Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) is the highest incidence of chronic liver disease worldwide, seriously endangering human health, and its pathogenesis is still unclear. In the recent years, increasing evidence has shown that intestinal flora plays an important role in the occurrence and development of NAFLD. Synbiotics can alter gut microbiota and may be a treatment option for NAFLD in the future.
Objectives:
To systematically investigate the therapeutic effect of synbiotic supplementation on NAFLD patients.
Design:
A systematic review and meta-analysis were conducted.
Data sources and methods:
We conducted a search on four databases (PubMed, Embase, Cochrane Library, and Web of Science) to identify relevant studies. Eligible studies were then screened, and data from the included studies were extracted, combined, and analyzed.
Result:
This study analyzed 10 randomized controlled trials involving 634 patients with NAFLD. The results showed that synbiotic supplementation could significantly reduce the level of alanine aminotransferase (mean difference (MD) = −8.80; (95% CI [−13.06, −4.53]), p < 0.0001), aspartate aminotransferase (MD = −9.48; 95% CI [−12.54, −6.43], p < 0.0001), and γ-glutamyl transferase (MD = −12.55; 95% CI [−19.40, −5.69], p = 0.0003) in NAFLD patients. In the field of metabolism, synbiotic supplementation could significantly reduce the level of total cholesterol (MD = −11.93; 95% CI [−20.43, −3.42], p = 0.006) and low-density lipoprotein cholesterol (MD = −16.2; 95% CI [−19.79, −12.60], p < 0.0001) and increase the level of high-density lipoprotein cholesterol (MD = 1.56; 95% CI [0.43, 2.68], p = 0.007) in NAFLD patients. In addition, synbiotic supplementation could significantly reduce liver stiffness measurement indicator (MD = −1.09; 95% CI [−1.87, −0.30], p = 0.006) and controlled attenuation parameter indicator (MD = −37.04; 95% CI [−56.78, −17.30], p = 0.0002) in NAFLD patients.
Conclusion:
Based on the current evidence, synbiotic supplementation can improve liver function, adjust lipid metabolism, and reduce the degree of liver fibrosis in patients with NAFLD, but these effects need to be confirmed by further studies.
Keywords: CAP, lipid metabolism, liver enzyme, LSM, NAFLD, synbiotic
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a metabolic stress-induced liver injury closely related to insulin resistance (IR) and genetic susceptibility. The disease-grading spectrum includes nonalcoholic hepatic steatosis, nonalcoholic steatohepatitis, liver cirrhosis, and hepatocellular carcinoma. 1 NAFLD is the most prevalent chronic liver disease in the world, affecting 25.2% of the population. Although the complex pathogenesis of NAFLD is challenging to elucidate fully, numerous studies have established a significant correlation between NAFLD and diabetes mellitus, metabolic syndrome (MetS), and other diseases. 1 The global prevalence of NAFLD in diabetes patients is 55.5%, rising to 68.0% in Europe. 2 Furthermore, the proportion of MetS in NAFLD patients (56.3%) was significantly higher than that in the control group (16.3%). Importantly, studies have confirmed that both diabetes and MetS considerably increase risk of advanced NAFLD fibrosis, liver-related mortality, and all-cause mortality. 3
The gut–liver axis refers to the bidirectional relationship between the gut microbiota and the liver, which is influenced by dietary, genetic, and environmental factors. This interaction is mainly through the portal vein, which enables the direct transport of visceral-derived products to the liver and promotes hepatic feedback to reach the intestine. The liver receives various signals from the gut, including bacterial products, environmental toxins, and food antigens, allowing nutrients to enter the circulation and reach the liver, limiting the systemic spread of toxins, and maintaining the balance of the immune system. As a part of this two-way communication, controlling the microbial community is essential to maintain the homeostasis of the gut–liver axis. Even in the absence of pathogens, changes in gut microbiota can disrupt intestinal homeostasis, leading to immune disorders and various liver diseases.4,5 Many studies have confirmed that NAFLD patients are prone to intestinal flora imbalance, microbial metabolic dysfunction in the digestive tract, and a significant increase in pathogenic bacteria. This provides the possibility of using synbiotics to improve intestinal flora and thus treat NAFLD.6–8
The World Health Organization defines probiotics as live microorganisms that provide health benefits to their hosts. 9 On the other hand, it is generally accepted that prebiotics are non-digestible food additives that support the growth of probiotics by stimulating their activity. 9 Synbiotics are a potent combination of prebiotics and probiotics that provide beneficial food for gut microbiota and improve the survival and activity of beneficial microorganisms in the gut. 10 Numerous previous studies have shown that probiotics, prebiotics, and synbiotics (PPS) can improve the metabolic function of the liver and delay the progression of liver steatosis and fibrosis by improving the intestinal flora and alleviating the risk factors of NAFLD.10–14 In this meta-analysis, we aim to explore whether synbiotics can improve metabolic function, liver fibrosis, and steatosis in NAFLD patients by including relevant studies from the last decade.
