Abstract
Purpose
This work focused on determining the highly efficient nodal classification system from American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) classification (eighth edition), positive lymph node, log odds of positive lymph nodes (LODDS), lymph node ratio, examined lymph node, and establishing the new nomogram for predicting cancer-specific survival in colon neuroendocrine tumors (CNETs).
Methods
From the Surveillance, Epidemiology, and End Results database, 943 CNET cases undergoing radical operation were enrolled, and randomized as training (n = 663) or validation set (n = 280). For the above 5 lymph node classification systems, their prediction performances were compared with C-index, Akaike information criterion (AIC), and area under the receiver operating characteristic curve. Univariate together with multivariate regression was carried out for identifying independent risk factors. Afterward, this work established 1 nomogram and confirmed its accuracy based on C-index, calibration curves, together with the area under the curve value. Besides, it was compared with the AJCC TNM classification system with regard to model prediction performance.
Results
LODSS achieved the greatest area under the curve and C-index, whereas the smallest AIC. Upon multivariate regression, age, histologic grade, T stage, M stage, and LODDS independently predicted the risk of CNETs. For the validation set, the C-index of the nomogram was 0.794, and the area under the curves at 1, 3, and 5 years was 0.826, 0.857, and 0.870, separately. Additionally, as revealed by the C-index, AIC, decision curve analysis, as well as Kaplan–Meier analysis, our nomogram had superior performance to the AJCC TNM classification system.
Conclusions
For postoperative patients with CNETs, the LODDS might achieve the best prediction performance. Moreover, the LODDS-based nomograms might show superior survival prediction performance to the AJCC TNM classification system (eighth edition).
Keywords: colon neuroendocrine tumors, positive lymph node, log odds of positive lymph nodes, examined lymph nodes, lymph node ratio, nomogram
Introduction
Neuroendocrine cells have been widely distributed within the human body, as a result, neuroendocrine tumors (NETs) may develop within diverse organs, and most of them are in the gastrointestinal (GI) tract or the bronchopulmonary system. 1 In the US, colonic NETs (CNETs) show an increasing prevalence from 0.02 out of 100,000 to 0.2 out of 100,000 during 1997 to 2004. 2 CNETs are associated with growing incidence rates in the past several decades, probably because of early detection, improved awareness, together with extensive application of enteroscopy for the colon. GI NETs are classified by the World Health Organization (WHO) as well, lowly differentiated, and mixed NETs in 2017 according to the differentiation degree. 3 However, due to the infrequent occurrence and morphological heterogeneity, including the lack of clinical data, it is difficult to perform an accurate risk and prognostic assessment of patients with CNETs.
The American Joint Committee on Cancer (AJCC) classification system (eighth edition) is proven for its performance in predicting CNETs prognosis. 4 In this system, the lymph node metastasis (LNM) stage classification has recently been improved from simple N0 (nonregional LNM) and N1 (regional LNM) to N0 (positive lymph node [PLN] = 0), N1 (1 ≤ PLN ≤ 3), and N2 (PLN ≥ 4), but evidence supporting its use as the optimal threshold for predicting all tumor prognosis is lacking. In addition, staging regional LNM according to PLN and neglecting the real examined lymph node (ELN) number intraoperatively and PLN metastasis is determined by surgery selection as well as cautious pathologist examination, which can induce great bias. Therefore, as far as oral squamous cell cancer, 5 colorectal cancer, 6 breast cancer, 7 and gastric cancer 8 are concerned, lymph nodes ratio (LNR) and log odds of positive lymph nodes (LODDS) have been suggested to be the superior classification systems for LNM compared with N stage. However, the applicability of this method to the prognosis evaluation of all cancers remains controversial. 9
Therefore, the present work focused on comparing whether N stage, PLN, ELN, LNR, and LODDS were of prognostic values, and selecting the highly efficient factor for the prediction of cancer-specific survival (CSS) among colon neuroendocrine tumor (CNET) cases undergoing surgical treatment. In addition, by combining additional independent risk factors, this work built 1 new nomogram prognosis staging model. At last, our nomogram prediction ability was tested based on the validation set and compared against the AJCC tumor node metastasis (TNM) classification system.
Materials and Methods
Data Extraction From the Surveillance, Epidemiology, and End Results (SEER) Database
The present retrospective work consecutively enrolled CNET cases undergoing curative surgical treatment during 2004 to 2015. Raw data utilized in the present work were obtained in SEER 18 registry research plus database (SEER*Stat 8.4.0.1), which covered about 28% of the US population. 10 Due to the retrospective nature, no institutional review board approval or patient consent was needed. SEER database has been developed as a public database that reports cancers, where private information can be freely obtained. Data from this database were obtained (ID:19277-Nov2021). All patient details have been de-identified for this research. The reporting of this study conforms to RECORD guideline. 11
This work identified CNET cases in line with “Site record ICD-O-3/WHO 2008” (Colon excluding Rectum) together with “ICD-O-3 Hist/behave” (8242/3, 8244/3, 8246/2, 8246/3, 8249/3, 8574/3). Patients conforming to the criteria below were included: (a) CNET cases identified in line with site records and histologic codes; (b) those diagnosed at the age of over 18 years; (c) those with sufficient records on lymph node biopsies; (d) those undergoing curative surgical treatment; and (e) those with over 1-month survival. Additionally, cases conforming to the criteria below were excluded: (a) those not diagnosed based on histopathological analysis; (b) those with insufficient clinicopathological information; (c) those dying of a cause apart from CNETs or with an unclear cause; and (d) those with incomplete or absent information about CSS or additional features.
