Abstract
Background
In patients with ischemic stroke, low hemoglobin-to-red blood cell distribution width ratio (HRR) was associated with an increased risk of mortality. However, it was unknown in the non-traumatic subarachnoid hemorrhage (SAH) population. The purpose of this study was to examine the association between baseline HRR and in-hospital mortality in patients with non-traumatic SAH.
Methods
Non-traumatic SAH patients were screened out of the Medical Information Mart for Intensive IV (MIMIC-IV) database between 2008 and 2019. The Cox proportional hazard regression models were utilized to analyze the association between baseline HRR and in-hospital mortality. Restricted cubic splines (RCS) analysis was utilized to determine the relationship curve between hospital mortality and the HRR level and examine the threshold saturation effect. We further applied Kaplan–Meier survival curve analysis to examine the consistency of these correlations. The interaction test was used to identify subgroups with differences.
Results
A total of 842 patients were included in this retrospective cohort study. Compared with individuals with lower HRR Q1 ( ≤ 7.85), the adjusted HR values in Q2 (7.86–9.15), Q3 (9.16–10.16), and Q4 (≥10.17) were 0.574 (95% CI: 0.368–0.896, p = 0.015), 0.555 (95% CI: 0.346–0.890, p = 0.016), and 0.625 (95% CI: 0.394–0.991, p = 0.045), respectively. The association between the HRR level and in-hospital mortality exhibited a non-linear relationship (p < 0.05). The threshold inflection point value of 9.50 was calculated using RCS analysis. When the HHR level was lower than 9.50, the risk of in-hospital mortality rate decreased with an adjusted HR of 0.79 (95% CI: 0.70–0.90, p = 0.0003). When the HRR level was higher than 9.50, the risk of in-hospital mortality almost hardly increased with the increase in the HRR level (adjusted HR = 1.18, 95% CI: 0.91–1.53, p = 0.2158). K-M analysis showed that patients with low HRR levels had significantly higher in-hospital mortality (p < 0.001).
Conclusion
There was a non-linear connection between the baseline HRR level and in-hospital mortality. A low level of HRR could increase the risk of death in participants with non-traumatic SAH.
Keywords: hospital mortality, hemoglobin-to-red blood cell distribution width ratio, non-traumatic subarachnoid hemorrhage, intensive care unit, MIMIC-IV
Introduction
Subarachnoid hemorrhage (SAH) is a common form of stroke in the intensive care unit (ICU) and a potentially devastating illness (1). It affects nearly 10 percent of every 1,00,000 individuals and accounts for nearly 5% of all strokes each year (2, 3). However, long-term disability and mortality from SAH accounted for 27% of all stroke-related potential years of life lost before the age of 65 years (4). Although the optimal management of SAH has improved, hospital mortality and severe disability are still high (5).
Inflammatory reactions play an important role during the early brain injury induced by SAH and significantly affect the outcomes (6). Experiments have suggested that the mechanism leading to this situation may be extravasated red blood cells in the subarachnoid space undergoing degradation, releasing a host of bioactive and pro-inflammatory properties including hemoglobin (Hb), methemoglobin, and bilirubin (7, 8). Vasoactive factors and inflammation are released, exacerbating brain edema, oxidative stress damage, and cell apoptosis, leading to disruption of the blood–brain barrier (9, 10). Hemoglobin reflects the patient's degree of anemia, and red-blood-cell distribution width (RDW) reflects the heterogeneity in the sizes of erythrocytes (11, 12). In the inflammatory state, the lifespan of red blood cells is shortened, leading to anemia and an increase in RDW.
Previous studies have shown that RDW was a parameter reflecting inflammation (13, 14) and related to the outcomes of SAH patients (15–19). In addition, Hb is an important component of the complete blood count and related to nutritional status (20) and immune response (21). However, the level of RDW and Hb may be affected by many factors, such as medications, nutritional status, oxidative stress, and blood transfusion (13, 22–24). Therefore, Hb/RDW (HRR) is a relatively good parameter for reducing the impact of the factors mentioned. HRR is an easily obtained parameter. Previous investigations have shown that HRR was associated with inflammation (25–27). In recent years, more and more evidence has shown that low level of HRR was closely related to significantly deteriorating prognosis in many critically ill patients, such as coronary heart disease, sepsis, and ischemic stroke (28–30). However, the relationship between HRR and mortality in patients with non-traumatic SAH is lacking.
In this study, we aim to test the association between baseline HRR and hospital mortality among critically ill patients with non-traumatic SAH.
Materials and methods
Data sources
The study data were downloaded freely from a large publicly accessed database called Medical Information Mart for Intensive Care (MIMIC-IV) (31). This database contains information on patients admitted to the Beth Israel Deaconess Medical Center (BIDMC) between 2008 and 2019. Posterior to the completion of the National Institutes of Health (NIH) training course and the Protecting Human Research Participants test, one researcher Junhong Wang obtained approval to exploit the database (Record ID, 45677587). The study was carried out following the Helsinki Declaration guidelines and was reviewed and approved by the Massachusetts Institute of Technology and the Institutional Review Board of BIDMC. All data were anonymous to protect patient privacy, and the need for informed consent was waived. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (32).
