Abstract
Morphine and codeine, two of the most common opioids, are widely used in the clinic for different types of pain. Morphine is one of the most potent agonists for the μ-opioid receptor, leading to the strongest analgesic effect. However, due to their association with serious side effects such as respiratory depression, constriction, euphoria, and addiction, it is necessary for derivatives of morphine and codeine to be developed to overcome such drawbacks. The development of analgesics based on the opiate structure that can be safe, orally active, and non-addictive is one of the important fields in medicinal chemistry. Over the years, morphine and codeine have undergone many structural changes. The biological investigation of semi-synthetic derivatives of both morphine and codeine, especially morphine, shows that studies on these structures are still significant for the development of potent opioid antagonists and agonists. In this review, we summarize several decade-long attempts to synthesize new analogues of morphine and codeine. Our summary placed a focus on synthetic derivatives derived from ring A (positions 1, 2, and 3), ring C (position 6), and N-17 moiety.
Keywords: semi-synthesis, opiate, alkaloid, antinociceptive, narcotic, natural product
1. Introduction
Natural products and their derivatives have historically played a significant role in drug discovery [1]. In 1805, the German chemist F. W. A. Sertürner isolated morphine from Papaver somniferum for the first time, which became a widely used medication to alleviate pain in the clinic [2]. In 1925, Robert Robinson proposed the chemical structure of morphine, and it was finally confirmed by X-ray crystallography in 1968. Codeine was isolated in 1832 by P. Robiquet [3]. Morphine is regarded as the “gold-standard” treatment for chronic and severe pain to which all other μ-agonists are compared. Morphine (4–20%) and codeine (0.7–5%) are the two major components of the opium poppy. Later, it was discovered that the latex of opium poppies contains other significant alkaloids, such as thebaine, papaverine, oripavine, and noscapine [4]. The structures of the most common alkaloids in opium poppy are depicted in the Figure 1.
Figure 1.
Structures of the most common opium alkaloids.
As other phenanthrene alkaloids, morphine and codeine belong to the 4,5-epoxymorphinan class.
This review provides an overview of the analogues of morphine and codeine, in which the modification of the three main moieties, including ring A, ring C, and N-17, have been investigated. The biological effects of the most potent analogues are also mentioned.
Morphine, the main alkaloid in the mixture of over 20 alkaloids in opium, could act as the prototype μ-receptor agonist in the central nervous system (CNS), which is responsible for opium’s analgesic and sedative activities. Morphine activates three types of opioid receptors: the MOR (Mu Opioid Receptors, μ), KOR (Kappa Opioid Receptor, κ), and DOR (Delta Opioid Receptor, δ) receptors. They are all G-protein-coupled receptors (GPCRs) that activate Gi or Go signal proteins [5,6]. The highest analgesic and sedative effects are produced when the μ-receptors are activated; however, this receptor is also associated with the strongest side effects. There are many side effects associated with morphine, such as respiratory depression, vomiting, constriction, nausea, euphoria, and addiction [7].
Through phase II conjugation, morphine is mainly metabolized into morphine-3-glucuronide (M3G, 60%), morphine-6-β-glucuronide (M6G, 9%), and a smaller amount of N-demethylated metabolite (3%). Two uridine-diphospho-glucuronosyltransferase (UGT) enzymes are involved in morphine glucuronidation. Morphine is metabolized by UGT2B7 to both M3G and M6G, whereas UGT1A3 forms only MG3 [8]. Morphine-3-sulfate (M3S) and morphine-6-sulfate (M6S) have been detected in plasma of newborns. In general, during fetal life, sulfation is known as a significant pathway, while in adults, glucuronidation is much more important [9,10,11]. There are also some minor metabolism routes, such as diglucuronidation to morphine 3, 6-diglucuronide, and morphine ethereal sulfate (Figure 2) [12]. M3G has shown low affinity for μ receptors [13], while M6G, as a potent μ opioid receptor activator [14], has shown more analgesic activity than morphine with significantly less side effects [15,16]. Since morphine is relatively polar, it is not efficiently absorbed from the gut. Moreover, when administered intravenously, a vast percentage is blocked by the blood–brain barrier due to its quaternary ammonium salt form at physiological pH [17]. Prodrugs can be prepared by masking the polar functional groups of morphine, which allows them to cross the blood–brain barrier and enter the central nervous system more effectively [18]. Codeine, as a prodrug of morphine, has an analgesic effect about 10- to 12-fold lower than morphine and is prepared by methylation of the phenolic OH group. Most codeine is metabolized in the liver to its glucuronidated form and around 10% to morphine via O-demethylation by the CYP2D6 (CYP2D6 encodes the Cytochrome P450 family 2 subfamily D member 6 enzyme) pathway. There are also two other metabolites, codeine-6-glucuronide and norcodeine (Figure 2) [19,20,21].
Figure 2.
Metabolism of morphine and codeine.
The functional groups that are important for the binding interactions of morphine with the active site at an opioid receptor include the phenol OH group, which is essential for hydrogen bond formation, the protonated amine to form an ionic interaction, and the aromatic ring for the Van der Waals interaction with the hydrophobic position. There have been attempts to modify the structure of the opiate alkaloids in the past, and now several semi-synthetic derivatives of morphine and their congeners, such as naltrexone, naloxone, buprenorphine, and nalbuphine, are developed and used in the clinic as drugs.
2. Modifications on Ring A
2.1. Modifications on Position 1
Halogenation of C1 and C2 of morphine alkaloids has been noticed since the middle of the 20th century. Furthermore, halogenated morphinans have been known as valuable intermediates in the synthesis of tritium-labeled radioligands [22,23]. Halogenation at C1 of codeine was reported in 1925 by Speyer and Rosenfeld [24]. Hydrogen bromide was used in the presence of hydrogen peroxide at high temperatures to yield 1-bromocodeine 10.
Singh et al. reported the synthesis of 1-chloromorphine (7) and 1-bromomorphine (8) in 1982 [25]. For the synthesis of 7 and 8, morphine was treated with HCl or HBr solutions, respectively, in the presence of an oxidizing agent (KIO3 or H2O2). The results demonstrated that the bromination of morphine with this method directed bromine to the meta position of the phenolic hydroxyl group. We will discuss another method of directing halogen at the ortho position of morphine in the next section. 3-O-Methylation of 7 and 8 with diazomethane led to the formation of 1-chlorocodeine (9) and 1-bromocodeine (10), respectively. However, 7 and 8 can also be synthesized from the direct halogenation of codeine by the method explained above.
The same group investigated the synthesis of halogenated morphine and codeine from their corresponding nitro compounds, which will be discussed further in the following. Synthesis of the 1-aminocodeine 12 intermediate is an appropriate method to attach a variety of substituents in this position. Direct nitration of codeine [26] followed by reduction of the nitro group of 11 yielded 1-aminocodeine (12). Compound 12 underwent a Sandmeyer reaction to produce 1-chlorocodeine 10 (Scheme 1).
Scheme 1.
Reagents and conditions: (i) HX (Cl or Br), KIO3, or H2O2; (ii) diazomethane, methanol/ether (1:1); (iii) HNO3, glacial acetic acid; (iv) SnCl2, HCl; (v) NaNO2, HCl; (vi) CuCl.
Moreover, a facile, safe, and yield-improving method has been developed for the synthesis of 1-bromocodeine 10 with N-bromoacetamide reagent in the presence of trifluoroacetic anhydride (Scheme 2) [27].
Scheme 2.
Reagents and conditions: (i) HBr, 30%, HCOOH, 30%. H2O2, 80 °C. 53%; (ii) NBA (1.08 eq.), TFAA (1.0 eq), THF, 0–15 °C, 24 h, 81%.
Braenden et al. reported the analgesic activity of the halogenated codeine analogues 9 and 10, which demonstrated to have about 50% potency as compared to the parent compounds [28]. The hot plate test effectiveness of 8 was three times lower than morphine, but the potency of 7 was comparable to that of morphine [29]. Two hypotheses were considered to explain this decrease in pharmacological effect: the influence of steric effects on the proper attachment of the molecule to the receptor binding site and the effect of charge distribution on the aromatic ring. The first hypothesis was substantiated by the synthesis of 1-fluorocodeine (16) by Lousberg and Weiss in 1974 and the investigation of its biological properties in comparison with 9 and 10 [30]. Fluorine has been known as a prominent feature in drug design and development [31,32,33,34]. Replacement of hydrogen with fluorine can influence the pharmacokinetic properties of the molecules and affect their stability and bioavailability. According to the antinociceptive hot plate method, 16 had an ED50 of 7.9 mg/kg, and codeine 2 had an ED50 of 7.5 mg/kg. Comparison of other parameters of these two compounds did not show any significant difference neither in this method nor in the binding to the receptor of rat brain. In view of the mentioned results, since fluorine has a high electronegativity compared to chlorine and bromine, the second hypothesis was rejected.
1-Fluorocodeine (16) was similarly synthesized from codeine via 1-aminocodeine in six steps [35]. First, codeine 2 was converted to 6-O-acetylcodeine (13) by reaction with acetic anhydride. Then, glacial acetic acid and nitric acid were added slowly to the solution of 6-O-acetylcodeine 13. After the pH was adjusted to 9 with ammonia solution, 14 was precipitated. In this step, C6-ester was hydrolyzed with NaOH to obtain 1-nitrocodeine (11), which was then reduced to the corresponding amino derivative 12 with Stannous chloride/HCl. Treatment of 12 with tetrafluoroboric acid in anhydrous ethanol, followed by adding sodium nitrite solutions, led to the formation of diazonium salt 15 in 100% yield. In the last step, codeine-1-diazonium tetrafluoroborate salt 15 was heated in the presence of MgO based on the Makleit–Dubina method [36], which led to 1-fluorocodeine (16) (Scheme 3).
Scheme 3.
Reagents and conditions: (i) Ac2O, reflux; (ii) HNO3, glacial acetic acid, (1) 5 °C, 1 h, (2) room temperature, 1 h; (iii) 10% NaOH, ethanol, reflux, 1 h; (iv) SnCl2, HCl, r.t, 2 h; (v) 48% aqueous HBF4, EtOH, 2 M NaNO2, −15 °C to r.t; (vi) MgO, 170 °C, 1 h, in vacuo.
Aromatic iodides are chemically more reactive than chlorides and bromides. They are ideal intermediates for the synthesis of organometallic compounds or derivatization involving palladium-catalyzed reactions. In the past decades, several types of research have been conducted to synthesize novel 1-iodomorphine and codeine derivatives for nuclear medical imaging and radioimmunoassay [22,37,38].
In 1997, Liebman and co-workers reported the synthesis of 1-iodomorphine 18 and 1-iodocodeine 17 [39]. Aiming to obtain 1-iodocodeine (17), a solution of codeine 2 in HCl was added to sodium iodide and chloramine T. Under these conditions, no obvious reaction occurred with morphine. Therefore, 1-iodomorphine (18) was synthesized through an O-demethylation of codeine derivative 17 with BBr3. Compound 19 was obtained after acetylation of 17 with acetic anhydride, and its structure was confirmed by crystallography (Scheme 4).
Scheme 4.
Reagents and conditions: (i) NaI, chloramine T, 0.1 M hydrochloric acid; (ii) Ac2O, reflux, 4 h; (iii) BBr3, CH2Cl2, r.t, 1 h.
Several attempts were made to introduce different groups taking advantage of the halogen on 1-bromocodeine 10 via palladium-catalyzed coupling reactions. Davies et al. synthesized novel 1-vinyl, -acyl, and -alkyl codeine derivatives through Heck-type reactions [40]. To prevent any side reactions, the allylic hydroxyl was protected by tert-butyldimethylsilyl chloride to yield 20. Treatment of 20 with a variety of vinyl compounds in the presence of Pd(OAc)2, phosphine ligand, and NEt3 led to the formation of 1-vinylcodeine derivatives. Methyl acrylate, styrene, methyl vinyl ketone, and methyl methacrylate were used as vinyl substrates, followed by deprotection of the silyl group of 21 with TBAF, which led to the formation of 22a–d, respectively. These derivatives 22a–d were identified as a single diastereoisomer with (E)-geometry at the double bond via 1H-NMR analysis based on the coupling constants (Scheme 5).
Scheme 5.
Reagents and conditions: (i) TBDMSCl (1.2 eq.), imidazole (1.2 eq.), DMF; (ii) Pd(OAc)2 (2 mol%), methyl acrylate (4 eq.), tri-o-tolylphosphine (8 mol%), NEt3 (1.4 eq.), DMF; (iii) TBAF (2 eq.), THF, r.t.
In the second approach, 1-acetylcodeine (23) was synthesized with a similar methodology. The synthesis procedure was performed by the involvement of either ethyl vinyl ether or (α-ethoxyvinyl)tributyltin in different types of palladium-catalyzed reactions. The results showed that the use of the Stille-type coupling with (α-ethoxyvinyl)tributyltin compared to the Heck process with ethyl vinyl ether increased the reaction yield from 39% to 85% (Scheme 6).
Scheme 6.
Reagents and conditions: (i) Pd(OAc)2 (2 mol%), ethyl vinyl ether (4 eq.), tri-o-tolylphosphine (8 mol%), NEt3 (1.4 eq.), DMF, then HCl (aq), 39%; (ii) Pd(OAc)2 (2 mol%), (α-ethoxyvinyl)tributyltin (4 eq.), PPh3 (8 mol%), NEt3 (1.4 eq.), DMF, then HCl, 85% (aq); (iii) PdCl2 (2 mol%), PPh3 (8 mol%), CO (3 atm), NEt3–EtOH; (iv) TBAF (2 eq.), THF.
To better investigate the reactivity of compound 20, 1-carboethoxy and 1-alkyl derivatives were synthesized as well. 1-Ethoxycarbonylcodeine (24) was obtained in 50% yield by carbonylation in the presence of carbon monoxide followed by silyl deprotection. Palladium (II) chloride was used as a source of palladium in this reaction (Scheme 6).
By the same authors, the production of 1-alkylation of codeine by a modified Stille procedure was also attempted [41]. Therefore, the methyl derivative 25a was synthesized by reacting 12 with tetramethyltin in the presence of Pd(OAc)2. In the following, some differently substituted R-tributyltins were used to produce four other 1-substituted codeine derivatives 25b–e in good yields (Scheme 7). Part of this work was conducted by Davies and Pyatt in 1989 [42].
Scheme 7.
Reagents and conditions: (i) Pd(OAc)2 (2 mol%), tri-o-tolylphosphine (8 mol%), Me4Sn (1.2 eq.), NEt3 (1.4 eq.), DMF; (ii) Pd(OAc)2 (2 mol%), PPh3 (8 mol%), Bu3SnR (1.2 eq.), NEt3; (iii) TBAF (2 eq.), THF, r.t.
1-Aminocodeine (12) is a key intermediate towards synthesizing several azo, thiazole, and hydrazine derivatives which have structures that are attractive probes in medicinal chemistry.