Materials and methods
Search strategy
This meta-analysis was carried out in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) statement. 15 Two independent authors (JC and DJ) conducted a literature search using four databases (PubMed, Embase, Cochrane Library, and Web of Science) to identify trials published until 1 September 2022. We established the following search strings to identify trials: ‘Non alcoholic Fatty Liver Disease or NAFLD or Nonalcoholic Fatty Liver Disease or Fatty Liver, Nonalcoholic or Fatty Livers, Nonalcoholic or Liver, Nonalcoholic Fatty or Livers, Nonalcoholic Fatty or Nonalcoholic Fatty Liver or Nonalcoholic Fatty Livers or Nonalcoholic Steatohepatitis or Nonalcoholic Steatohepatitides or Steatohepatitides, Nonalcoholic or Steatohepatitis, Nonalcoholic’ and ‘symbiotic or synbiotic’ in All Text.
Inclusion and exclusion criteria
Inclusion criteria
(1) Randomized controlled trial (RCT).
(2) Patients were diagnosed with NAFLD without the limitation in age and gender.
(3) The intervention was synbiotic or symbiotic, and the intervention duration time was more than 4 weeks.
(4) The treatment of the control group was the same as the intervention group except synbiotic intervention.
(5) RCTs reported change in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) or γ-glutamyl transferase (GGT) or total cholesterol (TC) or triglycerides (TG) or low-density lipoprotein cholesterol (LDL) or high-density lipoprotein cholesterol (HDL) or glucose or IR or insulin or liver stiffness measurement (LSM) or controlled attenuation parameter (CAP).
Exclusion criteria
(1) Patients with alcoholic steatohepatitis, alcoholic fatty liver, cirrhosis, or liver cancer
(2) Patients received additional intervention
(3) Articles published over 10 years
(4) Conference papers or abstracts
(5) Articles not written in English
Data extraction
Two authors (JC and DJ) independently used the inclusion and exclusion criteria before evaluating the titles and abstracts of identified articles to exclude irrelevant studies. Moreover, we utilized the predefined table to record the following detail from included RCTs: (1) basic characteristics: first author, publication date, and case numbers; (2) intervention supplementation: synbiotic ingredients, intervention duration, and type of control (placebo/non-placebo); and (3) basic traits of patients: age, gender, and body mass index (BMI).
Risk of bias and study quality assessment
Two independent authors (JC and DJ) used The Cochrane Collaboration’s Risk of Bias tool to evaluate bias in the included RCTs. 16 The level of bias was rated as ‘low risk’ or ‘high risk’ or ‘unclear risk’, and the study was excluded if more than one high risk existed. We also used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) profiler software (GradePro Version 3.6.1). The quality of evidence was categorized into four levels: very low, low, moderate, and high in the GRADE system. Funnel plots and Egger’s test were used to analyze whether there was publication bias.
Statistical analysis
This meta-analysis was performed using the RevMan Version 5.3 software (The Cochrane Collaboration, Copenhagen, Denmark). We calculated the mean difference (MD) for studies that used precisely the same measurement methods or units for similar outcomes. In comparison, we calculated the standard mean difference (SMD) for studies that used different measurement methods or units for similar outcomes. Data analyses were conducted by using the I2 statistic to detect heterogeneity. To search for potential sources of interstudy heterogeneity, we performed a subgroup analysis based on intervention duration (⩽12 weeks and >12 weeks), the diagnostic method of NAFLD (ultrasonic examination or liver biopsy), and baseline BMI (BMI ⩾ 30 and BMI < 30). Besides, we conducted meta-regression analysis to find the source of heterogeneity. When I2 value was <50%, we would use the fixed-effects model; otherwise, the random-effects model was used. All data were analyzed with 95% CI. The results were described using forest plots, with p values less than 0.05 considered statistically significant.
Result
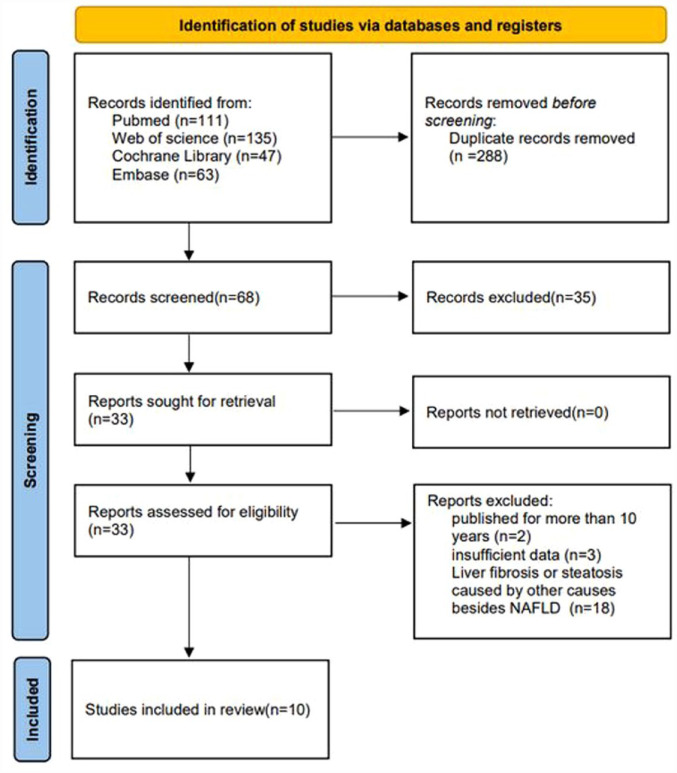
The process of literature retrieval is shown in Figure 1. According to the established strategy, 356 articles were preliminarily retrieved up to 1 September 2022, and 10 RCTs were finally included. The synbiotic group included 316 patients, while the placebo group included 308 patients.