At last, the present work included 943 CNET cases and randomized them as training (n = 663) or validation (n = 280) set at the 7:3 ratio (Table 1). The following information was collected, including age at diagnosis, sex, race, marital status, tumor size, primary site, grade, T/N/M stage, TNM stage, radiotherapy, chemotherapy, ELN, PLN, cause-specific death classification, survival status, and survival times. The primary site was categorized as right-sided colon NETs (RCNETs) or left-sided colon NETs (LCNETs). RCNETs included tumors in the cecum, ascending colon, transverse colon as well as hepatic flexure of the colon. LCNETs included tumors in the splenic flexure of the colon, sigmoid colon, and descending colon. The stage of each case was reidentified by the 8th AJCC classification system in line with the codes below: derived AJCC T/N/M/stage group, 6th edition (2004+); collaborative stage tumor size (2004+); and derived AJCC T/N/M/stage group, 6th edition (2010+). LNR and LODDS were calculated below, LNR = (PLN/ELN); LODDS = log[(PLN + 0.05)/(ELN-PLN + 0.05)]. This study set CSS as the end event, which indicated the duration between diagnosis and death due to CNETs.
Table 1.
Demographics and Clinicopathologic Characteristics of Patients in the Training and Validation Cohorts.
| Variables | Total | Training | Validation | P-value |
|---|---|---|---|---|
| n = 943 | n = 663 | n = 280 | ||
| Age | .830 | |||
| <60 | 316 (33.5) | 220 (33.2) | 96 (34.3) | |
| 60–79 | 451 (47.8) | 316 (47.7) | 135 (48.2) | |
| >79 | 176 (18.7) | 127 (19.2) | 49 (17.5) | |
| Sex | .158 | |||
| Female | 509 (54.0) | 348 (52.5) | 161 (57.5) | |
| Male | 434 (46.0) | 315 (47.5) | 119 (42.5) | |
| Race | .617 | |||
| White | 790 (83.8) | 557 (84.0) | 233 (83.2) | |
| Black | 106 (11.2) | 71 (10.7) | 35 (12.5) | |
| Others | 47 (5.0) | 35 (5.3) | 12 (4.3) | |
| Marital status | .610 | |||
| Married | 523 (55.5) | 369 (55.7) | 154 (55.0) | |
| Unmarried | 117 (12.4) | 86 (13.0) | 31 (11.1) | |
| Others | 303 (32.1) | 208 (31.4) | 95 (33.9) | |
| Laterality | .853 | |||
| RCNETs | 818 (86.7) | 576 (86.9) | 242 (86.4) | |
| LCNETs | 125 (13.3) | 87 (13.1) | 38 (13.6) | |
| Tumor size (cm) | .881 | |||
| <3.0 | 226 (24.0) | 157 (23.7) | 69 (24.6) | |
| 3.0–6.6 | 480 (50.9) | 341 (51.4) | 139 (49.6) | |
| >6.6 | 237 (25.1) | 165 (24.9) | 72 (25.7) | |
| Grade | .210 | |||
| I | 197 (20.9) | 149 (22.5) | 48 (17.1) | |
| II | 148 (15.7) | 97 (14.6) | 51 (18.2) | |
| III | 450 (47.7) | 312 (47.1) | 138 (49.3) | |
| IV | 148 (15.7) | 105 (15.8) | 43 (15.4) | |
| T stage | .403 | |||
| T1 | 38 (4.0) | 26 (3.9) | 12 (4.3) | |
| T2 | 77 (8.2) | 56 (8.4) | 21 (7.5) | |
| T3 | 546 (57.9) | 373 (56.3) | 173 (61.8) | |
| T4 | 282 (29.9) | 208 (31.4) | 74 (26.4) | |
| N stage | .302 | |||
| N0 | 186 (19.7) | 136 (20.5) | 50 (17.9) | |
| N1 | 479 (50.8) | 326 (49.2) | 153 (54.6) | |
| N2 | 278 (29.5) | 201 (30.3) | 77 (27.5) | |
| M stage | .870 | |||
| M0 | 589 (62.5) | 413 (62.3) | 176 (62.9) | |
| M1 | 354 (37.5) | 250 (37.7) | 104 (37.1) | |
| TNM stage | .975 | |||
| I | 32 (3.4) | 22 (3.3) | 10 (3.6) | |
| II | 120 (12.7) | 86 (13.0) | 34 (12.1) | |
| III | 437 (46.3) | 305 (46.0) | 132 (47.1) | |
| IV | 354 (37.5) | 250 (37.7) | 104 (37.1) | |
| Radiotherapy | .712 | |||
| No | 913 (96.8) | 641 (96.7) | 272 (97.1) | |
| Yes | 30 (3.2) | 22 (3.3) | 8 (2.9) | |
| Chemotherapy | .987 | |||
| No | 589 (62.5) | 414 (62.4) | 175 (62.5) | |
| Yes | 354 (37.5) | 249 (37.6) | 105 (37.5) | |
| ELN | .411 | |||
| ELN1 | 728 (77.2) | 507 (76.5) | 221 (78.9) | |
| ELN2 | 215 (22.8) | 156 (23.5) | 59 (21.1) | |
| PLN | .984 | |||
| PLN1 | 283 (30.0) | 200 (30.2) | 83 (29.6) | |
| PLN2 | 534 (56.6) | 375 (56.6) | 159 (56.8) | |
| PLN3 | 126 (13.4) | 88 (13.3) | 38 (13.6) | |
| LNR | .640 | |||
| LNR1 | 495 (52.5) | 351 (52.9) | 144 (51.4) | |
| LNR2 | 341 (36.2) | 234 (35.3) | 107 (38.2) | |
| LNR3 | 107 (11.3) | 78 (11.8) | 29 (10.4) | |
| LODDS | .534 | |||
| LODDS1 | 530 (56.2) | 376 (56.7) | 154 (55.0) | |
| LODDS2 | 301 (31.9) | 205 (30.9) | 96 (34.3) | |
| LODDS3 | 112 (11.9) | 82 (12.4) | 30 (10.7) | |
| CSS | .648 | |||
| Alive | 454 (48.1) | 316 (47.7) | 138 (49.3) | |
| Dead | 489 (51.9) | 347 (52.3) | 142 (50.7) | |
| Survival months | 44.65 ± 43.43 | 44.81 ± 43.62 | 44.28 ± 43.06 | .478 |
Abbreviations: CSS, cancer-specific survival; ELN, examined lymph node; LCNET, left-sided colon neuroendocrine tumor; LNR, lymph nodes ratio; LODDS, log odds of positive lymph nodes; PLN, positive lymph node; RCNET, right-sided colon neuroendocrine tumor.
