Abstract
The aim of this systematic review is twofold: (i) to examine the effects of micronutrient intake on athletic performance and (ii) to determine the specific micronutrients, such as vitamins, minerals, and antioxidants, that offer the most significant enhancements in terms of athletic performance, with the goal of providing guidance to athletes and coaches in optimizing their nutritional strategies. The study conducted a systematic search of electronic databases (i.e., PubMed, Web of Science, Scopus) using keywords pertaining to micronutrients, athletic performance, and exercise. The search involved particular criteria of studies published in English between 1950 and 2023. The findings suggest that vitamins and minerals are crucial for an athlete’s health and physical performance, and no single micronutrient is more important than others. Micronutrients are necessary for optimal metabolic body’s functions such as energy production, muscle growth, and recovery, which are all important for sport performance. Meeting the daily intake requirement of micronutrients is essential for athletes, and while a balanced diet that includes healthy lean protein sources, whole grains, fruits, and vegetables is generally sufficient, athletes who are unable to meet their micronutrient needs due to malabsorption or specific deficiencies may benefit from taking multivitamin supplements. However, athletes should only take micronutrient supplements with the consultation of a specialized physician or nutritionist and avoid taking them without confirming a deficiency.
Keywords: vitamins, minerals, sport, performance
1. Introduction
Optimal performance is a primary objective for many athletes, and this can be attained by following a suitable exercise protocol and ensuring proper nutrition [1]. Food is composed of nutrients that are essential for growth, repair, and energy generation depending on the amount that meets the body’s need [2]. Nutrients are typically categorized into two main groups: micronutrients and macronutrients [3]. When it comes to athletic performance, the importance of micronutrients should not be neglected [4]. Athletes are prone to consuming insufficient amounts of micronutrients due to inappropriate dietary habits, especially if they are not matching their physical activity requirements [5]. By making sure they are receiving adequate levels of micronutrients, athletes can give themselves a competitive edge and maximize the potential of their training [6]. Micronutrients may boost mental performance, help balance hormones, and keep cognitive performance at its peak [7].
It is noteworthy to emphasize that sports nutrition is not a one-size-fits-all solution, as each athlete has specific nutritional needs [7]. Therefore, athletes, nutritionists, and coaches must work together to customize nutritional plans for each athlete to ensure that their athletes/players’ needs are considered properly and they are receiving the sufficient level of nutrients they need to aid in the adaptation to their training and ultimately support optimal athletic performance. The evaluation of the evidence of the impact of micronutrients on the performance of athletes is the main purpose of this comprehensive systematic review paper.
2. Methods and Search Strategy
A comprehensive literature search was conducted using the Web of Science, Scopus, and PubMed databases. In order to retrieve relevant studies on the topic, our search strategy incorporated keywords including “micronutrients”, “vitamins”, “minerals”,” antioxidants”, “athletes”, “sport performance”, “training”, and “exercise”. Original research articles involving human subjects, English-language publications, human subjects, and a focus on micronutrients and athletic performance were the inclusion criteria. For the systematic review procedure, the Synthesis without meta-analysis (SWIM) recommendations were followed. The search was limited to articles published between January 1950 and 31 March 2023. The studies that made the cut for the review had to measure outcomes related to athletic performance, have a sample size of at least 10 participants, and use an intervention involving a micronutrient supplement. Studies that concentrated on macronutrients—such as carbohydrates and protein—were disregarded.
2.1. Data Extraction
Studies’ titles and abstracts located by the search were examined by two independent reviewers. After that, full-text articles were examined to see if they qualified for inclusion in the review. Data on the study design, sample size, intervention protocol, micronutrient supplements used, athletic performance outcomes assessed, and outcomes were extracted from the eligible studies.
2.2. Data Synthesis
To summarize the conclusions of the included studies, a narrative synthesis was carried out. The studies’ findings were categorized by micronutrient supplement and athletic performance outcome measures. A description of each study’s design, sample size, intervention strategy, and findings was included in the synthesis. It is important to ensure the accuracy and reliability of a systematic review by ensuring that all information is extracted in a standardized and consistent manner. In this study, two authors independently extracted all information from each paper to minimize the risk of bias and errors. This approach helps to ensure the validity of the review’s findings and strengthens the overall quality of the study. By having two authors independently extract information from each paper, the review can ensure data accuracy and increase confidence in the conclusions drawn from the analysis. It is a rigorous method that is commonly used in systematic reviews and emphasizes the importance of transparency and objectivity in research [8].
3. Results
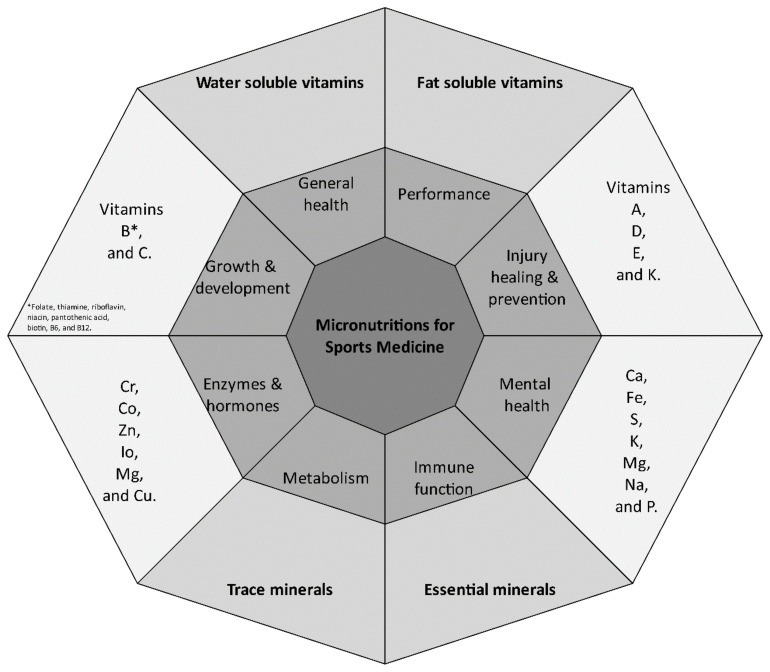
A total of 231 articles were obtained involving 18,683 athletes. Table 1 provides a summary of the main micronutrients researched in sports medicine. Table 2 provides a summary of all available 217 research articles about micronutrients and sports performance. Figure 1 simplified the model of micronutrients’ main functions in sports medicine.
Table 1.
Dietary Difference Intake and Top Sources of Vitamins and Minerals.
| Type of Micronutrient | DRI | Top Rich Food Sources | Role in Exercise Performance | Deficiency Effect on Performance |
|---|---|---|---|---|
| Vitamin A | 900 µg for males 700 µg for females [9] |
Beef liver Sweet potato Carrot [10] |
|
Vitamin A has an oxidation potency which protects athletes against induced and intense exercise free radicals, contributes to the elimination of ROS, and prevents muscle damage and the onset of diseases, despite the higher demand for vitamin A in athletes. Its deficiency is not directly linked to performance impairment, unlike other micronutrients such as iron and others [12]. |
| Vitamin E | 15 µg [13] | Sunflower seeds Almonds Cereals ready to eat, RALSTON [14] |
Decreased performance, recovery, immunity, and blood flow [15] | |
| Vitamin D | 1500–2200 IU [16] | Brown mushrooms oily fish, such as salmon, sardines, and cod liver oil [17] |
|
Deficient vitamin D concentration seemed to have an unpleasant influence on muscle power, strength, and stamina and elevated musculoskeletal damage, including stress fracture and other injuries impacting inflammation and severe muscle injuries occurring post-intensive exercises [17] |
| Vitamin K | 120 µg for males 90 µg for females [9] |
Kale Spinach Parsley [19] |
|
Insufficient consumption of vitamin K may be associated with a raised fracture risk [19] |
| Thiamin (B1) | 1.2 mg for males 1.1 mg for females [20] |
Fortified breakfast cereals Bacon Sunflower seed [21] |
|
Increased oxidative stress [22] |
| Riboflavin (B2) | 1.3 mg for males 1.1 mg for females [23] |
Dairy products, meat, egg [21] | Does not have an effect on athletic performance [21] | |
| Niacin (B3) | 16 mg for males 14 mg for females [10] |
Whole grains, dairy products, milk, and meat [21] |
|
May increases exercise capacity [9] |
| Pantothenic acid (B5) | 5 mg [20] |
|
No proven benefit, so deficiency does not cause any effect [7] | |
| Pyridoxine (B6) | 1.3 mg [9] | Fish, beef liver, and other organ meats [24] |
|
No effect |
| Cyano-cobalamin (B12) | 2.4 µg [20] | Fish, meat, poultry, eggs [26] |
|
May cause higher odds of anxiety [3] |
| Folic Acid | 400 µg [20] | Dark green leafy vegetables, fruits, nuts, and beans [28] |
|
Megaloblastic anemia, impairing red blood cells, tingling in hands and feet, tiredness, fatigue, weakness, loss of coordination, and weight loss [28] |
| Vitamin C | 90 mg for males 75 mg for females [13] |
Citrus fruits, tomatoes, green peppers, kiwifruit [25] | May have a higher chance of getting sick and missing performances; increased wound repair time [13]. | |
| Iron (Fe) | 8 mg for males [29] 18 mg for females |
Lean meat and seafood, nuts, beans [30] |
|
Iron deficiency, whether combined with anemia or not, can lead to muscle impaired function and limited endurance capacity, which affect athletic performance and training adaptation negatively [31]. |
| Calcium (Ca) | 1500 mg [32,33] | Dairy products, sardines and salmon, kale, broccoli [34] |
|
Calcium plays a crucial role in maintenance, growth regulating muscle contraction, normal blood clotting, and the conduction of nerve and bone tissue repair. The possibility of stress fracture and low bone-mineral density is elevated by low available levels of energy. In certain cases, such as female athletes, insufficient calcium intake combined with menstrual dysfunction increases the risk ratio [36] |
| Potassium (K) | 3500 mg for males [36] 2500 mg for females |
Dried fruits (raisins, apricots) Beans, lentils Potatoes [37] |
|
Whole body and muscle fatigue such as inappropriate exercise performance could be the result of the acute depletion of the trans-sarcolemma k+ [36] |
| Magnesium (Mg) | 400 mg for males 310 mg for females [38] |
Whole grains and dark-green, leafy vegetables, dried beans, and legumes [39]. |
|
Athletes who are insufficient in magnesium levels are not protected from inflammatory reactions, which may increase the risk of hypertension, atherosclerosis, diabetes mellitus, osteoporosis, and cancer occurrence [39] |
| Zinc (Zn) | 8 mg for males 11 mg for females [32] |
Meat, fish, seafood [40] |
|
Deficient zinc levels in athletes reduced endurance, led to a significant reduction in body weight, and latened fatigue with impaired endurance and osteoporosis risk [41] |
| Selenium (Se) | 55 mg [42] | Brazil nuts, seafoods, and organ meats [43] |
|
Insufficient Se levels may raise exercise-induced oxidative stress over time [44] |
| Manganese (Mn) | 2.3 mg for males 1.8 mg for females [45] |
Whole grains, oysters, mussels, nuts [46] |
|
The deficiency of manganese was indicated as an etiological agent in joint diseases and hip abnormalities development [45] |
Abbreviations: µg: microgram; DNA: deoxyribonucleic acid; IU: international unit; mg: milligram; ROS: reactive oxygen species.
Table 2.
Comprehensive overview of articles about micronutrients and sport performance.
| SN | Author | Year | Study Design | Micronutrient | Source of the Micronutrient | Sample Size | Mean Age | Gender | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Bezuglov E. [47] | 2023 | Cohort | Vit D | Natural source | 68 | M = 18.2 ± 1.9 F = 17.3 ± 2.6 |
M = 23 F = 45 |
No correlation was found between serum Vit D level and strength, speed characteristics, total testosterone concentration, performance in the 20 m and 30 m sprint, countermovement jump, and broad jump. |
| 2 | Pallante P.I. [48] | 2023 | Clinical trial | Vit D, Mg, and Zn | Natural source | 30 | F = 18–22 | F = 30 | No association was found between Mg and Zn intake and PMS. However, lower Vit D intake tended to be associated with presenting PMS in female athletes. |
| 3 | Zhang J. [49] | 2023 | Cross-sectional | B Vits (B1, B2, B3, B5, B6, B9, and B12) | Supplement | 427 | All = 27.65 ± 3.78 M = 27.47 ± 3.87 F = 27.81 ± 3.70 |
M = 210 F = 217 |
The supplementation of B vitamins and pectin may be beneficial for exercise performance and post-exercise recovery. |
| 4 | Mastali V.P. [50] | 2022 | Quasi-experimental | Vit D | Supplement | 24 | Group 1 = 24.33 ± 2.7 Group 2 = 25.83 ± 3.18 |
M = 24 | Short-term Vitamin D supplementation could prevent myocytes and hepatocytes damage induced by EAE. |
| 5 | Martínez-Ferrán M. [51] | 2022 | Double-blind randomized controlled trial | Vit C and Vit E | Supplement | 18 | Group 1 = 47.90 ± 5.75 Group 2 = 46.76 ± 4.60 |
M = 18 | Vit C and E supplementation did not seem to help with EIMD in endurance-trained individuals. |
| 6 | AL-Qurashi T.M. [9] | 2022 | Experimental trial | Mineral water | Natural source | 20 | M = 21.7 ± 3.21 | M = 20 | Rehydration with mineral water such as zamzam is unlikely to impair cardiorespiratory fitness, even with an intake equal to 100% of the loss in body weight. |
| 7 | Mesquita E.D.D.L. [10] | 2022 | Cross-sectional | Vit D | Natural source | 75 | N/A | N/A | Only adolescents with a combination of sports participation and higher serum concentrations of Vit D showed better bone geometry, indicating the relevance of the combination of both factors to bone health. |
| 8 | Chen L.-Y. [11] | 2022 | Cross-sectional | Vit D | Supplement | 50 | M = 21.14 ± 1.95 | M = 50 | Vit D may play a significant role in cardiovascular function that influences endothelial and smooth muscle cell function. Vit D deficiency may increase the risk of incident cardiovascular events after acute exhaustive exercise, even in healthy and active adults. |
| 9 | Książek A. [13] | 2022 | Cross-sectional | Vit D | Natural source | 40 | Group 1 = 22.5 ± 2.9 Group 2 = 21.9 ± 3.7 |
M = 40 | There was a significant correlation between Vit D metabolites and handgrip strength and vertical jump variables in indoor players. |
| 10 | Sone R. [14] | 2022 | Experimental trial | Antioxidant | Supplement | 7 | M = 22.6 ± 1.3 | M = 7 | Mineral-rich antioxidant supplements did not directly affect oxidative stress markers in the blood but suggested that performance (lactate) and salivary nitric oxide could be improved. |
| 11 | Marley A. [16] | 2022 | Randomized doubled-blind controlled trial | Vit D3 | Supplement | 27 | M = 25 ± 5 | M = 27 | Supplementing 50,000 IU of Vit D3 per week for six weeks combined with six weeks of SIT may improve markers of aerobic and anaerobic performance in recreational male combat sport athletes. |
| 12 | Rockwell M.S. [17] | 2022 | Cross-sectional | Vit D | Natural source | 53 | M = 19.9 ± 0.4 F = 19.7 ± 0.3 |
M = 25 F = 28 |
The bioavailable Vit D concentration was associated with a higher total, ap spine, and hip bone mineral density (bmd), but the total Vit D concentration was not related to total bmd and was negatively associated with ap spine and hip bmd |
| 13 | Brzeziański M [17] | 2022 | Experimental trial | Vit D3 | Supplement | 25 | M = 17.5 ± 0.7 | M = 25 | Vit D3 supplementation in a dose of 6000 IU/d increased its serum concentration in all the study groups of young athletes, causing the equalization of a suboptimal supply of Vit D3 in the serum. The study was not able to prove the ability of Vit D3 supplemented in the proposed dose to influence IL-6 or CRP concentrations in athletes. |
| 14 | de Brito E. [52] | 2022 | Randomized clinical trial | Vit C and Vit E | Supplement | 14 | All = 26.2 ± 5 | N/A | The association of Vits (C and E) with cryotherapy attenuated the inflammatory response and pain, favoring recovery after an acute resistance exercise session. |
| 15 | Şenışık S. [26] | 2022 | Cross-sectional | Vit D | Natural source | 256 | All = 13.2 ± 2.2 | N/A | The frequency of Vit D deficiency and insufficiency is higher in indoor athletes and is especially associated with the risk of bone injuries |
| 16 | Most A. [30] | 2021 | Cross-sectional | Vit D | Natural source | 112 | M = 26.1 ± 5.2 | M = 112 | Vit D insufficiency was associated with lower maximal aerobic power, as assessed with a standardized exhaustive cycling ergometer test. The Vit D level was the only independent predictor of maximal aerobic power in these athletes, highlighting the impact of Vit D on physical performance. A Vit D level of less than 30 ng/mL should be maintained to ensure optimal physical performance in these athletes. |
| 17 | Nikniaz L. [47] | 2021 | Randomized controlled trial | Vit D | Supplement | 40 | M = 30.40 ± 4.08 | M = 40 | Aerobic exercise combined with Vit D supplementation can reduce serum inflammatory factors and anti-inflammatory proteins and improve lung function after four weeks of intervention. The combination of aerobic exercise and Vit D supplementation remarkably reduced TNF-α, IL-6, and CC16. Aerobic exercise alone and the combination of aerobic exercise and Vit D supplementation significantly increased FEV1 and FVC. |
| 18 | Kawashima I. [53] | 2021 | Prospective cohort study | Vit D | Supplement | 42 | M = 20 ± 1 | M = 42 | Vit D supplementation of 25 μg/day significantly increased the serum Vit D level in elite male collegiate athletes. Vit D supplementation may play a role in maintaining athletes’ body fat percentage under circumstances where sports activity has decreased. |
| 19 | Mieszkowski J. [50] | 2021 | Cross-sectional | Vit D | Natural source | 32 | Group 1 = 20.6 ± 3.3 Group 2= 19.9 ± 1.0 |
M = 32 | Vit D metabolites affect the anaerobic performance and bone turnover markers at rest and after exercise |
| 20 | Janssen J.J.E. [54] | 2021 | Experimental trial | Vit B2 | Natural source | 31 | Group 1 = 24.0 Group 2 = 21.8 |
F = 31 | A single bout of exercise significantly increased egr activity but did not affect egrac values, indicating that a single bout of exercise did not affect Vit B2 status. |
| 21 | Ali A. [55] | 2021 | Clinical trial (crossover study) | Vit C | Natural source (sungold kiwifruit) | 10 | F = 30.92 ± 7.32 | F = 10 | Consuming liquid Vit C prior to high-intensity cycling appears to be more effective than eating kiwifruit in ameliorating exercise-induced stress in recreationally active women of reproductive age |
| 22 | Valtueña J. [56] | 2021 | Longitudinal study | Vit D | Natural source and supplement | 95 | M = 27.3 ± 4.6 | M = 95 | A positive interaction with supplementation existed in two different directions; outdoor training improves the Vit D status only in supplemented team players, and supplementation has a positive influence on the Vit D status only in individuals with adequate sun exposure. Vit D deficiency might affect team players’ overall health and performance |
| 23 | Wilson-Barnes S.L. [57] | 2021 | Longitudinal study | Vit D | Natural source | 50 | Group 1 = 20.8 ± 1.9 Group 2 = 24.8 ± 4.2 |
M = 24 F = 26 |
Predictors of physical performance were not associated with Vit D status within both groups or during both seasons. |
| 24 | Kamińska J. [58] | 2021 | Randomized controlled trial | (Ca2+, Na+, Mg2+, K+, Hco3−, So42−, Cl−, and F−) | Mineral in the fluids | 14 | F = 21.9 ± 2.3 | F = 14 | The osmolarity of consumed fluids does not significantly affect the indicators of the water–electrolyte balance and the acid–base balance during exercises; such an effect is only noticeable after consuming an isotonic drink. The degree of mineralization of the water consumed by female field hockey players did not affect the indicators of the water–electrolyte and acid–base balance in the blood and urine. |
| 25 | Kawashima I. [53] | 2021 | Cross-sectional | Vit D | Natural source | 48 | M = 19.8 ± 0.9 | M = 48 | Vit D is insufficiently widespread among indoor elite athletes, with the majority of them suffering from Vit D deficiency. Outdoor players had a sufficient Vit D level. Vit D insufficiently had significantly higher body fat percentages than sufficient Vit D athletes. |
| 26 | Alves J. [59] | 2021 | Cross-sectional | Antioxidant Vits (Vit C and Vit E) | 84 | M = 23.2 ± 3.25 | M = 84 | A maximum incremental test did not produce any changes in plasma Vits in athletes. However, it increased the levels of Vit C in erythrocytes and decreased malondialdehyde values in plasma and Vit E in erythrocytes. The levels of malondialdehyde, Vit C, and Vit E were related to performance parameters. |
|
| 27 | Martusevich A.K. [60] | 2021 | Randomized controlled trial | Vit-Mineral Complex (Vits C, E, A, D, Group B, Minerals and Trace Elements, Β-Carotene (1.5 Mg), Lutein (4.5 Mg), and L-Carnitine) | Supplement | 74 | N/A | N/A | The use of an individually prescribed Vit-mineral complex may allow for optimizing the state of the oxidative metabolism of athletes’ blood plasma. |
| 28 | Sasaki C.A.L. [61] | 2021 | Cross sectional | All Micronutrient | Natural Source and Supplement | 101 | All = 33.32 ± 9.88 | M = 82 F = 19 |
The prevalence of inadequacy for Vit D, calcium, Vit A, thiamine, riboflavin, and zinc was significantly higher in para-athletes. The prevalence of the risk for iron deficiency was recorded in female para-athletes. |
| 29 | Marley A. [16] | 2021 | Single-blind crossover | Vit D3 | Supplement | 27 | M = 24 ± 4 | M = 27 | Given the endurance adaptations from Vit D supplementation and the importance of endurance for combat performance, recreational combat athletes should supplement at 50,000 IU per week for six weeks. There is no additional benefit of increasing the dose above 50,000 IU Vit D per week. |
| 30 | Pilch W. [62] | 2020 | Randomized control trial | Vit D | Supplement | 60 | M = 20–24 | M = 60 | The plasma Vit D level is considered a significant indicator for reducing muscle cell damage induced by eccentric exercise. |
| 31 | Crewther B. [63] | 2020 | Cross-sectional | Vit D | Natural source | 88 | N/A | M = 88 | Serum Vit D was a poor predictor of exercise performance, but it did moderate (with cortisol) the testosterone link to muscle power. |
| 32 | Shalaby M.N. [64] | 2020 | Experimental trial | Vit D3 | Supplement | 20 | All = 18.64 ± 0.43 | N/A | Vit D supplementation may affect the muscle function and health of the athlete. It is recommended that Vit D levels should be checked on an annual basis in all athletes for their health. |
| 33 | Aminaei M. [65] | 2020 | Quasi-experimental | Vit D and Calcium | Supplement | 40 | F = 28.1 ± 2.7 | F = 40 | Eight weeks of TRX training with Vit D and calcium supplementation improved BMI and HDL serum levels. The intensity and duration of training and supplementation probably have positive effects on lipids profiles. |
| 34 | Bauer P. [66] | 2020 | Cross-sectional | Vit D | Supplement | 120 | M = 26 ± 5 | M = 120 | Athletes with sufficient Vit D achieved a higher maximum systolic BP and a higher maximum power output. Better performance was recoded among athletes with sufficient Vit D. |
| 35 | Molina-López J. [67] | 2020 | Clinical trial | MultiVit\Mineral; Vit A, Vit E, Vit C, Vit B, Vit B2, Vit B6, Vit B12, Vit D, Biotin, Floate, Niacin, Pantothenic Acid, Calcium, Phosphorous, Magnesium, Iron, Iodine, Cooper, Manganese, Selenium, Zinc | Supplement | 26 | Group 1 = 22.9 ± 2.7 Group 2 = 20.9 ± 2.8 |
M = 26 | Elite handball athletes showed a different expression profile in reference to key genes implicated in several sports’ performance-related functions compared to the sedentary controls, in addition to the modulation of gene expression after multiVit/mineral supplementation. |
| 36 | Krzywański J. [68] | 2020 | Clinical trial | Vit B12 | Injection | 243 | Group 1 = 23 ± 1 Group 2 = 25 ± 1 |
N/A | A weak positive correlation between the Vit B12 concentration and Hb and between MCH and Hct were found. Higher values of hemoglobin and hematocrit were observed after B12 injections in endurance athletes. |
| 37 | Sekel N.M. [69] | 2020 | A quasi-experimental trial | Vit D | Supplement | 20 | All = 20.25 ± 0.85 | M = 7 F = 13 |
A daily intake of 10,000 IU Vit D was not sufficient to recover the deficiency, while it protected against seasonal declines. An intake of 5000 IU daily was insufficient and failed to attenuate against seasonal decline. |
| 38 | Millward D. [70] | 2020 | Cohort study | Vit D | Supplement | 802 | M = 18.7 ± 1.2 F = 18.6 ± 1.2 |
M = 498 F = 305 |
Correcting low serum Vit D levels reduced the risk of stress fracture. |
| 39 | Pradita D.K. [71] | 2020 | Cross-sectional | Iron | Natural source | 70 | F = 12–21 | F = 70 | Significant relationships were observed between iron deficiency based on serum ferritin and muscle mass with bone density in young female athletes. |
| 40 | Shafiee S.E. [72] | 2020 | Cross-sectional | Vit D | Supplement | 100 | M = 28 ± 6 | M = 100 | Lower serum concentrations of Vit D are associated with the risk of ACLI in male athletes. |
| 41 | Ashtary-Larky D. [73] | 2020 | Non-randomized crossover design | Vit D | Injection | 14 | M = 24.3 ± 2.4 | M = 14 | Among resistance-trained males who suffer from Vit D deficiency, a single injection of Vit D reduced the insulin concentration and blood glucose levels and improved insulin resistance. Single injections of 300,000 IU Vit D had no impact on muscle damage improvement and inflammatory response. Improvement in the Vit D level had no impact on the resting metabolic rate nor inflammatory and cardiovascular biomarkers. |
| 42 | Farapti F [74] | 2019 | Cross-sectional study | Vit E, Vit C, Vit A, Zn, and Cu | Natural source | 40 | All = 23.08 ± 4.32 M = 22.96 ± 3.98 F = 23.29 ± 5.03 |
M = 26 F = 14 |
A low intake of nutrients might deplete the Vit/mineral status, especially in Vit C status among female athletes. |
| 43 | Kim D.K. [75] | 2019 | Retrospective study | Vit D | Natural source | 52 | M = 23.2 ± 4.5 | M = 52 | Although Vit D insufficiency was not associated with isokinetic muscle weakness, monitoring its levels is very important for musculoskeletal health; especially, the deficiency was common among elite volleyball players. |
| 44 | Bauer P. [76] | 2019 | Cross-sectional study | Vit D | Natural source | 50 | M = 26 ± 5 | M = 50 | Vit D insufficiency was associated with a significant increase in central systolic and diastolic blood pressure among elite athletes. |
