Abstract
Patients suffering bone fractures in different parts of the body require implants that will enable similar function to that of the natural bone that they are replacing. Joint diseases (rheumatoid arthritis and osteoarthritis) also require surgical intervention with implants such as hip and knee joint replacement. Biomaterial implants are utilized to fix fractures or replace parts of the body. For the majority of these implant cases, either metal or polymer biomaterials are chosen in order to have a similar functional capacity to the original bone material. The biomaterials that are employed most often for implants of bone fracture are metals such as stainless steel and titanium, and polymers such as polyethene and polyetheretherketone (PEEK). This review compared metallic and synthetic polymer implant biomaterials that can be employed to secure load-bearing bone fractures due to their ability to withstand the mechanical stresses and strains of the body, with a focus on their classification, properties, and application.
Keywords: orthopedic, bone, biodegradable, corrosion resistance, biocompatibility
1. Introduction
Biomaterials have been utilized for the treatment of human diseases since ancient times. For example, as early as 2000 BC, the Egyptians used ivory to replace lost teeth [1], [2], and employed wood to replace missing bones such as legs and toes [3]. They also used braces and splints to support and protect fractured bones after surgical procedures [4]. During the same period, copper was used to replace missing bony parts of the human body, but these implants failed due to the toxic effects of copper ions. The ancient Indian text from the Vedic period (1800–1500 BC) mentioned the use of teeth, eyes, and artificial legs. Autogenous tissues, or tissues from the patient’s own body, were also employed during this period to replace missing body parts [3].
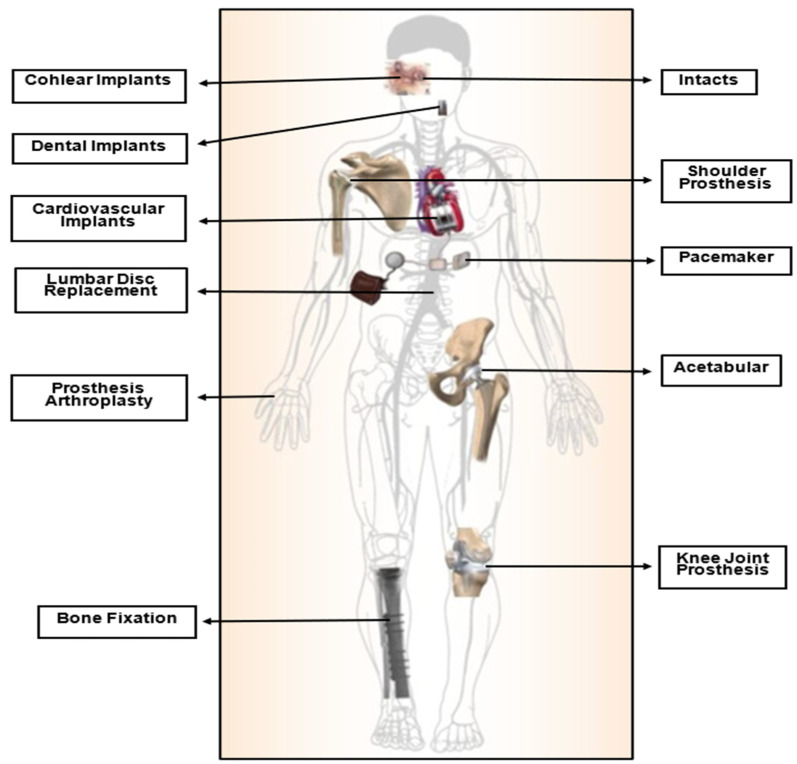
Biomaterials have become increasingly crucial in modern times, serving various applications as a result of advances in medicine and material processing in recent decades. They are extensively utilized in various fields, including orthopedics, dentistry, cardiovascular devices, drug delivery, and skin tissue engineering. These materials are specifically designed to interact with biological systems to repair, assess, or replace damaged or malfunctioning bodily tissues, organs, or systems [2,5,6,7], as shown in Figure 1. They are engineered for medical use either independently or as part of a biocompatible system with the body’s tissues and organs [8,9].
Figure 1.
Bio-devices utilized in the human body [10].
Biocompatibility is a term commonly used in biomaterials science to describe the interactions between foreign matter and the body [11]. Specifically, a substance’s biocompatibility is evaluated based on its ability to perform its intended medical function while eliciting an appropriate response from the host in a given application. It also encompasses the substance’s ability to interact with living systems without triggering any adverse reactions such as immune rejection, toxicity, or infection. Ultimately, the biomaterials must not produce any unwanted or unsuitable local or systemic effects [12,13]. Two major factors determine the biocompatibility of material: (i) the host’s reaction induced by the biomaterial and (ii) the degradation of the substance in the body’s environment. Often, both factors should be considered. One of the prime factors controlling biocompatibility is the material’s resistance to corrosion, which impacts the mechanical characteristics of biomaterials such as the specific weight and elastic modulus [5].
The majority of commercially utilized bio-implant materials are conventionally fabricated permanent metals and their alloys, such as stainless steel, titanium, and Co-Cr alloys [14]. They offer a stress-protective impact despite having excellent mechanical strength, biocompatibility, and acceptable wear resistance qualities [15,16] because the elasticity modulus of these bio-implants differs significantly from that of normal bone [17,18,19]. As a result, the bone can bear a much lower load, which progressively causes re-fracture and implant loosening as well as a considerable reduction in bio-efficacy. In addition, when the fracture is healed, a second surgery is necessary to remove the implant [14,20], causing colossal suffering to the patient. In addition, such an operation is quite expensive. According to studies, these follow-up surgeries, which are performed to remove permanent implants, account for approximately 30% of all orthopedic surgical procedures [14,21].
Corrosion plays a significant role in the design and selection of metals and alloys for use in vivo. During corrosion processes, allergenic, toxic/cytotoxic, or carcinogenic species such as Ni, Co, and Cr, may be released into the body. Moreover, different mechanisms of corrosion can contribute to implant loosening and failure [22,23,24]. Consequently, bio-implants often undergo corrosion and/or solubility testing before receiving approval from regulatory bodies [5].
Given the intricate nature of biomaterials and the significant responsibility involved in their use, a multidisciplinary approach is necessary for their design and development. Therefore, the involvement of professionals from diverse fields, such as chemists, biologists, engineers, histopathologists, and surgeons, is crucial to achieve the desired outcomes that would benefit patients with various pathologies [25].
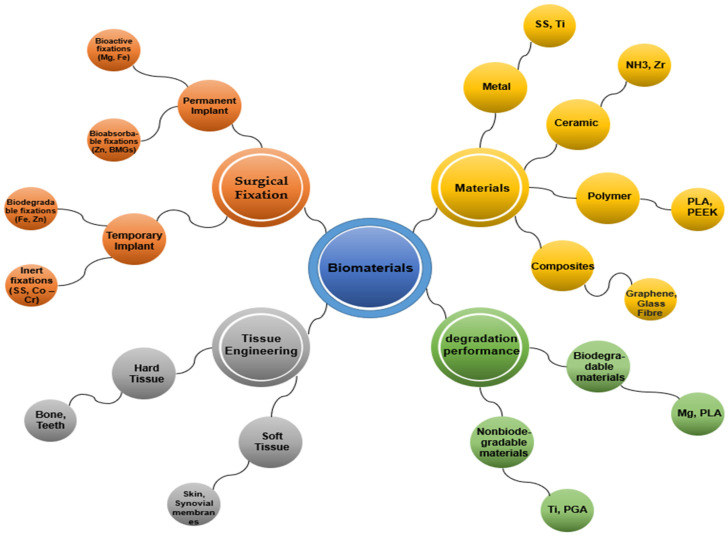
Biomaterials are categorized into several types, including metals (e.g., stainless steel, titanium, gold, iron, magnesium), polymers (e.g., PLLA, PGA, PDS, nylon, silicone, polyester), ceramics (e.g., hydroxyapatite, alumina, zirconia), and composites, which combine materials from the above-mentioned categories [2,6]. Furthermore, they can be categorized into two main groups: (i) biodegradable materials that degrade and are absorbed into the surrounding tissue after implantation, and (ii) non-biodegradable materials that do not degrade or become absorbed. Inactive biomaterials exhibit limited or no interaction with tissues, while bioactive substances promote interactions with surrounding tissues. Degradable materials gradually release their mass into the surrounding tissues and may eventually disintegrate. Metals are generally inert; ceramics can be absorbable, inert, or bioactive; and polymers can be either inert or absorbable [26]. In tissue engineering applications, biomaterials can be further divided into two major classes based on their applications in hard tissues (such as bone, teeth, cartilage, and nails) and soft tissues (including skin, synovial membranes, ligaments, and fibrous tissues), with or without mineral constituents. Additionally, commonly used biomaterials can be classified as permanent or temporary implants depending on the required surgical fixation [2,27], as shown in Figure 2.
Figure 2.
Classification of biomaterials.
Metallic, polymeric, and ceramic materials contribute significantly to advancing orthopedic disease treatment. Since calcium phosphate ceramics have numerous disadvantages, such as mechanical weakness and poor resistance to crack growth, they can only be used in non-load-bearing applications [20,28]. For alumina- and zirconia-based bio-ceramics in high-load-bearing applications, static and cyclic fracture, the phenomena of slow crack growth, poor toughness, loss of toughness over time, stress corrosion, and susceptibility to tensile stresses are all major causes of worry; Therefore, designs have to be constrained concerning confined tensile loads or compressive loading [29,30]. Moreover, due to these issues, ceramics have not yet been utilized for fracture fixing [30]. Consequently, this review systemically compared the characteristics of biodegradable and non-biodegradable metals and synthetic polymers. Their potential for load-bearing bone fixations was evaluated in terms of biodegradability, mechanical properties, and biocompatibility.
2. Biomaterials in Orthopedics
Bone is a living tissue that serves as a natural complex, consisting of approximately 30% matrix, 60% minerals, and 10% water [2,31]. It serves multiple functions in the body, including providing mechanical support as the skeletal structure; serving as attachment sites for muscles, ligaments, and tendons; and protecting vital organs [32]. Bone also acts as a mineral store, contributing to blood production and overall bone health, which plays a crucial role in maintaining overall well-being [33,34].
Bone fractures are a prevalent type of traumatic injury globally, and their treatment often imposes a significant economic burden on society [35,36]. Despite the availability of advanced therapeutic strategies, complications such as delayed fracture healing or non-union occur in approximately 10% of cases, leading to prolonged recovery periods and increased hardship for patients. High-risk groups, such as those with osteoporosis, the elderly, malnourished individuals, post-menopausal women, or those with an impaired blood supply, are particularly susceptible to developing fracture healing disorders [35]. Additionally, individuals with joint diseases such as osteoarthritis and rheumatoid arthritis may require surgical interventions such as hip and knee joint replacements. Temporary fracture fixation appliances and components such as plates, screws, wires, and nails are among the orthopedic implants used in these cases [37]. Table 1 illustrates the locations of various human body fractures that demand some form of temporary fixture until they heal.
Table 1.
Locations of various human body fractures that demand different temporary fixation implants [14,38].
| Fracture Site | Internal Fixators | |
|---|---|---|
| Head | Fracture of the skull | Plates, wires, and pins |
| Craniofacial fracture | Plates, wires, and pins | |
| Trunk | Fracture of the clavicle | Plates and intramedullary nails |
| Fracture of the scapular | Plates and screws | |
| Fracture of the pelvis | External fixators, screws, and plates | |
| Spinal fracture | Fixation implant contains plates, pedicle screws, and rods | |
| Upper limb fracture | Fracture of the humerus | Plates and screws for open reduction and an intramedullary nail for closed reduction |
| Fracture of the radius or ulnar | Plates and screws for open reduction and an intramedullary nail for closed reduction | |
| Fracture of the phalanges and metacarpal fracture | External fixators for close reduction and intramedullary nails, plates, and screws for open reduction | |
| Lower limb fracture | Femoral fracture | Plates and screws for open reduction and an intramedullary nail for closed reduction |
| Tibial and fibular fracture | Plates and screws for open reduction and an intramedullary nail for closed reduction | |
| Fracture of the metatarsus | Plates and screws for open reduction and an intramedullary nail for closed reduction | |
| Calcaneal fracture | Wires and screws for close reduction |
Continuous advancements are being made in the field of orthopedic implants to improve their interaction with the surrounding bone tissue, leading to positive and satisfactory outcomes for patients. The advantageous biological interaction between the implant and the surrounding bone is influenced by physical, mechanical, and topological characteristics [2,39]. Optimal fracture healing relies on achieving complete fixation of the fracture and promoting the preservation and growth of bony segments through local vascular remodeling [2,40]. To better understand the processes of adequate or impaired fracture healing, various in vivo, ex vivo, or in vitro models are available, providing opportunities for targeted and focused scientific research in both basic and translational settings [41].
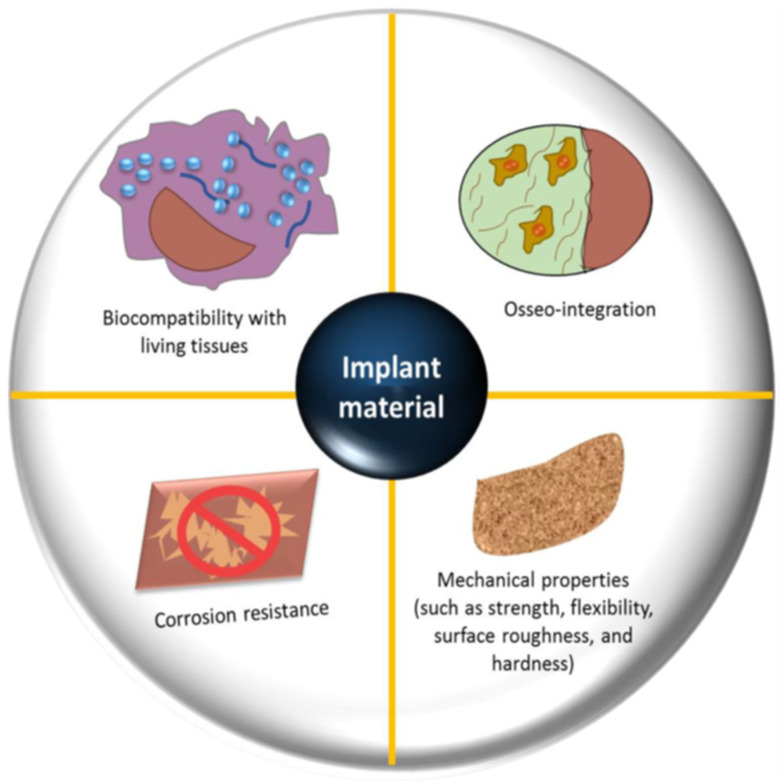
Biomaterials are used in orthopedics to restore the structural integrity of damaged bone or to substitute it. Every biomaterial must fulfill multiple essential criteria, such as possessing suitable mechanical properties (e.g., precise weight and elastic modulus), demonstrating good biostability (resistance to hydrolysis, oxidation, and corrosion), ensuring biocompatibility, particularly in the case of bone prostheses (promoting osseointegration), exhibiting high bio-inertness (non-toxic and non-irritant characteristics), demonstrating high wear resistance, and enabling easy application in practical settings [30,33,42,43]; see Figure 3.
Figure 3.
Factors that need to be considered in the selection and design of an orthopedic bio-implant [30].
The mechanical demands of an implant are determined by its intended medical function, with factors such as strength for bearing loads and elasticity for withstanding shear stress playing a crucial role. In orthopedics, implant materials must withstand repetitive loading and unloading cycles under various forces such as bending, twisting, and shearing stress. Additionally, implant devices are exposed to corrosive environments over extended periods, potentially impacting their properties. Therefore, it is essential to accurately evaluate the mechanical properties of these materials to ensure fracture reduction and maintain optimal performance [44]. The assessment of mechanical properties involves examining the deformation (strain) generated by an applied force (stress). This evaluation provides valuable insights into the material’s ability to withstand and adapt to external forces, guiding the design and selection of implants that can meet the mechanical requirements of their intended applications [45].
In terms of biocompatibility, most metals employed as biomaterials have a relatively low intrinsic osteogenic and osteoimmune modulating potential [46], especially when compared to polymers such as polylactic acid (PLA). The presence of metallic materials as foreign bodies in the human body can often be a dangerous factor leading to chronic inflammation [47]. If these permanent implants are not promptly removed, they can cause serious allergic issues due to the accumulation of ions around the fracture site, resulting in osteolysis and impeding the formation of new bone [14]. In addition to metallic biomaterials, polymeric biomaterials are also employed for load-bearing applications. Several polymeric biomaterials have garnered significant attention in this field due to their ability to endure significant physiological stresses without fracturing or distorting [48]. In regenerative medicine applications, synthetic biopolymers offer several advantages over other non-biodegradable materials. They can be produced with consistently high quality and purity and can be shaped into various forms with desired bulk and surface properties [2,49]. Table 2 illustrates the mechanical characteristics, corrosion resistance, and biocompatibility of metallic biomaterials widely used in the medical field.
Table 2.
