Abstract
Limitations of dairy products, such as lactose intolerance, problems related to a high cholesterol intake in diet, malabsorption, and the requirement for cold storage facilities, as well as an increasing demand for new foods and tastes, have initiated a trend in the development of non-dairy probiotic products. The possibility of producing beverages based on soy milk, sea buckthorn powder, and fermented by Bifidobacterium bifidus (Bb-12®, Bb) strain at different temperatures (30 °C and 37 °C) was examined. Strain viability, pH, and titratable acidity were measured during the fermentation period while the viability, pH, titratable acidity, and water holding capacity were determined during the storage time at 4 °C ± 1 °C within 14 days. Additionally, the survival and stability of Bb-12®, inoculated into a functional beverage when exposed to simulated gastrointestinal tract conditions, were assessed. The results obtained in this study revealed that the content of potent bioactive compounds in fermented soy milk and sea buckthorn powder depends on the processing conditions, the bacteria used in the fermentation step, and storage time.
Keywords: health, probiotic, Bifidobacterium bifidus, beverage, soy milk, sea buckthorn powder
1. Introduction
Currently, functional foods are the most promising in the field of nutrition. These foods are considered interesting from the consumer’s point of view for health and disease prevention because they use natural foods as part of a regular diet. The explanation for the increased interest in functional foods lies in the fact that these foods contain components with multiple health benefits, such as vitamins and minerals, prebiotics, and probiotics [1]. For centuries, traditionally, fermented foods and beverages have been produced in regions of Europe, Asia, America, and Africa using locally available raw materials [2]. One of the largest manufacturing sectors is the beverage industry, contributing to local economies in terms of providing value-added products but also a large workforce. Exports of food and beverages have doubled in European countries, obtaining receipts of more than EUR 90 billion, but they have also contributed to a balance of almost EUR 30 billion [3]. Fermented drinks and foods are obtained by spontaneous fermentation or by adding starter cultures or probiotics.
One of the great challenges of the food industry is the development of vegan products with improved nutritional, functional, and sensory characteristics, because in 2023, worldwide, the number of vegans will be approximately 88 million, which is around 1.1% of the world’s population [4]. For this reason, the scientific community is investigating natural and sustainable ingredients for the creation of innovative foods [5]. The functional beverage market is the fastest growing segment of the functional food sector. This market was valued at USD 25 billion in 2005 [6], and in the U.S., sales of functional beverages accounted for approximately 59% of the total market in 2012 [7]; by 2025, functional beverages are expected to represent 40% of total consumer demand [8]. Due to socio-cultural and socio-demographic factors, the differences between consumers and the acceptance of functional products have made the trends of the global market of functional drinks heterogeneous, and growth and development to be at different rates within and across countries [9].
A safe and cheap method of increasing the shelf life, nutritional, and organoleptic qualities of food is lactic acid fermentation [10]. The addition of active bacterial cultures can be considered a high-quality marker for functional foods. It is currently recognized that the consumption of probiotics has beneficial effects on the prevention of several diseases [11], and, in this context, many studies have demonstrated that probiotics can reduce blood pressure and hypertension [12], reduce symptoms associated with gastrointestinal disorders [13], help manage diabetes [14], and improve blood cholesterol levels [15], especially as metabolic syndrome associates obesity, dyslipidemia, hypertension, hyperglycemia, and the future risk of developing diabetes, cardiovascular complications, and stroke [16]. Likewise, probiotics reduce the risk of degenerative diseases [17], but they also prevent various allergies, and single-microbial-strain or multi-strain probiotics can serve to boost immunity against COVID-19 [18]. However, the minimum viable cell concentration, during consumption, for a probiotic food product has been estimated to be approximately 106–107 CFU/mL [19]. Typically, in the production of probiotic juices of non-lactic origin, the probiotics used are Lactobacillus and Bifidobacteria (L. casei, L. paracasei, L. johnsonii, Lactobacillus acidophilus, L. helveticus, L. reuteri, L. delbrueckii subsp. bulgaricus, L. rhamnosus, B. bifidum, B. breve, B. lactis, B. longum) but also other species, such as Enterococcus faecium, Leuconostoc spp., Saccharomyces cerevisiae var. Boulardii, Weissella spp., or mixed cultures of these microorganisms because they can provide different formulations in food matrices [20].
For healthy purposes, several raw materials can be used, soy being among those with the greatest potential [21]. In recent decades, soya (Glycine max) has been considered one of the cheapest and most important agricultural commodities due to its chemical composition; it has a high protein content (about 40%) compared to other leguminous species (20–30%) and cereals (8–15%) [22]. For this reason, interest in plant-based proteins is increasing, with them being used as substitutes for animal proteins [23], but also for their potential health benefits [24]. Nedele et al., 2021 [24] stated that soy-based products that include soy drink are considered a suitable substitute for milk, especially for vegans, people with allergies to milk proteins, and people with lactose intolerance. A good substrate for the growth of lactic acid bacteria, and especially Bifidobacteria, is soy milk [25]. In fact, soy milk is an aqueous extract from soybeans. It is considered an excellent source of dietary fiber, has a variety of micronutrients and phytochemicals, and a low fat content [26]. By fermenting soy milk, the aroma and the texture of the products is improved [25], soy milk being considered a good layer for the development and production of functional foods. It was also observed that the fermentation of soy milk with probiotic bacteria reduces the level of oligosaccharides and increases the level of free isoflavones [27].
Sea buckthorn (Hippophae rhamnoides L.) is a deciduous shrub, and according to research, it has lived on earth for approximately 200 million years [28]. Additionally, The World Health Organization has declared that sea buckthorn ranks first among the top ten raw materials used in health products [28]. Sea buckthorn fruits are a rich source of many nutrients and substances that promote health, such as polyphenols, carotenoids, tocopherols, ascorbic acid [29], and compounds belonging to the B vitamin complex, as well as minerals [30] and fatty acids [31]. It is also said that sea buckthorn berries provide a vast supply of compounds necessary for human nutrition because the content of multiple nutrients is superior compared to other common fruits (e.g., oranges or peaches) [30]. Due to the fact that, nowadays, as a rule, probiotic foods fall into the category of dairy products, which are unsuitable for people with lactose intolerance, it is appropriate to look for new formulations to expand the range of probiotic products with non-dairy foods. From this point of view, sea buckthorn is not sufficiently researched, but studies on other fruits have indicated the possibility of formulating probiotics from non-dairy products [30] (for example, probiotic juices of blueberries and blackberries [32], orange and pineapple juices [33], pomegranate juice [34], and sweet lemon [35]).
To better understand the action of biological compounds from sea buckthorn powder, the present study was conducted to investigate the effects of the addition of this powder on the microbiological and biochemical characteristics of probiotic soy beverages for possible use as a functional and lactose-free product. Thus, in this context, our study was designed to assess the functionality of Bifidobacterium bifidus when added to soy milk and sea buckthorn powder beverage matrices during fermentation, as well as during the storage period and gastrointestinal simulation.
2. Materials and Methods
2.1. Materials
2.1.1. Soy Milk
The commercial soy beverage originated by Inedit Company, Romania, was purchased from a food store (Galati, Romania). The raw materials of the soy beverage were water and 95% soy from ecologic farming, with 1.9% lipids, 0.0% sugars, and 1.1% proteins. The soy milk product used was a sterilized product.
2.1.2. Plant Material for Sea Buckthorn Powder
The sea buckthorn berries harvest period was September 2022 from the region of Moldavia (Romania). After reaching the laboratory, they were immediately stored in a freezer at −20 °C prior to the experiments. Mature and intact sea buckthorn was previously washed with distilled water to remove dust and surface impurities. After being washed, sea buckthorn berries were sorted and cleaned. Frozen sea buckthorn berries were thawed at room temperature for 12 h before they were squeezed with the help of a mixer. To obtain sea buckthorn powder, the collected juice was then freeze-dried with a Freeze-dryer Alpha 1–4 LDplus (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany).
2.1.3. Starter Culture (Probiotic Bacteria)
Probiotic lactic acid bacteria Bifidobacterium bifidus was used as a test organism for the fortification in the beverage matrices. The strain was procured from Christian Hansen (Hørsholm, Denmark) as a starter culture with the commercial name Bb-12®, Bb. The culture was maintained as frozen stocks in 50% glycerol and stored at −80 °C.
2.2. Fermentation for Production of Probiotic Fermented Beverage Based on Soy Milk and Sea Buckthorn Powder
To obtain functional beverage products, tests were carried out in the laboratory phase by fermenting soy milk and sea buckthorn powder (1% and 3% w/v) which were added in line with the variation in the fermentation temperature. After mixing the soy milk with sea buckthorn powder, the mixtures were homogenized separately with an Ultra Turrax blender (IKA, Merck, Germany) at 15,000 rpm until all the ingredients were dissolved in the soy milk. Then, 250 mL mixtures were inoculated with a starter culture and were fermented for 10 h at 30° and 37 °C. Samples were taken during the incubation at 0, 2, 4, 6, 8, and 10 h to test during and at the end of the fermentation; the samples were stored for 14 days at 4 ± 1 °C.