Study population
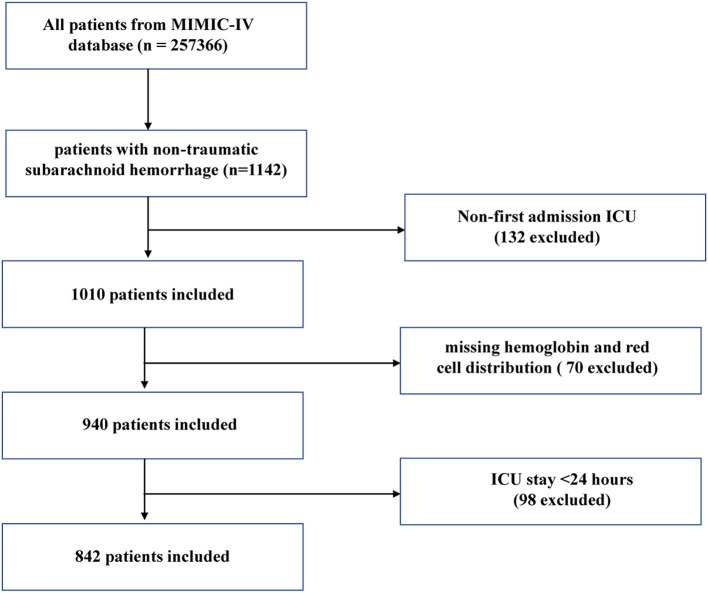
A total of 1,142 patients with non-traumatic SAH were selected based on the record of ICD-9 code 430 and ICD-10 code I60. Patients who met the requirements were selected to undergo analysis: (1) first ICU admission; (2) age > 18 years. The exclusion criteria were as follows: (1) ICU patients with a length of stay < 24 h and (2) participants who had missing hemoglobin or red blood cell distribution width value. Finally, 842 patients were included in this study (Figure 1).
Figure 1.
Flow chart of the study. MIMC-IV, Medical Information Mart for Intensive Care IV; ICU, intensive care unit.
Data extraction
Structured Query Language (SQL) and PostgreSQL are used to extract the following variables from the MIMIC-IV database in our study: (1) demographic variables include sex, age, and ethnicity. (2) vital signs include the heart rate, respiratory rate (RR), mean blood pressure (MBP), systolic blood pressure (SBP), diastolic blood pressure (DBP), percutaneous oxygen saturation (SpO2), and temperature. (3) Comorbidities include myocardial infarction, congestive heart failure, chronic pulmonary disease, paraplegia, hypertension, diabetes, and sepsis. (4) Laboratory variables include glucose, platelet, white blood cell (WBC) count, calcium, mean corpuscular hemoglobin (MCH), prothrombin time (PT), activated partial thromboplastin time (APTT), Hb, and RDW. The first test of RDW and Hb were included within 24 h after entering the ICU. (5) Severity at admission was identified via the Glasgow coma score (GCS) and World Federation of Neurosurgical Societies (WFNS). (6) Delayed cerebral ischemia (DCI), hydrocephalus, Length of ICU stay, length of the hospital, and in-hospital mortality. (7) HRR was calculated using the following formula: HRR = Hb (g/L)/RDW (%) (29, 30, 33).
Endpoints
The outcome was in-hospital mortality, which was described by the patient's survival status at the moment of discharge from the hospital.
Statistical analysis
The continuous variates were displayed as average ± standard deviation (SD) or mid-value (interquartile range). The Student's t-test or Mann–Whitney U-test was used according to the normality of the distribution. Categorical variates were displayed as case quantity (%), and the chi-square test (or Fisher's exact approach) was utilized for analyses of the difference between the different HRRs (four quantiles) (29).
To reduce the interference of potential confounding factors on in-hospital mortality, univariate and multivariate Cox proportional hazard regression analyses were performed. The screening of confounders was based on the following criteria: (1) The factor had a significant impact (>10%) on the research variate. (2) Certain factors may have a significant impact on the outcome variate based on previous experiences. (3) For univariate analysis, our team modified the variates (p < 0.05). In the multivariate case, we performed several statistical models to ensure that the results were stable. In the crude model, no variables were adjusted. In model I, age, sex, and ethnicity variables were adjusted, while model II further adjusted other 16 variables, including heart rate, RR, WBC, PT, APTT, congestive heart failure, Charlson comorbidity index, endovascular therapy, sepsis, norepinephrine, vasopressin, ventilation, GCS, WFNS, DCI, and hydrocephalus.
Restricted cubic spline analysis was employed to determine whether there was a curve relationship between hospital mortality. If the non-linear correlation was observed, a two-piecewise linear regression model was performed to calculate the threshold effect of the HRR on hospital mortality in terms of the smoothing plot (34). The turning point for the HRR was determined using “exploratory” analyses, which is to move the trial turning point along the pre-defined interval and pick up the one which gave maximum model likelihood. We also performed a log-likelihood ratio test and compared the one-line linear regression model with the two-piece-wise linear model (35). We used the bootstrap resampling method to calculate the 95% CI for the turning point, as described in the previous analysis (30, 34, 36).
Furthermore, interactions and stratified analyses were conducted using age (< 65 and ≥65 years old), sex, WBC counts, myocardial infarction, congestive heart failure, chronic pulmonary disease, renal disease, paraplegia, hypertension, sepsis, endovascular therapy of aneurysm, and GCS (< 8 and ≥8), as previously described. Missing values were filled with mean values (37). The details of the missing values are shown in Supplementary Table 1.
A two-tailed p-value of < 0.05 was considered to be statistically significant in all analyses. All statistical analyses were carried out using EmpowerStats (http://www.empowerstats.com, version 3.6.1 R software package) software (35).
Results
Baseline characteristics of the study patients
After the screening, 1,010 patients were admitted to the ICU due to non-traumatic SAH for the first time, of whom 842 were selected for the final data analysis (see the flowchart in Figure 1). The distribution of the baseline characteristics of the population according to baseline HRR levels in quartiles is described in Table 1 (Q1: ≤ 7.85, Q2: 7.86–9.15, Q3: 9.16–10.16, and Q4: ≥10.17). The mean age was 61.2 ± 14.9 years, and ~55.9% of these were women. The demographics, vital signs, comorbidities, laboratory variables and scoring, and other related data according to HRR levels are presented in Table 1. According to Table 1, we found that there were significant differences in age, sex, ethnicity, heart rate, DBP, MBP, RR, congestive heart failure, renal disease, sepsis, WBC, PT, APTT, GCS, and WFNS. Compared with patients in the Q1 group, patients with HRR in Q2, Q3, and Q4 groups were at lower risk of in-hospital mortality and ICU mortality.
Table 1.
