Abstract
French Guiana (FG), a French overseas territory in South America, is susceptible to tropical diseases, including arboviruses. The tropical climate supports the proliferation and establishment of vectors, making it difficult to control transmission. In the last ten years, FG has experienced large outbreaks of imported arboviruses such as Chikungunya and Zika, as well as endemic arboviruses such as dengue, Yellow fever, and Oropouche virus. Epidemiological surveillance is challenging due to the differing distributions and behaviors of vectors. This article aims to summarize the current knowledge of these arboviruses in FG and discuss the challenges of arbovirus emergence and reemergence. Effective control measures are hampered by the nonspecific clinical presentation of these diseases, as well as the Aedes aegypti mosquito’s resistance to insecticides. Despite the high seroprevalence of certain viruses, the possibility of new epidemics cannot be ruled out. Therefore, active epidemiological surveillance is needed to identify potential outbreaks, and an adequate sentinel surveillance system and broad virological diagnostic panel are being developed in FG to improve disease management.
Keywords: arbovirus, French Guiana, Dengue virus, Chikungunya virus, Zika virus, Yellow fever virus, Mayaro virus, Tonate virus, Oropouche virus
1. Introduction
French Guiana (FG) is a French overseas territory located on the Northeastern coast of South America and is part of the outermost regions of Europe. Due to its geographical location, it is prone to tropical diseases, including both endemic and epidemic arboviruses, but it also benefits from health facilities with European standards. Arthropod-borne viruses (arbovirus) refer to viruses that are maintained in nature through biological transmission between a susceptible vertebrate host and a hematophagous arthropod. Some of these viruses cause zoonoses that rely on a reservoir of non-human animal species for sustenance [1]. Tropical areas have a history of being heavily impacted by arboviruses, as the environment is favorable for the proliferation and establishment of the vectors involved in transmission. In some tropical areas, poor housing conditions or sanitation may increase the level of contact between human recipients and vectors, particularly in urban areas [2]. While the tropical climate may support the growth and diversity of arthropods, the epidemic transmission of arbovirus still depends on the combination of the arbovirus and its competent vector (anthropophilic and urban) [3].
Arbovirus infections occur in two forms: endemic (with sporadic cases) and epidemic forms (often imported). In 2014 and 2016, Chikungunya (CHIKV) and Zika (ZIKV) viruses were imported to the Americas, including FG, and caused large outbreaks. Millions of people were infected. On the other hand, some autochthonous arboviruses, known for many years, have managed to maintain themselves without ever giving rise to large-scale outbreaks on the French territory, such as the Oropouche virus (OROV) or Mayaro virus (MAYV). Of course, these manifestations, determined by the distribution and behavior of their vectors, may differ, which makes epidemiological surveillance more complex [4].
During the last decade, seven arboviruses involving human cases have particularly shed light on them in a more or less striking fashion: Dengue virus (DENV), CHIKV, ZIKV, Yellow fever virus (YFV), MAYV, Tonate virus (TONV), and OROV. The objective of this article was to summarize the current and most recent knowledge on these arboviruses in FG focusing on local specificities, and to discuss the future challenges of arbovirus infection epidemiology.
2. State of the Art on the Seven Main Arboviruses in French Guiana
2.1. Dengue Virus
DENV belongs to the Flaviviridae family and the genus Flavivirus. Currently, Aedes aegypti is the primary vector identified for DENV transmission in FG [5]. When looking at the past dengue fever epidemics recorded in FG, the etiology should be considered with a certain distrust; indeed, until the 1980s, dengue diagnosis was based only on clinical manifestations. In fact, it was recently shown that several epidemics described in the 19th century attributed to DENV were possibly due to another arthritis-inducing Alphavirus such as CHIKV, the only known pandemic Alphavirus, or MAYV [6]. Historically, the first confirmed DENV epidemic occurred in 1969–1970: Dengue 2 virus was isolated and serological conversions were noted in three patients [7]. From 1970 to 1977, Dengue virus was not isolated. Since then, dengue epidemics have followed with an ever increasing number of cases [8,9]. Since the early 2000s, dengue has transitioned from an endemic to a hyperendemic mode in FG, with the co-circulation of different serotypes. Regular outbreaks occur every 3–5 years, although this periodicity was slightly extended during the successive emergence of CHIKV and ZIKV (Table 1). The dominant serotypes change from one epidemic to another: all the DENV serotypes have been circulating in French Guiana, with a lower representation of DENV4. DENV1 has been predominant in 2009 and 2020–2021, DENV2 in 2013, and DENV3 during the 2005–2006 epidemics. In April 2023, some cases were reported, mainly DENV3 [10]. Notably, the four serotypes of DENV were detected through reverse transcriptase polymerase chain reaction (rtPCR) in wild mammals order Rodentia, Marsupialia, and Chiroptera in sites with and without human dengue cases investigated in FG [11].
Table 1.
Summary of seroprevalence and incidence data for the 7 main arboviruses of interest in French Guiana.
| Arbovirus (Acronym) |
Family (Genus) |
Main Vectors |
Sero- Prevalence |
Epidemiological Data (Number of Cases) |
|---|---|---|---|---|
|
Yellow fever (YFV) |
Flaviviridae
(Flavivirus) |
Haemagogus and Sabethes +/− Aedes aegypti |
95.0% | 1 in 1998 2 deaths in 2017–2018 2 deaths in 2020 |
|
Dengue (DENV) |
Flaviviridae
(Flavivirus) |
Ae.aegypti | 73.1% | 2005–2006 (DENV3 > DENV2): 13,700–16,200 2009 (DENV1 > DENV 4): ~7800 2013 (DENV2 > >DENV4): ~16,000 2020–2021 (DENV1 > >DENV2): ~10,000 |
|
Zika (ZIKV) |
Flaviviridae
(Flavivirus) |
Ae.aegypti | 23.3% | 2015–2017: ~9700 |
|
Chikungunya (CHIKV) |
Togaviridae
(Alphavirus) |
Ae.aegypti | 20.3% | 2014–2015: ~16,000 |
|
Mayaro (MAYV) |
Togaviridae
(Alphavirus) |
Haemagogus spp. Ae.aegypti |
3.3% | 17 from 2003 to 2019 ~15 in 2020 |
|
Tonate (TONV) |
Togaviridae
(Alphavirus) |
Culex portesi | 11.9% | 45 from 2003 to 2016 |
|
Oropouche (OROV) |
Peribunyaviridae
(Orthobunyavirus) |
Culicoides spp. Culicoides paraensis Culex quinquefasciatus? |
ND | 41 to 58 in August September 2020 in Saül |
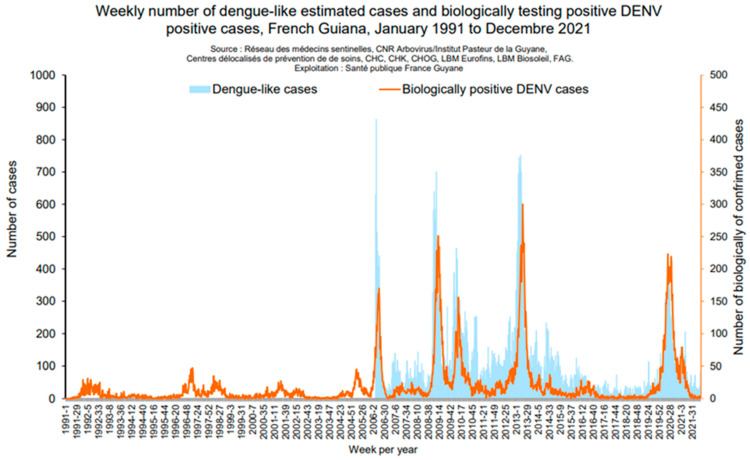
During the non-epidemic phases, particularly between 2013 and 2019, reported cases were based on positive anti-DENV IgM only, and no autochthonous case was diagnosed through PCR. This highlights that endemic circulation appears to be very limited because cases diagnosed through IgM serodiagnosis are not confirmed and may reflect past infections with persistent detectable IgM, immunological reactivations caused by a nonspecific acute infectious syndrome, or cross-reactivity with other flaviviruses such as YFV or ZIKV. These cross-reactivity phenomena may be amplified in populations with a history of exposure to flavivirus through vaccinations or infections [12]. In recent years, outbreak frequency has been rising as shown by the occurrence of outbreaks in 2006, 2010, 2013, and 2020–2021. The number of clinically suggestive cases ranged from 10,000 to 18,000 depending on the outbreak. The latest outbreak started in January 2020 and ended in June 2021 and was primarily caused by DENV-1. This outbreak was concurrent with the SARS-CoV-2 pandemic which settled in FG from May 2020. The number of suggestive cases was around 10,891 (against 13,240 in 2013), for an incidence rate of 38/1000 inhabitants. During this outbreak, 282 patients were hospitalized (against 701 in 2013) and 3 deaths were recorded, 2 of which were indirectly linked to dengue (Figure 1). There has probably been a significant underestimation of the number of dengue cases in French Guiana during the 2020–2021 epidemic. Indeed, at that time, the slightest fever was considered to be a SARS-CoV-2 infection until proven otherwise, with extensive protective measures for health care staff which meant that blood samples were only taken on a drip basis. Access to care was limited due to the shutdown of many hospital activities, and the fever pathway to test patients for both dengue and coronavirus disease 2019 struggled to be established. It is difficult to know whether the lockdown has contributed to the dengue epidemic. It can be assumed that patients, forced to stay at home, were more susceptible to attacks by Aedes growing in the peri-domestic breeding grounds. In addition, the mosquito control service of the Collectivité Territoriale de French Guiana reports the continuity of activities during the coronavirus disease 2019 period. It is therefore difficult to support a decrease in mosquito control activity, in view of this report, as the consumption of deltamethrin in 2020 was noted higher than in previous years, greater than in 2014 and equivalent to 2016.
Figure 1.
Epidemic curves for DENV epidemics (Santé Publique France).
The seroprevalence in 2017 was estimated at 73.1% in the general French Guianese population, all serotypes combined [13]. FG, as with many tropical regions, is affected by infectious diseases that share many common clinical and biological features at some point in their natural history, such as Dengue, Chikungunya, and Zika virus infections, malaria, primary HIV infection, Q fever, leptospirosis, and hantavirus. Therefore, a specific biological assessment is mandatory to confirm diagnoses and discard differential diagnoses that would justify specific treatment. Studies have recently shown that a C-reactive protein (CRP) > 50 mg/l is highly suggestive of a diagnosis other than dengue, such as leptospirosis or malaria [14,15]. Rapid diagnostic tests are also useful tools which are already used in remote areas of FG where physicians face unspecified febrile illness and do not have easy access to laboratory facilities. To date, no dengue vaccination program has been implemented in FG yet. A recent statement by the French High Authority for Health has specified that FG is a high endemic area and that DENV vaccination with the live attenuated vaccine DENGVAXIA (SANOFI) can be proposed to immunocompetent people aged between 6 and 45 years old, following the extension in December 2021 of the marketing authorization granted by the European Commission, if a previous DENV infection can be demonstrated to avoid a risk of primoimmunization. However, because no robust and validated test for the diagnosis of past DENV exists yet, there is no recommendation for a large vaccination program in FG, as well as in other overseas French regions. The European Medicines Agency gave a positive opinion on 14 October 2022. A marketing authorization was granted by the European Commission on 8 December 2022 for use in people aged 4 years and older, regardless of previous exposure to the infection. QDENGA is a live attenuated chimeric recombinant vaccine, consisting of four recombinant viruses constructed on the basis of Dengue virus 2 and expressing the surface proteins of the four Dengue viruses (DEN 1 to DEN 4). During its development, it was shown to be effective in preventing infection and severe forms of the disease requiring hospitalization. The protective efficacy against these severe forms has been estimated at 90.4% and is maintained at over 80% 4.5 years after vaccination. The vaccine was generally well tolerated and no significant risks were identified throughout the trials. Remarkably, no worsening of dengue cases was observed in vaccinated individuals, a phenomenon that led to limitations in the use of the DENGVAXIA vaccine produced by Sanofi Pasteur, licensed since 2018. The vaccine regimen consists of two subcutaneous injections of the QDENGA vaccine 3 months apart. For France, it is now up to the High Authority for Health to specify the place and recommendations for the use of this new vaccine.