| 45 | Schaad K.A. [77] | 2019 | Retrospective review of records | Vit D | Natural source | 381, 818 | All = 18–64 | M = 329, 085 F = 52, 733 |
Individuals living in a northerly latitude might be more prone for Vit D deficiency and at a higher risk for the diagnosis of depression. Vit D status monitoring was very important among military athletes’ members to prevent the risk for depression. |
| 46 | Seo M.-W. [78] | 2019 | Cross-sectional study | Vit D | Natural Source and Supplement | 47 | M = 16.7 ± 0.84 | M = 47 | Anaerobic capacity was correlated poorly with Vit D status. Mechanisms were not clear for how Vit D influence anaerobic performance. During growth periods, it is important to consider the importance of Vit D regarding health benefits. |
| 47 | de Oliveira D.C.X. [28] | 2019 | Double-blind, controlled clinical trial | Vit C and Vit E | Supplement | 21 | M = 19.9 ± 0.3 | M = 21 | Antioxidant supplementation reduced oxidative stress only among young athlete football players. It has no ergogenic aids on muscle damage or muscle soreness due to acute exercise. |
| 48 | Higgins M.F. [79] | 2019 | A double-blind, placebo-controlled crossover trial | Mineral | Deep ocean mineral | 9 | M = 22 ± 1 | M = 9 | The deep ocean mineral content of minerals and trace elements had many health recovery benefits for active male soccer players who have a prolonged high-intensity running capacity in thermoneutral environmental conditions. |
| 49 | Aydın C.G. [80] | 2019 | Cross-sectional study | Vit D | Natural source and supplement | 555 | All = 5–52 | M = 229 F = 326 |
Participating athletes’ performance benefited from improving Vit D levels, especially during the winter season. |
| 50 | Alkoot M.J. [81] | 2019 | Cross-sectional study | Vit D | Supplement | 250 | M ≥ 21 | M = 250 | Vit D deficiency was common among professional athletes. Eight winter weeks of supplementation with cholecalciferol increases Vit D serum levels, but not enough for professional athletes. |
| 51 | Michishita R. [82] | 2019 | Cross-sectional | Sodium, Potassium, and Vit E | Natural source | 302 | All = 48.4 ± 11.3 | M = 64 F = 238 |
Subjects who do not suffer from hypertension diseases would benefit from the dietary sodium, potassium, and antioxidant Vit intake. |
| 52 | Bauer P. [83] | 2019 | Prospective, non-interventional study | Vit D | Supplement | 70 | N/A | M = 70 | Professional handball athletes suffered from Vit D insufficiency even in summer. Their insufficiency level negatively impacted their physical performance, which is a risk for musculoskeletal injuries and infections. |
| 53 | Umarov J. [84] | 2019 | Prospective, non-interventional, observational | Vit D | N/A | 70 | N/A | N/A | Vit D insufficiency is common among elite athletes engaged in synchronized swimming and swimmers. It is accompanied by a decrease in ifn-γ, an increase in tnf-α, IL-4, and IL-6 levels, and an elevation of urti morbidity. Seasonal monitoring and correction of the Vit D level for the normalization of the cytokine profile and a decrease in urti morbidity is definitely advised |
| 54 | Wrzosek M. [85] | 2019 | Cross-sectional | Vit D, Calcium | Natural source | 593 | F = 18–50 | F = 593 | It is important to educate women about the necessity to provide the body with proper calcium and Vit D intake levels in a diet to avoid health problems, resulting from the deficit of the nutrients. |
| 55 | Alimoradi K. [27] | 2019 | Randomized controlled clinical trial | Vit D | Supplement | 70 | Group 1 = 24.09 ± 5.06 Group 2 = 22.71 ± 4.07 |
M = 36 F = 33 |
Weekly supplementation with 50,000 IU Vit D resulted in a nearly 17 ng/mL increment in circulating calcidiol. This increase was associated with a significant improvement in power leg press and sprint tests in D-supplemented group. |
| 56 | Carswell A.T. [86] | 2018 | Study 1: prospective cohort Study 2: double-blind, randomized, placebo-controlled trail |
Vit D | Natural sources | Study 1 = 967 | Study 1 = 22 ± 3 Study 2 = 22 ± 3 |
Study 1: M = 621; F = 364 Study 2: M = 173 |
The Vit D status was associated with endurance performance but not strength or power in a prospective cohort study. Achieving Vit D sufficiency via safe, simulated summer sunlight or oral Vit D3 supplementation did not improve exercise performance. |
| 57 | Jung H.C. [87] | 2018 | Double-blind, randomized, and placebo-controlled design | Vit D3 | Supplement | Study 2 = 173 | All = 20.1 ± 0.15 | N/A | Correcting Vit D insufficiency improves some but not all aspects of performance. Thus, the efficacy of Vit D supplementation to enhance performance remains unclear. |
| 58 | Orysiak J. [88] | 2018 | Cross-sectional | Iron, Vit D | Natural source | 35 | M = 17.2 ± 0.9 | M = 50 | Vit D insufficiency is highly prevalent in ice hockey players, but the Vit D level was not associated with exercise performance or indices of iron status. |
| 59 | Malczewska-Lenczowska J. [89] | 2018 | Cross-sectional | Iron, Vit D | Natural source | 50 | F = 20.0 ± 4.4 | F = 219 | The association between Vit D and iron status in female athletes is complex, and it is challenging to determine which nutrient exerts a stronger influence over the other. |
| 60 | Radzhabkadiev R.M. [90] | 2018 | Cross-sectional | Vit A, B, B1, B2, C | Supplement | 18 | M = 21.7 ± 0.8 F = 23.1 ± 1.5 |
M = 92 F = 67 |
To maintain the optimal Vit status of the athlete’s organism, it was inappropriate to use excessive doses of Vits C (>200–300 mg/day), E (>50 mg TE/day), and A (>1500 μg RE/day). |
| 61 | Wei C.-Y. [91] | 2017 | Double-blind placebo-controlled crossover | Trace element, Deep Ocean Mineral (Dom) | Supplement (dom) | 159 | Group 1 = 21.2 ± 0.4 Group 2 = 46.8 ± 1.4 |
M = 21 | Minerals and trace elements from deep oceans possess great promise in developing supplements to increase the cerebral hemodynamic response against a physical challenge and during post-exercise recovery for middle-aged men. |
| 62 | DiSilvestro R.A. [92] | 2017 | Randomized controlled trial | Minerals (Iron, Zinc, Copper) | Supplement | 21 | F = 18–30 | F = 76 | A combination of micronutrients can improve aerobic exercise performance in one set of circumstances. |
| 63 | Wardenaar F. [93] | 2017 | Cross-sectional | All Vits and minerals | Supplement and Natural Source | 26 | M = 23.5 ± 11.5 F = 22.0 ± 7.6 |
M = 327 F = 226 |
Both users and non-users of nutritional supplements reported inadequate intake of micronutrients. For most micronutrients, the use of nutritional supplements does not completely compensate for intakes below recommendation. |
| 64 | Backx E. [94] | 2017 | A longitudinal study | Vit D | Supplement | 553 | All = 22 ± 4 | M = 22 F = 30 |
A sufficient Vit D concentration in summer did not guarantee a sufficient status in winter. Coaches and medical professionals should monitor athletes’ Vit D concentration regularly to prevent Vit D deficiency. |
| 65 | Owens D.J. [94] | 2017 | Randomized clinical trial | Vit D | Supplement | 52 | M = 26 ± 3 | M = 46 | Frequent low doses of Vit D intake and gradual supplementation withdrawing were more preferable than the opposite. |
| 66 | Dahlquist D.T. [95] | 2017 | Randomized, placebo-controlled, single-blinded, triple-crossover study | Vits D3 and K2 | Supplement | 46 | M = 26.9 ± 6.4 | M = 10 | Vit D and K2 had no significant impact on hepcidin-25, IL-6, Hb, hematocrit, serum ferritin, or serum iron. |
| 67 | Nayir T. [96] | 2017 | Cross-sectional study | Vit D | Natural source | 10 | N/A | M = 679 F = 447 |
Vit D insufficiency is common in long-lasting sports. |
| 68 | Cheng-Shiun He,. [97] | 2016 | Randomized controlled trial | Vit K2 | Supplement | 76 | N/A | N/A | Vit K2 supplementation had been reported to improve cardiovascular function in diseased patients. |
| 69 | Hildebrand R.A. [98] | 2016 | Cross-sectional study | Vit D | Serum 25-oh d | 1126 | All ≥ 18 | N/A | Vit D insufficiency and deficiency were common among collegiate athletes, which affect their muscular strength and power. It is important to consider the benefits of Vit D for optimal training to maximize performance, especially in muscular strength anaerobic power exercise. |
| 70 | Cassity E.P. [99] | 2016 | Randomized controlled trial | Vit D | Supplement | 113 | All ≥ 18 | M = 19 F = 13 |
There was an inverse correlation between BMI and 4000 IU of Vit D supplementation in athletes. Normal-BMI athletes demanded less than the upper limit of Vit D supplementation to sustain sufficient Vit D status. High-bone-turnover athletes lost a significant amount of Vit D during training. |
| 71 | Darr R.L. [100] | 2016 | Double blinded, randomized clinical trial | Vit D3 | Supplement | 32 | Group 1 = 42.0 ± 10.7 Group 2 = 36.4 ± 6.9 |
M = 13 | Vit D supplementation post-low or -moderate exercise but not resistance exercise enhances IGFBP3, which promotes the delivery of IGF1 to tissues. |
| 72 | Krzywanski J. [101] | 2016 | Retrospective | Vite D | Sun Exposure and Supplement | 13 | N/A | M = 228 F = 181 |
Polish elite athletes suffer from an insufficient Vit D status that affecst their health and performance negatively, especially among indoor sports. Hence, it is recommended to be exposed to sunlight combined with an oral Vit D supplement. |
| 73 | Capó X. [102] | 2016 | Controlled clinical trial | Vit E | Beverage supplementation | 409 | Group 1 = 22.8 ± 3.8 Group 2 = 45.6 ± 1.6 |
M = 10 | To improve the pro-inflammatory circulating in young athletes, functional beverage supplementation is recommended during exercise. |
| 74 | Backx E.M.P. [103] | 2016 | Randomized, double blind, dose-response study | Vit D | Supplement | 10 | All = 8–32 | M = 54 F = 48 |
Intake of 2200 IU/day of Vit D can recover the deficiency among athletes. |
| 75 | Todd J. [104] |
2016 | Cross-sectional | Vit D | Supplement | 128 | All = 25 ± 5 | M = 46 F = 46 |
Irish athletes recovered from Vit D insufficiency due to supplementation program-targeted elite sports. |
| 76 | Maruyama-Nagao A. [105] | 2016 | Cross-sectional | Vit D | Sunlight exposure | 92 | F = 20–22 | F = 30 | Vit D insufficiency is common among indoor sports athletes compared to outdoor sports athletes, mostly during winter, which could influence bone mineralization over the three months. |
| 77 | Wyon M.A. [106] | 2016 | Randomized, placebo-controlled, double-blind trial | Vits D3 and K2 | Supplement | 30 | Group 1 = 29.6 ± 10.6 Group 2 = 26.6 ± 7.4 |
M = 22 | Among elite indoor athletes who suffer from insufficient Vit D, a shot of 150,000 IU showed a favorable impact on muscle function and Vit D level. |
| 78 | Keen D.A. [107] | 2016 | Randomized experimental study | Multimineral | Deep ocean mineral water (dom) | 22 | All = 23 ± 1.2 | N/A | The intake of kona showed a positive impact on exercise performance, especially when consumed during the recovery strategy of rehydration and post-exercise. |
| 79 | Veskoukis A.S. [108] | 2016 | A randomized, placebo-controlled, double-blind trial | Vit C | Supplement | 8 | M = 21.1 ± 3.1 | M = 30 | The intake of Vit C at rest showed a positive effect on reducing the oxidative stress, enhancing the antioxidant capacity, and altering the redox state and inflammation biomarkers. |
| 80 | Heffler E. [109] | 2016 | Cross-sectional | Vit D | Supplement and Natural source | 30 | All = 13–25 | M = 24 F = 13 |
Vit D deficiency negatively affected athletic performance by altering calcium homeostasis among young athletes who lived above the 40th parallel north. |
| 81 | Kaul A. [110] | 2016 | Cohort | Vit D | Natural source | 37 | All = 20–79 | M = 677 F = 700 |
Vit D levels correlated positively with physical performance among healthy subjects. |
| 82 | He C.-S. [111] | 2016 | N/A | Vit D | N/A | 1377 | N/A | N/A | Sunlight exposure in the summer combined with everyday supplementation of 1000 iu in the winter is the practical proposal to achieve Vit D sufficiency. |
| 83 | He C.-S. [111] | 2016 | Randomized controlled trial | Vit D3 | Supplement | N/A | M = 20.4 ± 1.9 | M = 39 | To improve respiratory infections resistance, it is recommended that athletes consume a daily dose of 5000 IU Vit D supplement by monitoring the expression of sIgA and Cathelicidins. |
| 84 | Chenoweth L.M. [112] | 2015 | Randomized, single-blinded, crossover design study | Vit C, Vit E, Zinc, Selenium, Copper, and Manganese | Supplement | 39 | All = 22 ± 1 | M = 5 F = 5 |
Among subjects who do not eat enough servings of fruits and vegetables, the intake of Vits and minerals supplements during exercise increased TAS, improved resting expiratory flow rates, and reduced EFL during exercise. |
| 85 | Caruana H. [113] | 2015 | Cross-over study | Vit C | Supplement | 10 | M = 21.1 ± 0.84 | M = 10 | In young men, hyperemia post-strenuous muscle contraction is reduced by ROS. |
| 86 | Fitzgerald J.S. [114] | 2015 | A cross-sectional design | Vit D | Serum level | 10 | M = 20.1 ± 1.5 | M = 53 | Among young male ice hockey players, the Vit D level was positively associated with the strength of the upper body but not the lower body force or the production of power. |
| 87 | Veasey R.C. [115] | 2015 | Placebo-controlled, double-blind, randomized, balanced cross-over study | Vit D | Supplement | 53 | M = 21.4 ± 3.0 | M = 40 | Pre-moderate intensity exercise and the intake of Vit and mineral complex supplements with guarana promote memory performance in active males. |
| 88 | Price O.J. [116] | 2015 | Single-blind, placebo-controlled trial | Vit D | Supplement | 40 | All = 35 ± 8 | M = 9 F = 1 |
Vit D and omega-3 PUFA supplementation does not ease the reduction in lung function post-EVH. |
| 89 | Allison R.J. [117] | 2015 | Cross-sectional | Vit D | Supplement and Natural source | 10 | Group 1 = 23.9 ± 5.0 Group 2 = 23.9 ± 4.4 Group 3 = 24.6 ± 4.6 Group 4 = 25.2 ± 4.6 |
M = 950 | Among male athletes, no association was found between Vit D and BMD and T-score. |
| 90 | Allison R.J. [118] | 2015 | Cross-sectional | Vit D | Natural source | 950 | Group 1 = 21.7 ± 4.8 Group 2 = 22.3 ± 5.2 Group 3 = 24.0 ± 5.5 Group 4 = 23.3 ± 5.3 |
M = 750 | Severe Vit D-deficient athletes show significantly fewer cardiac structural parameters than insufficient and sufficient athletes. |
| 91 | Heller J.E. [119] | 2015 | Cross-sectional | Vit D | Supplement and natural source | 750 | All = 20.7 ± 1.6 | M = 24 F = 18 |
Athletes with a large body size and/or excess adiposity may be at a higher risk for Vit D insufficiency and deficiency. |
| 92 | Popovic L.M. [120] | 2015 | Clinical trial | Vit C | Supplement | 42 | Group 1 = 22.5 ± 1.5 Group 2 = 24.5 ± 2.5 |
M = 60 | Vit C supplementation can suppress the lipid peroxidation process during exercise but cannot affect the neutrophil inflammatory response in either exercise group. |
| 93 | Turchaninov D.V. [121] | 2015 | Clinical trial | N/A | Fortified fermented milk product | 60 | All = 12–17 | N/A | It is recommended among sport-active adolescents to improve their intake with the dairy products “bifidin” and “prolacta” hypoVitosis and micro-elementoses. |
| 94 | Díaz V. [122] | 2015 | Clinical trial | Vit C, Vit E | Supplement | 94 | M = 26.9 ± 6.7 | M = 10 | Intakes of 500 mg/day Vit C and 400 IU/day Vit E for 28 days had no effect on the hepcidin level due to inflammatory and iron signals. |
| 95 | Theodorou A.A. [123] | 2014 | Clinical trial | Vit C | Supplement | 10 | Group 1 = 2.6 ± 0.9 Group 2 = 22.8 ± 1.1 |
M = 20 | Oxidative stress is decreased post-exercise, while it is increased with antioxidant stimulus exposure. |
| 96 | Fitzgerald J.S. [124] | 2014 | Cross-sectional | Vit D | Natural source | 20 | M = 20.1 ± 1.5 | M = 53 | Throughout the skate treadmill gxt, the level of Vit D was not significantly assoiciated with any physiological or physical parameter. |
| 97 | Karakilcik A.Z. [125] | 2014 | Cross-sectional | Vit C | Supplement | 52 | M = 23.50 ± 0.59 | M = 22 | In young soccer players, the intake of Vit C combined with exercise decreases TBARS-levels and may affect the values of PLT, MPV, PCT, and RDW. |
| 98 | Koundourakis N.E. [126] | 2014 | Cross-sectional | Vit D | N/A | 22 | M = 25.6 ± 6.2 | M = 67 | The concentration of Vit D was significantly associated with the pre- and post-experimental performance parameters. |
| 99 | Aguiló A. [127] | 2014 | Double-blinded study | Vit C | Supplement | 67 | Group 1 = 39.5 ± 5.6 Group 2 = 37.2 ± 5.4 |
M = 31 | In non-exhaustive exercise, the intake of 250 mg Vit C twice a day for 15 days had no impact on IL-6 and IL-10, while it increased the production of IL-6 and IL-10 during the 2 h post-exercise recovery. |
| 100 | Shanely R.A. [128] | 2014 | Cross-sectional | Vit D | Supplementation with Portobello Mushroom Powder | 31 | Group 1 = 15.9 ± 0.29 Group 2 = 16.6 ± 0.23 |
M = 33 | Among high school athletes, the intake of 600 IU/day of Vit D raised the Vit D level without any impact on muscular function or damage post-exercise. |
| 101 | Beketova N.A. [129] | 2014 | Cross-sectional | Vits A, E, C, B2, and Beta-Carotene | Supplement | 33 | Group 1 = 18.5 ± 0.3 Group 2 = 26.8 ± 0.7 |
N/A | It is important to enrich all athletes’ diets with supplements, considering age and gender variation. |
| 102 | Soria M. [130] | 2014 | Prospective, simple blind, placebo-controlled trial | Sulfur | Sulfurous mineral water (smw) | 169 | M = 27.3 ± 4.1 | M = 30 | To prevent muscle damage post-exercise, it is recommended to intake smw supplements for 3 weeks. |
| 103 | Barker T. [131] | 2014 | Cross-sectional | Vit D | Natural source | 30 | N/A | M = 13 | Vit D sufficiency increases the anti-inflammatory cytokine response to muscular injury. |
| 104 | Nieman D.C. [132] | 2013 | Double-blind experimental design | Vit D2 | Supplement and natural source | 13 | Group 1 = 27.3 ± 0.9 Group 2 = 27.1 ± 1.5 |
N/A | In crew athletes, the intake of 3800 iu/day for 6 weeks of Vit D increased the Vit D level significantly, with no impact on muscle function tests post-eccentric exercise. |
| 105 | Barker T. [133] | 2013 | Randomized, double-blind, placebo-controlled experimental design | Vit D | Supplement | 28 | Group 1 = 31.0 ± 5 Group 2 = 30.0 ± 6 |
M = 28 | To promote skeletal muscle strength recovery, Vit D supplement intake is recommended, especially after intense exercise in physically active adults. |
| 106 | He C.-S. [134] | 2013 | Cross-sectional | Vit D | Natural source | 28 | All = 21 ± 3 | M = 184 F = 83 |
A low Vit D level was associated with lower pro-inflammatory cytokine production by monocytes and lymphocytes. Low Vit D levels increase the risk of systemic immunity in endurance athletes. |
| 107 | Askari G. [135] | 2013 | Randomized, placebo-controlled, double-blind clinical trial | Vit C | Supplement | 225 | M = 21.0 ± 1.6 | M = 60 | Quercetin and Vit C supplementation may not be beneficial in lipid profile improvement, although it may reduce induced muscle damage and the body fat percentage. |
| 108 | Taghiyar M. [136] | 2013 | Randomized, double-blind clinical trial | Vit C, Vit E | Supplement | 60 | Group 1 = 31.3 ± 1.8 Group 2 = 38.5 ± 1.6 Group 3 = 33.9 ± 1.5 Group 4 = 38.1 ± 1.4 |
F = 64 | Vits C and E supplementation can be beneficial in reducing muscle damage indices during aerobic exercises. |
| 109 | Magee P.J. [137] | 2013 | Observational study | Vit D | Supplement | 64 | All ≥ 18 | M = 84 | Vit D supplement is recommended during the winter and early spring months to overcome insufficiency in athletes. |
| 110 | Peeling P. [138] | 2013 | Cross-sectional | Vit D | Natural source | 84 | All = 16 ± 4 | M = 43 F = 29 |
Vit D deficiency is common among athletes; hence, coaches are encouraged to recommend a stretching warm-up routine in an outdoor setting during the winter season specifically. |
| 111 | Sureda A. [139] | 2013 | Randomized, double blind clinical trial | Vit C, Vit E | Supplement | 72 | Group 1 = 32.7 ± 9.2 Group 2 = 36.4 ± 9.7 |
M = 14 | Neutrophils protein oxidation-induced exercise is reduced due to Vit E and Vit C antioxidant supplement intake proteins. Vit E and Vit C intake did not alter the adaptive response of the antioxidant and increased the gene expression of catalase and glutathione peroxidase. |
| 112 | Garelnabi M. [140] | 2012 | Randomized clinical trial | Vit E | Supplement | 14 | M = 32.4 ± 8.7 F = 34.2 ± 10.0 |
M = 195 F = 260 |
Vit E had no impacts on exercise oxidative stress and inflammation. |
| 113 | Spradley B.D. [141] | 2012 | Randomized, double-blind, placebo-controlled cross-over design | B Vits | Supplement | 60 | M = 28 ± 5 | M = 12 | The performance reaction and endurance of lower body muscles are enhanced significantly after consuming supplements pre-strenuous exercise. Moreover, spare energy and reduced fatigue delay fatigue. |
| 114 | Czaja J. [142] | 2011 | Clinical trial | Magnesium and B6 | Supplement | 12 | Supplementation food with magnesium is recommended | ||
| 115 | Patlar S. [143] | 2011 | Clinical trial | Vit E | Supplement | NR | M = 22.1 ± 0.5 | M = 7 | There is a significant interference of mineral and electrolyte metabolism due to the intake of Vit E in elite athletes. |
| 116 | Halliday T.M. [144] | 2011 | Clinical trial | Vit D | Natural source | 7 | M = 20.1 ± 1.9 F = 19.9 ± 1.5 |
M = 18 F = 23 |
Among university athletes, the intake of Vit D decreases the risk of frequent illness. |
| 117 | Abolghasem R. [145] | 2011 | Clinical trial | Vit C | Supplement | 41 | N/A | N/A | The level of serum CPK decreased significantly with the Vit C intake. |
| 118 | Louis J. [146] | 2010 | Clinical trial | Vit-Mineral Complexes | Supplement | N/A | Group 1 = 50.8 ± 6.5 Group 2 = 47.7 ± 6.3 |
N/A | Micronutrient supplements decrease muscle inflammation, which improves strength training. |
| 119 | Chatterjee P. [147] | 2010 | Clinical trial | Vit E | Supplement | 20 | F = 21–25 | F = 25 | Among inactive women and during phases of the menstrual cycle, the intake of 400 mg/day for 1 week of a Vit E supplement improved VO2max, maximum voluntary ventilation, oxygen pulse, and endurance capacity. Vit E supplementation should be considered to improve the endurance performance of females. |
| 120 | Fage N. [148] | 2010 | Clinical trial | Vit D | Supplement | 25 | All = 40.6 ± 12.1 | M = 15 F = 5 |
During anaerobic metabolism, there was no association between Vit D serum status and MAV. |
| 121 | Zaǐtseva I.P. [149] | 2010 | Clinical trial | Vit–Mineral Complexes | Supplement | 20 | All = 18–22 | N/A | A higher dose of minerals decreased the absorption percentage of iron, copper, and magnesium and increased fecal and urinary ME excretion. |
| 122 | Dalbo V.J. [150] | 2010 | Double-blind, randomized crossover design | Mineral Antioxidant Complex (Mac) | Supplement | 49 | M = 23.6 ± 3.7 | M = 15 | The performance of aerobic exercise and the lactate response did not differ due to the mineral antioxidants’ complex intake. |
| 123 | Karandish M. [151] | 2008 | Double blind, randomized controlled trial | Vit C | Supplement | 15 | F = 20–33 | F = 49 | Among healthy young women who engage in moderate-intensity exercise, the intake of 500 mg/day for two weeks of a Vit C supplement had no impact on oxidative stress markers. |
| 124 | Al-Khalidi M.J.M. [152] | 2009 | Clinical trial | Vit D3 | Supplement | 219 | N/A | F = 18 | The biomechanical variables studied helped the researchers to determine the strengths and weaknesses of the sample in the skill of spike. Applications of resistance exercises of all types with the aids with doses of Vit D had the effect of improving the performance of the skill of spike. |
| 125 | Sureda A. [153] | 2008 | Randomized clinical trial | Vit C, Vit E | Supplement | 49 | M = 32–36 | M = 14 | A moderate amount of Vits antioxidants supplements reduced oxidative damage due toexercise and lipid peroxidation due to intense exercise and maintained the exercise cellular adaptation. |
| 126 | Nakhostin-Roohi B. [154] | 2008 | Double-blind, placebo-controlled trial | Vit C | Supplement | 14 | Group 1 = 21.5 ± 0.8 Group 2 = 22.1 ± 0.6 |
M = 16 | The intake of Vit C prevented muscle damage and lipid peroxidation post-endurance exercise but had no impact on inflammatory markers. |
| 127 | Cholewa J. [155] | 2008 | Clinical trial | Vit C | Supplement | 16 | All = 23.9 ± 2.6 | N/A | Among basketball players, the levels of blood antioxidants and VO2max were not affected by the intake of a Vit C supplement. |
| 128 | Machefer G. [156] | 2007 | Cross-sectional | Antioxidant Vits (A-Tocopherol, Vc, Β-Carotene, Retinol) | Natural source | 21 | M = 41.4 ± 1.8 | M = 19 | Among ultra-endurance athletes, a low intake of antioxidant Vit led to insufficient energy intake. |
| 129 | Disilvestro R.A. [42] | 2007 | Clinical trial | Vit D, Calcium | Supplement | 19 | F = 18–24 | F = 24 | To improve bone health among young adult women, it is recommended to consider exercise along with the intake of micronutrients. |
| 130 | Gaeini A.A. [44] | 2006 | Randomized controlled trial | Vit E | Supplement | 24 | N/A | M = 20 | Student athletes’ performance was not affected by Vit E supplementation. |
| 131 | Johnston C.S. [157] | 2006 | Preliminary study | Vit C | Natural source | 20 | All = 18–38 | N/A | Fatigue might be reflected due to fat oxidation inhibition, which is related to a low Vit C status during submaximal exercise. |
| 132 | Davison G. [158] | 2006 | Clinical trial | Vit C | Supplement | 78 | M = 26 ± 2 | M = 9 | Post-endurance exercise neutrophil depression was not prevented by the intake of Vit C supplements when consumed for 2 weeks. |
| 133 | Fischer C.P. [159] | 2006 | Randomized controlled trial | Vit C, Vit E | Supplement | 9 | Group 1 = 25.6 Group 2 = 22.3 Group 3 = 24.1 |
M = 21 | During an acute exercise program, the intake of Vit E and Vit C for 28 days suppressed the heat shock protein. |
| 134 | Machefer G. [160] | 2006 | Cross-sectional | Vit E, Vit C, Beta Caroten, Retinol | Natural source | 21 | All = 41.4 ± 1.8 | N/A | Athletes suffered from an inadequate intake of antioxidant Vits. |
| 135 | Bryer S.C. [161] | 2006 | Randomized control trial | Vit C | Supplement | 19 | Group 1 = 24.4 ± 1.7 Group 2 = 21.4 ± 0.8 |
