Abstract
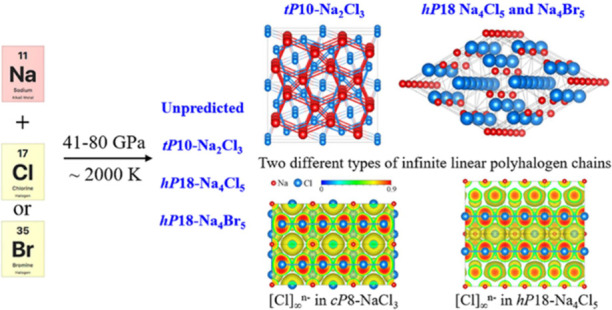
The field of polyhalogen chemistry, specifically polyhalogen anions (polyhalides), is rapidly evolving. Here, we present the synthesis of three sodium halides with unpredicted chemical compositions and structures (tP10-Na2Cl3, hP18-Na4Cl5, and hP18-Na4Br5), a series of isostructural cubic cP8-AX3 halides (NaCl3, KCl3, NaBr3, and KBr3), and a trigonal potassium chloride (hP24-KCl3). The high-pressure syntheses were realized at 41–80 GPa in diamond anvil cells laser-heated at about 2000 K. Single-crystal synchrotron X-ray diffraction (XRD) provided the first accurate structural data for the symmetric trichloride Cl3– anion in hP24-KCl3 and revealed the existence of two different types of infinite linear polyhalogen chains, [Cl]∞n– and [Br]∞n–, in the structures of cP8-AX3 compounds and in hP18-Na4Cl5 and hP18-Na4Br5. In Na4Cl5 and Na4Br5, we found unusually short, likely pressure-stabilized, contacts between sodium cations. Ab initio calculations support the analysis of structures, bonding, and properties of the studied halogenides.
Keywords: halogen bonding, polyhalogen anions, alkali halides, high-pressure chemistry, X-ray diffraction
1. Introduction
High pressure (HP) dramatically changes the chemistry of materials.1 Quantum-chemical calculations predict unusual but stable stoichiometries of alkali halides, such as, for example, NaCl3 and NaCl7,2 Na2Cl, Na3Cl2, and Na4Cl3,2,3 LinI (n = 2–5),4 and CsFm (m = 2–6),5,6 and suggest uncommon properties. Despite the numerous predictions, very few of them have been experimentally confirmed.
So far, the syntheses of the compounds with the stoichiometries AX3, A3X, and AX5 (A is an alkali metal, and X is a halogen) have been reported at high pressures. The list includes, for example, polymorphs of NaCl3 (space groups Pnma and Pm3̅n),2 KCl3 (Pm3̅n and P3̅c1),7 KBr3 (Pnma and P3̅c1),8 CsI3 (Pnma, P3̅c1, and Pm3̅n),9,10 and Na3Cl (P4/mmm)2 and KBr5 (P21)8 compounds. The majority of them were characterized in a diamond anvil cell (DAC) using powder X-ray diffraction (XRD) and Le Bail analysis, and only the two polymorphs of CsI3 (Pnma and P3̅c1)10 were studied using single-crystal XRD (SCXRD). Although the synergy of powder XRD and ab initio structure predictions is very helpful to obtain a structure solution, the interpretation of some powder XRD remains ambiguous (e.g., those of KBr3 and KBr5).8
SCXRD provides both a precise structure determination and the chemical composition of the products of reactions in a laser-heated DAC (LHDAC) and has become the ultimate method of HP chemical crystallography.11−14 In this work, using SCXRD on samples in LHDACs, we have studied chemical reactions in the four A–X systems (Na–Cl, Na–Br, K–Cl, and K–Br) at different pressures (see Table S1). As a result, a number of cP8-AX3 isostructural cubic (Pm3̅n) compounds (NaCl3, KCl3, NaBr3, and KBr3) and a trigonal (P3̅c1) hP24-KCl3 were synthesized; their structures were solved and refined. Sodium and potassium tribromides were previously unknown. New polyhalides of sodium, Na4Cl5 and Na4Br5, and a sodium sesquichloride, Na2Cl3, with hitherto unpredicted compositions and structures, were obtained and fully characterized using SCXRD. Our ab initio calculations reveal electronic properties and chemical bonding in novel compounds.
2. Experimental Method
Experiments have been performed in the pressure range of 41–80 GPa (for details see Supplementary Methods). As starting materials, high-purity, well-dried NaCl, NaBr, and KBr were used. A chosen alkali halide was loaded into a DAC along with either liquid carbon tetrachloride (CCl4) or solid carbon tetrabromide (CBr4), which decompose under laser heating above 40 GPa15−17 and serve as sources of the halogen atoms. As far as carbon from diamond anvils is unavoidably present in the pressure chamber,18 carbon-containing CCl4 and CBr4 do not introduce extra contamination of the system. The samples were compressed to the target pressures and laser-heated. Then SCXRD was collected in situ at room temperature. A summary of all LHDAC experiments is provided in Table S1.
3. Results and Discussion
3.1. Observation of the AX3 Compounds
The formation of cP8-AX3 isostructural halides (Pearson symbol cP8; space group Pm3̅n, #223), NaCl3, KCl3, KBr3, and NaBr3–x, was observed in the corresponding A–X systems. Their structures were solved and refined; full crystallographic data and refinement details are provided in Tables S2–S6. Na (or K) occupies the 2a Wyckoff position (000) in the nodes of the bcc lattice. Cl (or Br) atoms in the 6d Wyckoff position (1/4 1/2 0) form linear chains (Figure S1a). cP8-KBr3 and cP8-NaBr3–x were experimentally observed for the first time. According to the structure refinement, the occupancy of the Br1 atomic position in cP8-NaBr3–x is partial and equal to 0.72(8) and 0.757(17) at 46 and 73 GPa, respectively. The refinement of the structure model with the full occupancy of the Br1 atomic position results in larger values of the agreement factors (R1) (Table S6). A Hamilton significance test19 allowed us to judge that the improvement of the agreement factors for the model with the partial occupancy may be considered significant. According to the Hamilton test, the structure model with the partial occupancy of the Br1 atomic position is preferable with the 90–95% confidence level at 46 GPa and more than 99.5% at 73 GPa. The results of our density functional theory (DFT) calculations show that the parameters of the DFT-relaxed structures agree well with the experimental data (Tables S2–S5).
