Abstract
Human alveolar echinococcosis (HAE), caused by the metacestode stage of Echinococcus multilocularis, has emerged in many European countries over the last two decades. Here, we report the first data on the new HAE focus with increasing incidence in central Croatia, describe its clinical presentation and outcomes in diagnosed patients, and provide an update on the prevalence and geographic distribution of Echinococcus multilocuaris in red foxes. After the initial case in 2017 from the eastern state border, from 2019 to 2022, five new autochthonous HAE cases were diagnosed, all concentrated in the Bjelovar-Bilogora County (the county incidence in 2019 and 2021: 0.98/105, in 2022: 2.94/105/year; prevalence for 2019–2022: 4.91/105). The age range among four female and two male patients was 37–67 years. The patients’ liver lesions varied in size from 3.1 to 15.5 cm (classification range: P2N0M0–P4N1M0), and one patient had dissemination to the lungs. While there were no fatalities, postoperative complications in one patient resulted in liver transplantation. In 2018, the overall prevalence of red foxes was 11.24% (28/249). A new focus on HAE has emerged in central continental Croatia, with the highest regional incidence in Europe. Screening projects among residents and the implementation of veterinary preventive measures following the One Health approach are warranted.
Keywords: human alveolar echinococcosis, Echinococcus multilocularis, epidemiology, clinical characteristic, red fox, emergence, Croatia
1. Introduction
The causative agent of human alveolar echinococcosis (HAE)—Echinococcus multilocularis—was described for the first time as a different species (Cestoda: Taeniidae) by Leuckart in 1863. Although rare, HAE is one of the most pathogenic zoonoses in Europe. If untreated, it is associated with high mortality, up to 90%, within 10–15 years of diagnosis [1]. While humans are an accidental intermediate host (metacestode stage of infection), red foxes (Vulpes vulpes), as definitive hosts, present the main source of infection.
Recently, a geographical spread of HAE across Europe and an increase in case numbers has been reported. Moreover, from 2001 to 2018, nine European countries reported the first case of autochthonous HAE [2]. Although endemic in neighboring Slovenia and Hungary, Croatia was considered HAE-free until the first case was diagnosed from Vukovar, on the eastern Croatian border, in 2017 and published in 2020 [3]. E. multilocularis infection in red foxes in Croatia has been reported previously, with a mean prevalence of 7.5% and 6.6% in 2015 and 2016, respectively [4].
Croatia is administratively divided into 21 counties, among which, with 2.652 square km located in central continental Croatia, the Bjelovar-Bilogora County occupies 3.03% of the whole state territory. Here, we report data on the new focus and increasing incidence of HAE in Bjelovar-Bilogora County; we analyzed disease outcomes and can provide an update on the prevalence and geographic distribution of the parasite among red foxes. A significant increase in its mean prevalence in red foxes has been noticed, especially in the County where new human cases appeared recently, suggesting the spread of this disease from eastern to central continental Croatia, where one of the southernmost focuses of HAE in the Balkan region is emerging. Educational and screening projects among residents and the implementation of veterinary preventive measures should be initiated to prevent the spreading and decrease morbidity of this serious parasitic zoonosis.
2. Materials and Methods
2.1. Human Alveolar Echinococcosis
Patients included in this study were treated at the University Hospital for Infectious Diseases in Zagreb, which is a national referral center for infectious diseases. One additional patient from Split (Dalmatia) was diagnosed with HAE in the period studied but was not included in this case series since the disease was not autochthonous but probably imported from South Germany. According to the official data from the Croatian Institute of Public Health, there were no other patients with a diagnosis of AE reported in Croatia up to April 2023. The patientsʹ data were collected from medical documentation and analyzed retrospectively. The disease was classified based on morphological characteristics of the lesions obtained by multi-slice computed tomography (MSCT) using the echinococcosis PNM staging system [5].
The disease incidence and prevalence data for Bjelovar-Bilogora County were recalculated based on a population of 101,879 inhabitants, according to a recent population census [6].