M = 18 | The intake of Vit C before exercise would inhibit muscle stress, postpone creatine kinase release, and suppress the oxidation of bloodglutathione, with a minor impact on the loss of muscle function. |
| 136 | Fry A.C. [162] | 2006 | Randomized control trial | Vits (A, B1, B2, B3, B6, B5, B9, B12, Biotin, C, D, E) Minirals (Calcium, Chromium, Iodine, Iron, Magnesium, Manganese, Potassium Selenium, Sodium, Zinc) | Supplement and natural source | 18 | Group 1 = 25 ± 4 Group 2 = 23 ± 2 |
M = 14 | The performance post-short-term anaerobic exercise is enhanced by the intake of a micronutrients supplement, but not in well-trained individuals consuming an adequate diet. |
| 137 | Senturk U.K. [163] | 2005 | Clinical trial | Vits (C, E, A) | Supplement | 14 | Group 1 = 20.6 ± 0.33 Group 2 = 19.8 ± 0.44 |
M = 18 | In relation to a series of exhausting exercises, the risk of hemorheological and exercise-induced death would be prevented by antioxidant Vit treatment. |
| 138 | Davison G. [164] | 2005 | Clinical trial | Vit C | Supplement | 18 | M = 25 ± 2 | M = 6 | In prolonged exercise, high doses of Vit C with or without carbohydrates were traced to a minor impact on the hormonal, interleukin-6, or immune response. |
| 139 | Herrmann M. [165] | 2005 | Case-control | Vit B12, Folate | Natural source | 6 | Group 1 = 38 ± 7 Group 2 = 38 ± 9 |
N/A | Among recreational athletes, mma (methylmalonic acid) was not associated with Vit B12 metabolism. |
| 140 | Rousseau A.-S. [166] | 2004 | Cross-sectional study | Vits (E, C), B-Carotene, Carotenoids | Natural source | 118 | All = 26.8 ± 6.8 | M = 84 F = 34 |
Carotenoids as exogenous antioxidants have important protective roles and should be considered by athletes. |
| 141 | Cui J.-H. [167] | 2004 | Randomized control trial | Vit Tablet | Supplement | 118 | N/A | M = 40 | To delay sport fatigue and lipid peroxidation after hypoxia condition exercise, it is recommended to consume acetazolamide, highland-Vit-tablets, and redbull beverages. |
| 142 | Viitala P.E. [168] | 2004 | Clinical trial | Vit E | Supplement | 40 | Group 1 = 23.3 ± 3.8 Group 2 = 24.2 ± 3.7 |
M = 15 F = 12 |
No significant difference between Vit E supplements and placebo in reducing oxidative damage and lipid peroxidation measures between the trained and untrained groups. |
| 143 | Thompson D. [169] | 2004 | Experimental design | Vit C | Supplement | 27 | Group 1 = 25.3 ± 1.4 Group 2 = 22.6 ± 1.7 |
M = 14 | The intake of a Vit C supplement post-eccentric exercise has no impact on the interleukin-6 level. |
| 144 | McAnulty S.R. [170] | 2004 | Randomized, double-blind, crossover design | Vit C, Polyphenols | Vit C: supplement, polyphenols: natural source | 14 | M = 23.8 ± 2.5 | M = 9 | Blueberries supplement increases the level of rooh but not the f2-isoprostane level more than Vit C. |
| 145 | Avery N.G. [171] | 2003 | Clinical trial | Vit E | Supplement | 9 | N/A | M = 18 | The intake of Vit E supplement post-resistance concentric/eccentric exercise did not prevent oxidative stress, membrane damage, and low performance. |
| 146 | Bryant R.J. [172] | 2003 | Clinical trial | Vitc, Vit E | Supplement | 18 | M = 22.3 ± 2 | M = 7 | The intake of Vit E (400 IU/day) decreases the tissue damage but not the performance more than Vit C. To prevent the damage effect of exercise, it is recommended that athletes consume Vit E and Vit C via their diet. |
| 147 | Tauler P. [173] | 2003 | Experimental study | Vits (A, B1, B2, B6, B12, C, D, E, Niacin, Folic Acid) and Minerals (Sodium, Potassium, Calcium, Phosphorous, Magnesium, Iron, Zinc, and Iodine). | Vit C: supplement; dietary intake for all micronutrients | 7 | Group 1 = 25.0 ± 1.5 Group 2 = 24.4 ± 1.1 |
M = 16 | The excessive antioxidant nutrients’ intake, which contains Vit C, increases ascorbate in order to prevent negative impacts on rbc and body tissue due to post-exercise oxidative stress. |
| 148 | Schneider M. [174] | 2003 | Placebo controlled, cross-over | Vit E, Vit C | Supplement and natural source | 16 | M = 26.5 ± 0.9 | M = 13 | With moderate oxidative stress, Vit E supplement intake would not be able to enhance the level of Vit C. |
| 149 | Sacheck J.M. [175] | 2003 | Clinical trial | Vit E | Supplement | 13 | Group 1 = 26.4 ± 3.3 Group 2 = 71.1 ± 4.0 |
M = 32 | Vit E supplement would prevent the oxidative stress due to eccentric exercise. |
| 150 | König D. [176] | 2003 | Cross-sectional | B12, Folate | Blood sample | 32 | M = 27.1 ± 5.3 | M = 39 | High leveld of plasma folate among athletes decrease hcy levels due to the highest training volume. |
| 151 | Mel’nikov A.A. [177] | 2003 | 39 | ||||||
| 152 | Thompson D. [178] | 2003 | Clinical trial | Vit C | Supplement | NR | Group 1 = 23.6 ± 1.4 Group 2 = 24.3 ± 1.7 |
M = 16 | The immediate intake of Vit C post-exercise is not proper for promoting recovery. |
| 153 | Tauler P. [179] | 2002 | Clinical trial | Vit E, Vit C, and Beta-Carotene | Supplement | 16 | All = 23.3 ± 2.0 | N/A | The activity of superoxide dismutase and catalase antioxidant enzymes in neutrophils is enhanced by antioxidant supplementation. |
| 154 | Childs A. [180] | 2001 | Double-blind, placebo-controlled | Vit C | Supplement | 20 | M = 24.4 6 3.6 | M = 14 | The immediate intake of Vit C and NAC supplement after injury would have a negative effect on tissue damage and oxidative stress. |
| 155 | Krause R. [181] | 2001 | Clinical trial | Vit C | Supplement | 14 | M = 29 ± 3 | M = 10 | Post-strenuous exercise, neutrophil dysfunction is corrected due to the intake of Vit C supplement. |
| 156 | Thompson D. [182] | 2001 | Clinical trial | Vit C | Supplement | 10 | Group 1 = 23 ± 2 Group 2 = 25 ± 2 |
M = 16 | Long-term Vit C supplement intake would have a reasonable and beneficial impact on the recovery phase due to the unusual exercise protocol. |
| 157 | Akova B. [183] | 2001 | Clinical trial | Vit E | Supplement | 16 | Group 1 = 26 ± 6 Group 2 = 27 ± 8 |
F = 18 | Oestradiol protects from oxidative injury, more so than Vit E. Both had no impact on exhausted muscle performance. |
| 158 | Petersen E. [184] | 2001 | Clinical trial | Vit C, Vit E | Supplement | 18 | Group 1 = 28 Group 2 = 26 |
M = 20 | The level of Vit C and Vit E is raised significantly due to the supplements intake, although its level does not affect the immune indicators of cytokine, lymphocyte responses, or muscle enzymes due to exercise. |
| 159 | Sacheck J.M. [185] | 2000 | Experimental | Vit E, Vit C, Beta Carotene | Natural source | 20 | F = 18–25 | F = 22 | Vit E intake, even not from supplements, would be sufficient to protect from the oxidative stress which is yielded due to the moderate-intensity exercise. |
| 160 | Kawai Y. [186] | 2000 | Clinical trial | Vit E | Supplement | 22 | F = 21.2 ± 0.5 | F = 10 | The priority of Vit E intake is RBC protection against oxidative damage. The sufficiency level of serum Vit E promotes its shifting from the serum to a steady RBC-α-tocopherol level due to exercise. |
| 161 | Chung T.-W. [187] | 2000 | Clinical trial | Vit C | Supplement from fruit | 10 | N/A | M = 20 | A minor increase in the antioxidant level when athletes consume 500 mg of Vit C from fruits during moderate/high-intensity endurance training; however, it did not balance the oxidative stress. Athletes who engage in long-term endurance-training would benefit from Vit C extracted from fruit. |
| 162 | Beshgetoor D. [188] | 2000 | Prospective, observational study | Calcium | Natural source | 20 | F = 49.6 ± 7.9 | F = 30 | No significant interaction effect of the sport and dietary calcium intake was noted for bmd at any site. |
| 163 | Monnat A. [189] | 2000 | N/A | N/A | 30 | N/A | N/A | N/A | |
| 164 | Sürmen-Gür E. [190] | 1999 | Clinical trial | Vit E | Supplement | N/A | M = 12–24 | M = 36 | Vit E supplementation had an insufficient impact on plasma lipid peroxidation after exercise. |
| 165 | Krumbach C.J. [191] | 1999 | Cross-sectional | Multi-Vits + Minerals | Supplement and natural source | 36 | All ≥ 19 | M = 266 F = 145 |
Calcium and iron supplementation are commonly consumed by females, while males consume Vit B12 and Vit A supplements. There were some variations in the Vit/mineral supplement habits of the athletes by gender, ethnicity, and sport. |
| 166 | Virk R.S. [192] | 1999 | Nonrandomized clinical trial | Vit B6 | Supplement | 411 | N/A | M = 11 | Through intensive endurance exercise, Vit B6 supplementation can alter plasma FFA and amino acid levels. |
| 167 | Savino F. [193] | 1999 | Comparative study | Vits | Supplement | 11 | Group 1 = 6–12 Group 2 = 9 |
N/A | Subjects who are at risk of Vit deficiency or highly demanding would benefit from supplements intake. |
| 168 | Rourke K.M. [194] | 1998 | Double-blind clinical trial | Calcium | Supplement | 40 | F= 18–22 Year | F = 30 | Higher calcium intakes promote some benefit to bone mineral density. |
| 169 | Alessio H.M. [195] | 1997 | Clinical trial | Vit C | Supplement | 30 | M = 33.0 ± 2.6 | M = 9 | Vit C supplementation for 1 day/2 weeks reduces the oxidative stress induced by exercise. |
| 170 | Oostenbrug G.S. [196] | 1997 | Double-blind randomized | Vit E | Supplement | 9 | M = 19–42 | M = 24 | Endurance performance is not improved by fish oil supplements. Due to the increase in oxidative stress post-endurance exercise, fish oil may act as an antioxidant. |
| 171 | Hartmann A. [197] | 1995 | Clinical trial | Vit E | Supplement | 10 | M = 29–34 | M = 8 | Vit E prevents exercise-induced DNA damage and indicates that dna breakage occurs in WBC after exhaustive exercise as a consequence of oxidative stress |
| 172 | Sobal J. [198] | 1994 | Cross-sectional | MultiVites + Minerals | Supplement and natural source | 8 | N/A | N/A | Supplement use by these adolescents appears to be motivated more by health reasons than by sports performance. It is suggested that it may be useful to assess Vit/mineral supplement use by adolescents and to provide education and counseling about diet, nutrition, and exercise for those who use them as ergogenic aids to improve athletic performance. |
| 173 | Men’shikov I.V. [199] | 1994 | Comparative study | Vit E | Supplement | 742 | N/A | N/A | The use of Vit E resulted in a decrease in the energy value of exercises performed by the athletes under normo- and hyperthermic conditions as well as changes in blood and erythrocyte membrane lipid composition and blood calcium ion concentration. |
| 174 | Rokitzki L. [200] | 1994 | Cross-section | Vit B6 | Supplement | N/A | Bodybuilding = 25.3 ± 6.4 Wrestling = 20.6 ± 2.7 Basketball = 26.1 ± 4.7 Soccer = 23.5 + 2.8 Handball = 23.8 ± 7.9 |
M = 45 F = 12 |
Athletes’ Vit B6 level is not yet assessed due to the absence of generally valid reference values. |
| 175 | Rokitzki L. [201] | 1994 | Clinical trial | Vit B6 | Supplement | 57 | M = 35.6 ± 9.8 | M = 13 | With a balanced diet, the intake of exogenous Vit B6 is not necessary. |
| 176 | Rokitzki L. [202] | 1994 | Cross-section | ViteB2 | Supplement | 13 | N/A | Athletes Group: M = 50 F = 12 |
B2 Vit is favored among performance athletes. |
| 177 | Lorino A.M. [203] | 1994 | Single-blind crossover | Vit E | Supplement | 78 | M = 22.0 ± 1.0 | M = 7 | Vit E intake did not reduce the lung clearance exercise-induced increase. |
| 178 | Jakemanl P. [204] | 1993 | Double-blind clinical trial | Vit E and Vit C | Supplement | 7 | All = 19.6 | M = 16 F = 8 |
There was a reduction in the loss of contractile function post-eccentric exercise and in the first 24 h of recovery in the group supplemented with Vit C but not Vit E. Prior supplementation with Vit C can attenuate eccentric exercise-induced muscle damage. It is proposed that the effect of Vit C supplementation on contractile function, particularly LFF, could be to protect vital cell structures such as the SR from oxidative stress and free radical injury. |
| 179 | Nasolodin V.V. [205] | 1993 | N/A | Vit C, Vit P, and Vit Complex (Ascorutine, Thiamine, Riboflavin, Pyridoxine, Cyan Cobalamin, Folic Acid) | N/A | 24 | N/A | N/A | The metabolism of iron, copper, and manganese is affected by the intake of Vit c or Vit complex (ascorutine, thiamine, riboflavin, pyridoxine, cyan cobalamin, folic acid). |
| 180 | Bazzarre T.L. [206] | 1993 | Cross-sectional | Vit-Mineral Supplement | Supplement | N/A | N/A | N/A | Subjects who intake supplements might reflect healthy lifestyle practices. |
| 181 | Klausen T. [207] | 1993 | Cross-sectional | Vit D and Calcium | Natural source | 91 | M = 41–50 | M = 9 | Endurance training impacts the plasma Vit D level and pth. |
| 182 | Fogelholm M. [208] | 1993 | Clinical trial | B-Complex | Supplement | 9 | All = 18–32 | M = 24 F = 18 |
Activation coefficients acs (Vit B1, B2, and B6) were not associated with blood lactate. |
| 183 | Maxwell S.R.J. [209] | 1993 | Clinical trial | Vit E and Vit C | Supplement | 42 | All = 19.6 ± 0.3 | M = 16 F = 8 |
Vits supplementation enhanced the plasma capacity of antioxidants due to one hour of eccentric exercise. |
| 184 | Meydani M. [210] | 1993 | Double-blind clinical trial | Vit E | Supplement | 24 | Young Group = 22–29 Adult Group = 55–74 |
M = 21 | Vit E dietary supplementation for 48 days diminished free radical-mediated exercise oxidative damage, reduced oxidative injury, and increased muscle a-tocopherol. |
| 185 | Mikalauskaǐte D.A. [211] | 1992 | 21 | ||||||
| 186 | MEYDANI M. [212] | 1992 | NR | ||||||
| 187 | Telford R.D. [213] | 1992 | Clinical trial | Vits (B1, B2, B6, C, E, A, B12, Folate) and Six Minerals (Cu, Mg, Zn, Ca, P, Al) | Supplement | NR | M = 17.3 ± 1.4 F = 17.3 ± 1.1 |
M = 50 F = 36 |
Micronutrients supplementation for 7–8 months promotes blood Vits levels but not mineral levels, and some blood nutritional indicators may vary according to sex (the values generally being higher in females, with significant differences for Vits B2, C, and E and copper and aluminum). |
| 188 | Fogelholm M. [214] | 1992 | Cross-sectional | Vits (B1, B2, B6), Magnesium, Iron, and Zinc | Natural source | 86 | Group 1 = 24.0 ± 0.6 Group 2 = 26.0 ± 0.6 |
F = 39 | A protocol of 24-week fitness exercise effectively increased VO2max, while it did not affect thiamin, riboflavin, magnesium, iron, and zinc status. |
| 189 | Deuster P.A. [215] | 1991 | 39 | ||||||
| 190 | Colgan M. [216] | 1991 | Double-blind crossover trial | All Vits and Minerals | Supplement | NR | N/A | M = 12 F = 11 |
Among endurance intense training athletes, RDA is enough, along with iron supplements. |
| 191 | Pieralisi G. [217] | 1991 | Double-blind, randomized, crossover | Supplement | 23 | M = 21–47 | M = 50 | Ginseng preparation improved muscular oxygen utilization and hence enhanced work capacity. | |
| 192 | Miric M. [218] | 1991 | 50 | ||||||
| 193 | Cannon J.G. [219] | 1991 | Double-blind placebo-controlled protocol | Vit E | Supplement | NR | Group 1 = 25 ± 3 Group 2 = 65 ± 2 |
M = 21 | Vit E supplement significantly affected IL-1 and IL-6 production, with no significant impact on exercise-related changes |
| 194 | Faber M. [220] | 1991 | Cross-sectional | Calcium, Iron, Magnesium, Phosphorus, Vit A, Thiamin, Riboflavin, Nicotinic Acid, Vit B6, Folic Acid, Vit B12, Ascorbic Acid | Natural source | 21 | M = 22.1 ± 3.8 F = 22.3 ± 2.9 |
M = 20 F = 10 |
With a sufficient energy intake, males consumed an adequate amount of all micronutrients, while females digested insufficiently for calcium, iron, and magnesium. |
| 195 | Cannon J.G. [221] | 1990 | Clinical trial | Vit E | Supplement | 30 | Group 1 = 22–29 Group 2 = 55–74 |
M = 21 | Vit E supplementation had a positive impact on damaged tissue, as it promotes the accumulation of neutrophil. |
| 196 | Van Erp-Baart A.M.J. [222] | 1989 | Survey research | Calcum, Iron, Vites (C, A, B1, B2, B6) | Natural source and supplement | Nr | N/A | N/A | A sufficient intake of Vit and minerals was notice when the energy intake ranged between 10 and 20 mj/day. There was a positive correlation between calcium and iron intake and energy intake. Hence, low energy intakes challenge ca and iron. |
| 197 | Satoshi S. [223] | 1989 | Experimental trial | Vit E | Supplement | N/A | M = 20.3 ± 0.3 | M = 21 | Post-exercise Vit E supplementation significantly reduces the malondialdehyde level, the leakage of enzymes, and lipid peroxidation. |
| 198 | Guilland J.-C. [224] | 1989 | Clinical trial | Vit B1, B2, B6, C, A, and E | Natural source and supplement | 21 | Group 1 = 19.6 ± 0.56 Group 2 = 22.5 ± 0.4 |
M = 55 | Daily Vits supplements for one month significantly improve serum Vit C, plasma plp, and erythrocyte tpp concentrations, etk and egr basal activities, east basal activities (only in young athletes), and etk, egr, and east activation values. |
| 199 | Weight L.M. [225] | 1988 | Double-blind, placebo-controlled crossover study | Vits (E, D, C, A, B1, B2, B6) and Minerals (Selenium, Iodine, Phosphorus, Ca, Iron, Zn, Cu, K, Mg) | Supplement | 55 | M = 20–45 | M = 30 | A normal diet intake is enough, and there is no need for supplementation. |
| 200 | Weight L.M. [225] | 1988 | Placebo-controlled crossover | Multi-Vits and Minerals | Supplement | 30 | M = 31.9 ± 10.6 | M = 30 | |
| 201 | Manore M.M. [226] | 1988 | Clinical trial | B6 | Natural source | 30 | Group 1 = 25.6 ± 4 Group 2 = 24.4 ± 3.2 Group 3 = 55.8 ± 4.8 |
F = 15 | Among females, the intake of Vit B6 may alter serum fuel substrates during exercise, depending on her age. |
| 202 | Bell N.H. [227] | 1988 | Clinical trial | Calcium, Phosphorus, Sodium, Potassium, Magnesium | Natural source | 15 | Group 1 = 19–36 Group 2 = 26 ± 1 |
M = 28 | |
| 203 | Klepping J. [228] | 1988 | Clinical trial | Minerals (P, Mg, Ca, Fe) and Vits (C, B1, B2, B6) | Natural source | NR | Group 1 = 15.16 ± 1.6 Group 2 = 23.91 ± 4.85 |
M = 265 | Even with a high energy intake, deficient micronutrient intakes were common among many athletes. |
| 204 | Manore M.M. [229] | 1987 | Comparative study | B6 | Natural source | N/A | Group 1 = 25.6 ± 4 Group 2 = 24.4 ± 3.2 Group 3 = 55.8 ± 4.8 |
F = 15 | Vit B6 metabolism did not change due to exercise, nor training. |
| 205 | Zuliani U. [230] | 1985 | N/A | Mineral Salts | N/A | NR | N/A | N/A | N/A |
| 206 | Butturini U. [231] | 1984 | N/A | ||||||
| 207 | Walter M.C. [232] | 1984 | NR | ||||||
| 208 | Grandjean A.C. [12] | 1983 | N/A | N/A | N/A | NR | N/A | N/A | N/A |
| 209 | Rusin V.I. [233] | 1982 | N/A | ||||||
| 210 | Borisov I.M. [234] | 1980 | Comparative study | Vit C, Vit P | Supplement | NR | N/A | N/A | N/A |
| 211 | Helgheim I. [235] | 1979 | Double-blind experimental design | Vit E | Supplement | 1203 | All = 19–24 | M = 2 F = 24 |
Post-exercise intake of Vit E had no impact on serum enzyme levels. |
| 212 | Leklem J.E. [236] | 1979 | 26 | ||||||
| 213 | Laricheva K.A. [237] | 1979 | N/A | Vits (A, B1, B2, B6, Pp, C) | N/A | NR | N/A | N/A | N/A |
| 214 | Dam B.V. [238] | 1978 | Double-blind test | B1, B2, and B6 | Supplement (granulated multi-Vit electrolyte preparation) | N/A | F = 18.3 ± 3.0 M = 18.9 ± 3.5 |
M = 33 F = 7 |
B Vits deficiency is noticed due to the high rate of energy metabolism, the high body core temperature, and sweat loss. |
| 215 | Haralambie G. [239] | 1976 | Clinical trial | Vit B | Supplement | 40 | M = 17–38 | M = 25 | An intake of 10 mg of riboflavin lowers neuromuscular irritability. |
| 216 | Shephard R.J. [240] | 1974 | Matched-pair trial under near-double-blind conditions | Vit E | Supplement | 25 | M = 20.3 ± 1.6 | M = 16 | Vit E had no advantage for swimmers. |
| 217 | Bailey D.A. [241] | 1970 | Double-blind | Vit C | Supplement | NR | M = 24.5 ± 3.5 | M = 40 | There was no significant impact of Vit C on respiratory and oxygen utilization before, during, and after exercise in smoking and nonsmoking subjects. |
Figure 1.
Model of micronutrients’ main functions in sports medicine.
4. Vitamins
Vitamins are organic essential compounds that cannot be synthesized by the human body [29]. They play a vital role in numerous functions that are relevant to the athlete’s performance [7]. Their functions are evident in co-enzymes, hormones, and autoxidation, as well as their contribution to energy production [242]. There are thirteen various kinds of vitamins currently divided into two major groups due to their chemical and biological functions, four of which are fat-soluble vitamins (FSV) including vitamins A, D, E, and K, and the rest are water-soluble vitamins (WSV) including B complex vitamins and vitamin C [243]. Table 1 summarizes the recommendation requirements of vitamins along with the rich sources and their roles in exercise performance.
5. Fat-Soluble Vitamins (FSV)
Vitamin A
Vitamin A plays a significant role in promoting the overall wellbeing of athletes, as it aids in the formation of healthy tissues and improves oxygen access throughout the body, thereby supporting the maintenance of an adequate level of physical activity [3]. It also has a crucial influence on vision, skin health, and immune system functioning [29]. Moreover, vitamin A is a potent antioxidant that helps in neutralizing free radicals generated by oxidative stress during advanced physical training. The sufficient consumption of vitamin A may help alleviate the reactive oxygen species and avoid the onset of illnesses such as heart failure and muscle damage [244], as mentioned in many studies in Table 2.
Vitamin A mainly exists in two forms: animal source (retinol) and plant-based provitamin A (carotenoids). The intake of sufficient amounts of beef liver, eggs, dairy products, and seafood as well as dark leafy green vegetables may ensure meeting the dietary requirements for athletes. It is noteworthy that athletes may benefit from supplementation with multivitamins that contain vitamin A, as a part rather than its own sole use [29].
Vitamin A has been proved to enhance and support various body functions, including reaction time, muscles recovery [245,246], and protein synthesis, which is essential for muscle growth and recovery and can be important for those competing in events requiring fast reflexes [247]. Furthermore, vitamin A can help protect athletes against injuries by increasing healing times and promoting the formation of healthy connective tissues [3]. Finally, vitamin A may help fight off colds, flu, and other illnesses, which can be particularly helpful for traveling athletes.
To evaluate the effect of crocetin on fatigue, a study test was conducted by athletes to measure stamina using a bicycle ergometer at a standard workload for 120 min twice. They also performed non-workload tests of 10 s at 30 min at a maximum velocity (MV) [242]. A difference in MV from 30 to the 210 min test was remarkably reported only in men who consumed crocetin when compared to their counterpart who used a placebo (p < 0.05) [248]. No difference was observed with the consumption of ascorbic acid in all candidates with the same period [248]. The daily consumption of crocetin would reduce physical exhaustion in men, according to these results [179]. The attenuating effect of saffron carotenoids on muscle fatigue is due to their provitamin A activity [248]. Athletes should meet their daily recommended intake of vitamin A to guarantee a perfect peak physical condition [249].
The Recommended Dietary Allowance (RDA) of retinol activity equivalents (mcg RAE), as shown in Table 1, is 900 micrograms for men aged 19 years old and older, equal to 3000 international units (IU), and 700 mcg RAE for women aged 19 years old and older, equivalent to 2333 (IU). However, the adherence to a maximum Tolerable Upper Limit (UL) of 10,000 IU (3000 mcg)/day for adults is important to avoid any dangerous effect. It is also important to emphasize that toxicity may occur when frequent doses of more than 25,000 IU are taken daily [3].
β-carotene is a member of the carotenoid family that is thought to provide numerous health benefits, including immunity system boosting, antioxidation properties, and performance enhancement [250]. β-carotene is an essential antioxidant, meaning that it helps prevent the harmful effect of free radicals on cells [251]. This is important for athletes, as free radicals are generated during strenuous exercise and can lead to fatigue and soreness [176]. Several studies have reported similar findings, highlighting the ability of vitamin A to potentially reduce recovery time from exercise [7,250]
Sumac juice drink was tested in a study to evaluate its impact on pain scores through post-exercise intervals. Forty healthy candidates involved in an aerobic training protocol for four weeks received a dose of placebo or sumac juice consumption two times/day for a month. The results revealed that participants of the sumac juice group had a lower pain score increment and even a better enhancement during pain relief. The potency of protecting muscles might be due to the sumac juice antioxidant potency of β-carotene-linoleic acid components. These results highlight the possibility of sumac juice consumption in improving muscle performance among athletes [252]. Nevertheless, future studies of athletes are warranted.
Athletes often push their bodies to the limit, therefore compromising their immune systems and making them more susceptible to infections and illness [176]. Taking a supplement with β-carotene may have potential benefits for the immune system, helping to prevent illness and potentially enabling athletes to train harder and longer [250,251]. Apparently healthy nonsmoker adult males were involved in consuming placebo or 15 mg/day of β-carotene for 26 days. After oral administration, significant increases in the monocytes percentage representing the major histocompatibility complex class II molecule human leukocyte antigen DR isotype (HLA-DR) and the adhesion molecules intercellular adhesion molecule-1 and leukocyte function-associated antigen-3 were observed. Furthermore, tumor necrosis factor-alpha (TNF-alpha) was notably elevated due to the dose intake, arguing that a slight increment in the consumption of dietary β-carotene can enhance the responses of immune cells within the short term, supporting the process of the carcinogenic potency [253].