In 2008, Sipos et al. reported the synthesis of A-ring-fused 2-aminothiazole from 12 [43]. As a starting point, codeine was again converted to 11 [44]. For the reduction of the nitro analogue 11 to the corresponding amino compound 12, formamidinesulfinic acid (FSA) was used instead of the older method of using hydrogen sulfide. An attempt to synthesize the compound 26 using bromine with potassium thiocyanate in acetic acid (Kaufman method) was associated with low yield [45]. The possibility of radical intermediates in such reactions led the scientists to apply high temperature and microwave (MW) induction as successful optimizations (Scheme 8), and a mechanism was proposed for this reaction with radical intermediates, supported by DFT (Density Functional Theory) calculations.
Scheme 8.
Reagents and conditions: (i) FSA, NaOH; (ii) KSCN, Br2, AcOH, 60 W, 130 °C, 15 min.
With the aim to synthesize azo and hydrazone derivatives of codeine, Cankar et al. used 11 as a starting material [46]. Initially, a novel nitration process was developed using bismuth nitrate pentahydrate (instead of nitric acid) as a mild nitrating agent with a 69% yield. Then, the nitro group was reduced to an amino group (Scheme 9). In this study, the structures of 11 and 12 were confirmed by X-ray analysis. Diazonium salt 27 was synthesized by the reaction of 12 with sodium tetrafluoroborate. 2-Pyridylacetonitrile and 2-naphthol were reacted with 27 in the presence of a base to produce a mixture of E/Z isomer 29 (67%) and the E isomer of 28 (97%), respectively (Scheme 10). In the subsequent steps, intending to synthesize heterocyclic derivatives, compound 27 underwent reaction with malononitrile to afford hydrazone 30, followed by reaction of 30 with hydrazine to yield diaminopyrazole 31. Another analogue was obtained through a coupling reaction of 27 with (2-cyanoacetyl)carbamate, which cyclized directly to 6-azauracil 33 (Scheme 10).
Scheme 9.
Reagents and conditions: (i) Bi(NO3)3·5H2O, CH3COOH, r.t, 6 h, 69%; (ii) SnCl2, 35% HCl, r.t, 2 h, 86%.
Scheme 10.
Reagents and conditions: (i) NaNO2, HCl, NaBF4, H2O, 5–7 °C, 15 min; (ii) 2-Naphthol, NaOH, H2O, 5–7 °C, 18 h, 97%; (iii) 2-(pyridin-2-yl)acetonitrile, CH3COONa, H2O, 5–7 °C, 18 h, 67%; (iv) malononitrile, CH3COONa, H2O, 3–5 °C, 3 h; (v) N2H4·H2O, MeOH, 65 °C, 3 h, 48% (over two steps); (vi) ethyl (2-cyanoacetyl)carbamate, NaOH, H2O, 3–5 °C, 1 h; (vii) 47% (over two steps).
2.2. Modifications on Position 2
Halogenation at position 2 was achieved by converting morphine (1) to 2-aminomorphine (35) [25,44]. Initially, 2-nitromorphine (34) was prepared with the nitration being regioselective for position 2 due to the presence of the free hydroxyl group at the 3-position. Tin and hydrochloric acid [44], electrolytic reduction [44], and reduction utilizing formamidinesulfinic acid [44] were the three main methods for the reduction of nitro derivatives to the corresponding amino analogues. 2-Chloromorphine (36) was prepared through the Sandmeyer reaction from 35. Furthermore, treatment of 36 with ethereal diazomethane in methanol led to the formation of 2-chlorocodeine (37) (Scheme 11) [24].
Scheme 11.
Reagents and conditions: (i) Sn/HCl, FSA, or electrolytic reduction; (ii) HCl, NaNO2; (iii) CuCl; (iv) ethereal diazomethane, MeOH.
The formation of the 2-aminothiazole ring (26) from 1-nitrocodeine (12) has already been explained in the previous section. The same group demonstrated that a similar methodology can be applied to the synthesis of ring A-fused 2-aminothiazole 38 starting from 2-nitromorphine (35) (Scheme 12) [43].
Scheme 12.
Reagents and conditions: (i) KSCN, Br2, AcOH, 60 W, 130 °C, 15 min.
2.3. Modifications on Position 3
According to several studies, the 3-hydroxy group in morphine is necessary to form a hydrogen bond in its target binding site. Researchers have studied the interaction of these derivatives with opioid receptors over the years by substituting different groups in this position.
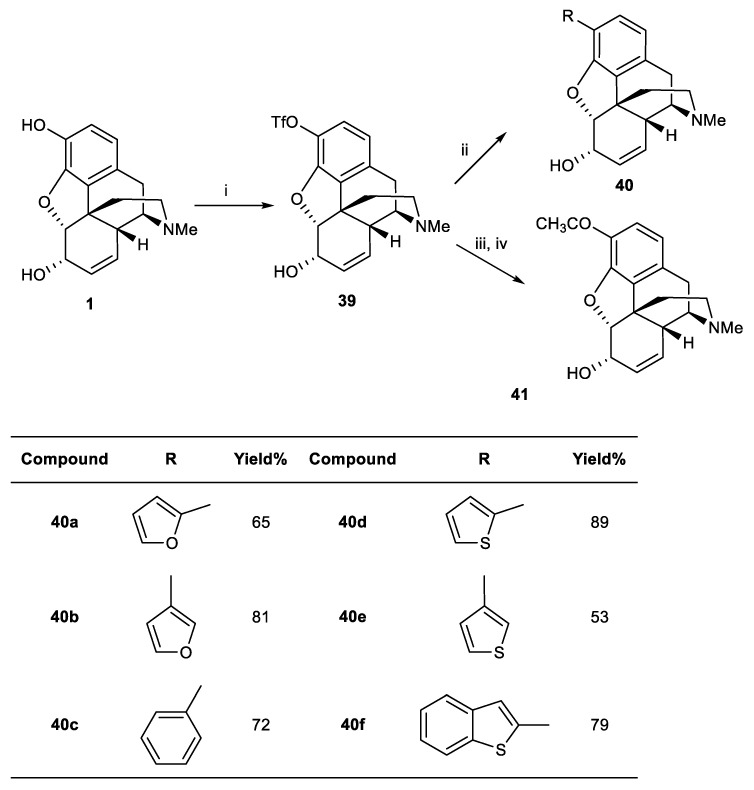
The palladium-catalyzed reaction was used to synthesize new C3-substituted derivatives of morphine [47]. As expected, substitution at the C3 position showed no improvement in morphine potency at any opioid receptors. However, the selectivity of 3-furyl analogue 40b at μ-receptors was close to that of morphine.
Treatment of morphine with N-phenyltrifluoromethanesulfonimide and triethylamine led to the formation of triflate derivative 39. Palladium-catalyzed coupling reaction of 39 with (1-ethoxy)vinyltributyltin and hydrolysis resulted in methylketone 41. By the Suzuki–Miyaura reaction of the different arylboronic acids with 39 in the presence of palladium-tetrakis(triphenylphosphine), C3-aryl-substituted analogues 40a–f were obtained in 53–89% yields (Scheme 13). The affinity of novel derivatives for opioid receptors was lower than morphine; however, the selectivity of compound 40b was comparable to that of morphine (Table 1).
Scheme 13.
Reagents and conditions: (i) N-phenyltrifluoromethanesulfonimide, CH2Cl2; (ii) RB(OH)2, Pd(PPh3)4, LiCl, 2M Na2CO3, EtOH, DME; (iii) CH2=C(OC2H5)-SnBu3, PPh3, PdCl2(PPh3)2, DMF, 2,6-di-t-butyl-4-methylphenol; (iv) THF, 1 M HCl.
Table 1.
Potencies of morphine and analogues at μ, δ, and κ-Opioid receptor recognition sites labeled by [3H]DAMGO, [3H]DPDPE, and [3H]U69593, respectively, in the guinea pig brain minus cerebellum (μ and δ) and the guinea pig cerebellum (κ).
| IC50 (nM) | |||
|---|---|---|---|
| Compound | [3H]DAMGO (µ) | [3H]DPDPE (δ) | [3H]U69593 (κ) |
| Morphine | 8.5 | 430 | 130 |
| 40a | 2600 | 31% at 10 μM | 9500 |
| 40b | 200 | 7400 | 910 |
| 40c | 43% at 10 μM | 16% at 10 μM | 76,000 |
| 40d | 49% at 10 μM | 17% at 10 μM | 25,000 |
| 40e | 3300 | 10% at 10 μM | 15,000 |
| 40f | 41% at 10 μM | 14% at 10 μM | 126,000 |
| 41 | 640 | 7900 | 5100 |
Previous work of Wentland and co-workers on cyclazocine analogues [48] had shown that mono N-substitution on the 8-OH group was found to have an affinity for opioid receptors, and it seems that replacing phenolic OH by NH can enhance the binding affinity. It can be demonstrated that NH2 can play an isostere role for the 3-OH of morphine for opioid receptors.
The 3-OH group of morphine can be replaced by the amino group using the Pd-catalyzed amination Buchwald methodology starting from aryl triflates [49]. Wentland et al. reported new morphine analogues based on this method [50]. In the first step, 3,6-hydroxy groups were both protected to form 3,6-bis-tert-butyldiphenylsilyl ether 42 through the reaction of morphine (1) with imidazole and excess tert-butyldiphenylsilyl chloride (TBDPSCl). To produce the monoprotected 6-tert-butyldiphenylsilyl ether 43, 42 was reacted with tetrabutylammonium fluoride. The best results for high selectivity 3-TBDPS deprotection of compound 42 were obtained with 0.25 eq of TBAF (1 M in THF containing 5% H2O). In this reaction, water may play a crucial role in catalyzing the reaction by converting the HF (resulting from hydrolyzing TBDPSF produced by 3-TPDPS cleavage) to TBAF, which can desilylate another 42 on the catalytic cyclic. Compound 43 was converted to the 3-triflate 44 using a standard method.
Two known Pd-catalyzed amination reactions were used for synthesizing derivatives 45c–f [49]. In one of them, the combination of Pd(OAc)2 with BINAP was used, and in the other method, Pd2(dba)3 with DPPF were used. Hydrolysis in the last step gave the desired derivatives 46b–e in 79–96% yields. To obtain compound 46a, 45a was converted to 45b under imine exchange conditions, followed by deprotection of the 6-hydroxyl group (Scheme 14).
Scheme 14.
Reagents and conditions: (i) TBDPSCl, imidazole, CH2Cl2, 25 °C, 1 h, quant; (ii) 0.25 eq. TBAF, THF/H2O, 25 °C, 1 h, 84%; (iii) Tf2O, pyr, CH2Cl2, 0 °C, 1 h; (iv) RR’NH, t-BuONa, toluene, 80 °C, Pd(OAc)2, BINAP; (v) Pd2(dba)3, DPPF, 26–84%; (vi) 1.5 eq.TBAF, THF/H2O, 25 °C, 6–12 h, 79–96%; (vii) NH2OH.HCl/NaOAc, MeOH, 97%.
Subsequently, binding affinity and selectivity of the synthesized derivatives 46a–e on μ, δ, and κ opioid receptors in guinea pig membranes were investigated in comparison to morphine. All derivatives showed significant decrease in affinity for µ (38–273-fold), δ (11–41-fold), and κ (10–141-fold) opioid receptors. As a consequence of substituting hydrogen in 46a with methyl (46c), the affinity for μ receptors decreased, although no significant difference was observed between 46c and 46d. The analogue 46e showed the highest μ affinity and highest µ:δ and µ:κ selectivity ratios in the series (Table 2). The results showed that replacement of the 3-OH group of morphine with -NH2 as a bioisoster generally had a favorable effect on the activity.
Table 2.
Opioid receptor binding data for compounds 46a–e.
| Ki (nM ± SE) | Receptor Selectivity a | |||||
|---|---|---|---|---|---|---|
| Compound | [3H]DAMGO (µ) | [3H]naltrindole (δ) | [3H]U69593 (κ) | µ:δ | µ:κ | κ:δ |
| Morphine | 0.88 ± 0.14 | 140 ±18 | 24 ± 2.3 | 156 | 27 | 6 |
| 46a | 53 ± 3.0 | 2400 ± 190 | 740 ± 75 | 45 | 14 | 3 |
| 46b | 63 ± 15 | 5700 ± 1100 | 2800 ± 420 | 90 | 44 | 2 |
| 46c | 240 ± 16 | 1600 ± 110 | 290 ± 8.1 | 7 | 1 | 6 |
| 46d | 59 ± 3.7 | 1500 ± 100 | 240 ± 23 | 25 | 4 | 61 |
| 46e | 33 ± 5.1 | 5500 ± 190 | 3400 ± 540 | 67 | 103 | 2 |
a Receptor selectivity is expressed as the ratio of the corresponding Ki values.
The A-ring-fused 2-aminothiazole derivative 50 was reported as a bioisostere of phenolic hydroxyl [51]. Morphine 1 was reacted with PhNTf2 to selectively convert the phenolic hydroxyl group to the triflate 39. After protection of the allylic hydroxyl with TBDPS, compound 47 was obtained. The next amination step to achieve 48 was the same as the method reported by Wentland et al. [50]. Treatment of 48 with KSCN and Br2, followed by deprotection of allylic hydroxyl group, led to the formation of 50 (Scheme 15). This heterocyclic bioisostere, aminothiazole, has been successfully applied to dopamine agonists, for instance, B-HT 920 [52] and PD 118440 [53], to improve their pharmacological properties. In comparison to the high affinity of morphine 1 at the µ receptors, this aminothiazole derivative (50) displayed a 34-fold (Ki = 30 nM) and a 9-fold (Ki =220 nM) decrease in affinity against μ and κ receptors, respectively, and a 4-fold (Ki = 32 nM) increase in affinity at δ receptors compared to the phenol, levorphanol (Ki (μ) = 0.21 nM, Ki (δ) = 4.2 nM, Ki (κ) =2.3 nM). Compound 50 also showed a 7-fold higher affinity at κ receptors than morphine (Ki = 24 nM), and at μ and δ receptors, no significant difference was observed (Table 3).
Scheme 15.
Reagents and conditions: (i) PhNTf2, Et3N; (ii) TBDMSCl, imidazole, THF; (iii) Pd(OAc)2, BINAP, Ph2C=NH, Cs2CO3; (iv) NaOAc, NH2OH.HCl; (v) KSCN, Br2, AcOH, 23.4%; (vi) TBAF, THF, 62.2%.
Table 3.
Ki value inhibition of δ, κ, and µ opioid binding to Chinese hamster ovary membranes by compound 50 a.
| Ki ± SEM (nM) | Selectivity | ||||
|---|---|---|---|---|---|
| Compound | [3H]DAMGO (µ) | [3H]naltrindole (δ) | [3H]U69593 (κ) | µ:κ | δ:κ |
| Morphine | 0.88 ± 0.14 | 140 ±18 | 24 ± 2 | 0.04 | 5.8 |
| Levorphanol | 0.21 ± 0.02 | 4.2 ± 2.3 | 2.3 ± 0.3 | 0.09 | 2 |
| 50 | 30 ± 3 | 32 ± 3 | 220 ± 10 | 0.13 | 0.14 |
a Chinese hamster ovary membrane, 0.5 mg of protein/sample, were incubated with 12 different concentrations of the compound in the presence of receptor-specific radioligands at 25 °C, in a final volume of 1 mL of 50 mM Tris-HCl, pH 7.5.