Figure 1.
Flowchart of the study selection process.
Table 1 reports the characteristics of the included RCTs. The risk of bias was assessed by the tool from the Cochrane Collaboration. As shown in Figure 2, the overall risk of the included studies was medium and low.
Table 1.
Basic traits and characteristics of the included studies.
| First author | Publication years | Case number (effect/control) | Intervention time | Synbiotic ingredients | BMI (effect/control) | Diagnostic method | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Abhari | 2020 | 23 | 22 | 12 weeks | 1 × 109 CFU of Bacillus coagulans (GBI-30) + 0.4 g inulin | 32.2 ± 6.72 | 33.6 ± 5.06 | Ultrasound or biopsy | ALT/AST/GGT (IU/L), TG/TC/LDL/HDL (mg/dL), glucose (mg/dL), insulin (uU/ml), IR LSM (kPa) CAP |
| Asgharian | 2017 | 38 | 36 | 8 weeks | 1 × 109 CFU of 7 bacterial strains + FOS | 29.58 ± 0.76 | 28.18 ± 0.68 | Ultrasonic examination | ALT/AST (IU/L) |
| Bakhshimoghaddam | 2018 | 34 | 34 | 24 weeks | 1 × 108 CFU of Bifidobacterium animalis + 1.5 g inulin | 30.5 ± 4.6 | 31.9 ± 5.1 | Ultrasonic examination | ALT/AST/GGT (IU/L), insulin (mU/L) IR |
| Ekhlasi | 2016 | 15 | 15 | 8 weeks | 2 × 108 CFU of 7 bacterial strains + FOS + magnesium stearate + hydroxypropyl methyl cellulose | 27.28 ± 2.21 | 27.84 ± 1.96 | Ultrasonic examination | ALT/AST (IU/L), TG/TC/LDL/HDL (mg/dL), glucose (mg/dL), insulin (uU/L) IR |
| Eslamparast | 2014 | 26 | 26 | 28 weeks | 4 × 108 CFU of 7 bacterial strains + FOS + magnesium stearate + hydroxypropyl methyl cellulose | 32.1 ± 2.4 | 31.3 ± 2.3 | Ultrasonic examination | ALT/AST/GGT (IU/L), insulin (mU/ml) IR LSM (kPa) |
| Ferolla | 2016 | 27 | 23 | 12 weeks | 1 × 108 CFU of Lactobacillus reuteri + 4 g hydrolyzed guar gum and inulin | 32.5 ± 4.0 | 32.5 ± 4.0 | Liver biopsy | TG/TC/LDL/HDL (mg/dL) |
| Javadi | 2017 | 17 | 19 | 12 weeks | 2 × 107 CFU of Bifidobacterium longum and Lactobacillus acidophilus + 10 g inulin | 32.30 ± 4.78 | 30.38 ± 2.88 | Liver biopsy | ALT/AST/GGT (IU/L) |
| Mofidi | 2017 | 21 | 21 | 28 weeks | 2 × 108 CFU of 7 bacterial strains + FOS + magnesium stearate + hydroxypropyl methyl cellulose | 23.17 ± 1.01 | 23.20 ±1 .07 | Ultrasonic examination | ALT/AST/GGT (IU/L), TG/TC/LDL/HDL (mg/dL), glucose (mg/dL) insulin (mU/L), IR LSM (kPa) CAP |
| Sayari | 2018 | 70 | 68 | 16 weeks | 1 × 109 CFU of 7 bacterial strains + FOS + magnesium stearate + hydroxypropyl methyl cellulose | 29.72 ± 3.62 | 29.54 ± 3.71 | Ultrasound or biopsy | ALT/AST (IU/L), TG/TC/LDL/HDL (mg/dL), glucose (mg/dL) |
| Scorletti | 2020 | 45 | 44 | 10–14 Months | 1 × 1010 CFU of B. animalis + FOS | 32.9 ± 5.5 | 33.2 ± 4.9 | Ultrasound or biopsy | ALT/AST/GGT (IU/L), TG/TC/LDL/HDL (mmol/L), glucose (mmol/L) insulin (uU/ml), LSM (kPa) CAP |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; GBI:B. coagulans; CFU, colony-forming unit; FOS, fructo-oligosaccharides; GGT, γ-glutamyl transferase; HDL, high-density lipoprotein cholesterol; IR, insulin resistance; LDL, low-density lipoprotein cholesterol; LSM, liver stiffness measurement; TC, total cholesterol; TG, triglycerides.
Figure 2.
Risk-of-bias graph.
Effect on liver enzyme
We carried out a meta-analysis on the the three liver enzyme indicators: ALT, AST GGT and futher performed subgroup analyses. The results are shown in Supplemental Figures S1–S9.