Lastly, β-carotene has been found to improve physical performance [254]. Studies have shown that β-carotene supplementation may lead to endurance strength improvement and injury risk prevention [3,150]. Similarly, a meta-analysis of nine studies including participants from both genders, with a total 190,545 candidates, emphasized that β-carotene leads to a significant enhancement in overall performance [29]. There was a 95% possibility that β-carotene consumption attenuates the possibility of hip fracture and other different fracture types by over 20% [251]. According to research, despite the uncertain effect of the antioxidant’s supplementation, consuming β-carotene and combining antioxidants or not still has an effective impact in reducing exercise-induced peroxidation [33]. This may enhance athletes’ endurance in the long term [251,254,255].
6. Vitamin E
As research progresses, the potential advantages of vitamin E for athletes are becoming increasingly apparent. Vitamin E helps in protecting athletes’ bodies and may improve both performance and recovery [75]. Overtraining and intense exercise are associated with reactive oxygen species (ROSs) production, which aids in enhanced muscular and endurance adaptation to exercise through the upregulation of endogenous antioxidant enzymes [256]. However, excess accumulation of ROS accompanied by the inability of the body to scavenge these compounds is harmful to the body’s cell components which is associated with fatigue, delayed recovery, and reduced performance [257]. Accordingly, research suggests the possible protective effects of vitamin E supplements against chronic stress associated with exercise [257,258]. This vitamin possesses antioxidant properties by neutralizing free radicals, protecting cells and tissues [257,258].
Studies showed controversial results of vitamin E supplementation in athletes [44]. It was shown that vitamin E supplements neither inhibit exercise-induced oxidative stress nor impact endurance running performance [259]. Moreover, these results were supported by the randomized controlled trial on athletic students where vitamin E oral consumption was found to not influence exercise endurance [260]. On the other hand, among healthy individuals, vitamin E (alpha-tocopherol) consumption inhibits the exercise-induced reduction in blood paraoxonase 1/arylesterase activity [6,44,147,260]. Excess doses of supplements have been shown in studies to inhibit the signaling reactions required for adaptations to exercise, creating an interference effect [3].
A meta-analysis revealed that vitamin E supplements have a beneficial and protective effect, particularly at low doses (≤500 IU/day), in reducing biomarkers associated with exercise-induced muscle damage and oxidative stress. Beneficial effects of the antioxidant characteristics of vitamin E were observed among exercise-induced side-effects [257]; both animal and human studies have indicated that Vitamin E has the potential to enhance immune function and provide protection against various infectious diseases [6]. Vitamin E reduces PGE2 production and inhibits COX2 activity, likely by decreasing nitric oxide production [261]. Furthermore, it improves T cells immune synapse formation and activation signals, and lastly, it helps in modulating the T (Th1/Th2) balance [52]. This is particularly beneficial for athletes, who are often susceptible to illness and injury due to the intense physical demands of their sport. By supplementing with vitamin E, athletes can help increase the body’s natural immunity, thus reducing the risk of infection and promoting recovery [262].
Vitamin E may assist in improving blood flow, which is essential for athletes [242]. γ-tocopherol, which is one of the compounds that contain vitamin E, increases cardiovascular functions. γ-tocopherol expands the activity of nitric oxide synthase, which in turn produces nitric oxide, aiding in vessels relaxation and thus improving blood flow [29]. Adequate levels of nutrients and oxygen concentrations in muscles indicate a raised blood flow rate, which may help improve performance [263]. Additionally, vitamin E improves red blood cells’ flow and flexibility [264]. This is important for athletes, as improved blood flow means better performance in delivering nutrients and oxygen to the muscles, allowing them to perform at their best [176].
Free radicals such as superoxide, nitric oxide, and hydrogen peroxide are known to be of significant importance, as there must be a balance between antioxidants and free radicals in order to obtain physiological muscle adaptation in response to exercise [25]. Few studies suggested that antioxidants supplementation may be beneficial under specific circumstances, such as overtraining, high-altitude training, or hypoxic training, and claimed that antioxidant usage such as the intake of vitamin E or vitamin C may have no benefit at all or may even cause harm [6,7,29,176,256]. Misusing or consuming excessive amounts of vitamins can lead to muscle fatigue and impede the recovery process due to the inactivation of the gene expression regulator Nrf2 (Nuclear factor erythroid 2-related factor 2), which plays a role in the response to cellular stress and contributes to enhancing exercise performance [265]. It is worth noting that Vitamin E toxicity may cause increases in mortality risk factors, since there has been a positive relation accompanied by a high-sensitivity C reactive protein indicative of inflammation [266].
7. Vitamin D
Vitamin D plays a cooperative role in the synthesis of various hormones in the body [3]. Dairy products, egg yolk, and fatty fish are the rich dietary intake sources [2]. Moreover, it can be synthesized in vivo and be activated by sunlight within a duration of 15 to 20 min of exposure [44]. It also plays an important role in calcium homeostasis and constant healthy bone [30], functions of improving the immune system, musculoskeletal system, power, and force output [45].
Vitamin D supplementation has been increased among athletes [45]. Unfortunately, the widespread vitamin D insufficiency has been clearly stated in elite male athletes, with evidence of a deficit in women [46]. Percentages of insufficiency in elite athletes were above 50%, and the deficiency in other studies was 70–90%, as reported by Harju et al. [47]. Certain circumstances impact vitamin D status, such as indoor training, pigmented skin, and living in a high-altitude region [48]. Studies have reported that athletes with vitamin D deficiency may experience ergogenic benefits when taking vitamin D supplements [103].
There was a direct relation between the concentrations of vitamin D and athletes’ performance, such as speed, jumps’ height, power muscle tone, and strength of handgrip [50]. Moreover, the addition of calcium to vitamin D supplements exhibited a reduction in the stress fracture rate [106,138].
In a study conducted among 70 athletes randomly assigned for 8 weeks to either vitamin D oral supplements of 50,000 IU/week group or a control group, a significant improvement in the test of the strength leg press in both groups was reported [267]. However, the results emphasized that the enhancement in the supplemented group was obviously more noted than that in the control group (p = 0.034). Moreover, when the sprint test was conducted, within-group enhancement had been noticed in the supplemented group only (p = 0.030). The results showed that regular weekly vitamin D supplementation with a dose of 50,000 IU increased the levels of circulating calcidiol (major circulating form of vitamin D) by approximately 17 ng/mL. This increment was related to a notable enhancement in sprint and power leg examinations in the vitamin D group [138]
Additionally, vitamin D is also thought to improve the body’s utilization of carbohydrates during exercise, providing the body with increased energy, which can help to enhance performance [1]. Twenty-two male adult athletes were allocated into two groups for 8 days: a one-shot dose of 150,000 IU vitamin D group and a placebo group. The vitamin D group showed a significant elevation in muscle power in the period from day 1 up to day 8, suggesting that a single dose of 150 000 IU vitamin D had a beneficial impact on serum 25-hydroxy vitamin D (25(OH)D) levels and the muscle’s role [268].
To maintain sufficient vitamin D levels, the most appropriate way is to spend time in outdoor direct sunlight for several minutes each day, as obviously indicated by many studies’ conclusions (see Table 2). This prescription for sun exposure should also be combined with foods that are rich in vitamin D, such as dairy products, fatty fish, and fortified foods [101]. Additionally, athletes may also benefit from daily multivitamin supplements that contain vitamin D to ensure that their body receives the best possible nutrition. The recommended daily dose of vitamin D, as shown in Table 1, varies depending on age; a daily dose of 600 IU (equivalent to 15 micrograms (mcg)) is considered sufficient for the age of 19 and above in both genders, and for adults over 70 years old, an 800 IU (20 mcg) daily dose would be sufficient [7,25]. Vitamin D-deficient athletes would require 50,000 IU of vitamin D per week for 8 weeks [138,258].
A previous study was conducted for 12 weeks among 53 youth athlete swimmers who suffered from insufficient levels of vitamin D to evaluate the influence of vitamin D oral supplementation on physical performance by taking 2000 IU/day of vitamin D or placebo. No notable difference was observed in performance between the supplemented and placebo groups [269]. The results concluded that there was no remarkable correlation found between Vitamin D levels and the evaluated criteria including strength or swimming performance and even the age-adjusted balance. Although the oral administration of vitamin D had raised the concentration of Vitamin D compared to the placebo group, no significant physical performance enhancement was reported [269].
8. Vitamin K
Vitamin K is essential for blood coagulation [163]. It may also impact bone metabolism in postmenopausal women, according to a few previous studies [3,7,270]. In elite female athletes, the oral intake of vitamin K at a dose of 10 mg/day has been shown to improve bone remodeling [254] by increasing the calcium-binding capacity of osteocalcin, promoting bone formation, and reducing bone resorption [176]. Moreover, the intake of vitamin K improved cardiovascular function [18,231]. Table 1 summarized the recommendation and the role of vitamin K in exercise performance.
9. Water-Soluble Vitamins (WSV)
Vitamin B
B-complex vitamins are essential for athletes to maintain optimal health and performance [19]. B-complex vitamins help athletes manage stress and anxiety, aid in muscle recovery, and reduce fatigue, which may adversely affect performance if left unchecked [2]. B-complex vitamins help in blood pressure regulation [271]. Moreover, B-complex vitamins aid in maintaining a healthy sleep schedule by regulating levels of the sleep-regulating hormone melatonin, helping athletes fall in a deep continuous sleep [5]. This is essential for athletes, as the lack of sleep can affect an athlete’s performance [52]. B-complex vitamins also contribute to maintaining optimal health and performance in athletes, supporting improved brain functioning, concentration, sleep quality, and energy levels [19]. Thus, athletes need to ensure that they are receiving enough vitamin B through their diet or supplements [244].
Thiamine (B1) is a water-soluble vitamin that must be consumed regularly from the diet [6]. Although free thiamine is stable at acidic pH, it is destroyed by ultraviolet (UV) and gamma irradiation and is heat-sensitive [29]. Whole grains, bread, and nuts are the most common thiamine food sources, while milled wheat flour, polished rice, vegetables, and fruits contain less thiamin [272]. The large intestine’s bacteria in the human body are able to produce thiamine and thiamine pyrophosphate (TPP) [52]. Thiamine leaches into the water due to its solubility and is inevitably lost in any discarded soaking or cooking water, as well as destroyed by heating during culinary methods [7].
Thiamine, in its active state (TPP), is a cofactor of numerous important enzymes involved in the metabolism of carbohydrates and branched-chain amino acids [7]. Moreover, it is necessary for several other cellular functions, including the development of nucleic acid precursors, myelin, and neurotransmitters (such as acetylcholine), as well as antioxidant defense [272]. A deficiency of this vitamin leads to a decline in oxidative metabolism [265]. The biochemical outcomes include a failure to create adenosine triphosphate (ATP), lactic acidosis resulting in a greater lactic acid generation, and a reduction in neurotransmitter synthesis (e.g., acetylcholine, glutamate, aspartate, and gamma-aminobutyric acid (GABA)) [6]. The major causes of thiamin deficiency are either the insufficient intake, poor absorption or metabolism, or an increase the body demand [265]. Furthermore, diuretics and diarrhea lead to thiamine deficiency [272]. Regarding thiamin and exercise, research suggests that thiamin availability in the diet appears to influence exercise capacity when athletes consume the recommended amount [254].
Riboflavin (B2) is the second vitamin from the B-complex vitamins [270]. It appears as a yellow-orange chemical molecule that is water-soluble [273]. Riboflavin is relatively heat- and oxygen-stable, especially in an acidic environment [19]. It is very light-sensitive, destroyed by reducing agents, and unstable in alkaline solutions [4]. Riboflavin is essential and must be obtained from food sources [2]. Riboflavin is abundant in almonds, beef liver, sardines, mushrooms, cheddar cheese, and eggs [271]. When athletes consume a typical amount of riboflavin, their exercise capability would be optimum [254].
Niacin (B3) is the third water-soluble member of the B vitamins family [176]. Humans can partially convert the essential amino acid tryptophan to nicotinamide, which is a dietary supply of niacin [270]. However, the conversion cannot meet the demands for niacin, so dietary niacin supplies around 50% of the daily niacin requirement [256]. Meat, whole grains, milk, and dairy products are good sources of niacin [242]. Niacin is abundant in peanuts, seafood, mushrooms, and yeasts [29]. Food items high in tryptophan-containing proteins, such as milk, cheese, and eggs, are good sources of niacin [7]. Its roles include reduction and oxidation (redox) processes, as well as acting as a ligand for a range of purine receptors [243].
It is hypothesized that this vitamin lowers cholesterol, improves thermoregulation, and improves oxidative metabolism [274]. In hypercholesteremic individuals, a niacin intake of 100–500 mg/day may help lower blood lipid levels while increasing homocysteine levels [176]. Nevertheless, consuming 280 mg of niacin during exercise has been demonstrated to reduce exercise capacity by moderating fatty acid mobilization [275].
Pantothenic acid (B5) is a water-soluble vitamin that is widely available in the diet [3]. It is often provided as calcium pantothenate, which is more stable against light, heat, and oxygen, but is unstable in both alkaline and acidic circumstances [273]. Sodium pantothenate is also available, but its use is restricted due to its hygroscopicity [272]. Pantothenic acid functions as a coenzyme for acetyl coenzyme A (acetyl CoA), implying its importance in aerobic or oxygen-based energy systems [2]. Acetyl CoA supplementation has not been shown to increase aerobic performance in studies [25,52,202,276]. Yet, one study found a reduction in the lactic acid buildup, but no benefit in performance was concluded [277].
Pyridoxine (B6) is marketed as a supplement that increases muscular growth, strength, and aerobic capacity in the lactic acid and oxygen systems [141]. It might additionally have a relaxing effect, which has been related to increased mental power [24]. Surprisingly, research showed that pyridoxine did not increase the capacity of aerobic exercise or the accumulation of lactic acid in well-nourished athletes [23]. However, when paired with vitamins B1 and B12, it has been shown to raise serotonin levels and enhance motor abilities, which are required in sports such as pistol shooting and archery [24]. Moreover, vitamin B6, thiamin, and pantothenic acid showed inverse relationships with stress risk and anxiety [25]. Another study revealed that after a month of vitamin B6 intake, young adult athletes reported feeling less anxiety [25]. Table 2 presents studies that investigated the effect of vitamin B6 intake either as part of a multivitamin supplement or as a sole intake on exercise performance. Most of the studies reported a positive impact on exercise performance, particularly in cases of vitamin B6 deficiency.
Cyano-cobalamin (B12) is a coenzyme required for the synthesis of deoxyribonucleic acid (DNA) and serotonin [176]. In theory, it would enhance muscular mass and blood oxygen-carrying capacity and lessen anxiety [273]. However, no ergogenic impact has been documented in well-nourished athletes [242]. Interestingly, it may enhance pistol shooting performance due to the stimulation of serotonin production, which reduces anxiety [2]. A cross-sectional research work studied 100 amnestic mild cognitive impairment (MCI) patients characterized by low-normal and high-normal vitamin B12 levels, who were then enrolled in an Auditory Verbal Learning test to evaluate their memory’s function. The results showed that those with low-normal B12 concentrations had notable defects in learning and recognition abilities and even in memory performance due to the low microstructure integrity of the hippocampus [23]. It is important to acknowledge that vitamin B12 is crucial for proper brain functioning, as it aids in faster information processing and enhances concentration levels [21]. This has been demonstrated in patients with mild cognitive impairment (MCI) who had low-normal levels of vitamin B12 [262]. This is especially important for athletes, as improved brain functioning may help improve performance in many ways, from learning new techniques to continuous focus maintenance during long competitions [24].
Folic acid (folate) is a coenzyme that aids in the synthesis of DNA and red blood cells [278]. An increased red blood cell count enhances oxygen supply to muscles during exercise [21,79]. It is thought to be crucial in preventing birth abnormalities and may lower homocysteine levels, which is a risk factor for heart disease [22]. Folic acid supplements did not increase exercise performance among malnourished athletes with folate deficiency [254].
10. Vitamin C
Vitamin C (also known as ascorbic acid) can be found in many types of food, including oranges, strawberries, broccoli, and sweet potatoes [15]. Athletes require more vitamin C than the average person since their bodies are working harder and being pushed to the limits [15,279]. Therefore, they need to receive enough of this vital nutrient to perform at their best. Researchers have reported that the intake of vitamin C supplements does not boost physical performance in well-nourished athletes [52]. Nevertheless, athletes are recommended to receive an adequate amount of vitamin C from their balanced diet.
The crucial role of vitamin C in neutralizing free radicals has been raised from its antioxidative potency [69], thereby improving the immune system [15] and reducing the risk of illnesses such as colds and other viruses [280]. It plays an important role in immunity by enhancing the differentiation and proliferation of B and T lymphocytes and increasing antibodies levels [25]. Furthermore, vitamin C has been reported to modulate cytokine production and decrease histamine levels [169,281]. Studies have also shown that vitamin C can eliminate fatigue, improve coordination, and increase endurance [15].
Vitamin C has a crucial role to play in wound healing and collagen production [3]. It helps boost energy levels and protects the body from illnesses and injuries [242]. Vitamin C works as a co-factor to produce collagen for the propyl and lysol hydroxyls enzymes, which stabilize the structure of collagen [29]. Furthermore, vitamin C also enhances collagen gene expression in fibroblasts [7], contributing to the strength and integrity of joints and muscles, which is essential for the success of any athlete. This is supported by the fact that vitamin C is crucial in protecting against ROS damage, enhancing keratinocyte differentiation, lipid synthesis, fibroblast proliferation, and migration, which has been seen to shorten the time of wound healing [28,282].
However, high levels of vitamin C can, in turn, act as a pro-oxidant rather than an antioxidant [29]. The overconsumption of vitamin C supplement decreases exercise-induced adaptation, delays post-exercise recovery, increases lipid peroxidation, and diminishes mitochondrial biogenesis [127]. These effects can hinder skeletal muscle adaptation to exercise [3].
11. Minerals
Numerous physiological and metabolic processes in the human body involve minerals [283]. Minerals have physiological effects on the body during exercise, including maintaining a normal heartbeat, oxygen transportation, antioxidation activity, healthy bone, and immune system enhancement [283]. Sufficient levels of minerals are required for optimal performance because many of these processes are enhanced during sports activity [284]. For athletes to perform at their best, maintaining a healthy body necessitates the intake of a variety of nutrients. Some minerals make weightlifting more effective by enhancing athletic performance; these are the minerals that degrade faster when used in sports endeavors [285] and thus need to be replaced routinely among athletes to sustain their performance. Table 1 summarized the recommendation requirements of minerals along with the rich sources and their roles in exercise performance.
12. Iron
Iron (Fe) is a crucial mineral for physical performance, and its importance cannot be overstated [286]. When it comes to peak performance, an adequate intake of iron can make all the difference [149]. It helps the body produce red blood cells, which are necessary for transporting oxygen to the muscles [71]. Without enough iron, athletes and other physically active individuals may suffer from fatigue and lethargy as the body struggles to meet the increased demands [71]. A huge part of the pool of plasma iron (almost 80%) is utilized by the bone marrow; this is equivalent to a 20–30 mg/day dose to ensure the efficient production of erythrocyte [31].
In addition to red blood cells production, iron is also important for energy metabolism [31]. It is necessary for converting food into energy, and it helps to ensure that the body can use energy efficiently for physical activities [280]. Iron also helps the body in regulating its temperature, making it an essential nutrient for athletes competing in warm climates or hot weather [149]. Finally, it is important for other bodily functions, such as the immune system, growth, and hormone production [284]. When considering physical performance, it is important to ensure that iron intake is adequate and balanced. The human physiological mechanism preserves the maximum iron [29]. Based on the total compulsory iron depletion that occurs daily and the average of 10% absorption and bioavailability, the World Health Organization (WHO) and other national institutes have estimated iron-recommended doses depending on several characteristics including gender, age, and race. The recommended dietary intake for females is 18 mg, and for males, it is 8 mg [31]. Poorly planned diets, coupled with inadequate levels of exercise, can lead to anemia and other problems associated with low iron levels [286]. This may cause fatigue, poor performance, and a decreased ability to perform physical activities [285]. Therefore, it is necessary to consume a high-quality variant diet that involves iron-rich sources [284].
It is also important to make sure that athletes have enough time to rest and recover between workouts. Iron helps to replenish energy stores and reduce fatigue, so it is important to give the body time to absorb the nutrient [287]. Additionally, certain supplements may also help in providing additional iron to meet the demands of physical performance [205]. Athletic training can result in alterations including higher vascularization (creation of new blood vessels), elevated hematocrit, and higher erythrocyte awareness in the blood, which may lead to an increase in iron needs [122]. A shortage of iron may result from hemorrhages, gastric blood loss, and/or urinary tract bleeding, especially among high-intensity sports [121]. Professionals are predicted to have 70% higher iron needs than non-professionals [287]. Iron deficiency anemia can impede progress in an athlete’s training by reducing oxygen delivery [283]. Lastly, most research concluded that iron supplements do not enhance aerobic performance, unless there is a specific depletion and/or anemia reported [149].
13. Calcium
Athletes must be in peak physical condition to perform at their best and make sure their diets are balanced, which is an important part of their training regimen [288]. Calcium (Ca) is among the many nutrients that athletes need to remain healthy [289]. It not only helps to keep bones and muscles strong, but it has also been linked to improved performance in athletes [32]. However, insufficient Ca consumption and elevated Ca depletion may expose a person to osteoporosis [194]. Athletes should make sure to consume an adequate amount of Ca each day as part of their balanced diet, which would achieve around 1500 mg/d [32,188]. The optimal Ca requirement is 1200 mg/day for adolescents and youth, 1000 mg/day for females aged 25 to 50 years old, and 1500 mg/day for postmenopausal females who are not treated with estrogen replacement therapy [33]. With the right diet and exercise routine, they may capitalize on the benefits that Ca has to offer and maximize their performance [32,188].
Numerous studies have indicated that the adequate and consistent consumption of Ca can potentially enhance physical performance in athletes [289], as it plays a crucial role in maintaining muscle strength, which is a key element for exercise performance [35,289]. Additionally, it may help reduce injuries and improve recovery time [32]. It is also known that Ca may protect the bones and joints from stress caused by continuous physical activity [32]. Conversely, improving Ca status with 2000 mg of Ca supplementation has been shown to reduce the risk of developing a stress fracture [289]. Calcium also helps to convert carbohydrates and fat into energy, which can contribute to performance improvement [78,288]. It also helps in reducing fatigue and delaying the onset of muscle soreness [290].
Calcium can be found in many common foods including milk, yogurt, cheese, and dark leafy greens. Other sources include tofu, nuts, fish, and fortified cereals [289]. Additionally, athletes may consider Ca supplements if they are unable to receive the recommended daily intake from their diet. It is important to note that the amount of Ca an athlete needs daily may vary depending on their weight and activity level [289]. Skeletal muscles’ ability to contract and relax depends in part on Ca [291]. The importance of it binding to troponin C for the contraction of muscles has the potential to influence performance [33]. While it is true that training leads to higher Ca loss, primarily through perspiration, the foundations of bone mineralization are Ca, vitamin D, and physical activity [292]. However, in rare circumstances, especially if the diet is low in its nutrient density, physical activity might endanger bones [65]. Every athlete should place a high priority on developing and maintaining optimal bone health, since vigorous physical activity increases the stress fractures risk [85,188].
14. Potassium
When it comes to athletes’ health and performance, one mineral that is essential to success is potassium (K) [293]. It is a required nutrient for human health and is necessary for many physiological processes [293]. Adults should not exceed the consumption of 2000 mg sodium/day (Na) or 5 g of salt and have a minimum dose of 3510 mg potassium/day, according to new guidelines established by the WHO [14,281]. It has a crucial role in muscle contractions and helps the body regulate fluid balance, blood pressure, and the heart rate [281]. In addition to its role in muscle contractions, it is also involved in nerve functions and proper electrolyte balance [59], which may be beneficial to athletes who may be sweating during a long practice or game [29]. Furthermore, proper potassium levels can help prevent injuries and help athletes maintain their energy levels [177,230].
Potassium is a great source of energy for athletes [293]. It helps to reduce the amount of lactic acid stored in the muscles [59], which may lead to fatigue as well as maintain a healthy metabolism [35]. It is also involved in the breakdown of carbohydrates, which helps keep energy levels high during intense physical activity [177]. It is unknown if potassium supplementation reduces the occurrence of muscular cramping in athletes. It should be acknowledged that there have been no reports of ergogenic effects [58].
15. Magnesium
Magnesium (Mg) is an essential mineral that is recognized for its critical role in athletic performance and overall health [294]. Magnesium helps to improve energy levels, reduce fatigue, and even increase muscle performance, making it a vital nutrient for athletes [36]. With its numerous benefits, magnesium is being increasingly taken by athletes to help them reach peak performance and maintain their physical health [294]. Magnesium helps to improve energy levels by raising the ATP availability, which is best defined as the gold energy stores of cells [283]. Deficiency may cause ATP levels to be depleted, resulting in fatigue and overall reduced performance [34]. The regular consumption of Mg can improve ATP production, providing athletes with increased energy and improved endurance [295]. The mineral is also important for maintaining muscle performance and reducing fatigue [296]. It is known to support muscle contraction and relaxation, allowing for better muscle control and improved performance [142]. It also works to reduce lactic acid buildup in muscles, which may help reduce pain during exercise and improve recovery time [297].
Moreover, Mg has numerous other benefits that support physical wellbeing. It helps to improve sleep quality, regulate blood sugar, reduce stress, and even support the cardiovascular system [294,297]. By regularly taking Mg, athletes may benefit from improved energy production, reduced fatigue, and improved physical health, allowing them to reach their maximum performance potential [36]. The Recommended Dietary Allowance (RDA) is 400 to 420 and 310 to 320 mg/day for 14 to over 70 years of age among males and females, respectively [294].
Magnesium is a versatile mineral that is important for recovery and is found in over 300 enzymes that are involved in energy metabolism [297]. It is linked to strength training and cardiorespiratory processes, showing a reciprocal relationship between exercise and Mg in the human body [142]. Exercise controls Mg distribution and usage [296]. Training triggers Mg to be transferred to areas where energy is produced [37]. For instance, during prolonged activity, serum Mg may be transferred from serum blood to red blood cells (RBCs) or muscle to support exercise. On the other hand, short-term exercise may result in a reduction in the plasma/serum volume and a rise in serum Mg levels [297].
Magnesium contributes to the metabolism of energy and supports typical muscular contraction and relaxation [283]. In male athletes, serum Mg levels are favorably correlated with muscular performance [36]. Additionally, research suggests a possible connection between Mg deficit and muscle cramps by demonstrating how it might alter neuromuscular function [297]. Physically active people might need more magnesium to sustain their peak exercise performance than inactive people do [283]. Low Mg levels may cause ineffective energy metabolism and decreased endurance in individuals who are engaged in a weight training program [294]. Higher Mg consumption is linked to enhanced cardiorespiratory function and lower oxygen demand during aerobic exercise [34]. Most studies reported little impact of 500 mg/day Mg on exercise performance in athletes, unless there is a deficit [37,142,296]. A study of 16 physically active men who were assigned to 300 mg/day for 14 days of Mg supplementation or a control group concluded no direct impact on exercise performance while using Mg supplementation [296].
16. Zinc
Athletes of all ages and skill levels rely on zinc (Zn) to keep their bodies performing at their peak [298]. It is an essential mineral that our bodies need for metabolic functions such as cell repair, immune system functioning, hormone production, and healthy skin [38]. Unfortunately, not all athletes receive enough zinc from food intake, which may leave athletes at a disadvantage [38]. According to the UK National Diet and Nutrition Survey (NDNS), current daily intakes are 9.5 mg and 7.6 mg for men and women, respectively [299]. The survey has also shown that 6% of men and 7% of women do not receive enough zinc in their diet, putting them at a greater risk of deficiency [300]. Fortunately, there are many benefits athletes may reap from adding zinc supplements to their routines [300].
