Abstract
Neuropeptides are abundant and essential signaling molecules in the nervous system involved in modulating neural circuits and behavior. Neuropeptides are generally released extrasynaptically and signal via volume transmission through G-protein coupled receptors (GPCR). Although substantive functional roles of neuropeptides have been discovered, many questions on neuropeptide transmission remain poorly understood, including the local diffusion and transmission properties in the brain extracellular space. To address this challenge, intensive efforts are required to develop advanced tools for releasing and detecting neuropeptides with high spatiotemporal resolution. Owing to the rapid development of biosensors and materials science, emerging tools are beginning to provide a better understanding of neuropeptide transmission. In this perspective, we summarize the fundamental advances in understanding neuropeptide transmission over the past decade, highlight the tools for releasing neuropeptides with high spatiotemporal solution in the brain, and discuss open questions and future directions in the field.
Keywords: neuropeptide transmission, neuropeptide release, neuropeptide sensor, light, brain
What we know and don’t know about neuropeptide volume transmission
Neuropeptides are a diverse class of endogenous molecules that are synthesized, stored, and secreted by neurons in the central and peripheral nervous systems.1 They have attracted great interest over the years owing to their unique structure and function in a wide range of physiological processes.2 Neuropeptides are usually packaged into dense-core vesicles and can be released in non-specialized synaptic sites.3 In contrast to small molecule transmitters such as glutamate or GABA, neuropeptides can diffuse over a long distance to act far from the release site. This widespread mode of intercellular communication is referred to as volume transmission.1, 4 Although over 100 neuropeptides have been discovered and probably more would be identified from genomic data,5 many fundamental questions remain. For example, what is the release pattern and how far does a particular neuropeptide act relative to its release site? What constraints limit the spread of neuropeptides?
In a pioneering work nearly ten years ago, Banghart and Sabatini measured the spatial profile of enkephalinergic volume transmission in acute brain slices.6 They found that enkephalin (LE) could diffuse as far as 150 μm to activate the opioid receptor in rat locus coeruleus of acute slices. In other words, the enkephalin signal spread rapidly through approximately 70,000 μm2 of tissue, which is approximately 200-fold larger than the area of release. Recently, Xiong et al. used new optical tools and cell-based sensors to determine the spatiotemporal scale of somatostatin-14 (SST) volume transmission in the mouse cortex in vivo.7 They revealed reduced but synchronized SST transmission within 130 μm, and delayed, reduced transmission at longer distances. The maximal diffusion distance of SST to activate the receptor was approximately 220 μm. Note that similar diffusion distance constraints were observed for LE and SST; however, the onset (0.25-1 s for 0-150 μm) of the evoked currents for LE diffusion is much shorter than the time of peak response (5-20 s for 0-150 μm) of cell-based sensors for SST diffusion. This difference in kinetics could be due to the different detection methods (electrophysiology vs downstream Ca2+ imaging) and the different preparations (slices vs in vivo). These measurements provide new insights into neuropeptide volume transmission in the brain of living animals.
Unlike primary neurotransmitters that are actively recycled, neuropeptides are released and signal until they are degraded. What are the factors limit that the diffusion of neuropeptides in the brain extracellular space? First, it is well known that neuropeptides are subject to degradation by peptidases. However, the degradation rate of neuropeptides in the brain extracellular space is largely unknown. To date, based on radioimmunoassays, the half-lives of oxytocin and vasopressin in cerebrospinal fluid is around 20 min.8 Banghart and Sabatini measured somatic currents evoked by uncaging LE in acute slices of rat locus coeruleus and found that peptidases limit the peptide signaling released in large volumes (>70 μm), while diffusion is dominant in limiting the spread in smaller release sites.6 Xiong et al. compared the distance-dependent SST signaling measurements in mouse cortex with a theoretical point-source diffusion model and estimated the loss rate of SST due to peptide degradation and binding in the range of 0.023 s−1–0.048 s−1. Although some progress has been made, additional studies are needed to better elucidate the effect of peptidase degradation on neuropeptide transmission. Second, the extracellular space (ECS) and extracellular matrix (ECM) can hinder extrasynaptic molecular diffusion,9, 10 and play an important role in neuropeptide transmission. For example, brain tissue with a chemically-degraded extracellular matrix provides less hindrance to peptide transmission.7 However, there are many factors including the charge and size of the peptide that can lead to very different transmission profiles.
Tools for controlled release of neuropeptide in vivo
Despite its importance in brain function, very few measurements of peptide release are available due to the lack of tools. Rough estimates suggest that each dense core vesicle contains ~104 peptides, and hundreds of vesicles are released per neuron over seconds (103 vesicles per second in hypothalamic neurons).5, 11 This suggests that a neuron releases ~106 peptide molecules per second upon stimulus, possibly higher in the hypothalamic neurons (107 molecules per second).
Tools to control the timing and spatial release of neuropeptides in vivo are needed to investigate the transmission and function of neuropeptides. The optogenetic approach is an elegant method to control the neurotransmitter release, such as acetylcholine,12 serotonin,13 and dopamine,14, 15 with millisecond precision and cell type-specific resolution. Dao et al. successfully induced and inhibited the SST release from SST-positive neurons in acute slices using optogenetics as validated by an enzyme-linked immunosorbent assay.16 Interestingly and importantly, Al-Hasani et al. measured the endogenous opioid peptide release (absolute concentration in dialysate: 0.13-8.69 pM) in freely moving rodents with a customized optogenetic-microdialysis probe.17 They controlled cell-type selective opioid release in different brain regions and detected several opioids such as dynorphin A1-8 and LE (leu- and met-). The new approach moves the field forward; however, one complication to optogenetic-driven neuropeptide release is the co-release of other small molecule transmitters (such as dopamine, GABA and glutamate),18, 19 which requires more specific detection of neuropeptides in real-time to measure the diffusion.