2.3. Analysis of Probiotic Fermented Beverage Based on Soy Milk and Sea Buckthorn Powder
2.3.1. pH—Was Measured Using a pH Meter-MP2000 (Mettler Toledo, Greifensee, Switzerland)
For the titratable acidity assay, 10.0 mL of centrifuged beverage supernatant was titrated against 0.1 N NaOH using phenolphthalein as an indicator (pH 8.3). Titration was initiated by adding 2–3 drops of phenolphthalein to the sample, followed by the dropwise addition of 0.1 N NaOH with continuous swirling. The appearance of a stable light pink color indicated the point of neutrality, i.e., the end point of the titration. Titratable acidity was expressed in grams of lactic acid per 100 mL of the fermented product. The cell viability, pH, and titratable acidity of the samples were measured before and after the incubation period.
2.3.2. Determination of Probiotic Viability
The counts of probiotic strain (Bifidobacterium bifidus) were conducted using the standard plate count method. Serial dilutions (with 0.9% NaCl solution) of fermented beverages were prepared. Aliquots of 1 mL of dilution were plated in MRS agar plates using the spread plate method. Then, the plates were incubated at 37 °C for 48 h under anaerobic conditions (Anaerocult® A kit anaerobic incubator, Merck KGaA, Darmstadt, Germany). Plates containing from 25 to 250 colonies were chosen for enumeration, which was expressed in CFU/mL (colony-forming units per milliliter of product). Analyses were performed in triplicate.
2.4. In Vitro Survival Analysis for Simulated Gastrointestinal Conditions
In-vitro-simulated gastric juice (SGJ) was performed according to the procedure of the USP [36], national formulary: 2.0 g of NaCl, 3.2 g of pepsin, and 3.0 mL of concentrated HCl were diluted to 1 L and the pH was adjusted to 2.0 with concentrated HCl or sterile 0.1 mol L−1 NaOH.
Simulated intestinal juices (SIJs) were prepared by suspending pancreatin USP (P-1500) in sterile sodium chloride solution (0.5%, w/v) to a final concentration of 1 g L−1, with 4.5% bile salts (Oxoid, Merck, Germany) and adjusting the pH to 8.0 with sterile 0.1 mol L−1 NaOH.
For sterilization, both solutions were filtered through a 0.22 μm membrane. To replicate the peristaltic bowel movements and temperature of the human body, the experiment was carried out in a shaker incubator at 37 °C. Briefly, 0.2 mL from each type of beverage was taken and added in a flask and mixed with 10 mL of SGJ and incubated for 5, 30, 60, and 120 min and 60, 90, and 120 min, respectively, in the SIJs at 37 °C. Shaking was maintained at 50 rpm in each step. After the process was complete, the samples were evaluated for the viability of bacteria using pour plate techniques in MRS agar by anaerobic incubation at 37 °C for 3 days. The data are expressed as the means from three independent experiments with three replicates.
2.5. Water Holding Capacity (WHC)
The water holding capacity (WHC) was measured according to the method described by [37] with the same modification. Briefly, 10 g of beverage was transferred into a 20 mL glass tube and was centrifuged (2500 rpm for 10 min at 20 °C). The expelled water was removed and weighed. The percentage of the WHC was defined according to the equation:
| WHC = [(Sample weight − Expelled water)/Sample weight] × 100 | (1) |
2.6. Statistical Analysis
All measurements and analyses were conducted on three prepared samples, and the results are presented as means ± standard deviations (SD). The data were analyzed using Statgraphics plus v.5.1 package (Manugistics Inc., Rockville, MA, USA) by a one-way analysis of variance, and a comparison of the means was conducted using Duncan’s multiple range test (DMRT) with significance levels of p < 0.05.
3. Results
3.1. Viable Cell Counts and Physicochemical Properties during Fermentation
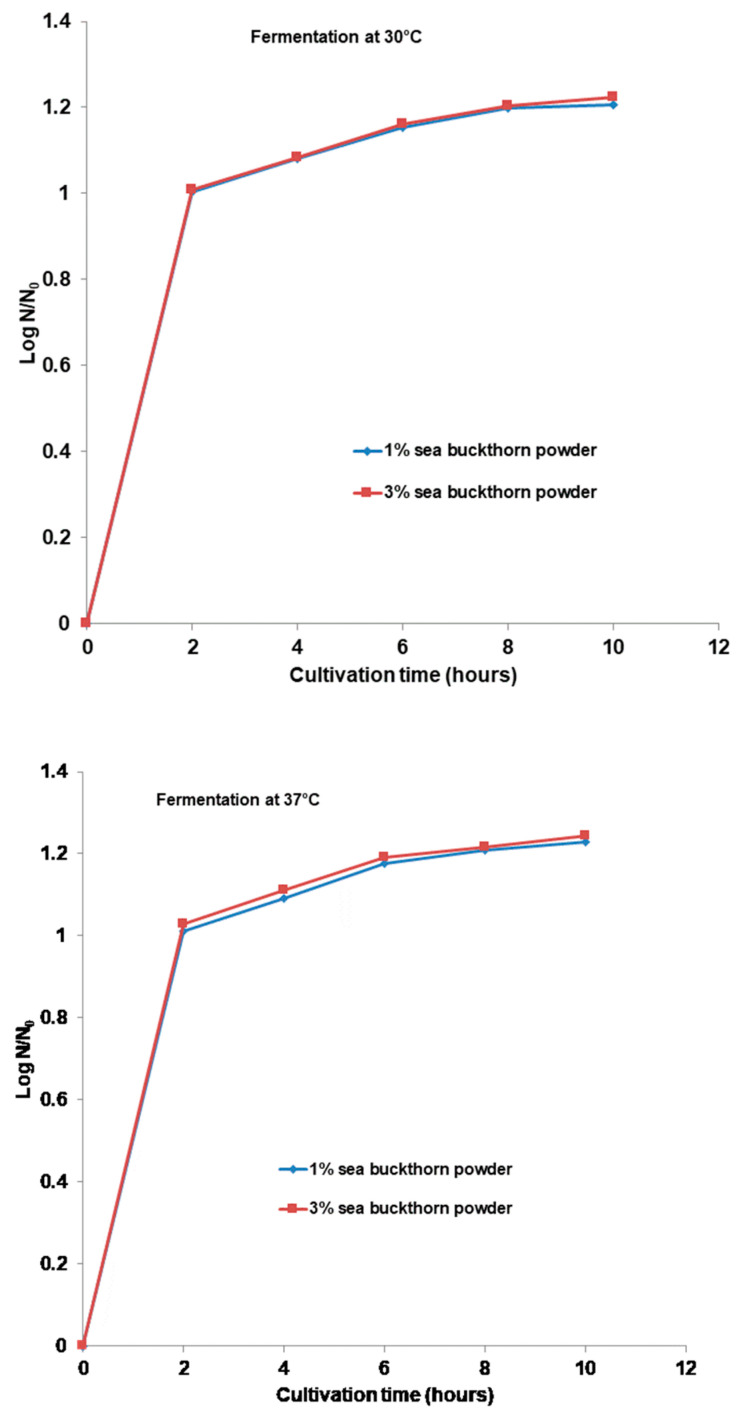
To identify the effect of sea buckthorn powder on Bifidobacterium bifidus (Bb-12®) viability in fermented soy drinks, samples were analyzed at the beginning (time 0) and at the end of the fermentation process (10 h) using the plate method (Figure 1). At time 0, there were no significant differences in the drinks (p < 0.05), with a value of 4.3 × 106 CFU·mL−1. The influence of sea buckthorn powder on Bifidobacteria could be observed after 10 h of fermentation at both temperatures (30° and 37 °C).
Figure 1.
Cell viability profile of the fermentation with Bifidobacteria. Values for Bb-12® viable cell growth is displayed as mean values, CFU·mL−1, n = 3.
As the fermentation progressed, the number of CFU of Bb-12® increased slightly in growth throughout the entire fermentation period to both temperatures of fermentation. At the end of the fermentation period, the number of viable cells of Bb-12® was around 1.2 × 108 CFU·mL−1.
