Abstract
Callistemon is an aromatic genus of flowering plants belonging to family Myrtaceae. The essential oils of C. subulatus leaves were collected in four seasons and analyzed using GC/MS. The oils demonstrated monoterpenes as the predominant class. Eucalyptol was the main component in all seasons; summer (66.87%), autumn (58.33%), winter (46.74%) and spring (44.63%), followed by α-pinene; spring (31.41%), winter (28.69%), summer (26.34%) and autumn (24.68%). Winter oil, the highest yield (0.53 mL/100g), was further investigated for its inhibitory activity against enzymes associated with ageing; elastase and acetylcholinesterase. It remarkably inhibited elastase and acetylcholinesterase with IC50 values of 1.05 and 0.20 µg/ml, respectively. A molecular docking study was conducted for the major oil components on the active sites of target enzymes. Eucalyptol revealed the best binding affinity for both enzymes. C. subualtus oil could be used as supplement for management of ageing disorders like skin wrinkles and dementia.
Keywords: Callistemon subulatus, seasonal variation, Elastase, acetylcholinesterase, molecular docking
GRAPHICAL ABSTRACT
Introduction
Historically, plants have recorded a great importance for utilisation in different health purposes in various countries such as Mesopotamia, Egypt, Greek, and Islamic cultures1–3. Medicinal plants continuously offer new and promising lead compounds with various pharmacological effects in different disorders such as ageing, Alzheimer, cancer, malaria, nephritis and hepatitis4–6.
Callistemon (Myrtaceae) is mainly found in tropic and sub-tropic areas around the world. It contains more than 35 species of shrubs or small trees with evergreen leaves and flower spikes that have a traditional bottle brush shape7. Different parts of the Callistemon plant have traditionally been used as anthelmintic8, anti-inflammatory9, antiviral, antimicrobial, pleasant-smelling tea substitute and in ornamental horticulture10.
Callistemon subulatus is a species native to south eastern Australia and recently cultivated in Egypt for ornamental purposes11. It is a short shrub with densely arranged leaves and dark crimson filaments. It flowers in late spring and early summer. Previous studies have shown that the leaves are rich source of volatile oils11 and polyphenolics12, and showed the ability of C. subulatus to be cytotoxic11, antimicrobial13, anti-nociceptive, anti-inflammatory, antidiarrheal, and antioxidant12,14.
The skin ageing symptoms are represented by loss of integrity and the appearance of wrinkles in the skin caused by a gradual decrease of skin proteins’ production such as elastin, and their increased damage by the action of degrading enzymes such as elastase, these enzymes are upregulated by different impacts as ageing, UV radiation and reactive oxygen species15,16. Elastase overexpression also contribute to other inflammatory conditions such as rheumatoid arthritis, cyclic neutropenia, sepsis, cystic fibrosis, asthma and atherosclerosis17.
Alzheimer disease is a neurodegenerative disease associated with disturbance in cholinergic transmission, resulting in memory loss, behavioural changes and cognitive impairment18. Acetylcholinesterase (AChE) inhibitors are one of the most effective therapeutics for Alzheimer disease, as they alter the excess of synaptic AChE and increase acetylcholine levels19.
Essential oils, containing compounds of relatively small molecular size and high lipophilicity, have a high ability to be absorbed through skin layers and to penetrate blood brain barrier20–22, which could be an effective approach for skin repair and for management of brain disorders. In addition, several studies reported the anti-elastase and anti- acetylcholinesterase activities of several plants belonging to the genus Callistemon and their metabolites23–25. This directs the search for other Callistemon species with potential towards these enzymes.
Therefore, the aim of the work is to identify the chemical constituents of C. subulatus essential oils collected in the four seasons of the year by GC-MS analysis to reveal their seasonal variation and to evaluate the ability of the highest yield oil (winter oil) to inhibit both elastase and AChE enzymes to unravel its potential as anti-ageing supplement. In addition, molecular docking studies for the major oil constituents were performed on these enzymes to support the observed results. It should be noted that the seasonal variation and anti-ageing evaluation of C. subulatus essential oil was carried out for the first time herein.
Materials and methods
Plant material and isolation of oil
Fresh leaves of Callistemon subulatus were harvested during the four seasons, in January, April, June and October 2021 (500 g each), from El-Orman Garden in Giza, Egypt. The plant was identified by Mrs. Treize Labib, the taxonomist at El-Orman Botanical Garden. The voucher specimen, PHG-P-CS-416, was placed in the Herbarium of Pharmacognosy Department, faculty of Pharmacy, Ain Shams University. Leaves collected in each season were hydro-distilled by a Clevenger apparatus for 6 h. The collected oils were then refrigerated and chemically evaluated by GC/MS analysis.
Gas Chromatography-Mass spectrometry (GC/MS) analysis
Analysis was performed by TRACE GC-Ultra gas chromatography, conjugated with thermal mass spectrometer (MS) detector (THERMO Scientific Corp., USA, and ISQ Single Quadrupole Mass Spectrometer). It was supplied with DB-5 column (30 m × 0.25 mm × 0.25 μm film thickness), a carrier gas was helium (flow rate of 1.0 mL/min). The injector temperature was 250 °C with split ratio of 1 to 15, about 0.2 μL of diluted oil samples (1: 10 in hexane) were injected. The oven temperature program was 80 °C for 2 min; then rising of 5.0 °C/min till 300 °C with hold for 5 min. Electron Ionisation (EI) MS has a spectral range of 20 to 500 m/z. The resulting peaks were assigned by matching retention indices (RI) with those found in the literature21,26–28 and online libraries such as NIST.
