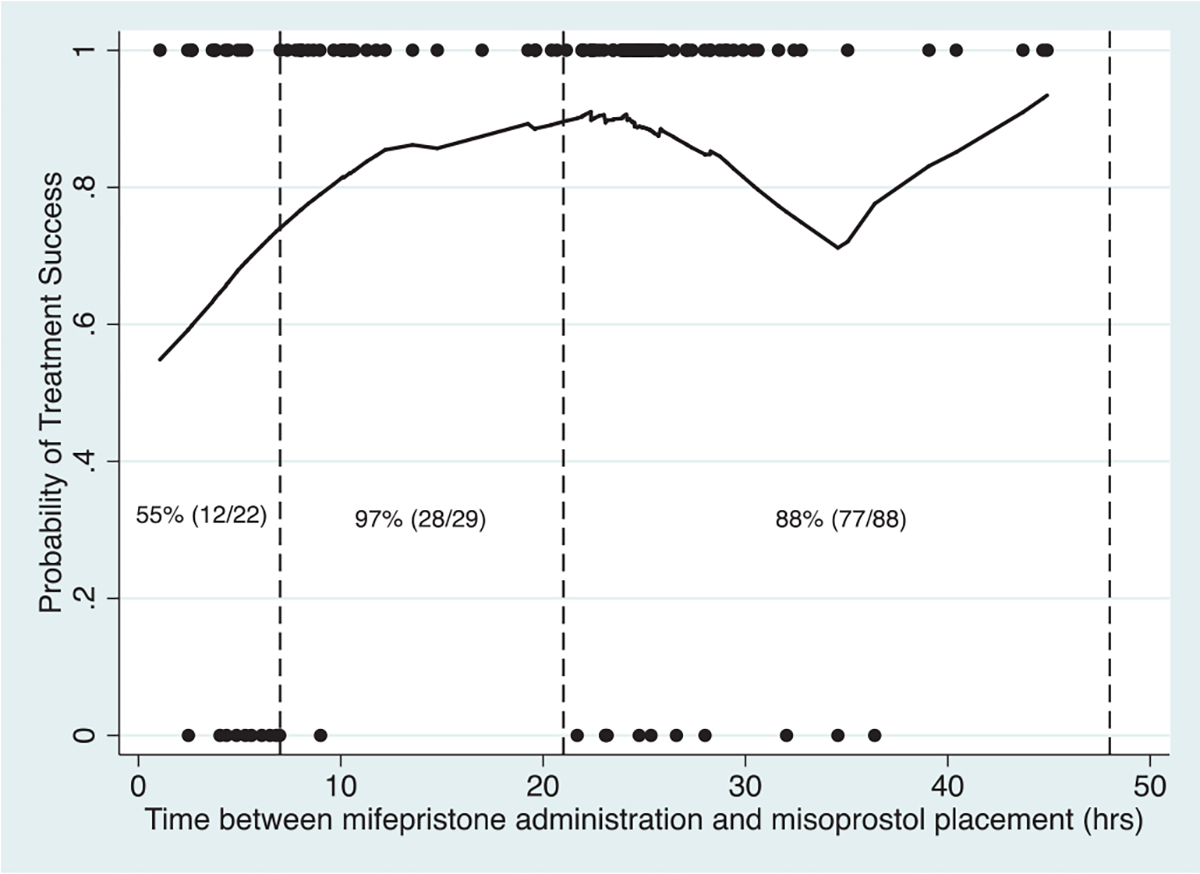
A Locally Weighted Scatterplot Smoothing (LOWESS) curve for early pregnancy loss treatment success by mifepristone and misoprostol administration time interval. Dots indicate observed treatment success (1) or failure (0). The solid line is a sliding average estimate of the probability of treatment success.
Table 1.
Baseline demographic characteristics in a clinical trial of medical management of early pregnancy loss, by mifepristone and misoprostol administration time interval cohorts.