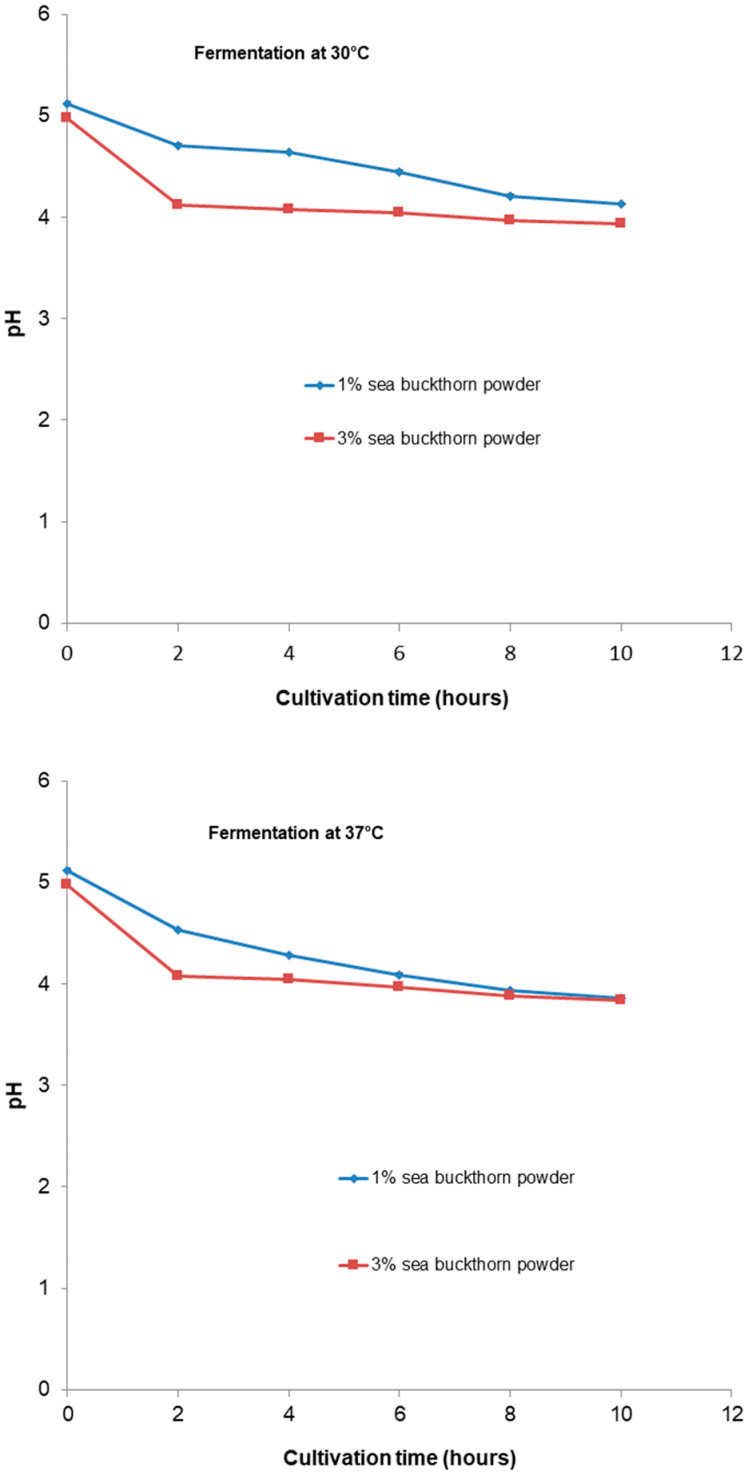
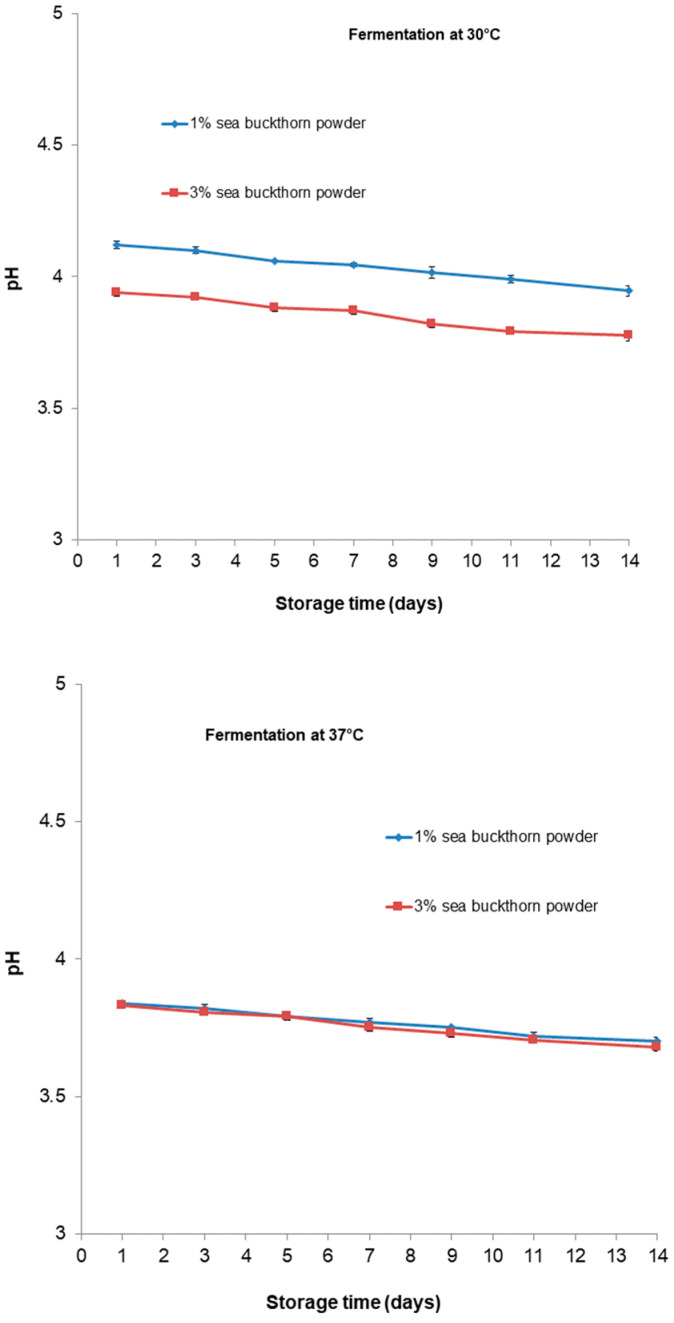
Throughout fermentation, the pH decreased, suggesting that the fermentation proceeded normally and began the production of organic acids by bifidobacterial. All formulations exhibited a pH decrease (p < 0.05) throughout the fermentation period (Figure 2).
Figure 2.
Changes in pH of functional beverages during fermentation period.
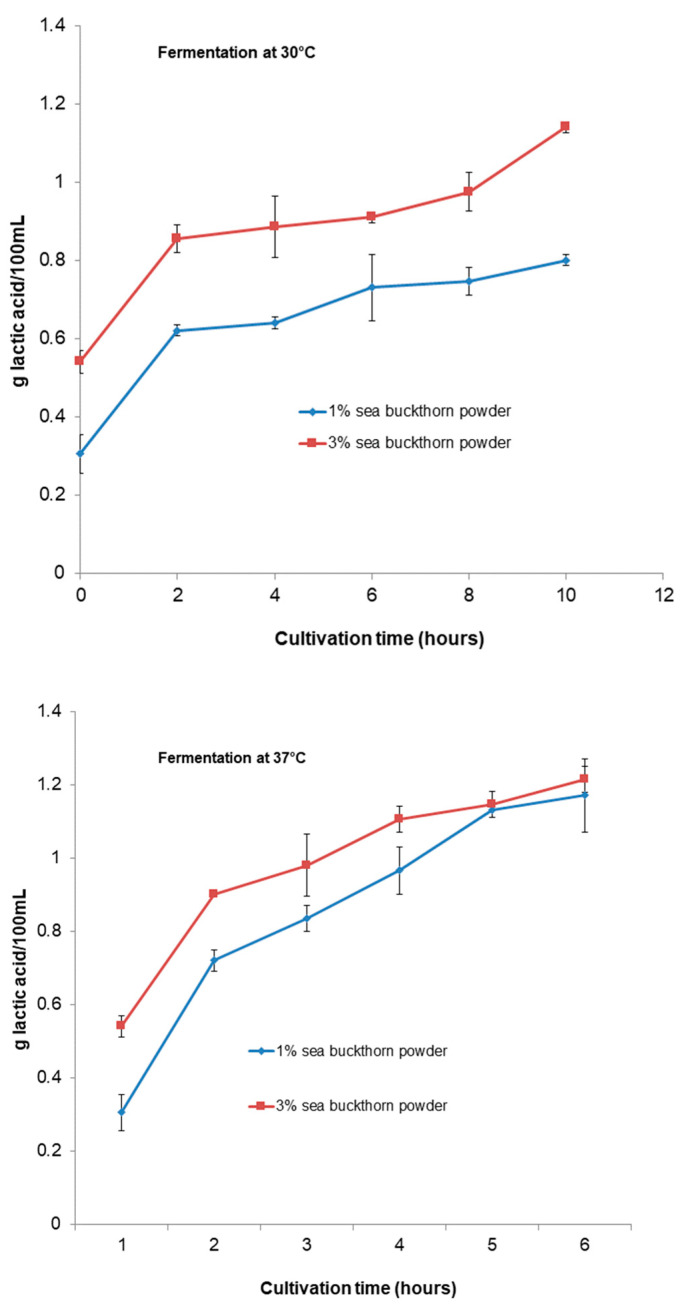
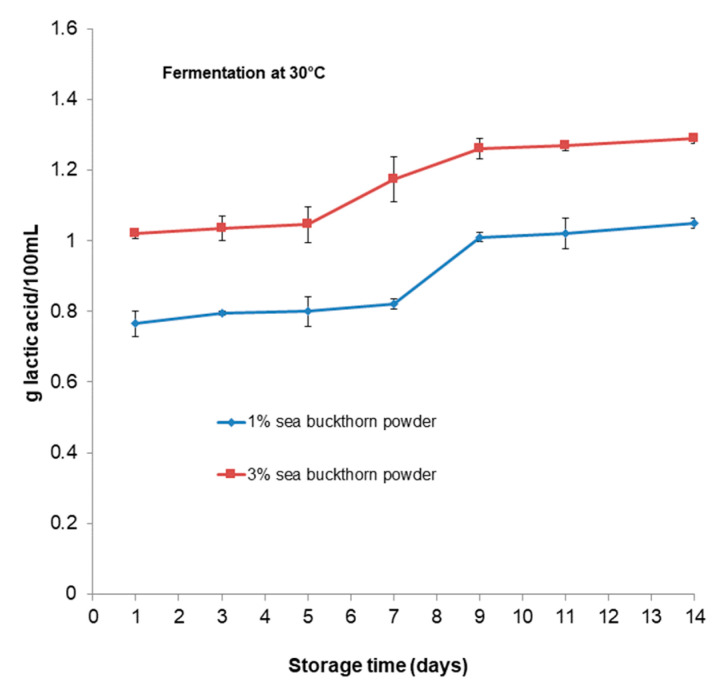
Figure 3 represents the changes in titratable acidity during the fermentation time. At the end of the fermentation process, the product had a titratable acidity of 0.8 g of lactic acid 100 mL−1 for the sample with 1% sea buckthorn powder and 1.14 g of lactic acid 100 mL−1 for the sample with 3% sea buckthorn powder for fermentation at 30 °C and 1.17 and 1.22 g of lactic acid 100 mL−1, respectively, for fermentation at 37 °C. The acidity changed significantly (p < 0.05) from all samples.