Best Thresholds for Variables
X-tile software was utilized to calculate optimum thresholds for age, tumor size, PLN, ELN, LNR, and LODDS (version 3.6.1), according to the minimal p-value and maximal chi-squared value (Supplemental Figure S1). As a result, optimal thresholds of age were 59 and 79 years, and age was divided into 25–59, 60–79, and 80+ years groups. Optimal thresholds of tumor sizes were 2.9 and 6.7 cm, and tumor sizes were divided into 0.1–2.9, 3.0–6.6, and 6.7–44 cm groups. Due to the optimal threshold of ELN of 22, we classified ELN as ELN1 (≤22) and ELN2 (>23) groups. Because of optimal thresholds of PLN being 1 and 11, PLN was divided into PLN1 (≤1), PLN2 (2–11), and PLN3 (≥12) groups. Due to the optimal threshold of LNR being 0.29 and 0.81, LNR was classified as LNR1 (0–0.29), LNR2 (0.30–0.81), and LNR3 (0.81–1) groups. Given the optimal thresholds of LODDS being −0.28 and 0.54, LODDS was classified as LODDS1 (≤−0.28), LODDS2 (−0.28–0.54), and LODDS3 (0.54–2.01) groups.
Statistical Analysis
R for Windows (Version 4.2.1; https://www.R-project.org/) and SPSS24.0 (SPSS, Inc., Chicago, IL) were employed for statistical analyses. We divided patients into training and validation cohorts with a ratio of 7:3 using the R studio software “crate” package to ensure that the outcome events (death or alive) were randomly distributed between the 2 cohorts. Categorical variables were compared by Fisher's test or chi-squared test. Prognosis prediction performances of N stage, PLN, ELN, LNR, and LODDS were compared by log-rank tests and Kaplan–Meier (KM) curve analysis. Additionally, C-index, Akaike information criterion (AIC), together with the area under the receiver operating characteristic curve (AUC) were adopted for comparing the prognosis prediction abilities of the above five classification systems. Univariate, as well as multivariate Cox regression, was carried out for identifying factors independently predicting CSS risk. The present work also obtained hazard ratios (HRs) together with 95% confidence intervals (CIs). According to multivariate regression analysis, we built 1 nomogram for predicting CSS at 1, 3, and 5 years. Additionally, nomogram prediction ability was verified through C-index, AIC, calibration curve, and AUC, which was also compared against the AJCC TNM classification system. Risk scores were calculated on the nomogram, and X-tile software was utilized to stratify the risk into 3 groups (low, middle, or high risk). The difference in survival was compared by log-rank tests and KM curves among different groups. P < .05 (two-sided) stood for statistical significance. Besides, variables satisfying p < .2 upon univariate analysis were chosen to be potential variables for multivariate regression.
Ethics Statement
The authors state that this article does not contain any studies with human participants or animals so exempt from institutional review board approval. Informed consent from study participants was not required as SEER is an anonymized database that is open to the public.
Results
Clinicopathological Characteristics
From 2004 to 2015, this work enrolled 943 CNET cases and randomized them into the training (n = 663) or validation (n = 280) group at the 7:3 ratio (Figure 1). Table 1 displays clinicopathological features for CNET cases. Data between the 2 groups were comparable, as revealed by the chi-squared test. Median survivals were 44.81 ± 43.62 and 44.28 ± 43.06 months for training and validation sets, separately. Table 1 displays additional clinicopathological characteristics.
Figure 1.
Flowchart of patient selection.
Abbreviation: CNET, colon neuroendocrine tumor.
