Abstract
Studies with adults found a memory bias for disgust, such that memory for disgusting stimuli was enhanced compared to neutral and frightening stimuli. We investigated whether this bias is more pronounced in females and whether it is already present in children. Moreover, we analyzed whether the visual exploration of disgust stimuli during encoding is associated with memory retrieval. In a first recognition experiment with intentional learning, 50 adults (mean age; M = 23 years) and 52 children (M = 11 years) were presented with disgusting, frightening, and neutral pictures. Both children and adults showed a better recognition performance for disgusting images compared to the other image categories. Males and females did not differ in their memory performance. In a second free recall experiment with eye-tracking, 50 adults (M = 22 years) viewed images from the categories disgust, fear, and neutral. Disgusting and neutral images were matched for color, complexity, brightness, and contrast. The participants, who were not instructed to remember the stimuli, showed a disgust memory bias as well as shorter fixation durations and longer scan paths for disgusting images compared to neutral images. This “hyperscanning pattern” correlated with the number of correctly recalled disgust images. In conclusion, we found a disgust-related memory bias in both children and adults regardless of sex and independently of the memorization method used (recognition/free recall; intentional/incidental).
Keywords: disgust, memory bias, visual, eye-tracking, children, adults
An evolutionary perspective on disgust proposes that this basic emotion evolved to protect humans from infectious diseases (e.g., Curtis et al., 2004; Rozin et al., 2008; Tybur et al., 2013). Disgust is part of the “behavioral immune system” (Schaller & Duncan, 2007) that motivates individuals to avoid and eliminate pathogens (e.g., via hygiene behaviors). Core disgust elicitors, such as spoiled food and body secretions (e.g., blood, excrements) are warning signals of possible pathogen presence (Curtis et al., 2004). It is important to memorize the sensory properties (e.g., visual, olfactory) of these disgust elicitors to effectively avoid them in the future and to prevent infectious disease.
In line with this view, research has provided evidence of a mnemonic advantage for disgust. Disgust stimuli are better remembered than neutral stimuli. For example, Duesenberg et al. (2016) found that participants recognized disgust words better than neutral words. In a study by Prokop et al. (2014), an instructor delivered two lectures on parasites and hormones. The students retained more knowledge about the disgusting lecture contents. In a series of experiments, Fernandes et al. (2017) administered images depicting neutral objects that were either touched by sick people or healthy people. In each of the experiments, the contaminated objects were remembered better. Other investigations identified a memory enhancement for faces presented in disgusting contexts compared to neutral contexts (Bell & Buchner, 2010).
The mnemonic advantage for disgust is not only present when compared to neutral information, but also when compared to fear (e.g., Charash & McKay, 2002). Several experiments revealed a superior memory performance for disgusting images relative to frightening images (Chapman et al., 2013; Chapman, 2018; Croucher et al., 2011; Dandan et al., 2019; Ferré et al., 2018). This finding is noteworthy because fear and disgust are both negative, avoidance-oriented basic emotions. In some experiments (e.g., Chapman et al., 2013), the visual disgust stimuli and fear stimuli were matched in terms of arousal (level of elicited excitement) and valence (level of unpleasantness). Additionally, visual properties (e.g., visual salience, conceptual distinctiveness) were comparable between the two emotion categories (Chapman, 2018; Chapman et al., 2013). Thus, both categories of stimuli only differed in the type of elicited emotion, and no other characteristic known to influence their memorability. These findings point to a specific mnemonic advantage for disgust stimuli which is possibly rooted in an evolutionary-based disease avoidance mechanism (e.g., Curtis et al., 2004).
To collect evidence that the disgust memory bias is indeed a common mechanism in humans, the present investigation was conducted. A first experiment aimed at demonstrating that both sexes and different age groups (children, adults) show this bias. Research on sex differences in disgust processing has revealed very consistently that females report more intense disgust feelings than males. On average, females rate disgust images as more disgusting than males (e.g., Curtis et al., 2004; Schienle et al., 2005). Curtis et al. (2004) have argued that the disease-avoidance emotion disgust is more pronounced in females since they play a double role in protecting both self and offspring from infectious disease. This elevated disgust sensitivity could lead to a more pronounced disgust memory bias in women, which has not yet been investigated.
Disgust responses are only weakly developed in early childhood but become more and more pronounced in middle childhood with the development of cognitive structures necessary to conceptualize the concept of contamination (for a review see Rottman, 2014). For example, in a study by Rozin et al. (1985), children of different age groups (3–6, 6–9, 9–12 years) were asked to drink juice stirred by a comb. The responses to this contamination manipulation were very different. From the youngest group, almost all children (80%) drank the juice, whereas the oldest group showed pronounced contamination sensitivity (90% refused to drink the juice). Similarly, in a study by Stevenson et al. (2010) younger children (2.5-year-olds) showed less avoidance behavior during various revolting tasks (e.g., eat candy from the bottom of a new toilet) than older children (7-year olds). Leutgeb et al. (2010) found that children (8–12 years old) diagnosed with spider phobia displayed elevated disgust propensity. These children showed excessive disgust responses to spiders. Based on these findings, it can be expected that the disgust memory bias is already present in middle childhood.
But what is the underlying mechanism of the disgust memory bias? How is it possible that disgust images are better memorized than fear images, even when the two picture types do not differ in emotional intensity and visual properties? (e.g., Chapman et al., 2013). Research has provided evidence that disgust stimuli are not only associated with a memory bias but also with an attentional bias (Charash & McKay, 2002; for a review see Knowles et al., 2019). Disgust stimuli capture automatic visual attention and are associated with a specific viewing pattern. An eye-tracking study by Schienle et al. (2016) showed that disgust pictures prompted more and shorter visual fixations (fixations = absence of substantial eye movements and changes in gaze direction) compared to frightening and neutral pictures. The participants quickly inspected many details within the disgust pictures (“hyperscanning”), whereas their gaze was maintained on only a few areas within the other pictures (neutral, frightening). The authors suggested that this detail-oriented visual inspection of disgust stimuli supports the fast identification of health-threatening information. Whether this viewing pattern of disgust stimuli facilitates the encoding in memory has not been investigated thus far. However, a study by Wells et al. (2010) demonstrated that hyperscanning was associated with an increased recognition performance for angry faces.