Figure 3.
Changes in titratable acidity of functional beverages during fermentation period.
3.2. Changes in Cell Viability, pH, Titratable Acidity, and Water Holding Capacity during Storage
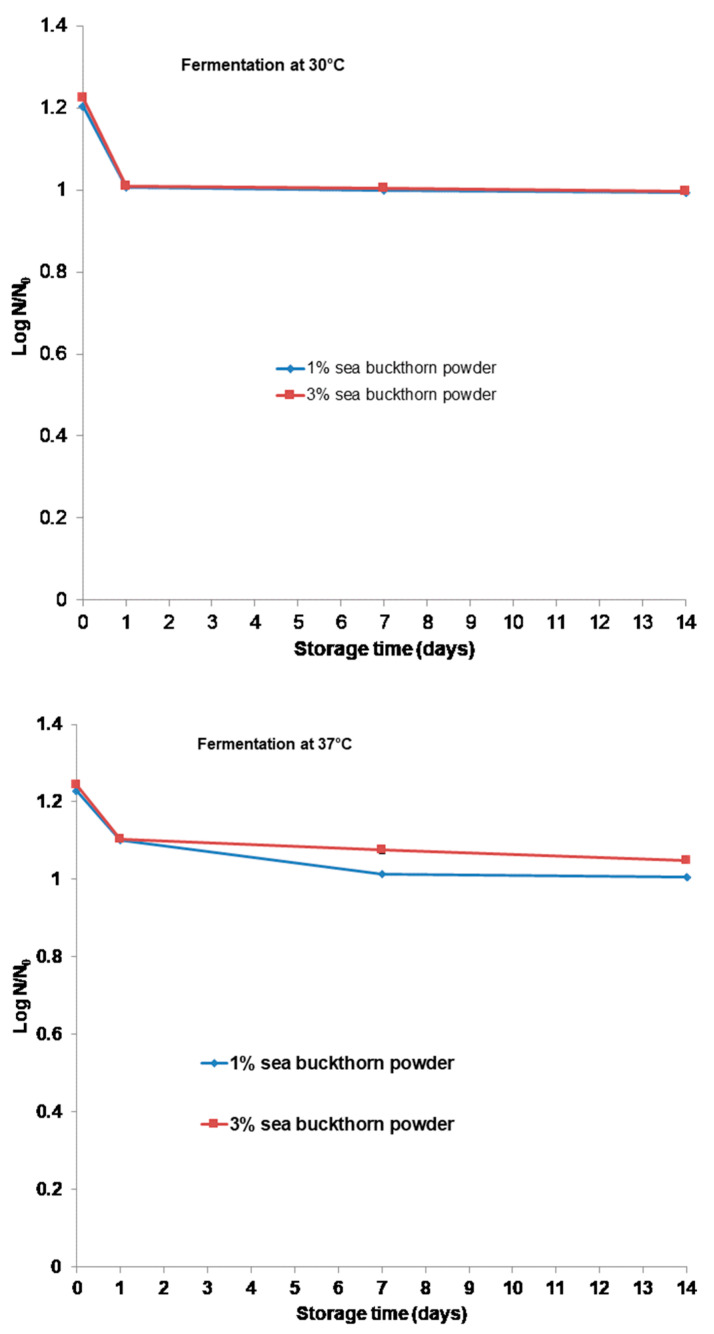
Figure 4 presents the changes in the cell viability of the Bb-12® strain (p < 0.05) for the 14 days of cold storage at 4 ± 1 °C. This evaluation began after the 10 h fermentation process ended. The total viable cells of Bb-12® were decreased moderately to 0.99 × 107 for the beverage with 1% and 3% sea buckthorn powder, fermented at 30 °C, and 1.01 × 108 and 1.05 × 108 CFU·mL−1 for the beverage with 1% and 3% sea buckthorn powder, respectively, fermented at 37 °C at the end of the storage period. However, the functional beverage with 1% and 3% sea buckthorn powder fermented at 37 °C presented a bigger population of Bb-12® compared to all the formulations (p < 0.05). After the storage period, the viability of Bb-12® remained at an acceptable level (over 107 CFU mL−1).
Figure 4.
Survivability of probiotics in functional beverage during storage.
The changes in the pH values of functional samples during storage are shown in Figure 5. The pH values of the beverage from different treatments slightly significantly decreased (p < 0.05) during storage. The lowest pH value of 3.68 belonged to the beverage with 3% sea buckthorn which was fermented at 37 °C; however, in general, the pH values of all the beverages were around four. By contrast, the acidity values increased (p < 0.05) in all formulations after 14 days of the storage period. In Figure 6, the values for all beverages after their storage time are shown. The acidity values range between 1.05 and 1.29 g of lactic acid 100 mL−1 for the sample with 1% and 3% sea buckthorn powder, respectively, for fermentation at 30 °C. For fermentation at 37 °C, the values range between 1.25 and 1.38 g of lactic acid 100 mL−1 for the sample with 1% and 3% sea buckthorn powder, respectively.
Figure 5.
Changes in pH of functional beverages during storage period.
Figure 6.
Changes in titratable acidity of functional beverages during storage period.
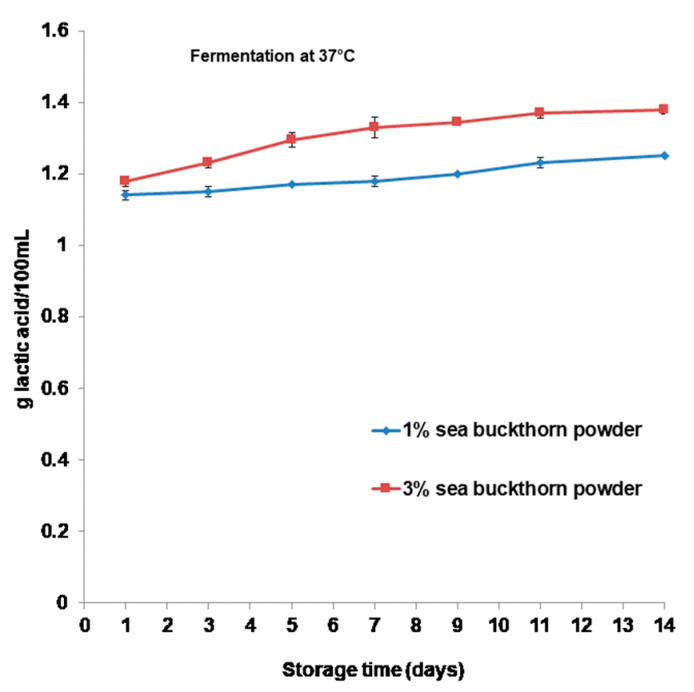
Figure 7 illustrates the effects of different levels of sea buckthorn powder on the WHC. During the period of storage, the WHC was higher for all beverages. As shown in Figure 7, the WHC of the beverage with 3% sea buckthorn powder fermented at 37 °C was significantly higher than that of other groups (p < 0.05). As can be seen from Figure 7, the WHC of all beverages varied between 74 and 76% at the end of the refrigeration period.
