Abstract
Among all available antimicrobials, antibiotics hold a prime position in the treatment of infectious diseases. However, the emergence of antimicrobial resistance (AMR) has posed a serious threat to the effectiveness of antibiotics, resulting in increased morbidity, mortality, and escalation in healthcare costs causing a global health crisis. The overuse and misuse of antibiotics in global healthcare setups have accelerated the development and spread of AMR, leading to the emergence of multidrug-resistant (MDR) pathogens, which further limits treatment options. This creates a critical need to explore alternative approaches to combat bacterial infections. Phytochemicals have gained attention as a potential source of alternative medicine to address the challenge of AMR. Phytochemicals are structurally and functionally diverse and have multitarget antimicrobial effects, disrupting essential cellular activities. Given the promising results of plant-based antimicrobials, coupled with the slow discovery of novel antibiotics, it has become highly imperative to explore the vast repository of phytocompounds to overcome the looming catastrophe of AMR. This review summarizes the emergence of AMR towards existing antibiotics and potent phytochemicals having antimicrobial activities, along with a comprehensive overview of 123 Himalayan medicinal plants reported to possess antimicrobial phytocompounds, thus compiling the existing information that will help researchers in the exploration of phytochemicals to combat AMR.
Keywords: antimicrobial resistance, antimicrobials, multidrug resistance, phytochemicals, phytocompounds, plant secondary metabolites
1. Introduction
Among the antimicrobials available for medication to clinicians globally, antibiotics hold the prime position. The history of antibiotics dates back to 1904, when the first antibiotic, Arsphenamine, was discovered and commercialized as “Salvarsan” in 1910, which was used to treat syphilis [1,2]. This was followed by the discovery of sulphanilamide precursor prontosil red in 1927, which was active against Streptococci and Staphylococci. However, the accidental discovery of penicillin in 1928 from Penicillium notatum sparked a quest for antibiotics derived from microbes, leading to the discoveries of streptomycin in 1944 from Streptomyces griseus, tetracycline from Streptomyces rimosus, and chloramphenicol from Streptomyces venezuelae by the end of 1953 [3,4]. Although the search for and discovery of antibiotics started earlier in the 1900s, however, the period from 1940 to 1962 is considered the “golden era” of antibiotic discovery because most antibiotics were discovered in that period, and those antibiotics have significantly contributed to the general health of humans via infectious disease modulation [3,4,5].
The usage of antibiotics proved extremely beneficial for treating infectious diseases, which were otherwise very difficult to cure, but it also exposed microbes to toxic conditions imposed by antibiotics, thus generating a selection pressure, which microbes must overcome to survive [6]. In response to this newly introduced selection pressure, microbes evolved mechanisms to circumvent the lethal effects of antibiotics. This selection pressure grew further due to extensive and improper use of antibiotics, which resulted in the emergence of microbes resistant to these antimicrobials [6,7]. The emergence of antimicrobial resistance (AMR) further compelled the usage of higher dosages or multiple combinations of antibiotics for treatment, all of which results in enhanced usage of antibiotics, thus further accelerating the pace of AMR emergence in bacteria [8,9]. Bacteria evolve comparatively faster than other organisms, primarily due to the small genome size, fast reproduction cycle, and a high rate of development of mutants, resulting in the evolution of resistant phenotypes [10,11].
Almost all antibiotics currently available for medication were discovered in the last century, while those introduced after the year 2000 are only the members of previously discovered antibiotic classes. However, resistance has emerged against almost all of these antibiotics [3]. The emergence of AMR against existing antibiotics is one of the major contributors to worldwide mortalities [4]. According to a review commissioned by the UK government, AMR could kill 10 million people per year by 2050 [12]. This has necessitated the introduction of novel antimicrobials, which has significantly slowed down in the last few decades and thus has created a void in the options available for the treatment of infectious diseases, along with the exploration of alternative sources of medicine.
Plant-based medicines are natural lucrative alternatives, as during the pre-antibiotic era, herbal medicines were extensively used for the treatment of various diseases. Plants, being easily available, were always a choice for the treatment of infectious diseases. Plants produce secondary metabolites for defensive purposes, many of which have antimicrobial properties, and are still commonly used in traditional medicine [10]. Further, plants produce a plethora of structurally and functionally diverse phytochemicals, many of which are effective against pathogenic microbes and hence could be explored for developing novel antimicrobials [13,14,15]. Systematic screening of phytochemical reservoirs of medicinal plants, known to possess antimicrobial properties, could lead to the identification of novel antimicrobial phytochemicals with unique mechanisms of action. Such novel phytochemicals could act by killing bacteria on their own, by inhibiting their molecular targets within the cell, necessary for cellular growth and division, or by acting synergistically with existing antibiotics by inhibiting the resistance determinants in antimicrobial-resistant bacteria. In both cases, the goal of reviving antimicrobial therapies and sensitizing the AMR bacteria could be achieved.
In India, around 17,000 species of higher plants have been discovered, of which 7500 plant species have been found to have medicinal properties [16,17]. The Indian Himalayas foster around 10,000 species of higher plants, of which 1748 species reportedly have medicinal properties [18,19]. Though vast literature is available citing the antimicrobial activity of Himalayan medicinal plants, reports describing their associated bioactive compounds and their molecular mechanisms of action are limited. In an attempt to bridge this gap, the present study focuses on summarizing the emergence of and mechanisms employed by microbes to gain antimicrobial resistance against existing antibiotics, followed by the description of potent phytochemicals, such as alkaloids, phenols, organosulfur compounds, and terpenes, that possess antimicrobial activities, along with their reported mechanisms of action. Finally, this study provides a comprehensive overview of 123 Himalayan medicinal plants having antimicrobial properties along with their bioactive compounds and target pathogens. This review provides an overall picture of the present state of antibiotic discovery and the emergence of antimicrobial resistance, along with the potential of phytochemicals possessing antimicrobial activity, which could be harnessed to screen and develop novel antibacterials that complement present antibiotic therapy by acting as a source of alternate medicine.
Emergence of Antimicrobial Resistance
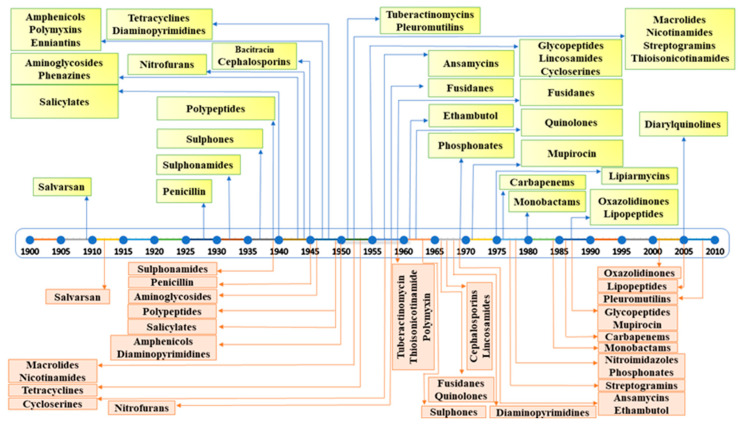
Numerous microbe-derived antimicrobials have been bought to the market since the introduction of antibiotics in 1930. However, extensive and inappropriate usage of antibiotics has imposed additional selection pressure, under which microbes have evolved to engage various strategies to resist the effects of antibiotics. Although evolution is a natural process, the wide usage of antibiotic regimes, further compounded by overuse, has accelerated the emergence of AMR in bacteria, which has proven to be a serious problem for the treatment of infectious diseases all around the world [6]. The first penicillin-hydrolyzing enzyme (β-lactamases) was discovered by Chain and Abraham in 1940, soon after the discovery of the first β-lactam antibiotic, penicillin, in 1928 [20]. Similarly, streptomycin, an aminoglycoside antibiotic, was discovered in 1943, and by 1946, resistance against it was reported in bacteria such as Mycobacterium tuberculosis [21]. The first tetracycline antibiotic, aureomycin, was discovered in 1945, and resistance against it was observed in Escherichia coli by 1948 [22]. Chloramphenicol was introduced in 1947, but resistance against it emerged in the microbial world by 1950 [23]. To cope with resistance against penicillin, methicillin, a semi-synthetic penicillin derivative, was introduced in 1959, but soon after, methicillin-resistant Staphylococcus aureus (MRSA) emerged in 1961 [24]. A summary of antibiotic discovery followed by the emergence of antimicrobial resistance is depicted in Figure 1.
Figure 1.
Timeline of introduction of antibiotics (shown in yellow) and the emergence of antimicrobial resistance (shown in light brown).
It is evident from the examples cited above that the introduction of novel antibiotics was always followed by the emergence of resistance against them. This strongly suggests that the usage of antibiotics itself is responsible for the emergence of antimicrobial resistance among bacteria [25]. The widespread use of antibiotic regimens worldwide has significantly added to the selection pressure during natural bacterial evolution and has accelerated the emergence of resistant strains. Bacteria thrive in harsh environments, reproduce quickly, and have small genomes, which allows them to evolve comparatively faster than other organisms due to faster accumulation of mutations and, thus, acquisition of new phenotypes [10]. In bacteria, the acquisition of an antimicrobial-resistant phenotype involves both intrinsic and extrinsic factors, which include the development and accumulation of de novo mutations in existing genes, resulting in altered phenotypes, as well as the acquisition of resistance-conferring genes through vertical or horizontal gene transfer from other resistant strains [10,11,26]. The most common mechanisms through which bacteria demonstrate resistance involve increased activity of efflux pumps, enzymatic modification or degradation of antibiotics, modifications of target sites, and alteration of membrane permeability through porin modifications [10,25]. These mechanisms are briefly discussed in the following sections.
2. Mechanisms of Antimicrobial Resistance
2.1. Bacterial Efflux Pumps
Although bacteria can employ different adaptive mechanisms to develop resistance against antimicrobials, efflux pumps hold key importance [27]. Recent data and laboratory studies have demonstrated that efflux pumps not only contribute to AMR but also play a substantial role in microbial adaptive potential and virulence [28]. Bacterial efflux pumps commonly belong to one of two major superfamilies: the widely prevalent secondary transporters that use proton motive force (PMF) as a source of energy and ATP-binding cassette (ABC) multidrug transporters [27,29]. The first superfamily comprises four subfamilies. These include multidrug and toxic compound extrusion (MATE), the major facilitator superfamily (MFS), resistance–nodulation–cell division (RND), and the small multidrug resistance (SMR) family [10,27,30]. Although efflux pumps from all the superfamilies and subfamilies enable a bacterial strain to exhibit increased AMR, RND pumps in particular show activity against a variety of compounds with diverse chemical structures, including bile salts, detergents, organic solvents, antimicrobial peptides, biocides, detergents, and organic solvents [31]. Gram-negative bacteria exhibit complex efflux pumps, which form a tripartite protein channel comprising a transporter protein found in the cytoplasmic membrane, an efflux protein in the outer membrane, and a membrane fusion protein that travels through the periplasm. AcrA-AcrB-TolC of Escherichia coli and MexA-MexB-OprM of Pseudomonas aeruginosa are two of the most well-studied tripartite systems [28]. Gram-positive bacteria have relatively simple efflux pumps comprising a single transporter, embedded in cytoplasmic membranes, and belong to the ABC, MFS, or SMR families [10,32]. Table 1 summarizes various efflux pumps belonging to ESKAPE pathogens, which include Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and members of Enterobacteriaceae. As a whole, these efflux pumps enable bacteria to lower the concentration of various antimicrobials inside the cell, to the point that is not lethal to the bacteria.
Table 1.
List of efflux pump families and representative candidates from selected candidates of ESKAPE pathogens.
| Pathogenic Bacteria | Efflux Pump Family |
Representative of Efflux Pump | Antibiotic Effluxed | References |
|---|---|---|---|---|
| Enterococcus faecium | ABC | EfrAB | Acriflavine, ciprofloxacin, daunomycin, doxorubicin, doxycycline, norfloxacin, tetraphenylphosphonium | [33] |
| Staphylococcus aureus | ABC | Isa(E) | Linosamide, pleuromutilin, streptogramin A | [34] |
| Msr(A) | Macrolides, telithromycin | [34] | ||
| MATE | MepA | Biocides, ethidium bromide, fluoroquinolones | [35] | |
| MFS | NorA | Fluoroquinolones | [36,37] | |
| QacA | Acriflavine, chlorhexidine, ethidium bromide, quaternary ammonium compounds | [37] | ||
| Klebsiella pneumoniae | MATE | KetM | 4, 6 Diamidino- 2- phenyl indole | [38] |
| MFS | KpnGH | Ceftazidime, cefepime, streptomycin, tetracycline | [39] | |
| RND | OqxAB | Chloramphenicol, fluoroquinolones | [40] | |
| SMR | KnpEF | Benzalkonium chloride, cefepime, chlorhexidine, erythromycin, streptomycin, tetracycline, triclosan | [39] | |
| Acinetobacter baumannii | ABC | MacAB- TolC | Macrolides | [41] |
| MATE | AbeM | Acriflavine, aminoglycosides, daunomycin, doxorubicin, fluoroquinolone | [42] | |
| MFS | CraA | Chloramphenicol | [43] | |
| RND | AdeABC, AdeFGH, AdeIJK | Aminoglycosides, beta-lactams, fluoroquinolones, macrolides, tetracycline, biocides | [44,45] | |
| Pseudomonas aeruginosa | RND | MexAB- OprM, | Aminoglycosides, beta-lactams, chloramphenicol, fluoroquinolones, macrolides, sulfonamides, tetracyclines, tigecycline | [46] |
| MexXY- OprM/A, MexCD- OprJ, MexEF- OprN | Trimethoprim, biocides, ethidium bromide | [47] | ||
| Escherichia coli | ABC | MacAB- TolC | Macrolides | [48] |
| MFS | MdfA | Chloramphenicol, doxorubicin, norfloxacin, tetracycline | [49] | |
| QepA/QepA2 | Fluoroquinolones | [50] | ||
| RND | AcrAB- TolC | β-lactams, chloramphenicol, fluoroquinolones, macrolides, novobiocin, tetracycline, tigecycline | [51,52] | |
| OqxAB | Chloramphenicol, fluoroquinolones | [40] | ||
| SMR | EmrE | Acriflavine, ethidium bromide, quaternary ammonium compounds | [53,54] |
2.2. Enzymatic Modification and Degradation of Antibiotics
Ever since the introduction of antimicrobials for the treatment of infectious diseases, resistance against these compounds had also emerged, which limits the options available for the treatment of disease. Resistance can be active, which results from selection pressure against a specific antibiotic/class of antibiotic, or passive, which results from a generalized adaptive process [55]. Production of enzymes, which changes the chemical structure of an antibiotic and renders it ineffective, is one of the most crucial strategies for developing resistance to antibiotics. Both Gram-positive as well as Gram-negative bacteria produce enzymes that bring about chemical alteration or modification of antibiotics. The most frequently encountered alteration mechanism involves group transfers, such as adenylation, acetylation, and phosphorylation, brought about by transferases. Moreover, bacteria also produce enzymes that specifically break down antibiotics. For instance, many pathogens secrete β-lactamases, which degrade antibiotics with β-lactam rings by specifically hydrolysing their amide bonds, rendering the antibiotic ineffective [56,57]. The following section discusses these enzyme classes in detail, which are listed in Table 2.
Table 2.
List of enzymes and their classes involved in the modification and inactivation of antibiotics.
| Enzyme Class | Type | Substrate Antibiotic Class | Representative |
|---|---|---|---|
| Hydrolases | β-lactamases | β-lactam | Penicillin |
| Cephalosporin | |||
| Carbapenem | |||
| Macrolide esterases | Macrolide | Erythromycin | |
| Roxithromycin | |||
| Azithromycin | |||
| Epoxidases | Epoxide | Fosfomycin | |
| Transferases | Acetyltransferases | Aminoglycoside | Gentamicin |
| Kanamycin | |||
| Amikacin | |||
| Chloramphenicol | Chloramphenicol | ||
| Streptogramin | Group A streptogramins | ||
| Phosphotransferases | Aminoglycoside | Gentamicin | |
| Kanamycin | |||
| Amikacin | |||
| Macrolide | Erythromycin | ||
| Roxithromycin | |||
| Azithromycin | |||
| Rifamycin | Rifampin | ||
| Rifabutin | |||
| Rifapentine | |||
| Peptide | Colistin | ||
| Polymixin B | |||
| Thiol S-transferases | Epoxide | Fosfomycin | |
| Nucleotidyltransferases | Aminoglycoside | Gentamicin | |
| Kanamycin | |||
| Amikacin | |||
| Lincosamide | Lincomycin | ||
| Clindamycin | |||
| Pirlimycin | |||
| ADP-ribosyltransferases | Rifamycin | Rifampin | |
| Rifabutin | |||
| Rifapentine | |||
| Glycosyltransferases | Macrolide | Erythromycin | |
| Roxithromycin | |||
| Azithromycin | |||
| Rifamycin | Rifampin | ||
| Rifabutin | |||
| Rifapentine | |||
| Redox enzymes | Monooxygenases | Tetracycline | Tetracycline |
| Oxytetracycline | |||
| Doxycycline | |||
| Rifamycin | Rifampin | ||
| Rifabutin | |||
| Rifapentine | |||
| Streptogramin | Group A streptogramins | ||
| Lyases | Lyases (Virginiamycin B lyase) |
Streptogramin | Group B streptogramins |
2.2.1. Hydrolysis
Several antibiotics possess chemical bonds that are vital for their activity and surprisingly hydrolytically susceptible, e.g., esters and amides, which are susceptible to enzymes that cleave these chemical bonds, rendering the antibiotics inactive. Among these enzymes, amidases, specifically those referred to as β-lactamases, hydrolyse the amide bond of the β-lactam ring present in penicillin, cephalosporin, and carbapenem classes of antibiotics. Other enzymes include esterases and epoxidases, which cleave the macrolide lactone rings and fosfomycin oxirane rings, respectively [55].
β-Lactamases
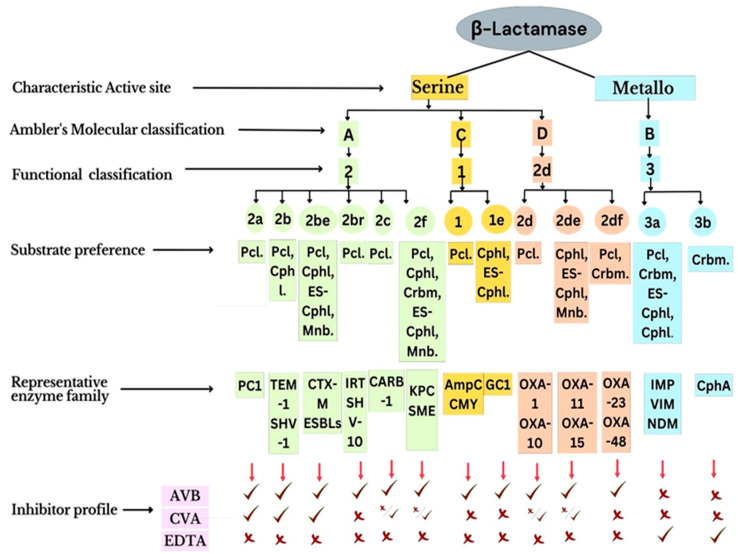
β-Lactamases constitute an enzyme superfamily, with around 2000 members, and hydrolyse the amide linkage of the β-lactam ring, which is a common structural and functional moiety of all β-lactam antibiotics [58]. β-Lactamases are categorized into four molecular classes (A, B, C, and D), based on amino acid sequence homology [59]. Class B constitutes metalloenzymes, while classes A, C, and D enzymes are serine hydrolases. β-lactamases of class A (such as CTX-M, KPC, SHV, and TEM) are the most common ones found in and around human settlements [60,61]. Among class A β-lactamases, TEM and SHV are more prone to mutational variability [61]. They can hydrolyse members of second-to-fourth-generation cephalosporins due to crucial mutations in the active site [62]. These mutational variants are referred to as extended-spectrum β-lactamases (ESBLs) [63]. β-Lactamases belonging to class C effectively hydrolyse cephalosporins. Primarily, the members of this class were encoded by chromosomally located genes, with inducible expression. However, later, these genes were also found to be located on mobile genetic elements [64]. β-lactamases of class D include OXA-type carbapenemases, with the highest structural diversity among all serine hydrolases. Finally, class B comprises all metallo-β-lactamases (MBLs), having one or two zinc ions in their active site, which are required for catalysis [63,65]. Except for monobactams, MBLs hydrolyse practically all β-lactam antibiotics and are inhibited by EDTA, dipicolinic acid, and phenanthroline, which are metal-ion-chelating agents.
A dedicated database of β-lactamases containing the models generated, their classification, and their characterization along with the associated available literature has been developed and maintained at www.bldb.eu (Accessed on 15 November 2022) [66]. Figure 2 elaborates on the molecular and functional classification, substrate specificity, and inhibition profiles of β-lactamases.
Figure 2.
Molecular and functional classification of β-lactamases. Adapted with permission from [60]. [Pcl—Penicillin, Cphl—Cephalosporin, ES-Cphl—Extended-spectrum cephalosporins, Mnb—Monobactams, Crbm—Carbapenem, AVB—Avibactam, CVA—Clavulanic acid, EDTA—Ethylenediamine tetra acetic acid].
Macrolide Esterases
Esterases are responsible for the development of resistance to macrolide antibiotics, which catalyse the hydrolysis of macrolide lactone rings [67,68]. However, the 16-membered macrolides such as spiramycin and tylosin are not their preferred substrates. EreA and EreB are erythromycin esterases of crucial clinical relevance. Compared to EreA, EreB has a wider substrate profile. Except for telithromycin, EreB confers resistance to practically all 14–15-membered macrolides, including roxithromycin and azithromycin. The genes encoding macrolide esterases are located on the plasmids in association with other antibiotic resistance determinants [67,69].
Epoxidases
Resistance to antibiotics, such as fosfomycin, is caused due to enzymatic opening of an epoxide ring mediated by a thiol-containing co-substrate or water. The presence of orthologues of this enzyme’s gene in bacterial chromosomes suggests that resistance behaviour due to epoxidases may be common in the environment [63].
2.3. Group Transfer
Transferases are the most varied and hence the largest class of resistance-conferring enzymes. They chemically modify antimicrobial drugs by transferring different chemical groups through covalent modification, thus altering the physical properties of the drug [55,70,71]. The members of different groups differ in terms of specificity towards different substrates, types of group transfer, and catalytic mechanisms. The chemical strategies employed by the enzymes to modify antibiotics include N- and O-acylation (aminoglycoside, chloramphenicol, and type A streptogramins), O-phosphorylation (aminoglycoside, macrolide, rifamycin, and peptides), O-nucleotidylation (aminoglycoside and lincosamides), O-ribosylation (rifamycin), O-glycosylation (macrolide and rifamycin), and transfer of thiol (fosfomycin). To carry these reactions, a co-substrate such as ATP, acetyl-CoA, NAD+, UDP glucose, or glutathione is required, which acts as a group donor, to bring about covalent modifications. As a result, these enzymes are predominantly active in the cytosol [55,70].
2.4. Miscellaneous Mechanisms of Antibiotic Degradation
2.4.1. Redox Enzymes
As the name indicates, redox enzymes oxidise or reduce antibiotics. Among the most well-studied examples is TetX-catalysed tetracycline oxidation [72,73]. Though TetX is a flavoprotein that requires oxygen for function, paradoxically, a tetX gene was found on a plasmid in obligate anaerobe bacteria. Therefore, tetracycline resistance was discovered phenotypically only when tetX was cloned into Escherichia coli [72,74]. TetX causes tetracycline monohydroxylation, which destroys the metal ion (Mg2+) binding site necessary for its antibacterial activity.
2.4.2. Lyases
Lyases are enzymes that cause non-oxidative or non-hydrolytic cleavage of carbon–carbon, carbon–sulfur, carbon–oxygen, and carbon–nitrogen bonds. The best-studied enzyme that brings about this cleavage is virginiamycin B lyase (Vgb), responsible for the resistance of type B streptogramins [75]. The VgB lyase was first cloned from streptogramin-resistant Staphylococci. Streptogramins are hexadepsipeptides or heptadepsipeptides, cyclized through an ester bond. The enzyme Vgb catalyses the lysis of an ester bond, leading to the opening of the antibiotic ring, rendering it ineffective [75,76].
2.5. Target Site Modification
One of the most common and generalized mechanisms employed by bacteria to gain resistance against antibiotics is modification of their binding sites, which is effective for almost all antibiotic classes. The target alterations involve modification of binding sites by enzymatic addition of chemical groups (e.g., methyl groups), modification of the target site by introducing point mutations in the gene itself, and replacement of the original target by an altered one [26].
2.5.1. Enzymatic Alteration of Target Site
One of the best examples of enzymatic target site modification is the methylation of ribosomes, which is caused by an enzyme that is expressed by the erythromycin ribosomal methylases (erm) gene, resulting in the emergence of macrolide resistance [69]. The addition of 1 or 2 methyl groups to an adenine residue (A2058) of 23S rRNA belonging to the 50S ribosomal unit brings about biochemical changes that impair the binding of antimicrobial agents to their target sites [26,77]. The erm gene is reported to confer resistance to what is known as the MLSB (macrolides, lincosamides, and streptogramin B) group of antimicrobials. The underlying reason for this cross-resistance is the overlapping binding sites of these antibiotics in the 23S rRNA [26,78,79].
2.5.2. Replacement or Bypass of Antimicrobial Binding Site
The acquisition of methicillin resistance by Staphylococcus aureus (MRSA) is a classic example of this mechanism. Penicillin-binding proteins (PBPs) are essential for the transpeptidation and transglycosylation of peptidoglycan units, to which β-lactam antibiotics bind, causing their inhibition and thus preventing bacterial cell wall synthesis. Methicillin resistance is developed due to the acquisition of a mecA gene, often found to be located on staphylococcal chromosomal cassette mec (SCC mec), which codes PBP2a, an altered variant of PBP that has low affinity for all β-lactam antibiotics, including penicillins, cephalosporins (all generations except fourth and fifth generation), and carbapenems [80].
Similar to β-lactam antibiotics, glycopeptides (vancomycin and teicoplanin) also inhibit bacterial cell wall biosynthesis. In contrast to β-lactams, glycopeptides attach to the acyl-D-Ala-D-Ala residues of the developing cell wall rather than PBPs, thus blocking PBP- mediated cross-linking of peptidoglycan and subsequently inhibiting cell wall production [77]. Resistance to vancomycin is particularly prevalent in Enterococci (especially Enterococcus faecium) and is generally mediated through the acquisition of van gene clusters. Genes in these clusters encode enzymes, which modify the synthesis of peptidoglycans through two routes: (i) replacement of final D-Ala of the polypeptide with either D-lactate or D-serine and (ii) destruction of “regular” D-Ala-D-Ala ending precursors, thus preventing interaction of vancomycin with the precursors of cell wall [81].