In the K–Cl system, we observed not only a cubic but also a trigonal polymorph of KCl3 (hP24, P3̅c1, #165) (see Table S7 for full crystallographic data). Its structure was previously known,7 but we refined it based on SCXRD analysis (Table S7 and Figure S1b). It consists of rows of potassium atoms oriented along the c direction and isolated linear [Cl3]− ions. The appearance of hP24-KCl3 after a chemical reaction in the sample (KCl + CCl4 + Cgraphite), pressurized in DAC #2 to 41 GPa, and then laser-heated, was especially remarkable. Before heating, the sample looked gray and translucent (due to graphite used as a laser light absorber), but after laser heating, it turned red and transparent (Figure S2a, left). Upon further compression to 50 GPa, the color became much darker (Figure S2b, left) that can be explained by the decreasing of the band gap of the hP24-KCl3 semiconductor material as revealed by our ab initio calculations (Figure S2a,b, right). For hP24-KCl3 and all cP8-AX3 compounds (cP8-NaCl3, cP8-KCl3, and cP8-KBr3), our experimental data on the pressure dependence of the volume per atom (V0/atom) fit well with the results of our DFT calculations (Figure S3a,b).
3.2. High-Pressure Synthesis of Na2Cl3, Na4Cl5, and Na4Br5: Crystal Structures, Stability, and Raman Spectra
SCXRD analysis using the DAFi program12 revealed three other sodium chlorine and sodium bromine compounds which have never been predicted from ab initio calculations: the isostructural hP18-Na4Cl5 and hP18-Na4Br5 (space group P63/mcm, #193) and tP10-Na2Cl3 (space group P4/mbm, #127) (see Tables S8–S10 for crystallographic details). Their chemical formulas resulted from a structure solution and refinement.
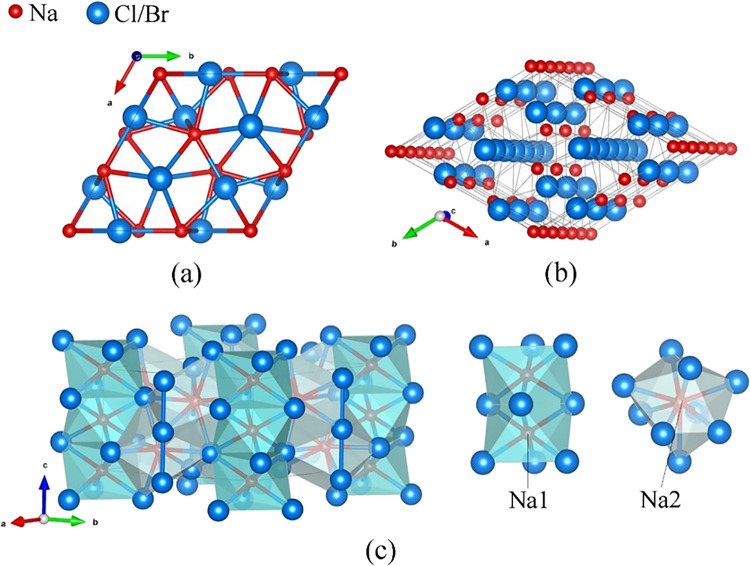
The hP18-Na4Cl5 compound was first synthesized at 50 GPa and 2100 K. At 50 GPa, it has the following lattice parameters: a = 7.329(2) Å and c = 4.776(18) Å. In its crystal structure (see Figure 1 and Table S8, and the CIF deposited at CSD 2224055), Na1 and Na2 are in the 2b and 6g Wyckoff sites, and Cl1 and Cl2 are in 4d and 6g, respectively. Cl1 atoms form linear chains aligned along the c direction with the Cl1–Cl1 distance of 2.3879(9) Å. Na1 atoms also form linear chains aligned along the c direction with the Na1–Na1 distance of 2.3879(9) Å (Figure 1b). Na1 atoms are coordinated by six Cl2 atoms forming an octahedron with an edge length of 3.1314(17) Å. The face-sharing Na1Cl26 octahedra form columns in the polyhedral model of the hP18-Na4Cl5 structure (Figure 1c). Na2 is coordinated by both the Cl1 and Cl2 atoms with the coordination number CN = 9, forming Na2Cl9 polyhedra (distorted capped square antiprisms) filling the space between the columns of the face-sharing Na1Cl26 octahedra (Figure 1c).
Figure 1.
Structure of the novel isostructural sodium polychloride hP18-Na4Cl5 and sodium polybromide hP18-Na4Br5 synthesized in this work. (a) Unit cell viewed along the c direction; Na atoms are red, and Cl (or Br) atoms are blue; (b) perspective view of the structure highlighting the linear polyhalogen chains along the c direction; and (c) polyhedral model of the structure; coordination polyhedra for Na1 and Na2 atoms are shown separately.
hP18-Na4Br5, observed at 48 and 73 GPa (see Table S1), was found to be isostructural to hP18-Na4Cl5. For crystallographic details, see Table S9 and the CIF deposited at CSD 2224057.
