Abstract
Satureja macrostema is a plant that is located in various regions of Mexico and is used in a traditional way against illness. Essential oils (EOs) were obtained from leaves Satureja macrostema and the chemical composition was evaluated by gas chromatography–mass spectrometry (GC-MS). The antioxidant effect of the oil was assayed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and by Trolox Equivalent Antioxidant Capacity (TEAC). In vitro antibacterial activity against Escherichia coli and Staphylococcus aureus was determined using a broth microdilution assay and thin layer chromatography–direct bioautography (TLC-DB) to identify active antibacterial compounds. The EOs analysis showed 21 compounds, 99% terpenes, and 96% oxygenated monoterpenes, with trans-piperitone epoxide (46%), cis-piperitone epoxide (22%), and piperitenone oxide (11%) as more abundant compounds. Likewise, S. macrostema EOs showed an antioxidant activity of DPPH = 82%, with 50% free radical scavenging (IC50) = 7 mg/mL and TEAC = 0.005, an antibacterial effect against E. coli of 73% inhibition, and 81% over S. aureus at dose of 100 µL of undiluted crude oil. The TLC-DB assay showed that the most active compounds were derived from piperitone. The comparison with other studies on S. macrostema shows variability in the compounds and their abundances, which can be attributed to climatic factors and the maturity of plants with similar antioxidant and antibacterial activities.
Keywords: piperitone derivatives, direct bioautography, TEAC, Lamiaceae, chemotypes
1. Introduction
The genus Satureja (Family Lamiaceae, tribe Mentheae) contains about 200 species, with a presence in the midwest of the European Mediterranean, West Asia, North Africa, and South America [1]. Satureja macrostema (Moc. and Sessé ex Benth.) Briq. (Lamiaceae), commonly called nurite or nurhitini (purepecha from Michoacan), poleo, tea bush, or tuché (Mixe from Oaxaca state), has been used since pre-Hispanic times in traditional herbalism. It is a Mexican medicinal plant of great importance in various mountain areas with temperate or semi-cold climates in the western part of the country, such as Michoacán, Oaxaca, Jalisco, and San Luis Potosi [2]. The region includes mountainous, volcanic, and forested areas of high biodiversity and floristic composition, with various plants with nutritional or medicinal properties occurring in the so-called “purepecha plateau” in Michoacan or in the central valleys of Oaxaca, where S. macrostema grows naturally and is discontinuously distributed below the main plant canopy (pine and oak forests) as part of the undergrowth [3]. It is a shrub with the scent of mint when squeezed; it stands 1 to 2 m high with erect stems and arched branches. The leaves are 1–4 cm long by 0.6–1.5 cm wide, with sharp apices and serrated and rounded bases. The flowers are solitary, corolla red or orange, and 2–3.5 cm length. The plant flowers from July to October and bears fruit from September to November [2]. This species is also known by the synonyms Melissa macrostema Moc. and Sessé ex Benth., Calamintha macrostema (Moc. and Sessé ex Benth.) Benth., and Clinopodium macrostemun (Moc. and Sessé ex Benth.) [4]. The leaves, flowers, and stems, and especially the dried leaves, of S. macrostema are used in traditional medicine as an infusion, called nurité tea, which is said to be effective against muscle aches, nausea, diarrhea, infectious diseases, and even against infertility and hangovers [5]. Phytochemical studies have revealed the presence of phenolic compounds, sterols, and volatile essential oils such as carvacrol and thymol in Satureja [6]. A characteristic of this genus of aromatic plants is their content of essential oils (EOs) (>0.5%). Terpenes are the main component, as in medicinal plants, and have antioxidant properties [7], but with different chemical compositions among the subspecies [8]. There are studies in both extracts and essential oils of the various species of Satureja which evaluate, above all, the antioxidant activity [9,10]. Likewise, the EOs are affected by extraction methods such as conventional hydrodistillation (HD), steam distillation (SD), and solvent extraction, as well as the modern techniques of supercritical carbon dioxide (scCO2) and microwave-assisted extraction (MAE). The commonly used techniques are HD, SD, and MAE due to costs, system simplicity, and extraction durations, but these have the disadvantages of low yield, low quality, and high temperature [11]. To obtain the EOs in this work, we used HD due to its simplicity and frequent use, while recognizing the advantages of the most efficient modern techniques. There are few studies on S. macrostema essential oils (S. macrostema EOs) and their biological activity, so the objective of this study was to determine the chemical composition of S. macrostema collected in the wild under environmental conditions on the Purepecha plateau. We also evaluated the effect on E. coli and S. aureus, determining the chemical compounds responsible for this microbicidal activity.