In all patients, the diagnosis was confirmed by the PCR and sequencing of two gene targets either from formalin-fixed, paraffin-embedded liver tissue or from fresh cysts collected during explorative surgery. Paraffin-embedded tissue was dewaxed with xylene, washed three times with 99.6% ethanol, and digested at 56 °C for 8 h, while, from fresh cysts, this step was skipped. DNA was extracted using a QIAamp DNA Mini QIAcube Kit according to the protocol for blood and body fluids on an automated QIAcube system.
In patient No 1, serology (genus and species-specific ELISAs and EUROLINE-IgG Western Blot system) was performed at the University of Zürich (Zürich, Switzerland) [3], while other patients were serologically tested at the Croatian Institute of Public Health, Department of Parasitology (Zagreb, Croatia). In patient No 2, NovaLisa Echinococcus IgG ELISA was used for the qualitative determination of IgG class antibodies against Echinococcus spp. in human serum or plasma (manufacturer NovaTec Immundiagnostica GmbH, Dietzenbach, Germany; by manufacturer-declared sensitivity: 97.22%, and specificity: 98.82%). In addition, an Echinococcus Western Blot IgG (manufacturer LDBIO Diagnostics, Lyon, France), a qualitative test for the serological diagnosis of alveolar and hydatid echinococcosis, was used as a confirmatory test. In patients No 3–6, besides the aforementioned tests, an E. multilocularis ELISA test (manufacturer Bordier Affinity Products, Crissier, Switzerland; by manufacturer declared sensitivity: 83%, and specificity: 98%) was used for the quantitative detection of IgG antibodies against Em2 and Em18 specific antigens of E. multilocularis.
2.2. E. multilocularis Infection in Red Foxes
Fecal samples were collected from 249 carcasses of red foxes, as part of the rabies control program in different regions of Croatia, from January to March 2018. Carcasses were delivered to the veterinary services, and the approximate location, based on the hunters’ mandatory reporting, was recorded for each sample. After collection, fecal samples were stored at −80 °C for at least 3 weeks prior to further processing. DNA was extracted directly from 200 mg of fecal samples using the QIAamp® DNA Stool Mini Kit (Qiagen, Hildesheim, Germany). All samples were analyzed using conventional E. multilocularis PCR reactions that amplified a 200-bp region in the mitochondrial gene nad1 [7] or a 395-bp region in the 12S rRNA gene [8]. Amplicons were purified, sequenced in both directions with the same primers as used in PCRs, assembled using SeqMan Pro, and edited with EditSeq (Lasergene, DNASTAR).
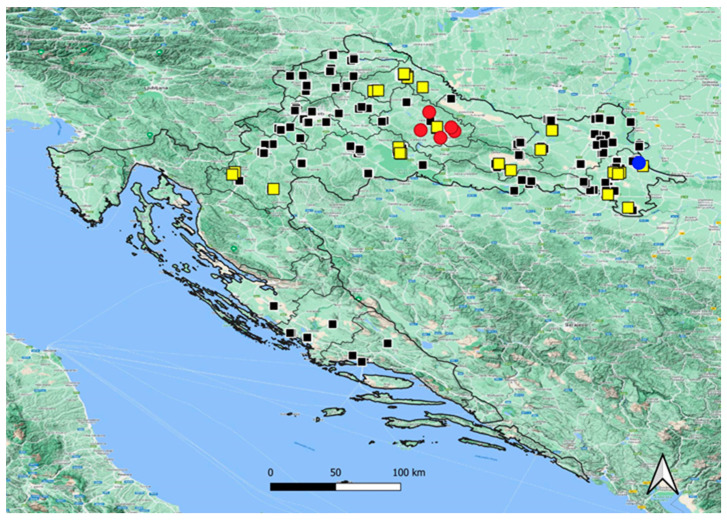
For better visibility of the obtained results, all samples were mapped using QGIS software version 3.30.0 RC, and Figure 1 was created using the QGIS program [9].
Figure 1.
Geographic distribution of autochthonous human alveolar echinococcosis cases from 2017 to 2022, and distribution of localities with Echinococcus multilocularis positive red foxes (Vulpes vulpes), tested from January to March 2018 in Croatia. Yellow squares—locations of positive foxes; Black squares—locations of negative foxes; Blue dot—first human case from 2017; Red dots—positive human cases from 2019 to 2022.
