Abstract
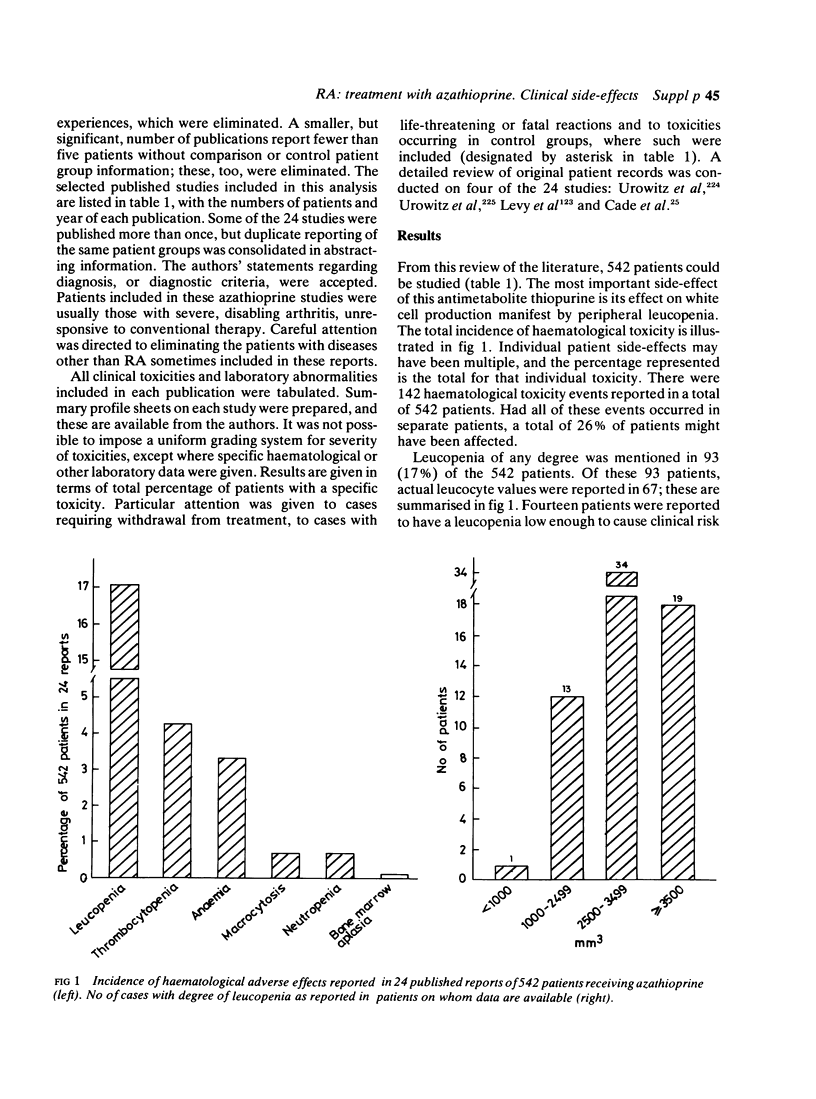
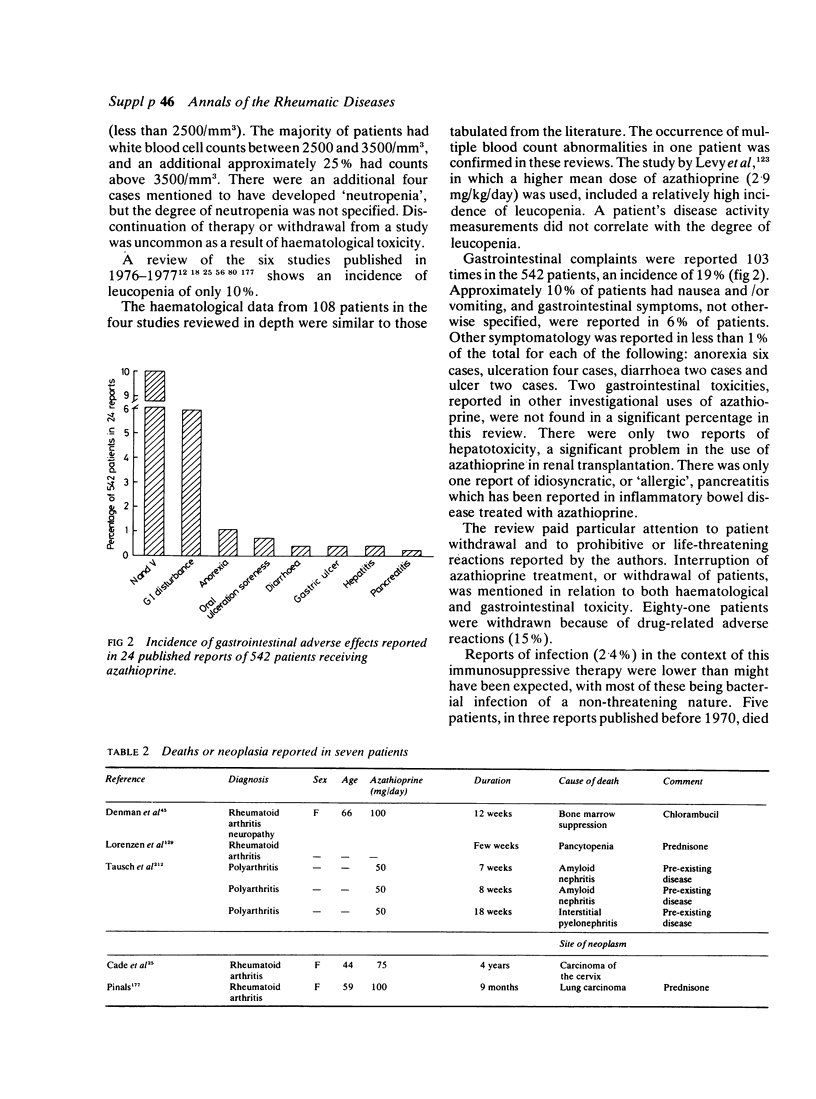
A retrospective review of the literature has been carried out to determine laboratory abnormalities occurring in patients with rheumatoid arthritis (RA) treated with azathioprine, in order to establish a profile for this agent in the treatment of this disease. A total of 542 patients in 24 studies, reported in the literature, were given a range of doses of azathioprine for up to four years. Fifteen percent of patients were withdrawn because of toxicity. The two major toxic effects were gastrointestinal symptoms and alteration in blood counts. Clinically significant leucopenia (less than 2500/mm3) occurred in 14 of the total of 93 patients reported to have developed leucopenia. Some adverse reactions, which would have been expected from the use of azathioprine in other diseases, were uncommon, namely significant infections, hepatotoxicity and pancreatitis. Adverse experience with azathioprine in rheumatoid arthritis compares well with other slow-acting, or disease modifying, drugs.
Full text
PDF