2.2. Chikungunya Virus
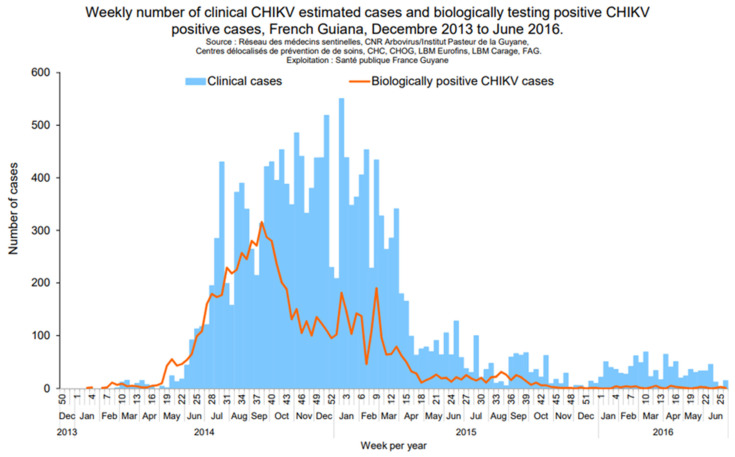
CHIKV belongs to the Togaviridae family and the Alphavirus genus. As DENV, Ae. aegypti is the main vector identified in FG. CHIKV infection is characterized by a dengue-like syndrome associated with joint pain and swelling. In French Guiana, the biological diagnosis relies on PCR at the early stage (D0 to D7) and on IgM detection (from D5). Between 2014 and 2015, the American continent faced an unprecedented epidemic of infection by CHIKV. Two lineages were identified during the epidemic: Asian and ECSA lineage; the latter is currently only established in Brazil and Paraguay and has not been identified in FG [16]. After the first autochthonous cases of CHIKV infection reported in Saint Martin (French West Indies) at the end of 2013, the first autochthonous cases from the South American continent were reported in FG in February 2014. The estimated total number of cases recorded by Santé Publique France was about 16,000 cases (Figure 2). No autochthonous case has been reported in French Guiana since April 2015.
Figure 2.
Epidemic curves for CHIK epidemics (Santé Publique France).
In 2021, the seroprevalence in the general French Guianese population was estimated at 20.3% [13]. This gap between recorded cases and the seroprevalence (that estimates around 55,000 people had CHIKV infections) highlights that only 25% of CHIKV infection cases were reported by the surveillance system. This was probably due to asymptomatic or paucisymptomatic cases, cases considered as dengue by clinicians, as well as a low level of healthcare use in the epidemic’s context, or renouncing care, a significant problem in FG [17]. The reported symptoms were relatively similar to those described in La Reunion Island, Indian Ocean, with a picture of high-grade fever and distal joint pain at the onset of the disease. However, atypical and/or severe cases were notified with neurological forms, encephalitis or Guillain-Barré syndrome, septic shock due to CHIKV or thrombotic thrombocytopenic purpura [5,18]. Although CHIKV has not been reported in FG and the West Indies since 2016, apart from rare, imported cases, the risk of a new epidemic in the mid-term remains real. Indeed, Brazil has been facing new epidemics since 2020 with several tens of thousands of cases recorded (mainly due to the ECSA lineage) [19]. Thus, any fever with arthralgia on return from FG should raise suspicion of arbovirus infection and CHIKV should be investigated. In addition, a virus similar to Chikungunya, the Mayaro virus (see below), which belongs to the same viral genus, has a very similar clinical presentation and is endemic in FG, and must be evoked in the event of a “CHIKV-like” attack.
2.3. Zika Virus
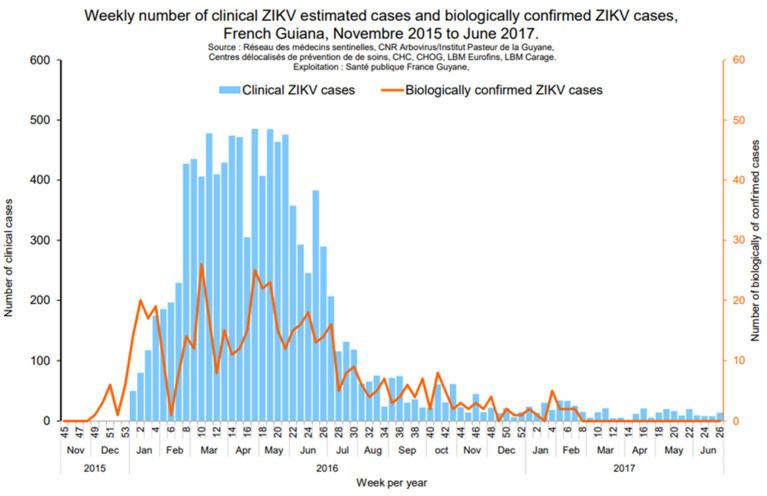
The ZIKV epidemic, which began in the Pacific in 2013, spread to Brazil and then to the entire American continent by 2015. After DENV-related outbreaks in 2013 and CHIKV-related outbreaks in 2014–2015, an outbreak due to the emerging ZIKV (Flavivirus genus) and transmitted by Ae. aegypti occurred in FG, in 2015–2017, where 23% of the population was infected, with only 26% reporting symptoms [20] (Figure 3). ZIKV infection seemed completely benign until 2014, when more worrying forms were described, first in French Polynesia and then in Brazil [21]. These severe forms included neurological disorders in adults, with an over-incidence of Guillain-Barré syndrome [22], and fetal and congenital damage, illustrated by severe microcephaly in fetuses or newborns whose mothers were infected during pregnancy [23]. While the initial data were extremely alarming, it would appear that the burden of ZIKV was lower than initially described. In fact, while significant, with around 15% of children exposed in utero to ZIKA, the complication rates appear similar to those of other congenital infections such as cytomegalovirus (CMV) infection [24]. The Saint Laurent du Maroni hospital, in western FG, has been actively sharing their experience on the maternal–infant impact of ZIKV infection [25]. A recently published study, in which 24 of the 546 investigated pregnant women lived in FG, assessed the risk of adverse pregnancy and early childhood likely related to in utero ZIKV exposure and found that among the 555 fetuses and infants, the overall risk was 15.7% (9.4% for mild abnormalities and 3.6% for severe sequelae or fatal outcomes) [26]. In another study assessing the long-term neurological development of children diagnosed with congenital ZIKV infection at birth in FG, 129 children exposed to ZIKV in utero, born and managed in western FG were followed for up to 3 years of life. Compared to those who tested negative at birth, infected neonates had a higher risk of adverse neonatal and early infantile outcomes (aRR 10.1 [3.5–29.0]), neurological impairment, neurosensory alterations or delays in motor acquisition (aRR 6.7 [2.2–20.0]), and suspected neurodevelopmental delay by three years of life (aRR 4.4 [1.9–10.1]) [25]. In 2015–2017, routine serologic screening and enhanced ultrasound surveillance were recommended during pregnancy. In case of confirmed infection, a close postnatal follow-up was implemented. Of note, no case of ZIKV infection has been detected in FG since March 2017 despite regular molecular screening in patients with dengue-like cases. Indeed, ZIKV PCR and/or IgM are systematically screened when a request for an arbovirus diagnosis is sent to the associated National Reference Centre for arboviruses located at the Institut Pasteur in French Guiana in Cayenne. However, it is no longer systematically sought in the three main hospitals of Cayenne, Kourou and Saint Laurent du Maroni. No imported case has been reported, and, according to our knowledge, currently the ZIKV does not appear to be circulating in neighboring regions.
Figure 3.
Epidemic curves for ZIKV epidemics (Santé Publique France).
2.4. Yellow Fever Virus
YFV can lead to a serious infection manifested by a hepatorenal hemorrhagic syndrome. It is a Flavivirus native to Africa and imported through triangular trade and circulating in both Africa (95%) and South America where it has become endemic [27]. It can be responsible for large epidemics, for example, those in Brazilian Southeast states, such as Minas Gerais, Espírito Santo, Rio de Janeiro, and São Paulo in 2016–2018, or more recently in 2021 in Venezuela [28,29]. An international certificate of immunization against Yellow fever is required for residents of FG and travelers wishing to go there. The certificate is valid for life after a single injection (WHO 11 July 2016), with the exception of immunocompromised persons, persons traveling to a country where active circulation of the virus is reported, women who have been vaccinated during pregnancy, and children over the age of 6 who received their first injection before the age of 2 [30]. The vaccine is recommended from 9 months of age for children traveling to or living in countries at risk. In French Guiana, the yellow fever vaccine Stamaril® (Sanofi Pasteur) is used. In 2017, a vaccine coverage survey was conducted among 2697 individuals from the 22 municipalities of FG. YFV vaccination coverage was estimated at 95.0% (95% CI: 93.4–96.2) but with spatial heterogeneity. The lowest coverage levels were in the western part of the territory along the Surinamese cross-border region, particularly in children not enrolled in school, immigrant adults, and people with low resources [31].
There is no proof that yellow fever occurred in FG before 1763, and thus the introduction of the virus was through the slave trade with the forced arrival in the Americas of people coming from Africa [27]. The first report of “vomito negro” appears during the Kourou expedition in 1763. From 1763 to 1888, yellow fever was reported repeatedly [32]. Epidemics were observed in 1765, 1793–1796, 1802, 1850, 1855 (with 27.2% mortality), 1873–1874, 1876, 1877, and 1885. In 1902, an epidemic started in Saint-Jean-du-Maroni and reached Cayenne in a few months: 471 cases were recorded with 139 deaths. Ae. aegypti was likely the main vector during these epidemics but the roles played by Haemagogus and Sabethes during a sylvatic cycle should not be discarded. No other sylvatic Aedes species have been implicated in the sylvatic transmission of YFV in our territory. After that, no human cases of yellow fever were reported from 1902 to 1998, when the first fatal case was reported in a Wayana Amerindian woman in the upper Maroni River [33]. No cases were reported for 20 years until 2017. Four cases have been reported since then: two cases in 2017 and 2020 in Brazilian illegal gold miners working in forest camps, one case in 2020 in a Wayana Amerindian teenager from the upper Maroni River who was vaccinated against yellow fever in childhood but did not receive any booster thereafter and was co-infected with coronavirus disease 2019, and, finally, one case in 2018 in a Swiss citizen who was not vaccinated [34,35]. All of these cases were fatal, which suggests that the circulation of this virus is under-detected, due to its sylvatic cycle, and that milder cases are not detected and reported.
