Abstract
Background
Recent large clinical trials have demonstrated cardiovascular benefits of similar overall magnitude for sodium–glucose cotransporter-2 inhibitor (SGLT-2i) and glucagon-like peptide-1 receptor agonist (GLP-1RA) therapy in subjects with type 2 diabetes. We sought to identify subgroups based on baseline characteristics with a differential response to either SGLT-2i or GLP-1RA.
Methods
PubMed, Cochrane CENTRAL, and EMBASE were searched from 2008 to 2022 for SGLT-2i or GLP-1RA randomized trials that reported 3-point major adverse cardiovascular events (3P-MACE). Baseline clinical and biochemical characteristics included age, sex, body mass index (BMI), HbA1c, estimated glomerular filtration rate (eGFR), albuminuria, preexisting cardiovascular disease (CVD), and heart failure (HF). Absolute and relative risk reductions (ARR and RRR) regarding incidence rates for 3P-MACE with a 95% confidence interval were calculated. The association of average baseline characteristics in each study with the ARR and RRR for 3P-MACE was investigated by meta-regression analyses (random-effects model, assuming inter-study heterogeneity). Meta-analysis was also conducted to investigate whether the efficacy of SGLT-2i or GLP-1RA on 3P-MACE reduction could differ according to the patient’s characteristics (e.g., HbA1c above/below cutoff).
Results
After a critical assessment of 1,172 articles, 13 cardiovascular outcome trials with a total of 111,565 participants were selected. In meta-regression analysis, the more patients with reduced eGFR in the studies, the greater ARR by SGLT-2i or GLP-1RA therapy. Similarly, in the meta-analysis, SGLT-2i therapy tended to be more effective in reducing 3P-MACE in people with eGFR < 60 ml/min/1.73 m2 than in those with normal renal function (ARR − 0.90 [–1.44 to − 0.37] vs. − 0.17 [–0.34 to − 0.01] events/100 person-years). Furthermore, people with albuminuria tended to respond better to SGLT-2i therapy than those with normoalbuminuria. However, this was not the case for the GLP-1RA treatment. Other factors including age, sex, BMI, HbA1c, and preexisting CVD or HF did not affect the efficacy of either SGLT-2i or GLP-1RA treatment on the ARR or RRR of 3P-MACE.
Conclusions
Because decreased eGFR [significant] and albuminuria [trend] were found to predict a better efficacy for SGLT-2i in 3P-MACE reduction, this class of drug should be preferred in such patients. However, GLP-1RA may be considered for patients with normal eGFR because it showed better efficacy than SGLT-2i in this subgroup [trend].
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01877-6.
Keywords: Sodium–glucose cotransporter-2 inhibitor; Glucagon-like peptide 1 receptor agonist; Major adverse cardiovascular events; Meta-regression; Meta-analysis; Diabetes mellitus, type 2
Background
Diabetes mellitus (DM) is one of the major causes of death in humans and doubles the risk of cardiovascular disease (CVD) in the United States (US) [1]. The 2008 US Food and Drug Administration antidiabetic drug guidance mandated that novel antihyperglycemic medications should demonstrate cardiovascular safety through large cardiovascular outcome trials (CVOTs) [2]. Accordingly, CVOTs comparing dipeptidyl peptidase-4 inhibitors, sodium–glucose cotransporter-2 inhibitors (SGLT-2is), and glucagon-like peptide-1 receptor agonists (GLP-1RAs) with placebo on a background of standard of care have been conducted [3, 4]. Among them, certain SGLT-2i and GLP-1RA compounds have shown not only safety but superiority in their effects on cardiovascular outcomes [5].
Of note, there are some concerns about adverse events in SGLT-2i and GLP-1RA therapy in clinical practice. In the early days, many doctors were reluctant to use SGLT-2i in patients with reduced renal function or older patients, particularly because of concerns about adverse effects on the kidney. However, recent trials with SGLT-2i in patients with chronic kidney disease have proven significant efficacy on composite renal outcomes [6–8]. Moreover, there was no difference in the cardiovascular benefits from SGLT-2i therapy between younger and older patients [9]. Moreover, SGLT-2i therapy seemed to be more effective regarding composite cardiovascular outcomes in patients with preexisting CVD [10, 11].
Physicians tend to avoid prescribing GLP-1RA to old people and those with low body mass index (BMI) because of its gastrointestinal adverse events [12]. However, more evidence is needed to support this practice pattern. On the contrary, it was speculated that people with overweight or obesity respond well to GLP-1RA because the therapy can reduce weight. In addition, some GLP-1RA types, such as dulaglutide and liraglutide, showed benefits in composite renal outcomes in the CVOTs [13, 14]. However, distinctive beneficial effects of GLP-1RA on cardiovascular outcomes in patients with obesity or those with renal impairment vs. those without these conditions have not been established yet.
Taken together, there is a clinical interest in determining patient factors that predict a differential therapeutic response to SGLT-2i and GLP-1RA (in the sense that subgroups are identified who respond better to one and worse to the alternative treatment).
To the best of our knowledge, no systematic approach has ever been taken to identify the patient characteristics related to the effectiveness of these two agents. Therefore, the current study was designed to compare the 3-point major adverse cardiovascular event (3P-MACE) risk reduction with SGLT-2i and GLP-1RA in subgroups of patients participating in the large CVOTs. Through this approach, we aimed to suggest recommendations for individualizing the choice between these two cardioprotective medications in patients with type 2 DM (T2DM).
Methods
Data sources and study selection
We conducted a systematic review and meta-analysis following the updated guidance in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020) [15]. Neither ethics approval nor patient consent was required for this analysis. The review was registered in PROSPERO (CRD42021235989).
Databases searched included MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials. The latest searches were conducted in December 2022, focusing on the period from January 2008 to December 2022. The search terms included the following keywords: ‘type 2 diabetes’ for the population; ‘sodium-glucose cotransporter 2 inhibitor’ and ‘glucagon-like peptide-1 receptor agonist’ (or the individual compounds in these classes) for the study intervention; ‘major adverse cardiovascular event’ and ‘cardiovascular event’ for outcomes. Searches were restricted to clinical trials and those reported in the English language.
We included trials using the following inclusion criteria: (1) evaluation of an antidiabetic agent compared with placebo; (2) report of 3P-MACE for the overall population and subgroups (grouped by patient characteristics); (3) a minimum number of 1,000 T2DM subjects enrolled; and (4) multinational randomized controlled trials (RCTs) with a wide representation of ethnic backgrounds.
Outcomes
The main outcomes were the relative and absolute risk reductions (RRR and ARR) for 3P-MACE by subgroup (based on patient characteristics presented at baseline) comparing SGLT-2i and GLP-1RA treatment, aiming to identify differential responses. The baseline characteristics included age, sex, BMI, initial HbA1c level, baseline renal function (estimated glomerular filtration rate, eGFR), presence of albuminuria, and preexisting CVD or heart failure (HF): 3P-MACE combined cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.