Mechanical characteristics, biocompatibility, and corrosion resistance of metallic and polymeric biomaterials that are extensively utilized in orthopedics.
| Materials | Density (g/cm3) | Yield Strength (Mpa) | Tensile Strength (Mpa) | Elongation at Break (%) | Elastic Modulus (Gpa) | Biocompatibility | Corrosion-Resistance | Refs. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Metal | Non-biodegradable | 316 L steel | 7.9 | 290 | 579 | 40 | 193 | Poor | Reasonable | [50,51,52,53] |
| Ti-6Al-4V | 4.43 | 850–900 | 960–970 | 14 | 110 | Fair | Excellent | |||
| CoCr20Ni15Mo7 | 7.8 | 240–450 | 450–960 | 50 | 195–230 | Poor | Excellent | |||
| Biodegradable | Pure Mg | 1.74–2 | 65–100 | 90–190 | 2–10 | 41–45 | Excellent | Poor | [50,53] | |
| Fe20Mn alloy | 7.73 | 420 | 700 | 8 | 207 | |||||
| Zn-Al-Cu (Zn based alloy) | 5.79 | 171 | 210 | 1 | 90 | |||||
| Polymer | Non-biodegradable | UHMWPE | 0.931–0.949 | 21.4–27.6 | 38.6–48.3 | 3.5–5.25 | 0.894–0.963 | Good | Excellent | [54,55] |
| PMMA | 1.18 | - | 72 | 5 | 310 | Good | Excellent | [56,57,58,59,60] | ||
| PEEK | 1.23–1.32 | 87–95.2 | 70.3–103 | 0.3–1.5 | 3.76–3.95 | Good | Excellent | [61,62,63,64] | ||
| Biodegradable | PLA | 1.21–1.25 | 60 | 21–60 | 6 | 0.35–3.5 | Excellent | poor | [2,65] | |
| PLGA | 1.30–1.34 | 3.8–26.6 | 13.9–16.7 | 5.7 | - | Excellent | Poor | [2,50] | ||
| PLC | 1.11–1.14 | 8.37–14.6 | 20.7–42 | 22.8–28.3 | 0.21–0.44 | Excellent | Poor | |||
2.1. Metals and Alloys
Metallic biomaterials are essential for repairing or replacing damaged bone tissue due to their high mechanical strength and fracture toughness, making them better suited for load-bearing applications than ceramics or polymeric materials. In recent years, non-biodegradable metals such as titanium, titanium alloys, stainless steel, nitinol (nickel–titanium alloys), and cobalt-based alloys have been the most widely used biomaterials for medical implant devices [66].
2.1.1. Non-Biodegradable Metals
Stainless Steel and Its Alloys
Since the 1930s, stainless steel (SS) has been a commonly used material for creating bone fixation plates. Stainless steel refers to a range of iron-based alloys that contain a significant amount of chromium (11–30wt %) and varying levels of nickel [67]. This versatile material is favored for its exceptional mechanical properties and cost-effectiveness when compared to other metals such as titanium alloys. Even today, stainless steel remains a crucial choice for temporary devices such as bone plates, fixating screws, and permanent orthopedic implants due to its widespread availability and desirable characteristics [68].
Compared to conventional steel, specifically 316 L austenitic stainless steel, stainless steel offers superior corrosion resistance. This property makes it a suitable choice for manufacturing prosthetic joints and bone plates [69]. Austenitic stainless steels, including 316 L, exhibit high stability and are less prone to hydrogen embrittlement compared to low-alloy steels, carbon steels, and less stable austenitic stainless steels. The presence of internal hydrogen enhances the yield and tensile strength while reducing ductility in the more stable 316 L austenitic stainless steels. The effect of hydrogen on fatigue properties depends on the internal hydrogen content [70].
Co–Cr Alloys
Cobalt–chromium-based alloys have significantly higher hardness and strength and better wear and corrosion resistance than Ti alloys. Co–Cr-based alloys for biomedical applications have previously been principally fabricated by molding and milling manners; However, portions fabricated by these traditional techniques required extra processing because of their poor dimensional and shaped precision [71].
Co–Cr–Mo alloy is one of the most vastly employed biomaterials for manufacturing orthopedic implants because of its superb compound of corrosion resistance, biocompatibility, and mechanical properties [72,73]. Nevertheless, Co–Cr–Mo alloy has lower automation, and it is challenging to manufacture some complicated orthopedic portions such as synthetic vertebrae utilizing the usual manufacturing manner [74].
Titanium (Ti) and Its Alloys
Titanium and its alloys have become the most widely used materials in the manufacture of biomaterial implants for fixing bone fractures due to their high biocompatibility and excellent mechanical properties [75,76]. However, the biological inertness of titanium alloys often results in poor and/or delayed osseointegration [77]. Two approaches are ordinarily commonly utilized to ameliorate the bioactivity of titanium-based alloys by targeting the substance’s compositional design or surface functionality [78].
The design of synthetic materials has great potential in fabricating bio-implants with incorporated structural steadiness and a desired biological function, which can help to avoid matrix coating interface problems or the mismatch of its properties due to surface operation [79]. In the evolution of biocompatible titanium alloys, biotoxicity and a low modulus of elasticity are important considerations. Some researchers have explored Ti–Al–V-based alloys with an extremely low modulus, but these alloys release toxic ions such as aluminum (Al) and vanadium (V), which can have long-term health effects [80]. The use of vanadium (V) compounds, for example, has been associated with DNA damage in blood cells, altered neurobehavioral functions, weight loss, and various toxicities [81,82]. Conversely, researchers have investigated a wide range of titanium alloys with different elemental compositions to achieve compatibility with various bone types. Parameters such as a low elastic modulus, non-toxicity, and biocompatibility have been extensively studied in the literature. Magnesium (Mg) is particularly renowned for its biodegradability.
Ti–Mg has emerged as a promising candidate for orthopedic implants due to its desirable characteristics, including a low elastic modulus, high strength-to-weight ratio, and good biocompatibility [83]. Currently, beta-type titanium alloys are being successfully used in orthopedic implants. These alloys are renowned for their very low Young’s modulus, high biocompatibility, excellent physical properties, and non-toxic nature, meeting the requirements for orthopedic applications. They also contain β-stabilizing elements such as Nb, Ta, Zr, Mo, and Hf [84]. Table 3 summarizes the advantages and disadvantages of various metallic materials used in orthopedic applications, along with their respective applications.
Table 3.
Diverse types of bio-metal materials employed in orthopedic implants with their applications, advantages, and disadvantages [85].
| Metal and Alloys | Particular Alloys | Major Applications | Advantages | Disadvantages |
|---|---|---|---|---|
| Stainless steel | 316 L Stainless steel | Surgical implements, stents, fracture fixation | High wear resistance | The modulus is increased compared to bone allergy due to Co, Cr, and Ni |
| Titanium-based alloys | CP–Ti | Dental implants, fracture fixation, bone and joint replacement, pacemaker encapsulation | Low density, excellent biocompatibility, high corrosion resistance, low Young’s modulus | Weak tribological characteristics, the toxic impact of V and Al with long-term use |
| Ti–Al–Nb | ||||
| Ti–6Al–4V | ||||
| Ti–13Nb–13Zr | ||||
| Ti– Mo–Zr–Fe | ||||
| Co and chromium alloys | Co–Cr–Mo | Dental implants and restorations, heart valves, joint and bone replacement | Excellent wear resistance | The modulus is increased compared to bone allergy due to Co, Cr, and Ni |
| Cr–Ni– Cr–Mo | ||||
| Others | Ni–Ti | Orthodontic wires, fracture fixation plates, stents | Low Young’s modulus | Allergy due to Ni |
| Platinum Pt–Ir | Electrodes | Excellent corrosion resistance under maximum voltage potential and charge transfer conditions | - | |
| Hg–Ag–Sn amalgam | Dental restorations | Easily moldable in situ into a desired shape that is resistant to corrosion in the oral environment | Concerns related to Hg toxicity |
2.1.2. Biodegradable Metals
Over the past two decades, there has been significant research and exploration of biodegradable metallic implants, specifically those composed of magnesium (Mg) and its alloys, iron (Fe) and its alloys, as well as zinc (Zn) and its alloys. These materials have garnered considerable interest due to their ability to degrade naturally, making them promising candidates for orthopedic implant applications [86,87,88,89,90,91,92,93,94,95,96,97].
Magnesium and Its Alloys
Magnesium and its alloys are considered promising biomaterials for orthopedic devices due to their excellent mechanical properties, biodegradability, and biocompatibility with human physiology [98]. However, one challenge associated with magnesium alloys is their susceptibility to corrosion in a biological environment. The significant corrosion rate and low bioactivity of magnesium implants pose a challenge that needs to be addressed before they can be used in clinical applications [99].
Magnesium alloys possess a similar elasticity to human bone, mitigating the negative effects of stress shielding in bone composition. Consequently, they are currently employed as temporary implants in the field of biomaterials. Within the biological environment, these alloys undergo complete degradation and are gradually substituted by newly regenerated bone, obviating the requirement for surgical intervention to extract the implant. This characteristic makes them highly desirable for metallic biomaterial implants used in bone regeneration applications that require temporary support [100].
However, there is a drawback: magnesium alloys degrade rapidly in the biological environment, necessitating effective control of the corrosion rate through bone tissue regeneration processes [101]. The rapid corrosion process can have adverse effects on the implant, including a decline in mechanical properties and the release of toxic by-products due to side reactions and corrosion accumulation.
As a result, there are significant implications in terms of cost and the overall health of the patient. Therefore, it is crucial to develop corrosion-resistant magnesium alloys in order to address these concerns in medical applications [100].
Fe and Its Alloys
Fe-based biodegradable materials as new-generation orthopedic implants draw increasing attention owing to their controllable internal pore structure, excellent mechanical characteristics, customizable complicated geometry, appropriate biocompatibility, and self-degradation feature [102,103]. In comparison to Mg-based alloys, pure Fe and its alloys possess high mechanical strength without causing hydrogen release during degradation. However, their degradation rates are considered too slow to align with bone growth, which is an issue requiring immediate attention [102,104,105,106,107,108,109].
Furthermore, Fe is a vital and scarce element in living organisms, playing a critical role in various physiological functions, including the formation, transportation, and storage of oxygen through hemoglobin, as well as the reduction in dinitrogen and ribonucleotides, and DNA installation [105,108,110,111,112,113]. Due to their combination of excellent strength and moderate corrosion rates, Fe and Fe-based bio-implants have been recognized as promising options for potential bone replacement or osteosynthesis materials [103,104,105,106,107]. Compared to Mg and its alloys, and Zn and its alloys, Fe combines superb compressive properties and tensile quality (compressive strength of 752 ± 13 MPa; tensile yield strength of 135 ± 15 MPa) [114,115]. These impressive mechanical characteristics make Fe well-suited for load-bearing devices, as highlighted in Table 4 [116].
Table 4.
Physical properties of metallic alloys compared to human cortical bone [116].
| Material | Young’s Modulus (GPa) | 0.2% Offset Yield Point (MPa) | Compressive Strength at 20% Strain (MPa) |
|---|---|---|---|
| Cast Fe | 203 | 157 | 498 |
| Cast Mg | 30–40 | 20–30 | 100–180 |
| Cast Zn | 100 | 95 | 200 |
| Cast Fe–35Mn | - | 240 | 440 |
| Human cortical bone | 1–35 | 1–20 | 103–140 |
However, the slow degradation rate of pure Fe in osteogenic environments (0.16 mm/year) [105] necessitates the incorporation of additional elements such as Mn, C, Si, Zn, and Pd. This strategic addition aims to improve the bio-absorption rate of iron-based materials and reduce their magnetic susceptibility, thereby enhancing their practicality for biodegradable applications [104,105,115,117,118,119]. Previous research has shown that the bone-forming capacity of Fe is similar to that of bio-inert stainless steel, indicating the need for improvement in the surface bioactivity of iron [115,120].
Zinc and Its Alloys
Over the past two decades, the development of biodegradable metal implants has predominantly centered around two types of metals: iron-based alloys and magnesium-based alloys. Iron-based alloys, despite their remarkable biocompatibility and high mechanical characteristics [117,118,121,122], are prone to significant corrosion, leading to the formation of a bulky iron oxide layer that can trigger inflammation [123]. On the other hand, magnesium-based alloys exhibit excellent biocompatibility [124,125], but the release of hydrogen gas during corrosive degradation can result in tissue separation and, in severe cases, gas embolism [126,127].
The limitations associated with magnesium and iron as biodegradable implant materials have prompted researchers to explore zinc and its alloys as alternatives. Zinc, being an essential trace element in the human body, offers acceptable corrosion rates and biocompatibility, making it suitable for orthopedic and other medical applications, including cardiovascular interventions; Zinc plays a critical role in various physiological processes such as nucleic acid metabolism, gene expression, and signal transduction [122,128,129,130,131]. However, it is important to note that excessive levels of zinc in the body can have detrimental effects, impairing normal growth and causing anemia by interfering with iron absorption [132,133].
Although laboratory studies have shown promising results for zinc-based materials as biodegradable implants [134,135,136], their performance in vivo remains significantly uncertain [137,138]. However, in vivo investigations have revealed that pure zinc exhibits useful properties, such as antiatherogenic properties and sufficient mechanical strength in stent devices. However, pure zinc has some limitations, including lower corrosion rates in vivo and relatively inferior mechanical characteristics [136,139].
Surface treatments of zinc-based biomaterials are needed to regulate their biodegradation rate [122]. To improve performance, different alloy elements are integrated into zinc, increasing the liquidity of the molten metal and improving the mechanical properties of the alloy [140]. In orthopedic surgery, zinc-based biomaterials are designed for long-term retention in order to achieve their intended purpose, as relatively low biodegradation rates appear. However, the over-release of Zn2+ during the decomposition process can lead to cytotoxic effects in the laboratory and delay bone integration within the body [122].
2.1.3. Nanocrystalline Metallic Materials
The orthopedic field has witnessed significant efforts to develop enhanced biomaterials for a range of applications. These applications encompass temporal osteosynthesis implants utilized in the treatment of bone fractures or critical defects, as well as permanent implants such as total knee replacement prostheses [141,142]. While bulk properties, specifically mechanical resistance and load-bearing capacity, are essential for bone-substituting implants [143,144,145], surface-related properties play a critical role in achieving optimal performance. These properties include osseointegration, osteosynthesis performance, infection prevention, and corrosion resistance [141].
Titanium oxide coatings have been extensively studied due to their demonstrated biocompatibility and excellent osseointegration in dental implants, which can be attributed to the native oxide of Ti-based implants [141,146,147,148]. However, concerns have arisen regarding the extensive use of Ti-based and TiO2 materials, including their limited bioactivity, reduced corrosion resistance in media containing F− or Cl− over prolonged periods, and the resulting adverse effects of titanium accumulation on the human body [149,150,151]. Reports of allergic reactions and hypersensitivity associated with titanium necessitate research into alternative materials [152,153].
Other biocompatible transition metal oxides have exhibited promising biological properties, including osseointegration, enhanced cell adhesion and proliferation, reduced inflammatory response, antibacterial effects, and remarkable corrosion and wear resistance [154,155,156,157]. However, there has been limited research into the biological response of potential oxides such as Nb2O5 and Ta2O5 [158,159,160,161]. Another viable alternative is zirconium oxide (ZrO2), which offers suitable mechanical strength, corrosion resistance, and a favorable biological response for intraosseous applications [162,163].
2.2. Polymers
The word “polymer” is a Greek word derived from “poly” and “meros”, meaning “many” and “parts”, respectively [164,165]. A polymer comprises several reprised subunits. Natural and synthetic polymers play vital and ubiquitous roles due to their diverse functions in daily life [166]. Synthetic and natural polymers are the current and future biomaterials for orthopedic devices and bone tissue engineering. With the progress in technology, they can imitate the natural extracellular matrix (ECM) [2,167].
A polymer was employed as a biomaterial by coincidence when surgeons noted that World War II pilots who were injured by fragments of polymethylmethacrylate (PMMA) due to cockpit damage did not suffer any established deleterious reactions from the presence of Perspex shards inside their eyes [2,168,169,170]. Since then, PMMA has been employed in various medical applications. PMMA was the first synthetic polymer employed as a base for dentures in 1939 [171] and corneal replacement in the 1940s [172].
The new generation of degradable and bioresorbable medical implant biomaterials does not have any poisonous or mutagenic impacts. However, they do have some problems, such as limitations in strength and mechanical stiffness, unfavorable tissue responses, foreign body reactions, late tissue degradation reactions, and the potential for infection due to their crystallinity and hydrophobicity [173].
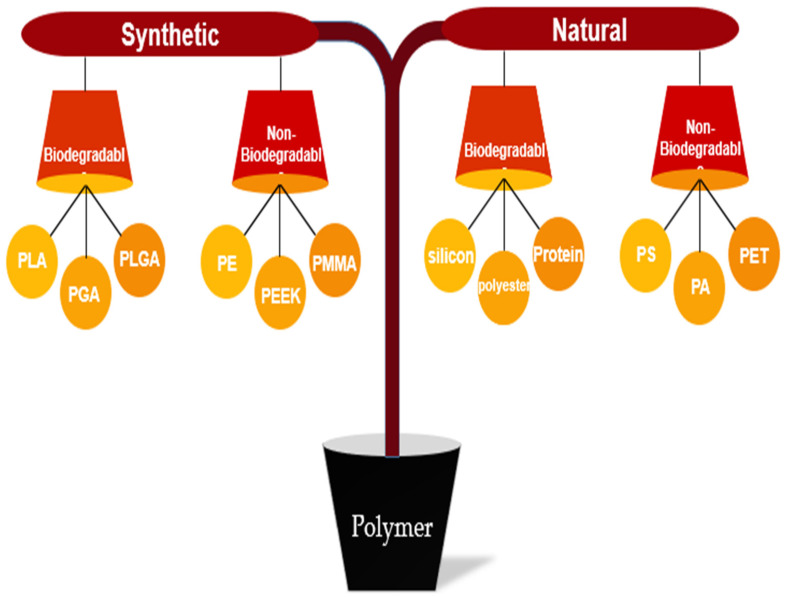
Polymers are classified into two main categories: synthetic and natural. Each section is further divided into two parts: biodegradable and non-biodegradable [174,175,176]. Figure 4 illustrates the categorization of polymers, including some examples.