Controlled release or uncaging of exogenously supplied neuropeptide has the advantage of releasing a certain amount of a specific neuropeptide. A widely used method of using light to release specific neurotransmitters is to ‘cage’ the neurotransmitter with a photo-cleavable group. Caged compounds, such as caged glutamate or GABA,20, 21 have been used to study cell signaling or physiology under one-photon or two-photon stimulation. Banghart and Sabatini measured the LE transmission using the caged-LE (CYLE) modified with carboxynitrobenzyl (CNB) chromophore, which responds to UV light illumination (Figure 1A).6 The binding affinity of CYLE to the delta and mu receptors was decreased by 100- to 500-fold with respect to LE, while CYLE enabled rapid (onset of response: ~350 ms) and robust delivery of LE under photolysis. Taking advantage of the high spatial resolution (2 μm light spot), the photoactivatable opioid peptide provides a useful tool to investigate the spatiotemporal dynamics of peptidergic signaling. Later, the same group synthesized a new caged analog of LE (N-MNVOC-LE) to reduce the residual activity and demonstrated the feasibility in brain slices of rat locus coeruleus.22 These can be extended to other caged analogs, including dynorphin,6 gastrin-releasing peptide, oxytocin,23 cholecystokinin, and substance P. However, caged peptides have some limitations. First, each caged compound requires a separate optimization process to ensure biological inertness, solubility in physiological pH, resistance to aqueous hydrolysis, fast uncaging speed, and high uncaging efficiency.21 For example, it is difficult to control the position and number of protecting groups since peptides may contain multiple reactive groups.24 Second, caged compounds offer good spatial and temporal control but may be limited by one-photon uncaging of ortho-nitrobenzyl photolabile protecting groups with UV or blue light.25 Furthermore, caged peptides are also subject to peptidase degradation which can limit their in vivo application.6 Thus, other approaches to uncage or photo-release neuropeptides are needed to better understand their transient and localized effects in the nervous system.
Figure 1.
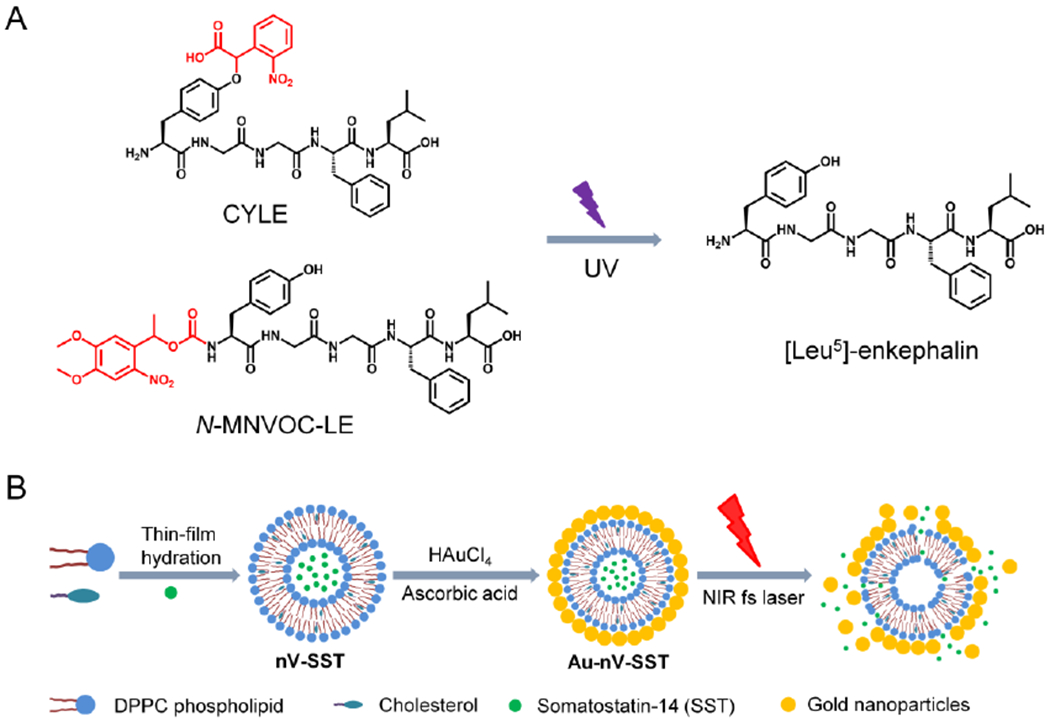
(A) Chemical structures change of caged-peptides after UV light irradiation. CYLE: carboxynitrobenzyl modified [Leu5]-enkephalin; N-MNVOC-LE N-(α-methyl-6-nitroveratryloxycarbonyl) modified [Leu5]-enkephalin. The caging groups are indicated in red. (B) Schematic of preparation of somatostatin-encapsulated photosensitive nanovesicles (Au-nV-SST) and photorelease by the near-infrared laser pulses. The illustration of Au-nV-SST was adapted with permission from Ref [7].