Data extraction
Data were extracted independently by two authors (M.S. and S.L.). The following data were extracted for the included studies: name of the trial, year of publication, number of patients, patient characteristics, comorbidity status, and concurrent medication. To check the population risks for CVDs, event rates of 3P-MACE were collected from each trial. The outcomes included the event rate, event number, and sample size of the group.
Data synthesis and analysis
Data were analyzed using R software (version 4.1.0; R Development Core Team, Vienna, Austria) with the ‘metafor’ package. Included trials were assessed for the risk of bias using the Cochrane risk of bias 2 tool. The I2 statistic was used to assess the overall heterogeneity of all the comparisons, with values of under 25%, 50%, and 75% corresponding to mild, moderate, and high heterogeneity, respectively.
ARR and RRR were calculated using the number of events and patient-years of observation [16], and then used for pooled estimates and their 95% confidence intervals (CIs). If these data were not explicitly reported, they were approximated: event rate = [(number of people with the event [n]) / ([total person-years of observation] × 100]).
We performed a meta-regression analysis of the selected trials to assess the relationship between the patient characteristics at baseline (based on mean values, e.g., for BMI, or proportions with a characteristic of interest, e.g., patients with preexisting CVD) and the corresponding ARR and RRR for 3P-MACE. We also estimated the strength of the association of the characteristics by estimating R2. The ‘rma’ function was used to examine the effects of drug types as moderators. The outcomes among different baseline subgroups or therapies were compared using frequentist meta-analysis with random-effects models.
Quality of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) method was used to assess the quality and strength of the evidence for each subgroup. Two authors (M.S. and S.L.) rated the quality of the evidence for each outcome independently. We used GRADEpro software (McMaster University and Evidence Prime Inc., Hamilton, Ontario, Canada) to generate evidence profile tables. An I2 value over 50% was regarded as an indication of serious inconsistency. Imprecision was assessed as serious if the reported subgroups were less than half and as very serious when only one trial reported the results. In terms of 3P-MACE, none of the meta-analyses were considered to have serious indirectness.
Results
Results of the search and study characteristics
We identified 1,172 articles. After critically assessing these papers, 13 CVOTs (6 SGLT-2i trials and 7 GLP-1RA trials) fulfilled the inclusion criteria (Supplementary Figure S1), representing 111,565 participants. However, the ELIXA trial was excluded because subgroup data for 3P-MACE were not reported.
The characteristics of the included trials and the recruited patients are presented in Table 1. The mean follow-up time range was 1.0–5.5 years. The mean age range of participants was 62–69 years, and the proportion of men varied between 53.7% and 71.5%. The percentage of patients with preexisting CVD ranged from 31.5 to 100%. The eGFR < 60 ml/min/1.73 m2 at baseline ranged from 7.4 to 100%. The 3P-MACE event rates in individual studies were 2.4–6.8 per 100 person-years with placebo and 2.3–5.4 per 100 person-years with active treatment.
Table 1.
Baseline Characteristics of Cardiovascular Outcome Trials with SGLT-2is or GLP-1RAs in Patients with Type 2 Diabetes
| Trial | Mean Follow-up | N | Participants’ characteristics | Characteristics of diabetes | Characteristics of comorbidities | Concurrent medication | Risk of events* | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
CANVAS program [25] (Canagliflozin) |
3.6 yr | 10,142 |
Age: 63.3 yr Men: 64.2% |
BMI: 32 kg/m2 SBP: 136.6 mmHg LDLc: 2.3 mmol/L |
HbA1c: 8.2% DM duration: 13.5 yr |
HTN: 90.0% CVD: 65.6% HF: 14.4% |
eGFR < 60: 20.1% ACR ≥ 30 mg/g: 29.8% |
Statin 74.9% Antithrombotics 73.6% RAAS blockers 80.0% |
3P-MACE 2.7/3.2 (100PY) |
|
|
CREDENCE [35] (Canagliflozin) |
2.6 yr | 4,401 |
Age: 63 yr Men: 66.1% |
BMI: 31.3 kg/m2 SBP: 140.0 mmHg LDLc: 2.5 mmol/L |
HbA1c: 8.3% DM duration: 15.8 yr |
HTN: 96.8% CVD: 50.4% HF: 14.8% |
eGFR < 60: 59.8% ACR ≥ 30 mg/g: 99.3% |
Statin 69.0% Antithrombotics 59.6% RAAS blockers 99.9% |
3P-MACE 3.9/4.9 (100PY) |
|
|
DECLARE-TIMI 58 [36] (Dapagliflozin) |
4.2 yr† | 17,160 |
Age: 63.9 yr Men: 62.6% |
BMI: 32.1 kg/m2 SBP: 135.8 mmHg LDLc: 2.3 mmol/L |