The zoonotic circulation of YFV has been documented in FG, although such studies are rare and date back to the 1990s. A wildlife rescue operation at the Petit Saut hydroelectric dam in 1994–1995 involved collecting blood samples from 574 individuals of 27 species, under veterinary control. The results showed that 10 species of various animal orders had yellow fever sero-neutralizing antibodies, including golden-handed tamarin, white-faced saki, and red howler monkeys (primates), agouti and porcupine (rodents), peccary (artiodactyl), tayra (carnivore), two-toed and three-toed sloths, and anteater (xenarthrans). Among the species tested, howler monkeys had the highest exposure to YFV, with a sero-neutralizing antibody prevalence of 18%. All of the infected animals were adults, and no obvious clinical effects were observed [36].
2.5. Mayaro Virus
MAYV is an Alphavirus of the Togaviridae family related to CHIKV and was first described in Trinidad in 1954. Its main vector is a sylvatic Haemagogus mosquito but Ae. aegypti has also been implicated in transmission to the human host. Ae. aegypti is a competent vector of MAYV under laboratory conditions [37], and has been found naturally infected in parks and gardens within urban areas [38]. Research has also shown that Aedes albopictus, another important human disease vector, is able to transmit MAYV to mice in the laboratory [39]. Other mosquito species from the genus Mansonia, Culex, Sabethes, Aedes, and Psorophora have also been implicated as potential vectors of MAYV [40]. MAYV has circulated in Latin America, causing several outbreaks in the Amazon region of Venezuela, Peru, Bolivia, and Brazil. It was first isolated in FG in 1996 [41]. A country-scale study recently showed a seroprevalence ranging from 1% in Cayenne to 23.5% in some isolated communes of the upper Oyapock River and upper Maroni River [13]. A retrospective study identified 17 human cases between 2003 and 2019, mostly acquired in the deep forest [42]. The clinical and biological picture was similar to CHIKV infection with fever and arthralgia. One patient had acute meningoencephalitis, and four had persistent arthralgias. On 13 October 2020, the French health authorities officially reported 13 laboratory-confirmed cases of Mayaro fever in FG. In September 2020, the Institut Pasteur de la Guyane (IPG) (associated laboratory of the French National Reference Center for arboviruses) identified two cases of MAYV infection in patients with joint paints confirmed by rtPCR and one probable case found positive for MAYV antibodies. rtPCR was positive in 13 out of 97 tested samples collected from patients with dengue-like symptoms between 15 July and early October. The onset of symptoms for the 13 confirmed cases ranged from 18 July to 29 September 2020. Of the 13 confirmed cases, 11 lived in the urban coastal area including 9 from the Cayenne area (Cayenne: 1, Rémire: 4, Matoury: 4), 1 from Kourou, and 1 from Montsinery-Tonnegrande. Only two cases lived in a rural/sylvatic area, both in Roura (including one in the village of Cacao, located further in the interior). The age of these cases ranged from 11 to 68 years old (median = 40 years old) and the male to female sex ratio was 1.2:1. The detection of 13 confirmed cases within less than 3 months was thus unusual, as well as the transmission in urban settings in 11 out of 13 (85%) of the identified cases. MAYV must therefore be evoked in the presence of febrile arthralgia in patients living in or returning from Latin America [43]. This arbovirus had also been mentioned as being a virus with potential epidemic bursts [44].
2.6. Tonate Virus
TONV is also an Alphavirus of the Togaviridae family. This virus is regularly detected in FG and belongs to sub-type IIIb of the Venezuelan equine encephalitis virus complex. It was first described in 1973 in FG, in a bird, the crested oropendola (Psarocolius decumanus), in the village of Tonate, near Cayenne, and then found in several species in FG and Suriname, including mosquitoes (Anopheles, Culex) and phlebotomine sand flies (Lutzomyia) [45]. It has been isolated in specimens of Cliff Swallow nest bugs (Oeciacus vicarius) and in nestling House Sparrows (Passer domesticus) and Cliff Swallows (Petrochelidon pyrrhonota), which are nestling birds in Colorado and South Dakota [46]. More recently, a study allowed the detection of TONV neutralizing antibodies in four species of bats and the isolation of a TONV strain from a fringe-lipped bat (Trachops cirrhosis) serum sample [47]. Although there are a few human publications in Guianese patients, the first of which were two cases reported in 1973 and 1975, this human infection has never been reported outside FG. Two serological studies in the population, one in the 1970s and the other in the 1990s, showed average seroprevalence rates of around 11–14%, with very wide geographical variations from 0 to 35%, the highest rates being found on the coastal plains [48,49]. A retrospective study was carried out on 45 cases identified at the National Reference Center for Arbovirus that were managed in Cayenne Hospital and health centers in remote areas between 2003 and 2016 [50]. The infection mainly affected young men and the symptoms most frequently observed were fever, chills, headache, and diffuse pain. As with MAYV, the biological work-up was not very specific, with lymphopenia found in about 20% of cases, and a CRP above 50 mg/L in 20% of cases. An acute meningoencephalitis with pleomorphic fluid associated with hyperproteinorachia at 1.52 g/L, and a spontaneously favorable evolution was reported. No deaths were reported. The only serious case in the literature is a fatal encephalitis in a 2-month-old child published in 1998 [50]. Recently, the Saint-Laurent-du-Maroni team reported for the first time a case of vertical transmission of the Tonate virus in a pregnant woman in FG. The fetus presented severe necrotic and hemorrhagic lesions in the brain and spinal cord [51]. TONV should therefore be considered in the presence of a dengue-like picture, as well as in the presence of an undocumented central nervous system infection.
2.7. Oropouche Virus
OROV is an arbovirus of the Peribunyaviridae family first identified in humans in 1955 in Trinidad and Tobago and usually transmitted by biting midges of the genus Culicoides. Several epidemics have been reported in Latin America, particularly in Brazil, Peru, and Ecuador [52]. In August and September 2020, in the midst of the coronavirus disease 2019 epidemic, around 50 cases were reported for the first time in FG, among the inhabitants of the small village of Saül, located in the heart of the Amazonian forest, with an estimated attack rate of between 43 and 61% [53]. The symptomatology was aspecific, accompanied by fever, headache, and diffuse pain, rarely leading to serious cases. This OROV epidemic resulted in hospital admission for three people, including one for acute lymphocytic meningitis; all of them had a favorable outcome. The reason for the emergence of this arbovirus is not known. One of the advanced hypotheses is the possible presence of Brazilian gold panners, originating from Brazilian states where OROV circulates more regularly, and is present in greater numbers around the village, due to the interruption of anti-panning patrols because of the confinement linked to coronavirus disease 2019. Nevertheless, this virus circulates regularly in Brazil, which indicates a well-established sylvatic cycle; this occurrence cannot be excluded in FG in areas far from inhabited zones, with possible spillover being less frequent than in Brazil. The entomologist’s initial investigation following the outbreak did not allow the identification of the potential vector of this cluster. Only one individual of the main known vector, Culicoides paraensis (Goeldi, 1905), was found during this entomological investigation, which was not performed immediately after the epidemic. At that time, numerous Culex quinquefasciatus (Say, 1823) were found, and suspected as vectors of this arbovirus. Indeed, although the main vector of OROV is Cx. paraensis, mosquitoes of the genus Culex and notably Cx. quinquefasciatus, which is very present on the littoral, have shown a slight degree of vectorial competence in the laboratory. Finally, two years later, at the same season as the outbreak, the presence of abundant Culicoides paraensis was proven within the village (Institut Pasteur de la Guyane’ works), confirming its probable past vector role [54].
3. Challenges for the Future
3.1. Increase in Factors Favoring Arbovirus Epidemics
The population of FG is increasing rapidly with a birth rate of 3.53 children per woman (vs. 1.8 children per woman in France). Thus, combined with growing poverty (53% of people living below the poverty line in FG in 2020) and a massive urbanization of FG, this demographic expansion will be challenging for the public authorities to avoid, or at least limit the expansion of anthropophilic vectors such as Ae. aegypti. Therefore, specific actions to reduce the number of larvae breeding sites, promote access to drinking water in order to limit water storage, offer efficient household waste collection services, and improve the awareness through efficient educational programs within communities or at school are important [55]. We can fear regular epidemics due to the decrease in the collective immunity of the population below the thresholds, allowing the occurrence of epidemics. Moreover, FG has a highly mobile population making the risk of introducing new arboviruses into the country real, as we have experienced in recent years [56]. It is important to identify them in order to pursue an effective and adapted health watch.
3.2. Despite Common Characteristics, a Great Heterogeneity
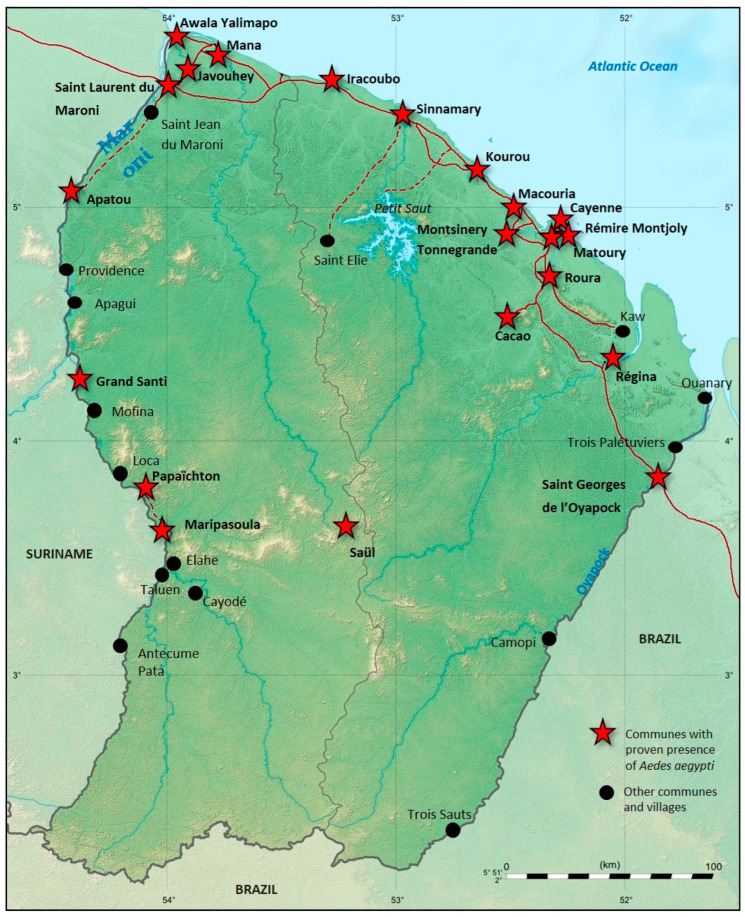
“Cosmopolitan” arbovirus epidemics reported in FG in the last decade have all shared common characteristics. Thus, DENV, CHIKV, and ZIKV were transmitted by a common vector, Ae. aegypti, which is an urban, anthropophilic vector. The distribution of the latter in FG is wide and expanding and present in almost all FG locations where people live (except for Camopi and Trois-Sauts) (Figure 4). Rural communities within the FG territory were spared the presence of Ae. Aegypti until the mid-2000s, when the first DENV cases were described [57]. A recent seroprevalence study has shown that the populations living along the northern part of the Maroni banks, which were safe from these arboviruses for a long time, have been strongly affected during the past years by DENV, CHIKV, and ZIKV [13,57]. On the other hand, the older arboviruses likely established for centuries on the territory (MAYV, TONV, and YFV) seem to have more varied transmission cycles (sylvatic and urban) and animal reservoirs that are still not well known. The vectors are thus affected and seem to be more diverse (Aedes, Haemagogus for MAYV and YFV, Anopheles, Culex for TONV). This heterogeneity may impact the dynamics of new epidemics caused by previously reported arboviruses, such as DENV, but also by new introductions and emerging arboviruses. Finally, this heterogeneity of the vector distribution is probably one of the main drivers of the arbovirus seroprevalence heterogeneity observed recently in FG [13].