The present investigation consisted of two memory experiments. In a first recognition experiment with intentional learning, we presented children (boys and girls, mean age = 11 years) and adults (males and females, mean age = 23 years) with disgusting, frightening, and neutral images. This was done to compare the disgust memory bias between the two age groups and sex groups. It was predicted that both children and adults would display this bias, which would be more pronounced in females (e.g., Curtis et al., 2004). Experiment 2 with eye-tracking focused on the association of the visual exploration of disgust images and the recall performance in adults. It was predicted that disgust images would prompt a “hyperscanning pattern” (as reflected by the inspection of many image details) compared to neutral pictures that had been matched for basic visual properties (e.g., complexity). This detail-oriented inspection (e.g., number of visual fixations) should be positively correlated with disgust memory performance (e.g., Wells et al., 2010).
Experiment 1
Methods
Participants
Adults: Twenty-five males and 25 females (all Caucasian) with a mean age of 23.4 years (SD = 2.74; range: 20–32 years) participated in the study. All of them had a high school diploma; 84% were university students, the others were white-collar workers. All participants provided written informed consent.
Children: Twenty-seven boys and 25 girls (all Caucasian) with a mean age of 11.4 years (SD = 0.89; range: 10–13 years) participated in the study. Teachers, parents, and children provided written informed consent.
The study was approved by the ethics committee of the University and performed following the Declaration of Helsinki. Exclusion criteria were reported diagnoses of mental disorders and the intake of psychotropic medication.
A previous study on the disgust memory bias (Chapman, 2018) in a free recall task with disgusting, frightening, and neutral pictures observed a large effect ( = 0.33 [f = 0.70]; Experiment 1, p. 1222). To determine the sample sizes for the present investigation (adults, children), we assumed a moderate effect (f = 0.30) between the groups (males, females) and the three picture conditions (disgusting, frightening, neutral). Then, a total sample size of 42 participants is sufficient (probability of 1–β = .95; α = .01, r = .50; see G*Power Version 3.1.9.2; Faul et al., 2007).
Stimuli
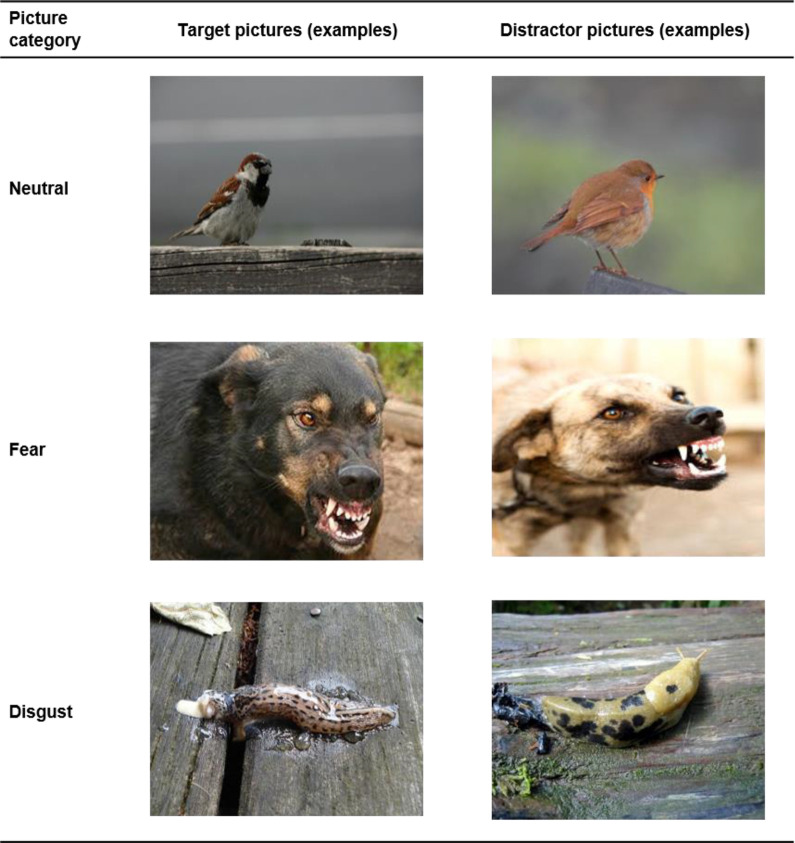
Adults and children viewed a total of 48 images from three categories (disgust, fear, neutral; see Figure 1). The neutral category comprised images of fish, birds, clocks, and glasses. Fear pictures showed aggressive dogs, sharks, guns, and car accidents. Disgust images depicted blowflies, slugs, excrements, and garbage. Each category comprised images of two different animal species and two different object types. The pictures were taken from the International Affective Picture System (Lang & Bradley, 2007), and the internet.
Figure 1.
Pictures (examples) of Experiment 1.
Procedure
The testing was performed in groups with 10–15 participants in the classroom (children) or a room at the university (adults).
The task consisted of an encoding phase, a delay phase, a recognition phase, and a picture-rating phase. During the encoding phase, 24 images (eight per category) were projected onto the wall of the room (image size: 1.2 m × 0.9 m; distance 4 m) for 3 s each in a randomized sequence. The participants were instructed to look at the pictures and to memorize them.