ALT
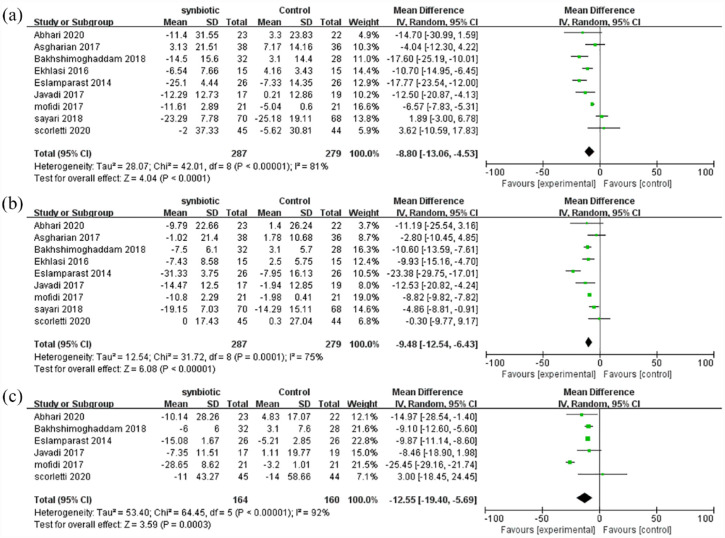
As shown in Figure 3(a), ALT was reported in nine studies involving 566 patients. The combined estimated value was MD = −8.80 (95% CI [−13.06, −4.53], p < 0.0001, I2 = 81%, random-effects model), and the difference was statistically significant, suggesting that synbiotic supplementation can significantly reduce ALT levels in NAFLD patients.
Figure 3.
Forest plot analyzing the effect of synbiotic on liver enzyme: (a) mean change in ALT, (b) mean change in AST, and (c) mean change in GGT.
AST
As shown in Figure 3(b), AST was reported in nine studies involving 566 patients. The combined estimated value was MD = −9.48 (95% CI [−12.54, −6.43], p < 0.0001, I2 = 75%, random-effects model), and the difference was statistically significant, suggesting that synbiotic supplementation can significantly reduce AST levels in NAFLD patients.
GGT
As shown in Figure 3(c), GGT was reported in six studies involving 324 patients. The combined estimated value was MD = −12.55 (95% CI [−19.40, −5.69], p = 0.0003, I2 = 92%, random-effects model), and the difference was statistically significant, suggesting that synbiotic supplementation can significantly reduce GGT levels in NAFLD patients.
Effect on lipid metabolism
We carried out a meta-analysis on the four lipid metabolism indicators: TG, TC, LDL, HDL and futher performed subgroup analyses. Subgroup analysis of the included studies revealed a statistically significant difference in TC and LDL between the two groups according to BMI (⩾30 and <30); the other results are shown in Supplemental Figures S19–S28.
TG
As shown in Figure 4(a), TG was reported in six studies involving 394 patients. The combined estimated value was MD = −2.47 (95% CI [−24.7, 1.74], p = 0.09, I2 = 57%, random-effects model). The result shows no significant difference in TG levels after the synbiotic supplementation group was compared with the control group.
Figure 4.
Forest plot analyzing the effect of synbiotic on lipid metabolism: (a) mean change in TG, (b) subgroup analyses of TC, (c) subgroup analyses of LDL, and (d) mean change in HDL.
TC
As shown in Figure 4(b), TC was reported in six studies involving 394 patients. The combined estimated value was MD = −11.93 (95% CI [−20.43, −3.42], p = 0.006, I2 = 62%, random-effects model), and the difference was statistically significant, suggesting that synbiotic supplementation can significantly reduce TC levels in NAFLD patients. And subgroup analysis showed a statistically significant decrease in TC levels in the subgroup of BMI < 30 (MD = −18.12; 95% CI [−24.64 to −11.59], p < 0.0001, I2 = 40%), whereas there was no significant change in the subgroup of BMI ⩾ 30 (MD = 0.01; 95% CI [−11.28 to 11.29], p = 1.00, I2 = 0%).
LDL
As shown in Figure 4(c), LDL was reported in six studies involving 394 patients. The combined estimated value was MD = −11.93 (95% CI [−19.79, −12.60], p < 0.0001, I2 = 50%, fixed-effects model), and the difference was statistically significant, suggesting that synbiotic supplementation can significantly reduce LDL levels in NAFLD patients. And the subgroup analysis showed a statistically significant decrease in LDL levels in the subgroup of BMI < 30 (MD = −18.16; 95% CI [−22.03, −14.30], p < 0.0001, I2 = 0%), whereas there was no significant change in the subgroup of BMI ⩾ 30 (MD = −3.57; 95% CI [−13.35 to 6.21], p = 0.47, I2 = 0%).
HDL
As shown in Figure 4(d), HDL was reported in six studies involving 394 patients. The combined estimated value was MD = 1.56 (95% CI [0.43, 2.68], p = 0.007, I2 = 0%, fixed-effects model), and the difference was statistically significant, suggesting that synbiotic supplementation can significantly improve HDL levels in NAFLD patients.
