Abstract
It is puzzling why countries do not all implement stringent behavioral control measures to prevent the spread of COVID-19 even though preventive behaviors have been proven to be the only effective means to stop the pandemic. We provide a novel evolutionary life history explanation whereby pathogenic and parasitic prevalence represents intrinsic rather than extrinsic mortality risk that drives slower life history strategies and the related disease control motivation in all animals but especially humans. Our theory was tested and supported based on publicly available data involving over 150 countries. Countries having a higher historical prevalence of infectious diseases are found to adopt slower life history strategies that are related to prompter COVID-19 containment actions by the government and greater compliance by the population. Findings could afford governments novel insight into the design of more effective COVID-19 strategies that are based on enhancing a sense of control, vigilance, and compliance in the general population.
Keywords: historical pathogen prevalence, fast and slow life history, COVID-19 containment, disease control efforts
Preventive measures implemented by governments in response to the coronavirus disease 2019 (COVID-19) pandemic (e.g., mask wearing, social distancing, home quarantine, lockdown, travel bans, curfews and/or movement restrictions) and the reactions of the population to these measures have varied between countries. Asian and African countries generally seemed to have implemented behavioral control measures swiftly, and their populations have apparently cooperated without much controversy (e.g., Blackbox Research & Toluna, 2020; Rupiva, 2020; Shaw et al., 2020; Wadvalla, 2020). Many western nations did not appear to be as prompt or restrictive in their preventive measures, while people in these countries also seemed less supportive of them (Betsch, 2020; Sanchez, 2020; Shokoohi et al., 2020). Notably, Sweden and the United Kingdom have considered adopting a policy of achieving herd immunity by forgoing the enforcement of severe public health restrictions. The United States government was likewise more eager to reopen the economy and schools than to close them down, while the masses often echoed similar anti-lockdown sentiments (Betsch, 2020; Ward, 2020).
Why do countries differ in how promptly and vigilantly preventive measures to control COVID-19 were implemented by the government and were observed by the population? Here we propose an evolutionary life history (LH) explanation that uses two constructs, historical pathogen prevalence and slow LH strategies, to account for variations in COVID-19 preventive efforts. Throughout the animal kingdom, parasitic and pathogenic prevalence would lead to heightened awareness and preventive effort among the animals in controlling the spread of diseases (Sarabian et al., 2018), whereas intensity and spread of diseases fluctuate depending on individual animals’ containment effort (Hart, 2011). Over time, disease prevention becomes part of the species’ means of survival, informing the slower LH components of each animal’s LH strategies. Among humans, pathogenic stress, in particular, induces slower LH strategies, including heightened risk aversion and conscientiousness (Schaller & Murray, 2008; Wu & Chang, 2012), and the greater adoption of social learning and group-focused behaviors like conformity, compliance, and respect for authority (Chang et al., 2011). We expect countries with slower LH adaptations, because of higher historical pathogen prevalence, to be prompter and more vigilant in their COVID-19 mitigation attempts.
Historical Pathogen Prevalence and COVID-19 Response Tendencies
The global transmission of pathogens, such as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which causes COVID-19, is a novel zoonotic phenomenon in evolutionary terms, because pathogens and infectious diseases have historically spread locally rather than globally, creating regional differences in pathogenic stress in the past (e.g., differences in historical pathogen prevalence). According to a widely used measure based on epidemiological atlases of the world from 1940 to 1960 (Murray & Schaller, 2009), the historical pathogen prevalence has ranged between 1.03 and −.34 (M = .49, SD = .38; higher scores indicate higher pathogen load) for Asia and between 1.17 and −.09 (M = .78, SD = .34) for Africa. Europe has scored much lower at between .33 and −.98 (M = −.43; SD = .39). Among the lowest scoring countries are the United States at −.89, and Sweden at −.98. These historical pathogenic stress levels seem to correspond closely with the reported efforts of the aforementioned countries and their populations in implementing and observing COVID-19 related preventive measures, respectively (e.g., Sanchez, 2020; Shaw et al., 2020; Shokoohi et al., 2020). The coupling of parasitic prevalence with behavioral control effort by the host is widely observed in other species. Most animals take prophylactic as well as therapeutic actions in the form of residence cleaning, sanitation, and fumigation (Bush & Clayton, 2018), social distancing and the quarantine or peripheralization of sick conspecifics (Behringer et al., 2006; Hart, 2011), the engagement in body maintenance behaviors including preening or grooming, sun and water bathing, and the external and internal application of antibacterial plants (Hart & Hart, 2018; Villalba et al., 2014), and the adoption of an overall behavioral style that is risk-aversive, cautious, and vigilant (Barber & Dingemanse, 2010; Kortet et al., 2010). The intensity of these behavioral controls increases as a function of pathogenic and parasitic prevalence (Hart, 2011).