One notable benefit of zinc supplementation is the improvement in athletic performance [281], as it reduces blood viscosity and enhances oxygen delivery, thereby boosting aerobic endurance [38,281]. Zinc helps to increase strength and endurance, so athletes may push their bodies to the limit while still receiving the nutrients they need [298]. A double-blinded cross-over study featuring 16 female athletes was conducted to estimate muscle strength and endurance [38]. The participants consumed 135 mg/day of Zn for 14 days and showed remarkably higher dynamic isokinetic strength and angular speed [29]. Additionally, Zn may help to reduce inflammation and soreness, which can accelerate recovery time and reduce the risk of injury [59]. Zinc may also help in improving attention and focus [60]. This can assist athletes in staying focused on their tasks and performing at their best. Zinc is a vital mineral for athletes of all ages and abilities [273]. Including it as a supplement to their regimen may help enhance strength, endurance, and focus while also reducing inflammation and supporting the immune system [92].
In addition, Zn may help to boost the immune system, making it easier for athletes to fight off colds and other illnesses that can stall their progress by increasing neutrophils’ ability to produce ROS after exercise [7]. It has been indicated that Zn oral consumption of 25 mg/day while exercising can reduce exercise-induced changes in immune function to the minimum [298]. Moreover, Zn may help support healthy vision and keep skin healthy, both of which are important for optimal performance [258]. Zn impacts the formation and efficient functioning of the skin and mucous membranes [298]. It helps maintain skin cell membranes, and it plays a part in cell mitosis and differentiation; moreover, it has an essential role in the survival of keratinocytes [215] and even in protecting skin against induced UV radiation damage [301]. Lastly, taking Zn supplements may help athletes meet their nutritional goals without having to increase their caloric intake, making it an ideal supplement for those who are trying to stay lean [6]. Despite Zn supplements being popular among athletes, there is limited proof regarding athletic performance improvement in a period of 1–6 weeks, as shown in the study of Polat, 2011 [302].
To detect the actual impact of zinc oral consumption on the hematological parameters, a study included 24 male kickboxing athletes, who were separated to form the three following groups: the EZ group, meaning they were exercising and consuming 2.5 mg/kg Zn supplement daily; the SZ group, who were supplemented without exercising; and the E group, who were exercising without being supplemented. After the period of 8 weeks, the results showed a significant increase in the erythrocyte count of the EZ group compared to the two other groups (p < 0.001). The hemoglobin and hematocrit levels increased in the EZ group (p < 0.05). These results revealed that the combination of exercise and Zn supplementation has a beneficial impact on the hematological parameters of athletes, which may result in enhanced performance and increased stamina [303]. Low levels of Zn in the muscles may diminish exercise endurance because it is necessary for the activity of energy metabolism enzymes [304]. Due to the influence of this enzyme on skeletal muscular exercise, lactic dehydrogenase (a Zn-containing enzyme) may facilitate the conversion of lactic acid to pyruvate [38]. This finding contradicts the commonly misinterpreted results of previous studies [59,303], which demonstrated that lactate accumulation does not directly cause fatigue [305].
17. Selenium
One possible approach for athletes to achieve their goals is by including selenium (Se) in their diet, as this mineral can be found in a variety of foods [40]. Selenium, when consumed in proper amounts, will help to boost an athlete’s performance, improve mental focus, and reduce inflammation, thereby contributing to overall health and fitness [40,41,257].
Selenium can be found in certain plant and animal products, and it may also be artificially added to processed foods [42]. It is advantageous for athletes due to its powerful antioxidant characteristics that boost the body’s defenses against cell damage, hence increasing endurance, strength, and overall performance [40]. Additionally, Se may increase mental focus, which improves an athlete’s ability to concentrate on tasks and stay motivated, even if the competition gets tougher [41]. It can also help to reduce levels of inflammation and support anti-inflammatory mechanisms, which may boost recovery times and minimize the risk of injuries [95]. Low levels of serum Se are associated with high serum levels of C-reactive protein (CRP), the inflammation biomarker [306] It is well addressed that Se increases glutathione peroxidase production, which prevents the effect of oxidative stress in response to exercise [303]. On the other hand, Se deficiency reduces glutathione peroxidase activity indirectly through controlling the Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) [257,307]. In the CHIANTI cohort study that assessed coordination performances among 1012 candidates aged 65 years or older, the authors found a reduction in neurological performance that was significantly associated with the low levels of Se [308].
Incorporating Se into an athlete’s diet may be as simple as consuming more foods that are naturally rich in Se or taking it in supplemental form. When consuming the recommended amounts, it may increase the overall health and performance of athletes [306,307,309]. Induced excessive mitochondrial oxidative stress could be caused by Se supplements overdose and may lead to serious health problems [273] marked by organelle damage and dysfunction [310]. Hence, it is important to integrate it into a balanced diet in appropriate doses rather than consuming mega doses [311]. A systematic review of oral Se supplementation of 180 µg/day or 240 µg/day (selenium methionine) and 200 µg/day (Sodium Selenite) reported a significant drop in lipid hydroperoxide levels and an increase in glutathione peroxidase (GPx) in plasma, erythrocyte, and muscle [306]. The authors concluded that the consumption of Se supplements has no impact on aerobic or anaerobic performance [306]. In addition, the study revealed that Se supplementation may inhibit Se deficiencies induced by high-volume and -intensity exercise, but it has no impact on anaerobic and aerobic athletic performance as well as creatine kinase activity, exercise training-induced adaptations, and testosterone hormone levels [303,306].
18. Manganese
As athletes struggle to achieve their best performance, they often look to improve their health and performance. One mineral that has been gaining recognition for its potential benefits is manganese (Mn) [312]. Mn is essential for several bodily functions, including energy production, bone formation, and enzyme activity [264]. Early studies have shown that it may help improve various aspects of athlete health and performance, but little is known about the exact benefits of Mn for athletes [205,303].
Manganese plays an important role in energy production, as it is involved in the breaking-down of carbohydrates, proteins, and fats needed for energy production [205]. It also helps the body to utilize energy more efficiently, which may result in improved endurance during long-term workouts and competitions [304]. Additionally, it aids in the production of important neurotransmitters, which may improve mental focus and coordination during physical activities [304]. Due to its crucial role in bone formation, several studies reported the relationship between Mn and bone health [43] It helps in the development of strong and healthy bones [43], which is crucial for athletes to prevent injury and speed up recovery time. Low serum Mn levels have been reported among osteoporotic women compared to healthy subjects [312]. Studies have also suggested that Mn helps to protect cells from damage caused by ROS, which is important during periods of strenuous exercise [95,304]. It is crucial for scavenging ROS in mitochondrial oxidative stress, as it involves the Mn superoxide dismutase (MnSOD) component [95].
In a clinical trial, it was found that athletes had significantly higher concentrations of basal Mn levels vs. sedentary individuals, as observed through blood and urine measurements [7]. Conversely, sedentary participants exhibited higher urine levels of Mn, which could be attributed to the possibility of iron deficiency in athletes, leading to increased Mn absorption [313]. There is limited evidence regarding Mn and athletic performance; however, athletes should be evaluated periodically for micronutrients deficiencies. Although more research needs to be conducted, the current evidence suggested that Mn may be beneficial for athletes who are looking to optimize their performance and health [205,304]. Adding Mn to an athlete’s diet could be useful for maximizing their performance [95].
19. Micronutrients Deficiency and Energy Deficiency’s Impact on an Athlete’s Performance
Pathways for utilizing energy are significantly influenced by vitamins and minerals [93]. A variety of physiological systems depend on micronutrients, which also have an impact on general health and athletic performance [23]. According to the widespread opinion on dietary guidelines for sports, a healthy athlete does not need to exceed RDA values if they consume an adequate number of nutrient-dense foods [267]. Unfortunately, many athletes do not meet the RDA requirements for most micronutrients [6,267]. Micronutrients would logically be impacted by poor macronutrient consumption [25]. It is common among many athletes who are not aware about their exercise energy demands and, on the contrary, suffer from being on a negative energy balance [2]. Negative energy balance due to increased or decreased calorie intake or a combination of both is a powerful disruptor of the endocrine milieu [123]. Additionally, it was associated with increased fatigue and mental disorders [14], reduced fertility, poor bone quality, a higher likelihood of sports injuries, endothelial dysfunction, a poor lipid profile, gastrointestinal disturbances, inflammatory processes, psychiatric conditions (such as emotional state changes/irritability), and poor athletic performance [93,108,154,314].
One common energy deficiency condition among athletes is the female athlete triad, characterized by disordered eating, negative energy balance, and irregular or absent menstrual cycles [315]. This condition predisposes women to menstrual dysfunction (amenorrhea) [316], diminished bone mineral density, and premature osteoporosis [45]. Each defect of the triad represents a significant medical concern, and if occurring together, the possibility of health concerns becomes even more serious and can often cause potential threats to life [317]. Medical adverse consequences associated with disordered eating involve decreased levels of glycogen in its stores, reduced lean body mass, long-term fatigue, dehydration, micronutrient deficiencies, electrolyte and acid-base imbalances, anemia, gastrointestinal diseases, parotid gland enlargement, reduced bone density, and tooth enamel erosion [300]. Osteoporosis can make adolescent female athletes prone to early bone loss and the improper formation of bone, resulting in low bone mineral density [318] and an elevated risk of stress fractures [319]. Bone mineral density lost because of amenorrhea may be totally or, at least partially, irreversible, even with the resumption of menses, calcium supplementation, and estrogen replacement therapy [256,320]. The dispensable role of supplementary vitamins and minerals is a concern of the Dietitians of Canada and the American College of Sports Medicine (ACSM) for ensuring adequate energy requirements are met from a varied and balanced diet with supplementations enrichment. Equally importantly, sports medicine experts may recommend the use of vitamin and mineral supplements in specific conditions such as energy intake restriction, the adoption of a plant-based diet, the presence of illness, or recovery from injuries [242,263,301,321,322]. It is worth noting that individual needs vary, and a personalized approach is crucial when making supplement recommendations.
20. Conclusions
Vitamins and minerals are crucial for an athlete’s health and performance, none more so than others. Micronutrients are essential to achieving optimal health and performance. They participate in many metabolic processes in the body, including energy production, muscle growth, and recovery. Athletes need to ensure they consume sufficient quantities of micronutrients to improve their physical activity and performance. A balanced diet that includes a variety of fruits, vegetables, whole grains, and lean proteins may help them meet their micronutrient needs. Additionally, they may benefit from taking a multivitamin supplement if they are not meeting their micronutrient requirements or suffer from malabsorption or specific deficiencies in certain vitamins. However, athletes must avoid taking micronutrient supplements without first ensuring there is a deficiency. It is important to consult with a physician or a dietitian to determine if supplementation is necessary and to obtain a proper prescription.
Abbreviations
| 25(OH)D | 25-hydroxy vitamin d |
| acetyl CoA | acetyl coenzyme a |
| ACSM | american college of sports medicine |
| ATP | adenosine triphosphate |
| B1 | thiamine |
| B12 | cyano-cobalamin |
| B2 | riboflavin |
| B3 | niacin |
| B5 | pantothenic acid |
| B6 | pyridoxine |
| BCKDH | branched-chain-keto acid dehydrogenase complex |
| BMD | bone mineral density |
| Ca | calcium |
| COX | cyclooxygenases |
| COX2 | cyclooxygenase-2 |
| CrI | credible interval |
| CRP | c-reactive protein |
| DNA | deoxyribonucleic acid |
| DPPH | 1,1-diphenyl-2-picryl-hydrazyl |
| DXA | dual-energy x-ray absorptiometry |
| EA | energy availability |
| EAE | exhaustive aerobic exercise |
| EIMD | exercise-induced muscle damage |
| F | female |
| Fe | iron |
| FSV | fat soluble vitamins |
| GABA | gamma-aminobutyric acid |
| GPx | plasma glutathione peroxidase |
| HLA-DR | human leukocyte antigen—dr |
| IU | international unit |
| K | potassium |
| LEA | low energy availability |
| LOX | lipoxygenase |
| M | male |
| MAPK | mitogen-activated protein kinase |
| mcg | micrograms |
| Mcg RAE | micrograms of retinol activity equivalents |
| Mg | magnesium |
| Mn | manganese |
| Mn SOD | mn superoxide dismutase |
| MV | maximum velocity |
| N/A | not available |
| Na | sodium |
| NDNS | the uk national diet and nutrition survey |
| NF-κB | the nuclear factor kappa-light-chain-enhancer of activated b cells |
| NR | not reported |
| PGE2 | prostaglandin e2 |
| PMS | premenstrual syndrome |
| RAE | retinol activity equivalent |
| RBCs | red blood cells |
| RDA | the recommended daily allowance |
| RED-S | relative energy deficit in sport |
| ROSs | reactive oxygen species |
| RR | risk ratio |
| Th1/Th2 | t helper type 1/t helper type 2 |
| TMIN | trace minerals, contained in a single tablet taken once/day |
| TNF-alpha | tumor necrosis factor-alpha |
| TPK1 | thiamine pyrophosphokinase |
| TPP | thiamine pyrophosphate |
| UK | United Kingdom |
| UL | tolerable upper intake level |
| UV | ultraviolet |
| WHO | world health organization |
| WSV | water-soluble vitamins |
| Zn | zinc |
| β-carotene | β-carotene |
Author Contributions
Conceptualization, H.J. and H.A.G.; Methodology, H.J.; Software, H.J.; Formal Analysis, H.J.; Writing—Original Draft Preparation, M.A.H., K.M.R., K.K.A., R.O.A., M.J., S.A. and R.A.A.; Writing—Review & Editing, H.A.G., K.T. and H.J.; Funding Acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
All data are available in the manuscript.
Conflicts of Interest
No conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yerzhanova Y.Y., Sabyrbek Z.B., Milašius K. Comparative evaluation of actual nutrition practices and macro- and micronutrients consumption of athletes in a range of sport types. Novosib. State Pedagog. Univ. Bull. 2018;8:205–222. doi: 10.15293/2226-3365.1801.13. [DOI] [Google Scholar]
- 2.Allendorf M. The Essential and the Nonessential. Electrochem. Soc. Interface. 2006;15:7. doi: 10.1149/2.002062if. [DOI] [Google Scholar]
- 3.Brancaccio M., Mennitti C., Cesaro A., Fimiani F., Vano M., Gargiulo B., Caiazza M., Amodio F., Coto I., D’alicandro G., et al. The Biological Role of Vitamins in Athletes’ Muscle, Heart and Microbiota. Int. J. Environ. Res. Public Health. 2022;19:1249. doi: 10.3390/ijerph19031249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong P., Harris L.M. The sporting body: Body image and eating disorder symptomatology among female athletes from leanness focused and nonleanness focused sports. J. Psychol. Interdiscip. Appl. 2015;149:141–160. doi: 10.1080/00223980.2013.846291. [DOI] [PubMed] [Google Scholar]
- 5.Potgieter S. Sport nutrition: A review of the latest guidelines for exercise and sport nutrition from the American College of Sport Nutrition, the International Olympic Committee and the International Society for Sports Nutrition. S. Afr. J. Clin. Nutr. 2013;26:6–16. doi: 10.1080/16070658.2013.11734434. [DOI] [Google Scholar]
- 6.Beavers K.M., Serra M.C., Greenwood M., Kalman D.S., Antonio J. Nutritional Supplements in Sports and Exercise. Springer; Berlin/Heidelberg, Germany: 2008. Essential and Nonessential Micronutrients and Sport; pp. 121–165. [DOI] [Google Scholar]
- 7.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;89:105906. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grout A., McClave S.A., Jampolis M.B., Krueger K., Hurt R.T., Landes S., Kiraly L. Basic Principles of Sports Nutrition. Curr. Nutr. Rep. 2016;5:213–222. doi: 10.1007/s13668-016-0177-3. [DOI] [Google Scholar]
- 9.Al-Qurashi T.M., Aljaloud K.S., Aldayel A., Alsharif Y.R., Alaqil A.I., Alshuwaier G.O. Effect of Rehydration with Mineral Water on Cardiorespiratory Fitness Following Exercise-Induced Dehydration in Athletes. J. Men’s Health. 2022;18:206. doi: 10.31083/j.jomh1810206. [DOI] [Google Scholar]
- 10.Mesquita E.D.D.L., Exupério I.N., Agostinete R.R., Luiz-De-Marco R., da Silva J.C.M., Maillane-Vanegas S., Kemper H.C.G., Fernandes R.A. The Combined Relationship of Vitamin D and Weight-Bearing Sports Participation on Areal Bone Density and Geometry Among Adolescents: ABCD—Growth Study. J. Clin. Densitom. 2022;25:674–681. doi: 10.1016/j.jocd.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Chen L.-Y., Wang C.-W., Chen L.-A., Fang S.-H., Wang S.-C., He C.-S. Low Vitamin D Status Relates to the Poor Response of Peripheral Pulse Wave Velocity Following Acute Maximal Exercise in Healthy Young Men. Nutrients. 2022;14:3074. doi: 10.3390/nu14153074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandjean A.C. Vitamins, diet, and the athlete. Clin. Sport. Med. 1983;2:105–114. doi: 10.1016/S0278-5919(20)31440-X. [DOI] [PubMed] [Google Scholar]
- 13.Książek A., Zagrodna A., Słowińska-Lisowska M., Lombardi G. Relationship Between Metabolites of Vitamin D, Free 25-(OH)D, and Physical Performance in Indoor and Outdoor Athletes. Front. Physiol. 2022;13:1211. doi: 10.3389/fphys.2022.909086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sone R., Nakazawa S., Ohishi K. Efficacy of mineral-rich antioxidant supplements on oxidative stress markers and exercise performance. Gazz. Med. Ital. -Arch. Sci. Med. 2022;181:295–302. doi: 10.23736/S0393-3660.20.04499-X. [DOI] [Google Scholar]
- 15.Rowe S., Carr A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients. 2020;12:2008. doi: 10.3390/nu12072008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marley A., Grant M.C., Babraj J. Weekly Vitamin D3 supplementation improves aerobic performance in combat sport athletes. Eur. J. Sport Sci. 2020;21:379–387. doi: 10.1080/17461391.2020.1744736. [DOI] [PubMed] [Google Scholar]
- 17.Rockwell M.S., Kostelnik S.B., Mcmillan R.P., Lancaster M., Larson-Meyer D.E., Hulver M.W. An Association between Bioavailable 25-Hydroxyvitamin D and Bone Mineral Density in a Diverse Cohort of Collegiate Athletes. Med. Sci. Sport. Exerc. 2021;54:371–376. doi: 10.1249/MSS.0000000000002807. [DOI] [PubMed] [Google Scholar]
- 18.Kurnatowska I., Grzelak P., Masajtis-Zagajewska A., Kaczmarska M., Stefańczyk L., Vermeer C., Maresz K., Nowicki M. Effect of vitamin K. Pol. Arch. Med. Wewn. 2015;125:631–640. doi: 10.20452/pamw. [DOI] [PubMed] [Google Scholar]
- 19.Hrubša M., Siatka T., Nejmanová I., Vopršalová M., Krčmová L.K., Matoušová K., Javorská L., Macáková K., Mercolini L., Remião F., et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients. 2022;14:484. doi: 10.3390/nu14030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brzezianski M., Pastuszak-Lewandoska D., Migdalska-Sek M., Jastrzebski Z., Radziminski L., Jastrzebska J., Brzezianska-Lasota E., Kiszalkiewicz J.M., Sewerynek E. Effect of Vitamin D3 Supplementation on Interleukin 6 and C-Reactive Protein Profile in Athletes. J. Nutr. Sci. Vitaminol. 2022;68:359–367. doi: 10.3177/jnsv.68.359. [DOI] [PubMed] [Google Scholar]
- 21.Higgins M.R., Izadi A., Kaviani M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public Health. 2020;17:8452. doi: 10.3390/ijerph17228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Sousa M.V., Lundsgaard A.M., Christensen P.M., Christensen L., Randers M.B., Mohr M., Nybo L., Kiens B., Fritzen A.M. Nutritional optimization for female elite football players—Topical review. Scand. J. Med. Sci. Sport. 2022;32:81–104. doi: 10.1111/sms.14102. [DOI] [PubMed] [Google Scholar]
- 23.Jordan S.L., Albracht-Schulte K., Robert-McComb J.J. Micronutrient deficiency in athletes and inefficiency of supplementation: Is low energy availability a culprit? PharmaNutrition. 2020;14:100229. doi: 10.1016/j.phanu.2020.100229. [DOI] [Google Scholar]
- 24.Mahdavifar B., Hosseinzadeh M., Salehi-Abargouei A., Mirzaei M., Vafa M. Dietary intake of B vitamins and their association with depression, anxiety, and stress symptoms: A cross-sectional, population-based survey. J. Affect. Disord. 2021;288:92–98. doi: 10.1016/j.jad.2021.03.055. [DOI] [PubMed] [Google Scholar]
- 25.Li S., Fasipe B., Laher I. Potential harms of supplementation with high doses of antioxidants in athletes. J. Exerc. Sci. Fit. 2022;20:269–275. doi: 10.1016/j.jesf.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Şenışık S., Köyağasıoğlu O., Denerel N. Vitamin D levels on sports injuries in outdoor and indoor athletes: A cross-sectional study. Physician Sportsmed. 2022;50:164–170. doi: 10.1080/00913847.2021.1969217. [DOI] [PubMed] [Google Scholar]
- 27.Neyestani T.R., Alimoradi K., Nikooyeh B., Ravasi A.A., Zahedirad M., Shariatzadeh N., Kalayi A. Efficacy of Vitamin D supplementation in physical performance of Iranian elite athletes. Int. J. Prev. Med. 2019;10:100. doi: 10.4103/ijpvm.IJPVM_227_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Oliveira D.C.X., Rosa F.T., Simões-Ambrósio L., Jordao A.A., Deminice R. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrition. 2019;63–64:29–35. doi: 10.1016/j.nut.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Carlsohn A., Braun H., Großhauser M., König D., Lampen A., Mosler S., Nieß A., Oberritter H., Schäbethal K., Schek A., et al. Position of the working group sports nutrition of the german nutrition society (Dge): Minerals and vitamins in sports nutrition. Dtsch. Z. Sportmed. 2020;71:208–215. doi: 10.5960/dzsm.2020.454. [DOI] [Google Scholar]
- 30.Most A., Dörr O., Nef H., Hamm C., Bauer T., Bauer P. Influence of 25-Hydroxy-Vitamin D Insufficiency on Maximal Aerobic Power in Elite Indoor Athletes: A Cross-Sectional Study. Sport. Med.—Open. 2021;7:74. doi: 10.1186/s40798-021-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed J.L. Ph.D. Thesis. The Pennsylvania State University; State College, PA, USA: 2012. The Role of Low Energy Availability in Predicting an Energy Deficiency and Menstrual Disturbances in Recreational and Competitive Female Athletes. [Google Scholar]
- 32.Chauhan R.C. Calcium as a boon or bane for athlete: A review. Asian J. Res. Mark. 2022;11:1–8. doi: 10.5958/2277-6621.2022.00003.2. [DOI] [Google Scholar]
- 33.Kunstel K. Calcium Requirements for the athletes. Curr. Sports Med. Rep. 2005;4:203–206. doi: 10.1097/01.CSMR.0000306208.56939.01. [DOI] [PubMed] [Google Scholar]
- 34.Hunt G., Sukumar D., Volpe S.L. Magnesium and Vitamin D Supplementation on Exercise Performance. Transl. J. Am. Coll. Sport. Med. 2021;6:e000179. doi: 10.1249/TJX.0000000000000179. [DOI] [Google Scholar]
- 35.Toamah W.O., Fadhil A.K., Alzamily I.A. Study the Effect of Testosterone Activated Hormone on the level of Concentration of Chlorine, Potassium, Calcium, and Sodium in the Blood of Bodybuilder Athletes. J. Drug Deliv. Technol. 2021;11:93–97. doi: 10.25258/ijddt.11.1.16. [DOI] [Google Scholar]
- 36.Song J., She J., Chen D., Pan F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020;8:1–41. doi: 10.1016/j.jma.2020.02.003. [DOI] [Google Scholar]
- 37.Pollock N., Chakraverty R., Taylor I., Killer S.C. An 8-year Analysis of Magnesium Status in Elite International Track & Field Athletes. J. Am. Coll. Nutr. 2019;39:443–449. doi: 10.1080/07315724.2019.1691953. [DOI] [PubMed] [Google Scholar]