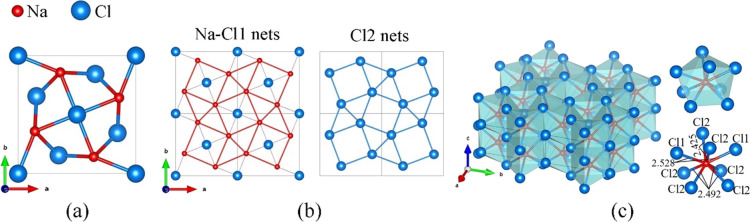
The chemical reaction of NaCl with CCl4 in DAC #1 (Table S1) at 50 GPa and 2100 K led to the formation of numerous good-quality single-crystal domains, which were identified as a sesquichloride of sodium, tP10-Na2Cl3 (space group P4/mbm, #127), also observed at 56 GPa (see Tables S1 and S10 and the CIF deposited at CSD 2224059). This compound has not been observed or predicted so far. In the crystal structure of tP10-Na2Cl3 (Figure 2), Na atoms occupy the 4g Wyckoff site, and Cl1 and Cl2—the 2a and 4h sites, respectively. Its lattice parameters at 50(1) GPa are a = 6.569(2) Å and c = 3.076(16) Å. The unit cell, as viewed along the c direction, is shown in Figure 2a. The structure can be easily visualized as an alternation of Na–Cl1 and Cl2 nets along the c direction (Figure 2b). In the Na–Cl1 nets, Cl1 atoms are located in the centers of squares of the 32.4.3.4 2D tiling formed by Na atoms in the ab plane (Figure 2b, left), which has the same topology as Cl2 nets (Figure 2b, right). For a polyhedral presentation, one should consider that Na has the CN = 8 with respect to Cl atoms forming a distorted square antiprism (Figure 2c) with an average Na–Cl distance of 2.484(3) Å. These antiprisms belong to the class of hendecahedrons (polyhedra with 11 faces) which, by sharing common faces, make a 3D tiling of the whole space (Figure 2c). The structure of tP10-Na2Cl3 can be viewed as “inverse” of that predicted for Na3Cl22 if sodium and chlorine would swap their positions.
Figure 2.
Structure of the novel sodium sesquichloride tP10-Na2Cl3 synthesized in this work. (a) Unit cell viewed along the c direction; Na atoms are red, and Cl atoms are blue; (b) Na–Cl1 nets and Cl2 nets, which alternate in the c direction (Na and Cl2 form the 2D tiling of the same 32.4.3.4 topology); and (c) polyhedral model of the structure; the Na coordination polyhedron is shown separately.
Our DFT calculations reproduced the crystal structures of hP18-Na4X5 (X = Cl or Br) and tP10-Na2Cl3 (Tables S8–S10). The pressure dependences of the volume per atom for these compounds (based on the pressure–volume relations from our DFT calculations), in comparison with experimental data, are shown in Figure S3c,d. The parameters of the third-order Birch–Murnaghan (BM3) equations of states (EOSes) are provided in Table S11. Our DFT results confirmed the dynamical stability of hP18-Na4Cl5 and hP18-Na4Br5 above 10 and 30 GPa, respectively (Figure S4), and indicated their thermodynamic stability on the convex hull diagram (see Figure 3 for hP18-Na4Cl5 and Figure S5 for hP18-Na4Br5).
Figure 3.
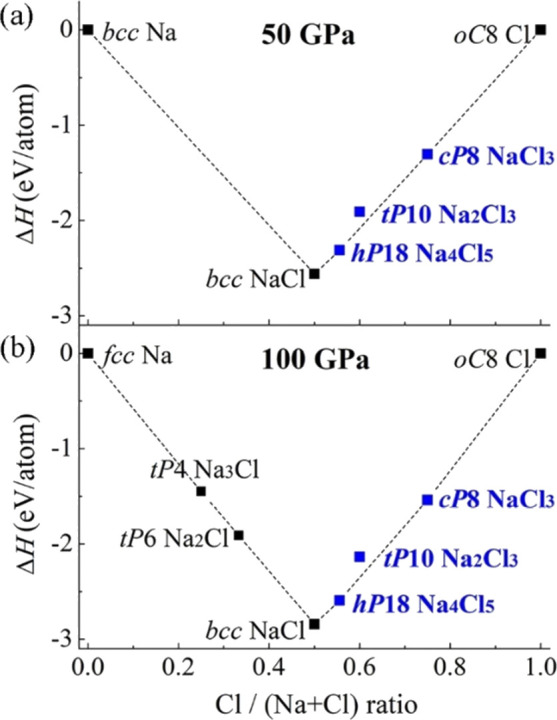
Convex hull diagrams for the Na–Cl system at different pressures. (a) 50 GPa; (b) 100 GPa. The compounds synthesized in this work are highlighted in blue bold font.
Considering the stability of tP10-Na2Cl3, we have found that at its synthesis pressure of about 50 GPa, the corresponding point is located 111 meV/atom above the static convex hull simulated at 0 K (Figure 3a). Harmonic phonon dispersion calculated at 50 GPa shows imaginary modes (Figure S6a), which contradicts our experimental observations. The finite-temperature calculations of phonon dispersion relations at T = 300 K and at P = 50 GPa, including thermal and anharmonic effects, are found to weaken the instability (see Figure S6b) though it does not remove it completely. Increasing the pressure further stabilizes tP10-Na2Cl3: in calculations carried out at 100 GPa, the phase is dynamically stable in the harmonic approximation already at T = 0 K (Figure S6c). The disagreement between theoretical calculations and experiment on the pressure at which tP10-Na2Cl3 is stabilized might be due to limitations of DFT calculations and require further studies. However, it does not influence the principal conclusions of this work and would be reported elsewhere.