CSS-Related Prognosis Prediction Factors for CNET Cases
As revealed by univariate regression in the training set, age, laterality, tumor size, histological grade, T/N/M stage, TNM stage, radiotherapy, chemotherapy, PLN, LNR, and LODDS showed close relation with CSS in CNET cases (Table 2). Additionally, based on the log-rank test and KM analysis, N stage, PLN, LNR, and LODDS performed well in stratifying CNET patient prognosis (P < .001, Supplemental Figure S2).
Table 2.
Univariate Analysis of Prognostic Factors Associated with Cancer-Specific Survival for CNET Patients in the Training Cohorts.
| Variables | Univariate analysis | |
|---|---|---|
| silver HR (95% CI) | silver P-value | |
| Age | <.001 | |
| <60 | Reference | |
| 60–79 | 1.300 (1.014–1.666) | .038 |
| >79 | 1.825 (1.354–2.459) | <.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.091 (0.884–1.347) | .418 |
| Race | .529 | |
| White | Reference | |
| Black | 0.861 (0.547–1.354) | .516 |
| Others | 0.732 (0.419–1.281) | .274 |
| Marital | .509 | |
| Married | Reference | |
| Unmarried | 1.006 (0.723–1.402) | .970 |
| Others | 1.143 (0.906–1.441) | .259 |
| Laterality | ||
| RCNETs | Reference | |
| LCNETs | 1.387 (1.039–1.850) | .026 |
| Tumor size (cm) | <.001 | |
| <3.0 | Reference | |
| 3.0–6.6 | 2.899 (2.068–4.064) | <.001 |
| >6.6 | 4.539 (3.170–6.500) | <.001 |
| Grade | <.001 | |
| I | Reference | |
| II | 2.611 (1.585–4.301) | <.001 |
| III | 6.337 (4.204–9.553) | <.001 |
| IV | 10.071 (6.453–15.717) | <.001 |
| T stage | <.001 | |
| T1 | Reference | |
| T2 | 2.600 (0.753–8.980) | .131 |
| T3 | 5.766 (1 .842–18.045) | .003 |
| T4 | 10.004 (3.186–31.409) | <.001 |
| N stage | <.001 | |
| N0 | Reference | |
| N1 | 2.128 (1.511–2.996) | <.001 |
| N2 | 3.714 (2.619–5.266) | <.001 |
| M stage | ||
| M0 | Reference | |
| M1 | 3.931 (3.168–4.879) | <.001 |
| TNM stage | <.001 | |
| I | Reference | |
| II | 6.449 (0.871–47.752) | .068 |
| III | 11.731 (1.639–83.944) | .014 |
| IV | 38.546 (5.399–275.184) | <.001 |
| Radiotherapy | ||
| No | Reference | |
| Yes | 1.893 (1.191–3.010) | .007 |
| Chemotherapy | ||
| No | Reference | |
| Yes | 1.936 (1.567–2.393) | <.001 |
| ELN | ||
| ELN1 | Reference | |
| ELN2 | 1.201 (0.943–1.530) | .137 |
| PLN | <.001 | |
| PLN1 | Reference | |
| PLN2 | 1.882 (1.438–2.463) | <.001 |
| PLN3 | 5.377 (3.847–7.517) | <.001 |
| LNR | <.001 | |
| LNR1 | Reference | |
| LNR2 | 2.097 (1.658–2.651) | <.001 |
| LNR3 | 5.076 (3.755–6.861) | <.001 |
| LODDS | <.001 | |
| LODDS1 | Reference | |
| LODDS2 | 2.181 (1.722–2.764) | <.001 |
| LODDS3 | 5.136 (3.827–6.894) | <.001 |
Abbreviations: ELN, examined lymph node; HR, hazard ratio; LCNET, left-sided colon neuroendocrine tumor; LNR, lymph nodes ratio; LODDS, log odds of positive lymph nodes; PLN, positive lymph node; RCNET, right-sided colon neuroendocrine tumor.
Table 3 shows comparisons of prognosis prediction abilities among N stage, PLN, ELN, LNR, and LODDS based on the training set. C-index values for the above five classification systems were 0.6231, 0.6374, 0.5193, 0.6535, and 0.6541, separately, while their AIC values were 4154.555, 4129.739, 4217.884, 4114.321, and 4106.655, separately; their AUCs in predicting 1-year CSS were 0.660, 0.684, 0.525, 0.709, and 0.707, separately; their AUCs in predicting 3-year CSS were 0.658, 0.672, 0.537, 0.696, and 0.699, separately; while in predicting 5-year CSS were 0.663, 0.664, 0.524, 0.678, and 0.686, separately (Supplemental Figure S3). To sum up, LODDS achieved the greatest AUC and C-index, whereas the smallest AIC, although some AUCs were equivalent to or slightly higher than LNR, which indicated the superior CSS prediction ability of LODDS to N stage, PLN, ELN, and LNR among CNET cases. According to univariate regression plus identical confounders, this work included N stage, PLN, ELN, LNR, and LODDS in 5 respective Cox regression models. As revealed by multivariate regression, age, grade, T/N/M stage, PLN, LNR, and LODDS were the independent prognostic factors of CSS (Supplemental Table S1). However, laterality, tumor size, radiotherapy, chemotherapy, and ELN showed no relation to CNET prognosis.
Table 3.