Figure 4.
Classification of synthetic and natural polymer biomaterials.
2.2.1. Natural Polymer
Natural polymers were employed as the first biodegradable biomaterials in medical applications due to their improved biological performance, excellent biodegradability, and high chemical versatility compared to traditional synthetic materials [2,177]. Hyaluronic acid, chitosan, collagen, gelatin, silk, cellulose, and alginate are among the most commonly used natural polymers. Specifically, chitosan, collagen, and chitin are the predominant natural polymers employed in medicine, particularly in the field of bone tissue engineering [2,167].
2.2.2. Synthetic Polymer
Non-Biodegradable Synthetic Polymer
Most non-biodegradable synthetic polymers are biologically inert [178]. These substances were developed to minimize the host’s response to the biomaterial, aiming to reduce it to the lowest possible level. They serve as the foundation for numerous medical applications, including fracture fixators and orthopedic implants. Despite their customizable mechanical characteristics and high biological inertness, orthopedic implants made from non-biodegradable synthetic polymers and non-biodegradable orthopedic cement often experience high failure rates due to issues at the interface. These issues can arise from infections, poor integration with the surrounding tissue, or bone resorption caused by stress shielding [176].
-
i.
Poly (Methyl Methacrylate) (PMMA)
Poly (methyl methacrylate) (PMMA) is extensively utilized as a nonmetallic implant material in orthopedics and bone grafting, specifically for fixing orthopedic prosthetics in the shoulders, knees, and hips. It is a non-biodegradable polyacrylate that possesses properties such as stiffness, thermos-plasticity, biological inertness, hydrophobicity, and biocompatibility. PMMA can be obtained through solution polymerization or the polymerization of methyl methacrylate using emulsion or mass methods. Its introduction into orthopedic surgery took place in the mid-1950s [179,180,181,182,183].
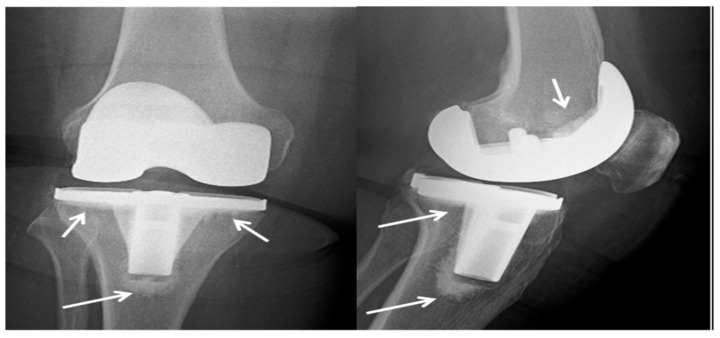
While bone cement based on PMMA is commonly employed and enables rapid primary stabilization of the bone, it lacks a biologically and mechanically stable interface with the bone. Additionally, it is prone to bacterial adhesion and the development of infections [184,185]. To modify treatment kinetics and impart mechanical properties, PMMA-based bone cement can be blended with bioactive glass or inorganic ceramics. In the 1970s, antibiotic-loaded PMMA cements were introduced to reduce the risk of prosthesis-related infections. However, self-curing PMMA cements have significant drawbacks, including their non-biodegradability, monomer toxicity, and the potential for necrosis of surrounding tissues due to high curing temperatures. Furthermore, PMMA cements exhibit limited interactions with the surrounding bone [185,186]. Figure 5 illustrates an X-ray image of a total knee arthroplasty performed on the right knee of a 73-year-old woman. The procedure involved the use of cement (PMMA). This X-ray was taken after a six-month follow-up period, and the arrows indicate the presence of the cement in the image.
Figure 5.
X-ray of total knee arthroplasty made from cement (PMMA) in the right knee of a 73-year-old woman after a six-month follow-up period, where the arrows point to the cement [184].
-
ii.
Polyetheretherketone (PEEK)
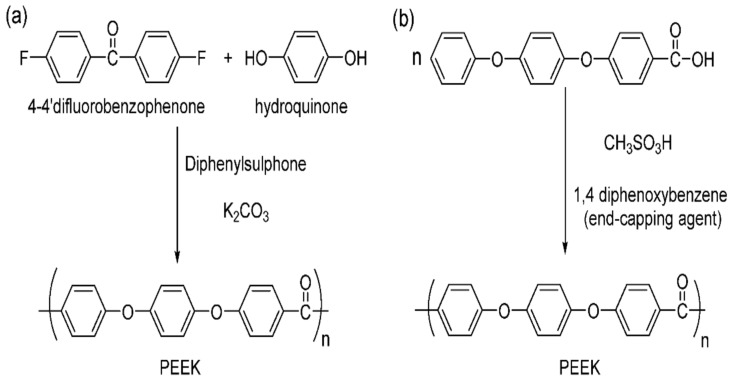
Polyetheretherketone (PEEK) is a thermoplastic polymer known for its high strength-to-weight ratio [187], excellent thermal stability [188], resistance to chemical and biological attacks [189,190], high melting temperatures [191], and glass transition [192]. At room temperature, it is chemically inert and insoluble in most solvents. There are two methods for producing PEEK: electrophilic reactions and nucleophilic displacement reactions, as illustrated in Figure 6 [193].
Figure 6.
(a) Nucleophilic and (b) electrophilic polyetheretherketone structure [183].
Despite its chemical resistance and biocompatibility, PEEK is biologically inert, resulting in limited interaction with the surrounding bone tissues and a potential risk of implant failure. Several studies have explored strategies to transform PEEK into a bioactive material and enhance its compatibility with bone implants [194,195,196,197,198,199,200,201]. One approach involves modifying the surface of PEEK, while another approach focuses on creating bioactive compounds based on PEEK [202].
When a biomaterial is implanted in vivo, water molecules are the first to reach the surface of the implant. Subsequently, proteins interact with the implant, a process influenced by the adsorbed water molecules. Following this, cells adhere to the adsorbed proteins, thereby impacting tissue growth [203,204]. However, PEEK’s low surface energy hinders cellular adherence since the implant initially interacts with water molecules. To promote cellular attachment and spreading, it is crucial to have a substrate with high surface energy, creating a hydrophilic surface. Surfaces with higher energy stimulate faster cell attachment and spreading compared to surfaces with lower energy. Thus, modifying the surface energy of a polymer alters its surface reactions, resulting in an optimal surface for the intended application [205,206].
Wang et al. employed plasma immersion ion implantation to modify the surface of PEEK. In addition, hydroxyl groups were grafted onto the PEEK surface to impart hydrophilic properties to the modified material. The cytocompatibility of both pure PEEK and the modified PEEK was also evaluated. The study revealed that the modified surface significantly enhances osteoblast adhesion, spreading, and proliferation, which can accelerate bone maturation around the implant [207].
-
iii.
Polythene (PE)
Polyethylene (PE) is a thermoplastic polymer that undergoes melting at a specific temperature and solidifies upon cooling in a reversible process. This characteristic allows for repeated cycles of melting and solidification without significant degradation. Due to this property, PE is widely used, enabling injection molding and rapid shaping into desired sizes and shapes. PE exhibits semi-crystalline behavior due to its symmetric molecular structure, which can lead to crystallization and subsequently affect density and chemical stability. Most types of polyethylene are chemically resistant, with only specific formulations being soluble in high-temperature solvents such as toluene, xylene, trichlorobenzene, or trichloroethane [208]. PE has a density ranging from 0.88 to 0.96 g/cm3, and varying molecular weights and branching. The American Society for Testing and Materials recognizes five major types of PE: low-density polyethylene (LDPE), linear low-density polyethylene (LLDPE), medium-density polyethylene (MDPE), cross-linked polyethylene (XPE), and high-density polyethylene (HDPE) [209].
Ultra-high-molecular-weight polyethylene (UHMWPE) is a specific type of polyethylene (PE) that is linear and semicrystalline. It has a high molecular weight. In orthopedics, UHMWPE typically has a molecular weight ranging from 3.5 to 6 million g/mol and a crystallinity degree of 50–55% [210]. Worldwide, approximately three million bone joint replacement surgeries are performed annually, with the majority involving the use of UHMWPE implants [211]. UHMWPE has an elastic modulus closer to that of bone compared to other commonly used prosthetic materials such as Ti–6Al–4V and Co–Cr–Mo metal alloys (Table 5). Prostheses with a significantly different elastic modulus than natural bone can lead to stress shielding, resulting in a reduced mechanical load on the bone due to the bone–implant interaction, which may lead to bone loss around the implant [183].
Table 5.
The differences in elastic modulus values between bone and materials commonly used in prosthetic manufacturing [183].
| Material | Elastic Modulus (GPa) | Tensile Strength (MPa) |
|---|---|---|
| Trabecular bone | 0.02–0.05 | 1–5 |
| Cortical bone trabecular | 3–30 | 50–151 |
| UHMWPE | 0.9–2.7 | 50–151 |
| PMMA | 1.88–3.3 | 68 |
| PEEK | 3.5–4.0 | 118 |
| Co–Cr–Mo alloy | 210–232 | 1173 |
| Ti–6Al–4V alloy | 116 | 1018 |
Biodegradable Synthetic Polymer
Compared with natural polymers, biodegradable synthetic polymers possess superior mechanical properties and thermal stability. Extensive research has been conducted on the group of synthetic (α-hydroxy) polymeric biomaterials. Among them, the most frequently employed polymers are polyglycolic acid, polylactic-co-glycolic acid, and polylactic acid. These polymers are capable of biodegradation or absorption under in vivo conditions, making them ideal matrices for applications in regenerative medicine [2,212].
-
i.
Poly (Glycolic Acid) (PGA)
Since the 1970s, polyglycolide acid (PGA) has been the pioneering biodegradable polymer used for orthopedic fixation, specifically in screws, nails, and plates. It is a robust and dense polymer with a molecular weight ranging from 2.0 × 104 to 1.45 × 105 g·mol−1, a glass transition temperature (Tg) of 35 to 40 °C, and a melting point (TM) of approximately 224 °C [3]. PGA exhibits a highly crystalline structure, but its degradation time is relatively short, lasting from 6 to 12 months. Due to its rapid degradation properties, PGA demonstrates an early decline in mechanical strength in vivo, occurring approximately 4 to 7 weeks after the implantation procedure. Moreover, the use of PGA in bone surgery for orthopedic fixation can potentially lead to adverse side effects such as sinus wound formation, swelling, and fluid accumulation, primarily due to the increased presence of glycolic acid [213].
-
ii.
Poly (Lactic Acid) (PLA)
Polylactic acid (PLA) is derived from natural organic lactic acid and belongs to the category of biodegradable thermoplastic aliphatic polyesters [214]. PLA possesses several distinct qualities that make it an environmentally and economically attractive biopolymer, including its excellent rigidity, superb transparency, excellent processability, and glossy appearance. However, it does have some limitations, such as intrinsic brittleness, poor toughness, and a slow degradation rate [215]. Nevertheless, PLA has shown great potential as a biomaterial in various medical applications, including regenerative medicine, orthopedics, and tissue engineering. It has also gained significant importance as a printable biopolymer for 3D printing [216]. PLA is particularly well-suited for bone fixation devices, including absorbable plates and screws. By manipulating the polymer’s stereochemical structure and molecular weight, it is possible to adjust the ratio and degree of crystallization, thereby influencing its mechanical properties, degradation behavior, and processing temperatures. PLA exists in two stereoisomers: poly (L-lactide) (PLLA) and poly (D-lactide) (PDLA). Due to the racemic combination of monomers, PDLA is amorphous, leading to a disruption of crystallinity and consequently faster erosion compared to PLLA. In vitro and in vivo comparisons between PLLA and PDLA under physiological conditions have shown that highly crystalline PLLA may take two to five years to degrade, while amorphous poly (D,L-lactic acid) loses its integrity within two months and completely degrades within twelve months [217]. As a result, polymeric biodegradable plates and screws have been employed in various surgical procedures, including maxillofacial surgery, pediatric surgery, and orthognathic surgery (Table 6) [213].
Table 6.
The system of polymeric biodegradable implants, including plates and screws, utilized for bone fixation [213].
| Product Name | Manufacturer | Polymer Composition | Degradation Time |
|---|---|---|---|
| Biofix® SR-PGA | Bionx Implants, Tampere, Finland | SR-PGA | 6 weeks |
| Biofix® SR-PLLA | Bionx Implants, Tampere, Finland | SR-PLLA | 5–7 years |
| Resomer® LR708 | Evonik Industries, Darmstadt, Germany | PLLA (70%) + PDLLA (30%) | 2–3 years |
| MacroPore® | MacroPore Biosurgery Inc., San Diego, CA, USA | PLLA (70%) + PDLLA (30%) | 2–3 years |
| Macrosorb® | MacroPore Biosurgery Inc., San Diego, CA, USA | PLLA (70%) + PDLLA (30%) | 2–3 years |
| Biosorb FX® | Linvatec Biomaterials Ltd., Tampere, Finland | PLLA (70%) + PDLLA (30%) | 2–3 years |
| Resorb X® | KLS Martin Group, Tuttlingen, Germany | PLLA (50%) + PDLLA (50%) | 12–30 months |
| PolyMax® | Synthes, Oberdorf, Switzerland | PLLA (70%) + PDLLA (30%) | 2 years |
| PolyMax® RAPID | Synthes, Oberdorf, Switzerland | PLLA (85%) + PGA (15%) | 12 months |
| Rapidsorb® | DePuy Synthes, West Chester, PA, USA | PLLA (85%) + PGA (15%) | 12 months |
| Lactosorb® | Lorenz, Jacksonville, FL, USA | PLLA (82%) + PGA (18%) | 12 months |
| Delta® | Stryker Leibinger Corp., Kalamazoo, MI, USA | PLLA (85%), PGA (10%), PDLA (5%) | 8–13 months |
| Inion CPS® | Inion Inc., Tampere, Finland | PLLA, PGA, TMC–proportion varies | 2–4 years |
| Inion CPS® baby | Inion Inc., Tampere, Finland | PLLA, PGA, TMC–proportion varies | 2–3 years |
| Osteotrans-MX® | TEIJIN Medical Corp., Osaka, Japan | PLLA (60–70 wt%), u-HA (30–40 wt%) | 4.5–5.5 years |
SR, self-reinforced; PGA, polyglycolic acid; PLLA, poly-l-lactic acid; PDLLA, poly-d-l-lactic acid; TMC, trimethylene carbonate; u-HA, unsintered hydroxyapatite; USA, United States of America.
-
iii.
Poly (Lactide-Co-Glycolide)
This copolymer combines the desirable characteristics of PGA and PLLA, including adjusted degradability, biocompatibility, and hydrophilicity [2,218]. PLGA is hydrolytically degraded to the principal acidic constituents, lactic and glycolic acids, which are biologically removed to avert complications. However, these acidic degradation products, present at higher concentrations in PLGA interference screws, can alter the behavior of osteoblasts. These molecules impede cell proliferation and accelerate differentiation, thereby hindering the healing process around the degrading polymer implant in vivo. To mitigate the release of acidic by-products, PLGA has been combined with ceramics such as beta-tricalcium phosphate and hydroxyapatite [2,219,220].
-
iv.
Poly (Caprolactone)
PCL is a stiff, aliphatic, semi-crystalline, and biodegradable non-toxic polyester. It demonstrates sufficient biocompatibility and can be categorized into three groups based on a single glass transition temperature (Tg) to determine mechanical compatibility. Notably, poly (ε-caprolactone) stands out with its low melting temperature (59–64 °C) and low glass transition temperature (60 °C) [2,221,222,223]. These properties make PCL suitable for various applications, such as orthopedics, tissue engineering scaffolds, drug delivery systems, and sutures [224]. However, PCL does have some limitations, including its slow degradation process, which can take up to 3 or 4 years, and its hydrophobic nature, which hampers cell adhesion and penetration [175]. Nevertheless, PCL offers advantages over polyhydroxy acids (PHA) due to its cost-effectiveness, wide availability, and greater stability. By employing copolymerization or blending it with other polymers, the properties of PCL can be modified [224].
3. Enhanced Bone–Implant Biocompatibility due to Osteogenic Factors
While minerals, ceramics, and polymers are available to meet diverse industry needs, the biomedical sector has witnessed the significant adoption of metals and metal alloys for medical implant applications. However, despite their unique mechanical properties, metals do not exhibit favorable biocompatibility when implanted in the body. Researchers have expressed concerns regarding the potential toxicity effects of various implant metals, including titanium, aluminum, stainless steel, and iron. These metals possess a high modulus, which can result in stress shielding, triggering inflammatory or foreign body reactions. Additionally, they have been associated with various infections, such as yellow bile syndrome caused by titanium ions and osteomalacia due to the leaching of aluminum ions, among others [225].
Modern biomaterials have revolutionized our understanding of the intricate interactions occurring within biological systems, operating at both the cellular and molecular levels. The ultimate objective of these investigations is to develop materials and products that are better suited for diverse applications in biomedicine. Significant progress has been made in recent years in creating such materials through the utilization of nano-coatings, grafted polymer brushes, nanotubes, hydrogels, and organic and inorganic nanoparticles, among others. Polymer materials often undergo modifications to enhance their biological properties. These modifications encompass various components, such as drugs and biomolecules absorbed on or loaded into the scaffold’s surface, incorporation of inorganic micro- and nanoparticles onto or within the scaffold, as well as the application of coatings onto the scaffold’s surface. These advancements have been introduced to address the aforementioned concerns [226,227].