Figure S7 shows the Raman spectrum taken from the sample in DAC #1 at 50 GPa and room temperature after a NaCl + CCl4 + Cgraphite mixture was laser heated to ∼2000 K. The spatial distribution of the reaction products, which were formed upon heating, was determined using XRD mapping of the whole sample chamber. It revealed an uneven distribution of B2-NaCl, hP18-Na4Cl5, tP10-Na2Cl3, and cP8-NaCl3 phases formed in DAC #1. We calculated the positions of the Brillouin-zone-center optical phonons for cP8-NaCl3 and hP18-Na4Cl5 and could conclude that all phases might contribute to the Raman spectrum we observed. The Raman peak at 323 cm–1 (marked with an asterisk in Figure S7) can be assigned to the Ag mode of oC8 chlorine.20,21 A similar Raman spectrum was reported in ref (2), but the authors assigned it to a pure Pnma NaCl3 phase.2 Raman spectra taken from the samples in DAC #2 (Figure S8a), DAC #3 (Figure S8b), and DACs #4 and #5 (Figure S8c) give evidence of chemical reactions after heating, but we did not analyze them in detail because of the insufficient resolution of individual Raman bands.
3.3. Unraveling the Bonding Complexity of Polyhalogen Anions: Geometrical Similar but Different Infinite Linear Polyhalogen [X]∞n– Chains in cP8-NaX3 vs hP18-Na4X5
Experimental structural data and theoretical calculations provide a basis for discussing crystal chemistry and the nature of chemical bonding in the novel alkali halides. The discrete trichloride anions [Cl3]− in the structure of hP24-KCl3 were found to be linear and symmetric (at 41 GPa, the intramolecular Cl1–Cl2 distances are of 2.301(3) Å, while the shortest intermolecular ones are of 2.800(5) Å (Figure S1b)). The [Cl3]− anion in hP24-KCl3 is an example of 22-valence electron systems, which are well known in inorganic chemistry22−25 (XeF2 and trihalide anions being among them). As several so far known trichloride [Cl3]− anions are asymmetric,22,26 the [Cl3]− anion in hP24-KCl3 provides the first example of a symmetric trichloride anion, similar to the symmetric [Br3]− and [I3]− known at ambient pressure.22,27 This agrees with the empirical rule of high-pressure crystal chemistry—elements behave at HP like the elements below them in the periodic table at lower pressure.28
The cP8-AX3 (A = Na or K, X = Cl or Br) and hP18-Na4X5 phases also contain polyhalogen anions, but of a different type, infinite linear chains [Cl]∞n– and [Br]∞n–, hitherto unknown for any polyhalides at ambient condition.22 In the cP8-AX3 compounds, Cl–Cl and Br–Br distances depend on the cation. For example, according to the experimental data, at 50 GPa, Cl–Cl distances in cP8-NaCl3 and cP8-KCl3 are of 2.3535(2) and 2.4097(6) Å, respectively. This gives a difference of 0.056(1) Å. At the same pressure, in ionic high-pressure sodium and potassium chlorides, B2-NaCl and B2-KCl, they are of 2.91 and 3.04 Å, respectively, with a difference twice larger.29,30 In sodium and potassium compounds, such as NaN3/KN3, NaC2/KC2, and NaO2/KO2, featuring molecular anions with strong covalent bonds (N3–, C2–, and O2–), the intramolecular distances are almost similar31−33 (1.177 and 1.183 Å in N3– at ambient pressure, for instance32). Thus, the common crystal chemical analysis suggests that in cP8-AX3 compounds, there is a covalent interaction between halogen atoms in infinite chains but much weaker than in molecular anions.
Comparing halogen–halogen distances in the [Cl]∞n– and [Br]∞n– chains in pairs cP8-NaCl3 vs hP18-Na4Cl5 and cP8-NaBr3 vs hP18-Na4Br5, one can see that in each pair, the difference is of about 0.06 Å (∼2.38 vs ∼2.44 Å and ∼2.53 vs ∼2.60 Å in the corresponding chlorides and bromides at 40 GPa). Such a dissimilarity in the separation of halogen atoms in chains of different kind may mean an appreciable variation of formal charges if there is a correlation similar to that found for pernitrides, in which the charge of N2n– anions varies by one electron unit if its length changes by ∼0.05 Å.31
In order to further analyze the chemical bonding in [Cl]∞n– and [Br]∞n– polyhalogen anions, we performed DFT calculations of total and projected electron densities of states (TDOS and PDOS), the electron localization function (ELF), partial charge density across Fermi energy (Ef), the crystal orbital Hamilton population (COHP),34 and the crystal orbital bond index (COBI),35 as well as the integrated values of the latter two (ICOHP and ICOBI) (see Figures 4, S9, and S10 for [Cl]∞n– and Figure S11 for [Br]∞n–).
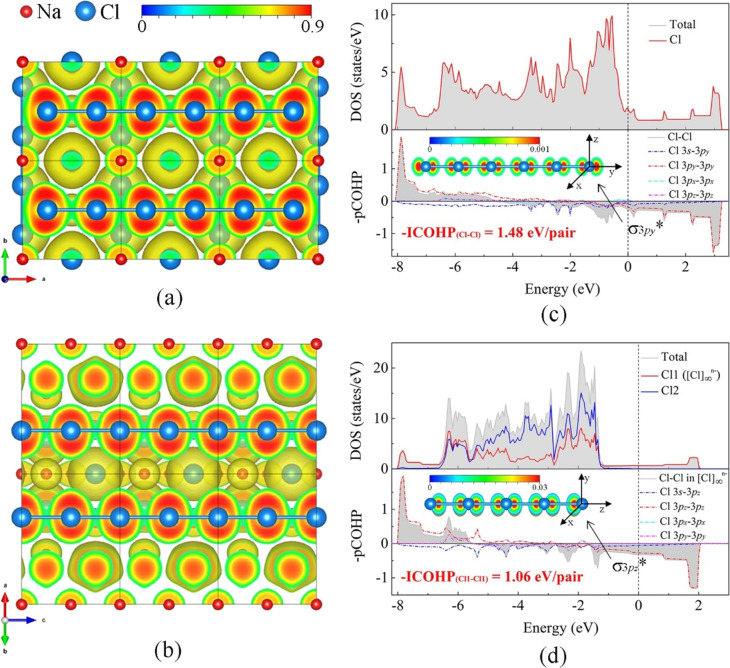
Figure 4.
