Abstract
Cutaneous fungal infection of the skin and nails poses a significant global public health challenge. Dermatophyte infection, mainly caused by Trichophyton spp., is the primary pathogenic agent responsible for skin, hair, and nail infections worldwide. The epidemiology of these infections varies depending on the geographic location and specific population. However, epidemiological pattern changes have occurred over the past decade. The widespread availability of antimicrobials has led to an increased risk of promoting resistant strains through inappropriate treatment. The escalating prevalence of resistant Trichophyton spp. infections in the past decade has raised serious healthcare concerns on a global scale. Non-dermatophyte infections, on the other hand, present even greater challenges in terms of treatment due to the high failure rate of antifungal therapy. These organisms primarily target the nails, feet, and hands. The diagnosis of cutaneous fungal infections relies on clinical presentation, laboratory investigations, and other ancillary tools available in an outpatient care setting. This review aims to present an updated and comprehensive analysis of the epidemiology, clinical manifestations, and diagnostic testing methods for cutaneous fungal infections caused by dermatophytes and non-dermatophytes. An accurate diagnosis is crucial for effective management and minimizing the risk of antifungal resistance.
Keywords: clinical, cutaneous fungal infection, dermatophyte, diagnosis, epidemiology, microsporum, non-dermatophyte, onychomycosis, tinea, trichophyton
1. Introduction
Cutaneous fungal infections, caused by dermatophyte and non-dermatophyte fungi, pose significant challenges to global public health. Dermatophyte infections primarily affect the skin, hair, and nails. In recent years, there has been a concerning rise in the prevalence of antifungal-resistant species within certain ethnic groups, further complicating the management of these infections. One example is the emergence of Trichophyton indotineae in the Indian population. Non-dermatophyte infections require specific diagnostic criteria to differentiate them from contamination. These organisms can infect the nails and skin on the hands and feet, presenting as potential pathogens. The occurrence of these two major pathogenic fungal infections is not uncommon.
Clinical manifestations can provide valuable guidance in identifying the specific fungal pathogen and initiating management. This is particularly relevant in regions where conventional techniques for fungal identification remain widely used, such as in middle to low-income countries.
This review aims to provide an updated literature analysis focusing on the epidemiology, clinical manifestations, and diagnostic testing of dermatophyte and non-dermatophyte cutaneous fungal infections.
2. Epidemiology
2.1. Dermatophyte Infections
Cutaneous fungal infections have been reported worldwide, affecting an estimated 20–25% of the global population [1]. In geographic regions with high prevalence, the incidence of dermatophytosis can reach as high as 40–60% [2,3]. Various factors, such as age, sex, climate, urban environment, socioeconomic level, and cultural habits can contribute to the occurrence of dermatophytosis.
In 1934, dermatophytes were classified by Chester Emmons into three genera based on spore morphology and accessory organs: Trichophyton, Microsporum, and Epidermophyton [4]. However, with advancements in phylogenetic analysis, a total of nine genera have now been identified: Trichophyton, Epidermophyton, Nannizia, Paraphyton, Lopophyton, Microsporum, Arthroderma, Ctenomyces, and Guarromyces [5]. Nevertheless, the primary pathogens that predominantly infect humans belong to the four genera of Trichophyton, Microsporum, Epidermophyton, and Nannizzia. Additionally, these organisms can be further classified based on their primary habitat into anthropophilic, zoophilic, and geophilic species (see Table 1).
Table 1.
| Habitat | Anthropophilic Dermatophytes |
Zoophilic Dermatophytes |
Geophilic Dermatophytes |
|---|---|---|---|
| Dermatophyte species | Trichophyton concentricum | Trichophyton benhamiae | Nannizzia aenygmaticum |
| Trichophyton indotineae | Trichophyton bullosum | Nannizzia corniculata | |
| Trichophyton interdigitale | Trichophyton equinum | Nannizzia fulva | |
| Trichophyton rubrum | Trichophyton eriotrephon | Nannizzia gypsea | |
| Trichophyton schoenleinii | Trichophyton erinacei | Nannizzia incurvata | |
| Trichophyton soudanense | Trichophyton mentagrophytes | Nannizzia praecox | |
| Trichophyton tonsurans | Trichophyton quinckeanum | Paraphyton cookei | |
| Trichophyton violaceum | Trichophyton simii | Paraphyton cookiellum | |
| Epidermophyton floccosum | Trichophyton verrucosum | Arthroderma ciferrii | |
| Microsporum andouinii | Nannizzia nana | Arthroderma cuniculi | |
| Microsporum ferrugineum | Nannizzia persicolor | Arthroderma curreyi | |
| Arthroderma onychocola | Paraphyton mirabile | Arthroderma eboreum | |
| Lophophyton gallinae | Arthroderma gertleri | ||
| Microsporum canis | Arthroderma gloriae | ||
| Arthroderma amazonicum | Arthroderma insingulare | ||
| Arthroderma flavescens | Arthroderma lenticulare | ||
| Arthroderma redellii | Arthroderma melis | ||
| Arthroderma vespertilii | Arthroderma multifidum | ||
| Arthroderma phaseoliforme | |||
| Arthroderma quadrifidum | |||
| Arthroderma thuringiensis | |||
| Arthroderma tuberculatum | |||
| Arthroderma uncinatum |
In the 20th century, Microsporum audouinii and Trichophyton schoenleinii were the primary pathogenic organisms responsible for tinea capitis in the British Isles, Northern and Western Europe, and the Americas before 1950 [7]. During that period, Trichophyton mentagrophytes were the leading cause of tinea pedis and tinea corporis. By the late 20th century, T. rubrum had emerged as the predominant global agent, with a prevalence of over 40–70% in Central and North European countries, followed by T. mentagrophytes [8,9]. This trend was similar in the United States of America and Southeast Asia [10,11]. Southern Europe, on the other hand, had Microsporum canis and Trichophyton verrucosum as the most commonly isolated zoophilic dermatophytes [8]. In Western Asia, the most frequently isolated organism was Epidermophyton floccosum [12], while Trichophyton violaceum was the chief causative agent in Africa [13].
Moving into the 21st century, anthropophilic dermatophytes such as E. floccosum, M. audouinii, and T. schoenleinii saw a decline in prevalence in European countries, being replaced by various Trichophyton species [14]. T. rubrum emerged as the predominant organism worldwide, affecting Europe, South America, Asia, and Africa. The incidence of T. rubrum infection has significantly increased since the 20th century, predominantly affecting individuals between the ages of 20 and 60, with a tendency to infect older patients in more recent years [15]. T. rubrum is responsible for a wide range of fungal infections, including tinea pedis, onychomycosis, tinea cruris, tinea corporis, and tinea manuum [3,15,16,17,18,19,20,21,22]. The most commonly affected areas are the feet and toenails. Several risk factors contribute to T. rubrum infection, including the use of occlusive footwear and hot, humid weather conditions, which are particularly conducive to the spread of the infection during the summer season [15]. Other causative organisms vary depending on the site of infection and regional factors, as outlined in Table 2 [3,16,17,18,19,20,21,22].
2.2. Non-Dermatophyte Infections
In addition to dermatophyte infections, non-dermatophytes have long been acknowledged as saprophytic organisms prevalent in soil and the environment. They are renowned for their ability to flourish in fungal culture media. However, recent reports have identified them as causative pathogens in cases of onychomycosis, tinea pedis, and tinea manuum. To differentiate these organisms as genuine pathogens rather than mere contaminants, precise diagnostic criteria have been established [23,24,25]. Notably, non-dermatophytes have increasingly been recognized as significant contributors to the development of onychomycosis. The prevalence of non-dermatophyte infections is detailed in Section 3.7 below. Table 3 provides an overview of non-dermatophyte distribution across different countries.
2.3. Resistant Cutaneous Dermatophytosis
Over the past decade, there has been a noticeable increase in the prevalence of cutaneous dermatophytosis in India, with rates ranging from 6.0% to 61.5% depending on the specific region [2]. Notably, there is mounting evidence of the T. mentagrophytes complex replacing T. rubrum as the dominant species [26,27]. Of particular global concern is the emergence of Trichophyton indotineae, a newly identified species within the T. mentagrophytes complex (T. mentagrophytes ITS type VIII) [28]. A multicenter study conducted in India, involving clinically diagnosed dermatophytosis patients and molecular analysis of 351 specimens, reported that T. indotineae was the most prevalent species (90%), followed by T. rubrum (5%) and T. mentagrophytes/T. interdigitale (5%) [29]. T. indotineae has demonstrated reduced susceptibility to azole drugs and resistance to terbinafine, with over 50% of the strains exhibiting resistance [29,30]. Reports of T. indotineae have also emerged in the Middle East (Iran), Asia, and Europe in recent years [31]. Furthermore, other species within the T. mentagrophytes complex as well as T. rubrum have exhibited evidence of terbinafine-resistant and azole-resistant mechanisms attributed to SQLE gene mutations [32,33,34,35].
Table 2.
Epidemiology of positive dermatophyte fungal isolations from cutaneous dermatophytosis in the 21st century.
