Abstract
Insects are a significant source of food for millions of people worldwide. Since ancient times, insects in medicine have been contributing to the treatment of diseases in humans and animals. Compared to conventional animal farming, the production of insects for food and feed generates significantly less greenhouse gas emissions and uses considerably less land. Edible insects provide many ecosystem services, including pollination, environmental health monitoring, and the decomposition of organic waste materials. Some wild edible insects are pests of cash crops. Thus, harvesting and consuming edible insect pests as food and utilizing them for therapeutic purposes could be a significant progress in the biological control of insect pests. Our review discusses the contribution of edible insects to food and nutritional security. It highlights therapeutic uses of insects and recommends ways to ensure a sustainable insect diet. We stress that the design and implementation of guidelines for producing, harvesting, processing, and consuming edible insects must be prioritized to ensure safe and sustainable use.
Keywords: edible insects, entomophagy, entomotherapy, food security, insect-based foods, ecosystem function
1. Introduction
The global population is rising exponentially, and so is the societal difficulty of meeting nutritional needs, which drives up the worldwide demand for meat (1). As a result, dietary diversity, biofortification, supplementation, and commercial food fortification, are all approaches that are beneficial in combating malnutrition (2–5). Thousands of insect species are consumed annually, mostly in developing nations (6). About 2.5 billion people worldwide rely on insects as a supplementary food source (7). Over the past decade, edible insects have gained popularity as healthy and environmentally friendly substitutes for traditional meat and dairy products. The global edible insect industry will be worth over $3 billion by 2030 (8). However, the consumption of insects is still unusual in most western populations if not considered a taboo (9–11). Many nations in Asia, Africa, Europe, and Latin America consume whole, easily recognizable edible insects as a typical snack or even as their primary source of protein (12). These insects are often prepared by being boiled, dried, toasted, or fried before being used in cooking (13).
Edible insects can solve many environmental and health issues, including climate change, hunger, and environmental degradation caused by agro-industrial production (14). The growing population of the world, along with the resulting shifts in demographics regarding lifestyle, dietary preference, and income, and the resulting expectation of increased access to a wide variety of lifestyle options has led to a heightened awareness of the global sustainability challenges humanity faces today (15). Sustainable development and commercialization require multi-disciplinary research into edible species and documentation of the species’ preparation process and therapeutic characteristics (16).
Though consuming edible insects for food and using them for treating animal and human diseases have received greater attention, there needs to be more information on other benefits associated with production, marketing and harvesting. For instance, the African coconut beetle Oryctes monoceros (Olivier), Asiatic rhinoceros beetles Oryctes rhinoceros (Linnaeus), and African palm weevil Rhychophorus phoenicis (L.) attack and kill economically important crops, such as palms, banana, and pineapple (17–20). However, many people in Sub-Saharan Africa consume the same insects because of their nutritional properties (21, 22).
This review discusses wild edible insects as agricultural pests of cash crops and how harvesting these insect pests could contribute to their management. Moreover, our review examines edible insects as a long-term solution to global food security by considering their nutritional properties, ecosystem services, and environmental impacts. We highlight the potential of wild edible insects as reservoirs for pathogens harmful to plants, animals, and humans. Furthermore, we discuss some edible insects with therapeutic properties for treating diseases. We highlight options for designing and implementing guidelines for using insects as food and the need to prioritize harvesting and consumption to ensure safe and sustainable use.
2. Methodology
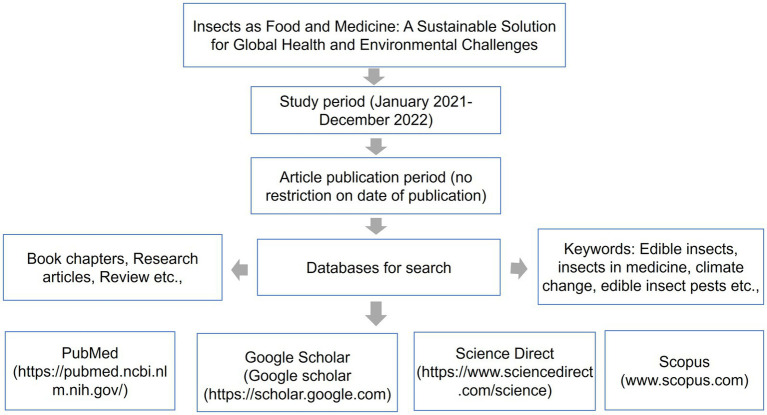
In this review, we sourced articles from these databases: Semantic Scholar1, Google Scholar2, Scopus3, Science Direct4, and SciELO5. The review started from January 2022 to December 2022. Articles published in indexed scientific journals and books were considered without limitations on the year of publication. We selected only articles published in English. To be more specific in our search, the keywords used, included “edible insects,” “the bioactivity of edible insects,” “edible insects and climate change,” “pathogens of edible insects,” “insects in medicine,” “nutritional benefits of insects,” “insects as food and feed,” “ecological benefits of edible insects,” “edible insect pests,” and “edible insects as vectors of diseases in plants, animals and crops.” A detailed representation of the search of articles for the review is illustrated in Figure 1.
Figure 1.
A schematic diagram showing the methodology followed during the review.
3. Food and nutritional benefits of edible insects
Food security measures the availability and accessibility to safe, nutritious, and sufficient food. One billion people rely on livestock for their livelihood, and 70% of the 880 million rural poor who earn less than USD $1.00 a day rely on livestock at least in part for their income and food security (23). Nevertheless, the prevalence of undernourishment has increased by 1.5% points in recent years, representing a midpoint of about 720 to 811 million people suffering from hunger in the first year of the COVID-19 pandemic (24). The rise was estimated to be 446 million in Africa, 57 million in Asia, and 14 million in Latin America and the Caribbean (23–25). Substantial dietary changes are required to achieve global food security goals. Edible insects could serve as an alternative source of nutrients and are currently considered as significant food sources.
About 5.5 million insect species are available worldwide, of which approximately 1 million have been described (26). Of this number, approximately 2,100 species are edible (27). Among these edible insects are beetles, caterpillars, ants, bees, wasps, grasshoppers, true bugs, dragonflies, termites, and cockroaches (27). In Africa, for instance, the most critical edible insect orders include Lepidoptera (30.93%), Orthoptera (22.80%), Coleoptera (19.70%), Heteroptera (9.32%), Blattodea (7.40%), Hymenoptera (6.78%), Diptera (1.06%), Dictyoptera (0.85%), Odonata (0.64%), and Ephemenoptera (0.42%), with Odonata and Ephemeroptera forming relatively lower percentages (28).
Generally, it is difficult to determine the nutritional profile of edible insects due to the considerable differences encountered across species, country, insect feed composition, insect rearing mode, and developmental stage of insects, all of which may affect the nutritional profile. However, proteins, lipids, chitin, minerals, and vitamins form significant components of nutrients in edible insects. Protein represents the major component of nutrient composition in edible insects, followed by lipids (29). In terms of dry matter, the protein content of edible insects ranges between 35.3 and 61.3% for Blattodea (termites) and Orthoptera (crickets, grasshoppers, locusts), respectively (30). The latter exhibits the lowest lipid content of about 13.41% dry matter, whereas beetles, termites, and fly larvae depict high contents of approximately 33.40% dry matter. The former includes palmitic, stearic, palmitoleic, oleic, linoleic, α-linolenic and γ-linolenic acids. Chitin, the main polysaccharide component of the insect exoskeleton, protects insects from harsh environmental conditions. Furthermore, even though edible insects are generally rich in copper, iron, magnesium, manganese, phosphorous, selenium, and zinc, little is known about the nutritional profile of vitamins and minerals (31). The potential contribution of insects to food and nutritional security is crucial worldwide. However, much more knowledge is essential for the quantitative nutritional assessment of insects, especially vitamins and minerals. The nutritional profile of insects used as animal feed also needs more research.
4. Medicinal benefits of edible insects
In many cultures worldwide, traditional medicine has used insects for a long time to treat stomach aches, respiratory issues, and wound healing. However, most of the research on the functional significance of edible insects has been on their ability to act as antioxidants in cell models or in vitro (32). Additionally, there is scant research on how edible insects affect platelet aggregation, anti-inflammation, lipid modulation, and glucose metabolism (33–35). However, there has been a recent uptick in research on the potential health benefits of edible insects from a theoretical and practical standpoint (32). Recent advances have investigated the biological activities of common insect orders, such as Blattodea, Coleoptera, Diptera, and Hemiptera (Table 1).
Table 1.
Common edible insects, isolated compounds and biological activity.