Evaluation of antiaging activities
Anti-elastase activity
The elastase inhibitor activity of the tested oil was screened using EnzChek® Elastase Assay Kit (E-12056), including N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone, as a standard elastase inhibitor. The assay was done according to the provided manual. The concentration of oil causing 50% elastase inhibition (IC50) was calculated from the graph correlating the concentration and % of enzyme inhibition.
Anti-cholinesterase activity
The evaluation of anticholinesterase activity of collected oil of C. subulatus had been done by Bio-vision AChE inhibitor screening kit (Catalog # K197-100). The kit contained an inhibitor control donepezil (20 µM), colorimetric substrate for the reaction; Acetylthiocholine iodide and (5,5′-dithio-bis(2-nitrobenzoic) acid (DTNB) for assessment of AChE activity. The graph correlating the oil concentration and the percentage of AChE inhibition gives IC50 value of the oil.
Molecular docking study
The major identified compounds by GC-MS analysis in the highest yield (winter) oil of C. subulatus leaves (eucalyptol, α-pinene and α-phellandrene) were docked into the active sites of elastase and acetylcholinestrase (AChE) enzymes. The software implicated was autodock Vina. Docking was done as per reported methodology29,30. The crystal structures of both enzymes co-crystallized with ligands were downloaded from the protein data bank with PDB-IDs as follows (6qeo for elastase and 7d9o for acetylcholinesterase). The compound structures were drawn using ChemDraw Ultra 8.0 and Amber12: EHT force field was applied for energy minimisation. Conformers of lowest energies are saved by the program. Docking validation was carried out by re-docking the co-crystallized ligands into the active sites of their respective enzymes. Following revealing of the active sites, the identified compounds of the oil were docked separately into them and their docking was analysed by Biovia Discovery Studio visualiser to show their binding interaction diagrams.
Result and discussion
Chemical composition of leaf essential oil and seasonal variation
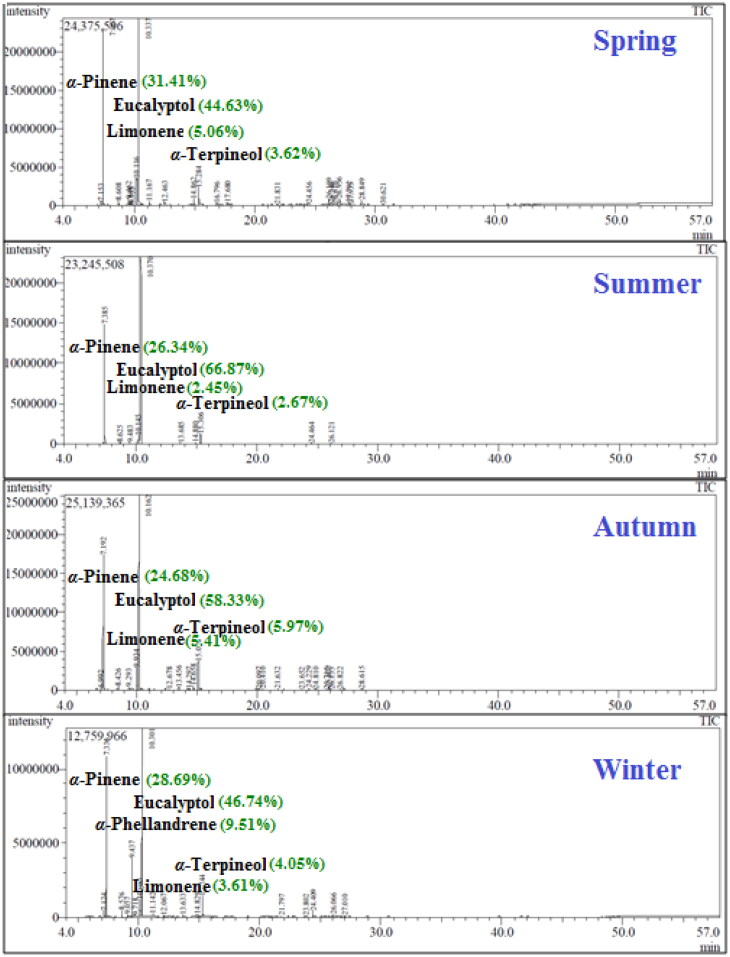
The GC/MS analysis showed the presence of 31 compounds in C. subulatus leaf essential oils, the spring oil contained the largest number of compounds; 23 compounds (98.96%), followed by autumn; 22 compounds (100%), then winter; 18 compounds (99.93%), and finally summer has only has 10 compounds (99.99%), so the highest chemical diversity appeared in spring and autumn. The chemical constituents of the oil and the yield in each season have been illustrated in (Table 1, Figure 1).
Table 1.