The picture presentation was followed by a delay period (20 min), during which answer sheet forms (paper-pencil-assessment) were handed to the participants and the evaluation procedure was explained (recognition test, affective ratings). After the delay period, the second picture presentation was conducted. The participants viewed 48 images (16 per category). Half of the images (24) were new distractor pictures and the other half (24) target pictures. Each image was presented for 3 s. Then the question “Have you seen this picture before?” appeared on the screen (answer mode: yes/ no). When all participants indicated to have answered the question, the picture presentation was continued. All participants viewed the same targets and distractors. Targets and assigned distractors did not differ in their content, complexity, t(23) = 1.56, p = .13, d = 0.32; brightness, t(23) = .01, p = .99, d < 0.01; color, red: t(23) = 1.06, p = .30, d = 0.22; green: t(23) = .49, p = .63, d = 0.10; blue: t(23) = 1.94, p = .06, d = 0.40; and contrast, t(23) = .33, p = .75, d < .07 (analyzed via a Matlab script provided by Blechert et al., 2014).
After the recognition phase, a picture-rating phase was conducted. All participants rated a selection of the same 12 target pictures on the answer sheet form (four from each category) regarding experienced disgust and fear on 9-point Likert-type scales (1 = not disgusted/not afraid; 9 = very disgusted/very afraid). We did not show all pictures again to prevent boredom and fatigue.
The participants were seated with sufficient distance between each other, so they were not able to see the answers of the other participants. They were instructed not to talk to each other during the experiment.
Statistical Analyses
The data were analyzed with mixed model analyses of variance (ANOVAs) to test the effects of PICTURE CATEGORY (neutral, fear, disgust), SEX (male, female), and AGE GROUP (children, adults) on “hits” (correctly recognized target images), “false alarms” (incorrectly recognized distractor images as targets), and “disgust” and “fear” elicited by the pictures. Effect sizes are expressed by partial eta squared ( ). If violations of sphericity occurred, Greenhouse-Geisser corrections were used. Significant effects were followed up by Holm-adjusted pairwise comparisons.
Based on the signal detection theory (e.g., Wixted, 2007), we calculated a sensitivity measure (d′) for each of the three picture categories. This measure reflects both the probability of a “hit” and the probability of a “false alarm”: d′ = z(Phit) − z(PFA). When the difference between the proportion of hits and false alarms is large, d′ is large and indicates a high subjective sensitivity (recognition accuracy).
Results
Recognition Performance
Hits
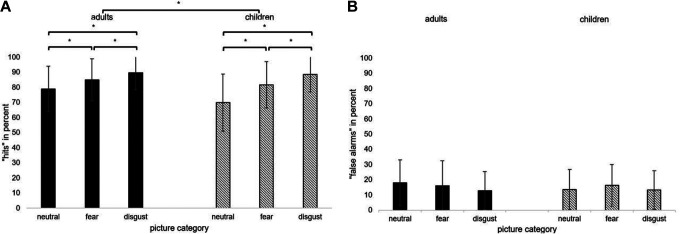
The conducted ANOVA revealed significant main effects for PICTURE CATEGORY (p < .001) and AGE GROUP (p < .05; see Table 1). Disgust pictures (M = 89%, SD = 11) were better recognized than fear pictures (M = 83%; SD = 15; t = −4.13, p < .001, d = 0.41), and fear pictures were better recognized than neutral pictures (M = 74%, SD = 17; t(101) = −4.90, p < .001; d = 0.49 see Figure 2). This pattern was the same for children and adults (children: all p < .001; adults: all p < .023; see Figure 2).
Table 1.
F(df) Values, p Values, and Values of the ANOVAs With the Within-Subjects Factor Picture Category and the Between-Subjects Factors Sex and Age Group for the Dependent Variables Hits, False Alarms, Disgust Ratings, and Fear Ratings.
| Effects | F (df) | p | ||
|---|---|---|---|---|
| Hits | ||||
| PICTURE CATEGORY | 39.68 (1.85, 181.15) | <.001 | .288 | |
| AGE GROUP | 3.99 (1, 98) | .048 | .039 | |
| SEX | 0.96 (1, 98) | .329 | .010 | |
| AGE GROUP × SEX | 0.27 (1, 98) | .602 | .003 | |
| PICTURE CAT. × AGE GROUP | 3.06 (1.85, 181.15) | .053 | .030 | |
| PICTURE CAT. × SEX | 0.94 (1.85, 181.15) | .388 | .009 | |
| PICTURE CAT. × AGE GROUP × SEX | 0.60 (1.85, 181.15) | .539 | .006 | |
| False alarms | ||||
| PICTURE CATEGORY | 2.42 (1.96, 139.9) | .093 | .024 | |
| AGE GROUP | 0.47 (1, 98) | .493 | .005 | |
| SEX | 0.10 (1, 98) | .921 | <.001 | |
| AGE GROUP × SEX | 11.39 (1, 98) | .001 | .104 | |
| PICTURE CAT. × AGE GROUP | 1.27 (1.96, 139.9) | .282 | .013 | |
| PICTURE CAT. × SEX | 1.48 (1.96, 139.9) | .230 | .015 | |
| PICTURE CAT. × AGE GROUP × SEX | 2.88 (1.96, 139.9) | .060 | .029 | |
| Disgust ratings | ||||
| PICTURE CATEGORY | 536 (1.40, 137.13) | < .001 | .846 | |
| AGE GROUP | 5.66 (1, 98) | .019 | .055 | |
| SEX | 0.03 (1, 98) | .871 | < .001 | |
| AGE GROUP × SEX | 0.07 (1, 98) | .795 | .001 | |
| PICTURE CAT. × AGE GROUP | 1.54 (1.40, 137.13) | .221 | .015 | |
| PICTURE CAT. × SEX | 1.45 (1.40, 137.13) | .237 | .015 | |
| PICTURE CAT. × AGE GROUP × SEX | 0.62 (1.40, 137.13) | .619 | .485 | |
| Fear ratings | ||||
| PICTURE CATEGORY | 245 (1.21, 118.16) | < .001 | .714 | |
| AGE GROUP | 1.96 (1, 98) | .165 | .020 | |
| SEX | 0.27 (1, 98) | .605 | .003 | |
| AGE GROUP × SEX | 0.37 (1, 98) | .544 | .004 | |
| PICTURE CAT. × AGE GROUP | 7.28 (1.21, 118.16) | .005 | .069 | |
| PICTURE CAT. × SEX | 3.03 (1.21, 118.16) | .077 | .030 | |
| PICTURE CAT. × AGE GROUP × SEX | 0.28 (1.21, 118.16) | .643 | .003 | |
Figure 2.