| Insect | Common name | Compound isolated from | Isolated compound | Biological activity | References |
|---|---|---|---|---|---|
| Blattodea | |||||
| Periplaneta americana (Linnaeus) | American cockroach | Adult | Isocoumarins periplatins A–D | Cytotoxic activities against human liver (HepG2) and breast cancer (MCF-7) cells | (36) |
| Polyphaga plancyi (Bolivar) | Chinese cockroach | Not determined | Plancyamide A Plancypyrazine A Plancypyrazine B Plancyol A |
Antiproliferative | (37) |
| Macrotermes spp. | Termites | Body surface | Roseoflavin, 8-methylamino-8- demethyl-D-riboflavin Natalamycin Termisoflavones A-C |
Antibacterial Antifungal |
(38, 39) |
| Coleoptera | |||||
| Blaps japanensis | Adult | Blapsols A–D | Anti-inflammatory | (40) | |
| Holotrichia diomphalia (Hope) | Larvae | Tricin, palmitinic acid eicosane | Antifungal | (41) | |
|
Hycleus oculatus (Thunberg) H. tinctus (Walsingham) H. lunata (Smith) |
African blister beetles | Adult | (R)- (+)-palasonin Palasonimide Cantharimide Palasonin |
Anticancer | (42, 43) |
| Diptera | |||||
| Hermetia Illucens (L.) | Black Soldier Fly | Larvae | α-pyrone diketopiperazine |
Antibacterial | (44) |
| Hemiptera | |||||
|
Aspongopus Chinensis (Dallas) |
Chinese stinkbug | Not determined | (±)-Aspongamide A | Therapeutic agents for chronic kidney disease | (45, 46) |
|
Dactylopius coccus (Costa) |
Not determined | Carminic acid | Antioxidant | (40) | |
| Hymenoptera | |||||
| Perga affinis (Kirby) | Australian sawfly | Larvae | Macrocarpal and grandinol | Antimicrobial against Bacillus subtilis | (47) |
| Polyrhachis dives (Smith) | Chinese black ants | Adult | (±)-Polyrhadopamine A, (±)-Polyrhadopamine B, (±)-Polyrhadopamine C, trolline, (±)-Polyrhadopamine C β-carboline-3-carboxamide 5-(3-indolylmethyl)-nicotinsaureamide |
Used to treat rheumatoid and osteo arthritis, inflammatory diseases, and central nervous system. Antiproliferative against T-lymphocytes |
(48, 49) |
| Tetraponera rufonigra (Jerdon) | Iron ant | Adult | Tetraponerines | Neurotoxic Antiproliferative |
(50) |
| Apis mellifera (Linnaeus) | Brazilian red propolis | Not determined | Lupeol, lupenone, lupeol acetate | Antitumor | (51) |
| Lepidoptera | |||||
| Byasa polyeuctes (Doubleday) | Taiwan butterfly | Adult | Papilistatin | Antibacterial Anticancer |
(52) |
| Orthoptera | |||||
| Brachystola magna (Girard) | Texas grasshopper | Adult | Pancratistatin Narciclasine Ungeremine |
Anticancer | (40) |
| Schistocerca gregaria (Forsskål) | Desert locust | Adult gut | Desmosterol, (3β, 5α) cholesta-8, 14, 24-trien-3-ol, 4, 4-dimethyl, (3β, 20R) cholesta-5, 24-dien-3, 20-diol | Antimicrobial properties under investigation | (53) |
Numerous therapeutic qualities, such as antioxidant and anti-platelet aggregation action, have been examined in vitro in various edible insects (32). The expression of anti-tryptic and chymotryptic activity, as well as the inhibition of pancreatic lipase, and dipeptidyl peptidase-4 activity, have all been studied in vitro (32). Moreover, a recent study showed that flour from Tenebrio molitor (Linnaeus) affected the growth of Lactobacillus and Bifidobacterium by improving short-chain fatty acid production and viability in nutritive stress conditions (54).
There have been parallel efforts in cellular and ex vivo models. Human umbellar vein endothelial cells (HUVECs) exhibited lower levels of thrombin, plasminogen activator inhibitor, and factor Xa after exposure to indole alkaloids derived from Protaetia brevitaris seulensis (Kolbe) (55). The ethanolic extract from Gryllus bimaculatus (De Geer), Oxya chinensis sinuosa (Mishchenko), and Protaetia brevitaris seulensis reduced intracellular lipid accumulation and triacylglycerol in liver hepatocellular carcinoma (HepG2) (55). Lipid accumulation was also reduced in L-02 cells, a human fetal hepatocyte line, by polyunsaturated fatty acids and α-linolenic acid from Bombyx mori (L.) (35). Again, tetrahydroquinolines isolated from Allomyrna dichotoma caused a reduction in vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 levels, as well as adherence of monocytes to HUVECs monolayers and migration of human neutrophils (56).
Moreover, there have been investigations into the effects of edible insects through animal models. For instance, Zebrafish Danio rerio (F. Hamilton) fed with Hermetia illucens (L.) showed a significant increase in growth rate (57). Tetrahydroquinolines from Allomyrina dichotoma (L.) administered to septic C57BL/6 mice increased their survival rate (56). Also, wheat noodles enriched with B. mori powder significantly reduced post-prandial blood glucose, glucose peak, and area under the curve of glucose and glucose index (58).
5. Contribution of edible insects to climate change mitigation
Rapid global climate change continues to threaten the existence of humanity on earth (59). Nevertheless, the production of livestock is solely responsible for more than 14.5% of all greenhouse gas (GHG) emissions (CO2, CH4, and NO2), 64% of the world’s NH4 emissions, water pollution, and biodiversity loss (7). Livestock, a significant driver of environmental degradation, calls for an alternative protein source, such as consuming edible insects known for their low contribution to GHG (60). For example, the emissions of greenhouse gases per kilogram of mass and NH3 of three edible insects T. molitor, A. domesticus, and Locusta migratoria (L.) were lower than pigs and far lower than cattle (61). Also, the global warming potential per kg of Protaetia brevitarsis seulensis production (15.93 kgCO2eq) was lower than the conventional meat sources, such as chicken (18–36 kgCO2eq), pork (21–53 kgCO2eq), and beef (75–170 kgCO2eq) (62). Furthermore, the methane output of cockroaches and beetle larvae was more than 20 times lower than that of cattle and was similar to or slightly lower than that of pigs (62). Using the lifecycle assessment (LCA) method, GHG emission from producing 1 kg of mealworm was far lower than that of chicken, pork, or beef (63). Also, the LCA for black soldier fly production was more sustainable than that of fresh chicken meat (64).
6. Contribution of edible insects to ecosystem services
Edible insects provide direct and indirect ecosystem services like cultural, provision, maintenance, and regulation as per the definition by the Millennium Ecosystem Services (65). One of the ecological services that insects may give is the decomposition of organic waste. Insects are frequently employed to break down agricultural and culinary waste when raising insects in large numbers for food. The black soldier fly, Mealworms, houseflies, and crickets are the most effective bio converters (7, 66, 67). These insects can simultaneously make valuable commodities, like biomass from insects, cosmetics, lubricants, medicines, biofuels, surfactants and fertilizers (68). These insects also render regulatory services through the control of crop pests and pollination (Table 2).
Table 2.
Examples of insects’ species and the ecosystem services they render.
| Order | Family | Species | Pollination | Decomposition | Reduction of food waste | Food chain or food web | Reference |
|---|---|---|---|---|---|---|---|
| Blatodea | Termitidae | Macrotermes spp. | √ | √ | (69) | ||
| Blattidae | Periplaneta americana (L.) | √ | √ | ||||
| Coleoptera | Curculionidae | Rhynchophorus ferrugineus (Olivier) | √ | √ | √ | (70–72) | |
| Dytiscidae | Schoolhouse acupunctatus (Gyllenhal) | √ | √ | √ | |||
| Dytiscus latissimus (L.) | √ | √ | √ | ||||
| Hydrophilidae | Cybister spp. | √ | √ | √ | |||
| Curculionidae | Metamasius spp. | √ | √ | √ | |||
| Scarabaeidae | Anoplognathus viridiaeneus (Donovan) | √ | √ | √ | |||
| Phyllophaga spp. | √ | √ | √ | ||||
| Oryctes spp. | √ | √ | |||||
| Tenebrionidae | Tenebrio spp. | √ | √ | √ | |||
| Zophobas morio (Fabr.) | √ | √ | √ | ||||
| Lucanidae | Lucanus cervus (L.) | √ | √ | √ | |||
| Lepidoptera | Cossidae | Comadia spp. | √ | √ | √ | √ | (73, 74) |
| Redtenbacheri spp. | √ | √ | √ | √ | |||
| Hesperiidae | Aegiale hesperiaris (Walker) | √ | √ | √ | √ | ||
| Saturniidae | Cirina butyrospermi (Vuillet) | √ | √ | √ | √ | ||
| Gonimbrasia belina (Westwood) | √ | √ | √ | √ | |||
| Psychidae | Psychidae gen. | √ | √ | √ | √ | ||
| Crambidae | Omphisa fuscidentalis (Hampson) | √ | √ | √ | √ | ||
| Sphingidae | Macroglossum stellatarum (L.) | √ | √ | √ | √ | ||
| Bombycidae | Bombyx mori (L.) | √ | √ | √ | √ | ||
| Hymenoptera | Vespidae | Vespula vulgaris (L.) | √ | (75) | |||
| Formicidae | Oecophylla smaragdina (Fabr.) | √ | √ | √ | (76, 77) | ||
| Camponotus inflatus (Lubbock) | √ | √ | √ | ||||
| Oecophylla longinoda | √ | √ | √ | ||||
| (Latreille) | √ | √ | √ | ||||
| Myrmelachista schumanni (Emery) | √ | √ | √ | ||||
| Atta spp. | √ | √ | |||||
| Apidae | Apis spp. | √ | √ | ||||
| Bombus spp. | √ | √ | |||||
| Xylocopa spp. | √ | √ | |||||
| Trigona spp. | √ | √ | |||||
| Orthoptera | Pyrgomorphidae | Sphenarium purpurascens (Charpentier) | √ | (78–81) | |||
| Acrididae | Locusta migratoria (L.) | √ | |||||
| Gryllidae | Oxya hyla (Serville) | √ | |||||
| Acheta domestica (L.) | √ | √ | |||||
| Gryllidae | Gryllus veletis (Alexander & Bigelow) | √ | √ | ||||
| Tettigonioidea | Tettigonia viridissima (L.) | √ | √ | ||||
| Hemiptera | Aphididae | Aphididae spp. | √ | (71) | |||
| Cicadidae | Magicicada spp. | √ |
*√ indicates the service rendered.
6.1. Pollination
Globally, insects are relied on extensively for pollination in agriculture. This ecosystem service is of great economic value. The Apidae family of bees is considered the most significant edible insect pollinators. Honey bees are also noted to increase the yield of about 96% of crops, with recent literature indicating that wild bees might be even better pollinators than honey bees (82). The study further indicated that, in the United States, bee pollination services are valued at 3.07 billion US dollars. Therefore, the limitation on pollination by bees poses a significant risk to yield stability and food security (82). Butterflies and moths are also important pollinators of crops. Agrius convolvuli (L.), a Hawk moth, is a critical papaya pollinator in Kenya and Southeast Asia (64).