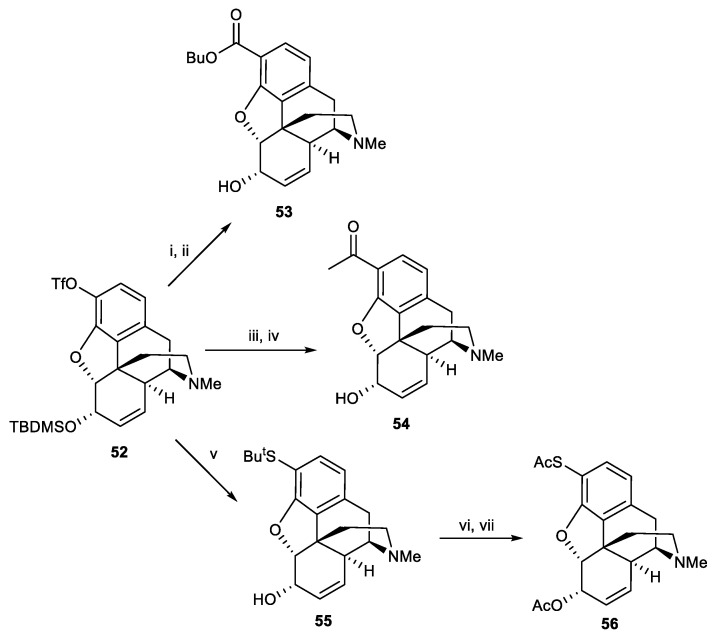
The synthesis of some derivatives in position 1 of codeine through the palladium-catalyzed reactions was mentioned in the previous section. The same group used this reaction to synthesize new 3-morphine analogues [40]. Initially, compound 51 was obtained in 87% yield from the protection of two hydroxyl groups of morphine with tert-butyldimethylsilyl chloride. Towards 52, first the phenolic group of 3,6-di-O-tert-butyldimethylsilylmorphine 51 was selectivity deprotected by TBAF to yield 47, followed by conversion to triflate 52 (Scheme 16). Compounds 53 and 54 were synthesized from 52 in the presence of Pd(OAc)2 through the Stille and Pd-catalyzed carobonylation reactions, respectively. Substitution of sulfur at the 3-position resulted in the formation of 56. Triflate 52 was reacted with tributylstannyl tert-butyl sulfide and Pd(PPh3)4 to produce 55. The tert-butyl group deprotected with mercuric acetate and TFA and then treated with hydrogen sulfide gas and acetic anhydride led to the formation of 56 in 47% overall yield (Scheme 17).
Scheme 16.
Reagents and conditions: (i) TBDMSCl (3 eq.), imidazole (5.0 eq.), THF; (ii) TBAF (1 eq.), THF, 0 °C; (iii) trifuoromethanesulfonic anhydride (1.2 eq.), 2,6-dimethylpyridine (1 eq.), DCM, 0 °C.
Scheme 17.
Reagents and conditions: (i) CO (3 atm), n-BuOH, Pd(OAc)2 (4 mol%), 1,3-bis(diphenylphosphino)propane (4 mol%), heat (ii) TBAF, THF, 76% (overall two step); (iii) (α-ethoxyvinyl)tributylstannane, Pd(OAc)2 (4 mol%), PPh3 (8 mol%), LiCl, DMF, heat; (iv) HCl(aq), r.t, 80% (overall two steps); (v) Pd(PPh3)4 (0.5 eq.), tributylstannyl tert-butyl sulfide (2 eq.), LiCl (3 eq.), DMF, 83%; (vi) Hg(OAc)2 (1.05 eq.), anisole, TFA, 0 °C, then H2S; (vii) acetic anhydride, NEt3, DMAP, DCM, 47% (overall two step).
In 2012, Salvatella et al. synthesized two lipophilic analogues of M3G by forming an amide bond between a primary alkylamine and carboxylic acid group of M3G [54]. The synthesis procedures were explained in detail in the review of sugar derivatives of morphine by Jash and Gorai [55]. A surprising result of biological activity in rats and mice showed that increasing lipophilicity by an aliphatic octyl chain produced highly toxic derivative 58. The opioid antagonist activity of 57 showed the same properties as naloxone without being toxic (Figure 3).
Figure 3.
Structures of morphine-3-O-octylglucuronamide and morphine-3-O-glucuronamide.
3. Modifications on Position 6
α-Halogenated-codides and -morphides are known to be key intermediates for substituting with nucleophiles at this allylic position. α-Chlorocodide (59a) was synthesized through the reaction of codeine 2 with thionyl chloride by the method of Wieland and Kappelmeier [56]. Stork and Clarke showed that the α-chlorocodide reacts with nucleophiles such as piperidine and thioethanol by an SN2 mechanism [56]. In 1976, the synthesis of α-chloromorphine 59b from the reaction of morphine hydrate with dimethylchloroformiminium chloride (Vilsmeier reagent) was reported (Scheme 18) [57]. The measurement of the analgesic potency and toxicity has shown that α-chloromorphine was about 10–15 times more potent than morphine, but in vivo test showed it was significantly more toxic, again compared to morphine.
Scheme 18.
Reagents and conditions. (i) Dimethylchloroformiminium chloride, DMF; (ii) PPh3, diethyl azodicarboxylate (DEAD), or diisopropyl azodicarboxylate (DIAD), benzene or toluene, r.t, 1 h.
Compounds 59a–c were also synthesized via the Mitsunobu reaction [29,58]. The hydrochloride salts of codeine and morphine were reacted with azocarboxylates (DEAD or DIAD) in the presence of triphenylphosphine. It was shown that the hydrogen halide produced in situ in this reaction can act as an acid component. The reaction proceeded with chloride and bromide with an inversion of configuration at the C6 position to obtain 59a–c (Scheme 18).
In 1988, Fujii et al. reported the novel analogues of 6-β-thiomorphine [59]. The C6-hydroxyl group was replaced with the thiol group. Two independent procedures were examined to synthesize compounds 60 and 61. In the first step, morphine was treated with thioacetic acid and N,N-dimethylformamide dineopentyl acetal. This reaction resulted in the formation of 61 as the major product. In the second procedure, morphine underwent the Mitsunobu reaction with thioacetic acid and triphenylphosphine (PPh3) in the presence of diisopropyl azodicarboxylate (DIAD). In this reaction compound 60 was prepared in 73% yield as the major product (Scheme 19). The stereochemistry of 60 and 61 at the C6 position was assigned as the β-orientation based on 1H-NMR spectroscopy. Compound 63 underwent the reaction with L-cysteine methyl ester followed by ethyl chloroformate and K2CO3, producing carbamate 64 (Scheme 20). It has been demonstrated that this sulfhydryl morphine derivative 63 binds to the μ-receptors from the disulfide bond [60]. Furthermore, the analgesic activity of compound 60 was five times higher than morphine in rats.
Scheme 19.
Reagents and conditions: (i) AcSH, N,N-dimethylformamide dineopentyl acetal, dry toluene, 80 °C; (ii) AcSH, 2-nitrobenzenesulfenyl chloride, PPh3, dry THF, 0 °C.
Scheme 20.
Reagents and conditions: (i) 0.2 N KOH, EtOH, N2 (96%); (ii) 2-nitrobenzenesulfenyl chloride, CH3CN, 0 °C (78%); (iii) L-Cys-OMe.HCl, EtOH, or DMF; (iv) ethyl chloroformate (3 eq), K2CO3 (anh), CH3Cl, 0 °C.
By converting the C6-hydroxyl of morphinans to amine, a group of compounds called aminomorphinans have been produced. The synthesis of pharmacologically active analogues of aminomorphinans through the Mitsunobu reaction led to the development of a variety of derivatives [61,62,63].
The Mitsunobu reaction has gained much attention in the field of natural products [64,65,66]. Several researchers have reported the application of this method for the synthesis of novel morphine derivatives.
In 1968, Makleit and Bognár reported the new derivative of morphine and codeine that obtained much attention in the following years [67]. 6-Deoxy-6-azido-dihydroisomorphine (azidomorphine 67a) and d 6-deoxy-6-azido-dihydroisocodeine (azidocodeine 67b) were synthesized. It was demonstrated that azidomorphine has 40 to 300 times, and azidocodeine has about 13 times, more potency than morphine in animal [68] and human [69] clinical trial studies. The ED50 of 67a and 67b in the tail flick and hot plate test were reported in Table 4 [70]. It has been demonstrated that the incidence of side effects was significantly decreased in patients who were treated with azidomorphine. The reaction of 65 with methanesulfonyl chloride and pyridine yielded compound 66. Treatment of mesylates 66 with sodium azide through an SN2 reaction with inversion of configuration followed by removal of the 3-acetyl group led to the formation of azidomorphine 67a (Scheme 21) [71]. The dependence liability and tolerance of azidomorphine in mice and rats were much lower than that of morphine [72].
Table 4.
The relative analgesic activity of azidomorphine and azidocodeine.
| Compound | Hot Plate Test ED50 (mg/kg) |
Tail Flick Test ED50 (mg/kg) |
|---|---|---|
| Morphine | 4.7 | 1.8 |
| (2.9–7.8) a | (1.08–3.15) | |
| Codeine | 14 | 22 |
| (6.7–29.4) | (15.3–28.8) | |
| 67a | 0.016 | 0.012 |
| (0.007–0.038) | (0.009–0.016) | |
| 67b | 0.36 | 0.61 |
| (0.16–0.79) | (0.4.1–0.79) |
a Figures in parentheses indicate 95% confidence limits.
Scheme 21.
Reagents and conditions: (i) mesyl chloride, pyridine, 47.5%; (ii) aq.sodium azide, DMF, 24 h, 100 °C, 50.3%.
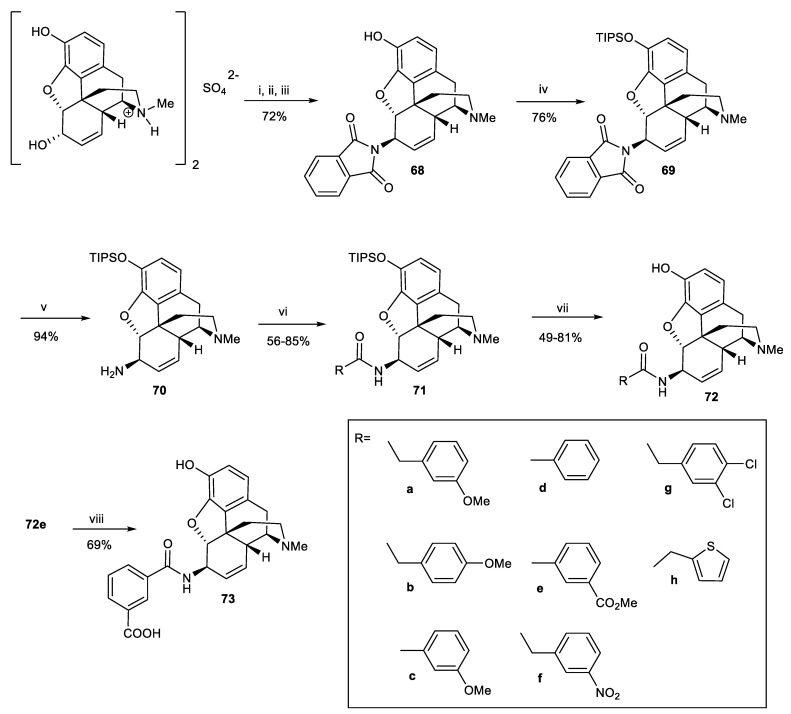
In 2004, MacDougall and co-workers attempted to synthesize novel 6-arylamidomorphine derivatives as morphine-6-glucuronide (M6G) analogues by introducing different aryl groups instead of saccharides [73]. First, the 6-α-hydroxyl group was converted to 6-β-phthalimide 68 through the Mitsunobu reaction. The phenolic hydroxyl group of morphine was selectively acetylated, and the resultant allylic alcohol subsequently reacted with DIAD and phthalimide in the presence of Ph3P. This was followed by the removal of the C-3 acetyl group, affording compound 68. Treatment of 6-β-phthalimidomorphine 68 with imidazole and triisopropylsilyl chloride (TIPSCl) followed by deprotection of the phthalimide group in the presence of hydrazine hydrate led to the formation of primary amine 70. The amide derivatives 71a–h were obtained from the reaction of 70 with a variety of arylmethyl and heteroarylmethyl acid chlorides. Deprotection with TBAF produced the desired 6-β-amidomorphines 72a–h. Subsequently, hydrolysis of 72e with lithium hydroxide gave the isophthalic acid derivative (73) (Scheme 22).
Scheme 22.
Reagents and conditions: (i) Ac2O, NaHCO3, H2O; (ii) PPh3, i-PrO2CN=NCO2i-Pr, phthalimide; (iii) NH2OH.HCl, EtOH, 55 °C; (iv) TIPSCl, imidazole, DMF; (v) NH2NH2, EtOH, 55 °C; (vi) RCOCl, NEt3, CH2Cl2; (vii) TBAF, THF; (viii) LiOH.
Analogues 72a–h exhibited 21–64-fold higher affinity for μ receptors compared to M6G and 1.9–5.5-fold higher affinity than morphine. The affinity of compound 73 for the μ receptors was significantly lower than other derivatives. The [35S]GTPγS assay was used to evaluate the potency or affinity of derivatives for the opioid receptors. [35S]GTPγS binding was maximal with full agonists and reduced with partial agonists. Based on the EC50 values, all derivatives showed full μ agonist effects except 72b and 73, which were partial μ agonists. Compound 73 was chosen for the tail flick test in rats, and it showed weak antinociceptive activity with ED50 = 12.6 mg/kg at 60 min. These results showed well the pharmacological effects of substituting arylamide groups with saccharide portion in M6G. Part of these biological data are summarized in Table 5.
Table 5.