Osteogenic factors can help enhance bone–implant biocompatibility when implant toxicity is a concern. Implant toxicity refers to the adverse effects caused by the materials used in the implant, which can impede the healing process and integration with the surrounding bone. Osteogenic factors can mitigate these toxic effects and promote better biocompatibility. Examples that illustrate this concept are as follows:
A study was conducted to evaluate the biocompatibility and osteogenic potential of various calcium-silicate-based cements (CSCs) when combined with an enamel matrix derivative (Emdogain) using human-bone-marrow-derived mesenchymal stem cells. The results demonstrated that the incorporation of CSCs as retrograde filling materials and the administration of additional Emdogain resulted in significant improvements in bone regeneration and prognosis for apical microsurgery procedures [228].
Concentrated growth factors (CGF) refer to a product obtained from autologous blood through the centrifugation of venous blood. In a study, researchers investigated the potential of CGF to stimulate the osteogenic differentiation of human bone marrow stem cells (hBMSC) in an in vitro setting. The findings unequivocally demonstrated that CGF alone possesses the ability to induce osteogenic differentiation of hBMSC [229].
Another study focused on the utilization of exosomes, which are nano-sized extracellular vesicles containing proteins, nucleic acids, and lipids, as therapeutic nanoparticles for treating diseases. The researchers developed a cell-free tissue engineering system by employing functional exosomes instead of seed cells. They achieved this by constructing gene-activated engineered exosomes that encapsulated the VEGF gene derived from ATDC5 cells. Effective integration of the engineered exosomes with 3D-printed porous bone scaffolds was accomplished using a specific exosomal anchor peptide known as CP05. The study demonstrated that these engineered exosomes play a dual role: they act as an osteogenic matrix, inducing the osteogenic differentiation of mesenchymal stem cells, and also function as gene vectors, allowing controlled release of the VEGF gene to remodel the vascular system [230].
A study was conducted to investigate the potential of 3D-printed β-tricalcium phosphate (β-TCP) scaffolds in promoting the osteogenesis of bone marrow stem cells (BMSCs) through N6-methyladenosine (m6A) modification. The results revealed that β-TCP exhibited excellent biocompatibility and demonstrated osteoinductive properties. Furthermore, the study observed an increase in methyltransferase-like 3 (METTL3), which resulted in an elevated m6A level of RUNX2. Consequently, this led to a more stable level of RUNX2 mRNA [231].
In a conducted study, researchers aimed to develop two self-assembling supramolecular hydrogels by utilizing an osteogenic growth peptide (OGP) and evaluate their impact on proliferation and osteogenesis in both in vitro and in vivo settings. The hydrogels, known as F-sequence and G-sequence hydrogels, exhibited remarkable biocompatibility and demonstrated the ability to enhance cell proliferation. Additionally, the hydrogels effectively stimulated the upregulation of crucial osteogenic factors, including RUNX2, BMP2, OCN, and OPN, thus promoting the process of osteogenic differentiation. The findings of this study provided significant insights into the underlying mechanism involved in hydrogel-mediated repair of bone defects [232].
4. Additive Manufacturing (AM)
Additive manufacturing, also known as 3D printing, is a manufacturing technique that creates 3D objects by adding material layer by layer. It is considered the third pillar of overall manufacturing technology, alongside subtractive manufacturing techniques such as milling or lathing, and formative manufacturing techniques such as casting or forging. Additive manufacturing was formerly known as rapid prototyping (RP) and is now commonly referred to as 3D printing. In 1984, Chuck Hull developed stereolithography, the first additive process for polymers, which was later commercialized. He coined the term “stereolithography” and patented the technology in 1986 [233,234]. There are various additive manufacturing techniques, including fused-deposition modeling, 3D inkjet printing, stereolithography, direct powder extrusion, and selective laser sintering. These techniques involve digitally controlled layer-by-layer deposition of materials to create different geometries of printlets [235].
Additive manufacturing has emerged as a prominent research topic in the past decade due to its low cost, ease of use, and the reliability of 3D printing equipment. It enables the straightforward and customized production of complex 3D structures and components through the layer-by-layer deposition of materials, eliminating the need for specialized tools or molds [234,236].
Additive manufacturing offers a unique opportunity for fabricating personalized dosage forms, which is crucial in addressing the diverse medical needs of patients. Despite having been in existence for four decades, AM has only recently gained wider usage in both surgical and non-surgical fields [48].
Furthermore, 3D printing is becoming a popular method of producing medical devices for orthopedic applications, tissue engineering, and the rehabilitation of patients suffering from disabling neurological diseases such as spinal cord injuries and amyotrophic lateral sclerosis. This is due to 3D printing enabling the creation of patient-specific designs, highly complex structural elements, and affordable on-demand manufacture [237]. Moreover, human organs can be manufactured according to the principles of AM using specialized 3D bioprinters [238]. Techniques of 3D printing have great potential for fabricating porous, complex-formed substances, and forms with highly complex internal structures. As a result, 3D printing technology allows the creation of hierarchical substances with mechanical qualities (strength and elastic Young’s modulus) and porous structures comparable to natural bone, while reducing the stress-shielding impact created by orthopedic implants [239,240,241].
Although 3D printing enables researchers to create parts that meet these requirements, the majority of clinical work in orthopedics focuses on metallic biomaterials, and most commercial representation is centered around metal-related approaches. However, polymers and polymeric composites receive significant attention in bone engineering applications due to the strong similarities between their thermomechanical properties and those of tissues, as well as their biodegradability and biocompatibility [242]. Furthermore, 3D printing technologies offer several advantages, including mass production capability, economic efficiency, and repeatability [243]. Moreover, when combined with computer-aided design (CAD) [244], 3D technology can be used to create completely patient-specific implants [245,246].
Despite the significant advances that have been made in 3D printing technology, there are still notable problems to overcome. These include software design, standardization and integration of a comprehensive bio-fabrication platform, repeatability, limitations of 3D printers’ capabilities, biomaterial characterization, regulatory hurdles, and quality by design. Addressing these challenges is crucial for 3D printing to be recognized as a traditional bio-manufacturing method in medicine and to gain access to the medical market. Among these challenges, the lack of heterogeneous biomaterials that would enable their reliable clinical utilization is the main obstacle [237].
4.1. Additive Manufacturing of Metallic Implants
Over the years, porous metal biomaterials have been fabricated using conventional manufacturing techniques, primarily based on powder metallurgy, such as metal injection molding and spacer processes. Although these fabrication techniques have made remarkable progress, certain limitations still exist, including the inability to precisely control pore shape and distribution, as well as dimensional inaccuracies [247]. Additionally, implants manufactured using conventional processing methods, known as standard-type implants, cannot match the structure, performance, and required physical and chemical characteristics for addressing specific bone flaws. This limitation restricts the therapeutic efficacy and longevity of implants [52].
On the other hand, additive manufacturing (AM) of metallic biomaterials demonstrates excellent medical potential, encompassing prostheses, implants, drug delivery systems, scaffolds, and stents. The process of AM involves creating and manufacturing 3D designs, which can be achieved using a CAD program. Furthermore, these 3D designs can be manufactured using various techniques such as fused deposition modeling (FDM), selective laser melting (SLM), and selective laser sintering (SLS) [248].
AM technology offers several advantages, including more precise fabrication and greater flexibility in designing both the internal and external macro- and micro-architectures of orthopedic implants [249]. The geometrical and topological porosity qualities of metallic biomaterials can be accurately tuned through controlled AM manufacturing techniques, improving their mechanical properties to mimic bone [250,251]. This leads to improved rates of bone tissue regeneration [252,253,254], altered biodegradation kinetics [255,256], and the formation of a vast, interconnected osteocyte lacuno-canalicular network [257,258].
However, certain characteristics such as wear resistance, hardness, anti-ferromagnetic properties, or antibacterial characteristics cannot be easily modified through geometrical design alone, as these properties require modifications to the underlying base material(s) prior to AM processing [247].
4.2. Additive Manufacturing of Polymeric Implants
Polymers play a crucial role in 3D printing manufacture due to their versatility, excellent processability, and compatibility with various AM processes [48,259]. They possess notable characteristics such as surface detailing, high precision, temperature resistance, accuracy, and improved strength [260]. In the realm of additive manufacturing, polymers contribute significantly, accounting for 51% of the polymer parts produced, 29% of metal and polymer combinations, and 19.8% of metal products [261]. Reactive monomers, thermoplastic filaments, powder, and resin are commonly utilized forms of polymers in AM techniques [259].
Despite the wide array of available AM techniques, advancements in polymer printing primarily focus on three key strategies: (1) powder bed fusion processes such as selective laser sintering (SLS), (2) deposition-on-demand processes, including extrusion-based technologies such as fused deposition modeling (FDM) and direct-ink-write printing [262], as well as inkjet or drop-wise deposition methods, and (3) photo-polymer-based printing techniques, such as stereolithography (SLA). These printing methods have successfully incorporated various polymers as raw materials [259,263].
The production of polymer composites through 3D printing entails both advantages and drawbacks, with each method having specific requirements regarding the polymer’s structure, state (liquid or solid), and physical characteristics (melting temperature and viscosity) [264]. When it comes to load-bearing applications, the range of materials that can be employed for polymer AM is comparatively limited compared to other applications. For instance, commonly used polymers in SLS processes for load-bearing applications include PEEK, UHMWPE, PMMA, PLA, PCL, polypropylene (PP), polyvinyl alcohol (PVA), and polyamide (PA) [48].
5. Future Direction
The field of medical science is advancing rapidly to meet the increasing demand for biomedical implants used in the treatment of injuries and traumas [265]. This progress is driven by the development of new materials and the integration of three-dimensional fabrication techniques, enabling the production of complex geometric implants [227]. However, to ensure the safety and efficacy of these implants, it is crucial to conduct toxicity analysis and biocompatibility studies on the alloying elements used in metallic implants that undergo controlled degradation.
The interfacial properties of implants in a biological environment can be improved through the utilization of advanced surface modification techniques and coatings. Understanding the chemistry of coating materials, conducting corrosion analysis, and optimizing fabrication techniques and parameters are essential for achieving efficient and accurate outcomes.
The development of novel composite materials holds great potential in enhancing the mechanical performance of implants, providing flexibility, and introducing new functionalities. Non-biodegradable implants present challenges such as stress shielding, toxicity, and the generation of harmful byproducts. To address these issues, biodegradable implants are being developed, which support tissue growth and self-sustainability. Future research into biodegradable materials aims to focus on strategies for controlling impurity levels, optimizing coatings and alloying elements, functionalizing implant materials, optimizing the biodegradation rate at the implant/tissue interface, and exploring new degradable materials.
In tissue engineering, the design of polymeric scaffolds is being optimized to elicit timely and desirable responses. The demand for tissue and organ replacements drives advancements and efforts in tissue engineering [266]. Polymeric scaffold materials offer the ability to control various physical and chemical properties for tissue engineering applications [267]. However, further research and development are needed to overcome limitations related to porosity, bioactivity, and mechanical properties. The study of a wide range of materials and fabrication techniques is crucial in addressing these limitations.
Overall, the continuous exploration of new materials, fabrication techniques, and surface modifications is essential for the advancement of biomedical implants and tissue engineering. Through interdisciplinary research and collaboration, the field can overcome the existing limitations and pave the way for safer, more efficient, and effective implant solutions [266].
6. Conclusions
Bone fractures present complex challenges for scientists and orthopedic surgeons. In orthopedic surgery, both metals and synthetic polymers have been utilized and compared. Metal implants, such as titanium and its alloys, and stainless steel and its alloys are commonly used for fracture repair due to their excellent mechanical properties, strength, and toughness. However, these materials exhibit limited biocompatibility, leading to foreign-body interactions such as poisoning, inflammation, swelling at the surgical site, and high corrosion rates, often requiring additional surgeries for removal.
On the other hand, polymeric implants have emerged as an alternative to metal implants, offering high biocompatibility, non-toxicity, and biodegradability. However, their mechanical properties are comparatively poor. Polylactic acid is a promising biopolymer due to its unique qualities, including its excellent rigidity, transparency, processability, and glossy appearance. It is an ecologically and economically attractive material. To enhance the mechanical strength and biocompatibility of biodegradable polymer implants, it is necessary to develop controlled absorption rates and explore methods for increasing their mechanical properties.
Additive manufacturing plays a significant role in the production of scaffolds and orthotic devices. It allows for customized designs for individual patients, cost-effective on-demand manufacturing, and the creation of complex porous structures with internal features similar to natural bone. This technique enables the fabrication of graded materials with varying porosities and mechanical properties. Additionally, special 3D biological printers can be utilized to produce composite human organs, following the principles of additive manufacturing.
The field of orthopedic implants and tissue engineering is exploring a range of materials and manufacturing techniques to address the challenges associated with bone fractures. The use of biodegradable polymers, additive manufacturing, and advanced 3D printing technologies offers promising solutions for improving biocompatibility, mechanical properties, and customization in the development of implants and tissue engineering constructs.
Acknowledgments
The authors would like to acknowledge Universiti Putra Malaysia (UPM) Research Grant (UPM/GP-IPB/2020/9688700), for providing necessary resources. This work was supported by the research grant from the Chuongbong Academic Research Fund of Jeju National University in 2022. Thank you for making this publication possible.