Prognostic Efficiency of Different Lymph Node Staging Systems
| Systems | C-index | AIC | AUC | ||
|---|---|---|---|---|---|
| 1-year CSS | 3-year CSS | 5-year CSS | |||
| N stage | 0.6231 | 4154.555 | 0.660 | 0.658 | 0.663 |
| PLN | 0.6374 | 4129.739 | 0.684 | 0.672 | 0.664 |
| ELN | 0.5193 | 4217.884 | 0.525 | 0.537 | 0.524 |
| LNR | 0.6535 | 4114.321 | 0.709 | 0.696 | 0.678 |
| LODDS | 0.6541 | 4106.655 | 0.707 | 0.699 | 0.686 |
Abbreviations: AIC, Akaike information criterion; AUC, area under the curve; CSS, cancer-specific survival; ELN, examined lymph node; LNR, lymph nodes ratio; LODDS, log odds of positive lymph nodes; PLN, positive lymph node.
CSS Prognosis Prediction Nomogram Establishment and Verification
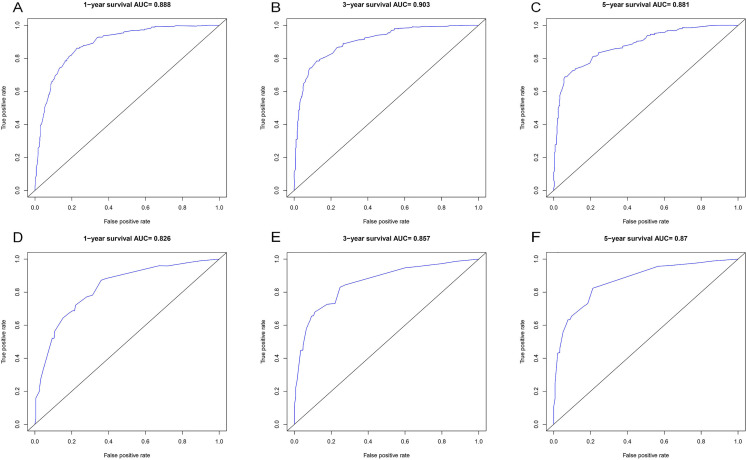
This work built 1 CSS prognosis prediction nomogram based on the above 5 risk factors: age, grade, T stage, M stage, and LODDS (Figure 2). Nomogram AUCs of CSS at 1, 3, and 5 years were 0.888, 0.903, and 0.881, separately, for the training set (Figure 3A to C); similarly, those for the validation set were 0.826, 0.857, and 0.870, separately (Figure 3D to F). Additionally, our nomogram-predicted CSS at 1, 3, and 5 years were well consistent with the real measurements, as revealed by calibration plots for the training set (Figure 4A to C) and validation set (Figure 4D to F).
Figure 2.
Nomogram for predicting the 1-, 3-, and 5-year cancer-specific survival in patients with colon neuroendocrine tumors (CNETs).
Figure 3.
AUC value of ROC predicting: (A) 1-year CSS of the nomogram in the training set; (B) 3-year CSS of the nomogram in the training set; (C) 5-year CSS of nomogram in the training set; (D) 1-year CSS of the nomogram in the validation set; (E) 3-year CSS of the nomogram in the validation set, and (F) 5-year CSS of nomogram in the validation set.
Abbreviations: AUC, area under the receiver operating characteristic curve; ROC, receiver operating characteristic; CSS, cancer-specific survival.
Figure 4.
The calibration curve for predicting cancer-specific survival (CSS): (A) at the 1st year in the training set; (B) at the 3rd year in the training set; (C) at the 5th year in the training set; (D) at the 1st year in the validation set; (E) at the 3rd year in the validation set; (F) at the 5th year in the validation set.
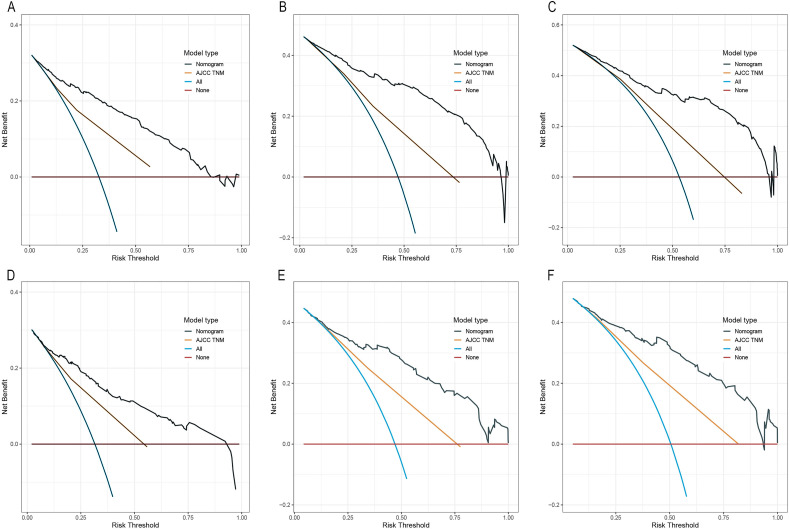
Performance was then compared in our constructed nomogram with the AJCC TNM classification system (Table 4). As a result, the C-index of our constructed nomogram in predicting CSS for the training set was 0.810 (95% CI, 0.790–0.830), remarkably elevated relative to the AJCC TNM stage (0.698 [95% CI, 0.675–0.818]). Additionally, our nomogram achieved an AIC value of 3835.481, which significantly decreased relative to the AJCC TNM system (4044.157). Similarly, our nomogram C-index was 0.794 for the validation set (95% CI, 0.757–0.831), dramatically elevated relative to the AJCC TNM stage (0.703 [95% CI, 0.661–0.735]), besides, our nomogram AIC was 1355.123, significantly decreased relative to AJCC TNM 1409.552. To sum up, our nomogram performed well in accurately predicting prognosis compared with the AJCC TNM system. Additionally, this work carried out decision curve analysis (DCA) for determining clinical applicability, which revealed the superior performance of our nomogram to the TNM stage system, since it achieved increased net benefits compared with the TNM classification system for almost every threshold probability for the training set and validation set (Figure 5).