The evolutionary explanation follows that, as a recurrent challenge in evolutionary history, pathogenic stress was instrumental in shaping animal behavior, and particularly human behavioral responses to infectious diseases known as the human behavioral immune system (Murray & Schaller, 2009; Wu & Chang, 2012). The human behavioral immune system consists of self-perceived vulnerability to disease transmission, sensitivity and aversion to infection, and vigilance/caution regarding adherence to preventive/treatment-related behaviors. In addition, because engaging in a trial and error approach (i.e., individual learning style; Boyd & Richerson, 1988) may be fatal when dealing with infectious diseases, a social learning style (i.e., copying existing solutions; Boyd & Richerson, 1988), together with its underlying group-focused attributes such as conformity, compliance, and respect for authority, is a more effective approach and has been more prevalently adopted in populations with higher levels of pathogenic stress (Chang et al., 2011). These factors could explain the differences in governmental implementation of, and populational responses to, COVID-19 related preventive measures. In particular, we expect governments and populations in regions with higher historical pathogen prevalence to exert more effort in implementing and adhering to COVID-19 containment measures. The opposite is predicted for countries with less exposure to pathogens in the past.
LH Theorizations and Predictions
Pathogenic stress also shapes LH strategies that regulate a range of behaviors (Del Giudice et al., 2015; Ellis et al., 2009; Figueredo et al., 2018), including one’s responses to the COVID-19 pandemic (Corpuz et al., 2020). LH theory distinguishes between the intrinsic component of mortality risk, which relates to mortality-causing threats that an organism has some control in overcoming (e.g., thorough somatic investment or behavioral change), and extrinsic mortality risk, which relates to threats resulting in age-specific mortality and morbidity despite individual organisms’ survival efforts (Ellis et al., 2009; Stearns, 1992; Williams, 1957). Intrinsic risk is associated with a slow LH strategy that is future oriented via the prioritization of somatic development over reproduction, caution over risk taking, and rational thinking and planning over impulsivity and emotionality because the organism has some control over the environment and can therefore attempt to maximize future fitness gains. Extrinsic risk is linked to a fast LH strategy that favors early and active mating, impulsive and carefree behavior, and immediate reward in order to capitalize on residual fitness before extrinsic mortality and morbidity strike (Ellis et al., 2009; Figueredo et al., 2018). Most parasites and pathogens represent intrinsic risks because they do not cause species-wide adult mortalities but are differentially tolerated or resisted by individuals of the host population, which leads to individual differences in disease susceptibility or defensibility (Schmid-Hempel, 2003). As reviewed earlier, much of disease defense is achieved through conscious behavioral control efforts that are generally trade-offs of faster growth and reproduction. For example, great tits (Parus major) attempt to behaviorally control hen flea (Ceratophyllus gallinae) infestation by waiting for the hematophagous adults of the previous season to leave the nests. Field experiments show that, compared to great tits assigned to clean nests, those from infested ones delayed egg laying and hatching by 11 days (Oppliger et al., 1994), and both parents but especially fathers from infested nests would increase parental investment (e.g., increasing feeding trips) to achieve fewer breeding failures, larger offspring size, and a greater number of first-year-grand-offspring (Heeb et al., 1998). The animal’s behavioral control of ectoparasites represents the cognitive and behavioral aspects of slow LH strategies that resulted in delayed reproduction but uncompromised or improved fitness (Figueredo et al., 2018).
In these and earlier examples, the animals traded current reproduction for disease prevention. This behavioral manifestation of a slow LH strategy draws a contrast to the adoption of an internally mediated fast LH strategy (e.g., the predictive adaptive response model, Gluckman et al., 2005; Nettle et al., 2013) when an animal contracts a fatal infectious disease. For example, marine snails (Cerithidea califomica) that were suffering from long-term parasitic infections would mature more quickly (Lafferty, 1993), and young female Tasmanian devils (Sarcophilus harrisii) would breed precoitally when infected with a deadly cancer on the face (Jones et al., 2008). As shown in these examples, the animals would as a last resort respond with fast or super-fast LH strategies once they have succumbed to a lethal or unrecoverable infection. However, before succumbing to a disease, the animals’ predictive adaptive response seems to represent cognitive and behavioral manifestations of a slow LH strategy whereby the animals would first try various prophylactic methods to control the disease (Sarabian et al., 2018), with this slow LH preventive effort being a function of disease prevalence (Hart, 2011).
Pathogen prevalence is expected to be even more strongly associated with slow LH strategies in humans as compared to other animals because human disease control efforts and abilities that contribute to nonuniform disease susceptibility in the adult population (Van Sluijs et al., 2017) are more advanced and have more intraspecific variations than other animals. Compared to other animals, infectious diseases are even less likely to cause uniform adult mortalities in humans but, instead, could create more selective pressure on the slow LH evolution of disease control strategies and related psychological mechanisms. In a manner similar to, but in a more advanced form than other animals, humans engage in disease control via the adoption of external preventive and interventional strategies such as traditional herbal medicine, which is present in almost all ancestral human groups (e.g., Petrovska, 2012; Sneader, 2005), and via the development of an elaborate behavioral immune system, including attitudes and beliefs about disease control as well as the relevant temperament and personalities to facilitate implementation (Chang et al., 2011). The current COVID-19 related preventive measures such as the quarantining of at-risk individuals, travel restrictions, and the caring for the sick have been systematically practiced by human ancestors ranging from Homo erectus to prehistorical human groups (Conti & Gensini, 2007; Fincher & Thornhill, 2008; Walker & Shipman, 1997). Associated with these disease control efforts are cognitive and behavioral manifestations of slow LH strategies such as the inclinations to preserve life and to conserve energy, to exercise caution and to gain insight and control, and to maintain an affiliative, cooperative, and altruistic relationship with conspecifics (Chang et al., 2019b; Figueredo et al., 2018). As with other animals, these disease control endeavors are a function of disease prevalence that modulates the cost-benefit ratio in relation to LH trade-off decisions (Hart, 2011; Richner, 1998). According to this theoretical framework, populations in regions with higher levels of pathogenic stress would adopt slower LH strategies, while those in regions with lower levels of pathogenic stress would instead pursue faster LH strategies. We hypothesize a positive association between slow LH strategies and greater concerted efforts in containing the COVID-19 pandemic.