An alternative approach to chemical caging is to encapsulate neuropeptides in the photosensitive nanovesicles (Au-nV, Figure 1B). These are 100~200 nanometer-sized structures that consist of a natural phospholipid membrane that surrounds an aqueous core. To control the release of neuropeptides, nanovesicles are coated with small gold particles (3–5 nm),26 which enables near-infrared light-triggered photo-release. Ultrashort laser pulses (picosecond or femtosecond) can activate gold nanoparticles to generate nanoscale cavitation bubbles that are effective to burst the vesicle, releasing the encapsulated neuropeptides.26, 27 Physiological concentrations of SST (~100 nM, 1.2×108) could be photoreleased from nanovesicles at a depth of 200 μm in the mouse cortex,7 which is close to the level of endogenous released peptides from ~104 dense core vesicles or 10 neurons per second (~104 peptides per vesicle, ~103 vesicles per neuron).5 The photosensitive nanovesicles (Au-nV) have several features that are complementary to current methodologies for neuropeptide release. First, nanovesicles allow in vivo measurement by protecting peptides from rapid enzymatic degradation. Second, the in vivo optical stimulation provides a high spatial (μm, or fL volume) and temporal (sub-second) resolution to control neuropeptide release by the laser power and duration using a two-photon microscope. Furthermore, our recent work on photoswtichable nanovesicles demonstrated that it is possible to switch on and off the photorelease so providing the opportunity to consecutively release the molecules over several cycles.28 Third, photo-stimulation of nanovesicles releases a bolus of a specific neuropeptide instead of a mixture with co-released transmitters. Furthermore, near-infrared light is more accessible for in vivo studies due to the deep tissue penetration and reduced photo-damage29 and the photosensitive liposomes are suitable to package a wide range of neuropeptides.30 However, since the Au-nV are much larger than the narrow width of the brain extracellular space (40-60 nm),31 large Au-nV have limited penetration or diffusion in the brain. The development of small and brain-penetrating nanovesicles could allow investigation of neuropeptide transmission across a large brain region with a single minimally invasive injection.32
Integrating neuropeptide release with neuropeptide sensing
Integrating neuropeptide release with neuropeptide monitoring would provide a powerful set of tools to study neuropeptide transmission. Monitoring the neurotransmitters or neuropeptides by optical approaches is appealing to neuroscientists due to the high spatiotemporal resolution compared to analytic chemical methods such as fast scanning cyclic voltammetry and microdialysis.33, 34 Cell-based fluorescent sensors can detect nM concentrations of neuropeptides in vivo by the co-expression of specific GPCR and Ca2+ indicators (Figure 2A).7, 35–38 With a new SST2 CNiFERs (cell-based neurotransmitter fluorescent engineered reporter) to detect SST in vivo in real-time,7 Xiong et al. integrated the SST-encapsulated plasmonic nanovesicles and CNiFEERs (PACE) and probed the neuropeptide transmission in vivo by the synchronization of NIR stimulation and two-photon imaging. Since SST2 CNiFERs provide a proxy for G protein activation via increases in intracellular Ca2+, PACE provides new insights into neuropeptide extrasynaptic volume transmission, as it includes neuropeptide release, extracellular diffusion, GPCR binding, and intracellular downstream signaling. PACE is an excellent example of integrating neuropeptide release with neuropeptide monitoring to map neuropeptide transmission. The development of genetically encoded GPCR-based fluorescent sensors for neuropeptides provides another new tool for probing neuropeptide signaling and diffusion. Recently, several groups have reported new genetically encoded sensors to monitor the release and dynamics of dynorphin,39 orexin,40 and oxytocin41, 42 in living animals, respectively. Li’s group reported a toolkit of G protein-coupled receptor activation–based (GRAB) sensors for several neuropeptides, including SST, cholecystokinin, corticotropin-releasing factor, neuropeptide Y, neurotensin, and vasoactive intestinal peptide.43 CNiFERs with FRET-based Ca2+ indicators have a high signal-to-noise ratio in vivo, but require multiple implantations at different distances for the diffusion measurement. Genetically encoded GPCR-based fluorescent sensors are simpler to implement and may have a higher spatial resolution for measuring neuropeptide diffusion. Since genetically encoded sensors are more widely used, GPCR-based sensors for neuropeptides are under rapid development and will likely play a more important role in future diffusion measurements. With the photorelease technique and brighter and more sensitive genetically encoded GPCR-based sensors for neuropeptides (Figure 2B), the integrated approach will allow for a better understanding of the neuropeptide volume transmission in the brain at a cellular resolution.
Figure 2.
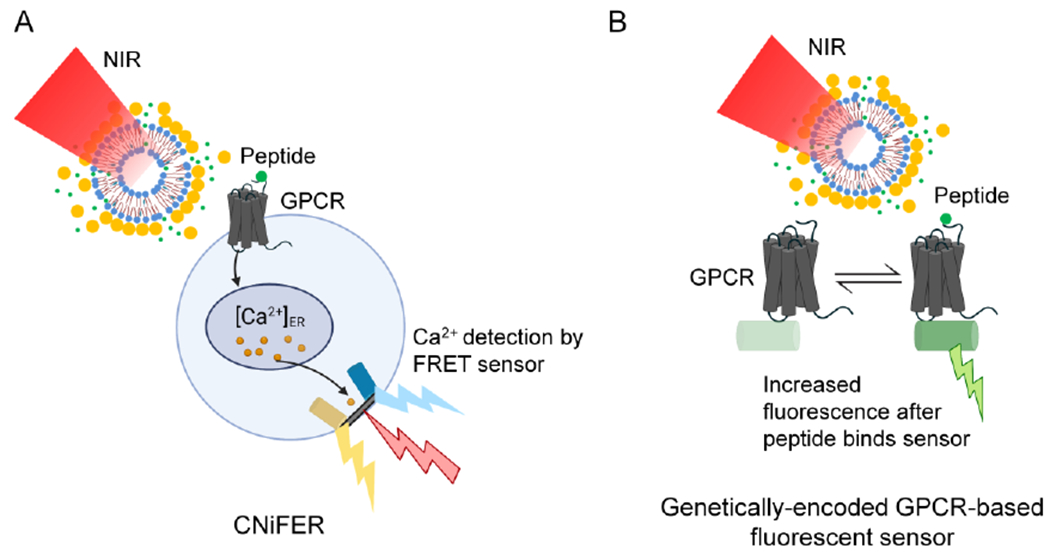
Integrating neuropeptide release and sensing to probe neuropeptide transmission. (A) Schematic of cell-based neurotransmitter fluorescent engineered reporter (CNiFERs) for neuropeptide detection. (B) Schematic of genetically encoded GPCR-based sensors for neuropeptide detection.