Figure 4.
Mapping the distribution of Ae. aegypti in French Guiana. Map produced by combining DDAS data with GBIF data (Page et al.). Red cross indicates the presence of Ae. aegypti.
After the CHIKV and ZIKV major epidemics that followed their introduction to the Americas in 2014 and 2015, respectively, the active surveillance of these arboviruses was maintained which confirmed that there was no detectable current endemic circulation of these viruses. It is worth remembering that CHIKV has been established in Brazil since 2013 and always manifests itself in epidemic form [58]. The risk of endemicity should be considered.
Like many infectious diseases, vector-borne diseases have a strong impact on the most vulnerable populations. Indeed, poverty has been described as a risk factor for ZIKV and CHIKV infection, particularly in the early stages of an outbreak [59,60]. This population is often more exposed to vectors and remote from prevention campaigns or actions. Specific actions targeting this at-risk population have been settled, such as the WASH project developed by the French Red Cross to fight against waterborne and vector-borne diseases in disadvantaged areas [61].
3.3. Vector Control
The breeding site removal to prevent larvae and the spatial and domiciliary insecticide spraying to prevent adult mosquitoes are historically the foundations of vector control [62]. Concerning the dengue vector, Ae. aegypti, several other strategies are used such as governmental vector control programs, enhanced community engagement, or individual protection campaigns. While several insecticidal molecules and application methods have been used over the last 60 years to maintain the efficiency of vector control, FG, as the French department in the Americas, follows European regulations, which is very unique in South America [63]. As such, only pyrethroids compounds are available against adult mosquitoes, contrary to other South American countries where organophosphates and carbamates can be used. Deltamethrin is the sole insecticide available to spray against adult Ae. aegypti in FG, while Bacillus thuringiensis var israelensis H14 (Bti) treatments along with the mechanical removal of Ae. aegypti breeding sites are used to reduce larval densities.
The intensive and successive use of several families of insecticides these last decades (i.e., organophosphates and pyrethroids) has led to the resistance of mosquito populations, especially Ae. aegypti, reducing the efficiency of the treatments. The high resistance to insecticides in mosquitoes [64] as well as the description of the adverse effects of most of insecticides, such as impact on biodiversity and cancerogenic effects [65], have led to the need for alternative methods to be tested in FG, such as wolbachia-infected mosquitoes and sterile male methods [66].
While Aedes is the major vector of arboviruses, and the only vector involved in large-scale epidemics, particular attention should be paid to Culex and Haemagogus, the vectors involved in the transmission of autochthonous arboviruses.
It is important to keep in mind that most of the vector control measurements mentioned are only effective in urban settings where mosquito populations exploit artificial habitats for breeding. Those measurements don’t extrapolate to sylvatic settings where mosquito habitats and behavior differ. Therefore, antibody surveillance is essential in human populations and sentinel surveillance may help to detect early epidemics of new or incoming arboviruses.
3.4. Risks of Introduction of Aedes Albopictus in French Guiana
Aedes albopictus (Skuse, 1894) is one of the most invasive mosquito species in the world. In the last decades, this species has spread from its native range in Asia to the most temperate and tropical inhabited areas of the world, including the Americas [67]. So far, the geographical area known as the Guiana Shield has been largely saved from the spread of the Asian Tiger mosquito. However, in recent years, this species has been documented in the main island of Trinidad and Tobago [68] and in the Brazilian city of Macapá in the state of Amapá [69,70]. The location is only an 8 h drive (600 km) from the eastern border of FG, where the introduction of Ae. albopictus is likely to happen through human and trade interactions. This event is likely to occur and remaining questions are when and where [71]. Currently, entomological surveys dedicated to the early detection of Ae. albopictus in FG are conducted by the Direction de la Démoustication et des Actions de Santé (DDAS) at the international Cayenne—Félix Eboué Airport, the major maritime ports, and the main human settlements situated at the borders with Suriname and Brazil. The establishment of Ae. albopictus in FG would probably lead to interspecific larval competition with Ae. aegypti which share the same environmental niches [72]. As already suggested in North and South America, the introduction of Ae. albopictus could result in geographical displacement and the population reduction in Ae. aegypti [73]. Given the differences in vectorial competence between the species, a modification in the overall transmission dynamics and patterns of DENV, CHIKV, and ZIKV in urban areas is possible but would need to be assessed locally. The higher adaptability of Ae. albopictus, especially to forested areas near human dwellings, could facilitate the movement of rare zoonotic arboviruses from rural to urban areas, thereby increasing the risk of new infectious diseases emerging in FG.
3.5. Zoonotic Arboviruses
Concerning the MAYV, the reservoir and the vector seem well identified in FG. So far, their location in primary forest limits the risk of transmission to humans. However, MAYV belongs to the same family as the CHIKV, which went from being a forest virus to an urban virus. Such a possibility should not be ruled out for MAYV, as well as an adaptation to an animal reservoir closer to humans. Seroprevalence surveillance studies in domestic animals should therefore be regularly performed to assess any change. Similarly, the vector competence of Ae. aegypti and possibly Ae. albopictus against MAYV should be regularly performed on newly isolated strains or strains whose genome differs significantly from older strains. TONV should be tested regularly on the same principles. The vectors of the OROV certainly need to be better explored in FG in order to better assess the risk for the population.
3.6. Potential Emergence of New Arbovirus
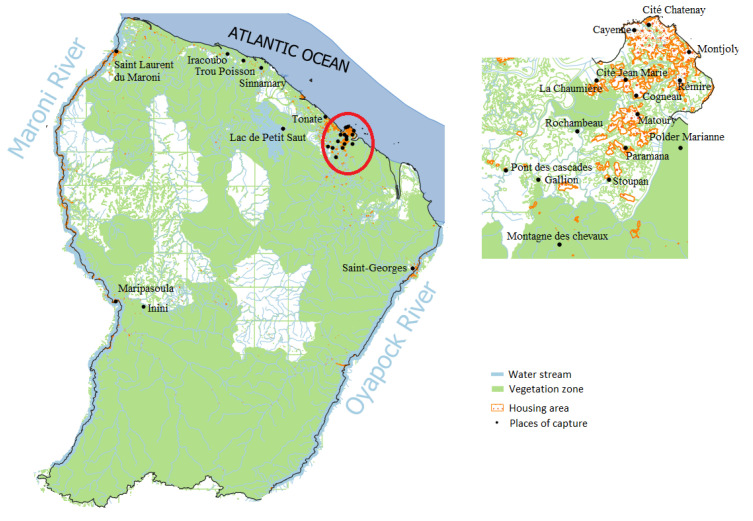
In 1966, virology became a crucial aspect of the activities at the Institut Pasteur in FG. The U79 INSERM Group was established in 1968 to conduct research on arbovirus diseases [8]. The group carried out investigations (Figure 5) on YFV and identified several viruses, some of which were already known while others were previously unknown in mosquitoes, wildlife, birds, and humans such as Tonate, Mucambo, Cabassou, Una, and Aura viruses in the Togaviridae family, or Saint-Louis Encephalitis and Ilheus viruses in the Flaviviridae family [8,45,48,74,75,76]. Nevertheless, the most frequently identified virus family was the Peribunyaviridae family with the Murutucu virus; Oriboca and Caraparu Orthobunyaviruses from the serogroup C; Guama, Bimiti, and Catu Orthobunyaviruses from the Guama group; Inini virus from the Simbu group; Guaroa Orthobunyavirus from the California group; and Maguari and Wyeomyia Orthobunyaviruses from the Bunyamwera group. Finally, a few ungrouped viruses, Itaporanga and Aruac viruses, have been detected [77]. At the time of detection, Culex portesi appeared to be the mosquito involved in the sylvatic circulation of the largest number of arboviruses in the region. For each virus, details on the host, the number, and the date of isolation are presented in Table 2 below with the reference of the corresponding publication.
Figure 5.
Map illustrating the distribution of mosquito sampling locations during investigations carried out on arboviruses, presented in Table 2.
Table 2.
Summary of knowledge on identification of rare arboviruses from publications in French Guiana by virus isolation, molecular biology, as well as common arboviruses in the wild fauna.