Population characteristics by quartiles of the baseline HRR level.
| Variables | Quartiles of HRR | p-value | ||||
|---|---|---|---|---|---|---|
| Total (n = 842) | Q1 ( ≤ 7.85) (n = 211) | Q2 (7.86–9.15) (n = 209) | Q3 (9.16–10.16) (n = 211) | Q4 (≥10.17) (n = 211) | ||
| Demographic | ||||||
| Female, n (%) | 471 (55.9) | 136 (64.5) | 151 (72.2) | 111 (52.6) | 73 (34.6) | < 0.001 |
| Age, years | 61.2 ± 14.9 | 64.4 ± 15.2 | 63.3 ± 16.0 | 59.8 ± 13.5 | 57.3 ± 13.5 | < 0.001 |
| Ethnicity, n (%) | 0.022 | |||||
| Asian | 32 (3.8) | 8 (3.8) | 9 (4.3) | 8 (3.8) | 7 (3.3) | |
| White | 507 (60.2) | 109 (51.7) | 128 (61.2) | 135 (64) | 135 (64) | |
| Black | 68 (8.1) | 30 (14.2) | 16 (7.7) | 13 (6.2) | 9 (4.3) | |
| Other | 235 (27.9) | 64 (30.3) | 56 (26.8) | 55 (26.1) | 60 (28.4) | |
| Vital signs | ||||||
| Heart rate, beats/min | 78.7 ± 13.1 | 81.5 ± 14.3 | 77.3 ± 12.4 | 77.2 ± 12.4 | 78.7 ± 12.7 | < 0.001 |
| SBP, mmHg | 125.3 ± 12.9 | 123.4 ± 14.3 | 126.1 ± 12.6 | 125.2 ± 13.6 | 126.5 ± 10.8 | 0.069 |
| DBP, mmHg | 64.3 ± 8.9 | 61.7 ± 9.2 | 62.2 ± 8.6 | 65.7 ± 7.7 | 67.5 ± 8.7 | < 0.001 |
| MBP, mmHg | 82.5 ± 8.6 | 80.2 ± 8.9 | 81.9 ± 8.6 | 83.9 ± 8.3 | 84.1 ± 8.0 | < 0.001 |
| RR, times/min | 18.2 ± 3.2 | 18.9 ± 3.5 | 17.9 ± 2.9 | 17.7 ± 3.1 | 18.0 ± 3.2 | < 0.001 |
| Temperature, °C | 37.0 ± 0.5 | 37.0 ± 0.7 | 37.0 ± 0.4 | 37.0 (36.8, 37.3) | 37.0 (36.8, 37.3) | 0.383 |
| SpO2, % | 97.5 ± 1.9 | 97.8 ± 2.0 | 97.8 ± 1.8 | 97.2 ± 2.1 | 97.2 ± 1.7 | < 0.001 |
| Comorbidities, n (%) | ||||||
| Myocardial infarction | 61 (7.2) | 21 (10) | 17 (8.1) | 11 (5.2) | 12 (5.7) | 0.203 |
| Congestive heart failure | 70 (8.3) | 34 (16.1) | 13 (6.2) | 16 (7.6) | 7 (3.3) | < 0.001 |
| Chronic pulmonary disease | 122 (14.5) | 38 (18) | 36 (17.2) | 26 (12.3) | 22 (10.4) | 0.072 |
| Paraplegia | 139 (16.5) | 44 (20.9) | 38 (18.2) | 32 (15.2) | 25 (11.8) | 0.075 |
| Renal disease | 54 (6.4) | 35 (16.6) | 9 (4.3) | 5 (2.4) | 5 (2.4) | < 0.001 |
| Hypertension | 423 (50.2) | 105 (49.8) | 117 (56) | 101 (47.9) | 100 (47.4) | 0.269 |
| Diabetes | 39 (4.6) | 15 (7.1) | 10 (4.8) | 9 (4.3) | 5 (2.4) | 0.142 |
| Sepsis | 424 (50.4) | 127 (60.2) | 116 (55.5) | 98 (46.4) | 83 (39.3) | < 0.001 |
| Charlson comorbidity index | 4.0 (3.0, 6.0) | 5.0 (4.0, 8.0) | 5.0 (3.0, 6.0) | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.0) | < 0.001 |
| Laboratory results | ||||||
| Platelets, 109/L | 230.0 (187.0, 280.8) | 219.0 (161.5, 284.5) | 238.0 (188.0, 279.0) | 232.0 (191.5, 276.0) | 231.0 (193.5, 281.5) | 0.186 |
| WBC, 109/L | 12.9 (9.9, 16.5) | 12.9 (8.9, 17.2) | 12.5 (10.0, 15.9) | 12.7 (10.0, 15.3) | 14.0 (10.8, 17.3) | 0.046 |
| Hemoglobin, g/L | 12.2 ± 1.9 | 9.9 ± 1.4 | 11.7 ± 0.8 | 12.8 ± 0.7 | 14.2 ± 1.1 | < 0.001 |
| RDW, % | 13.9 ± 1.6 | 15.6 ± 2.1 | 13.6 ± 0.8 | 13.3 ± 0.7 | 12.9 ± 0.8 | < 0.001 |
| Glucose, mg/dl | 130.9 (113.4, 153.5) | 128.6 (110.6, 158.7) | 135.6 (114.0, 150.8) | 129.0 (113.2, 151.0) | 131.8 (114.6, 154.0) | 0.756 |
| Calcium, mg/dl | 8.7 (8.3, 9.1) | 8.6 (8.2, 9.1) | 8.7 (8.3, 9.1) | 8.7 (8.4, 9.0) | 8.9 (8.6, 9.2) | < 0.001 |
| MCH, pg | 30.5 (29.2, 31.8) | 29.5 (27.1, 30.9) | 30.4 (29.3, 31.6) | 30.7 (29.6, 31.8) | 31.2 (29.9, 32.4) | < 0.001 |
| PT, s | 12.6 (11.7, 13.8) | 13.6 (12.2, 15.8) | 12.5 (11.8, 13.4) | 12.3 (11.5, 13.1) | 12.4 (11.6, 13.4) | < 0.001 |
| APTT, s | 28.8 (25.9, 33.1) | 29.6 (26.9, 36.7) | 28.4 (25.6, 32.8) | 27.8 (25.6, 31.6) | 29.1 (26.4, 32.0) | 0.003 |
| Therapy, n (%) | ||||||
| Norepinephrine | 30 (3.6) | 17 (8.1) | 5 (2.4) | 4 (1.9) | 4 (1.9) | < 0.001 |
| Vasopressin | 8 (1.0) | 4 (1.9) | 0 (0) | 1 (0.5) | 3 (1.4) | 0.193 |
| Ventilation | 429 (51.0) | 130 (61.6) | 111 (53.1) | 95 (45) | 93 (44.1) | < 0.001 |
| Clipping of aneurysm | 35 (4.2) | 6 (2.8) | 10 (4.8) | 13 (6.2) | 6 (2.8) | 0.410 |