7. Decomposition
Edible insects like ants and termites are vital in soil formation and the cycling of nutrients through the decomposition of organic matter. The direct consumption of organic matter and the indirect effects of insect activities, such as creating larval tunnels in woody materials, result in their decomposition in the tropical forest, thereby increasing soil fertility. An example is the palm weevil which deposits its eggs on the trunk or directly on the inner tissues of falling trees. The emergent larvae then accelerate the decomposition of the logs by burrowing through and feeding on the inner tissues. Twenty-nine percent of deadwood’s carbon flux emanates from insects’ net effects, making it impossible to rule out the functional importance of edible insects in decomposition (83).
7.1. Reduction of food waste
The loss and wastage of food threaten the sustainability of our food system. Millions of tonnes of food waste are generated annually, with research confirming up to 50% waste along the food supply chain (84). Insect-based bioconversions through a novel approach could be an immediate approach to reducing food waste (85). Edible insects can convert low-value food waste such as brewery grains, potato peels, and expired food into biomass and frass for other purposes (84). Using food waste to rear insects is an alternative means to close the gap in the food value chain. Edible insects reared on food waste enter the food chain, with their residues serving as a nutrient source for crop production (86).
7.2. Food chain/web
Insects are considered rich in essential nutrients and have recently attracted attention as food and feed for terrestrial livestock or fish (87). Edible insects like H. illucens can transform lost nutrients into the food chain as protein-rich human food, animal feed, and even fertilizer (88). Although these edible insects render such tremendous ecosystem services, the fact that they are part of the food chain cannot be ignored. Therefore, they can be harvested and consumed cautiously without overexploiting them beyond their regeneration capacity.
8. Economic benefits
Even though edible insects are for human consumption, it is critical to note that the food industry has historically been a significant driver of economic growth and employment creation, making insect farming a promising strategy for alleviating poverty. Insects can be a source of income for even the lowest sections of society because they are easy to harvest, cultivate, rear, and process (89–92). The market for edible insects is expected to expand from $400 million to 1.2 billion in 5 years (2018–2023) (93). More specifically, in the Asia Pacific, the market is likely to exceed $270 million by 2024 (94).
In Africa, lepidoptera is the most consumed order of insects (95). They provide proteins, fats, and essential micronutrients. In South Africa, Uganda, and Nigeria, rearing and selling caterpillars generate income in rural areas (95). In East Africa, insect rearing is rapidly growing and becoming a sustainable option as opposed to the current farming options, which demand arable water and land (96). The authors further indicated the ongoing trends in insect farming, the important insect species, the nutrients derived from insects, and the marketing and regulatory frameworks associated (96). They highlighted how insect farming had created microenterprises in East African countries, including Kenya, Uganda, and Tanzania (96). Insect rearing can reduce poverty as it demands labor. In Africa, after palms have been cut down in a labor-intensive process to extract sap, the cut trunks are revisited to extract the larvae of the palm weevil, R. phoenicis (7).
As a result of insect farming practices, new employment opportunities have emerged. Producing powders from insects can generate jobs and financial gain (97). The European Union has recently permitted the use of processed insects as feed for fish (97). Several species of insects can be farmed: Musca domestica (L.), Alphitobius diaperinus (Panzer), H. illucens, Gryllodes sigillatus (Walker), Gryllus assimilisit (Fabr.), T. molitor (L.), A. domesticus (L.) (97). Due to rising food prices, the European Union plans to encourage insect rearing and expand the number of insects that can legally be added to fish food. Some African countries, including Uganda and Kenya, feed their poultry with insects (97). If other African countries followed suit, the outcome for the continent would be sustainable development within the animal farming sector.
A recent study showed how insects create socioeconomic changes, mitigate societal challenges, create healthier food, and reduce animal waste production and consumption (98). In Thailand, insect rearing has been revolutionized by disseminating knowledge and improving rearing methods as opposed to the previous practice of collecting insects in the wild (98). Globally, insect rearing has the potential to help humanity achieve the seventeen Sustainable Development Goals (SDGs) proposed by the United Nations. Some of the goals, include zero hunger, good health and well-being. The increasing popularity of eating insects suggests that they could be further promoted as a healthy and sustainable food option.
9. Edible insects as pests of crops
Some insect pests are simultaneously considered a vital source of micro-nutrient and protein and thus are consumed by humans. The most common insect pests considered edible include species belonging to the order Coleoptera, Lepidoptera, Hemiptera, and Orthoptera (Table 3) (99, 102). The most well-known edible insect pests are Schistocerca gregaria (Forsskål), L. migratoria, Locusta napardalina (Walker), Zonocerus variegatus (L.) and Nomadacris septemfasciata (Audinet-Serville) (102). These insect pests can cause significant yield losses to host crops exposing bare ground to soil erosion and impacting ~10% of humans (102). In addition, yam, banana, cassava, cocoa, citrus, cowpea, maize, and soybeans are all targets of these polyphagous feeders. Because of their high nutritional value, locusts are collected and used as food and feed in 65 countries, primarily in Africa and Asia, during outbreaks (53). Oryctes rhinoceros, O. monoceros, O. boas, and R. phoenicis are considered an economically important pest of Elaei guineensis, Phoenix dactylifera, Raphia spp. and Cocos nucifera. The larvae of Orycte boas destroy the crops and cause low yields. Moreover, R. phoenicis are voracious feeders, and with their hard mouth parts, they penetrate and damage the plant tissues, causing the leaves to die (106). The larvae of these pests are considered edible insects mainly in Africa (107). The fall army worm Spodoptera frugiperda (Smith) and S. exempta can destroy entire crops by feeding on the early stages of the maize plant. The larvae of the pest are consumed in Zambia (102). Although insect pests are considered a significant constraint of crop production, edible insect pests have an exceptionally high potential to contribute to a more sustainable and socially equitable global food security. Also, consuming these insects could be an environmentally friendly strategy for biological control.
Table 3.
Common insect pests used as food.
| Insect species | Common name | Consumption stage | Host plant | Reference |
|---|---|---|---|---|
| Coleoptera | ||||
| Heteroligus mele (Billberg) | Yam beetles | Adult | Dioscorea spp. | (99) |
|
Rhynchophorus phoenicis Fabr.) Oryctes monoceros (Oliv.) |
African palm weevil Coconut beetle |
Larvae |
Elaeis guineensis Phoenix dactylifera Raphia spp., and Cocos nucifera |
(100) |
| Lepidoptera | ||||
| Leuconodes laisalis Walker | Egg fruit borer | Larvae | Solanum anguivi, Solanum incanum, Solanum linnaeanum, Solanum macrocarpon, Solanum melongenum, Lycopersicon esculentum and Capsicum annuum | (101) |
|
Spodoptera frugiperda (Smith) Spodoptera littoralis (Boisduval) |
The fall armyworm African cotton leafworm |
Larvae Larvae |
Zea mays and
Ricinus communis |
(102, 103) |
| Cirina forda | Larvae | Vitellaria paradoxa | (102) | |
| Hemiptera | ||||
| Agonoscelis versicolor (Fabr.) | Sudan millet bug | Adult | Sorghum spp. | (104) |
| Orthoptera | ||||
|
Ruspolia spp. R. differens (Serville) R. nitidulis vicinus (Walker) |
Long-horned grasshopper | Adult | Ageratum conyzoides (L.), Citrus depressa Hayata, Cynodon dactylon (L.), D. gayana, Eragrostis mexicana Hornem, Eucalyptus saligna SM., Indigofera arrecta Hochst. ex A. Rich., Persicaria nepalensis (L.), and Sorghum halepense (L.) | (13) |
| Kraussaria angulifera (Krauss) | Senegalese grasshopper | Nymph and adult | Cenchrus americanus | (105) |
| Nomadacris septemfasciata (Audinet-Serville) | Red locust | Adult | Echinochloa pyramidalis, Cynodon dactylon and Cyperus spp. | (13) |
| Schistocerca gregaria (Forsskål) | Desert locust | Adult | Sorghum spp., Vigna unguiculata, Manihot esculenta, Abelmoschus esculentus and Zea mays |
(28) |
| Acrida turrita (L.) | Long-headed grasshopper |
Adult |
Vigna unguiculata,
Manihot esculenta and Abelmoschus esculentus |
(28) |
| Zonocerus variegatus (L.) | Variegated grasshopper | Adult |
Chromolaena odorata,
Vigna unguiculata, Manihot esculenta and Abelmoschus esculentus |
(102) |
10. Edible insects as a reservoir of diseases
Insects as food and feed have recently attracted tremendous attention due to their high-quality nutrient contents, ability to upcycle low-grade organic substrates into high-quality insect biomass, and reduction in environmental footprint (94). Despite the rapidly expanding insect farming industry, there has been a significant focus on the potential for disease outbreaks in insect colonies and their spread to humans, animals, and plants.
Insect-borne pathogens threaten the health of humans, animals, and insects as they can cause disease or even death and eventually collapse an entire insect colony (108). A recent study characterized bacterial communities associated with A. domesticus and G. assimilis and compared populations associated with the surface and whole body of crickets to uncover potentially beneficial and pathogenic microorganisms. Findings from the study support the use of probiotics composed of microorganisms already present in the human digestive system (109). In contrast, some potentially dangerous microorganisms were in the samples.
A recent study by Gałęcki and Sokół (110) identified parasites colonizing mealworms, house crickets, cockroaches, and migrating locusts in Central Europe household farms and pet stores (Table 4). The study revealed parasites in 244 out of 300 examined insect farms. Interestingly, 206 of the cases had parasites that were pathogenic for insects only; 106 had parasites pathogenic for animals; and in 91 cases, parasites were pathogenic for humans (6). However, in humans, before being consumed, edible insects must first undergo one of four common processes: boiling, drying, toasting, or frying (103), which can kill pathogens associated with the edible insect.
Table 4.