| <7 hours n (%) | 7–20 hours n (%) | 21–48 hours n (%) | |
|---|---|---|---|
|
| |||
| Race | |||
| Black | 16 (72.7) | 19 (65.5) | 25 (28.4) |
| Non-Black | 6 (27.3) | 10 (34.5) | 63 (71.6) |
| Gestational age | |||
| <7 wk | 5 (22.7) | 13 (44.8) | 39 (44.3) |
| 7–8 wk 6 d | 15 (68.2) | 12 (41.4) | 33 (37.5) |
| 9–12 wk 6 d | 2 (9.1) | 4 (13.8) | 16 (18.2) |
| Diagnosis | |||
| Embryonic/fetal demise | 12 (54.6) | 22 (75.9) | 69 (78.4) |
| Anembryonic gestation | 10 (45.5) | 7 (24.1) | 19 (21.6) |
| Bleeding at presentation | |||
| No | 19 (86.4) | 29 (100.0) | 77 (87.5) |
| Yes | 3 (13.6) | 0 (0.0) | 11 (12.5) |
| Gravidity | |||
| 1 | 4 (18.2) | 5 (17.2) | 25 (28.4) |
| 2 | 8 (36.4) | 7 (24.1) | 19 (21.6) |
| 3+ | 10 (45.5) | 17 (58.6) | 44 (50.0) |
| Parity | |||
| 0 | 9 (40.9) | 11 (37.9) | 39 (44.3) |
| 1+ | 13 (59.1) | 18 (62.1) | 49 (55.7) |
| Insurance type | |||
| Private | 6 (28.6) | 9 (31.0) | 51 (58.0) |
| Nonprivate | 15 (71.4) | 20 (69.0) | 37 (42.1) |
| Enrollment site | |||
| University of Pennsylvania | 21 (95.5) | 24 (82.8) | 31 (35.2) |
| University of California, Davis | 1 (4.6) | 0 (0.0) | 35 (39.8) |
| Montefiore Hospital and Albert Einstein College of Medicine | 0 (0.0) | 5 (17.2) | 22 (25.0) |
Treatment was successful in 96.6% of participants (n = 28) in the 7- to 20-hour cohort compared to 54.6% of participants (n = 12) and 87.5% of participants (n = 77) in the 0 to 6 hours and 21 to 48 hours cohorts, respectively. When adjusting for race, gestational age, diagnosis, bleeding at presentation, insurance status, and enrollment site, participants administering misoprostol between 0 and 6 hours (adjusted risk ratio 0.58, 95% CI 0.40–0.85) and 21 to 48 hours (adjusted risk ratio 0.91, 95% CI 0.72–0.99) had a lower risk of success when compared to participants administering 7 to 20 hours after mifepristone (Table 2).
Table 2.
Associations of predictors of interest with early pregnancy loss treatment success.
| RR | aRR | 95% CI | |
|---|---|---|---|
|
| |||
| Timing interval | |||
| 0–6 hours | 0.56 | 0.58 | 0.40–0.85 |
| 7–20 hours | Ref. group | - | |
| 21–48 hours | 0.91 | 0.85 | 0.72–0.99 |
| Race | |||
| Non-Black | Ref. group | - | |
| Black | 0.92 | 1.03 | 0.89–1.20 |
| Gestational age | |||
| <7 wk | Ref. group | - | |
| 7–8 wk 6 d | 0.99 | 1.08 | 0.93–1.25 |
| 9–12 wk 6 d | 0.93 | 1.03 | 0.86–1.23 |
| Diagnosis | |||
| Embryonic/fetal demise | Ref. group | - | |
| Anembryonic gestation | 0.94 | 0.99 | 0.84–1.18 |
| Bleeding at presentation | |||
| No | Ref. group | - | |
| Yes | 1.06 | 1.01 | 0.82–1.24 |
| Insurance | |||
| Nonprivate | Ref. group | - | |
| Private | 1.14 | 1.09 | 0.90–1.32 |
| Enrollment site | |||
| University of Pennsylvania | Ref. group | - | |
| University of California, Davis | 1.15 | 1.14 | 0.92–1.40 |
| Montefiore Hospital and Albert Einstein College of Medicine | 1.04 | 1.05 | 0.84–1.30 |
aRR, adjusted risk ratio from multivariable estimated from a generalized linear model with a log link and robust Poisson error variance; Ref. group, reference group; RR, risk ratio.
4. Discussion
The purpose of this study was to explore the relationship between medication timing and EPL treatment success. Other factors that have been previously shown to modulate the success of medical management of EPL were accounted for within our adjusted model including gestational age, diagnosis, and bleeding at the time of presentation [14, 15]. We found that the variability in success rates remained according to medication timing even after adjusting for these covariates. Therefore, we conclude that medical management of EPL is most likely to be effective when misoprostol is self-administered between 7 and 20 hours after ingestion of mifepristone.
We hypothesized that the 24-hour time interval between misoprostol and mifepristone could be shortened based on previous medical abortion research. In a randomized control trial comparing misoprostol timing in medical abortion, women were pretreated with mifepristone and randomized to self-administered vaginal misoprostol at 6 to 8 hours or 23 to 25 hours later. Completed abortion rates were equivalent between the 6 to 8 hour group (503/525; 95.8%) and the 23 to 25 hour group (521/531; 98.1%), indicating that for medical abortion misoprostol can be taken sooner than 24 hours after mifepristone without compromising treatment efficacy [8]. Prior studies of mifepristone and misoprostol in the management of EPL have utilized protocols with time intervals of 36 to 48 hours [16, 17], but intervals shorter than 24 hours have not been studied. In the current study, participants in the 0- to 6hour group experienced the lowest frequency of treatment success, suggesting that mifepristone requires some time to act and effectively prime the uterus for misoprostol in EPL. Based on these exploratory data, simultaneous administration of mifepristone and misoprostol may not be effective for management of EPL.