3. Results
3.1. Human Alveolar Echinococcosis
In 2017, the first case of HAE was diagnosed in Croatia. From 2019 to 2022, five new autochthonous HAE cases were diagnosed, all concentrated in the rural eastern part of Bjelovar-Bilogora County in central continental Croatia (Figure 1). The incidence in this County in 2019 and 2021 was 0.98/105/year, and it rose to 2.94/105/year in 2022. In 2020 there were no cases recorded. The County prevalence for 2019–2022 was 4.91/105.
The basic demographic and clinical characteristics of the diagnosed autochthonous HAE patients are shown in Table 1.
Table 1.
Demographic and clinical characteristics of autochthonous human alveolar echinococcosis patients in Croatia from 2017 to 2022.
| Patient: Gender, Age, Year of Diagnosis |
Clinical Presentation (Duration of Symptoms before Diagnosis) |
PNM Classification (Clinical Stage) |
Diagnosis and Follow Up |
Therapy | Complications |
|---|---|---|---|---|---|
| No 1: male; 63 y.; 2017 |
Fever, dyspnea, cough, right-sided chest pain (3 y.) |
P4N1M1 (IV) |
MSCT Pathohistology Serology PCR FDG-PET |
Albendazole; Partial resection; Amphotericin B; Mefloquine |
Intraop. bleeding; Pancytopenia, agranulocytosis with sepsis—due to albendazole therapy |
| No 2: male; 64 y.; 2019 |
Upper right abdominal quadrant pain; jaundice (5 mo.) |
P4N1M0 (IV) |
MSCT Pathohistology PCR |
Liver lobectomy; Albendazole 2 y. postoperatively | No |
| No 3: female; 37 y.; 2021 |
Upper right abdominal quadrant pain (8 mo.) |
P4N0M0 (IIIb) |
MSCT Pathohistology Serology PCR FDG-PET |
Excision; Albendazole postoperatively; Liver transplantation |
Postexcisional: hepatic artery thrombosis; recurrent cholangitis |
| No 4: female; 58 y.; 2022 |
Upper right abdominal quadrant pain, nausea (2 mo.) |
P4N0M0 (IIIb) |
MSCT Pathohistology Serology PCR FDG-PET |
Albendazole Partial resection |
No |
| No 5: female; 67 y.; 2022 |
Upper right abdominal quadrant discomfort (8 y.) |
P3N0M0 (IIIa) |
MSCT Pathohistology Serology PCR FDG-PET |
Liver lobectomy; Albendazole postoperatively |
Leucopenia; Increased transaminases due to albendazole therapy |
| No 6: female; 53 y.; 2022 |
Upper abdominal discomfort, nausea (4 y.) |
P2N0M0 (II) |
MSCT Serology FDG-PET Pathohistology PCR |
Albendazole; Excision |
Increased transaminases and neutropenia due to albendazole therapy |
y.—years; mo.—months; MSCT—Multi-Slice Computed Tomography; PCR—Polymerase Chain Reaction; FDG-PET—Fluorodeoxyglucose-Positron Emission Tomography.