| Endovascular therapy of aneurysm | 201 (23.9) | 44 (20.9) | 60 (28.7) | 53 (25.1) | 44 (20.9) | 0.050 |
| Scores | ||||||
| GCS | 12.0 (7.0, 14.0) | 10.0 (7.0, 14.0) | 10.0 (6.0, 14.0) | 13.0 (8.0, 14.0) | 13.0 (7.5, 14.0) | < 0.001 |
| WFNS | 0.003 | |||||
| I | 127 (15.1) | 24 (11.4) | 27 (12.9) | 39 (18.5) | 37 (17.5) | |
| II | 172 (20.4) | 32 (15.2) | 33 (15.8) | 50 (23.7) | 57 (27) | |
| III | 115 (13.7) | 29 (13.7) | 26 (12.4) | 34 (16.1) | 26 (12.3) | |
| IV | 185 (22.0) | 51 (24.2) | 57 (27.3) | 39 (18.5) | 38 (18) | |
| V | 243 (28.9) | 75 (35.5) | 66 (31.6) | 49 (23.2) | 53 (25.1) | |
| Outcomes | ||||||
| Delayed cerebral ischemia | 64 (7.60%) | 7 (3.32%) | 14 (6.70%) | 19 (9.00%) | 24 (11.37%) | 0.014 |
| Hydrocephalus | 77(9.14) | 19 (9.00%) | 18 (8.61%) | 16 (7.58%) | 24 (11.37%) | 0.581 |
| Length of ICU stay, days | 7.0 (2.9, 12.9) | 5.9 (2.7, 11.9) | 7.5 (2.9, 13.8) | 6.8 (3.2, 13.8) | 7.1 (2.6, 12.7) | 0.408 |
| Length of hospital stay, days | 11.3 (6.7, 18.7) | 11.8 (6.0, 19.9) | 12.1 (7.4, 19.3) | 10.8 (6.2, 17.1) | 10.5 (6.7, 16.4) | 0.275 |
| In-hospital mortality | 166 (19.7) | 71 (33.6) | 35 (16.7) | 29 (13.7) | 31 (14.7) | < 0.001 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; RR, respiratory rate; SpO2, percutaneous oxygen saturation; HRR, hemoglobin/red cell distribution width; WBC, white blood cell; MCH, mean corpuscular hemoglobin; PT, prothrombin time; APTT, activated partial thromboplastin time; GCS, Glasgow coma score; WFNS, World Federation of Neurosurgical Societies; ICU, Intensive care unit; SAH, subarachnoid hemorrhage.
The non-survivor group presented lower HRR (median: 8.5 vs. 9.3, p < 0.001). Compared with the survivor group, the non-survivor group was older (66.8 ± 14.9 vs. 59.8 ± 14.5 years old, p < 0.001) and presented a higher comorbidity incidence, such as congestive heart failure, sepsis, chronic pulmonary disease, Charlson comorbidity index, as well as lower GCS scores (all p-values < 0.05) (Supplementary Table 2).
The association between baseline HRR and in-hospital mortality
The univariate analysis demonstrated that age, heart rate, RR, WBC, PT, APTT, sepsis, Charlson comorbidity index, norepinephrine, vasopressin, ventilation, GCS, WFNS were associated with in-hospital mortality (Supplementary Table 3). Table 2 showed an unadjusted and a multivariable-adjusted association between HRR and in-hospital mortality. In model I, age, sex, and ethnicity variables were adjusted, while model II further adjusted other 16 variables, including heart rate, RR, WBC, PT, APTT, congestive heart failure, Charlson comorbidity index, endovascular therapy, sepsis, norepinephrine, vasopressin, ventilation, GCS, WFNS, DCI, hydrocephalus. When HRR was used as a continuous variable, the results showed that HRR was associated with in-hospital mortality (non-adjusted model: HR = 0.861, 95% CI: 0.798–0.929, p = 0.0001; Model I: HR = 0.895, 95% CI: 0.828–0.968, p = 0.0052; Model II: HR = 0.887, 95% CI: 0.797–0.964, p = 0.007). The in-hospital mortality of non-traumatic SAH decreased with a 1-unit increase in HRR. Moreover, as a classification variable, patients with lower HRR levels had significantly increased in-hospital mortality. Compared to the reference group (Q1 ≤ 7.85), the adjusted HR values for individuals in Q2 (7.86–9.15), Q3 (9.16–10.16), and Q4 (≥10.17) were 0.574 (95% CI: 0.368–0.896, p = 0.015), 0.555 (95% CI: 0.346–0.890, p = 0.016), and 0.625 (95% CI: 0.394–0.991, p = 0.045), respectively (p for trend = 0.015). Regarding sensitivity analysis, HRR levels were assessed as a continuous and categorical variable, respectively, with in-hospital mortality, yielding consistent results. In addition, the K-M curves contrasting the four groups were displayed in Figure 2. The figure indicated that the survival rate of group Q1 was lower than groups Q2, Q3, and Q4 (p < 0.0001).