Open questions about neuropeptide transmission and future directions
There are several important unanswered questions about neuropeptide transmission. First, how do the diverse physicochemical properties of neuropeptides impact their transmission in the brain? Factors such as molecule size or weight,31, 44, 45 the strength of transient binding interactions,46 and charge47 can also affect diffusion in the brain extracellular space. As such, it is likely that neuropeptides’ physicochemical properties could affect how they diffuse through brain extracellular spaces. There are nearly 300 unique human neuropeptides recorded in the NeuroPep database that exhibit a broad range of physicochemical properties (Figure 3A).48 Coupled with differences in peptide structure and folding (Figure 3B), such differences in neuropeptide physicochemical properties could potentially contribute to differential diffusion and volume transmission in the brain. Currently, there are very limited data on the diffusion and transmission properties and thus requires future work in this direction. We will likely need high throughput methods to investigate these important questions.
Figure 3.
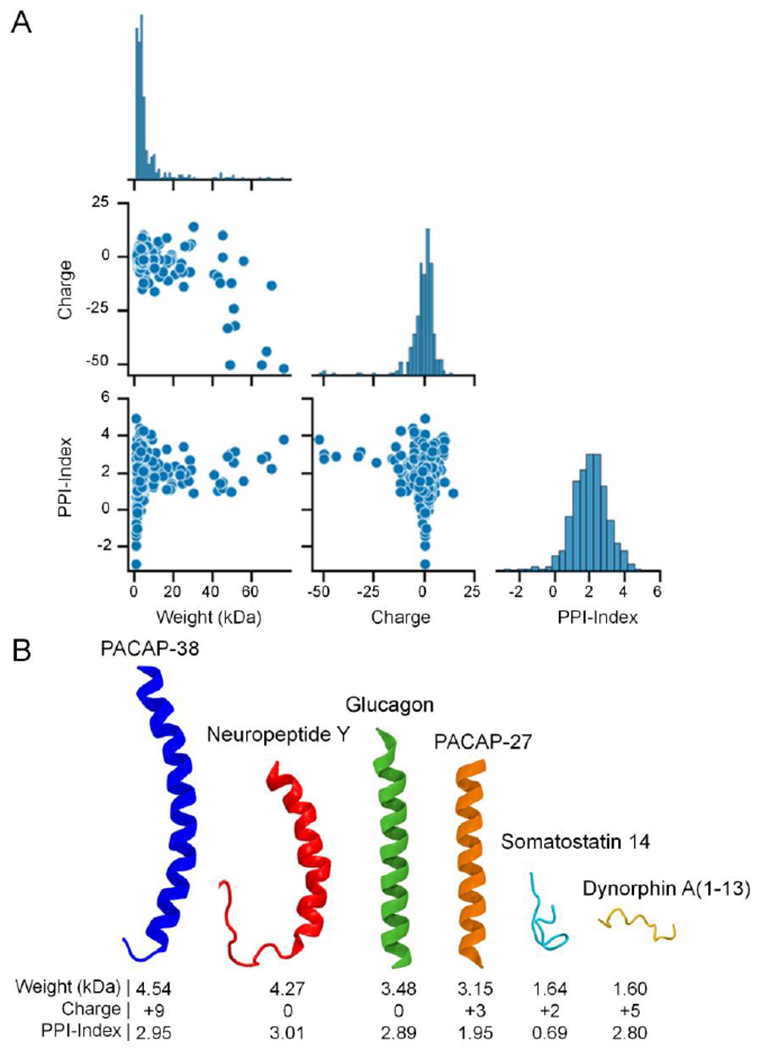
The diverse physicochemical properties of neuropeptides. (A) Pair plot comparing the molecular weight (kDa), theoretical net charge at a physiologic pH of 7.4, and the Potential Protein Interaction Index (PPI-Index), a predictor of a polypeptide’s propensity to bind other proteins/receptors,49 for all 283 human neuropeptides in the NeuroPep database.48 The properties were estimated from the peptide sequences using the peptides.py package (https://github.com/althonos/peptides.py).50 Note that the diagonal edge of the pair plot shows the distributions of each property. (B) A selection of human neuropeptide structures collected from the RCSB Protein Data Bank51 and AlphaFold Protein Structure Database52, 53 highlighting the diversity of neuropeptide structure and physicochemical properties, including the 38 amino acid variant (blue) of pituitary adenylate cyclase-activating peptide (PACAP), Neuropeptide Y (red), glucagon (green), the 27 amino acid variant of PACAP (orange), Somatostatin 14 (light blue), and Dynorphin A (1-13) (yellow). The neuropeptide structures were rendered using the Visual Molecular Dynamics (VMD) software.54
Second, how different is the transmission across the brain regions? Both electron microscopy of chemically fixed tissue and the super-resolution imaging of living brain slices demonstrate that ECS is diverse and heterogeneous.55–57 The measurements from cation tetramethylammonium (TMA+) diffusion directly revealed that the tortuosity of ECS is heterogenous in different brains.9 For example, the cerebellum exhibits significant heterogeneity between the molecular layer and granule cell layer, and both the tortuosity and volume fraction are different in the two layers.58 There is also increasing evidence that diffusion is anisotropic in several regions. For instance, TMA+ diffuses more readily along an axon bundle than across it, as observed in the myelinated corpus callosum.59 The anisotropic diffusion in the brain has also been confirmed by magnetic resonance imaging (MRI).60, 61 Furthermore, the neuropeptide GPCR expression levels are anticipated to differ significantly across brain regions.62, 63 Therefore, neuropeptide transmission across different brain regions is expected to be heterogeneous but has yet to be experimentally confirmed. Future work could explore this aspect of neuropeptide transmission and determine how it impacts the function of different brain regions.