All HAE patients were from rural areas and were exposed to rural soil and home garden-grown vegetables near the wood; however, some of them were additionally engaged in forest activities (wood and mushroom collecting). The duration of symptoms before the final diagnosis ranged from two months to eight years. The patient’s liver lesions varied in size from 3.1 to 15.5 cm in diameter. The patient with the largest lesion (No 5) was followed up for 8 years under the diagnosis of “atypical hemangioma”. Only in patient No 1 was a spread in the infection spread found outside the liver to the lungs. All patients were surgically treated: two by partial resection (patient No 1 with the disseminated disease and No 4 with numerous parasitic lesions which permeated virtually the whole liver) and four by total excision. In three of the six patients, who were initially misdiagnosed as having a neoplastic tumor of the liver, preoperative albendazole therapy was not applied. One severe and two moderate reactions to albendazole were recorded, which led to albendazole discontinuation in three patients. The most serious adverse effect of albendazole occurred in patient No 1, with disseminated disease to the lungs, for whom one year after palliative surgery, albendazole had to be discontinued due to significant pancytopenia and sepsis triggered by agranulocytosis. In this patient, “salvage therapy” with amphotericin B (deoxycholate) was given three times weekly for four weeks and once a week for the next two years. Afterward, due to a rise in serum creatinine and gamma-glutamyl transferase, the therapy was switched to mefloquine, 1 × 250 mg once per week. After 1.5 years, mefloquine therapy had to be discontinued due to malaise, leucopenia, and a rise in serum creatinine and gamma-glutamyl transferase. Despite intolerance to multiple therapies, there were no signs of disease progression during the 5-year long follow-up period in this patient, and the last FDG-PET scan in March 2022 showed no metabolic activity in the remaining parasitic lesions. In patient No 3, the post-excisional clinical course was complicated by hepatic artery thrombosis, and consequent episodes of severe recurrent septic cholangitis were indications for liver transplantation, which was successfully performed. In our cohort, there were no fatal outcomes.
3.2. Prevalence of E. multilocularis Infection in Red Foxes
In the observed period from January to March 2018, the fecal samples of 249 red foxes from different parts of continental Croatia were examined by PCR for the presence of E. multilocularis genes. Among them, 28/249 samples were positive, with a calculated mean prevalence of 11.24% (95% CI: 7.89–15.77). An almost identical prevalence of 11.7% (6/51; 95% CI: 5.51–23.38) was found on location in Eastern Croatia, from where the first HAE case was diagnosed. In Bjelovar-Bilogora County, where new human cases recently emerged, local prevalence among red foxes reached 28.57% (2/7; 95% CI: 8.22–64.11). The locations from which the fecal samples of red foxes were analyzed and the distribution of locations with E. multilocularis positive and negative findings are shown in Figure 1.
Sequences from humans and red foxes obtained in the current study were identical to each and to Croatian sequences submitted earlier to GenBank under the accession numbers MG755265 and MG755266.
4. Discussion
The incidence for HAE of 2.94/105/year in 2022 and prevalence of 4.91/105 for 2019–2022 in Bjelovar-Bilogora County in central continental Croatia exceeded the overall incidence reported for Central Europe of 0.03–0.26/105 [10], and the national mean incidence of 0.09/105/year in our neighboring country Slovenia (2001–2005) [11]. It also exceeded the highest reported European regional incidence of 1.9/105 in the Austrian Federal State of Vorarlberg in 2011 by more than double [12]. In addition to an increase in the red fox population in Europe, related to the elimination of rabies and their increased infection rates with E. multilocularis, the increasing urbanization of fox habitats has been proposed as one of the important factors associated with an increase in the incidence of HAE cases [2].
The high County prevalence of HAE in Bjelovar-Bilogora County is in accordance with a high observed County prevalence among red foxes of 28.57% recorded in 2018. As in Croatia, in the neighboring countries, a rise in E. multilocularis infection prevalence among red foxes has been observed: in Slovenia, it increased from 2.6% (2010) [13] to 29.1% (2019–2022) [14]. In Hungary, the mean prevalence of red foxes gradually increased, and it was 5%, 10.7%, 7.9%, and 12.5% in 2002, 2008–2009, 2012–2013, and 2018–2020, respectively [15,16,17]. In the Vojvodina Province of Serbia, a prevalence of 17.9% was recorded in 2016 [18].
Recently (2022), the first positive red fox was reported from Bosnia and Herzegovina (B&H) [19], caught close to the westernmost state border with Croatia, in a belt of the Dinaric Mountains that spread continuously from Slovenia to Albania and where from the Croatian territory two positive red foxes were reported in 2015–2016 [4].