Calculated properties of cP8-NaCl3 and hP18-Na4Cl5 at 50 GPa. (a) Electron localization function (ELF) calculated in the (001) plane for cP8-NaCl3 and (b) in the (110) plane for hP18-Na4Cl5. The isosurface value is set as 0.3. Na and Cl atoms are shown in red and blue colors, respectively. Calculated TDOS and PDOS curves for (c) cP8-NaCl3 and (d) hP18-Na4Cl5 along with the −pCOHP and −ICOHP for the Cl–Cl bond in the linear [Cl]∞n– chains. The vertical dashed line indicates the Fermi energy. The insets in panels (c) and (d) show the partial charge density distributions around the Fermi level (−1 eV < E – EF < 0 eV), which indicate the occupation of σ3p* antibonding orbitals in cP8-NaCl3 (σ3py*) and hP18-Na4Cl5 (σ3pz*).
The ELFs confirm that [Cl]∞n– and [Br]∞n– are conjugated 1D electronic systems forming polyanions (Figures 4 and S11a,b). There are, however, obvious differences in the ELFs of polyanions in cP8-NaX3 trichlorides and hP18-Na4X5: in the cP8-NaX3 compounds, the electron distribution in the pairs of halogen atoms has a shape of hourglass (Figures 4a and S11a), whereas in hP18-Na4X5, the electron distribution around each atom is mirror symmetric (Figures 4b and S11b). Both the Löwdin and Bader charges of halogen atoms in the chains of cP8-NaX3 and hP18-Na4X5 compounds are different (Table S12). The analysis of ICOBI and −ICOHP confirms the existence of chemical bonds between halogen atoms in polyanion chains. The bonding character is intermediate between ionic (ICOBI = 0) and covalent (ICOBI = 1): for cP8-NaCl3 at 50 GPa, ICOBI = 0.285, and for hP18-Na4Cl5, ICOBI = 0.197 (Table S13). Thus, both qualitative crystal-chemical analysis and quantitative data based on our DFT calculations lead to the conclusion that despite geometrical similarities, [X]∞n– chains in the cP8-NaX3 and in the hP18-Na4X5 compounds are different halogen polyanions. Nevertheless, there are common features in the chemical bonding and electron properties of these different [X]∞n– chains. The analysis of the −pCOHP curves (Figure 4c,d) shows the occupation of the antibonding orbitals (both π* and σ*) by halogens’ electrons in both cP8-NaCl3 (Figure 4c) and hP18-Na4Cl5 (Figure 4d). These delocalized electrons contribute to the conduction bands making a σ-conjugated system in these materials metallic (see the inserts in Figures 4c,d and S9). In contrast, the [Cl3]− ion in hP24-KCl3, which is isoelectronic to XeF2 with the 4-electron 3-center (4e–3c) hypervalent bonding system,23−25 is obviously non-metallic (Figure S12). In addition, the −pCOHP curves explain the difference in the ELFs of cP8-NaX3 and hP18-Na4X5. The two 3p−π interactions (Cl 3px–3px and Cl 3py–3py) in hP18-Na4Cl5 are similar, so that the corresponding curves in Figure 4d coincide (see also Figure S10b for details). The −pCOHP curves for Cl 3px–3px and Cl 3pz–3pz in cP8-NaCl3 are different (Figures 4c and S10a) which implies a tilting of the two Cl 3p orbitals in the xz plane for cP8-NaCl3.
3.4. Unusually Short Contacts between Sodium Cations in Na4Cl5 and Na4Br5
The interactions between A and X in hP24-KCl3 and cP8-AX3 compounds are ionic since the interatomic A–X distances and Löwdin/Bader charges are similar to those in the corresponding B2-AX salts. That is also confirmed by the analysis of ICOBI values (for example, for cP8-NaCl3, the ICOBI of the Na–Cl bond is equal to 0.047, which is comparable to the value for B2-NaCl of 0.057, Tables S12 and S13). For the hP18-Na4X5 compounds, the analysis of the Löwdin/Bader charges (Table S12) and ICOBI (Table S13) also points out that both structurally distinct Na atoms are in an ionic state. One of the sodium atoms (Na2 in Figure 1c), located in the first coordination sphere of [X]∞n– ions in hP18-Na4X5, is at the distance characteristic for ionic contacts. The second type of Na atoms (see Na1 in Figure 1c) is surrounded by six halogen atoms forming octahedra with a short cation–anion distance (2.351(2) Å in hP18-Na4Cl5 at 50 GPa from experimental data). These Na1 atoms form chains with very short interatomic contacts (2.3879(9) Å in hP18-Na4Cl5 at 50 GPa, see Figure 1b). Distances between Na1 atoms in the chains are shorter than in pure metallic Na at the same pressure (2.617 Å at 50 GPa, see Figure 5). Short interatomic contacts result in higher atomic density (for example, from DFT calculations at 50 GPa, the volume per atom of 12.55 Å3 for hP18-Na4Cl5 is smaller than that of 12.77 Å3 for cP8-NaCl3; see Figure S3c,d). According to our DFT calculations, there is no orbital overlapping between Na1 atoms in the chains, or any contribution of sodium’s electrons at the Fermi level (Figure S9b), as well as there are no signs that these may be electrides. Thus, our finding suggests that HP can drive contacts between cations (particularly Na+) much closer than previously known (Figure 5).
Figure 5.