Effect on sugar metabolism
We carried out a meta-analysis on the three sugar metabolism indicators: glucose, IR, insulin and futher performed subgroup analyses. We also performed subgroup analyses, and the results are shown in Supplemental Figures S10–S18.
Glucose
As shown in Figure 5(a), glucose was reported in five studies involving 344 patients. The combined estimated value was MD = −8.06 (95% CI [−16.33, 0.21], p = 0.06, I2 = 89%, random-effects model). The result shows no significant difference in glucose level after synbiotic supplementation group compared with the control group.
Figure 5.
Forest plot analyzing the effect of synbiotic on sugar metabolism: (a) mean change in glucose, (b) mean change in IR, and (c) mean change in insulin.
IR
As shown in Figure 5(b), IR was reported in five studies involving 229 patients. The combined estimated value was MD = −0.03 (95% CI [−0.54, 0.48], p = 0.91, I2 = 92%, random-effects model).The result shows no significant difference in IR level after the synbiotic supplementation group was compared with the control group.
Insulin
As shown in Figure 5(c), insulin was reported in five studies involving 266 patients. The combined estimated value was SMD = −0.50 (95% CI [−1.17, 0.17], p = 0.14, I2 = 85%, random-effects model). The result shows no significant difference in insulin level after the synbiotic supplementation group was compared with the control group.
Effect on liver degeneration
We carried out a meta-analysis on the two liver degeneration indicators of LSM, CAP and futher performed subgroup analyses. We also conducted subgroup analyses of LSM. The results are shown in Supplemental Figures S29–S31. Because there are only three RCTs that include a CAP indicator, we did not perform subgroup analyses of CAP.
LSM
As shown in Figure 6(a), LSM was reported in four studies involving 228 patients. The combined estimated value was MD = −1.09 (95% CI [−1.87, −0.30], p = 0.006, I2 = 58%, random-effects model), and the difference was statistically significant, suggesting that synbiotic supplementation can significantly reduce LSM in NAFLD patients.
Figure 6.
Forest plot analyzing the effect of synbiotic on liver degeneration: (a) mean change in LSM and (b) mean change in CAP.
CAP
As shown in Figure 6(b), CAP was reported in three studies involving 176 patients. The combined estimated value was MD = −37.04 (95% CI [−56.78, −17.30], p = 0.0002, I2 = 61%, random-effects model), and the difference was statistically significant, suggesting that synbiotic supplementation can significantly reduce CAP in NAFLD patients.
Discussion
This study systematically evaluated the effect of synbiotic supplementation on liver function, lipid and sugar metabolism, and changes in liver stiffness and steatosis in NAFLD patients. The results indicated that synbiotic supplementation can alleviate liver stiffness and steatosis, significantly reduce the level of ALT, AST, GGT, TG, and LDL, while increasing the level of HDL. However, our study showed no significant influence on glucose, TG, insulin, and IR levels. There was no statistical difference in subgroup analysis based on intervention duration (⩽12 and >12 weeks), the diagnostic method of NAFLD (ultrasonic examination or liver biopsy), and baseline BMI (⩾30 and <30). Although the subgroup analysis based on the diagnostic method suggested significant differences between groups in AST and TG, only one study was included in the liver biopsy group, so we did not show them in Figures 3 and 4 (the details can be found in Supplemental Figures S5 and S20). Subgroup analysis of GGT level based on BMI was similarly handled (the details can be found in Supplemental Figure S9). Due to variations in clinical input data, this study exhibited high heterogeneity. Subgroup analysis results suggest that the source of this high heterogeneity may be attributed to differences in the diagnostic methods used (the details can be found in Supplemental Figures S11, S20, and S23 and Figure 4(b)).
Elevations in ATL, AST, and GGT are common manifestation in patients with NAFLD and are often used as indicators for early NAFLD screening and liver function evaluation. 17 In this study, the improvement of liver function indicators in NAFLD patients after synbiotic supplementation was taken as the primary marker to evaluate the supplementation effect. We found synbiotic supplementation could significantly reduce the level of ALT (MD = −8.80; 95% CI [−13.06, −4.53], p < 0.0001), AST (MD = −9.48; 95% CI [−12.54, −6.43], p < 0.0001), and GGT (MD = −12.55; 95% CI [−19.40, −5.69], p = 0.0003) in NAFLD patient. This is consistent with the results of other meta-analyses.18,19 In NAFLD patients, intestinal flora metabolism disorders usually occur, thus stimulating liver Kupffer cells and stellate cells, leading to liver cell death and release of liver enzyme into the blood, accompanied by increases in inflammatory factors such as tumor necrosis factor-α (TNF-α), high-sensitivity C-reactive protein (hs-CRP), and interleukin-6 (IL-6).20,21 TNF-α is a multipotent cytokine that increases the activity of Kupffer cells by activating the nuclear factor kappa-B and mitogen-activated protein kinase pathways, leading to hepatocyte apoptosis, hepatic stellate cell activation, and hepatocyte aggregation. IL-6 is a potent activator of the liver signal transduction and transcription activator 3 pathway and is widely involved in different aspects of liver pathophysiology. In this meta-analysis, the inflammatory indicators were not analyzed because of an insufficient number of RCTs on TNF-α, hs-CRP, and IL-6. However, previous studies have confirmed that synbiotic supplementation can reduce their levels in patients with NAFLD, thus alleviating the progression of liver inflammation.22,23 Loman et al. 24 and Hadi et al. 25 performed meta-analyses and found that synbiotic supplementation significantly reduced TNF-α and CPR levels, but there was no significant change in IL-6 levels, which may be related to the limited number of studies included in their analysis. Further studies are needed to investigate the effects of synbiotic supplementation on inflammatory factors in NAFLD patients.