List of diseases/pathogens transmitted by edible insects.
| Vector | Pathogen group | Type of pathogen | Pathology notes | Reference |
|---|---|---|---|---|
| Crickets | Bacteria | Acinetobacter, Enterococcus | Water- or food-borne disease in humans | (109) |
| Crickets | Bacteria | Bacillus cereus | listed as biologically hazardous in edible insects | (111) |
| Crickets and locust | Gordiidae | Intestinal parasites | (114) | |
| Crickets and locusts | Fungi | Nosema spp. | Decrease dry matter consumption, increase mortality in mass-reared insect colonies and reduce profitability | |
| Cockroaches | Protozoans | Gregarine spp.; Nyctotherus spp. | Deprive insects of nutrients and compromise the immune system, reproduction and lifespan | |
| Cockroaches | Nematode | Thelastoma spp.; Hammerschmidtiella diesigni (Hammerschmidt) | Lower fat content of insect body | |
| Crickets and locusts | – | Steinernema spp. | Have a special structure for storing the bacteria. Once inside the insect’s body, the bacteria are released that produce toxins, which kill the insect. | |
| Mealworms, house crickets, cockroaches and locusts | Protozoans | Cryptosporidium spp. | Cause chronic diarrhoea in reptiles | |
| Mealworms, house crickets, cockroaches and locusts | Protozoans | Isospora spp. | Cause isosporiasis in both immunosuppressed humans and in animals who ingest oocytes | |
| Mealworms, cockroaches and locusts | Protozoans (Ciliates) | Balantidium spp. | Can cause balantidiasis in humans and animals | (17) |
| Mealworms, cockroaches and locusts | Amoeboids | Entamoeba spp. (E. histolytica and E. invadens) | Can cause dysentery in humans, animals, reptiles, and amphibians | (17) |
| Mealworms, house crickets, cockroaches and locusts | – | Cestoda | Colonize insects and aids in transmitting tapeworms to birds, insectivorous animals, and humans | (110) |
| Mealworms and cockroaches | Nematodes | Pharyngodon spp. | Colonize wild and captive animals, e.g., lizards | |
| Mealworms, house crickets, cockroaches and locusts | Nematodes | Physaloptera spp. | Influence insect behavior | |
| Mealworms and cockroaches | – | Spiruroidea | Colonize mainly animals, but can also infec thumans who consume infected intermediate hosts | |
| Mealworms and cockroaches | Thorny-headed worms | Acanthocephala | Decreases immune reactivity in cockroaches. Can compromise glycogen and lipid levels in crustaceans | |
| Cockroaches | Arthropods | Pentastomida | Causes pentastomiasis in wild and captive reptiles | |
| Black soldier fly larvae/rearing substrates | Bacteria | Staphylococcus aureus, Clostridium, Escherichia coli, Salmonella and Enterococcus | Could be pathogenic to livestock fed contaminated feed supplements (larval meal) | (112) |
| Frass of black soldier fly larvae | Phytopathogenic fungi | Cercospora | Cause irregular and unhealthy leaf appearance in lettuce plants | (113) |
Often, organic side streams are used in rearing Black Soldier Fly (BSF) larvae which are a potential source of food-safety-related microbes (112, 115, 116). In plants, Dzepe et al. (113) identified phytopathogenic fungi (see Table 4) in the leaves of lettuce Lactuca sativa when grown in frass-exposed soil. The presence of pathogens in a rearing substrate could be problematic when the resulting residues are used as fertilizers since phytopathogenic fungi can negatively impact crop yield (117). Thus, pathogen contamination of rearing substrates could pose multiple risks when the insects are reared for food and feed and the residue for soil enrichment. Interestingly, BSF larvae can reduce pathogen abundance in substrates during rearing. This pathogen inhibitory effect has been observed for different food-safety-related bacteria, including Escherichia coli and Salmonella species (115, 118). Kuznetsova et al. (116) found a complete elimination of mycelial fungi from feed substrates when BSF larvae were reared on food substrates.
So far, adult BSF has not been reported as a disease vector. However, van Huis (7) warns against a possible susceptibility to infections following the sector’s rapid growth. Adult House fly (HF) is a nuisance to humans, animals, and vectors of about a hundred pathogens, including bacteria, protozoans, helminths, and viruses (7). Although insect-borne pathogenic viruses are largely host-specific (119), their effects can be felt in humans and other animals that are exposed to disease-ridden material. For instance, adult HF can potentially act as a vector of the orf virus Ecthyma contagiosum (Poxviridae), which causes ecthyma in sheep and goats and humans exposed to disease-infected animals (120). Like BSF, HF larvae can reduce the microbial load in manure (14). Furthermore, bacterial endospores have been identified in yellow mealworms and house crickets. A high endospore count of 5.0 log (c.f.u. g − 1) was recorded in cricket samples (121). The study hinted at a possible food safety risk since this endospore count surpassed the lower threshold for Bacillus cereus in edible insects. However, no legal microbiological criteria existed specifically for edible insects (121). Therefore, edible insects can sufficiently contribute to diversifying and securing global food and feed. However, the potential of this mini-livestock to harbor and transmit diseases cannot be neglected as they can pose both direct and indirect health risks to humans, animals, and plants.
11. Environmental impacts of edible insect production
Safe food and water supply, unpolluted air, safe use of chemicals, sound agricultural practices, and preservation of natural resources are all attributes of a healthy environment and align very well with the United Nations Sustainable Development Goals (SDGs), especially SDGs 6, 12–15 (122). Providing food for everyone now and more so in the future is one of the most significant challenges directly involving SDGs 1 and 2 (98). Our existing food systems are heavily involved in many environmental issues, such as greenhouse gas emissions, eutrophication of freshwater resources, and biodiversity loss (123).
Farming insects for food and feed has recently received considerable attention as a sustainable alternative to conventional food production models, providing food for humans and animals with minimal environmental footprint (Table 5) (97). To understand the comparative advantage of insect farming over conventional livestock farming, Skrivervik (124) re-echoed the criticism meted out on meat production due to its negative impact on the ecosystem. For instance, farming livestock takes up considerable agricultural land, and the emission of nitrous oxide is concerning, thus making livestock production highly eco-degrading (124). Insects have a smaller feed conversion ratio than cattle and require less space to farm. House crickets are known to be about four times more efficient feed converters than pigs and over 12 times better than cattle (124). Almost (in most cases 100%) all of the insect body is consumed, compared to lower than 50% for cattle, which may translate to less food wastage in favor of insects (124). Furthermore, producing insects in areas with near-optimal environmental conditions, such as the tropics, could benefit energy use reduction. Insects are poikilothermic and adjust their body temperature to that of their surroundings, hence less demand for external energy inputs.
Table 5.
Advantages of insect farming over livestock farming.
| Criteria | Insect farming | Livestock farming |
|---|---|---|
| Land use | Low requirements for land | High requirements for land |
| Water use | Low requirements for water | High requirements for water |
| Pollutants | Low production of greenhouse gases | Greenhouse gases such as carbon dioxide and methane are emitted |
| Recycling | Transforms low-value matter into nutritious insects by feeding | Some livestock such as cattle do not feed on low-value matter |
| Use as feed | Insects can be used as fishmeal | Most livestock cannot be used as fishmeal |
Madau et al. (97).
Among several factors considered in assessing environmental impact is methane release, which results from the fermentation of Methano-bacteriaceae in the gut, food conversion, and reproduction rate, all typical in beef cattle, poultry, and pigs. This far, cockroaches, termites, and beetles are known to release methane, and less so for other edible insects. For instance, the yellow mealworm is not known to produce methane and thus retains a low global warming potential relative to other livestock products (125). Greenhouse gas emissions from mealworms, house crickets, black soldier flies, and houseflies; feed conversion efficiency, organic waste reduction, and; fishmeal replacement by insect meal in animal feed are all topics that have been examined in the context of conventional livestock production (61).
Despite the several benefits of insect farming, the fast-growing and innovative sector is not without negative effects. Quang Tran et al. (126) suggests a trade-off in using insect meal from BSF, HF, mealworm, and (Z. variegatus) as an aquafeed. In their analysis, Quang Tran et al. (126) showed that the inclusion of insect meals in aquafeeds led to higher values of global warming potential, and water and energy use than those obtained in diets without insects. They, however, attribute this impact to the insufficiency of production technology and scalability. Nikkhah et al. (62) also found positive impacts of farming Protaetia, brevitarsis seulensis larvae, noting beneficial environmental effects on land use, mineral extraction, and aquatic and terrestrial ecotoxicity when insects were reared on bio-waste. However, several reports found negative environmental effects of insect farming associated with global warming (62, 125).
Edible insect farming can contribute to a sustainable food and feed system, given that some insect species can thrive on low-grade organic streams. However, the safety of derived food and feed cannot be guaranteed without careful monitoring and implementation of preventive measures while closely checking the effects of technological advancements (61).
Edible insect farming has the potential to benefit humanity and the environment; however, farming should be critically reviewed so as not to damage the environment. Farmed insects are numerous, and the order with the highest number of edible insects belongs to Coleoptera (127). There are currently twelve living orders of aquatic insects, six of which are considered edible. Unfortunately, these insects are not being harvested sustainably and are exposed to overexploitation and extinction (127). Dragonflies, for example, are edible aquatic insects, and their over-exploitation and extinction can affect the environment due to an ecosystem balance. It is important to emphasize how farming insects help improve conservation directly and indirectly. Dragonflies feed on mosquitoes, and the former is considered the natural enemy of the latter. The balance between these natural enemies and the pests enhances the environmental health and sustainability of ecosystems and guarantees that there is food at the different trophic levels of the food chain.
The United Nations has reported the urgency of reducing greenhouse gases to combat climate change and improve environmental health (98). Livestock production accounts for about 14.5% of all greenhouse gas emissions (128). Insects raised from human waste can help clean waterways, one of many ways to improve environmental health (98). Several greenhouse gases are currently contributing to the damage of ozone layer. Even though some insects, such as cockroaches, termites, and beetles, release methane into the atmosphere, insect rearing can reduce climate change as it is more environmentally friendly source of protein (98). Even though pig, poultry, and beef products are currently the preferred source of animal proteins, insect rearing has a better impact on the environment regarding reproductive rate, food conversion efficiency, and methane production. The yellow mealworm, T. molitor, is considered environmentally benign due to its high reproductive rate and lack of methane production (125).
Many studies have shown that raising insects positively affects the planet (97). Their requirements for arable land and water are relatively low compared to fish or poultry farming (97). In addition, insect rearing has a low environmental cost for producing greenhouse gases such as carbon dioxide (97). Furthermore, insects’ nutritional quality is relatively high compared to the trade-off regarding the effect on the environment. Proteins and minerals like calcium, iron, and zinc are a few nutrients that can be obtained from eating insects. Finally, just as beekeeping has been promoted over several centuries to increase honey production and environmental conservation (129), there is a need for sustainable rearing of edible insects.