Lastly, how is neuropeptide transmission under different brain states? It has been reported that sleep induces an increase of ECS volume fraction and drives metabolite clearance in mice and humans.64–66 MRI of healthy adult brains also provides evidence for the reduction in ECS volume of large parts of human white matter after wakefulness.67 Therefore, it is reasonable to hypothesize that the sleep/wakefulness state modulates the extracellular diffusion of neuropeptides and their actions in brain circuits. The diffusion properties in pathological brain states are also of high interest since they can serve as an indicator of pathological processes and perhaps offer insights into their underlying mechanisms. Several studies have shown that the diffusion coefficients of TMA+ vary in brain diseases such as ischemia, spreading depression, and Alzheimer’s disease.9 Changes in the ECM and ECS in these diseased states might affect neuropeptide transmission.68–70
To address these questions, we need to build a database for the parameters of neuropeptide transmission in the brain. First and foremost, the toolkit to control the timing and spatial release of neuropeptides and in vivo sensors to monitor neuropeptides in real-time needs to be expanded. With the rapid development of optogenetics, the photo-stimulation of the endogenous genetically encoded neurons is more widely used by neuroscientists to investigate the release and dynamics of neuropeptides. However, co-transmission has been inescapable until now. the caged compound or photosensitive nanovesicles focuses on the transient release of neuropeptides (sub-second), while endogenous neuropeptide release in the physiological conditions could last longer. The photoswitchable release from liposomes with azobenzene-containing phosphatidylcholine shows promise for controlling the neuropeptide release in seconds.28 We anticipate that integration of these new opto-chemical tools with newly developed genetically encoded neuropeptide sensors (e.g., Light, GRAB) will significantly advance our understanding of neuropeptide signaling in the brain.
Acknowledgments
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH) under Award Number RF1NS110499 (Z.Q., P.A.S.) and National Science Foundation under award number 2123971 (Z.Q., P.A.S.), National Institute of Mental Health of NIH under award number R01MH111499 (P.A.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Competing interests
The authors declare that they have no competing financial interests.
References
- (1).van den Pol AN Neuropeptide Transmission in Brain Circuits. Neuron 2012, 76 (1), 98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Guillaumin MCC; Burdakov D, Neuropeptides as Primary Mediators of Brain Circuit Connectivity. Front Neurosci 2021, 15 (229). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ding K; Han Y; Seid TW; Buser C; Karigo T; Zhang S; Dickman DK; Anderson DJ Imaging Neuropeptide Release at Synapses with a Genetically Engineered Reporter. Elife 2019, 8, e46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Katherine HT; Robin AH Volume Transmission in the Brain: Beyond the Synapse. J. Neuropsychiatry Clin. Neurosci 2014, 26 (1), iv–4. [DOI] [PubMed] [Google Scholar]
- (5).Russo AF Overview of Neuropeptides: Awakening the Senses? Headache 2017, 57 Suppl 2 (Suppl 2), 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Banghart MR; Sabatini BL Photoactivatable Neuropeptides for Spatiotemporally Precise Delivery of Opioids in Neural Tissue. Neuron 2012, 73 (2), 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Xiong H; Lacin E; Ouyang H; Naik A; Xu X; Xie C; Youn J; Wilson BA; Kumar K; Kern T; Aisenberg E; Kircher D; Li X; Zasadzinski JA; Mateo C; Kleinfeld D; Hrabetova S; Slesinger PA; Qin Z Probing Neuropeptide Volume Transmission In Vivo by Simultaneous Near-Infrared Light-Triggered Release and Optical Sensing. Angew. Chem. Int. Ed 2022, 61 (34), e202206122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Mens WB; Witter A; Greidanus TBVW Penetration of Neurohypophyseal Hormones from Plasma into Cerebrospinal Fluid (CSF): Half-times of Disappearance of these Neuropeptides from CSF. Brain Res. 1983, 262 (1), 143–149. [DOI] [PubMed] [Google Scholar]
- (9).Syková E; Nicholson C Diffusion in Brain Extracellular Space. Physiol. Rev 2008, 88 (4), 1277–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Nicholson C; Hrabětová S Brain Extracellular Space: The Final Frontier of Neuroscience. Biophys. J 2017, 113 (10), 2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ludwig M; Leng G Dendritic Peptide Release and Peptide-dependent Behaviours. Nat. Rev. Neurosci 2006, 7 (2), 126–136. [DOI] [PubMed] [Google Scholar]