| Disease | Europe | South America | ||||
|---|---|---|---|---|---|---|
| Switzerland [17] (2001–2018) |
Ireland [18] (2001–2020) |
Slovakia [36] (2014–2016) |
Germany [37] (2014–2016) |
Brazil [19] (2011–2019) |
Argentina [38] (2002–2007) |
|
| N = 10,958 | N = 2263 | N = 2103 | N = 1252 | N = 10,396 | N = 1313 | |
| Tinea capitis | n = 830 | n = 100 | n = 44 (including tinea faciei) | n = 28 | n = 435 | n = 269 |
| 1. T. violaceum | 1. T. tonsurans | 1. T. mentagrophytes | 1. T. mentagrophytes | 1. T. tonsurans | 1. M. canis | |
| 2. M. audouinii | 2. M. canis | 2. M. canis | 2. M. canis | 2. M. canis | 2. T. mentagrophytes | |
| 3. T. soudanense | 3. T. rubrum | 3. M. audouinii | 3. T. benhamiae | 3. N. gypsea | 3. N. gypsea | |
| Tinea faciei | n = 283 | n = 10 | - | n = 14 | n = 151 | - |
| 1. T. mentagrophytes | 1. T. tonsurans | 1. T. rubrum | 1. T. rubrum | |||
| 2. T. benhamiae | 2. T. verrucosum | 2. T. benhamiae | 2. N. gypsea | |||
| 3. T. rubrum | - | 3. M. canis | 3. T. interdigitale | |||
| Tinea corporis | n = 1006 | n = 64 | n = 169 | n = 185 | n = 1148 | n = 202 |
| 1. T. mentagrophytes | 1. T. rubrum | 1. T. tonsurans | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. rubrum | 2. M. canis | 2. T. rubrum | 2. T. benhamiae | 2. M. canis | 2. T. mentagrophytes | |
| 3. M. canis | 3. T. tonsurans | 3. T. mentagrophytes | 3. T. interdigitale | 3. T. tonsurans | 3. M. canis | |
| Tinea manuum | n = 169 | n = 19 | n = 100 | n = 48 | n = 231 | n = 26 |
| 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. mentagrophytes | |
| 2. T. mentagrophytes | 2. T. verrucosum | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. interdigitale | 2. T. rubrum | |
| 3. T. benhamiae | - | 3. T. tonsurans | 3. T. benhamiae | 3. T. tonsurans | 3. Trichophyton spp. | |
| Tinea cruris | n = 427 | n = 6 | n = 245 | - | n = 588 | n = 53 |
| 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | ||
| 2. T. mentagrophytes | 2. E. floccosum | 2. T. interdigitale | 2. T. interdigitale | 2. M. canis | ||
| 3. M. canis | - | 3. E. floccosum | 3. T. tonsurans | 3. T. mentagrophytes | ||
| Tinea pedis | n = 2439 | n = 134 | n = 649 | n = 398 | n = 3222 | n = 77 |
| 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. interdigitale | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. interdigitale | 2. T. interdigitale | 2. T. interdigitale | |
| 3. E. floccosum | 3. T. interdigitale | 3. T. mentagrophytes | 3. T. mentagrophytes | 3. E. floccosum | 3. Trichophyton spp. | |
| Onychomycosis | n = 5803 | n = 1617 | n = 896 | n = 579 | n = 4621 | n = 671 |
| 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. interdigitale | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. interdigitale | 2. T. interdigitale | 2. T. interdigitale | |
| 3. T. soudanense | 3. T. interdigitale | 3. T. tonsurans | - | 3. T. mentagrophytes | 3. Trichophyton spp. | |
| Diseases | Asia | |||||
|
Iran [39] (2008–2010) |
Iran [40] (2010–2014) |
India [26] (2014–2015) |
China [21] (2004–2014) |
Japan [20] (2016) |
Thailand [16] (2014–2016) |
|
| N = 777 | N = 1535 | N = 66 | N = 588 | N = 1268 | N = 2350 | |
| Tinea capitis | n = 15 | n = 80 | n = 5 | n = 109 | n = 15 | n = 19 |
| 1. M. canis | 1. T. tonsurans | 1. T. tonsurans | 1. M. canis | 1. M. canis | 1. T. rubrum | |
| 2. T. tonsurans | 2. T. mentagrophytes | 2. T. violaceum | 2. T. mentagrophytes | 2. T. rubrum | 2. T. mentagrophytes | |
| 3. T. interdigitale | 3. T. rubrum | - | 3. T. violaceum | 3. T. tonsurans | 3. M. canis | |
| Tinea faciei | n = 9 | - | - | n = 22 | - | n = 50 |
| 1. T. tonsurans | 1. T. mentagrophytes | 1. T. rubrum | ||||
| 2. M. canis | 2. T. rubrum | 2. T. mentagrophytes | ||||
| 3. T. interdigitale | 3. M. canis | 3. M. canis | ||||
| Tinea corporis | n = 131 | n = 242 | n = 20 | n = 61 | n = 188 | n = 276 |
| 1. T. interdigitale | 1. T. tonsurans | 1. T. interdigitale | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. rubrum | 2. T. rubrum | 2. T. tonsurans | 2. M. canis | 2. M. canis | 2. M. canis | |
| 3. M. canis | 3. E. floccosum | 3. M. gypseum | 3. M. gypseum | 3. T. interdigitale | 3. T. mentagrophytes | |
| Tinea manuum | n = 16 | n = 155 | - | n = 20 | n = 19 | n = 54 |
| 1. T. interdigitale | 1. T. tonsurans | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | ||
| 2. T. rubrum | 2. E. floccosum | 2. M. canis | 2. T. interdigitale | 2. T. mentagrophytes | ||
| - | 3. T. verrucosum | - | - | 3. M. canis | ||
| Tinea cruris | n = 171 | n = 457 | n = 35 | n = 72 | n = 90 | n = 198 |
| 1. E. floccosum | 1. E. floccosum | 1. T. interdigitale | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. rubrum | 2. T. rubrum | 2. T. tonsurans | 2. M. gypseum | 2. E. floccosum | 2. T. mentagrophytes | |
| 3. T. interdigitale | 3. T. mentagrophytes | 3. T. rubrum | 3. T. mentagrophytes | 3. N. gypsea | 3. E. floccosum | |
| Tinea pedis | n = 353 | n = 466 | n = 4 | n = 105 | n = 665 | n = 716 |
| 1. T. interdigitale | 1. T. mentagrophytes | 1. T. interdigitale | 1. T. rubrum | 1. T. rubrum | 1. T. mentagrophytes | |
| 2. T. rubrum | 2. T. rubrum | 2. T. tonsurans | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. rubrum | |
| 3. E. floccosum | 3. E. floccosum | - | - | 3. E. floccosum | 3. E. floccosum | |
| Onychomycosis | n = 82 | n = 135 | n = 2 | n = 199 | n = 290 | n = 1137 |
| 1. T. rubrum | 1. T. rubrum | 1. T. interdigitale | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. interdigitale | 2. T. mentagrophytes | - | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. mentagrophytes | |
| 3. E. floccosum | 3. E. floccosum | - | - | 3. M. canis | 3. T. tonsurans | |
Note: Some studies identified Trichophyton mentagrophytes by conventional techniques whereas the others can distinguish Trichophyton interdigitale by molecular diagnosis.
Table 3.
Prevalence of non-dermatophyte onychomycosis in various countries during the 21st century.
| Organisms | Europe | South America | Africa | Asia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Switzerland [17] (2001–2018) |
Greece [41,42] (2004–2015) (2015–2017) |
Serbia [43] (2012–2014) |
Guatemala [44] (2008–2011) |
French Guiana [45] (2006–2009) |
Ethiopia [46] (2015–2019) |
Morocco [47] (2006–2010) |
Iran [48] (2007–2014) |
Israel [49] (2001–2015) |
China [50] (2001–2020) |
Thailand [51] (2014–2019) |
|
| N = 17,175 * | N = 1450 ** | N = 190 ** | N = 4220 ** | N = 205 * | N = 571 * | N = 1335 * | N = 648 * | N = 27,093 * | N = 32,190 * | N = 2740 ** | |
| Acremonium spp. | 1078 *** | 41 | - | 2 | - | 1 | - | 3 | 26 | - | - |
| Alternaria spp. | - | - | - | 1 | - | 3 | - | 3 | 15 | - | - |
| Aspergillus spp. | - | 1 | 2 | 11 | 2 | 28 | 14 | 108 | 53 | 958 | - |
| Cladosporium spp. | - | - | - | 3 | - | 14 | - | 2 | 1 | - | - |
| Fusarium spp. | 1078 *** | 10 | 1 | 1 | 5 | 14 | 6 | 7 | 14 | 106 | 253 |
| Neoscytalidium dimidiatum | - | - | 2 | - | 29 | 11 | 27 | - | - | - | 360 |
| Penicillium spp. | - | - | - | - | 1 | 14 | - | 3 | 2 | 496 | - |
| Scopulariopsis brevicaulis | - | 48 | 2 | 8 | 2 | 4 | - | 8 | 22 | 45 | - |
| Other molds | 6996 | - | 1 | 6 | 5 | - | 20 | 9 | 24 | 522 | - |
| Total | 8074 (47%) |
100 (6.9%) |
8 (4.2%) |
32 (0.8%) |
44 (21.5%) |
89 (15.6%) |
67 (5%) |
143 (22%) |
157 (0.6%) |
2127 (6.6%) |
N/A |
* Number of positive fungal isolations from onychomycosis without the mention of complete criteria for non-dermatophyte diagnosis. ** Number of patients diagnosed with onychomycosis with repetitive culture for non-dermatophyte infection. *** Number including both Acremonium spp. and Fusarium spp.
Although supporting evidence is limited, a previous study suggested that factors such as patient nonadherence, the widespread availability of topical antifungal-corticosteroid combinations, and improper use of antifungal agents may contribute to the promotion of antifungal resistance [52]. Another proposed mechanism of drug resistance in dermatophyte and non-dermatophyte infections is the formation of biofilms within the extracellular matrix. This phenomenon results in antimicrobial resistance, compromised function of host immune cells, and the formation of dermatophytomas on the nail plate [53].
3. Body Sites, Dermatophyte Species and Their Geographical Distribution
3.1. Tinea Capitis
Tinea capitis, a fungal infection of the scalp, is more commonly observed in school-age children than in adults. However, adult-onset tinea capitis has been associated with immunocompromised individuals [54]. This condition represents a major global public health concern, with varying prevalence rates across countries. The frequency of the disease ranges from 0.4% to 87.7% in Africa, 0.2% to 74.0% in America, 0.04% to 78.6% in Europe, and 0.01% to 91.2% in Asia [55].
In the African continent, tinea capitis exhibits regional variations in the predominant pathogens. In the northern region, T. violaceum and M. canis have been identified as the primary pathogens [56]. In the eastern and southern regions, T. violaceum and M. audouinii prevail, while in the western region, Trichophyton soudanense and M. audouinii are commonly observed [3].
In the Americas, Trichophyton tonsurans is the predominant causative organism in the United States, while M. canis is more prevalent in Mexico and Central America. These two organisms also dominate in South America [55]. European countries exhibit a predominance of zoophilic dermatophytosis, with M. canis and T. mentagrophytes being common across regions. T. soudanense and T. tonsurans are significant agents in France and Ireland, respectively, while T. violaceum is reported as the most prevalent species in Switzerland, Scotland, and Sweden [55].
Across Asia, there is considerable variation in the main pathogens. T. violaceum and T. tonsurans predominate in India, while T. mentagrophytes, T. violaceum, T. verrucosum, and M. canis have been identified as the primary agents in Western Asia. Eastern Asia shows a predominance of M. canis and T. violaceum, while Southeast Asia is characterized by M. canis as the chief pathogen. Notably, Thailand differs from other Southeast Asian countries, with T. rubrum and T. mentagrophytes being the predominant organisms [16,55,57].
3.2. Tinea Faciei
The prevalence and causative agents of tinea faciei vary across regions. The reported isolation rates range from 0.4% to 4.2% in various parts of the world [16,17,18,19,20,21,26,36,37,38,39,40,58,59], with more than 50% of cases observed in patients younger than 12 years of age [37,60]. In Europe, a study conducted in Portugal identified M. audouinii, T. soudanense, and T. rubrum as the main pathogenic agents [60]. Other European countries have reported zoophilic dermatophytes as the primary causative agents, such as T. mentagrophytes in Switzerland and Slovakia, and M. canis in Italy and Greece [17,36,61,62]. T. rubrum was predominant in Germany and Brazil [19,37]. In Asia, Trichophyton spp., including T. rubrum, T. mentagrophytes, and T. tonsurans, were identified as the main pathogenic agents [16,21,39,63].