2. Results
2.1. Chemical Characterization of Essential Oils
A total of 21 compounds were detected in the EOs according to the essential oil MS library, and these are presented in Table 1. The table consists of eight columns, and the abbreviations are described in the footer of the table. The seventh column (% relative area) indicates the relative concentration of the compounds. The last column (S.D.) consists of the standard deviation of three replicates. The components are listed in order of elution. The nomenclature is in accordance with NIST (National Institute of Standards and Technology) [12]. The identification methods of the compounds were MS library matching [12,13] and linear retention indices (LRI) [13]. The identified compounds were also compared for their coincidence with the IR of bibliographic reports [14,15,16]. The percentage of identified compounds was 99% (1% unidentified). The compounds with the highest presence were the oxygenated monoterpenes derived from trans-piperitone epoxide ketones (C10H16O2) (46%), cis-piperitone epoxide (22%), piperitone oxide (11%), 3-octanol acetate (6%), linalool (5%), pulegone (3%), and menthone (3%).
Table 1.
Chemical composition of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. essential oil.
| No. | Compound | LRI (Calculated) |
LRI (Literature) |
CAS # | Retention Time (Min) | % Relative Area | S.D. |
|---|---|---|---|---|---|---|---|
| 1 | 2E-hexenal | 845 | 846 | 6728-26-3 | 4.2 | 0.08 | ±0.0 |
| 2 | α-pinene | 928 | 932 | 99-83-2 | 6.3 | 0.1 | ±0.0 |
| 3 | β-thujene | 967 | 968 | 28634-89-1 | 7.8 | 1 | ±0.0 |
| 4 | β-pinene | 972 | 974 | 127-91-3 | 8.0 | 0.3 | ±1.0 |
| 5 | 3-octanol | 994 | 988 | 589-98-0 | 8.8 | 0.3 | ±0.1 |
| 6 | limonene | 1021 | 1024 | 138-86-3 | 10.6 | 0.4 | ±0.1 |
| 7 | linalool | 1094 | 1095 | 78-70-6 | 16.1 | 5 | ±1.0 |
| 8 | 1-octen-3-yl-acetate | 1105 | 1110 | 2442-10-6 | 17.1 | 0.2 | ±0.0 |
| 9 | 3-octanol, acetate | 1116 | 1120 | 4864-61-3 | 18.4 | 6 | ±0.2 |
| 10 | menthone | 1145 | 1148 | 89-80-5 | 21.9 | 3 | ±0.0 |
| 11 | iso-menthone | 1154 | 1150 | 491-07-6 | 22.9 | 0.2 | ±0.1 |
| 12 | neoiso-menthol | 1160 | 1184 | 491-02-1 | 23.6 | 0.1 | ±0.1 |
| 13 | pulegone | 1226 | 1233 | 89-82-7 | 31.7 | 3 | ±0.4 |
| 14 | cis-piperitone epoxide | 1240 | 1250 | 4713-37-5 | 33.4 | 22 | ±1.4 |
| 15 | trans-piperitone epoxide | 1243 | 1252 | 57130-28-6 | 33.7 | 46 | ±4.2 |
| 16 | acetic acid, 2-phenylethyl ester | 1246 | 1254 | 103-45-7 | 34.1 | 1 | ±0.0 |
| 17 | piperitenone oxide | 1360 | 1366 | 35178-55-3 | 47 | 11 | ±1.1 |
| 18 | caryophyllene | 1404 | 1417 | 87-44-5 | 50.5 | 1 | ±0.1 |
| 19 | bicyclogermacrene | 1486 | 1500 | 24703-35-3 | 54.0 | 0.2 | ±0.0 |
| 20 | spathulenol | 1568 | 1577 | 6750-60-3 | 56.8 | 0.3 | ±0.1 |
| 21 | cyclocolorenone | 1747 | 1759 | 489-45-2 | 61.5 | 1 | ±0.5 |
| Not identified | 0.7 | ||||||
| Oxygenated Hydrocarbons | 0.3 | ||||||
| Monoterpenes | 1 | ||||||
| Oxygenated monoterpenes | 96 | ||||||
| Sesquiterpenes | 1 | ||||||
| Oxygenated sesquiterpenes | 1 | ||||||
| Total | 100 |
Note: Components are listed in order of elution, and nomenclature is in accordance with NIST (National Institute of Standards and Technology). LRI (Calculated): The calculated linear retention index was obtained by comparison to an alkane C7–C20 standard mixture analyzed in the same conditions as the samples on a non-polar column. LRI (Literature): linear retention index in literature. CAS #: registry number assigned by the Chemical Abstracts Service. S.D.: standard deviation.