Ki inhibition values of μ, δ, and κ opioid binding to CHO membranes, and EC50 values of stimulation of [35S]GTPγS binding by amides 72a–h and 73 mediated by μ, δ, and κ opioid receptors.
| Ki ± SEM (nM) | EC50 | |||||
|---|---|---|---|---|---|---|
| Compound | [3H]DAMGO (µ) | [3H]DPDPE (δ) | [3H]U69593 (κ) | µ | δ | κ |
| Morphine | 1.1 ± 0.1 | 140 ± 2 | 46.9 ± 14 | |||
| M6G | 12.85 ± 0.95 | 160.96 ± 0.73 | 4058.75 ± 230 | 72.3 ± 26.7 | 190.35 ± 22.9 | >10K |
| 72a | 0.35 ± 0.08 | 9.5 ± 2.6 | 0.96 ± 0.2 | 1.7 ± 0.2 | 18.8 ± 2.2 | 4.8 ± 0.1 |
| 72b | 0.59 ± 0.19 | 8.89 ± 2.1 | 2.84 ± 0.96 | 4.8 ± 2.1 | 25.2 ± 3.0 | 5.0 ± 1.0 |
| 72c | 0.21 ± 0.07 | 8.96 ± 1.95 | 2.65 ± 1.25 | 2.8 ± 0.2 | 37.8 ± 1.9 | 9.8 ± 0.02 |
| 72d | 0.40 ± 0.03 | 24.95 ± 2.33 | 4.13 ± 1.21 | 5.5 ± 0.9 | 62.7 ± 24.0 | 5.3 ± 1.3 |
| 72e | 0.23 ± 0.05 | 3.39 ± 0.03 | 1.53 ± 0.2 | 2.4 ± 0.3 | 6.20 ± 0.42 | 11.22 ± 3.51 |
| 72f | 0.20 ± 0.04 | 18.0 ± 5.63 | 2.63 ± 1.1 | 1.9 ± 0.2 | 24.5 ± 3.0 | 1.2 ± 0.1 |
| 72g | 0.20 ± 0.04 | 0.94 ± 0.02 | 0.75 ± 0.2 | 0.1 ± 0.0 | 1.3 ± 0.3 | 0.03 ± 0.02 |
| 72h | 0.33 ± 0.02 | 14.42 ± 1.26 | 0.58 ± 0.17 | 6.0 ± 2.1 | 32.4 ± 2.1 | 4.5 ± 0.2 |
| 73 | 19.92 ± 4.29 | 50.78 ± 8.86 | >10K | 65.1 ± 21.8 | 74.0 ± 12.7 | 5096 |
Following the efforts to synthesize new analogues of M6G in 2013, Váradi et al. synthesized novel 6β-acylamides of morphine 1 and codeine 2 [74]. Initially, 6β-amino derivatives 74 and 76 were synthesized using the Mitsunobu reaction. Treatment of 6β-amino morphine or codeine in NEt3 with thionyl chloride and appropriate carboxylic acids led to the formation of the desired 75a–e and 77a–e derivatives, respectively (Scheme 23).
Scheme 23.
Reagents and conditions: (i) acetic anhydride, NaHCO3, H2O, r.t, 1 h; (ii) phthalimide, DIAD, Ph3P, benzene, r.t, 2h; (iii) hydrazine hydrate, EtOH; (iv) 1.1 eq. thionyl chloride, RCOOH, Et3N, CH2Cl2, r.t, 2 h; (v) Na2CO3, H2O, MeOH, r.t, 12 h.
In vitro binding studies showed that cinnamoyl morphinamines had a moderate to high affinity for MOR-1 (Table 6). In contrast, the affinity of the corresponding codeine derivatives was significantly lower than the cinnamoyl morphinamine analogues. The analgesic potency of 75a (ED50 = 3.13 ± 1.09 mg/kg) as the most effective analogue was similar to that of morphine (ED50 = 4.96 ± 0.97 mg/kg) but without causing respiratory depression, which has been the main side effect of traditional opiates. The [35S]GTPγS binding assay for the most potent derivative (75a) showed that it acts as a full agonist for all opioid receptors (MOR-1 agonist DAMGO, KOR-1 agonist U50,488H, and DOR-1 agonist DPDPE).
Table 6.
Receptor binding and in vivo analgesia data of selected 6β-acylaminomorphinans (75a–d) a.
| Affinity (Ki (nM) ± SEM) | Analgesia (ED50, mg/kg, s.c.) |
|||
|---|---|---|---|---|
| Compound | MOR-1 | KOR-1 | DOR-1 | |
| Morphine | 4.60 ± 1.81 | 4.96 ± 0.96 | ||
| 75a | 0.10 ± 0.02 | 2.90 ± 0.66 | 10.26 ± 6.76 | 3.13 ± 1.09 |
| 75b | 0.15 ± 0.03 | 1.97 ± 0.01 | 9.38 ± 1.53 | >10 |
| 75c | 0.19 ± 0.09 | 10.52 ± 0.90 | 14.52 ± 6.82 | >10 |
| 75d | 0.74 ± 0.12 | 5.43 ± 1.38 | 15.26 ± 1.74 | >10 |
| 75e | 0.12 ± 0.006 | 0.81 ± 0.09 | 5.15 ± 0.75 | >10 |
a Competition studies were performed with the indicated compounds against 125I-BNtxA (0.1 nM) in membranes from CHO cells stably expressing the indicated cloned mouse opioid receptors.
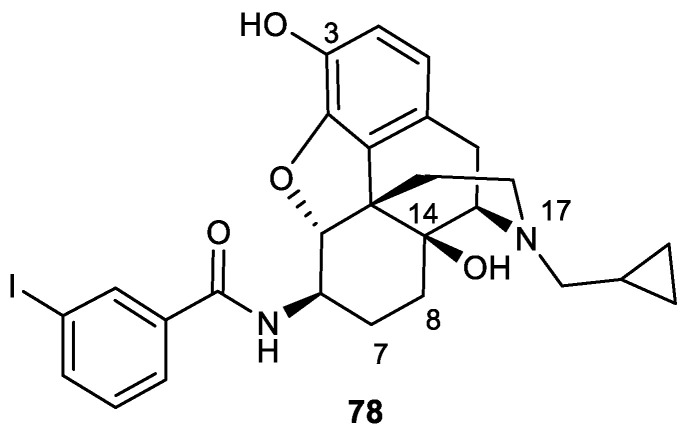
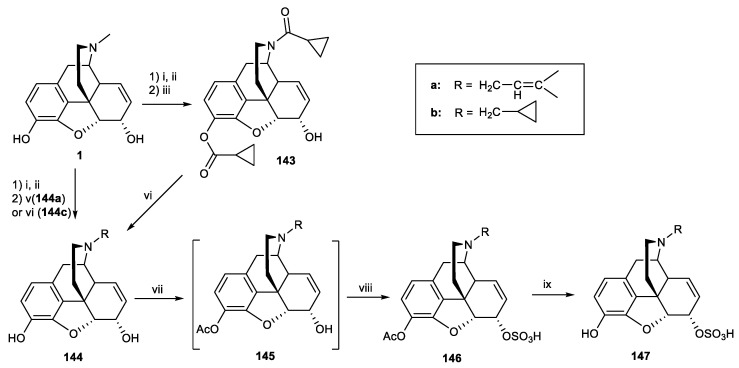
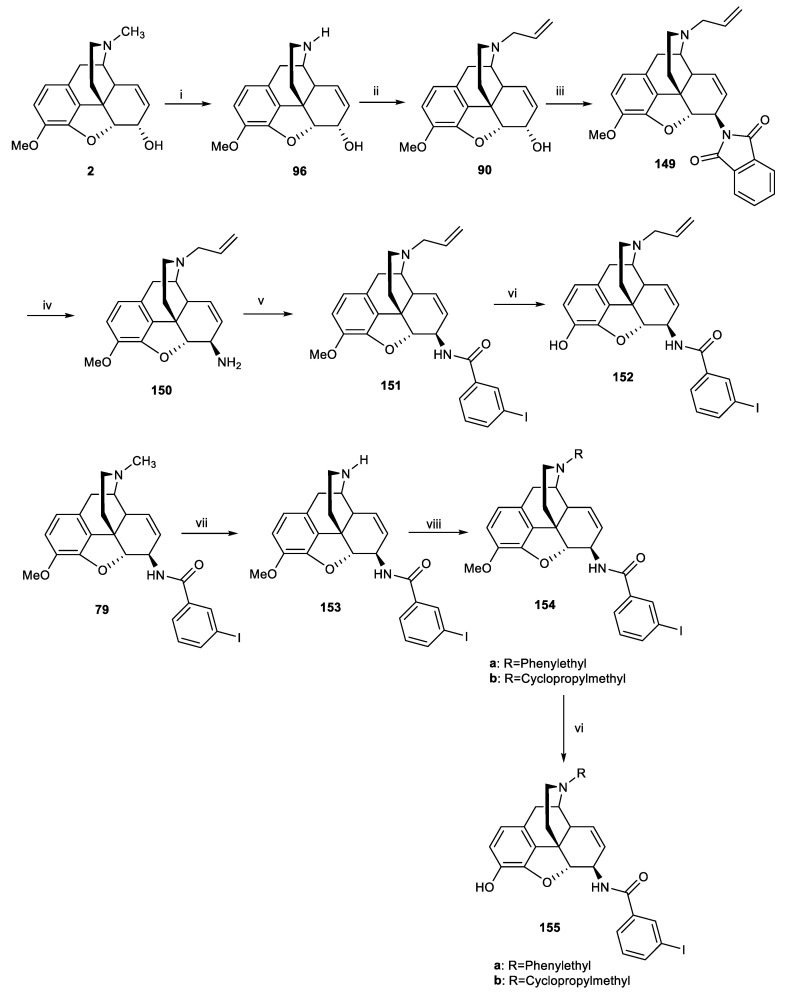
In 2015, the same group synthesized five analogues of 3-iodobenzoyl naltrexamine (IBNtxA) 78, a potent aminomorphinan antinociceptive drug (ED50 = 0.39 mg/kg) [75]. However, it had a lower affinity for the target site, 6TM/E11, compared to traditional opioid receptors. The 6-transmembrane (6-TM) is a novel opioid target that is known as a novel exon 11-associated splice variant of the μ-opioid receptor gene. An attempt was made to modify these structures by removing the 14-hydroxy group, substituting different alkyls at the N-17 position, and adding a double bond at positions 7–8 (Figure 4). Derivatives were synthesized starting from codeine. First, 6β-aminocodeine 76 was synthesized from 2, and the amine derivative was subsequently coupled with 3-iodobenzoic acid in the presence of HATU to produce compound 79. O-Demethylation of compound 79 with BBr3 led to the formation of 80 (Scheme 24).
Figure 4.
Structure of 3-iodobenzoyl naltrexamine.
Scheme 24.
Reagents and conditions: (i) phthalimide, Ph3P, dry THF, DIAD in dry toluene, 16 h, r.t, 93%; (ii) hydrazine hydrate, cis-2-penten-1-ol, MeOH, 16 h, r.t, 100%; (iii) 3-iodobenzoic acid, 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU), N,N-diisopropylethylamine (DIEA), dimethyl formamide (DMF), 15 min, r.t, 76%; (iv) BBr3, DCM, 30 min, 0 °C to r.t, 55%.
The affinity of compound 79 for 6TM/E11 and DOR-1 was moderate, whereas it showed high affinity for MOR-1 (1.2 nM) and KOR-1 (10 nM) receptors. Analogue 80 demonstrated a high affinity for all opioid receptors (Table 7). This analogue, in contrast with the corresponding 14-OH-epoxymorphinan, exhibited moderate affinity (27 nM) for 6TM/E11 and analgesic potency in vivo. Although incorporating a double bond at the 7–8-positions increased the affinity to MOR, KOR, and DOR, it reduced the selectivity to the 6TM/E11 site. The modification of the N-17 position will be explained in the next section.
Table 7.
Binding and analgesia of 79 and 80.
| Ki (nM) ± SEM a | Tail Flick Analgesia (CI) b, ED50 [mg/kg] | ||||
|---|---|---|---|---|---|
| Compound | MOR-1 | KOR-1 | DOR-1 | 6TM/E11 | |
| Morphine | 4.6 ± 1.81 | >1000 | 4.96 ± 0.96 | ||
| IBNtxA | 0.11 ± 0.02 | 0.03 ± 0.001 | 0.24 ± 0.05 | 0.16 ± 0.04 | 0.39 (0.15, 0.58) |
| 79 | 1.2 ± 0.48 | 10 ± 3.5 | 49 ± 6.4 | 61 ± 7.7 | >10 |
| 80 | 0.037 ± 0.0051 | 0.21 ± 0.046 | 0.88 ± 0.08 | 27 ± 18 | 6.31 (4.6, 8.5) |
a [125I]BNtxA (0.1 nM) competition binding assays were performed in membranes prepared from CHO cells expressing mouse MOR-1, DOR-1, or KOR-1, or mouse brain in the presence of CTAP, DPDPE, and U50,488 blockers at 200 nM. b Assays were performed at least two times, and means ± SEM of replicates or CIs were reported.
After synthesizing a very potent and selective μ opioid receptor (compound 81) (Figure 5) derived from 80 in 2009 [76], it was selected as a lead compound for further study. In 2017, a novel series of 6β-heteroarylamidomorphinanes was synthesized to modify some parts of 81 to investigate structure–activity relationship of this potent analogue [71].
Figure 5.
Structure of compound 81.
Treatment of 6β-aminomorphine 74 or the corresponding dihydromorphine analogue 82 with isonicotinic and nicotinic acid chlorides led to the formation of the 6β-aminomorphinans 84a–d, respectively. A similar reaction was performed between 6β-aminocodeine 76 and isonicotinic acid chloride to produce 85 (Scheme 25).
Scheme 25.
Reagents and conditions: (i) acetic anhydride, aq. NaHCO3, r.t, 2 h; (ii) Ph3P, phthalimide, DIAD, benzene, r.t, 1 h; (iii) ethanol/hydrazine monohydrate, heating, 3 h; (iv) hydrazine monohydrate/RANEY nickel, ethanol, r.t., 2 h; (v) acyl chloride, Et3N, DCM; (vi) K2CO3, methanol, heating, 4 h.
Based on in vitro studies, the codeine analogue 85 showed significantly lower binding affinity compared to morphine derivatives 84a–d. This illustrated the importance of the free hydroxyl group at the C-3 position. Derivatives 84a and 84b revealed the highest affinity to the MOR compared with the analogues bearing 14-hydroxyl group and a saturated C7-C8. Based on the [35S]GTPγS binding assay of compounds 84a–d, all derivatives were full agonists, and only the ED50 value for 84d was determined. The [35S]GTPγS assay is summarized in Table 8. Saturating the double bonds in these derivatives decreased both affinity and activity, despite the literature indicating that saturation of double bonds at positions 7–8 increased morphine derivative affinity. According to the tail flick test, 84b (ED50 = 0.51 μg) was as potent as morphine (ED50 = 0.53 μg). The 84c and 84d with saturated double bonds, on the other hand, exhibited long-lasting analgesic effects.
Table 8.
In vitro efficacy of the compounds 82a–d in the [35S]GTPγS assay a.
| MOR | KOR | |||
|---|---|---|---|---|
| Compound | EC50 (nM) | %Emax | EC50 (nM) | %Emax |
| 82a | 4.62 ± 0.28 | 114.36 ± 7.06 | nd b | nd b |
| 82b | 2.83 ± 0.41 | 113.63 ± 4.54 | nd b | nd b |
| 82c | 8.16 ± 2.64 | 114.85 ± 1.26 | nd b | nd b |
| 82d | 7.98 ± 2.35 | 117.16 ± 1.98 | 58.83 ± 7.49 | 87.88 ± 4.9 |
| DAMGO | 19 ± 7.0 | nd b | nd b | nd b |
| U50,488H | nd b | nd b | 17 ± 6.1 | nd b |
a Functional properties were studied using agonist-induced stimulation of the [35S]GTPγS binding assay. Potency is represented as EC50 (nM) and efficacy as percent maximal stimulation (%Emax) relative to standard agonist DAMGO (MOR), or U50,488H (KOR)at 100 nM. b Not determined.
As mentioned before, M6G is one of the metabolites of morphine in the body. This potent analgesic is four times more active than, and twice as long-lasting, as morphine. Due to the low bioavailability of M6G, scientists are interested in developing new glycosylated derivatives of morphine at the C6 position. Many attempts have been made to synthesize new C6-glycosylated morphine, and most of them were collected in the earlier review of S. K. J. Dilip Gorai [55]. Here, we have only mentioned some more potent analogues.