Method
Measurement
Historical pathogen prevalence
Based on epidemiological atlases of the world from 1940 to 1960, Murray and Schaller (2009) compiled a 9-disease index (i.e., leishmanias, schistosomes, trypanosomes, leprosy, malaria, typhus, filariae, dengue, and tuberculosis) representing historical pathogen prevalence for 160 countries and regions. The 9 diseases were each rated on a 4-point scale (0 = completely absent or never reported, 1 = rarely reported, 2 = sporadically or moderately reported, 3 = present at severe levels or epidemic levels at least once) and were combined and standardized into one z-score. This variable is normally distributed (M = .15; SD = .65; Skewness (Sk) = −.37; Kurtosis (Kurt) = −.76) in the present sample of 154 countries.
Slow LH strategy
The Arizona Life History Battery (ALHB, Figueredo et al., 2007) consisted of 199 items that were adopted and adapted from existing psychological instruments. As a measure of slow LH, ALHB has rarely been used due to its vast number of items. Two shorter versions of ALHB, the mini-K (Figueredo et al., 2006), a 20 item-scale that has captured the meaning of slow LH but is not a subset of ALHB, and the K-SF-42 (Figueredo et al., 2017), a 42-item scale with items selected from ALHB, are widely utilized in the literature. All three versions, ALHB, Mini-K, and K-SF-42, measure behavioral and cognitive aspects of LH strategies on a single continuum in the direction of slow LH (Figueredo et al., 2017). We have identified 17 items from the World Value Survey (WVS, 2014), that are conceptually similar to items in both the mini-K and the K-SF-42, for use in this study. The LH items were rated on different scale points ranging from 2 to 6 points. We equated all 17 items on a 4-point scale, which is the most frequently used scale point among the 17 items. We used the same items previously to measure slow LH strategy (Zhu & Chang, 2020). The items which are grouped by the ALHB subscales (Figueredo et al., 2007) are presented in the Online Supplemental Material.
WVS (2014) has been conducted in about 90 countries using nationally representative samples of over 1,000 adults per country or region. Since its commencement in 1981, WVS has gone through seven cycles with Wave 7 still currently in progress. The latest publicly available data is from Wave 6 (conducted between 2010 and 2014). The survey was conducted either via face-to-face or telephone interviews using the same set of questionnaires for all the participating countries. However, not all countries have participated in each wave, nor had participants answered all the questionnaires when they have participated. We have mainly used the LH items from Wave 6. In the event that a country’s data was unavailable from Wave 6, data from the preceding Waves were used instead. We were able to obtain data from 93 countries that have addressed at least some of the 17 questions of interest to this study. We conducted a confirmatory factor analysis on the items, which extracted a single factor with acceptable psychometric properties. The internal consistency reliability estimate was acceptable (α = .66) and the variable was normally distributed (M = 2.98; SD = .27; Sk = −.37; Kurt = .45).
Time taken by government to implement mobility restrictions
We have obtained this measure from data provided by the Oxford COVID-19 Government Response Tracker (OxCGRT, 2020) and Our World in Data Coronavirus Pandemic (Roser et al., 2020). These datasets provide information about governmental responses to the pandemic from more than 160 countries. The variable measures the number of days that have elapsed from the first identified local COVID-19 case before the government started implementing restrictions on population movements. A CFA found that the variable was moderately positively skewed (M = 22.68; SD = 19.57; Sk = .72; Kurt = −.03). A linear transformation yielded similar results and hence the original data was retained.
Percentage change in people’s visits to public places
This measure was based on the COVID-19 Community Mobility Reports Data Set (Google LLC, 2020a). This is a composite score of differences regarding mobility (percentage changes in the number of visits to parks, retail shops, recreational places, and public transit stations), where the median values of the most recent 5-week span of time with available data (i.e., from July 22 to August 25, 2020) are compared with those of the initial 5-week span (i.e., from January 30, 2020 to February 6, 2020) which Google has set as the baseline representing a “recent period, before widespread disruption as communities responded to COVID-19” (Google LLC, 2020a, 2020b). The variable was found to be normally distributed (M = −61.43; SD = 91.29; Sk = .28; Kurt = −.14) with negative numbers representing negative percentage change or decrease in visits to public places.