Author Contributions
Conceptualization, D.B., F.D.A.-S. and A.H.M.A.; methodology, M.K.A.M.A., C.L.S.K. and A.H.M.A.; resources, A.H.M.A. and D.-W.J.; data curation, M.K.A.M.A., C.L.S.K. and M.O.A.-O.; writing—original draft preparation, F.D.A.-S., D.B., C.L.S.K.; writing—review and editing, M.K.A.M.A., A.H.M.A. and D.-W.J.; visualization, F.D.A.-S. and A.H.M.A.; supervision, A.H.M.A., M.K.A.M.A., C.L.S.K., D.B. and D.-W.J.; project administration, A.H.M.A. and F.D.A.-S.; funding acquisition, A.H.M.A. and D.-W.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Universiti Putra Malaysia (UPM) Research Grant (UPM/GP-IPB/2020/9688700), and the Chuongbong Academic Research Fund of Jeju National University in 2022.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Marin E., Boschetto F., Pezzotti G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. A. 2020;108:1617–1633. doi: 10.1002/jbm.a.36930. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shalawi F.D., Hanim M.A., Ariffin M., Kim C.L.S., Brabazon D., Calin R., Al-Osaimi M.O. Biodegradable synthetic polymer in orthopaedic application: A review. Mater. Today Proc. 2023;74:540–546. doi: 10.1016/j.matpr.2022.12.254. [DOI] [Google Scholar]
- 3.Yousef J., Leow S.K.H., Morrison W. Plastic surgery in antiquity: An examination of ancient documents. Eur. J. Plast. Surg. 2021;44:419–428. doi: 10.1007/s00238-020-01763-4. [DOI] [Google Scholar]
- 4.Shahar F.S., Sultan M.T.H., Lee S.H., Jawaid M., Shah A.U.M., Safri S.N.A., Sivasankaran P.N. A review on the orthotics and prosthetics and the potential of kenaf composites as alternative materials for ankle-foot orthosis. J. Mech. Behav. Biomed. Mater. 2019;99:169–185. doi: 10.1016/j.jmbbm.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Eliaz N. Corrosion of metallic biomaterials: A review. Materials. 2019;12:407. doi: 10.3390/ma12030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharadwaj A. An Overview on Biomaterials and Its Applications in Medical Science. IOP Conf. Ser. Mater. Sci. Eng. 2021;1116:012178. doi: 10.1088/1757-899X/1116/1/012178. [DOI] [Google Scholar]
- 7.Ali S., Rani A.M.A., Baig Z., Ahmed S.W., Hussain G., Subramaniam K., Hastuty S., Rao T.V. Biocompatibility and corrosion resistance of metallic biomaterials. Corros. Rev. 2020;38:381–402. doi: 10.1515/corrrev-2020-0001. [DOI] [Google Scholar]
- 8.Im G.I. Biomaterials in orthopaedics: The past and future with immune modulation. Biomater. Res. 2020;24:7–10. doi: 10.1186/s40824-020-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams D.F. Challenges with the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019;7:127. doi: 10.3389/fbioe.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manivasagam G., Dhinasekaran D., Rajamanickam A. Biomedical Implants: Corrosion and its Prevention—A Review. Recent Pat. Corros. Sci. 2010;2:40–54. doi: 10.2174/1877610801002010040. [DOI] [Google Scholar]
- 11.Winkler T., Sass F.A., Duda G.N., Schmidt-Bleek K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018;7:232–243. doi: 10.1302/2046-3758.73.BJR-2017-0270.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghasemi-Mobarakeh L., Kolahreez D., Ramakrishna S., Williams D. Key terminology in biomaterials and biocompatibility. Curr. Opin. Biomed. Eng. 2019;10:45–50. doi: 10.1016/j.cobme.2019.02.004. [DOI] [Google Scholar]
- 13.Thomas S., Balakrishnan P., Sreekala M.S. Fundamental Biomaterials: Ceramics. Woodhead Publishing; Sawston, UK: 2018. [Google Scholar]
- 14.Bairagi D., Mandal S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magnes. Alloy. 2022;10:627–669. doi: 10.1016/j.jma.2021.09.005. [DOI] [Google Scholar]
- 15.Seal C.K., Vince K., Hodgson M.A. Biodegradable surgical implants based on magnesium alloys—A review of current research. IOP Conf. Ser. Mater. Sci. Eng. 2009;4:012011. doi: 10.1088/1757-899X/4/1/012011. [DOI] [Google Scholar]
- 16.Sumner D.R. Long-term implant fixation and stress-shielding in total hip replacement. J. Biomech. 2015;48:797–800. doi: 10.1016/j.jbiomech.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Hermawan H., Dubé D., Mantovani D. Developments in metallic biodegradable stents. Acta Biomater. 2010;6:1693–1697. doi: 10.1016/j.actbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Hermawan H., Dubé D., Mantovani D. Degradable metallic biomaterials: Design and development of Fe–Mn alloys for stents. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010;93:1–11. doi: 10.1002/jbm.a.32224. [DOI] [PubMed] [Google Scholar]
- 19.Mani G., Feldman M.D., Patel D., Agrawal C.M. Coronary stents: A materials perspective. Biomaterials. 2007;28:1689–1710. doi: 10.1016/j.biomaterials.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Alizadeh-Osgouei M., Li Y., Wen C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019;4:22–36. doi: 10.1016/j.bioactmat.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acklin Y.P., Michelitsch C., Sommer C. Elective implant removal in symptomatic patients after internal fixation of proximal humerus fractures improves clinical outcome. BMC Musculoskelet. Disord. 2016;17:119. doi: 10.1186/s12891-016-0977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matusiewicz H., Richter M. Local release of metal ions from endovascular metallic implants in the human biological specimens: An overview of in vivo clinical implications. World J. Adv. Res. Rev. 2021;11:91–102. doi: 10.30574/wjarr.2021.11.1.0326. [DOI] [Google Scholar]
- 23.Chin P.Y.Y., Cheok Q., Glowacz A., Caesarendra W. A review of in-vivo and in-vitro real-time corrosion monitoring systems of biodegradable metal implants. Appl. Sci. 2020;10:3141. doi: 10.3390/app10093141. [DOI] [Google Scholar]
- 24.Matusiewicz H., Richter M. Potentially toxic metallic wear nanoparticles and trace metal ions release from metal-on-metal orthopedic implants in the human biological specimens: An Overview of in vivo and ex vivo clinical studies. World J. Adv. Res. Rev. 2020;8:242–292. doi: 10.30574/wjarr.2020.8.3.0469. [DOI] [Google Scholar]
- 25.Gómez-Gil V., Pascual G., Bellón J.M. Biomaterial implants in abdominal wall Hernia Repair: A review on the importance of the peritoneal interface. Processes. 2019;7:105. doi: 10.3390/pr7020105. [DOI] [Google Scholar]
- 26.Hudecki A., Kiryczyński G., Łos M.J. Biomaterials, definition, overview. Stem Cells Biomater. Regen. Med. 2018;7:85–98. doi: 10.1016/B978-0-12-812258-7.00007-1. [DOI] [Google Scholar]
- 27.Munir K.S., Wen C., Li Y. Carbon Nanotubes and Graphene as Nanoreinforcements in Metallic Biomaterials: A Review. Adv. Biosyst. 2019;3:1800212. doi: 10.1002/adbi.201800212. [DOI] [PubMed] [Google Scholar]
- 28.Yang L. Nanotechnology-Enhanced Orthopedic Materials: Fabrications, Applications and Future Trends. Woodhead Publishing; Sawston, UK: 2015. [Google Scholar]
- 29.Hulbert S.F. An Introduction to Bioceramics. World Scientific; Singapore: 1993. The use of alumina and zirconia in surgical implants; pp. 25–40. [Google Scholar]
- 30.Kumar S., Nehra M., Kedia D., Dilbaghi N., Tankeshwar K., Kim K.H. Nanotechnology-based biomaterials for orthopaedic applications: Recent advances and future prospects. Mater. Sci. Eng. C. 2020;106:110154. doi: 10.1016/j.msec.2019.110154. [DOI] [PubMed] [Google Scholar]
- 31.Bommala V.K., Krishna M.G., Rao C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloy. 2019;7:72–79. doi: 10.1016/j.jma.2018.11.001. [DOI] [Google Scholar]
- 32.Pajarinen J., Lin T., Gibon E., Kohno Y., Maruyama M., Nathan K., Lu L., Yao Z., Goodman S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89. doi: 10.1016/j.biomaterials.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M.Q. Recent Advances and Perspective of Nanotechnology-Based Implants for Orthopedic Applications. Front. Bioeng. Biotechnol. 2022;10:878257. doi: 10.3389/fbioe.2022.878257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J.W., Du C.F., Yuchi C.X., Zhang C.Q. Application of Biodegradable Materials in Orthopedics. J. Med. Biol. Eng. 2019;39:633–645. doi: 10.1007/s40846-019-00469-8. [DOI] [Google Scholar]
- 35.Pfeiffenberger M., Damerau A., Lang A., Buttgereit F., Hoff P., Gaber T. Fracture Healing Research-Shift towards In Vitro Modeling? Biomedicines. 2021;9:748. doi: 10.3390/biomedicines9070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H., Wu S., Chen W., Hu Y., Geng Z., Su J. Bone/cartilage targeted hydrogel: Strategies and applications. Bioact. Mater. 2023;23:156–169. doi: 10.1016/j.bioactmat.2022.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin W., Chu P.K. Orthopedic implants. Encycl. Biomed. Eng. 2019;1:425–436. doi: 10.1016/B978-0-12-801238-3.10999-7. [DOI] [Google Scholar]
- 38.Tian L., Tang N., Ngai T., Wu C., Ruan Y.C., Huang L., Qin L. Hybrid fracture fixation systems developed for orthopaedic applications: A general review. J. Orthop. Transl. 2019;16:1–13. doi: 10.1016/j.jot.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartolomeu F., Dourado N., Pereira F., Alves N., Miranda G., Silva F.S. Additive manufactured porous biomaterials targeting orthopedic implants: A suitable combination of mechanical, physical and topological properties. Mater. Sci. Eng. C. 2020;107:110342. doi: 10.1016/j.msec.2019.110342. [DOI] [PubMed] [Google Scholar]
- 40.Dhandapani R., Krishnan P.D., Zennifer A., Kannan V., Manigandan A., Arul M.R., Jaiswal D., Subramanian A., Kumbar S.G., Sethuraman S. Additive manufacturing of biodegradable porous orthopaedic screw. Bioact. Mater. 2020;5:458–467. doi: 10.1016/j.bioactmat.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J.L., Xu J.K., Hopkins C., Chow D.H.K., Qin L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020;7:1902443. doi: 10.1002/advs.201902443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huzum B., Puha B., Necoara R.M., Gheorghevici S., Puha G., Filip A., Sirbu P.D., Alexa O. Biocompatibility assessment of biomaterials used in orthopedic devices: An overview (Review) Exp. Ther. Med. 2021;22:1315. doi: 10.3892/etm.2021.10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cvrček L., Horáková M. Non-Thermal Plasma Technology for Polymeric Materials. Elsevier; Amsterdam, The Netherlands: 2019. Plasma modified polymeric materials for implant applications; pp. 367–407. [Google Scholar]
- 44.Filip N., Radu I., Veliceasa B., Filip C., Pertea M., Clim A., Pinzariu A.C., Drochioi I.C., Hilitanu R.L., Serban I.L. Biomaterials in Orthopedic Devices: Current Issues and Future Perspectives. Coatings. 2022;12:1544. doi: 10.3390/coatings12101544. [DOI] [Google Scholar]
- 45.Silver F.H., Shah R. Measurement of mechanical properties of natural and engineered implants. Adv. Tissue Eng. Regen. Med. 2016;1:20–25. doi: 10.15406/atroa.2016.01.00004. [DOI] [Google Scholar]
- 46.Xiao D., Zhang J., Zhang C., Barbieri D., Yuan H., Moroni L., Feng G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020;106:22–33. doi: 10.1016/j.actbio.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Wooley P.H., Hallab N.J. Wound healing, chronic inflammation, and immune responses. Met. Met. Bear. A Clin. Pract. 2014:109–133. [Google Scholar]
- 48.Nouri A., Shirvan A.R., Li Y., Wen C. Additive manufacturing of metallic and polymeric load-bearing biomaterials using laser powder bed fusion: A review. J. Mater. Sci. Technol. 2021;94:196–215. doi: 10.1016/j.jmst.2021.03.058. [DOI] [Google Scholar]
- 49.Mourey T.H., Schunk T.C. Synthetic Polymers. Elsevier Inc.; Amsterdam, The Netherlands: 1992. [DOI] [Google Scholar]
- 50.Al-Amin M., Abdul-Rani A.M., Danish M., Rubaiee S., Mahfouz A.B., Thompson H.M., Ali S., Unune D.R., Sulaiman M.H. Investigation of coatings, corrosion and wear characteristics of machined biomaterials through hydroxyapatite mixed-edm process: A review. Materials. 2021;14:3597. doi: 10.3390/ma14133597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majumdar T., Eisenstein N., Frith J.E., Cox S.C., Birbilis N. Additive Manufacturing of Titanium Alloys for Orthopedic Applications: A Materials Science Viewpoint. Adv. Eng. Mater. 2018;20:1800172. doi: 10.1002/adem.201800172. [DOI] [Google Scholar]
- 52.Bai L., Gong C., Chen X., Sun Y., Zhang J., Cai L., Zhu S., Xie S.Q. Additive manufacturing of customized metallic orthopedic implants: Materials, structures, and surface modifications. Metals. 2019;9:1004. doi: 10.3390/met9091004. [DOI] [Google Scholar]
- 53.Al-Amin M., Rani A.M.A., Aliyu A.A.A., Razak M.A.A., Hastuty S., Bryant M.G. Powder mixed-EDM for potential biomedical applications: A critical review. Mater. Manuf. Process. 2020;35:1789–1811. doi: 10.1080/10426914.2020.1779939. [DOI] [Google Scholar]
- 54.Material Properties of UHMW Polyethylene—Thermoplastic. Jul 25, 2022. [(accessed on 21 February 2023)]. Available online: https://dielectricmfg.com/knowledge-base/uhmw/
- 55.Qu S., Liu Y., Gong K. UHMWPE Biomaterials for Joint Implants: Structures, Properties and Clinical Performance. Springer; Singapore: 2019. Drug-Loaded UHMWPE to Inhibit Wear Particle-Induced Osteolysis: Processing, Characterization, and Biological Evaluation; pp. 151–190. [Google Scholar]
- 56.Jin W., Hao Q., Peng X., Chu P.K. Enhanced corrosion resistance and biocompatibilty of PMMA-coated ZK60 magnesium alloy. Mater. Lett. 2016;173:178–181. doi: 10.1016/j.matlet.2016.03.071. [DOI] [Google Scholar]
- 57.Szczęsny G., Kopec M., Politis D.J., Kowalewski Z.L., Łazarski A., Szolc T. A Review on Biomaterials for Orthopaedic Surgery and Traumatology: From Past to Present. Materials. 2022;15:3622. doi: 10.3390/ma15103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trentin A., Gasparini A.D.L., Faria F.A., Harb S.V., dos Santos F.C., Pulcinelli S.H., Santilli C.V. Barrier properties of high performance PMMA-silica anticorrosion coatings. Prog. Org. Coat. 2020;138:105398. doi: 10.1016/j.porgcoat.2019.105398. [DOI] [Google Scholar]
- 59.Harb S.V., Trentin A., de Souza T.A.C., Magnani M., Pulcinelli S.H., Santilli C.V., Hammer P. Effective corrosion protection by eco-friendly self-healing PMMA-cerium oxide coatings. Chem. Eng. J. 2020;383:123219. doi: 10.1016/j.cej.2019.123219. [DOI] [Google Scholar]
- 60.Harb S.V., Bassous N.J., de Souza T.A., Trentin A., Pulcinelli S.H., Santilli C.V., Webster T.J., Lobo A.O., Hammer P. Hydroxyapatite and β-TCP modified PMMA-TiO2 and PMMA-ZrO2 coatings for bioactive corrosion protection of Ti6Al4V implants. Mater. Sci. Eng. C. 2020;116:111149. doi: 10.1016/j.msec.2020.111149. [DOI] [PubMed] [Google Scholar]
- 61.Material Properties of Thermoplastic PEEK—Polyetheretherketone. 25 July 2022. [(accessed on 21 February 2023)]. Available online: https://dielectricmfg.com/knowledge-base/peek/
- 62.Zhao Y., Zhao K., Li Y., Chen F. Mechanical characterization of biocompatible PEEK by FDM. J. Manuf. Process. 2020;56:28–42. doi: 10.1016/j.jmapro.2020.04.063. [DOI] [Google Scholar]
- 63.Ma H., Suonan A., Zhou J., Yuan Q., Liu L., Zhao X., Lou X., Yang C., Li D., Zhang Y.-G. PEEK (Polyether-ether-ketone) and its composite materials in orthopedic implantation. Arab. J. Chem. 2021;14:102977. doi: 10.1016/j.arabjc.2020.102977. [DOI] [Google Scholar]
- 64.Wang J., Sun Y., Bi W., Jiang Z., Zhang M., Pang J. High-strength corrosion resistant membranes for the separation of oil/water mixtures and immiscible oil mixtures based on PEEK. J. Memb. Sci. 2020;616:118418. doi: 10.1016/j.memsci.2020.118418. [DOI] [Google Scholar]
- 65.Travieso-Rodriguez J.A., Jerez-Mesa R., Llumà J., Traver-Ramos O., Gomez-Gras G., Rovira J.J.R. Mechanical Properties of 3D-Printing Polylactic Acid Parts subjected to Bending Stress and Fatigue Testing. Materials. 2019;12:3859. doi: 10.3390/ma12233859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mozafari M., Bordbar-Khiabani A., Yarmand B. Emerging magnesium-based biomaterials for orthopedic implantation. Emerg. Mater. Res. 2020;8:305–319. doi: 10.1680/jemmr.18.00048. [DOI] [Google Scholar]
- 67.Li J., Qin L., Yang K., Ma Z., Wang Y., Cheng L., Zhao D. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020;36:190–208. doi: 10.1016/j.jmst.2019.07.024. [DOI] [Google Scholar]
- 68.Balestriere M.A., Schuhladen K., Seitz K.H., Boccaccini A.R., Cere S.M., Ballarre J. Sol-gel coatings incorporating borosilicate bioactive glass enhance anti corrosive and surface performance of stainless steel implants. J. Electroanal. Chem. 2020;876:114735. doi: 10.1016/j.jelechem.2020.114735. [DOI] [Google Scholar]