Table 2.
Multivariate cox regression analyses for in-hospital mortality in non-traumatic subarachnoid hemorrhage patients.
| Exposure | Non-adjust model | Model I | Model II | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| HRR quartiles | ||||||
| Q1 ( ≤ 7.85) | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||
| Q2 (7.86–9.15) | 0.500 (0.333, 0.751) | < 0.001 | 0.522 (0.347, 0.786) | 0.002 | 0.574 (0.368, 0.896) | 0.015 |
| Q3 (9.16–10.16) | 0.435 (0.282, 0.671) | < 0.001 | 0.489 (0.315, 0.759) | 0.001 | 0.555 (0.346, 0.890) | 0.015 |
| Q4 (≥10.17) | 0.447 (0.292, 0.686) | < 0.001 | 0.534 (0.342, 0.834) | 0.006 | 0.625 (0.394, 0.991) | 0.045 |
| p for trend | 0.742 (0.643, 0.855) | < 0.001 | 0.784 (0.676, 0.909) | 0.001 | 0.872 (0.780, 0.974) | 0.015 |
| HRR (per 1 increases) | 0.861 (0.798, 0.929) | 0.001 | 0.895 (0.828, 0.968) | 0.005 | 0.877 (0.797, 0.964) | 0.007 |
Non-adjusted: no covariates were adjusted.
Model I: adjusted for age, sex, and ethnicity.
Model II: adjusted for age, sex, ethnicity, heart rate, RR, WBC, PT, APTT, congestive heart failure, Charlson comorbidity index, endovascular therapy, sepsis, Norepinephrine, vasopressin, ventilation, GCS, WFNS, delayed cerebral ischemia, hydrocephalus.
RR, respiratory rate; WBC, white blood cell; INR, international normalized ratio; PT, prothrombin time; APTT, activated partial thromboplastin time; GCS, Glasgow coma score; WFNS, World Federation of Neurosurgical Societies; HRR, hemoglobin/red cell distribution width; HR, hazard ratio; CI, confidence interval; Ref, reference.
Figure 2.

Kaplan–Meier survival curves for critically ill patients with non-traumatic SAH based on the baseline HRR level. X-Axis: survival time (days). Y-Axis: survival probability. HRR, hemoglobin/red cell distribution width; SAH, subarachnoid hemorrhage.
Analysis of the non-linear relationship between the baseline HRR and in-hospital mortality
After adjusting the variables in Model II, a curve-fitting equation for baseline HRR and death during hospitalization was established using restricted cubic spline analysis. We observed that the association between the HRR level and in-hospital mortality exhibited a non-linear curve (Figure 3). In the threshold analysis, we used a two-piecewise model to fit the link between the baseline HRR level and in-hospital mortality. We found an inflection point at 9.50 (Table 3). On the left side of the inflection point, the HR of HRR was 0.79 (95% CI: 0.70–0.90, p = 0.0003). This meant that the risk of in-hospital mortality was reduced by 21% per 1 unit increase. On the right side of the inflection point, the HR was 1.18 (95% CI: 0.91–1.53, p = 0.2158). It suggested that the association between HRR and in-hospital mortality was not statistically significant when the level of HRR was more than 9.50. This meant that the risk of in-hospital no longer decreased with increasing HRR. In our study, the p-value for the log-likelihood ratio test was 0.025 (Table 3).
Figure 3.

Non-linear relationship observed between in-hospital mortality and the baseline HRR level, and the slope changes evidently, which may have a threshold saturation effect. The solid red line represents the smooth curve fit between variables. Blue bands present the 95% confidence interval. The data were adjusted for the variables in Model II in Table 2.
Table 3.
Threshold-effect analysis of the relationship between the baseline HRR level and in-hospital mortality in non-traumatic SAH patients.
| Models | Per-unit increase | ||
|---|---|---|---|
| HR | 95%CI | p-value | |
| Model I | |||
| One line effect | 0.88 | 0.80–0.96 | 0.0066 |
| Model II | |||
| Turning point (K) | 9.50 | ||
| Baseline HRR levels < K | 0.79 | 0.70–0.90 | 0.0003 |
| Baseline HRR levels > K | 1.18 | 0.91–1.53 | 0.2158 |
| p-value for LRT test* | 0.025 | ||
Model I, one-line linear regression model; Model II, two-piece wise linear regression model. Adjusted for age, sex, ethnicity, heart rate, RR, WBC, PT, APTT, congestive heart failure, Charlson comorbidity index, endovascular therapy, sepsis, Norepinephrine, vasopressin, ventilation, GCS, WFNS, delayed cerebral ischemia, hydrocephalus.
HR, Hazard Ratio; CI, confidence interval; LRT, logarithm likelihood ratio test.
*p < 0.05 indicates that Model II is significantly different from Model I.
Subgroup analysis
The subgroup analysis was conducted to reveal the correlation between HRR and in-hospital mortality across age (< 65 and ≥65 years old), sex, WBC counts, myocardial infarction, congestive heart failure, chronic pulmonary disease, renal disease, paraplegia, hypertension, sepsis, endovascular therapy of aneurysm, GCS (< 8 and ≥8), and the results are shown in Figure 4. The interaction between the HRR and all subgroup factors was analyzed, and significant interactions were not observed (p for interaction > 0.05).
Figure 4.
Subgroup analyses of the effect of hospital mortality. Adjusted for age, sex, ethnicity, HR, RR, WBC, PT, APTT, congestive heart failure, Charlson comorbidity index, endovascular therapy, sepsis, norepinephrine, vasopressin, ventilation, GCS, WFNS, except the subgroup variable.