Results
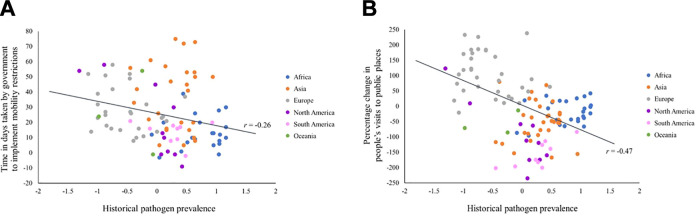
We computed zero-order correlations (see Figure 1) between historical pathogen prevalence (Murray & Schaller, 2009) and the two COVID-19-related mobility restriction measures. As expected, the correlations were both negative and significant (Time taken by government to implement mobility restrictions: r = −.26, p < .05, n = 94 countries; Percentage change in people’s visits to public places: r = −.47, p < .001, n = 114 countries), suggesting that countries in regions with higher historical pathogenic stress have responded to the current pandemic more vigilantly (i.e., governments have implemented mobility restrictions sooner and people have reduced visits to public places to a greater extent).
Figure 1.
Correlation between historical pathogen prevalence and (A) time taken by government to implement mobility restrictions, (B) percentage change in people’s visits to public places.
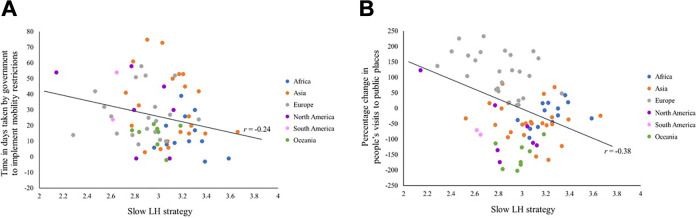
We also computed the zero-order correlations between the 17-item slow LH scale and the two COVID-19 preventive measures and we found that the zero-order correlations (see Figure 2) with both time taken by government to implement mobility restrictions (r = −.24; p < .05, n = 70 countries) and percentage change in people’s visits to public places (r = −.38, p < .001, n = 81 countries) were negative and significant. The results suggested that LH strategies can account for differences in COVID-19 preventive behaviors, where slower LH strategies are linked to more timely and vigilant responses from both the government and the population.
Figure 2.
Correlation between slow LH strategy and (A) time taken by government to implement mobility restrictions, (B) percentage change in people’s visits to public places.
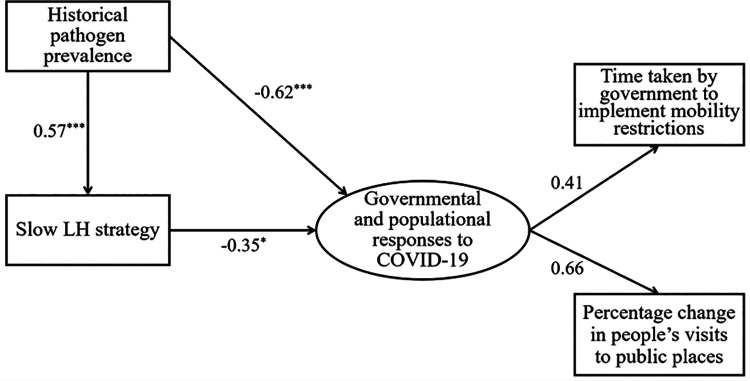
A path analysis was subsequently conducted (see Figure 3) to examine the proposed relationship between historical pathogen prevalence and LH strategies in the same model. For the path analysis, we have combined the two COVID-19 related measures into one latent construct, termed “governmental and populational responses to COVID-19.” The results were consistent with the zero-order correlations, supporting our evolutionary theorization that historical pathogenic stress would shape slower LH strategies and that both variables could engender population characteristics that are likely to facilitate the management of the COVID-19 pandemic.
Figure 3.
Path analysis of the associations among historical pathogen prevalence, slow LH strategy, and governmental and populational responses to COVID-19.
We conducted additional data analyses, reported in the Online Supplemental Material, to validate our results. We first computed zero-order correlations of the two predictor variables with two additional COVID-19 preventive measures (i.e., time taken to lockdown and time taken to close schools, both obtained from the Oxford COVID-19 Government Response Tracker and Our World in Data Coronavirus Pandemic). The correlations were equally negative and statistically significant, supporting our hypotheses. We then conducted path analysis by accounting for other potential social (i.e., Democratic Index), economic (i.e., GDP; Health expenditure as percentage of GDP), and health-related confounding variables (i.e., Total fertility rate per woman; Age-standardized mortality of all causes per 100,000 population) that are likely relevant to COVID-19. After controlling for all these variables, all our hypothesized predictions involving both historical pathogen prevalence and slow LH strategy remain supported.
Discussion
The findings suggest that historical pathogen levels and associated slow LH strategies are predictive of how swiftly and strictly governments and populations have been attempting to manage the COVID-19 pandemic. Most pathogens and parasites do not cause indiscriminate adult casualties but are differentially tolerated by the individuals of the host population in part because individuals exert different levels of disease control effort. Representing intrinsic rather than extrinsic mortality risk (Williams, 1957), local pathogen prevalence thus drives the adoption of slow LH strategies that include and are reinforced by different forms of prophylactic as well as therapeutic behaviors observable in almost all animals (Sarabian et al., 2018). These disease control behaviors would intensify as a function of pathogen prevalence and they represent the expanded cognitive repertoire of slow LH strategies (e.g., conservative sociosexual attitudes, cognitive insight, control, planning, and perseverance, and the inclination toward an inclusive and mutualistic sociality, Figueredo et al., 2018). For most animals and especially humans, pathogen and disease prevalence are major drivers of LH evolution, with disease control effort driving slower LH development (Richner, 1998) while not exercising control or losing the sense of control and succumbing to infections would promote faster LH strategies (Chang et al., 2019a). As shown by the findings of the present study, nations and populations from regions that were historically high in pathogen prevalence (e.g., China) are more vigilant about coronavirus containment efforts and their vigilance represents a slow LH inclination that results from and further promotes disease control efforts and successes. The opposite is true with countries and populations that have a lower historical pathogen prevalence and are less vigilant about containing the spread of COVID-19 (e.g., United States). The findings could afford governments with evolutionary insights into designing more effective responses in containing the current, and any future, pandemic. Specifically, strategies to control the COVID-19 pandemic should include efforts that are designed to encourage the perception of the pandemic as an intrinsic risk that is controllable through personal actions. This kind of risk perception might induce slower LH inclinations and related behaviors that are conducive to the vigilant management of a pandemic.