2.2. DPPH and TEAC Free Radical Scavenging Assay
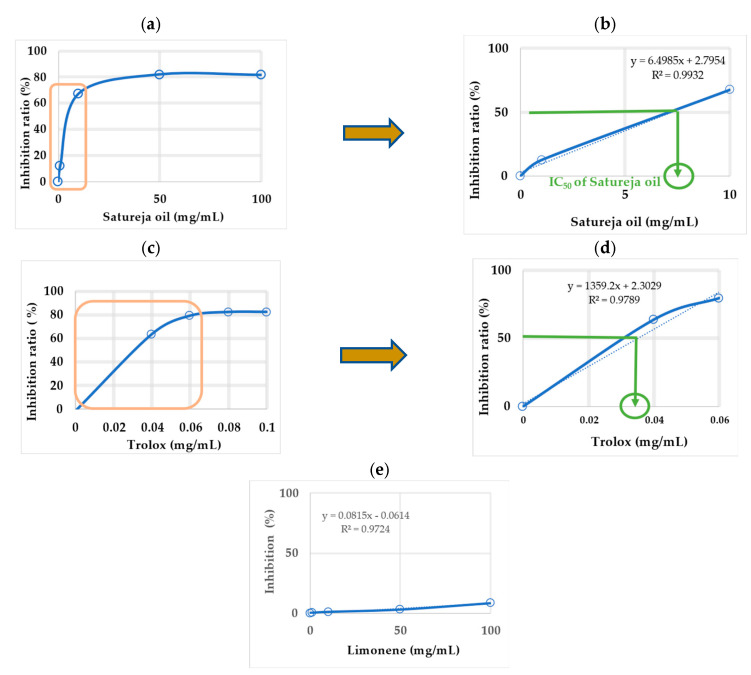
Table 2 shows the antioxidant activity of S. macrostema essential oils, with limonene as a control (since it is a component of essential oils with antioxidant activity) and Trolox as a reference. Limonene (an antioxidant monoterpene) and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, a compound with strong antioxidant activity) are included as references. In addition, the data of Table 2 were plotted and shown in Figure 1 before the zone of 50% inhibition was located. The data from Table 2 were plotted as shown in Figure 1a,c,d. Next in Figure 1 graphs a and b, the straightest area in which 50% of the initialization is found (orange box) was located to replot this area as shown in Figure 1b,d and then obtain the equation of the line y the half-maximal inhibitory concentration (IC50). These were as follows: IC50 S. macrostema EOs (y = 6.4985x + 2.7954, R2 = 0.9932) = 7.3 mg/mL; IC50 limonene (y = 0.0815x − 0.0614, R2 = 0.9724) = 614 mg/mL; IC50 Trolox (y = 1359.2x + 2.3029, R2 = 0.98) = 0.03 mg/mL. The DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) value was expressed in Trolox equivalents (TEAC = IC50Trolox (mg/mL)/IC50 sample (mg/mL)): TEAC S. macrostema EOs = 0.005 y TEAC limonene = 0.00006. High TEAC (Trolox Equivalent Antioxidant Capacity) values signify higher DPPH free radical scavenging or scavenging activity.
Table 2.
In vitro antioxidant activity of essential oils of Satureja macrostema. (Moc. and Sessé ex Benth.) Briq.
| mg/mL | |||
|---|---|---|---|
| Essential Oil | Limonene | Trolox | |
| 0.00 | nd | nd | nd |
| 0.04 | nd | nd | 51 ± 2.3 |
| 0.06 | nd | nd | 79 ± 1.5 |
| 0.08 | nd | nd | 82 ± 0.2 |
| 0.1 | nd | 0.3 ± 0.16 | 83 ± 0.1 |
| 1 | 12 ± 0.4 | 0.2 ± 0.1 | nr |
| 10 | 67 ± 1.7 | 1 ± 0.8 | nr |
| 50 | 82 ± 0.2 | 3 ± 1.1 | nr |
| 100 | 82 ± 0.2 | 9 ± 0.9 | nr |
nd = not detectable; nr = not carried out.
Figure 1.