Discussion
In the retrospective observational study, we investigated the relationship between the baseline HRR level and in-hospital mortality among non-traumatic SAH patients and made several important findings. First, we demonstrated that HRR was inversely associated with in-hospital mortality after adjusting for possible confounding factors. Second, we also found a threshold effect. When the HRR was < 9.50, the risk of in-hospital mortality decreased with the increase in HRR level. However, patients with progressively higher HRRs above this level did not show any further trend of mortality decreasing. Third, we observed no obvious interaction between the baseline HRR and in-hospital mortality, which indicated that HRR was independently associated with in-hospital mortality in different subgroups, even considering the surgical requirements.
HRR is easily obtained from a routine laboratory database without any external technology or cost and is significantly related to the level of inflammatory reactions (25–27). The HRR itself is not associated with mortality but rather the degree of inflammation that it represents. To the best of our knowledge, the relationship between the level of inflammation and mortality rate has long been a concern of non-traumatic SAH patients. The relationship between HRR and in-hospital mortality remains unknown, which prompted us to carry out the current study. A series of previous studies have demonstrated that a low level of HRR was associated with poor outcomes among multiple malignant diseases (25, 33, 38–41). In recent years, there was mounting evidence that a lower level of HRR was strongly associated with significantly worse outcomes in many critically ill patients, such as coronary heart disease (28), sepsis (29), ischemic stroke (30), and so on. One study with 6,046 participants found that a lower HRR (HRR < 10.25) in patients with percutaneous coronary atherosclerotic heart disease was associated with a 1.47-fold increased risk of long-term all-cause mortality. Qu et al. revealed an inverse association between lower HRR (< 9.76) and the risk of frailty in elderly patients with coronary heart disease, and HRR was identified as a stronger predictor of frailty than RDW or Hb. In our previous survey, a lower HRR (< 5.877) was also observed to be strongly associated with an increased risk of all-cause mortality in individuals with sepsis (29). Moreover, Qin et al. used the MIMIC-IV to determine the relationship between HRR and all-cause mortality in ischemic stroke patients and found that a lower HRR was associated with increased mortality in these patients (30). All the above results indicated that a lower HRR was associated with worse outcomes than a higher HRR. According to the findings above, we hypothesize that low HRR levels may increase the risk of mortality in patients with non-traumatic SAH. More experiments are needed to validate our hypothesis.
Several hypothesized mechanisms have been proposed to explain the reason why lower HRR leads to adverse outcomes in non-traumatic SAH patients. First, higher RDW in the normal range may mean red blood cell disruption or ineffective erythropoiesis (42). Furthermore, a higher RDW level also reflects an underlying inflammatory state and is related to adverse outcomes (43, 44). Forhecz et al. performed a retrospective cohort study involving 195 patients suffering chronic heart failure and found the correlation between RDW and inflammatory markers such as C-reactive protein, interleukin-6, soluble tumor necrosis factor (TNF) receptor I and II (45). Inflammation reactions will damage iron metabolism and inhibit the secretion of erythropoietin, resulting in a decrease in the hemoglobin level (46). In addition, after SAH, it usually triggers a physiological stress response, and increased release of endogenous catecholamine and cortisol, which may lead to secondary brain injury and potential inflammatory complications. Second, the oxygen-carrying capacity mainly depends on the hemoglobin level. The decrease in the hemoglobin value indicates that the oxygen supply of cerebral vessels is significantly reduced, and the oxygen supply of brain tissue is limited, which may lead to vasospasm. Moreover, extravasated red blood cells in the subarachnoid space undergo degradation, releasing a host of bioactive and potentially toxic molecules including hemoglobin, methemoglobin, and bilirubin, which have long been associated with the development of cerebral vasospasm and outcome (7, 8). Vasospasm will seriously affect the prognosis of patients with SAH (11, 12). Third, previous studies have shown that HRR was associated with the risk of frailty risk (26). Dysphagia, systemic infection, or anemia were the main causes of frailty in the early stage of SAH, which may aggravate the condition. Among individuals with lower HRR levels, we consistently noted a higher incidence of sepsis, which also explains this.
Our research has the following strengths: (1) This is the first study to examine the association between baseline HRR levels and in-hospital mortality in participants with non-traumatic SAH. (2) The study used real-world data to design a large-scale and diverse population study. The missing value of HRR was lower, which may reduce the selection bias. (3) We used a 2-piecewise Cox proportional risk regression model to analyze the threshold effect of the relationship between HRR and all-cause mortality. In addition, our findings may help clinicians identify high-risk participants with non-traumatic SAH.
However, the study has some limitations. First, due to its retrospective observational design, and thus, we were only able to provide the association between HRR and hospital mortality, and it is difficult to distinguish the cause and effect. Second, this single-center cohort may not fully represent the general patient population with non-traumatic SAH. Third, due to the limitations of the MIMIC database, missing information that could have affected the model was not collected, such as medications and acute stress. However, it should be noted that the potential results from these variables would bias toward the null, resulting in an undervaluation of the connection between HRR levels and hospital mortality. Four, we analyzed the first HRR record collected during ICU admission, and therefore, the results are limited to a confined period during which HRR was measured. We only collected data on hospitalization, so we only evaluated short-term results. Long-term results should be evaluated through further research. Nevertheless, the relationship between low HRR levels and hospital mortality was revealed.
Conclusions
Therefore, patients with low HRRs should be given more attention, and their in-hospital mortality rate may be higher. This would benefit clinicians and contribute to better decisions.
Data availability statement
The data analyzed in this study was obtained from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, the following licenses/restrictions apply: To access the files, users must be credentialed users, complete the required training (CITI Data or Specimens Only Research) and sign the data use agreement for the project. Requests to access these datasets should be directed to Physionet, https://doi.org/10.13026/7vcr-e114.