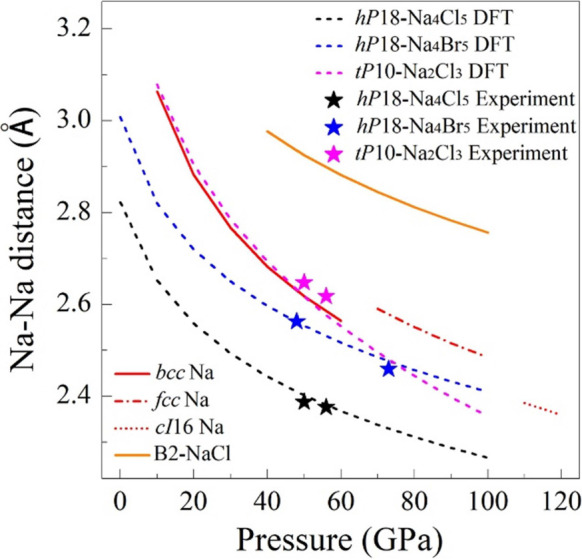
Pressure dependences of the shortest Na–Na distances in different allotropes of sodium36,37 and B2-NaCl,30 and in hP18-Na4Cl5, hP18-Na4Br5, and tP10-Na2Cl3.
3.5. Electronic Structure of tP10-Na2Cl3
The crystal chemistry of tP10-Na2Cl3 is obviously different from that of the cP8-NaCl3 and hP18-Na4Cl5 phases—its structure does not possess polychlorine anions (Figure S13a). The shortest Na–Cl contacts are quite short (2.425(3) vs 2.52 Å in B2-NaCl30 at 50 GPa), indicating that chemical bonds are not simple ionic and/or formal charges of Cl ions are smaller than 1 (in absolute value), similarly to those in FeO2.38 Our calculations show that Cl atoms in tP10-Na2Cl3 are not chemically connected with each other and exist as Cl ions with partially occupied p orbitals (Figure S13b).
4. Conclusions
To summarize, we experimentally studied chemical reactions in the four systems, Na–Cl, Na–Br, K–Cl, and K–Br, at HP and HT. SCXRD analysis revealed AX3 compounds in all of the studied systems (isostructural cP8 NaCl3, NaBr3, KCl3, and KBr3, and hP24-KCl3). Three previously unpredicted novel phases were synthesized in the Na–Cl (tP10-Na2Cl3 and hP18-Na4Cl5 at 50 and 56 GPa) and Na–Br (hP18-Na4Br5 at 48 and 73 GPa) systems. On the basis of the structural data and the results of ab initio calculations, we have characterized the chemical bonding of polyhalogen anions and chains and have found that infinite linear halogen chains, [Cl]∞n– and [Br]∞n–, in hP18-Na4Cl5 and hP18-Na4Br5 are different from those in sodium and potassium tribromides, cP8-NaCl3 and cP8-NaBr3. These results give a new insight into the chemistry of polyhalides.
Acknowledgments
The authors acknowledge the Deutsches Elektronen-Synchrotron (DESY, PETRA III) and the European Synchrotron Radiation Facility (ESRF) for provision of beamtime at the P02.2, and ID11 and ID27 beamlines, respectively. Y.Y. acknowledges the financial support provided by the China Scholarship Council (CSC) during her visit to the University of Bayreuth. N.D. and L.D. thank the Federal Ministry of Education and Research, Germany (BMBF, grant no. 05K19WC1), and the Deutsche Forschungsgemeinschaft (DFG; projects DU 954-11/1, DU 393-9/2, and DU 393-13/1) for financial support. N.D. also thanks the Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University (Faculty grant SFO-Mat-LiU no. 2009 00971). M.B. acknowledges the support of Deutsche Forschungsgemeinschaft (DFG Emmy-Noether project BY112/2-1). D.L. thanks the UKRI Future Leaders Fellowship (MR/V025724/1) for financial support. N.J., F.T., and I.A.A. acknowledge support by the Knut and Alice Wallenberg Foundation (Wallenberg Scholar grant no. KAW-2018.0194). Support from the Swedish Research Council (VR) grant no. 2019-05600 and the Swedish Government Strategic Research Areas in Materials Science on Functional Materials at Linköping University (Faculty grant SFO-Mat-LiU no. 2009 00971) is gratefully acknowledged. DFT calculations were enabled by resources provided by the National Academic Infrastructure for Supercomputing in Sweden (NAISS) at National Supercomputer Center partially funded by the Swedish Research Council through grant agreement no. 2022-06725. For the purpose of open access, the author has applied a creative commons attribution (CC BY) license to any author accepted manuscript version arising from this submission.
Data Availability Statement
CSD 2224055, CSD 2224057, and CSD 2224059 contain the supplementary crystallographic data for the novel compounds presented. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.3c00090.