| Genus | Virus | Reference | Date | Number of Isolations | Place | Vector | Vertebrate Hosts | Humans | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Alphavirus | Mucambo virus | [45] | 1972 | 1 | Montsinéry |
Phlebotominae Lutzomyia sp. |
|||
| [8,45] | 1973 | 5 | Maripasoula |
Birds
Monasa atra Tachyphonus cristatus Trogon violaceus Turdus nudigenis |
|||||
| [45] | NA | NA | NA |
Culicidae Aedes sp. Culex portesi Culex sp. Haemagogus sp. Mansonia sp. Sabethini sp. Wyeomyia sp. |
Primates Cebus apella (used as sentinel) |
||||
| [45] | NA | NA | Maripasoula |
Birds 4 birds sp. |
|||||
| [45] | 1972 | NA | Cayenne |
Phlebotominae Lutzomyia sp. |
|||||
| [45] | 1973 | 3 | Cayenne | Four laboratory workers who had handled the reference strain. Symptoms: sudden onset of high fever, myalgias, headaches, nausea or vomiting, very high asthenia lasting 15 days |
Since March 1973, it has never been isolated again and seems to have been replaced by another virus of subtype III, Tonate | ||||
| Tonate virus | [8,45,74] | 1973 | NA | Tonate |
Culicidae (n = 71 from 1973 to 1978) Anopheles sp. Coquillettidia sp. Culex portesi Mansonia sp. Uranotaenia sp. Wyeomyia sp. Phlebotominae Lutzomyia sp. |
Birds
Psarocolius decumanus |
See main paragraph | ||
| [45] | 1973 | NA | Gallion Paramana Stoupan Trou Poissons |
Culicidae
Culex portesi Mansonia titillans |
|||||
| [45] | 1975 | NA | Cité Chatenay Gallion La Chaumière Matoury Paramana Tonate Trou Poissons |
Culicidae
Coquillettidia venezuelensis Culex portesi Culex spissipes Culex zeteki Wyeomyia melanocephala Wyeomyia occulta |
|||||
| [45] | 1973–1977 | 16 (birds) 72 (mosquitoes) |
Gallion La Chaumière Matoury Paramana Sinnamary Tonate Trou-Poissons |
Culicidae Anopheles braziliensis Anopheles mediopunctatus * Coquillettidia albicosta (as species of Culex) Coquillettidia venezuelensis Culex nigripalpus Culex portesi Culex spissipes Culex zeteki Mansonia pseudotitillans Mansonia titillans Uranotaenia geometrica Wyeomyia melanocephala Wyeomyia occulta Wyeomyia pseudopecten Phlebotominae Lutzomyia sp. |
Birds Ardeola ibis Chiroxiphia pareola Elaenia chiriquensis Glyphorynchus spirurus Leucopternis albicollis Myiozetetes cayanensis Nycticorax violacea Oxyura dominica Psarocolius decumanus Ramphocelus carbo Sakesphorus canadenses Sporophila lineola Tachyphonus rufus Tolmomyias poliocephalus Turdus nudigenis Rodents Sentinel mice |
||||
| [47] | 2011 | 1 |
Bats
Trachops cirrhosus |
Isolation of TONV | |||||
| Cabassou virus | [45] | 1972 | 1 | Paramana |
Culicidae
Culex portesi |
(n = 15 from 1974 to 1980) | |||
| [8] | NA | NA | Saint Laurent du Maroni |
Bats Chiroptera sp. |
|||||
| [8,74,78] | 1973–1980 | 2 (birds) 3 (mammals) |
Cité Jean-Marie¤ Gallion La Chaumière Matoury Montagne des chevaux Paramana Tonate Sinnamary Trou-Poissons |
Culicidae
Anopheles peryassui Coquillettidia venezuelensis Culex nigripalpus Culex portesi Limatus pseudomethysticus Mansonia titillans Wyeomyia occulta |
Birds Tolmomyias poliocephalus Turdus nudigenis Marsupials Didelphis marsupialis Philander oppossum Rodents Sentinel mice Bats Chiroptera sp. |
||||
| [45] | 1974–1975 | 2 | Gallion |
Culicidae
Culex portesi |
|||||
| Una virus | [8,45] | 1973 | 1 | Montjoly |
Culicidae
Psorophora ferox |
(n = 5 from 1973 to 1977) | |||
| [45] | 1973 | 1 | Sinnamary |
Culicidae
Coquillettidia venezuelensis |
|||||
| [45] | 1973 | 1 | Paramana |
Culicidae
Coquillettidia albicosta |
|||||
| [8,45] | 1975 | 2 | Maripasoula |
Culicidae
Psorophora lutzii |
|||||
| [78] | 1977 | NA | Cité Jean-Marie¤ |
Culicidae
Anopheles nimbus |
|||||
| 1973 | 1 | Inini |
Birds
Campephilus rubricollis |
||||||
| Aura virus | [8,78] | 1973 | 2 | Inini |
Culicidae
Aedes serratus |
||||
| [45] | NA | Polder Marianne |
Culicidae
Mansonia titillans |
||||||
| [45] | 1974 | 1 | Cayenne | One woman with high fever and severe jaundice who died quickly after admission. | The definite proof that the virus was the cause of disease is lacking. | ||||
| Flavivirus | Ilheus virus | [8,45] | 1973 | 3 | Iracoubo Maripasoula Montsinéry |
Birds
Leistes militaris Molothrus bonariensis Momotus momota Piaya minuta |
|||
| [78] | 1977 | 1 | Gallion |
Culicidae
Coquilletidia venezuelensis |
|||||
| [45,79] | 1973 | 2 | Macouria | From the blood of a patient suffering a “dengue-like” syndrome, with fever, headaches, chills and co-infection with Plasmodium falciparum | |||||
| Saint-Louis Encephalitis virus | [8] | 1967 | 1 | NA |
Culicidae Culex sp. |
||||
| [8] | 1977 | 1 | NA |
Birds
Anhinga anhinga |
|||||
| Dengue virus | [11,80] | 2006 | 5 | Saint Georges de l’Oyapock |
Rodents
Proechimys cuvieri |
5/72 (7%), DENV3 3/72 and DENV4 2/72 | |||
| [11,80] | 2001–2007 | 16 (rodents) 39 (Marsupials) 19 (bats) |
Camp du Tigre |
Rodents Mesomys hispidus (1/5 DENV3) Oecomys spp. (13/28 DENV1 and DENV2) Oryzomys megacephalus (2/8 DENV1 and DENV2) Proechymys cayennensis (7/45 DENV and 2 and 3) Marsupials Caluromys philander (1/14 DENV1) Didelphis marsupialis (12/88 DENV2, 3 and 4) Marmosops parvidens (3/7 DENV3) Marmosa murina (16/96 DENV1, 2 and 3) Micoureus demerarae (4/45 DENV1, 2, 3 and 4) Philander opossum (3/37 DENV1 and 2) Bats Artibeus planirostris (14/42 DENV1, 2 and 3) Carollia perspicillata (5/63 DENV1 and 3) |
16% (87/543) of the animals were infected on the site Distribution of DENV-1, -2, -3, and -4 was 41% (36/87), 20%, 33%, and 6%, respectively Chiroptera 4% (19/543), Rodentia 5%, and Marsupialia 7% |
||||
|
Orthobunyavirus
(Serogroup C) |
Murutucu virus | [8] | 1972–1975 | NA | Cayenne Gallion |
Culicidae
Anopheles peryassui Culex portesi |
|||
| [78] | 1980 | 2 | Montagne des Chevaux |
Culicidae
Culex portesi |
|||||
| [8,78] | 1973 | 3 | Cayenne | Three patients with fever, headache and myalgia. | |||||
| Oriboca virus | [8] | 1975 | 1 | Gallion |
Culicidae
Culex portesi |
||||
| Caraparu virus | [8] | 1975 | 1 | La Chaumière |
Culicidae
Culex portesi |
||||
| [78] | 1975 | 1 | Gallion |
Culicidae
Culex spissipes |
|||||
| [78] | 1974 | 1 | Tonate |
Culicidae
Mansonia titillans |
|||||
| [8] | NA | NA | NA | Limatus durhami | |||||
| Group C undifferentiated (Muturucu, Oriboca, Caraparu) | [78] | 1973–1980 | 38 | Gallion La Chaumière Matoury Montagne des chevaux Paramana Tonate Sinnamary Trou-Poissons |
Culicidae Aedes arborealis Anopheles peryassui Coquillettidia albicosta Coquillettidia venezuelensis Culex portesi Culex spissipes Mansonia titillans Trichoprosopon digitatum Trichoprosopon sp. Wyeomyia occulta |
Marsupials Philander oppossum Rodents Sentinel mice |
|||
|
Orthobunyavirus
(Bunyamwera group) |
Guaroa virus | [8,78] | 1973 | 1 | Gallion |
Culicidae
Anopheles peryassui |
|||
| Maguari virus | [8,78] | 1978 | 3 | Cité Jean-Marie Matoury Paramana |
Culicidae Wyeomyia sp. Culex portesi Wyeomyia aphobema Wyeomyia occulta |
||||
| [78] | 1979 | NA | Gallion |
Culicidae
Anopheles nimbus |
|||||
| Wyeomyia virus | [8,78] | 1975 | 1 | Pont des Cascades |
Culicidae
Wyeomyia occulta |
||||
| [78] | 1977 | 1 | Cabassou |
Culicidae
Coquillettidia albicosta |
|||||
| [8] | NA | NA | NA |
Culicidae Aedes taeniorhynchus Culex portesi Johnbelkinia longipes ** |
|||||
| [78] | 1979 | 6 | Paramana Gallion |
Culicidae
Anopheles nimbus |
|||||
|
Orthobunyavirus
(Guama group) |
Guama group undifferentiated (Bimiti, Catu, Guama.) | [8,78] | 1972–1980 | 107 (mosquitoes) | Cité jean-Marie Gallion La Chaumière Matoury Montagne aux chevaux Paramana Rochambeau Sinnamary Tonate Trou-Poissons |
Culicidae Coquillettidia venezuelensis Culex portesi Anopheles braziliensis Anopheles darlingi Culex spissipes Culex taeniopus $ Culex sp. Mansonia titillans Wyeomyia splendida £ Wyeomyia occulta Psorophora ferox Sabethes undosus Trichoprosopon digitatum ** Trichoprosopon longipes Johnbelkinia longipes Phlebotominae Lutzomyia sp. |
Rodents Sentinel mice |
||
| [78] | 1976–1978 | 19 | Cogneau Inini La Chaumière Paramana Tonate Trou Poisson |
Birds Attila cinnamoneus Columba plumbea Elaenia chiriquensis Galbula dea Galbula galbula Myiarchus ferox Pitangus sulphuratus Platirynchus sp. Querula purpurata Ramphocelus carbo Tamnomanes ardesiacus Thruapis episcopus Thraupis palmarum Turdus fumigatus |
|||||
| [78] | 1976 | 5 | La Chaumière Inini Paramana |
Marsupials
Didelphis Marsupialis Rodents Proechimys guyanensis/ cuvieri |
|||||
| [78] | 1977 | 1 | NA | Strain isolated from patient serum | |||||
| Guama virus (Guama group) | [8] | 1974–1975 | 3 | Gallion La Chaumière Paramana |
Culicidae Culex portesi Johnbelkinia longipes ** |
||||
| [78] | 1975–1976 | 2 | Matoury |
Marsupials
Didelphis marsupialìs |
|||||
| Bimiti virus (Guama group) | [8,78] | 1972–1976 | 12 | Gallion Iracoubo Matoury Paramana Trou Poisson |
Culicidae Culex sp. Culex portesi Culex taeniopus $ Coquillettidia venezuelensis |
||||
| [8,78] | 1972 | 1 | Matoury |
Birds
Galbula dea |
|||||
| Catu virus (Guama group) | [8,78] | 1973–1976 | 4 | Gallion Paramana Pont des Cascades |
Culicidae
Culex portesi Culex spissipes |
||||
| [78] | 1975 | 1 | La Chaumière |
Marsupials
Didelphis marsupialìs |
|||||
| Inini virus (Simbu group) | [45] | 1973 | 1 | Maripasoula |
Birds
Pteroglossus aracari |
It shows some antigenic relationships with Mermet and Ingwavuma viruses | |||
| Bunyamwera like | Itaporanga virus (Phlebotomus group) | [8,45] | 1977–1978 | 2 | Cité Jean-Marie¤Paramana |
Culicidae
Culex albinensis Culex spissipes |
|||
| [78] | 1977 | 1 | Iracoubo |
Birds
Nycticorax violacea |
|||||
| Ungrouped | Aruac virus | [8] | NA | NA | NA |
Culicidae Coquillettidia albicosta Coquillettidia venezuelensis Culex sp. |
|||
| Rochambeau virus/Paramana virus | [8,78] | 1973 | 1 | Paramana |
Culicidae
Coquillettidia albicosta |
||||
| NA | 1 | Paramana |
Culicidae
Culex portesi |
||||||
| [78] | 1974 | 1 | Paramana |
Birds
Tyrannus dominicensis |
NA: data not available, Taxonomic names were ordered alphabetically by taxonomic groups and spelled exactly as published, and thus may not agree with currently accepted names. * Anopheles mediopunctatus has been confused with Anopheles costai and Anopheles forattinii in French Guiana [81]. Therefore, the viral isolations attributed to An. mediopunctatus could be attributed to An. costai or An. forattinii. ** Johnbelkinia longipes is the currently valid combination for this species [81]. $ Culex taeniopus has been widely confused with Culex. pedroi on the coastal plain of French Guiana [82]. Therefore, the viral isolations attributed to Culex. taeniopus could be attributed to Cx. pedroi. £ Wyeomyia splendida is currently the correct scientific name for this species [81].