Ethics statement
The studies involving human participants were examined and approved by Beth Israel Deaconess Medical Center. To protect patient privacy, all data were de-identified; therefore, the Ethical Committee of the Beth Israel Deaconess Medical Center waived the requirement for informed consent. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, and supervision: JW and JL. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1180912/full#supplementary-material
References
- 1.Connolly ES, Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2012) 43:1711–37. 10.1161/STR.0b013e3182587839 [DOI] [PubMed] [Google Scholar]
- 2.Petridis AK, Kamp MA, Cornelius JF, Beez T, Beseoglu K, Turowski B, et al. Aneurysmal subarachnoid hemorrhage. Dtsch Arztebl Int. (2017) 114:226–36. 10.3238/arztebl.2017.0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rincon F, Rossenwasser RH, Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. (2013) 73:217–22; discussion 2-3. 10.1227/01.neu.0000430290.93304.33 [DOI] [PubMed] [Google Scholar]
- 4.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. (1998) 50:1413–8. 10.1212/WNL.50.5.1413 [DOI] [PubMed] [Google Scholar]
- 5.Velly LJ, Bilotta F, Fabregas N, Soehle M, Bruder NJ, Nathanson MH, et al. Anaesthetic and ICU management of aneurysmal subarachnoid haemorrhage: a survey of European practice. Eur J Anaesthesiol. (2015) 32:168–76. 10.1097/EJA.0000000000000163 [DOI] [PubMed] [Google Scholar]
- 6.Geraghty JR, Davis JL, Testai FD. Neuroinflammation and microvascular dysfunction after experimental subarachnoid hemorrhage: emerging components of early brain injury related to outcome. Neurocrit Care. (2019) 31:373–89. 10.1007/s12028-019-00710-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon MS, Woo SK, Kurland DB, Yoon SH, Palmer AF, Banerjee U, et al. Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. Int J Mol Sci. (2015) 16:5028–46. 10.3390/ijms16035028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucke-Wold BP, Logsdon AF, Manoranjan B, Turner RC, McConnell E, Vates GE, et al. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci. (2016) 17:497. 10.3390/ijms17040497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hugelshofer M, Sikorski CM, Seule M, Deuel J, Muroi CI, Seboek M, et al. Cell-free oxyhemoglobin in cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: biomarker and potential therapeutic target. World Neurosurg. (2018) 120:e660–e6. 10.1016/j.wneu.2018.08.141 [DOI] [PubMed] [Google Scholar]
- 10.Mayberg MR, Okada T, Bark DH. The role of hemoglobin in arterial narrowing after subarachnoid hemorrhage. J Neurosurg. (1990) 72:634–40. 10.3171/jns.1990.72.4.0634 [DOI] [PubMed] [Google Scholar]
- 11.Barrera-Reyes PK, Tejero ME. Genetic variation influencing hemoglobin levels and risk for anemia across populations. Ann N Y Acad Sci. (2019) 1450:32–46. 10.1111/nyas.14200 [DOI] [PubMed] [Google Scholar]
- 12.Parizadeh SM, Jafarzadeh-Esfehani R, Bahreyni A, Ghandehari M, Shafiee M, Rahmani F, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors. (2019) 45:507–16. 10.1002/biof.1518 [DOI] [PubMed] [Google Scholar]
- 13.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. (2015) 52:86–105. 10.3109/10408363.2014.992064 [DOI] [PubMed] [Google Scholar]
- 14.Hu ZD, Lippi G, Montagnana M. Diagnostic and prognostic value of red blood cell distribution width in sepsis: a narrative review. Clin Biochem. (2020) 77:1–6. 10.1016/j.clinbiochem.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Chugh C, Nyirjesy SC, Nawalinski KP, Sandsmark DK, Frangos S, Maloney-Wilensky E, et al. Red blood cell distribution width is associated with poor clinical outcome after subarachnoid hemorrhage: a pilot study. Neurocrit Care. (2015) 23:217–24. 10.1007/s12028-015-0117-x [DOI] [PubMed] [Google Scholar]
- 16.Huang YL, Han ZJ, Hu ZD. Red blood cell distribution width and neutrophil to lymphocyte ratio are associated with outcomes of adult subarachnoid haemorrhage patients admitted to intensive care unit. Ann Clin Biochem. (2017) 54:696–701. 10.1177/0004563216686623 [DOI] [PubMed] [Google Scholar]
- 17.Siegler JE, Marcaccio C, Nawalinski K, Quattrone F, Sandsmark DK, Maloney-Wilensky E, et al. Elevated red cell distribution width is associated with cerebral infarction in aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2017) 26:26–33. 10.1007/s12028-016-0306-2 [DOI] [PubMed] [Google Scholar]
- 18.Fontana V, Bond O, Spadaro S, Annoni F, Nobile L, Badenes R, et al. Red cell distribution width after subarachnoid hemorrhage. J Neurosurg Anesthesiol. (2018) 30:319–27. 10.1097/ANA.0000000000000459 [DOI] [PubMed] [Google Scholar]
- 19.Song SY, Hua C, Dornbors D, III, Kang RJ, Zhao XX, Du X, et al. Baseline red blood cell distribution width as a predictor of stroke occurrence and outcome: a comprehensive meta-analysis of 31 studies. Front Neurol. (2019) 10:1237. 10.3389/fneur.2019.01237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakersain B, Santoni G, Faxen-Irving G, Rizzuto D, Fratiglioni L, Xu W. Nutritional status and survival among old adults: an 11-year population-based longitudinal study. Eur J Clin Nutr. (2016) 70:320–5. 10.1038/ejcn.2015.109 [DOI] [PubMed] [Google Scholar]
- 21.Srikantia SG, Prasad JS, Bhaskaram C, Krishnamachari KA. Anaemia and immune response. Lancet. (1976) 1:1307–9. 10.1016/S0140-6736(76)92647-7 [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Lee K, Kim I, Jung S, Kim MJ. Red cell distribution width and early mortality in elderly patients with severe sepsis and septic shock. Clin Exp Emerg Med. (2015) 2:155–61. 10.15441/ceem.15.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulus EM, Weinberg JA, Magnotti LJ, Sharpe JP, Schroeppel TJ, Fabian TC, et al. Admission red cell distribution width: a novel predictor of massive transfusion after injury. Am Surg. (2014) 80:685–9. 10.1177/000313481408000724 [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Chaparro MA, Calvo-Bonacho E, Gonzalez-Quintela A, Cabrera M, Sainz JC, Fernandez-Labandera C, et al. Higher red blood cell distribution width is associated with the metabolic syndrome: results of the Ibermutuamur CArdiovascular RIsk assessment study. Diabetes Care. (2010) 33:e40. 10.2337/dc09-1707 [DOI] [PubMed] [Google Scholar]