- 69.Gawad S.A., Nasr A., Fekry A.M., Filippov L.O. Electrochemical and hydrogen evolution behaviour of a novel nano-cobalt/nano-chitosan composite coating on a surgical 316L stainless steel alloy as an implant. Int. J. Hydrog. Energy. 2021;46:18233–18241. doi: 10.1016/j.ijhydene.2021.03.018. [DOI] [Google Scholar]
- 70.Komatsu A., Fujinami M., Hatano M., Matsumoto K., Sugeoi M., Chiari L. Straining-temperature dependence of vacancy behavior in hydrogen-charged austenitic stainless steel 316L. Int. J. Hydrog. Energy. 2021;46:6960–6969. doi: 10.1016/j.ijhydene.2020.11.148. [DOI] [Google Scholar]
- 71.Kwak T., Yang J., Heo Y., Kim S., Kwon S., Kim W., Lim D. Additive manufacturing of a porous titanium layer structure Ti on a Co-Cr alloy for manufacturing cementless implants. J. Mater. Res. Technol. 2021;10:250–267. doi: 10.1016/j.jmrt.2020.11.080. [DOI] [Google Scholar]
- 72.Xiang D., Wang P., Tan X., Chandra S., Wang C., Nai M., Tor S., Liu W., Liu E. Anisotropic microstructure and mechanical properties of additively manufactured Co–Cr–Mo alloy using selective electron beam melting for orthopedic implants. Mater. Sci. Eng. A. 2019;765:138270. doi: 10.1016/j.msea.2019.138270. [DOI] [Google Scholar]
- 73.Duan J., Yang Y., Zhang E., Wang H. Co-Cr-Mo-Cu alloys for clinical implants with osteogenic effect by increasing bone induction, formation and development in a rabbit model. Burn. Trauma. 2020;8:tkaa036. doi: 10.1093/burnst/tkaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gong X., Li Y., Nie Y., Huang Z., Liu F., Huang L., Jiang L., Mei H. Corrosion behaviour of CoCrMo alloy fabricated by electron beam melting. Corros. Sci. 2018;139:68–75. doi: 10.1016/j.corsci.2018.04.033. [DOI] [Google Scholar]
- 75.He X., Reichl F.-X., Milz S., Michalke B., Wu X., Sprecher C.M., Yang Y., Gahlert M., Röhling S., Kniha H., et al. Titanium and zirconium release from titanium- and zirconia implants in mini pig maxillae and their toxicity in vitro. Dent. Mater. 2020;36:402–412. doi: 10.1016/j.dental.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 76.Hourmand M., Sarhan A.A.D., Sayuti M., Hamdi M. A comprehensive review on machining of titanium alloys. Arab. J. Sci. Eng. 2021;46:7087–7123. doi: 10.1007/s13369-021-05420-1. [DOI] [Google Scholar]
- 77.Ren B., Wan Y., Liu C., Wang H., Yu M., Zhang X., Huang Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C. 2021;118:111505. doi: 10.1016/j.msec.2020.111505. [DOI] [PubMed] [Google Scholar]
- 78.Tshephe T.S., Akinwamide S.O., Olevsky E., Olubambi P.A. Additive manufacturing of titanium-based alloys- A review of methods, properties, challenges, and prospects. Heliyon. 2022;8:e09041. doi: 10.1016/j.heliyon.2022.e09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang L., Huang Q., Wu H., Ouyang Z., Liu T., He H., Xiao J., Lei G., Zhou K. Stimulation of in vitro and in vivo osteogenesis by Ti-Mg composite materials with the sustained-release function of magnesium ions. Colloids Surf. B Biointerfaces. 2021;197:111360. doi: 10.1016/j.colsurfb.2020.111360. [DOI] [PubMed] [Google Scholar]
- 80.Cordeiro J.M., Nagay B.E., Ribeiro A.L.R., da Cruz N.C., Rangel E.C., Fais L.M., Vaz L.G., Barão V.A. Functionalization of an experimental Ti-Nb-Zr-Ta alloy with a biomimetic coating produced by plasma electrolytic oxidation. J. Alloys Compd. 2019;770:1038–1048. doi: 10.1016/j.jallcom.2018.08.154. [DOI] [Google Scholar]
- 81.Zwolak I. Protective Effects of Dietary Antioxidants against Vanadium-Induced Toxicity: A Review. Oxid. Med. Cell Longev. 2020;2020:1490316. doi: 10.1155/2020/1490316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Engwa G.A., Ferdinand P.U., Nwalo F.N., Unachukwu M.N. Poisoning in the Modern World-New Tricks for an Old Dog? Volume 5. IntechOpen; London, UK: 2019. Mechanism and health effects of heavy metal toxicity in humans; pp. 77–100. [Google Scholar]
- 83.Verma R.P. Titanium based biomaterial for bone implants: A mini review. Mater. Today Proc. 2020;26:3148–3151. doi: 10.1016/j.matpr.2020.02.649. [DOI] [Google Scholar]
- 84.Çaha I., Alves A.C., Rocha L.A., Toptan F. A Review on Bio-functionalization of β-Ti Alloys. J. Bio-Tribo-Corros. 2020;6:135. doi: 10.1007/s40735-020-00432-0. [DOI] [Google Scholar]
- 85.Hussein M.A., Mohammed A.S., Al-Aqeeli N. Wear Characteristics of Metallic Biomaterials: A Review. Materials. 2015;8:2749–2768. doi: 10.3390/ma8052749. [DOI] [Google Scholar]
- 86.Shuai C., Li S., Peng S., Feng P., Lai Y., Gao C. Biodegradable metallic bone implants. Mater. Chem. Front. 2019;3:544–562. doi: 10.1039/C8QM00507A. [DOI] [Google Scholar]
- 87.Kraus T., Moszner F., Fischerauer S., Fiedler M., Martinelli E., Eichler J., Witte F., Willbold E., Schinhammer M., Meischel M., et al. Biodegradable Fe-based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014;10:3346–3353. doi: 10.1016/j.actbio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Ulum M.F., Nasution A.K., Yusop A.H., Arafat A., Kadir M.R.A., Juniantito V., Noviana D., Hermawan H. Evidences of in vivo bioactivity of Fe-bioceramic composites for temporary bone implants. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103:1354–1365. doi: 10.1002/jbm.b.33315. [DOI] [PubMed] [Google Scholar]
- 89.Yang Y., Yang M., He C., Qi F., Wang D., Peng S., Shuai C. Rare earth improves strength and creep resistance of additively manufactured Zn implants. Compos. B Eng. 2021;216:108882. doi: 10.1016/j.compositesb.2021.108882. [DOI] [Google Scholar]
- 90.Yang Y., Cheng Y., Peng S., Xu L., He C., Qi F., Zhao M., Shuai C. Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold. Bioact. Mater. 2021;6:1230–1241. doi: 10.1016/j.bioactmat.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H.F., Shi Z.Z., Wang L.N. Opportunities and challenges of biodegradable Zn-based alloys. J. Mater. Sci. Technol. 2020;46:136–138. doi: 10.1016/j.jmst.2019.12.014. [DOI] [Google Scholar]
- 92.Yang Y., Cheng Y., Yang M., Qian G., Peng S., Qi F., Shuai C. Semicoherent strengthens graphene/zinc scaffolds. Mater. Today Nano. 2022;17:100163. doi: 10.1016/j.mtnano.2021.100163. [DOI] [Google Scholar]
- 93.Liu L., Ma H., Gao C., Shuai C., Peng S. Island-to-acicular alteration of second phase enhances the degradation resistance of biomedical AZ61 alloy. J. Alloys Compd. 2020;835:155397. doi: 10.1016/j.jallcom.2020.155397. [DOI] [Google Scholar]
- 94.Kang M.-H., Lee H., Jang T.-S., Seong Y.-J., Kim H.-E., Koh Y.-H., Song J., Jung H.-D. Biomimetic porous Mg with tunable mechanical properties and biodegradation rates for bone regeneration. Acta Biomater. 2019;84:453–467. doi: 10.1016/j.actbio.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 95.Zhu D., Cockerill I., Su Y., Zhang Z., Fu J., Lee K.-W., Ma J., Okpokwasili C., Tang L., Zheng Y., et al. Mechanical strength, biodegradation, and in vitro and in vivo biocompatibility of Zn biomaterials. ACS Appl. Mater. Interfaces. 2019;11:6809–6819. doi: 10.1021/acsami.8b20634. [DOI] [PubMed] [Google Scholar]
- 96.Li S., Mo Y., Gao C., Shuai C., Peng S. A dual redox system for enhancing the biodegradability of Fe-C-Cu composite scaffold. Colloids Surf. B Biointerfaces. 2022;213:112431. doi: 10.1016/j.colsurfb.2022.112431. [DOI] [PubMed] [Google Scholar]
- 97.Prokoshkin S., Pustov Y., Zhukova Y., Kadirov P., Dubinskiy S., Sheremetyev V., Karavaeva M. Effect of Thermomechanical Treatment on Functional Properties of Biodegradable Fe-30Mn-5Si Shape Memory Alloy. Met. Mater. Trans. A Phys. Met. Mater. Sci. 2021;52:2024–2032. doi: 10.1007/s11661-021-06217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Banerjee P.C., Al-Saadi S., Choudhary L., Harandi S.E., Singh R. Magnesium implants: Prospects and challenges. Materials. 2019;12:136. doi: 10.3390/ma12010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kamrani S., Fleck C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. BioMetals. 2019;32:185–193. doi: 10.1007/s10534-019-00170-y. [DOI] [PubMed] [Google Scholar]
- 100.Tsakiris V., Tardei C., Clicinschi F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloy. 2021;9:1884–1905. doi: 10.1016/j.jma.2021.06.024. [DOI] [Google Scholar]
- 101.Gao X., Dai C.Y., Jia Q., Zhai C., Shi H., Yang Y., Zhao B.C., Cai H., Lee E.-S., Jiang H.B. In vivo corrosion behavior of biodegradable magnesium alloy by MAF treatment. Scanning. 2021;2021:5530788. doi: 10.1155/2021/5530788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li S., Ren J., Wang X., Ding Y., Li P., Hu Y., Yang Y. Dilemmas and countermeasures of Fe-based biomaterials for next-generation bone implants. J. Mater. Res. Technol. 2022;20:2034–2050. doi: 10.1016/j.jmrt.2022.07.089. [DOI] [Google Scholar]
- 103.Xu C., Yu S., Wu W., Liu Q., Ren L. Direct ink writing of Fe bone implants with independently adjustable structural porosity and mechanical properties. Addit. Manuf. 2022;51:102589. doi: 10.1016/j.addma.2021.102589. [DOI] [Google Scholar]
- 104.Heiden M., Walker E., Stanciu L. Magnesium, iron and zinc alloys, the trifecta of bioresorbable orthopaedic and vascular implantation-a review. J. Biotechnol. Biomater. 2015;5:1. [Google Scholar]
- 105.Purnama A., Hermawan H., Couet J., Mantovani D. Assessing the biocompatibility of degradable metallic materials: State-of-the-art and focus on the potential of genetic regulation. Acta Biomater. 2010;6:1800–1807. doi: 10.1016/j.actbio.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 106.Hermawan H. Biodegradable Metals. Springer; Berlin/Heidelberg, Germany: 2012. Biodegradable metals: State of the art; pp. 13–22. [Google Scholar]
- 107.Wegener B., Sievers B., Utzschneider S., Müller P., Jansson V., Rößler S., Nies B., Stephani G., Kieback B., Quadbeck P. Microstructure, cytotoxicity and corrosion of powder-metallurgical iron alloys for biodegradable bone replacement materials. Mater. Sci. Eng. B. 2011;176:1789–1796. doi: 10.1016/j.mseb.2011.04.017. [DOI] [Google Scholar]
- 108.Gorejová R., Haverová L., Oriňaková R., Oriňak A., Oriňak M. Recent advancements in Fe-based biodegradable materials for bone repair. J. Mater. Sci. 2019;54:1913–1947. doi: 10.1007/s10853-018-3011-z. [DOI] [Google Scholar]
- 109.Liu B., Zheng Y.F., Ruan L. In vitro investigation of Fe30Mn6Si shape memory alloy as potential biodegradable metallic material. Mater. Lett. 2011;65:540–543. doi: 10.1016/j.matlet.2010.10.068. [DOI] [Google Scholar]
- 110.Vojtech D., Kubasek J., Capek J., Pospisilova I. Comparative mechanical and corrosion studies on magnesium, zinc and iron alloys as biodegradable metals. Mater. Tehnol. 2015;49:877–882. doi: 10.17222/mit.2014.129. [DOI] [Google Scholar]
- 111.Cheng J., Liu B., Wu Y.H., Zheng Y.F. Comparative in vitro study on pure metals (Fe, Mn, Mg, Zn and W) as biodegradable metals. J. Mater Sci. Technol. 2013;29:619–627. doi: 10.1016/j.jmst.2013.03.019. [DOI] [Google Scholar]
- 112.Zhang E., Chen H., Shen F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010;21:2151–2163. doi: 10.1007/s10856-010-4070-0. [DOI] [PubMed] [Google Scholar]
- 113.Jia P., Wang Z., Zhang Y., Zhang D., Gao W., Su Y., Li Y., Yang C. Selective sensing of Fe3+ ions in aqueous solution by a biodegradable platform based lanthanide metal organic framework. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020;230:118084. doi: 10.1016/j.saa.2020.118084. [DOI] [PubMed] [Google Scholar]
- 114.Kabir H., Munir K., Wen C., Li Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021;6:836–879. doi: 10.1016/j.bioactmat.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ulum M., Arafat A., Noviana D., Yusop A., Nasution A., Kadir M.A., Hermawan H. In vitro and in vivo degradation evaluation of novel iron-bioceramic composites for bone implant applications. Mater. Sci. Eng. C. 2014;36:336–344. doi: 10.1016/j.msec.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 116.Carluccio D., Xu C., Venezuela J., Cao Y., Kent D., Bermingham M., Demir A.G., Previtali B., Ye Q., Dargusch M. Additively manufactured iron-manganese for biodegradable porous load-bearing bone scaffold applications. Acta Biomater. 2020;103:346–360. doi: 10.1016/j.actbio.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 117.Schinhammer M., Hänzi A.C., Löffler J.F., Uggowitzer P.J. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010;6:1705–1713. doi: 10.1016/j.actbio.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 118.Liu B., Zheng Y.F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011;7:1407–1420. doi: 10.1016/j.actbio.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 119.Sezer N., Evis Z., Kayhan S.M., Tahmasebifar A., Koç M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018;6:23–43. doi: 10.1016/j.jma.2018.02.003. [DOI] [Google Scholar]
- 120.Wang S., Xu Y., Zhou J., Li H., Chang J., Huan Z. In vitro degradation and surface bioactivity of iron-matrix composites containing silicate-based bioceramic. Bioact. Mater. 2017;2:10–18. doi: 10.1016/j.bioactmat.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feng Q., Zhang D., Xin C., Liu X., Lin W., Zhang W., Chen S., Sun K. Characterization and in vivo evaluation of a bio-corrodible nitrided iron stent. J. Mater. Sci. Mater. Med. 2013;24:713–724. doi: 10.1007/s10856-012-4823-z. [DOI] [PubMed] [Google Scholar]
- 122.Yuan W., Xia D., Wu S., Zheng Y., Guan Z., Rau J.V. A review on current research status of the surface modification of Zn-based biodegradable metals. Bioact. Mater. 2022;7:192–216. doi: 10.1016/j.bioactmat.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pierson D., Edick J., Tauscher A., Pokorney E., Bowen P., Gelbaugh J., Stinson J., Getty H., Lee C.H., Drelich J., et al. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100:58–67. doi: 10.1002/jbm.b.31922. [DOI] [PubMed] [Google Scholar]
- 124.Dunne C.F., Levy G.K., Hakimi O., Aghion E., Twomey B., Stanton K.T. Corrosion behaviour of biodegradable magnesium alloys with hydroxyapatite coatings. Surf. Coat. Technol. 2016;289:37–44. doi: 10.1016/j.surfcoat.2016.01.045. [DOI] [Google Scholar]
- 125.Levy G., Aghion E. Effect of diffusion coating of Nd on the corrosion resistance of biodegradable Mg implants in simulated physiological electrolyte. Acta Biomater. 2013;9:8624–8630. doi: 10.1016/j.actbio.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 126.Song G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007;49:1696–1701. doi: 10.1016/j.corsci.2007.01.001. [DOI] [Google Scholar]
- 127.Aghion E., Levy G. The effect of Ca on the in vitro corrosion performance of biodegradable Mg-Nd-Y-Zr alloy. J. Mater. Sci. 2010;45:3096–3101. doi: 10.1007/s10853-010-4317-7. [DOI] [Google Scholar]
- 128.Hennig B., Toborek M., Mcclain C.J. Antiatherogenic Properties of Zinc: Implications in Endothelial Cell Metabolism. Nutrition. 1996;12:711–717. doi: 10.1016/S0899-9007(96)00125-6. [DOI] [PubMed] [Google Scholar]
- 129.Plum L.M., Rink L., Hajo H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hambidge K.M., Krebs N.F. Zinc Deficiency: A Special Challenge. J. Nutr. 2007;137:1101–1105. doi: 10.1093/jn/137.4.1101. [DOI] [PubMed] [Google Scholar]
- 131.Jia B., Yang H., Han Y., Zhang Z., Qu X., Zhuang Y., Wu Q., Zheng Y., Dai K. In vitro and in vivo studies of Zn-Mn biodegradable metals designed for orthopedic applications. Acta Biomater. 2020;108:358–372. doi: 10.1016/j.actbio.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 132.Piao F., Yokoyama K., Ma N., Yamauchi T. Subacute toxic effects of zinc on various tissues and organs of rats. Toxicol. Lett. 2003;145:28–35. doi: 10.1016/S0378-4274(03)00261-3. [DOI] [PubMed] [Google Scholar]
- 133.Drelich A.J., Bowen P.K., LaLonde L., Goldman J., Drelich J.W. Importance of oxide film in endovascular biodegradable zinc stents. Surf. Innov. 2016;4:133–140. doi: 10.1680/jsuin.16.00014. [DOI] [Google Scholar]
- 134.Levy G.K., Leon A., Kafri A., Ventura Y., Drelich J.W., Goldman J., Vago R., Aghion E. Evaluation of biodegradable Zn-1%Mg and Zn-1%Mg-0.5%Ca alloys for biomedical applications. J. Mater Sci. Mater. Med. 2017;28:174. doi: 10.1007/s10856-017-5973-9. [DOI] [PubMed] [Google Scholar]
- 135.Levy G.K., Goldman J., Aghion E. The prospects of zinc as a structural material for biodegradable implants—A review paper. Metals. 2017;7:402. doi: 10.3390/met7100402. [DOI] [Google Scholar]