- (12).Venkatesan S; Lambe EK Chrna5 is Essential for a Rapid and Protected Response to Optogenetic Release of Endogenous Acetylcholine in Prefrontal Cortex. J. Neurosci 2020, 40 (38), 7255–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Xiao N; Privman E; Venton BJ Optogenetic Control of Serotonin and Dopamine Release in Drosophila Larvae. ACS Chem. Neurosci 2014, 5 (8), 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bass CE; Grinevich VP; Vance ZB; Sullivan RP; Bonin KD; Budygin EA Optogenetic Control of Striatal Dopamine Release in Rats. J. Neurochem 2010, 114 (5), 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lu Y; Driscoll N; Ozden I; Yu Z; Nurmikko A Modulating Dopamine Release by Optogenetics in Transgenic Mice Reveals Terminal Dopaminergic Dynamics. Neurophotonics 2015, 2 (3), 031207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Dao NC; Brockway DF; Crowley NA In Vitro Optogenetic Characterization of Neuropeptide Release from Prefrontal Cortical Somatostatin Neurons. Neuroscience 2019, 419, 1–4. [DOI] [PubMed] [Google Scholar]
- (17).Al-Hasani R; Wong J-MT; Mabrouk OS; McCall JG; Schmitz GP; Porter-Stransky KA; Aragona BJ; Kennedy RT; Bruchas MR In Vivo Detection of Optically-evoked Opioid Peptide Release. eLife 2018, 7, e36520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Nusbaum MP; Blitz DM; Marder E Functional Consequences of Neuropeptide and Small-Molecule Co-transmission. Nat. Rev. Neurosci 2017, 18 (7), 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Apergis-Schoute J; Burnstock G; Nusbaum MP; Parker D; Morales MA; Trudeau L-E; Svensson E Editorial: Neuronal Co-transmission. Front. Neural Circuits 2019, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ellis-Davies GCR Caged Compounds: Photorelease Technology for Control of Cellular Chemistry and Physiology. Nat. Methods 2007, 4 (8), 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ellis-Davies GCR Useful Caged Compounds for Cell Physiology. Acc. Chem. Res 2020, 53 (8), 1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Banghart MR; He XJ; Sabatini BL A Caged Enkephalin Optimized for Simultaneously Probing Mu and Delta Opioid Receptors. ACS Chem. Neurosci 2018, 9 (4), 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ahmed I; Liu J; Gieniec K; Bair-Marshall C; Adewakun A; Hetzler B; Arp C; Khatri L; Vanwalleghem G; Seidenberg A Optopharmacological Tools For Precise Spatiotemporal Control of Oxytocin Signaling In the Central Nervous System And Periphery. bioRxiv 2022, 10.1101/2022.11.10.516001. [DOI] [Google Scholar]
- (24).Shigeri Y; Tatsu Y; Yumoto N Synthesis and Application of Caged Peptides and Proteins. Pharmacol. Ther 2001, 91 (2), 85–92. [DOI] [PubMed] [Google Scholar]
- (25).Hansen MJ; Velema WA; Lerch MM; Szymanski W; Feringa BL Wavelength-selective Cleavage of Photoprotecting Groups: Strategies And Applications In Dynamic Systems. Chem. Soc. Rev 2015, 44 (11), 3358–3377. [DOI] [PubMed] [Google Scholar]
- (26).Li X; Che Z; Mazhar K; Price TJ; Qin Z Ultrafast Near-Infrared Light-Triggered Intracellular Uncaging to Probe Cell Signaling. Adv. Funct. Mater 2017, 27 (11), 1605778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Xiong H; Li X; Kang P; Perish J; Neuhaus F; Ploski JE; Kroener S; Ogunyankin MO; Shin JE; Zasadzinski JA; Wang H; Slesinger PA; Zumbuehl A; Qin Z Near-Infrared Light Triggered-Release in Deep Brain Regions Using Ultra-photosensitive Nanovesicles. Angew. Chem. Int. Ed 2020, 59 (22), 8608–8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Xiong H; Alberto KA; Youn J; Taura J; Morstein J; Li X; Wang Y; Trauner D; Slesinger PA; Nielsen SO; Qin Z Optical Control of Neuronal Activities With Photoswitchable Nanovesicles. Nano Res. 2023, 16 (1), 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Yaroslavsky A; Schulze P; Yaroslavsky I; Schober R; Ulrich F; Schwarzmaier H Optical Properties of Selected Native and Coagulated Human Brain Tissues In Vitro In the Visible and Near Infrared Spectral Range. Phys. Med. Biol 2002, 47 (12), 2059. [DOI] [PubMed] [Google Scholar]
- (30).Niu Z; Conejos-Sánchez I; Griffin BT; O’Driscoll CM; Alonso MJ Lipid-based Nanocarriers for Oral Peptide Delivery. Adv. Drug Deliv. Rev 2016, 106, 337–354. [DOI] [PubMed] [Google Scholar]
- (31).Thorne RG; Nicholson C In Vivo Diffusion Analysis with Quantum Dots and Dextrans Predicts the Width of Brain Extracellular Space. Proc. Natl. Acad. Sci 2006, 103 (14), 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Nance EA; Woodworth GF; Sailor KA; Shih T-Y; Xu Q; Swaminathan G; Xiang D; Eberhart C; Hanes J A Dense Poly (ethylene glycol) Coating Improves Penetration of Large Polymeric Nanoparticles within Brain Tissue. Sci. Transl. Med 2012, 4 (149), 149ra119–149ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Sabatini BL; Tian L Imaging Neurotransmitter and Neuromodulator Dynamics In Vivo with Genetically Encoded Indicators. Neuron 2020, 108 (1), 17–32. [DOI] [PubMed] [Google Scholar]