Figure 7.
Changes in WHC of functional beverages during storage period.
3.3. Gastrointestinal Simulation
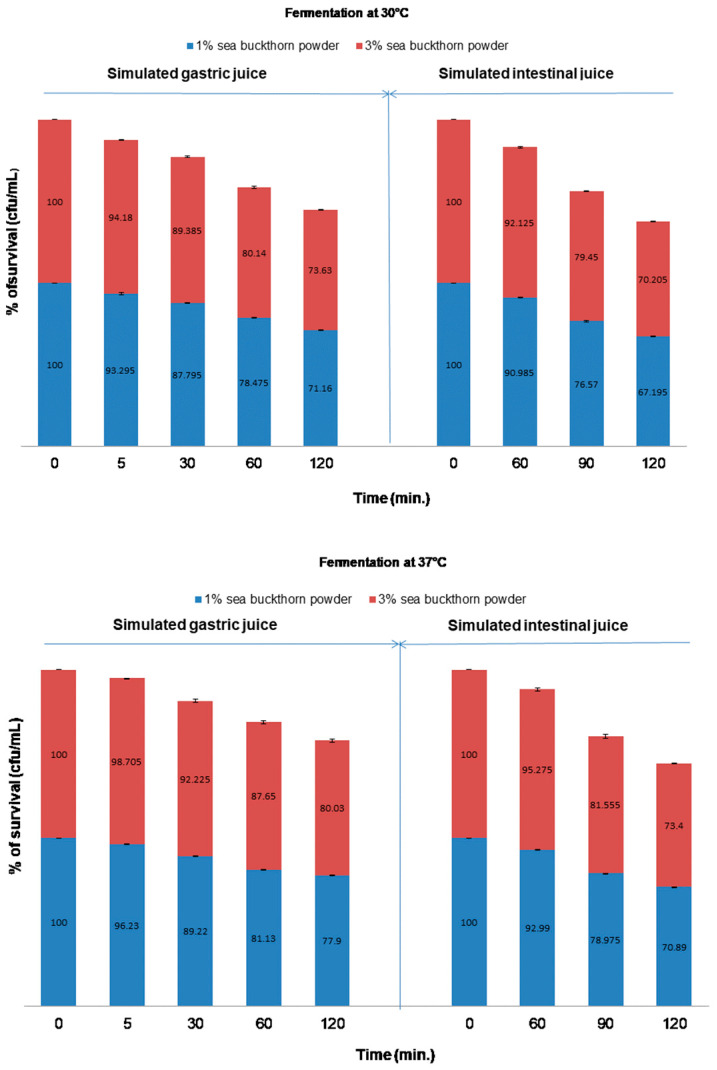
In order to follow the bio-accessibility of the Bifidobacteria in functional beverages, the samples were subjected to gastric and intestinal phases of the in vitro digestion protocol. Figure 8 shows the pre- and post-digestion counts of Bb-12® in soy drinks, with 1% and 3% sea buckthorn powder fermented at 30 °C and 37 °C, respectively.
Figure 8.
Survival rates of Bb-12® incorporated into the beverages after continuous gastrointestinal simulations.
During the digestion process, the bacterial count in all the samples was reduced significantly (p < 0.05). There were lower reductions after the gastric simulation compared with the intestinal simulation for all samples. The positive effects of the concentration of sea buckthorn powder in the gastric simulation can be observed in the functional drink, where the soy drinks with 3% sea buckthorn powder were significantly higher (73.63% for sample fermented at 30 °C and 80.03% for sample fermented at 37 °C, respectively) than 1% sea buckthorn powder beverages (71.16% for sample fermented at 30 °C and 77.9% for sample fermented at 37 °C, respectively) (p < 0.05). The same positive effects of the concentration of sea buckthorn powder in the intestinal simulation for all samples can be observed in the functional beverages: 70.21% for sample fermented at 30 °C and 73.4% for sample fermented at 37 °C, respectively, for sample with 3% sea buckthorn powder compared with 67.2% for sample fermented at 30 °C and 70.9% for sample fermented at 37 °C, respectively, for sample with 1% sea buckthorn powder.
4. Discussion
During the development of non-dairy probiotic products, important attention should be granted at the formulation to the food matrix for probiotic delivery to obtain the highest efficiency. The beneficial impact of probiotics for health is not only due to the type of strain used but is also mostly dependent on the matrix environment. In conclusion, it is very important that the food matrix planned for use as a probiotic vehicle supports the high survival of probiotics for prolonged time periods. Hence, we have evaluated several in vivo and in vitro factors to assess the effect of the food matrix on Bifidobacterium bifidus (Bb-12®) strain. This study examined the possibility of producing a functional beverage based on soy milk, sea buckthorn powder in different concentrations, and fermented by the Bb-12®strain.
The cells viability of Bb-12® was not affected by either the time, concentration of sea buckthorn powder, or the temperature. At the 10th h of fermentation, its number increased, and the results indicated that the presence of different components such as sugars and proteins in the beverage could have enhanced the growth by providing nutrition to the probiotic Bifidobacteria in all samples. The increase in cell numbers was higher at 37 °C for the beverages with 1 and 3% sea buckthorn powder compared to 30 °C. As shown in Figure 1, supplementation with different amounts of sea buckthorn powder in the beverage affects the growth of the Bb-12®strain; the cell numbers increased as the samples with the amount of sea buckthorn powder increased.
During storage, the cell numbers of the Bb-12® slightly decreased and remained around 1 × 107 and 1 × 108 CFU mL−1 for the beverage with 3% sea buckthorn powder fermented at 30 °C and 37 °C, respectively, (Figure 4). Generally, during storage, the cell numbers of Bb-12® decreased slightly for all the beverages at both temperatures of fermentation. The analysis of variance for the probiotic counts showed that supplementation with different amounts of sea buckthorn powder in the beverage and different temperatures of fermentation affect the growth of Bb-12®. The cell numbers increased as the samples with the amount of sea buckthorn powder during fermentation increased and slightly decreased during the storage period. An increase in the maximum viability was observed in the sample with 3% sea buckthorn powder fermented at 37 °C, and a decrease in the minimum viability during the storage period was observed in the same beverage. Therefore, sea buckthorn powder and soy milk positively affected the development of Bifidobacteria, providing them with a favorable environment for growth. Inoguchi et al. [38] and Hara et al. [39] declared that soy milk contains soybean oligosaccharides that are utilized by Bifidobacteria. Additionally, [40] declared that the fermentation temperature is one of the important factors which affects the viability of probiotic microorganisms and other qualitative parameters of probiotic fermented products, and the favorable temperature for the growth of most probiotic strains is in the range of 37–43 °C. Our results comply with the study conducted by [41] where soy milk was fermented at two different temperatures (37 °C and 42 °C) using ABTS culture (Lactobacillus acidophilus, Bifidobacterium spp, Streptococcus thermophilus). They reported that Bifidobacterium grew better during fermentation at 42 °C than at 37 °C. Additionally, [42] reported that Lactobacillus casei grew significantly better at 37 °C than at 30 °C for 12 h of fermentation. The growth results obtained in this work for the analyzed Bifidobacteria are comparable to those reported by other authors for species and strains of another bacterial groups and soy milk [27,43,44,45]. Therefore, we can declare that it is very important to test the compatibility between probiotics, fermentation conditions, and the food matrix to provide a positive interaction capable of increasing the microbial viability during fermentation and the storage period.
The intended health benefits of probiotics can only be obtained when the food (dairy and non-dairy beverages) contains the required minimum viable microorganism count at the time of consumption. Generally, the food industry has declared that the minimum recommended level is 106 CFU mL−1 at the time of consumption [46], but and US FDA has recommended that the minimum probiotic count in a probiotic food should be at least 106 CFU mL–1. Knorr [47] declared that a daily intake of 108–109 probiotic, depending on the amount ingested and considering the effect of storage on probiotic viability, is essential to achieve probiotic benefits in the human organism. In our study, the viable cells of Bb-12® remained at the level mentioned, which means that our beverage is a functional drink. Additionally, we can conclude that the beverage based on soy milk, sea buckthorn powder, and Bb-12® could act as a trigger for new research to identify new balanced matrices.
Throughout fermentation, the pH values of all the samples decreased from the initial pH after 10 h of fermentation and increased in terms of cell count. All samples showed nearly the same final pH, around 3.90, except for the sample with 1% sea buckthorn powder which was fermented at 30 °C (pH = 4.13). As a result of the continuing metabolic activities of Bifidobacteria toward the end of fermentation, the pH lowered to around 3.7 for all samples, except for the sample with 1% sea buckthorn powder which was fermented at 30 °C (pH = 3.95). In accordance with our results, [48] declared that for a probiotic, Prunus mume puree had a final pH of 3.0 from an initial pH of 3.5 after 10 days of storage at room temperature. Additionally, insignificant changes in pH, from an initial pH of 5.5 to 5.7, were observed for a probiotic cheese-like product over a period of 4 weeks [49].