- 38.Maret W., Sandstead H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Michalak M., Pierzak M., Kręcisz B., Suliga E. Bioactive Compounds for Skin Health: A Review. Nutrients. 2021;13:203. doi: 10.3390/nu13010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rayman M.P. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 41.Barchielli G., Capperucci A., Tanini D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants. 2022;11:251. doi: 10.3390/antiox11020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiSilvestro R.A., Crawford B., Zhang W., Shastri S. Effects of micronutrient supplementation plus resistance exercise training on bone metabolism markers in young adult woman. J. Nutr. Environ. Med. 2007;16:26–32. doi: 10.1080/13590840701343673. [DOI] [Google Scholar]
- 43.Rondanelli M., Faliva M.A., Peroni G., Infantino V., Gasparri C., Iannello G., Perna S., Riva A., Petrangolini G., Tartara A. Essentiality of Manganese for Bone Health: An Overview and Update. Nat. Prod. Commun. 2021;16:1934578X211016649. doi: 10.1177/1934578X211016649. [DOI] [Google Scholar]
- 44.Gaeini A.A., Rahnama N., Hamedinia M.R. Effects of vitamin E supplementation on oxidative stress at rest and after exercise to exhaustion in athletic students. J. Sport. Med. Phys. Fit. 2006;46:458. [PubMed] [Google Scholar]
- 45.Ghazzawi H.A., Amawi A.T., Alduraidi H., Juweid M., Alhawari H.H., Al-Abbadi M.A., Alabbadi A.M., AlNemer L.S.S. The Preventable Effect of Taekwondo Sport among Cadets and Junior’ Bone Mineral Density: DEXA Assessment. [(accessed on 1 March 2023)];Children. 2023 10:170. doi: 10.3390/children10010170. Available online: https://www.mdpi.com/2227-9067/10/1/170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cobb K.L., Bachrach L.K., Greendale G., Marcus R., Neer R.M., Nieves J., Sowers M.F., Brown B.W., Jr., Gopalakrishnan G., Luetters C., et al. Disordered Eating, Menstrual Irregularity, and Bone Mineral Density in Female Runners. Med. Sci. Sport. Exerc. 2003;35:711–719. doi: 10.1249/01.MSS.0000064935.68277.E7. [DOI] [PubMed] [Google Scholar]
- 47.Nikniaz L., Ghojazadeh M., Nateghian H., Nikniaz Z., Farhangi M.A., Pourmanaf H. The interaction effect of aerobic exercise and vitamin D supplementation on inflammatory factors, anti-inflammatory proteins, and lung function in male smokers: A randomized controlled trial. BMC Sport. Sci. Med. Rehabil. 2021;13:102. doi: 10.1186/s13102-021-00333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bezuglov E., Shoshorina M., Lazarev A., Emanov A., Koroleva E., Anishchenko I., Waśkiewicz Z., Butovskiy M., Morgans R. Does vitamin D affect strength and speed characteristics and testosterone concentration in elite young track and field athletes in the North European summer? Nutr. J. 2023;22:16. doi: 10.1186/s12937-023-00848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J., Chen J., Sui X., Drenowatz C., Wang Q. Association between Different Types of Exercise and Intake of Nutrients including Carbohydrate, Fat, Protein, and B Vitamins in Young Adults. Nutrients. 2023;15:806. doi: 10.3390/nu15040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mieszkowski J., Kochanowicz A., Piskorska E., Niespodziński B., Siódmiak J., Buśko K., Stankiewicz B., Olszewska-Słonina D., Antosiewicz J. Serum levels of bone formation and resorption markers in relation to vitamin D status in professional gymnastics and physically active men during upper and lower body high-intensity exercise. J. Int. Soc. Sport. Nutr. 2021;18:29. doi: 10.1186/s12970-021-00430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martínez-Ferrán M., Cuadrado-Peñafiel V., Sánchez-Andreo J.M., Villar-Lucas M., Castellanos-Montealegre M., Rubio-Martín A., Romero-Morales C., Casla-Barrio S., Pareja-Galeano H. Effects of Acute Vitamin C plus Vitamin E Supplementation on Exercise-Induced Muscle Damage in Runners: A Double-Blind Randomized Controlled Trial. Nutrients. 2022;14:4635. doi: 10.3390/nu14214635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Brito E., Teixeira A.D.O., Righi N.C., Paulitcth F.D.S., da Silva A.M.V., Signori L.U. Vitamins C and E Associated With Cryotherapy in the Recovery of the Inflammatory Response After Resistance Exercise: A Randomized Clinical Trial. J. Strength Cond. Res. 2022;36:135–141. doi: 10.1519/JSC.0000000000003342. [DOI] [PubMed] [Google Scholar]
- 53.Kawashima I., Hiraiwa H., Ishizuka S., Kawai R., Hoshino Y., Kusaka Y., Tsukahara T. Comparison of vitamin D sufficiency between indoor and outdoor elite male collegiate athletes. Nagoya J. Med Sci. 2021;83:219–226. doi: 10.18999/nagjms.83.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janssen J.J.E., Lagerwaard B., Nieuwenhuizen A.G., Timmers S., de Boer V.C.J., Keijer J. The effect of a single bout of exercise on vitamin B2 status is not different between high-and low-fit females. Nutrients. 2021;13:4097. doi: 10.3390/nu13114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ali A., Mehta S., Starck C., Wong M., O’Brien W.J., Haswell C., McNabb W., Rutherfurd-Markwick K., Nasef N.A. Effect of SunGold Kiwifruit and Vitamin C Consumption on Ameliorating Exercise-Induced Stress Response in Women. Mol. Nutr. Food Res. 2021;65:2001219. doi: 10.1002/mnfr.202001219. [DOI] [PubMed] [Google Scholar]
- 56.Valtueña J., Aparicio-Ugarriza R., Medina D., Lizarraga A., Rodas G., González-Gross M., Drobnic F. Vitamin D Status in Spanish Elite Team Sport Players. Nutrients. 2021;13:1311. doi: 10.3390/nu13041311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson-Barnes S.L., Hunt J.E.A., Mendis J., Williams E.L., King D., Roberts H., Lanham-New S.A., Manders R.J.F. The relationship between vitamin D status, intake and exercise performance in UK University-level athletes and healthy inactive controls. PLoS ONE. 2021;16:e0249671. doi: 10.1371/journal.pone.0249671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamińska J., Podgórski T., Rachwalski K., Pawlak M. Does the Minerals Content and Osmolarity of the Fluids Taken during Exercise by Female Field Hockey Players Influence on the Indicators of Water-Electrolyte and Acid-Basic Balance? Nutrients. 2021;13:505. doi: 10.3390/nu13020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrientos G., Alves J., Pradas F., Robles M.C., Muñoz D., Maynar M. Association between Parameters Related to Oxidative Stress and Trace Minerals in Athletes. Sustainability. 2020;12:4966. doi: 10.3390/su12124966. [DOI] [Google Scholar]
- 60.Martusevich A.K., Karuzin K.A. Metabolic estimation of efficiency of vitamin and mineral complexes in qualified athletes. Vopr. Pitan. 2021;90:94–101. doi: 10.33029/0042-8833-2021-90-1-94-101. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki C.A., da Costa T.H. Micronutrient deficiency in the diets of para-athletes participating in a sports scholarship program. Nutrition. 2020;81:110992. doi: 10.1016/j.nut.2020.110992. [DOI] [PubMed] [Google Scholar]
- 62.Pilch W., Kita B., Piotrowska A., Tota Ł., Maciejczyk M., Czerwińska-Ledwig O., Krepa E.S., Kita S., Pałka T. The effect of vitamin D supplementation on the muscle damage after eccentric exercise in young men: A randomized, control trial. J. Int. Soc. Sport. Nutr. 2020;17:53. doi: 10.1186/s12970-020-00386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crewther B., Cook C., Fitzgerald J., Starczewski M., Gorski M., Orysiak J. Vitamin D and Cortisol as Moderators of the Relationship Between Testosterone and Exercise Performance in Adolescent Male Athletes. Pediatr. Exerc. Sci. 2020;32:204–209. doi: 10.1123/pes.2019-0229. [DOI] [PubMed] [Google Scholar]
- 64.Shalaby M., Sakoury M.M.A., Harthi S.M., Alshalawi F.M., Alhajji M.M., Alshaikh Z.H., Aljaber A.H. Vitamin D3 for health and muscle functions of athletes. Syst. Rev. Pharm. 2020;11:851–854. doi: 10.31838/srp.2020.9.122. [DOI] [Google Scholar]
- 65.Aminaei M., Shamsi E.H., Nikoei R. The impact of eight weeks of calcium intake and vitamin D along with TRX exercise on body composition and lipid profiles of overweight women. Obes. Med. 2020;19:100249. doi: 10.1016/j.obmed.2020.100249. [DOI] [Google Scholar]
- 66.Bauer P., Kraushaar L., Dörr O., Bauer T., Nef H., Hamm C.W., Most A. Association of 25-hydroxy vitamin D level with the blood pressure response to a maximum exercise test among professional indoor athletes. Eur. J. Appl. Physiol. 2020;120:1931–1941. doi: 10.1007/s00421-020-04421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molina-López J., Ricalde M.A.Q., Hernández B.V., Planells A., Otero R., Planells E. Effect of 8-week of dietary micronutrient supplementation on gene expression in elite handball athletes. PLoS ONE. 2020;15:e0232237. doi: 10.1371/journal.pone.0232237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krzywański J., Mikulski T., Pokrywka A., Młyńczak M., Krysztofiak H., Frączek B., Ziemba A. Vitamin B12 Status and Optimal Range for Hemoglobin Formation in Elite Athletes. Nutrients. 2020;12:1038. doi: 10.3390/nu12041038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sekel N.M., Gallo S., Fields J., Jagim A.R., Wagner T., Jones M.T. The Effects of Cholecalciferol Supplementation on Vitamin D Status Among a Diverse Population of Collegiate Basketball Athletes: A Quasi-Experimental Trial. Nutrients. 2020;12:370. doi: 10.3390/nu12020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Millward D., Root A.D., Dubois J., Cohen R.P., Valdivia L., Helming B., Kokoskie J., Waterbrook A.L., Paul S. Association of Serum Vitamin D Levels and Stress Fractures in Collegiate Athletes. Orthop. J. Sport. Med. 2020;8:2325967120966967. doi: 10.1177/2325967120966967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pradita D., Dieny F., Kurniawati D., Tsani A., Widyastuti N., Fitranti D., Rahadiyanti A. The relationship between iron deficiency and bone mineral density in young female athletes. Food Res. 2020;4:99–108. doi: 10.26656/fr.2017.4(S3).S24. [DOI] [Google Scholar]
- 72.Shafiee S.E., Partovi G., Eslami P., Shayestehazar M. Associations between Vitamin D Levels and Risk of Anterior Cruciate Ligament Injury in Male Athletes. J. Maz. Univ. Med. Sci. 2020;30:131–136. [Google Scholar]
- 73.Ashtary-Larky D., Kheirollah A., Bagheri R., Ghaffari M.A., Mard S.A., Hashemi S.J., Mir I., Wong A. A single injection of vitamin D3 improves insulin sensitivity and β-cell function but not muscle damage or the inflammatory and cardiovascular responses to an acute bout of resistance exercise in vitamin D-deficient resistance-trained males. Br. J. Nutr. 2019;123:394–401. doi: 10.1017/S0007114519002770. [DOI] [PubMed] [Google Scholar]
- 74.Adiningsih S., Rifqi M.A. Erythrocyte-superoxide dismutase (SOD1) among elite combat sport athletes running an intensive training program and the association with micronutrient intake. Biochem. Cell. Arch. 2019;19((Suppl. S2)):4703–4711. doi: 10.35124/bca.2019.19.S2.4703. [DOI] [Google Scholar]
- 75.Kim D.K., Park G., Kuo L.-T., Park W.H. The Relationship between Vitamin D Status and Rotator Cuff Muscle Strength in Professional Volleyball Athletes. Nutrients. 2019;11:2768. doi: 10.3390/nu11112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bauer P., Henni S., Dörr O., Bauer T., Hamm C.W., Most A. High prevalence of vitamin D insufficiency in professional handball athletes. Phys. Sportsmed. 2019;47:71–77. doi: 10.1080/00913847.2018.1520055. [DOI] [PubMed] [Google Scholar]
- 77.Schaad K.A., Bukhari A.S., Brooks D.I., Kocher J.D., Barringer N.D. The relationship between vitamin D status and depression in a tactical athlete population. J. Int. Soc. Sport. Nutr. 2019;16:40. doi: 10.1186/s12970-019-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo M.-W., Song J.K., Jung H.C., Kim S.-W., Kim J.-H., Lee J.-M. The Associations of Vitamin D Status with Athletic Performance and Blood-borne Markers in Adolescent Athletes: A Cross-Sectional Study. Int. J. Environ. Res. Public Health. 2019;16:3422. doi: 10.3390/ijerph16183422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Higgins M.F., Rudkin B., Kuo C.-H. Oral Ingestion of Deep Ocean Minerals Increases High-Intensity Intermittent Running Capacity in Soccer Players after Short-Term Post-Exercise Recovery: A Double-Blind, Placebo-Controlled Crossover Trial. Mar. Drugs. 2019;17:309. doi: 10.3390/md17050309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aydın C.G., Dinçel Y.M., Arıkan Y., Taş S.K., Deniz S. The effects of indoor and outdoor sports participation and seasonal changes on vitamin D levels in athletes. SAGE Open Med. 2019;7:2050312119837480. doi: 10.1177/2050312119837480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alkoot M.J., Boland F., Brugha R., Biesma R. The prevalence and risk factors of vitamin D inadequacy among male athletes in Kuwait: A cross-sectional study. J. Steroid Biochem. Mol. Biol. 2018;187:76–81. doi: 10.1016/j.jsbmb.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Michishita R., Ohta M., Ikeda M., Jiang Y., Yamato H. An exaggerated blood pressure response to exercise is associated with the dietary sodium, potassium, and antioxidant vitamin intake in normotensive subjects. Clin. Exp. Hypertens. 2018;41:152–159. doi: 10.1080/10641963.2018.1451539. [DOI] [PubMed] [Google Scholar]
- 83.Bauer P., Kraushaar L., Hölscher S., Tajmiri-Gondai S., Dörr O., Nef H., Hamm C., Most A. Elite athletes as research model: Vitamin D insufficiency associates with elevated central blood pressure in professional handball athletes. Eur. J. Appl. Physiol. 2019;119:2265–2274. doi: 10.1007/s00421-019-04210-w. [DOI] [PubMed] [Google Scholar]
- 84.Umarov J., Kerimov F., Toychiev A., Davis N., Osipova S. Association of the 25(OH) vitamin D status with upper respiratory tract infections morbidity in water sports elite athletes. bioRxiv. 2019:559278. doi: 10.23736/S0022-4707.19.09834-7. [DOI] [PubMed] [Google Scholar]
- 85.Wrzosek M., Woźniak J., Kozioł-Kaczorek D., Włodarek D. The Assessment of the Supply of Calcium and Vitamin D in the Diet of Women Regularly Practicing Sport. J. Osteoporos. 2019;2019:9214926. doi: 10.1155/2019/9214926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carswell A.T., Oliver S.J., Wentz L.M., Kashi D.S., Roberts R., Tang J., Izard R.M., Jackson S., Allan D., Rhodes L., et al. Influence of Vitamin D Supplementation by Sunlight or Oral D3 on Exercise Performance. Med. Sci. Sport. Exerc. 2018;50:2555–2564. doi: 10.1249/MSS.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung H.C., Seo M.W., Lee S., Jung S.W., Song J.K. Correcting Vitamin D Insufficiency Improves Some But Not All Aspects of Physical Performance During Winter Training in Taekwondo Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018;28:635–643. doi: 10.1123/ijsnem.2017-0412. [DOI] [PubMed] [Google Scholar]
- 88.Orysiak J., Mazur-Różycka J., Fitzgerald J., Starczewski M., Malczewska-Lenczowska J., Busko K. Vitamin D status and its relation to exercise performance and iron status in young ice hockey players. PLoS ONE. 2018;13:e0195284. doi: 10.1371/journal.pone.0195284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malczewska-Lenczowska J., Sitkowski D., Surała O., Orysiak J., Szczepańska B., Witek K. The Association between Iron and Vitamin D Status in Female Elite Athletes. Nutrients. 2018;10:167. doi: 10.3390/nu10020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Radzhabkadiev R.M., Vrzhesinskaya O., A Beketova N., Kosheleva O.V., Vybornaya K.V., Kodentsova V.M. Content of some vitamins in food ration and blood serum of professional athletes. Vopr. Pitan. 2018;87:43–51. doi: 10.24411/0042-8833-2018-10052. [DOI] [PubMed] [Google Scholar]
- 91.Wei C.-Y., Chen C.-Y., Liao Y.-H., Tsai Y.-S., Huang C.-Y., Chaunchaiyakul R., Higgins M.F., Kuo C.-H. Deep Ocean Mineral Supplementation Enhances the Cerebral Hemodynamic Response during Exercise and Decreases Inflammation Postexercise in Men at Two Age Levels. Front. Physiol. 2017;8:1016. doi: 10.3389/fphys.2017.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DiSilvestro R.A., Hart S., Marshall T., Joseph E., Reau A., Swain C.B., Diehl J. Enhanced aerobic exercise performance in women by a combination of three mineral Chelates plus two conditionally essential nutrients. J. Int. Soc. Sport. Nutr. 2017;14:42. doi: 10.1186/s12970-017-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wardenaar F., Brinkmans N., Ceelen I., Van Rooij B., Mensink M., Witkamp R., De Vries J. Micronutrient Intakes in 553 Dutch Elite and Sub-Elite Athletes: Prevalence of Low and High Intakes in Users and Non-Users of Nutritional Supplements. Nutrients. 2017;9:142. doi: 10.3390/nu9020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Owens D.J., Tang J.C., Bradley W.J., Sparks S.A., Fraser W.D., Morton J.P., Close G.L. Efficacy of High Dose Vitamin D Supplements for Elite Athletes. [(accessed on 1 March 2023)];Med. Sci. Sports Exerc. 2016 doi: 10.1249/MSS.0000000000001105. Available online: http://researchonline.ljmu.ac.uk/id/eprint/4786/ [DOI] [PubMed] [Google Scholar]
- 95.Dahlquist D.T., Stellingwerff T., Dieter B.P., McKenzie D.C., Koehle M. Effects of macro- and micronutrients on exercise-induced hepcidin response in highly trained endurance athletes. Appl. Physiol. Nutr. Metab. 2017;42:1036–1043. doi: 10.1139/apnm-2017-0207. [DOI] [PubMed] [Google Scholar]
- 96.Nayir T., Aydın C.G., Eroglu A. Does the regular sports activities affect vitamin D levels at young athletes: A cross sectional study. Acta Med. Medit. 2017;33:913–919. [Google Scholar]
- 97.He C.-S., Fraser W.D., Tang J., Brown K., Renwick S., Rudland-Thomas J., Teah J., Tanqueray E., Gleeson M. The effect of 14 weeks of vitamin D3 supplementation on antimicrobial peptides and proteins in athletes. J. Sport. Sci. 2016;34:67–74. doi: 10.1080/02640414.2015.1033642. [DOI] [PubMed] [Google Scholar]
- 98.Hildebrand R.A., Miller B., Warren A., Hildebrand D., Smith B.J. Compromised vitamin D status negatively affects muscular strength and power of collegiate athletes. Int. J. Sport Nutr. Exerc. Metab. 2016;26:558–564. doi: 10.1123/ijsnem.2016-0052. [DOI] [PubMed] [Google Scholar]
- 99.Cassity E.P., Redzic M., Teager C.R., Thomas D.T. The effect of body composition and BMI on 25 (OH) D response in vitamin D-supplemented athletes. Eur. J. Sport Sci. 2016;16:773–779. doi: 10.1080/17461391.2015.1125952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Darr R.L., Savage K.J., Baker M., Wilding G.E., Raswalsky A., Rideout T., Browne R.W., Horvath P.J. Vitamin D supplementation affects the IGF system in men after acute exercise. Growth Horm. IGF Res. 2016;30–31:45–51. doi: 10.1016/j.ghir.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Krzywanski J., Mikulski T., Krysztofiak H., Mlynczak M., Gaczynska E., Ziemba A. Seasonal Vitamin D Status in Polish Elite Athletes in Relation to Sun Exposure and Oral Supplementation. PLoS ONE. 2016;11:e0164395. doi: 10.1371/journal.pone.0164395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Capó X., Martorell M., Sureda A., Riera J., Drobnic F., Tur J.A., Pons A. Effects of almond-and olive oil-based Docosahexaenoic-and vitamin E-enriched beverage dietary supplementation on inflammation associated to exercise and age. Nutrients. 2016;8:619. doi: 10.3390/nu8100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.EBackx E.M.P., Tieland M., Maase K., Kies A.K., Mensink M., van Loon L.J.C., de Groot L.C.P.G.M. The impact of 1-year vitamin D supplementation on vitamin D status in athletes: A dose–response study. Eur. J. Clin. Nutr. 2016;70:1009–1014. doi: 10.1038/ejcn.2016.133. [DOI] [PubMed] [Google Scholar]
- 104.Todd J., Madigan S., Pourshahidi K., McSorley E., Laird E., Healy M., Magee P. Vitamin D status and supplementation practices in elite Irish athletes: An update from 2010/2011. Nutrients. 2016;8:485. doi: 10.3390/nu8080485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maruyama A., Sakuraba K., Suzuki Y. Seasonal variations in vitamin D status in indoor and outdoor female athletes. Biomed. Rep. 2016;5:113–117. doi: 10.3892/br.2016.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wyon M.A., Wolman R., Nevill A.M., Cloak R., Metsios G.S., Gould D., Ingham A., Koutedakis Y. Acute effects of vitamin D3 supplementation on muscle strength in judoka athletes: A randomized placebo-controlled, double-blind trial. Clin. J. Sport Med. 2016;26:279–284. doi: 10.1097/JSM.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 107.Keen D.A., Constantopoulos E., Konhilas J.P. The impact of post-exercise hydration with deep-ocean mineral water on rehydration and exercise performance. J. Int. Soc. Sports Nutr. 2016;13:17. doi: 10.1186/s12970-016-0129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veskoukis A.S., Goutianos G., Paschalis V., Margaritelis N.V., Tzioura A., Dipla K., Zafeiridis A., Vrabas I.S., Kyparos A., Nikolaidis M.G. The rat closely mimics oxidative stress and inflammation in humans after exercise but not after exercise combined with vitamin C administration. Eur. J. Appl. Physiol. 2016;116:791–804. doi: 10.1007/s00421-016-3336-8. [DOI] [PubMed] [Google Scholar]
- 109.Heffler E., Bonini M., Brussino L., Solidoro P., Guida G., Boita M., Nicolosi G., Bucca C. Vitamin D deficiency and exercise-induced laryngospasm in young competitive rowers. Appl. Physiol. Nutr. Metab. 2016;41:735–740. doi: 10.1139/apnm-2015-0517. [DOI] [PubMed] [Google Scholar]
- 110.Kaul A., Gläser S., Hannemann A., Schäper C., Nauck M., Felix S.B., Bollmann T., Ewert R., Friedrich N. Vitamin D is associated with cardiopulmonary exercise capacity: Results of two independent cohorts of healthy adults. Br. J. Nutr. 2016;115:500–508. doi: 10.1017/S000711451500464X. [DOI] [PubMed] [Google Scholar]
- 111.He C.-S., Yong X.H.A., Walsh N.P., Gleeson M. Is there an optimal vitamin D status for immunity in athletes and military personnel? Exerc. Immunol. Rev. 2016;22:42–64. [PubMed] [Google Scholar]
- 112.Chenoweth L.M., Smith J.R., Ferguson C.S., Downey A.E., Harms C.A. The effects of antioxidant vitamin supplementation on expiratory flow rates at rest and during exercise. Eur. J. Appl. Physiol. 2015;115:2049–2058. doi: 10.1007/s00421-015-3183-z. [DOI] [PubMed] [Google Scholar]
- 113.Caruana H., Marshall J.M. Effects of modest hyperoxia and oral vitamin C on exercise hyperaemia and reactive hyperaemia in healthy young men. Eur. J. Appl. Physiol. 2015;115:1995–2006. doi: 10.1007/s00421-015-3182-0. [DOI] [PubMed] [Google Scholar]
- 114.Fitzgerald J.S., Peterson B.J., Warpeha J.M., Johnson S.C., Ingraham S.J. Association between vitamin D status and maximal-intensity exercise performance in junior and collegiate hockey players. J. Strength Cond. Res. 2015;29:2513–2521. doi: 10.1519/JSC.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 115.Veasey R.C., Haskell-Ramsay C.F., Kennedy D.O., Wishart K., Maggini S., Fuchs C.J., Stevenson E.J. The effects of supplementation with a vitamin and mineral complex with guaraná prior to fasted exercise on affect, exertion, cognitive performance, and substrate metabolism: A randomized controlled trial. Nutrients. 2015;7:6109–6127. doi: 10.3390/nu7085272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Price O.J., Hull J.H., Howatson G., Robson-Ansley P., Ansley L. Vitamin D and omega-3 polyunsaturated fatty acid supplementation in athletes with exercise-induced bronchoconstriction: A pilot study. Expert. Rev. Respir. Med. 2015;9:369–378. doi: 10.1586/17476348.2015.1036032. [DOI] [PubMed] [Google Scholar]
- 117.Allison R.J., Farooq A., Hamilton B., Close G.L., Wilson M.G. No association between vitamin D deficiency and markers of bone health in athletes. Med. Sci. Sports Exerc. 2015;47:782–788. doi: 10.1249/MSS.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 118.Allison R.J., Close G.L., Farooq A., Riding N.R., Salah O., Hamilton B., Wilson M.G. Severely vitamin D-deficient athletes present smaller hearts than sufficient athletes. Eur. J. Prev. Cardiol. 2015;22:535–542. doi: 10.1177/2047487313518473. [DOI] [PubMed] [Google Scholar]
- 119.Heller J.E., Thomas J.J., Hollis B.W., Larson-Meyer D.E. Relation between vitamin D status and body composition in collegiate athletes. Int. J. Sport Nutr. Exerc. Metab. 2015;25:128–135. doi: 10.1123/ijsnem.2013-0250. [DOI] [PubMed] [Google Scholar]
- 120.Popovic L.M., Mitic N.R., Miric D., Bisevac B., Miric M., Popovic B. Influence of vitamin C supplementation on oxidative stress and neutrophil inflammatory response in acute and regular exercise. Oxid. Med. Cell Longev. 2015;23 doi: 10.1155/2015/295497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Turchaninov D.V., Bovarskaya L.A., Bogdashin I.V., Bagrova L.V., Gotwald A.R., Kozubenko O.V. Influence of the regular intake of fermented milk products enriched by micronutrients on some indices of iron metabolism in adolescents involved in sports. Gig. Sanit. 2015;94:76–79. [PubMed] [Google Scholar]
- 122.Díaz V., Peinado A.B., Barba-Moreno L., Altamura S., Butragueño J., González-Gross M., Alteheld B., Stehle P., Zapico A.G., Muckenthaler M.U., et al. Elevated hepcidin serum level in response to inflammatory and iron signals in exercising athletes is independent of moderate supplementation with vitamin C and E. Physiol. Rep. 2015;3:e12475. doi: 10.14814/phy2.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Theodorou A.A., Paschalis V., Kyparos A., Panayiotou G., Nikolaidis M.G. Passive smoking reduces and vitamin C increases exercise-induced oxidative stress: Does this make passive smoking an anti-oxidant and vitamin C a pro-oxidant stimulus? Biochem. Biophys. Res. Commun. 2014;454:131–136. doi: 10.1016/j.bbrc.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 124.Fitzgerald J.S., Peterson B.J., Warpeha J.M., Wilson P.B., Rhodes G.S., Ingraham S.J. Vitamin D Status and V [Combining Dot Above] O2peak During a Skate Treadmill Graded Exercise Test in Competitive Ice Hockey Players. J. Strength Cond. Res. 2014;28:3200–3205. doi: 10.1519/JSC.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 125.Karakilcik A.Z., Halat R., Zerin M., Celik H., Nazligul Y. Effects of vitamin C and exercise on lipid profile, platelet and erythrocyte indices in young soccer players. J. Sport. Med. Phys. Fit. 2014;54:665–671. [PubMed] [Google Scholar]