2.5.3. Mutations in the Genes Encoding Target Sites
Resistance to rifamycin is a suitable example of mutational resistance. Rifamycin binds to bacterial DNA-dependent RNA polymerase, thus blocking transcription. The binding site of rifamycin is located in a pocket in the β-subunit of RNA polymerase, encoded by the rpoB gene. Once bound, the antibiotic hinders transcription by blocking the path of newly synthesized RNA [82]. Resistance to rifamycin emerged due to mutation in the rpoB gene, resulting in single-amino-acid substitution, which not only decreased the affinity of the antibiotic towards its target site but also did not affect the catalytic proficiency of the polymerase [83].
2.6. Porin Modification
Gram-negative bacteria exhibit resistance to antibiotics primarily due to the presence of an outer membrane, which acts as a permeability barrier and confers intrinsic resistance to particular antibiotic drugs. Porins are the main sites of entrance for several hydrophilic antimicrobials including β-lactams, chloramphenicol, fluoroquinolones, and tetracyclines to cross the bacterial outer membrane. However, any change in the permeability of porins leads to acquired resistance against antibiotics that were previously effective [25]. Decreased porin expression or any mutation altering their structure or function can contribute to the development of acquired resistance. Changes in porin expression typically result in low-level antibiotic resistance. However, the coexistence of other resistance determinants, combined with the changes in porin expression, has been reported to enhance the level of resistance [25]. In essence, the effect of low porin expression resulting in less antibiotic intake, coupled with the action of already-existing resistance mechanisms such as efflux pumps or antibiotic-degrading enzymes, results in a high level of resistance [25].
3. Antimicrobial Resistance Modulation via Natural Products
Infectious diseases constitute one of the major contributing factors towards high mortalities worldwide, and the slow discovery of novel antibiotics has created a void in the available treatment options, which has necessitated the need to revisit and explore natural resources [84]. In the pre-antibiotic era, since people were completely dependent on natural resources for all their needs, including medicines, these resources significantly contributed to the treatment of various diseases. Among natural products, microbes and plant products hold prime importance. Since the discovery rate of microbially derived antimicrobials is at its lowest since the golden age of antimicrobials and the emergence of AMR has further narrowed down the treatment options, exploration of alternative medicine based on plant products has become necessary [85]. Plants, being easily available and easy to handle, were the first to be used as treatment options for infectious diseases, which continues even today in many tribal communities as an alternative to modern antibiotics. Plants produce hundreds and thousands of structurally and functionally diverse phytochemicals that exert a multitargeted impact on pathogenic microbes, ensuring their death and no further resistance development [13,14,85,86]. Given the availability of huge phytochemical reserves in the plant kingdom, exploring them for antimicrobial agents seems promising.
3.1. Multiple-Compound Synergy vs. Single-Compound Therapy
Plants, as living organisms, are complex systems that are self-organising and environmentally adaptive. These complex adaptive traits are a function of the complex chemical matrix that works in synergy to give rise to complex systems such as plants [13]. Plants thrive in diverse habitats, which are vulnerable to pathogenic attacks, and unlike animals, plants do not possess an adaptive immune system. Therefore, they produce structurally and functionally diverse chemicals known as plant secondary metabolites (PSM), which are functionally so diverse that they not only kill pathogenic microbes but also ensure that there is no resistance development anytime soon [87,88,89]. For instance, a recent study conducted on Artemisia annua L. crude extracts (herbal tea) and pure artemisinin resulted in a 6–18-fold reduction in plasmodial IC50 in the case of crude extract as compared to purified artemisinin [90]. This phenomenon is explained by the existence of interacting and potentiating compounds in the crude extract that enhance its activity in comparison to the single active compound [87,88,89]. Further insights are needed to decipher the interaction of phytocompounds in a mixture to devise efficient antimicrobials from plant secondary metabolites.
3.2. Plant Secondary Metabolites as Antimicrobials
Since the advent of antibiotics in 1930, many classes of microbe-derived antimicrobials have been introduced in the antibiotics market. However, over time, cross-resistance to these antibiotics has proved to be a grave issue for infection treatment worldwide, particularly in developing countries [7,8]. Over-the-counter availability and ignorant consumption of antibiotics have significantly contributed to the evolution of multidrug resistance among microbes [7,25].
With the growing antimicrobial resistance in microbes, recent years have shown a great shift towards alternative therapies, compared to conventional antibiotics, including increasing use of natural products. There has been a growing need for the use of alternate therapies, especially those derived from plants [91,92,93,94]. Plant metabolites are being used directly or as precursors for new synthetic products [95]. Due to their having almost no side effects, most people worldwide prefer biological components for maintaining their health [96]. The first phytocompound used as medicine was morphine, which was discovered from opium poppy (Papaver somniferum L.) [97]. Since then, chemicals found in plants that have the potential to treat disease have been widely used, usually in crude forms. However, the period after the 1980s saw a dramatic shift in pharmaceutical firms towards synthetic chemistry or, most appropriately, towards combinatorial chemistry for more efficient and economical drug development options [98,99]. The effectiveness of plant secondary metabolites as herbal formulations and antimicrobial agents has prioritized the use of phytocompounds in drug development against multidrug-resistant microbes [15,100,101]. However, despite extensive research, the Food and Drug Administration (FDA) has authorised only a few phytochemicals, such as capsaicin, codeine, paclitaxel, reserpine, and colchicine, as antimicrobial agents against drug-resistant microbes [10,57]. Crude methanolic extracts of several plants such as lemongrass, neem, Aloe vera L., oregano, rosemary, thyme, and tulsi have demonstrated effective antimicrobial activities, which were attributed to the presence of flavonoids and tannins in their crude extracts [102].
Apart from crude extracts, which represent the synergistically cooperating mixture of various phytochemicals, several active formulae in their purified states have shown activity against MDR pathogens along with their molecular targets. To name a few, baicalein found in Thymus vulgaris L., Scutellaria baicalensis Georgi, and Scutellaria lateriflora L. has shown antimicrobial activity against MRSA, which can be attributed to its potential to inhibit the NorA efflux pump of MRSA [103,104]. Berberine, isolated from Berberis L. sp., has demonstrated antimicrobial activity by inhibiting bacterial gyrase/topoisomerase, RNA polymerase, and cell division [105]. Magnolol, isolated from the bark of Magnolia officinalis Rehder & E.H.Wilson, has demonstrated synergistic activity with meropenem by inhibiting New Delhi metallo-β-lactamase (NDM-1), thereby restoring its activity against NDM-1 expressing Escherichia coli [106]. Plasmid-mediated antimicrobial resistance is one of the underlying reasons for bacteria exhibiting resistance behaviour in response to antibiotics. Several phytochemicals, such as 8-epidiosbulbin-E-acetate isolated from Dioscorea bulbifera Russ. ex Wall., have been reported to possess curing efficiency against resistance plasmids of Enterococcus faecalis, Shigella sonnei, Pseudomonas aeruginosa, and Escherichia coli [107].
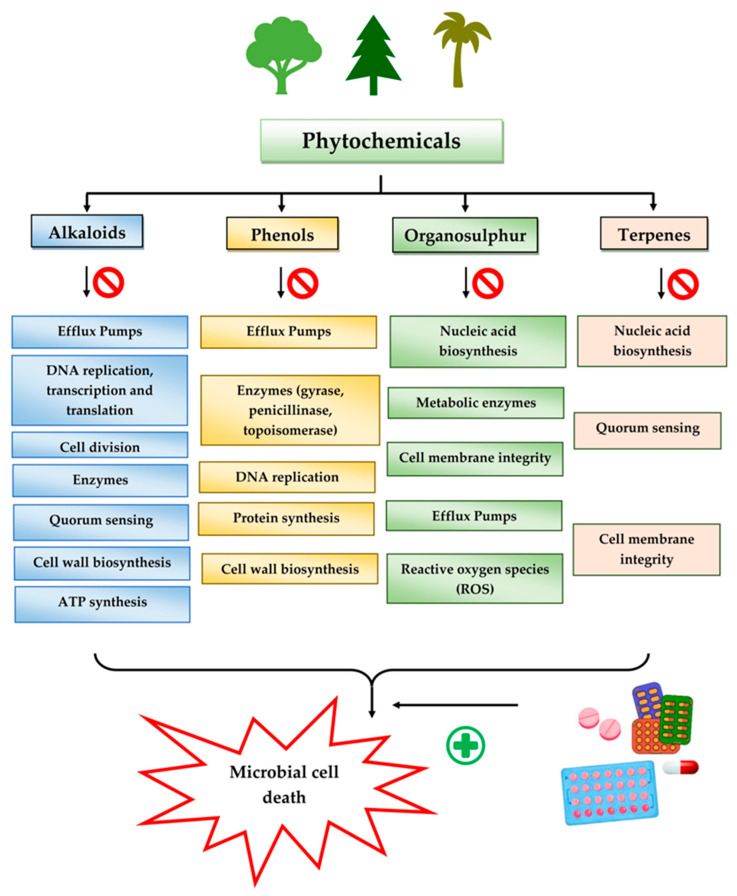
Given the promising results of plant-based bioactive compounds against antibiotic-resistant strains and the slow discovery of new and efficient antibiotics, it has become highly imperative to explore the vast repository of phytocompounds to overcome the looming catastrophe of antimicrobial resistance. The antimicrobial activities and mechanisms of action hence employed by phytochemicals such as alkaloids, flavonoids, organosulfur compounds, and terpenes are discussed in the following sections, and a summary is depicted in Figure 3.
Figure 3.
Flowchart of plant-derived phytochemicals and their mechanistic role as antimicrobials.
3.2.1. Alkaloids
Alkaloids are heterocyclic nitrogenous compounds, biosynthetically derived from amino acids, and show variability in chemical structures [108,109]. The activity of alkaloids against microbial infections is mainly attributed to their inhibitory effects against efflux pumps. Many alkaloid compounds have been reported to have marked significance in the treatment of microbial infections.
Berberine is an isoquinoline alkaloid found in the bark of the stem and roots of Berberis L. species and is found to possess antimicrobial activity against various microbes including bacteria, fungi, protozoa, and viruses [92]. The mode of antimicrobial activity of berberine is attributed to DNA intercalation, inhibition of RNA polymerase, and inhibition of DNA gyrase and Topoisomerase IV [57,110]. Further, it was also shown to inhibit FtsZ (filamenting temperature-sensitive mutant Z) protein, thus inhibiting cell division [57,92].
Another isoquinoline alkaloid, ungeremine, isolated from methanolic fractions of Pancratium Illyricum L., was found to possess significant antibacterial activity, as it inhibits bacterial topoisomerase, leading to DNA cleavage [92,111].
Piperine, isolated from Piper nigrum L. (black pepper) and Piper longum L. (Indian long pepper), is a piperidine alkaloid that has demonstrated antimicrobial activity against Staphylococcus aureus and synergistically reduced the minimum inhibitory concentration (MIC) values when administered along with fluoroquinolone antibiotics [112]. Its inhibitory effect against MRSA was due to the inhibition of NorA efflux pumps. The synergism of piperine and aminoglycoside antibiotic, namely gentamicin when administered as nano-liposomes, demonstrated high effectiveness against MRSA infection [92,113,114]. Apart from naturally occurring piperine, its synthetic analogues such as 5-(2,2-dimethyl-chroman-6-yl)-4-methyl-penta-2,4-dienoic acid ethyl ester and 5-(2,2-Dimethyl-chroman-6-yl)-4-methyl-2E,4E-pentadienoic acid pyrrolidine were also found to inhibit NorA efflux pumps expressed in Staphylococcus aureus [27,115].
Maculine, kokusagine, and dictamine belong to the quinolone class of alkaloids, are primarily found in the stem bark of Teclea afzelii Engl., and have demonstrated significant antimicrobial activity. The mode of action of both natural and synthetic quinoline alkaloids involves the inhibition of type II topoisomerase leading to the inhibition of DNA replication [116]. Reserpine, isolated from Rauvolfia serpentina (L.) Benth. ex Kurz, is an indole alkaloid, was found to inhibit efflux pumps, and was reported to decrease the fluoroquinolone resistance in Stenotrophomonas maltophilia, which was earlier resistant due to over-expression of efflux pumps [117].
The steroidal alkaloids tomatidine and conessine possess antibacterial activities due to potentiating other antibiotics when used in synergism. When used alone or in conjunction with aminoglycoside antibiotics, tomatidine, which is derived from plants belonging to the Solanaceae family such as tomato, brinjal, and potato, has demonstrated antimicrobial activity against Staphylococcus aureus [118]. It could be used as a potentiator for many antibiotics of different classes, such as ampicillin, cefepime, ciprofloxacin, and gentamicin, when used to treat infections caused by Pseudomonas aeruginosa, Staphylococcus aureus, or Enterococcus faecalis bacteria [92]. Conessine has demonstrated synergistic activity when administered along with antibiotics [92,119]. It has demonstrated resistance-modifying activity against Acinetobacter baumannii by inhibiting the AdeIJK efflux pump [120].
Sanguinarine is an alkaloid constituent of many plants including Argemone Mexicana L., Chelidonium majus L., Macleaya cordata (Willd.) R.Br., and Sanguinaria canadensis L. It has shown antibacterial activity against MRSA strains, and its mechanism involves cell lysis brought about by the release of autolytic enzymes [121]. It was also reported to act as an effective inhibitor of bacterial replication and transcription [122]. Furthermore, Sanguinarine exhibits potent antimycobacterial activities against Mycobacterium aurum and Mycobacterium smegmatis [123].
Caffeine, a xanthine alkaloid, has shown anti-quorum-sensing activity against Pseudomonas aeruginosa by interacting with the quorum-sensing proteins such as LasR and LasI and down-regulating the secretion of its virulence factors [124].
Plant secondary metabolites (PSM) can have additive, antagonistic, or synergistic effects on conventional antibiotics. However, the synergistic effect of PSM with antibiotics is the most preferable interaction in terms of antimicrobial therapies. Two drugs are said to be synergistic when the combined effect they produce is greater than the sum of their individual effects (the phenomenon where the combined effect equals the sum is known as additive effect). Synergistic interaction between two drugs is preferred in the case of antimicrobial therapies, as it allows the use of lower doses of the combination constituents, which not only reduces the duration of antimicrobial therapy but also reduces the chances of dose-dependent toxicity, if any [89]. Many PSMs have been found to have synergistic activities with antibiotics against pathogenic infections. Chanoclavine, an ergot alkaloid, has shown synergistic activity with tetracycline against resistant strains of Escherichia coli [125]. Furthermore, 1-4-napthoquinone has demonstrated antimicrobial activity for both Gram-negative and Gram-positive bacteria [126]. It has exhibited synergistic behaviour with carbapenems (imipenem) and cephalosporins (cefotaxime and cefuroxime) against MRSA [13,127].
Alkaloids found in the plant kingdom are structurally very diverse and thus show variability in scale and mode of activity. However, irrespective of the diversification in the mechanism of action, plant alkaloids can be developed into potent antimicrobials, which would not only revive the treatment options but also ensure the development of further resistance is prevented. Table 3 summarizes some of the important plant-derived alkaloids, their target pathogens, and their modes of action.
Table 3.
List of important alkaloid classes of plant-based antimicrobial agents reported and their sources, pathogenic targets, and mechanisms of action.
| Bioactive Compound | Chemical Formula | PubChem CID | Chemical Structure * | Plant Source | Target Pathogen | Mode of Action | References |
|---|---|---|---|---|---|---|---|
| Conessine | C24H40N2 | 441082 |
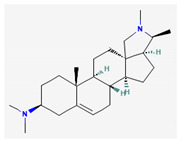
|
Holarrhena antidysenterica (G. Don) Wall. ex A. DC., Holarrhena floribunda (G.Don) T. Durand & Schinz, Holarrhena pubescens Wall. ex G. Don, Funtumia elastica (Preuss) Stapf. |
Pseudomonas aeruginosa | Efflux pump inhibitor | [120,128] |
| Piperine | C17H19NO3 | 638024 |
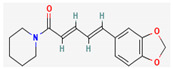
|
Piper sylvaticum Roxb. | Methicillin- resistant Staphylococcus aureus (MRSA) | Efflux pump inhibitor | [92,114] |
| Berberine | C20H18NO4+ | 2353 |
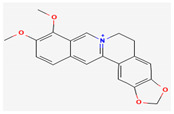
|
Berberis lycium Royle | Escherichia coli | Cell division inhibitor, protein, and DNA synthesis inhibitor | [129,130] |
| Lysergol | C16H18N2O | 14987 |
|
Convolvulaceae Juss. | Escherichia coli | Efflux pump inhibitor | [125] |
| 8-epidiosbulbin E-acetate | C21H24O7 | 131751666 |
|
Dioscorea bulbifera L. | Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Shigella. | Plasmid curing (R-plasmids in Escherichia coli and Enterococcus faecalis) |
[57,107] |
| Reserpine | C33H40N2O9 | 5770 |
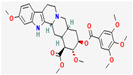
|
Rauvolfia serpentina (L.) Benth. ex Kurz | Staphylococcus sp., Streptococcus sp. | Efflux pump inhibitor | [131] |
| Tomatidine | C27H45NO2 | 65576 |
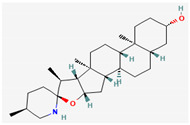
|
Solanum L. sp. | Listeria, Bacillus and Staphylococcus sp. | ATP synthase inhibitor | [118,132] |
| Dictamnine | C12H9NO2 | 68085 |
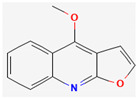
|
Teclea afzelii (Engl.) | Escherichia coli, Microsporum audorium, Bacillus subtilis, Mycobacterium smegmatis | Inhibition of Type II topoisomerase enzyme and inhibition of DNA replication | [92] |
| Kokusagine | C13H9NO4 | 5318829 |
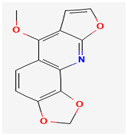
|
Teclea afzelii (Engl.) | |||
| Maculine | C13H9NO4 | 68232 |
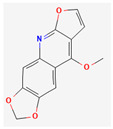
|
Teclea afzelii (Engl.) | |||
| Sanguinarine | C20H14NO4+ | 5154 |
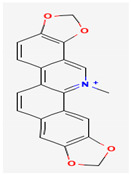
|
Chelidonium majus L., Sanguinaria canadensis L., Macleaya cordata (Willd.) R. Br. | MRSA, Mycobacterium aurum and Mycobacterium smegmatis | Compromising cytoplasmic membrane, cell lysis, replication, and transcription inhibition | [92] |
| Chanoclavine | C16H20N2O | 5281381 |
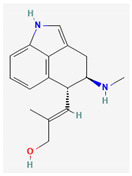
|
Ipomoea muricata (L.) Jacq. | MDR Escherichia coli | Efflux pump inhibition | [92] |
| Caffeine | C8H10N4O2 | 2519 |
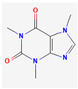
|
Camellia sinensis (L.) Kuntze | Pseudomonas aeruginosa | Inhibition of quorum-sensing proteins LasR and LasI and inhibition of bacterial virulence factors |
[124] |
| Caranine | C16H17NO3 | 441589 |
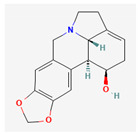
|
Clivia miniata (Lindl.) Verschaff., Crinum bulbispermum (Burm.f.) Milne-Redh. & Schweick. |
Candida dubliniensis | NA | [133] |
| Evodiamine | C19H17N3O | 442088 |
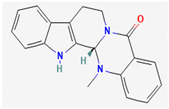
|
Evodia aromatica (Sonn.) Pers. | Streptococcus pneumoniae | Inhibition of ATP-dependent MurE ligase of Mycobacterium tuberculosis, an enzyme required for the biosynthesis of peptidoglycan | [134] |
| Chanoclavine | C16H20N2O | 5281381 |
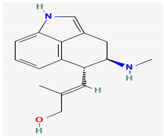
|
Ipomoea muricata (L.) Jacq. | MDR Escherichia coli | Efflux pump inhibition | [92] |
| Evocarpine | C23H33NO | 5317303 |
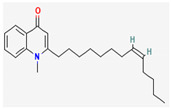
|
Evodia aromatica (Sonn.) Pers. | Streptococcus pneumoniae | Inhibition of ATP-dependent MurE ligase of Mycobacterium tuberculosis, an enzyme required for the biosynthesis of peptidoglycan | [134] |
| Voacafricines A & B | Fruits of Voacanga Africana Stapf. | Staphylococcus aureus | NA | [133] | |||
| Thalicfoetine | Roots of Thalictrum foetidum L. | Bacillus subtilis | NA | [135,136] |
* Chemical structures of compounds have been taken from PubChem; www.pubchem.ncbi.nlm.nih.gov (Accessed on 10 December 2022).
3.2.2. Phenols
Due to their wide range of pharmacological activities and strong pharmacological effects, plant phenolics are recognised as important bioactive compounds. Plant-derived phenols can be found in simple or polymerized forms and contain an aromatic ring structure with one or more hydroxyl groups. Plant phenolics are categorized into many classes such as simple phenols, phenolic acids, quinones, flavonoids, and tannins. Phenols have proven to be potent against a wide range of diseases such as bacterial infections, cancers, diabetes, and cardiovascular diseases [137,138,139,140]. Plant phenolics have exhibited antimicrobial potency against a variety of microbes by sensitizing them against antibiotics and tuning down the efflux pump activity by acting as potent efflux pump inhibitors.
Simple phenols such as catechol and pyrogallol, which are allelochemicals synthesized by plants, have shown antibacterial activities against three bacterial strains: Corynebacterium xerosis, Pseudomonas putida, and Pseudomonas pyocyanea. Moreover, catechol was found to have an antifungal effect on Fusarium oxysporum and Penicillium italicum [141]. Furthermore, 4-(4-Hydroxyphenethyl) phen-1,2-diol (2a), a derivative of catechol and pyrogallol, was found to inhibit Helicobacter pylori urease enzyme [142]. Resorcinol, isolated from Ainsliaea bonatii Beauverd, was found to be effective against MRSA and ESBL Staphylococcus aureus. The mode of action was reported to be cell wall disintegration, leading to increased permeability and leakage of intracellular constituents, negatively influencing gene expression and leading to decreased protein synthesis [143]. Resveratrol, a natural phenolic compound, exhibited efflux pump inhibitory activity against various bacterial strains such as CmeABC, a multidrug efflux system of Campylobacter jejuni, and efflux pumps of Mycobacterium smegmatis [144].
Gallic acid and ferulic acid have been reported to possess significant antimicrobial activities against Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, and Pseudomonas aeruginosa, and the mode of action was found to be the disruption of cell membrane via changes in membrane potential [145]. Furthermore, 3-p-trans-coumaroyl-2-hydroxyquinic acid, isolated from Cedrus deodara (Roxb. ex D.Don) G.Don, has shown effective antibacterial activity against Staphylococcus aureus, and the mechanism of action involves damage to cytoplasmic membrane due to membrane hyperpolarization and loss of membrane integrity, which results in subsequent discharge of intracellular constituents [146]. Chebulinic acid, primarily isolated from Terminalia chebula Retz., has been reported to inhibit DNA gyrase of quinolone-resistant Mycobacterium tuberculosis [147]. However, the whole study was in silico based, and further insights are needed to unravel its significance as a DNA gyrase inhibitor and anti-tuberculosis agent [92].
Quercetin and apigenin belong to the flavonoid class of plant phenols, which act as antibacterial agents against Helicobacter pylori and Escherichia coli, and the mechanism of action involves inhibition of d-alanine:d-alanine ligase, an enzyme important for bacterial cell wall assembly [148].
Baicalein is a flavone, primarily isolated from Scutellaria baicalensis Georgi, Scutellaria lateriflora L., and Thymus vulgaris L. It inhibits NorA efflux pumps, thus increasing the efficacy of antibiotics such as β-lactams, ciprofloxacin, and tetracycline against methicillin-resistant Staphylococcus aureus. When co-administered with tetracycline, baicalein also shows a synergistic effect against Escherichia coli due to inhibition of the efflux pump [103,104].
Biochanin A, an isoflavone, has inhibitory activity against MRSA and has been found to inhibit MRSA efflux pumps by reducing NorA protein expression [149].
Kaempferol, an active flavonoid, has shown potent antimicrobial activity against triazole-resistant Candida albicans and MRSA [150,151]. Kaempferol inhibits NorA efflux pump, as does its naturally occurring glycoside derivative, kaempferol rhamnoside, which has a potentiating effect on ciprofloxacin against NorA pumps of Staphylococcus aureus [150].
Catechins found in green tea form the basis of the antimicrobial potential of tea extracts. The antimicrobial activity of catechins is attributed to their hydrogen peroxide generation, which ultimately leads to bacterial cell membrane damage [152]. Epigallocatechin gallate (EGCG) is yet another phenolic compound that exhibits antimicrobial activity against MRSA by inhibiting NorA efflux pump [27,92,98]. EGCG has been shown to inhibit DNA gyrase by blocking its β-subunit at the ATP binding site, bacterial efflux pump, and inhibition of chromosomal penicillinases, owing to its multitargeted action against pathogenic microbes [153].
Tannins have been reported to have much more effective antimicrobial action on Gram-positive bacteria than Gram-negative ones. This difference in activity is because of the mode of action of tannins. Tannins pass through the bacterial cell wall and interfere with the metabolism of bacterial cell. On the other hand, double-layered cell walls of Gram-negative bacteria offer much resistance for the tannins to pass through, hence the reduced activity [154]. Curcumin, abundantly found in Curcuma longa L., has demonstrated antimicrobial activity against Escherichia coli and Staphylococcus aureus. The antibacterial activity is attributed to its capacity to damage the membrane by penetrating through the bilayer and increasing the membrane permeability [155].
Phenolics have shown diverse mechanisms against different bacteria ranging from inhibition of efflux pumps, cellular membrane disruption, and inhibition of cell wall synthesis to inhibition of key enzyme biosynthesis. The observed traits of phenolics as antibacterials make them desirable candidates for further in vitro studies. The most significant phenolics with antibacterial activities have been summarized in Table 4.
Table 4.