GC-MS analysis of C. subulatus leaf oils in the four seasons.
| No. | Compound name | Retention time | Molecular formula | RI exp | RI lit | Relative abundance (%) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | ||||||
| 1. | α-Thujene | 7.155 | C10H16 | 908 | 906 | 0.64 | – | 0.25 | 0.98 |
| 2. | α-Pinene | 7.365 | C10H16 | 917 | 917 | 31.41 | 26.34 | 24.68 | 28.69 |
| 3. | β-Pinene | 8.576 | C10H16 | 962 | 963 | 0.72 | 0.25 | 0.52 | 0.97 |
| 4. | β-Myrcene | 9.057 | C10H16 | 978 | 978 | – | – | – | 0.45 |
| 5. | α-Phellandrene | 9.460 | C10H16 | 993 | 994 | 1.03 | 0.32 | 0.64 | 9.51 |
| 6. | 2-Carene | 9.635 | C10H16 | 999 | 1000 | 0.10 | – | – | – |
| 7. | 3-Carene | 9.755 | C10H16 | 1003 | 1004 | 0.17 | – | – | 0.38 |
| 8. | Limonene | 10.115 | C10H16 | 1016 | 1018 | 5.06 | 2.45 | 5.41 | 3.61 |
| 9. | Eucalyptol | 10.335 | C10H18O | 1022 | 1022 | 44.63 | 66.87 | 58.33 | 46.74 |
| 10. | γ-Terpinene | 11.165 | C10H16 | 1049 | 1049 | 0.76 | – | – | 0.58 |
| 11. | α-Terpinolene | 12.067 | C10H16 | 1090 | 1085 | 0.74 | – | – | 0.34 |
| 12. | Linalool | 12.678 | C10H18O | 1113 | 1113 | – | – | 0.14 | – |
| 13. | Pinocarveol | 13.633 | C10H16O | 1138 | 1138 | – | 0.23 | 0.45 | 0.42 |
| 14. | Borneol | 14.297 | C10H18O | 1166 | 1166 | – | – | 0.16 | – |
| 15. | Terpinen-4-ol | 14.860 | C10H18O | 1168 | 1168 | 1.27 | 0.43 | 0.89 | 0.60 |
| 16. | α-Terpineol | 15.285 | C10H18O | 1181 | 1181 | 3.62 | 2.67 | 5.97 | 4.05 |
| 17. | β-Citral | 16.795 | C10H16O | 1232 | 1232 | 0.63 | – | – | – |
| 18. | α-Citral | 17.680 | C10H16O | 1263 | 1267 | 1.27 | – | – | – |
| 19. | Eugenol | 20.097 | C10H12O2 | 1366 | 1366 | – | – | 0.20 | – |
| 20. | Geranyl acetate | 20.410 | C12H20O2 | 1372 | 1377 | – | – | 0.15 | – |
| 21. | Caryophyllene | 21.797 | C15H24 | 1409 | 1409 | 0.27 | – | 0.18 | 0.26 |
| 22. | Ledene | 23.652 | C15H24 | 1500 | 1495 | – | – | 0.12 | 0.26 |
| 23. | δ-Cadinene | 24.455 | C15H24 | 1512 | 1515 | 0.56 | 0.22 | 0.34 | 1.20 |
| 24. | flavesone | 24.810 | C14H20O4 | 1547 | 1546 | – | – | 0.17 | – |
| 25. | Spathulenol | 26.066 | C15H24O | 1577 | 1577 | 1.39 | – | 0.14 | 0.50 |
| 26. | Viridiflorol | 26.121 | C15H26O | 1586 | 1590 | 0.61 | 0.21 | 0.57 | – |
| 27. | Guaiol | 26.610 | C15H26O | 1596 | 1600 | 0.44 | – | 0.18 | – |
| 28. | Caryophyllene oxide | 27.010 | C15H24O | 1613 | 1615 | 2.34 | – | 0.20 | 0.39 |
| 29. | Guaia-3,9-diene-11-ol | 27.790 | C15H24O | 1647 | 1649 | 0.57 | – | – | – |
| 30. | Neointermedeol | 27.935 | C15H26O | 1653 | 1656 | 0.39 | – | – | – |
| 31. | Germacrone | 28.850 | C15H22O | 1693 | 1696 | 1.40 | – | 0.32 | – |
| Monoterpene hydrocarbons | 40.66 | 29.36 | 31.5 | 45.51 | |||||
| Oxygenated monoterpenes | 51.72 | 70.2 | 66.29 | 51.81 | |||||
| Sesquiterpene hydrocarbons | 0.83 | 0.22 | 0.64 | 1.72 | |||||
| Oxygenated Sesquiterpene | 7.14 | 0.21 | 1.58 | 0.89 | |||||
| Total identified (%) | 98.96 | 99.99 | 100 | 99.93 | |||||
| Yield (mL/100 gm) | 0.17 | 0.26 | 0.34 | 0.53 | |||||
The major oil components are written in bold.
Figure 1.
GC chromatograms of C. subulatus leaves essential oils showing their seasonal variation.