Supplementary methods, SCXRD measurements and crystallographic details, and equation of states of all synthesized compounds, stability analyses, Raman spectra, Löwdin and Bader charge, electronic structures, and COHP and COBI analyses (PDF)
Author Contributions
L.D., N.D., and Y.Y. designed the study. L.D. and N.D. supervised the research. Y.Y., S.K., T.F., E.B., D.L., M.B., A.A., F.I.A., L.D., K.G., G.G., C.G., and E.L.B. conducted the experiments. Theoretical calculations were performed by Y.Y., A.A., B.W., N.J., F.T., and I.A.A. Y.Y., L.D., and N.D. wrote the paper with contributions from all authors. A.A., D.L., A.A., and Z.J. reviewed and edited the draft. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Miao M. S.; Sun Y. H.; Zurek E.; Lin H. Q. Chemistry under high pressure. Nat. Rev. Chem. 2020, 4, 508–527. 10.1038/s41570-020-0213-0. [DOI] [Google Scholar]
- Zhang W.; Oganov A. R.; Goncharov A. F.; Zhu Q.; Boulfelfel S. E.; Lyakhov A. O.; Stavrou E.; Somayazulu M.; Prakapenka V. B.; Konopkova Z. Unexpected stable stoichiometries of sodium chlorides. Science 2013, 342, 1502–1505. 10.1126/science.1244989. [DOI] [PubMed] [Google Scholar]
- Saleh G.; Oganov A. R. Alkali subhalides: high-pressure stability and interplay between metallic and ionic bonds. Phys. Chem. Chem. Phys. 2016, 18, 2840–2849. 10.1039/C5CP06026E. [DOI] [PubMed] [Google Scholar]
- Botana J.; Brgoch J.; Hou C.; Miao M. Iodine Anions beyond -1: Formation of LinI (n = 2-5) and Its Interaction with Quasiatoms. Inorg. Chem. 2016, 55, 9377–9382. 10.1021/acs.inorgchem.6b01561. [DOI] [PubMed] [Google Scholar]
- Miao M. S. Caesium in high oxidation states and as a p-block element. Nat. Chem. 2013, 5, 846–852. 10.1038/nchem.1754. [DOI] [PubMed] [Google Scholar]
- Zhu Q.; Oganov A. R.; Zeng Q. Formation of stoichiometric CsFn compounds. Sci. Rep. 2015, 5, 7875. 10.1038/srep07875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Oganov A. R.; Zhu Q.; Lobanov S. S.; Stavrou E.; Goncharov A. F. Stability of numerous novel potassium chlorides at high pressure. Sci. Rep. 2016, 6, 26265. 10.1038/srep26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. N.; Verma A. K.; Mishra A. K.; Sunder M.; Sharma S. M. The synthesis of unconventional stoichiometric compounds in the K-Br system at high pressures. Phys. Chem. Chem. Phys. 2017, 19, 7996–8007. 10.1039/C7CP00340D. [DOI] [PubMed] [Google Scholar]
- Patel N. N.; Sunder M.; Garg A. B.; Poswal H. K. Pressure-induced polymorphism in hypervalent CsI3. Phys. Rev. B 2017, 96, 174114 10.1103/PhysRevB.96.174114. [DOI] [Google Scholar]
- Poreba T.; Racioppi S.; Garbarino G.; Morgenroth W.; Mezouar M. Investigating the Structural Symmetrization of CsI3 at High Pressures through Combined X-ray Diffraction Experiments and Theoretical Analysis. Inorg. Chem. 2022, 61, 10977. 10.1021/acs.inorgchem.2c01690. [DOI] [PubMed] [Google Scholar]
- Dubrovinskaia N.; Dubrovinsky L. Crystallography taken to the extreme. Phys. Scr. 2018, 93, 062501 10.1088/1402-4896/aabf25. [DOI] [Google Scholar]
- Aslandukov A.; Aslandukov M.; Dubrovinskaia N.; Dubrovinsky L. Domain Auto Finder (DAFi) program: the analysis of single-crystal X-ray diffraction data from polycrystalline samples. J. Appl. Crystallogr. 2022, 55, 1383–1391. 10.1107/S1600576722008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovinsky L.; Khandarkhaeva S.; Fedotenko T.; Laniel D.; Bykov M.; Giacobbe C.; Lawrence Bright E.; Sedmak P.; Chariton S.; Prakapenka V.; Ponomareva A. V.; Smirnova E. A.; Belov M. P.; Tasnádi F.; Shulumba N.; Trybel F.; Abrikosov I. A.; Dubrovinskaia N. Materials synthesis at terapascal static pressures. Nature 2022, 605, 274–278. 10.1038/s41586-022-04550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykov M.; Chariton S.; Bykova E.; Khandarkhaeva S.; Fedotenko T.; Ponomareva A. V.; Tidholm J.; Tasnadi F.; Abrikosov I. A.; Sedmak P.; Prakapenka V.; Hanfland M.; Liermann H. P.; Mahmood M.; Goncharov A. F.; Dubrovinskaia N.; Dubrovinsky L. High-Pressure Synthesis of Metal-Inorganic Frameworks Hf4 N20 N2 , WN8 N2 , and Os5 N28 3 N2 with Polymeric Nitrogen Linkers. Angew. Chem., Int. Ed. Engl. 2020, 59, 10321–10326. 10.1002/anie.202002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravica M.; Sneed D.; Wang Y.; Smith Q.; Subrahmanyam G. Carbon tetrachloride under extreme conditions. J. Chem. Phys. 2014, 140, 194503. 10.1063/1.4876220. [DOI] [PubMed] [Google Scholar]
- Pravica M.; Sneed D.; Smith Q.; Bai L. High pressure X-ray photochemical studies of carbon tetrachloride: Cl2 production and segregation. Chem. Phys. Lett. 2013, 590, 74–76. 10.1016/j.cplett.2013.10.056. [DOI] [Google Scholar]
- Fedotenko T. In personal communication, 2022.