List of important phenolic classes of plant-based antimicrobial agents reported and their sources, pathogenic targets, and mechanisms of action.
| Bioactive Compound | Chemical Formula | PubChem CID | Chemical Structure * | Plant Source | Target Pathogen | Mode of Action | References |
|---|---|---|---|---|---|---|---|
| Myricetin | C15H10O8 | 5281672 |
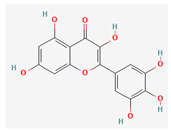
|
Myricaceae Rich. ex Kunth., Anacardiaceae R.Br., Polygonaceae Juss., Pinaceae Spreng. ex F.Rudolphi., Primulaceae Batsch ex Borkh. |
Mycobacterium smegmatis | Efflux pump inhibitor | [144] |
| Baicalein | C15H10O5 | 5281605 |
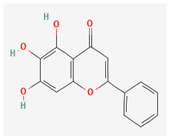
|
Thymus vulgaris L., Scutellaria baicalensis Georgi, Scutellaria lateriflora L. |
Methicillin-resistant Staphylococcus aureus | Inhibition of the NorA efflux Pump | [103,104] |
| 4′,6′-Dihydroxy-3′,5′-dimethyl-2′-methoxychalcone | C18H18O4 | 10424762 |
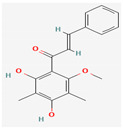
|
Dalea versicolor Zucc. |
Staphylococcus aureus,
Bacillus cereus. |
Inhibition of NorA efflux pump | [92,156] |
| Epigallocatechin gallate | C22H18O11 | 65064 |
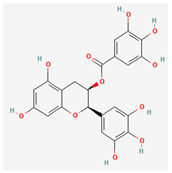
|
Camellia sinensis (L.) Kuntze | Methicillin-resistant Staphylococcus aureus | Inhibition of NorA efflux pump, inhibition of chromosomal penicillinase and DNA gyrase | [98,153] |
| Chebulinic acid | C41H32O27 | 72284 |
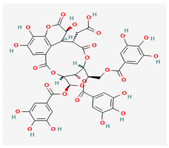
|
Terminalia chebula Retz. | Quinolone-resistant mutants of Mycobacterium tuberculosis | Inhibition of DNA gyrase | [147] |
| Emodin | C15H10O5 | 3220 |
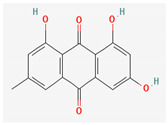
|
Rheum palmatum L. | Methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium | Inhibition of DNA gyrase | [157] |
| Curcumin | C21H20O6 | 969516 |
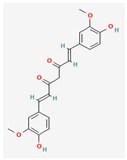
|
Curcuma longa L. | Staphylococcus aureus, Escherichia coli. | Enhancing membrane permeability, inhibition of enzyme sortase A | [155] |
| Quercetin | C15H10O7 | 5280343 |
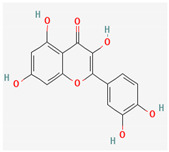
|
Vitaceae Juss. Brassicaceae Burnett, Amaryllidaceae J.St.-Hil., Rutaceae Juss. |
Staphylococcus aureus, Escherichia coli, Helicobacter pylori | Efflux pump inhibitor, inhibition of d-alanine:d-alanine ligase in Helicobacter pylori and Escherichia coli | [148,158] |
| Kaempferol | C15H10O6 | 5280863 |
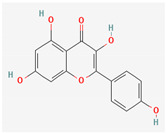
|
Alpinia calcarata–Roscoe. | Methicillin-resistant Staphylococcus aureus, Candida albicans | Efflux pump inhibitor | [150,151] |
| Resveratrol | C14H12O3 | 445154 |
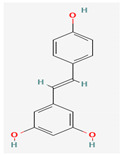
|
Vitis vinifera L. | Campylobacter jejuni | Efflux pump inhibitor | [159] |
| Taxifolin/dihydroquercetin | C15H12O7 | 439533 |
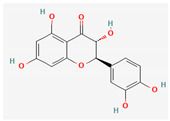
|
Conifers-like Larix sibirica Ledeb., Pinus roxburghii Sarg., Cedrus deodara (Roxb. ex D.Don) G.Don, Taxus chinensis (Pilg.) Rehder. | Methicillin-resistant Staphylococcus aureus, Enterococcus faecalis | Cysteine transpeptidase sortase A (SrtA) inhibitor, β-ketoacyl acyl carrier protein synthase inhibitor |
[160,161] |
| Osthole | C15H16O3 | 10228 |
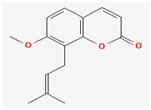
|
Prangos hulusii (Şenol, Yıldırım & Seçmen), Cnidium monnieri (L.) Cusson ex Juss., Angelica pubescens Maxim. | Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumoniae | DNA gyrase inhibitor, MCR-1 inhibitor | [162,163] |
| Galbanic acid | C24H30O5 | 7082474 |
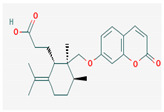
|
Ferula szowitsiana DC. | Staphylococcus aureus | Efflux pump inhibitor | [164] |
| Asphodelin A | C15H10O6 | 54679752 |
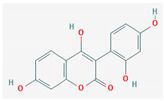
|
Asphodelus microcarpus Rchb. | Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, Botrytis cinerea | DNA gyrase inhibitor | [165] |
| Aegelinol | C14H14O4 | 600671 |
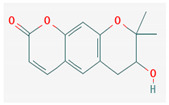
|
Phlojodicarpus villosus (Turcz. ex Fisch. & C.A.Mey.) Turcz. ex Ledeb., Peucedanum praeruptorum Dunn, Ferulago galbanifera (Mill.) W.D.J.Koch |
Salmonella enterica serovar typhi, Enterobacter aerogenes, Enterobacter cloacae, Staphylococcus aureus | DNA gyrase inhibitor | [92,166] |
| 3,4,5-trihydroxybenzoic acid (Gallic acid) | C7H6O5 | 370 |
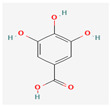
|
Mimosa bimucronata (DC.) Kuntze, Punica granatum L. | Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa | Cell membrane disintegration | [145] |
| Ferulic acid | C10H10O4 | 445858 |
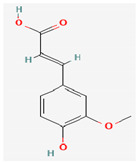
|
Commelinaceae Mirb. | Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa | Cell membrane disintegration | [145] |
| Apigenin | C15H10O5 | 5280443 |
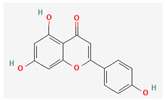
|
Matricaria chamomilla L. | Pseudomonas aeruginosa | NA | [134,148] |
| Genistein | C15H10O5 | 5280961 |
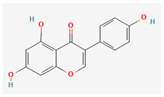
|
Glycine max (L.) Merr. | Pseudomonas aeruginosa | NA | [167] |
| Eriodictyol | C15H12O6 | 440735 |
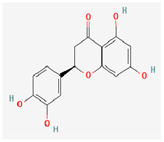
|
Eriodictyon californicum (Hook. & Arn.) Decne. |
Enterococcus
faecalis |
NA | [161] |
| Agasyllin | C19H20O5 | 15596603 |
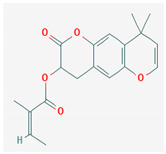
|
Ferulago galbanifera (Mill.) W.D.J.Koch | Campylobacter species | DNA gyrase inhibitor | [166] |
* Chemical structures of compounds have been taken from PubChem; www.pubchem.ncbi.nlm.nih.gov (Accessed on 10 December 2022).
3.2.3. Organosulfur Compounds
Organosulfur compounds are sulfur-containing organic molecules that are responsible for the strong aromas of Allium vegetables such as onions and garlic. They are also present in cruciferous vegetables such as cabbage and broccoli. Several organosulfur compounds such as allicin, ajoene, dialkenyl sulfides, S-allyl cysteine, and isothiocyanates were found to be effective against both Gram-positive as well as Gram-negative bacteria [168,169,170,171]. Investigations have revealed that high-concentration polysulfide-containing plants possess broad-spectrum antibacterial activities [172].
Diallyl thiosulfinate, commonly known as ‘allicin’, is an organosulfur compound that is isolated from Allium sativum L. Its antibacterial action has been seen against a variety of pathogenic microbes, including MRSA, Pseudomonas aeruginosa, Streptococcus agalactiae, Staphylococcus epidermidis, and oral pathogens that can cause periodontitis [168,173]. Allicin mainly causes the suppression of sulfhydryl-dependent enzymes, including alcohol dehydrogenase, thioredoxin reductase, and RNA polymerase, which is the primary mechanism of its antibacterial activity. Further, allicin has also been shown to partially inhibit protein and nucleic acid synthesis [174,175].
Ajoene, another organosulfur compound, is not as functionally diverse as allicin. However, it exhibits potency against both Gram-positive as well as Gram-negative bacteria along with some fungal strains, including Aspergillus niger and Candida albicans. The mechanism of action is the same as that of allicin, as ajoene is also a sulfhydryl-dependent enzyme inhibitor [168].
Isothiocyanates (ITCs) are exclusively abundant in members of the family Brassicaceae Burnett. such as broccoli, cabbage, cauliflower, and mustard, and they show activity against oral pathogens as well as Helicobacter pylori [170,176,177]. The antimicrobial mechanism of ITCs is not fully understood yet. However, it is speculated that their activity might be due to their reaction with cellular proteins and enzymes, which then hamper the biochemical processes inside the cell. Due to the high electrophilicity of an ITC carbon atom, it can react with amines, thiols, and hydroxyl groups of cellular proteins [170]. Table 5 lists some of the important organosulfur compounds that have been found to have antimicrobial activities against different pathogenic microbes.
Table 5.
List of important organosulfur/isothiocyanate classes of plant-based antimicrobial agents reported and their sources, pathogenic targets, and mechanisms of action.
| Bioactive Compound | Chemical Formula | PubChem CID | Chemical Structure * | Plant Source | Target Pathogen | Mode of Action | References |
|---|---|---|---|---|---|---|---|
| Allicin | C6H10OS2 | 65036 |
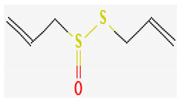
|
Allium sativum L. |
Salmonella typhimurium, Staphylococcus aureus, Bacillus subtilis, Bacillus typhosus, Bacillus paratyphosus, Morganella morganii, Bacillus enteritidis, Shigella dysenteriae, Vibrio cholera, Escherichia. coli, Listeria monocytogenes, Helicobacter pylori, drug-resistant strains of Mycobacterium tuberculosis |
Sulfhydryl-dependent enzyme inhibitor, DNA/RNA synthesis inhibitor, inhibitor of acetyl-CoA synthases in yeasts |
[169,174,178,179] |
| Ajoene | C9H14OS3 | 5386591 |
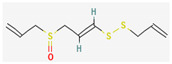
|
Allium sativum L. | Campylobacter jejuni, Staphylococcus aureus, Escherichia coli, Helicobacter pylori | Sulfhydryl-dependent enzyme | [168,180] |
| Sulforaphane | C6H11NOS2 | 5350 |
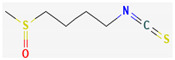
|
Brassicaceae Burnett. |
Bacillus cereus, Escherichia coli | Membrane destruction, ATP synthase inhibitor, DNA/protein synthesis inhibitor | [181] |
| Allyl isothiocyanates (AITCs) | C4H5NS | 5971 |
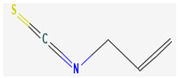
|
Armoracia rusticana G.Gaertn., B.Mey., & Scherb. | Oral pathogens, Helicobacter pylori, Escherichia coli | Inhibition of urease, reducing the inflammatory component of Helicobacter infections, inhibition of ATP binding sites of P-ATPase in bacteria | [92,170] |
| Benzyl isothiocyanate (BITC) | C8H7NS | 2346 |
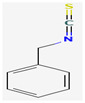
|
Alliaria petiolata (M.Bieb.) Cavara & Grande | Methicillin-resistant Staphylococcus aureus | Disruption of membrane integrity | [176] |
| Phenethyl isothiocyanate(PEITC) | C9H9NS | 16741 |
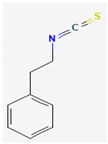
|
Brassica campestris L., Brassica rapa L. |
Gram-positive bacteria | Intracellular accumulation of reactive oxygen species (ROS), depolarization of mitochondrial membrane | [92,182] |
| Berteroin | C7H13NS2 | 206037 |

|
Brassica oleracea L. | Bacillus subtilis, Escherichia coli, Helicobacter pylori | NA | [93] |
| Cheirolin | C5H9NO2S2 | 10454 |
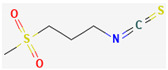
|
Cheiranthus cheiri L. | Helicobacter pylori | NA | [134] |
| Alyssin | C7H13NOS2 | 206035 |
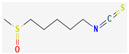
|
Alyssum L. sp. | Helicobacter pylori | NA | [134] |
* Chemical structures of compounds have been taken from PubChem; www.pubchem.ncbi.nlm.nih.gov (Accessed on 10 December 2022).
3.2.4. Terpenes
Terpenes are aromatic compounds found in many plants and are responsible for the characteristic smell of many plants, such as cannabis, pine, and lavender, as well as fresh orange peel. Terpenes are commonly distributed in nature, in nearly all living forms, and perform a variety of functions in cells. Apart from being primary structural components of cells (cholesterol and steroids in cellular membranes), they also act as functional molecules such as carotenoids, quinones, and retinal in photosynthesis, electron transport, and vision, respectively [183].
Normally, terpenes have demonstrated more potent activity for Gram-positive than Gram-negative bacteria and bring about their antibacterial effects mainly via lipophilic features. Monoterpenes change membrane structure by changing their composition, which increases fluidity and permeability and causes changes in the topology of membrane proteins, causing disruptions throughout the respiratory chain [184]. Carvacrol is commonly found in the essential oils of Thymus vulgaris L., Lepidium flavum Torr., Citrus aurantium (Spreng.), Balle ssp. Bergamia, and Origanum vulgare L., among other plants. It has demonstrated antibiofilm development activity against Staphylococcus aureus and Salmonella typhimurium and is reported to have activity against tobacco mosaic virus and cucumber mosaic virus [185,186]. Carvacrol has also been shown to be effective against food-borne pathogens such as Escherichia coli, Salmonella, and Bacillus cereus [124].
Thymol, found as an essential oil component of Thymus vulgaris L, has shown antibacterial effects on tetracycline-resistant Salmonella typhimurium and Escherichia coli, penicillin-resistant Staphylococcus aureus, and erythromycin-resistant Streptococcus pyogenes. The mechanism of action, as per many studies, involves disintegration of cell membranes [187,188].
Ursolic acid, a pentacyclic triterpene, possess broad-spectrum antibacterial activity. It was shown that ursolic acid has disorganising effects on Escherichia coli membrane [189]. Eugenol and cinnamaldehyde are yet more important terpenes present in plant essential oils and have shown activity against a wide range of pathogens including Helicobacter pylori, causing damage to the cell membrane [190,191]. Eugenol has been shown to inhibit biofilm formation by MRSA and MSSA clinical strains as well as the synthesis of virulence factors by Pseudomonas aeruginosa [190,192]. The mechanism of eugenol action involves damage to bacterial membrane, followed by leakage of cellular contents. As for cinnamaldehyde, the compound works by damaging the membrane, decreasing the membrane potential, and alterations in metabolic activity [193]. Some of the most significant terpenes having antibacterial effects are listed in Table 6.
Table 6.
List of important terpene classes of plant-based antimicrobial agents reported and their sources, pathogenic targets, and mechanisms of action.
| Bioactive Compound | Chemical Formula | PubChem CID | Chemical Structure * | Plant Source | Target Pathogen | Mode of Action | References |
|---|---|---|---|---|---|---|---|
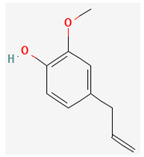
| Eugenol | C10H12O2 | 3314 |
|
Syzygium aromaticum (L.) Merr. & L.M.Perr, Cinnamomum zeylanicum Blume. | Helicobacter pylori, Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive Staphylococcus aureus, Pseudomonas aeruginosa | Inhibits biofilm construction, interrupts cell-to-cell communication, eradicates the pre-established biofilms, and kills the bacteria in biofilms | [190,192] |
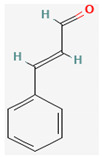
| Cinnamaldehyde | C9H8O | 637511 |
|
Cinnamomum verum J. Presl. | Escherichia coli, Staphylococcus aureus | Membrane damage | [193] |
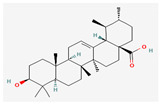
| Ursolic acid | C30H48O3 | 64945 |
|
Salvia rosmarinus Spenn., Salvia officinalis L. | Escherichia coli | Cell membrane disturbance | [189] |
| Farnesol | C15H26O | 445070 |
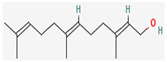
|
Vachellia farnesiana (L.) Wight & Arn. | Staphylococcus aureus including MRSA | Membrane damage | [194] |
| Carvacrol | C10H14O | 10364 |
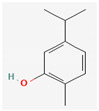
|
Thymus capitatus (L.) Hoffmanns. & Link., Thymus vulgaris L. | Escherichia coli | Cell membrane damage | [167,195] |
| Nerolidol | C15H26O | 5284507 |
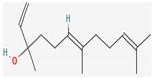
|
Cannabis sativa L. | Staphylococcus aureus including MRSA | Cell membrane damage | [194] |
| Thymol | C10H14O | 6989 |
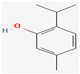
|
Thymus capitatus (L.) Hoffmanns. & Link. | Staphylococcus aureus including MRSA | NA | [195,196] |
* Chemical structures of compounds have been taken from PubChem; www.pubchem.ncbi.nlm.nih.gov (Accessed on 10 December 2022).
4. Himalayan Medicinal Plants as a Reservoir of Phytochemicals for Novel Antimicrobial Drug Discovery
4.1. Plant Diversity of Indian Himalayas
The Indian Himalayas are one of the thirty-six designated biodiversity hotspots globally [16]. Spread over an area of 3000 km from Northern Pakistan to North East India, the region spans incredible variations in climate across its course. Geographically, the entire mountain range has been divided into two regions: the Eastern Himalayas, which span from Nepal, Tibet, Bhutan, West Bengal, Assam, and Arunachal Pradesh to Northern Myanmar; and the Western Himalayas, which include parts of Uttarakhand, Northwest Kashmir, and Northern Pakistan [16].
The Indian Himalayan region is home to an estimated 10,000 species of vascular plants, out of which 3160, accounting for almost 1/3rd of the total plant species found, are endemic to the region [197]. Additionally, 71 genera and 5 plant families are also endemic to the area. The endemic plant families include Trochodendraceae Eichler, Hamamelidaceae R. Br., Butomaceae Mirb., and Stachyuraceae J. Agardh. The largest family of flower-bearing plants in the region is Orchidaceae Juss., with an estimated number of 750 species [197]. Among the 5725 species of angiosperms endemically found in India, 3471 species are hosted by the Himalayas themselves. Moreover, among the 147 genera of angiosperms that are endemic to India, 71 are found exclusively in the Himalayan region [198]. The Himalayas host all the conifer (gymnosperms) flora of India except for Podocarpus wallichianus C.Presl and Podocarpus neriifolius D.Don, which are found in peninsular India and the Andamans, respectively. Among the gymnosperm shrubs, Ephedra gerardiana Wall. ex Klotzsch & Garcke is exclusively distributed in the Himalayas and is highly revered as a medicinal plant due to its alkaloid ephedrine [199]. Among the pteridophytes, the Eastern Himalayas contain about 847 taxa in 179 genera, followed by the Western Himalayas, which contain 340 taxa in 101 genera of pteridophytes [200]. Of the 2000 species of mosses (bryophyte) found in India, the Eastern Himalayas contain 1030 species and 751 species are distributed in the Western Himalayas [201]. About 30% of the total liverwort population is maximally distributed in the Eastern Himalayas followed by the Western Himalayas and the Western Ghats [202].
Around 30% of India’s land area, including biodiversity hotspots such as the Himalayas, the Western Ghats, and the Nicobar Islands, is still unexplored and unrecorded in terms of its floral richness. Therefore, our understanding of the delicate ecosystems of these hotspots is still insufficient [16].
4.2. Medicinal Plant Resources of Himalayas and Alternate Systems of Medicine
The Indian subcontinent possesses one of the oldest and most well-structured medical systems, which originated more than 5000 years ago [203]. The vast information on medicine is backed by different traditional medicinal practices such as Ayurveda and Unani and various literary manuscripts such as Charak Samhita, Sushruta Samhita, Dhanvantri, and Nighatu [204,205]. These scriptures provide a solid foundation for traditional medicinal practices in India [206]. Various communities in India, both tribal and urban, rely on traditional medicine, and it has long been an important element in the treatment of diseases and disorders. Around 25000 phytocompounds are used as herbal formulations in rural Indian traditional medicine, particularly in tribal populations [207]. Of these phytocompounds, only 5–10% have been confirmed scientifically [208]. Due to the rising interest in adopting traditional medicine globally, government institutions in India have made attempts to validate the therapeutic efficiency of the drugs used in traditional medicine [209]. The Himalayan region is home to many endemic human populations, and due to the remoteness of the area, the people have been relying on forest products for multiple needs, including the ethnomedicinal use of plants for disease treatment, as a result of which the people of the Himalayas have a strong belief in traditional herbal medicine [210,211].
The Indian Himalayas foster around 10,000 species of higher plants, of which 1748 species reportedly have medicinal properties [18,19]. Medicinal plants of the region have played fundamental roles in the disease treatment of the people living in and around the Himalayan mountain range [19]. The vegetation of the area is determined by the climate and weather conditions of the area. For instance, the North-Western Himalayas, including the areas of Ladakh and Gilgit, have weather conditions ranging from mild summers to severely cold winters, and the medicinal flora are represented by Achillea millefolium L., Bunium persicum (Boiss.) B. Fedtsch., Picrorhiza kurroa Royle ex Benth., Juniperus communis L., and Ephedra gerardiana Wall. ex Klotzsch & Garcke [212]. The Western Himalayan region, including Jammu and Kashmir, Himachal Pradesh, Garhwal, and Kumaon Himalaya, experiences warm humid summers and cold humid winters, and the medicinal flora are primarily represented by Saussurea costus (Falc.) Lipsch., Colchucum luteum Baker, Atropa acuminata Royle ex Lindl., and Physochlaina praealta (Decne.) Miers. On the other hand, the Eastern Himalayas, comprising areas such as Darjeeling, parts of Assam, Sikkim, and Arunachal Pradesh, are characterized by warm summer and cool winter. Hence, the vegetation is represented predominantly by Aquilaria malaccensis Benth., Coptis teeta Wall., and Panax pseudoginseng Wall. [16]. In the adjoining Himalayan region of north-western Pakistan, medicinal plants such as Berberis lyceum Royle, Achillea millefolium L., Bergenia ciliata (Royle) A.Braun ex Engl., and Aloe vera L. have been reported to be used against urinary tract infections due to their antimicrobial activity against Staphylococcus aureus and Escherichia coli [140]. Further, medicinal plants such as Impatiens glandulifera Royle, Artemisia scoparia Waldst. & Kit., Ageratum conyzoides L., and Achillea millefolium L. have been reported to be used as treatment options for various ailments such as urinary tract infections, cardiac diseases, baldness, abortion and miscarriage jaundice, hepatitis, typhoid, fever, and tuberculosis [211].
In India, around 17,000 species of higher plants have been discovered, of which 7500 plant species have been found to have medicinal properties, which is the highest total-plants-to-medicinal-plants proportion so far reported [16,17]. The maximum population of medicinal plants (1717 species) has been reported at an elevation of 1800 m. Traditional medical practices of the Indian subcontinent use many medicinal plants, and Ayurveda alone has reported 2000 medicinal plant species. One of the oldest written documents on herbal medicine, the Charak Samhita, documents 340 herbal drug productions and their aboriginal uses [213]. The rich diversity of medicinal plants in the Himalayas gave rise to the traditional medicine practices such as Ayurveda and Unani. Apart from the widely followed systems of traditional medicine, various local systems of practices based on the cultural demography have also developed. For instance, the traditional healers of Ladakh region (North-Western Himalayas) are known as “amchies”, those who practice in Kashmir Valley are known as “hakeems”, and those in Jammu are called “veds”. These traditional practices came into existence primarily because of the absence of modern medicine in past times and are still carried forward to this date as a part of tradition [214].
5. Antimicrobial Profile of Himalayan Medicinal Plants
One of the main causes of clinical mortality in humans has been infectious diseases. Moreover, with the emergence of multidrug-resistant microbes, the existing antimicrobial therapies have been rendered inactive, which has made the development of new antimicrobials necessary [26]. In the pursuit of novel antimicrobials, plants blessed with a plethora of secondary metabolites offer a vast array of phytochemicals to be screened for novel antimicrobials and developed into new antimicrobial therapies [102]. Humans have been using plants for remedial measures against various ailments for generations, as a result of which many forms of traditional medicines came into existence. These herbal medicines constitute a major part of traditional medical practices [206,211]. The Indian Himalayan region comprises 31% native, 15.5% endemic, and 14% threatened plant species [204]. The floristically rich Himalayan region is a potential source of many drug-yielding plants [215]. Many of the medicinal plants in the Himalayas have shown potent antimicrobial activity against pathogenic microbes [14,216].
Angiosperms such as Acorus calamus L. (asarone), Aegle marmelos (L.) Corrêa (rutacin), Arnebia euchroma (Royle ex Benth.) I.M.Johnst. (shikonin), Berberis L. sp. (berberine), Callicarpa macrophylla Vahl (sesquiterpenes and triterpenes), Curcuma caesia Roxb. (cinnamate), Hedychium spicatum G.Lodd. (limonene, linalool), Inula racemosa Hook.f. (isoalantolactone), Jasminum officinale L. (jasminol, lupeol), Myrsine semiserrata Wall. (embelic acid), Nardostachys jatamansi (D.Don) DC. (jatamansic acid), and Piper longum L. (piperine) are a few of the candidate phytochemicals that have shown potent antimicrobial activities. Prunus cornuta (Wall. ex Royle) Steud. and Quercus semecarpifolia Sm. have shown antibacterial activity against Acinetobacter baumannii, Salmonella enterica, and Escherichia coli [212].
Gymnosperm plants such as the species of Cycas L. and Ginkgo L., Sabina chinensis (L.) Antoine, Cedrus deodara (Roxb. ex D.Don) G.Don, Pinus bungeana Zucc. ex Endl., Platycladus orientalis (L.) Franco, and Torreya grandis Fortune ex Lindl. have shown antimicrobial activities. The essential oil ‘turpentine’ obtained from plants such as Abies balsamea (L.) Mill., Pinus brutia Ten., and Pinus roxburghii Sarg. has demonstrated antimicrobial activity against MRSA [199].
Among the pteridophytes, Adiantum philippense L., Adiantum caudatum L., Adiantum incisum C. Presl., and Adiantum venustum D.Don have shown strong antimicrobial activity against pathogens, causing food-borne infections [217]. Members of the genus Dryopteris have shown activity against Pseudomonas aeruginosa [218]. Equisetum arvense L. has shown activity against Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella enteritidis, Aspergillus niger, and Candida albicans [219].
Many bryophytes have been used traditionally for inflammation, heart disease, digestive problems, lung, and skin diseases [220]. However, some bryophytes (mosses) have shown antimicrobial properties [221]. Marchatia polymorpha L. has demonstrated antimicrobial activity against Escherichia coli, Staphylococcus aureus, Proteus mirabilis, Aspergillus niger, Aspergillus flavus, and Candida albicans [222]. Some antimicrobial bioactive compounds such as polygodial, norpiguisone, and lunularin have been isolated from Porella platyphylloidea (L.) Pfeiff., Conocephalum conicum (L.) Dumort, and Lunularia cruciate (L.) Dumort. ex Lindb.