Graphs of the antioxidant effects at different concentrations of antioxidant compounds and IC50 determination of the DPPH antioxidant assay. (a,c): plots of the inhibition ratio (y) vs. the sample concentration (x) of S. macrostema EOs and Trolox; (b,d): plots of the zone containing 50% free radical scavenging (IC50) of S. macrostema essential oils and Trolox; (e) antioxidant effect of limonene (in this case, IC50 was obtained by extrapolation).
2.3. Antimicrobial Activity In Vitro
The in vitro antibacterial activity data of the essential oils of S. macrostema at five different dilution levels (undiluted or direct up to 1:1000) are presented in Table 3. During the tests, 100 µL of each dilution of S. macrostema EOs was applied over E. coli and S. aureus, and the level of inhibition of bacterial growth was measured. The greatest effect was obtained by applying the essential oils directly, undiluted, with 73% inhibition on E. coli and 81% on S. aureus. Observing the data in the Table 3 shows a greater antimicrobial effect on S. aureus than on E. coli.
Table 3.
In vitro bacterial growth inhibition of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. essential oils over E. coli and S. aureus.
| Dilution, v/v, % | % Inhibition, |
|
|---|---|---|
| E. coli | S. aureus | |
| Direct (100%) | 73 ± 1.6 | 81 ± 1.7 |
| 1:10 (10%) | 51 ± 2.2 | 46 ± 3.2 |
| 1:100 (1%) | 31 ± 1.4 | 28 ± 2.5 |
| 1:500 (0.5%) | 6 ± 0.9 | 20 ± 2.9 |
| 1:1000 (0.1%) | nd | 6 ± 2.1 |
nd = not detectable.
2.4. Thin Layer Chromatography–Direct Bioautography (TLC-DB)
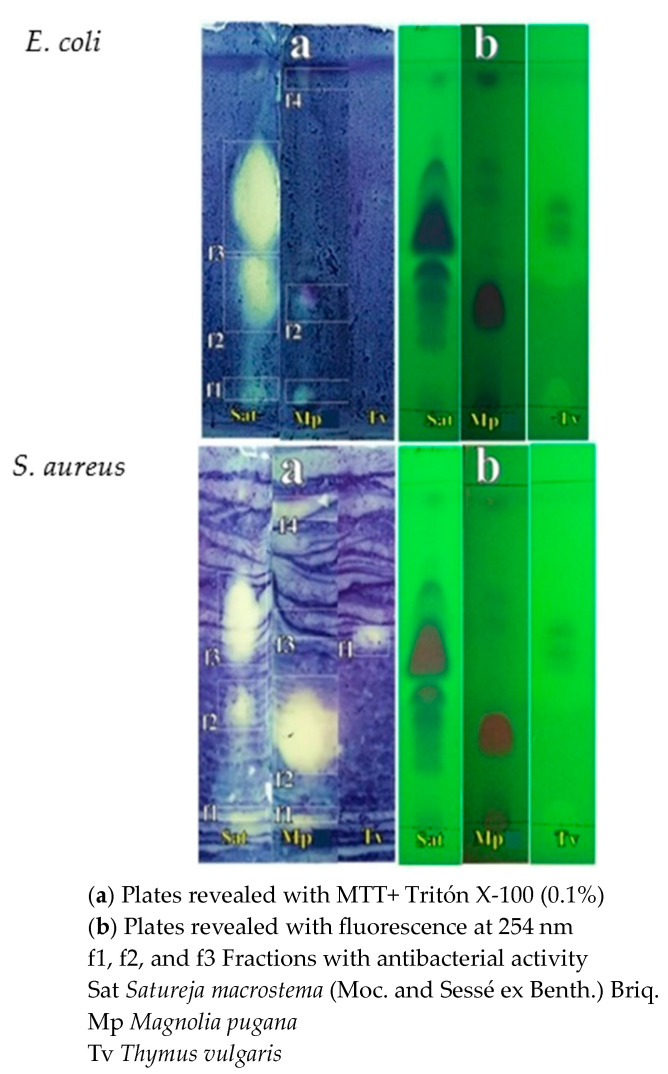
Figure 2 shows the results of the activity of the EOs from S. macrostema (expressed as Sat in the chromatographic plate) on E. coli and on S. aureus. Two different techniques revealed that double verification of the antimicrobial zones on the TLC plates was required. In the TLC-DB trial (Figure 2) EOs from the plants Magnolia pugana (Mp) (Magnoliaceae) and Thymus vulgaris (Tv) (Lamiaceae) were also tested to compare the antimicrobial effect of these plants with medicinal properties with the S. macrostema EOs. The antibacterial effect manifested itself in the form of light or dark spots, and a larger surface area was interpreted as indicating a greater intensity of the antibacterial effect. In Figure 2, it can be appreciated that the f1, f2, and f3 fractions represented the spots with the largest areas on the chromatographic plate, and, thus, were analyzed by HPLC-MS to evaluate the compounds that caused the antibacterial activity. The results are presented in Table 4, where the major inhibition compounds (>) found were piperitone oxide, trans-piperitone epoxide, and linalool, in addition to other compounds such as piperitenone, cis-piperitone epoxide, trans-thujene, benzaldehyde, caryophyllene, spathulenol, iso-menthone, and 2-phenyl ethyl acetate.