Table 4.
Prognostic Efficiency of Nomogram Prognostic Model and the AJCC TNM
| Systems | C-index | AIC | ||
|---|---|---|---|---|
| Training cohort (n = 663) | Validation cohort (n = 280) | Training cohort (n = 663) | Validation cohort (n = 280) | |
| Nomogram | 0.810 (0.790–0.830) | 0.794 (0.757–0.831) | 3835.481 | 1355.123 |
| AJCC TNM | 0.698 (0.675–0.818) | 0.703 (0.661–0.735) | 4044.157 | 1409.552 |
Abbreviations: AIC, Akaike information criteria; AJCC, American Joint Committee on Cancer; TNM, tumor node metastasis.
Figure 5.
DCA of nomogram and TNM staging system for predicting 1-, 3-, and 5-year CSS in the training set (A–C) and validation set (D–F).
Abbreviations: DCA, decision curve analysis; TNM, tumor node metastasis; CSS, cancer-specific survival.
Nomogram-Based Risk Stratification
According to our constructed nomogram, this work utilized X-tile for determining the best thresholds and establishing the risk classification system. The cases were divided into low-risk (risk score: 1–34.57), middle-risk (risk score: 34.71–83.65), and high-risk (risk score: 85.68–507.06). According to KM analysis, the risk classification system performed well in stratifying and differentiating training from validation sets compared with the AJCC TNM classification system (Figure 6).
Figure 6.
Kaplan–Meier CSS curves categorized by different staging systems: (A) AJCC TNM staging system in the training set; (B) AJCC TNM staging system in the validation set; (C) nomogram staging system in the training set; (D) nomogram staging system in the validation set.
Abbreviations: CSS, cancer-specific survival; AJCC, American Joint Committee on Cancer; TNM, tumor necrosis metastasis.
Discussion
Regional LNM in cancers is widely used to be the key factor for predicting tumor prognosis. However, there is still some controversy about which staging criteria for LNM have the highest predictive effects on the 8th AJCC N stage classification, PLN, ELN, LNR, and LODDS. In these cases, some researchers have evaluated them and found that the predictive power of different staging criteria of regional lymph metastasis may be different in various tumors. In this study, the predictive efficiency of different staging criteria was compared, where LODDS showed independent relation with long-time CSS and had better prognostic power than other classification criteria among patients with CNETs. In addition, the nomogram was constructed by incorporating age, grade, T stage, M stage, and LODDS, which showed better prediction ability than the eighth AJCC classification system.
With regard to different classification criteria for LNM, the univariate and multivariate analysis results in this study revealed that N stage, LNR, PLN, and LODDS showed significant relation with CSS among CNET cases, but not ELN. Although some studies have shown that ELN has an ideal effect on predicting tumor prognosis, it is still unable to avoid the drawback caused by the impact of surgical procedures.12,13 Currently, the eighth AJCC N stage classification system is extensively adopted to predict the prognosis of cancer patients.4,14,15 Nonetheless, its effectiveness remains controversial, although the N stage has been further subdivided into N0 (PLN = 0), N1 (PLN = 1–3), and N2 (PLN ≥ 4) from the seventh AJCC N stage (N0 and N1). According to PLN number, X-tile software was adopted for calculating the optimal threshold of PLN and subdividing it into PLN1 (PLN < 2), PLN2 (PLN = 2-11), and PLN3 (PLN ≥ 12). Interestingly, our division did not conform to the eighth AJCC N stage system used in CNETs; besides, our further analysis results, including C-index, AIC, and AUC, demonstrated that PLN stage classification performed superior to the N stage for predicting CSS among CNET cases. 16 The PLN and N stage classification systems depend on PLN numbers alone, and factors affecting ELN numbers remain unclear. In the case of identical PLN numbers, patients showing inadequate ELN numbers may exhibit dismal prognostic outcomes, while such deficiency is referred to as staging migration. 17 Therefore, PLN and ELN numbers are considered in LNR and LODDS. Martin et al 18 divided LNR into LNR ≤ 0.2, 0.2 < LNR ≤ 0.5, and LNR > 0.5, in CSS analysis, as a result, the difference was of statistical significance in gastroenteropancreatic NETs) cases across the 3 groups. However, LNR loses its inherent ability when PLNs cannot retrieve or if positive intraoperative lymph nodes are retrieved, whereas negative lymph nodes are not retrieved, and the LNR is 0 or 1. Therefore, certain scholars construct the LODDS for evaluating nodal status. LODDS takes comprehensive consideration of PLN as well as negative lymph node number into consideration, suggesting that it may be adopted if LNR can hardly be stratified, thereby reducing the staging migration risk. In our study, through X-tile software stratification and KM analysis, we found that the LODDS stage showed a close relation with CNET prognosis; in addition, it represents a critical factor for stratifying risk of CNETs, with an increased LODDS stage indicating the decreased CSS. In line with the evaluation standards involving C-index, AUC, and AIC, the LODDS stage was identified to exhibit superior prognosis prediction ability to the N stage, PLN, and LNR stage. Such results conformed to numerous prior works, however, the LODDS classification value remains unclear, possibly because of the diverse aggressiveness and the diverse LNM patterns within distinct tumors.19–22
Moreover, based on multivariate regression, apart from the LODDS stage, age, historic grade, T stage, and M stage independently predicted the risk of CNETs as well, and their prognosis prediction performances have been confirmed in diverse cancers such as CNETs. 23 According to those 5 risk factors, this work established 1 novel prognosis prediction nomogram for evaluating prognosis in CNET cases. Xu et al 23 demonstrated that the survival prediction model constructed based on the N stage had some predictive effect on CNETs, but our results had shown that the N stage performed significantly weaker than the LODDS stage in predicting the survival of CNETs. According to our results, our constructed nomogram performed well in predicting CSS of CNET cases relative to the conventional eighth AJCC TNM classification system according to C-index, AIC, and DCA results. The LODDS-based nomogram model for survival prediction of NETs was also applicable to lung and biliary tract sites, and its predictive power was also shown to be superior to the 8th AJCC TNM or SEER classification system by C-index or DCA, net reclassification index, and integrated discrimination improvement (IDI).24,25 In addition, our KM analysis results also proved that it assists clinicians to make individual treatment decisions for improving CNET prognosis.