Medicinal plants are still being used in domestic households for many infectious diseases. For instance, paste of Rheum emodi Wall. is used to cure abscesses and boils in many parts of the North-Western Himalayas, particularly in Kashmir Valley; a fermented product of Viola odorata L. is used to treat respiratory tract infections; and roots of Juglans regia L. are used to treat gum infections [19]. Despite the availability of modern antibiotics, many parts of the Himalayan region, particularly the tribal population, still practice and prefer herbal medicine over modern antibiotics. Although many plant species of Himalayan medicinal plants have been investigated for their antimicrobial activities, given the medicinal plant diversity of the Himalayas, extensive research is needed to explore the untapped reserve of phytochemicals produced by the medicinal plants. The phytochemicals could act as novel antimicrobials, antibiotic potentiators, or resistance breakers. Table 7 summarizes selected medicinal plants of the Indian Himalayas that have shown potency as novel antimicrobials.
Table 7.
List of plants found in the Indian Himalayan region and their reported antimicrobial bioactive compounds.
| Plant Name | Bioactive Compounds | Target Pathogen | References |
|---|---|---|---|
| Abrus precatorius L. | 6-propionyloxymethyl-4′,5,7-trihydroxyisoflavanone |
Bacillus cereus, Escherichia coli |
[223] |
| Abutilon theophrasti Medik. | Rutin, |
Salmonella enterica, Escherichia coli, Streptococcus pneumoniae, Staphylococcus aureus |
[224] |
| quercetin 7-o-β-glucoside, | |||
| kaempferol 3-o-α-rhamnopyranosyl (1→6)-β-glucopyranoside, | |||
| luteolin, | |||
| apigenin 7-o-β-diglucoside, | |||
| poncirin, | |||
| tiliroside | |||
| Achillea millefolium L. | Camphor, germacrene-d, (e)-nerolidol, sabinene, (e)-p-mentha-2,8-dien-1-ol, 1,8-cineole |
Salmonella typhimurium, Salmonella agona | [225] |
| Achyranthes aspera L. | Achyranthine, betaine, betanin, isobetanin | Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | [226] |
|
Aconitum violaceum Jacq. ex Stapf. |
Ethyl acetate fraction |
Escherichia coli, Shigella flexneri, Bacillus subtilis, Staphylococcus aureus |
[227] |
|
Aconitum heterophyllum Wall. ex Royle |
6-dehydroacetylsepaconitine, | Staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa | [228] |
| 13-hydroxylappaconitine, lycoctonine, | |||
| lappaconitine | |||
| Acorus calamus L. | Asarone | Aspergillus niger, Candida albicans | [229] |
|
Adiantum capillus-veneris L. |
3-p-coumaroylquinic acid, kaempferol 3-o-glucoside |
Staphylococcus aureus, Staphylococcus epidermidis, β-hemolytic Streptococcus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa | [230] |
|
Adiantum
pedatum |
Ethyl and acetone extracts | Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli | [231] |
|
Aegle marmelos (L.) Corrêa |
Limonene, β-ocimene, germacrene, α-phellandrene | Caenorhabditis elegans | [232] |
| Ageratum houstonianum Mill. | Ageratochromene, demothoxyageratochromene, β-caryophyllene | Micrococcus luteus, Rhodococcus rhodochrous | [233] |
| Ajuga parviflora Benth. | Ajugin A, ajugin B | Citrobacter sp., Pseudomonas aeruginosa | [234] |
|
Allamanda cathartica L. |
Silver nanoparticles of flower aqueous extracts | Salmonella typhimurium, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae | [235] |
|
Allium cepa L. |
Allicin | Salmonella typhimurium, Staphylococcus aureus, Escherichia coli | [236] |
|
Allium sativum L. |
Allicin, | Aspergillus versicolor, Penicillium citrinum, Burkholderia cepacia, Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Penicillium funiculosum, Candida albicans, Helicobacter pylori | [179] |
| diallyl sulfide, | |||
| diallyl disulfide, | |||
| diallyl trisulfide, | |||
| e/z-ajoene, | |||
| s-allyl-cysteine, | |||
| s-allyl-cysteine sulfoxide. | |||
|
Amaranthus caudatus L. |
Ferulic acid | Escherichia coli | [145] |
| Amaranthus viridis L. | Rutin, quercetin, spinosterol, amasterol | Staphylococcus aureus, Escherichia coli, c, Rhizopus oligosporus, Colletotrichum musae, Fusarium solani | [237] |
| Amomum subulatum Roxb. | 1,8-cineole, α-terpineol, α-pinene, β-pinene | Aspergillus niger | [238] |
| Angelica glauca Edgew. | β-phellandrene, (z)-ligustilide methyl octane, limonene, β-phellandrene, β-pinene, (z)-3-butylidene-phthalide, (z)-ligustilide, (e)-ligustilide, citronellyl acetate | Clostridium difficile, Clostridium perfringens, Enterococcus faecalis, Eubacterium limosum, Peptostreptococcus anaerobius, Candida albicans | [239] |
|
Arctium lappa L. |
Chlorogenic acid, caffeic acids |
Pseudomonas aeruginosa, Bacillus cereus |
[240] |
|
Arnebia benthamii (Wall. ex G.Don) I.M.Johnst. |
Shikonin, | Escherichia coli, Pseudomonas aeruginosa, Shigella flexneri, Klebsiella pneumoniae, Salmonella typhimurium, Staphylococcus aureus | [241] |
| alkanin hoslundal, | |||
| artemidiol, | |||
| ganoderiol, | |||
| 2-hexaprenyl-6-hydroxyphenol | |||
|
Artemisia dubia Wall. ex Besser |
Chrysanthenone, coumarin, camphor | Aspergillus niger | [242] |
| Artemisia indica Willd. | Isoascaridole, trans-p-mentha-2,8-dien-1-ol, trans-verbenol, artemisia ketone, germacrene B, borneol, cis-chrysanthenyl acetate, davanone, β-pinene. |
Bacillus subtilis, Staphylococcus epidermidis, Pseudomonas aeruginosa, Salmonella typhi, Klebsiella pneumoniae, Penicillium chrysogenum, Aspergillus niger | [242,243] |
|
Asparagus racemosus Willd. |
Catecholic tannin, saponin, gallic tannin |
Escherichia coli, Salmonella typhimurium, Bacillus subtilis, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, Enterococcus faecalis, Saccharomyces cerevisiae |
[244] |
|
Atropa acuminata Royle ex Lindl. |
Aqueous extract |
Bacillus Subtilis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhimurium, Staphylococcus aureus |
[245] |
|
Atropa bella-donna L. |
Ethanolic extracts |
Staphylococcus aureus, Escherichia coli |
[246] |
|
Bacopa monnieri (L.) Wettst. |
Luteolin | Staphylococcus aureus, Alternaria alternate, Fusarium acuminatum | [247] |
|
Baliospermum montanum (Willd.) Müll.Arg. |
Leaf (methanolic and aqueous extract), callus (acetone and ethanolic extract) |
Bacillus subtilis, Klebsiella pneumoniae, Staphylococcus aureus, Escherichia coli | [248] |
| Berberis lyceum Royle | Berberine |
Streptococcus agalactiae, Staphylococcus aureus, Streptococcus mutans, Streptococcus pyogenes, Corynebacterium diphtheriae |
[110] |
|
Bergenia ciliate (Haw.) Sternb. |
Pyrogallol, | Staphylococcus aureus, Bacillus subtilis, Bacillus megaterium, Escherichia coli, Serratia marcescens, Nocardia tenerifensis, Streptomyces sp., Aspergillus niger, Fusarium oxysporum | [249] |
| rutin, | |||
| morin, | |||
| bergenin, | |||
| catechin, | |||
| gallic acid | |||
| Betula utilis D.Don | Geranic acid, |
Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli |
[250] |
| β-seleneol, | |||
| β-linalool, | |||
| β-sesquiphellendrene, | |||
| champacol, | |||
| 1,8-cineol. | |||
|
Bidens biternate (Lour.) Merr. & Sherff |
Methanolic extract |
Escherichia coli, Klebsiella pneumoniae, Pseudomonas sp., Staphylococcus aureus, Staphylococcus epidermidis |
[251] |
| Blumea lacera (Burm.f.) DC. | Lachnophyllum ester, lachnophyllic acid, germacrene d, β-farnesene. | Staphylococcus aureus, Candida albicans, Aspergillus niger | [252] |
|
Bridelia retusa (L.) A.Juss. |
Ethanolic extract |
Pseudomonas aeruginosa, Escherichia coli |
[253] |
|
Calendula officinalis L. |
Selenium nanoparticles of methanolic extract of flowers |
Serratia marcescens, Enterobacter cloacae, Alcaligenes faecalis |
[254] |
| Calotropis procera (Aiton) W.T.Aiton | α-amyrin, lupeol acetate, phytol, hexadecanoic acid, stigmasterol, linolenic acid, gombasterol A | Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli | [255] |
|
Caltha palustris L. |
Methanolic extract |
Staphylococcus epidermidis, Proteus vulgaris |
[256] |
|
Cannabis sativa L. |
Cannabidiol (cannabinoids) | Staphylococcus aureus (MDR, MRSA), Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus pyogenes, Enterococcus faecium, Cutibacterium acnes, Clostridium difficile, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, Serratia marcescens, Proteus mirabilis, Salmonella typhimurium | [257] |
| Cassia fistula L. | Eugenol, phytol, camphor, linonene, salicyl alcohol, 4-hydroxybenzyl alcohol | Aspergillus niger, Candida albicans | [229] |
| Cassia tora L. | Elemol, linalool, palmitic acid | Bacillus cereus, Staphylococcus aureus | [229] |
| Cedrus deodara (Roxb. ex D.Don) G.Don | Wikstromal, matairesinol, dibenzylbutyrolactol, berating, isopimpillin, lignans 1, 4 diaryl butane, benzofuranoid neo lingam, isohemacholone, sesquiterpenes, deodarone, atlantone, deodarin, deodardione, limonenecarboxylic acid, α-himacholone, β-himacholone, α-pinene, β-pinene, myrcene, cedrin (6-methyldihydromyricetin), taxifolin, cedeodarin (6-methyltaxifolin), dihydromyricetin and cedrinoside | Escherichia coli | [258] |
| Chaerophyllum villosum Wall. ex DC. | γ-terpinene, p-cymene, carvacrol methyl ether, myristicin, thymol | Staphylococcus aureus, Streptococcus mutans, Candida albicans, Candida glabrata | [259] |
|
Chenopodium ambrosioides L. |
Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside) | Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, Paenibacillus apiarius, Paenibacillus thiaminolyticus | [260] |
|
Cichorium intybus L. |
Triterpenois, cichoridiol, intybusoloid, lupeol, fridelin, β- sitosterol, sigmasterol, betulinic acid, betunaldehyde, syringic acid, vanilic acid |
Pseudomonas aeruginosa, Staphylococcus aureus |
[260] |
| Cinnamomum glanduliferum (Wall.) Meisn. | 1,8-cineole, α-pinene, α-terpineol, germacrene d-4-ol, α-thujene | Micrococcus luteus, Escherichia coli, Pseudomonas aeruginosa, Aeromonas salmonicida | [261] |
| Cissampelos pareira L. | Bis-benzylisoquinoline, benzylisoquinoline, tropoloisoquinoline, aporphine, azafluoranthene, protoberberine | Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus vulgaris | [262] |
|
Convolvulus arvensis L. |
Butanolic extract | Staphylococcus aureus, Acinetobacter junii, Klebsiella pneumoniae, Acinetobacter baumannii, Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Salmonella dysenteriae, Vibrio cholera, Proteus mirabilis, Salmonella paratyphi, Serratia marcescens, Enterobacter cloacae | [263] |
|
Coriandrum sativum L. |
β-linalool (essential oil) |
Bacillus subtilis, Stenotrophomonas maltophilia |
[264] |
| Crocus sativus L. | Crocin, safranal semi-synthetic safranal derivatives |
Helicobacter pylori, Staphylococcus aureus, Listeria spp. Bacillus subtilis, Bacillus cereus, Salmonella enterica, Shigella dysenteriae, Escherichia coli |
[265] |
| Curcuma Longa L. | α-turmerone, β-turmerone, α-phellandrene, 1,8-cineole, p-cymene, terpinolene | Bacillus cereus, Staphylococcus aureus, Aspergillus niger | [266] |
| Cuscuta reflexa Roxb. | cis-3-butyl-4-vinylcyclopentane, limonene, (e)-nerolidol | Aspergillus niger | [267] |
|
Cymbopogon citratus (DC.) Stapf |
α-citral, | Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Bacillus subtilis | [268] |
| β-citral, | |||
| myrcene | |||
|
Cyperus rotundus L. |
α.-pinene, camphene, D-limonene, |
Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus (ARSA) |
[269,270] |
| camphenol, p-mentha-1,5-dien-8-ol, | |||
| thymol, myrtenal, carveol, copaene, | |||
| caryophyllene, naphthalene, | |||
| 1,6-dimethyl-4-(1-methylethyl). | |||
| Datura metel L. | Daturaolone |
Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, Staphylococcus epidermidis |
[271] |
|
Datura stramonium L. |
Chloroform extracts | Staphylococcus aureus | [272] |
|
Daucus carota L. |
Methylisoeugenol | Campylobacter jejuni | [273] |
|
Dioscorea bulbifera L. |
Bafoudiosbulbins, 2,7-dihydroxy-4-methoxyphenanthrene |
Escherichia coli, Enterobacter aerogenes, Klebsiella pneumoniae, Pseudomonas aeruginosa |
[274] |
| Dodecadenia grandiflora Nees | Germacrene D, furanodiene | Staphylococcus aureus, Pasteurella multocida | [275] |
| Dodonaea viscosa Jacq. | Hautriwaic acid, dodonoside B, dodonic acid, kaempferol, sakuranetin, dehydrohautriwaic acid, hautriwaic lactone, alizarin, penduletin, 3,5,7-trihydroxy-4′-methoxyflavone, isorhamnetin-3-rhamnosylgalactoside, donoside a, 5- hydroxy-3,6,7,4′-tetra methoxy flavone | Streptococcus pyogenes, Escherichia coli, Klebsiella pneumonia, Pseudomonas fluorescens, Staphylococcus aureus, Bacillus subtilis | [276] |
| Epimedium grandiflorum C. Morren | Hydroethanolic extract | Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Acinetobacter sp., Pseudomonas sp., Salmonella sp. | [277] |
|
Equisetum diffusum D. Don |
Aqueous extract |
Escherichia coli, Micrococcus luteus, Pseudomonas aeruginosa, Bacillus pumilus, Bacillus cereus, Bacillus licheniformis, Salmonella typhi, Streptococcus mutans |
[278] |
| Eupatorium adenophorum Spreng. | p-cymene, bornyl acetate, amorph-4-en-7-ol, camphene | Arthrobacter protophormiae, Escherichia coli, Micrococcus luteus, Rhodococcus rhodochrous, Staphylococcus aureus | [233] |
|
Euphorbia helioscopia L. |
Euphoheliosnoid E | Streptococcus mutans, Actinomyces viscosus | [279] |
|
Euphorbia wallichii Hook. f. |
Acorenone B, cycloisosativene, β-cedrene, copaene, 3β-hydroxy-5α-androstane, palmitic acid |
Staphylococcus aureus | [279] |
|
Foeniculum vulgare Mill. |
Dillapional | Bacillus subtilis | [280] |
|
Fritillaria roylei Hook. |
Peonidin |
Escherichia coli, Klebsiella pneumoniae, Micrococcus luteus, Staphylococcus pneumonia, Haemophilus influenza, Neisseria mucosa |
[281] |
| Fumaria indica (Hausskn.) Pugsley | n-octacosan-7β-ol |
Leishmania donovani promastigotes, Staphylococcus epidermidis, Escherichia coli, Candida albicans, Aspergillus niger |
[282] |
| Galium aparine L. | Chlorogenic acid, p-coumaric acid, ferulic acid, luteolin, rutin | Staphylococcus aureus, Listeria monocytogenes | [283] |
| Gentiana kurroo Royle | Flavonoids and phenols |
Proteus mirabilis, Streptococcus faecalis, Escherichia coli, Salmonella enteritidis, Micrococcus luteus, Enterobacter cloacae |
[284] |
|
Geranium wallichianum D.Don ex Sweet |
Leaf extracts conjugated with zinc oxide nanoparticles |
Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae |
[285] |
|
Girardinia diversifolia (Link) Friis |
β-sitosterol, 3-hydroxystigmast-5-en-7-one, 7-hydroxysitosterol |
Bacillus pumilus, Escherichia coli, Staphylococcus aureus |
[286] |
| Gaultheria fragrantissima Wall. | Methyl salicylate | Staphylococcus aureus | [259] |
|
Hedychium spicatum G.Lodd. |
Hedychenone, spicatanol, 6-endo-hydroxycineole |
Shigella boydii, Shigella sonnei, Shigella flexneri, Bacillus cereus, Vibrio cholera, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae | [287,288] |
|
Holarrhena antidysenterica Wall. |
Conessine |
Acinetobacter baumannii, Pseudomonas aeruginosa |
[120] |
|
Hyoscyamus niger L. |
Non-alkaloidal seed extract |
Bacillus subtilis, Escherichia coli, Staphylococcus aureus |
[289] |
|
Hypericum perforatum L. |
Hypericin | Methicillin-resistant Staphylococcus aureus, methicillin-sensitive Staphylococcus aureus, Escherichia coli | [290] |
| Inula cappa (Buch.-Ham. ex D.Don) DC. | β-caryophyllene, cis-dihydro-mayurone, β-bisabolene, (E)-β-farnesene | Enterococcus faecalis, Klebsiella pneumoniae, Xanthomonas phaseoli and Bacillus subtilis | [291] |
|
Inula racemose Hook.f. |
Isoalantolactone | Bacillus subtilis, Escherichia coli, Pseudomonas fluorescens, Staphylococcus lentus, Staphylococcus aureus | [292] |
| Iris ensata Thunb. | Methanolic extracts | Bacillus cereus, Pseudomonas aeruginosa, Proteus vulgaris, Escherichia coli | [293] |
|
Iris kashmiriana Baker |
Irigenin, iridin, junipeginin-c, |
Bacillus subtilis, Staphylococcus epidermidis, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus typhimurium, Escherichia coli, Shigella dysenteriae, Klebsiella pneumoniae |
[293] |
| resveratrol, piecid, resveratroloside, | |||
| isorhamnetin-3-oneohesperidoside | |||
|
Iris nepalensis D.Don |
Methanolic extract |
Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa |
[294] |
|
Jasminum officinale L. |
Ethanolic extract | Methicillin-resistant Staphylococcus aureus | [295] |
| Juglans regia L. | α-pinene, β-pinene, β-caryophyllene, germacrene d, limonene, eugenol, methyl salicylate, germacrene d, (e)-β-farnesene | Bacillus subtilis, Staphylococcus epidermidis, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella typhi, Escherichia coli, Shigella dysenteriae, Klebsiella pneumoniae | [296] |
| Juniperus macropoda Boiss. | Sabinene, terpinen-4-ol, cedrol, β-elemene, trans-sabinene hydrate, α-cubebene, α-thujone, biformene | Candida albicans, Colletotrichum fragariae, Colletotrichum gloeosporioides | [297] |
| Lagenaria siceraria (Molina) Standl. | β-carotene, 22-deoxocurcubitacin-d, 22-deoxoisocurcubitacin D, avenasterol, codisterol, elesterol, isofucasterol, stigmasterol, sitosterol, compesterol, spinasterol, 7-0-glucosyl-6-c-glucoside apigenin, 6-c-glucoside apigenin, 6-cglucoside luteolin, 7,4′-o-diglucosyl- 6-c-glucoside, apigenin | Staphylococcus aureus, Pseudomonas sp., Escherichia coli, Bacillus subtilis, Candida sp., Aspergillus niger | [298] |
| Lantana camara (Hayek) R.W.Sanders | Germacrene B, β-caryophyllen, 3,7,11-trimethyl-1,6,10-dodecatriene, β-caryophyllene, zingiberene, γ-curcumene, davanone, (E)-nerolidol | Arthrobacter protophormiae, Micrococcus luteus, Rhodococcus rhodochrous, Staphylococcus aureus | [233] |
|
Lavandula stoechas L. |
1,8-cineole, | Methicillin-resistant Staphylococcus aureus, Klebsiella pneumoniae, Salmonella typhimurium |
[299] |
| fenchone, | |||
| camphor | |||
| Lindera neesiana (Wall. ex Nees) Kurz | Geranial, neral, citronellal, 1,8-cineole, α-pinene, β-pinene, methyl chavicol, safrole | Staphylococcus aureus, Candida albicans | [300] |
| Lindera pulcherrima (Nees) Benth. ex Hook.f. | Curzerenone, furanodienone | Staphylococcus aureus, Salmonella enterica | [275] |
| Mallotus philippensis (Lam.) Müll.Arg. | Bergenin, mallotophilippinens, rottlerin, and isorottlerin | Bacillus cereus var mycoides, Bacillus pumilus, Bacillus subtilis, Bordetella bronchiseptica, Micrococcus luteus, Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, Candida albicans, Saccharomyces cerevisiae | [301,302] |
| Malva neglecta Wallr. | Hydrotyrosol, coumaroylhexoside, kaempferol-3-(p-coumaroyldiglucoside)-7-glucoside, quercetin-3-o-rutinoside, epicatechin-3-o-(4-o-methyl)-gallate, oleic acid, taurine, ethylene dimercaptan, isoeugenol, patchoulane, methyl 12-methyltetradecanoate, isopropyl myristate | Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Salmonella typhi, Bacillus subtilis, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Fusarium solani | [303] |
|
Marrubium vulgare L. |
Methanolic extract |
Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Proteus vulgaris, Candida albicans |
[304] |
| Melia azedarach L. | Crude extract | Bacillus subtilis, Proteus mirabilis, Shigella flexneri, Proteus mirabilis, Shigella flexneri, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Shigella flexneri | [305] |
| Morina longifolia Wall. ex DC. | Germacrene d, α-pinene, bicyclogermacrene, α-cadinol, (e)-citronellyl tiglate, β-phellandrene | Escherichia coli, Staphylococcus aureus, Proteus vulgaris, Klebsiella pneumoniae, Bacillus subtilis, Pseudomonas aeruginosa, Alternaria alternata, Aspergillus flavus, Aspergillus fumigatus, Fusarium solani | [306,307] |
|
Nepeta cataria L. |
Nepetalactone, |
Neisseria subflava, Citrobacter freundii, Branhamella ovis, Aeromonas caviae, Escherichia coli, Serratia marcescens, Enterococcus species, Staphylococcus aureus |
[308,309] |
| β-caryophyllene, | |||
| thymol | |||
| Nardostachys jatamansi (D.Don) DC. | β-gurjunene, valerena-4,7(11)-diene (7.1%), nardol a, 1(10)-aristolen-9β-ol, jatamansone | Bacillus cereus, Escherichia coli, Candida albicans | [310] |
|
Oxalis corniculate L. |
Methanolic extract |
Staphylococcus aureus, Escherichia coli, Shigella dysenteriae, Shigella flexneri, Shigella boydii, Shigella sonnei |
[311] |
| Paeonia emodi Royle | Leaf extract nanoparticles |
Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Klebsiella pneumoniae |
[312] |
| Phoebe lanceolata (Nees) Nees | 1,8-cineole, β-caryophyllene | Escherichia coli | [275] |
| Persicaria hydropiper (L.) Delarbre | Confertifolin, polygodial | Enterococcus faecalis, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Salmonella enterica, Epidermophyton floccosum, Curvularia lunata, Scopulariopsis sp., Candida albicans, Candida utilis, Candida krusei, Cryptococcus neoformans, Saccharomyces cerevisiae, Epidermophyton floccosum, Trichophyton mentagrophytes, Penicillium marneffei | [313] |
| Plantago lanceolata L. | Luteolin 7-glucoside, hispidulin 7-glucuronide, luteolin 7-diglucoside, apigenin 7-glucoside, nepetin 7-glucoside and luteolin 6-hydroxy 4′-methoxy 7-galactoside, oleanolic acid, sitosterol acid, 18β-glycyrrhetinic, plantamajoside, verbacoside, 10-hydroxymajoroside, 10-acetoxymajoroside | Bacillus subtilis, Staphylococcus aureus, Candida albicans, Candida tropicalis, Escherichia coli, Streptococcus pneumoniae | [314] |
| Podophyllum hexandrum Royle | Phthalic acid, |
Bacillus megaterium, Pseudomonas aeruginosa, Aspergillus flavus, Fusarium solani, Staphylococcus aureus, Salmonella typhi, Klebsiella pneumoniae, Enterococcus faecalis |
[315,316] |
| di-isobutyl ester, | |||
| 1,2-benzenedicarboxylic acid, | |||
| diisooctyl ester, | |||
| polyneuridine, | |||
| podophyllotoxin, | |||
| β-sitosterol | |||
|
Punica granatum L. |
Punicalagin |
Pseudomonas aeruginosa, Salmonella enteritidis, Escherichia coli, Staphylococcus epidermidis, Staphylococcus xylosus, Staphylococcus aureus, Bacillus cereus, Enterococcus faecium, Enterococcus faecalis |
[317] |
|
Prunus domestica L. |
Quercetin-3-o-galactoside |
Campylobacter jejuni, Salmonella typhimurium, Escherichia coli, Staphylococcus aureus, Listeria monocytogenes |
[318] |
|
Rheum emodi Wall. |
Emodin, rhein, chrysophanol dimethyl ether, resveratrol, revandchinone-4 |
Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Bacillus subtilis, Pseudomonas aeruginosa, Klebsiella aerogenes, Bacillus sphaericus, Chromobacterium violaceum |
[319] |
| Rhododendron anthopogon D.Don | α-pinene, β-pinene, limonene, δ-cadinene | Bacillus subtilis, Mycobacterium tuberculosis, Candida pseudotropicalis | [320] |
| Rumex dentatus L. | Musizin, torachrysone-glucoside, 2-methoxystypandrone |
Escherichia coli, Klebsiella pneumoniae, Salmonella typhi, Pseudomonas aeruginosa, Bacillus subtilis, Streptococcus pneumoniae, Listeria monocytogenes, Staphylococcus epidermidis, Staphylococcus aureus, Bacillus cereus |
[321] |
| Salix alba L. | Anthocyanins, p-hydroxybenzoic, gallic acid, gentisic acid, sisymbrifolin, catechol |
Pseudomonas aeruginosa, Escherichia coli, Salmonella enterica, Staphylococcus aureus | [322] |
| Salvia sclarea L. | Essential oil |
Escherichia coli, Staphylococcus aureus, Methicillin-resistant Staphylococcus epidermidis |
[323] |
| Sambucus wightiana Wall. ex Wight & Arn. | Gold nanoparticles of whole-plant extract | Escherichia coli, Staphylococcus epidermidis, Salmonella enteritidis | [324] |
| Saussurea lappa (Decne.) Sch.Bip. | Sesquiterpene lactones, zinc oxide nanoparticles of rhizome methanolic extract |
Staphylococcus aureus, Sphingobacterium thalpophilum, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Sphingobacterium sp., Acinetobacter sp., Ochrobactrum sp. |
[325] |
| Skimmia laureola (DC.) Decne. | Linalyl acetate, linalool, limonene, α-terpineol, geranyl acetate | Staphylococcus aureus, Staphylococcus epidermidis, Aspergillus niger, Penicillium chrysogenum | [326] |
| Solanum tuberosum L. | Potide-g, afp-j, potamin-1 or pg-2 |
Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, Candida albicans |
[327] |
| Sonchus arvensis L. | Phenols and flavonoids | Escherichia coli, Salmonella enterica, Vibrio parahaemolyticus, Staphylococcus aureus | [328] |
| Stephania glabra (Roxb.) Miers | Glabradine | Staphylococcus aureus, Streptococcus. mutans, Microsporum gypseum, Microsporum canis, Trichophyton rubrum | [329] |
| Taraxacum officinale F.H.Wigg. | 9-hydroxyoctadecatrienoic acid, 9-hydroxyoctadecadienoic acid, vanillin, coniferaldehyde, p-methoxyphenylglyoxylic acid | Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus, Bacillus cereus | [330] |
| Terminalia arjuna (Roxb. ex DC.) Wight & Arn. | Silver nanoparticles of bark extract | Escherichia coli | [331] |
| Terminalia chebula Retz. | 1,2,6-tri-o-galloyl-β-d-glucopyranose |
Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, Proteus mirabilis, Acinetobacter baylyi, Bacillus megaterium |
[332,333,334] |
| Valeriana jatamansi (D.Don) Wall. | Maaliol, 3-methylvaleric acid, β-gurjunene | Microsporum canis, Fusarium solani | [335] |
|
Verbascum Thapsus L. |
1-hexzanol 2-hexene |
Klebsiella pneumoniae, Staphylococcus aureus, Escherichia coli, Mycobacterium phlei, methicillin-resistant Staphylococcus aureus | [336] |
|
Viola odorata L. |
3-(2′,4′,6′,6′-tetramethylcyclohexa-1′,4′-dienyl) acrylic acid |
Haemophilus influenzae, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae |
[337] |
|
Viscum album L. |
Hydroxycinnamic acids |
Xanthomonas campestris, Clavibacter michiganensis, Alternaria alternate, Fusarium oxysporum |
[338] |
| Vitex negundo L. | Methanolic extract | Vibrio cholerae, Vibrio parahaemolyticus, Vibrio mimicus, Escherichia coli, Shigella sp., Aeromonas sp. | [339] |
6. Challenges of Using Phytochemicals as Medicine
6.1. Effects of Climate Change
The productivity of medicinal plants is susceptible to changes in climatic conditions, leading to varied responses among different species. This could result in decreased biomass production along with changes in the production of secondary metabolites, thus impacting the quality and safety of herbal medicinal products [340]. Abiotic stresses such as extreme temperatures, increased CO2 concentration, and drought conditions influence secondary metabolite production [341]. Since the production of phytochemicals greatly depends on the physiological condition of a plant, the plant response to any of these stress conditions would therefore result in the increase or decrease in the production of phytochemicals from its normal value and hence the compromised efficacy and safety of the prepared herbal formulation [342]. Climate change can severely affect the composition as well as the production of secondary metabolites by the plant, which in the long term may compromise or altogether abrogate the medicinal value of the plant. Further, climate change along with unsustainable harvesting practices can drive plant species to extinction. Therefore, in order to minimize the long-term effects of climate change, conservation efforts, sustainable harvesting practices, preservation of traditional knowledge, and climate change mitigation measures are highly recommended [340].