- 25.Sun P, Zhang F, Chen C, Bi X, Yang H, An X, et al. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: a retrospective study from southern China. Oncotarget. (2016) 7:42650–60. 10.18632/oncotarget.9516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu J, Zhou T, Xue M, Sun H, Shen Y, Chen Y, et al. Correlation analysis of hemoglobin-to-red blood cell distribution width ratio and frailty in elderly patients with coronary heart disease. Front Cardiovasc Med. (2021) 8:728800. 10.3389/fcvm.2021.728800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JL, Wu JN, Lv XD, Yang QC, Chen JR, Zhang DM. The value of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and hemoglobin-to-red blood cell distribution width ratio in the progression of non-small cell lung cancer. PLoS ONE. (2020) 15:e0237947. 10.1371/journal.pone.0237947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiu WJ, Zheng YY, Wu TT, Hou XG, Yang Y, Ma YT, et al. Hemoglobin-to-red-cell distribution width ratio is a novel predictor of long-term patient outcomes after percutaneous coronary intervention: a retrospective cohort study. Front Cardiovasc Med. (2022) 9:726025. 10.3389/fcvm.2022.726025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Chen Z, Yang H, Li H, Chen R, Yu J. Relationship between the hemoglobin-to-red cell distribution width ratio and all-cause mortality in septic patients with atrial fibrillation: based on propensity score matching method. J Cardiovasc Dev Dis. (2022) 9:400. 10.3390/jcdd9110400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin Z, Liao N, Lu X, Duan X, Zhou Q, Ge L. Relationship between the hemoglobin-to-red cell distribution width ratio and all-cause mortality in ischemic stroke patients with atrial fibrillation: an analysis from the MIMIC-IV database. Neuropsychiatr Dis Treat. (2022) 18:341–54. 10.2147/NDT.S350588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson ABL, Pollard T, Horng S, Celi LA, Mark R. MIMIC-IV (version 1.0). PhysioNet. (2021). 10.13026/7vcr-e114 [DOI] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 33.Petrella F, Casiraghi M, Radice D, Cara A, Maffeis G, Prisciandaro E, et al. Prognostic value of the hemoglobin/red cell distribution width ratio in resected lung adenocarcinoma. Cancers. (2021) 13:710. 10.3390/cancers13040710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X, Chen J, Li Y, Liu H, Hou C, Zeng Q, et al. Threshold effects of moderately excessive fluoride exposure on children's health: a potential association between dental fluorosis and loss of excellent intelligence. Environ Int. (2018) 118:116–24. 10.1016/j.envint.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 35.Lin L, Chen CZ, Yu XD. The analysis of threshold effect using Empower Stats software. Zhonghua Liu Xing Bing Xue Za Zhi. (2013) 34:1139–41. [PubMed] [Google Scholar]
- 36.Yu X, Cao L, Yu X. Elevated cord serum manganese level is associated with a neonatal high ponderal index. Environ Res. (2013) 121:79–83. 10.1016/j.envres.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Annal. Transl. Med. (2016) 4:30. 10.3978/j.issn.2305-5839.2015.12.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Z, Zhang X, Luo Y, Chen Y, Yuan Y. The value of hemoglobin-to-red blood cell distribution width ratio (Hb/RDW), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) for the diagnosis of nasopharyngeal cancer. Medicine. (2021) 100:e26537. 10.1097/MD.0000000000026537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su YC, Wen SC, Li CC, Su HC, Ke HL, Li WM, et al. Low hemoglobin-to-red cell distribution width ratio is associated with disease progression and poor prognosis in upper tract urothelial carcinoma. Biomedicines. (2021) 9:672. 10.3390/biomedicines9060672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu F, Yang S, Tang X, Liu W, Chen H, Gao H. Prognostic value of baseline hemoglobin-to-red blood cell distribution width ratio in small cell lung cancer: a retrospective analysis. Thoracic Cancer. (2020) 11:888–97. 10.1111/1759-7714.13330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yilmaz H, Yilmaz A, Demirag G. Prognostic significance of hemoglobin-to-red cell distribution width ratio in patients with metastatic renal cancer. Future Oncol. (2021) 17:3853–64. 10.2217/fon-2021-0040 [DOI] [PubMed] [Google Scholar]
- 42.Zheng YY, Wu TT, Chen Y, Hou XG, Yang Y, Ma X, et al. Gamma-glutamyl transferase-to-platelet ratio as a novel predictor of long-term adverse outcomes in patients after undergoing percutaneous coronary intervention: a retrospective cohort study. Thromb Haemost. (2019) 119:1021–30. 10.1055/s-0039-1681103 [DOI] [PubMed] [Google Scholar]
- 43.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. (2001) 344:1959–65. 10.1056/NEJM200106283442601 [DOI] [PubMed] [Google Scholar]
- 44.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. (2003) 290:2945–51. 10.1001/jama.290.22.2945 [DOI] [PubMed] [Google Scholar]
- 45.Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. (2009) 158:659–66. 10.1016/j.ahj.2009.07.024 [DOI] [PubMed] [Google Scholar]
- 46.Cornelissen A, Guo L, Sakamoto A, Virmani R, Finn AV. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine. (2019) 47:598–606. 10.1016/j.ebiom.2019.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study was obtained from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, the following licenses/restrictions apply: To access the files, users must be credentialed users, complete the required training (CITI Data or Specimens Only Research) and sign the data use agreement for the project. Requests to access these datasets should be directed to Physionet, https://doi.org/10.13026/7vcr-e114.