12. Implication of harvesting wild edible insects on their conservation
Over-exploitation of insects significantly contributes to the decline of many edible insect species, threatening wild insects (73). Usually, insects are sourced through wild harvesting, farming, and semi-domestication of the wild species. However, literature shows that about 92% of these insect species are harvested from the wild (28). Wild harvest of edible insects by humans brings about direct competition with other predators, eventually undermining their population viability. Quite many edible insect species are hosts or prey to other organisms. Hence, overexploitation of edible insects from the different trophic levels beyond regeneration capacity may adversely affect the population of other organisms and, consequently, the provision of some essential ecosystem services (130). Over a decade of research has revealed that the population of widely consumed Mopane worm (Imbrasia belina) keeps declining in South Africa and Zimbabwe due to increased commercialization and overexploitation (131). The global trade in insect species continues to grow due to the need to feed a world population approaching 8 billion people (132). During periods of meat protein shortage, insects constitute nearly a third of their protein intake, which threatens the edible insect species population since it often exceeds their regeneration capacity. Also, the collection practices have become less selective and sustainable (133). A decline in the population of some edible insect predators or parasitoids has been reported in western and northern Europe and New Zealand (134).
13. Conclusion and future perspectives
Using insects as food and feed has a long history since ancient times and continues to provide food for millions of people worldwide. Edible insects contain essential nutrients, such as carbohydrates, proteins, vitamins, and minerals, which have antimicrobial properties. Apart from these benefits, edible insects require a smaller space for production than livestock, which need more extensive land to produce the same amount of energy. Edible insects provide many ecosystem services, such as decomposition, pollination, reduction of food waste and support of food chain or web, and monitoring of environmental health. Utilizing insects as food and feed, we can alter beef, fish, and poultry consumption and the life cycle assessment, thereby reducing greenhouse gas emissions, ammonia emissions, and carbon footprints. Several activities associated with edible insect production and marking generate jobs, and income, thereby ensuring poverty reduction and zero hunger, especially in developing countries. Edible insects can potentially improve our global food security significantly, but they also have several challenges that need addressing. Some wild edible insects harbour pathogens of plants, animals, and humans. For instance, migratory locusts are consumed by amphibians, reptiles, and humans, mainly in parts of Africa and Asia. Nosema spp. and Gregarine spp., which cause severe losses to bee colonies worldwide, are the common parasites of Locusts. Acheta domesticus harbors Nosema spp., Gregarine spp., and Steinernema spp. (135). Though A. domesticus are often consumed in powdery form or protein extracts, the insect can also be consumed directly (136, 137). Recent scientific research demonstrates that the bacteria levels and anti-nutrient components in edible insects are reduced using preservation procedures, primarily thermal treatments, employed in cooking or processing (135). Specifically, these methods suggest proper preparation by boiling, drying, toasting, or frying edible insects to ensure a safe diet. Rearing edible insect pests of horticultural and forest crops like termites, locusts, and grasshoppers requires an appropriate procedure to avoid possible introduction outside the farming facility. Moreover, with proper rules and policies, these alternative protein sources may offer a solution to problems of availability and accessibility of conventional proteins sources. Furthermore, the problem of overexploitation of edible insect resources can be curbed by laying rules to control their consumption and, more importantly, by educating people on the need to move from wild harvesting to farming and semi-domesticating wild species. In farming/rearing edible insects, there would be a need to encourage the use of food leftovers to save production costs and solve the problem of loss and wastage of food.
Author contributions
OFA: Study – conceived and designed, writing – original draft, and review and editing. JO-O, KA, AKD, BOB, SKD, KDN, and SYC: writing – original draft, review and editing. SAS: review and editing. All authors contributed to proofreading of the final version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors appreciate the comments from Ito Fernando for the improvement of this article.
Footnotes
References
- 1.Acosta-Estrada BA, Reyes A, Rosell CM, Rodrigo D, Ibarra-Herrera CC. Benefits and challenges in the incorporation of insects in food products. Front Nutr. (2021) 8:344. doi: 10.3389/fnut.2021.687712, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhaliwal SS, Sharma V, Shukla AK, Verma V, Kaur M, Shivay YS, et al. Biofortification—a frontier novel approach to enrich micronutrients in field crops to encounter the nutritional security. Molecules. (2022) 27:1340. doi: 10.3390/molecules27041340, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel A, Desai SS, Mane VK, Enman J, Rova U, Christakopoulos P, et al. Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases. Trends Food Sci Technol. (2022) 120:140–53. doi: 10.1016/j.tifs.2022.01.006 [DOI] [Google Scholar]
- 4.Usman MA, Haile MG. Market access, household dietary diversity and food security: evidence from eastern Africa. Food Policy. (2022) 113:102374. doi: 10.1016/j.foodpol.2022.102374 [DOI] [Google Scholar]
- 5.Wade M, Hoelle J. A review of edible insect industrialization: scales of production and implications for sustainability. Environ Res Lett. (2022) 15:12–123013. doi: 10.1088/1748-9326/aba1cl [DOI] [Google Scholar]
- 6.van Itterbeeck J, Pelozuelo L. How many edible insect species are there? A not so simple question. Diversity. (2022) 14:2–143. doi: 10.3390/d14020143 [DOI] [Google Scholar]
- 7.van Huis A. Edible insects: challenges and prospects. Entomol Res. (2022) 52:161–77. doi: 10.1111/1748-5967.12582 [DOI] [Google Scholar]
- 8.IPIFF . An overview of the European market of insects as feed. International Platform of Insects for Food and Feed, Brussels. (2021). https://ipiff.org/wp-content/uploads/2021/04/Apr-27-2021-IPIFF_The-European-market-of-insects-as-feed.pdf [Google Scholar]
- 9.Baiano A. Edible insects: an overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci Technol. (2020) 100:35–50. doi: 10.1016/j.tifs.2020.03.040 [DOI] [Google Scholar]
- 10.Dossey AT, Tatum JT, McGill WL. Modern insect-based food industry: current status, insect processing technology, and recommendations moving forward In: Insects as sustainable food ingredients. Cambridge, MA: Academic Press; (2016). 113–54. [Google Scholar]
- 11.Siddiqui SA, Zannou O, Karim I, Kasmiati Awad NMH, Gołaszewski J, Heinz V, et al. Consumer acceptability of plant-, seaweed-, and insect-based foods as alternatives to meat: a critical compilation of a decade of research. Crit Rev Food Sci Nutr. (2022b):1–22. doi: 10.1080/10408398.2022.2036096, PMID: [DOI] [PubMed] [Google Scholar]
- 12.van Huis A, Halloran A, Van Itterbeeck J, Klunder H, Vantomme P. How many people on our planet eat insects: 2 billion? J Insects Food Feed. (2022) 8:1–14. doi: 10.3920/JIFF2021.x010 [DOI] [Google Scholar]
- 13.Ishara J, Ayagirwe R, Karume K, Mushagalusa GN, Bugeme D, Niassy S, et al. Inventory reveals wide biodiversity of edible insects in the Eastern Democratic Republic of Congo. Sci Rep. (2022) 12:1576–13. doi: 10.1038/s41598-022-05607-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Huis A, Oonincx DGAB, Rojo S, Tomberlin JK. Insects as feed: house fly or black soldier fly? J Insects Food Feed. (2020) 6:221–9. doi: 10.3920/jiff2020.x003 [DOI] [Google Scholar]
- 15.Kavle RR, Pritchard ETM, Bekhit AEDA, Carne A, Agyei D. Edible insects: a bibliometric analysis and current trends of published studies (1953–2021). Int J Trop Insect Sci. (2022) 52:161–77. doi: 10.1111/1748-5967.12582 [DOI] [Google Scholar]
- 16.Puzari M. Prospects of entomophagy. Int J Trop Insect Sci. (2021) 41:1989–92. doi: 10.1007/s42690-020-00317-2 [DOI] [Google Scholar]
- 17.Gałęcki R, Sokół R. A parasitological evaluation of edible insects and their role in the transmission of parasitic diseases to humans and animals. PLoS One. (2019) 14:e0219303–3. doi: 10.1371/journal.pone.0219303, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aidoo OF. Ding F, Ma T, Jiang D, Wang D, Hao M, et al. Determining the potential distribution of Oryctes monoceros and Oryctes rhinoceros by combining machine-learning with high-dimensional multidisciplinary environmental variables. Sci Rep. (2022a) 12:17439. doi: 10.1038/s41598-022-21367-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aidoo OF. Hao M, Ding F, Wang D, Jiang D, Ma T, et al. The impact of climate change on potential invasion risk of Oryctes monoceros worldwide. Front Ecol Evol. (2022b) 10:10. doi: 10.3389/fevo.2022.895906 [DOI] [Google Scholar]