6.2. Toxicity of Herbal Medicine
While herbal medicines are generally considered safer than synthetic counterparts, they can still pose cellular toxicity risks and cause adverse effects on the human system. Toxicity issues may arise from the specific effects of certain phytochemicals in addition to the issues of self-medication, unqualified practitioners, sub-standard products, and improper dosages [343]. Different toxicity issues have been reported with herbal formulations, such as nephrotoxicity induced by a diterpenoid epoxide produced by Tripterygium wilfordii Hook., renal calcification caused by Guaiacum officinale L., and Arctostaphylos uva-ursi L. [344]. Neurotoxicity has been observed with plant species such as Catharanthus roseus (L.) G. Don, Papaver somniferum L., and Cannabis indica Mure [345,346]. Cardiotoxicity has been reported with plants such as Ephedra distachya L., Mandragora officinarum L., and Aconitum napellus L., [347]. Similarly, hepatotoxicity has been associated with Teucrium chamaedrys L., Scutellaria baicalensis Georgi, and Larrea tridentata (DC.) [348].
Apart from inherent toxicity issues, related contamination could occur during product development, which includes heavy metal and microbial contamination, plant misidentification, and economically motivated adulteration [349]. Therefore, to assess the safety of herbal formulations, different techniques could be employed such as predictive toxicology approaches involving in silico modelling. Omics approaches for toxicology studies including toxicogenomics, toxicometabolomics, and toxicoproteomics could be used for early prediction of toxicity in product development [349].
6.3. Other Challenges and Regulation
The introduction of phytochemicals in modern medicine poses a number of challenges in terms of standardizing plant extracts to ensure the consistent quality of the end product. The identification and isolation of bioactive compounds, followed by the elucidation of their mechanisms of action, is yet another challenging task due to the presence of diverse compounds in them. Different phytochemicals can interact to work in synergy to bring about their antimicrobial effects, an understanding of which will be crucial in the development of combination therapies. Insufficient scientific evidence and intellectual property protection pose additional hindrances to the integration of herbal formulations in modern medicine. Most of these challenges could be overcome by creating proper checks and balances in the form of comprehensive regulatory bodies, quality control measures, conduction of clinical trials, intellectual property protection, and integration of traditional knowledge with scientific advancements [350].
To set forth a proper safety assessment plan and regulatory laws for manufacturing herbal medicine, different regulatory bodies around the globe such as the International Life Sciences Institute, Washington, DC, USA; International Union of Pure and Applied Chemistry, North Carolina, USA; European Medicines Agency, Amsterdam, The Netherlands; and European Food Safety Authority, Parma, Italy, have issued guidance documents for the assessment of safety of herbal medicine [351]. In the USA, the sale and purchase of herbal medicines is regulated by the Dietary Supplement Health and Education Act of 1994 [352]. In the European Union, the production and marketing of herbal drugs are regulated by various national regulatory bodies such as the Committee on Herbal Medicinal Products (HMPC), which is a part of the European Medicines Agency [349]. Similarly, Canada has Natural Health Products Regulations (NHPR), and Australia has the Therapeutic Goods Administration (TGA) as the regulatory authority to assess and ensure the manufacturing and marketing of herbal drugs [353,354]. In India, the Ministry of AYUSH is the regulatory authority, which provides licenses for the manufacturing and marketing of herbal drugs [355].
7. Methodology
In the present study, three databases, namely PubMed, DOAJ, and Google Scholar, were searched by using specific keywords: “Antimicrobial resistance”, “plant antimicrobials”, and “Himalayan medicinal plants”. Collectively, a total number of 4878 articles, both reviews as well as original research articles, published from the year 1940 to 2023, were identified. The selection of articles was rigorously conducted as per the focus of the review article, and only those articles published in peer-reviewed journals were included in the study to ensure the quality of the work.
Furthermore, the chemical structures and formulae of phytocompounds were sourced from PubChem by using their common as well as IUPAC names (wherever necessary), and their respective PubChem IDs were assigned to the compounds in the tabular form. The botanical names of the plants mentioned in the study have been cross-verified with the International Plant Names Index (IPNI).
8. Conclusions and Future Prospects
Since the advent of antibiotics, the development of antibiotic resistance and subsequent pursuit of novel antimicrobials has always been a run-and-chase scenario. The discovery of every antibiotic was followed by its resistance development in bacteria. To overcome the resistance against antimicrobials, several anti-resistance measures were taken up; one of the approaches was to develop inhibitors. Clavulanic acid, produced by Streptomyces clavuligerus, though far less effective as an antimicrobial agent by itself, proved to be very efficient in augmenting the activity of antibiotics in combination therapies against bacteria producing serine β-lactamases. However, the inefficiency of clavulanic acid in inhibiting metallo-β-lactamases made it a weak option for overcoming the multifaceted mechanisms of antimicrobial resistance. Plant secondary metabolites, on the other hand, are structurally and functionally dynamic entities that have shown broad-spectrum antimicrobial activities.
This study summarizes the timeline of antibiotic discovery and the subsequent emergence of resistance among microbes, along with the mechanistic details of various resistance determinants expressed by multidrug-resistant pathogens. This study further shows the need to explore plants as alternative sources of antimicrobials and sums up the mechanistic effects of various phytochemicals on microbial strains, including those expressing resistance phenotypes. Plant phytochemicals have shown multifaceted effects on microbial cells, such as alkaloids imposing their antimicrobial effects by inhibiting efflux pumps, nucleic acid synthesis, enzyme activities, ATP synthesis, cell-to-cell communication, cell wall biosynthesis, and jeopardizing the cell division machinery. Phenols act as antimicrobials by inhibiting metabolically important enzymes, efflux pumps, and cell wall biosynthesis; inactivating enzymes such as DNA gyrase and penicillinases; and increasing the membrane permeability, eventually leading to cell death. Organosulfur compounds inhibit nucleic acid synthesis and act as potent enzyme inhibitors, while terpenes mostly exert their action by compromising the membrane integrity of microbial cells.
Plant-based antimicrobials have attracted significant attention due to the reduced potency and increasing toxicity of synthetic antimicrobials. Plant-based antimicrobial formulations have emerged as a boon in medical sciences, as they are easily available and have almost no side effects. The limited target specificity of existing synthetic antibiotics can be overcome by the broad-spectrum antibacterial action of phytochemicals. The poor adaptability of bacteria, fungi, and viruses to a plant-based antimicrobial regime might be the reason for the efficacy of herbal treatment against diseases. Therefore, synergistic combinations of synthetic antimicrobials and chemically defined phytochemicals will not only help to deal with global antimicrobial resistance but will also assure no further resistance development. This study provides a comprehensive overview of the medicinal plants of the Indian Himalayan region. The region, as discussed, possesses a mammoth repository of medicinal plants. The Indian Himalayan region happens to be one of the world’s 36 biodiversity hotspots. With such boundless plant resources available, the tribal communities in particular have developed traditional ways to use these plants for medicinal purposes. However, detailed studies deciphering the medicinal importance of these plants in the region have been scarce. In this study, 123 plants, native to the Himalayan region, with antimicrobial properties have been described along with their reported bioactive compounds and the target pathogens against which they are active. However, details on their mechanistic roles as antimicrobials are not available in every case where the effects of their crude extracts have been described. Further research is required on the identification of active substances and the underlying mechanisms of action, and efficacy analysis during in vivo applications is highly necessary to assist the pursuance of potent antimicrobials. This not only will allow the development of novel plant-based antimicrobials but also opens up the possibilities for developing combination therapies as multitarget solutions, which will greatly help to combat antimicrobial resistance in bacterial strains.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group research project under grant number (RGP2/90/44). The authors extend their gratitude to their fellow colleagues, who helped in improving the contents of this manuscript.
Author Contributions
Conceptualization, M.V.A., S.P. and S.A.; writing—original draft preparation, M.V.A., M.A.H.K. and S.A.; writing—review and editing, S.P., A.A.S., M.J., H.W.S.A., S.S. and S.A.; visualization, M.V.A. and M.A.H.K.; supervision, A.A.S. and S.A.; project administration, S.A.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group research project under grant number (RGP2/90/44).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Aminov R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010;1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen S.B. Drugs that changed society: History and current status of the early antibiotics: Salvarsan, sulfonamides, and β-lactams. Molecules. 2021;26:6057. doi: 10.3390/molecules26196057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro da Cunha B., Fonseca L.P., Calado C.R.C. Antibiotic discovery: Where have we come from, where do we go? Antibiotics. 2019;8:45. doi: 10.3390/antibiotics8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchings M.I., Truman A.W., Wilkinson B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019;51:72–80. doi: 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Cook M.A., Wright G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022;14:eabo7793. doi: 10.1126/scitranslmed.abo7793. [DOI] [PubMed] [Google Scholar]
- 6.Uddin T.M., Chakraborty A.J., Khusro A., Zidan B.R.M., Mitra S., Bin Emran T., Dhama K., Ripon K.H., Gajdács M., Sahibzada M.U.K., et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health. 2021;14:1750–1766. doi: 10.1016/j.jiph.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Said M.S., Saleem I., Hashmi A.M., Ullah I., Khan A.H. Rational use of antibiotics and requisition of pharmacist. Int. J. Nat. Med. Health Sci. 2022;1:21–24. doi: 10.52461/ijnms.v1i3.916. [DOI] [Google Scholar]
- 8.Aslam B., Khurshid M., Arshad M.I., Muzammil S., Rasool M., Yasmeen N., Shah T., Chaudhry T.H., Rasool M.H., Shahid A., et al. Antibiotic resistance: One health one world outlook. Front. Cell. Infect. Microbiol. 2021;11:1153. doi: 10.3389/fcimb.2021.771510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samreen, Ahmad I., Malak H.A., Abulreesh H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021;27:101–111. doi: 10.1016/j.jgar.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Kongkham B., Prabakaran D., Puttaswamy H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia. 2020;147:104762. doi: 10.1016/j.fitote.2020.104762. [DOI] [PubMed] [Google Scholar]
- 11.Larsson D.G.J., Flach C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022;20:257–269. doi: 10.1038/s41579-021-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manesh A., Varghese G.M. CENDRIC Investigators and Collaborators. Rising antimicrobial resistance: An evolving epidemic in a pandemic. Lancet Microbe. 2021;2:419–420. doi: 10.1016/S2666-5247(21)00173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaou N., Stavropoulou E., Voidarou C., Tsakris Z., Rozos G., Tsigalou C., Bezirtzoglou E. Interactions between medical plant-derived bioactive compounds: Focus on antimicrobial combination effects. Antibiotics. 2022;11:1014. doi: 10.3390/antibiotics11081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arsalan H.M., Javed H., Farheen N. Antibacterial activity of some medicinal plants against human pathogens. Int. J. Nat. Med. Health Sci. 2022;1:82–88. doi: 10.52461/ijnms.v1i2.855. [DOI] [Google Scholar]
- 15.Anand U., Jacobo-Herrera N., Altemimi A., Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9:258. doi: 10.3390/metabo9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhetri D.R. Medicinal Plants of the Himalaya: Production Technology and Utilization. 1st ed. Agrobios; Jodhpur, India: 2015. [Google Scholar]
- 17.Shiva M.P. Inventory of Forest Resources for Sustainable Management & Biodiversity Conservation with Lists of Multipurpose Tree Species Yielding Both Timber & Non-Timber Forest Products (Ntfps), and Shrub & Herb Species of Ntfp Importance. 1st ed. Indus Publishing Company; New Delhi, India: 1998. [Google Scholar]
- 18.Singh D.K., Pusalkar P.K. Floristic diversity of the Indian Himalaya. In: Dar G.H., Khuroo A.A., editors. Biodiversity of the Himalaya: Jammu and Kashmir State. Volume 18. Springer; Singapore: 2020. pp. 93–126. [Google Scholar]
- 19.Bhat M.N., Singh B., Surmal O., Singh B., Shivgotra V., Musarella C.M. Ethnobotany of the Himalayas: Safeguarding medical practices and traditional uses of Kashmir regions. Biology. 2021;10:851. doi: 10.3390/biology10090851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham E.P., Chain E. An enzyme from bacteria able to destroy penicillin. Nature. 1940;146:837. doi: 10.1038/146837a0. [DOI] [PubMed] [Google Scholar]
- 21.Woodruff H.B., Selman A. Waksman, winner of the 1952 Nobel prize for physiology or medicine. Appl. Environ. Microbiol. 2014;80:2–8. doi: 10.1128/AEM.01143-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F., Myers A.G. Development of a platform for the discovery and practical synthesis of new tetracycline antibiotics. Curr. Opin. Chem. Biol. 2016;32:48–57. doi: 10.1016/j.cbpa.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Wiest D.B., Cochran J.B., Tecklenburg F.W. Chloramphenicol toxicity revisited: A 12-year-old patient with a brain abscess. J. Pediatr. Pharmacol. Ther. 2012;17:182–188. doi: 10.5863/1551-6776-17.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee A.S., de Lencastre H., Garau J., Kluytmans J., Malhotra-Kumar S., Peschel A., Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers. 2018;4:18033. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 25.Christaki E., Marcou M., Tofarides A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Molecular Evolution. 2020;88:26–40. doi: 10.1007/s00239-019-09914-3. [DOI] [PubMed] [Google Scholar]
- 26.Munita J.M., Arias C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016;4:481–511. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shriram V., Khare T., Bhagwat R., Shukla R., Kumar V. Inhibiting bacterial drug efflux pumps via phyto-therapeutics to combat threatening antimicrobial resistance. Front. Microbiol. 2018;9:2990. doi: 10.3389/fmicb.2018.02990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du D., Wang-Kan X., Neuberger A., van Veen H.W., Pos K.M., Piddock LJ V., Luisi B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018;16:523–539. doi: 10.1038/s41579-018-0048-6. [DOI] [PubMed] [Google Scholar]
- 29.Putman M., van Veen H.W., Konings W.N. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 2000;64:672–693. doi: 10.1128/MMBR.64.4.672-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J., Deng Z., Yan A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014;453:254–267. doi: 10.1016/j.bbrc.2014.05.090. [DOI] [PubMed] [Google Scholar]
- 31.Poole K. Efflux-Mediated Multiresistance in Gram-Negative Bacteria. Clin. Microbiol. Infect. 2004;10:12–26. doi: 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 32.Stavri M., Piddock L.J.V., Gibbons S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007;59:1247–1260. doi: 10.1093/jac/dkl460. [DOI] [PubMed] [Google Scholar]
- 33.Lee E.W., Huda M.N., Kuroda T., Mizushima T., Tsuchiya T. EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis. Antimicrob. Agents Chemother. 2003;47:3733–3738. doi: 10.1128/AAC.47.12.3733-3738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendlandt S., Kadlec K., Feßler A.T., Schwarz S. Identification of ABC transporter genes conferring combined pleuromutilin–lincosamide–streptogramin A resistance in bovine methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococci. Vet. Microbiol. 2015;177:353–358. doi: 10.1016/j.vetmic.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Kaatz G.W., McAleese F., Seo S.M. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob. Agents Chemother. 2005;49:1857–1864. doi: 10.1128/AAC.49.5.1857-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida H., Bogaki M., Nakamura S., Ubukata K., Konno A.M. Nucleotide sequence and characterization of the Staphylococcus aureus NorA gene, which confers resistance to quinolones. J. Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Littlejohn T.G., Paulsen I.T., Gillespie M.T., Tennent J.M., Midgley M., Jones I.G., Purewal A.S., Skurray R.A. Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 1992;95:259–265. doi: 10.1111/j.1574-6968.1992.tb05376.x. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa W., Minato Y., Dodan H., Onishi M., Tsuchiya T., Kuroda T. Characterization of MATE-type multidrug efflux pumps from Klebsiella pneumoniae MGH78578. PLoS ONE. 2015;10:e0121619. doi: 10.1371/journal.pone.0121619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srinivasan V.B., Singh B.B., Priyadarshi N., Chauhan N.K., Rajamohan G. Role of novel multidrug efflux pump involved in drug resistance in Klebsiella Pneumoniae. PLoS ONE. 2014;9:e96288. doi: 10.1371/journal.pone.0096288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen L.H., Johannesen E., Burmølle M., Sørensen A.H., Sørensen S.J. Plasmid-encoded multidrug efflux pump conferring resistance to olaquindox in Escherichia coli. Antimicrob. Agents Chemother. 2004;48:3332–3337. doi: 10.1128/AAC.48.9.3332-3337.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada U., Yamashita E., Neuberger A., Morimoto M., van Veen H.W., Murakami S. Crystal Structure of tripartite-type ABC transporter MacB from Acinetobacter baumannii. Nat. Commun. 2017;8:1336. doi: 10.1038/s41467-017-01399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su X.Z., Chen J., Mizushima T., Kuroda T., Tsuchiya T. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob. Agents Chemother. 2005;49:4362–4364. doi: 10.1128/AAC.49.10.4362-4364.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roca I., Marti S., Espinal P., Martínez P., Gibert I., Vila J. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009;53:4013–4014. doi: 10.1128/AAC.00584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coyne S., Rosenfeld N., Lambert T., Courvalin P., Périchon B. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010;54:4389–4393. doi: 10.1128/AAC.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajamohan G., Srinivasan V.B., Gebreyes W.A. Novel role of Acinetobacter baumannii RND efflux transporters in mediating decreased susceptibility to biocides. J. Antimicrob. Chemother. 2009;65:228–232. doi: 10.1093/jac/dkp427. [DOI] [PubMed] [Google Scholar]
- 46.Poole K., Gotoh N., Tsujimoto H., Zhao Q., Wada A., Yamasaki T., Neshat S., Yamagishi J.-I., Li X.-Z., Nishino T. Overexpression of the MexC-MexD-OprJ efflux operon in NfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 47.Köhler T., Pechère J.-C., Plésiat P. Bacterial antibiotic efflux systems of medical importance. Cell. Mol. Life Sci. 1999;56:771–778. doi: 10.1007/s000180050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi N., Nishino K., Yamaguchi A. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 2001;183:5639–5644. doi: 10.1128/JB.183.19.5639-5644.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishino K., Latifi T., Groisman E.A. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2006;59:126–141. doi: 10.1111/j.1365-2958.2005.04940.x. [DOI] [PubMed] [Google Scholar]
- 50.Cattoir V., Poirel L., Nordmann P. Plasmid-mediated quinolone resistance pump QepA2 in an Escherichia coli isolate from France. Antimicrob. Agents Chemother. 2008;52:3801–3804. doi: 10.1128/AAC.00638-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swick M.C., Morgan-Linnell S.K., Carlson K.M., Zechiedrich L. Expression of multidrug efflux pump genes AcrAB-TolC, MdfA, and NorE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance. Antimicrob. Agents Chemother. 2011;55:921–924. doi: 10.1128/AAC.00996-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lennen R.M., Politz M.G., Kruziki M.A., Pfleger B.F. Identification of transport proteins involved in free fatty acid efflux in Escherichia coli. J. Bacteriol. 2013;195:135–144. doi: 10.1128/JB.01477-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuldiner S. EmrE, a model for studying evolution and mechanism of ion-coupled transporters. Biochim. Et Biophys. Acta Proteins Proteom. 2009;1794:748–762. doi: 10.1016/j.bbapap.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 54.Beketskaia M.S., Bay D.C., Turner R.J. Outer membrane protein OmpW participates with small multidrug resistance protein member EmrE in quaternary cationic compound efflux. J. Bacteriol. 2014;196:1908–1914. doi: 10.1128/JB.01483-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005;57:1451–1470. doi: 10.1016/j.addr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Tooke C.L., Hinchliffe P., Bragginton E.C., Colenso C.K., Hirvonen V.H.A., Takebayashi Y., Spencer J. β-Lactamases and β-Lactamase inhibitors in the 21st Century. J. Mol. Biol. 2019;431:3472–3500. doi: 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khare T., Anand U., Dey A., Assaraf Y.G., Chen Z.-S., Liu Z., Kumar V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021;12:720726. doi: 10.3389/fphar.2021.720726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonomo R.A. β-Lactamases: A focus on current challenges. Cold Spring Harb. Perspect. Med. 2017;7:a025239. doi: 10.1101/cshperspect.a025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall B.G., Barlow M. Revised ambler classification of β-lactamases. J. Antimicrob. Chemother. 2005;55:1050–1051. doi: 10.1093/jac/dki130. [DOI] [PubMed] [Google Scholar]
- 60.Bush K. Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 2018;62:e01076-18. doi: 10.1128/AAC.01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bush K. Proliferation and significance of clinically relevant β-lactamases. Ann. N. Y. Acad. Sci. 2013;1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 62.Orencia M.C., Yoon J.S., Ness J.E., Stemmer W.P.C., Stevens R.C. Predicting the emergence of antibiotic resistance by directed evolution and structural analysis. Nat. Struct. Biol. 2001;8:238–242. doi: 10.1038/84981. [DOI] [PubMed] [Google Scholar]
- 63.Egorov A.M., Ulyashova M.M., Rubtsova M.Y. Bacterial enzymes and antibiotic resistance. Acta Nat. 2018;10:33–48. doi: 10.32607/20758251-2018-10-4-33-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Philippon A., Arlet G., Jacoby G.A. Plasmid-determined AmpC-type β-Lactamases. Antimicrob. Agents Chemother. 2002;46:1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bebrone C. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharmacol. 2007;74:1686–1701. doi: 10.1016/j.bcp.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 66.Naas T., Oueslati S., Bonnin R.A., Dabos M.L., Zavala A., Dortet L., Retailleau P., Iorga B.I. β-Lactamase Database (BLDB)–structure and function. J. Enzym. Inhib. Med. Chem. 2017;32:917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morar M., Pengelly K., Koteva K., Wright G.D. Mechanism and diversity of the erythromycin esterase family of enzymes. Biochemistry. 2012;51:1740–1751. doi: 10.1021/bi201790u. [DOI] [PubMed] [Google Scholar]