Our protocol stipulated that participants use misoprostol 24 hours after mifepristone, but despite this nearly half of participants used misoprostol prior to that time. In particular, participants who self-identified as Black/African-American and who lacked private insurance were significantly more likely to self-administer misoprostol at a shorter time interval. Participants at the University of Pennsylvania were also more likely to administer misoprostol prior to 21 hours. However, all participants received standardized counseling across the 3 sites and a reminder note on their daily diary card of the planned time to insert misoprostol. Future research is needed to better understand patient priorities during medical management of EPL in order to provide both effective and patient-centered care.
This was a secondary analysis in which we examined data from a clinical trial to identify a possible relationship between effectiveness and medication timing interval. One limitation of this study design was our reliance upon participant self-report of medication use, which may have been inaccurate due to social desirability bias or recall bias. In addition, due to the high efficacy of the mifepristone pretreatment regimen, the total number of clinical failures was small (n = 22), which reduced our power to evaluate a more refined evaluation of timing on treatment success. Future prospective research in a larger cohort might compare medication time intervals defined a priori to confirm the optimal time interval between mifepristone and misoprostol in the management of EPL.
Mifepristone leads to improved efficacy of medical management of EPL and should be offered whenever medical management is considered. These preliminary data suggest that administration of misoprostol 7 to 20 hours after ingesting mifepristone is most associated with successful treatment.
Funding:
This work was supported in part by the National Institute of Health (R01 HD071920–0, Dr Schreiber).
Footnotes
Declaration of Competing Interest: Dr Schreiber has received consulting fees from Danco laboratories. No other potential conflict of interest relevant to this article was reported.
References
- [1].Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep 2012;60(7):1–21. [PubMed] [Google Scholar]
- [2].Trinder J, Brocklehurst P, Porter R, Read M, Vyas S, Smith L. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (miscarriage treatment (MIST) trial). BMJ 2006;332(7552):1235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schreiber CA, Creinin MD, Atrio J, Sonalkar S, Ratcliffe SJ, Barnhart KT. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med 2018;378(23):2161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Prager S, Dalton V, Allen RH, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 200: early pregnancy loss. Obstet Gynecol 2018;132(5):e197–207. [DOI] [PubMed] [Google Scholar]
- [5].Gemzell-Danielsson K, Bygdeman M, Aronsson A. Studies on uterine contractility following mifepristone and various routes of misoprostol. Contraception 2006;74(1):31–5. [DOI] [PubMed] [Google Scholar]
- [6].Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med 1997;48:129–56. [DOI] [PubMed] [Google Scholar]
- [7].Heikinheimo O, Kekkonen R, Lahteenmaki P. The pharmacokinetics of mifepristone in humans reveal insights into differential mechanisms of antiprogestin action. Contraception 2003;68(6):421–6. [DOI] [PubMed] [Google Scholar]
- [8].Creinin MD, Fox MC, Teal S, Chen A, Schaff EA, Meyn LA. A randomized comparison of misoprostol 6 to 8 hours versus 24 hours after mifepristone for abortion. Obstet Gynecol 2004;103(5 I):851–9. [DOI] [PubMed] [Google Scholar]
- [9].Schmidt-Hansen M, Lord J, Hasler E, Cameron S. Simultaneous compared to interval administration of mifepristone and misoprostol for medical abortion up to 10(+0) weeks’ gestation: a systematic review with meta-analyses. BMJ Sex Reprod Health 2020;46(4):270–8. [DOI] [PubMed] [Google Scholar]
- [10].Li YT, Hsieh JC, Hou GQ, Chen TH, Chu YC, Lin TC, et al. Simultaneous use of mifepristone and misoprostol for early pregnancy termination. Taiwan J Obstet Gynecol 2011;50(1):11–14. [DOI] [PubMed] [Google Scholar]
- [11].Kulier R, Kapp N, Gülmezoglu AM, Hofmeyr GJ, Cheng L, Campana A. Medical methods for first trimester abortion. Cochrane Database Syst Rev 2011(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schreiber CA, Creinin MD, Reeves MF, Harwood BJ. Mifepristone and misoprostol for the treatment of early pregnancy failure: a pilot clinical trial. Contraception 2006;74(6):458–62. [DOI] [PubMed] [Google Scholar]
- [13].Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74(368):829–36. [Google Scholar]
- [14].Creinin MD, Huang X, Westhoff C, Barnhart K, Gilles JM, Zhang J. Factors related to successful misoprostol treatment for early pregnancy failure. Obstet Gynecol 2006;107(4):901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Robledo C, Zhang J, Troendle J, Barnhart K, Creinin MD, Westhoff C, et al. Clinical indicators for success of misoprostol treatment after early pregnancy failure. Int J Gynaecol Obstet 2007;99(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nielsen S, Hahlin M, Platz-Christensen J. Randomised trial comparing expectant with medical management for first trimester miscarriages. BJOG 1999;106(8):804–7. [DOI] [PubMed] [Google Scholar]
- [17].Wagaarachchi PT, Ashok PW, Narvekar N, Smith NC, Templeton A. Medical management of early fetal demise using a combination of mifepristone and misoprostol. Hum Reprod 2001;16(9):1849–53. [DOI] [PubMed] [Google Scholar]