The production of organic acids during fermentation is linked to a decrease in the pH, as was also observed in this study, and a very low pH value increases the concentration of undissociated organic acids in fermented products, thereby enhancing the bactericidal effect of these acids [50]. Additionally, [50] suggested that beverages (such as fruit juices) with low pH values present a major challenge to probiotics. The optimum value of pH for the growth of Bifidobacteria is in the range of 6.0–7.0 [51]. Dunne et al. [52] declared that Bifidobacteria species are noted to be less acid-tolerant, and a pH level below 4.6 is against their survival. The minimal pH reduction in samples stored under refrigerated conditions can be attributed to the metabolic activity of Bb-12®.
The titratable acidity increased with a decrease in the pH for all functional beverages during fermentation for both temperatures. The sample with 1% sea buckthorn powder fermented at 30 °C had a lower value of titratable acidity compared with all the beverages during fermentation and the storage period. However, the titratable acidity increased as the pH decreased during fermentation for both temperatures and for all beverages. The titratable acidity increased, as has been revealed by several studies [53,54,55,56,57]. In this study, the increased acidity of the formulations can be the result of post-acidification because of continued fermentation by Bb-12® during storage. The pH and titratable acidity values observed in our study can be indirect indicators of starter metabolic activity during storage, and they confirm our results regarding the viability of Bb-12® and organic acid content observed during the refrigerated period. We can conclude that the acid tolerance of Bb-12® depends upon the strain of the species and characteristics of the substrate. For example, [40] reported that B. longum survived best in the presence of acids and bile salts, and B. lactis in fermented milks. Additionally, Sheehan et al. [33] observed extensive differences in the acid resistance of Lactobacillus and Bifidobacterium when added to juices (orange, pineapple, and cranberry juices). All the strains survived better in orange and pineapple juices compared to cranberry juice [33].
Syneresis in the fermented soy milk decreased for both temperatures during storage. The WHC values obtained on the first day of sampling were higher than those found during storage, and they decreased with the decreasing sea buckthorn powder concentrations. The storage period did not significantly (p < 0.05) affect the WHC of the functional beverages. The results indicate that despite the increased acidification over the storage period, there was no tendency to release water by the functional beverage. Our results agree with the results reported by [58], on the contrary to the results observed by other authors who have studied fermented dairy beverages [59].
In our study, during the gastrointestinal simulation, a reduction in the total viable count of Bb-12® in all the samples was observed. Considering the results previously presented in the literature, the survival of the SGJs of the Bb-12® strain after 7 days of storage of fermented products has been analyzed. The drop in viable count throughout the passage from the stomach to the large and small intestine observed in this study is a major finding due to the extreme conditions, such as the low pH of stomach acid, HCl, and bile juice, encountered by the Bb-12® which restrict probiotic activity in the gut. The results show that the sea buckthorn powder concentration and fermentation temperature had a significant effect on the survivability rate of Bb-12® cells during exposure to acidic conditions. This finding indicates a protective effect of sea buckthorn powder on Bb-12® cells and the possible migration of prebiotic constituents to the final product that stimulated survival through gastrointestinal simulation. Our results agree with the results reported by [60,61,62].
The viability of cells in the SIJs decreased easily after the first hour and then decreased gradually in the next hour. Chen et al. [63] suggested that bile salt can traverse the bacterial cell membrane which can cause bacterial death. These results agree with the results reported by [64].
Leahy et al. [65] declared that Bifidobacterium is a predominant member of the intestinal microbiota of humans, and it is also essential for maintaining the intestinal environment of the host. Likewise, Bifidobacterium exerts various beneficial effects such as in the synthesis of vitamins, those involved in the regulation of the intestinal environment, in the alleviation of constipation, in digestion and absorption, diarrhea, and infection, in enhancements to the immune system, in the amelioration of lactose intolerance, in the inhibition of carcinogenesis, in therapy and prophylaxis for inflammatory bowel disease, in reductions in serum cholesterol [65], and it has been used as a probiotic [66]; soybean oligosaccharides are also regarded as capable prebiotics and Bifidobacterium growth promoters [67]. Uriot et al. [68] suggests that the stomach is considered a biological and chemical barrier. Because of its low pH and the presence of proteolytic enzymes that promote an unfavorable environment to microorganisms, the difference in the pH in the intestine, combined with pancreatic fluids and bile salts, affects the chemical and stability of cell membranes (proteins and phospholipids). For this reason, this can cause cell disruption and homeostasis malfunction [68].
The results obtained in this study suggest the potential of soy milk and sea buckthorn powder as a functional food. From the point of view of the results obtained in this study, we can declare that the content of potent bioactive compounds in fermented soy milk and sea buckthorn powder depends on the processing conditions, the bacteria used in the fermentation step, and storage time. Finally, we wish to specify that it was very difficult to make comparisons to the literature because, presently, to the best of our knowledge, there have not been any more studies published about the behavior of the Bb-12® strain when incorporated in a food matrix based on soy milk and sea buckthorn powder.
5. Conclusions
This research showed that the addition of 1 and 3% sea buckthorn powder in a fermented soy-milk-based product at 30 °C and 37 °C positively influences the growth and development of Bb-12®. The viability of these Bifidobacteria increased significantly after 10 h of fermentation, and the pH decreased at values of around 3.90. The titratable acidity increased with a decrease in the pH for all functional beverages during fermentation and the storage period for both temperatures, and the WHC values obtained decreased along with decreasing sea buckthorn powder concentrations. The Bb-12® strain was able to survive in vitro in the human gastrointestinal tract, and the results obtained reveal that the Bb-12® strain could be a potential probiotic which could be used in a soy-milk- and sea-buckthorn-powder-based beverage. According to the results obtained after digestion, the supportive impact of sea buckthorn powder depends on the bacteria used and temperature of fermentation. Thus, enriching soy beverages with sea buckthorn powder and fermenting with Bb-12® presents a functional improved product. A further investigation into probiotics is required to understand the components responsible for the effective distribution of bacteria through the gastrointestinal tract, and the next step will be to verify whether these in vitro findings also apply to an in vivo situation because the potential probiotics must compete for mucosa receptors and nutrients with a plethora of intestinal microorganisms. Our research proves that there are opportunities to develop a new functional beverage and a market of functional foods based on fermented soy milk and sea buckthorn powder that incorporates health benefits.
Acknowledgments
The authors acknowledge the research staff of “Dunarea de Jos” University” Galati for the financial support of publishing our study.
Author Contributions
Conceptualization; N.-M.M., E.L.L. and A.-V.I.; methodology; N.-M.M., T.V.G. and A.Y.R.-V.; software; N.-M.M., A.-V.I. and T.V.G.; validation; N.-M.M., A.M.E. and E.L.L.; formal analysis; N.-M.M., T.V.G. and A.Y.R.-V.; investigation; N.-M.M., A.-V.I. and A.M.E.; resources; N.-M.M., E.L.L. and A.-V.I.; data curation; N.-M.M. and T.V.G.; writing—original draft preparation; N.-M.M. and A.Y.R.-V.; writing—review and editing; N.-M.M., E.L.L. and A.M.E.; visualization; N.-M.M., A.M.E. and T.V.G.; supervision; N.-M.M., A.Y.R.-V. and A.M.E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the “Dunarea de Jos” University of Galati, and The APC was paid by the “Dunarea de Jos” University of Galati, VAT 27232142.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mantzourani I., Kazakos S., Terpou A., Alexopoulos A., Bezirtzoglou E., Bekatorou A., Plessas S. Potential of the probiotic Lactobacillus Plantarum ATCC 14917 strain to produce functional fermented pomegranate juice. Foods. 2019;8:4. doi: 10.3390/foods8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamang J.P., Watanabe K., Holzapfel W.H. Review: Diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016;7:377. doi: 10.3389/fmicb.2016.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Internal Market, Industry, Entrepreneurship and SMEs-Food and Drink Industry. [(accessed on 14 April 2023)]. Available online: https://ec.europa.eu/growth/sectors/food-and-drink-industry_en.
- 4.Data Hub: The Cost of Being a Vegan in 2023. [(accessed on 31 May 2023)]. Available online: https://www.cashlady.com/your-cash/cost-of-being-a-vegan.