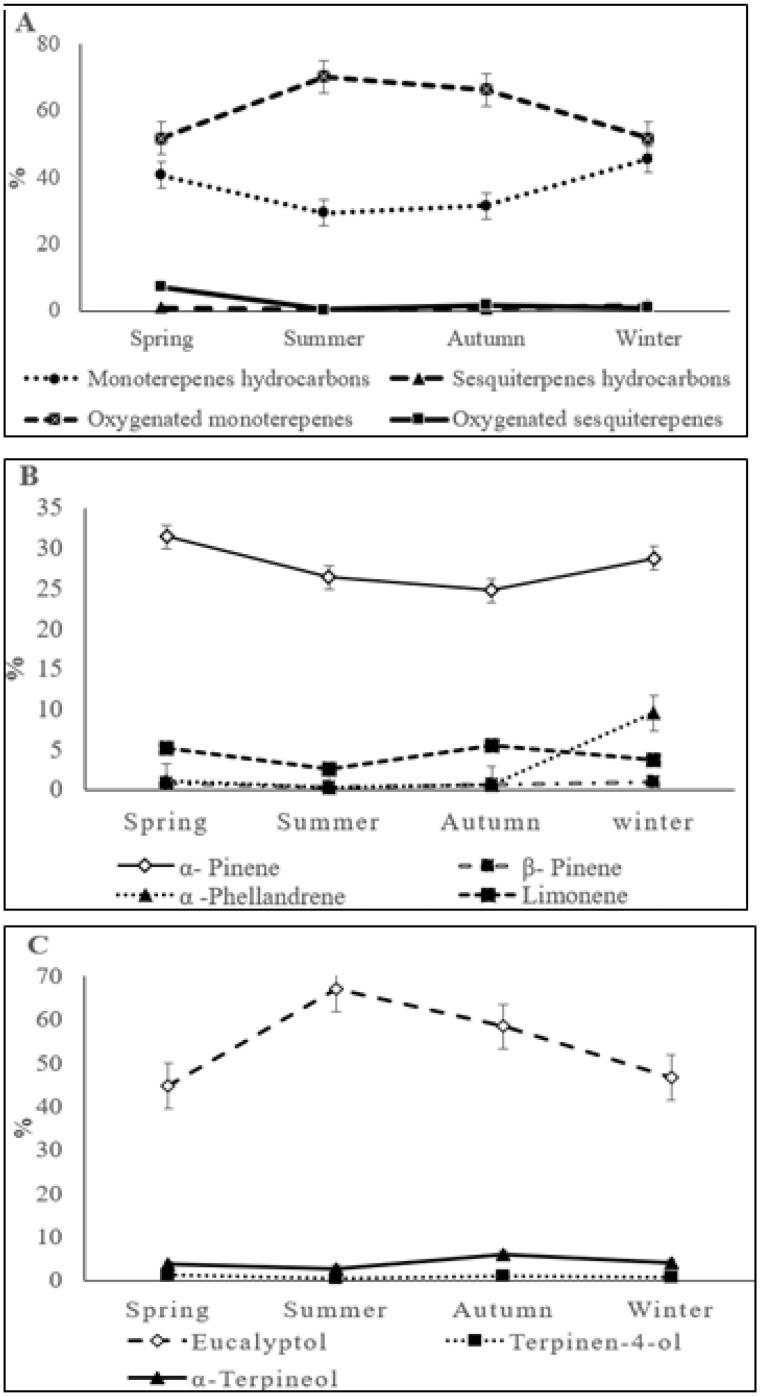
Of the 31 compounds identified, 20 are monoterpenes and 11 are sesquiterpenes, the monoterpenes also found in higher relative abundance than sesquiterpenes. In the summer oil, the monoterpenes (99.56%) are up to 230 times of the sesquiterpenes (0.43%), but this ratio in the spring oil decreased to only 11 times since the abundance of sesquiterpenes in this oil reached 8% (Figure 2).
Figure 2.
Seasonal variation in the major compounds of C. subulatus leaves oil, showing seasonal variation of the total classes of compounds (A), the four major monoterpenes hydrocarbons (B) and the three major oxygenated monoterpenes (C).
The main components of the plant essential oils are terpene hydrocarbons and oxygenated terpenes, terpenoids31. In C. subulatus essential oil, the highest content of monoterpene hydrocarbons (MTHC) was in winter (45.51%) while it was the lowest in the summer (29.36%), it has been previously explained that these MTHC compounds are light, small and thermolabile so they are more fixed to leaves in less hot and more humid climates, decreasing their volatilisation32. The identified oxygenated terpenes in C. subulatus oil were 18 compounds, which are monoterpenoids and sesquiterpenoids. Ten monoterpenoids identified in C. subulatus oil that were equal to the number of monoterpene hydrocarbons, the number of identified sesquiterpenoids was 8 while only 3 sesquiterpenes hydrocarbons were found. The relative abundances of monoterpenoids in all seasons was more than that of monoterpene hydrocarbons, they reached more than doubled in summer and autumn. While the sesquiterpenoids were more abundant than sesquiterpenes hydrocarbons in both spring and autumn, they were less abundant in winter and about the same in summer.
The major compound in all-seasons oils was eucalyptol (1,8 cineol); oxygenated monoterpene, and in summer it exceeds 70% of the entire oil content, this is consistent with what has been published that 1,8 cineol has been the main terpene in most of chemical analysed Callistemon oils33. Second major compound was α-pinene, a monoterpene hydrocarbon, which accounts for about a third of spring and winter oils. Then followed by α-phellandrene (9.51%) in winter; α-terpineol in autumn and summer (5.97 and 2.67%, respectively); and limonene in spring (5.6%).