The perception of extrinsic risk and uncontrollability, on the other hand, might activate a fast LH and related carefree mentality that might be oblivious to the challenges of the ongoing pandemic. The extent to which one commits to disease control efforts corresponds to the nature of the encountered risk being controllable through personal effort or uncontrollable in affecting the adult population indifferently. Behavioral control and prevention efforts (e.g., committing to social quarantine instead of going on a date) represent extra survival effort that slows down other aspects of life such as growth and reproduction. Uncontrollable or extrinsic risk increases investment in fertility and reduces investment in survival (Williams, 1957). From this perspective, fertility effort may also be seen as a form of disease control for the next generation through the recruitment of good genes. The existing literature lends support to this contention. Pathogen stress is associated with more polygynous marriage practice that is gene-based and non-sororal (Low, 1990). Gene-based and nonsororal polygyny serves to recruit good genes and to increase genetic variations both to improve offspring’s immune system and overall health. Similarly, both country-level communicable disease and experimentally induced pathogen stress on individuals are correlated with a visual preference for symmetric and healthy faces for both men and women (e.g., Ainsworth & Maner, 2019; DeBruine et al., 2010; Little et al., 2011). Ultimately, both slow and fast LH strategies attempt to control infectious disease either by increasing behavioral immune efforts or by enhancing the physical immune effectiveness of the next generation.
Our findings and theoretical framework could also lay the foundations for some potentially useful post-COVID-19 predictions. If we can soon medically-control COVID-19 effectively, faster LH adaptations in the form of less restricted sociosexual attitudes and behaviors, greater consumer spending (especially on luxury products), and accelerated economic growth and productivity are predicted to occur (Ellis et al., 2009). This will likely be a global trend led by relatively historically pathogen-free regions (e.g., United States). If COVID-19 is not effectively controlled quickly enough and living with the coronavirus becomes the new normal for an extended period, slow to super-slow LH adaptations will prevail. Governments will likely intensify COVID-19-related measures, and people will become more compliant with them. Possibly influenced by historically pathogen-afflicted regions (e.g. China), this global LH strategy should result in the rise of conservative sociosexual values/behaviors, generally slower but more sustainable forms of economic development, and greater international/interpersonal cooperation and conflict resolution.x However, some populations, especially those in historically relatively pathogen-free regions, could predictably persist in pursuing faster LH strategies, thereby polarizing worldviews and disrupting intergroup relations. Altogether, we surmise that, with the lengthening of the pandemic, the strength of the faster LH prediction will gradually decrease in tandem with a gradual increase in the strength of the slower LH prediction, as attempts at controlling otherwise uncontrollable extrinsic risks would conceivably induce ever-slower LH strategies (Chang et al., 2019b; Del Giudice et al., 2015; Ellis et al., 2009).
There are two sets of limitations with the present study. On a theoretical level, LH applications in psychology, like the present study, are criticized for investigating specific, often independent ideas induced from observations, whereas LH research is said to be based on mathematically explicit models from which to deduce predictions (Frankenhuis & Nettle, 2020). More specifically, LH research in psychology assumes and attempts to measure within-species LH trait variations, whereas the overall assumption for within-species trait variations is questionable (Stearns & Rodrigues, 2020), and assuming and measuring such individual differences in humans are questioned (Međedović, 2020; Zietsch & Sidari, 2020). In light of these criticisms, we acknowledge that the present study attempts to test the specific idea that disease history and life history are correlated with efforts to control COVID-19 but we do not claim to have deduced our predictions from formal models or to have aligned them fully consistent with biological expositions of LH theory. However, psychological LH research does not have to be conceived as “psychology imitating biology” but should develop its own theoretical framework and methodological approach. Assuming, measuring, and testing latent trait variations from observed variables such as questionnaires is the unique psychological approach that fits humans as the unique speaking animal species and should be used even though the obtained LH constructs may not be fully consistent with the original formulations as long as the potential limitations and delimitations are acknowledged. The mini-K and K-SF-42 items used in the present study are meant to be “a set of cognitive and behavioral indicators of LH strategy” (Figueredo et al., 2017, p. 4) that is narrower in meaning than the original construct derived from biological as well as behavioral observations of other animals in biological research. However, this is not a serious limitation because our aim is not on physical but on behavioral immunity and disease control that involve primarily cognitive and behavioral systems for all animals. Future psychological LH researchers should make more deductive efforts in developing psychological predictions within the LH theoretical framework and be mindful of the fact that the theoretical framework is derived from between-species, higher taxonomic observations, but should not entirely abandon the latent trait and psychometric approach. Anything that differs in amount or kind can be measured (Thorndike, 1918). When human research subjects speak unlike other animals, there is no reason not to ask them questions directly. LH strategy that can only be derived from patterns of LH traits for other animals can also be directly observed or reported with humans. Efforts can be made to refine and redefine the underlying constructs, which are always imbedded in the method by which they are derived from.