- 20.Hao M, Aidoo OF. Qian Y, Wang D, Ding F, Ma T, et al. Global potential distribution of Oryctes rhinoceros, as predicted by boosted regression tree model. Glob Ecol Conserv. (2022) 37:e02175–5. doi: 10.1016/j.gecco.2022.e02175 [DOI] [Google Scholar]
- 21.Anaduaka EG, Uchendu NO, Osuji DO, Ene LN, Amoke OP. Nutritional compositions of two edible insects: Oryctes rhinoceros larva and Zonocerus variegatus. Heliyon. (2021) 7:e06531–1. doi: 10.1016/j.heliyon.2021.e06531, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debrah SK, Anankware PJ, Asomah S, Ofori DO. Challenges associated with Rhynchophorus phoenicis Fabricius (Coleoptera: Curculionidae) farming: a case study of the Ejisu-Juaben municipality. J Insects Food Feed. (in press) 9:15–24. doi: 10.3920/JIFF2021.0219 [DOI] [Google Scholar]
- 23.Neely C, Bunning S, Wilkes A. Review of evidence on drylands pastoral systems and climate change. Rome, FAO. (2009) 23–123 [Google Scholar]
- 24.FAO, IFAD, UNICEF, WFP, WHO . The State of Food Security and Nutrition in the World 2018. Building climate resilience for food security and nutrition. FAO Licence: CC BY-NC-SA 30 IGO, Rome. (2018) 134–342
- 25.FAO . The state of food security and nutrition in the world 2021. Transforming food systems for food security, improved nutrition and affordable healthy diets for all, in brief. FAO. (2021):78–98. doi: 10.4060/cb5409en [DOI] [Google Scholar]
- 26.Stork NE. How many species of insects and other terrestrial arthropods are there on earth? Annu Rev Entomol. (2018) 63:31–45. doi: 10.1146/annurev-ento-020117-043348, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Jongema Y. Worldwide list of recorded edible insects. Wageningen, The Netherlands: Department of Entomology, Wageningen University & Research. (2017). [Google Scholar]
- 28.Jongema Y. List of edible insect species of the world. Wageningen: Laboratory of Entomology, Wageningen University; (2017). [Google Scholar]
- 29.van Huis A, Rumpold B, Maya C, Roos N. Nutritional Qualities and Enhancement of Edible Insects. Annu Rev Nutr. (2021) 41:551–76. doi: 10.1146/annurev-nutr-041520-010856 [DOI] [PubMed] [Google Scholar]
- 30.Rumpold BA, Schlüter OK. Insect-based protein sources and their potential for human consumption: nutritional composition and processing. Anim Front. (2015) 5:20–4. [Google Scholar]
- 31.Kulma M, Kouˇrimska L, Homolkova D, Božik M, Plachy V, Vrabec V. Effect of developmental stage on the nutritional value of edible insects. A case study with Blaberus craniifer and Zophobas morio. J Food Compos Anal. (2020) 92:103570–14. doi: 10.1016/j.jfca.2020.103570 [DOI] [Google Scholar]
- 32.D’Antonio V, Battista N, Sacchetti G, Di Mattia C, Serafini M. Functional properties of edible insects: a systematic review. Nutr Res Rev. (2021a) 36:98–119. doi: 10.1017/S0954422421000366 [DOI] [PubMed] [Google Scholar]
- 33.Hwang BB, Chang MH, Lee JH, Heo W, Kim JK, Pan JH, et al. The edible insect Gryllus bimaculatus protects against gut-derived inflammatory responses and liver damage in mice after acute alcohol exposure. Nutrients. (2019) 11:857. doi: 10.3390/nu11040857, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao JG, Wang HY, Wei ZG, Zhang YQ. Therapeutic effects of ethanolic extract from the green cocoon shell of silkworm Bombyx mori on type 2 diabetic mice and its hypoglycaemic mechanism. Toxicol Res. (2019) 8:407–20. doi: 10.1039/C8TX00294K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Y, Wang L, Lv Y, Wu X, Hou C, Li J. Regulation mechanism of silkworm pupa oil PUFAs on cholesterol metabolism in hepatic cell L-02. J Sci Food Agric. (2020) 100:1418–25. doi: 10.1002/jsfa.10115, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Luo SL, Huang HJ, Wang Y, Jiang RW, Wang L, Bai LL. Isocoumarins from American cockroach (Periplaneta americana) and their cytotoxic activities. Fitoterapia. (2014) 95:115–20. doi: 10.1016/j.fitote.2014.03.004, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Zhu HJ, Yan YM, Tu ZC, Luo JF, Liang R, Yang TH. Compounds from Polyphaga plancyi and their inhibitory activities against JAK3 and DDR1 kinases. Fitoterapia. (2016) 114:163–7. doi: 10.1016/j.fitote.2016.09.005, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Kang HR, Lee D, Benndorf R, Jung WH, Beemelmanns C, Kang KS, et al. Termisoflavones A–C, isoflavonoid glycosides from termite-associated Streptomyces sp. RB1. J Nat Prod. (2016) 79:3072–8. doi: 10.1021/acs.jnatprod.6b00738, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Zhou LF, Wu J, Li S, Li Q, Jin LP, Yin CP, et al. Antibacterial potential of termite-associated Streptomyces sp. ACS Omega. (2021) 6:4329–34. doi: 10.1021/acsomega.0c05580, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seabrooks L, Hu L. Insects: an underrepresented resource for the discovery of biologically active natural products. Acta Pharm Sinica B. (2017) 7:409–26. doi: 10.1016/j.apsb.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong QF, Wang JL, Zhang SF, Wang Z, Zhang CX, Gao H, et al. Antifungal activity of crude extracts and fat-soluble constituents of Holotrichia diomphalia larvae. Bioresour Technol. (2008) 99:8521–3. doi: 10.1016/j.biortech.2008.03.010 [DOI] [PubMed] [Google Scholar]
- 42.Mebs D, Pogoda W, Schneider M, Kauert G. Cantharidin and demethylcantharidin (palasonin) content of blister beetles (Coleoptera: Meloidae) from southern Africa. Toxicon. (2009) 53:466–8. doi: 10.1016/j.toxicon.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 43.Dettner K, Schramm S, Seidl V, Klemm K, Gäde G, Fietz O. Occurrence of terpeneanhydride Palasonin and Palasoninimide in blister beetle Hycleus lunata (Coleoptera: Meloidae). Biochem Syst Ecol. (2003) 31:203–5. doi: 10.1016/S0305-1978(02)00069-8 [DOI] [Google Scholar]
- 44.Mudalungu CM, Tanga CM, Kelemu S, Torto B. An overview of antimicrobial compounds from African edible insects and their associated microbiota. Antibiotics. (2021) 10:621. doi: 10.3390/antibiotics10060621, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan YM, Ai J, Shi YN, Zuo ZL, Hou B, Luo J. Aspongamide A, an N-acetyldopaminetrimer isolated from the insect Aspongopus chinensis, is an inhibitor of p-Smad3. Org Lett. (2010) 16:532–5. doi: 10.1021/ol403409v, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Shi YN, Tu ZC, Wang XL, Yan YM, Fang P, Zuo ZL. Bioactive compounds from the insect Aspongopus chinensis. Bioorg Med Chem Lett. (2014) 24:5164–9. doi: 10.1016/j.bmcl.2014.09.083, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Vihakas MA, Kapari L, Salminen JP. New types of flavonol oligoglycosides accumulate in the hemolymph of birch-feeding sawfly larvae. J Chem Ecol. (2010) 36:864–72. doi: 10.1007/s10886-010-9822-2, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Tang JJ, Zhang L, Jiang LP, Di L, Yan YM, Tu ZC. Dopamine derivatives from the insect Polyrhachis dives as inhibitors of ROCK1/2 and stimulators of neural stem cell proliferation. Tetrahedron. (2014) 70:8852–7. doi: 10.1016/j.tet.2014.09.095 [DOI] [Google Scholar]
- 49.Tang JJ, Fang P, Xia HL, Tu ZC, Hou BY, Yan Y. Constituents from the edible Chinese black ants (Polyrhachis dives) showing protective effect on rat mesangial cells and anti- inflammatory activity. Food Res Int. (2015) 67:163–8. doi: 10.1016/j.foodres.2014.11.022 [DOI] [Google Scholar]
- 50.Bosque I, Gonzalez-Gomez JC, Loza MI, Brea J. Natural tetraponerines: a general synthesis and antiproliferative activity. J Org Chem. (2014) 79:3982–91. doi: 10.1021/jo500446f, PMID: [DOI] [PubMed] [Google Scholar]
- 51.De Carvalho FM, Schneider JK, de Jesus CVF, de Andrade LN, Amaral RG, David JM, et al. Brazilian red propolis: extracts production, physicochemical characterization, and cytotoxicity profile for antitumor activity. Biomol Ther. (2020) 10:5–726. doi: 10.3390/biom10050726, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettit TJ, Fiksel J, Croxton KL. Ensuring supply chain resilience: development of a conceptual framework. J Business Log. (2010) 31:1–21. doi: 10.1002/j.2158-1592.2010.tb00125.x [DOI] [Google Scholar]
- 53.Cheseto X, Kuate SP, Tchouassi DP, Ndung'u M, Teal PE, Torto B. Potential of the desert locust Schistocerca gregaria (Orthoptera: Acrididae) as an unconventional source of dietary and therapeutic sterols. PLoS One. (2015) 10:10–e0127171. doi: 10.1371/journal.pone.0127171, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Antonio V, Serafini M, Battista N. Dietary modulation of oxidative stress from edible insects: a mini-review. Front Nutr. (2021b) 2021:642551: 8–64255. doi: 10.3389/fnut [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J, Lee W, Kim MA, Hwang JS, Na M, Bae JS. Inhibition of platelet aggregation and thrombosis by indole alkaloids isolated from the edible insect Protaetia brevitarsis seulensis (Kolbe). J Cell Mol Med. (2017) 21:1217–27. doi: 10.1111/jcmm.13055, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park I, Lee W, Yoo Y, Shin H, Oh J, Kim H, et al. Protective effect of tetrahydroquinolines from the edible insect Allomyrina dichotoma on LPS-induced vascular inflammatory responses. Int J Mol Sci. (2020) 21:10–3406. doi: 10.3390/ijms21103406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarantoniello M, Randazzo B, Gioacchini G, Truzzi C, Giorgini E, Riolo P, et al. Zebrafish (Danio rerio) physiological and behavioural responses to insect-based diets: a multidisciplinary approach. Sci Rep. (2020) 10:1–16. doi: 10.1038/s41598-020-67740-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suk W, Kim J, Kim DY, Lim H, Choue R. Effect of wheat flour noodles with Bombyx mori powder on glycemic response in healthy subjects. Prev Nutr Food Sci. (2016) 21:165–70. doi: 10.3746/pnf.2016.21.3.165, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park SJ, Kim KY, Baik MY. Sericulture and the edible-insect industry can help humanity survive: insects are more than just bugs, food, or feed. Food Sci Biotechnol. (2022) 31:657–68. doi: 10.1007/s10068-022-01090-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siddiqui SA, Alvi T, Sameen A, Khan S, Blinov AV, Nagdalian AA, et al. Consumer acceptance of alternative proteins: a systematic review of current alternative protein sources and interventions adapted to increase their acceptability. Sustainability. (2022c) 14:22–15370. doi: 10.3390/su142215370 [DOI] [Google Scholar]