Tinea barbae, a relatively rare form of tinea faciei, refers to a fungal infection affecting the hair follicles in the beard area of adult men. The main causative agents identified include Trichophyton spp., such as T. mentagrophytes, T. rubrum, T. tonsurans, and Trichophyton benhamiae [64,65,66,67]. Cases have been reported in Europe, the Americas, and Asia.
3.3. Tinea Corporis
Tinea corporis, a fungal infection of the body, poses a significant global public health challenge. The primary causative agents of tinea corporis worldwide in recent years are Trichophyton spp., with T. rubrum being the most common, followed by the T. mentagrophytes complex, M. canis, T. tonsurans, and T. benhamiae [16,17,18,19,20,21,37,39]. In Africa, the estimated prevalence ranges from 2% to 41% [3]. In other regions, the prevalence of tinea corporis among isolated dermatophytes is estimated to be between 2.8% and 30.3% (Table 2) [16,17,18,19,20,21,26,36,37,38,39,40,58,59]. Notably, in India, there has been a shift in prevalence over the past two decades, with T. mentagrophytes emerging as a replacement for T. rubrum [27,68].
Tinea corporis manifests in various clinical variants. Majocchi granuloma, which primarily affects females in a ratio of 3:1 and occurs in the age range of 20–35 years, represents a deep dermatophyte folliculitis. Trauma (such as leg shaving) and immunosuppression are associated factors. While T. rubrum is the usual causative agent, other species of Trichophyton spp. or Microsporum spp. have been reported [69].
Tinea gladiatorum is a dermatophytosis commonly observed in contact sports athletes, wrestlers, and their family members. The prevalence of dermatophytosis in wrestlers varies from 2.4% to 100%, as reported in the United States, Iran, and Japan, with over 90% of cases being asymptomatic carriers [70,71]. The primary pathogenic agent in tinea gladiatorum is T. tonsurans. Factors associated with this condition include excessive sweating, poor hygiene, and contaminated training mats [70].
Tinea imbricata presents a unique clinical appearance characterized by concentric annular rings with flaking skin. It is caused by Trichophyton concentricum and has been reported in Asia, Oceania, the Middle East, and South America [72]. The prevalence of tinea imbricata varies across countries and regions. The highest prevalence (18.3–20.1%) has been documented among indigenous people or tribes in Malaysia and Indonesia [73,74].
3.4. Tinea Cruris
Tinea cruris, a common fungal infection affecting the groin region, is prevalent worldwide, particularly in warm and humid areas [58]. The estimated prevalence of cutaneous fungal infections ranges from 0.3% to 53.0% [16,17,18,19,20,21,26,36,37,38,39,40,58,59]. High prevalence rates exceeding 20% have been documented in regions such as Asia and the Middle East, including countries such as China and Iran [39,58]. In India, tinea cruris has been reported as the most prevalent form of chronic and recurrent dermatophytosis, affecting up to 80% of cases [75].
The primary causative agent worldwide is T. rubrum, followed by other common pathogens such as T. mentagrophytes and E. floccosum. Interestingly, the epidemiology of tinea cruris exhibits regional variations. Evidence suggests a decline in the prevalence of E. floccosum in certain regions, such as Germany, Chile, and Thailand, where the prevalence has decreased from 12.6% to 2.7% over 30 years [8,11,16,76]. However, in Iran, E. floccosum continues to be the chief pathogen responsible for tinea cruris [39,40]. Major predisposing factors include excessive sweating, diabetes, and obesity [77]. The development of the disease can be influenced by autoinfection from tinea pedis and onychomycosis, as these conditions share similar causative agents.
3.5. Tinea Manuum
Tinea manuum is a cutaneous fungal infection affecting the hand. The estimated prevalence of cutaneous dermatophytosis is 0.8% to 12.6% [16,17,18,19,20,21,26,36,37,38,39,40,58,59]. The most common pathogen worldwide is T. rubrum, followed by zoophilic dermatophytes such as T. mentagrophytes and M. canis. In recent years, there have been emerging case reports of Trichophyton erinacei from various regions across the globe [78,79,80,81]. This particular agent is frequently isolated from hedgehogs and can cause widespread infection with increased virulence among immunocompromised patients [82].
3.6. Tinea Pedis
Tinea pedis, commonly known as fungal foot infection, typically affects the feet, primarily involving the sole, interdigital toe web, and dorsal surface of the foot. Major risk factors for this condition include older age, male sex, obesity, low level of education, low income, physical disability, and specific populations exposed to sweating, occlusive footwear, or contaminated floors in communal settings [83,84,85,86]. The estimated prevalence of tinea pedis among cutaneous dermatophytosis ranges from 5.9% to 52.4% [16,17,18,19,20,21,26,36,37,38,39,40,58]. Dermatophytes, particularly Trichophyton species, account for the majority of causative agents, comprising approximately 36.8% to 70.5% [87,88,89]. T. rubrum is the most common pathogen worldwide, followed by T. mentagrophytes/T. interdigitale. The prevalence of these T. mentagrophytes complex has shown an increasing trend in the past 20 years [85]. Previous studies in specific populations, such as naval cadets, have reported T. mentagrophytes/T. interdigitale as the most prevalent species [86,90]. Non-dermatophytes have been reported in 8.02% to 57.9% of cases, with higher rates observed in tropical regions such as Africa and Southeast Asia [87,88,89]. Common agents causing non-dermatophyte infections on the feet include Neoscytalidium dimidiatum and Fusarium species. Tinea pedis is also frequently observed as a concomitant infection with onychomycosis [85,91].
3.7. Onychomycosis
Onychomycosis is a chronic or relapsing fungal infection of the nail, predominantly affecting geriatrics. The global prevalence in the general population is 5.5%, varying depending on population heterogeneity [92]. In the United States, the estimated prevalence ranges from 1.6% to 13.8% [93,94]. A systematic review reported a mean prevalence of 4.3% based on population-based studies in Europe and North America, while hospital-based studies showed a mean prevalence of 8.9% [95]. Recent studies on cutaneous fungal infections have identified onychomycosis as having the highest prevalence among other cutaneous dermatophytosis, ranging from 3.0% to 82.9% [16,17,18,19,20,21,26,36,37,38,39,40,58].
The dermatophyte group is the most commonly isolated agent worldwide and is responsible for 60% to 70% of cases. T. rubrum is the predominant species (accounting for approximately 50% or more), followed by T. mentagrophytes and T. interdigitale [95,96]. Although M. canis is a rare etiological agent, it has been reported in cases of fingernail onychomycosis, particularly in younger patients with a history of contact with an infected pet [97]. Non-dermatophyte onychomycosis is more challenging to treat than dermatophyte infections due to the high failure rate of antifungal therapy [51,53].
Non-dermatophytes are globally recognized as causative agents, with their prevalence ranging from 0.8% to 65.8% [44,95,98,99,100]. In specific regions, such as Brazil [99], Sri Lanka [101], and Thailand [88], non-dermatophytes have been detected in up to 51.6% to 68.2% of cases. In contrast, Europe reports a lower prevalence, ranging from 4% to 7% [41,42,43]. The spectrum of pathogenic agents also varies across regions. In Europe, Scopulariopsis brevicaulis, Aspergillus spp., Acremonium spp., and Fusarium spp. are the most commonly identified non-dermatophytes [41,42,43,102], with Fusarium spp. accounting for a prevalence of up to 7.5% to 9.2% of onychomycosis cases [51,102,103,104]. In tropical regions such as Thailand, N. dimidiatum is the primary pathogen [88,98], reported with a prevalence of 13% in onychomycosis cases [51]. Table 3 provides an overview of other pathogenic non-dermatophytes observed across countries.
Risk factors associated with onychomycosis include the following [92]:
Patients’ medical attributes: advanced age, genetic susceptibility, foot deformities, and comorbidities such as diabetes, immunosuppression, venous insufficiency, peripheral arterial diseases, malignancy, and obesity.
Dermatologic conditions: previous or concurrent tinea pedis, psoriasis, and hyperhidrosis.
Exogenous factors: trauma, poor nail grooming, participation in sports activities, occupational exposure, smoking, and wearing occlusive footwear.
A previous study on diabetes patients found that older age, agricultural-related activities, family history of dermatophytosis, and comorbidities such as coronary heart disease were associated with onychomycosis and tinea pedis [90].
4. Clinical Presentations
4.1. Tinea Capitis
Tinea capitis presents various clinical manifestations depending on the causative agents and the severity of the disease. It predominantly affects children aged 6 months to 12 years [105]. The clinical variants are categorized into three forms based on the level of hair shaft invasion, and in outbreak infections, a mixed-type clinical pattern may be observed [106]. In adult patients, nearly 40% exhibit concurrent dermatophytosis at other sites [57].
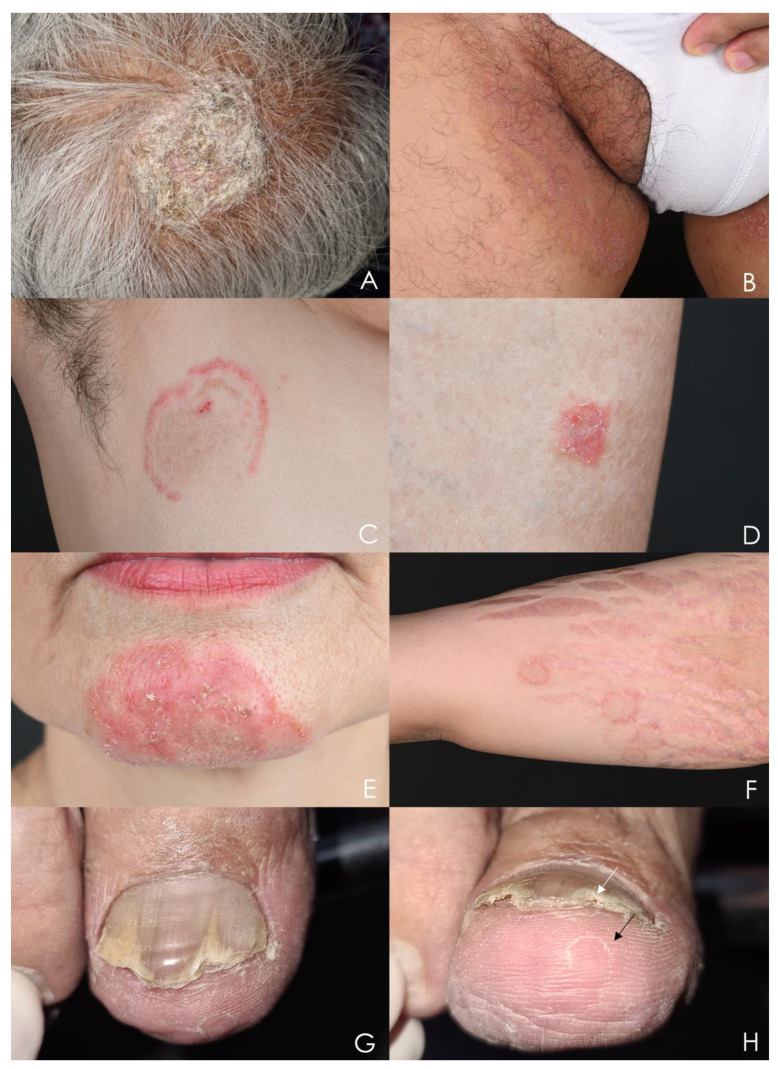
The ectothrix form is caused by fungal pathogens that invade the mid-follicle level of the hair shaft and form a sheath around the hair [105]. Clinical presentations include solitary or multiple gray patches with scales and circular alopecia patches with breaking hair shafts above the scalp level (Figure 1A). The causative agents are Microsporum spp. and certain Trichophyton spp. In M. audouinii infection, the degree of inflammation is minimal, resulting in only fine scales. Zoophilic organisms such as M. canis or Trichophyton verrucosum commonly lead to greater inflammation and can present with pustules, furuncles, or kerion. Kerion is a severe, painful inflammatory mass that can cause scarring alopecia.