- (34).Wu Z; Lin D; Li Y Pushing the Frontiers: Tools for Monitoring Neurotransmitters and Neuromodulators. Nat. Rev. Neurosci 2022, 23, 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Soria-Gomez E; Pagano Zottola AC; Mariani Y; Desprez T; Barresi M; Bonilla-del Río I; Muguruza C; Le Bon-Jego M; Julio-Kalajzić F; Flynn R; Terral G; Fernández-Moncada I; Robin LM; Oliveira da Cruz JF; Corinti S; Amer YO; Goncalves J; Varilh M; Cannich A; Redon B; Zhao Z; Lesté-Lasserre T; Vincent P; Tolentino-Cortes T; Busquets-García A; Puente N; Bains JS; Hebert-Chatelain E; Barreda-Gómez G; Chaouloff F; Lohman AW; Callado LF; Grandes P; Baufreton J; Marsicano G; Bellocchio L Subcellular Specificity of Cannabinoid Effects In Striatonigral Circuits. Neuron 2021, 109 (9), 1513–1526.e11. [DOI] [PubMed] [Google Scholar]
- (36).Zaelzer C; Gizowski C; Salmon CK; Murai KK; Bourque CW Detection of Activity-Dependent Vasopressin Release from Neuronal Dendrites and Axon Terminals Using Sniffer Cells. J. Neurophysiol 2018, 120 (3), 1386–1396. [DOI] [PubMed] [Google Scholar]
- (37).Gizowski C; Zaelzer C; Bourque CW Clock-Driven Vasopressin Neurotransmission Mediates Anticipatory Thirst Prior to Sleep. Nature 2016, 537 (7622), 685–688. [DOI] [PubMed] [Google Scholar]
- (38).Piñol RA; Jameson H; Popratiloff A; Lee NH; Mendelowitz D Visualization of Oxytocin Release that Mediates Paired Pulse Facilitation In Hypothalamic Pathways to Brainstem Autonomic Neurons. PloS one 2014, 9 (11), e112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Abraham AD; Casello SM; Schattauer SS; Wong BA; Mizuno GO; Mahe K; Tian L; Land BB; Chavkin C Release of Endogenous Dynorphin Opioids In the Prefrontal Cortex Disrupts Cognition. Neuropsychopharmacology 2021, 46 (13), 2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Duffet L; Kosar S; Panniello M; Viberti B; Bracey E; Zych AD; Radoux-Mergault A; Zhou X; Dernic J; Ravotto L; Tsai Y-C; Figueiredo M; Tyagarajan SK; Weber B; Stoeber M; Gogolla N; Schmidt MH; Adamantidis AR; Fellin T; Burdakov D; Patriarchi T A Genetically Encoded Sensor for In Vivo Imaging of Orexin Neuropeptides. Nat. Methods 2022, 19 (2), 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Ino D; Tanaka Y; Hibino H; Nishiyama M A Fluorescent Sensor for Real-time Measurement of Extracellular Oxytocin Dynamics In the Brain. Nat. Methods 2022, 19 (10), 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Qian T; Wang H; Wang P; Geng L; Mei L; Osakada T; Wang L; Tang Y; Kania A; Grinevich V; Stoop R; Lin D; Luo M; Li Y A Genetically Encoded Sensor Measures Temporal Oxytocin Release From Different Neuronal Compartments. Nat. Biotechnol 2023, 10.1038/s41587-022-01561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Wang H; Qian T; Zhao Y; Zhuo Y; Wu C; Osakada T; Chen P; Ren H; Yan Y; Geng L; Fu S; Mei L; Li G; Wu L; Jiang Y; Qian W; Peng W; Xu M; Hu J; Chen L; Tang C; Lin D; Zhou J-N; Li Y A Toolkit of Highly Selective And Sensitive Genetically Encoded Neuropeptide Sensors. bioRxiv 2022, 10.1101/2022.03.26.485911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Xiao F; Nicholson C; Hrabe J; Hrabĕtová S Diffusion of Flexible Random-Coil Dextran Polymers Measured in Anisotropic Brain Extracellular Space by Integrative Optical Imaging. Biophys. J 2008, 95 (3), 1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Nicholson C; Tao L Hindered Diffusion of High Molecular Weight Compounds in Brain Extracellular Microenvironment Measured with Integrative Optical Imaging. Biophys. J 1993, 65 (6), 2277–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Thorne RG; Lakkaraju A; Rodriguez-Boulan E; Nicholson C In Vivo Diffusion of Lactoferrin In Brain Extracellular Space Is Regulated by Interactions with Heparan Sulfate. Proc. Natl. Acad. Sci 2008, 105 (24), 8416–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Hrabětová S; Masri D; Tao L; Xiao F; Nicholson C Calcium Diffusion Enhanced After Cleavage of Negatively Charged Components of Brain Extracellular Matrix by Chondroitinase ABC. J. Physiol 2009, 587 (16), 4029–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Wang Y; Wang M; Yin S; Jang R; Wang J; Xue Z; Xu T NeuroPep: A Comprehensive Resource of Neuropeptides. Database 2015, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Boman HG Antibacterial Peptides: Basic Facts and Emerging Concepts. J. Intern. Med 2003, 254 (3), 197–215. [DOI] [PubMed] [Google Scholar]
- (50).Larralde M, “peptides.py: Physicochemical properties, indices and descriptors for amino-acid sequences” (v0.2.1), https://pypi.org/project/peptides/0.2.1, 2022. [Google Scholar]