- 61.van Huis A, Oonincx DGAB. The environmental sustainability of insects as food and feed. a review. ASD. (2017) 37:37. doi: 10.1007/s13593-017-0452-8 [DOI] [Google Scholar]
- 62.Nikkhah A, Van Haute S, Jovanovic V, Jung H, Dewulf J, Velickovic TC, et al. Life cycle assessment of edible insects (Protaetia brevitarsis seulensis larvae) as a future protein and fat source. Sci Rep. (2021) 11:14030. doi: 10.1038/s41598-021-93284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oonincx D, De Boer I. Environmental impact of the production of mealworms as a protein source for humans: a life cycle assessment. PLoS One. (2012) 7:7–e51145. doi: 10.1371/journal.pone.0051145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smetana S. Avoiding food neophobia and increasing consumer acceptance of new food trends—a decade of research. Sustainability. (2022a) 14:16–10391. doi: 10.3390/su141610391 [DOI] [Google Scholar]
- 65.Payne CL, van Itterbeeck J. Ecosystem services from edible insects in agricultural systems: a review. Insects. (2017) 8:24. doi: 10.3390/insects8010024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng Z, Yu L, Li H, Xu X, Yang A. Use of housefly (Musca domestica L.) larvae to bioconversion food waste for animal nutrition and organic fertilizer. ESPR. (2021) 35:4892–48928. doi: 10.1007/s11356-021-14118 [DOI] [PubMed] [Google Scholar]
- 67.Siddiqui SA, Ristow B, Rahayu T, Putra NS, Widya Yuwono N. Nisa’ K, Mategeko B, Smetana S, Saki M, Nawaz A, Nagdalian A. Black soldier fly larvae (BSFL) and their affinity for organic waste processing. Waste Manag. (2022d) 140:1–13. doi: 10.1016/j.wasman.2021.12.044, PMID: [DOI] [PubMed] [Google Scholar]
- 68.Fowles TM, Nansen C. Insect-based bioconversion: Value from food waste. Food waste management. Palgrave Macmillan, Cham. (2020) 321–346 [Google Scholar]
- 69.Sileshi GW, Nyeko P, Nkunika POY, Sekematte BM, Akinnifesi FK, Ajayi OC. Integrating ethno-ecological and scientific knowledge of termites for sustainable termite management and human welfare in Africa. Ecol Soc. (2009) 14:48. doi: 10.5751/es-02877-140148 [DOI] [Google Scholar]
- 70.Choo J, Zent EL, Simpson BB. The importance of traditional ecological knowledge for palm-weevil cultivation in the Venezuelan Amazon. J Ethnobiol. (2009) 29:113–28. doi: 10.2993/0278-0771-29.1.113 [DOI] [Google Scholar]
- 71.Choulamany X. Traditional use and availability of aquatic biodiversity in rice-based ecosystems. III. Xieng Khouang and Houa Phanh provinces, Lao PDR In: Halwart M, Bartley D, Funge-Smith S, editors. Aquatic biodiversity in rice-based ecosystems. Studies and Reports from Cambodia, China, Lao People’s Democratic Republic and Vietnam Rome (Italy): FAO; (2005) [Google Scholar]
- 72.Nonaka K. Resource use in wetland and paddy field in Vientiane plain, Lao PDR. Tropics. (2008) 17:325–34. doi: 10.3759/tropics.17.325 [DOI] [Google Scholar]
- 73.Ramos-Elorduy J. Threatened edible insects in Hidalgo, Mexico and some measures to preserve them. J Ethnobiol Ethnomed. (2006) 2:1–10. doi: 10.1186/1746-4269-2-51, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Badanaro F, Amevoin K, Lamboni C, Amouzou KS. Edible Cirina forda (Westwood, 1849) (Lepidoptera: Saturniidae) caterpillar among Moba people of the Savannah region in North Togo: from collector to consumer. Asian J Appl Sci Eng. (2014) 3:13–24. doi: 10.15590/ajase/2014/v3i8/54479 [DOI] [Google Scholar]
- 75.Nonaka K. Cultural and commercial roles of edible wasps in Japan. Edible Forest Insects: Humans Bite Back, Proceedings of a Workshop on Asia-Pacific Resources and Their Potential for Development, Chiang Mai, Thailand, 19–21 February 2008 Durst P.B., Johnson D.V., Leslie R.N., Shono K., Rome: FAO; (2010) [Google Scholar]
- 76.Van Mele P. A historical review of research on the weaver ant Oecophylla in biological control. Agric For Entomol. (2008) 10:13–22. [Google Scholar]
- 77.Hölldobler B, Wilson EO. The leafcutter ants: civilization by instinct. New York: WW Norton & Company; (2010). [Google Scholar]
- 78.Cerritos R, Cano-Santana Z. Harvesting grasshoppers Sphenarium purpurascens in Mexico for human consumption: a comparison with insecticidal control for managing pest outbreaks. J Crop Prot. (2008) 27:473–80. doi: 10.1016/j.cropro.2007.08.001 [DOI] [Google Scholar]
- 79.Mohamed EHA. Determination of nutritive value of the edible migratory locust Locusta migratoria, Linnaeus, 1758 (Orthoptera: Acrididae). IJAPBC. (2016) 4:144–8. [Google Scholar]
- 80.Pemberton R. The revival of rice-field grasshoppers as human food in South Korea. Pan-Pacific Entomol. (1994) 70:323–7. [Google Scholar]
- 81.Hanboonsong Y, Durst PB. Edible insects in Lao PDR: building on tradition to enhance food security. Bangkok: FAO; (2014). [Google Scholar]
- 82.Reilly JR, Artz DR, Biddinger D, Bobiwash K, Boyle NK, Brittain C, et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc R Soc B. (2020) 287:20200922–2. doi: 10.1098/rspb.2020.0922, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seibold S, Rammer W, Hothorn T, Seidl R, Ulyshen MD, Lorz J, et al. The contribution of insects to global forest deadwood decomposition. Nature. (2021) 597:77–81. doi: 10.1038/s41586-021-03740-8, PMID: [DOI] [PubMed] [Google Scholar]
- 84.Ites S, Smetana S, Toepfl S, Heinz V. Modularity of insect production and processing as a path to efficient and sustainable food waste treatment. J Clean Prod. (2020) 248:119248–8. doi: 10.1016/j.jclepro.2019.119248 [DOI] [Google Scholar]
- 85.Pahmeyer MJ, Siddiqui SA, Pleissner D, Gołaszewski J, Heinz V, Smetana S. An automated, modular system for organic waste utilization using Hermetia illucens larvae: design, sustainability, and economics. J Clean Prod. (2022) 379:134727–7. doi: 10.1016/j.jclepro.2022.134727 [DOI] [Google Scholar]
- 86.Ojha S, Bußler S, Schlüter OK. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. (2020) 118:600–9. doi: 10.1016/j.wasman.2020.09.010, PMID: [DOI] [PubMed] [Google Scholar]
- 87.Hawkey KJ, Lopez-Viso C, Brameld JM, Parr T, Salter AM. Insects: a potential source of protein and other nutrients for feed and food. Annu Rev Anim Biosci. (2021) 9:333–54. doi: 10.1146/annurev-animal-021419-083930, PMID: [DOI] [PubMed] [Google Scholar]
- 88.Siddiqui SA, Brunner TA, Tamm I, van der Read P, Patekar G, Alim Bahmid N, et al. Insect-based dog and cat food: a short investigative review on market, claims and consumer perception. J Asia-Pacific Entomol. (2022e) 26:102020. doi: 10.1016/j.aspen.2022.102020 [DOI] [Google Scholar]
- 89.Steinfeld H, Gerber P, Wassenaar TD, Castel V, Rosales M, Rosales M, et al. Livestock's long shadow: Environmental issues and options. Rome: FAO; (2006). [Google Scholar]
- 90.Oonincx DGAB, van Itterbeeck J, Heetkamp MJW, van den Brand H, van Loon J, van Huis A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS One. (2010) 5:12–e14445. doi: 10.1371/journal.pone.0014445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuyper E, Vitta B, Dewey K. Novel and underused food sources of key nutrients for complementary feeding. A&T Tech Brief. (2013) 6:1–8. [Google Scholar]
- 92.Rumpold BA, Schlüter OK. Nutritional composition and safety aspects of edible insects. Mol Nutr Food Res. (2013) 57:802–23. doi: 10.1002/mnfr.201200735 [DOI] [PubMed] [Google Scholar]
- 93.Tavares PP, dos Santos Lima M, Pessôa LC, de Andrade Bulos RB, de Oliveira TT, da Silva Cruz LF, et al. Innovation in Alternative Food Sources: A Review of a Technological State-of-the-Art of Insects in Food Products. Foods. (2022) 11:3792. doi: 10.3390/foods11233792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guiné RPF, Correia P, Coelho C, Costa CA. The role of edible insects to mitigate challenges for sustainability. Open Agric. (2021) 6:24–36. doi: 10.1515/opag-2020-0206 [DOI] [Google Scholar]
- 95.Hlongwane ZT, Slotow R, Munyai TC. Nutritional composition of edible insects consumed in Africa: a systematic review. Nutrients. (2020) 12:12–2786. doi: 10.3390/nu12092786, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tanga CM, Egonyu JP, Beesigamukama D, Niassy S, Emily K, Magara HJ, et al. Edible insect farming as an emerging and profitable enterprise in East Africa. Curr Opin Insect Sci. (2021) 48:64–71. doi: 10.1016/j.cois.2021.09.007, PMID: [DOI] [PubMed] [Google Scholar]
- 97.Madau FA, Arru B, Furesi R, Pulina P. Insect farming for feed and food production from a circular business model perspective. Sustainability. (2020) 12:5418. doi: 10.3390/su12135418 [DOI] [Google Scholar]