At least some of them have been anecdotally detected in humans [45]: Mucambo virus infected four laboratory workers who reported a sudden onset of high fever, myalgia, headache, nausea or vomiting, and very high asthenia lasting 15 days, after handling the reference strain in 1973; Aura virus was incriminated in the infection of a woman with high fever and severe jaundice who died quickly after admission in 1974; Ilheus virus was detected in a patient suffering from fever, headaches, and chills in the 1970s; and Murutucu virus (group C) was described in three patients with fever, headache, and myalgia in 1973. The U7 9 INSERM Group ended its activities in 1979, and, after that, the focus shifted to dengue fever surveillance until recently. This explains the high number of new arboviruses reported or discovered during this period. The risk of emergence of autochthonous arboviruses is real and aggravated by the rapid urbanization of the FG (destruction of ecosystems, vulnerability to new vectors, or emergence of new vector reservoirs).
The risk of introduction of new arboviruses is not negligible in FG, and may come from either Brazil or from the North-East of South America (Venezuelan equine encephalitis virus…). The development of multiplex tools to target all arboviruses, which is most likely to be introduced in the territory, is certainly a major development axis for the coming months in order to respond as soon as possible in the case of febrile syndrome that is not diagnosed with conventional tools. The vectorial competence of the vectors most present in FG should also be tested with respect to a certain number of viruses prevailing in neighboring countries.
3.7. Potential Introduction of Arboviruses from French Guiana to Europe and the West Indies
Some of the viruses reported here have a real potential for introduction in Europe. France is particularly at risk because of its special links with its French territories in the Americas, the French West Indies, because of the numerous population exchanges, especially for tourism, and FG to a lesser extent. The risk for the introduction of dengue in Europe is no longer needing to be demonstrated due to the ever-increasing presence of Ae. albopictus throughout Europe. The French experience of the summer of 2022 is particularly telling, as the number of reported autochthonous cases in metropolitan France was greater than the total number of cases reported over the period 2010–2021 [83]. Nevertheless, the cases identified in 2022 did not originate from the French territories of America but instead mainly Cuba, the Ivory Coast, and Mexico. CHIKV and ZIKV are also known to have the potential for introduction in Europe, but the current absence of epidemics in FG and the French West Indies can limit the risk. Furthermore, while autochthonous cases have been described in Europe for CHIKV, this has not been reported for ZIKV to our knowledge. It should be noted that some European scientific societies still recommend additional restrictions in the case of medically assisted reproductive assessments in people from Latin America, despite the disappearance of the epidemic more than 3 years ago. To our knowledge, no case of TONV has been reported out of FG, and no case of OROV out of Latin America. On the other hand, several cases of MAYV infection have been described in travelers from Latin America, and, in particular, from FG. Although there is a potential for transmission of this virus by Ae. aegypti, no autochthonous case has ever been described outside endemic areas. However, it is important to remain vigilant about this virus that some experts consider as having a pandemic potential.
Concerning the West Indies, exchanges occur daily between FG and the French West Indies, and, through them, with all the West Indies including Haiti. Therefore, some viruses may strike the West Indies as well as FG because of the presence of Ae. aegypti (DENV, CHIKV, ZIKV); however, the others have not been described, with the exception of MAYV in Haiti (its autochthonous origin remains to be demonstrated). However, it is important to remain vigilant, especially for YFV, since the competence of Ae. aegypti strains from the French West Indies has been demonstrated. The risk of an urban epidemic following the introduction of a YFV cannot be ruled out. Vaccination of the inhabitants of the West Indies before a stay in FG should therefore remain mandatory.
4. Conclusions
French Guiana is home to a high diversity of infectious and tropical diseases, illustrated here by several arboviruses. The nonspecific clinical picture implies a good knowledge of the local epidemiology but also an easy access to advanced biology to catch the correct bug. Many of the arboviruses described here have a significant epidemic potential within FG but also abroad. The example of FG allows us to distinguish the viruses with endemic circulation (YFV, MAYV and TONV) from viruses with an epidemic form (DENV, CHIKV, ZIKV) and that are transmitted by Ae. aegypti. The resistance of Ae. aegypti to insecticides makes control very difficult, and the development of alternative methods, and effective vaccines, is urgent.
Concerning the risk of relapse of certain arbovirus epidemics, can we be reassured today that CHIKV and ZIKV will not return, given that herd immunity or any other unclear phenomenon is sufficient for no epidemic to return for a generation? It cannot be said that a seroprevalence of 20%, like that of CHIKV in FG, provides collective protection. A new epidemic circulation cannot be ruled out, even in the relatively near future, hence the need for surveillance.
The origin of the OROV epidemic remains unknown and underscores the need for active epidemiological surveillance, in FG as well as throughout the Amazon basin. Furthermore, older publications indicate that many poorly known arboviruses may be circulating in the area, causing infection in humans, although they have not been identified in recent decades, due to the lack of an adequate sentinel surveillance system and a sufficiently broad virological diagnostic panel. Such a surveillance network is currently being set up in FG under the coordination of health authorities, with sentinel doctors, private and hospital laboratories, and the NRC for arbovirus of the IPG and with a diagnostic algorithm extended to other arboviruses such as several orthobunyaviruses.
Acknowledgments
Paul Le Turnier is currently benefiting from a grant from the Agence Nationale de Recherche sur le SIDA, les Hépatites et Maladies Infectieuses Emergentes (ANRS-MIE).
Author Contributions
Conceptualization, T.B., P.L.T. and L.E.; methodology, T.B., P.L.T. and L.E.; data curation, visualization, S.R., writing—original draft preparation, T.B., P.L.T. and L.E.; writing—review and editing, Y.E., L.C., B.D.T., F.D., J.-B.D., P.D., A.L., R.M., M.N., S.T., A.T., A.E. and D.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Musso D., Rousset D., Peyrefitte C. Special Issue “Endemic Arboviruses”. Viruses. 2022;14:645. doi: 10.3390/v14030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Thoisy B., Duron O., Epelboin L., Musset L., Quénel P., Roche B., Binetruy F., Briolant S., Carvalho L., Chavy A., et al. Ecology, Evolution, and Epidemiology of Zoonotic and Vector-Borne Infectious Diseases in French Guiana: Transdisciplinarity Does Matter to Tackle New Emerging Threats. Infect. Genet. Evol. 2021;93:104916. doi: 10.1016/j.meegid.2021.104916. [DOI] [PubMed] [Google Scholar]
- 3.Esser H.J., Mögling R., Cleton N.B., van der Jeugd H., Sprong H., Stroo A., Koopmans M.P.G., de Boer W.F., Reusken C.B.E.M. Risk Factors Associated with Sustained Circulation of Six Zoonotic Arboviruses: A Systematic Review for Selection of Surveillance Sites in Non-Endemic Areas. Parasites Vectors. 2019;12:265. doi: 10.1186/s13071-019-3515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gould E., Pettersson J., Higgs S., Charrel R., de Lamballerie X. Emerging Arboviruses: Why Today? One Health. 2017;4:1–13. doi: 10.1016/j.onehlt.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonifay T., Godaert L., Epelboin Y., Rousset D., Douine M., Hilderal H., Clavel C., Abel S., Najioullah F., Fagour L., et al. Contribution of Research in the West Indies and Northeast Amazonia to Knowledge of the 2014–2015 Chikungunya Epidemic in the Americas. Curr. Trop. Med. Rep. 2021;8:164–172. doi: 10.1007/s40475-021-00242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonifay T., Walter G., Abboud P., Epelboin L., Bellaud G., Bleibtreu A., Dournon N., Henn A., Lemaignen A., Nguyen L.B.L., et al. Analyse rétrospective des épidémies d’arbovirus du XIXe siècle: Alors dengue ou chikungunya? Méd. Mal. Infect. 2019;49:S120–S121. doi: 10.1016/j.medmal.2019.04.289. [DOI] [Google Scholar]
- 7.Fouque F., Reynes J.M., Moreau J.P. Dengue in French Guiana, 1965–1993. Bull. Pan Am. Health Organ. 1995;29:147–155. [PubMed] [Google Scholar]
- 8.Robin Y., Lhuillier M., Girault G., Pajot F., Dégallier N. Dengue Viruses and Other Arboviruses in French Guiana; Proceedings of the Internacional Simposium on Tropical Arboviruses and Haemorrhagic Fevers, Academia Brasiliera de ciencias; Rio de Janeiro, Brazil. 14 April 1980. [Google Scholar]
- 9.L’Azou M., Taurel A.-F., Flamand C., Quénel P. Recent Epidemiological Trends of Dengue in the French Territories of the Americas (2000–2012): A Systematic Literature Review. PLoS Negl. Trop. Dis. 2014;8:e3235. doi: 10.1371/journal.pntd.0003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santé Publique France Situation Epidémiologique de la Dengue en Guyane. Point au 9 Février 2023. [(accessed on 16 May 2023)]. Available online: https://www.santepubliquefrance.fr/regions/guyane/documents/bulletin-regional/2023/situation-epidemiologique-de-la-dengue-en-guyane.-point-au-9-fevrier-2023.