Methodologically, there are limitations in using pre-existing data to measure constructs and test their associations. In general, this kind of study has lower statistical power than those that have collected primary data in a bid to test particular hypotheses. Specifically, for example, in calculating percentage changes regarding people’s visits to public places, Google has used the same time period, between January 3rd and February 6th, as the baseline for all countries. Where our analyses are concerned, this would potentially reduce variance and could attenuate the statistical strength of expected associations because countries around the world could have encountered their first known local case of COVID-19 (and have therefore taken preventive measures) on different dates or time periods. Attenuation of effects and other statistical errors may also result from using a limited number of items from the WVS to measure cognitive and behavioral aspects of a slow LH strategy and aggregating these individual-level items to perform country-level analyses. However, aggregate data are widely used especially when the concept under consideration is relevant only at the individual level as is the case with the present study, and we have conducted a confirmatory factor analysis to extract a single slow LH factor that meets psychometric requirements (Zhu & Chang, 2020). Finally, using publicly available data also means leaving out variables that are unavailable. Many macro- and microenvironmental factors, in addition to pathogenic stress, could shape LH strategies. In the present study, we have focused only on historical pathogen prevalence as a correlate of slow LH and COVID-19 preventive behaviors. We were unable to and did not intend to investigate any other LH or pathogenrelated questions, and our predictions prevailed after controlling many potential confounding variables. We are therefore confident that, despite these and other limitations, the demonstrated associations based on our analyses of different public datasets were not incidental but are instead a reflection of the effects both disease history and life history have on how governments and populations respond to the COVID-19 pandemic.
Supplemental Material
Supplemental Material, sj-pdf-1-evp-10.1177_14747049211000714 for Disease History and Life History Predict Behavioral Control of the COVID-19 Pandemic by Hui Jing Lu, Yuan Yuan Liu, Jiaqing O, Shaolingyun Guo, Nan Zhu, Bin Bin Chen, Jennifer E. Lansford and Lei Chang in Evolutionary Psychology
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work is supported by a General Research Fund (Project Number: 15608415) from the Research Grants Council of the Hong Kong Special Administrative Region and a Multiyear Research Grant (MYRG2018-00100-FSS) from the University of Macau.
ORCID iD: Hui Jing Lu  https://orcid.org/0000-0003-4025-3160
https://orcid.org/0000-0003-4025-3160
Lei Chang  https://orcid.org/0000-0001-6457-0254
https://orcid.org/0000-0001-6457-0254
Supplemental Material: Supplemental material for this article is available online.
References
- Ainsworth S. E., Maner J. K. (2019). Pathogen avoidance mechanisms affect women’s preference for symmetrical male faces. Evolutionary Behavioral Sciences, 13, 265. [Google Scholar]
- Barber I., Dingemanse N. J. (2010). Parasitism and the evolutionary ecology of animal personality. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 4077–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer D. C., Butler M. J., Shields J. D. (2006). Avoidance of disease by social lobsters. Nature, 441(7092), 421–421. [DOI] [PubMed] [Google Scholar]
- Betsch C. (2020). How behavioural science data helps mitigate the COVID-19 crisis. Nature Human Behavior, 4, 438–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackbox Research & Toluna. (2020). The world in crisis. A global public opinion survey across 23 countries (summary report). https://issuu.com/blackbox4/docs/world_in_crisis_final_report?fr=sZTM1ODEyNzA0Nzc
- Boyd R., Richerson P. J. (1988). An evolutionary model of social learning: The effects of spatial and temporal variation. In Zentall T., Galef B. G. (Eds.), Social learning: A psychological and biological approaches (pp. 29–48). Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Bush S. E., Clayton D. H. (2018). Anti-parasite behaviour of birds. Philosophical Transactions of the Royal Society B, 373(1751), 20170196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. Lu H. J. Lansford J. E. Bornstein M. H. Steinberg L. Chen B. Skinner A. T. Dodge K. A. Deater-Deckard K. Bacchini D. Pastorelli C. Alampay L. P. Tapanya S. Sorbring E. Oburu P. Al-Hassan S. Di Giunta L. Malone P. S. Uribe Tirado L. M.…Yotanyamaneewong S. (2019. b). External environment and internal state in relation to life history behavioural profiles of adolescents in nine countries. Proceedings of the Royal Society B: Biological Sciences, 286(1917), 20192097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Lu H. J., Lansford J. E., Skinner A. T., Bornstein M. H., Steinberg L., Dodge K., Chen B. B., Tian Q., Bacchini D., Deater-Deckard K., Pastorelli C., Alampay L. P., Sorbring E., Al-Hassan S. M., Oburu P., Malone P. S., Di Giunta L., Uribe Tirado L. M.…Tapanya S. (2019. a). Environmental harshness and unpredictability, life history, and social and academic behavior of adolescents in nine countries. Developmental Psychology, 55(4), 890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Mak M. C. K., Li T., Wu B. P., Lu H. J., Chen B. B. (2011). Cultural adaptations to environmental variability: An evolutionary account of East-West differences. Educational Psychology Review, 23(1), 99–129. [Google Scholar]