Figure 1.
Clinical presentations of cutaneous fungal infection. (A) Tinea capitis: Gray patch on the vertex of the scalp. (B) Tinea cruris: Multiple annular scaly erythematous patches on the groin. (C) Tinea incognito: Ring-within-a-ring appearance. (D) Tinea corporis: Annular scaly erythematous macule with inflammation on the right leg caused by Microsporum canis. (E) Tinea faciei: Multiple scaly erythematous concentric rings on the chin caused by Microsporum canis. (F) Tinea corporis: Multiple red-rubber-ring macules on the right arm caused by Trichophyton mentagrophytes. (G) Dorsal view of the right big toenail: Onychomycosis caused by Trichophyton mentagrophytes, showing dermatophytoma and longitudinal striae adjacent to the dermatophytoma. (H) Hyponychium view of the right big toenail of the same patient: Sulfur-nugget-like subungual debris (white arrow) concurrent with tinea pedis presenting as an annular scaly macule (black arrow).
The endothrix form typically occurs when hyphae invade the hair shaft without damaging the cortex and cuticle [105]. This form is characterized by multiple alopecia patches with broken hairs at the level of the hair follicle opening, resulting in the appearance of black dots. Common causes include T. tonsurans, T. soudanense, and T. violaceum.
Favus form is a fungal infection caused by T. schoenleinii. It is characterized by fungal hyphae growing longitudinally along the hair shaft, with air spaces within [105,107]. Favus presents with scutula, which are yellow cup-shaped crusts containing the hyphae, on the follicular opening of the hair shaft. The lesions can sometimes enlarge, forming a confluent mass of pale powdery crusts. Without treatment, chronic disease progression can lead to scarring alopecia.
4.2. Tinea Coporis, Tinea Faciei, and Tinea Cruris
The clinical presentation of superficial fungal infections of the skin is characteristic and typical. Tinea corporis, tinea faciei, and tinea cruris typically manifest as solitary or multiple annular scaly erythematous macules and patches that progress with central clearing (Figure 1B). An additional sign of anthropophilic cutaneous dermatophytosis is a ring-within-a-ring appearance (Figure 1C). In contrast, zoophilic dermatophytosis exhibits more inflammation and rapid progression (Figure 1D,E). Clues that may indicate zoophilic dermatophytosis include a history of contact with pets, involvement of exposed areas, presence of vesicles or pustular lesions, and a red-rubber-ring appearance (Figure 1F) [108].
Other forms of tinea corporis present with distinct clinical manifestations. Majocchi granulomas appear as erythematous or violaceous papules, plaques, or nodules, typically on the legs and forearms. These lesions are often painful or pruritic and exhibit more inflammation despite primarily being caused by anthropophilic dermatophytes [69]. Tinea imbricata starts as a solitary concentric, ring-like scaly macule with slow progression into a patch or plaque with or without pruritus. The rash is commonly found on the trunk or extremities [72]. Patients who have previously applied topical corticosteroids may develop a condition known as tinea incognito. Using steroids can modify the rash, resulting in the disappearance of an annular scaly border and the progression to a diffuse erythematous rash or follicular papules or pustules that mimic other dermatologic conditions [109]. Although approximately half of the patients may present with a ring-within-a-ring appearance, the difference is insignificant compared to patients without prior topical steroid usage [108].
4.3. Tinea Manuum, Tinea Pedis
Dermatophytosis on the hands and feet exhibits distinct clinical features depending on the sites of infection. The symptoms and signs of dorsal surface infection are similar to those observed in tinea corporis due to the similarities in skin structure [110]. However, the thick keratinized epidermis and numerous eccrine glands on the palms and soles give rise to different manifestations [107].
Tinea manuum commonly presents as dry, scaly macules or patches, sometimes accentuated in the flexural creases of the palms. These lesions can be localized or diffuse and may affect one or both hands. In cases of zoophilic dermatophytosis infection, more inflammatory lesions, such as erythematous, pustular, or vesicular formations, may occur. Tinea manuum can also manifest as two feet-one hand syndrome, indicating concurrent tinea pedis and onychomycosis [111].
Tinea pedis, or athlete’s foot, exhibits three clinical subtypes and can involve one or both feet. The moccasin type is a chronic mild form characterized by small collarette scaly macules or diffuse, dry, scaly lesions with or without inflammation. This type is commonly associated with anthropophilic dermatophytosis, particularly T. rubrum infection. The vesicular type resembles a dyshidrotic reaction and spontaneously regresses with collarette scaling. In more severe cases, inflammatory bullous lesions may develop. This more inflammatory type is usually caused by zoophilic dermatophytosis. The last clinical subtype is interdigital tinea pedis, which presents as scaly whitish patches with fissuring or moist maceration in the interdigital toe web spaces. The fourth interdigital web space is commonly affected. Concurrent onychomycosis, especially at the same site affected by tinea pedis, is common, highlighting the need for toenail examination in most cases [107].
4.4. Onychomycosis
Onychomycosis primarily affects the toenails in geriatrics, with the great toenail being the most commonly involved [92]. However, fingernail onychomycosis has a higher prevalence among younger individuals [112]. Distal lateral subungual onychomycosis is the most frequently observed clinical subtype, followed by total nail dystrophy, proximal subungual onychomycosis, and superficial white onychomycosis [50]. Typical presentations are onycholysis, subungual hyperkeratosis, and nail discoloration [92]. Chronic and long-standing onychomycosis can lead to extensive onychodystrophy.
In distal lateral subungual onychomycosis, a characteristic feature called dermatophytoma can be observed. It presents as a fungal abscess, appearing as a white- or yellow-colored, triangular, longitudinal streak in the nail plate (Figure 1G). However, dermatophytosis-like traumatic onychodystrophy can also occur without fungal infection. Differentiation between dermatophytoma onychomycosis and other conditions can be guided by specific characteristics. Dermatophytoma onychomycosis typically exhibits inhomogeneous colored, longitudinal striae adjacent to the dermatophytoma, as well as sulfur-nugget-like subungual debris visible on hyponychium view (Figure 1H) [113]. Approximately one-third of patients with onychomycosis also have concomitant tinea pedis (Figure 1H) [85]. Therefore, considering the presence of tinea pedis can assist in the clinical diagnosis, and simultaneous treatment of both conditions is necessary for complete resolution.
One of the challenges in managing onychomycosis lies in distinguishing dermatophyte from non-dermatophyte infections through physical examination alone. Unfortunately, there are no significant differences in clinical nail characteristics. However, it is worth noting that patients with non-dermatophyte onychomycosis typically lack fungal glabrous skin infections in areas other than the feet [98]. For instance, compared to Neoscytalidium onychomycosis, Fusarium onychomycosis is more commonly associated with a history of pedicure, predominant lateral nail involvement, and the absence of concurrent foot infection [51].
5. Diagnostic Testing
5.1. Direct Microscopic Examination
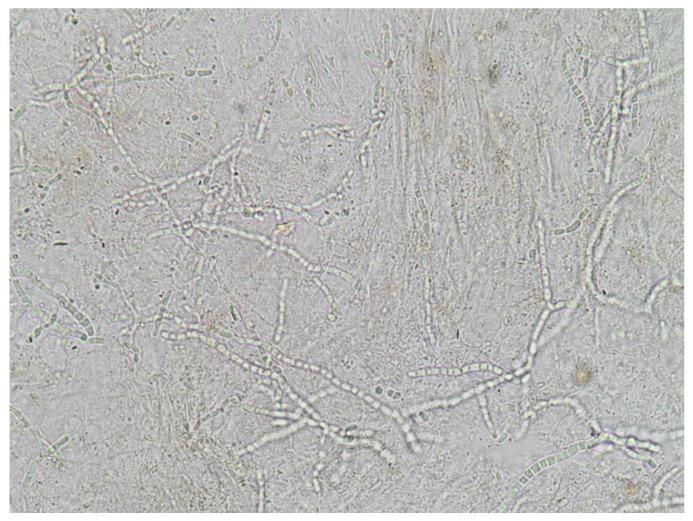
A mycological examination is essential for a definitive diagnosis prior to initiating treatment. This method has proven cost-effective, considering the potential risks associated with systemic drug use [114]. Potassium hydroxide (KOH) examination is a simple bedside technique that involves collecting a sample by scraping the keratin from an active border area of the rash or nail. The sample is then prepared with KOH and examined under a light microscope. Branching septate hyphae can be observed on the slide (Figure 2). Although the accuracy of this method relies on the experience of technicians [115], conducting repeated examinations can improve diagnostic potential [116]. The sensitivity ranges from 67% to 93%, while the specificity ranges from 38% to 78% [92].
Figure 2.
Potassium hydroxide examination with the 40× objective revealing branching septate hyphae under a light microscope.
5.2. Histopathology
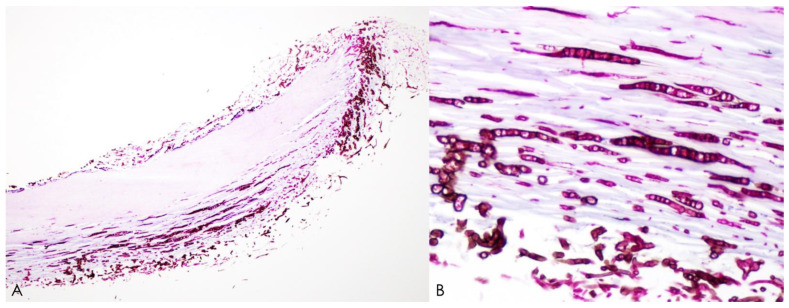
Histopathology with Periodic acid-Schiff or Grocott methenamine silver stains obtained from nail clipping is the convenient method, and high sensitivity is reported (92–98.8%) [43]. The fungal hyphae penetrating into the nail plate is obvious, allowing for diagnosis (Figure 3). However, both KOH examination and histology cannot identify whether the fungus is viable or not.
Figure 3.
Histopathological analysis of nail clippings from Neoscytalidium dimidiatum-induced onychomycosis using periodic acid–Schiff staining. Microscopic examination at low power with the 4× objective (A) and high power with the 40× objective (B) revealing black-brown fungal hyphae invading the nail plate.