NAFLD is closely associated with diabetes mellitus and MetS, also considered to be the liver manifestation of MetS. 2 The lipid metabolism disorder is an important feature of NAFLD, which is marked by an increase in TC, TG, and LDL and a decrease in HDL. 26 Many studies suggest that synbiotic supplementation may alter the gene expression of adipogenic enzymes and reduce the synthesis of fatty acids in the liver, thus reducing the accumulation of triglycerides in the liver. In addition, synbiotic supplementation has been shown to inhibit the carbohydrate response element binding protein transcription and activate the peroxisome proliferator-activated receptor α (PPARα) encoding gene transcription and increase the content of butyric acid in the liver, which promotes fasting-induced adipose factor and reduces the accumulation of free fatty acids, ultimately leading to an improvement in the lipid metabolism of NAFLD patients.27,28 We found synbiotic supplementation could significantly reduce the level of TC (MD = −11.93; 95% CI [−20.43, −3.42], p = 0.006) and LDL (MD = −16.20; 95% CI [−19.79, −12.60], p < 0.0001) and increase the level of HDL (MD = 1.56; 95% CI [0.43, 2.68], p = 0.007) in NAFLD patients. Besides, we found a significant decrease in TC and LDL levels in the subgroup of BMI <30. This may be related to the degree of metabolic disorder; reports of NAFLD patients with BMI ⩾30 stated levels were more severe than those with BMI <30; therefore, synbiotic treatment was less effective. Further studies are needed to investigate the effects of synbiotic treatment on metabolic regulation in NAFLD patients with different values of BMI. Although there was no statistically significant change in the TG level (MD = −11.48; 95% CI [−24.7, 1.74], p = 0.09), the overall effect would suggest that synbiotic supplementation could reduce TG level to some degree, supporting the belief that synbiotic supplementation can improve lipid metabolism.
In addition to lipid metabolism disorders, NAFLD patients often suffer from glucose metabolism disorders. Stem cell growth factor-beta can induce the differentiation of M1 classically activated macrophages, and their infiltration in adipose tissue can lead to inflammation and inducing IR, which is the core of hepatic steatosis in NAFLD. CRP and IL-6 levels were confirmed to predict the level of SCGF-β, which is positively correlated with the severity of IR and hepatic steatosis. 29 IR results in chronically elevated levels of insulin and blood glucose in the body, which can reduce glucose uptake by muscle tissue and increase the decomposition of peripheral adipose tissue. This process finally leads to long-term accumulation of fatty acids in liver and glucose metabolism disorders. 30 Studies have confirmed that synbiotic supplementation can improve IR and promote blood glucose absorption by improving gut microbiota, increasing fecal pH, promoting the release of the glucose-dependent insulinotropic polypeptide and glucagon-like peptides, and reducing intestinal permeability to endotoxin. In addition, synbiotics can indirectly reduce insulin concentration by improving glucose metabolism. 31 In this study, we found that synbiotic supplementation had no significant effect on the level of blood glucose (MD = −8.06; 95% CI [−16.33, 0.21], p = 0.06), IR (MD = −0.03; 95% CI [−0.54, 0.48], p = 0.91), and insulin (SMD = −0.50; 95% CI [−1.17, 0.17], p = 0.14). Hadi et al. 25 performed meta-analysis and found that synbiotic supplementation could significantly reduce IR and levels of glucose and insulin in NAFLD patients. Subgroup analysis showed that the level of glucose decreased significantly in the ultrasonography or biopsy subgroups compared to the ultrasound subgroups (the details can be found in Supplemental Figure S11). Considering the large difference in sample size between the two subgroups in sample size, we did not include it in Figure 5. This may be related to the insufficient number of studies we included, and further research is needed in the future.
In addition to monitoring liver function and metabolism indicators in patients with NAFLD, it is crucial to evaluate the degree of hepatic steatosis and fibrosis. LSM based on FibroScan is the preferred imaging method for clinical diagnosis of NAFLD, as well as an important tool for early detection of NAFLD, which can accurately reflect the degree of liver inflammation and fibrosis. 26 In the study, we found that synbiotic supplementation could significantly reduce LSM (MD = −1.09; 95% CI [−1.87, −0.30], p = 0.006) in NAFLD patients, which was consistent with other meta-analyses.18,19,32 CAP is a novel ultrasound-based technique for quantitatively diagnosing fatty liver. It can detect hepatic steatosis greater than 5% and accurately differentiate between mild and moderate-to-severe hepatic steatosis. We also found that synbiotic supplementation could significantly reduce CAP indicator (MD = −37.04; 95% CI [−56.78, −17.30], p = 0.0002) in NAFLD patients. These results imply that synbiotic supplementation may be an effective method to slow down the progression of hepatic steatosis and fibrosis in patients with NAFLD, presenting a promising option for the treatment of NAFLD.