Our results regarding the chemical composition of C. subulatus came in agreement with previously published data. Brophy et al. reported that eucalyptol was the most abundant terpene identified in the leaves of Australian C. subualtus34, representing 61.3%. Also, it was reported to be the major constituents of Egyptian chemotype (48.97%)11. α-Pinene (31.59%), α-terpineol (7.84%) and limonene (5.27%) were reported in C. subulatus oil, in agreement with the autumn and summer essential oils of the current study. However, α-pinene (47.06%) was the major in the C. subulatus oil in another study, then eucalyptol (16.13%) and α-terpineol (15.27%)13.
Assessment of antiaging activities
Anti-elastase activity
Elastin is a skin protein that plays a vital role in maintaining the healthy appearance and integrity of the skin15. Activation of elastase by ageing or exposure to UV rays and free radicals leads to the destruction of cutaneous elastin and thus loss of skin elasticity. The C. subulatus leaf oil of winter showed an inhibition to elastase activity with IC50 value of 1.05 ± 0.05 µg/mL, compared to the standard N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone (IC50 = 0.55 ± 0.03 µg/mL). The essential oil of Illicium anisatum containing eucalyptol as a major component (36.7%) was able to inhibit elastase (IC50 = 1.79 mg/mL)35. Eucalyptol was one of the most abundant terpenes (11.82%) in Mentha viridis essential oil that showed elastase inhibition by IC50 = 114.24 ± 1.22 µg/mL36.
Anti-cholinesterase activity
The inhibition of AChE was introduced as therapeutic strategy for Alzheimer management via prevention of acetylcholine degradation causing enhancement in cholinergic transmission37. The winter leaf oil showed AChE inhibitory activity with IC50 value of 0.208 ± 0.011 µg/mL, this compared to the standard donepezil (IC50 = 0.045 ± 0.002 µg/mL). Other Callistemon species showed potential for neuroprotection through AChE inhibitory activity, as the essential oil of C. citrinus aerial part, containing eucalyptol (55.40%) as the major component, showed a strong AChE inhibitory with an IC50 value of 6.335 μg/mL, slightly lower than that of galanthamine (IC50 = 6.652 µg/mL)25. Commercially available 1,8-cineole and α-pinene showed AChE inhibitory activities with IC50 values of 0.015 and 0.022 mg/mL, respectively that were 0.017–0.025 fold of galantamine38. The major components of Salvia lavandulaefolia essential oil: eucalyptol and α-pinene, showed dose-dependent inhibition of AChE with IC50 values of 0.05, and 0.06 mg/mL, respectively39.
Molecular docking study
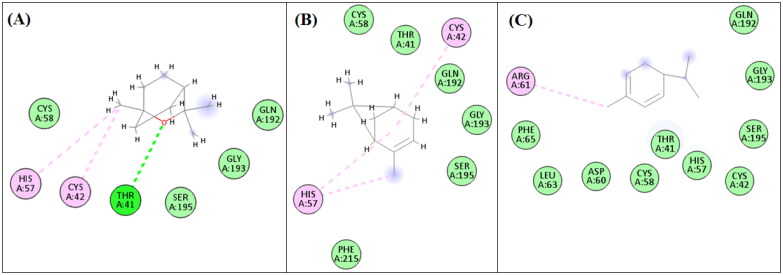
Molecular docking has been indispensable in recent researches, to display binding affinities of bioactive molecules in the target enzymes and to validate their proposed mechanism of bioactivities40,41. As shown in Figure 3, eucalyptol revealed hydrogen bonding with ThR A:41 amino acid moiety present in the active site of elastase enzyme, in addition to two alkyl and Pi-alkyl interactions with HIS A:57 and CYS A:42 as well as van der Waals interactions with CYS A:58, SER A:195, GLY A:193 and GLN A:192 amino acids. Similarly, α-pinene revealed three alkyl and Pi-alkyl interactions with HIS A:57 and CYS A:42 amino acids and numerous van der Waals interactions. This was also observed to a lower extent in α-phellandrene binding interactions.
Figure 3.
2D-Binding diagrams of eucalyptol (A), α-pinene (B) and α-phellandrene (C) on the active site of elastase enzyme.
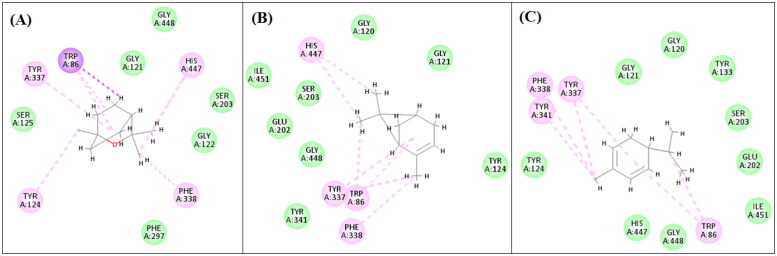
Regarding acetylcholinesterase enzyme (Figure 4), eucalyptol showed one Pi-Sigma interaction with TRP A:86, six Pi-Alkyl interactions with HIS A: 447, TRP A: 86, TYR A:337, TYR A:124, PHE A:338, and van der Waals interactions. α-Pinene showed seven Pi-alkyl interactions with HIS A:447, TYR A:337, TRP A:86, PHE A:338, in addition to van der Waals interactions. α-Phellandrene showed many van der Waals interactions and six Pi-alkyl interactions with TYR A:337, TYR A:341, PHE A:338, and TRP A:86 amino acid moieties.