5.3. Fungal Culture
Fungal culture is a definitive method for identifying fungi and detecting viable organisms. Sabouraud dextrose agar is commonly used as a culture medium for non-dermatophyte growth, while Sabouraud dextrose agar containing cycloheximide is used for dermatophyte growth [92]. This technique is widely available in dermatology clinics worldwide. The fungal species can be identified by observing the distinct morphological colonies on the culture media and examining the characteristics of conidia under a light microscope. However, there are limitations: the time required for fungal identification from colony cultures, false negative results, and the potential growth of various non-dermatophyte contaminants, especially in nail samples [117]. The overall sensitivity ranges from 23.8% to 79.3%, while the specificity ranges from 83% to 100% [92,118].
For the diagnosis of non-dermatophyte onychomycosis, it has been proposed that at least three of the following six criteria be met [102]:
identification of fungal hyphae in KOH examination
isolation of non-dermatophytes in culture
repeated isolation of the same non-dermatophytes in fungal culture
growth of the inoculum in at least 5 out of 20 fragments
absence of dermatophyte isolation in culture
supporting evidence of onychomycosis from histology
While these criteria are widely used for diagnosis, a previous study reported that mixed dermatophyte and non-dermatophyte infections are common, with a prevalence ranging from 20% to 40% in onychomycosis [119]. The most common mixed organisms were T. mentagrophytes and N. dimidiatum (53.2%), followed by T. rubrum and N. dimidiatum (21.3%). Treatment for mixed infections usually requires a longer duration than pure dermatophyte infections.
5.4. Molecular Diagnosis
Molecular diagnosis has become a well-known technique in scientific research, aiming to enhance the speed, cost-effectiveness, and convenience of identifying fungi with minimal technical effort [120]. Various molecular techniques have been developed: conventional polymerase chain reaction (PCR), real-time PCR, nested PCR, multiplex PCR, PCR enzyme-linked immunosorbent assay, and PCR-restricted fragment length polymorphism. Notably, significant advancements have been made in molecular characterizations, such as DNA extraction and DNA sequencing, utilizing internal transcribed spacer polymorphisms within the ITS rDNA regions. These advancements have enabled reliable differentiation of various species, particularly in cases of T. rubrum and other dermatophyte infections [121,122]. These methods offer high sensitivity (95%) and specificity (100%) for dermatophyte species identification [92]. Furthermore, advances have been made in identifying non-dermatophyte pathogens [35,120]. The application of multiplex PCR for the diagnosis of Fusarium spp. onychomycosis has demonstrated high efficiency, sensitivity, and specificity [123]. However, challenges remain, including the requirement for specialized laboratory equipment and the difficulty in differentiating true pathogens from contaminants [102]. Nevertheless, commercial PCR kits have shown promise through their cost-effectiveness and availability, with increasing usage in several countries [92].
5.5. Dermoscopy
Dermoscopy is a valuable tool for dermatologic examinations at the bedside, aiding the diagnosis of onychomycosis and tinea capitis. Dermoscopy findings also help diagnose tinea capitis and distinguish it from other types of alopecia [113]. The hyponychium view reveals subungual hyperkeratosis with sulfur nuggets, characterized by yellow clumping debris with a crumbled appearance, supporting the diagnosis of onychomycosis [124]. On the dorsal view, distinct features such as distal subungual longitudinal streaks, longitudinal striae adjacent to dermatophytoma, spikes at the proximal margin of an onycholytic area, and brown and black nail discoloration are indicative of onychomycosis [124,125].
Dermoscopy findings also help diagnose tinea capitis and distinguish it from other types of alopecia. Multiple comma-shaped hairs, dystrophic and elbow-shaped hairs, varying levels of broken hair resembling Morse code, and other specific hair patterns strongly support the diagnosis of tinea capitis [105].
5.6. Wood’s Light Examination
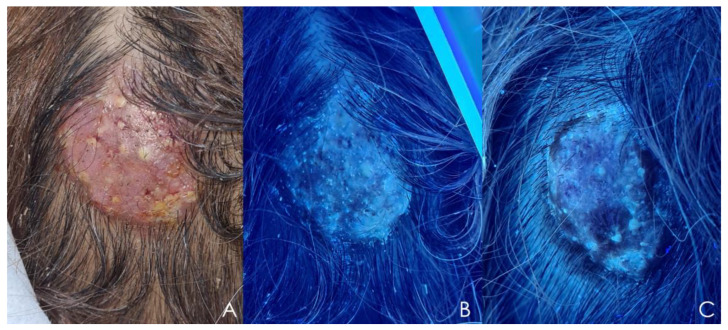
Wood’s light examination can support the diagnosis of cutaneous dermatophytosis, particularly tinea capitis caused by Microsporum spp. and T. schoenleinii infections [105]. Microsporum spp. emit a blue-green fluorescence (Figure 4B), while T. schoenleinii exhibits a light-blue fluorescence under Wood’s light [126].
Figure 4.
Kerion caused by Microsporum canis. (A) Clinical presentation: Solitary inflammatory mass with alopecia. (B) Wood’s light examination of the same patient: Blue-greenish fluorescence. (C) Wood’s light examination after 2 weeks of fluconazole treatment: Disappearance of fluorescence.
6. Conclusions
Cutaneous fungal infections from both dermatophyte and non-dermatophyte pathogens pose a significant global public health concern. The epidemiology of these infections is dynamic, with an increasing prevalence of non-dermatophyte infections and the emergence of resistant strains of dermatophytosis. This review provides an updated overview of the epidemiology, clinical manifestations, and diagnostic methods, offering valuable insights for an accurate diagnosis before initiating treatment. Achieving a precise diagnosis is crucial for effective management and can contribute to the prevention of antifungal resistance. Further research on resistant superficial fungal infections is imperative to enhance global prevention strategies and address this evolving healthcare challenge.
Acknowledgments
The authors gratefully acknowledge Penvadee Pattanaprichakul for histopathology-photo support. The authors are also indebted to David Park for the English-language editing of this paper.
Author Contributions
Conceptualization, P.C., S.B. and C.L.; data collection, P.C., S.B. and C.L.; writing original draft, P.C.; editing and supervision, S.B. and C.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research did not receive specific grants from public, commercial or nonprofit funding agencies.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Havlickova B., Czaika V.A., Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51((Suppl. 4)):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 2.Verma S.B., Panda S., Nenoff P., Singal A., Rudramuruthy S.M., Uhrlass S., Das S., Bisherwal K., Shaw D., Vasani R. The unprecedented epidemic-like scenario of dermatophytosis in India: I. Epidemiology, risk factors and clinical features. Indian J. Dermatol. Venereol. Leprol. 2021;87:154–175. doi: 10.25259/IJDVL_301_20. [DOI] [PubMed] [Google Scholar]
- 3.Coulibaly O., L’Ollivier C., Piarroux R., Ranque S. Epidemiology of human dermatophytoses in Africa. Med. Mycol. 2018;56:145–161. doi: 10.1093/mmy/myx048. [DOI] [PubMed] [Google Scholar]
- 4.Emmons C.W. Dermatophytes: Natural grouping based on the form of the spores and accessory organs. Arch. Derm. Syphilol. 1934;30:337–362. doi: 10.1001/archderm.1934.01460150003001. [DOI] [Google Scholar]
- 5.de Hoog G.S., Dukik K., Monod M., Packeu A., Stubbe D., Hendrickx M., Kupsch C., Stielow J.B., Freeke J., Göker M., et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5–31. doi: 10.1007/s11046-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhrlaß S., Verma S.B., Gräser Y., Rezaei-Matehkolaei A., Hatami M., Schaller M., Neoff P. Trichophyton indotineae-an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide-a multidimensional perspective. J. Fungi. 2022;8:757. doi: 10.3390/jof8070757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philpot C.M. Geographical distribution of the dermatophytes: A review. Epidemiol. Infect. 1978;80:301–313. doi: 10.1017/S0022172400053663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seebacher C., Bouchara J.-P., Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335–352. doi: 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- 9.Monod M., Jaccoud S., Zaugg C., Léchenne B., Baudraz F., Panizzon R. Survey of dermatophyte infections in the Lausanne area Switzerland. Dermatology. 2002;205:201–203. doi: 10.1159/000063913. [DOI] [PubMed] [Google Scholar]
- 10.Weitzman I., Chin N.-X., Kunjukunju N., Della-Latta P. A survey of dermatophytes isolated from human patients in the United States from 1993 to 1995. J. Am. Acad. Dermatol. 1998;39:255–261. doi: 10.1016/S0190-9622(98)70085-4. [DOI] [PubMed] [Google Scholar]
- 11.Imwidthaya S., Thianprasit M., Omcharoen V. Prevalence of dermatophytosis in Siriraj Hospital. J. Med. Assoc. Thai. 1987;70:331–334. [PubMed] [Google Scholar]
- 12.Chadeganipour M., Shadzi S., Dehghan P., Movahed M. Prevalence and aetiology of dermatophytoses in Isfahan, Iran. Mycoses. 1997;40:321–324. doi: 10.1111/j.1439-0507.1997.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 13.Ellabib M.S., Khalifa Z., Kavanagh K. Dermatophytes and other fungi associated with skin mycoses in Tripoli, Libya. Mycoses. 2002;45:101–104. doi: 10.1046/j.1439-0507.2002.00731.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhan P., Liu W. The changing face of dermatophytic infections worldwide. Mycopathologia. 2017;182:77–86. doi: 10.1007/s11046-016-0082-8. [DOI] [PubMed] [Google Scholar]