HbA1c: 8.3% DM duration: 11.8 yr |
HTN‡: 89.4% CVD: 40.6% HF: 10.0% |
eGFR < 60: 7.4% ACR ≥ 30 mg/g: 30.3% |
Statin 75.0% Antithrombotics 61.1% RAAS blockers 81.3% |
3P-MACE 2.3/2.4 (100PY) |
|
|
EMPA-REG OUTCOME [11] (Empagliflozin) |
3.0 yr | 7,020 |
Age: 63.1 yr Men: 71.5% |
BMI: 30.6 kg/m2 SBP: 135.5 mmHg LDLc: 2.2 mmol/L |
HbA1c: 8.1% DM duration > 10 year: 57.1% |
HTN‡: 94.6% CVD: 100% HF: 10.1% |
eGFR < 60: 25.9% ACR ≥ 30 mg/g: 39.6% |
Statin 77.0% Antithrombotics 89.1% RAAS blockers 80.7% |
3P-MACE 3.7/4.4 (100PY) |
|
|
VERTIS-CV [37] (Ertugliflozin) |
3.5 yr | 8,246 |
Age: 64.4 yr Men: 70.0% |
BMI: 29.5 kg/m2 SBP: 133.4 mmHg LDLc: 2.3 mmol/L |
HbA1c: 8.2% DM duration: 13.0 yr |
HTN‡: 95.2% CVD: 100% HF: 23.7% |
eGFR < 60: 21.9% ACR ≥ 30 mg/g: 39.3% |
Statin 82.3% Antithrombotics 88.9% RAAS blockers 81.1% |
3P-MACE 3.9/4.0 (100PY) |
|
|
SCORED [8] (Sotagliflozin) |
1.3 yr† | 10,584 |
Age: 69 yr Men: 55.1% |
BMI: 31.8 kg/m2 SBP: 138.5 mmHg |
HbA1c: 8.3% |
CVD: 48.6% HF: 31.0% |
eGFR < 60: 100% ACR ≥ 30 mg/g: 65.0% |
RAAS blockers 88.5% |
3P-MACE 4.1/4.7 (100PY) |
|
|
EXSCEL [28] (Exenatide) |
3.3 yr† | 14,752 |
Age: 62 yr Men: 62.0% |
BMI: 32.7 kg/m2 SBP: 135.5 mmHg LDLc: 2.3 mmol/L |
HbA1c: 8.1% DM duration: 13.1 yr |
HTN‡: 90.3% CVD: 73.1% HF: 16.2% |
eGFR < 60: 21.6% ACR ≥ 30 mg/g: 15.9% |
Statin 73.5% Antithrombotics 73.4% RAAS blockers 85.0% |
3P-MACE 3.7/4.0 (100PY) |
|
|
LEADER [21] (Liraglutide) |
3.8 yr† | 9,340 |
Age: 64.3 yr Men: 64.3% |
BMI: 32.5 kg/m2 SBP: 135.9 mmHg LDLc: 2.3 mmol/L |
HbA1c: 8.7% DM duration: 12.7 yr |
HTN: 90.0% CVD: 81.3% HF: 17.8% |
eGFR < 60: 23.1% ACR ≥ 30 mg/g: 36.6% |
Statin 72.2% Aspirin 69.8% RAAS blockers 77.2% |
3P-MACE 3.4/3.9 (100PY) |
|
|
REWIND [13] (Dulaglutide) |
5.5 yr† | 9,901 |
Age: 66.2 yr Men: 53.7% |
BMI: 32.3 kg/m2 SBP: 137.2 mmHg LDLc: 2.6 mmol/L |
HbA1c: 7.3% DM duration: 10.5 yr |
HTN: 93.2% CVD: 31.5% HF: 8.6% |
eGFR < 60: 22.2% ACR ≥ 30 mg/g: 35.0% |
Statin 66.1% Antithrombotics 54.0% RAAS blockers 81.5% |
3P-MACE 2.4/2.7 (100PY) |
|
|
Harmony [19] (Albiglutide) |
1.6 yr† | 9,463 |
Age: 64.2 yr Men: 69.4% |
BMI: 32.3 kg/m2 SBP: 134.7 mmHg LDLc: 2.1 mmol/L |
HbA1c: 8.7% DM duration: 14.2 yr |
HTN: 86.5% CVD: 100% HF: 20.3% |
eGFR < 60: 23.5% |
Statin 84.0% Antithrombotics 84.1% RAAS blockers 81.6% |
3P-MACE 4.6/5.9 (100PY) |
|
|
SUSTAIN-6 [29] (Semaglutide) |
2.1 yr | 3,297 |
Age: 64.6 yr Men: 60.7% |
BMI: 32.8 kg/m2 SBP: 135.6 mmHg LDLc: 2.1 mmol/L |
HbA1c: 8.7% DM duration: 13.9 yr |
HTN: 92.8% CVD: 83.0% HF: 23.6% |
eGFR < 60: 28.5% ACR ≥ 30 mg/g: 42.0% |
Statin 72.8% Antithrombotics 76.3% RAAS blockers 83.5% |
3P-MACE 3.2/4.4 (100PY) |
|
|
PIONEER 6 [20] (Oral semaglutide) |
1.0 yr† | 3,183 |
Age: 66 yr Men: 68.4% |
BMI: 32.3 kg/m2 SBP: 135.6 mmHg LDLc: 2.2 mmol/L |
HbA1c: 8.2% DM duration: 14.9 yr |
HTN‡: 95.3% CVD: 84.7% HF: 12.2% |
eGFR < 60: 26.9% |
Lipid-lowering agents 85.2% Antithrombotics 79.4% |
3P-MACE 2.6/3.7 (100PY) |
|
|
AMPLITUDE-O [18] (Efpeglenatide) |
1.8 yr† | 4,076 |
Age: 64.5 yr Men: 67.0% |
BMI: 32.7 kg/m2 SBP: 134.9 mmHg LDLc: 2.1 mmol/L |
HbA1c: 8.9% DM duration: 15.4 yr |
HTN: 91.3% CVD: 89.5% HF: 18.1% |
eGFR < 60: 31.6% ACR ≥ 30 mg/g: 48.5% |
Statin 80.8% Aspirin 67.9% Other antiplatelets 25.7% RAAS blockers 80.0% |
3P-MACE 5.4/6.8 (100PY) |
|
BMI, body mass index; SBP, systolic blood pressure; LDLc, low-density lipoprotein cholesterol; HTN, hypertension; CVD, cardiovascular disease; HF, heart failure; eGFR, estimated glomerular filtration rate; ACR, albumin–creatinine ratio; RAAS, renin–angiotensin–aldosterone system; 3P-MACE, 3-point major adverse cardiovascular events; 100PY, 100 patient-year. * 3P-MACE event rate (100 patients-year) of active/placebo group. † Median and interquartile range converted to the mean value. ‡ Medically treated hypertension
Overall 3P-MACE and risk of Bias
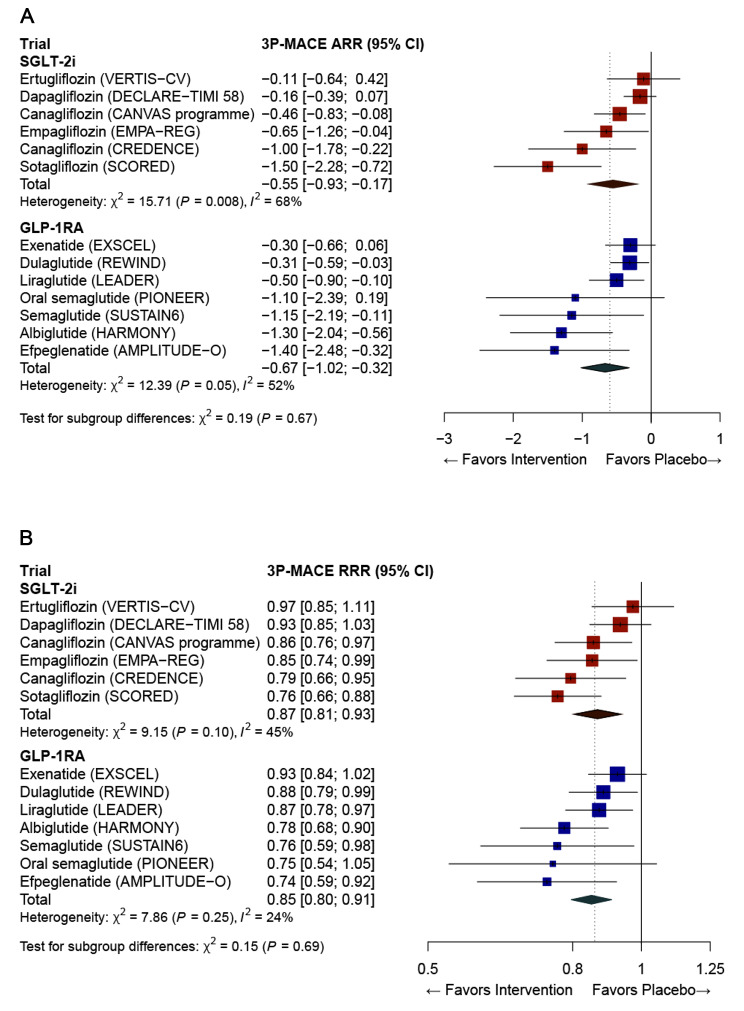
The ARR and RRR of 3P-MACE calculated from six SGLT-2i trials and seven GLP-1RA trials are shown in Fig. 1. For all trial participants, compared with placebo, the ARR for 3P-MACE with SGLT-2is was − 0.55 per 100 person-years of follow-up (95% CI: − 0.93, − 0.17), which was slightly less pronounced than the − 0.67 per 100 person-years of follow-up (95% CI: − 1.02, − 0.32) with GLP-1RAs. Similarly, the RRR for 3P-MACE was slightly less pronounced with SGLT-2i therapy (RRR 0.87, 95% CI: 0.81, 0.93) than that with GLP-1RA therapy (RRR 0.85, 95% CI: 0.80, 0.91). All included trials were found to have high quality with a low risk of bias when the Cochrane risk of bias tool was applied (Supplementary Figure S2).