- 11.de Thoisy B., Lacoste V., Germain A., Muñoz-Jordán J., Colón C., Mauffrey J.-F., Delaval M., Catzeflis F., Kazanji M., Matheus S., et al. Dengue Infection in Neotropical Forest Mammals. Vector Borne Zoonotic Dis. 2009;9:157–170. doi: 10.1089/vbz.2007.0280. [DOI] [PubMed] [Google Scholar]
- 12.Fritzell C., Rousset D., Adde A., Kazanji M., Kerkhove M.D.V., Flamand C. Current Challenges and Implications for Dengue, Chikungunya and Zika Seroprevalence Studies Worldwide: A Scoping Review. PLoS Negl. Trop. Dis. 2018;12:e0006533. doi: 10.1371/journal.pntd.0006533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailly S., Rousset D., Fritzell C., Hozé N., Ben Achour S., Berthelot L., Enfissi A., Vanhomwegen J., Salje H., Fernandes-Pellerin S., et al. Spatial Distribution and Burden of Emerging Arboviruses in French Guiana. Viruses. 2021;13:1299. doi: 10.3390/v13071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Turnier P., Bonifay T., Mosnier E., Schaub R., Jolivet A., Demar M., Bourhy P., Nacher M., Djossou F., Epelboin L. Usefulness of C-Reactive Protein in Differentiating Acute Leptospirosis and Dengue Fever in French Guiana. Open. Forum Infect. Dis. 2019;6:ofz323. doi: 10.1093/ofid/ofz323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epelboin L., Boullé C., Ouar-Epelboin S., Hanf M., Dussart P., Djossou F., Nacher M., Carme B. Discriminating Malaria from Dengue Fever in Endemic Areas: Clinical and Biological Criteria, Prognostic Score and Utility of the C-Reactive Protein: A Retrospective Matched-Pair Study in French Guiana. PLoS Negl. Trop. Dis. 2013;7:e2420. doi: 10.1371/journal.pntd.0002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Oliveira E.C., Fonseca V., Xavier J., Adelino T., Claro I.M., Fabri A., Macario E.M., Viniski A.E., Souza C.L.C., da Costa E.S.G., et al. Short Report: Introduction of Chikungunya Virus ECSA Genotype into the Brazilian Midwest and Its Dispersion through the Americas. PLoS Negl. Trop. Dis. 2021;15:e0009290. doi: 10.1371/journal.pntd.0009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Melle A., Cropet C., Parriault M.-C., Adriouch L., Lamaison H., Sasson F., Duplan H., Richard J.-B., Nacher M. Renouncing Care in French Guiana: The National Health Barometer Survey. BMC Health Serv. Res. 2019;19:99. doi: 10.1186/s12913-019-3895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifay T., Prince C., Neyra C., Demar M., Rousset D., Kallel H., Nacher M., Djossou F., Epelboin L., The Char Chik Working Group Atypical and Severe Manifestations of Chikungunya Virus Infection in French Guiana: A Hospital-Based Study. PLoS ONE. 2018;13:e0207406. doi: 10.1371/journal.pone.0207406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Silva Neto S.R., Tabosa de Oliveira T., Teixiera I.V., Medeiros Neto L., Souza Sampaio V., Lynn T., Endo P.T. Arboviral Disease Record Data—Dengue and Chikungunya, Brazil, 2013–2020. Sci. Data. 2022;9:198. doi: 10.1038/s41597-022-01312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flamand C., Bailly S., Fritzell C., Berthelot L., Vanhomwegen J., Salje H., Paireau J., Matheus S., Enfissi A., Fernandes-Pellerin S., et al. Impact of Zika Virus Emergence in French Guiana: A Large General Population Seroprevalence Survey. J. Infect. Dis. 2019;220:1915–1925. doi: 10.1093/infdis/jiz396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oehler E., Watrin L., Larre P., Leparc-Goffart I., Lastere S., Valour F., Baudouin L., Mallet H., Musso D., Ghawche F. Zika Virus Infection Complicated by Guillain-Barre Syndrome—Case Report, French Polynesia, December 2013. Eurosurveillance. 2014;19:20720. doi: 10.2807/1560-7917.ES2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 22.Cao-Lormeau V.-M., Blake A., Mons S., Lastère S., Roche C., Vanhomwegen J., Dub T., Baudouin L., Teissier A., Larre P., et al. Guillain-Barré Syndrome Outbreak Associated with Zika Virus Infection in French Polynesia: A Case-Control Study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musso D., Ko A.I., Baud D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019;381:1444–1457. doi: 10.1056/NEJMra1808246. [DOI] [PubMed] [Google Scholar]
- 24.Pomar L., Vouga M., Lambert V., Pomar C., Hcini N., Jolivet A., Benoist G., Rousset D., Matheus S., Malinger G., et al. Maternal-Fetal Transmission and Adverse Perinatal Outcomes in Pregnant Women Infected with Zika Virus: Prospective Cohort Study in French Guiana. BMJ. 2018;363:k4431. doi: 10.1136/bmj.k4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hcini N., Kugbe Y., Rafalimanana Z.H.L., Lambert V., Mathieu M., Carles G., Baud D., Panchaud A., Pomar L. Association between Confirmed Congenital Zika Infection at Birth and Outcomes up to 3 Years of Life. Nat. Commun. 2021;12:3270. doi: 10.1038/s41467-021-23468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant R., Flechelles O., Elenga N., Tressières B., Gaete S., Hebert J.-C., Schaub B., Djossou F., Mallard A., Delver L., et al. Consequences of In Utero Zika Virus Exposure and Adverse Pregnancy and Early Childhood Outcomes: A Prospective Cohort Study. Viruses. 2022;14:2755. doi: 10.3390/v14122755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chippaux J.-P., Chippaux A. Yellow Fever in Africa and the Americas: A Historical and Epidemiological Perspective. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018;24:20. doi: 10.1186/s40409-018-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldani L.Z. Yellow Fever Outbreak in Brazil, 2017. Braz. J. Infect. Dis. 2017;21:123–124. doi: 10.1016/j.bjid.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Morales A.J., Bonilla-Aldana D.K., Suárez J.A., Franco-Paredes C., Forero-Peña D.A., Mattar S., Villamil-Gómez W.E., Ruíz-Sáenz J., Cardona-Ospina J.A., Figuera M.E., et al. Yellow Fever Reemergence in Venezuela—Implications for International Travelers and Latin American Countries during the COVID-19 Pandemic. Travel. Med. Infect. Dis. 2021;44:102192. doi: 10.1016/j.tmaid.2021.102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matheron S., Nicand E., Rapp C., Chidiac C. Health recommendations for travellers, 2022 (for health professionals) Bull. Epidemiol. Hebdo. 2022;BEH Hors-Série:99. [Google Scholar]
- 31.Flamand C., Bailly S., Fritzell C., Fernandes Pellerin S., Toure A., Chateau N., Saout M., Linares S., Dubois F., Filleul L., et al. Vaccination Coverage in the Context of the Emerging Yellow Fever Threat in French Guiana. PLoS Negl. Trop. Dis. 2019;13:e0007661. doi: 10.1371/journal.pntd.0007661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garnier M.A. La Fievre Jaune à La Guyane Avant 1902 et L’épidémie de 1902. Octave Doin; Paris, France: 1903. [Google Scholar]
- 33.Heraud J.M., Hommel D., Hulin A., Deubel V., Poveda J.D., Sarthou J.L., Talarmin A. First Case of Yellow Fever in French Guiana since 1902. Emerg. Infect. Dis. 1999;5:429–432. doi: 10.3201/eid0503.990314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanna A., Andrieu A., Carvalho L., Mayence C., Tabard P., Hachouf M., Cazaux C.-M., Enfissi A., Rousset D., Kallel H. Yellow Fever Cases in French Guiana, Evidence of an Active Circulation in the Guiana Shield, 2017 and 2018. Eurosurveillance. 2018;23:1800471. doi: 10.2807/1560-7917.ES.2018.23.36.1800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas C., Michaud C., Gaillet M., de Thoisy B., Lacerda M., Duchemin J. Risques de Réémergence de La Fièvre Jaune Sur Le Plateau Des Guyanes: Une Série de Cas 1990–2020 et Une Revue de La Littérature. 5e Journée Des Travaux Scientifiques Des Soignants de Guyane. Med. Trop. Sante Int. 2022;2:mtsi.v2i2.2022.248. doi: 10.48327/mtsi.v2i2.2022.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Thoisy B., Dussart P., Kazanji M. Wild Terrestrial Rainforest Mammals as Potential Reservoirs for Flaviviruses (Yellow Fever, Dengue 2 and St Louis Encephalitis Viruses) in French Guiana. Trans. R. Soc. Trop. Med. Hyg. 2004;98:409–412. doi: 10.1016/j.trstmh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Long K.C., Ziegler S.A., Thangamani S., Hausser N.L., Kochel T.J., Higgs S., Tesh R.B. Experimental Transmission of Mayaro Virus by Aedes Aegypti. Am. J. Trop. Med. Hyg. 2011;85:750–757. doi: 10.4269/ajtmh.2011.11-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serra O.P., Cardoso B.F., Ribeiro A.L.M., dos Santos F.A.L., Slhessarenko R.D. Mayaro Virus and Dengue Virus 1 and 4 Natural Infection in Culicids from Cuiabá, State of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz. 2016;111:20–29. doi: 10.1590/0074-02760150270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith G.C., Francy D.B. Laboratory Studies of a Brazilian Strain of Aedes Albopictus as a Potential Vector of Mayaro and Oropouche Viruses. J. Am. Mosq. Control Assoc. 1991;7:89–93. [PubMed] [Google Scholar]
- 40.Diagne C.T., Bengue M., Choumet V., Hamel R., Pompon J., Missé D. Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens. 2020;9:738. doi: 10.3390/pathogens9090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talarmin A., Chandler L.J., Kazanji M., de Thoisy B., Debon P., Lelarge J., Labeau B., Bourreau E., Vié J.C., Shope R.E., et al. Mayaro Virus Fever in French Guiana: Isolation, Identification, and Seroprevalence. Am. J. Trop. Med. Hyg. 1998;59:452–456. doi: 10.4269/ajtmh.1998.59.452. [DOI] [PubMed] [Google Scholar]
- 42.Mutricy R., Matheus S., Mosnier É., Martinez-Lorenzi E., De Laval F., Nacher M., Niemetzky F., Naudion P., Djossou F., Rousset D., et al. Mayaro Virus Infection in French Guiana, a Cross Sectional Study 2003–2019. Infect. Genet. Evol. 2022;99:105243. doi: 10.1016/j.meegid.2022.105243. [DOI] [PubMed] [Google Scholar]
- 43.Mayaro Virus Disease—French Guiana, France. [(accessed on 4 April 2023)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/mayaro-virus-disease---french-guiana-france.