Mean percentages and standard deviations (error bars) of (A) “hits” and (B) “false alarms” per picture category (neutral, fear, and disgust) in children and adults (asterisks indicate p < .05).
Adults had a higher total hit rate (M = 85%; SD = 10) than children, M = 80%; SD = 12, t(100) = −2.04, p = .044, d = 0.40. The interaction PICTURE CATEGORY × AGE GROUP was marginally significant (p = .053). Adults showed a higher recognition performance for neutral pictures than children (adults: M = 79%, SD = 15; children: M = 70%, SD = 18; t(100) = −2.66, p = .009, d = .53) but not for fear (adults: M = 85%, SD = 14; children: M = 82%, SD = 15; t(100) = −1.13, p = .26, d = .22) and disgust pictures (adults: M = 90%, SD = 11; children: M = 89%, SD = 12; t(100) = −0.42, p = .68, d = .08). All other main effects and interaction effects were not statistically significant (all p > .10).
False alarms
The conducted ANOVA revealed no significant effects (all p > .05), except for the interaction AGE GROUP × SEX (see Table 1). Girls (M = 11.2%; SD = 5.86) showed less false alarms than boys (M = 17.5%; SD = 10.47). Men (M = 12.3%; SD = 10.65) showed less false alarms than women (M = 18.9%; SD = 10.71).
Recognition accuracy
In the adult sample, the recognition accuracy was higher for Disgust pictures (d′ = 2.41), than for Fear pictures (d′ = 2.03), and Neutral pictures (d′ = 1.73). The same pattern was found for children: Disgust (d′ = 2.36), Fear (d′ = 1.91), and Neutral (d′ = 1.60).
Affective Ratings
Disgust ratings
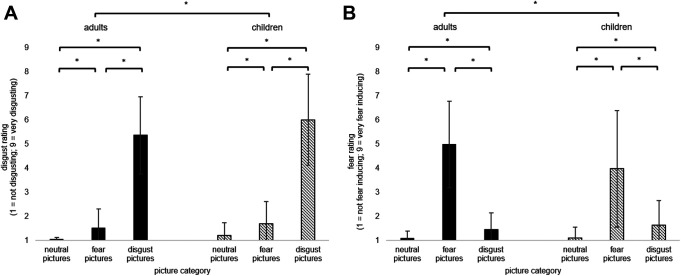
The ANOVA revealed significant main effects for PICTURE CATEGORY and AGE GROUP (see Table 1). Disgust ratings were higher for disgust pictures than fear pictures, t(101) = −22.84, p < .001, d = 2.26, and neutral pictures, t(101) = −26.08, p < .001, d = 2.58.
Disgust ratings across all categories were higher in the children sample (M = 2.97, SD = 0.75) than in the adult sample, M = 2.64, SD = 0.67; t(100) = 2.40, p = .018, d = 0.48; Figure 3. Adults (M = 5.37, SD = 1.58) and children (M = 6.01, SD = 1.88) did not differ in their disgust ratings for the Disgust pictures, t(100) = 1.87, p = .065, d = 0.37. All other main effects and interaction effects were not statistically significant (see Table 1).
Figure 3.
Mean percentages and standard deviations (error bars) of A) “disgust ratings” and B) “fear ratings” per picture category (neutral, fear, and disgust) in children and adults (asterisks indicate p < .05).
Fear ratings
The ANOVA showed a significant main effect PICTURE CATEGORY and a significant interaction PICTURE CATEGORY × AGE GROUP (see Table 1). Fear pictures received higher fear ratings (M = 4.47, SD = 2.20) than disgust pictures, M = 1.54, SD = 0.89, t(101) = 14.83, p < .001, d = 1.47; and neutral pictures (M = 1.10, SD = .36, t = −15.99, p < .001, d = 1.58).
Adults (M = 4.98, SD = 1.82) reported more fear for Fear pictures than children (M = 3.98, SD = 2.42, t(94.6) = −2.36, p = .020, d = 0.47). Adults and children did not differ in their fear ratings for neutral and disgusting pictures (all p > .05). All other main effects and interaction effects were not statistically significant (see Table 1). In summary, Experiment 1 identified a disgust memory bias in both children and adults independent of sex.
Experiment 2
The second experiment aimed at investigating attentional processes associated with the disgust memory bias. Eye-tracking was used to identify the relationship between the visual exploration of disgust images and memory performance in adults. The participants were not informed that they would have to recall the images before the eye-tracking investigation to allow free visual exploration of the pictures.
Method
Participants
A total of 50 university students (30 females, 20 males) with a mean age of 22 years (SD = 2.79) participated in the eye-tracking experiment. They had normal/corrected-to-normal vision and did not report any current somatic/mental disorders or intake of medication. All participants gave written informed consent. Experiment 2 was approved by the local ethics committee and was conducted following the Declaration of Helsinki.