Figure 2.
TLC-DB of the essential oils from Satureja macrostema (Moc. and Sessé ex Benth.) Briq. And their activity against E. coli and S. aureus, compared with essential oils from the leaves of Magnolia pugana (Mp) and Thymus vulgaris (Tv). White and gray spots show fractions with antimicrobial activity.
Table 4.
Active antibacterial fractions in the essential oils of Satureja macrostema. (Moc. And Sessé ex Benth.) Briq. Obtained via the TLC-DB analysis. F1, f2, and f3 are the zones of the thin layer in the TLC-DB test with bacterial activity, and Rf is the retention factor of each of these active zones. Compounds found by GC-MS from each of these areas are included.
| S. macrostema Essential Oils Fractions with Antimicrobial Properties | |||
|---|---|---|---|
| f1 | f2 | f3 | |
| Retention factor (Rf) values | |||
|
E. coli
S. aureus |
0.02 0.02 |
0.31 0.35 |
0.62 * 0.61 * |
| Detected compounds | |||
| linalool (>) caryophyllene trans-thujene piperitenone spathulenol |
pulegone piperitone oxide (>) |
cis-piperitone epoxide trans-piperitone epoxide (>) iso-menthone 2-phenil ethyl acetate |
|
* Antibacterial fraction with major inhibition; (>) compound with the highest presence inside the antibacterial fraction.
3. Discussion
The chemical composition, antioxidant effects, and in vitro antibacterial activity of essential oils from Satureja macrostema, an aromatic plant commonly used in some regions of Mexico for the treatment of various diseases, were evaluated. The essential oils of the S. macrostema leaves were isolated by hydrodistillation, with a dry basis yield of 0.8% (w/w). Table 1 shows the chemical composition of S. macrostema Eos, which include various volatile oils, such as monoterpene hydrocarbons, oxygenated monoterpenes, aldehydes, ketones, alcohols, phenols, epoxy compounds, oxides, sesquiterpenes (some with roles as non-steroidal anti-inflammatory drugs), oxygenated sesquiterpenes, and oxygenated hydrocarbons. A comparison of these results with those of other studies of S. macrostema from the same region (the Purepecha plateau of Michoacan, Mexico) shows differences in the amounts and types of compounds found in essential oils (EOs). The essential oils obtained from the aerial parts (leaves and stems) of S. macrostema a (Moc. And Sessé ex Benth.) Briq. Plants, which were attained by micropropagation from seeds collected in experimental plantations in Parangaricutiro Michoacan (19°25′23″ N, 02°07′47″ W), have the major compounds pulegone (25.5%), linalool (16.62%), thymol (14.64%), limonene (5.53%), caryophyllene (3.98%), and menthone (3.09%) [13]. This highlights the absence of piperitone compounds. On the other hand, in another study, the leaves of S. macrostema plants from an experimental field in Uruapan Michoacan were treated with a process of prolonged cold maceration and subsequent procurement of the terpene fractions of the essential oils during plant development (15, 30, 45 and 90 days). This showed variation in the presence of some compounds according to the maturity of the plant, with the most significant effect being a decrease in pulegone and an increase in limonene and piperitone. In the highest state of maturity, these plants contain mainly limonene (35.54%), piperitone oxide (25.71%), and piperitone (10.10%), and in lower concentrations, they contain menthol, pulegone, and menthone, among others [17]. In another study, EOs obtained from dry leaves of Clinopodium macrostemum (synonym of S. macrostema) were collected from the forest of the Mixe region of Oaxaca. They had a yield of 0.8% (w/v), with 32 compounds identified, among them being linalool (55.4%), caryophyllene (6.3%), menthone (5.8%), geraniol acetate (4.1%), terpineol (3.7%), and pulegone (2.8%) [18]. This variation in plant compounds from the same area of study is not strange or rare and has been documented in other investigations. This was the case of the study carried out on EOs from Satureja parvifolia leaves in five populations of Tucuman, Argentina, which showed variability in their chemical compositions, particularly with the presence of pulegone, carvacrol, and piperitone in samples from one region and their absence in samples from another region of the study area [19]. Another study [20] concerning EOs obtained from three wild species of Satureja from