In Croatian surroundings, autochthonous HAE cases were reported from southern and north-eastern parts of Slovenia—there were nine serologically and morphologically diagnosed cases between 2001 and 2005 [11]. In Hungary, a case series of 16 HAE patients (2003–2018) was published: four patients (25%) were cured by radical surgery and adjuvant albendazole therapy, five (31.3%) were unresectable but without progression under albendazole therapy, in seven (43.8%) the disease progressed, and there were three (18.8%) lethal outcomes recorded [20]. In comparison to these data, the data from our study suggest better disease outcomes (no disease progression, no lethal outcome) among our patients, although this conclusion could be biased due to a low number of cases. However, no reports of HAE from Serbia or B&H can be found in the literature yet, which could be the result of a potential misdiagnosis bias [19].
A meta-analysis from 2016 showed the highest pooled prevalence of the parasite among red foxes in Baltic and central European countries of 58.0, 36.8, 34.9, 29.2 and 27.3% in Lithuania (2001–2006), Latvia (2002–2008), Liechtenstein (1990–1992), Germany (2000–2012) and Slovakia (2000–2013), respectively [21]. Recent animal data from neighboring Slovenia [14] and data from Bjelovar-Bilogora County from this study, where parasite prevalence among examined red foxes in the observed regions reached nearly 30%, clearly show the spread of this infection in the main host; this has resulted in the local emergence of HAE cases in central continental Croatia. The low disease incidence and unfamiliarity of healthcare workers with alveolar echinococcosis have contributed to initial misdiagnosis in a substantial proportion of our patients.
5. Conclusions
Continental Croatia should be considered endemic for HAE. A new focus on HAE is emerging in Bjelovar-Bilogora County, where a high parasite prevalence in red foxes has been recently documented. The education of the general population and clinicians, screening projects among local inhabitants, the implementation of veterinary medicine preventive measures, and systematic surveillance of the parasite prevalence in red fox populations should be initiated.
Acknowledgments
The authors thank Arijana Pavelić for proofreading the manuscript.
Author Contributions
Conceptualization, M.B.T.; clinical data collection, disease staging and review of literature: K.V., T.F.K., S.J. and M.B.T.; geographic distribution exploration and serology diagnostics of human cases, M.S.; collecting of human materials for diagnostics, S.J.; molecular diagnostics on humans and foxes, D.J. and R.B.; writing—original draft preparation, M.B.T.; writing—review and editing, M.B.T., N.P. and R.B.; geographic distribution visualization, D.J. and R.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
An ethical review and approval were waived due to the retrospective and noninterventional nature of the study.
Informed Consent Statement
Patient consent was waived due to the retrospective and noninterventional nature of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Detailed patient data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Torgerson P.R., Schweiger A., Deplazes P., Pohar M., Reichen J., Ammann R.W., Tarr P.E., Halkic N., Müllhaupt B. Alveolar echinococcosis: From a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J. Hepatol. 2008;49:72–77. doi: 10.1016/j.jhep.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Baumann S., Shi R., Liu W., Bao H., Schmidberger J., Kratzer W., Li W. Worldwide literature on epidemiology of human alveolar echinococcosis: A systematic review of research published in the twenty-first century. Infection. 2019;47:703–727. doi: 10.1007/s15010-019-01325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dušek D., Vince A., Kurelac I., Papić N., Višković K., Deplazes P., Beck R. Human Alveolar Echinococcosis, Croatia. Emerg. Infect. Dis. 2020;26:364–366. doi: 10.3201/eid2602.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck R., Mihaljević Ž., Brezak R., Bosnić S., Janković I.L., Deplazes P. First detection of Echinococcus multilocularis in Croatia. Parasitol. Res. 2018;117:617–621. doi: 10.1007/s00436-017-5732-3. [DOI] [PubMed] [Google Scholar]
- 5.Brunetti E., Kern P., Vuitton D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Croatian Bureau of Statistics Census of Population. Population by Age and Sex, according to the Counties, Census 2021. [(accessed on 28 February 2023)]. Available online: https://podaci.dzs.hr/hr/podaci/stanovnistvo/popis-stanovnistva.