- 15.Lee W.J., Kim S.L., Jang Y.H., Lee S.J., Kim D.W., Bang Y.J., Jun J.B. Increasing prevalence of trichophyton rubrum identified through an analysis of 115,846 cases over the last 37 years. J. Korean Med. Sci. 2015;30:639–643. doi: 10.3346/jkms.2015.30.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunyaratavej S., Limphoka P., Kiratiwongwan R., Leeyaphan C. Survey of skin and nail fungal infections by subject age among Thai adults and the etiological organisms. Southeast Asian J. Trop. Med. Public Health. 2019;50:1132–1138. [Google Scholar]
- 17.Bontems O., Fratti M., Salamin K., Guenova E., Monod M. Epidemiology of dermatophytoses in Switzerland according to a survey of dermatophytes isolated in Lausanne between 2001 and 2018. J. Fungi. 2020;6:95. doi: 10.3390/jof6020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell J., Porter E., Field S., O’Connell N.H., Carty K., Dunne C.P. Epidemiology of dermatomycoses and onychomycoses in Ireland (2001–2020): A single-institution review. Mycoses. 2022;65:770–779. doi: 10.1111/myc.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Oliveira Pereira F., Gomes S.M., Lima da Silva S., Paula de Castro Teixeira A., Lima I.O. The prevalence of dermatophytoses in Brazil: A systematic review. J. Med. Microbiol. 2021;70:001321. doi: 10.1099/jmm.0.001321. [DOI] [PubMed] [Google Scholar]
- 20.Shimoyama H., Sei Y. Epidemiological survey of dermatomycoses in Japan. Med. Mycol. J. 2019;60:75–82. doi: 10.3314/mmj.19.007. [DOI] [PubMed] [Google Scholar]
- 21.Cai W., Lu C., Li X., Zhang J., Zhan P., Xi L., Sun J., Yu X. Epidemiology of superficial fungal infections in Guangdong, Southern China: A retrospective study from 2004 to 2014. Mycopathologia. 2016;181:387–395. doi: 10.1007/s11046-016-9986-6. [DOI] [PubMed] [Google Scholar]
- 22.Sacheli R., Cuypers L., Seidel L., Darfouf R., Adjetey C., Lagrou K., Hayette M.P. Epidemiology of dermatophytes in Belgium: A 5 years’ survey. Mycopathologia. 2021;186:399–409. doi: 10.1007/s11046-021-00542-4. [DOI] [PubMed] [Google Scholar]
- 23.Summerbell R.C., Kane J., Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989;32:609–619. doi: 10.1111/j.1439-0507.1989.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 24.Schechtman R.C. Nondermatophytic filamentous fungi infection in South America--reality or misdiagnosis? Dermatol. Clin. 2008;26:271–283. doi: 10.1016/j.det.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A.K., Summerbell R.C., Venkataraman M., Quinlan E.M. Nondermatophyte mould onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2021;35:1628–1641. doi: 10.1111/jdv.17240. [DOI] [PubMed] [Google Scholar]
- 26.Dabas Y., Xess I., Singh G., Pandey M., Meena S. Molecular identification and antifungal susceptibility patterns of clinical dermatophytes following CLSI and EUCAST Guidelines. J. Fungi. 2017;3:17. doi: 10.3390/jof3020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nenoff P., Verma S.B., Vasani R., Burmester A., Hipler U.C., Wittig F., Krüger C., Nenoff K., Wiegand C., Saraswat A., et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes—A molecular study. Mycoses. 2019;62:336–356. doi: 10.1111/myc.12878. [DOI] [PubMed] [Google Scholar]
- 28.Kano R., Kimura U., Kakurai M., Hiruma J., Kamata H., Suga Y., Harada K. Trichophyton indotineae sp. nov.: A new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia. 2020;185:947–958. doi: 10.1007/s11046-020-00455-8. [DOI] [PubMed] [Google Scholar]
- 29.Ebert A., Monod M., Salamin K., Burmester A., Uhrlaß S., Wiegand C., Hipler U.C., Krüger C., Koch D., Wittig F., et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses. 2020;63:717–728. doi: 10.1111/myc.13091. [DOI] [PubMed] [Google Scholar]
- 30.Kong X., Tang C., Singh A., Ahmed S.A., Al-Hatmi A.M.S., Chowdhary A., Nenoff P., Gräser Y., Hainsworth S., Zhan P., et al. Antifungal susceptibility and mutations in the squalene epoxidase gene in dermatophytes of the trichophyton mentagrophytes species complex. Antimicrob. Agents Chemother. 2021;65:e0005621. doi: 10.1128/AAC.00056-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khurana A., Agarwal A., Agrawal D., Panesar S., Ghadlinge M., Sardana K., Sethia K., Malhotra S., Chauhan A., Mehta N. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: A randomized clinical trial. JAMA. Dermatol. 2022;158:1269–1278. doi: 10.1001/jamadermatol.2022.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posso-De Los Rios C.J., Tadros E., Summerbell R.C., Scott J.A. Terbinafine resistant Trichophyton indotineae isolated in patients with superficial dermatophyte infection in canadian patients. J. Cutan. Med. Surg. 2022;26:371–376. doi: 10.1177/12034754221077891. [DOI] [PubMed] [Google Scholar]
- 33.Yamada T., Yaguchi T., Maeda M., Alshahni M.M., Salamin K., Guenova E., Feuermann M., Monod M. Gene amplification of CYP51B: A new mechanism of resistance to azole compounds in Trichophyton indotineae. Antimicrob. Agents Chemother. 2022;66:e0005922. doi: 10.1128/aac.00059-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kano R., Kimura U., Noguchi H., Hiruma M. Clinical isolate of a multi-antifungal-resistant Trichophyton rubrum. Antimicrob. Agents Chemother. 2022;66:e0239321. doi: 10.1128/aac.02393-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leeyaphan C., Makimura K., Yamanishi C., Bunyaratavej S., Hau C., Tada Y., Suthammarak W., Kaewsutthi S., Phaitoonwattanakij S., Watanab S. PCR-Based diagnosis of Neoscytalidium dimidiatum infection using internal transcribed spacer 1 region of ribosomal DNA primers. Siriraj Med. J. 2018;70:28–35. [Google Scholar]
- 36.Baranová Z., Kampe T., Dorko E., Rimárová K. Epidemiological and clinical aspects of dermatophytoses in Eastern Slovakia: A retrospective three-year study. Cent. Eur. J. Public Health. 2018;26:S72–S75. doi: 10.21101/cejph.a5279. [DOI] [PubMed] [Google Scholar]
- 37.Kromer C., Celis D., Hipler U.C., Zampeli V.A., Mößner R., Lippert U. Dermatophyte infections in children compared to adults in Germany: A retrospective multicenter study in Germany. J. Dtsch. Dermatol. Ges. 2021;19:993–1001. doi: 10.1111/ddg.14432. [DOI] [PubMed] [Google Scholar]
- 38.Mazza M., Refojo N., Davel G., Lima N., Dias N., Passos da Silva C.M.F., Canteros C.E. Epidemiology of dermatophytoses in 31 municipalities of the province of Buenos Aires, Argentina: A 6-year study. Rev. Iberoam. Micol. 2018;35:97–102. doi: 10.1016/j.riam.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Rezaei-Matehkolaei A., Makimura K., de Hoog S., Shidfar M.R., Zaini F., Eshraghian M., Naghan P.A., Mirhendi H. Molecular epidemiology of dermatophytosis in Tehran, Iran, a clinical and microbial survey. Med. Mycol. 2013;51:203–207. doi: 10.3109/13693786.2012.686124. [DOI] [PubMed] [Google Scholar]
- 40.Zamani S., Sadeghi G., Yazdinia F., Moosa H., Pazooki A., Ghafarinia Z., Abbasi M., Shams-Ghahfarokhi M., Razzaghi-Abyaneh M. Epidemiological trends of dermatophytosis in Tehran, Iran: A five-year retrospective study. J. Mycol. Med. 2016;26:351–358. doi: 10.1016/j.mycmed.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Maraki S., Mavromanolaki V.E. Epidemiology of onychomycosis in Crete, Greece: A 12-year study. Mycoses. 2016;59:798–802. doi: 10.1111/myc.12533. [DOI] [PubMed] [Google Scholar]
- 42.Gregoriou S., Mpali N., Vrioni G., Hatzidimitriou E., Chryssou S.-E., Rigopoulos D. Epidemiology of onychomycosis in an academic nail unit in South Greece during a three-year period. Ski. Appendage Disord. 2019;6:102–107. doi: 10.1159/000504812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubljanin E., Džamić A., Vujčić I., Grujičić S., Arsenijević V.A., Mitrović S., Čalovski I.C. Epidemiology of onychomycosis in Serbia: A laboratory-based survey and risk factor identification. Mycoses. 2017;60:25–32. doi: 10.1111/myc.12537. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Herrera E.O., Arroyo-Camarena S., Tejada-García D.L., Porras-López C.F., Arenas R. Onychomycosis due to opportunistic molds. An. Bras. Dermatol. 2015;90:334–337. doi: 10.1590/abd1806-4841.20153521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonnet C., Berger F., Gantier J.C. Epidemiology of superficial fungal diseases in French Guiana: A three-year retrospective analysis. Med. Mycol. 2011;49:608–611. doi: 10.3109/13693786.2011.558929. [DOI] [PubMed] [Google Scholar]
- 46.Araya S., Abuye M., Negesso A.E. Epidemiological characterization of dermatomycosis in Ethiopia. Clin. Cosmet. Investig. Dermatol. 2021;14:83–89. doi: 10.2147/CCID.S292286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halim I., El Kadioui F., Soussi Abdallaoui M. Onychomycosis in Casablanca (Morocco) J. Mycol. Med. 2013;23:9–14. doi: 10.1016/j.mycmed.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Chadeganipour M., Mohammadi R. Causative agents of onychomycosis: A 7-year study. J. Clin. Lab. Anal. 2016;30:1013–1020. doi: 10.1002/jcla.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segal R., Shemer A., Hochberg M., Keness Y., Shvarzman R., Mandelblat M., Frenkel M., Segal E. Onychomycosis in Israel: Epidemiological aspects. Mycoses. 2015;58:133–139. doi: 10.1111/myc.12287. [DOI] [PubMed] [Google Scholar]
- 50.Song G., Zhang M., Liu W., Liang G. Epidemiology of Onychomycosis in Chinese Mainland: A 30-year Retrospective Study. Mycopathologia. 2022;187:323–331. doi: 10.1007/s11046-022-00647-4. [DOI] [PubMed] [Google Scholar]
- 51.Phaitoonwattanakij S., Leeyaphan C., Lertrujiwanit K., Bunyaratavej S. Predisposing factors, clinical features and treatment outcomes of Fusarium onychomycosis and comparison of its characteristics with Neoscytalidium onychomycosis. J. Mycol. Med. 2021;31:101165. doi: 10.1016/j.mycmed.2021.101165. [DOI] [PubMed] [Google Scholar]