- 136.Bakhsheshi-Rad H.R., Hamzah E., Low H.T., Cho M.H., Kasiri-Asgarani M., Farahany S., Mostafa A., Medraj M. Thermal characteristics, mechanical properties, in vitro degradation and cytotoxicity of novel biodegradable Zn-Al-Mg and Zn-Al-Mg-xBi alloys. Acta Metall. Sin. 2017;30:201–211. doi: 10.1007/s40195-017-0534-2. [DOI] [Google Scholar]
- 137.Zhao S., Seitz J.-M., Eifler R., Maier H.J., Guillory R.J., Earley E.J., Drelich A., Goldman J., Drelich J.W. Zn-Li alloy after extrusion and drawing: Structural, mechanical characterization, and biodegradation in abdominal aorta of rat. Mater. Sci. Eng. C. 2017;76:301–312. doi: 10.1016/j.msec.2017.02.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Y.T., Fang Q., Zhang L.I., Tao H.W. Spatial Asymmetry and Short-Term Suppression Underlie Direction Selectivity of Synaptic Excitation in the Mouse Visual Cortex. Cereb. Cortex vol. 2018;28:2059–2070. doi: 10.1093/cercor/bhx111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bakhsheshi-Rad H., Hamzah E., Low H., Kasiri-Asgarani M., Farahany S., Akbari E., Cho M. Fabrication of biodegradable Zn-Al-Mg alloy: Mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater. Sci. Eng. C. 2017;73:215–219. doi: 10.1016/j.msec.2016.11.138. [DOI] [PubMed] [Google Scholar]
- 140.El-Khair M.T.A., Daoud A., Ismail A. Effect of different Al contents on the microstructure, tensile and wear properties of Zn-based alloy. Mater. Lett. 2004;58:1754–1760. doi: 10.1016/j.matlet.2003.10.058. [DOI] [Google Scholar]
- 141.Fernández-Lizárraga M., García-López J., Rodil S.E., Ribas-Aparicio R.M., Silva-Bermudez P. Evaluation of the biocompatibility and osteogenic properties of metal oxide coatings applied by magnetron sputtering as potential biofunctional surface modifications for orthopedic implants. Materials. 2022;15:5240. doi: 10.3390/ma15155240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sarraf M., Nasiri-Tabrizi B., Yeong C.H., Hosseini H.R.M., Saber-Samandari S., Basirun W.J., Tsuzuki T. Mixed oxide nanotubes in nanomedicine: A dead-end or a bridge to the future? Ceram. Int. 2021;47:2917–2948. doi: 10.1016/j.ceramint.2020.09.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tipan N., Pandey A., Mishra P. Selection and preparation strategies of Mg-alloys and other biodegradable materials for orthopaedic applications: A review. Mater. Today Commun. 2022;31:103658. doi: 10.1016/j.mtcomm.2022.103658. [DOI] [Google Scholar]
- 144.Wang L., Ding X., Feng W., Gao Y., Zhao S., Fan Y. Biomechanical study on implantable and interventional medical devices. Acta Mech. Sin. 2021;37:875–894. doi: 10.1007/s10409-021-01116-9. [DOI] [Google Scholar]
- 145.Yadav R., Meena A., Patnaik A. Biomaterials for dental composite applications: A comprehensive review of physical, chemical, mechanical, thermal, tribological, and biological properties. Polym. Adv. Technol. 2022;33:1762–1781. doi: 10.1002/pat.5648. [DOI] [Google Scholar]
- 146.Pesode P., Barve S. Surface modification of titanium and titanium alloy by plasma electrolytic oxidation process for biomedical applications: A review. Mater. Today Proc. 2021;46:594–602. doi: 10.1016/j.matpr.2020.11.294. [DOI] [Google Scholar]
- 147.Sanchez A.G., Katunar M.R., Pastore J.I., de la Hoz M.F.T., Ceré S. Evaluation of annealed titanium oxide nanotubes on titanium: From surface characterization to in vivo assays. J. Biomed. Mater. Res. A. 2021;109:1088–1100. doi: 10.1002/jbm.a.37101. [DOI] [PubMed] [Google Scholar]
- 148.Blendinger F., Seitz D., Ottenschlager A., Fleischer M., Bucher V. Atomic layer deposition of bioactive TiO2 thin films on polyetheretherketone for orthopedic implants. ACS Appl. Mater Interfaces. 2021;13:3536–3546. doi: 10.1021/acsami.0c17990. [DOI] [PubMed] [Google Scholar]
- 149.Hazwani M.R.S.N., Lim L.X., Lockman Z., Zuhailawati H. Fabrication of titanium-based alloys with bioactive surface oxide layer as biomedical implants: Opportunity and challenges. Trans. Nonferrous Met. Soc. China. 2022;32:1–44. doi: 10.1016/S1003-6326(21)65776-X. [DOI] [Google Scholar]
- 150.Prestat M., Thierry D. Corrosion of titanium under simulated inflammation conditions: Clinical context and in vitro investigations. Acta Biomater. 2021;136:72–87. doi: 10.1016/j.actbio.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 151.Vrchovecká K., Weiser A., Pribyl J., Kuta J., Holzer J., Pavkova-Goldbergova M., Sobola D., Dlouhy A. A release of Ti-ions from nanostructured titanium oxide surfaces. Surf. Interfaces. 2022;29:101699. doi: 10.1016/j.surfin.2021.101699. [DOI] [Google Scholar]
- 152.Xu A., Alhamad M., Ramachandran R.A., Shukla A., Barão V.A., Sukotjo C., Mathew M.T. Peri-Implantitis in Relation to Titanium Corrosion: Current Status and Future Perspectives. J. Bio-Tribo-Corros. 2022;8:46. doi: 10.1007/s40735-022-00644-6. [DOI] [Google Scholar]
- 153.Comino-Garayoa R., Brinkmann J.C.-B., Peláez J., López-Suárez C., Martínez-González J.M., Suárez M.J. Allergies to titanium dental implants: What do we really know about them? A scoping review. Biology. 2020;9:404. doi: 10.3390/biology9110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ding Z., Tang Y., Liu L., Ding Z., Tan Y., He Q. Improving the adhesive, mechanical, tribological properties and corrosion resistance of reactive sputtered tantalum oxide coating on Ti6Al4V alloy via introducing multiple interlayers. Ceram. Int. 2022;48:5983–5994. doi: 10.1016/j.ceramint.2021.11.134. [DOI] [Google Scholar]
- 155.Mani G., Porter D., Grove K., Collins S., Ornberg A., Shulfer R. A comprehensive review of biological and materials properties of Tantalum and its alloys. J. Biomed. Mater. Res. A. 2022;110:1291–1306. doi: 10.1002/jbm.a.37373. [DOI] [PubMed] [Google Scholar]
- 156.Fomina M., Koshuro V., Shumilin A., Voyko A., Zakharevich A., Skaptsov A., Steinhauer A., Fomin A. Functionally graded ‘Ti-base+(Ta, Ta2O5)-coatings’ structure and its production using induction heat treatment. Compos. Struct. 2020;234:111688. doi: 10.1016/j.compstruct.2019.111688. [DOI] [Google Scholar]
- 157.de Almeida Bino M.C., Eurídice W.A., Gelamo R.V., Leite N.B., da Silva M.V., de Siervo A., Pinto M.R., Buranello P.A.D.A., Moreto J.A. Structural and morphological characterization of Ti6Al4V alloy surface functionalization based on Nb2O5 thin film for biomedical applications. Appl. Surf. Sci. 2021;557:149739. doi: 10.1016/j.apsusc.2021.149739. [DOI] [Google Scholar]
- 158.Huang H.-L., Tsai M.-T., Chang Y.-Y., Lin Y.-J., Hsu J.-T. Fabrication of a Novel Ta (Zn) O Thin Film on Titanium by Magnetron Sputtering and Plasma Electrolytic Oxidation for Cell Biocompatibilities and Antibacterial Applications. Metals. 2020;10:649. doi: 10.3390/met10050649. [DOI] [Google Scholar]
- 159.Horandghadim N., Khalil-Allafi J., Urgen M. Effect of Ta2O5 content on the osseointegration and cytotoxicity behaviors in hydroxyapatite-Ta2O5 coatings applied by EPD on superelastic NiTi alloys. Mater. Sci. Eng. C. 2019;102:683–695. doi: 10.1016/j.msec.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 160.Fialho L., Grenho L., Fernandes M.H., Carvalho S. Porous tantalum oxide with osteoconductive elements and antibacterial core-shell nanoparticles: A new generation of materials for dental implants. Mater. Sci. Eng. C. 2021;120:111761. doi: 10.1016/j.msec.2020.111761. [DOI] [PubMed] [Google Scholar]
- 161.Alves C.F.A., Fialho L., Marques S., Pires S., Rico P., Palacio C., Carvalho S. MC3T3-E1 cell response to microporous tantalum oxide surfaces enriched with Ca, P and Mg. Mater. Sci. Eng. C. 2021;124:112008. doi: 10.1016/j.msec.2021.112008. [DOI] [PubMed] [Google Scholar]
- 162.Peron M., Cogo S., Bjelland M., Bin Afif A., Dadlani A., Greggio E., Berto F., Torgersen J. On the evaluation of ALD TiO2, ZrO2 and HfO2 coatings on corrosion and cytotoxicity performances. J. Magnes. Alloy. 2021;9:1806–1819. doi: 10.1016/j.jma.2021.03.010. [DOI] [Google Scholar]
- 163.Peron M., Bertolini R., Cogo S. On the corrosion, stress corrosion and cytocompatibility performances of ALD TiO2 and ZrO2 coated magnesium alloys. J. Mech. Behav. Biomed. Mater. 2022;125:104945. doi: 10.1016/j.jmbbm.2021.104945. [DOI] [PubMed] [Google Scholar]
- 164.Yenagolla P., Sandeep K., Sharma J.V.S. EPRA International Journal of Research and Development (IJRD) Natural Polymers and Its Applications—A Review. Epra Int. J. Res. Dev. (IJRD) 2022;7838:6–15. [Google Scholar]
- 165.Kumar V., Jule L.T., Ramaswamy K. Conducting Polymers for Advanced Energy Applications. CRC Press; Boca Raton, FL, USA: 2021. Conducting Polymers for Organic Solar Cell Application; p. 139. [Google Scholar]
- 166.Ilyas R.A., Sapuan S.M. The Preparation Methods and Processing of Natural Fibre Bio-polymer Composites. Curr. Org. Synth. 2020;16:1068–1070. doi: 10.2174/157017941608200120105616. [DOI] [PubMed] [Google Scholar]
- 167.Chahal S., Kumar A., Hussian F.S.J. Development of biomimetic electrospun polymeric biomaterials for bone tissue engineering. A review. J. Biomater. Sci. Polym. Ed. 2019;30:1308–1355. doi: 10.1080/09205063.2019.1630699. [DOI] [PubMed] [Google Scholar]
- 168.James D.M.B. Remodeling Life and Living—A Review of Advanced Polymeric Materials. Int. J. Res. Appl. Sci. Eng. Technol. 2020;8:396–400. doi: 10.22214/ijraset.2020.5066. [DOI] [Google Scholar]
- 169.John C.B. Sir Harold Ridley and the intraocular foreign body that made history. Kerala J. Ophthalmol. 2020;32:315–320. doi: 10.4103/kjo.kjo_160_20. [DOI] [Google Scholar]
- 170.Fitzgerald J.K. Advances in Biomaterials. CRC Press; Boca Raton, FL, USA: 2021. Silicone Contact Lenses; pp. 209–216. [Google Scholar]
- 171.Wijesinghe W.P.S.L., Mantilaka M.M.M.G.P.G., Karunarathne T.S.E.F., Rajapakse R.M.G. Synthesis of a hydroxyapatite/poly(methyl methacrylate) nanocomposite using dolomite. Nanoscale Adv. 2019;1:86–88. doi: 10.1039/C8NA00006A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sedlak J., Vocilka O., Slany M., Chladil J., Polzer A., Varhanik M. Design and production of eye prosthesis using 3D printing. MM Sci. J. 2020;2020:3806–3812. doi: 10.17973/MMSJ.2020_03_2019127. [DOI] [Google Scholar]
- 173.Matsuda Y., Karino M., Okui T., Kanno T. Complications of Poly-l-Lactic Acid and Polyglycolic Acid (PLLA/PGA) Osteosynthesis Systems for Maxillofacial Surgery: A Retrospective Clinical Investigation. Polymers. 2021;13:889. doi: 10.3390/polym13060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reddy M.S.B., Ponnamma D., Choudhary R., Sadasivuni K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers. 2021;13:1105. doi: 10.3390/polym13071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hassan M.E., Bai J., Dou D.Q. Biopolymers; Definition, classification and applications. Egypt. J. Chem. 2019;62:1725–1737. doi: 10.21608/ejchem.2019.6967.1580. [DOI] [Google Scholar]
- 176.Hacker M.C., Krieghoff J., Mikos A.G. Principles of Regenerative Medicine. Elsevier; Amsterdam, The Netherlands: 2019. Synthetic polymers; pp. 559–590. [Google Scholar]
- 177.Rodríguez G.R., Patrício T., López J.D. Natural Polymers for Bone Repair. 2nd ed. Elsevier Ltd.; Amsterdam, The Netherlands: 2019. [DOI] [Google Scholar]
- 178.Hench L.L., Polak J.M. Third-generation biomedical materials. Science. 2002;295:1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 179.Saha S., Pal S. Mechanical properties of bone cement: A review. J. Biomed. Mater. Res. 1984;18:435–462. doi: 10.1002/jbm.820180411. [DOI] [PubMed] [Google Scholar]
- 180.Kenny S.M., Buggy M. Bone cements and fillers: A review. J. Mater. Sci. Mater. Med. 2003;14:923–938. doi: 10.1023/A:1026394530192. [DOI] [PubMed] [Google Scholar]
- 181.Bettencourt A., Almeida A.J. Poly (methyl methacrylate) particulate carriers in drug delivery. J. Microencapsul. 2012;29:353–367. doi: 10.3109/02652048.2011.651500. [DOI] [PubMed] [Google Scholar]
- 182.Webb J.C.J., Spencer R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surg. Br. 2007;89:851–857. doi: 10.1302/0301-620X.89B7.19148. [DOI] [PubMed] [Google Scholar]
- 183.Senra M.R., Marques M.d.F.V. Synthetic polymeric materials for bone replacement. J. Compos. Sci. 2020;4:191. doi: 10.3390/jcs4040191. [DOI] [Google Scholar]
- 184.Bistolfi A., Ferracini R., Albanese C., Vernè E., Miola M. PMMA-based bone cements and the problem of joint arthroplasty infections: Status and new perspectives. Materials. 2019;12:4002. doi: 10.3390/ma12234002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bistolfi A., Massazza G., Verné E., Massè A., Deledda D., Ferraris S., Miola M., Galetto F., Crova M. Antibiotic-loaded cement in orthopedic surgery: A review. Int. Sch. Res. Not. 2011;2011:290851. doi: 10.5402/2011/290851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ferraris S., Miola M., Bistolfi A., Fucale G., Crova M., Masse’ A., Verné E. In vitro comparison between commercially and manually mixed antibiotic-loaded bone cements. J. Appl. Biomater. Biomech. 2010;8:166–174. doi: 10.5301/JABB.2010.6068. [DOI] [PubMed] [Google Scholar]
- 187.Chen B., Wang J., Yan F. Comparative investigation on the tribological behaviors of CF/PEEK composites under sea water lubrication. Tribol. Int. 2012;52:170–177. doi: 10.1016/j.triboint.2012.03.017. [DOI] [Google Scholar]
- 188.Patel P., Stec A.A., Hull T.R., Naffakh M., Diez-Pascual A.M., Ellis G., Safronava N., Lyon R.E. Flammability properties of PEEK and carbon nanotube composites. Polym. Degrad. Stab. 2012;97:2492–2502. doi: 10.1016/j.polymdegradstab.2012.07.013. [DOI] [Google Scholar]
- 189.Li E.Z., Guo W.L., Wang H.D., Xu B.S., Liu X.T. Research on Tribological Behavior of PEEK and Glass Fiber Reinforced PEEK Composite. Phys. Procedia. 2013;50:453–460. doi: 10.1016/j.phpro.2013.11.071. [DOI] [Google Scholar]
- 190.Kurtz S.M. PEEK Biomaterials Handbook. Elsevier; Amsterdam, The Netherlands: 2012. Chemical and radiation stability of PEEK; pp. 75–79. [Google Scholar]
- 191.Normand B., Takenouti H., Keddam M., Liao H., Monteil G., Coddet C. Electrochemical impedance spectroscopy and dielectric properties of polymer: Application to PEEK thermally sprayed coating. Electrochim. Acta. 2004;49:2981–2986. doi: 10.1016/j.electacta.2004.01.057. [DOI] [Google Scholar]
- 192.Zhang G., Li W.-Y., Cherigui M., Zhang C., Liao H., Bordes J.-M., Coddet C. Structures and tribological performances of PEEK (poly-ether-ether-ketone)-based coatings designed for tribological application. Prog. Org. Coat. 2007;60:39–44. doi: 10.1016/j.porgcoat.2007.06.004. [DOI] [Google Scholar]
- 193.Kurtz S.M. PEEK Biomaterials Handbook. Elsevier; Amsterdam, The Netherlands: 2012. Synthesis and processing of PEEK for surgical implants; pp. 9–22. [Google Scholar]
- 194.Zheng Y., Liu L., Ma Y., Xiao L., Liu Y. Enhanced osteoblasts responses to surface-sulfonated polyetheretherketone via a single-step ultraviolet-initiated graft polymerization. Ind. Eng. Chem. Res. 2018;57:10403–10410. doi: 10.1021/acs.iecr.8b02158. [DOI] [Google Scholar]
- 195.Ma R., Guo D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019;14:1–13. doi: 10.1186/s13018-019-1069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Johansson P., Jimbo R., Naito Y., Kjellin P., Currie F., Wennerberg A. Polyether ether ketone implants achieve increased bone fusion when coated with nano-sized hydroxyapatite: A histomorphometric study in rabbit bone. Int. J. Nanomed. 2016;11:1435. doi: 10.2147/IJN.S100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Khoury J., Selezneva I., Pestov S., Tarassov V., Ermakov A., Mikheev A., Lazov M., Kirkpatrick S.R., Shashkov D., Smolkov A. Surface bioactivation of PEEK by neutral atom beam technology. Bioact. Mater. 2019;4:132–141. doi: 10.1016/j.bioactmat.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Terpiłowski K., Wiącek A.E., Jurak M. Influence of nitrogen plasma treatment on the wettability of polyetheretherketone and deposited chitosan layers. Adv. Polym. Technol. 2018;37:1557–1569. doi: 10.1002/adv.21813. [DOI] [Google Scholar]