- 44.Acosta-Ampudia Y., Monsalve D.M., Rodríguez Y., Pacheco Y., Anaya J.-M., Ramírez-Santana C. Mayaro: An Emerging Viral Threat? Emerg. Microbes Infect. 2018;7:163. doi: 10.1038/s41426-018-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Digoutte J.P. Ecologie Des Arbovirus et Leur Rôle Pathogène Chez l’homme En Guyane Française. Institut Pasteur de la Guyane Française; Cayenne, French Guiana: 1975. Groupe Institut national de la santé et de la recherche médicale U79. [Google Scholar]
- 46.Monath T.P., Lazuick J.S., Cropp C.B., Rush W.A., Calisher C.H., Kinney R.M., Trent D.W., Kemp G.E., Bowen G.S., Francy D.B. Recovery of Tonate Virus (“Bijou Bridge” Strain), a Member of the Venezuelan Equine Encephalomyelitis Virus Complex, from Cliff Swallow Nest Bugs (Oeciacus Vicarius) and Nestling Birds in North America. Am. J. Trop. Med. Hyg. 1980;29:969–983. doi: 10.4269/ajtmh.1980.29.969. [DOI] [PubMed] [Google Scholar]
- 47.Fischer C., Pontier D., Filippi-Codaccioni O., Pons J.-B., Postigo-Hidalgo I., Duhayer J., Brünink S., Drexler J.F. Venezuelan Equine Encephalitis Complex Alphavirus in Bats, French Guiana. Emerg. Infect. Dis. 2021;27:1141–1145. doi: 10.3201/eid2704.202676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Digoutte J.P., Girault G. Résultats de l’étude chez la souris du pouvoir protecteur du virus Tonate et de deux souches de virus Cabassou contre la souche neurovirulente everglades du groupe VEE. Ann. Microbiol. 1976;127B:429–437. [PubMed] [Google Scholar]
- 49.Talarmin A., Trochu J., Gardon J., Laventure S., Hommel D., Lelarge J., Labeau B., Digoutte J.P., Hulin A., Sarthou J.L. Tonate Virus Infection in French Guiana: Clinical Aspects and Seroepidemiologic Study. Am. J. Trop. Med. Hyg. 2001;64:274–279. doi: 10.4269/ajtmh.2001.64.274. [DOI] [PubMed] [Google Scholar]
- 50.Mutricy R., Djossou F., Matheus S., Lorenzi-Martinez E., De Laval F., Demar M., Nacher M., Rousset D., Epelboin L. Discriminating Tonate Virus from Dengue Virus Infection: A Matched Case-Control Study in French Guiana, 2003–2016. Am. J. Trop. Med. Hyg. 2020;102:195–201. doi: 10.4269/ajtmh.19-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambert V., Enfissi A., Lefebvre M., Pomar L., Kedous S., Guimiot F., Carles G., Lavergne A., Rousset D., Hcini N. Tonate Virus and Fetal Abnormalities, French Guiana, 2019. Emerg. Infect. Dis. 2022;28:445–448. doi: 10.3201/eid2802.210884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakkas H., Bozidis P., Franks A., Papadopoulou C. Oropouche Fever: A Review. Viruses. 2018;10:175. doi: 10.3390/v10040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaillet M., Pichard C., Restrepo J., Lavergne A., Perez L., Enfissi A., Abboud P., Lambert Y., Ma L., Monot M., et al. Outbreak of Oropouche Virus in French Guiana. Emerg. Infect. Dis. 2021;27:2711–2714. doi: 10.3201/eid2710.204760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walsh C.E.S., Robert M.A., Christofferson R.C. Observational Characterization of the Ecological and Environmental Features Associated with the Presence of Oropouche Virus and the Primary Vector Culicoides Paraenesis: Data Synthesis and Systematic Review. Trop. Med. Infect. Dis. 2021;6:143. doi: 10.3390/tropicalmed6030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paixão M.M., Ballouz T., Lindahl J.F. Effect of Education on Improving Knowledge and Behavior for Arboviral Diseases: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2019;101:441–447. doi: 10.4269/ajtmh.19-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jolivet A., Cadot E., Florence S., Lesieur S., Lebas J., Chauvin P. Migrant Health in French Guiana: Are Undocumented Immigrants More Vulnerable? BMC Public Health. 2012;12:53. doi: 10.1186/1471-2458-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meynard J.-B., Ardillon V., Venturin C., Ravachol F., Basurko C., Matheus S., Gaborit P., Grenier C., Dussart P., Quénel P. First Description of a Dengue Fever Outbreak in the Interior of French Guiana, February 2006. Eur. J. Public Health. 2009;19:183–188. doi: 10.1093/eurpub/ckn137. [DOI] [PubMed] [Google Scholar]
- 58.Microbe T.L. The Lancet Microbe Chikungunya in Brazil… and Beyond? Lancet Microbe. 2023;4:e284. doi: 10.1016/S2666-5247(23)00120-9. [DOI] [PubMed] [Google Scholar]
- 59.Hallet E., Flamand C., Rousset D., Bonifay T., Fritzell C., Matheus S., Dueymes M., Ntab B., Nacher M. ZIKA Virus Infection in Pregnant Women in French Guiana: More Precarious-More at Risk. PLoS Negl. Trop. Dis. 2020;14:e0008193. doi: 10.1371/journal.pntd.0008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonifay T., Douine M., Bonnefoy C., Hurpeau B., Nacher M., Djossou F., Epelboin L. Poverty and Arbovirus Outbreaks: When Chikungunya Virus Hits More Precarious Populations Than Dengue Virus in French Guiana. Open Forum Infect. Dis. 2017;4:ofx247. doi: 10.1093/ofid/ofx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oberlis M., Renaud-Grappin E., Sevestre E., Bauza J.-L., Souleymane G., Cochet C. Projet « WASH » En Situation d’épidémie de COVID-19—5e Journée Des Travaux Scientifiques Des Soignants de Guyane. Med. Trop. Sante Int. 2022;2:mtsi.v2i2.2022.248. [Google Scholar]
- 62.Esu E., Lenhart A., Smith L., Horstick O. Effectiveness of Peridomestic Space Spraying with Insecticide on Dengue Transmission; Systematic Review. Trop. Med. Int. Health. 2010;15:619–631. doi: 10.1111/j.1365-3156.2010.02489.x. [DOI] [PubMed] [Google Scholar]
- 63.Epelboin Y., Chaney S.C., Guidez A., Habchi-Hanriot N., Talaga S., Wang L., Dusfour I. Successes and Failures of Sixty Years of Vector Control in French Guiana: What Is the next Step? Mem. Inst. Oswaldo Cruz. 2018;113:e170398. doi: 10.1590/0074-02760170398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dusfour I., Thalmensy V., Gaborit P., Issaly J., Carinci R., Girod R. Multiple Insecticide Resistance in Aedes Aegypti (Diptera: Culicidae) Populations Compromises the Effectiveness of Dengue Vector Control in French Guiana. Mem. Inst. Oswaldo Cruz. 2011;106:346–352. doi: 10.1590/S0074-02762011000300015. [DOI] [PubMed] [Google Scholar]
- 65.International Agency for Research on Cancer IARC Monographs Volume 112: Evaluation of Five Organophosphate Insecticides and Herbicides—IARC. [(accessed on 27 January 2023)]. Available online: https://www.iarc.who.int/news-events/iarc-monographs-volume-112-evaluation-of-five-organophosphate-insecticides-and-herbicides/
- 66.O’Neill S.L. The Use of Wolbachia by the World Mosquito Program to Interrupt Transmission of Aedes Aegypti Transmitted Viruses. Adv. Exp. Med. Biol. 2018;1062:355–360. doi: 10.1007/978-981-10-8727-1_24. [DOI] [PubMed] [Google Scholar]
- 67.Kraemer M.U.G., Sinka M.E., Duda K.A., Mylne A.Q.N., Shearer F.M., Barker C.M., Moore C.G., Carvalho R.G., Coelho G.E., Van Bortel W., et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and Ae. Albopictus. eLife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chadee D.D., Fat F.H., Persad R.C. First Record of Aedes Albopictus from Trinidad, West Indies. J. Am. Mosq. Control Assoc. 2003;19:438–439. [PubMed] [Google Scholar]
- 69.Saraiva J.F., Maitra A., Galardo A.K.R., Scarpassa V.M. First Record of Aedes (Stegomyia) Albopictus in the State of Amapá, Northern Brazil. Acta Amaz. 2019;49:71–74. doi: 10.1590/1809-4392201802771. [DOI] [Google Scholar]
- 70.Muller J.N., Galardo A.K.R., dos Santos W.M., Ferro E.P., Dias L.d.S., Corrêa A.P.S.d.A., Nunes M.C.d.L., Jesus J.S., Lima J.B.P. Expansion of Aedes (Stegomyia) Albopictus (Skuse, 1894) in Northern Brazil: New Records and Distribution in Urban Areas of Macapa City. CheckList. 2021;17:911–915. doi: 10.15560/17.3.911. [DOI] [Google Scholar]
- 71.Talaga S. Ph.D. Thesis. Université de Guyane; Cayenne, French Guiana, France: 2016. Ecologie, Diversité et Évolution Des Moustiques (Diptera Culicidae) de Guyane Française: Implications Dans l’invasion Biologique Du Moustique Aedes aegypti (L.) [Google Scholar]
- 72.Juliano S.A. Species Introduction and Replacement Among Mosquitoes: Interspecific Resource Competition or Apparent Competition? Ecology. 1998;79:255–268. doi: 10.1890/0012-9658(1998)079[0255:SIARAM]2.0.CO;2. [DOI] [Google Scholar]
- 73.Braks M.A.H., Honório N.A., Lourenço-De-Oliveira R., Juliano S.A., Lounibos L.P. Convergent Habitat Segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J. Med. Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- 74.Dégallier N., Digoutte J.P., Pajot F.-X., Kramer R., Claustre J., Bellony S., Chatenay G., Alfré E. Epidémiologie de deux arbovirus du complexe VEE en Guyane française: Données préliminaires sur les relations virus-vecteurs. Cah. ORSTOM Sér. Entomol. Méd. Parasitol. 1978;XVI:209–221. [Google Scholar]
- 75.Dégallier N., Digoutte J.P., Pajot F.-X., Kramer R., Claustre J., Bellony S., Chatenay G., Alfré E. Epidémiologie de Bunyavirus (arbovirus) des groupes C et Guama en Guyane française: Données préliminaires et comparaison avec les virus du complexe V.E.E. Cah. ORSTOM Sér. Entomol. Méd. Parasitol. 1979;XVII:3–11. [Google Scholar]
- 76.Panday R.S., Digoutte J.P. Tonate and Guama-Group Viruses Isolated from Mosquitoes in Both a Savannah and Coastal Area in Surinam. Trop. Geogr. Med. 1979;31:275–282. [PubMed] [Google Scholar]
- 77.Current ICTV Taxonomy Release|ICTV. [(accessed on 10 March 2023)]. Available online: https://ictv.global/taxonomy.
- 78.Dégallier N. Dr. Thesis. Université Pierre et Marie Curie; Paris, France: 1982. Les Arbovirus Selvatiques en Guyane Française et Leurs Vecteurs. [Google Scholar]
- 79.Panon G., Fauran P., Digoutte J.P. Isolation of Ilheus virus in french Guyana. Bull. Soc. Pathol. Exot. Fil. 1979;72:315–318. [PubMed] [Google Scholar]
- 80.Lavergne A., Lacoste V., Germain A., Matheus S., Dussart P., Deparis X., de Thoisy B. Dengue virus infection in neotropical forest mammals: Incidental hosts or potential reservoirs? Med. Trop. (Mars) 2009;69:345–350. [PubMed] [Google Scholar]
- 81.Talaga S., Dejean A., Carinci R., Gaborit P., Dusfour I., Girod R. Updated Checklist of the Mosquitoes (Diptera: Culicidae) of French Guiana. J. Med. Entomol. 2015;52:770–782. doi: 10.1093/jme/tjv109. [DOI] [PubMed] [Google Scholar]
- 82.Talaga S., Duchemin J.-B., Girod R., Dusfour I. The Culex Mosquitoes (Diptera: Culicidae) of French Guiana: A Comprehensive Review With the Description of Three New Species. J. Med. Entomol. 2021;58:182–221. doi: 10.1093/jme/tjaa205. [DOI] [PubMed] [Google Scholar]
- 83.Cochet A., Calba C., Jourdain F., Grard G., Durand G.A., Guinard A., Team I., Noël H., Paty M.-C., Franke F. Autochthonous Dengue in Mainland France, 2022: Geographical Extension and Incidence Increase. Eurosurveillance. 2022;27:2200818. doi: 10.2807/1560-7917.ES.2022.27.44.2200818. [DOI] [PMC free article] [PubMed] [Google Scholar]