- 5.Delgado S., Guadamuro L., Flórez A.B., Vázquez L., Mayo B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high -glucosidase activity. Innov. Food Sci. Emerg. Technol. 2019;51:148–155. doi: 10.1016/j.ifset.2018.03.018. [DOI] [Google Scholar]
- 6.Marete E.N., Jacquier J.C., O’Riordan D. Feverfew as a source of bioactives for functional foods: Storage stability in model beverages. J. Funct. Foods. 2011;3:38–43. doi: 10.1016/j.jff.2011.01.004. [DOI] [Google Scholar]
- 7.Sloan E., Adams Hutt C. Beverage trends in 2012 and beyond. Agro Food Ind. Hi-Tech. 2012;23:8. [Google Scholar]
- 8.Bagchi D., Nair S. Developing New Functional Food and Nutraceutical Products. Academic Press; Cambridge, MA, USA: 2016. [Google Scholar]
- 9.Nazir M., Arif S., Khan R.S., Nazir W., Khalid N., Maqsood S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019;88:513–526. doi: 10.1016/j.tifs.2019.04.011. [DOI] [Google Scholar]
- 10.Martelli F., Cirlini M., Lazzi C., Neviani E., Bernini V. Solid-State fermentation of Arthrospira platensis to implement new food products: Evaluation of stabilization treatments and bacterial growth on the volatile fraction. Foods. 2021;10:67. doi: 10.3390/foods10010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandylis P., Pissaridi K., Bekatorou A., Kanellaki M., Koutinas A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016;7:58–63. doi: 10.1016/j.cofs.2015.11.012. [DOI] [Google Scholar]
- 12.Khalesi S., Sun J., Buys N., Jayasinghe R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira P.M., Júnior B.R.D.C.L., Martins E.M.F., Martins M.L., Vieira E.N.R., de Barros F.A.R., Cristianini M., de Almeida Costa N., Ramos A.M. Mango and carrot mixed juice: A new matrix for the vehicle of probiotic lactobacilli. Int. J. Food Sci. 2021;58:98–109. doi: 10.1007/s13197-020-04518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikbakht E., Khalesi S., Singh I., Williams L.T., West N.P., Colson N. Effect of probiotics and synbiotics on blood glucose: A systematic review and meta-analysis of controlled trials. Eur. J. Nutr. 2016;57:95–106. doi: 10.1007/s00394-016-1300-3. [DOI] [PubMed] [Google Scholar]
- 15.Sun J., Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and metaanalysis of randomized controlled trials. Ann. Med. 2015;47:430–440. doi: 10.3109/07853890.2015.1071872. [DOI] [PubMed] [Google Scholar]
- 16.Pelin A.M., Matasaru S. Metabolic syndrome in obese children and adolescents. Rev. Med. Chir. Soc. Med. Nat. 2012;116:957–961. [PubMed] [Google Scholar]
- 17.Rostami F.M., Mousavi H., Mousavi M.R.N., Shahsafi M. Efficacy of probiotics in prevention and treatment of infectious diseases. Clin. Microbiol. Newsl. 2018;40:97–103. doi: 10.1016/j.clinmicnews.2018.06.001. [DOI] [Google Scholar]
- 18.Singh K., Rao A. Probiotics: A potential immunomodulator in COVID-19 infection management. Nutr. Res. 2021;87:1–12. doi: 10.1016/j.nutres.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plessas S., Bosnea L., Alexopoulos A., Bezirtzoglou E. Potential effects of probiotics in cheese and yogurt production: A review. Eng. Life Sci. 2012;12:433–440. doi: 10.1002/elsc.201100122. [DOI] [Google Scholar]
- 20.James A., Wang Y. Characterization, health benefits and applications of fruits and vegetable probiotics. CYTA J. Food. 2019;17:770–780. doi: 10.1080/19476337.2019.1652693. [DOI] [Google Scholar]
- 21.Šertović E., Mujić I., Jokić S., Alibabić V., Sarić Z. Effect of soybean cultivars on the content of isoflavones in soymilk. Rom. Biotechnol. Lett. 2012;17:7151–7159. [Google Scholar]
- 22.Kumari M., Kokkiligadda A., Dasriya V., Naithani H. Functional relevance and health benefits of soymilk fermented by lactic acid bacteria. J. Appl. Microbiol. 2022;133:104–119. doi: 10.1111/jam.15342. [DOI] [PubMed] [Google Scholar]
- 23.Sá A.G.A., Moreno Y.M.F., Carciofi B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci Technol. 2020;97:170–184. doi: 10.1016/j.tifs.2020.01.011. [DOI] [Google Scholar]
- 24.Nedele A.K., Gross S., Rigling M., Zhang Y. Reduction of green off-flavor compounds: Comparison of key odorants during fermentation of soy drink with Lycoperdon pyriforme. Food Chem. 2021;334:127591. doi: 10.1016/j.foodchem.2020.127591. [DOI] [PubMed] [Google Scholar]
- 25.Tsangalis D., Ashton J.F., McGill A.E.J., Shah N.P. Biotrasformation of isoflavones by bifidobacteria in fermented soymilk supplemented with D-glucose and L-cystein. J. Food Sci. 2003;68:623–631. doi: 10.1111/j.1365-2621.2003.tb05721.x. [DOI] [Google Scholar]
- 26.Ewe J.A., Yeo S.K. Beneficial Microorganisms in Food and Nutraceuticals. Springer; Cham, Switzerland: 2015. Fermented soymilk as a nutraceutical; pp. 133–159. [DOI] [Google Scholar]
- 27.Wei Q.K., Chen T.R., Chen J.T. Using of Lactobacillus and Bifidobacterium to product the isoflavone aglycones in fermented soymilk. Int. J. Food Microbiol. 2007;117:120–124. doi: 10.1016/j.ijfoodmicro.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Chena A., Fengc X., Dorjsurend B., Chimedtserenb C., Damdab T.A., Zhangc C. Traditional food, modern food and nutritional value of Sea buckthorn (Hippophae rhamnoides L.): A review. J. Future Foods. 2023;3:191–205. doi: 10.1016/j.jfutfo.2023.02.001. [DOI] [Google Scholar]
- 29.Ilhan G., Gundogdu M., Karlović K., Židovec V., Vokurka A., Ercişli S. Main Agro-Morphological and Biochemical Berry Characteristics of Wild-Grown Sea Buckthorn (Hippophae rhamnoides L. ssp. caucasica Rousi) Genotypes in Turkey. Sustainability. 2021;13:1198. doi: 10.3390/su13031198. [DOI] [Google Scholar]
- 30.Stobdan T., Chaurasia O.P., Korekar G., Yadav A., Singh S. Attributes of Seabuckthorn (Hippophae rhamnoides L.) to Meet Nutritional Requirements in High Altitude. Def. Sci. J. 2010;60:226–230. doi: 10.14429/dsj.60.344. [DOI] [Google Scholar]
- 31.Kallio H., Yang B., Peippo P., Tahvonen A.R., Pan R. Triacylglycerols, Glycerophospholipids, Tocopherols, and Tocotrienols in Berries and Seeds of Two Subspecies (ssp. sinensisandmongolica) of Sea Buckthorn (Hippophae rhamnoides) J. Agric. Food Chem. 2002;50:3004–3009. doi: 10.1021/jf011556o. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y., Li S., Tao Y., Li D., Han Y., Show P.L., Wen G., Zhou J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021;348:129083. doi: 10.1016/j.foodchem.2021.129083. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan V.M., Ross P., Fitzgerald G.F. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov. Food Sci. Emerg. Technol. 2007;8:279–284. doi: 10.1016/j.ifset.2007.01.007. [DOI] [Google Scholar]
- 34.Mousavi Z.E., Mousavi S.M., Razavi S.H., Hadinejad M., Emam-Djomeh Z., Mirzapour M. Effect of Fermentation of Pomegranate Juice by Lactobacillus plantarum and Lactobacillus acidophiluson the Antioxidant Activity and Metabolism of Sugars, Organic Acids and Phenolic Compounds. Food Biotechnol. 2013;27:1–13. doi: 10.1080/08905436.2012.724037. [DOI] [Google Scholar]
- 35.Hashemi S.M.B., Khaneghah A.M., Barba F.J., Nemati Z., Shokofti S.S., Alizadeh F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. J. Funct. Foods. 2017;38:409–414. doi: 10.1016/j.jff.2017.09.040. [DOI] [Google Scholar]
- 36.USP National Formulary. [(accessed on 12 March 2023)]. Available online: www.uspnf.com.