- 52.Dogra S., Ramam M. Difficult dermatophytosis. JAMA Dermatol. 2022;158:1243–1244. doi: 10.1001/jamadermatol.2022.3736. [DOI] [PubMed] [Google Scholar]
- 53.Gupta A.K., Foley K.A. Evidence for biofilms in onychomycosis. G. Ital. Dermatol. Venereol. 2018;154:50–55. doi: 10.23736/S0392-0488.18.06001-7. [DOI] [PubMed] [Google Scholar]
- 54.Khosravi A.R., Shokri H., Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371–378. doi: 10.1007/s11046-016-0004-9. [DOI] [PubMed] [Google Scholar]
- 55.Rodríguez-Cerdeira C., Martínez-Herrera E., Szepietowski J.C., Pinto-Almazán R., Frías-De-León M.G., Espinosa-Hernández V.M., Chávez-Gutiérrez E., García-Salazar E., Vega-Sánchez D.C., Arenas R., et al. A systematic review of worldwide data on tinea capitis: Analysis of the last 20 years. J. Eur. Acad. Dermatol. Venereol. 2021;35:844–883. doi: 10.1111/jdv.16951. [DOI] [PubMed] [Google Scholar]
- 56.Osman M., Kasir D., Rafei R., Kassem I.I., Ismail M.B., El Omari K., Dabboussi F., Cazer C., Papon N., Bouchara J.P., et al. Trends in the epidemiology of dermatophytosis in the middle east and north Africa region. Int. J. Dermatol. 2022;61:935–968. doi: 10.1111/ijd.15967. [DOI] [PubMed] [Google Scholar]
- 57.Udomphan P., Bunyaratavej S., Leeyaphan C., Matthapan L., Lertrujiwanit K., Pattanaprichakul P. Review of adult tinea capitis cases presenting to Siriraj hospital, Bangkok, Thailand. Southeast Asian J. Trop. Med. Public Health. 2019;50:905–911. [Google Scholar]
- 58.Song G., Zhang M., Liu W., Liang G. Changing face of epidemiology of dermatophytoses in Chinese Mainland: A 30 years nationwide retrospective study from 1991 to 2020. Mycoses. 2022;65:440–448. doi: 10.1111/myc.13425. [DOI] [PubMed] [Google Scholar]
- 59.Carrascal-Correa D.F., Zuluaga A., González A. Species distribution of the main aetiologic agents causing skin dermatophytosis in Colombian patients: A 23-year experience at a mycological reference center. Mycoses. 2020;63:494–499. doi: 10.1111/myc.13073. [DOI] [PubMed] [Google Scholar]
- 60.Borges A., Brasileiro A., Galhardas C., Apetato M. Tinea faciei in a central Portuguese hospital: A 9-year survey. Mycoses. 2018;61:283–285. doi: 10.1111/myc.12730. [DOI] [PubMed] [Google Scholar]
- 61.Atzori L., Aste N., Pau M. Tinea faciei due to Microsporum Canis in children: A survey of 46 cases in the district of Cagliari (Italy) Pediatr. Dermatol. 2012;29:409–413. doi: 10.1111/j.1525-1470.2011.01595.x. [DOI] [PubMed] [Google Scholar]
- 62.Maraki S., Mavromanolaki V.E. Epidemiology of dermatophytoses in Crete, Greece. Med. Mycol. J. 2016;57:e69–e75. doi: 10.3314/mmj.16-00008. [DOI] [PubMed] [Google Scholar]
- 63.Ebrahimi M., Zarrinfar H., Naseri A., Najafzadeh M.J., Fata A., Parian M., Khorsand I., Babič M.N. Epidemiology of dermatophytosis in northeastern Iran; A subtropical region. Curr. Med. Mycol. 2019;5:16–21. doi: 10.18502/cmm.5.2.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeshima R., Asahina Y., Yaguchii T., Sato T. Tinea barbae due to Trichophyton rubrum successfully treated using oral fosravuconazole l-lysine ethanolate. J. Dermatol. 2020;47:e254–e255. doi: 10.1111/1346-8138.15374. [DOI] [PubMed] [Google Scholar]
- 65.Kirsten H., Haiduk J., Nenoff P., Uhrlaß S., Ziemer M., Simon J.C. Tinea barbae profunda due to Trichophyton mentagrophytes: Case report and review. Hautarzt. 2019;70:601–611. doi: 10.1007/s00105-019-4407-7. [DOI] [PubMed] [Google Scholar]
- 66.Müller V.L., Kappa-Markovi K., Hyun J., Georgas D., Silberfarb G., Paasch U., Uhrlaß S., Nenoff P., Schaller J. Tinea capitis et barbae caused by Trichophyton tonsurans: A retrospective cohort study of an infection chain after shavings in barber shops. Mycoses. 2021;64:428–436. doi: 10.1111/myc.13231. [DOI] [PubMed] [Google Scholar]
- 67.Baeza-Hernández G., de la Soledad Vallejo-Ruiz M., Rubio-Aguilera R.F., Romero-Maté A., Martínez-Morán C. An unexpected dermatophyte? Two remarkable cases of tinea barbae by Trichophyton benhamiae. Dermatol. Pract. Concept. 2023;13:e2023037. doi: 10.5826/dpc.1301a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saxena V., Shenoy M.M., Devrari J.C., Pai V., Agrawal V. A mycological study of tinea corporis: A changing epidemiological trend from Trichophyton rubrum to Trichophyton mentagrophytes in India. Indian. J. Dermatol. Venereol. Leprol. 2020;86:607. doi: 10.4103/ijdvl.IJDVL_766_17. [DOI] [PubMed] [Google Scholar]
- 69.Castellanos J., Guillén-Flórez A., Valencia-Herrera A., Toledo-Bahena M., Ramírez-Cortés E., Toussaint-Caire S., Mena-Cedillos C., Salazar-García M., Bonifaz A. Unusual inflammatory tinea infections: Majocchi’s granuloma and deep/systemic dermatophytosis. J. Fungi. 2021;7:929. doi: 10.3390/jof7110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zalewski A., Goldust M., Szepietowski J.C. Tinea gladiatorum: Epidemiology, clinical aspects, and management. J. Clin. Med. 2022;11:4066. doi: 10.3390/jcm11144066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hiruma J., Ogawa Y., Hiruma M. Trichophyton tonsurans infection in Japan: Epidemiology, clinical features, diagnosis and infection control. J. Dermatol. 2015;42:245–249. doi: 10.1111/1346-8138.12678. [DOI] [PubMed] [Google Scholar]
- 72.Er Y.X., Lee S.C., Than L.T., Muslim A., Leong K.F., Kwan Z., Sayed I.M., Lim Y.A.L. Tinea imbricata among the indigenous communities: Current global epidemiology and research gaps associated with host genetics and skin microbiota. J. Fungi. 2022;8:202. doi: 10.3390/jof8020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polunin I. Tinea imbricata in Malaya. Br. J. Dermatol. 1952;64:378–384. doi: 10.1111/j.1365-2133.1952.tb15801.x. [DOI] [PubMed] [Google Scholar]
- 74.Budimulja U. Tinea imbricata in central Kalimantan. Mal. J. Dermatol. 1995;8:17–21. [Google Scholar]
- 75.Shenoy M.M., Rengasamy M., Dogra S., Kaur T., Asokan N., Sarveswari K.N., Poojary S., Aror D., Patil S., Das A., et al. A multicentric clinical and epidemiological study of chronic and recurrent dermatophytosis in India. Mycoses. 2022;65:13–23. doi: 10.1111/myc.13360. [DOI] [PubMed] [Google Scholar]
- 76.Cruz R., Carvajal L. Frequency of Epidermophyton floccosum in isolated dermatophytea laboratory from the region of Valparaiso, Chile. Time frame: 1990–2010. Rev. Chilena. Infectol. 2018;35:262–265. doi: 10.4067/s0716-10182018000300262. [DOI] [PubMed] [Google Scholar]
- 77.Dias M.F.R.G., Quaresma-Santos M.V.P., Bernardes-Filho F., Amorim A.G.D.F., Schechtman R.C., Azulay D.R. Update on therapy for superficial mycoses: Review article part I. An. Bras. Dermatol. 2013;88:764–774. doi: 10.1590/abd1806-4841.20131996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J., Tsuchihashi H., Hiruma M., Kano R., Ikeda S. Tinea corporis due to Trichophyton erinacei probably transmitted from a hedgehog the second case report from Japan. Med. Mycol. J. 2018;59:E77–E79. doi: 10.3314/mmj.18-00006. [DOI] [PubMed] [Google Scholar]
- 79.Choi E., Huang J., Chew K.L., Jaffar H., Tan C. Pustular tinea manuum from trichophyton erinacei infection. JAAD. Case Rep. 2018;4:18–20. doi: 10.1016/j.jdcr.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weishaupt J., Kolb-Mäurer A., Lempert S., Nenoff P., Uhrlaß S., Hamm H., Goebeler M. A different kind of hedgehog pathway: Tinea manus due to Trichophyton erinacei transmitted by an African pygmy hedgehog (Atelerix albiventris) Mycoses. 2014;57:125–127. doi: 10.1111/myc.12113. [DOI] [PubMed] [Google Scholar]
- 81.Perrier P., Monod M. Tinea manuum caused by Trichophyton erinacei: First report in Switzerland. Int. J. Dermatol. 2015;54:959–960. doi: 10.1111/ijd.12291. [DOI] [PubMed] [Google Scholar]
- 82.Phaitoonwattanakij S., Leeyaphan C., Bunyaratavej S., Chinhiran K. Trichophyton erinacei onychomycosis: The first to evidence a proximal subungual onychomycosis pattern. Case Rep. Dermatol. 2019;11:198–203. doi: 10.1159/000501424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moseley I., Ragi S.D., Ouellette S., Rao B. Tinea pedis in underrepresented groups: All of us database analysis. Mycoses. 2023;66:29–34. doi: 10.1111/myc.13522. [DOI] [PubMed] [Google Scholar]
- 84.Kiraz N., Metintas S., Oz Y., Koc F., Koku Aksu E.A., Kalyoncu C., Kasifoglu N., Cetin E., Arikan I. The prevalence of tinea pedis and tinea manuum in adults in rural areas in Turkey. Int. J. Environ. Health Res. 2010;20:379–386. doi: 10.1080/09603123.2010.484861. [DOI] [PubMed] [Google Scholar]
- 85.Ilkit M., Durdu M. Tinea pedis: The etiology and global epidemiology of a common fungal infection. Crit. Rev. Microbiol. 2015;41:374–388. doi: 10.3109/1040841X.2013.856853. [DOI] [PubMed] [Google Scholar]
- 86.Ongsri P., Bunyaratavej S., Leeyaphan C., Pattanaprichakul P., Ongmahutmongkol P., Komoltri C., Kulthanan K. Prevalence and clinical correlation of superficial fungal foot infection in Thai naval rating cadets. Mil. Med. 2018;183:e633–e637. doi: 10.1093/milmed/usx187. [DOI] [PubMed] [Google Scholar]
- 87.Toukabri N., Dhieb C., El Euch D., Rouissi M., Mokni M., Sadfi-Zouaoui N. Prevalence, etiology, and risk factors of tinea pedis and tinea unguium in Tunisia. Can. J. Infect. Dis. Med. Microbiol. 2017;2017:6835725. doi: 10.1155/2017/6835725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ungpakorn R., Lohaprathan S., Reangchainam S. Prevalence of foot diseases in outpatients attending the institute of dermatology, Bangkok, Thailand. Clin. Exp. Dermatol. 2004;29:87–90. doi: 10.1111/j.1365-2230.2004.01446.x. [DOI] [PubMed] [Google Scholar]