- 68.Fyfe C., Grossman T.H., Kerstein K., Sutcliffe J. Resistance to macrolide antibiotics in public health pathogens. Cold Spring Harb. Perspect. Med. 2016;6:a025395. doi: 10.1101/cshperspect.a025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomes C., Martínez-Puchol S., Palma N., Horna G., Ruiz-Roldán L., Pons M.J., Ruiz J. Macrolide resistance mechanisms in Enterobacteriaceae: Focus on azithromycin. Crit. Rev. Microbiol. 2017;43:1–30. doi: 10.3109/1040841X.2015.1136261. [DOI] [PubMed] [Google Scholar]
- 70.Morar M., Wright G.D. The genomic enzymology of antibiotic resistance. Annu. Rev. Genet. 2010;44:25–51. doi: 10.1146/annurev-genet-102209-163517. [DOI] [PubMed] [Google Scholar]
- 71.Schwarz S., Shen J., Kadlec K., Wang Y., Michael G.B., Feßler A.T., Vester B. Lincosamides, streptogramins, phenicols, and pleuromutilins: Mode of action and mechanisms of resistance. Cold Spring Harb. Perspect. Med. 2016;6:a027037. doi: 10.1101/cshperspect.a027037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park B.H., Levy S.B. The cryptic tetracycline resistance determinant on Tn4400 mediates tetracycline degradation as well as tetracycline efflux. Antimicrob. Agents Chemother. 1988;32:1797–1800. doi: 10.1128/AAC.32.12.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang W., Moore I.F., Koteva K.P., Bareich D.C., Hughes D.W., Wright G.D. TetX is a flavin-dependent monooxygenase conferring resistance to tetracycline antibiotics. J. Biol. Chem. 2004;279:52346–52352. doi: 10.1074/jbc.M409573200. [DOI] [PubMed] [Google Scholar]
- 74.Speer B.S., Salyers A.A. Characterization of a novel tetracycline resistance that functions only in aerobically grown Escherichia coli. J. Bacteriol. 1988;170:1423–1429. doi: 10.1128/jb.170.4.1423-1429.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Korczynska M., Mukhtar T.A., Wright G.D., Berghuis A.M. Structural basis for streptogramin B resistance in Staphylococcus aureus by virginiamycin B lyase. Proc. Natl. Acad. Sci. USA. 2007;104:10388–10393. doi: 10.1073/pnas.0701809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allignet J., Loncle V., Mazodier P., el Solh N. Nucleotide sequence of a Staphylococcal plasmid gene, Vgb, encoding a hydrolase inactivating the B components of virginiamycin-like antibiotics. Plasmid. 1988;20:271–275. doi: 10.1016/0147-619X(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 77.Luthra S., Rominski A., Sander P. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front. Microbiol. 2018;9:2179. doi: 10.3389/fmicb.2018.02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weisblum B. Minireview erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leclercq R. Mechanisms of resistance to macrolides and lincosamides: Nature of the Resistance Elements and Their Clinical Implications. Clin. Infect. Dis. 2002;34:482–492. doi: 10.1086/324626. [DOI] [PubMed] [Google Scholar]
- 80.Bush K., Bradford P.A. β-Lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016;6:a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller W.R., Munita J.M., Arias C.A. Mechanisms of antibiotic resistance in Enterococci. Expert Rev. Anti-Infect. Ther. 2014;12:1221–1236. doi: 10.1586/14787210.2014.956092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Campbell E.A., Korzheva N., Mustaev A., Murakami K., Nair S., Goldfarb A., Darst S.A. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell. 2001;104:901–912. doi: 10.1016/S0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 83.Floss H.G., Yu T.W. Rifamycin—Mode of action, resistance, and biosynthesis. Chem. Rev. 2005;105:621–632. doi: 10.1021/cr030112j. [DOI] [PubMed] [Google Scholar]
- 84.Michaud C.M. Global burden of infectious diseases. Encycl. Microbiol. 2009:444–454. doi: 10.1016/B978-012373944-5.00185-1. [DOI] [Google Scholar]
- 85.Iskandar K., Murugaiyan J., Hammoudi Halat D., el Hage S., Chibabhai V., Adukkadukkam S., Roques C., Molinier L., Salameh P., van Dongen M. Antibiotic discovery and resistance: The chase and the race. Antibiotics. 2022;11:182. doi: 10.3390/antibiotics11020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baessa M., Rodrigues M.J., Pereira C., Santos T., da Rosa Neng N., Nogueira J.M.F., Barreira L., Varela J., Ahmed H., Asif S., et al. A comparative study of the in vitro enzyme inhibitory and antioxidant activities of Butea monosperma (Lam.) Taub. and Sesbania grandiflora (L.) poiret from Pakistan: New sources of natural products for public health problems. South Afr. J. Bot. 2019;120:146–156. doi: 10.1016/j.sajb.2018.04.006. [DOI] [Google Scholar]
- 87.Rasoanaivo P., Wright C.W., Willcox M.L., Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011;10:S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choohan M.A., Jabeen R., Bibi N. Extraction and quantification of antimicrobial peptides from medicinal plants through TrisNaCl and PBS buffer. Int. J. Nat. Med. Health Sci. 2022;1:1–5. doi: 10.52461/ijnms.v1i3.892. [DOI] [Google Scholar]
- 89.Archana H., Geetha Bose V. Evaluation of phytoconstituents from selected medicinal plants and its synergistic antimicrobial activity. Chemosphere. 2022;287:132276. doi: 10.1016/j.chemosphere.2021.132276. [DOI] [PubMed] [Google Scholar]
- 90.Gruessner B.M., Cornet-Vernet L., Desrosiers M.R., Lutgen P., Towler M.J., Weathers P.J. It is not just Artemisinin: Artemisia sp. for treating diseases including malaria and schistosomiasis. Phytochem. Rev. 2019;18:1509–1527. doi: 10.1007/s11101-019-09645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams J.D. β-Lactamases and β-Lactamase inhibitors. Int. J. Antimicrob. Agents. 1999;12:S3–S7. doi: 10.1016/S0924-8579(99)00085-0. [DOI] [PubMed] [Google Scholar]
- 92.Khameneh B., Iranshahy M., Soheili V., Fazly Bazzaz B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019;8:118. doi: 10.1186/s13756-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hochma E., Yarmolinsky L., Khalfin B., Nisnevitch M., Ben-Shabat S., Nakonechny F. Antimicrobial effect of phytochemicals from edible plants. Processes. 2021;9:2089. doi: 10.3390/pr9112089. [DOI] [Google Scholar]
- 94.Adhikari B., Marasini B.P., Rayamajhee B., Bhattarai B.R., Lamichhane G., Khadayat K., Adhikari A., Khanal S., Parajuli N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother. Res. 2021;35:1298–1312. doi: 10.1002/ptr.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Houghton P.J. Old yet new—Pharmaceuticals from plants. J. Chem. Educ. 2001;78:175. doi: 10.1021/ed078p175. [DOI] [Google Scholar]
- 96.Mothana R.A.A., Lindequist U. Antimicrobial activity of some medicinal plants of the island Soqotra. J. Ethnopharmacol. 2005;96:177–181. doi: 10.1016/j.jep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 97.Schmitz R. Friedrich Wilhelm Sertürner and the discovery of Morphine. Pharm. Hist. 1985;27:61–74. [PubMed] [Google Scholar]
- 98.Gibbons S. Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem. Rev. 2005;4:63–78. doi: 10.1007/s11101-005-2494-9. [DOI] [Google Scholar]
- 99.Cragg G.M., Newman D.J. Natural products: A continuing source of novel drug leads. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu Z., Tang J., Khare T., Kumar V. The alarming antimicrobial resistance in ESKAPEE pathogens: Can essential oils come to the rescue? Fitoterapia. 2020;140:104433. doi: 10.1016/j.fitote.2019.104433. [DOI] [PubMed] [Google Scholar]
- 101.Mohamed T.A., Abd El Aty A.A., Shahat A.A., Abdel-Azim N.S., Shams K.A., Elshamy A.A., Ahmed M.M., Youns S.H.H., El-Wassimy T.M., El-Toumy S.A., et al. New antimicrobial metabolites from the medicinal herb Artemisia herba-alba. Nat. Prod. Res. 2021;35:1959–1967. doi: 10.1080/14786419.2019.1647430. [DOI] [PubMed] [Google Scholar]
- 102.Dahiya P., Purkayastha S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J. Pharm. Sci. 2012;74:443. doi: 10.4103/0250-474X.108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fujita M., Shiota S., Kuroda T., Hatano T., Yoshida T., Mizushima T., Tsuchiya T. Remarkable synergies between baicalein and Tetracycline, and Baicalein and β-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2005;49:391–396. doi: 10.1111/j.1348-0421.2005.tb03732.x. [DOI] [PubMed] [Google Scholar]
- 104.Chan B.C.L., Ip M., Lau C.B.S., Lui S.L., Jolivalt C., Ganem-Elbaz C., Litaudon M., Reiner N.E., Gong H., See R.H., et al. Synergistic effects of Baicalein with Ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and Inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011;137:767–773. doi: 10.1016/j.jep.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 105.Iwasa K., Moriyasu M., Yamori T., Turuo T., Lee D.-U., Wiegrebe W. In vitro cytotoxicity of the protoberberine-type alkaloids. J. Nat. Prod. 2001;64:896–898. doi: 10.1021/np000554f. [DOI] [PubMed] [Google Scholar]
- 106.Liu S., Zhou Y., Niu X., Wang T., Li J., Liu Z., Wang J., Tang S., Wang Y., Deng X. Magnolol restores the activity of meropenem against NDM-1-producing Escherichia coli by inhibiting the activity of metallo-beta-lactamase. Cell Death Discov. 2018;4:28. doi: 10.1038/s41420-018-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shriram V., Jahagirdar S., Latha C., Kumar V., Puranik V., Rojatkar S., Dhakephalkar P.K., Shitole M.G. A potential plasmid-curing agent, 8-Epidiosbulbin E Acetate, from Dioscorea bulbifera L. against multidrug-resistant bacteria. Int. J. Antimicrob. Agents. 2008;32:405–410. doi: 10.1016/j.ijantimicag.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 108.Verpoorte R., Heijden R., Schripsema J., Hoge J., Hoopen H. Plant cell biotechnology for the production of alkaloids: Present status and prospects. J. Nat. Prod. 2004;56:186–207. doi: 10.1021/np50092a003. [DOI] [Google Scholar]
- 109.Heinrich M., Mah J., Amirkia V. Alkaloids used as medicines: Structural phytochemistry meets biodiversity—An update and forward look. Molecules. 2021;26:1836. doi: 10.3390/molecules26071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malik T.A., Kamili A.N., Chishti M.Z., Ahad S., Tantry M.A., Hussain P.R., Johri R.K. Breaking the resistance of Escherichia coli: Antimicrobial activity of Berberis lycium Royle. Microb. Pathog. 2017;102:12–20. doi: 10.1016/j.micpath.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 111.Tse-Dinh Y.C. Targeting bacterial topoisomerase I to meet the challenge of finding new antibiotics. Future Med. Chem. 2015;7:459–471. doi: 10.4155/fmc.14.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aqil F., Khan M.S.A., Owais M., Ahmad I. Effect of certain bioactive plant extracts on clinical isolates of β-lactamase producing methicillin resistant Staphylococcus aureus. J. Basic Microbiol. 2005;45:106–114. doi: 10.1002/jobm.200410355. [DOI] [PubMed] [Google Scholar]
- 113.Kumar A., Khan I.A., Koul S., Koul J.L., Taneja S.C., Ali I., Ali F., Sharma S., Mirza Z.M., Kumar M., et al. Novel structural analogues of Piperine as inhibitors of the NorA efflux pump of Staphylococcus aureus. J. Antimicrob. Chemother. 2008;61:1270–1276. doi: 10.1093/jac/dkn088. [DOI] [PubMed] [Google Scholar]
- 114.Khameneh B., Diab R., Ghazvini K., Fazly Bazzaz B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016;95:32–42. doi: 10.1016/j.micpath.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 115.Chopra B., Dhingra K.A., Dhar K.L. Synthesis and characterization of Piperine analogues as potent Staphylococcus aureus NorA efflux pump inhibitors. Chem. Methodol. 2019;3:104–114. [Google Scholar]
- 116.Heeb S., Fletcher M.P., Chhabra S.R., Diggle S.P., Williams P., Cámara M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011;35:247–274. doi: 10.1111/j.1574-6976.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mahmood H.Y., Jamshidi S., Mark Sutton J.M., Rahman K. Current advances in developing inhibitors of bacterial multidrug efflux pumps. Curr. Med. Chem. 2016;23:1062–1081. doi: 10.2174/0929867323666160304150522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guay I., Boulanger S., Isabelle C., Brouillette E., Chagnon F., Bouarab K., Marsault E., Malouin F. Tomatidine and analog FC04-100 possess bactericidal activities against Listeria, Bacillus and Staphylococcus sp. BMC Pharmacol. Toxicol. 2018;19:7. doi: 10.1186/s40360-018-0197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao Y., Li H., Wei S., Zhou X., Xiao X. Antimicrobial effects of chemical compounds isolated from traditional chinese herbal medicine (TCHM) against drug-resistant bacteria: A review paper. Mini-Rev. Med. Chem. 2018;19:125–137. doi: 10.2174/1389557518666181017143141. [DOI] [PubMed] [Google Scholar]
- 120.Siriyong T., Chusri S., Srimanote P., Tipmanee V., Voravuthikunchai S.P. Holarrhena Antidysenterica extract and its steroidal alkaloid, Conessine, as resistance-modifying agents against extensively drug-resistant Acinetobacter baumannii. Microb. Drug Resist. 2016;22:273–282. doi: 10.1089/mdr.2015.0194. [DOI] [PubMed] [Google Scholar]
- 121.Obiang-Obounou B.-W., Kang O.-H., Choi J.-G., Keum J.-H., Kim S.-B., Mun S.-H., Shin D.-W., Woo Kim K., Park C.-B., Kim Y.-G., et al. The mechanism of action of Sanguinarine against methicillin-resistant Staphylococcus aureus. J. Toxicol. Sci. 2011;36:277–283. doi: 10.2131/jts.36.277. [DOI] [PubMed] [Google Scholar]
- 122.Al-Ani I., Zimmermann S., Reichling J., Wink M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine. 2015;22:245–255. doi: 10.1016/j.phymed.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 123.Newton S.M., Lau C., Gurcha S.S., Besra G.S., Wright C.W. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J. Ethnopharmacol. 2002;79:57–67. doi: 10.1016/S0378-8741(01)00350-6. [DOI] [PubMed] [Google Scholar]
- 124.Alibi S., Crespo D., Navas J. Plant-derivatives small molecules with antibacterial activity. Antibiotics. 2021;10:231. doi: 10.3390/antibiotics10030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maurya A., Dwivedi G.R., Darokar M.P., Srivastava S.K. Antibacterial and synergy of clavine alkaloid lysergol and its derivatives against nalidixic acid-resistant Escherichia coli. Chem. Biol. Drug Des. 2013;81:484–490. doi: 10.1111/cbdd.12103. [DOI] [PubMed] [Google Scholar]
- 126.Ravichandiran P., Sheet S., Premnath D., Kim A.R., Yoo D.J. 1,4-Naphthoquinone analogues: Potent antibacterial agents and mode of action evaluation. Molecules. 2019;24:1437. doi: 10.3390/molecules24071437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yap J.K.Y., Tan S.Y.Y., Tang S.Q., Thien V.K., Chan E.W.L. Synergistic antibacterial activity between 1,4-naphthoquinone and β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. Microb. Drug Resist. 2021;27:234–240. doi: 10.1089/mdr.2020.0178. [DOI] [PubMed] [Google Scholar]
- 128.Siriyong T., Srimanote P., Chusri S., Yingyongnarongkul B.E., Suaisom C., Tipmanee V., Voravuthikunchai S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017;17:405. doi: 10.1186/s12906-017-1913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boberek J.M., Stach J., Good L. Genetic evidence for inhibition of bacterial division protein FtsZ by Berberine. PLoS ONE. 2010;5:e13745. doi: 10.1371/journal.pone.0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zorić N., Kosalec I., Tomić S., Bobnjarić I., Jug M., Vlainić T., Vlainić J. Membrane of Candida albicans as a target of Berberine. BMC Complement. Altern. Med. 2017;17:268. doi: 10.1186/s12906-017-1773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sridevi D., Shankar C., Prakash P., Park J.H., Thamaraiselvi K. Inhibitory effects of Reserpine against efflux pump activity of antibiotic resistance bacteria. Chem. Biol. Lett. 2017;4:69–72. [Google Scholar]
- 132.Boulet M.L., Isabelle C., Guay I., Brouillette E., Langlois J.P., Jacques P.É., Rodrigue S., Brzezinski R., Beauregard P.B., Bouarab K., et al. Tomatidine is a lead antibiotic molecule that targets Staphylococcus aureus ATP synthase subunit C. Antimicrob. Agents Chemother. 2018;62:e02197-17. doi: 10.1128/AAC.02197-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan Y., Li X., Zhang C., Lv L., Gao B., Li M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics. 2021;10:318. doi: 10.3390/antibiotics10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gorlenko C.L., Kiselev H.Y., Budanova E.V., Zamyatnin A.A., Ikryannikova L.N. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: New heroes or worse clones of antibiotics? Antibiotics. 2020;9:170. doi: 10.3390/antibiotics9040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Misiurek J., Plech T., Kaproń B., Makuch-Kocka A., Szultka-Młyńska M., Buszewski B., Petruczynik A. Determination of Some Isoquinoline Alkaloids in Extracts Obtained from Selected Plants of the Ranunculaceae, Papaveraceae and Fumarioideae Families by Liquid Chromatography and In Vitro and In Vivo Investigations of Their Cytotoxic Activity. Molecules. 2023;28:3503. doi: 10.3390/molecules28083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ding C.F., Qin X.J., Yu H.F., Liu Y.P., Wang X.H., Luo X.D. Thalicfoetine, a novel isoquinoline alkaloid with antibacterial activity from Thalictrum foetidum. Tetrahedron. Lett. 2019;60:151135. doi: 10.1016/j.tetlet.2019.151135. [DOI] [Google Scholar]
- 137.Zacchino S.A., Butassi E., di Liberto M., Raimondi M., Postigo A., Sortino M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine. 2017;37:27–48. doi: 10.1016/j.phymed.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 138.Lima M.C., Paiva de Sousa C., Fernandez-Prada C., Harel J., Dubreuil J.D., de Souza E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019;130:259–270. doi: 10.1016/j.micpath.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 139.Ecevit K., Barros A.A., Silva J.M., Reis R.L. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022;2:460–498. doi: 10.3390/futurepharmacol2040030. [DOI] [Google Scholar]
- 140.Mehmood A., Javid S., Khan M.F., Ahmad K.S., Mustafa A. In vitro total phenolics, total flavonoids, antioxidant and antibacterial activities of selected medicinal plants using different solvent systems. BMC Chem. 2022;16:64. doi: 10.1186/s13065-022-00858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kocaçalışkan I., Talan I., Terzi I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. J. Biosci. 2006;61:639–642. doi: 10.1515/znc-2006-9-1004. [DOI] [PubMed] [Google Scholar]
- 142.Xiao Z.P., Ma T.W., Fu W.C., Peng X.C., Zhang A.H., Zhu H.L. The synthesis, structure and activity evaluation of pyrogallol and catechol derivatives as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem. 2010;45:5064–5070. doi: 10.1016/j.ejmech.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 143.Ma C., He N., Zhao Y., Xia D., Wei J., Kang W. Antimicrobial mechanism of hydroquinone. Appl. Biochem. Biotechnol. 2019;189:1291–1303. doi: 10.1007/s12010-019-03067-1. [DOI] [PubMed] [Google Scholar]
- 144.Lechner D., Gibbons S., Bucar F. Modulation of isoniazid susceptibility by flavonoids in Mycobacterium. Phytochem. Lett. 2008;1:71–75. doi: 10.1016/j.phytol.2008.01.002. [DOI] [Google Scholar]
- 145.Borges A., Ferreira C., Saavedra M.J., Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013;19:256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 146.Wu Y., Bai J., Zhong K., Huang Y., Qi H., Jiang Y., Gao H. Antibacterial activity and membrane-disruptive mechanism of 3-p-Trans-Coumaroyl-2-Hydroxyquinic acid, a novel phenolic compound from pine needles of Cedrus deodara, against Staphylococcus aureus. Molecules. 2016;21:1084. doi: 10.3390/molecules21081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patel K., Tyagi C., Goyal S., Jamal S., Wahi D., Jain R., Bharadvaja N., Grover A. Identification of chebulinic acid as potent natural inhibitor of Mycobacterium tuberculosis dna gyrase and molecular insights into its binding mode of action. Comput. Biol. Chem. 2015;59:37–47. doi: 10.1016/j.compbiolchem.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 148.Wu D., Kong Y., Han C., Chen J., Hu L., Jiang H., Shen X. D-Alanine:D-Alanine ligase as a new target for the flavonoids Quercetin and Apigenin. Int. J. Antimicrob. Agents. 2008;32:421–426. doi: 10.1016/j.ijantimicag.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 149.Zou D., Xie K., Wang H., Chen Y., Xie M. Inhibitory effects of Biochanin a on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA) Wei Sheng Wu Xue Bao. 2014;54:1204–1211. [PubMed] [Google Scholar]
- 150.Shao J., Zhang M., Wang T., Li Y., Wang C. The roles of CDR1, CDR2, and MDR1 in Kaempferol-induced suppression with fluconazole-resistant Candida albicans. Pharm. Biol. 2016;54:984–992. doi: 10.3109/13880209.2015.1091483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Randhawa H.K., Hundal K.K., Ahirrao P.N., Jachak S.M., Nandanwar H.S. Efflux pump inhibitory activity of flavonoids isolated from Alpinia calcarata against methicillin-resistant Staphylococcus aureus. Biologia. 2016;71:484–493. doi: 10.1515/biolog-2016-0073. [DOI] [Google Scholar]
- 152.Gopal J., Muthu M., Paul D., Kim D.H., Chun S. Bactericidal activity of green tea extracts: The importance of catechin containing nano particles. Sci. Rep. 2016;6:19710. doi: 10.1038/srep19710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gradišar H., Pristovšek P., Plaper A., Jerala R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J. Med. Chem. 2007;50:264–271. doi: 10.1021/jm060817o. [DOI] [PubMed] [Google Scholar]
- 154.Kaczmarek B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials. 2020;13:3224. doi: 10.3390/ma13143224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tyagi P., Singh M., Kumari H., Kumari A., Mukhopadhyay K. Bactericidal activity of Curcumin I is associated with damaging of bacterial membrane. PLoS ONE. 2015;10:e0121313. doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Belofsky G., Percivill D., Lewis K., Tegos G.P., Ekart J. Phenolic metabolites of Dalea Versicolor that enhance antibiotic activity against model pathogenic bacteria. J. Nat. Prod. 2004;67:481–484. doi: 10.1021/np030409c. [DOI] [PubMed] [Google Scholar]
- 157.Duan F., Xin G., Niu H., Huang W. Chlorinated Emodin as a natural antibacterial agent against drug-resistant bacteria through dual influence on bacterial cell membranes and DNA. Sci. Rep. 2017;7:12721. doi: 10.1038/s41598-017-12905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brown J.C., Wang J., Kasman L., Jiang X., Haley-Zitlin V. Activities of muscadine grape skin and Quercetin against Helicobacter pylori infection in mice. J. Appl. Microbiol. 2011;110:139–146. doi: 10.1111/j.1365-2672.2010.04870.x. [DOI] [PubMed] [Google Scholar]
- 159.Klančnik A., Šikić Pogačar M., Trošt K., Tušek Žnidarič M., Mozetič Vodopivec B., Smole Možina S. Anti-Campylobacter activity of Resveratrol and an extract from waste pinot noir grape skins and seeds, and resistance of Campylobacter jejuni planktonic and biofilm cells, mediated via the CmeABC efflux pump. J. Appl. Microbiol. 2017;122:65–77. doi: 10.1111/jam.13315. [DOI] [PubMed] [Google Scholar]
- 160.Wang L., Wang G., Qu H., Wang K., Jing S., Guan S., Su L., Li Q., Wang D. Taxifolin, an inhibitor of sortase a, interferes with the adhesion of methicillin-resistant Staphylococcal aureus. Front. Microbiol. 2021;12:686864. doi: 10.3389/fmicb.2021.686864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jeong K.W., Lee J.Y., Kang D.I., Lee J.U., Shin S.Y., Kim Y. Screening of flavonoids as candidate antibiotics against Enterococcus faecalis. J. Nat. Prod. 2009;72:719–724. doi: 10.1021/np800698d. [DOI] [PubMed] [Google Scholar]
- 162.Tan N., Yazıcı-Tütüniş S., Bilgin M., Tan E., Miski M. Antibacterial activities of pyrenylated coumarins from the roots of Prangos hulusii. Molecules. 2017;22:1098. doi: 10.3390/molecules22071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun M., Sun M., Zhang J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021;30:1767–1794. doi: 10.1007/s00044-021-02775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fazly Bazzaz B.S., Memariani Z., Khashiarmanesh Z., Iranshahi M., Naderinasab M. Effect of galbanic acid, a sesquiterpene coumarin from ferula szowitsiana, as an inhibitor of efflux mechanism in resistant clinical isolates of Staphylococcus aureus. Braz. J. Microbiol. 2010;41:574–580. doi: 10.1590/S1517-83822010000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.El-Seedi H.R. Antimicrobial arylcoumarins from Asphodelus microcarpus. J. Nat. Prod. 2007;70:118–120. doi: 10.1021/np060444u. [DOI] [PubMed] [Google Scholar]