Stimuli
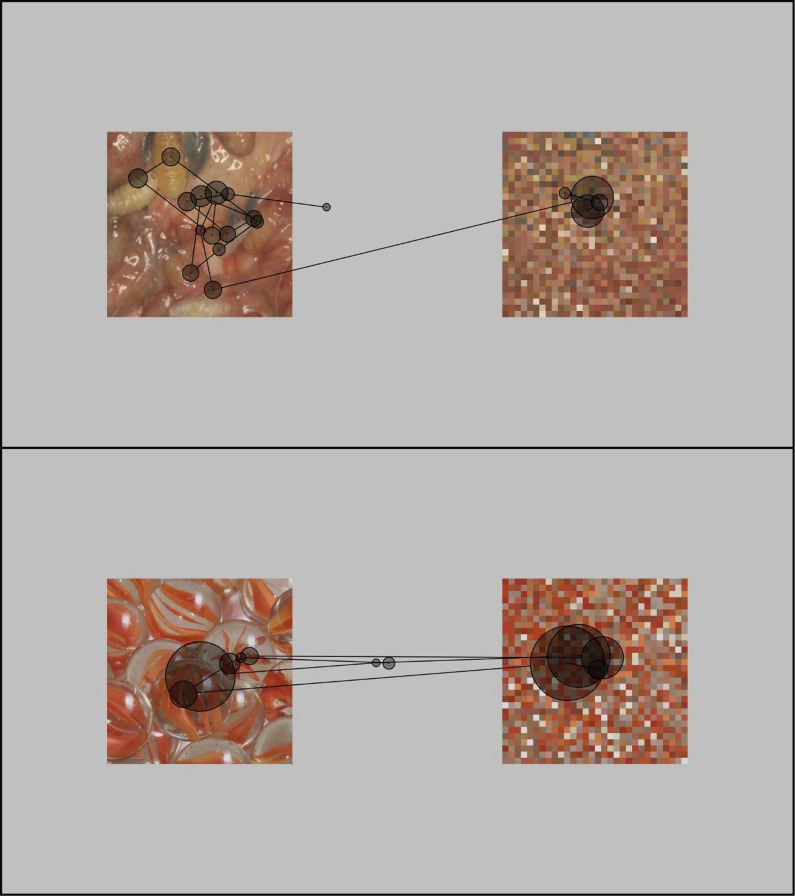
The stimulus material consisted of 24 images (450 × 450 pixels) from the categories Disgust (e.g., maggots, excrements), Fear (e.g., knife attack, tank), and Neutral (e.g., basket, glove). Each category contained eight pictures. The images were retrieved from the International Affective Picture System (Lang & Bradley, 2007) and online image databases. For each disgust image, a neutral image was selected that matched in color, R: t(7) = .659, p = .531; G: t(7) = .799, p = .451; B: t(7) = .189, p = .855, complexity, t(7) = .049, p = .962; brightness, t(7) = .004, p = .997; and contrast, t(7) = 1.231, p = .258; for the analysis see Blechert et al., 2014. For example, a picture with maggots had a corresponding neutral picture with marbles (see Figure 4). The Fear images were not visually matched with the other two categories (because it was not possible to find three visually matching pictures that induced three different emotional states).
Figure 4.
Example of scan paths from a disgust trial and a corresponding neutral trial. Lines: saccades, circles: fixations (larger circle = longer fixation).
Procedure
Each affective image (fear, disgust, neutral) was presented together with an image that depicted a random pattern of colored pixels (30 × 30 pixels; see Figure 4). Affective images and pixelated images were presented in the same size side by side on the computer screen (12° viewing angle at a viewing distance of 60 cm) on a gray background. We chose this display to allow the participants to look at the disgust pictures or to look at an alternative visual stimulus away from the disgust pictures (visual avoidance).
At the beginning of each trial, a fixation cross in the center of the screen had to be fixated for 1 s. Then the image pair was displayed for 6 s. Picture pairs were presented in a randomized order. For half of the pairs of each category, the affective image was presented on the left versus right side of the screen. Matched disgust and neutral images were presented on the same side of the screen. The arrangement of the pairs was counterbalanced across participants.
The participants were asked to look at the images as if they were watching TV. They were instructed that the study aimed at recording the pupil diameter during affective picture viewing. They were not informed about the memory experiment (incidental learning). Participants underwent a 10-min break between the eye-tracking session and the memory task. During this break, they answered questions concerning their age, sex, current/previous somatic/mental disorders, and medication. After the break, participants were asked to name all picture contents they remembered (free recall; time restriction: 5 min). Subsequently, the subjects rated each picture according to elicited fear, disgust, valence, and arousal on 9-point Likert-type scales (1 = not at all; 9 = extremely).
Gaze Data Recording and Data Analysis
Two-dimensional eye movements were recorded during the picture viewing using an SMI RED 250 mobile eye-tracker with a sampling rate of 250 Hz. To minimize head movements, a chin rest was used. Both eyes were calibrated and data from the eye that produced a better spatial resolution (< 0.35° visual angle) were used. Stimuli were presented on a 24-in. screen with a resolution of 1920 × 1080 pixels running at 60 Hz. The experiment was controlled via the SMI Experiment Center (Version 3.6.53). Data were exported using SMI BeGaze (Version 3.6.52) and customized PYTHON scripts. Gaze events were defined by the standard velocity based thresholds of BeGaze (saccade threshold: 40°/s, fixations = absence of blinks or saccades ≥ 50 ms).
The analysis of the eye-tracking data focused on fixations (time interval where the eye is kept aligned with an image detail) and saccades (eye movement used to move the fovea rapidly from one point of interest to another). The dependent variables were the number of fixations, fixation duration, length of saccades (distance between two fixated points), and scan path length (the total visual angle traveled by all saccades within the image; see Armstrong & Olatunji, 2012; Chen et al., 2015).
Statistical Analyses
Mixed model analyses of variance (ANOVAs) were computed to test the effect of PICTURE CATEGORY (neutral, fear, disgust) on the number of recalled pictures and affective ratings. Effect sizes are expressed by partial eta squared (part.η2). If violations of sphericity occurred, Greenhouse-Geisser correction was used.
To compare gaze patterns between disgust and neutral pictures, pairwise t tests were calculated. Fear stimuli were not visually comparable and hence not included in the analysis. Additionally, Pearson correlations were computed to test the association between the eye-tracking parameters (e.g., number/ duration of fixations) and memory performance (number of recalled images).