6-Morphinyl-R-D-mannopyranoside 86 (Figure 6) is one of the potent analogues which was synthesized by Arsequell and co-workers, which was twice as long-lasting when administered intraperitoneally to rats and was found to have a 100-fold naloxone-reversible antinociceptive action [77].
Figure 6.
Structure of potent C6-glycosylated derivatives.
Due to the low bioavailability of M6G and the fact that the S-glycoside linkage has more in vivo stability in comparison with O-glycosides in metabolic conjugates, MacDougall et al. synthesized novel 6-β-thiosaccharide derivatives of morphine and codeine [78]. Compounds 87a and 87b (Figure 6) were determined as full μ agonists and showed a slightly better potency (around 1.5-fold) on μ receptors compared to M6G in the [35S]GTPγS assay. Based on the tail flick test, the ED50 of 87a was 2.5 mg/kg, which was slightly lower than morphine (3 mg/kg). Due to the low selectivity of these derivatives for μ in comparison with δ and κ receptors, the same group synthesized two new amide-linked C-β-glycopyranoside derivatives of M6G [79]. It was found that compound 88 had a 3.7-fold greater affinity for μ receptors than M6G and had selectivity ratios of 76.7 and 166 for δ versus μ and κ versus μ receptors, respectively (Figure 6). Compound 88 also showed high stability at pH 2 and pH 7.4.
In 2020, Yang et al. synthesized the M6αG analogue and evaluated its biological activity compared to its isomer M6βG (M6G), an active metabolite of morphine [80]. M6αG was synthesized from the reaction of 3-O-acetylmorphine with methyl 2,3,4-tri-O-acetyl-D-glucopyranosyluronate bromide and ZnBr2 followed by hydrolyzing the acetyl group in 5% NaOH (Scheme 26). In the first step, a mixture of two isomers (α:β = 4:1 by HPLC) was obtained. After treating the mixture with hydrochloric acid, the M6αG 89 was crystallized in hot water. Both of these analogues demonstrated a nanomolar affinity for all μ, δ, and κ opioid receptors. However, in the hot plate test, compound M6αG showed lower analgesic activity than M6G.
Scheme 26.
Reagents and conditions; (i) methyl 2,3,4-tri-O-acetyl-D-glucopyranosyluronate bromide, ZnBr2, molecular sieves, CHCl3; (ii) NaOH 5%.
4. Modifications at N-17
In many alkaloids, particularly opiates, the tertiary N-methylamine group is a characteristic moiety. The removal and substitution of the N-methyl group in the natural products, especially in the alkaloids, is considered a significant step in the processes of semi-synthesis for further functionalization and modification to produce novel compounds with improved pharmacological and biological activities [81]. The methyl group in position 17 of the morphine scaffold is one of the most common sites for modification, and many derivatives of morphine and other structurally related analogues have been developed via the replacement of this group with other substituents. A wide variety of procedures for the N-demethylation of opiate alkaloids have been reported in the literature. The more conventional methods involve the use of reagents like cyanogen bromide (von Braun reaction), chloroformate esters, and dialkyl azodicarboxylates. The toxicity of cyanogen bromide is one of the major disadvantages of the von Braun reaction, which significantly limits the use of this method both in the laboratory and in the industry [81,82]. Recently, the modified non-classical Polonovski reaction has been employed for the N-demethylation of opiates in good to high yields through the formation of the corresponding N-oxide and its subsequent treatment with FeSO4.7H2O to afford the N-nor secondary amine opiates [83]. Furthermore, various efficient photochemical and biochemical methods have been successfully employed for N-demethylation of opiate alkaloids [84,85].
The methyl group in position 17 of the morphine scaffold is one of the most common sites for substitution and modification, and many derivatives of morphine and other structurally related analogues have been developed via the replacement of this group with other substituents.
N-Allylnorcodeine (nalodeine) 90a and N-allylnormorphine (nalorphine) 90b are notable as the first N-substituted analogues of morphine and codeine (Figure 7) [86]. It was demonstrated that nalorphine does not have analgesic activity in animals. In the writhing test, nalorphine-6-glucuronide and -sulfate showed higher analgesic activity that nalorphine. Nalorphine has both agonist and antagonist properties [87].
Figure 7.

Structures of nalorphine and nalodeine.
In the following years, many researchers synthesized hundreds of N-substituted normorphines to better understand their structure–activity relationship. Here, we have summarized some of these synthesized derivatives and, in some cases, their biological properties.
In 1953, Clark and co-workers synthesized several N-substituted normorphine 91a–r and dihydromorphine 92a–f analogues [88]. Some of these derivatives are summarized in Table 9 and Table 10. It was found that substitution of allyl, methallyl, n-propyl, or isobutyl in all series produced compounds that counteracted morphine’s analgesic effects.
Table 9.
N-substituted normorphine derivatives.
| Compound | R1 | R2 | R3 | |
|---|---|---|---|---|

|
a | CH2CH2CH3 | H | H |
| b | CH2CONH2 | H | H | |
| c | CH2COOEt | H | H | |
| d | CH2CH2OC6H5 | H | H | |
| e | CH2CH=CH2CH2Br | H | H | |
| f | CH2CHBr=CH2 | H | H | |
| g | CH2CH2CH3 | CH3 | H | |
| h | CH2(CH3)2 | H | H | |
| i | CH2(CH3)2 | CH3 | H | |
| j | CH2CH2CN | H | H | |
| k | CH2CHOHCH3 | H | H | |
| l | CH2CH2COOEt | H | H | |
| m | CH2C(CH3)=CH2 | H | H | |
| n | CH2CH(CH3)2 | CH3 | H | |
| o | CH2(CH2)3CH3 | H | H | |
| p | CH2C6H5 | H | H | |
| q | CH2COC6H5 | H | H | |
| r | CH2CH2C6H5 | H | H |
Table 10.
N-substituted dihydronormorphine derivatives.
| Compound | R1 | R2 | R3 | |
|---|---|---|---|---|

|
a | CH2CH=CH2 | CH3 | H |
| b | CH2CH=CH2 | Ac | Ac | |
| c | CH2CH2CH3 | CH3 | H | |
| d | CH2CH2CH3 | Ac | Ac | |
| e | CH2C(CH3)=CH2 | H | H | |
| f | CH2CH(CH3)2 | H | H |
In 1958, Small et al. synthesized N-phenethylnorcodeine [89]. Initially, norcodeine was reacted with phenylacetyl chloride, and then the resultant amide was reduced to the tertiary amine using lithium aluminum hydride to give the desired product 93a. N-Phenethylnormorphine 93b was synthesized in the same manner (Figure 8). Investigation of the analgesic potency of 93a showed that it provided analgesic action that was twice as long as its N-methyl counterpart.
Figure 8.
N-phenethyl derivative of morphine.
Ben Haddou et al. synthesized N-phenylpropylnormorphine (94) and compared the opioid receptor binding and antinociceptive activity of this analogue with N-phenethylnormorphine (93b) [90]. It was demonstrated that 94 has high affinity and selectivity at the μ opioid receptors (MOR) as a potent agonist (Ki = 0.93 nM) and displayed an affinity for the MOR that was seven times higher than morphine. Compound 94 had significantly reduced binding affinity and selectivity at μ receptors (Table 11).
Table 11.
Opioid receptor binding affinity and selectivity of compounds 93a and 94 at MOP, DOP, and KOP receptors.
| Ki ± SEM (nM) | Selectivity | ||||
|---|---|---|---|---|---|
| Compound | MOP | DOP | KOP | δ:μ | κ:μ |
| Morphine | 6.556 ± 0.74 | 2176 ± 19 | 1136 ± 9 | 33 | 17 |
| 93a | 0.936 ± 0.14 | 37.06 ± 5.5 | 1076 ± 18 | 40 | 115 |
| 94 | 79.56 ± 1.1 | 8696 ± 171 | 5656 ± 24 | 11 | 7 |
Binding assays were performed with membranes from rat brain (MOP and DOP receptors) and guinea pig brain (KOP receptors).
In 2020, Salehi et al. synthesized a new series of triazole-tethered derivatives of norcodeine [91]. N-Demethylation of codeine (96) was successfully carried out with a good yield via the Polonowski-type reaction in the presence of hydrogen peroxide and FeSO4.7H2O. Then, 96 was treated with propargyl bromide to produce N-propargylnorcodeine (97). From the CuAAC reaction of 97 with a variety of azides, the desired triazole derivatives 98a–r were prepared (Scheme 27). Radioligand Binding Assay showed that derivatives have an affinity to μ receptor. Compound 98d (Ki =159.9 nM; IC50 =363.1 nM) showed high affinity, and compound 98k (Ki = 850.6 nM; IC50 = 1931.0 nM) showed the lowest affinity compared to codeine (Ki = 144.2 nM; IC50 =327.4 nM). Compounds 98a, 98c, 98d, 98f, 98h, and 98r with high affinity were selected for tail flick analgesic test. The results showed that Compound 98d exhibited significant analgesic activity with an ED50 of 8.68 mg/kg compared to codeine (ED50: 9.55 mg/kg) as the best analogue (Table 12).
Scheme 27.
Reagents and conditions: (i) H2O2, acetonitrile, r.t, 4 h; (ii) FeSO4.7H2O, MeOH, −5–0 °C, 8h, 57%; (iii) propargyl bromide, K2CO3, acetone, r.t, 4 h, 86%; (iv) R-N3, CuSO4, sodium ascorbate, MeOH/DCM (1:1), r.t, 10–60 min.
Table 12.
Values of Ki and IC50 of various ligands in radioligand binding assay and ED50 by in vivo studies of synthetic compounds.
| Affinity | Analgesia | ||
|---|---|---|---|
| Compound | IC50
a (%95 CI b) (nM) |
Ki c (nM) | ED50
d (95% CI) (mg/kg) |
| Codeine | 327.4 | 144.2 | 9.55 |
| (152.5 to 702.9) | (67.1 to 309.6) | (8.50 to 10.74) | |
| 98d | 363.1 | 159.9 | 8.68 |
| (150.6 to 875.0) | (66.3 to 385.4) | (6.47 to 11.66) | |
| 98c | 442.7 | 195.0 | 17.05 |
| (255.3 to 767.7) | (112.4 to 338.1) | (13.72 to 22.49) | |
| 98a | 486.6 | 214.3 | 17.57 |
| (381.3 to 620.9) | (167.9 to 273.5) | (14.56 to 19.97) | |
| 98f | 559.3 | 246.3 | 26.14 |
| (299.0 to 1046.0) | (131.7 to 460.7) | (19.82 to 34.46) | |
| 98r | 536.7 | 236.4 | 30.27 |
| (349.3 to 824.6) | (153.8 to 363.2) | (19.41 to 47.21) | |
| 98h | 566.3 | 249.4 | 31.17 |
| (274.7 to 1167.0) | (121.0 to 514.0) | (22.98 to 42.28) | |
a IC50, the half maximal inhibitory concentration, b CI, confidence interval, c Ki, affinity of the ligand, d ED50, the dose (or concentration) causing 50 % of maximum effect.
5. Disubstituted Derivatives
5.1. Modifications on C3–C6
Previous results have demonstrated that glucuronidation is the main metabolism pathway of morphine, whereas less attention has been devoted to the sulfation pathway. According to the tail flick assay, M6S showed a 30-fold higher analgesic potency than morphine, similar to morphine-6β-glucuronide (M6G) [92,93]. Codeine-6-sulfate (C6S) showed similar potency ratios to that of M6S, whereas a high toxicity level for C6S was exhibited [94]. This issue grabbed the attention of scientists and led to the synthesis of various sulfated derivatives.
In 2006, Crooks et al. reported the synthesis of 6-O-sulfate ester derivatives starting from codeine and morphine and their corresponding dihydro analogues to investigate their binding affinity to several opioid receptors [95]. A variety of 3-O-acyl-6-O-sulfate derivatives were synthesized by treating codeine and 3-O-acyl analogues of morphine with pyridine-SO3 to produce 102a–i. The dihydro analogues 100a–d were synthesized in the same manner. Deacetylation of 100b and 102b led to the formation of 101 and 103, respectively. Treatment of two 3-O-acyl derivatives with iodomethane in dichloromethane yielded compounds 104a–b, and this was followed by sulfation with pyridine-SO3. Thus, betaines 105a–b were synthesized (Scheme 28).
Scheme 28.
Reagents and conditions: (i) pyridine-SO3, pyridine, 55 °C; (ii) 5% MeOH, aq. NaOH, r.t, 1 h; (iii) Pd-C, 5%, H2, 50psig; (iv) CH3I/CH2Cl2, r.t, 12–18 h.
The affinity of M6S to the μ (ki = 0.9 ± 0.01 nM), δ (ki = 18 ± 0.4 nM), and κ-3 (ki = 6.3 ± 0.3 nM) receptors improved slightly compared to morphine (ki = μ (2.5 ± 0.3 nM), δ (58 ± 3.8 nM), and κ-3 (13.9 ± 4.1 nM)). Furthermore, it showed low affinity to κ-1 receptors and no affinity for κ-2 receptors. Derivative 102a and its 7,8-dihydro analogue 100a demonstrated similar activity. As compared to M6S, codeine 6-O-sulfate 102i and its dihydro derivative 100d showed a significant decline in all opioid receptor affinity. A low affinity of betaines 105a and 105b for all receptors was also observed. The 3-O-propionyl 102c, 3-O-isobutyryl 102d, and 3-O-pivaloyl 102e analogues of M6S demonstrated high affinity for μ- receptors but low affinity for κ-1 receptors while 102c, as the best analogue, showed four times higher affinity than morphine for μ-receptors (Ki = 0.6 ± 0.15 nM). It should be noted that, nowadays, the κ-subtype, especially κ-3, no longer has approval [96].
In 2011, Váradi et al. synthesized several 3-O-sulfate and 6-O-sulfate ester derivatives of morphine, dihydromorphine, codeine, and dihydrocodeine [97]. The same procedure, as already explained, was used for the monosubstitution of ester analogues. Morphine-3,6-O-disulfate 114 was prepared directly from the reaction of morphine with N,N′-dicyclohexylcarbodiimide and concentrated sulfuric acid (Scheme 29). Compounds 112 and 113 were synthesized by alkylation (isopropyl and ethyl) at 3-hydroxyl, and then sulfated by pyridine-SO3. For C3-sulfation, first, the C3-acetyl of diacetylmorphine (116) was selectively hydrolyzed, then compound 117 was reacted with pyridine-SO3, followed by hydrolysis of C6-acetyl to yield 119 (Scheme 30). The structures of all derivatives are summarized in Table 13. 1H- and 13C-NMR, as well as CD/ORD (Circular Dichroism/Optical Rotatory Dispersion Spectroscopy) of all derivatives, were analyzed in detail. These data can be utilized to identify various sulfate esters in biological samples. Most sulfate ester derivatives exhibited selective peripheral analgesic effects.
Scheme 29.