- 37.Pyo Y.H., Song S.M. Physicochemical and sensory characteristics of a medicinal soy yogurt containing health-benefit ingredients. J. Agric. Food Chem. 2009;57:170–175. doi: 10.1021/jf8026952. [DOI] [PubMed] [Google Scholar]
- 38.Inoguchi S., Ohashi Y., Narai-Kanayama A., Aso K., Nakagaki T., Fujisawa T. Effects of nonfermented and fermented soybean milk intake on faecal microbiota and faecal metabolites in humans. Int. J. Food Sci. Nutr. 2012;63:402–410. doi: 10.3109/09637486.2011.630992. [DOI] [PubMed] [Google Scholar]
- 39.Hara H., Li S.T., Sasaki M., Maruyama T., Terada A., Ogata Y., Fujita K., Ishigami H., Hara K., Fujimori I., et al. Effective dose of lactosucrose on fecal flora and fecal metabolites of humans. Bifidobact. Microflora. 1994;13:51–63. doi: 10.12938/bifidus1982.13.2_8. [DOI] [Google Scholar]
- 40.Korbekandi H., Mortazavian A.M., Iravani S. Technology and Stability of Probiotic in Fermented Milks. In: Shah N., Cruz A.G., Faria J.A.F., editors. Probiotic and Prebiotic Foods: Technology, Stability and Benefits to the Human Health. Nova Science Publishers; New York, NY, USA: 2011. pp. 131–169. [Google Scholar]
- 41.Božanić R., Lovković S., Jeličić I. Optimising Fermentation of Soymilk with Probiotic Bacteria. Czech J. Food Sci. 2011;29:51–56. doi: 10.17221/97/2010-CJFS. [DOI] [Google Scholar]
- 42.Maftei N.-M., Aprodu I., Dinică R., Bahrim G. New fermented functional product based on soy milk and sea buckthorn syrup. CYTA J. Food. 2013;11:256–269. doi: 10.1080/19476337.2012.730554. [DOI] [Google Scholar]
- 43.Tsangalis D., Ashton J.F., McGill A.E.J., Shah N.P. Enzymic transformation of isoflavone phytoestrogens in soy milk by β-glucosidase producing bifidobacteria. J. Food Sci. 2002;67:3104–3113. doi: 10.1111/j.1365-2621.2002.tb08866.x. [DOI] [Google Scholar]
- 44.Chien H.L., Huang H.Y., Chou C.C. Transformation of isoflavone phytoestrogens during the fermentation of soy milk with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:772–778. doi: 10.1016/j.fm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Donkor O.N., Shah N.P. Production of β-glucosidase and hydrolysis of isoflavone phytoestrogens by Lactobacillus acidophilus, Bifidobacterium lactis, and Lactobacillus casei in soy milk. J. Food Sci. 2008;73:M15–M20. doi: 10.1111/j.1750-3841.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 46.Boylston T.D., Vinderola C.G., Ghoddusi H.B., Reinheimer J.A. Incorporation of Bifidobacteria into cheeses: Challenges and rewards. Int. J. Dairy Technol. 2004;14:375–387. doi: 10.1016/j.idairyj.2003.08.008. [DOI] [Google Scholar]
- 47.Knorr D. Technology aspects related to microorganisms in functional foods. Trends Food Sci. Technol. 1998;9:295–306. doi: 10.1016/S0924-2244(98)00051-X. [DOI] [Google Scholar]
- 48.Yu Y., Xiao G., Xu Y., Wu J., Zhang Y., Chen W. Changes of quality in the fruits of Prunus mume during deacidification by fermentation with Lactobacillus fermentium. J. Food Sci. 2015;80:405–410. doi: 10.1111/1750-3841.12769. [DOI] [PubMed] [Google Scholar]
- 49.Cichosz G., Aljewicz M., Nalepa B. Viability of the Lactobacillus rhamnosus hn001probiotic strain in Swiss-and Dutch-type cheese and cheese-like products. J. Food Sci. 2014;79:1181. doi: 10.1111/1750-3841.12458. [DOI] [PubMed] [Google Scholar]
- 50.Tripathi M.K., Giri S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods. 2014;9:225–241. doi: 10.1016/j.jff.2014.04.030. [DOI] [Google Scholar]
- 51.De Vuyst L. Technology aspects related to the application of functional starter cultures. Food Sci. Biotechnol. 2000;38:105–112. [Google Scholar]
- 52.Dunne C., O’Mahony L., Murphy L., Thornton G., Morrissey D., O’Halloran S., Feeney M., Flynn S. In vitro selection criteria for probiotic bacteria of human origin: Correlation with in vivo findings. Am. J. Clin. Nutr. 2001;73:386S–392S. doi: 10.1093/ajcn/73.2.386s. [DOI] [PubMed] [Google Scholar]
- 53.Obadina A., Akinola O., Shittu T., Bakare H. Effect of Natural Fermentation on the Chemical and Nutritional Composition of Fermented Soymilk Nono. Niger. Food J. 2013;31:91–97. doi: 10.1016/S0189-7241(15)30081-3. [DOI] [Google Scholar]
- 54.Kim H.J., Han M.J. The fermentation characteristics of soy yogurt with different content of d-allulose and sucrose fermented by lactic acid bacteria from Kimchi. Food Sci. Biotechnol. 2019;28:1155–1161. doi: 10.1007/s10068-019-00560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.da Silveira E.O., Lopes Neto J.H., da Silva L.A., Raposo A.E.S., Magnani M., Cardarelli H.R. The effects of inulin combined with oligofructose and goat cheese whey on the physicochemical properties and sensory acceptance of a probiotic chocolate goat dairy beverage. LWT Food Sci. Technol. 2015;62:445–451. doi: 10.1016/j.lwt.2014.09.056. [DOI] [Google Scholar]
- 56.Gautam N., Sharma N. Quality attributes of a novel cereal-based probiotic prepared by using food grade lactic acid bacteria. Indian J. Tradit. Knowl. 2014;13:525–530. [Google Scholar]
- 57.Shukla M., Jha Y.K., Admassu S. Development of probiotic beverage from whey and pineapple juice. J. Food Sci. Technol. 2013;4:206. doi: 10.4172/2157-7110.1000206. [DOI] [Google Scholar]
- 58.Aparicio-García N., Martínez-Villaluenga C., Frias J., Peñas E. Production and Characterization of a Novel Gluten-Free Fermented Beverage Based on Sprouted Oat Flour. Foods. 2021;10:139. doi: 10.3390/foods10010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin F.-M., Chiu C.-H., Tzu-Ming P. Fermentation of a milk–soymilk and Lycium chinense Miller mixture using a new isolate of Lactobacillus paracasei subsp. Paracasei NTU101 and Bifidobacterium longum. J. Ind. Microbiol. Biotechnol. 2004;31:559–564. doi: 10.1007/s10295-004-0184-z. [DOI] [PubMed] [Google Scholar]
- 60.Terpoua A., Papadaki A., Bosnea L., Kanellaki M., Kopsahelis N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT Food Sci. Technol. 2019;105:242–249. doi: 10.1016/j.lwt.2019.02.024. [DOI] [Google Scholar]
- 61.Romano N., Tymczyszyn E., Mobili P., Gomez-Zavaglia A. Chapter 10—Prebiotics as Protectants of Lactic Acid Bacteria. In: Watson R.R., Preedy V.R., editors. Probiotics, Prebiotics, and Synbiotics. Academic Press; Cambridge, CA, USA: 2016. pp. 155–163. [Google Scholar]
- 62.Terpou A., Bekatorou A., Kanellaki M., Koutinas A.A., Nigam P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochem. 2017;55:1–10. doi: 10.1016/j.procbio.2017.01.013. [DOI] [Google Scholar]
- 63.Chen H.Y., Li X.Y., Liu B.J., Meng X.H. Microencapsulation of Lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. J. Funct. Foods. 2017;29:248–255. doi: 10.1016/j.jff.2016.12.015. [DOI] [Google Scholar]
- 64.Homayouni-Rad A., Mortazavian A.M., Mashkani M.G., Hajipoura N., Pourjafar H. Effect of Alyssum homolocarpum mucilage and inulin microencapsulation on the survivability of Lactobacillus casei in simulated gastrointestinal and high temperature conditions. Biocatal. Agric. Biotechnol. 2021;35:102075. doi: 10.1016/j.bcab.2021.102075. [DOI] [Google Scholar]
- 65.Leahy S.C., Higgins D.G., Fitzgerald G.F., van Sinderen D. Getting better with bifidobacteria. J. Appl. Microbiol. 2005;98:1303–1315. doi: 10.1111/j.1365-2672.2005.02600.x. [DOI] [PubMed] [Google Scholar]
- 66.Fujisawa T., Ohashi Y., Shin R., Narai-Kanayama A., Nakagaki T. The effect of soymilk intake on the fecal microbiota, particularly Bifidobacterium species, and intestinal environment of healthy adults: A pilot study. Biosci. Microbiota Food Health. 2017;36:33–37. doi: 10.12938/bmfh.16-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wada K., Watabe J., Mizutani J., Tomoda M., Suzuki H., Saitoh Y. Effects of soybean oligosaccharides in a beverage on human fecal flora and metabolites. Nippon Nōgeikagaku Kaishi. 1992;66:127–135. doi: 10.1271/nogeikagaku1924.66.127. (In Japanese with English abstract) [DOI] [Google Scholar]
- 68.Uriot O., Galia W., Awussi A.A., Perrin C., Denis S., Chalancon S., Lorson E., Poirson C., Junjua M., Le Roux Y., et al. Use of the dynamic gastro-intestinal model TIM to explore the survival of the yogurt bacterium Streptococcus thermophilus and the metabolic activities induced in the simulated human gut. Food Microbiol. 2016;53:18–29. doi: 10.1016/j.fm.2015.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.