- 166.Basile A., Sorbo S., Spadaro V., Bruno M., Maggio A., Faraone N., Rosselli S. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae) Molecules. 2009;14:939–952. doi: 10.3390/molecules14030939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gutiérrez S., Morán A., Martínez-Blanco H., Ferrero M.A., Rodríguez-Aparicio L.B. The usefulness of non-toxic plant metabolites in the control of bacterial proliferation. Probiotics Antimicrob. Proteins. 2017;9:323–333. doi: 10.1007/s12602-017-9259-9. [DOI] [PubMed] [Google Scholar]
- 168.Nakamoto M., Kunimura K., Suzuki J., Kodera Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, Vinyldithiin, Ajoene and Diallyl Polysulfides (review) Exp. Ther. Med. 2019;19:1550–1553. doi: 10.3892/etm.2019.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dwivedi V.P., Bhattacharya D., Singh M., Bhaskar A., Kumar S., Fatima S., Sobia P., van Kaer L., Das G. Allicin Enhances Antimicrobial Activity of Macrophages during Mycobacterium tuberculosis infection. J. Ethnopharmacol. 2019;243:111634. doi: 10.1016/j.jep.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 170.Dufour V., Stahl M., Baysse C. The Antibacterial Properties of Isothiocyanates. Microbiology. 2015;161:229–243. doi: 10.1099/mic.0.082362-0. [DOI] [PubMed] [Google Scholar]
- 171.Sagdic O., Tornuk F. Dietary Phytochemicals and Microbes. Volume 9789400739260. Springer; Dordrecht, The Netherlands: 2012. Antimicrobial properties of organosulfur compounds; pp. 127–156. [Google Scholar]
- 172.Boghrati Z., Iranshahi M. Ferula species: A rich source of antimicrobial compounds. J. Herb. Med. 2019;16:100244. doi: 10.1016/j.hermed.2018.10.009. [DOI] [Google Scholar]
- 173.Reiter J., Levina N., der Linden M., Gruhlke M., Martin C., Slusarenko A.J. Diallylthiosulfinate (Allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules. 2017;22:1711. doi: 10.3390/molecules22101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Feldberg R.S., Chang S.C., Kotik A.N., Nadler M., Neuwirth Z., Sundstrom D.C. In vitro mechanism of inhibition of bacterial cell growth by allicin Antimicrob. Agents Chemother. 1988;32:1763–1768. doi: 10.1128/AAC.32.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Müller A., Eller J., Albrecht F., Prochnow P., Kuhlmann K., Bandow J.E., Slusarenko A.J., Leichert L.I.O. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016;291:11477–11490. doi: 10.1074/jbc.M115.702308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sofrata A., Santangelo E.M., Azeem M., Borg-Karlson A.K., Gustafsson A., Pütsep K. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against gram-negative bacteria. PLoS ONE. 2011;6:e23045. doi: 10.1371/journal.pone.0023045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Park H.-W., Choi K.-D., Shin I.-S. Antimicrobial activity of Isothiocyanates (ITCs) extracted from Horseradish (Armoracia rusticana) root against oral microorganisms. Biocontrol. Sci. 2013;18:163–168. doi: 10.4265/bio.18.163. [DOI] [PubMed] [Google Scholar]
- 178.Choo S., Chin V.K., Wong E.H., Madhavan P., Tay S.T., Yong P.V.C., Chong P.P. Review: Antimicrobial properties of Allicin used alone or in combination with other medications. Folia Microbiol. 2020;65:451–465. doi: 10.1007/s12223-020-00786-5. [DOI] [PubMed] [Google Scholar]
- 179.Kyung K.H. Antimicrobial properties of Allium species. Curr. Opin. Biotechnol. 2012;23:142–147. doi: 10.1016/j.copbio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 180.Rehman F., Mairaj S. Antimicrobial Studies of Allicin and Ajoene. Int. J. Pharm. Bio Sci. 2013;4:1095–1105. [Google Scholar]
- 181.Abukhabta S., Khalil Ghawi S., Karatzas K.A., Charalampopoulos D., McDougall G., Allwood J.W., Verrall S., Lavery S., Latimer C., Pourshahidi L.K., et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur. J. Nutr. 2021;60:1263–1276. doi: 10.1007/s00394-020-02322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen H., Yang N., Yu L., Li J., Zhang H., Zheng Y., Xu M., Liu Y., Yang Y., Li J. Synergistic Microbicidal effect of AUR and PEITC against Staphylococcus aureus skin infection. Front. Cell. Infect. Microbiol. 2022;12:741. doi: 10.3389/fcimb.2022.927289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oldfield E., Lin F.-Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Ed. 2012;51:1124–1137. doi: 10.1002/anie.201103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Paduch R., Kandefer-Szerszeń M., Trytek M., Fiedurek J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007;55:315. doi: 10.1007/s00005-007-0039-1. [DOI] [PubMed] [Google Scholar]
- 185.Knowles J.R., Roller S., Murray D.B., Naidu A.S. Antimicrobial action of Carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar Typhimurium. Appl. Env. Microbiol. 2005;71:797–803. doi: 10.1128/AEM.71.2.797-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Astani A., Reichling J., Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010;24:673–679. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Helander I.M., Alakomi H.-L., Latva-Kala K., Mattila-Sandholm T., Pol I., Smid E.J., Gorris L.G.M., von Wright A. Characterization of the action of selected essential oil components on gram-negative bacteria. J. Agric. Food Chem. 1998;46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- 188.Mahizan N.A., Yang S.K., Moo C.L., Song AA L., Chong C.M., Chong C.W., Abushelaibi A., Erin Lim S.H., Lai K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules. 2019;24:2631. doi: 10.3390/molecules24142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Broniatowski M., Mastalerz P., Flasiński M. Studies of the interactions of ursane-type bioactive terpenes with the model of Escherichia coli Inner Membrane—Langmuir Monolayer Approach. Biochim. Biophys. Acta Biomembr. 2015;1848:469–476. doi: 10.1016/j.bbamem.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 190.Yadav M.K., Chae S.W., Im G.J., Chung J.W., Song J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus Clinical Strain Biofilms. PLoS ONE. 2015;10:e0119564. doi: 10.1371/journal.pone.0119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ali S.M., Khan A.A., Ahmed I., Musaddiq M., Ahmed K.S., Polasa H., Rao L.V., Habibullah C.M., Sechi L.A., Ahmed N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005;4:20. doi: 10.1186/1476-0711-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rathinam P., Vijay Kumar H.S., Viswanathan P. Eugenol exhibits anti-virulence properties by competitively binding to quorum sensing receptors. Biofouling. 2017;33:624–639. doi: 10.1080/08927014.2017.1350655. [DOI] [PubMed] [Google Scholar]
- 193.Zhang Y., Liu X., Wang Y., Jiang P., Quek S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. doi: 10.1016/j.foodcont.2015.05.032. [DOI] [Google Scholar]
- 194.Togashi N., Hamashima H., Shiraishi A., Inoue Y., Takano A. Antibacterial activities against Staphylococcus aureus of terpene alcohols with aliphatic carbon chains. J. Essent. Oil Res. 2010;22:263–269. doi: 10.1080/10412905.2010.9700321. [DOI] [Google Scholar]
- 195.Althunibat O.Y., Qaralleh H., Yassin S., Al-Dalin A., Abboud M., Khleifat K., Majali I.S., Aldal’in H.K.H., Rayyan W.A., Jaafraa A. Effect of thymol and carvacrol, the major components of Thymus capitatus on the growth of Pseudomonas aeruginosa. Jr. Pure Appl. Microbiol. 2016;10:367–374. [Google Scholar]
- 196.Qiu J., Wang D., Xiang H., Feng H., Jiang Y., Xia L., Dong J., Lu J., Yu L., Deng X. Subinhibitory Concentrations of Thymol reduce enterotoxins A and B and α-Hemolysin production in Staphylococcus aureus isolates. PLoS ONE. 2010;5:e9736. doi: 10.1371/journal.pone.0009736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mittermeier R.A., Gil P.R., Hoffmann M., Pilgrim J., Brooks T., Mittermeier C.G., Lamourex J., Fonseca G.A.B. Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions. 1st ed. CEMEX; Mexico City, Mexico: 2004. p. 390. [Google Scholar]
- 198.Nayar M.P. Thiruvananthapuram. 1st ed. Tropical Botanic Garden and Research Institute; Trivandrum, India: 1996. Hot spots of endemic plants of India, Nepal and Bhutan; pp. 19–252. [Google Scholar]
- 199.Singh K.P., Gymnosperms M.V. In: Floristic Diversity and Conservation Strategies in India. Mudgal V., Hajra P.K., editors. Botanical Survey of India, Ministry of Environment and Forests; Calcutta, India: 1997. pp. 443–472. [Google Scholar]
- 200.Kholia B.S., Balkrishna A. Ferns. Springer; Singapore: 2022. Pteridophytes used by peoples of indian Himalayan region and Northern India: An overview; pp. 379–412. [DOI] [Google Scholar]
- 201.Poddar Sarkar M., Biswas Raha A., Datta J., Mitra S. Chemotaxonomic and evolutionary perspectives of Bryophyta based on multivariate analysis of fatty acid fingerprints of eastern Himalayan mosses. Protoplasma. 2022;259:1125–1137. doi: 10.1007/s00709-021-01723-0. [DOI] [PubMed] [Google Scholar]
- 202.Negi V.S., Pathak R., Thakur S., Joshi R.K., Bhatt I.D., Rawal R.S. Scoping the Need of Mainstreaming Indigenous Knowledge for Sustainable Use of Bioresources in the Indian Himalayan Region. Environ. Manag. 2021;72:135–146. doi: 10.1007/s00267-021-01510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hajar R. The medicine of old India. Heart Views. 2013;14:92. doi: 10.4103/1995-705X.115497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Samant S.S., Pant S. Diversity, distribution pattern and conservation status of the plants used in liver diseases/ailments in indian Himalayan region. J. Mt. Sci. 2006;3:28–47. doi: 10.1007/s11629-006-0028-6. [DOI] [Google Scholar]
- 205.Wani Z.A., Pant S. Ethnomedicinal study of plants used to cure skin diseases and healing of wounds in Gulmarg Wildlife Sanctuary (GWLS), Jammu & Kashmir. Indian Jr. Trad. Knowl. 2020;19:327–334. [Google Scholar]
- 206.Mukherjee P.K., Harwansh R.K., Bahadur S., Banerjee S., Kar A. From Ayurveda to Chinese Medicine. World Scientific Publishing Co. Pte Ltd.; Singapore: 2017. Evidence-based validation of Indian traditional medicine: Way forward; pp. 137–167. [Google Scholar]
- 207.Nema N.K., Dalai M.K., Mukherjee P.K. Ayush herbs and status quo in herbal industries. Pharma. Rev. 2011;141:148. [Google Scholar]
- 208.Ahmad S., Zahiruddin S., Parveen B., Basist P., Parveen A., Gaurav, Parveen R., Ahmad M. Indian medicinal plants and formulations and their potential against COVID-19 preclinical and clinical research. Front. Pharmacol. 2021;11:2470. doi: 10.3389/fphar.2020.578970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mukherjee P.K. Quality Control of Herbal Drugs—An Approach to Evaluation of Botanicals. 1st ed. Business Horizons; New Delhi, India: 2002. pp. 10–800. [Google Scholar]
- 210.Kunwar R.M., Nepal B.K., Kshhetri H.B., Rai S.K., Bussmann R.W. Ethnomedicine in Himalaya: A case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J. Ethnobiol. Ethnomed. 2006;2:27. doi: 10.1186/1746-4269-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ahmad K.S., Hamid A., Nawaz F., Hameed M., Ahmad F., Deng J., Akhtar N., Wazarat A., Mahroof S. Ethnopharmacological studies of indigenous plants in Kel village, Neelum valley, Azad Kashmir, Pakistan. J. Ethnobiol. Ethnomed. 2017;13:68. doi: 10.1186/s13002-017-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kausar F., Kim K.-H., Farooqi H.M.U., Farooqi M.A., Kaleem M., Waqar R., Khalil A.A.K., Khuda F., Abdul Rahim C.S., Hyun K., et al. Evaluation of antimicrobial and anticancer activities of selected medicinal plants of Himalayas, Pakistan. Plants. 2022;11:48. doi: 10.3390/plants11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Das Prajapati N., Purohit S.S., Sharma A.K., Kumar T. A Handbook of Medicinal Plants: A Complete Source Book. 1st ed. Agrobios; Jodhpur, India: 2003. p. 554. [Google Scholar]
- 214.Pandey M.M., Rastogi S., Rawat A.K.S. Indian traditional Ayurvedic system of medicine and nutritional supplementation. Evid.-Based Complement. Altern. Med. 2013;2013:376327. doi: 10.1155/2013/376327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bhat J.A., Kumar M., Bussmann R.W. Ecological status and traditional knowledge of medicinal plants in Kedarnath Wildlife Sanctuary of Garhwal Himalaya, India. J. Ethnobiol. Ethnomed. 2013;9:1. doi: 10.1186/1746-4269-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Heinrich M., Jiang H., Scotti F., Booker A., Walt H., Weckerle C., Maake C. Medicinal plants from the Himalayan region for potential novel antimicrobial and anti-inflammatory skin treatments. J. Pharm. Pharmacol. 2021;73:956–967. doi: 10.1093/jpp/rgab039. [DOI] [PubMed] [Google Scholar]
- 217.Adnan M., Patel M., Deshpande S., Alreshidi M., Siddiqui A.J., Reddy M.N., Emira N., de Feo V. Effect of Adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: An in vitro-in Silico Approach. Front. Microbiol. 2020;11:823. doi: 10.3389/fmicb.2020.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Alam F., Khan S.H.A., Asad M.H.H. bin. Phytochemical, antimicrobial, antioxidant and enzyme inhibitory potential of medicinal plant Dryopteris ramosa (Hope) C. Chr. BMC Complement. Med. Ther. 2021;21:197. doi: 10.1186/s12906-021-03370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Radulović N., Stojanović G., Palić R. Composition and antimicrobial activity of Equisetum arvense L. Essential Oil. Phytother. Res. 2006;20:85–88. doi: 10.1002/ptr.1815. [DOI] [PubMed] [Google Scholar]
- 220.Frahm J.-P. An evaluation of the Bryophyte flora of the Azores. Bryophyt. Divers Evol. 2005;26:57–79. doi: 10.11646/bde.26.1.12. [DOI] [Google Scholar]
- 221.Alam A. Some Indian Bryophytes known for their biologically active compounds. Int. J. Appl. Biol. Pharmceutical Technol. 2012;3:239–246. [Google Scholar]
- 222.Mewari N., Kumar P. Antimicrobial activity of extracts of Marchantia polymorpha. Pharm. Biol. 2008;46:819–822. doi: 10.1080/13880200802315725. [DOI] [Google Scholar]
- 223.Li Y., Wu J.-W., Tan H.-B., Li B.-L., Qiu S.-X. Three new pterocarpans from the aerial parts of Abrus precatorius. Nat. Prod. Res. 2020;34:1836–1844. doi: 10.1080/14786419.2018.1564293. [DOI] [PubMed] [Google Scholar]
- 224.Tian C., Zhang P., Yang C., Gao X., Wang H., Guo Y., Liu M. Extraction process, component analysis, and in vitro antioxidant, antibacterial, and anti-inflammatory activities of total flavonoid extracts from Abutilon Theophrasti Medic. Leaves. Mediat. Inflamm. 2018;2018:3508506. doi: 10.1155/2018/3508506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.El-Kalamouni C., Venskutonis P., Zebib B., Merah O., Raynaud C., Talou T. Antioxidant and antimicrobial activities of the essential oil of Achillea millefolium L. Grown in France. Medicines. 2017;4:30. doi: 10.3390/medicines4020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mishra D. Antibacterial activity of alkaloids present in plant Achyranthes aspera. Pharma Innov. J. 2018;7:147–153. [Google Scholar]
- 227.Khan F.A., Khan S., Khan N.M., Khan H., Khan S., Ahmad S., Rehman N., Aziz R., Khan F.A., Khan S., et al. Antimicrobial and antioxidant role of the aerial parts of Aconitum violaceum. J. Mex. Chem. Soc. 2021;65:84–93. doi: 10.29356/jmcs.v65i1.1310. [DOI] [Google Scholar]
- 228.Ahmad M., Ahmad W., Ahmad M., Zeeshan M., Obaidullah, Shaheen F. Norditerpenoid Alkaloids from the roots of Aconitum heterophyllum Wall with antibacterial activity. J. Enzyme Inhib. Med. Chem. 2008;23:1018–1022. doi: 10.1080/14756360701810140. [DOI] [PubMed] [Google Scholar]
- 229.Satyal P., Dosoky N.S., Poudel A., Setzer W.N. Essential oil constituents and their biological activities from the leaves of Cassia fistula growing in Nepal. Open Access J. Med. Aromat. Plants. 2017;3:1–4. [Google Scholar]
- 230.Yuan Q., Wang J., Ruan J. Screening for bioactive compounds from Adiantum capillus-veneris L. J. Chem. Soc. Pak. 2012;34:207. [Google Scholar]
- 231.Karthik M.R.M.G., Chandrappa C.P., Shilpashree C.B., Sadananda T.S. Antibacterial and antioxidant activities of Adiantum pedatum L. J. Phytol. 2011;3:26–32. [Google Scholar]
- 232.Verma R.S., Padalia R.C., Chauhan A. Essential oil composition of Aegle marmelos (L.) Correa: Chemotypic and seasonal variations. J. Sci. Food Agric. 2014;94:1904–1913. doi: 10.1002/jsfa.6510. [DOI] [PubMed] [Google Scholar]
- 233.Kurade N.P., Jaitak V., Kaul V.K., Sharma O.P. Chemical composition and antibacterial activity of essential oils of Lantana camara, Ageratum houstonianum and Eupatorium adenophorum. Pharm. Biol. 2010;48:539–544. doi: 10.3109/13880200903193336. [DOI] [PubMed] [Google Scholar]
- 234.Rahman N., Ahmad M., Riaz M., Jahan N., Ahmad R. Phytochemical, antimicrobial, insecticidal and brine shrimp lethality bioassay of the crude methanolic extract of Ajuga parviflora Benth. Pak. J. Pharm. Sci. 2013;26:751–756. [PubMed] [Google Scholar]
- 235.Karunakaran G., Jagathambal M., Gusev A., Torres J.A.L., Kolesnikov E., Kuznetsov D. Rapid biosynthesis of AgNPs using soil bacterium Azotobacter vinelandii with promising antioxidant and antibacterial activities for biomedical applications. JOM. 2017;69:1206–1212. doi: 10.1007/s11837-016-2175-8. [DOI] [Google Scholar]
- 236.Ahiabor C., Gordon A., Ayittey K., Agyare R. In vitro assessment of antibacterial activity of crude extracts of onion (Allium cepa L.) and shallot (Allium aescalonicum L.) on isolates of Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and Salmonella typhi (ATCC 19430) Int. J. Appl. Res. 2016;2:1029–1032. [Google Scholar]
- 237.Carminate B., Martin G.B., Barcelos R.M., Gontijo I., Almeida M.S., Belinelo V.J. Evaluation of antifungal activity of Amaranthus viridis L. (Amaranthaceae) on Fusariosis by Piper nigrum L. and on Anthracnose by Musa sp. Agric. J. 2012;7:215–219. [Google Scholar]
- 238.Satyal P., Dosoky N.S., Kincer B.L., Setzer W.N. Chemical compositions and biological activities of Amomum subulatum essential oils from Nepal. Nat. Prod. Commun. 2012;7:1233–1236. doi: 10.1177/1934578X1200700935. [DOI] [PubMed] [Google Scholar]
- 239.Joshi R.K. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; Cambridge, MA, USA: 2015. Angelica (Angelica glauca and Angelica archangelica) oils; pp. 203–208. [Google Scholar]
- 240.Petkova N., Hambarlyiska I., Tumbarski Y., Vrancheva R., Raeva M., Ivanov I. Phytochemical composition and antimicrobial properties of Burdock (Arctium lappa L.) roots extracts. Biointerface Res. Appl. Chem. 2022;12:2826–2842. [Google Scholar]
- 241.Shameem N., Kamili A.N., Parray J.A., Hamid R., Bandh S.A. Antimicrobial and antioxidant activity of methanol extracts of Arnebia benthamii (Wall Ex. G. Don) Johnston—A critically endangered medicinal plant of North Western Himalaya. J. Anal. Sci. Technol. 2015;6:36. doi: 10.1186/s40543-015-0076-z. [DOI] [Google Scholar]
- 242.Rashid S., Rather M.A., Shah W.A., Bhat B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013;138:693–700. doi: 10.1016/j.foodchem.2012.10.102. [DOI] [PubMed] [Google Scholar]
- 243.Haider S.Z., Mohan M., Andola H. Constituents of Artemisia indica Willd. from Uttarakhand Himalaya: A source of Davanone. Pharmacogn. Res. 2014;6:257. doi: 10.4103/0974-8490.132607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shrestha R., Shakya A., Kumar Shrestha K. Phytochemical screening and antimicrobial activity of Asparagus racemosus Willd. and Asparagus curillus Buch.-Ham. Ex Roxb. J. Nat. Hist. Mus. 2015;29:91–102. doi: 10.3126/jnhm.v29i0.19041. [DOI] [Google Scholar]
- 245.Rahman K., Khan S.U., Fahad S., Shinwari Z.K., Khan D., Kamal S., Ullah I., Anjum S.I., Man S., Khan A.J., et al. In vitro biological screening of a critically endangered medicinal plant, Atropa acuminata Royle Ex Lindl of North Western Himalaya. Sci. Rep. 2018;8:11028. doi: 10.1038/s41598-018-29231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Munir N., Iqbal A., Altaf I., Bashir R., Sharif N., Saleem F., Naz S. Evaluation of antioxidant and antimicrobial potential of two endangered plant species Atropa belladonna & Matricaria chamomilla. Afr. J. Tradit. Complement. Altern. Med. 2014;11:111. doi: 10.4314/ajtcam.v11i5.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bin Emran T., Rahman M.A., Uddin M.M.N., Dash R., Hossen M.F., Mohiuddin M., Alam M.R. Molecular docking and inhibition studies on the interactions of Bacopa monnieri’s potent phytochemicals against pathogenic Staphylococcus aureus. DARU J. Pharm. Sci. 2015;23:26. doi: 10.1186/s40199-015-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Raveesha H.R., Sushma B.K. Antimicrobial activity of Baliospermum montanum (Wild.) Muell. Arg. J. Mycopathol. Res. 2018;56:35–39. [Google Scholar]
- 249.Zafar R., Ullah H., Zahoor M., Sadiq A. Isolation of bioactive compounds from Bergenia ciliata (Haw.) Sternb rhizome and their antioxidant and anticholinesterase activities. BMC Complement. Altern. Med. 2019;19:296. doi: 10.1186/s12906-019-2679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pal M., Mishra T., Kumar A., Baleshwar, Upreti D.K., Rana T.S. Chemical constituents and antimicrobial potential of essential oil from Betula utilis growing in high altitude of Himalaya (India) J. Essent. Oil-Bear. Plants. 2015;18:1078–1082. doi: 10.1080/0972060X.2015.1036569. [DOI] [Google Scholar]
- 251.Zahara K., Bibi Y., Qayyum A., Nisa S. Investigation of antimicrobial and antioxidant properties of Bidens Biternata. Iranian J. Sci. Technol. Trans. A Sci. 2019;43:725–734. doi: 10.1007/s40995-018-0564-2. [DOI] [Google Scholar]
- 252.Satyal P., Chhetri B.K., Dosoky N.S., Shrestha S., Poudel A., Setzer W.N. Chemical composition of Blumea lacera essential oil from Nepal. biological activities of the essential oil and (Z)-Lachnophyllum ester. Nat. Prod. Commun. 2015;10:1749–1750. doi: 10.1177/1934578X1501001028. [DOI] [PubMed] [Google Scholar]
- 253.Owk A.K., Lagudu M.N. Bridelia retusa (L.) Spreng. Fruits: Antimicrobial efficiency and their phytochemical constituents. Not. Sci. Biol. 2016;8:33–36. doi: 10.15835/nsb819769. [DOI] [Google Scholar]
- 254.Hernández-Díaz J.A., Garza-García J.J., León-Morales J.M., Zamudio-Ojeda A., Arratia-Quijada J., Velázquez-Juárez G., López-Velázquez J.C., García-Morales S. Antibacterial activity of biosynthesized selenium nanoparticles using extracts of Calendula officinalis against potentially clinical bacterial strains. Molecules. 2021;26:5929. doi: 10.3390/molecules26195929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Saddiq A.A., Tag H.M., Doleib N.M., Salman A.S., Hagagy N. Antimicrobial, antigenotoxicity, and characterization of Calotropis procera and its rhizosphere-inhabiting Actinobacteria: In vitro and in vivo studies. Molecules. 2022;27:3123. doi: 10.3390/molecules27103123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mubashir S., Yousuf Dar M., Lone B.A., Zargar M.I., Shah W.A. Anthelmintic, antimicrobial, antioxidant and cytotoxic activity of Caltha palustris Var. Alba Kashmir, India. Chin. J. Nat. Med. 2014;12:567–572. doi: 10.1016/S1875-5364(14)60087-X. [DOI] [PubMed] [Google Scholar]
- 257.Blaskovich M.A.T., Kavanagh A.M., Elliott A.G., Zhang B., Ramu S., Amado M., Lowe G.J., Hinton A.O., Pham D.M.T., Zuegg J., et al. The Antimicrobial potential of Cannabidiol. Commun Biol. 2021;4:7. doi: 10.1038/s42003-020-01530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chaudhary A.K., Ahmad S., Mazumder A. Cedrus deodara (Roxb.) Loud.: A review on its ethnobotany, phytochemical and pharmacological profile. Pharmacogn. J. 2011;3:12–17. doi: 10.5530/pj.2011.23.2. [DOI] [Google Scholar]
- 259.Joshi S., Subedi P.C. Phytochemical and biological studies on essential oil and leaf extracts of Gaultheria fragrantissima Wall. Nepal J. Sci. Technol. 2013;14:59–64. doi: 10.3126/njst.v14i2.10416. [DOI] [Google Scholar]
- 260.Shaikh T., Rub R.A., Sasikumar S. Antimicrobial screening of Cichorium intybus seed extracts. Arab. J. Chem. 2016;9:S1569–S1573. doi: 10.1016/j.arabjc.2012.04.012. [DOI] [Google Scholar]
- 261.Singh C., Singh S., Pande C., Tewari G., Pande V., Sharma P. Exploration of antimicrobial potential of essential oils of Cinnamomum glanduliferum, Feronia elephantum, Bupleurum hamiltonii and Cyclospermum leptophyllum against foodborne pathogens. Pharm. Biol. 2013;51:1607–1610. doi: 10.3109/13880209.2013.805234. [DOI] [PubMed] [Google Scholar]
- 262.Ngoci N.S., Ramadhan M., Ngari M.S., Leonard O.P. Screening for antimicrobial activity of Cissampelos pareira L. methanol root extract. Eur. J. Med. Plants. 2012;4:45–51. doi: 10.9734/EJMP/2014/5464. [DOI] [Google Scholar]
- 263.Ghori N.-H., Khan M., Hayat M.Q. Phytochemical analyses for antibacterial activity and therapeutic compounds of Convolvulus arvensis L., collected from the salt range of Pakistan. Adv. Life Sci. 2015;2:83–90. [Google Scholar]