Reagents and conditions: (i) acetic anhydride, NaHCO3, H2O, r.t, 1 h; (ii) MeI, acetone, 40 °C, 4 h; (iii) pyridine-SO3, pyridine, 60 °C, 3.5 h; (iv) 20% aq. K2CO3 solution, r.t, 1 h; (v) 2-iodopropane, NaOEt, EtOH, reflux, 4 h; (vi) N,N′-dicyclohexylcarbodiimide, conc. sulfuric acid, DMF, 0 °C, 15 min.
Scheme 30.
Reagents and conditions: (i) hydroxylamine hydrochloride, EtOH, 60 °C, 45 min; (ii) pyridine-SO3, pyridine, 60 °C, 3.5 h; (iii) 10% aq. NaOH, MeOH, r.t, 1 h; (iv) MeI, acetone, 40 °C, 4 h; (v) 20% aq. K2CO3 solution, r.t, 1 h.
Table 13.
Structures of synthesized compounds.
| Compound | R1 | R2 | R3 | C7–C8 Bond | |
|---|---|---|---|---|---|

|
108 | H | SO3− | CH3 | Double |
| 109 | H | SO3− | CH3 | Single | |
| 110 | CH3 | SO3− | CH3 | Double | |
| 111 | CH3 | SO3− | CH3 | Single | |
| 112 | iPr | SO3− | H | Double | |
| 113 | Et | SO3− | H | Double | |
| 114 | SO3− | SO3− | H | Double | |
| 115 | CH3 | SO3− (6β) | H | Double | |
| 119 | SO3− | H | H | Double | |
| 120 | SO3− | H | H | Single | |
| 123 | SO3− | H | CH3 | Double | |
| 124 | SO3− | H | CH3 | Single |
5.2. Modifications on N-17, Together with Different Parts of the Structure
In 2015, Hosztafi and Marton [35] synthesized novel N-substituted-1-fluoronorcodeine 128a–b and dihydrocodeine derivatives 128c–d. 1-Fluoro-6-O-acetylnorcodeine (126a) was synthesized from the reaction of 1-fluorocodeine (125a) with acetic anhydride to protect the 6-hydroxyl group, and then the demethylation of compound 127a was performed with 1-chloroethyl chloroformate. Alkyl groups (propyl and allyl) were substituted in N-17 moiety to obtain 128a–b (Scheme 31). The corresponding dihydro analogues 128c–d were synthesized in a same way. These derivatives could be potential candidates in pharmacology and can be employed for fluorine-18 labeled morphinan radiotracers as standard references in imaging.
Scheme 31.
Reagents and conditions: (i) Ac2O, reflux, 4 h; (ii) (1) 1-chloroethyl chloroformate, NaHCO3, 1,2-dichloroethane, reflux, 8 h; (2) methanol, 50 °C, 1 h; (3) NaOH, ethanol, reflux, 1 h; (iii) RX (n-propylbromide or allyl bromide), NaHCO3, DMF, 95 °C, 18 h.
Novel N-propyl- and -allyl-substituted derivatives of 1-iodocodeine and their 7,8-dihydro analogues were synthesized in 2018 by Hosztafi et al. [98]. The synthesis of compound 17 was described in the previous section. The reaction of 17 with α-chloroethyl chloroformate followed by realkylation of 130 yielded the corresponding N-alkyl analogues 131a–d (Scheme 32). The newly developed derivatives can be applied as a tool for preparing 18F-labelled aryl fluorides and can be used as intermediates for the synthesis of palladium aryl complexes, arylstannanes, and aryl-pinacol boronic esters.
Scheme 32.
Reagents and conditions: (i) NaI, chloramine T, 0.1 M hydrochloric acid; (ii): Ac2O, reflux, 4 h; (iii): (1) α-chloroethyl chloroformate, NaHCO3, 1,2-dichloroethane; (2) methanol, 50 °C, 1 h; (3) 5% NaOH, ethanol, reflux, 1 h; (iv): RX (n-propylbromide or allyl bromide), NaHCO3, DMF, 95 °C, 18 h.
Shel’metsi et al. synthesized a new series of N-benzyl-3-O-benzyl- and 3-O-benzoylnormorphine analogues and also developed a novel selective method for the 3-O-alkylation of morphine, which resulted in the production of various N-aIkyl-3-O-alkyl-normorphine derivatives [99]. The first step for the preparation of these analogues involved the reaction of normorphine with a variety of substituted-benzyl halides, followed by the Claisen method, where 3-O-benzyl-N-substituted normorphine derivatives were selectively obtained by reacting the phenolic hydroxide group with alkyl halides in the presence of potassium carbonate in absolute acetone (Figure 9). Among these compounds, 3-(p-nitrobenzyl)-N-(p-nitrobenzyl)normorphine (132b) demonstrated the most potent analgesic effect without undesirable psychomimetic side effects.
Figure 9.
N-AIkyl-3-O-alkyl derivatives of normorphine.
By the use of the Mitsunobu reaction for the alkylation of cyclic imides (phthalimido and succinimido) with various alcohols, Simon et al. synthesized new 6β-aminomorphine and codeine analogues [100]. Through the reaction of 3-O-acetyl-N-substituted derivatives of morphine and codeine with phthalimide or succinimide utilizing triphenylphosphine and diethyl azodicarboxylate, 6β-phthalimido 134a–h or succinimido 138a–c derivatives were synthesized. Then, phthalimido-substituent derivatives 134a–h were converted into the corresponding primary amine compounds 136a–h by treatment with hydrazine hydrate in ethanol (Scheme 33). The Δ7,8 double bond in ring C of succinimido derivatives was hydrogenated with Pd/C to yield 139a–b (Scheme 34). Such a reaction was not possible with the corresponding phthalimido analogues.
Scheme 33.
Reagents and conditions: (i) PPh3, phthalimide, diethyl azodicarboxylate, benzene; (ii) hydroxylamine hydrochloride, EtOH, 50 °C, 10 min; (iii) 98% hydrazine hydrate, EtOH; (iv) Pd/C 10%, EtOH.
Scheme 34.
Reagents and conditions: (i) PPh3, succinimide, diethyl azodicarboxylate, benzene; (ii) Pd/C 10%, EtOH.
Isocodeine 142a and isomorphine 142g are the epimers of codeine and morphine, respectively, which have opposite stereochemistry at the C6 hydroxyl group. Several approaches have been reported to synthesize 142a and 142g. However, only few reports have been published on their novel derivatives. In 1991, Simon et al. reported novel analogues of isocodeine and isomorphine by the employment of the Mitsunobu reaction [101]. The reaction was started from different N-substituted analogues of isocodeine and isomorphine. In the first step, 140 reacted with triphenylphosphine and diethyl azodicarboxylate to obtain 141a–j. In the second step, the ester was hydrolyzed to hydroxy at the 6-position with good to excellent yield to obtain the final derivatives 142a–j (Scheme 35).
Scheme 35.
Reagents and conditions: (i) PPh3, benzoic acid, anhydrous benzene, diethyl azodicarboxylate, 1 h; (ii) 10% aqueous KOH, EtOH, reflux, 10 min.
It has already been proven that glucuronidation and sulfation of morphine at the 6-position dramatically improved the analgesic activity. On the other hand, it is noteworthy to explain what occurs when an allyl or a cyclopropylmethyl group is attached to nitrogen. Neither naltrexone nor naloxone has any analgesic effect, and nalorphine has only mild analgesic activity. In this regard, Hirano et al. synthesized two novel N-substituted normorphine derivatives 147a–b that conjugated to the sulfate group at the 6-position of the morphine scaffold (Scheme 36) and evaluated their analgesic and antagonistic activities [102]. Evaluation of analgesic activity demonstrated that N-cyclopropylmethylnormorphine-6-sulfate 147b and N-dimethylallylnormorphine-6-sulfate 147a had similar analgesic properties to N-cyclopropylmethylnormorphine 144b and N-dimethylallylnormorphine 144a due to the acetic acid writhing test in mice. Compounds 147a and 147b showed higher antagonistic activity than their parent compounds to morphine analgesia by tail pinch test in mice.
Scheme 36.
Reagents and conditions: (i) acetic anhydride; (ii) BrCN, HCl; (iii) cyclopropanecarbonyl chloride, CHCl3, triethylamine, reflux, 8 h; (iv) LiAlH4, THF, r, t; (v) dimethylallyl bromide, K2CO3, DMF, 90–95 °C, N2, 3 h; (vi) allyl bromide, Na2CO3, dry DMF, 16 h, 90 °C; (vii) Ac2O, aq.NaHCO3, 30 min; (viii) ClSO3H, dry pyridine; (ix) 5% NaOH-MeOH.
Simon et al. synthesized several N-substituted-C1-halogenated derivatives of morphine 148a–d, 148e–h codeine, and their dihydro analogues 148i–l [29]. The synthesis of C1-halogenation derivatives was explained in the previous sections. N-substituted (allyl and n-propyl) analogues were synthesized according to the von Braun–Olofson method (Table 14) [103].
Table 14.
Structures of 148a–l derivatives.
| Compound | X | R1 | R2 | C7–C8 Bond | |
|---|---|---|---|---|---|

|
a | Br | H | CH2CH=CH2 | Double |
| b | Cl | H | CH2CH=CH2 | Double | |
| c | Br | H | n-Pr | Double | |
| d | Cl | H | n-Pr | Double | |
| e | Br | CH3 | CH2CH=CH2 | Double | |
| f | Cl | CH3 | CH2CH=CH2 | Double | |
| g | Br | CH3 | n-Pr | Double | |
| h | Cl | CH3 | n-Pr | Double | |
| i | Br | H | CH2CH=CH2 | Single | |
| g | Cl | H | CH2CH=CH2 | Single | |
| k | Br | CH3 | CH2CH=CH2 | Single | |
| l | Cl | CH3 | CH2CH=CH2 | Single |
According to our discussion in the previous sections to modify the scaffold of IBNtxA (78) (Figure 4), Váradi et al. synthesized potent analgesic analogues of 3-iodobenzoyl naltrexamine (IBNtxA) by substituting a new alkyl and allyl group in nitrogen position 17 instead of a cyclopropylmethyl, along with the incorporation of a 7,8-double bond and removal of the 14-hydroxy group (Scheme 37) [75]. The affinity of compounds 155a and 155b for the 6TM/E11 site decreased compared with 78. However, both compounds showed an increased affinity for MOR-1, DOR-1, and KOR-1. The affinity of compound 152 for MOR-1, KOR-1, and DOR-1 receptors was higher than 6TM/E11. In addition, the affinity of 152 for these receptors compared to the similar derivative with the hydroxy group at position 14 was increased (Table 15). Compound 155b demonstrated 15-fold greater potency than morphine in tail flick analgesic tests (ED50 = 0.33 ± 0.09 mg/kg). Compound 155b was selected for [35S]GTPγS binding assay and was found to be a full agonist for MOR and a dual agonist at KOR-1 and DOR-1 receptors (EC50 = 0.027−0.41 nM for mentioned receptors). Biological studies also showed that pretreatment with compound 155b prevented cocaine-associated reward behaviors. In conclusion, N-17 alkylation, removal of the 14-OH, and incorporation of a 7, 8 double bond lead to dual kappa–delta agonism, which could be an ideal starting point for novel analgesics and treatments for cocaine addiction.
Scheme 37.
Reagents and conditions: (i) chloroethyl chloroformate, NaHCO3, 1,2-dichloroethane, 30 min, r.t, then 16 h, 85 °C; (ii) allyl bromide, Na2CO3, dry DMF, 16 h, 90 °C, 68%; (iii) phthalimide, Ph3P, dry THF, DIAD in dry toluene, 16 h, r.t, 68%; (iv) hydrazine hydrate, cis-2-penten-1-ol, MeOH, 16 h, r.t, 100%; (v) 3-iodobenzoic acid, HATU, N,N-diisopropylethylamine (DIEA), dimethyl formamide (DMF), 15 min, r.t, 70%; (vi) BBr3, DCM, 30 min, 0 °C to r.t, 50–60%; (vii) DIAD, acetonitrile, 20 h, 65 °C, then pyridine-HCl, 72 h, r.t, 54%; (viii) alkyl halide, Na2CO3, or Cs2CO3, DMF, 16 h, 90 °C.
Table 15.
Binding and Analgesia of compounds 152, 155a–b.
| Ki a (nM) ± SEM | Tail Flick Analgesia (CI) b, ED50 [mg/kg] | ||||
|---|---|---|---|---|---|
| Compound | MOR-1 | KOR-1 | DOR-1 | 6TM/E11 | |
| Morphine | 4.6 ± 1.81 | >1000 | 4.96 ± 0.96 | ||
| 152 | 0.034 ± 0.006 | 0.022 ± 0.0043 | 0.39 ± 0.0041 | 0.78 ± 0.1 | 0.53 (0.45, 0.59) |
| 155a | 0.088 ± 0.028 | 0.14 ± 0.022 | 0.13 ± 0.032 | 14 ± 3.2 | 1.3 (1.03, 2.2) |
| 155b | 0.021 ± 0.0034 | 0.0064 ± 0.002 | 0.08 ± 0.019 | 0.47 ± 0.009 | 0.33 ± 0.09 |
| IBNtxA | 0.11 ± 0.02 | 0.03 ± 0.001 | 0.24 ± 0.05 | 0.16 ± 0.04 | 0.39 (0.15, 0.58) |
a [125I]BNtxA (0.1 nM) competition binding assays were performed in membranes prepared from CHO cells expressing mouse MOR-1, DOR-1, or KOR-1, or mouse brain in the presence of CTAP, DPDPE, and U50,488 blockers at 200 nM. b Assays were performed at least two times, and means ± SEM of replicates or CIs were reported.
6. Conclusions
There have been extensive studies on the chemistry of morphine and codeine and their congeners for several decades. Efforts to modify their structures to obtain analogues with fewer undesirable side effects continue. These changes have led to the synthesis of potent analogues such as oxycodone, naltrexone, and buprenorphine, which are used as drugs. However, the semi-synthesis starting from morphine and codeine main scaffolds is still of interest. Some newly synthesized analogues have shown better biological effects than morphine with fewer side effects and, in some cases, changed morphine’s agonist activity to antagonist. There are many reviews about morphine and codeine metabolism in the body and related pharmaceutical studies. This review spotlights the majority of morphine and codeine derivatives synthesized to date and summarizes the most effective analogues in biological investigations. Several studies have demonstrated that the C3-hydroxyl group plays a significant role in interacting with opioid receptors, and substituting different groups at this position did not work in most cases. Even the substitution of amine with C3-hydroxyl decreased its affinity for μ, δ, and κ receptors. This explains why codeine derivatives do not provide better effects than morphine derivatives. However, structural changes in C6 and N-17 positions have helped to improve the properties of synthetic derivatives compared to codeine. The C6-hydroxyl and N-17 are considered significant parts of morphine and codeine’s structures. Modifications of these moieties can lead to the synthesis of much more potent analogues with decreased side effects. A review of the synthetic derivatives reported here indicated that replacing C6 hydroxyl with some groups, especially acrylamide groups, can enhance opioid receptor affinity as well as analgesic activity. The change in this position, along with the substitution of different groups in the N-17 position, have the ability to increase the analgesic effect. By further studying these structural changes, it can be of hope that in the future, novel derivatives of morphine will show higher analgesic activity and be devoid of some dangerous side effects of morphine. Hopefully, this review will lead to further synthesis of more potent analogues or more studies of the pharmacological properties of some reported derivatives.