- 89.Assadamongkol R., Lertwattanarak R., Wannachalee T., Bunyaratavej S., Leeyaphan C., Matthapan L. Prevalence, risk factors, and type of organism in fungal foot infection and toenail onychomycosis in Thai diabetic patients. J. Med. Assoc. Thai. 2016;99:659–664. [PubMed] [Google Scholar]
- 90.Ingordo V., Naldi L., Fracchiolla S., Colecchia B. Prevalence and risk factors for superficial fungal infections among Italian navy cadets. Dermatology. 2004;209:190–196. doi: 10.1159/000079888. [DOI] [PubMed] [Google Scholar]
- 91.Leeyaphan C., Bunyarata S., Chadchavalpanichaya N., Rujitharanawong C., Phaitoonwattanakij S., Matthapan L. Clinical and laboratory findings in trauma-induced nail dystrophy versus onychomycosis. Siriraj Med. J. 2018;70:490–495. [Google Scholar]
- 92.Lipner S.R., Scher R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatol. 2019;80:835–851. doi: 10.1016/j.jaad.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 93.Ghannoum M.A., Hajjeh R.A., Scher R., Konnikov N., Gupta A.K., Summerbell R., Sullivan S., Daniel R., Krusinski P., Fleckman P., et al. A large-scale North American study of fungal isolates from nails: The frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 2000;43:641–648. doi: 10.1067/mjd.2000.107754. [DOI] [PubMed] [Google Scholar]
- 94.Gold J.A.W., Wu K., Jackson B.R., Benedict K. Opportunities to improve guideline adherence for the diagnosis and treatment of onychomycosis: Analysis of commercial insurance claims data, United States. J. Am. Acad. Dermatol. 2023;88:683–686. doi: 10.1016/j.jaad.2022.06.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sigurgeirsson B., Baran R. The prevalence of onychomycosis in the global population: A literature study. J. Eur. Acad. Dermatol. Venereol. 2014;28:1480–1491. doi: 10.1111/jdv.12323. [DOI] [PubMed] [Google Scholar]
- 96.de Berker D. Clinical practice. Fungal nail disease. N. Engl. J. Med. 2009;360:2108–2116. doi: 10.1056/NEJMcp0804878. [DOI] [PubMed] [Google Scholar]
- 97.Limphoka P., Bunyaratavej S., Leeyaphan C. Fingernail onychomycosis caused by Microsporum canis in a teenager. Pediatr. Dermatol. 2021;38:524–525. doi: 10.1111/pde.14524. [DOI] [PubMed] [Google Scholar]
- 98.Bunyaratavej S., Prasertworonun N., Leeyaphan C., Chaiwanon O., Muanprasat C., Matthapan L. Distinct characteristics of scytalidium dimidiatum and non-dermatophyte onychomycosis as compared with dermatophyte onychomycosis. J. Dermatol. 2015;42:258–262. doi: 10.1111/1346-8138.12768. [DOI] [PubMed] [Google Scholar]
- 99.Cursi I.B., Freitas L.B., Neves Mde L., Silva I.C. Onycomychosis due to Scytalidium spp.: A clinical and epidemiologic study at a University Hospital in Rio de Janeiro, Brazil. An. Bras. Dermatol. 2011;86:689–693. doi: 10.1590/S0365-05962011000400010. [DOI] [PubMed] [Google Scholar]
- 100.Ebihara M., Makimura K., Sato K., Abe S., Tsuboi R. Molecular detection of dermatophytes and nondermatophytes in onychomycosis by nested polymerase chain reaction based on 28S ribosomal RNA gene sequences. Br. J. Dermatol. 2009;161:1038–1044. doi: 10.1111/j.1365-2133.2009.09249.x. [DOI] [PubMed] [Google Scholar]
- 101.Ranawaka R.R., Nagahawatte A., Gunasekara T.A., Weerakoon H.S., de Silva S.H. Randomized, double-blind, comparative study on efficacy and safety of itraconazole pulse therapy and terbinafine pulse therapy on nondermatophyte mold onychomycosis: A study with 90 patients. J. Dermatolog. Treat. 2016;27:364–372. doi: 10.3109/09546634.2015.1119781. [DOI] [PubMed] [Google Scholar]
- 102.Gupta A.K., Drummond-Main C., Cooper E.A., Brintnell W., Piraccini B.M., Tosti A. Systematic review of nondermatophyte mold onychomycosis: Diagnosis, clinical types, epidemiology, and treatment. J. Am. Acad. Dermatol. 2012;66:494–502. doi: 10.1016/j.jaad.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 103.Tosti A., Piraccini B.M., Lorenzi S. Onychomycosis caused by nondermatophytic molds: Clinical features and response to treatment of 59 cases. J. Am. Acad. Dermatol. 2000;42:217–224. doi: 10.1016/S0190-9622(00)90129-4. [DOI] [PubMed] [Google Scholar]
- 104.Guilhermetti E., Takahachi G., Shinobu C.S., Svidzinski T.I. Fusarium spp. as agents of onychomycosis in immunocompetent hosts. Int. J. Dermatol. 2007;46:822–826. doi: 10.1111/j.1365-4632.2007.03120.x. [DOI] [PubMed] [Google Scholar]
- 105.Hay R.J. Tinea capitis: Current status. Mycopathologia. 2017;182:87–93. doi: 10.1007/s11046-016-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bunyaratavej S., Leeyaphan C., Rujitharanawong C., Muanprasat C., Matthapan L. Clinical and laboratory characteristics of a tinea capitis outbreak among novice Buddhist monks. Pediatr. Dermatol. 2017;34:371–373. doi: 10.1111/pde.13102. [DOI] [PubMed] [Google Scholar]
- 107.Degreef H. Clinical forms of dermatophytosis (ringworm infection) Mycopathologia. 2008;166:257–265. doi: 10.1007/s11046-008-9101-8. [DOI] [PubMed] [Google Scholar]
- 108.Bunyaratavej S., Kiratiwongwan R., Komoltri C., Lertrujiwanit K., Leeyaphan C. Predictive equation to identify infection due to anthropophilic or zoophilic dermatophytes based on clinical features and risk factors: A ten-year retrospective study. Indian J. Dermatol. Venereol. Leprol. 2022;88:416–419. doi: 10.25259/IJDVL_1398_20. [DOI] [PubMed] [Google Scholar]
- 109.Romano C., Maritati E., Gianni C. Tinea incognito in Italy: A 15-year survey. Mycoses. 2006;49:383–387. doi: 10.1111/j.1439-0507.2006.01251.x. [DOI] [PubMed] [Google Scholar]
- 110.Chamorro M.J., House S.A. Tinea Manuum. StatPearls Publishing; Treasure Island, FL, USA: 2022. [PubMed] [Google Scholar]
- 111.Mizumoto J. Two feet-one hand syndrome. Cureus. 2021;13:e20758. doi: 10.7759/cureus.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gelotar P., Vachhani S., Patel B., Makwana N. The prevalence of fungi in fingernail onychomycosis. J. Clin. Diagnostic Res. 2013;7:250. doi: 10.7860/JCDR/2013/5257.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bunyaratavej S., Pattanaprichakul P., Sitthinamsuwan P., Pongkittilar B., Prasertsook S., Wongdama S., Yan C., Leeyaphan C. Clinical clues to differentiate between dermatophyte onychomycosis (DP-OM) and dermatophytoma-like traumatic onychodystrophy (DP-TO) Biomed. Res. Int. 2022;2022:8519376. doi: 10.1155/2022/8519376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gupta A.K., Versteeg S.G., Shear N.H. Confirmatory testing prior to initiating onychomycosis therapy is cost-effective. J. Cutan. Med. Surg. 2018;22:129–141. doi: 10.1177/1203475417733461. [DOI] [PubMed] [Google Scholar]
- 115.Bunyaratavej S., Pattanaprichakul P., Srisuma S., Leeyaphan C. Experiences and factors that influence potassium hydroxide examination by microscopists. Med. Mycol. J. 2016;57:E29–E34. doi: 10.3314/mmj.15-00022. [DOI] [PubMed] [Google Scholar]
- 116.Meireles T.E.F., Rocha M.F.G., Brilhante R.S.N., Cordeiro R.D.A., Sidrim J.J.C. Successive mycological nail tests for onychomycosis: A strategy to improve diagnosis efficiency. Braz. J. Infect. Dis. 2008;12:333–337. doi: 10.1590/S1413-86702008000400016. [DOI] [PubMed] [Google Scholar]
- 117.Wanat K.A., Dominguez A.R., Carter Z., Legua P., Bustamante B., Micheletti R.G. Bedside diagnostics in dermatology: Viral, bacterial, and fungal infections. J. Am. Acad. Dermatol. 2017;77:197–218. doi: 10.1016/j.jaad.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 118.Verrier J., Monod M. Diagnosis of dermatophytosis using molecular biology. Mycopathologia. 2017;182:193–202. doi: 10.1007/s11046-016-0038-z. [DOI] [PubMed] [Google Scholar]
- 119.Salakshna N., Bunyaratavej S., Matthapan L., Lertrujiwanit K., Leeyaphan C. A cohort study of risk factors, clinical presentations, and outcomes for dermatophyte, nondermatophyte, and mixed toenail infections. J. Am. Acad. Dermatol. 2018;79:1145–1146. doi: 10.1016/j.jaad.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 120.Begum J., Mir N.A., Lingaraju M.C., Buyamayum B., Dev K. Recent advances in the diagnosis of dermatophytosis. J. Basic. Microbiol. 2020;60:293–303. doi: 10.1002/jobm.201900675. [DOI] [PubMed] [Google Scholar]
- 121.Petrucelli M.F., Abreu M.H., Cantelli B.A.M., Segura G.G., Nishimura F.G., Bitencourt T.A., Marins M., Fachin A.L. Epidemiology and diagnostic perspectives of dermatophytoses. J. Fungi. 2020;6:310. doi: 10.3390/jof6040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Su H., Packeu A., Ahmed S.A., Al-Hatmi A.M.S., Blechert O., İlkit M., Hagen F., Gräser Y., Liu W., Deng S., et al. Species distinction in the Trichophyton rubrum complex. J. Clin. Microbiol. 2019;57:e00352-19. doi: 10.1128/JCM.00352-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koo S.H., Teoh Y.L., Koh W.L., Ochi H., Tan S.K., Sim D.M.F., Jiang B., Tan A.L., Tan T.Y., Lim S.P.R. Development and validation of a real-time multiplex PCR assay for the detection of dermatophytes and Fusarium spp. J. Med. Microbiol. 2019;68:1641–1648. doi: 10.1099/jmm.0.001082. [DOI] [PubMed] [Google Scholar]
- 124.Leeyaphan C., Suphatsathienkul P., Limphoka P., Kiratiwongwan R., Bunyaratavej S. Sulphur nuggets: A distinct dermoscopic feature of onychomycosis. Med. Mycol. J. 2021;62:63–65. doi: 10.3314/mmj.21-00006. [DOI] [PubMed] [Google Scholar]
- 125.Litaiem N., Nakouri I., Bouhlel S., Mansour Y., Bouchakoua M., Zegaloui F. Dermoscopic features of toenail onychomycosis. J. Am. Podiatr. Med. Assoc. 2020;110:Article_4. doi: 10.7547/18-102. [DOI] [PubMed] [Google Scholar]
- 126.Veasey J.V., Miguel B., Bedrikow R.B., Mota R.D.C., Jr., Buarque V. Wood’s lamp in dermatology: Applications in the daily practice. Surg. Cosmet. Dermatol. 2017;9:324–326. doi: 10.5935/scd1984-8773.201794964. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not applicable to this article.