Fig. 1.
Absolute and relative risk reduction (ARR and RRR) in the incidence of a 3-point major adverse cardiovascular event (3P-MACE) in cardiovascular outcome trials with SGLT-2 inhibitor (SGLT-2i) or GLP-1 receptor agonist (GLP-1RA). The diamond indicates the pooled estimates, and the boxes are each study with 95% confidence interval. (A) Absolute risk reduction in the incidence of 3P-MACE by SGLT-2i and GLP-1RA. (B) Relative risk reduction in the incidence of 3P-MACE by SGLT-2i and GLP-1RA
Effect of SGLT-2is or GLP-1RAs on 3P-MACE by baseline eGFR or Albuminuria Status
We investigated whether the effects of SGLT-2is or GLP-1RAs on 3P-MACE reduction would differ according to baseline renal function estimated by eGFR or albuminuria. For this, we conducted the meta-regression and meta-analyses with subgroups divided by the therapies (Figs. 2, 3 and 4 and Supplementary Figures S3–S5). All six SGLT-2i trials were included, but two GLP-1RA trials (REWIND [17] and AMPLITUDE-O [18]) were excluded because they did not report the 3P-MACE rate according to the baseline eGFR or albuminuria status.
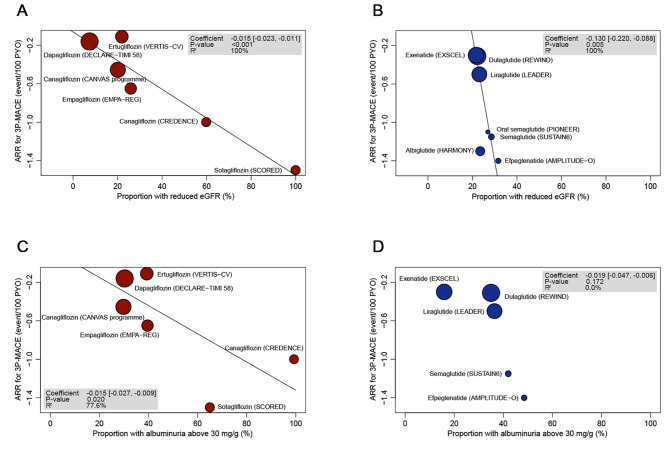
Fig. 2.
Meta-regression between ARR for 3P-MACE by SGLT-2i or GLP-1RA therapy and the proportion of patients with reduced eGFR (< 60 mL/min/1.73 m2) (A, B) or the proportion of patients with albuminuria (≥ 30 mg/g) (C, D). The coefficient represents the slope of the regression line, which is present when there is significance with P-value under 0.05. R2 indicates the strength of the association of the characteristics. ARR, absolute risk reduction; eGFR, estimated glomerular filtration ratio; GLP-1RA, glucagon-like peptide 1 receptor agonists; PYO, person-years of observation; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; 3P-MACE, 3-point major adverse cardiovascular events. (A) Meta-regression between ARR for 3P-MACE by SGLT-2i therapy and the proportion of patients with reduced eGFR (< 60 mL/min/1.73 m2). (B) Meta-regression between ARR for 3P-MACE by GLP-1RA therapy and the proportion of patients with reduced eGFR (< 60 mL/min/1.73 m2). (C) Meta-regression between ARR for 3P-MACE by SGLT-2i therapy and proportion of patients with albuminuria (≥ 30 mg/g). (D) Meta-regression between ARR for 3P-MACE by GLP-1RA therapy and proportion of patients with albuminuria (≥ 30 mg/g)
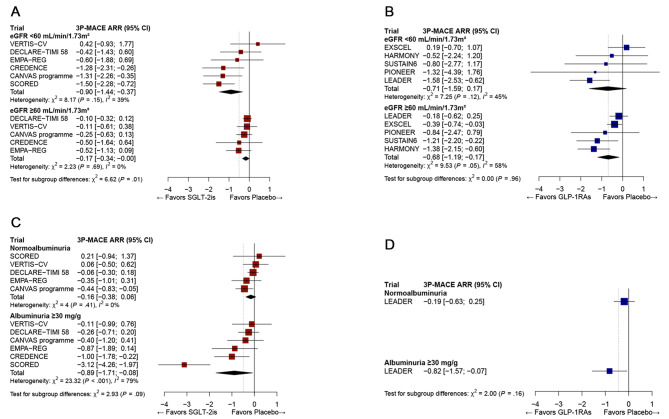
Fig. 3.
Comparison of absolute risk reduction for 3P-MACE according to baseline eGFR category and albuminuria status in SGLT-2i (A, C) or GLP-1RA (B, D) trials. The diamond indicates the pooled estimates, and the boxes are each study with 95% confidence interval. ARR, absolute risk reduction; eGFR, estimated glomerular filtration ratio; GLP-1RA, glucagon-like peptide 1 receptor agonists; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; 3P-MACE, 3-point major adverse cardiovascular events. (A) Efficacy comparison on ARR for 3P-MACE according to baseline eGFR category in SGLT-2i trials. (B) Efficacy comparison on ARR for 3P-MACE according to baseline eGFR category in GLP-1RA trials. (C) Efficacy comparison on ARR for 3P-MACE according to albuminuria status in SGLT-2i trials. (D) Efficacy comparison on ARR for 3P-MACE according to albuminuria status in GLP-1RA trials
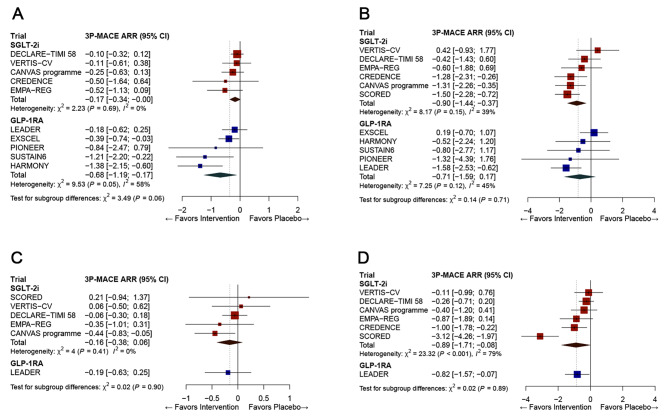
Fig. 4.