The use of PPS as part of the treatment of NAFLD has become a hot topic due to the prevalence of gut microbiota disorders in NAFLD patients. Clinical studies evaluating the effectiveness of PPS in NAFLD treatment have been conducted in recent years. While numerous studies have demonstrated that PPS can improve metabolic function and delay the progression of fibrosis and steatosis in NAFLD patients, some studies have reported different results. For example, Mohammed et al. 33 found that the oral administration of a multi-strain probiotic for 6 months did not significantly improve hepatic steatosis or fibrosis in NAFLD patients. This meta-analysis indicated that synbiotic supplementation may be beneficial for individuals with NAFLD. The findings are encouraging and suggest that synbiotics have a promising future in the treatment of NAFLD. This will enable more researchers to conduct related clinical experiments and apply synbiotic to treat NAFLD. While synbiotics show potential value for treating NAFLD, there are already many promising treatments available, such as PPAR agonists, Farnesoid X receptor agonists, and Thyroid hormone receptor-beta agonists.34,35 Hence, it means synbiotic need to be considered carefully and thoroughly tested to determine its value.
Our paper has several notable strengths. First, the measures included in our analysis were very comprehensive, including liver enzyme, lipid, and glucose metabolism, as well as liver fibrosis and steatosis assessment measures. In addition, through the subgroup analysis, we discovered that synbiotic supplementation had a significant effect on lipid metabolism in NAFLD patients with different BMI. Finally, to the best of our knowledge, this is the first study to investigate the effects of gut microbiome-targeted therapies on CAP in patients with NAFLD. This is a topic that has been largely overlooked in previous meta-analyses.
This study also has some limitations. (1) There were differences in the types of synbiotic ingredients, dosage, intervention time, and lifestyle management among the included RCTs. (2) The design of a few trials was not standardized, which affected the effectiveness of the evaluation. (3) The included studies involved the side effects of microbial therapy, and its unclear side effects on NAFLD need to be further studied. (4) Although the risk of bias in this study was medium to low, we could not determine the effect of this risk. (5) All the included literature and studies were RCTs, but some failed to accurately explain the random sampling method, distribution plan, and blinding method. (6) In the publication process, some results can be omitted, especially negative ones, which may lead to publication bias. Therefore, further randomized double-blind controlled trials are needed to confirm the therapeutic effect of synbiotics on NAFLD.
In conclusion, intestinal flora imbalance is a risk factor contributing to the development and progression of NAFLD. The ability of synbiotics to reverse gut dysbiosis has led to significant interest in synbiotics as a supplementation option for patients with NAFLD. Our study found that synbiotic supplementation significantly improved liver function, adjusted lipid metabolism, and delayed the progression of NAFLD. Further studies are needed to elucidate the role of synbiotics for NAFLD patients.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848231174299 for The effect of synbiotics in patients with NAFLD: a systematic review and meta-analysis by Jiacheng Cai, Jia Dong, Dahua Chen and Hua Ye in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iD: Jiacheng Cai  https://orcid.org/0000-0002-0222-4089
https://orcid.org/0000-0002-0222-4089
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jiacheng Cai, The Affiliated Lihuili Hospital of Ningbo University, Ningbo, Zhejiang, People’s Republic of China.
Jia Dong, The Affiliated Lihuili Hospital of Ningbo University, Ningbo, Zhejiang, People’s Republic of China.
Dahua Chen, The Affiliated Lihuili Hospital of Ningbo University, Ningbo, Zhejiang, People’s Republic of China.
Hua Ye, The Affiliated Lihuili Hospital of Ningbo University, Ningbo, Zhejiang 315040, People’s Republic of China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Jiacheng Cai: Data curation; Formal analysis; Methodology; Project administration; Resources; Software; Visualization; Writing – original draft.
Jia Dong: Data curation; Methodology; Resources; Software; Visualization; Writing – original draft.
Dahua Chen: Methodology; Software; Visualization; Writing – review & editing.
Hua Ye: Funding acquisition; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Zhejiang Provincial Natural Science Foundation of China (Nos. LGF19H030006 and LQ20H030001), Ningbo Science and Technology Project (No. 2019C50100), Ningbo Clinical Medicine Research Center Project (No. 2019A21003), and the Major Special Science and Technology Project of Ningbo City (2022Z128).