Figure 4.
2D-Binding diagrams of eucalyptol (A), α-pinene (B) and α-phellandrene (C) on the active site of acetylcholinestrase enzyme.
Eugenol showed the best results revealing good binding affinities to both enzymes and lowest binding energies as demonstrated in Table 2.
Table 2.
Docking binding energies of major identified compounds of C. subulatus winter oil with elastase and acetylcholinesterase enzymes.
| Compound | Binding energy (Kcal/mol) |
|
|---|---|---|
| Elastase | Acetylcholinesterase | |
| Eucalyptol | −8.6 | −9.7 |
| α-Pinene | −7.7 | −7.9 |
| α-Phellandrene | −7.1 | −7.3 |
Conclusions
The essential oil of C. subulatus leaves is rich in monoterpenes, especially monoterpenoids. On the contrary, sesquiterpenes are very low in the oil and their concentration varies according to the harvest season, being much lower in summer oil while reaching their highest concentration in spring oil. The essential oil of C. subulatus has shown antiaging prospects, so further research is needed to discover more specifically the constituent responsible for this activity and to be applied in skin care formulations and as adjuvant in Alzheimer therapy, enhancing memory and concentration.
Funding Statement
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups Project under grant number [RGP.2/311/44]. The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number [PNURSP2023R25], Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Younis T, Jabeen F, Hussain A, Rasool B, Raza Ishaq A, Nawaz A, El-Nashar HAS, El-Shazly M.. Antioxidant and pulmonary protective potential of Fraxinus xanthoxyloides Bark Extract against CCl(4) – induced toxicity in rats. Chem Biodivers. 2023;20(3):e202200755. doi: 10.1002/cbdv.202200755. [DOI] [PubMed] [Google Scholar]
- 2.Ishaq AR, El-Nashar HAS, Younis T, Mangat MA, Shahzadi M, Ul Haq AS, El-Shazly M.. Genus Lupinus (Fabaceae): a review of ethnobotanical, phytochemical and biological studies. J Pharm Pharmacol. 2022;74(12):1700–1717. doi: 10.1093/jpp/rgac058. [DOI] [PubMed] [Google Scholar]
- 3.Abdallah SH, Mostafa NM, Mohamed MAEH, Nada AS, Singab ANB.. UPLC-ESI-MS/MS profiling and hepatoprotective activities of Stevia leaves extract, butanol fraction and stevioside against radiation-induced toxicity in rats. Nat Prod Res. 2022;36(21):5619–5625. doi: 10.1080/14786419.2021.2015594. [DOI] [PubMed] [Google Scholar]
- 4.Mostafa NM, Edmond MP, El-Shazly M, Fahmy HA, Sherif NH, Singab ANB.. Phytoconstituents and renoprotective effect of Polyalthia longifolia leaves extract on radiation-induced nephritis in rats via TGF-β/smad pathway. Nat Prod Res. 2022;36(16):4187–4192. doi: 10.1080/14786419.2021.1961252. [DOI] [PubMed] [Google Scholar]
- 5.El-Nashar HA, Mostafa NM, El-Shazly M, et al. The role of plant-derived compounds in managing diabetes mellitus: a review of literature from 2014 To 2019. Curr Med Chem. 2021;28(23):4694–4730. doi: 10.2174/0929867328999201123194510. [DOI] [PubMed] [Google Scholar]
- 6.Edmond MP, Mostafa NM, El-Shazly M, Singab ANB.. Two clerodane diterpenes isolated from Polyalthia longifolia leaves: comparative structural features, anti-histaminic and anti-Helicobacter pylori activities. Nat Prod Res. 2021;35(23):5282–5286. doi: 10.1080/14786419.2020.1753048. [DOI] [PubMed] [Google Scholar]
- 7.Sowndhararajan K, Deepa P, Kim S.. A review of the chemical composition and biological activities of Callistemon lanceolatus (Sm.) Sweet. J App Pharm Sci. 2021;11(12):65– 73. doi: 10.7324/JAPS.2021.1101204. [DOI] [Google Scholar]
- 8.Gilbert Y, Dieudonné N, Ngnodandi Francois N.. InVivo anthelmintic activity of callistemon rigidus ethanolic extract on haemonchus contortus in goats. Afr J Biol Sci. 2020;02(03):103–112. doi: 10.33472/AFJBS.2.3.2020.103-112. [DOI] [Google Scholar]
- 9.Radulović NS, Randjelović PJ, Stojanović NM, Cakić ND, Bogdanović GA, Živanović AV.. Aboriginal bush foods: a major phloroglucinol from Crimson Bottlebrush flowers (Callistemon citrinus, Myrtaceae) displays strong antinociceptive and anti-inflammatory activity. Food Res Int. 2015;77:280–289. doi: 10.1016/j.foodres.2015.02.023. [DOI] [Google Scholar]