Efficacy comparison between SGLT-2i and GLP-1RA therapies on absolute risk reduction for 3P-MACE according to baseline eGFR category (A, B) and albuminuria status (C, D). The diamond indicates the pooled estimates, and the boxes are each study with 95% confidence interval. ARR, absolute risk reduction; eGFR, estimated glomerular filtration ratio; GLP-1RA, glucagon-like peptide 1 receptor agonists; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; 3P-MACE, 3-point major adverse cardiovascular events. (A) Comparison between SGLT-2i and GLP-1RA therapy on ARR for 3P-MACE in normal eGFR. (B) Comparison between SGLT-2i and GLP-1RA therapy on ARR for 3P-MACE in reduced eGFR. (C) Comparison between SGLT-2i and GLP-1RA therapy on ARR for 3P-MACE in normoalbuminuria. (D) Comparison between SGLT-2i and GLP-1RA therapy on ARR for 3P-MACE in albuminuria
The meta-regression analyses revealed significant negative associations, with strong strength (R2 = 100%), between the 3P-MACE incidence rates with SGLT-2i or GLP-1RA vs. placebo treatment and the proportions of the patients who had eGFR < 60 ml/min/1.73 m2 at baseline (Fig. 2 for ARR and Supplementary Figure S3 for RRR). This result suggests more favorable effects of two agents in people with reduced eGFR.
Notably, most study participants in GLP-1RA trials had a relatively good renal function: the participants with a baseline eGFR < 60 ml/min/1.73 m2 were fewer than 35%. The coefficient slopes relating the ARR in 3P-MACE to the proportion with eGFR < 60 ml/min/1.73 m2 were significantly steeper in the GLP-1RA studies than in the SGLT-2i studies.
Similarly, in the meta-analysis by eGFR subgroups, SGLT-2i therapy was more effective in reducing 3P-MACE in people with eGFR < 60 ml/min/1.73 m2, compared with those with ≥ 60 ml/min/1.73 m2 (ARR − 0.90, 95% CI: − 1.44, − 0.37 vs. ARR − 0.17, 95% CI: − 0.34, − 0.00; P = 0.01 for between-subgroup differences; Table 2). There was a trend for a greater effect of GLP-1RA therapy on 3P-MACE than that of SGLT-2i therapy in patients with normal eGFR: ARR − 0.68 (95% CI: − 1.19, − 0.17) vs. ARR − 0.17 (95% CI: − 0.34, − 0.00; P = 0.06; Fig. 4).
Table 2.
Comparisons of Reductions in Absolute and Relative Risk for 3P-MACE Incidence with SGLT-2i or GLP-1RA Therapy According to Patient Characteristics
| Subgroups | SGLT-2is | GLP-1RAs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean event rate in active / placebo groups (100 PYO) | Pooled ARR (95% CI) |
P | Pooled RRR (95% CI) | P | Mean event rate in active / placebo groups (100 PYO) | Pooled ARR (95% CI) |
P | Pooled RRR (95% CI) | P | ||
| Overall | 3.5 / 4.2 | –0.55 (–0.93, − 0.17) | 0.87 (0.81, 0.93) | 3.6 / 4.4 | –0.67 (–1.02, − 0.32) | 0.85 (0.80, 0.91) | |||||
| eGFR ≥60 mL/min/1.73 m2 | 2.8 / 3.1 | –0.17 (–0.34, − 0.01) | 0.01 | 0.93 (0.86, 0.99) | 0.09 | 3.2 / 4.0 | –0.68 (–1.19, − 0.17) | 0.96 | 0.83 (0.74, 0.94) | 0.64 | |
| eGFR < 60 mL/min/1.73 m2 | 4.4 / 5.2 | –0.90 (–1.44, − 0.37) | 0.83 (0.74, 0.92) | 5.1 / 5.9 | –0.71 (–1.59, 0.17) | 0.88 (0.74, 1.04) | |||||
| Normoalbuminuria | 2.9 / 3.0 | –0.16 (–0.38, 0.06) | 0.09 | 0.94 (0.87, 1.02) | 0.13 | 2.8 / 3.0 | –0.19 (–0.63, 0.25) | 0.16 | 0.94 (0.80, 1.09) | 0.34 | |
| Albuminuria ≥30 mg/g | 4.4 / 5.3 | –0.89 (–1.71, − 0.08) | 0.84 (0.75, 0.94) | 4.3 / 5.2 | –0.82 (–1.57, − 0.07) | 0.82 (0.72, 0.99) | |||||
| Age < 65 years | 2.7 / 3.0 | –0.16 (–0.37, 0.05) | 0.17 | 0.92 (0.85, 1.01) | 0.41 | 3.0 / 4.0 | –0.73 (–1.32, − 0.13) | 0.59 | 0.82 (0.70, 0.95) | 0.52 | |
| Age ≥65 years | 3.8 / 4.4 | –0.54 (–1.04, − 0.05) | 0.87 (0.78, 0.97) | 4.0 / 4.7 | –0.55 (–0.81, − 0.28) | 0.86 (0.81, 0.92) | |||||
| Men | 3.5 / 4.0 | –0.36 (–0.65, − 0.08) | 0.44 | 0.90 (0.83, 0.97) | 0.91 | 4.0 / 4.8 | –0.50 (–0.77, − 0.22) | 0.61 | 0.88 (0.83, 0.94) | 0.40 | |
| Women | 2.7 / 3.0 | –0.22 (–0.45, 0.02) | 0.89 (0.80, 0.99) | 2.7 / 3.4 | –0.40 (–0.65, − 0.16) | 0.84 (0.76, 0.92) | |||||
| BMI < 30 kg/m2 | 3.1 / 3.6 | –0.32 (–0.70, 0.06) | 0.99 | 0.90 (0.81, 0.99) | 0.99 | 3.4 / 4.6 | –0.92 (–1.64, − 0.20) | 0.30 | 0.80 (0.69, 0.94) | 0.45 | |
| BMI ≥30 kg/m2 | 3.3 / 3.7 | –0.33 (–0.60, − 0.05) | 0.90 (0.82, 0.98) | 3.6 / 4.1 | –0.52 (–0.74, − 0.30) | 0.86 (0.80, 0.92) | |||||
| Low HbA1c | 3.0 / 3.4 | –0.31 (–0.63, 0.01) | 0.90 | 0.90 (0.82, 0.99) | 0.96 | 3.2 / 3.8 | –0.31 (–0.56, − 0.06) | 0.07 | 0.90 (0.83, 0.98) | 0.16 | |
| High HbA1c | 3.4 / 3.9 | –0.28 (–0.67, 0.11) | 0.90 (0.82, 1.00) | 3.8 / 4.8 | –0.79 (–1.26, − 0.33) | 0.83 (0.76, 0.90) | |||||
| No CVD | 2.6 / 2.9 | –0.20 (–0.75, 0.34) | 0.41 | 0.91 (0.72, 1.16) | 0.81 | 1.9 / 2.3 | –0.21 (–0.73, 0.30) | 0.12 | 0.89 (0.71, 1.12) | 0.70 | |
| Previous CVD | 3.6 / 4.1 | –0.44 (–0.67, − 0.21) | 0.89 (0.83, 0.94) | 4.1 / 5.0 | –0.68 (–0.96, − 0.40) | 0.85 (0.80, 0.91) | |||||
| No HF | 2.8 / 3.3 | –0.29 (–0.52, − 0.06) | 0.66 | 0.89 (0.83, 0.96) | 0.28 | 3.4 / 3.9 | –0.42 (–0.67, − 0.17) | 0.63 | 0.88 (0.82, 0.95) | 0.97 | |
| Previous HF | 5.0 / 5.2 | –0.14 (–0.77, 0.48) | 0.97 (0.85, 1.10) | 5.7 / 6.7 | –0.81 (–2.38, 0.76) | 0.88 (0.71, 1.10) | |||||