Results
Eye Movements
Number and duration of fixations
The number of fixations did not differ between Disgust images (M = 8.31, SD = 2.55) and Neutral images, M = 7.87, SD = 2.38; t(49) = 1.52, p = .13, d = 0.22. The fixation duration was shorter for Disgust images (M = 417.7 ms, SD = 156.4) compared to Neutral images, M = 544.1, SD = 247.8; t(49) = −4.53, p < .001, d = −0.64; see Figure 4).
Length of scan path and saccades
The scan path was longer for Disgust images (M = 16.72°, SD = 6.20) compared to Neutral images (M = 15.04°, SD = 6.63; t(49) = 2.12, p = .039, d = 0.30). The saccade length did not differ between Disgust images and Neutral images, t(49) = −.96, p = .34, d = −0.14; disgust: M = 2.56°, SD = 0.48; neutral: M = 2.72°, SD = 0.48.
Rating and free recall
The affective pictures elicited the target emotions with sufficient intensity (Disgust images: Mdisgust = 6.90, SD = 1.93; Fear images: Mfear = 5.58, SD = 2.32) and specificity. Disgust pictures elicited more disgust than fear, t(49) = 17.50; p < .001; d = 2.47; and fear pictures elicited more fear than disgust, t(49) = 8.44; p < .001; d = 1.19.
The ANOVAs for the valence and arousal ratings revealed significant main effects for CATEGORY, valence: F(1.78, 87.27) = 59.33, p < .001, = .55); arousal: F(1.43, 70.12) = 52.69, p < .001, = .52). Holm-corrected pairwise comparisons showed that the neutral pictures were rated as most pleasant and least arousing (all p < .001).
For the free recall task, the ANOVA revealed a significant main effect CATEGORY, F(2,98) = 48.44, p < .001, = .50. Disgust pictures were remembered better than Fear pictures, t(49) = 3.43, p = .001, d = 0.49 and Neutral pictures, t(49) = 10.88, p < .001; d = 1.54. Fear pictures were remembered better than neutral pictures, t(49) = 6.00; p < .001; d = 0.85; M and SD see Table 2). The overall memory performance was on average 46% correctly recalled images.
Table 2.
Means (M), Standard Deviations (SD), and Observed Ranges for Free Recall Performance as well as Valence and Arousal Ratings for the Three Picture Categories (Disgust, Fear, Neutral).
| Effects | M | SD | Observed Range |
|---|---|---|---|
| Percentage of correctly recalled pictures | |||
| Disgust | 61% | 20% | 13%–100% |
| Fear | 48% | 21% | 0%–100% |
| Neutral | 28% | 21% | 0%–88% |
| Valence ratings (1 = very unpleasant; 9 = very pleasant) | |||
| Disgust | 3.34 | 2.04 | 1–9 |
| Fear | 4.12 | 1.42 | 1–8 |
| Neutral | 7.06 | 2.08 | 1–9 |
| Arousal ratings (1 = very relaxing; 9 = very arousing) | |||
| Disgust | 4.70 | 2.13 | 1–9 |
| Fear | 5.18 | 2.11 | 1–9 |
| Neutral | 2.08 | 2.04 | 1–9 |
The number of recalled disgust images was negatively correlated with the mean fixation duration for these images (r = −.33, p = .02). The affective ratings (valence, arousal, disgust, fear) were not significantly correlated with the number of recalled disgust pictures (p > .16).
Discussion
The present study focused on the disgust memory bias for visual stimuli. One main finding was that this bias was present in both children and adults, and males and females. Moreover, the mnemonic advantage for disgust was independent of the chosen learning procedure (intentional, incidental) and the used memory test (recognition, free recall). These findings support the claim that the disgust memory bias is indeed a common phenomenon in humans (Knowles et al., 2019).
In Experiment 1, both children and adults attained a higher hit rate for disgust images compared to the other two categories (neutral, frightening images) and showed a high disgust recognition accuracy (d′). Children and adults did not differ in the percentage of correctly recognized disgust pictures. The hit rate was excellent (adults: 90%, children: 89%). Thus, we were able to replicate findings on the disgust memory bias for visual stimuli in adults (e.g., Chapman et al., 2013; Chapman, 2018; Charash & McKay, 2002; Croucher et al., 2011) and showed for the first time that a similar bias exists in children (aged 10–13 years).
The recognition performance was independent of sex, which was not in line with our hypothesis. Interestingly, we found that adults’ recognition performance was slightly better than that of children. This effect was driven by a better recognition of neutral images (e.g., clocks, glasses), which indicates that these types of stimuli might be of different relevance or interest for both age groups.
The absence of a sex-related memory bias for disgust might be associated with the disgust ratings for the images, which did not differ between males and females. This is not in line with previous studies (e.g., Curtis et al., 2004; Schienle et al., 2005). For example, Curtis et al. (2004) conducted a web-based survey with over 40,000 individuals using photo stimuli. The participants viewed images with high versus low disease relevance and rated the intensity of experienced disgust (1–5; 5 = very high). Disease-salient images were rated as more disgusting by females (M = 3.5) than males (M = 3.2). This difference was highly statistically significant (p < .001); however, the meaningfulness of this small difference for disgust-motivated behavior seems questionable. Schienle et al. (2005) presented males and females with disgusting images during functional magnetic resonance imaging. This study also found sex differences in reported disgust. However, brain activation did not differ between males and females. These findings indicate that sex differences in disgust processing are smaller than previously assumed. This is in line with the basic function of disgust to prevent pathogen transmission (e.g., Rozin et al., 2008; Rozin et al., 2009; Tybur et al., 2013). If disgust is indeed a universal disease-avoidance mechanism, then it should be present in all individuals.