- 10.Zubair M, Hassan S, Rizwan K, Rasool N, Riaz M, Zia-Ul-Haq M, De Feo V.. Antioxidant potential and oil composition of Callistemon viminalis leaves. Sci World J. 2013;2013:1–8. doi: 10.1155/2013/489071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim N, Moussa AY.. Comparative metabolite profiling of Callistemon macropunctatus and Callistemon subulatus volatiles from different geographical origins. Ind Crops Prod. 2020;147:112222. doi: 10.1016/j.indcrop.2020.112222. [DOI] [Google Scholar]
- 12.Mady MS, Elsayed HE, El-Sayed EK, Hussein AA, Ebrahim HY, Moharram FA.. Polyphenolic profile and ethno pharmacological activities of Callistemon subulatus (cheel) craven leaves cultivated in Egypt. J Ethnopharmacol. 2022;284:114698. doi: 10.1016/j.jep.2021.114698. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim R, Ibrahim H, Moharram F.. Essential oil composition of two plants belonging family Myrtaceae grown in Egypt prepared by different methods and their antibacterial activity. J Pharm Sci Res. 2020;12(1):133–137. [Google Scholar]
- 14.Maneenet J, Tawila AM, Omar AM, Phan ND, Ojima C, Kuroda M, Sato M, Mizoguchi M, Takahashi I, Awale S, et al. Chemical constituents of Callistemon subulatus and their anti-pancreatic cancer activity against human PANC-1 cell line. Plants. 2022;11(19):2466. doi: 10.3390/plants11192466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Nashar HAS, El-Labbad EM, Al-Azzawi MA, Ashmawy NS.. A new xanthone glycoside from Mangifera indica L.: physicochemical properties and in vitro anti-skin aging activities. Molecules. 2022;27(9):2609. doi: 10.3390/molecules27092609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad S, Saleem M, Riaz N, Lee YS, Diri R, Noor A, Almasri D, Bagalagel A, Elsebai MF.. The natural polypeptides as significant elastase inhibitors. Front Pharmacol. 2020;11:688. doi: 10.3389/fphar.2020.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Younis MM, Ayoub IM, Mostafa NM, El Hassab MA, Eldehna WM, Al-Rashood ST, Eldahshan OA.. GC/MS profiling, anti-collagenase, anti-elastase, anti-tyrosinase and anti-hyaluronidase activities of a Stenocarpus sinuatus leaves extract. Plants. 2022;11(7):918. doi: 10.3390/plants11070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Nashar HAS, Abbas H, Zewail M, Noureldin MH, Ali MM, Shamaa MM, Khattab MA, Ibrahim N.. Neuroprotective effect of artichoke-based nanoformulation in sporadic Alzheimer’s Disease Mouse Model: focus on antioxidant, anti-inflammatory, and amyloidogenic pathways. Pharmaceuticals. 2022;15(10):1202. doi: 10.3390/ph15101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Nashar HAS, Adel M, El-Shazly M, Yahia IS, El Sheshtawy HS, Almalki AA, Ibrahim N.. Chemical composition, antiaging activities and molecular docking studies of essential oils from Acca sellowiana (Feijoa). Chem Biodivers. 2022;19(9):e202200272. doi: 10.1002/cbdv.202200272. [DOI] [PubMed] [Google Scholar]
- 20.Agatonovic-Kustrin S, Chan CKY, Gegechkori V, Morton DW.. Models for skin and brain penetration of major components from essential oils used in aromatherapy for dementia patients. J Biomol Struct Dyn. 2020;38(8):2402–2411. doi: 10.1080/07391102.2019.1633408. [DOI] [PubMed] [Google Scholar]
- 21.El-Nashar HAS, Eldehna WM, Al-Rashood ST, Alharbi A, Eskandrani RO, Aly SH.. GC/MS analysis of essential oil and enzyme inhibitory activities of Syzygium cumini (Pamposia) Grown in Egypt: chemical characterization and molecular docking studies. Molecules. 2021;26(22):6984. doi: 10.3390/molecules26226984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelghffar EAR, Mostafa NM, El-Nashar HAS, Eldahshan OA, Singab ANB.. Chilean pepper (Schinus polygamus) ameliorates the adverse effects of hyperglycaemia/dyslipidaemia in high fat diet/streptozotocin-induced type 2 diabetic rat model. Ind Crops Prod. 2022;183:114953. doi: 10.1016/j.indcrop.2022.114953. [DOI] [Google Scholar]
- 23.Adesina AB, Josephine OO.. Inhibitory effects of the volatile oils of Callistemon citrinus (Curtis) Skeels and Eucalyptus citriodora Hook (Myrtaceae) on the acetylcholine induced contraction of isolated rat ileum. Pak J Pharm Sci. 2012;25(2):435–439. [PubMed] [Google Scholar]
- 24.Kim JH, Byun JC, Bandi AKR, et al. Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J Med Plants Res. 2009;3:914–920. [Google Scholar]
- 25.Gogoi R, Begum T, Sarma N, Kumar Pandey S, Lal M.. Chemical composition of Callistemon citrinus (Curtis) Skeels aerial part essential oil and its pharmacological applications, neurodegenerative inhibitory, and genotoxic efficiencies. J Food Biochem. 2021; 45(7):e13767. doi: 10.1111/jfbc.13767. [DOI] [PubMed] [Google Scholar]