P for subgroup differences. ARR, absolute risk reduction, RRR: relative risk reduction; 3P-MACE, 3-point major adverse cardiovascular events; SGLT-2i, sodium–glucose cotransporter-2 inhibitor; GLP-1RA, glucagon-like peptide-1 receptor agonist; PYO, person-years of observation; CI, confidence interval
In terms of albuminuria status, meta-regression analyses showed a negative association between the ARR for 3P-MACE and the proportions of people who had albuminuria ≥ 30 mg/g at baseline in SGLT-2i trials, with strong strength (R2 = 77.6%; Fig. 2). In GLP-1RA trials, HARMONY [19] and PIONEER-6 [20] trials were excluded because they did not provide results by albuminuria status (≥ 30 vs. < 30 mg/g).
Similar results were found in the meta-analysis (Fig. 3). SGLT-2i therapy effectively reduced 3P-MACE in people with albuminuria ≥ 30 mg/g at baseline (ARR − 0.89, 95% CI: − 1.71, − 0.08) but not in those with normoalbuminuria (ARR − 0.16, 95% CI: − 0.38, 0.06). However, there was no difference between subgroups (P = 0.09). For the GLP-1RA class, the LEADER trial alone [21] reported the subgroup results according to the baseline albuminuria status. In this study, liraglutide therapy effectively reduced 3P-MACE in people with albuminuria at baseline but not in those without albuminuria, without a significant between-subgroup difference (P = 0.16).
Efficacy comparison between SGLT-2is and GLP-1RAs on 3P-MACE according to baseline eGFR category or Albuminuria Status
In the meta-analysis, GLP-1RA therapy was more effective in the ARR of 3P-MACE in patients with normal renal function than SGLT-2 therapy (Fig. 4). In contrast, the beneficial effects of SGLT-2i and GLP-1RA therapies on the ARR of 3P-MACE were not different in the patients with reduced renal function.
Concerning the albuminuria status, the beneficial effects of SGLT-2i and GLP-1RA therapies on the ARR of 3P-MACE did not differ according to this characteristic. However, only one study (LEADER study [21]) was included in the GLP-1RA class.
3P-MACE reduction with SGLT-2i and GLP-1RA by other baseline characteristics
The effects of SGLT-2i or GLP-1RA therapy on 3P-MACE by age, sex, BMI, baseline HbA1c level, preexisting CVD, and preexisting HF were also examined (Supplementary Figures S6–S11). Among the SGLT-2i studies, the SCORED trial [8] was not included in these subgroup meta-analyses because it did not report the results by these characteristics. All seven GLP-1RA trials were included in these subgroup meta-analyses. However, three GLP-1RA trials were included for the meta-analysis by preexisting HF.
Comparing subgroups defined by age, sex, BMI, HbA1c, and proportion with preexisting CVD or HF, both the ARR and RRR were not significantly different (Table 2; Supplementary Figures S6–S11). GLP-1RAs exhibit better efficacy for 3P-MACE reduction in patients with high HbA1c levels vs. those with low HbA1c levels without significance (Table 2). The ARR in 3P-MACE was greater with GLP-1RA therapy compared with SGLT-2i therapy in the patients under 65 years of age (–0.73, 95% CI: − 1.32, − 0.13 vs. − 0.16, 95% CI: − 0.37, 0.05 for 3P-MACE events per 100 person-years of observation; P = 0.08; Supplementary Figure S6C). This may suggest the better effect of GLP-1RA treatment at a young age.
Quality Assessment
Supplementary table S1 presents the GRADE evidence profiles for the subgroups. Nine meta-analyses had inconsistency with moderate heterogeneity. Limited reporting of results by the status of previous CVD and HF history resulted in downgrades in certainty. As only the LEADER trial reported 3P-MACE by albuminuria status, the analyses were downgraded two levels. Finally, four meta-analyses were given low certainty: (1–2) subgroups divided by albuminuria in GLP-1RA trials; (3) patients without previous CVD in SGLT-2i trials; and (4) patients with HF history in GLP-1RA trials.
Discussion
In CVOTs, SGLT-2i and GLP-1RA therapies were significantly and similarly effective in 3P-MACE reduction, providing benefits of 13% and 15% RRR and 0.55 and 0.67 ARR (event/100 person-years of observation), respectively. In the meta-regression of 13 randomized placebo-controlled trials examining SGLT-2i and GLP-1RA treatments, there was a negative association between poorer renal dysfunction (decreased eGFR) and greater ARR for 3P-MACE. The presence of albuminuria was also linked to a greater ARR with SGLT-2i therapy. In the meta-analysis by eGFR subgroups, SGLT-2i therapy was more effective in reducing 3P-MACE in people with eGFR < 60 ml/min/1.73 m2 compared with those with normal renal function (Fig. 3). At the same time, this difference was much less prominent with GLP-1RA therapy.
Both reduced eGFR and albuminuria are independently associated with a higher risk of cardiovascular events in people with T2DM [22]. Recent studies on people with established chronic renal failure have proven that SGLT-2i effectively reduces cardiovascular fatality and a composite renal outcome [6, 7]. Thus, in addition to the previously reported renal benefits of SGLT-2i therapy in patients with T2DM [23–25], our data support the use of SGLT-2i therapy in this subgroup at high risk for both cardiovascular and renal complications.