The memory bias was also found with the free recall task in experiment 2. The participants showed a better memory performance for disgust images than for the other two picture categories. In Experiment 2, 61% of the disgust images were recalled correctly, and the overall recall performance across all picture categories was 46%. These worse results compared to the findings of experiment 1 (with 90% correctly recognized disgust images by the adult participants and an overall hit rate of 85%) is possibly associated with two factors. On the one hand, free recall is a more difficult task than recognition (e.g., Balota & Neely, 1980). On the other hand, Experiment 2 used an incidental learning paradigm (where participants didn’t know they would have to recall the images later on). They were told that their pupil size would be registered during the picture viewing. This incidental learning approach however has high ecological validity. In everyday life, we do not have the intention to memorize certain affective information. Disgust learning typically occurs incidentally.
The observed memory bias in adults was associated with the visual exploration of the disgusting images, which prompted shorter fixations over a broader array compared to the neutral pictures. Thus, even though disgusting and neutral pictures had been matched for visual properties (complexity, brightness, color, contrast), the disgusting pictures were scanned differently. The participants showed a “hyperscanning pattern” (shorter fixations, greater distance between fixations). For example, participants tended to inspect each maggot in a piece of contaminated meat, while in a cluster of marbles, basically only one was fixated. This detailed-oriented exploration of all contaminated aspects of a disgusting stimulus was positively correlated with the recall performance. Thus, the gaze bias was related to the encoding of the information. Similar effects have been reported before. In a study by Wells et al. (2010), hyperscanning of angry faces was associated with increased memory performance. Moreover, eye-tracking studies have shown that visual search produces improvement in detection performance (Võ & Wolfe, 2012). Through an active search engagement, relevant information on a target object can be acquired which serves as an efficient guide when the same target is searched again. For disgust, it is crucial to quickly identify complex sensory features in the environment, such as the presence of small maggots in food (Alexander & Zelinsky, 2011; Cunningham & Wolfe, 2014). This skill requires a rapid interplay between perceptual, attentional, and memory systems (Eckstein, 2011).
We also need to mention the following limitations of this research. In Experiment 2, we conducted a free-viewing paradigm, which focuses on controlled visual attention processes. Earlier automatic attention capture that might also be relevant for the disgust memory bias cannot be assessed. Future studies should therefore employ additional physiological measurements, such as the electroencephalogram (EEG). For example, the P300 (a positive deflection in the EEG starting approximately 300 ms after stimulus onset) is associated with automatic attentional resource allocation and memory (Dandan et al., 2019). These automatic processes may also contribute to the mnemonic advantage for disgust. Moreover, the visual encoding of disgust-relevant images needs to be investigated via eye-tracking in a sample with children. A subsequent study should also attempt to match disgusting and frightening images in visual properties to demonstrate that the observed hyperscanning effects are due to the emotion of disgust per se, and not to the emotional load of the images. An alternative would be to use different affective contexts for stimuli (disgust vs. fear) to investigate whether these contexts influence the scanning pattern.
In summary, the results of the conducted experiments showed that both adults and children regardless of sex displayed a comparable memory bias for visual disgust stimuli. In adults, the disgust memory bias was identified with different memory paradigms (incidental/intentional learning, recognition/free recall) and associated with a specific visual exploration pattern (“hyperscanning”) during the encoding stage. The findings are in line with the assumption that the disgust memory bias is indeed a common phenomenon in humans.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed the following financial support for the research, authorship, and/or publication of this article: This publication was supported by the University of Graz.
ORCID iD: Anne Schienle  https://orcid.org/0000-0003-2173-6626
https://orcid.org/0000-0003-2173-6626
References
- Alexander R. G., Zelinsky G. J. (2011). Visual similarity effects in categorical search. Journal of Vision, 11(8). 10.1167/11.8.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T., Olatunji B. O. (2012). Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review, 32(8), 704–723. 10.1016/j.cpr.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota D. A., Neely J. H. (1980). Test-expectancy and word-frequency effects in recall and recognition. Journal of Experimental Psychology: Human Learning & Memory, 6(5), 576–587. 10.1037/0278-7393.6.5.576 [DOI] [Google Scholar]
- Bell R., Buchner A. (2010). Valence modulates source memory for faces. Memory & Cognition, 38(1), 29–41. 10.3758/MC.38.1.29 [DOI] [PubMed] [Google Scholar]
- Blechert J., Meule A., Busch N. A., Ohla K. (2014). Food-pics: An image database for experimental research on eating and appetite. Frontiers in Psychology, 5, 617. 10.3389/fpsyg.2014.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A. (2018). Enhanced recall of disgusting relative to frightening photographs is not due to organisation. Cognition & Emotion, 32(6), 1220–1230. 10.1080/02699931.2017.1394817 [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Johannes K., Poppenk J. L., Moscovitch M., Anderson A. K. (2013). Evidence for the differential salience of disgust and fear in episodic memory. Journal of Experimental Psychology: General, 142(4), 1100–1112. 10.1037/a0030503 [DOI] [PubMed] [Google Scholar]
