Abstract
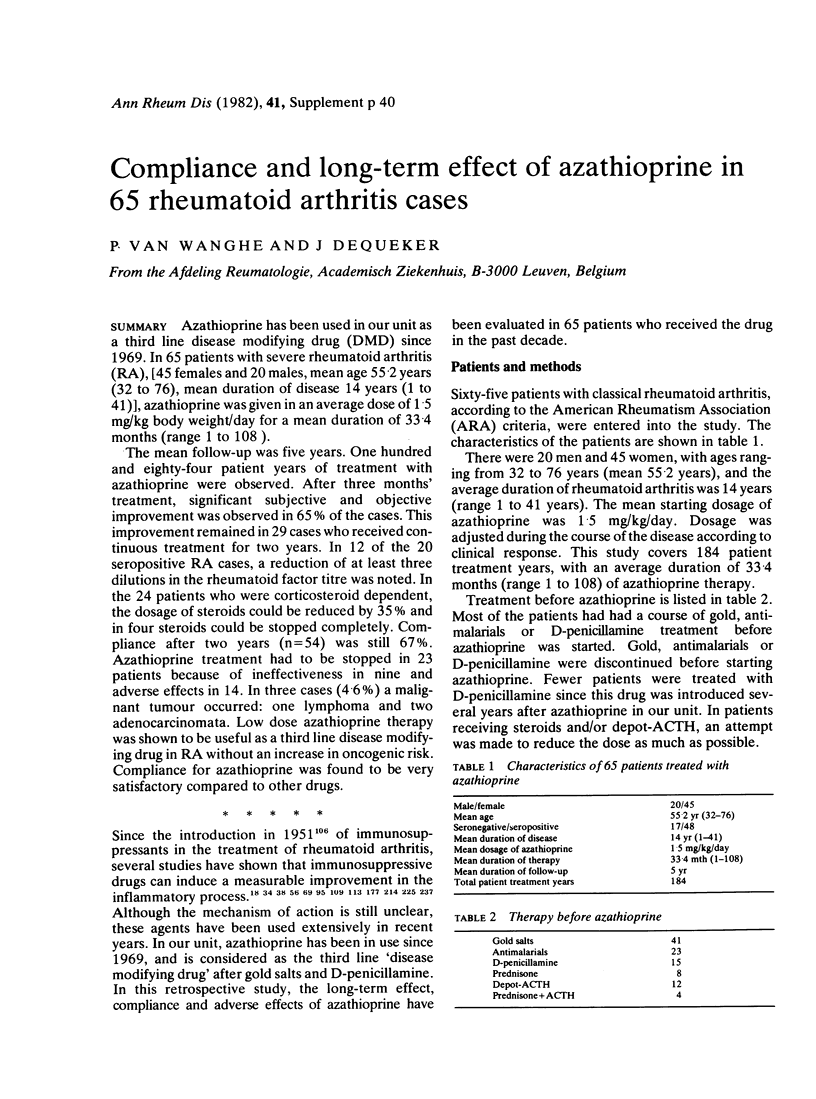
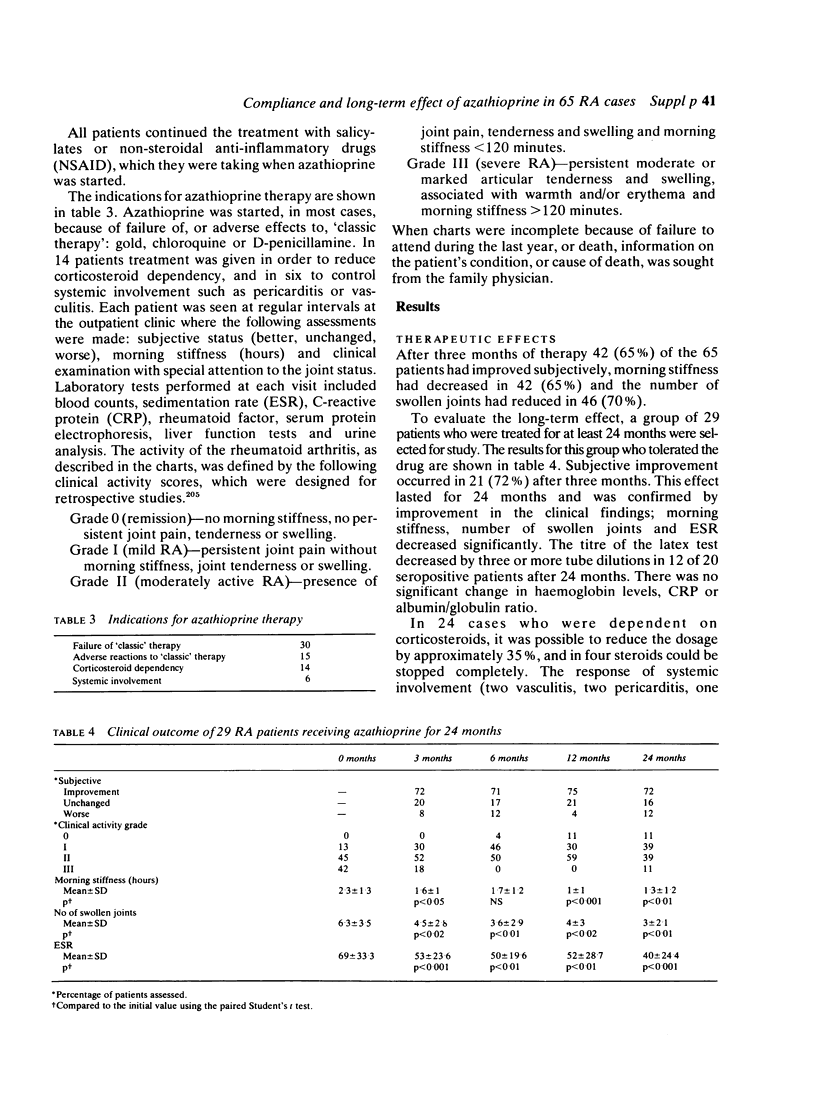
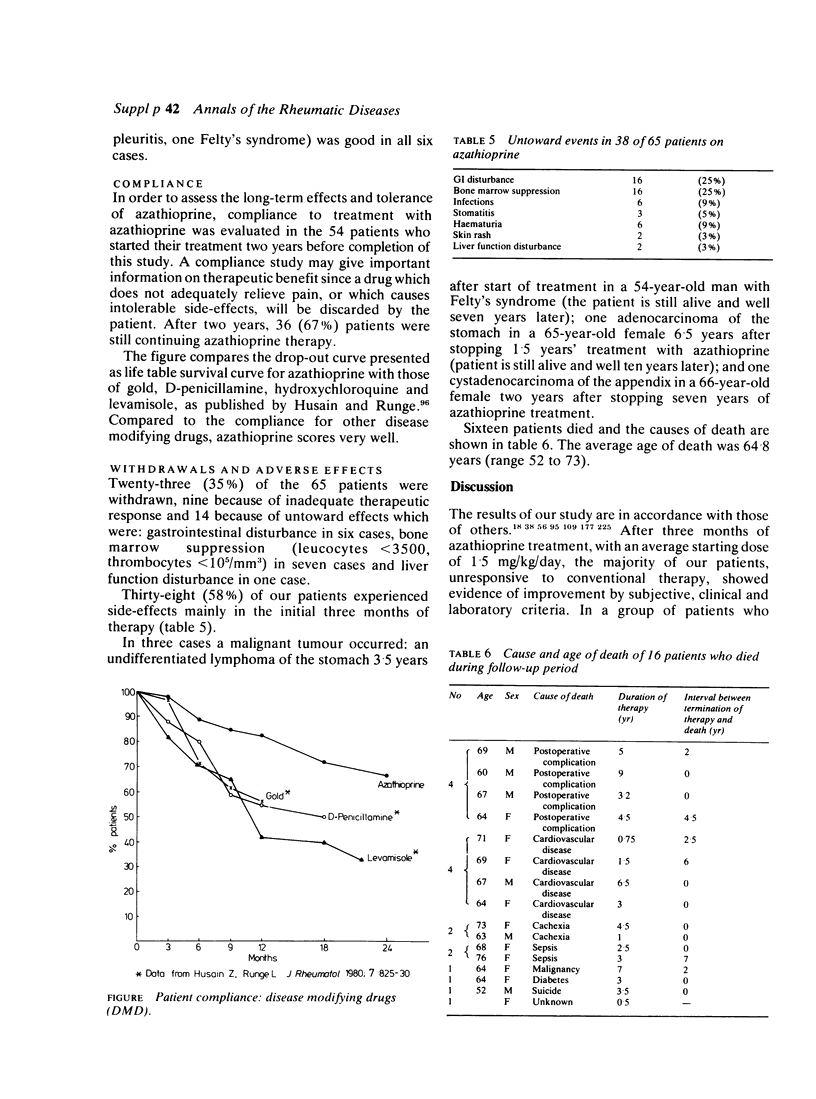
Azathioprine has been used in our unit as a third line disease modifying drug (DMD) since 1969. In 65 patients with severe rheumatoid arthritis (RA), [45 females and 20 males, mean age 55.2 years (32 to 76), mean duration of disease 14 years (1 to 41)], azathioprine was given in an average dose of 1.5 mg/kg body weight/day for a mean duration of 33.4 months (range 1 to 108). The mean follow-up was five years. One hundred and eighty-four patient years of treatment with azathioprine were observed. After three months' treatment, significant subjective and objective improvement was observed in 65% of the cases. This improvement remained in 29 cases who received continuous treatment for two years. In 12 of the 20 seropositive RA cases, a reduction of at least three dilutions in the rheumatoid factor titre was noted. In the 24 patients who were corticosteroid dependent, the dosage of steroids could be reduced by 35% and in four steroids could be stopped completely. Compliance after two years (n = 54) was still 67%. Azathioprine treatment had to be stopped in 23 patients because of ineffectiveness in nine and adverse effects in 14. In three cases (4.6%) a malignant tumour occurred: one lymphoma and two adenocarcinomata. Low dose azathioprine therapy was shown to be useful as a third line disease modifying drug in RA without an increase in oncogenic risk. Compliance for azathioprine was found to be very satisfactory compared to other drugs.
Full text
PDF