It is obvious that both SGLT-2i and GLP-1RA therapy help reduce 3P-MACE in patients with renal impairment, who generally exhibit a high risk for CVD [26]. Intriguingly, the ARR in people with normal renal function seemed to be larger with GLP-1RA therapy than with SGLT-2i therapy (–0.68 vs. − 0.17; P = 0.06; Fig. 3). However, it should be noted that GLP-1RA trials included a relatively narrow spectrum in the proportion of people with eGFR < 60 ml/min/1.73 m2 than SGLT-2i trials (21.6–31.6% vs. 7.4–100%, P < 0.05). Thus, it might have been difficult to identify the different effects of GLP-1RA on 3P-MACE reduction according to the eGFR level. On the contrary, the lack of benefit observed in GLP-1RA therapy in patients with low eGFR may have resulted from insufficient statistical power caused by the narrow range of eGFR in the study participants [27]. Taken together, our analysis data including the most available studies indicate that GLP-1RA therapy may be beneficial even for people with normal renal function, which is not the case for SGLT-2i therapy.
Liraglutide therapy in the LEADER trial reduced 3P-MACE significantly more in patients with eGFR < 60 ml/min/1.73 m2 than in those with ≥ 60 ml/min/1.73 m2 [21], while other GLP-1RAs such as exenatide, albiglutide, and semaglutide tended to reduce 3P-MACE more in patients with normal eGFR [19, 28, 29]. This finding suggests that individual GLP-1RAs might have varying effects on 3P-MACE reduction according to the baseline renal function, but data are not enough to draw a conclusion indicating a drug-specific efficacy of GLP-1RA rather than the class effect. More studies with participants with a wide spectrum of eGFR are needed.
Subjects with albuminuria at baseline showed a tendency of greater reduction in the 3P-MACE by both SGLT-2i and GLP-1RA therapies. Treatment with SGLT-2is is known to significantly reduce albuminuria [30, 31], and reduction in albuminuria in the first year was associated with long-term cardiovascular benefits [31]. Among SGLT-2i trials, sotagliflozin therapy in SCORED showed remarkable efficacy in 3P-MACE reduction in patients with albuminuria [8]. Since the SCORED trial included only patients with low eGFR, with 65.0% of them had albuminuria, it can be speculated that the protective effect of SGLT-2is could be more pronounced in such patients [32]. Only the LEADER trial reported the subgroup results by albuminuria, which hinders the gathering of important data regarding the potential influences of albuminuria on the cardioprotective effects of GLP-1RA class.
Regarding albuminuria, renin–angiotensin–aldosterone system (RAAS) blockers might be intertwined in response to SGLT-2i or GLP-1RA therapy. However, RAAS blockers were used in over 80% of all 13 trials without significant differences (Table 1). In addition, no associations between the use of RAAS blockers and the efficacy of SGLT-2i or GLP-1RAs on 3P-MACE were observed in the meta-regression analysis.
Other characteristics, including age, sex, BMI, HbA1c level at baseline, preexisting CVD, or preexisting HF status, did not significantly affect the effects of SGLT-2i and GLP-1RA therapies on the ARR or RRR of 3P-MACE. However, GLP-1RA therapy tended to be more effective in the ARR of 3P-MACE than SGLT-2i therapy, with borderline significance in the younger age group (Supplementary Figure S6). It is also noteworthy that, compared with the SGLT-2i therapy, GLP-1RA therapy tended to exhibit better efficacy for 3P-MACE reduction in people with uncontrolled diabetes vs. those with controlled diabetes (Table 2).
A previous study with a different approach reported that people with established CVD showed a greater reduction in 3P-MACE by GLP-1RA and SGLT-2i therapies compared with those with risk factors alone (difference in effect between patients with vs. without a history of CVD: P = 0.049) [33]. However, that study included CVOTs up to June 2019. In our study, which included the CVOTs up to 2022, CVD was associated with a greater reduction in 3P-MACE by SGLT-2i and GLP-1RA. There was no statistically significant difference in the between-group analysis. Taken together, it is obvious that more trials are needed to confirm such effects of patient characteristics. Still, our data do not support the differential use of SGLT-2is and GLP-1RAs with the expectation to improve the prognosis by individualizing treatment considering these baseline characteristics.
The present analysis could be improved in a number of ways. First, the relatively small number of studies is likely to reduce the power needed to find significance between subgroups, particularly in meta-regression analysis [34]. Thus, nonsignificant results cannot rule out the significant impact of certain characteristics on the efficacy of SGLT-2i or GLP-1RA therapy in 3P-MACE reduction. Second, studies that did not report the 3P-MACE results by subgroups were not included for the analysis, reducing the statistical power of the analysis. Some subgroups were limited in representation: e.g., studies with GLP-1RAs in patients with impaired renal function at baseline. Third, each compound was only tested against placebo, and there were no head-to-head comparison studies between SGLT-2is and GLP-1RAs regarding cardiovascular effects. Thus, all comparisons are indirect and may be confounded by differences in unidentified patients characteristics, such as concomitant medical therapy addressing hypertension, lipid abnormalities, and other conditions typically associated with T2DM. Nonetheless, we included all large CVOTs currently available, and we believe that the results obtained by meta-regression and meta-analysis provide meaningful information about the proper use of these two novel agents.
Conclusions
In the meta-regression and meta-analyses, SGLT-2i therapy was more effective in the ARR of 3P-MACE in patients with decreased renal function (significant) or albuminuria (trend) than in those without. In contrast, the beneficial effects of GLP-1RA therapy on the ARR of 3P-MACE did not differ for these subgroups. However, the effect of GLP-1RA therapy was more pronounced in patients with high HbA1c levels at baseline than in those without.
Both classes showed a significant positive association between the efficacy of the reduction of 3P-MACE and the proportion of patients with reduced renal function. SGLT-2i therapy was effective in the 3P-MACE in both groups of patients with normal and reduced eGFR, with greater efficacy in reduced eGFR. In contrast, the efficacy of GLP-1RA therapy of 3P-MACE was similar in both groups, but significant only in those with normal eGFR. Our findings support the use of SGLT-2i in patients with impaired renal function and GLP-1RA in patients with normal renal function for optimizing the differential prescription to prevent major adverse cardiovascular events.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- ARR
absolute risk reduction
- ASCVD
Atherosclerotic cardiovascular disease
- BMI
Body mass index
- CKD
Chronic kidney disease
- CI
Confidence interval
- CVD
Cardiovascular death
- CVOT
Cardiovascular outcome trials
- DM
Diabetes mellitus
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- GLP-1RA
Glucagon-like peptide-1 receptor agonist
- HF
Heart failure
- MACE
Major adverse cardiovascular events
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- RCT
Randomized control trials
- RR
Risk ratio
- RRR
relative risk reduction
- SGLT-2i
Sodium-glucose cotransporter-2 inhibitor
- T2DM
type 2 diabetes mellitus
- 3P-MACE
3-point major adverse cardiovascular events
Authors’ contributions
M.S. designed the study, collected the data, contributed to the statistical analysis, and served as the primary author of the manuscript. J.D., M.A.N. and S.L. designed the study, contributed to the statistical analysis, contributed to the writing of the manuscript, and provided critical feedback to shape the manuscript. All authors significantly contributed to the manuscript and approved the final version for publication.