- 264.Kačániová M., Galovičová L., Ivanišová E., Vukovic N.L., Štefániková J., Valková V., Borotová P., Žiarovská J., Terentjeva M., Felšöciová S., et al. Antioxidant, antimicrobial and antibiofilm activity of Coriander (Coriandrum sativum L.) essential oil for its application in foods. Foods. 2020;9:282. doi: 10.3390/foods9030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mykhailenko O., Kovalyov V., Goryacha O., Ivanauskas L., Georgiyants V. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry. 2019;162:56–89. doi: 10.1016/j.phytochem.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 266.Essien E., Newby J., Walker T., Setzer W., Ekundayo O. Chemotaxonomic characterization and in-vitro antimicrobial and cytotoxic activities of the leaf essential oil of Curcuma longa grown in Southern Nigeria. Medicines. 2015;2:340–349. doi: 10.3390/medicines2040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Paudel P., Satyal P., Maharjan S., Shrestha N., Setzer W.N. Volatile analysis and antimicrobial screening of the parasitic plant Cuscuta reflexa Roxb. from Nepal. Nat. Prod. Res. 2014;28:106–110. doi: 10.1080/14786419.2013.847440. [DOI] [PubMed] [Google Scholar]
- 268.Shah G., Shri R., Panchal V., Sharma N., Singh B., Mann A. Scientific basis for the therapeutic use of Cymbopogon citratus, Stapf (lemon grass) J. Adv. Pharm. Technol. Res. 2011;2:3. doi: 10.4103/2231-4040.79796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Eltayeib A.A., Um Ismaeel H. Extraction of Cyperus rotundus rhizomes oil, identification of chemical constituents and evaluation of antimicrobial activity of the oil in North Kordofan state. Int. J. Adv. Res. Chem. Sci. 2014;1:18–29. [Google Scholar]
- 270.Cheypratub P., Leeanansaksiri W., Eumkeb G. The synergy and mode of action of Cyperus Rotundus L. Extract plus ampicillin against ampicillin-resistant Staphylococcus aureus. Evid.-Based Complement. Altern. Med. 2018;2018:3438453. doi: 10.1155/2018/3438453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bawazeer S., Rauf A. In vitro antibacterial and antifungal potential of amyrin-type triterpenoid isolated from Datura metel Linnaeus. Biomed. Res. Int. 2021;2021:1543574. doi: 10.1155/2021/1543574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Solomon B., Nega B., Wagaw S., Lianzhong A. Antibacterial activity of Datura stramonium against standard and clinical isolate pathogenic microorganisms. J. Med. Plants Res. 2017;11:501–506. doi: 10.5897/JMPR2017.6381. [DOI] [Google Scholar]
- 273.Dedieu L., Brunel J.M., Lorenzi V., Muselli A., Berti L., Bolla J.M. Antibacterial mode of action of the Daucus carota essential oil active compounds against Campylobacter jejuni and efflux-mediated drug resistance in gram-negative bacteria. Molecules. 2020;25:5448. doi: 10.3390/molecules25225448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kuete V., BetrandTeponno R., Mbaveng A.T., Tapondjou L.A., Meyer J.J.M., Barboni L., Lall N. Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Complement. Altern. Med. 2012;12:228. doi: 10.1186/1472-6882-12-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Joshi S.C., Verma A.R., Mathela C.S. Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food Chem. Toxicol. 2010;48:37–40. doi: 10.1016/j.fct.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 276.Al-Snafi P.D.A.E. A review on Dodonaea viscosa: A potential medicinal plant. IOSR J. Pharm. 2017;7:10–21. doi: 10.9790/3013-0702011021. [DOI] [Google Scholar]
- 277.Munir N., Mahmood Z., Riaz M., Tahir I.M., Ali Shah S.M., Bacha U., Mehmood R., Hussain S. In vitro evaluation of antimicrobial and cytotoxic potential of Epimedium grandiflorum hydroethanolic extract as natural medicine. Int. J. Nat. Med. Health Sci. 2022;2:25–31. doi: 10.52461/ijnms.v2i1.1229. [DOI] [Google Scholar]
- 278.Mir M., Ashraf W., Mir B., Biomater Antimicrobial and antifungal and phytochemical analysis of various extracts of Equisetum diffusum. Biomater. Artif. Cells Artif. Organs. 2021;35:186–189. [Google Scholar]
- 279.Salehi B., Iriti M., Vitalini S., Antolak H., Pawlikowska E., Kręgiel D., Sharifi-Rad J., Oyeleye S.I., Ademiluyi A.O., Czopek K., et al. Euphorbia-derived natural products with potential for use in health maintenance. Biomolecules. 2019;9:337. doi: 10.3390/biom9080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kwon Y.S., Choi W.G., Kim W.J., Kim W.K., Kim M.J., Kang W.H., Kim C.M. Antimicrobial constituents of Foeniculum vulgare. Arch. Pharm. Res. 2002;25:154–157. doi: 10.1007/BF02976556. [DOI] [PubMed] [Google Scholar]
- 281.Bhat B.A., Mir W.R., Sheikh B.A., Rather M.A., Dar Tul H., Mir M.A. In Vitro and in silico evaluation of antimicrobial properties of Delphinium cashmerianum L., a medicinal herb growing in Kashmir, India. J. Ethnopharmacol. 2022;291:115046. doi: 10.1016/j.jep.2022.115046. [DOI] [PubMed] [Google Scholar]
- 282.Jameel M., Islamuddin M., Ali A., Afrin F., Ali M. Isolation, characterization and antimicrobial evaluation of a novel compound N-Octacosan 7β Ol, from Fumaria parviflora Lam. BMC Complement. Altern. Med. 2014;14:98. doi: 10.1186/1472-6882-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Vlase L., Mocana A., Hanganu D., Benedec D., Gheldiu A.-M., Crişan G. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four Galium species (Rubiaceae) Dig. J. Nanomater. Biostruct. 2014;9:1085–1094. [Google Scholar]
- 284.Skinder B., Ganai B., Wani A. Scientific study of Gentiana kurroo Royle. Medicines. 2017;4:74. doi: 10.3390/medicines4040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Abbasi B.A., Iqbal J., Ahmad R., Zia L., Kanwal S., Mahmood T., Wang C., Chen J.-T. Bioactivities of Geranium wallichianum leaf extracts conjugated with zinc oxide nanoparticles. Biomolecules. 2019;10:38. doi: 10.3390/biom10010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Njogu P.M., Thoithi G.N., Mwangi J.W., Kamau F.N., Kibwage I.O., Kariuki S.T., Yenesew A., Mugo H.N., Mwalukumbi J.M. Phytochemical and antimicrobial investigation of Girardinia diversifolia (Link) Friis (Urticaceae) East Cent. Afr. J. Pharm. Sci. 2011;14:89–94. [Google Scholar]
- 287.Rawat S., Jugran A.K., Bahukhandi A., Bahuguna A., Bhatt I.D., Rawal R.S., Dhar U. Anti-oxidant and anti-microbial properties of some ethno-therapeutically important medicinal plants of Indian Himalayan Region. 3 Biotech. 2016;6:154. doi: 10.1007/s13205-016-0470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Arora R., Mazumder A. Phytochemical screening and antimicrobial activity of rhizomes of Hedychium spicatum. Pharmacogn. J. 2017;9:s64–s68. doi: 10.5530/pj.2017.6s.159. [DOI] [Google Scholar]
- 289.Begum S., Saxena B., Goyal M., Ranjan R., Joshi V.B., Rao C.V., Krishnamurthy S., Sahai M. Study of anti-inflammatory, analgesic and antipyretic activities of seeds of Hyoscyamus niger and Isolation of a New Coumarinolignan. Fitoterapia. 2010;81:178–184. doi: 10.1016/j.fitote.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 290.Nazlı O., Baygar T., Demirci Dönmez Ç.E., Dere Ö., Uysal A.İ., Aksözek A., Işık C., Aktürk S. Antimicrobial and antibiofilm activity of polyurethane/Hypericum perforatum extract (PHPE) composite. Bioorg. Chem. 2019;82:224–228. doi: 10.1016/j.bioorg.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 291.Priydarshi R., Melkani A.B., Mohan L., Pant C.C. Terpenoid composition and antibacterial activity of the essential oil from Inula cappa (Buch-Ham. Ex. D. Don) DC. J. Essent. Oil Res. 2016;28:172–176. doi: 10.1080/10412905.2015.1090935. [DOI] [Google Scholar]
- 292.Liu C., Mishra A.K., He B., Tan R. Antimicrobial activities of Isoalantolactone, a major sesquiterpene lactone OfInula racemosa. Chin. Sci. Bull. 2001;46:498–501. doi: 10.1007/BF03187267. [DOI] [Google Scholar]
- 293.Wagay J.I., Jain K. Phytochemical analysis and antimicrobial activity of Iris Kashmiriana and Iris ensata extracts against selected microorganisms. J. Drug Deliv. Ther. 2018;8:28–34. doi: 10.22270/jddt.v8i6.1975. [DOI] [Google Scholar]
- 294.Zakir M., Kumar N. Phytochemical screening and antimicrobial activity of extracts of Iris nepalensis species growing in Karnah Vallay, Kupwara, Jammu and Kashmir, India. SSR Inst. Int. J. Life Sci. 2021;7:2754–2762. doi: 10.21276/SSR-IIJLS.2021.7.1.4. [DOI] [Google Scholar]
- 295.Gunasekara T., Radhika N., Ragunathan K., Gunathilaka D., Weerasekera M., Hewageegana H., Arawwawala L., Fernando S. Determination of antimicrobial potential of five herbs used in ayurveda practices against Candida albicans, Candida parapsilosis and methicillin resistant Staphylococcus aureus. Anc. Sci. Life. 2017;36:187. doi: 10.4103/asl.ASL_179_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Rather M.A., Dar B.A., Dar M.Y., Wani B.A., Shah W.A., Bhat B.A., Ganai B.A., Bhat K.A., Anand R., Qurishi M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine. 2012;19:1185–1190. doi: 10.1016/j.phymed.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 297.Stappen I., Tabanca N., Ali A., Wedge D.E., Wanner J., Kaul V.K., Lal B., Jaitak V., Gochev V.K., Schmidt E. Chemical composition and biological activity of essential oils from wild growing aromatic plant species of Skimmia laureola and Juniperus macropoda from Western Himalaya. Nat. Prod. Commun. 2015;10:1071–1074. doi: 10.1177/1934578X1501000669. [DOI] [PubMed] [Google Scholar]
- 298.Menpara D., Desai D., Rathod T., Chanda S. Evaluation of Nutraceutical Bottle Gourd (Lagenaria siceraria) as a potential source of natural antimicrobial agent. Am. J. Phytomed. Clin. Ther. 2014;2:375–389. [Google Scholar]
- 299.Insawang S., Pripdeevech P., Tanapichatsakul C., Khruengsai S., Monggoot S., Nakham T., Artrod A., D’Souza P.E., Panuwet P. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas Cultivars Grown in Thailand. Chem. Biodivers. 2019;16:e1900371. doi: 10.1002/cbdv.201900371. [DOI] [PubMed] [Google Scholar]
- 300.Comai S., Dall’Acqua S., Grillo A., Castagliuolo I., Gurung K., Innocenti G. essential Oil of Lindera neesiana Fruit: Chemical analysis and its potential use in topical applications. Fitoterapia. 2010;81:11–16. doi: 10.1016/j.fitote.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 301.Gangwar M., Goel R.K., Nath G. Mallotus philippinensis Muell. Arg (Euphorbiaceae): Ethnopharmacology and phytochemistry review. BioMed Res. Int. 2014;2014:213973. doi: 10.1155/2014/213973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Bilal M., Parveen A., Fiaz A., Mazhar M. In vitro phytochemical analysis, antimicrobial and antioxidant activity of Mallotus philippinensis. Int. J. Nat. Med. Health Sci. 2022;2:11–16. doi: 10.52461/ijnms.v2i1.1237. [DOI] [Google Scholar]
- 303.Saleem U., Khalid S., Zaib S., Anwar F., Ahmad B., Ullah I., Zeb A., Ayaz M. Phytochemical analysis and wound healing studies on ethnomedicinally important plant Malva neglecta Wallr. J. Ethnopharmacol. 2020;249:112401. doi: 10.1016/j.jep.2019.112401. [DOI] [PubMed] [Google Scholar]
- 304.Aćimović M., Jeremić K., Salaj N., Gavarić N., Kiprovski B., Sikora V., Zeremski T. Marrubium vulgare L.: A phytochemical and pharmacological overview. Molecules. 2020;25:2898. doi: 10.3390/molecules25122898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Khan A.V., Ahmed Q.U., Mir M.R., Shukla I., Khan A.A. Antibacterial efficacy of the seed extracts of Melia azedarach against some hospital isolated human pathogenic bacterial strains. Asian Pac. J. Trop. Biomed. 2011;1:452–455. doi: 10.1016/S2221-1691(11)60099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kumar A., Varshney V.K., Prasad R., Rawat MS M., Stashenko E.E. In vitro antioxidant, antifungal and antibacterial activities of essential oil of Morina longifolia Wall. leaves. J. Biol. Act. Prod. Nat. 2013;3:183–193. [Google Scholar]
- 307.Joshi R.K., Mathela C.S. Volatile oil composition of Morina longifolia Wall. Ex. Dc. from Himalayan region of Uttarakhand. Asian J. Res. Pharm. Sci. 2013;3:12–14. [Google Scholar]
- 308.Ghosh A., Zhu E.V., Wang H., Zurek L., Zhu J.J. Antibacterial activities of Nepetalactones against public health-related pathogens. Nat. Prod. Commun. 2021;16:1934578X211004875. doi: 10.1177/1934578X211004875. [DOI] [Google Scholar]
- 309.Adiguzel A., Ozer H., Sokmen M., Gulluce M., Sokmen A., Kilic H., Sahin F., Baris O. Antimicrobial and antioxidant activity of the essential oil and methanol extract of Nepeta cataria. Pol. J. Microbiol. 2009;58:69–76. [PubMed] [Google Scholar]
- 310.Satyal P., Chhetri B.K., Dosoky N.S., Poudel A., Setzer W.N. Chemical composition of Nardostachys grandiflora rhizome oil from Nepal– A contribution to the chemotaxonomy and bioactivity of Nardostachys. Nat. Prod. Commun. 2015;10:1067–1070. doi: 10.1177/1934578X1501000668. [DOI] [PubMed] [Google Scholar]
- 311.Mukherjee S., Koley H., Barman S., Mitra S., Datta S., Ghosh S., Paul D., Dhar P. Oxalis corniculata (Oxalidaceae) leaf extract exerts in vitro antimicrobial and in vivo anticolonizing activities against Shigella dysenteriae 1 (NT4907) and Shigella flexneri 2a (2457T) in induced diarrhea in suckling mice. J. Med. Food. 2013;16:801–809. doi: 10.1089/jmf.2012.2710. [DOI] [PubMed] [Google Scholar]
- 312.Ahmad M., Malik K., Tariq A., Zhang G., Yaseen G., Rashid N., Sultana S., Zafar M., Ullah K., Khan M.P.Z. Botany, ethnomedicines, phytochemistry and pharmacology of Himalayan Paeony (Paeonia emodi Royle.) J. Ethnopharmacol. 2018;220:197–219. doi: 10.1016/j.jep.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 313.Huq A.K.M.M., Jamal J.A., Stanslas J. Ethnobotanical, phytochemical, pharmacological, and toxicological aspects of Persicaria hydropiper (L.) Delarbre. Evid.-Based Complement. Altern. Med. 2014;2014:782830. doi: 10.1155/2014/782830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Adom M.B., Taher M., Mutalabisin M.F., Amri M.S., Abdul Kudos M.B., Wan Sulaiman M.W.A., Sengupta P., Susanti D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017;96:348–360. doi: 10.1016/j.biopha.2017.09.152. [DOI] [PubMed] [Google Scholar]
- 315.Kalam M.A., Malik A.H., Ganie A.H., Butt T.A. Medicinal importance of Papra (Podophyllum hexandrum Royle) in Unani system of medicine. J. Complement. Integr. Med. 2021;18:485–490. doi: 10.1515/jcim-2020-0178. [DOI] [PubMed] [Google Scholar]
- 316.Li M., Zhou L., Yang D., Li T., Li W. Biochemical composition and antioxidant capacity of extracts from Podophyllum hexandrum rhizome. BMC Complement. Altern. Med. 2012;12:263. doi: 10.1186/1472-6882-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Gosset-Erard C., Zhao M., Lordel-Madeleine S., Ennahar S. Identification of Punicalagin as the bioactive compound behind the antimicrobial activity of Pomegranate (Punica granatum L.) peels. Food Chem. 2021;352:129396. doi: 10.1016/j.foodchem.2021.129396. [DOI] [PubMed] [Google Scholar]
- 318.Silvan J.M., Michalska-Ciechanowska A., Martinez-Rodriguez A.J. Modulation of antibacterial, antioxidant, and anti-inflammatory properties by drying of Prunus domestica L. plum juice extracts. Microorganisms. 2020;8:119. doi: 10.3390/microorganisms8010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rolta R., Kumar V., Sourirajan A., Upadhyay N.K., Dev K. Bioassay guided fractionation of rhizome extract of Rheum emodi Wall as bio-availability enhancer of antibiotics against bacterial and fungal pathogens. J. Ethnopharmacol. 2020;257:112867. doi: 10.1016/j.jep.2020.112867. [DOI] [PubMed] [Google Scholar]
- 320.Innocenti G., Dall’Acqua S., Scialino G., Banfi E., Sosa S., Gurung K., Barbera M., Carrara M. Chemical composition and biological properties of Rhododendron anthopogon essential oil. Molecules. 2010;15:2326–2338. doi: 10.3390/molecules15042326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Mishra A.P., Sharifi-Rad M., Shariati M.A., Mabkhot Y.N., Al-Showiman S.S., Rauf A., Salehi B., Župunski M., Gusain P., Sharifi-Rad J. Bioactive compounds and health benefits of edible Rumex species-A review. Cell. Mol. Biol. 2018;64:27–34. doi: 10.14715/cmb/2018.64.8.5. [DOI] [PubMed] [Google Scholar]
- 322.Tawfeek N., Mahmoud M.F., Hamdan D.I., Sobeh M., Farrag N., Wink M., El-Shazly A.M. Phytochemistry, pharmacology and medicinal uses of plants of the genus Salix: An updated review. Front. Pharmacol. 2021;12:593856. doi: 10.3389/fphar.2021.593856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Cui H., Zhang X., Zhou H., Zhao C., Lin L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015;56:16. doi: 10.1186/s40529-015-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Khuda F., Ul Haq Z., Ilahi I., Ullah R., Khan A., Fouad H., Ali Khan Khalil A., Ullah Z., Umar Khayam Sahibzada M., Shah Y., et al. Synthesis of gold nanoparticles using Sambucus wightiana extract and investigation of its antimicrobial, anti-inflammatory, antioxidant and analgesic activities. Arab. J. Chem. 2021;14:103343. doi: 10.1016/j.arabjc.2021.103343. [DOI] [Google Scholar]
- 325.Omer R.E.E., Koua F.H.M., Abdelhag I.M., Ismail A.M. Gas chromatography/mass spectrometry profiling of the Costus plant Saussurea lappa (Decne.) CB Clarke root extracts and their anti-bacterial activity. J. Appl. Pharm. Sci. 2019;9:73–81. [Google Scholar]
- 326.Shah W.A., Dar M.Y., Zagar M.I., Agnihotri V.K., Qurishi M.A., Singh B. Chemical composition and antimicrobial activity of the leaf essential oil of Skimmia laureola growing wild in Jammu and Kashmir, India. Nat. Prod. Res. 2013;27:1023–1027. doi: 10.1080/14786419.2012.696252. [DOI] [PubMed] [Google Scholar]
- 327.Bártová V., Bárta J., Jarošová M. Antifungal and antimicrobial proteins and peptides of potato (Solanum tuberosum L.) tubers and their applications. Appl. Microbiol. Biotechnol. 2019;103:5533–5547. doi: 10.1007/s00253-019-09887-9. [DOI] [PubMed] [Google Scholar]
- 328.Xia D.Z., Yu X.F., Zhu Z.Y., Zou Z.D. Antioxidant and antibacterial activity of six edible wild plants (Sonchus sp.) in China. Nat. Prod. Res. 2011;25:1893–1901. doi: 10.1080/14786419.2010.534093. [DOI] [PubMed] [Google Scholar]
- 329.Semwal D., Rawat U. Antimicrobial Hasubanalactam alkaloid from Stephania glabra. Planta. Med. 2009;75:378–380. doi: 10.1055/s-0028-1112223. [DOI] [PubMed] [Google Scholar]
- 330.Kenny O., Brunton N.P., Walsh D., Hewage C.M., McLoughlin P., Smyth T.J. Characterisation of antimicrobial extracts from Dandelion root (Taraxacum officinale) using LC-SPE-NMR. Phytother. Res. 2015;29:526–532. doi: 10.1002/ptr.5276. [DOI] [PubMed] [Google Scholar]
- 331.Ahmed Q., Gupta N., Kumar A., Nimesh S. Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif. Cells Nanomed. Biotechnol. 2017;45:1192–1200. doi: 10.1080/21691401.2016.1215328. [DOI] [PubMed] [Google Scholar]
- 332.Bag A., Bhattacharyya S.K., Chattopadhyay R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013;3:244–252. doi: 10.1016/S2221-1691(13)60059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mandeville A., Cock I.E. Terminalia chebula Retz. fruit extracts inhibit bacterial triggers of some autoimmune diseases and potentiate the activity of tetracycline. Indian J. Microbiol. 2018;58:496–506. doi: 10.1007/s12088-018-0754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Kher M.N., Sheth N.R., Bhatt V.D. In vitro antibacterial evaluation of Terminalia chebula as an alternative of antibiotics against Bovine subclinical Mastitis. Anim. Biotechnol. 2019;30:151–158. doi: 10.1080/10495398.2018.1451752. [DOI] [PubMed] [Google Scholar]
- 335.Rather A.M., Nawchoo I.A., Ganie A.H., Singh H., Dutt B., Wani A.A. Bioactive compounds & medicinal properties of Valeriana jatamansi Jones-a review. Life Sci. J. 2012;9:847–850. [Google Scholar]
- 336.Mahdavi S., Amiradalat M., Babashpour M., Sheikhlooei H., Miransari M. The antioxidant, anticarcinogenic and antimicrobial properties of Verbascum thapsus L. Med. Chem. 2020;16:991–995. doi: 10.2174/1573406415666190828155951. [DOI] [PubMed] [Google Scholar]
- 337.Gautam S.S., Bithel N., Kumar S., Painuly D., Singh J. A new derivative of ionone from aerial parts of Viola odorata Linn. and its antibacterial role against respiratory pathogens. Clin. Phytosci. 2017;2:1–5. doi: 10.1186/s40816-016-0018-3. [DOI] [Google Scholar]
- 338.García-García J.D., Anguiano-Cabello J.C., Arredondo-Valdés R., Candido del Toro C.A., Martínez-Hernández J.L., Segura-Ceniceros E.P., Govea-Salas M., González-Chávez M.L., Ramos-González R., Esparza-González S.C. Phytochemical characterization of Phoradendron bollanum and Viscum album Subs. Austriacum as mexican mistletoe plants with antimicrobial activity. Plants. 2021;10:1299. doi: 10.3390/plants10071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kamruzzaman M., Bari S.M.N., Faruque S.M. In vitro and in vivo bactericidal activity of Vitex negundo leaf extract against diverse multidrug resistant enteric bacterial pathogens. Asian Pac. J. Trop. Med. 2013;6:352–359. doi: 10.1016/S1995-7645(13)60038-3. [DOI] [PubMed] [Google Scholar]
- 340.Applequist W.L., Brinckmann J.A., Cunningham A.B., Hart R.E., Heinrich M., Katerere D.R., van Andel T. Scientists’ Warning on Climate Change and Medicinal Plants. Planta Med. 2020;86:10–18. doi: 10.1055/a-1041-3406. [DOI] [PubMed] [Google Scholar]
- 341.Sobuj N., Virjamo V., Zhang Y., Nybakken L., Julkunen-Tiitto R. Impacts of elevated temperature and CO2 concentration on growth and phenolics in the sexually dimorphic Populus tremula (L. ). Environ. Exp. Bot. 2018;146:34–44. doi: 10.1016/j.envexpbot.2017.08.003. [DOI] [Google Scholar]
- 342.Arab L., Kreuzwieser J., Kruse J., Zimmer I., Ache P., Alfarraj S., Al-Rasheid K.A.S., Schnitzler J.-P., Hedrich R., Rennenberg H. Acclimation to heat and drought—Lessons to learn from the date palm (Phoenix dactylifera) Environ. Exp. Bot. 2016;125:20–30. doi: 10.1016/j.envexpbot.2016.01.003. [DOI] [Google Scholar]
- 343.Rehman S., Iqbal Z., Qureshi R., Ur Rahman I., Khan M.A., Elshaer M.M.A., Al Farraj D.A., Elshikh M.S., Younas M., Sakhi S., et al. Ethnogynaecological knowledge of traditional medicinal plants used by the indigenous communities of north waziristan, Pakistan. Evid. Based Complement. Alternat. Med. 2022;2022:6528264. doi: 10.1155/2022/6528264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Allard T., Wenner T., Greten H.J., Efferth T. Mechanisms of herb-induced nephrotoxicity. Curr. Med. Chem. 2013;20:2812–2819. doi: 10.2174/0929867311320220006. [DOI] [PubMed] [Google Scholar]
- 345.Wattanathorn J., Uabundit N., Itarat W., Mucimapura S., Laopatarakasem P., Sripanidkulchai B. Neurotoxicity of Coscinium fenestratum stem, a medicinal plant used in traditional medicine. Food Chem. Toxicol. 2006;l44:1327–1333. doi: 10.1016/j.fct.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 346.Parez B.E., Rodriquez O.R., Sanchez V.M.C. Toxic plants: Brugmansia (floripondio) neurotoxicity. Arch. Med. Urg. Mex. 2012;4:119–124. [Google Scholar]
- 347.Maffè S., Paffoni P., Laura Colombo M., Davanzo F., Dellavesa P., Cucchi L., Zenone F., Paino A.M., Franchetti Pardo N., Bergamasco L., et al. Herbs and cardiotoxic effects. G. Ital. Cardiol. 2013;14:445–455. doi: 10.1714/1280.14158. [DOI] [PubMed] [Google Scholar]
- 348.Andrade R.J., Robles M., Fernández-Castañer A., López-Ortega S., López-Vega M.C., Lucena M.I. Assessment of drug-induced hepatotoxicity in clinical practice: A challenge for gastroenterologists. World J. Gastroenterol. 2013;13:329–340. doi: 10.3748/wjg.v13.i3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Vinogradova N., Glukhov A., Chaplygin V., Kumar P., Mandzhieva S., Minkina T., Rajput V.D. The Content of Heavy Metals in Medicinal Plants in Various Environmental Conditions: A Review. Horticulturae. 2023;9:239. doi: 10.3390/horticulturae9020239. [DOI] [Google Scholar]
- 350.Roufogalis B.D. Challenges in integrating herbal medicine in healthcare systems. A Perspect. Focus Altern. Complement. Ther. 2015;20:34–35. doi: 10.1111/fct.12165. [DOI] [Google Scholar]
- 351.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sharma S. Current status of herbal product: Regulatory overview. Jr. Pharm. Bioallied Sci. 2015;7:293–296. doi: 10.4103/0975-7406.168030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Smith A., Jogalekar S., Gibson A. Regulation of natural health products in Canada. J. Ethanopharmacol. 2014;158 Pt B:507–510. doi: 10.1016/j.jep.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 354.Harnett J., McIntyre E., Adams J., Addison T., Bannerman H., Egelton L., Ma J., Zabakly L., Steel A. Prevalence and Characteristics of Australians Complementary Medicine Product Use, and Concurrent Use with Prescription and Over-the-Counter Medications-A Cross Sectional Study. Nutrients. 2023;15:327. doi: 10.3390/nu15020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sahoo N., Manchikanti P. Herbal drug regulation and commercialization: An Indian industry perspective. J. Alt. Comp. Med. 2013;19:957–963. doi: 10.1089/acm.2012.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.