- 126.Koundourakis N.E., Androulakis N.E., Malliaraki N., Margioris A.N. Vitamin D and Exercise Performance in Professional Soccer Players. PLoS ONE. 2014;9:e101659. doi: 10.1371/journal.pone.0101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aguiló A., Monjo M., Moreno C., Martinez P., Martínez S., Tauler P. Vitamin C supplementation does not influence plasma and blood mononuclear cell IL-6 and IL-10 levels after exercise. J. Sport. Sci. 2014;32:1659–1669. doi: 10.1080/02640414.2014.912759. [DOI] [PubMed] [Google Scholar]
- 128.Shanely R.A., Nieman D.C., Knab A.M., Gillitt N.D., Meaney M.P., Jin F., Sha W., Cialdella-Kam L. Influence of vitamin D mushroom powder supplementation on exercise-induced muscle damage in vitamin D insufficient high school athletes. J. Sport. Sci. 2014;32:670–679. doi: 10.1080/02640414.2013.847279. [DOI] [PubMed] [Google Scholar]
- 129.Beketova N.A., Kosheleva O.V., Pereverzeva O.G., Vrzhesinskaia O.A., Kodentsova V.M., Solntseva T.N., Khanfer’ian R.A. Vitamin-antioxidant sufficiency of winter sports athletes. Vopr Pitan. 2013;82:49–57. doi: 10.4103/0019-5545.102424. [DOI] [PubMed] [Google Scholar]
- 130.Soria M., González-Haro C., Esteva S., Escanero J.F., Pina J.R. Effect of sulphurous mineral water in haematological and biochemical markers of muscle damage after an endurance exercise in well-trained athletes. J. Sport. Sci. 2014;32:954–962. doi: 10.1080/02640414.2013.868921. [DOI] [PubMed] [Google Scholar]
- 131.Barker T., Martins T.B., Hill H.R., Kjeldsberg C.R., Dixon B.M., Schneider E.D., Henriksen V.T., Weaver L.K. Vitamin D sufficiency associates with an increase in anti-inflammatory cytokines after intense exercise in humans. Cytokine. 2014;65:134–137. doi: 10.1016/j.cyto.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 132.Nieman D.C., Gillitt N.D., Shanely R.A., Dew D., Meaney M.P., Luo B. Vitamin D2 Supplementation Amplifies Eccentric Exercise-Induced Muscle Damage in NASCAR Pit Crew Athletes. Nutrients. 2013;6:63–75. doi: 10.3390/nu6010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barker T., Schneider E.D., Dixon B.M., Henriksen V.T., Weaver L.K. Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise. Nutr. Metab. 2013;10:69. doi: 10.1186/1743-7075-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He C.-S., Handzlik M., Fraser W.D., Muhamad A., Preston H., Richardson A., Gleeson M. Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc. Immunol. Rev. 2013;19 [PubMed] [Google Scholar]
- 135.Askari G., Hajishafiee M., Ghiasvand R., Hariri M., Darvishi L., Ghassemi S., Iraj B., Hovsepian V. Does quercetin and vitamin C improve exercise performance, muscle damage, and body composition in male athletes? J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012;17:328. [PMC free article] [PubMed] [Google Scholar]
- 136.Taghiyar M., Ghiasvand R., Feizi A., Askari G., Shokri N. Effects of Vitamins C and E Supplementation on Muscle Damage and Oxidative Stress in Female Athletes: A Double-Blind Clinical Trial. J. Isfahan Med. Sch. 2013;30:2113–2124. [PMC free article] [PubMed] [Google Scholar]
- 137.Magee P.J., Pourshahidi L.K., Wallace J.M.W., Cleary J., Conway J., Harney E., Madigan S.M. Vitamin D status and supplementation in elite Irish athletes. Int. J. Sport Nutr. Exerc. Metab. 2013;23:441–448. doi: 10.1123/ijsnem.23.5.441. [DOI] [PubMed] [Google Scholar]
- 138.Peeling P., Fulton S.K., Binnie M., Goodman C. Training environment and Vitamin D status in athletes. Int. J. Sports Med. 2013;34:248–252. doi: 10.1055/s-0032-1321894. [DOI] [PubMed] [Google Scholar]
- 139.Sureda A., Ferrer M.D., Mestre A., Tur J.A., Pons A. Prevention of neutrophil protein oxidation with vitamins C and E diet supplementation without affecting the adaptive response to exercise. Int. J. Sport Nutr. Exerc. Metab. 2013;23:31–39. doi: 10.1123/ijsnem.23.1.31. [DOI] [PubMed] [Google Scholar]
- 140.Garelnabi M., Veledar E., White-Welkley J., Santanam N., Abramson J., Weintraub W., Parthasarathy S. Vitamin E differentially affects short term exercise induced changes in oxidative stress, lipids, and inflammatory markers. Nutr. Metab. Cardiovasc. Dis. 2012;22:907–913. doi: 10.1016/j.numecd.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spradley B.D., Crowley K.R., Tai C.-Y., Kendall K.L., Fukuda D.H., Esposito E.N., E Moon S., Moon J.R. Ingesting a pre-workout supplement containing caffeine, B-vitamins, amino acids, creatine, and beta-alanine before exercise delays fatigue while improving reaction time and muscular endurance. Nutr. Metab. 2012;9:28. doi: 10.1186/1743-7075-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Czaja J., Lebiedzińska A., Marszałł M., Szefer P. Evaluation for magnesium and vitamin B6 supplementation among Polish elite athletes. Rocz. Państwowego Zakładu Hig. 2011;62:413–418. [PubMed] [Google Scholar]
- 143.Patlar S., Boyali E., Baltaci A.K., Mogulkoc R., Günay M. Elements in Sera of Elite Taekwondo Athletes: Effects of Vitamin E Supplementation. Biol. Trace Elem. Res. 2010;139:119–125. doi: 10.1007/s12011-010-8648-7. [DOI] [PubMed] [Google Scholar]
- 144.Halliday T.M., Peterson N.J., Thomas J.J., Kleppinger K., Hollis B.W., Larson-Meyer D.E. Vitamin D Status Relative to Diet, Lifestyle, Injury, and Illness in College Athletes. Med. Sci. Sport. Exerc. 2011;43:335–343. doi: 10.1249/MSS.0b013e3181eb9d4d. [DOI] [PubMed] [Google Scholar]
- 145.Abolghasem R., Alireza S., Javad P.M. The Effect of Rest Duration and Vitamin C Consumption on Serum Lactate Dehydrogenase and Creatine Phosphokinase Concentration of Men Athletes during Interval Trainings. Biosci. Biotechnol. Res. Asia. 2016;8:119–123. doi: 10.13005/bbra/832. [DOI] [Google Scholar]
- 146.Louis J., Hausswirth C., Bieuzen F., Brisswalter J. Influence d’une supplémentation en vitamines sur performance musculaire maximale au cours d’un programme d’entraînement en force chez des athlètes masters. Sci. Sport. 2010;25:253–259. doi: 10.1016/j.scispo.2010.03.005. [DOI] [Google Scholar]
- 147.Chatterjee P., Maitra S., Bandyopadhyay A. Effects of vitamin-E supplementation on cardiorespiratory responses in female athletes during endurance exercise in different phases of menstrual cycle. Med. Sci. 2011;4:358–364. [Google Scholar]
- 148.Fage N., Deliac P., Germain P. Taux sanguin de vitamine D et performance sportive: Étude comparée entre sportifs et sédentaires (à propos de 20 sujets) Sci. Sport. 2010;25:201–203. doi: 10.1016/j.scispo.2010.03.007. [DOI] [Google Scholar]
- 149.Zaĭtseva I.P. Efficiency of using vitamin-mineral complexes in the prevention of iron-deficiency states in athletes. Gig. Sanit. 2010;4:66–69. [PubMed] [Google Scholar]
- 150.Dalbo V.J., Roberts M.D., Hassell S.E., Moon J.R., Kerksick C.M. Effects of a mineral antioxidant complex on clinical safety, body water, lactate response, and aerobic performance in response to exhaustive exercise. Int. J. Sport Nutr. Exerc. Metab. 2010;20:381–392. doi: 10.1123/ijsnem.20.5.381. [DOI] [PubMed] [Google Scholar]
- 151.Rahideh T., Karandish M., Moghaddam Z.A. Annals of Nutrition and Metabolism. Karger Allschwilerstrasse; Basel, Switzerland: 2009. Effect of vitamin c supplementation on oxidative stress markers following thirty minutes moderate intensity exercise in healthy young women; pp. 336–337. [DOI] [Google Scholar]
- 152.Al-Khalidi M.J.M., Al-Janbi D.T.H. Effect of Resistance Exercises by Means of Aids with Doses of Vitamin D3 in Some Biomechanical Variants to Develop the Spike Skill of Volleyball for Female Students. J. Diabetes Metab. Disord. 2009;18:232–331. doi: 10.1007/s40200-019-00416-z. [DOI] [Google Scholar]
- 153.Sureda A., Tauler P., Aguiló A., Cases N., Llompart I., Tur J.A., Pons A. Influence of an antioxidant vitamin-enriched drink on pre-and post-exercise lymphocyte antioxidant system. Ann. Nutr. Metab. 2008;52:233–240. doi: 10.1159/000140515. [DOI] [PubMed] [Google Scholar]
- 154.Nakhostin-Roohi B., Babaei P., Rahmani-Nia F., Bohlooli S. Effect of vitamin C supplementation on lipid peroxidation, muscle damage and inflammation after 30-min exercise at 75% VO^ sub 2max^. J. Sport. Med. Phys. Fit. 2008;48:217. [PubMed] [Google Scholar]
- 155.Cholewa J., Poprzęcki S., Zajac A., Waskiewicz Z. The influence of vitamin C on blood oxidative stress parameters in basketball players in response to maximal exercise. Sci. Sport. 2008;23:176–182. doi: 10.1016/j.scispo.2008.01.004. [DOI] [Google Scholar]
- 156.Machefer G., Groussard C., Zouhal H., Vincent S., Youssef H., Faure H., Malardé L., Gratas-Delamarche A. Nutritional and plasmatic antioxidant vitamins status of ultra endurance athletes. J. Am. Coll. Nutr. 2007;26:311–316. doi: 10.1080/07315724.2007.10719616. [DOI] [PubMed] [Google Scholar]
- 157.Johnston C.S., Corte C., Swan P.D. Marginal vitamin C status is associated with reduced fat oxidation during submaximal exercise in young adults. Nutr. Metab. 2006;3:35. doi: 10.1186/1743-7075-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Davison G., Gleeson M. The effect of 2 weeks vitamin C supplementation on immunoendocrine responses to 2.5 h cycling exercise in man. Eur. J. Appl. Physiol. 2006;97:454–461. doi: 10.1007/s00421-006-0196-7. [DOI] [PubMed] [Google Scholar]
- 159.Fischer C.P., Hiscock N.J., Basu S., Vessby B., Kallner A., Sjöberg L.-B., Febbraio M.A., Pedersen B.K. Vitamin E isoform-specific inhibition of the exercise-induced heat shock protein 72 expression in humans. J. Appl. Physiol. 2006;100:1679–1687. doi: 10.1152/japplphysiol.00421.2005. [DOI] [PubMed] [Google Scholar]
- 160.Machefer G., Malarde L., Groussard C., Gratas-Delamarche A. Antioxidant vitamin intakes of endurance athletes. Sci. Sport. 2006;21:107–109. doi: 10.1016/j.scispo.2005.06.007. [DOI] [Google Scholar]
- 161.Bryer S., Goldfarb A. Effect of High Dose Vitamin C Supplementation on Muscle Soreness, Damage, Function, and Oxidative Stress to Eccentric Exercise. Int. J. Sport Nutr. Exerc. Metab. 2006;16:270–280. doi: 10.1123/ijsnem.16.3.270. [DOI] [PubMed] [Google Scholar]
- 162.Fry A.C., Bloomer R.J., Falvo M.J., Moore C.A., Schilling B.K., Weiss L.W. Effect of A Liquid Multivitamin/Mineral Supplement on Anaerobic Exercise Performance. Res. Sport. Med. 2006;14:53–64. doi: 10.1080/15438620500528323. [DOI] [PubMed] [Google Scholar]
- 163.Senturk U.K., Yalcin O., Gunduz F., Kuru O., Meiselman H.J., Baskurt O.K. Effect of antioxidant vitamin treatment on the time course of hematological and hemorheological alterations after an exhausting exercise episode in human subjects. J. Appl. Physiol. 2005;98:1272–1279. doi: 10.1152/japplphysiol.00875.2004. [DOI] [PubMed] [Google Scholar]
- 164.Davison G., Gleeson M. Influence of Acute Vitamin C and/or Carbohydrate Ingestion on Hormonal, Cytokine, and Immune Responses to Prolonged Exercise. Int. J. Sport Nutr. Exerc. Metab. 2005;15:465–479. doi: 10.1123/ijsnem.15.5.465. [DOI] [PubMed] [Google Scholar]
- 165.Herrmann M., Obeid R., Scharhag J., Kindermann W., Herrmann W. Altered Vitamin B12 Status in Recreational Endurance Athletes. Int. J. Sport Nutr. Exerc. Metab. 2005;15:433–441. doi: 10.1123/ijsnem.15.4.433. [DOI] [PubMed] [Google Scholar]
- 166.Rousseau A.-S., Hininger I., Palazzetti S., Faure H., Roussel A.-M., Margaritis I. Antioxidant vitamin status in high exposure to oxidative stress in competitive athletes. Br. J. Nutr. 2004;92:461–468. doi: 10.1079/BJN20041222. [DOI] [PubMed] [Google Scholar]
- 167.Cui J.H., Wang Y.H., Zhan X.Z., Zhang X.Z., Xing G.X., Ha Z.D. Acetozolamide and highland-vitamin-tablet for improving the recovery after exhaustion exercise at high altitude. Chin. J. Clin. Rehabil. 2004;8:5100–5102. doi: 10.1089/ham.2006.7.290. [DOI] [Google Scholar]
- 168.E Viitala P., Newhouse I.J., Lavoie N., Gottardo C. The effects of antioxidant vitamin supplementation on resistance exercise induced lipid peroxidation in trained and untrained participants. Lipids Health Dis. 2004;3:14. doi: 10.1186/1476-511X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thompson D., Hill J., Hurst T., Powell J.R., Williams C., Bailey D.M. Prolonged vitamin C supplementation and recovery from eccentric exercise. Eur. J. Appl. Physiol. 2004;92:133–138. doi: 10.1007/s00421-004-1064-y. [DOI] [PubMed] [Google Scholar]
- 170.McAnulty S.R., McAnulty L.S., Nieman D.C., Dumke C.L., Morrow J.D., Utter A.C., Henson D.A., Proulx W.R., George G.L. Consumption of blueberry polyphenols reduces exercise-induced oxidative stress compared to vitamin C. Nutr. Res. 2004;24:209–221. doi: 10.1016/j.nutres.2003.10.003. [DOI] [Google Scholar]
- 171.Avery N.G., Kaiser J.L., Sharman M.J., Scheett T.P., Barnes D.M., Gómez A.L., Kraemer W.J., Volek J.S. Effects of Vitamin E Supplementation on Recovery From Repeated Bouts of Resistance Exercise. J. Strength Cond. Res. 2003;17:801–809. doi: 10.1519/1533-4287(2003)0172.0.co;2. [DOI] [PubMed] [Google Scholar]
- 172.Bryant R.J., Ryder J., Martino P., Kim J., Craig B.W. Effects of vitamin E and C supplementation either alone or in combination on exercise-induced lipid peroxidation in trained cyclists. J. Strength Cond. Res. 2003;17:792–800. doi: 10.1519/1533-4287(2003)0172.0.co;2. [DOI] [PubMed] [Google Scholar]
- 173.Tauler P., Aguiló A., Gimeno I., Fuentespina E., Tur J.A., Pons A. Influence of vitamin C diet supplementation on endogenous antioxidant defences during exhaustive exercise. Pflügers Archiv. 2003;446:658–664. doi: 10.1007/s00424-003-1112-1. [DOI] [PubMed] [Google Scholar]
- 174.Schneider M., Niess A.M., Rozario F., Angres C., Tschositsch K., Battenfeld N., Northoff H., Dickhuth H.-H., Fehrenbach E., Trommer W.E., et al. Vitamin E supplementation does not increase the vitamin C radical concentration at rest and after exhaustive exercise in healthy male subjects. Eur. J. Nutr. 2003;42:195–200. doi: 10.1007/s00394-003-0414-6. [DOI] [PubMed] [Google Scholar]
- 175.Sacheck J.M., Milbury P.E., Cannon J.G., Roubenoff R., Blumberg J.B. Effect of vitamin E and eccentric exercise on selected biomarkers of oxidative stress in young and elderly men. Free Radic. Biol. Med. 2003;34:1575–1588. doi: 10.1016/S0891-5849(03)00187-4. [DOI] [PubMed] [Google Scholar]
- 176.König D., Bissé E., Deibert P., Müller H.-M., Wieland H., Berg A. Influence of Training Volume and Acute Physical Exercise on the Homocysteine Levels in Endurance-Trained Men: Interactions with Plasma Folate and Vitamin B12. Ann. Nutr. Metab. 2003;47:114–118. doi: 10.1159/000070032. [DOI] [PubMed] [Google Scholar]
- 177.Mel’Nikov A.A., Vikulov A.D. Relationship between mineral metabolism and blood rheology in athletes. Fiziol. Cheloveka. 2003;29:48–56. [PubMed] [Google Scholar]
- 178.Thompson D., Williams C., Garcia-Roves P.M., McGregor S.J., McArdle F., Jackson M. Post-exercise vitamin C supplementation and recovery from demanding exercise. Eur. J. Appl. Physiol. 2003;89:393–400. doi: 10.1007/s00421-003-0816-4. [DOI] [PubMed] [Google Scholar]
- 179.Tauler P., Aguiló A., Fuentespina E., Tur J., Pons A. Diet supplementation with vitamin E, vitamin C and β-carotene cocktail enhances basal neutrophil antioxidant enzymes in athletes. Pflügers Arch. 2002;443:791–797. doi: 10.1007/s00424-001-0770-0. [DOI] [PubMed] [Google Scholar]
- 180.Childs A., Jacobs C., Kaminski T., Halliwell B., Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free. Radic. Biol. Med. 2001;31:745–753. doi: 10.1016/S0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 181.Krause R., Patruta S., Daxböck F., Fladerer P., Biegelmayer C., Wenisch C. Effect of vitamin C on neutrophil function after high-intensity exercise. Eur. J. Clin. Investig. 2001;31:258–263. doi: 10.1046/j.1365-2362.2001.00797.x. [DOI] [PubMed] [Google Scholar]
- 182.Thompson D., Williams C., McGregor S.J., Nicholas C.W., McArdle F., Jackson M.J., Powell J.R. Prolonged vitamin C supplementation and recovery from demanding exercise. Int. J. Sport Nutr. Exerc. Metab. 2001;11:466–481. doi: 10.1123/ijsnem.11.4.466. [DOI] [PubMed] [Google Scholar]
- 183.Akova B., Gur E., Gür H., Dirican M., Sarandöl E., Küçükoglu S. Exercise-induced oxidative stress and muscle performance in healthy women: Role of vitamin E supplementation and endogenous oestradiol. Eur. J. Appl. Physiol. 2001;84:141–147. doi: 10.1007/s004210000331. [DOI] [PubMed] [Google Scholar]
- 184.Petersen E.W., Ostrowski K., Ibfelt T., Richelle M., Offord E., Pedersen B.K., Febbraio M.A., Sacheck J.M., Cannon J.G., Hamada K., et al. Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. Am. J. Physiol. Physiol. 2001;280:C1570–C1575. doi: 10.1152/ajpcell.2001.280.6.C1570. [DOI] [PubMed] [Google Scholar]
- 185.Sacheck J.M., Decker E.A., Clarkson P.M. The effect of diet on vitamin E intake and oxidative stress in response to acute exercise in female athletes. Eur. J. Appl. Physiol. 2000;83:40–46. doi: 10.1007/s004210000252. [DOI] [PubMed] [Google Scholar]
- 186.Kawai Y., Shimomitsu T., Takanami Y., Murase N., Katsumura T., Maruyama C. Vitamin E level changes in serum and red blood cells due to acute exhaustive exercise in collegiate women. J. Nutr. Sci. Vitaminol. 2000;46:119–124. doi: 10.3177/jnsv.46.119. [DOI] [PubMed] [Google Scholar]
- 187.Chung T., Chen F., Liu J. The effect of vitamin C supplementation from fruit and endurance-training on the antioxidant status of athletes. Nutr. Sci. J. 2000;25:91–98. [Google Scholar]
- 188.Beshgetoor D., Nichols J.F., Rego I. Effect of training mode and calcium intake on bone mineral density in female master cyclists, runners, and non-athletes. Int. J. Sport Nutr. Exerc. Metab. 2000;10:290–301. doi: 10.1123/ijsnem.10.3.290. [DOI] [PubMed] [Google Scholar]
- 189.Monnat A., Grossen R., Bielinski R., Decombaz J., Gremion G., Moser C.A. De la pratique des injections de vitamines et perfusions en médecine du sport. Schweiz. Z. Fur Sportmed. Und Sport. 2000;48:133–136. [Google Scholar]
- 190.SuÈrmen-GuÈr E., OÈztuÈrk E., GuÈr H., Pündük Z., Tuncel P. Effect of vitamin E supplementation on post-exercise plasma lipid peroxidation and blood antioxidant status in smokers: With special reference to haemoconcentration effect. Eur. J. Appl. Physiol. Occup. Physiol. 1999;79:472–478. doi: 10.1007/s004210050539. [DOI] [PubMed] [Google Scholar]
- 191.Krumbach C.J., Ellis D.R., Driskell J.A. A report of vitamin and mineral supplement use among university athletes in a division I institution. Int. J. Sport Nutr. Exerc. Metab. 1999;9:416–425. doi: 10.1123/ijsn.9.4.416. [DOI] [PubMed] [Google Scholar]
- 192.Virk R.S., Dunton N.J., Young J.C., Leklem J.E. Effect of vitamin B-6 supplementation on fuels, catecholamines, and amino acids during exercise in men. Med. Sci. Sports Exerc. 1999;31:400–408. doi: 10.1097/00005768-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 193.Savino F., Bonfante G., Madon E. Use of vitamin supplements in children during convalescence and in children performing sports. Minerva Pediatr. 1999;51:1–9. [PubMed] [Google Scholar]
- 194.Rourke K., Bowering J., Turkki P., Buckenmeyer P., Keller B., Sforzo G. Effect of calcium supplementation on bone mineral density in female athletes. Nutr. Res. 1998;18:775–783. doi: 10.1016/S0271-5317(98)00063-3. [DOI] [Google Scholar]
- 195.Alessio H.M., Goldfarb A.H., Cao G. Exercise-Induced Oxidative Stress before and after Vitamin C Supplementation. Int. J. Sport Nutr. 1997;7:1–9. doi: 10.1123/ijsn.7.1.1. [DOI] [PubMed] [Google Scholar]
- 196.Oostenbrug G.S., Mensink R.P., Hardeman M.R., De Vries T., Brouns F., Hornstra G. Exercise performance, red blood cell deformability, and lipid peroxidation: Effects of fish oil and vitamin E. J. Appl. Physiol. 1997;83:746–752. doi: 10.1152/jappl.1997.83.3.746. [DOI] [PubMed] [Google Scholar]
- 197.Hartmann A., Nie A.M., Grünert-Fuchs M., Poch B., Speit G. Vitamin E prevents exercise-induced DNA damage. Mutat. Res. Lett. 1995;346:195–202. doi: 10.1016/0165-7992(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 198.Sobal J., Marquart L.F. Vitamin/mineral supplement use among high school athletes. Adolescence. 1994;29:835. [PubMed] [Google Scholar]
- 199.Men’shikov I.V., Ioffe L.A., Susekov V.E. Vitamin E and the aerobic work capacity of athletes under normo-and hyperthermia. Fiziol. Cheloveka. 1994;20:110–115. [PubMed] [Google Scholar]
- 200.Rokitzki L., Sagredos A.N., Reuß F., Büchner M., Keul J. Acute Changes in Vitamin B6, Status in Endurance Athletes before and after a Marathon. Int. J. Sport Nutr. 1994;4:154–165. doi: 10.1123/ijsn.4.2.154. [DOI] [PubMed] [Google Scholar]
- 201.Rokitzki L., Sagredos A.N., Reuss F., Cufi D., Keul J. Assessment of vitamin B6 status of strength and speedpower athletes. J. Am. Coll. Nutr. 1994;13:87–94. doi: 10.1080/07315724.1994.10718377. [DOI] [PubMed] [Google Scholar]
- 202.Rokitzki L., Sagredos A., Keck E., Sauer B., Keul J. Assessment of Vitamin B2 Status in Performance Athletes of Various Types of Sports. J. Nutr. Sci. Vitaminol. 1994;40:11–22. doi: 10.3177/jnsv.40.11. [DOI] [PubMed] [Google Scholar]
- 203.Lorino A.M., Paul M., Cocea L., Scherrer-Crosbie M., Dahan E., Meignan M., Atlan G. Vitamin E does not prevent exercise-induced increase in pulmonary clearance. J. Appl. Physiol. 1994;77:2219–2223. doi: 10.1152/jappl.1994.77.5.2219. [DOI] [PubMed] [Google Scholar]
- 204.Jakemanl P., Maxwell S. Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1993;67:426–430. doi: 10.1007/BF00376459. [DOI] [PubMed] [Google Scholar]
- 205.Nasolodin V.V., Gladkikh I.P., Dvorkin V.A. Effect of vitamin supplementation on the metabolism of iron, copper and manganese in athletes. Gig. Sanit. 1993;6:31–33. [PubMed] [Google Scholar]
- 206.Bazzarre T.L., Scarpino A., Sigmon R., Marquart L.F., Wu S.M., Izurieta M. Vitamin-mineral supplement use and nutritional status of athletes. J. Am. Coll. Nutr. 1993;12:162–169. doi: 10.1080/07315724.1993.10718297. [DOI] [PubMed] [Google Scholar]
- 207.Klausen T., Breum L., Sørensen H.A., Schifter S., Sonne B. Plasma levels of parathyroid hormone, vitamin D, calcitonin, and calcium in association with endurance exercise. Calcif. Tissue Int. 1993;52:205–208. doi: 10.1007/BF00298719. [DOI] [PubMed] [Google Scholar]
- 208.Fogelholm M., Ruokonen I., Laakso J.T., Vuorimaa T., Himberg J.-J. Lack of Association between Indices of Vitamin B1, B2, and B6, Status and Exercise-Induced Blood Lactate in Young Adults. Int. J. Sport Nutr. 1993;3:165–176. doi: 10.1123/ijsn.3.2.165. [DOI] [PubMed] [Google Scholar]
- 209.Maxwell S.R.J., Jakeman P., Thomason H., Leguen C., Thorpe G.H.G. Changes in Plasma Antioxidant Status During Eccentric Exercise and the Effect of Vitamin Supplementation. Free. Radic. Res. Commun. 1993;19:191–202. doi: 10.3109/10715769309111602. [DOI] [PubMed] [Google Scholar]
- 210.Meydani M., Evans W.J., Handelman G., Biddle L., Fielding R.A., Meydani S.N., Burrill J., Fiatarone M.A., Blumberg J.B., Cannon J.G. Protective effect of vitamin E on exercise-induced oxidative damage in young and older adults. Am. J. Physiol. J. Physiol. Regul. Integr. Comp. Physiol. 1993;264:R992–R998. doi: 10.1152/ajpregu.1993.264.5.R992. [DOI] [PubMed] [Google Scholar]
- 211.Mikalauskaĭte D.A., Skerniavichius I.P., Pechiukonene M.V., Valatkaĭte A.A., Ligeĭkene D.Z., Vilkas A.I., Spirichev V.B. The effect of the multivitamin premix 730/4 on the vitamin C allowance, physical development, work capacity and psychomotor indices of athletes. Vopr. Pitan. 1992;5–6:37–41. [PubMed] [Google Scholar]
- 212.Meydani M., Evans W., Handelman G., Fielding R.A., Meydani S.N., Fiatarone M.A., Blumberg J.B., Cannon J.G. Antioxidant Response to Exercise-induced Oxidative Stress and Protection by Vitamin E. Ann. N. Y. Acad. Sci. 1992;669:363–364. doi: 10.1111/j.1749-6632.1992.tb17124.x. [DOI] [PubMed] [Google Scholar]
- 213.Telford R.D., Catchpole E.A., Deakin V., McLeay A.C., Plank A.W. The Effect of 7 to 8 months of Vitamin/Mineral Supplementation on the Vitamin and Mineral Status of Athletes. Int. J. Sport Nutr. 1992;2:123–134. doi: 10.1123/ijsn.2.2.123. [DOI] [PubMed] [Google Scholar]
- 214.Fogelholm M. Micronutrient Status in Females during a 24-Week Fitness-Type Exercise Program. Ann. Nutr. Metab. 1992;36:209–218. doi: 10.1159/000177720. [DOI] [PubMed] [Google Scholar]
- 215.Deuster P., Kyle S.B., Singh A., Moser P.B., Bernier L.L., A Yu-Yahiro J., Schoomaker E.B. Exercise-induced changes in blood minerals, associated proteins and hormones in women athletes. J. Sport. Med. Phys. Fit. 1991;31:552–560. [PubMed] [Google Scholar]
- 216.Colgan M., Fiedler S., Colgan L. Micronutrient status of endurance athletes affects hematology and performance. J. Appl. Nutr. 1991;43:16–30. [Google Scholar]
- 217.Pieralisi G., Ripari P., Vecchiet L. Effects of a standardized ginseng extract combined with dimethylaminoethanol bitartrate, vitamins, minerals, and trace elements on physical performance during exercise. Clin. Ther. 1991;13:373–382. [PubMed] [Google Scholar]
- 218.Mirić M., Haxhiu M.A. Effect of vitamin C on exercise-induced bronchoconstriction. Plucne Boles. Cas. Udruz. Pneumoftiziol. Jugosl. J. Yugosl. Assoc. Phthisiology Pneumol. 1991;43:94–97. [PubMed] [Google Scholar]
- 219.Cannon J.G., Meydani S.N., Fielding R.A., Fiatarone M.A., Meydani M.O., Farhangmehr M.E., Orencole S.F., Blumberg J.B., Evans W.J. Acute phase response in exercise. II. Associations between vitamin E, cytokines, and muscle proteolysis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1991;260:R1235–R1240. doi: 10.1152/ajpregu.1991.260.6.R1235. [DOI] [PubMed] [Google Scholar]