- 7.Trachsel D., Deplazes P., Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- 8.Stieger C., Hegglin D., Schwarzenbach G., Mathis A., Deplazes P. Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology. 2002;124:631–640. doi: 10.1017/S0031182002001749. [DOI] [PubMed] [Google Scholar]
- 9.QGIS Development Team, v. 3.30.0, 2023. QGIS Geographic Information System. Open Source Geospatial Foundation Project. [(accessed on 30 May 2023)]. Available online: http://qgis.osgeo.org.
- 10.Baneth G., Thamsborg S.M., Otranto D., Guillot J., Blaga R., Deplazes P., Solano-Gallego L. Major Parasitic Zoonoses Associated with Dogs and Cats in Europe. J. Comp. Pathol. 2016;155:S54–S74. doi: 10.1016/j.jcpa.2015.10.179. [DOI] [PubMed] [Google Scholar]
- 11.Logar J., Soba B., Lejko-Zupanc T., Kotar T. Human alveolar echinococcosis in Slovenia. Clin. Microbiol. Infect. 2007;13:544–546. doi: 10.1111/j.1469-0691.2007.01701.x. [DOI] [PubMed] [Google Scholar]
- 12.Schneider R., Aspöck H., Auer H. Unexpected increase of alveolar echincoccosis, Austria, 2011. Emerg. Infect. Dis. 2013;19:475–477. doi: 10.3201/eid1903.120595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rataj A.V., Bidovec A., Žele D., Vengušt G. Echinococcus multilocularis in the red fox (Vulpes vulpes) in Slovenia. Eur. J. Wildl. Res. 2010;56:819–822. doi: 10.1007/s10344-010-0417-6. [DOI] [Google Scholar]
- 14.Bandelj P., Blagus R., Vengušt G., Žele Vengušt D. Wild Carnivore Survey of Echinococcus Species in Slovenia. Animals. 2022;12:2223. doi: 10.3390/ani12172223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sréter T., Széll Z., Egyed Z., Varga I. Echinococcus multilocularis: An emerging pathogen in Hungary and Central Eastern Europe? Emerg. Infect. Dis. 2003;9:384–386. doi: 10.3201/eid0903.020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolnai Z., Széll Z., Sréter T. Environmental determinants of the spatial distribution of Echinococcus multilocularis in Hungary. Vet. Parasitol. 2013;198:292–297. doi: 10.1016/j.vetpar.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Halász T., Nagy G., Nagy I., Csivincsik Á. Micro-Epidemiological Investigation of Echinococcus multilocularis in Wild Hosts from an Endemic Area of Southwestern Hungary. Parasitologia. 2021;1:158–167. doi: 10.3390/parasitologia1030017. [DOI] [Google Scholar]
- 18.Lalošević D., Lalošević V., Simin V., Miljević M., Čabrilo B., Čabrilo O.B. Spreading of multilocular echinococcosis in southern Europe: The first record in foxes and jackals in Serbia, Vojvodina Province. Eur. J. Wildl. Res. 2016;62:793–796. doi: 10.1007/s10344-016-1050-9. [DOI] [Google Scholar]
- 19.Omeragić J., Goletić T., Softić A., Goletić Š., Kapo N., Soldo D.K., Šupić J., Škapur V., Čerkez G., Ademović E., et al. First detection of Echinococcus multilocularis in Bosnia and Herzegovina. Int. J. Parasitol. Parasites Wildl. 2022;19:269–272. doi: 10.1016/j.ijppaw.2022.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dezsényi B., Dubóczki Z., Strausz T., Csulak E., Czoma V., Káposztás Z., Fehérvári M., Somorácz Á., Csilek A., Oláh A., et al. Emerging human alveolar echinococcosis in Hungary (2003–2018): A retrospective case series analysis from a multi-centre study. BMC Infect. Dis. 2021;21:168. doi: 10.1186/s12879-021-05859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oksanen A., Siles-Lucas M., Karamon J., Possenti A., Conraths F.J., Romig T., Wysocki P., Mannocci A., Mipatrini D., La Torre G., et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: A systematic review and meta-analysis. Parasites Vectors. 2016;9:519. doi: 10.1186/s13071-016-1746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Detailed patient data are not publicly available due to privacy or ethical restrictions.