- 26.Elhawary EA, Mostafa NM, Labib RM, Singab AN.. Metabolomic profiles of essential oils from selected Rosa varieties and their Antimicrobial activities. Plants. 2021;10(8):1721. doi: 10.3390/plants10081721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayoub N, Singab AN, Mostafa N, Schultze W.. Volatile constituents of leaves of Ficus carica Linn. grown in Egypt. J Essent Oil Bear Plants. 2010;13(3):316–321. doi: 10.1080/0972060X.2010.10643827. [DOI] [Google Scholar]
- 28.El-Kersh DM, Mostafa NM, Fayez S, Al-Warhi T, Abourehab MAS, Eldehna WM, Salem MA.. GC-MS metabolites profiling of anethole-rich oils by different extraction techniques: antioxidant, cytotoxicity and in-silico enzymes inhibitory insights. J Enzyme Inhib Med Chem. 2022;37(1):1974–1986. doi: 10.1080/14756366.2022.2097445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostafa NM, Mostafa AM, Ashour ML, Elhady SS.. Neuroprotective effects of Black Pepper cold-pressed oil on scopolamine-induced oxidative stress and memory impairment in rats. Antioxidants (Basel). 2021;10(12):1993. doi: 10.3390/antiox10121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mostafa NM. β-Amyrin rich Bombax ceiba leaf extract with potential neuroprotective activity against scopolamine-induced memory impairment in rats. RecNatProd. 2018;12(5):480–492. doi: 10.25135/rnp.47.17.10.062. [DOI] [Google Scholar]
- 31.Moussa AY, Mostafa NM, Singab ANB.. Pulchranin A: first report of isolation from an endophytic fungus and its inhibitory activity on cyclin dependent kinases. Nat Prod Res. 2020;34(19):2715–2722. doi: 10.1080/14786419.2019.1585846. [DOI] [PubMed] [Google Scholar]
- 32.Siebert DA, Tenfen A, Yamanaka CN, de Cordova CMM, Scharf DR, Simionatto EL, Alberton MD.. Evaluation of seasonal chemical composition, antibacterial, antioxidant and anticholinesterase activity of essential oil from Eugenia brasiliensis Lam. Nat Prod Res. 2015;29(3):289–292. doi: 10.1080/14786419.2014.958736. [DOI] [PubMed] [Google Scholar]
- 33.Abdelhady MI, Aly HA.. Antioxidant antimicrobial activities of Callistemon comboynensis essential oil. Free Radicals Antioxid. 2012;2(1):37–41. doi: 10.5530/ax.2012.2.8. [DOI] [Google Scholar]
- 34.Brophy JJ, Goldsack RJ, Forster PI, Craven LA, Lepschi BJ.. The leaf essential oils of the australian members of the genus callistemon (Myrtaceae). J Essent Oil Res. 1998;10(6):595–606. doi: 10.1080/10412905.1998.9700986. [DOI] [Google Scholar]
- 35.Min H-J, Kim C-S, Hyun H-J, Bae Y-S.. Essential oil analysis of Illicium anistum L. extracts. J Korean Wood Sci Technol. 2017;45(6):682–688. doi: 10.5658/WOOD.2017.45.6.682. [DOI] [Google Scholar]
- 36.Bouyahya A, Lagrouh F, El Omari N, Bourais I, El Jemli M, Marmouzi I, Salhi N, Faouzi MEA, Belmehdi O, Dakka N, et al. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal Agric Biotechnol. 2020;23:101471. doi: 10.1016/j.bcab.2019.101471. [DOI] [Google Scholar]
- 37.Marucci G, Buccioni M, Ben DD, Lambertucci C, Volpini R, Amenta F.. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology. 2021;190:108352. doi: 10.1016/j.neuropharm.2020.108352. [DOI] [PubMed] [Google Scholar]
- 38.Dohi S, Terasaki M, Makino M.. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J Agric Food Chem. 2009;57(10):4313–4318. doi: 10.1021/jf804013j. [DOI] [PubMed] [Google Scholar]
- 39.Savelev S, Okello E, Perry NSL, Wilkins RM, Perry EK.. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol Biochem Behav. 2003;75(3):661–668. doi: 10.1016/s0091-3057(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 40.El-Nashar HAS, Sayed AM, El-Sherief HAM, et al. Metabolomic profile, anti-trypanosomal potential and molecular docking studies of Thunbergia grandifolia. J Enzyme Inhib Med Chem. 2023;38(1):2199950. doi: 10.1080/14756366.2023.2199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shady NH, Mostafa NM, Fayez S, Abdel-Rahman IM, Maher SA, Zayed A, Saber EA, Khowdiary MM, Elrehany MA, Alzubaidi MA, et al. Mechanistic wound healing and antioxidant potential of Moringa oleifera seeds extract supported by metabolic profiling, in silico network design, molecular docking, and in vivo studies. Antioxidants. 2022;11(9):1743. doi: 10.3390/antiox11091743. [DOI] [PMC free article] [PubMed] [Google Scholar]