- 220.Faber M., Benade A.J.S. Mineral and vitamin intake in field athletes (discus-, hammer-, javelin-throwers and shotputters) Int. J. Sports Med. 1991;12:324–327. doi: 10.1055/s-2007-1024690. [DOI] [PubMed] [Google Scholar]
- 221.Cannon J.G., Orencole S.F., Fielding R.A., Meydani M.O., Meydani S.N., Fiatarone M.A., Blumberg J.B., Evans W.J. Acute phase response in exercise: Interaction of age and vitamin E on neutrophils and muscle enzyme release. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1990;259:R1214–R1219. doi: 10.1152/ajpregu.1990.259.6.R1214. [DOI] [PubMed] [Google Scholar]
- 222.Van Erp-Baart A., Saris W.H.M., Binkhorst R.A., Vos J.A., Elvers J.W.H. Nationwide survey on nutritional habits in elite athletes. Int. J. Sports Med. 1989;10:S3–S10. doi: 10.1055/s-2007-1024947. [DOI] [PubMed] [Google Scholar]
- 223.Sumida S., Tanaka K., Kitao H., Nakadomo F. Exercise-induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. Int. J. Biochem. 1989;21:835–838. doi: 10.1016/0020-711x(89)90280-2. [DOI] [PubMed] [Google Scholar]
- 224.Guilland J.-C., Penaranda T., Gallet C., Boggio V., Fuchs F., Klepping J. Vitamin status of young athletes including the effects of supplementation. Med. Sci. Sports Exerc. 1989;21:441–449. doi: 10.1249/00005768-198908000-00017. [DOI] [PubMed] [Google Scholar]
- 225.Weight L.M., Myburgh K.H., Noakes T.D. Vitamin and mineral supplementation: Effect on the running performance of trained athletes. Am. J. Clin. Nutr. 1988;47:192–195. doi: 10.1093/ajcn/47.2.192. [DOI] [PubMed] [Google Scholar]
- 226.Manore M.M., Leklem J.E. Effect of carbohydrate and vitamin B6 on fuel substrates during exercise in women. Med. Sci. Sports Exerc. 1988;20:233–241. doi: 10.1249/00005768-198806000-00004. [DOI] [PubMed] [Google Scholar]
- 227.Bell N.H., Godsen R.N., Henry D.P., Shary J., Epstein S. The effects of muscle-building exercise on vitamin D and mineral metabolism. J. Bone Miner. Res. 1988;3:369–374. doi: 10.1002/jbmr.5650030402. [DOI] [PubMed] [Google Scholar]
- 228.Klepping J., Fuchs F., Boggio V., Moreau D., Guilland J.C., Penaranda T. Evaluation of vitamin and mineral intakes in adolescent and adult athletes. Sci. Sports. 1988;3:157–172. doi: 10.1016/S0765-1597(88)80008-X. [DOI] [Google Scholar]
- 229.Manore M.N., Leklem J.E., Walter M.C. Vitamin B-6 metabolism as affected by exercise in trained and untrained women fed diets differing in carbohydrate and vitamin B-6 content. Am. J. Clin. Nutr. 1987;46:995–1004. doi: 10.1093/ajcn/46.6.995. [DOI] [PubMed] [Google Scholar]
- 230.Zuliani U., Azzali G.L., Solito F. Mineral salts and water in the diet of the athlete. Acta Biomed. Ateneo Parm. 1985;56:149–154. [PubMed] [Google Scholar]
- 231.Butturini U., Dei Cas L., Mineo F., Baroni M.C., Buia E., Crotti G., Bernardini B., Manca C., Delsignore R. K-strophanthin and vitamin E in the prevention of myocardiopathy caused by adriamycin. Noninvasive evaluation at rest and after isometric exercise. Clin. Ter. 1984;108:389–395. [PubMed] [Google Scholar]
- 232.Walter M.C., Manore M., Leklem J.E. Federation Proceedings. Federation of American Societies for Experimental Biology; Rockville, MD, USA: 1984. Effect of High and Normal Carbohydrate Diets and Vitamin-B-6 Supplementation on Fuel Metabolism During Exercise in Trained and Untrained Women; p. 869. [DOI] [Google Scholar]
- 233.Rusin V.I., Nasolodin V.V., Gladkikh I.P. Effectiveness of training young athletes by including vitamin and trace element supplements in the diet. Gig. Sanit. 1982;3:78–80. doi: 10.1139/h00-004. [DOI] [PubMed] [Google Scholar]
- 234.Borisov I.M. Vitamin P in the nutrition of athletes. Vopr. Pitan. 1980;1:35–38. [PubMed] [Google Scholar]
- 235.Helgheim I., Hetland Ø., Nilsson S., Ingjer F., Strømme S.B. The effects of vitamin E on serum enzyme levels following heavy exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1979;40:283–289. doi: 10.1007/BF00421520. [DOI] [PubMed] [Google Scholar]
- 236.Leklem J.E., Shultz T.D., Miller L.T. Federation Proceedings. Federation of American Societies for Experimental Biology; Rockville, MD, USA: 1979. Vitamin-B6 Metabolism during Exercise in Male Adolescents; p. 452. [DOI] [Google Scholar]
- 237.Laricheva K.A., Bogdanov N.G., Ialovaia N.I., Gvozdova L.G., Taraban’ko V.M. Characteristics of the vitamin status and of the individual indices of mineral metabolism and catecholamines in the students of sports colleges. Vopr. Pitan. 1979;1:40–43. [PubMed] [Google Scholar]
- 238.Dam B.V. Vitamins and sport. Br. J. Sport. Med. 1978;12:74–79. doi: 10.1136/bjsm.12.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Haralambie G. Vitamin B2 status in athletes and the influence of riboflavin administration on neuromuscular irritability. Ann. Nutr. Metab. 1976;20:1–8. doi: 10.1159/000175682. [DOI] [PubMed] [Google Scholar]
- 240.Shephard R.J., Campbell R., Pimm P., Stuart D., Wright G.R. Vitamin E, exercise, and the recovery from physical activity. Eur. J. Appl. Physiol. 1974;33:119–126. doi: 10.1007/BF00449513. [DOI] [PubMed] [Google Scholar]
- 241.Bailey D.A., Carron A.V., Teeoe R.G., Wehner H. Effect of vitamin C supplementation upon the physiological response to exercise in trained and untrained subjects. Int. Z. Fur Vitam. 1970;40:435–441. [PubMed] [Google Scholar]
- 242.Beck K.L., von Hurst P.R., O’Brien W.J., Badenhorst C.E. Micronutrients and athletic performance: A review. Food Chem. Toxicol. 2021;158:112618. doi: 10.1016/j.fct.2021.112618. [DOI] [PubMed] [Google Scholar]
- 243.Mason S.A., Trewin A.J., Parker L., Wadley G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020;35:101471. doi: 10.1016/j.redox.2020.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Vitale K., Hueglin S. Update on vegetarian and vegan athletes: A review. J. Phys. Fit. Sport. Med. 2021;10:1–11. doi: 10.7600/jpfsm.10.1. [DOI] [Google Scholar]
- 245.Dobrowolski H., Karczemna A., Włodarek D. Nutrition for Female Soccer Players—Recommendations. Medicina. 2020;56:28. doi: 10.3390/medicina56010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Purcell L., Society C.P. Paediatric Sports and Exercise Medicine Section Sport nutrition for young athletes. Paediatr. Child Health. 2013;18:200–202. doi: 10.1093/pch/18.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mizuma H., Tanaka M., Nozaki S., Mizuno K., Tahara T., Ataka S., Sugino T., Shirai T., Kajimoto Y., Kuratsune H., et al. Daily oral administration of crocetin attenuates physical fatigue in human subjects. Nutr. Res. 2009;29:145–150. doi: 10.1016/j.nutres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 248.Nirwandi, Hardiansyah S. 1st Progress in Social Science, Humanities and Education Research Symposium (PSSHERS 2019) Atlantis Press; Noord-Holland, The Netherlands: 2020. Relationship Between Nutritional Status and Students’ Physical Condition; pp. 318–321. [DOI] [Google Scholar]
- 249.Charkos T.G., Liu Y., Oumer K.S., Vuong A.M., Yang S. Effects of β-carotene intake on the risk of fracture: A Bayesian meta-analysis. BMC Musculoskelet. Disord. 2020;21:711. doi: 10.1186/s12891-020-03733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Alghadir A., Gabr S. Efficacy of Rhus coriaria (sumac) juice in reducing muscle pain during aerobic exercise. Acta Physiol. Hung. 2016;103:231–242. doi: 10.1556/036.103.2016.2.10. [DOI] [PubMed] [Google Scholar]
- 251.Hughes D.A., Wright A.J.A., Finglas P.M., Polley A.C.J., Bailey A.L., Astley S.B., Southon S. Effects of Lycopene and Lutein Supplementation on the Expression of Functionally Associated Surface Molecules on Blood Monocytes from Healthy Male Nonsmokers. J. Infect. Dis. 2000;182((Suppl. S1)):S11–S15. doi: 10.1086/315910. [DOI] [PubMed] [Google Scholar]
- 252.Journal O., Norsuriani S., Ooi F.K. A Review of Martial Arts and Bone Health Status in Young and Older Population. Sports Exerc. Med. Open J. 2018;4:58–62. doi: 10.17140/SEMOJ-4-162. [DOI] [Google Scholar]
- 253.Maughan R.J., Burke L.M., Dvorak J., Larson-Meyer D.E., Peeling P., Phillips S.M., Rawson E.S., Walsh N.P., Garthe I., Geyer H., et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sport. Med. 2018;52:439–455. doi: 10.1136/bjsports-2018-099027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Teixeira V.H., Valente H.F., Casal S.I., Marques A.F., Moreira P.A. Antioxidants Do Not Prevent Postexercise Peroxidation and May Delay Muscle Recovery. Med. Sci. Sport. Exerc. 2009;41:1752–1760. doi: 10.1249/MSS.0b013e31819fe8e3. [DOI] [PubMed] [Google Scholar]
- 255.Close G.L., Baar K., Sale C., Bermon S. Nutrition for the prevention and treatment of injuries in track and field athletes. Int. J. Sport Nutr. Exerc. Metab. 2019;29:189–197. doi: 10.1123/ijsnem.2018-0290. [DOI] [PubMed] [Google Scholar]
- 256.Nuti R., Martini G., Merlotti D., Valleggi F., De Paola V., Gennari L. Professional sport activity and micronutrients: Effects on bone mass. J. Endocrinol. Investig. 2005;28((Suppl. S10)):52–60. [PubMed] [Google Scholar]
- 257.Holzhauer P. Micronutrient therapy with selenium, vitamin C, and L carnitine in gynecological oncology: Areas of application and studies. Gynakologe. 2017;50:15–21. doi: 10.1007/s00129-016-3993-z. [DOI] [Google Scholar]
- 258.Keong C.C., Singh H.J., Singh R. Effects of palm vitamin e supplementation on exercise-induced oxidative stress and endurance performance in the heat. J. Sport. Sci. Med. 2006;5:629–639. [PMC free article] [PubMed] [Google Scholar]
- 259.Tsakiris S., Karikas G.A., Parthimos T., Tsakiris T., Bakogiannis C., Schulpis K.H. Alpha-tocopherol supplementation prevents the exercise-induced reduction of serum paraoxonase 1/arylesterase activities in healthy individuals. Eur. J. Clin. Nutr. 2009;63:215–221. doi: 10.1038/sj.ejcn.1602918. [DOI] [PubMed] [Google Scholar]
- 260.Campbell S.C., Wisniewski P.J. Nutrition in the Prevention and Treatment of Disease. Academic Press; Cambridge, MA, USA: 2017. Nutritional recommendations for athletes. [Google Scholar]
- 261.Heaton L.E., Davis J.K., Rawson E.S., Nuccio R.P., Witard O.C., Stein K.W., Baar K., Carter J.M., Baker L.B. Selected In-Season Nutritional Strategies to Enhance Recovery for Team Sport Athletes: A Practical Overview. Sport. Med. 2017;47:2201–2218. doi: 10.1007/s40279-017-0759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Antosik A., Czubak K., Cichon N., Nowak P., Zbikowska H. Vitamin E analogue protects red blood cells against storage-induced oxidative damage. Transfus. Med. Hemotherapy. 2018;45:347–354. doi: 10.1159/000486605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Maughan R.J. Role of micronutrients in sport and physical activity. Br. Med Bull. 1999;55:683–690. doi: 10.1258/0007142991902556. [DOI] [PubMed] [Google Scholar]
- 264.Li L., Yang X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018;2018:7580707. doi: 10.1155/2018/7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Peyravi A., Nayeri H., Yazdanpanahi N., Hosseini S.A. The Effect of Endurance Training with Crocin Consumption on the Levels of AMPK and NRF1 Gene Expression in the Soleus Muscles of Diabetic Rats. Ann. Mil. Health Sci. Res. 2021;19 doi: 10.5812/amh.113123. [DOI] [PubMed] [Google Scholar]
- 266.Bytomski J.R. Fueling for Performance. Sport. Health A Multidiscip. Approach. 2017;10:47–53. doi: 10.1177/1941738117743913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Serra M.C., Beavers K.M. Nutritional Supplements in Sports and Exercise. 2nd ed. Springer; Berlin/Heidelberg, Germany: 2015. [(accessed on 1 March 2023)]. Essential and nonessential micronutrients and sport. Available online: https://scholars.uthscsa.edu/en/publications/essential-and-nonessential-micronutrients-and-sport. [Google Scholar]
- 268.Dubnov-Raz G., Livne N., Raz R., Rogel D., Cohen A.H., Constantini N.W. Vitamin D concentrations and physical performance in competitive adolescent swimmers. Pediatr. Exerc. Sci. 2014;26:64–70. doi: 10.1123/pes.2013-0034. [DOI] [PubMed] [Google Scholar]
- 269.Erpenbach K., Erpenbach M.C., Mayer W., Hoffmann U., Mücke S. Level of Micronutrients in Elite Sports: Impact on Muscle Damage, Infections, Sleep Disorders and Fatigue. Adv. Orthop. Sport. Med. 2020;2020 doi: 10.37722/AOASM.20203. [DOI] [Google Scholar]
- 270.Frączek B., Pięta A., Burda A., Mazur-Kurach P., Tyrała F. Paleolithic Diet—Effect on the Health Status and Performance of Athletes? Nutrients. 2021;13:1019. doi: 10.3390/nu13031019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lucock M. Molecular Nutrition and Genomics. John Wiley & Sons; Hoboken, NJ, USA: 2007. Some Non-Micronutrient Essential and Nonessential Nutrients with Molecular and Possible Evolutionary Impact. [DOI] [Google Scholar]
- 272.Lohman R., Carr A., Condo D. Nutritional intake in Australian football players: Sports nutrition knowledge and macronutrient and micronutrient intake. Int. J. Sport Nutr. Exerc. Metab. 2019;29:289–296. doi: 10.1123/ijsnem.2018-0031. [DOI] [PubMed] [Google Scholar]
- 273.Heffernan S.M., Horner K., De Vito G., Conway G.E. The role of mineral and trace element supplementation in exercise and athletic performance: A systematic review. Nutrients. 2019;11:696. doi: 10.3390/nu11030696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Monthuy-Blanc J., Maïano C., Therme P. Prévalence des symptômes de troubles du comportement alimentaire chez des danseuses classiques et des joueuses de basket-ball ne pratiquant pas à un haut niveau: Une étude exploratoire et contrôlée auprès d’adolescentes fran. Rev. Epidemiol. Sante Publique. 2010;58:415–424. doi: 10.1016/j.respe.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 275.Teixidor-Batlle C., Ventura C., Andrés A. Eating Disorder Symptoms in Elite Spanish Athletes: Prevalence and Sport-Specific Weight Pressures. Front Psychol. 2021;11:3612. doi: 10.3389/fpsyg.2020.559832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Stach K., Stach W., Augoff K. Vitamin B6 in health and disease. Nutrients. 2021;13:3229. doi: 10.3390/nu13093229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bangsbo J., Hostrup M. Lactate production contributes to development of fatigue during intense exercise in humans. Ugeskr. Laeger. 2019;181:V10180669. [PubMed] [Google Scholar]
- 278.Shulpekova Y., Nechaev V., Kardasheva S., Sedova A., Kurbatova A., Bueverova E., Kopylov A., Malsagova K., Dlamini J.C., Ivashkin V. The concept of folic acid in health and disease. Molecules. 2021;26:3731. doi: 10.3390/molecules26123731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Neubauer O., Yfanti C. Antioxidants in Athlete’s Basic Nutrition: Considerations Towards a Guideline for the Intake of Vitamin C and Vitamin E. Taylor & Francis; Boca Raton, FL, USA: 2015. [PubMed] [Google Scholar]
- 280.Thompson S.H. Characteristics of the female athlete triad in collegiate cross-country runners. J. Am. Coll. Health. 2007;56:129–136. doi: 10.3200/JACH.56.2.129-136. [DOI] [PubMed] [Google Scholar]
- 281.Williams M.H. Dietary Supplements and Sports Performance: Minerals. [(accessed on 1 March 2023)];J. Int. Soc. Sports Nutr. 2005 2:1–7. doi: 10.1186/1550-2783-2-1-43. Available online: www.sportsnutritionsociety.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rossi K.A. Nutritional Aspects of the Female Athlete. Clin. Sport. Med. 2017;36:627–653. doi: 10.1016/j.csm.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 283.Lee N. A Review of Magnesium, Iron, and Zinc Supplementation Effects on Athletic Performance. Korean J. Phys. Educ. 2017;56:797–806. doi: 10.23949/kjpe.2017.01.56.1.59. [DOI] [Google Scholar]
- 284.IAlaunyte I., Stojceska V., Plunkett A. Iron and the female athlete: A review of dietary treatment methods for improving iron status and exercise performance. J. Int. Soc. Sport. Nutr. 2015;12:38. doi: 10.1186/s12970-015-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Vincent J.B., Neggers Y., McClung J. Nutrition and Enhanced Sports Performance. Elsevier; Amsterdam, The Netherlands: 2018. Roles of Chromium(III), Vanadium, Iron, and Zinc in Sports Nutrition; pp. 653–664. [DOI] [Google Scholar]
- 286.Pasricha S.-R., Tye-Din J., Muckenthaler M.U., Swinkels D.W. Iron deficiency. Lancet. 2021;397:233–248. doi: 10.1016/S0140-6736(20)32594-0. [DOI] [PubMed] [Google Scholar]
- 287.Sim M., Garvican-Lewis L.A., Cox G.R., Govus A., McKay A.K., Stellingwerff T., Peeling P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019;119:1463–1478. doi: 10.1007/s00421-019-04157-y. [DOI] [PubMed] [Google Scholar]
- 288.Jeoung B., Kim J. Analysis and evaluation of nutritional intake and nutrition quotient of Korean athletes with disabilities in the Tokyo paralympic games. Nutrients. 2021;13:3631. doi: 10.3390/nu13103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cormick G., Belizán J.M. Calcium intake and health. Nutrients. 2019;11:1606. doi: 10.3390/nu11071606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.McLoughlin G., Fecske C.W., Castaneda Y., Gwin C., Graber K. Sport participation for elite athletes with physical disabilities: Motivations, barriers, and facilitators. Adapt. Phys. Act. Q. 2017;34:421–441. doi: 10.1123/apaq.2016-0127. [DOI] [PubMed] [Google Scholar]
- 291.Ebert J.K. A Pilot Study of the Relationship of Calcium Intake and Frequency of Injuries In High School Athletes. 2010. [(accessed on 1 March 2023)]. Available online: https://digitalcommons.unl.edu/nutritiondiss/15/
- 292.Rheaume-Bleue K. Vitamin K2 and the Calcium Paradox: How a Little-Known Vitamin Could Save your Life. John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
- 293.Hinsinger P. Managing Global Resources and Universal Processes. CRC Press; Boca Raton, FL, USA: 2020. Potassium; pp. 315–321. [Google Scholar]
- 294.Volpe S.L. Magnesium and the Athlete. Curr. Sports Med. Rep. 2015;14:279–283. doi: 10.1249/JSR.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 295.Abbasi B., Kimiagar M., Sadeghniiat K., Shirazi M.M., Hedayati M., Rashidkhani B. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J. Res. Med. Sci. 2012;17:1161. [PMC free article] [PubMed] [Google Scholar]
- 296.Kass L.S., Skinner P., Poeira F. A pilot study on the effects of magnesium supplementation with high and low habitual dietary magnesium intake on resting and recovery from aerobic and resistance exercise and systolic blood pressure. J. Sport. Sci. Med. 2013;12:144–150. [PMC free article] [PubMed] [Google Scholar]
- 297.Setaro L., Santos-Silva P.R., Nakano E.Y., Sales C.H., Nunes N., Greve J.M., Colli C. Magnesium status and the physical performance of volleyball players: Effects of magnesium supplementation. J. Sports Sci. 2014;32:438–445. doi: 10.1080/02640414.2013.828847. [DOI] [PubMed] [Google Scholar]
- 298.Singh S., Prakash Dubey R., Shruti Singh C. Zinc, and its essentiality for human health. [(accessed on 1 March 2023)];Pharma Innov. J. 2019 8:137–139. Available online: https://en.m.wikipedia.org. [Google Scholar]
- 299.Ahrendt D.M. Ergogenic Aids: Counseling the Athlete. Am. Fam. Physician. 2001;63:913–923. [PubMed] [Google Scholar]
- 300.Ito T., Tanisawa K., Kawakami R., Usui C., Ishii K., Suzuki K., Sakamoto S., Muraoka I., Oka K., Higuchi M. Micronutrient intake adequacy in men and women with a healthy Japanese dietary pattern. Nutrients. 2020;12:6. doi: 10.3390/nu12010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Dairo M., Ige O. Supplementation of micronutrients in community micronutrient deficiency prevention programmes. Ann. Ib. Postgrad. Med. 2011;7:6–9. doi: 10.4314/aipm.v7i1.64053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Polat Y. Effects of zinc supplementation on hematological parameters of high performance athletes. Afr. J. Pharm. Pharmacol. 2011;5:1436–1440. doi: 10.5897/AJPP11.062. [DOI] [Google Scholar]
- 303.Maynar M., Grijota F.J., Siquier-Coll J., Bartolome I., Robles M.C., Muñoz D. Erythrocyte concentrations of chromium, copper, manganese, molybdenum, selenium and zinc in subjects with different physical training levels. J. Int. Soc. Sport. Nutr. 2020;17:35. doi: 10.1186/s12970-020-00367-4. Erratum in 2021, 21, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Muñoz D., Maynar M., Barrientos G., Siquier-Coll J., Bartolomé I., Grijota F.J., Robles M.C. Effect of an acute exercise until exhaustion on the serum and urinary concentrations of cobalt, copper, and manganese among well-trained athletes. Biol. Trace Elem. Res. 2019;189:387–394. doi: 10.1007/s12011-018-1500-1. [DOI] [PubMed] [Google Scholar]
- 305.Martinsen M., Bratland-Sanda S., Eriksson A.K., Sundgot-Borgen J. Dieting to win or to be thin? A study of dieting and disordered eating among adolescent elite athletes and non-athlete controls. Br. J. Sports Med. 2010;44:70–76. doi: 10.1136/bjsm.2009.068668. [DOI] [PubMed] [Google Scholar]
- 306.Fernández-Lázaro D., Fernandez-Lazaro C.I., Mielgo-Ayuso J., Navascués L.J., Martínez A.C., Seco-Calvo J. The role of selenium mineral trace element in exercise: Antioxidant defense system, muscle performance, hormone response, and athletic performance. A systematic review. Nutrients. 2020;12:1790. doi: 10.3390/nu12061790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Demircan K., Bengtsson Y., Sun Q., Brange A., Vallon-Christersson J., Rijntjes E., Malmberg M., Saal L.H., Rydén L., Borg Å., et al. Serum selenium, selenoprotein P and glutathione peroxidase 3 as predictors of mortality and recurrence following breast cancer diagnosis: A multicentre cohort study. Redox Biol. 2021;47:102145. doi: 10.1016/j.redox.2021.102145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Shahar D., Shai I., Vardi H., Shahar A., Fraser D. Diet and eating habits in high and low socioeconomic groups. Nutrition. 2005;21:559–566. doi: 10.1016/j.nut.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 309.Hammouh F., Zein S., Amr R., Ghazzawi H., Muharib D., Al Saad D., Subih H. Assessment of dietary selenium intake of Jordanian adults in Madaba: A cross sectional study. Nutr. Food Sci. 2020;51:494–506. doi: 10.1108/NFS-11-2019-0337. [DOI] [Google Scholar]
- 310.Hoch A.Z., Papanek P., Szabo A., Widlansky M.E., Schimke J.E., Gutterman D.D. Association Between the Female Athlete Triad and Endothelial Dysfunction in Dancers. 2011. [(accessed on 1 March 2023)]. Available online: www.cjsportmed.com. [DOI] [PMC free article] [PubMed]
- 311.Rauh M.J., Nichols J.F., Barrack M.T. Relationships Among Injury and Disordered Eating, Menstrual Dysfunction, and Low Bone Mineral Density in High School Athletes: A Prospective Study. [(accessed on 1 March 2023)]. Available online: www.nata.org/jat. [DOI] [PMC free article] [PubMed]
- 312.Erikson K.M., Aschner M. Manganese: Its role in disease and health. Met. Ions Life Sci. 2019;19:9783110527872-016. doi: 10.1515/9783110527872-016. [DOI] [PubMed] [Google Scholar]
- 313.Beals K.A., Beals K.A., Manore M.M. Disorders of the Female Athlete Triad / 281 Disorders of the Female Athlete Triad Among Collegiate Athletes. Int. J. Sport Nutr. Exerc. Metab. 2002;12:281–293. doi: 10.1123/ijsnem.12.3.281. [DOI] [PubMed] [Google Scholar]
- 314.Gomes-Santos J.A., Lambertucci R.H., Vardaris C.V., Passos M.E., Silva-Junior E.P., Hatanaka E., Gorjão R., McAnulty S.R., Souza-Junior T.P., Barros M.P. Early signs of inflammation with Mild oxidative stress in mixed martial arts athletes after simulated combat. J. Strength Cond. Res. 2022;36:180–186. doi: 10.1519/JSC.0000000000003383. [DOI] [PubMed] [Google Scholar]
- 315.Ghazzawi H.A., Alhaj O.A., Nemer L.S., Amawi A.T., Trabelsi K., Jahrami H.A. The Prevalence of ‘at Risk’ Eating Disorders among Athletes in Jordan. Sports. 2022;10:182. doi: 10.3390/sports10110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Thein-Nissenbaum J.M., Rauh M.J., Carr K.E., Loud K.J., Mcguine T.A. Associations between disordered eating, menstrual dysfunction, and musculoskeletal injury among high school athletes. J. Orthop. Sport. Phys. Ther. 2011;41:60–69. doi: 10.2519/jospt.2011.3312. [DOI] [PubMed] [Google Scholar]
- 317.Robinson L., Zhang Z., Jia T., Bobou M., Roach A., Campbell I., Irish M., Quinlan E.B., Tay N., Barker E.D., et al. Original investigation | psychiatry association of genetic and phenotypic assessments with onset of disordered eating behaviors and comorbid mental health problems among adolescents. JAMA Netw. Open. 2020;3:e2026874. doi: 10.1001/jamanetworkopen.2020.26874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nguyen V.H. Osteoporosis prevention and osteoporosis exercise in community-based public health programs. Osteoporos. Sarcopenia. 2017;3:18–31. doi: 10.1016/j.afos.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Prather H., Hunt D., McKeon K., Simpson S., Meyer E.B., Yemm T., Brophy R. Are Elite Female Soccer Athletes at Risk for Disordered Eating Attitudes, Menstrual Dysfunction, and Stress Fractures? PM R. 2016;8:208–213. doi: 10.1016/j.pmrj.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sale C., Elliott-Sale K.J. Nutrition and athlete bone health. Sport. Med. 2019;49((Suppl. S2)):139–151. doi: 10.1007/s40279-019-01161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Gorczyca A.M., Sjaarda L.A., Mitchell E.M., Perkins N.J., Schliep K.C., Wactawski-Wende J., Mumford S.L. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur. J. Nutr. 2016;55:1181–1188. doi: 10.1007/s00394-015-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Pallante P.I., Vega A.C., Escobar A., Hackney A.C., Rubin D.A. Micronutrient intake and premenstrual syndrome in female collegiate athletes. J. Sports Med. Phys. Fit. 2023;63:444–451. doi: 10.23736/S0022-4707.22.13829-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the manuscript.