To our knowledge, the present work first suggested that LODDS reliably classified disease stage compared with N stage, PLN, ELN, and LNR for patients with CNETs. At the same time, the accuracy and intuitiveness of our nomogram model based on LODDS were superior to the conventional AJCC TNM classification system. Nonetheless, some limitations should be noted in the present work. Firstly, due to the retrospective nature of this work, our conclusions should be further confirmed in prospective and multicenter studies with larger sample sizes, regardless of their large sample size. Secondly, the threshold of LODDS utilized in the present work might not be suitable for different articles, therefore, it is necessary to conduct a meta-analysis that contains different LODDS validation studies for the sake of determining the optimal threshold. Thirdly, the SEER database does not include certain possible CNETs prognostic factors such as resection status, CEA levels, as well as other clinical information affecting patient survival.
Conclusions
The present work indicated that LODDS independently predicted the risk of CSS among CNET cases, and its prognostic predictive ability might be superior to the 8th AJCC N stage, PLN, ELN, and LNR. Additionally, this work builds 1 prognosis prediction nomogram and confirms its performance based on age, grade, T stage, M stage, and LODDS. Our nomogram might exhibit superior survival prediction capacity to the 8th AJCC TNM classification system, which is the potential approach contributing to individualized decision-making in the clinic.
Supplemental Material
Supplemental material, sj-jpg-1-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment
Supplemental material, sj-jpg-2-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment
Supplemental material, sj-jpg-3-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment
Supplemental material, sj-jpg-4-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-5-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment
Acknowledgments
Our thanks should go to all the staff for their assistance and suggestions on the present work.
Abbreviations
- AIC
Akaike information criterion
- AJCC
American Joint Committee on Cancer
- AUC
area under the receiver operating characteristic curve
- CI
confidence interval
- CNET
colon neuroendocrine tumor
- CSS
cancer-specific survival
- DCA
decision curve analysis
- ELN
examined lymph node
- GI
gastrointestinal
- HR
hazard ratio
- IDI
integrated discrimination improvement
- KM
Kaplan–Meier
- LCNET
left-sided CNET
- LNM
lymph node metastasis
- LNR
lymph nodes ratio
- LODDS
log odds of positive lymph nodes
- PLN
positive lymph node
- RCNET
right-sided CNET
- ROC
receiver operating characteristic curve
- SEER
surveillance, epidemiology, and end results
- TNM
tumor node metastasis.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by funding from the Major Projects of Natural Science Research Projects in Anhui Universities (No.: KJ2020ZD202020) and the Anhui Province Translational Medicine Research Fund Project (No.: 2021zhyx-C54).