Acknowledgments
We acknowledge the KU Leuven (C14/19/078) in support of this work. P.S., M.K.Z.Z. and A.A.B.A. are grateful to the Research and Technology Council of Shahid Beheshti University for partial support.
Author Contributions
M.K.Z.Z.: Writing of first draft of manuscript (main), conceptualization; W.D.: Correcting manuscript, conceptualisation; P.S.: Correcting manuscript, conceptualisation, supervision; A.A.B.A.: Writing first draft (partly), correcting (partly). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by KU Leuven (C14/19/078).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Patridge E., Gareiss P., Kinch M.S., Hoyer D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today. 2016;21:204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 2.McCurdy C.R. Analogue-Based Drug Discovery. John Wiley & Sons; Hoboken, NJ, USA: 2006. Development of Opioid Receptor Ligands; pp. 259–276. [DOI] [Google Scholar]
- 3.Robiquet M. Neue Beobachtungen über die vorzüglichsten Produkte aus dem Opium. Eur. J. Org. Chem. 1833;5:82–111. doi: 10.1002/jlac.18330050109. [DOI] [Google Scholar]
- 4.EFSA Panel on Contaminants in the Food Chain (CONTAM) Knutsen H.K., Alexander J., Barregård L., Bignami M., Brüschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Edler L., et al. Update of the Scientific Opinion on opium alkaloids in poppy seeds. EFSA J. 2018;16:e05243. doi: 10.2903/j.efsa.2018.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spetea M., Schmidhammer H. Opioids and Their Receptors: Present and Emerging Concepts in Opioid Drug Discovery. Molecules. 2020;25:5658. doi: 10.3390/molecules25235658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law P.-Y. The Opiate Receptors. Humana Press; Totowa, NJ, USA: 2011. Opioid Receptor Signal Transduction Mechanisms; pp. 195–238. [DOI] [Google Scholar]
- 7.Patrick G. An Introduction to Medicinal Chemistry. Oxford University Press; Oxford, UK: 2023. p. 658. [Google Scholar]
- 8.Sverrisdóttir E., Lund T.M., Olesen A.E., Drewes A.M., Christrup L.L., Kreilgaard M. A review of morphine and morphine-6-glucuronide’s pharmacokinetic–pharmacodynamic relationships in experimental and clinical pain. Eur. J. Pharm. Sci. 2015;74:45–62. doi: 10.1016/j.ejps.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Andersson M., Björkhem-Bergman L., Ekström L., Bergqvist L., Lagercrantz H., Rane A., Beck O. Detection of morphine-3-sulfate and morphine-6-sulfate in human urine and plasma, and formation in liver cytosol. Pharmacol. Res. Perspect. 2014;2:e00071. doi: 10.1002/prp2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson M., Janosik T., Shirani H., Slätt J., Fischer A., Beck O. Synthesis and bioanalytical evaluation of morphine-3-O-sulfate and morphine-6-O-sulfate in human urine and plasma using LC-MS/MS. J. Sep. Sci. 2012;35:367–375. doi: 10.1002/jssc.201100739. [DOI] [PubMed] [Google Scholar]
- 11.Dinis-Oliveira R.J. Metabolism and metabolomics of opiates: A long way of forensic implications to unravel. J. Forensic Leg. Med. 2018;61:128–140. doi: 10.1016/j.jflm.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Smith H.S. Opioid Metabolism. Mayo Clin. Proc. 2009;84:613–624. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett S.E., Smith M.T. The apparent affinity of morphine-3-glucuronide at mu1-opioid receptors results from morphine contamination: Demonstration using HPLC and radioligand binding. Life Sci. 1995;57:609–615. doi: 10.1016/0024-3205(95)00311-S. [DOI] [PubMed] [Google Scholar]
- 14.Brown G.P., Yang K., Ouerfelli O., Standifer K.M., Byrd D., Pasternak G.W. 3H-morphine-6β-glucuronide binding in brain membranes and an MOR-1- transfected cell line. J. Pharmacol. Exp. Ther. 1997;282:1291–1297. [PubMed] [Google Scholar]
- 15.Peat S.J., Hanna M.H., Woodham M., Knibb A.A., Ponte J. Morphine-6-glucuronide: Effects on ventilation in normal volunteers. Pain. 1991;45:101–104. doi: 10.1016/0304-3959(91)90170-3. [DOI] [PubMed] [Google Scholar]
- 16.Thompson P.I., Joel S.P., John L., Wedzicha J.A., Maclean M., Slevin M.L. Respiratory depression following morphine and morphine-6-glucuronide in normal subjects. Br. J. Clin. Pharmacol. 1995;40:145–152. [PMC free article] [PubMed] [Google Scholar]
- 17.Noszal B., Hosztafi S. Physicochemical and Pharmacological Characterization of Permanently Charged Opioids. Curr. Med. Chem. 2017;24:3633–3648. doi: 10.2174/0929867324666170705112239. [DOI] [PubMed] [Google Scholar]
- 18.Pavan B., Dalpiaz A., Ciliberti N., Biondi C., Manfredini S., Vertuani S. Progress in Drug Delivery to the Central Nervous System by the Prodrug Approach. Molecules. 2008;13:1035–1065. doi: 10.3390/molecules13051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varghese V., Hudlicky T. A Short History of the Discovery and Development of Naltrexone and Other Morphine Derivatives. Nat. Prod. Med. Chem. 2014;60:225–250. doi: 10.1002/9783527676545.ch06. [DOI] [Google Scholar]
- 20.Ortiz de Montellano P.R. Cytochrome P450-activated prodrug. Future Med. Chem. 2013;2:213–228. doi: 10.4155/fmc.12.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lötsch J. Opioid Metabolites. J. Pain Symptom Manag. 2005;29:10–24. doi: 10.1016/j.jpainsymman.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Filer C.N. Morphinan alkaloids labeled with tritium: Synthesis and applications. J. Label. Compd. Radiopharm. 2013;56:639–648. doi: 10.1002/jlcr.3094. [DOI] [PubMed] [Google Scholar]
- 23.Toth G. Tritiated Opioid Receptor Ligands as Radiotracers. Curr. Pharm. Des. 2014;19:7461–7472. doi: 10.2174/138161281942140105170259. [DOI] [PubMed] [Google Scholar]
- 24.Speyer E., Rosenfeld H. Darstellung von Monobrom- und Monochlor-kodein und ihr Verhalten bei der katalytischen Reduktion. Ber. Dtsch. Chem. Ges. 1925;58:1110–1113. doi: 10.1002/cber.19250580622. [DOI] [Google Scholar]
- 25.Weiss U., Singh B.B., Chauhan R.S., Madyastha K.M., Bhatnagar S.P., Kirk K.L. 1- and 2-Chloromorphine. Halogenation of Morphine meta to the Free Phenolic Hydroxyl Group. Heterocycles. 1982;19:837. doi: 10.3987/R-1982-05-0837. [DOI] [Google Scholar]
- 26.Anderson T. Ueber die Constitution des Codeïns und seine Zersetzungsproducte. Eur. J. Org. Chem. 1851;77:341–382. doi: 10.1002/jlac.18510770309. [DOI] [Google Scholar]
- 27.Barmaki S., Ali A., Desaulniers J.-P., Bolshan Y. Bromination of codeine and its derivatives: Revisiting a 95 year old process. Tetrahedron Lett. 2020;61:152234. doi: 10.1016/j.tetlet.2020.152234. [DOI] [Google Scholar]
- 28.Braenden O.J., Eddy N.B., Halbach H. Synthetic substances with morphine-like effect; relationship between chemical structure and analgesic action. Bull. World Health Organ. 1955;13:937–998. [PMC free article] [PubMed] [Google Scholar]
- 29.Simon C., Hosztafi S., Makleit S. Application of the Mitsunobu reaction in the morphine series.: The reaction of 14β-chloro and 14β-bromocodeine with phthalimide and diphenylphosphoryl azide. Tetrahedron Lett. 1993;34:6475–6478. doi: 10.1016/0040-4039(93)85074-7. [DOI] [Google Scholar]
- 30.Lousberg R.J.J.C., Weiss U. The analgesic action of 1-fluorocodeine. Cell. Mol. Life Sci. 1974;30:1440–1441. doi: 10.1007/BF01919684. [DOI] [PubMed] [Google Scholar]
- 31.Ojima I. Fluorine in Medicinal Chemistry and Chemical Biology. John Wiley & Sons; Hoboken, NJ, USA: 2009. [DOI] [Google Scholar]
- 32.Hagmann W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 33.Purser S., Moore P.R., Swallow S., Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2007;37:320–330. doi: 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- 34.Gillis E.P., Eastman K.J., Hill M.D., Donnelly D.J., Meanwell N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015;58:8315–8359. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- 35.Hosztafi S., Marton J. Synthesis of 1-fluoro-substituted codeine derivatives. Chem. Pap. 2016;70:973–982. doi: 10.1515/chempap-2016-0033. [DOI] [Google Scholar]
- 36.Makleit S., Dubina V. A gram scale preparation of 1-fluorocodeine and 1-fluorodihydrocodeine. ACH-Model. Chem. 2000;137:447–449. [Google Scholar]
- 37.Enginar H., Unak P., Lambrecht F.Y., Müftüler F.Z.B., Medine E.I., Yolcular S., Yurt A., Seyitoglu B., Bulduk I. Radiolabeling of codeine with 131I and its biodistribution in rats. J. Radioanal. Nucl. Chem. 2009;280:363–370. doi: 10.1007/s10967-009-0528-2. [DOI] [Google Scholar]
- 38.Tafani J., Lazorthes Y., Danet B., Verdie J., Esquerre J., Simon J., Guiraud R. Human brain and spinal cord scan after intracerebroventricular administration of iodine-123 morphine. Int. J. Radiat. Appl. Instrum. Part B Nucl. Med. Biol. 1989;16:505–509. doi: 10.1016/0883-2897(89)90064-0. [DOI] [PubMed] [Google Scholar]
- 39.Kumada N., Kinomura N. Preparation and Crystal Structure of K2YNb5O15−δ. J. Solid State Chem. 1996;123:285–290. doi: 10.1006/jssc.1996.0180. [DOI] [Google Scholar]
- 40.Davies S.G., Goodwin C.J., Pyatt D., Smith A.D. Palladium catalysed elaboration of codeine and morphine. J. Chem. Soc. Perkin Trans. 1. 2001;2:1413–1420. doi: 10.1039/b102581n. [DOI] [Google Scholar]
- 41.Milstein D., Stille J.K. Palladium-catalyzed coupling of tetraorganotin compounds with aryl and benzyl halides. Synthetic utility and mechanism. J. Am. Chem. Soc. 1979;101:4992–4998. doi: 10.1021/ja00511a032. [DOI] [Google Scholar]
- 42.Davies S.G., Pyatt D. Synthesis of 1-Substituted Derivatives of Codeine from 1-Bromocodeine via Palladium Catalysed Coupling Reactions. Heterocycles. 1989;28:163. doi: 10.3987/COM-88-S71. [DOI] [Google Scholar]
- 43.Girán L., Berényi S., Sipos A. Formation of novel thiazolomorphinans and thiazoloaporphines. Tetrahedron. 2008;64:10388–10394. doi: 10.1016/j.tet.2008.08.069. [DOI] [Google Scholar]
- 44.Chatterjie N., Minar A., Clarke D.D. Synthesis of 2-Aminomorphine and 2-Aminocodeine. Reduction of Aromatic Nitro Groups with Formamidinesulfinic Acid. Synth. Commun. 1979;9:647–657. doi: 10.1080/00397917908066712. [DOI] [Google Scholar]
- 45.Kaufmann H.P., Oehring W., Clauberg A. Wissenschaftlicher Teil.: Die Bildung von Thiazol-Derivaten bei der Rhodanierung von Aminen. Arch. Pharm. 1928;266:197–218. doi: 10.1002/ardp.192800069. [DOI] [Google Scholar]
- 46.Cankař P., Popa I., Trávníček Z., Stýskala J., Hradil P., Slouka J. Synthesis of 1-Aminocodeine as a Synthon for Other Codeine Derivatives. Eur. J. Org. Chem. 2013;2013:6062–6068. doi: 10.1002/ejoc.201300701. [DOI] [Google Scholar]
- 47.Hedberg M.H., Johansson A.M., Fowler C.J., Terenius L., Hacksell U. Palladium-catalyzed synthesis of C3-substituted 3-deoxymorphines. Bioorganic Med. Chem. Lett. 1994;4:2527–2532. doi: 10.1016/S0960-894X(01)80277-8. [DOI] [Google Scholar]
- 48.Wentland M.P., Xu G., Cioffi C.L., Ye Y., Duan W., Cohen D.J., Colasurdo A.M., Bidlack J.M. 8-Aminocyclazocine analogues: Synthesis and structure–activity relationships. Bioorganic Med. Chem. Lett. 2000;10:183–187. doi: 10.1016/S0960-894X(99)00670-8. [DOI] [PubMed] [Google Scholar]
- 49.Louie J., Driver M.S., Hamann B.C., Hartwig J.F. Palladium-Catalyzed Amination of Aryl Triflates and Importance of Triflate Addition Rate. J. Org. Chem. 1997;62:1268–1273. doi: 10.1021/jo961930x. [DOI] [Google Scholar]
- 50.Wentland M.P., Duan W., Cohen D.J., Bidlack J.M. Selective Protection and Functionalization of Morphine: Synthesis and Opioid Receptor Binding Properties of 3-Amino-3-desoxymorphine Derivatives,1. J. Med. Chem. 2000;43:3558–3565. doi: 10.1021/jm000119i. [DOI] [PubMed] [Google Scholar]
- 51.Zhang A., Xiong W., Hilbert J.E., DeVita E.K., Bidlack J.M., Neumeyer J.L. 2-Aminothiazole-Derived Opioids. Bioisosteric Replacement of Phenols. J. Med. Chem. 2004;47:1886–1888. doi: 10.1021/jm049978n. [DOI] [PubMed] [Google Scholar]
- 52.Andén N.-E., Nilsson H., Ros E., Thornström U. Effects of B-HT 920 and B-HT 933 on Dopamine and Noradrenaline Autoreceptors in the Rat Brain. Acta Pharmacol. Toxicol. 1983;52:51–56. doi: 10.1111/j.1600-0773.1983.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 53.Jaen J.C., Wise L.D., Caprathe B.W., Tecle H.B.S., Humblet C.C., Heffner T.G., Meltzer L.T., Pugsley T.A. 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist proper-ties. J. Med. Chem. 1990;33:311–317. doi: 10.1021/jm00163a051. [DOI] [PubMed] [Google Scholar]