- 98.Moruzzo R, Mancini S, Guidi A. Edible insects and sustainable development goals. Insects. (2021) 12:557. doi: 10.3390/insects12060557, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amadi EN, Kiin-Kabari DB, William-West DP, Pepple GE. Microbiological flora and proximate composition of the yam beetle. Heteroligus meles IJCMAS. (2016) 5:370–5. doi: 10.20546/ijcmas.2016.512.040 [DOI] [Google Scholar]
- 100.van Huis A, van Gurp H, Dicke M. The insect cookbook: food for a sustainable planet. New York: Columbia University Press; (2014). [Google Scholar]
- 101.Kariyanna B, Prabhuraj A, Asokan R. Identification of suitable reference genes for normalization of RT-qPCR data in eggplant fruit and shoot borer (Leucinodes orbonalis Guenée). Biologia. (2020) 75:289–97. doi: 10.2478/s11756-019-00346-4 [DOI] [Google Scholar]
- 102.Mariod AA. African edible insects as alternative source of food, oil, protein and bioactive components. Cham, Switzerland: Springer Nature Switzerland AG; (2020). [Google Scholar]
- 103.Nyangen D, Mutungi C, Imathiu S, Kinyuru J, Aognon H, Ekesi S, et al. Effects of traditional processing techniques on the nutritional and microbiological quality of four edible insect species used for food and feed in East Africa. Foods. (2020) 9:5–574. doi: 10.3390/foods9050574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anankware PJ, Fening KO, Osekre E, Obeng-Ofori D. Insects as food and feed: a review. Int J Agric Res. (2015) 3:143–51. [Google Scholar]
- 105.Séré A, Bougma A, Judicaël Ouilly T, Traoré M, Sangaré H, Lykke AM, et al. Traditional knowledge regarding edible insects in Burkina Faso. J Ethnobiol Ethnomed. (2018) 14:59–9. doi: 10.1186/s13002-018-0258-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cloutier J. Edible insects in Africa: an introduction to finding, using and eating insects. Wageningen, Netherlands: CTA Publications. Agromisa. (2015) 978–990 [Google Scholar]
- 107.Anankware JP, Roberts BJ, Cheseto X, Osuga I, Savolainen V, Collins CM. The nutritional profiles of five important edible insect species from West Africa—an analytical and literature synthesis. Front Nutr. (2021) 8:792941. doi: 10.3389/fnut.2021.792941, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bertola M, Mutinelli F. A systematic review on viruses in mass-reared edible insect species. Viruses. (2018) 13:11–2280. doi: 10.3390/v13112280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aleknavičius D, Lukša J, Strazdaitė-Žielienė Ž, Servienė E. The bacterial microbiota of edible insects Acheta domesticus and Gryllus assimilis revealed by high content analysis. Foods. (2022) 11:11–1073. doi: 10.3390/foods11081073, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gałęcki R, Sokół R. A parasitological evaluation of edible insects and their role in the transmission of parasitic diseases to humans and animals. PLoS One. (2019) 14:e0219303. doi: 10.1371/journal.pone.0219303, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Belluco S, Mantovani A, Ricci A. Edible insects in a food safety perspective. Edible insects in sustainable food systems. Springer, Cham. (2018) 109–126 [Google Scholar]
- 112.Cifuentes Y, Glaeser SP, Mvie J, Bartz JO, Müller A, Gutzeit HO, et al. The gut and feed residue microbiota changing during the rearing of Hermetia illucens larvae. Antonie Van Leeuwenhoek. (2020) 113:1323–44. doi: 10.1007/s10482-020-01443-0, PMID: [DOI] [PubMed] [Google Scholar]
- 113.Dzepe D, Mbenda Théclaire K, Ngassa G, Mube H, Chia SY, Aoudou Y, et al. Application of black soldier fly frass, hermetia illucens (diptera: Stratiomyidae) as sustainable organic fertilizer for lettuce, lactuca sativa production. Open J Appl Sci. (2022) 12:1632–48. doi: 10.4236/ojapps.2022.1210111 [DOI] [Google Scholar]
- 114.Gałęcki R, Sokół R. Treatment of cryptosporidiosis in captive green iguanas (Iguana iguana). Vet Parasitol. (2018) 252:17–21. doi: 10.1016/j.vetpar.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 115.Gorrens E, van Looveren N, van Moll L, Vandeweyer D, Lachi D, De Smet J, et al. Staphylococcus aureus in substrates for black soldier fly larvae (Hermetia illucens) and its dynamics during rearing. Microbiol Spectr. (2021) 9:e0218321–1. doi: 10.1128/spectrum.02183-21, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuznetsova TA, Vecherskii MV, Khayrullin DR, Stepankov AA, Maximova IA, Kachalkin AV, et al. Dramatic effect of black soldier fly larvae on fungal community in a compost. J Sci Food Agric. (2021) 102:2598–603. doi: 10.1002/jsfa.11601, PMID: [DOI] [PubMed] [Google Scholar]
- 117.Peng Y, Li SJ, Yan J, Tang Y, Cheng JP, Gao AJ, et al. Research progress on phytopathogenic fungi and their role as biocontrol agents. Front Microbiol. (2021) 12:670135. doi: 10.3389/fmicb.2021.670135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elhag O, Zhang Y, Xiao X, Cai M, Zheng L, Jordan HR, et al. Inhibition of zoonotic pathogens naturally found in pig manure by black soldier fly larvae and their intestine bacteria. Insects. (2022) 13:1–66. doi: 10.3390/insects13010066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Niassy S, Omuse E, Roos N, Halloran A, Eilenberg J, Egonyu J, et al. Safety, regulatory and environmental issues related to breeding and international trade of edible insects in Africa. Rev Sci Tech. (2022) 41:117–31. doi: 10.20506/rst.41.1.3309, PMID: [DOI] [PubMed] [Google Scholar]
- 120.Raele DA, Stoffolano JG, Jr, Vasco I, Pennuzzi G, Nardella La Porta MC, Cafiero MA. Study on the role of the common house fly, Musca domestica, in the spread of ORF virus (Poxviridae) DNA under laboratory conditions. Microorganisms. (2021) 9:9–2185. doi: 10.3390/microorganisms9112185, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vandeweyer D, Lievens B, Van Campenhout L. Identification of bacterial endospores and targeted detection of foodborne viruses in industrially reared insects for food. Nat Food. (2020) 1:511–6. doi: 10.1038/s43016-020-0120-z, PMID: [DOI] [PubMed] [Google Scholar]
- 122.Chia SY, Tanga CM, van Loon JJA, Dicke M. Insects for sustainable animal feed: inclusive business models involving smallholder farmers. COSUST. (2019) 41:23–30. doi: 10.1016/j.cosust.2019.09.003 [DOI] [Google Scholar]
- 123.Ritchie H, Roser M. Environmental impacts of food production. Our world in data. (2020). Available at: https://ourworldindata.org/environmental-impacts-of-food#water-use
- 124.Skrivervik E. Insects’ contribution to the bioeconomy and the reduction of food waste. Heliyon. (2020) 6:6–e03934. doi: 10.1016/j.heliyon.2020.e03934, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Illa J, Yuguero O. An analysis of the ethical, economic, and environmental aspects of entomophagy. Cureus. (2022) 14:e26863. doi: 10.7759/cureus.26863, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Quang Tran H, Van Doan H, Stejskal V. Environmental consequences of using insect meal as an ingredient in aquafeeds: a systematic view. Rev Aqua. (2021) 14:237–51. doi: 10.1111/raq.12595 [DOI] [Google Scholar]
- 127.Williams D, Williams S, Huis A. Can we farm aquatic insects for human food or livestock feed? J Insects Food Feed. (2021) 7:121–7. doi: 10.3920/JIFF2021.x002 [DOI] [Google Scholar]
- 128.Tagawa K, Hosoya T, Hyakumura K, Suzuki D, Yoshizawa S, Praxaysombath B. The effects of season, geography, and urbanization on the diversity of edible insects at food markets in Laos. PLoS One. (2022) 17:e0267307–7. doi: 10.1371/journal.pone.0267307, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Flores FF, Hilgert NI, Zamudio F, Fabbio F, Lupo LC. Pollen analysis of honeys from Apis mellifera and Tetragonisca fiebrigi (Hymenoptera: Apidae) in the Upper Paraná Atlantic Forest, Argentina. Rodriguésia. (2021) 72:72. doi: 10.1590/2175-7860202172100 [DOI] [Google Scholar]
- 130.Lange KW, Nakamura Y. Edible insects as future food: chances and challenges. J Foods Soc. (2021) 1:38–46. doi: 10.1016/j.jfutfo.2021.10.001 [DOI] [Google Scholar]
- 131.Musundire R, Ngonyama D, Chemura A, Ngadze RT, Jackson J, Matanda MJ, et al. Stewardship of wild and farmed edible insects as food and feed in Sub-Saharan Africa: a perspective. Front Vet Sci. (2021) 8:8. doi: 10.3389/fvets.2021.601386, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sage RF. Global change biology: a primer. Glob Chang Biol. (2020) 26:3–30. doi: 10.1111/gcb.14893, PMID: [DOI] [PubMed] [Google Scholar]
- 133.Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, et al. Scientists’ warning to humanity on insect extinctions. Biol Conserv. (2020) 242:108426. doi: 10.1016/j.biocon.2020.108426 [DOI] [Google Scholar]
- 134.Wagner DL. Insect declines in the anthropocene. Annu Rev Entomol. (2020):65. doi: 10.1146/annurev-ento-011019-025151 [DOI] [PubMed] [Google Scholar]
- 135.Aguilar-Toalá JE, Cruz-Monterrosa RG, Liceaga AM. Beyond human nutrition of edible insects: health benefits and safety aspects. Insects. (2022) 13:1007. doi: 10.3390/insects13111007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhong A. Product development considerations for a nutrient rich bar using cricket (Acheta domesticus) protein. Long Beach: California State University; (2017). [Google Scholar]
- 137.Hanboonsong Y, Jamjanya T, Durst PB. Six-legged livestock: edible insect farming, collection and marketing in Thailand. Thailand: Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific Bangkok; (2013). [Google Scholar]