- (51).Berman HM; Westbrook J; Feng Z; Gilliland G; Bhat TN; Weissig H; Shindyalov IN; Bourne PE The protein data bank. Nucleic Acids Res. 2000, 28 (1), 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Jumper J; Evans R; Pritzel A; Green T; Figurnov M; Ronneberger O; Tunyasuvunakool K; Bates R; Žídek A; Potapenko A; Bridgland A; Meyer C; Kohl SAA; Ballard AJ; Cowie A; Romera-Paredes B; Nikolov S; Jain R; Adler J; Back T; Petersen S; Reiman D; Clancy E; Zielinski M; Steinegger M; Pacholska M; Berghammer T; Bodenstein S; Silver D; Vinyals O; Senior AW; Kavukcuoglu K; Kohli P; Hassabis D Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596 (7873), 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Varadi M; Anyango S; Deshpande M; Nair S; Natassia C; Yordanova G; Yuan D; Stroe O; Wood G; Laydon A; Žídek A; Green T; Tunyasuvunakool K; Petersen S; Jumper J; Clancy E; Green R; Vora A; Lutfi M; Figurnov M; Cowie A; Hobbs N; Kohli P; Kleywegt G; Birney E; Hassabis D; Velankar S AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-sequence Space with High-accuracy Models. Nucleic Acids Res. 2021, 50 (D1), D439–D444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Humphrey W; Dalke A; Schulten K VMD: Visual Molecular Dynamics. J. Mol. Graph 1996, 14 (1), 33–38. [DOI] [PubMed] [Google Scholar]
- (55).Korogod N; Petersen CCH; Knott GW Ultrastructural Analysis of Adult Mouse Neocortex Comparing Aldehyde Perfusion with Cryo Fixation. eLife 2015, 4, e05793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Godin AG; Varela JA; Gao Z; Danné N; Dupuis JP; Lounis B; Groc L; Cognet L Single-nanotube Tracking Reveals the Nanoscale Organization of the Extracellular Space In the Live Brain. Nat. Nanotechnol 2017, 12 (3), 238–243. [DOI] [PubMed] [Google Scholar]
- (57).Tønnesen J; Inavalli VVGK; Nägerl UV Super-Resolution Imaging of the Extracellular Space in Living Brain Tissue. Cell 2018, 172 (5), 1108–1121.e15. [DOI] [PubMed] [Google Scholar]
- (58).Rice ME; Okada YC; Nicholson C Anisotropic and Heterogeneous Diffusion in the Turtle Cerebellum: Implications for Volume Transmission. J. Neurophysiol 1993, 70 (5), 2035–2044. [DOI] [PubMed] [Google Scholar]
- (59).Voříšek I; Syková E Evolution of Anisotropic Diffusion in the Developing Rat Corpus Callosum. J. Neurophysiol 1997, 78 (2), 912–919. [DOI] [PubMed] [Google Scholar]
- (60).Soria FN; Miguelez C; Peñagarikano O; Tønnesen J Current Techniques for Investigating the Brain Extracellular Space. Front Neurosci 2020, 14, 570750–570750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Shimony JS; McKinstry RC; Akbudak E; Aronovitz JA; Snyder AZ; Lori NF; Cull TS; Conturo TE Quantitative Diffusion-tensor Anisotropy Brain MR Imaging: Normative Human Data and Anatomic Analysis. Radiology 1999, 212 (3), 770–784. [DOI] [PubMed] [Google Scholar]
- (62).Smith SJ; Sümbül U; Graybuck LT; Collman F; Seshamani S; Gala R; Gliko O; Elabbady L; Miller JA; Bakken TE Single-cell Transcriptomic Evidence for Dense Intracortical Neuropeptide Networks. Elife 2019, 8, e47889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Zhong W; Barde S; Mitsios N; Adori C; Oksvold P; Feilitzen KV; O’Leary L; Csiba L; Hortobágyi T; Szocsics P; Mechawar N; Maglóczky Z; Renner É; Palkovits M; Uhlén M; Mulder J; Hökfelt T The Neuropeptide Landscape of Human Prefrontal Cortex. Proc. Natl. Acad. Sci 2022, 119 (33), e2123146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Ding F; O’donnell J; Xu Q; Kang N; Goldman N; Nedergaard M Changes In the Composition of Brain Interstitial Ions Control the Sleep-Wake Cycle. Science 2016, 352 (6285), 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Xie L; Kang H; Xu Q; Chen MJ; Liao Y; Thiyagarajan M; O’Donnell J; Christensen DJ; Nicholson C; Iliff JJ Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342 (6156), 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Fultz NE; Bonmassar G; Setsompop K; Stickgold RA; Rosen BR; Polimeni JR; Lewis LD Coupled Electrophysiological, Hemodynamic, and Cerebrospinal Fluid Oscillations in Human Sleep. Science 2019, 366 (6465), 628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Voldsbekk I; Maximov II; Zak N; Roelfs D; Geier O; Due-Tønnessen P; Elvsåshagen T; Strømstad M; Bjørnerud A; Groote I Evidence for Wakefulness-related Changes to Extracellular Space In Human Brain White Matter from Diffusion-weighted MRI. NeuroImage 2020, 212, 116682. [DOI] [PubMed] [Google Scholar]
- (68).Lau LW; Cua R; Keough MB; Haylock-Jacobs S; Yong VW Pathophysiology of the Brain Extracellular Matrix: A New Target for Remyelination. Nat. Rev. Neurosci 2013, 14 (10), 722–729. [DOI] [PubMed] [Google Scholar]
- (69).Winkler J; Abisoye-Ogunniyan A; Metcalf KJ; Werb Z Concepts of Extracellular Matrix Remodelling In Tumour Progression and Metastasis. Nat. Commun 2020, 11 (1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Soria FN; Paviolo C; Doudnikoff E; Arotcarena M-L; Lee A; Danné N; Mandal AK; Gosset P; Dehay B; Groc L; Cognet L; Bezard E Synucleinopathy Alters Nanoscale Organization and Diffusion In the Brain Extracellular Space through Hyaluronan Remodeling. Nat. Commun 2020, 11 (1), 3440. [DOI] [PMC free article] [PubMed] [Google Scholar]