- 199.Lu T., Qian S., Meng F., Ning C., Liu X. Enhanced osteogenic activity of poly ether ether ketone using calcium plasma immersion ion implantation. Colloids Surf. B Biointerfaces. 2016;142:192–198. doi: 10.1016/j.colsurfb.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 200.Zhang J., Wei W., Yang L., Pan Y., Wang X., Wang T., Tang S., Yao Y., Hong H., Wei J. Stimulation of cell responses and bone ingrowth into macro-microporous implants of nano-bioglass/polyetheretherketone composite and enhanced antibacterial activity by release of hinokitiol. Colloids Surf. B Biointerfaces. 2018;164:347–357. doi: 10.1016/j.colsurfb.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 201.Zheng Y., Liu L., Xiao L., Zhang Q., Liu Y. Enhanced osteogenic activity of phosphorylated polyetheretherketone via surface-initiated grafting polymerization of vinylphosphonic acid. Colloids Surf. B Biointerfaces. 2019;173:591–598. doi: 10.1016/j.colsurfb.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 202.Ma R., Tang T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014;15:5426–5445. doi: 10.3390/ijms15045426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kasemo B. Biological surface science. Surf. Sci. 2002;500:656–677. doi: 10.1016/S0039-6028(01)01809-X. [DOI] [Google Scholar]
- 204.Almasi D., Iqbal N., Sadeghi M., Sudin I., Kadir M.R.A., Kamarul T. Preparation methods for improving PEEK’s bioactivity for orthopedic and dental application: A review. Int. J. Biomater. 2016;2016:8202653. doi: 10.1155/2016/8202653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Poulsson A.H.C., Eglin D., Richards R.G. PEEK Biomaterials Handbook. Elsevier; Amsterdam, The Netherlands: 2019. Surface modification techniques of PEEK, including plasma surface treatment; pp. 179–201. [Google Scholar]
- 206.Poulsson A.H.C., Richards R.G. PEEK Biomaterials Handbook. Elsevier; Amsterdam, The Netherlands: 2012. Surface modification techniques of polyetheretherketone, including plasma surface treatment; pp. 145–161. [Google Scholar]
- 207.Wang H., Lu T., Meng F., Zhu H., Liu X. Enhanced osteoblast responses to poly ether ether ketone surface modified by water plasma immersion ion implantation. Colloids Surf. B Biointerfaces. 2014;117:89–97. doi: 10.1016/j.colsurfb.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 208.Visakh P.M. Polyaniline Blends, Composites, and Nanocomposites. Elsevier; Amsterdam, The Netherlands: 2018. Polyaniline-Based Blends, Composites, and Nanocomposites; pp. 1–22. [DOI] [Google Scholar]
- 209.Paxton N.C., Allenby M.C., Lewis P.M., Woodruff M.A. Biomedical applications of polyethylene. Eur. Polym. J. 2019;118:412–428. doi: 10.1016/j.eurpolymj.2019.05.037. [DOI] [Google Scholar]
- 210.Sobieraj M.C., Rimnac C.M. Ultra high molecular weight polyethylene: Mechanics, morphology, and clinical behavior. J. Mech. Behav. Biomed. Mater. 2009;2:433–443. doi: 10.1016/j.jmbbm.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kurtz S.M. 1-A primer on UHMWPE. UHMWPE Biomaterials Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices. Volume 1. ELSEVIER; London, UK: 2016. pp. 1–6. [Google Scholar]
- 212.Budak K., Sogut O., Sezer U.A. A review on synthesis and biomedical applications of polyglycolic acid. J. Polym. Res. 2020;27:208. doi: 10.1007/s10965-020-02187-1. [DOI] [Google Scholar]
- 213.On S.W., Cho S.W., Byun S.H., Yang B.E. Bioabsorbable osteofixation materials for maxillofacial bone surgery: A review on polymers and magnesium-based materials. Biomedicines. 2020;8:300. doi: 10.3390/biomedicines8090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liu Y., Cao H., Ye L., Coates P., Caton-Rose F., Zhao X. Long-chain branched poly (lactic acid)-b-poly (lactide-co-caprolactone): Structure, viscoelastic behavior, and triple-shape memory effect as smart bone fixation material. Ind. Eng. Chem. Res. 2020;59:4524–4532. doi: 10.1021/acs.iecr.9b06514. [DOI] [Google Scholar]
- 215.Siakeng R., Jawaid M., Ariffin H., Sapuan S.M., Asim M., Saba N. Natural fiber reinforced polylactic acid composites: A review. Polym. Compos. 2019;40:446–463. doi: 10.1002/pc.24747. [DOI] [Google Scholar]
- 216.DeStefano V., Khan S., Tabada A. Applications of PLA in modern medicine. Eng. Regen. 2020;1:76–87. doi: 10.1016/j.engreg.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Naseem R., Tzivelekis C., German M.J., Gentile P., Ferreira A.M., Dalgarno K. Strategies for enhancing polyester-based materials for bone fixation applications. Molecules. 2021;26:992. doi: 10.3390/molecules26040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shirazi R.N., Aldabbagh F., Ronan W., Erxleben A., Rochev Y., McHugh P. Effects of material thickness and processing method on poly (lactic-co-glycolic acid) degradation and mechanical performance. J. Mater. Sci. Mater. Med. 2016;27:154. doi: 10.1007/s10856-016-5760-z. [DOI] [PubMed] [Google Scholar]
- 219.Ramos D.M., Dhandapani R., Subramanian A., Sethuraman S., Kumbar S.G. Clinical complications of biodegradable screws for ligament injuries. Mater. Sci. Eng. C. 2020;109:110423. doi: 10.1016/j.msec.2019.110423. [DOI] [PubMed] [Google Scholar]
- 220.Danhier F., Ansorena E., Silva J.M., Coco R., Le Breton A., Préat V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 221.Lopes M.S., Jardini A.L., Filho R.M. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012;42:1402–1413. doi: 10.1016/j.proeng.2012.07.534. [DOI] [Google Scholar]
- 222.Middleton J.C., Tipton A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–2346. doi: 10.1016/S0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 223.Woodruff M.A., Hutmacher D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35:1217–1256. doi: 10.1016/j.progpolymsci.2010.04.002. [DOI] [Google Scholar]
- 224.Chocholata P., Kulda V., Babuska V. Fabrication of scaffolds for bone-tissue regeneration. Materials. 2019;12:568. doi: 10.3390/ma12040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Aggarwal D., Kumar V., Sharma S. Drug-loaded biomaterials for orthopedic applications: A review. J. Control. Release. 2022;344:113–133. doi: 10.1016/j.jconrel.2022.02.029. [DOI] [PubMed] [Google Scholar]
- 226.Lishchynskyi O., Stetsyshyn Y., Raczkowska J., Awsiuk K., Orzechowska B., Abalymov A., Skirtach A.G., Bernasik A., Nastyshyn S., Budkowski A. Fabrication and impact of fouling-reducing temperature-responsive poegma coatings with embedded caco3 nanoparticles on different cell lines. Materials. 2021;14:1417. doi: 10.3390/ma14061417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sebbe N.P.V., Fernandes F., Sousa V.F.C., Silva F.J.G. Hybrid Manufacturing Processes Used in the Production of Complex Parts: A Comprehensive Review. Metals. 2022;12:1874. doi: 10.3390/met12111874. [DOI] [Google Scholar]
- 228.Kim H.M., Lee D., Kim S.Y. Biocompatibility and osteogenic potential of calcium silicate-based cement combined with enamel matrix derivative: Effects on human bone marrow-derived stem cells. Materials. 2021;14:7750. doi: 10.3390/ma14247750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rochira A., Siculella L., Damiano F., Palermo A., Ferrante F., Carluccio M.A., Calabriso N., Giannotti L., Stanca E. Concentrated growth factors (CGF) induce osteogenic differentiation in human bone marrow stem cells. Biology. 2020;9:370. doi: 10.3390/biology9110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zha Y., Li Y., Lin T., Chen J., Zhang S., Wang J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2020;11:397–409. doi: 10.7150/thno.50741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jiao X., Sun X., Li W., Chu W., Zhang Y., Li Y., Wang Z., Zhou X., Ma J., Xu C., et al. 3D-Printed β-Tricalcium Phosphate Scaffolds Promote Osteogenic Differentiation of Bone Marrow-Deprived Mesenchymal Stem Cells in an N6-methyladenosine-Dependent Manner. Int. J. Bioprint. 2022;8:31–44. doi: 10.18063/ijb.v8i2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhao Y., Xing Y., Wang M., Huang Y., Xu H., Su Y., Zhao Y., Shang Y. Supramolecular Hydrogel Based on an Osteogenic Growth Peptide Promotes Bone Defect Repair. ACS Omega. 2022;7:11395–11404. doi: 10.1021/acsomega.2c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bandyopadhyay A., Gualtieri T., Heer B., Bose S. Additive Manufacturing. CRC Press; Boca Raton, FL, USA: 2019. Introduction to Additive Manufacturing; pp. 1–23. [Google Scholar]
- 234.Eckert J., Grieße T. Additive Manufacturing at Montanuniversität Leoben. Adv. Eng. Mater. 2023;25:2300179. doi: 10.1002/adem.202300179. [DOI] [Google Scholar]
- 235.Muhindo D., Elkanayati R., Srinivasan P., Repka M.A., Ashour E.A. Recent advances in the applications of additive manufacturing (3D printing) in drug delivery: A comprehensive review. AAPS PharmSciTech. 2023;24:57. doi: 10.1208/s12249-023-02524-9. [DOI] [PubMed] [Google Scholar]
- 236.Appel J., Ho D., Dobyns B.M., Reichert W.M.M., Duranty E.R. Electrochemical Society Meeting Abstracts 242. The Electrochemical Society, Inc.; Philadelphia, PA, USA: 2022. Additive Manufacturing of Biopolymers Via Modified FDM 3D Printing Enabled By the Dissolution Properties of Hydrophilic Ionic Liquids; p. 2068. [Google Scholar]
- 237.Pugliese R., Beltrami B., Regondi S., Lunetta C. Polymeric biomaterials for 3D printing in medicine: An overview. Ann. 3d Print. Med. 2021;2:100011. doi: 10.1016/j.stlm.2021.100011. [DOI] [Google Scholar]
- 238.Skylar-Scott M.A., Uzel S.G., Nam L.L., Ahrens J.H., Truby R.L., Damaraju S., Lewis J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019;5:eaaw2459. doi: 10.1126/sciadv.aaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang B., Pei X., Zhou C., Fan Y., Jiang Q., Ronca A., D’Amora U., Chen Y., Li H., Sun Y., et al. The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4V scaffold for load-bearing bone reconstruction. Mater. Des. 2018;152:30–39. doi: 10.1016/j.matdes.2018.04.065. [DOI] [Google Scholar]
- 240.Söhling N., Neijhoft J., Nienhaus V., Acker V., Harbig J., Menz F., Ochs J., Verboket R.D., Ritz U., Blaeser A., et al. 3D-printing of hierarchically designed and osteoconductive bone tissue engineering scaffolds. Materials. 2020;13:1836. doi: 10.3390/ma13081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pei X., Ma L., Zhang B., Sun J., Sun Y., Fan Y., Gou Z., Zhou C., Zhang X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication. 2017;9:045008. doi: 10.1088/1758-5090/aa90ed. [DOI] [PubMed] [Google Scholar]
- 242.Weems A.C., Pérez-Madrigal M.M., Arno M.C., Dove A.P. 3D Printing for the Clinic: Examining Contemporary Polymeric Biomaterials and their Clinical Utility. Biomacromolecules. 2020;21:1037–1059. doi: 10.1021/acs.biomac.9b01539. [DOI] [PubMed] [Google Scholar]
- 243.Attaran M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017;60:677–688. doi: 10.1016/j.bushor.2017.05.011. [DOI] [Google Scholar]
- 244.Stepniak K., Ursani A., Paul N., Naguib H. Novel 3D printing technology for CT phantom coronary arteries with high geometrical accuracy for biomedical imaging applications. Bioprinting. 2020;18:e00074. doi: 10.1016/j.bprint.2020.e00074. [DOI] [Google Scholar]
- 245.Jardini A.L., Larosa M.A., Filho R.M., de Carvalho Zavaglia C.A., Bernardes L.F., Lambert C.S., Calderoni D.R., Kharmandayan P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio-Maxillofac. Surg. 2014;42:1877–1884. doi: 10.1016/j.jcms.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 246.Mobbs R.J., Coughlan M., Thompson R., Sutterlin C.E., Phan K. The utility of 3D printing for surgical planning and patient-specific implant design for complex spinal pathologies: Case report. J. Neurosurg. Spine. 2017;26:513–518. doi: 10.3171/2016.9.SPINE16371. [DOI] [PubMed] [Google Scholar]
- 247.Putra N.E., Mirzaali M.J., Apachitei I., Zhou J., Zadpoor A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020;109:1–20. doi: 10.1016/j.actbio.2020.03.037. [DOI] [PubMed] [Google Scholar]
- 248.Chua K., Khan I., Malhotra R., Zhu D. Additive manufacturing and 3D printing of metallic biomaterials. Eng. Regen. 2021;2:288–299. doi: 10.1016/j.engreg.2021.11.002. [DOI] [Google Scholar]
- 249.Bose S., Vahabzadeh S., Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater. Today. 2013;16:496–504. doi: 10.1016/j.mattod.2013.11.017. [DOI] [Google Scholar]
- 250.Campoli G., Borleffs M.S., Yavari S.A., Wauthle R., Weinans H., Zadpoor A.A. Mechanical properties of open-cell metallic biomaterials manufactured using additive manufacturing. Mater. Design. 2013;49:957–965. doi: 10.1016/j.matdes.2013.01.071. [DOI] [Google Scholar]
- 251.Yavari S.A., Wauthlé R., van der Stok J., Riemslag A.C., Janssen M., Mulier M., Kruth J.-P., Schrooten J., Weinans H., Zadpoor A.A. Fatigue behavior of porous biomaterials manufactured using selective laser melting. Mater. Sci. Eng. C. 2013;33:4849–4858. doi: 10.1016/j.msec.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 252.van Bael S., Chai Y.C., Truscello S., Moesen M., Kerckhofs G., Van Oosterwyck H., Kruth J.-P., Schrooten J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012;8:2824–2834. doi: 10.1016/j.actbio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 253.Fukuda A., Takemoto M., Saito T., Fujibayashi S., Neo M., Pattanayak D.K., Matsushita T., Sasaki K., Nishida N., Kokubo T., et al. Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta Biomater. 2011;7:2327–2336. doi: 10.1016/j.actbio.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 254.Taniguchi N., Fujibayashi S., Takemoto M., Sasaki K., Otsuki B., Nakamura T., Matsushita T., Kokubo T., Matsuda S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C. 2016;59:690–701. doi: 10.1016/j.msec.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 255.Li Y., Zhou J., Pavanram P., Leeflang M., Fockaert L., Pouran B., Tümer N., Schröder K.-U., Mol J.M.C., Weinans H., et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018;67:378–392. doi: 10.1016/j.actbio.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 256.Li Y., Jahr H., Lietaert K., Pavanram P., Yilmaz A., Fockaert L.I., Leeflang M.A., Pouran B., Gonzalez-Garcia Y., Weinans H., et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018;77:380–393. doi: 10.1016/j.actbio.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 257.Shah F.A., Snis A., Matic A., Thomsen P., Palmquist A. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016;30:357–367. doi: 10.1016/j.actbio.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 258.Shah F.A., Omar O., Suska F., Snis A., Matic A., Emanuelsson L., Norlindh B., Lausmaa J., Thomsen P., Palmquist A. Long-term osseointegration of 3D printed CoCr constructs with an interconnected open-pore architecture prepared by electron beam melting. Acta Biomater. 2016;36:296–309. doi: 10.1016/j.actbio.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 259.Ngo T.D., Kashani A., Imbalzano G., Nguyen K.T.Q., Hui D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018;143:172–196. doi: 10.1016/j.compositesb.2018.02.012. [DOI] [Google Scholar]
- 260.Bhushan J., Grover V. Biomanufacturing. Springer International Publishing; Cham, Switzerland: 2019. Additive manufacturing: Current concepts, methods, and applications in oral health care; pp. 103–122. [DOI] [Google Scholar]
- 261.Mohan D., Teong Z.K., Bakir A.N., Sajab M.S., Kaco H. Extending cellulose-based polymers application in additive manufacturing technology: A review of recent approaches. Polymers. 2020;12:1876. doi: 10.3390/polym12091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lewis J.A. Direct ink writing of 3D functional materials. Adv. Funct. Mater. 2006;16:2193–2204. doi: 10.1002/adfm.200600434. [DOI] [Google Scholar]
- 263.Bikas H., Stavropoulos P., Chryssolouris G. Additive manufacturing methods and modeling approaches: A critical review. Int. J. Adv. Manuf. Technol. 2016;83:389–405. doi: 10.1007/s00170-015-7576-2. [DOI] [Google Scholar]
- 264.Park S., Shou W., Makatura L., Matusik W., Fu K.K. 3D printing of polymer composites: Materials, processes, and applications. Matter. 2022;5:43–76. doi: 10.1016/j.matt.2021.10.018. [DOI] [Google Scholar]
- 265.Rajpurkar P., Chen E., Banerjee O., Topol E.J. AI in health and medicine. Nat. Med. 2022;28:31–38. doi: 10.1038/s41591-021-01614-0. [DOI] [PubMed] [Google Scholar]
- 266.Zwawi M. Recent advances in bio-medical implants; mechanical properties, surface modifications and applications. Eng. Res. Express. 2022;4:032003. doi: 10.1088/2631-8695/ac8ae2. [DOI] [Google Scholar]
- 267.Jafari M., Paknejad Z., Rad M.R., Motamedian S.R., Eghbal M.J., Nadjmi N., Khojasteh A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017;105:431–459. doi: 10.1002/jbm.b.33547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.