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Xue Zhang MD, PhDhttps://orcid.org/0000-0001-8336-5256
References
- 1.Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12(8):791-807. doi: 10.4251/wjgo.v12.i8.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplin M, Sundin A, Nillson O, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95(2):88-97. doi: 10.1159/000335594 [DOI] [PubMed] [Google Scholar]
- 3.Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Shang L, Zhang PP, et al. Clinicopathological features and prognostic validity of the European Neuroendocrine Tumor Society (ENETS) and American Joint Committee on Cancer (AJCC) 8th staging systems in colonic neuroendocrine neoplasms. Cancer Med. 2019;8(11):5000-5011. doi: 10.1002/cam4.2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltramini GA, Belloni LM, Fusco N, et al. Comparing prognostic utility between the 8th edition of TNM staging system and the lymph node ratio for oral squamous cell carcinoma. Head Neck. 2021;43(10):2876-2882. doi: 10.1002/hed.26769 [DOI] [PubMed] [Google Scholar]
- 6.Occhionorelli S, Andreotti D, Vallese P, et al. Evaluation on prognostic efficacy of lymph nodes ratio (LNR) and log odds of positive lymph nodes (LODDS) in complicated colon cancer: The first study in emergency surgery. World J Surg Oncol. 2018;16(1):186. doi: 10.1186/s12957-018-1483-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Li Y, Wu J, et al. Nomograms for prediction of overall and cancer-specific survival in young breast cancer. Breast Cancer Res Treat. 2020;184(2):597-613. doi: 10.1007/s10549-020-05870-5 [DOI] [PubMed] [Google Scholar]
- 8.Agnes A, Biondi A, Cananzi FM, et al. Ratio-based staging systems are better than the 7th and 8th editions of the TNM in stratifying the prognosis of gastric cancer patients: a multicenter retrospective study. J Surg Oncol. 2019;119(7):948-957. doi: 10.1002/jso.25411 [DOI] [PubMed] [Google Scholar]
- 9.Gao B, Zhou D, Qian X, et al. Number of positive lymph nodes is superior to LNR and LODDS for predicting the prognosis of pancreatic neuroendocrine neoplasms. Front Endocrinol (Lausanne). 2021;12:613755. doi: 10.3389/fendo.2021.613755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronin KA, Ries LA, Edwards BK. The surveillance, epidemiology, and end results (SEER) program of the National Cancer Institute. Cancer. 2014;120(Suppl 23):3755-3757. doi: 10.1002/cncr.29049 [DOI] [PubMed] [Google Scholar]
- 11.Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Shen J, Xing Z, et al. Significance of examined lymph-node count in accurate staging and long-term survival in patients undergoing radical prostatectomy: a population-based study. Int Urol Nephrol. 2020;52(2):271-278. doi: 10.1007/s11255-019-02300-4 [DOI] [PubMed] [Google Scholar]
- 13.Bagante F, Tran T, Spolverato G, et al. Perihilar cholangiocarcinoma: number of nodes examined and optimal lymph node prognostic scheme. J Am Coll Surg. 2016;222(5):750-759 e2. doi: 10.1016/j.jamcollsurg.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong P, Chen C, Wang Z, et al. Prognostic significance for colorectal carcinoid tumors based on the 8th edition TNM staging system. Cancer Med. 2020;9(21):7979-7987. doi: 10.1002/cam4.3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Lian CL, Zhou P, et al. The prognostic and predictive value of the 8th American Joint Committee on Cancer (AJCC) staging system among early breast cancer patients aged <50 years. Gland Surg. 2021;10(1):233-241. doi: 10.21037/gs-20-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1(2):120-125. [PubMed] [Google Scholar]
- 17.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604-1608. doi: 10.1056/NEJM198506203122504 [DOI] [PubMed] [Google Scholar]
- 18.Martin JA, Warner RRP, Aronson A, Wisnivesky JP, Kim MK. Lymph node metastasis in the prognosis of gastroenteropancreatic neuroendocrine tumors. Pancreas. 2017;46(9):1214-1218. doi: 10.1097/MPA.0000000000000921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J, Jiang S, Gao L, et al. Construction and validation of a nomogram based on the log odds of positive lymph nodes to predict the prognosis of medullary thyroid carcinoma after surgery. Ann Surg Oncol. 2021;28(8):4360-4370. doi: 10.1245/s10434-020-09567-3 [DOI] [PubMed] [Google Scholar]
- 20.Cai H, Xu T, Zhuang Z, et al. Value of the log odds of positive lymph nodes for prognostic assessment of colon mucinous adenocarcinoma: analysis and external validation. Cancer Med. 2021;10(23):8542-8557. doi: 10.1002/cam4.4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Xiao W, Ren P, et al. The prognostic performance of the log odds of positive lymph nodes in patients with esophageal squamous cell carcinoma: A population study of the US SEER database and a Chinese single-institution cohort. Cancer Med. 2021;10(17):6149-6164. doi: 10.1002/cam4.4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao E, Zhou C, Chen S. Prognostic nomogram based on log odds of positive lymph nodes for gastric carcinoma patients after surgical resection. Future Oncol. 2019;15(36):4207-4222. doi: 10.2217/fon-2019-0473 [DOI] [PubMed] [Google Scholar]
- 23.Xu R, Zhou B, Hu P, et al. Development and validation of prognostic nomograms for patients with colon neuroendocrine neoplasms. World J Surg Oncol. 2021;19(1):233. doi: 10.1186/s12957-021-02338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Wei J, Guo Y, et al. Construction and validation of nomograms based on the log odds of positive lymph nodes to predict the prognosis of lung neuroendocrine tumors. Front Immunol. 2022;13:987881. doi: 10.3389/fimmu.2022.987881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang S, Yang F, Zhang L, et al. A prognostic nomogram based on log odds of positive lymph nodes to predict the overall survival in biliary neuroendocrine neoplasms (NENs) patients after surgery. J Endocrinol Invest. 2022;45(12):2341-2351. doi: 10.1007/s40618-022-01874-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment
Supplemental material, sj-jpg-2-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment
Supplemental material, sj-jpg-3-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment
Supplemental material, sj-jpg-4-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-5-tct-10.1177_15330338231180776 for A Nomogram Based on the Log Odds of Positive Lymph Nodes Predicts the Prognosis of Patients with Colon Neuroendocrine Tumors After Surgery: A Surveillance, Epidemiology, and End Results Population-Based Study by Xue Zhang, Kui Zhang, Su Li and Aman Xu in Technology in Cancer Research & Treatment