- Charash M., McKay D. (2002). Attention bias for disgust. Journal of Anxiety Disorders, 16(5), 529–541. 10.1016/S0887-6185(02)00171-8 [DOI] [PubMed] [Google Scholar]
- Chen N. T. M., Thomas L. M., Clarke P. J. F., Hickie I. B., Guastella A. J. (2015). Hyperscanning and avoidance in social anxiety disorder: The visual scanpath during public speaking. Psychiatry Research, 225(3), 667–672. 10.1016/j.psychres.2014.11.025 [DOI] [PubMed] [Google Scholar]
- Croucher C. J., Calder A. J., Ramponi C., Barnard P. J., Murphy F. C. (2011). Disgust enhances the recollection of negative emotional images. PLoS One, 6(11), e26571. 10.1371/journal.pone.0026571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. A., Wolfe J. M. (2014). The role of object categories in hybrid visual and memory search. Journal of Experimental Psychology: General, 143(4), 1585–1599. 10.1037/a0036313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis V., Aunger R., Rabie T. (2004). Evidence that disgust evolved to protect from risk of disease. Proceedings. Biological Sciences, 271(Suppl 4), S131–S133. 10.1098/rsbl.2003.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandan Z., Yiqin L. I. N., Yunzhe L. I. U., Yuejia L. U. O., Donghong J. (2019). Memory encoding, retention and retrieval of disgusting and fearful faces. Acta Psychologica Sinica, 51(1), 36. 10.3724/SP.J.1041.2019.00036 [DOI] [Google Scholar]
- Duesenberg M., Weber J., Schaeuffele C., Fleischer J., Hellmann-Regen J., Roepke S., Moritz S., Otte C., Wingenfeld K. (2016). Effects of hydrocortisone on false memory recognition in healthy men and women. Behavioral Neuroscience, 130(6), 635–642. 10.1037/bne0000170 [DOI] [PubMed] [Google Scholar]
- Eckstein M. P. (2011). Visual search: A retrospective. Journal of Vision, 11(5). 10.1167/11.5.14 [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Fernandes N. L., Pandeirada J. N. S., Soares S. C., Nairne J. S. (2017). Adaptive memory: The mnemonic value of contamination. Evolution and Human Behavior, 38(4), 451–460. 10.1016/j.evolhumbehav.2017.04.003 [DOI] [Google Scholar]
- Ferré P., Haro J., Hinojosa J. A. (2018). Be aware of the rifle but do not forget the stench: Differential effects of fear and disgust on lexical processing and memory. Cognition & Emotion, 32(4), 796–811. 10.1080/02699931.2017.1356700 [DOI] [PubMed] [Google Scholar]
- Knowles K. A., Cox R. C., Armstrong T., Olatunji B. O. (2019). Cognitive mechanisms of disgust in the development and maintenance of psychopathology: A qualitative review and synthesis. Clinical Psychology Review, 69, 30–50. 10.1016/j.cpr.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P., Bradley M. M. (2007). The International Affective Picture System (IAPS) in the study of emotion and attention. Handbook of Emotion Elicitation and Assessment, 29, 70–73. [Google Scholar]
- Leutgeb V., Schäfer A., Köchel A., Scharmüller W., Schienle A. (2010). Psychophysiology of spider phobia in 8- to 12-year-old girls. Biological Psychology, 85(3), 424–431. 10.1016/j.biopsycho.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Prokop P., Fančovičová J., Fedor P. (2014). Parasites enhance self-grooming behaviour and information retention in humans. Behavioural Processes, 107, 42–46. 10.1016/j.beproc.2014.07.017 [DOI] [PubMed] [Google Scholar]
- Rottman J. (2014). Evolution, development, and the emergence of disgust. Evolutionary Psychology, 12(2). 10.1177/147470491401200209 [DOI] [PubMed] [Google Scholar]
- Rozin P., Fallon A., Augustoni-Ziskind M. (1985). The child’s conception of food: The development of contamination sensitivity to “disgusting” substances. Developmental Psychology, 21(6), 1075–1079. 10.1037/0012-1649.21.6.1075 [DOI] [Google Scholar]
- Rozin P., Haidt J., Fincher K. (2009). Psychology. From oral to moral. Science (New York, N.Y.), 323(5918), 1179–1180. 10.1126/science.1170492 [DOI] [PubMed] [Google Scholar]
- Rozin P., Haidt J., McCauley C. R. (2008). Disgust. In Handbook of emotions (3rd ed., pp. 757–776). The Guilford Press. [Google Scholar]
- Schaller M., Duncan L. A. (2007). The behavioral immune system: Its evolution and social psychological implications. In Sydney symposium of social psychology. Evolution and the social mind: Evolutionary psychology and social cognition (pp. 293–307). Routledge/Taylor & Francis Group. [Google Scholar]
- Schienle A., Schäfer A., Stark R., Walter B., Vaitl D. (2005). Gender differences in the processing of disgust- and fear-inducing pictures: An fMRI study. NeuroReport, 16(3). https://journals.lww.com/neuroreport/Fulltext/2005/02280/Gender_differences_in_the_processing_of_disgust_.15.aspx [DOI] [PubMed] [Google Scholar]
- Schienle A., Übel S., Gremsl A., Schöngassner F., Körner C. (2016). Disgust proneness and the perception of disgust-evoking pictures. Journal of Psychophysiology, 30(3), 124–129. 10.1027/0269-8803/a000162 [DOI] [Google Scholar]
- Stevenson R. J., Oaten M. J., Case T. I., Repacholi B. M., Wagland P. (2010). Children’s response to adult disgust elicitors: Development and acquisition. Developmental Psychology, 46(1), 165–177. 10.1037/a0016692 [DOI] [PubMed] [Google Scholar]
- Tybur J. M., Lieberman D., Kurzban R., DeScioli P. (2013). Disgust: Evolved function and structure. Psychological Review, 120(1), 65–84. 10.1037/a0030778 [DOI] [PubMed] [Google Scholar]
- Võ M. L.-H., Wolfe J. M. (2012). When does repeated search in scenes involve memory? Looking at versus looking for objects in scenes. Journal of Experimental Psychology: Human Perception and Performance, 38(1), 23–41. 10.1037/a0024147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T. T., Beevers C. G., Robison A. E., Ellis A. J. (2010). Gaze behavior predicts memory bias for angry facial expressions in stable dysphoria. Emotion (Washington, D.C.), 10(6), 894–902. 10.1037/a0020022 [DOI] [PubMed] [Google Scholar]
- Wixted J. T. (2007). Signal-detection theory and the neuroscience of recognition memory. In Nairne J. S. (Ed.), The foundations of remembering: Essays in honor of H.L. Roediger, III (pp. 67–82). Psychology Press. [Google Scholar]