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1.Lonardo A, Leoni S, Alswat KA, et al. History of nonalcoholic fatty liver disease. Int J Mol Sci 2020; 21: 5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019; 71: 793–801. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019; 69: 2672–2682. [DOI] [PubMed] [Google Scholar]
- 4.Albillos A, de Gottardi A, Rescigno M.The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol 2020; 72: 558–577. [DOI] [PubMed] [Google Scholar]
- 5.Milosevic I, Vujovic A, Barac A, et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: a review of the literature. Int J Mol Sci 2019; 20: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fianchi F, Liguori A, Gasbarrini A, et al. Nonalcoholic fatty liver disease (NAFLD) as model of gut-liver axis interaction: from pathophysiology to potential target of treatment for personalized therapy. Int J Mol Sci 2021; 22: 6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji Y, Yin Y, Sun L, et al. The molecular and mechanistic insights based on gut-liver axis: nutritional target for non-alcoholic fatty liver disease (NAFLD) improvement. Int J Mol Sci 2020; 21: 3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safari Z, Gerard P.The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci 2019; 76: 1541–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai YL, Lin TL, Chang CJ, et al. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci 2019; 26: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mounir M, Ibijbijen A, Farih K, et al. Synbiotics and their antioxidant properties, mechanisms, and benefits on human and animal health: a narrative review. Biomolecules 2022; 12: 1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpi RZ, Barbalho SM, Sloan KP, et al. The effects of probiotics, prebiotics and synbiotics in non-alcoholic fat liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. Int J Mol Sci 2022; 23: 8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moszak M, Szulinska M, Walczak-Galezewska M, et al. Nutritional approach targeting gut microbiota in NAFLD-To date. Int J Environ Res Public Health 2021; 18: 1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrouz V, Aryaeian N, Zahedi MJ, et al. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: a randomized clinical trial. J Food Sci 2020; 85: 3611–3617. [DOI] [PubMed] [Google Scholar]
- 14.Chong CYL, Orr D, Plank LD, et al. Randomised double-blind placebo-controlled trial of inulin with metronidazole in non-alcoholic fatty liver disease (NAFLD). Nutrients 2020; 12: 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021; 10: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Association for the Study of the L, European Association for the Study of D and European Association for the Study of O. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 18.Khan MY, Mihali AB, Rawala MS, et al. The promising role of probiotic and synbiotic therapy in aminotransferase levels and inflammatory markers in patients with nonalcoholic fatty liver disease - a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2019; 31: 703–715. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Li P, Liu Y, et al. Efficacy of probiotics and synbiotics in patients with nonalcoholic fatty liver disease: a meta-analysis. Dig Dis Sci 2019; 64: 3402–3412. [DOI] [PubMed] [Google Scholar]
- 20.Org E, Blum Y, Kasela S, et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol 2017; 18: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceccarelli S, Panera N, Mina M, et al. LPS-induced TNF-alpha factor mediates pro-inflammatory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget 2015; 6: 41434–41452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cakir M, Aksel Isbilen A, Eyupoglu I, et al. Effects of long-term synbiotic supplementation in addition to lifestyle changes in children with obesity-related non-alcoholic fatty liver disease. Turk J Gastroenterol 2017; 28: 377–383. [DOI] [PubMed] [Google Scholar]
- 23.Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci 2012; 57: 545–553. [DOI] [PubMed] [Google Scholar]
- 24.Loman BR, Hernandez-Saavedra D, An R, et al. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev 2018; 76: 822–839. [DOI] [PubMed] [Google Scholar]
- 25.Hadi A, Mohammadi H, Miraghajani M, et al. Efficacy of synbiotic supplementation in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis of clinical trials: synbiotic supplementation and NAFLD. Crit Rev Food Sci Nutr 2019; 59: 2494–2505. [DOI] [PubMed] [Google Scholar]
- 26.Cusi K, Isaacs S, Barb D, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American association for the study of liver diseases (AASLD). Endocr Pract 2022; 28: 528–562. [DOI] [PubMed] [Google Scholar]
- 27.Wa Y, Yin B, He Y, et al. Effects of single probiotic- and combined probiotic-fermented milk on lipid metabolism in hyperlipidemic rats. Front Microbiol 2019; 10: 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabirin F, Lim SM, Neoh CF, et al. Hepatoprotection of probiotics against non-alcoholic fatty liver disease in vivo: a systematic review. Front Nutr 2022; 9: 844374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarantino G, Citro V, Balsano C, et al. Could SCGF-beta levels be associated with inflammation markers and insulin resistance in male patients suffering from obesity-related NAFLD? Diagnostics (Basel) 2020; 10: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, Fang L.Research progress on the mechanism of acupuncture treatment for nonalcoholic fatty liver disease. Gastroenterol Res Pract 2022; 2022: 5259088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Liu J, Wang Z, et al. The promising role of probiotics/prebiotics/synbiotics in energy metabolism biomarkers in patients with NAFLD: a systematic review and meta-analysis. Front Public Health 2022; 10: 862266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharpton SR, Maraj B, Harding-Theobald E, et al. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr 2019; 110: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohamad Nor MH, Ayob N, Mokhtar NM, et al. The effect of probiotics (MCP((R)) BCMC((R)) strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients 2021; 13: 3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rong L, Zou J, Ran W, et al. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front Endocrinol (Lausanne) 2022; 13: 1087260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negi CK, Babica P, Bajard L, et al. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism 2022; 126: 154925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848231174299 for The effect of synbiotics in patients with NAFLD: a systematic review and meta-analysis by Jiacheng Cai, Jia Dong, Dahua Chen and Hua Ye in Therapeutic Advances in Gastroenterology