- 54.Salvatella M., Arsequell G., Valencia G., Rodríguez R.E. A highly toxic morphine-3-glucuronide derivative. Bioorganic Med. Chem. Lett. 2004;14:905–908. doi: 10.1016/j.bmcl.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 55.Jash S.K., Gorai D. Sugar Derivatives of Morphine: A New Window for the Development of Potent Anesthetic Drugs. Nat. Prod. Bioprospecting. 2015;5:111–127. doi: 10.1007/s13659-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stork G., Clarke F.H. The SN2’ Reaction. III. Structure and SN2′ Reactions of the Halocodides. J. Am. Chem. Soc. 1956;78:4619–4624. doi: 10.1021/ja01599a026. [DOI] [Google Scholar]
- 57.Yeh H.J., Wilson R.S., Klee W.A., Jacobson A.E. α- and β-halomorphides: Stereochemistry, analgesic potency, toxicity, and interaction with narcotic receptors in vitro. J. Pharm. Sci. 1976;65:902–904. doi: 10.1002/jps.2600650624. [DOI] [PubMed] [Google Scholar]
- 58.Simon C., Hosztafi S., Makleit S. The First Preparation of 6β-Bromo Codeine and Morphine Derivatives. Kinetic vs. Thermodynamic Control. J. Chem. Res. 1997:437. doi: 10.1039/a704502f. [DOI] [Google Scholar]
- 59.Fujii I., Togame H., Yamamoto M., Kanematsu K., Takayanagi I., Konno F. Design and synthesis of sulfur-containing morphine and an opioid receptor probe. Chem. Pharm. Bull. 1988;36:2282–2285. doi: 10.1248/cpb.36.2282. [DOI] [PubMed] [Google Scholar]
- 60.Kanematsu K., Naito R., Shimohigashi Y., Ohno M., Ogasawara T., Kurono M., Yagi K. Design and synthesis of an opioid receptor probe: Mode of binding of S-activated (-)-6.BETA.-sulfhydryldihydromorphine with the SH group in the .MU.-opioid receptor. Chem. Pharm. Bull. 1990;38:1438–1440. doi: 10.1248/cpb.38.1438. [DOI] [PubMed] [Google Scholar]
- 61.Kumagai H., Ebata T., Takamori K., Muramatsu T., Nakamoto H., Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: A Phase III, randomized, double-blind, placebo-controlled study. Nephrol. Dial. Transplant. 2009;25:1251–1257. doi: 10.1093/ndt/gfp588. [DOI] [PubMed] [Google Scholar]
- 62.Sayre L.M., Larson D.L., Takemori A.E., Portoghese P.S. Design and synthesis of naltrexone-derived affinity labels with nonequilibrium opioid agonist and antagonist activities. Evidence for the existence of different .mu. receptor subtypes in different tissues. J. Med. Chem. 1984;27:1325–1335. doi: 10.1021/jm00376a018. [DOI] [PubMed] [Google Scholar]
- 63.Majumdar S., Burgman M., Haselton N., Grinnell S., Ocampo J., Pasternak A.R., Pasternak G.W. Generation of novel radiolabeled opiates through site-selective iodination. Bioorganic Med. Chem. Lett. 2011;21:4001–4004. doi: 10.1016/j.bmcl.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitsunobu O. The Use of Diethyl Azodicarboxylate and Triphenylphosphine in Synthesis and Transformation of Natural Products. Synthesis. 1981;1981:1–28. doi: 10.1055/s-1981-29317. [DOI] [Google Scholar]
- 65.Simon C., Hosztafi S., Makleit S. Application of the mitsunobu reaction in the field of alkaloids. J. Heterocycl. Chem. 1997;34:349–365. doi: 10.1002/jhet.5570340201. [DOI] [Google Scholar]
- 66.Munawar S., Zahoor A.F., Ali S., Javed S., Irfan M., Irfan A., Kotwica-Mojzych K., Mojzych M. Mitsunobu Reaction: A Powerful Tool for the Synthesis of Natural Products: A Review. Molecules. 2022;27:6953. doi: 10.3390/molecules27206953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bognár R., Makleit S. Conversions of tosyl and mesyl derivatives of morphine group. 2. aminomorphides and aminocodides preliminary communication. Acta Chim. Acad. Sci. Hung. 1968;58:203–205. [Google Scholar]
- 68.Elliott H., Meredith P., Vincent J., Reid J. Clinical pharmacological studies with doxazosin. Br. J. Clin. Pharmacol. 1986;21:27S–31S. doi: 10.1111/j.1365-2125.1986.tb02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knoll J., Furst S., Kelemen K. The pharmacology of azidomorphine and azidocodeine. J. Pharm. Pharmacol. 1973;25:929–939. doi: 10.1111/j.2042-7158.1973.tb09982.x. [DOI] [PubMed] [Google Scholar]
- 70.Knoll J. Azidomorphine and rymazolium an approach to the ideal analgesic. Pharmacol. Res. Commun. 1973;5:175–191. doi: 10.1016/S0031-6989(73)80035-9. [DOI] [Google Scholar]
- 71.Urai Á., Váradi A., Szőcs L., Komjáti B., Le Rouzic V., Hunkele A., Pasternak G.W., Majumdar S., Hosztafi S. Synthesis and pharmacological evaluation of novel selective MOR agonist 6β-pyridinyl amidomorphines exhibiting long-lasting antinociception. MedChemComm. 2016;8:152–157. doi: 10.1039/C6MD00450D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knoll J. Azidomorphines: A new family of potent analgesics with low dependence capacity. Prog. Neuro-Psychopharmacol. 1979;3:95–108. doi: 10.1016/0364-7722(79)90074-2. [DOI] [PubMed] [Google Scholar]
- 73.MacDougall J.M., Zhang X.-D., Polgar W.E., Khroyan T.V., Toll L., Cashman J.R. Synthesis and biological evaluation of some 6-arylamidomorphines as analogues of morphine-6-glucuronide. Bioorganic Med. Chem. 2004;12:5983–5990. doi: 10.1016/j.bmc.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 74.Váradi A., Hosztafi S., Le Rouzic V., Tóth G., Urai Á., Noszál B., Pasternak G.W., Grinnell S.G. Novel 6β-acylaminomorphinans with analgesic activity. Eur. J. Med. Chem. 2013;69:786–789. doi: 10.1016/j.ejmech.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Váradi A., Marrone G.F., Eans S.O., Ganno M.L., Subrath J.J., Le Rouzic V., Hunkele A., Pasternak G.W., McLaughlin J.P., Majumdar S. Synthesis and Characterization of a Dual Kappa-Delta Opioid Receptor Agonist Analgesic Blocking Cocaine Reward Behavior. ACS Chem. Neurosci. 2015;6:1813–1824. doi: 10.1021/acschemneuro.5b00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li G., Aschenbach L.C., Chen J., Cassidy M.P., Stevens D.L., Gabra B.H., Selley D.E., Dewey W.L., Westkaemper R.B., Zhang Y. Corrections to Design, Synthesis, and Biological Evaluation of 6α- and 6β-N-Heterocyclic Substituted Naltrexamine Derivatives as μ Opioid Receptor Selective Antagonists. J. Med. Chem. 2009;52:8057. doi: 10.1021/jm9016224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arsequell G., Salvatella M., Valencia G., Fernández-Mayoralas A., Fontanella M., Venturi C., Jiménez-Barbero J., Marrón E., Rodríguez R.E. Synthesis, Conformation, and Biological Characterization of a Sugar Derivative of Morphine that is a Potent, Long-Lasting, and Nontolerant Antinociceptive. J. Med. Chem. 2009;52:2656–2666. doi: 10.1021/jm8011245. [DOI] [PubMed] [Google Scholar]
- 78.MacDougall J.M., Zhang X.-D., Polgar W.E., Khroyan T.V., Toll L., Cashman J.R. Design, Chemical Synthesis, and Biological Evaluation of Thiosaccharide Analogues of Morphine- and Codeine-6-Glucuronide. J. Med. Chem. 2004;47:5809–5815. doi: 10.1021/jm049554t. [DOI] [PubMed] [Google Scholar]
- 79.MacDougall J.M., Zhang X.-D., Polgar W.E., Khroyan T.V., Toll L., Cashman J.R. Synthesis and in vitro biological evaluation of a carbon glycoside analogue of morphine-6-glucuronide. Bioorganic Med. Chem. Lett. 2005;15:1583–1586. doi: 10.1016/j.bmcl.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 80.Yang J., Lu G., Zhang G., Wang X., Wen H., Huang C., Yin J., Li J. Morphine-6-Glucuronide Isomers-Synthesis and Biological Evaluation. Bull. Korean Chem. Soc. 2020;41:1073–1079. doi: 10.1002/bkcs.12112. [DOI] [Google Scholar]
- 81.Thavaneswaran S., Mccamley K., Scammells P.J. N-Demethylation of Alkaloids. Nat. Prod. Commun. 2006;1:2006. doi: 10.1177/1934578X0600101008. [DOI] [Google Scholar]
- 82.Machara A., Hudlický T. Advances in N- and O-demethylation of opiates. Targets Heterocycl. Syst. 2016;20:113–138. doi: 10.17374/TARGETS.2017.20.113. [DOI] [Google Scholar]
- 83.McCamley K., Ripper J.A., Singer R.D., Scammells P.J. Efficient N-Demethylation of Opiate Alkaloids Using a Modified Nonclassical Polonovski Reaction. J. Org. Chem. 2003;68:9847–9850. doi: 10.1021/jo035243z. [DOI] [PubMed] [Google Scholar]
- 84.Ripper J.A., Tiekink R.T., Scammells P.J. Photochemical N -Demethylation of Alkaloids. Bioorg. Med. Chem. Lett. 2001;11:443–445. doi: 10.1016/S0960-894X(00)00690-9. [DOI] [PubMed] [Google Scholar]
- 85.Chaudhary V., Leisch H., Moudra A., Allen B., De Luca V., Cox D.P., Hudlický T. Biotransformations of morphine alkaloids by fungi: N-demethylations, oxidations, and reductions. Collect. Czechoslov. Chem. Commun. 2009;74:1179–1193. doi: 10.1135/cccc2009025. [DOI] [Google Scholar]
- 86.Morton A.A., Olson A.R., Blattenberger J.W. Thioanilides of Malonic Acids. J. Am. Chem. Soc. 1941;63:314–315. doi: 10.1021/ja01846a505. [DOI] [Google Scholar]
- 87.Unna K. Antagonistic effect of N-allyl-normorphine upon morphine. J. Pharmacol. Exp. Ther. 1943;79:28–31. doi: 10.1016/0014-2999(84)90254-1. [DOI] [Google Scholar]
- 88.Clark R.L., Pessolano A.A., Weijlard J., Pfister K., 3rd N-Substituted Epoxymorphinan. J. Am. Chem. Soc. 1953;75:4963–4967. doi: 10.1021/ja01116a024. [DOI] [Google Scholar]
- 89.Small L., Eddy N., Ager J., May E. Notes: An Improved Synthesis of N-Phenethylnormorphine and Analogs. J. Org. Chem. 1958;23:1387–1388. doi: 10.1021/jo01103a615. [DOI] [Google Scholar]
- 90.Ben Haddou T., Béni S., Hosztafi S., Malfacini D., Calo G., Schmidhammer H., Spetea M. Pharmacological Investigations of N-Substituent Variation in Morphine and Oxymorphone: Opioid Receptor Binding, Signaling and Antinociceptive Activity. PLoS ONE. 2014;9:e99231. doi: 10.1371/journal.pone.0099231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gharehnaghadeh S., Salehi P., Bararjanian M., Pecio Ł., Babanezhad-Harikandei K., Khoramjouy M., Shahhosseini S., Faizi M. Novel Triazole-Tethered Derivatives of Nor-codeine: Synthesis, Radioligand Binding Assay, Docking Study and Evaluation of Their Analgesic Properties. Chemistryselect. 2020;5:14753–14758. doi: 10.1002/slct.202003684. [DOI] [Google Scholar]
- 92.Brown C.E., Roerig S.C., Burger V.T., Cody R.B., Fujimoto J.M. Analgesic Potencies of Morphine 3- and 6-Sulfates After Intracerebroventricular Administration in Mice: Relationship to Structural Characteristics Defined by Mass Spectrometry and Nuclear Magnetic Resonance. J. Pharm. Sci. 1985;74:821–824. doi: 10.1002/jps.2600740804. [DOI] [PubMed] [Google Scholar]
- 93.Houdi A.A., Kottayil S., Crooks P.A., Butterfield D. 3-O-Acetylmorphine-6-O-sulfate: A potent, centrally acting morphine derivative. Pharmacol. Biochem. Behav. 1996;53:665–671. doi: 10.1016/0091-3057(95)02067-5. [DOI] [PubMed] [Google Scholar]
- 94.Zuckerman A., Bolan E., de Paulis T., Schmidt D., Spector S., Pasternak G.W. Pharmacological characterization of morphine-6-sulfate and codeine-6-sulfate. Brain Res. 1999;842:1–5. doi: 10.1016/S0006-8993(99)01766-7. [DOI] [PubMed] [Google Scholar]
- 95.Crooks P.A., Kottayil S.G., Al-Ghananeem A.M., Byrn S.R., Butterfield D.A. Opiate receptor binding properties of morphine-, dihydromorphine-, and codeine 6-O-sulfate ester congeners. Bioorganic Med. Chem. Lett. 2006;16:4291–4295. doi: 10.1016/j.bmcl.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 96.Dietis N., Rowbotham D., Lambert D. Opioid receptor subtypes: Fact or artifact? Br. J. Anaesth. 2011;107:8–18. doi: 10.1093/bja/aer115. [DOI] [PubMed] [Google Scholar]
- 97.Váradi A., Gergely A., Béni S., Jankovics P., Noszál B., Hosztafi S. Sulfate esters of morphine derivatives: Synthesis and characterization. Eur. J. Pharm. Sci. 2011;42:65–72. doi: 10.1016/j.ejps.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 98.Hosztafi S., Kraszni M., Tóth G., Marton J. Synthesis of 1-Iodo-substituted Codeine Derivatives. Lett. Org. Chem. 2018;15:1012–1020. doi: 10.2174/1570178615666171222163442. [DOI] [Google Scholar]
- 99.Shel’Metsi D., Slavik L., Kashkete Z., Lilek L.-M., Matsko I. Synthesis of new morphine derivatives. Pharm. Chem. J. 1968;2:302–306. doi: 10.1007/BF00759723. [DOI] [Google Scholar]
- 100.Simon C., Hosztafi S., Makleit S. Application of the Mitsunobu Reaction for Morphine Compounds. Preparation of 6β-Aminomorphine and Codeine Derivatives. Synth. Commun. 1992;22:913–921. doi: 10.1080/00397919208020855. [DOI] [Google Scholar]
- 101.Simon C., Hosztafi S., Makleit S. Application of the Mitsunobu Reaction for the Preparation of Isomorphine and Isocodeine Derivatives. Synth. Commun. 1991;21:407–412. doi: 10.1080/00397919108016763. [DOI] [Google Scholar]
- 102.Hirano T., Oguri K., Yoshimura H. Synthesis and Pharmacological Activity of Sulfate Conjugates at 6-Position of N-Substituted Normorphine Derivatives. Chem. Pharm. Bull. 1991;39:2000–2004. doi: 10.1248/cpb.39.2000. [DOI] [PubMed] [Google Scholar]
- 103.Olofson R.A., Martz J.T., Senet J.P., Piteau M., Malfroot T. A new reagent for the selective, high-yield N-dealkylation of tertiary amines: Improved syntheses of naltrexone and nalbuphine. J. Org. Chem. 1984;49:2081–2082. doi: 10.1021/jo00185a072. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.