Funding
None.
Data Availability
Data are included in the tables and figures.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
M.A.N. has been member on advisory boards or has consulted with Boehringer-Ingelheim, Berlin-Chemie/ Menarini, Eli Lilly & Co., Merck Sharp & Dohme, Novo Nordisk, Shou-Ti/Gasherbrum, Regor Pharmaceuticals, Sun Pharma. He has received grant support from Merck Sharp & Dohme and Novo Nordisk. He has also served on the speakers’ bureau of Berlin-Chemie/ Menarini, Eli Lilly & Co., Medical Learning Institute, Medscape, Merck Sharp & Dohme, Novo Nordisk, Sanofi, and Sun Pharma. S.L. has been a member of advisory boards, or has consulted, for Merck, Sharp & Dohme, and Novo Nordisk. He has received grant support from AstraZeneca, Merck, Sharp & Dohme, and Astellas. He has also served on the speakers’ bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Merck, Sharp & Dohme, and Novo Nordisk.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael A. Nauck, Email: michael.nauck@rub.de
Soo Lim, Email: limsoo@snu.ac.kr.
References
- 1.Rosenquist KJ, Fox CS et al. Mortality Trends in Type 2 Diabetes. In: Diabetes in America Edited by rd, Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E. Bethesda (MD); 2018. [PubMed]
- 2.Zannad F, Stough WG, Lipicky RJ, Tamargo J, Bakris GL, Borer JS, Alonso Garcia Mde L, Hadjadj S, Koenig W, Kupfer S, et al. Assessment of cardiovascular risk of new drugs for the treatment of diabetes mellitus: risk assessment vs. risk aversion. Eur Heart J Cardiovasc Pharmacother. 2016;2(3):200–5. doi: 10.1093/ehjcvp/pvw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin DS, Lee JK, Hung CS, Chen WJ. The efficacy and safety of novel classes of glucose-lowering drugs for cardiovascular outcomes: a network meta-analysis of randomised clinical trials. Diabetologia. 2021;64(12):2676–86. doi: 10.1007/s00125-021-05529-w. [DOI] [PubMed] [Google Scholar]
- 4.Lim S, Kim KM, Nauck MA. Glucagon-like Peptide-1 receptor Agonists and Cardiovascular events: Class Effects versus individual patterns. Trends Endocrinol Metab. 2018;29(4):238–48. doi: 10.1016/j.tem.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Giugliano D, Longo M, Signoriello S, Maiorino MI, Solerte B, Chiodini P, Esposito K. The effect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: a network meta-analysis of 23 CVOTs. Cardiovasc Diabetol. 2022;21(1):42. doi: 10.1186/s12933-022-01474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group E-KC, Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2022.
- 7.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 9.Evans M, Morgan AR, Davies S, Beba H, Strain WD. The role of sodium-glucose co-transporter-2 inhibitors in frail older adults with or without type 2 diabetes mellitus. Age Ageing 2022, 51(10). [DOI] [PMC free article] [PubMed]
- 10.Neal B, Perkovic V, Matthews DR. Canagliflozin and Cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099. doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 11.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, Cardiovascular Outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 12.Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19:336–47. doi: 10.1111/dom.12824. [DOI] [PubMed] [Google Scholar]
- 13.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Botros FT, Riddle MC, Ryden L, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–8. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 14.Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornoe K, Zinman B, Buse JB, Committee LS, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–48. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumpston MS, McKenzie JE, Welch VA, Brennan SE. Strengthening systematic reviews in public health: guidance in the Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. J Public Health (Oxf) 2022. [DOI] [PMC free article] [PubMed]
- 17.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Ryden L, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 18.Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, Lam CSP, Khurmi NS, Heenan L, Del Prato S, et al. Cardiovascular and renal outcomes with Efpeglenatide in Type 2 diabetes. N Engl J Med. 2021;385(10):896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Sr., Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 20.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al. Oral Semaglutide and Cardiovascular Outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 21.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and Cardiovascular Outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosenzon O, Bain SC, Heerspink HJL, Idorn T, Mann JFE, Persson F, Pratley RE, Rasmussen S, Rossing P, von Scholten BJ, et al. Cardiovascular and renal outcomes by baseline albuminuria status and renal function: results from the LEADER randomized trial. Diabetes Obes Metab. 2020;22(11):2077–88. doi: 10.1111/dom.14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 24.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–17. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 25.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and Cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 26.Jankowski J, Floege J, Fliser D, Bohm M, Marx N. Cardiovascular Disease in chronic kidney Disease Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143(11):1157–72. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arshad A, Sarween N, Sharif A. Systematic review of Cardiovascular outcome trials using New Antidiabetic Agents in CKD stratified by estimated GFR. Kidney Int Rep. 2021;6(9):2415–24. doi: 10.1016/j.ekir.2021.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, et al. Effects of once-weekly Exenatide on Cardiovascular Outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and Cardiovascular Outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 30.Mosenzon O, Wiviott SD, Heerspink HJL, Dwyer JP, Cahn A, Goodrich EL, Rozenberg A, Schechter M, Yanuv I, Murphy SA, et al. The Effect of Dapagliflozin on Albuminuria in DECLARE-TIMI 58. Diabetes Care. 2021;44(8):1805–15. doi: 10.2337/dc21-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waijer SW, Xie D, Inzucchi SE, Zinman B, Koitka-Weber A, Mattheus M, von Eynatten M, Inker LA, Wanner C, Heerspink HJL. Short-term changes in Albuminuria and Risk of Cardiovascular and renal outcomes in type 2 diabetes Mellitus: a Post Hoc Analysis of the EMPA-REG OUTCOME Trial. J Am Heart Assoc. 2020;9(18):e016976. doi: 10.1161/JAHA.120.016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amin AP, Whaley-Connell AT, Li S, Chen SC, McCullough PA, Kosiborod MN, Investigators K. The synergistic relationship between estimated GFR and microalbuminuria in predicting long-term progression to ESRD or death in patients with diabetes: results from the kidney early evaluation program (KEEP) Am J Kidney Dis. 2013;61(4 Suppl 2):12–23. doi: 10.1053/j.ajkd.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Andrea E, Kesselheim AS, Franklin JM, Jung EH, Hey SP, Patorno E. Heterogeneity of antidiabetic treatment effect on the risk of major adverse cardiovascular events in type 2 diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2020;19(1):154. doi: 10.1186/s12933-020-01133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hempel S, Miles JN, Booth MJ, Wang Z, Morton SC, Shekelle PG. Risk of bias: a simulation study of power to detect study-level moderator effects in meta-analysis. Syst Rev. 2013;2:107. doi: 10.1186/2046-4053-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 36.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and Cardiovascular Outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 37.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 diabetes. N Engl J Med. 2020;383(15):1425–35. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are included in the tables and figures.