- Conti A., Gensini G. F. (2007). The historical evolution of some intrinsic dimensions of quarantine. Journal of History of Medicine, 19(1), 173–188. [PubMed] [Google Scholar]
- Corpuz R., D’Alessandro S., Adeyemo J., Jankowski N., Kandalaft K. (2020). Life history orientation predicts COVID-19 precautions and projected behaviors. Frontiers in Psychology, 11, 1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruine L. M., Jones B. C., Crawford J. R., Welling L. L., Little A. C. (2010). The health of a nation predicts their mate preferences: cross-cultural variation in women’s preferences for masculinized male faces. Proceedings of the Royal Society B: Biological Sciences, 277, 2405–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M., Gangestad S. W., Kaplan H. S. (2015). Life history theory and evolutionary psychology. In Buss D. M. (Ed.), The handbook of evolutionary psychology (pp. 88, 114). Wiley. [Google Scholar]
- Ellis B. J., Figueredo A. J., Brumbach B. H., Schlomer G. L. (2009). Fundamental dimensions of environmental risk. Human Nature, 20(2), 204–268. [DOI] [PubMed] [Google Scholar]
- Figueredo A. J., Garcia R. A., Menke J. M., Jacobs W. J., Gladden P. R., Bianchi J., Patch E. A., Beck C. J., Kavanagh P. S., Sotomayor-Peterson M., Jiang Y., Li N. P. (2017). The K-SF-42: A new short form of the Arizona life history battery. Evolutionary Psychology, 15(1), 1474704916676276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueredo A. J., Jacobs W. J., Gladden P. R., Bianchi J., Patch E. A., Kavanagh P. S., Beck C. J. A., Sotomayor-Peterson M., Jiang Y., Li N. P. (2018). Intimate partner violence, interpersonal aggression, and life history strategy. Evolutionary Behavioral Sciences, 12(1), 1–31. [Google Scholar]
- Figueredo A. J., Vásquez G., Brumbach B. H., Schneider S. M. (2007). The K-factor, covitality, and personality. Human Nature, 18(1), 47–73. [DOI] [PubMed] [Google Scholar]
- Figueredo A. J., Vásquez G., Brumbach B. H., Schneider S. M., Sefcek J. A., Tal I. R., Hill S., Wenner C., Jacobs W. J. (2006). Consilience and life history theory: From genes to brain to reproductive strategy. Developmental Review, 26(2), 243–275. [Google Scholar]
- Fincher C. L., Thornhill R. (2008). Assortative sociality, limited dispersal, infectious disease and the genesis of the global pattern of religion diversity. Proceedings of the Royal Society B: Biological Sciences, 275(1651), 2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhuis W. E., Nettle D. (2020). Current debates in human life history research. Evolution and Human Behavior, 41, 469–473. [Google Scholar]
- Gluckman P. D., Hanson M. A., Spencer H. G. (2005). Predictive adaptive responses and human evolution. Trends in Ecology & Evolution, 20(10), 527–533. [DOI] [PubMed] [Google Scholar]
- Google LLC. (2020. a). Google COVID-19 community mobility reports. https://www.google.com/covid19/mobility/
- Google LLC. (2020. b). Community Mobility Reports Help. https://support.google.com/covid19-mobility/answer/9824897?hl=en-GB&ref_topic=9822927
- Hart B. L. (2011). Behavioural defences in animals against pathogens and parasites: Parallels with the pillars of medicine in humans. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1583), 3406–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B. L., Hart L. A. (2018). How mammals stay healthy in nature: The evolution of behaviours to avoid parasites and pathogens. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1751), 20170205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb P., Werner I., Kölliker M., Richner H. (1998). Benefits of induced host responses against an ectoparasite. Proceedings of the Royal Society of London. Series B: Biological Sciences, 265(1390), 51–56. [Google Scholar]
- Jones M. E., Cockburn A., Hamede R., Hawkins C., Hesterman H., Lachish S., Mann D., McCallum H., Pemberton D. (2008). Life-history change in disease-ravaged Tasmanian devil populations. Proceedings of the National Academy of Sciences, 105(29), 10023–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortet R., Hedrick A. V., Vainikka A. (2010). Parasitism, predation and the evolution of animal personalities. Ecology Letters, 13(12), 1449–1458. [DOI] [PubMed] [Google Scholar]
- Lafferty K. D. (1993). The marine snail. Cerithidea Californica, matures at smaller sizes where parasitism is high. Oikos, 68(1), 3–3. [Google Scholar]
- Little A. C., DeBruine L. M., Jones B. C. (2011). Exposure to visual cues of pathogen contagion changes preferences for masculinity and symmetry in opposite-sex faces. Proceedings of the Royal Society B: Biological Sciences, 278, 2032–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. S. (1990). Marriage systems and pathogen stress in human societies. American Zoologist, 30, 325–340. [Google Scholar]
- Međedović J. (2020). On the incongruence between psychometric and psychosocial-biodemographic measures of life history. Human Nature, 31, 341–360. [DOI] [PubMed] [Google Scholar]
- Murray D. R., Schaller M. (2009). Historical prevalence of infectious diseases within 230 geopolitical regions: A tool for investigating origins of culture. Journal of Cross-Cultural Psychology, 41(1), 99–108. [Google Scholar]