- Aprilis G.; Kantor I.; Kupenko I.; Cerantola V.; Pakhomova A.; Collings I. E.; Torchio R.; Fedotenko T.; Chariton S.; Bykov M.; Bykova E.; Koemets E.; Vasiukov D. M.; McCammon C.; Dubrovinsky L.; Dubrovinskaia N. Comparative study of the influence of pulsed and continuous wave laser heating on the mobilization of carbon and its chemical reaction with iron in a diamond anvil cell. J. Appl. Phys. 2019, 125, 095901 10.1063/1.5067268. [DOI] [Google Scholar]
- Hamilton W. C. Significance tests on the crystallographic R factor. Acta Crystallogr. 1965, 18, 502–510. 10.1107/S0365110X65001081. [DOI] [Google Scholar]
- Johannsen P. G.; Holzapfel W. B. Effect of pressure on Raman spectra of solid chlorine. J. Phys. C: Solid State Phys. 1983, 16, L1177–L1179. 10.1088/0022-3719/16/33/001. [DOI] [Google Scholar]
- Dalladay-Simpson P.; Binns J.; Pena-Alvarez M.; Donnelly M. E.; Greenberg E.; Prakapenka V.; Chen X. J.; Gregoryanz E.; Howie R. T. Band gap closure, incommensurability and molecular dissociation of dense chlorine. Nat. Commun. 2019, 10, 1134. 10.1038/s41467-019-09108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg K.; Mann L.; Redeker F. A.; Schmidt B.; Riedel S. Polyhalogen and Polyinterhalogen Anions from Fluorine to Iodine. Angew. Chem., Int. Ed. Engl. 2020, 59, 5464–5493. 10.1002/anie.201903197. [DOI] [PubMed] [Google Scholar]
- Braida B.; Hiberty P. C. The essential role of charge-shift bonding in hypervalent prototype XeF(2). Nat. Chem. 2013, 5, 417–422. 10.1038/nchem.1619. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Moore K. B. 3rd; Hill J. G.; Peterson K. A.; Schaefer H. F. 3rd; Hoffmann R. Alkali-Metal Trihalides: M(+)X3(−) Ion Pair or MX-X2 Complex?. J. Phys. Chem. B 2018, 122, 3339–3353. 10.1021/acs.jpcb.7b10005. [DOI] [PubMed] [Google Scholar]
- Munzarova M. L.; Hoffmann R. Electron-rich three-center bonding: role of s,p interactions across the p-block. J. Am. Chem. Soc. 2002, 124, 4787–4795. 10.1021/ja010897f. [DOI] [PubMed] [Google Scholar]
- Keil H.; Sonnenberg K.; Muller C.; Herbst-Irmer R.; Beckers H.; Riedel S.; Stalke D. Insights into the Topology and the Formation of a Genuine ppsigma Bond: Experimental and Computed Electron Densities in Monoanionic Trichlorine [Cl3]. Angew. Chem., Int. Ed. Engl. 2021, 60, 2569–2573. 10.1002/anie.202013727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichierri F. Structure and bonding in polybromide anions Br–(Br2)n (n=1–6). Chem. Phys. Lett. 2011, 515, 116–121. 10.1016/j.cplett.2011.09.003. [DOI] [Google Scholar]
- Prewitt C. T.; Downs R. T. High-pressure crystal chemistry. Rev. Mineral. 1998, 37, 284–318. [Google Scholar]
- Dewaele A.; Belonoshko A. B.; Garbarino G.; Occelli F.; Bouvier P.; Hanfland M.; Mezouar M. High-pressure–high-temperature equation of state of KCl and KBr. Phys. Rev. B: Condens. Matter Mater. Phys. 2012, 85, 214105 10.1103/PhysRevB.85.214105. [DOI] [Google Scholar]
- Sata N.; Shen G.; Rivers M. L.; Sutton S. R. Pressure-volume equation of state of the high-pressureB2phase of NaCl. Phys. Rev. B: Condens. Matter Mater. Phys. 2002, 65, 104114 10.1103/PhysRevB.65.104114. [DOI] [Google Scholar]
- Laniel D.; Winkler B.; Fedotenko T.; Aslandukova A.; Aslandukov A.; Vogel S.; Meier T.; Bykov M.; Chariton S.; Glazyrin K.; Milman V.; Prakapenka V.; Schnick W.; Dubrovinsky L.; Dubrovinskaia N. High-pressure Na3(N2)4, Ca3(N2)4, Sr3(N2)4, and Ba(N2)3 featuring nitrogen dimers with noninteger charges and anion-driven metallicity. Phys. Rev. Mater. 2022, 6, 023402 10.1103/PhysRevMaterials.6.023402. [DOI] [Google Scholar]
- Eremets M. I.; Popov M. Y.; Trojan I. A.; Denisov V. N.; Boehler R.; Hemley R. J. Polymerization of nitrogen in sodium azide. J. Chem. Phys. 2004, 120, 10618–10623. 10.1063/1.1718250. [DOI] [PubMed] [Google Scholar]
- Laniel D.; Trybel F.; Yin Y.; Fedotenko T.; Khandarkhaeva S.; Aslandukov A.; Aprilis G.; Abrikosov A. I.; Bin Masood T.; Giacobbe C. J. N. C. Aromatic hexazine [N6] 4– anion featured in the complex structure of the high-pressure potassium nitrogen compound K9N56. Nat. Chem. 2023, 15, 641–646. 10.1038/s41557-023-01148-7. [DOI] [PubMed] [Google Scholar]
- Dronskowski R.; Bloechl P. E. Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. 10.1021/j100135a014. [DOI] [Google Scholar]
- Müller P. C.; Ertural C.; Hempelmann J.; Dronskowski R. Crystal Orbital Bond Index: Covalent Bond Orders in Solids. J. Phys. Chem. C 2021, 125, 7959–7970. 10.1021/acs.jpcc.1c00718. [DOI] [Google Scholar]
- Gregoryanz E.; Lundegaard L. F.; McMahon M. I.; Guillaume C.; Nelmes R. J.; Mezouar M. Structural diversity of sodium. Science 2008, 320, 1054–1057. 10.1126/science.1155715. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Eremets M.; Oganov A. R.; Xie Y.; Trojan I.; Medvedev S.; Lyakhov A. O.; Valle M.; Prakapenka V. Transparent dense sodium. Nature 2009, 458, 182–185. 10.1038/nature07786. [DOI] [PubMed] [Google Scholar]
- Koemets E.; Leonov I.; Bykov M.; Bykova E.; Chariton S.; Aprilis G.; Fedotenko T.; Clement S.; Rouquette J.; Haines J.; Cerantola V.; Glazyrin K.; McCammon C.; Prakapenka V. B.; Hanfland M.; Liermann H. P.; Svitlyk V.; Torchio R.; Rosa A. D.; Irifune T.; Ponomareva A. V.; Abrikosov I. A.; Dubrovinskaia N.; Dubrovinsky L. Revealing the Complex Nature of Bonding in the Binary High-Pressure Compound FeO2. Phys. Rev. Lett. 2021, 126, 106001 10.1103/PhysRevLett.126.106001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CSD 2224055, CSD 2224057, and CSD 2224059 contain the supplementary crystallographic data for the novel compounds presented. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.