- Nettle D., Frankenhuis W. E., Rickard I. J. (2013). The evolution of predictive adaptive responses in human life history. Proceedings of the Royal Society Biological Science, 280(1766), 20131343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppliger A., Richner H., Christe P. (1994). Effect of an ectoparasite on lay date, nest-site choice, desertion, and hatching success in the great tit (Pants major). Behavioral Ecology, 5(2), 130–134. [Google Scholar]
- Oxford Coronavirus Government Response Tracker. (2020). https://www.bsg.ox.ac.uk/research/research-projects/coronavirus-government-response-tracker
- Petrovska B. B. (2012). Historical review of medicinal plants’ usage. Pharmacognosy Reviews, 6(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner H. (1998). Host-parasite interactions and life-history evolution. Zoology, 101(4), 333–344. [Google Scholar]
- Roser M., Ritchie H., Ortiz-Ospina E., Hasell J. (2020). Coronavirus pandemic (COVID-19). Published online at OurWorldInData.org. https://ourworldindata.org/coronavirus
- Rupiva M. (2020, April23). At war with Covid-19: Opportunities for Africa? Mail & Guardian. https://mg.co.za/article/2020-04-23-at-war-with-covid-19-opportunities-for-africa/
- Sanchez G. H. (2020, March23). 24 Pictures of Americans failing horribly at social distancing during the Coronavirus outbreak. BuzzFeedNews. https://www.buzzfeednews.com/article/gabrielsanchez/americans-coronavirus-social-distancing-shelter-in-place
- Sarabian C., Curtis V., McMullan R. (2018). Evolution of pathogen and parasite avoidance behaviors. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1751), 20170256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M., Murray D. R. (2008). Pathogens, personality, and culture: Disease prevalence predicts worldwide variability in sociosexuality, extraversion, and openness to experience. Journal of Personality and Social Psychology, 95(1), 212–221. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. (2003). Variation in immune defence as a question of evolutionary ecology. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1513), 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R., Kim Y. K., Hua J. (2020). Governance, technology and citizen behavior in pandemic: Lessons from COVID-19 in East Asia. Progress in Disaster Science, 6, 100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokoohi M., Osooli M., Stranges S. (2020). COVID-19 pandemic: What can the West learn from the East. International Journal of Health Policy and Management, 9(10), 436–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneader W. (2005). Drug discovery: A history. John Wiley & Sons. [Google Scholar]
- Stearns S. C. (1992). The evolution of life histories. Oxford University. [Google Scholar]
- Stearns S. C., Rodrigues A. M. (2020). On the use of “life history theory” in evolutionary psychology. Evolution and Human Behavior, 41, 474–485. [Google Scholar]
- Thorndike E. L. (1918). The nature, purposes, and general methods of measurements of educational products. In Whipple G. M. (Ed.), The Seventeenth yearbook of the National Society for Study of Education. Part II. The Measurement of Educational Products, Vol. 2 (pp. 16–24). Public School Publishing Co. [Google Scholar]
- Van Sluijs L., Pijlman G. P., Kammenga J. E. (2017). Why do individuals differ in viral susceptibility? A story told by model organisms. Viruses, 9(10), 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba J. J., Miller J., Ungar E. D., Landau S. Y., Glendinning J. (2014). Ruminant self-medication against gastrointestinal nematodes: Evidence, mechanism, and origins. Parasite, 21, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadvalla B. A. (2020). Covid-19: Decisive action is the hallmark of South Africa’s early success against coronavirus. BMJ, 369, m1623. [DOI] [PubMed] [Google Scholar]
- Walker A., Shipman P. (1997). The wisdom of the bones: In search of human origins. Vintage. [Google Scholar]
- Ward A. (2020). Anti-lockdown protests aren’t just an American thing. They’re a global phenomenon . https://www.vox.com/2020/5/20/21263919/anti-lockdown-protests-coronavirus-germany-brazil-uk-chile
- Williams G. C. (1957). Pleiotropy, natural selection, and the evolution of senescence. Evolution, 11(4), 398–411. [Google Scholar]
- World Values Survey Association. (2014). World values survey: All rounds-country-pooled datafile 1981–2014. JD Systems Institute. http://www.worldvaluessurvey.com [Google Scholar]
- Wu B. P., Chang L. (2012). The social impact of pathogen threat: How disease salience influences conformity. Personality and Individual Differences, 53(1), 50–54. [Google Scholar]
- Zhu N., Chang L. (2020). An evolutionary life history explanation of sexism and gender inequality. Personality and Individual Difference, 157, 109806. [Google Scholar]
- Zietsch B. P., Sidari M. J. (2020). A critique of life history approaches to human trait covariation. Evolution and Human Behavior, 41, 527–535. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-evp-10.1177_14747049211000714 for Disease History and Life History Predict Behavioral Control of the COVID-19 Pandemic by Hui Jing Lu, Yuan Yuan Liu, Jiaqing O, Shaolingyun Guo, Nan Zhu, Bin Bin Chen, Jennifer E. Lansford and Lei Chang in Evolutionary Psychology