the middle of the Atlas Mountains of Morocco showed variability in the chemical composition of the EOs according to the species. S. briquetti essential oils showed borneol (27.64%), β-bisabolene (9.58%), α-pinene, and linalool as its main compounds, while S. atlántica showed piperitone oxide (27.74%%), limonene (20.57%), pulegone (16.88%), and cis-piperitone oxide (15.55%). Finally, the analysis of S. alpina presented pulegone (87.74%) as the main compound. It is known that the variation in the chemical composition of plants of the Lamiaceae family is due to environmental factors, geographic location, light intensity, soil type, altitude, and phenology, since the degree of vegetative development favors the expression of certain compounds which increase with the age of the plant [21]. This highlights the differences in the chemical composition of the EOs of Satureja subspecies, with great variation due to the genetic inheritance of each species and also to environmental conditions [20]. This also seems to be the case for the samples of S. macrostema from the Purepecha plateau.
Data regarding the antioxidant properties of S. macrostema EOs are shown in Table 2 and in Figure 1. Inspecting these data allows us to observe an increasing effect up to a concentration of around 50 mg/mL after which a plateau occurred in the curve of Figure 1a, in which the antioxidant effect remained almost constant, with only small increases in this effect with increasing dose of S. macrostema EOs. The maximum value of the DPPH antioxidant effect was 82% at a dose of 50 mg/mL of EOs. Limonene is a characteristic terpene of essential oils, and in this test, it showed a DPPH antioxidant effect of 9% with a dose of 100 mg/mL (Table 2 and Figure 1e), which was lesser than the antioxidant effect of S. macrostema EOs. Moreover, Trolox, a strong antioxidant often used as a reference, was the compound with the highest DPPH antioxidant effect value of 83% at a 0.1 mg/mL dose (Figure 1c). In a study on S. macrostema [14], the EOs demonstrated in vitro DPPH antioxidant activity of 53.11% (IC50) with a dose of 1 mg/mL, lower than the IC50 obtained in this study. Likewise, extracts of green tea and black tea with known antioxidant activity showed better antioxidant effectiveness than S. macrostema EOs, with DPPH greater than 80% at a lower dose of 0.5 mg/mL [22].
Further, essential oils from S. macrostema showed positive antimicrobial activity against the tested pathogenic microbial strains. The greatest effect (Table 3) on bacterial growth was with the direct application (without dilution) of 100 µL of essential oil extract, which led to 81% inhibition of S. aureus and 73% inhibition of E. coli. Bibliographic references indicate that the minimum inhibitory concentration (MIC) of EOs of Satureja hortensis that absolutely suppressed the visible microbial growth of several bacteria and fungi, including S. aureus and E. coli, was 0.2 µL EOs/mL methanol for the S. aureus strain [23], which is a lower dose of EOs than that used in this study. In another study on the EOs of Satureja spicigera, the MIC was of 1.5 mg/mL for S. aureus and 6 mg/mL for E. coli [24].
Essential oils are generally biologically active, with antioxidant, antibacterial, and fungicidal properties. In general, the production of essential oils and metabolites by plants is due to self-protection against the environment and against pathogens, which is why they show antibacterial activity of varying intensity depending on their origin, concentration, composition, extraction, and processing, as well as the type of microorganism [20].
The Lamiaceae family, to which the genus Satureja belongs, contains aromatic plants, many of them medicinal, with various compounds of biological interest [25]. In this study, the most active fractions in the TLC-DB test, identified as f1, f2, and f3 in Figure 2, were analyzed by gas chromatography–mass spectrometry (GC/MS). The result is shown in the Table 4, in which one can observe higher proportions of cis-piperitone epoxide, trans-piperitone epoxide, and piperitenone oxide, which were also the most abundant in the crude essential oil. Furthermore, in Table 4, it can be seen that linalool, trans-thujene, and iso-menthone are present. Since these compounds are present in the active fractions, a correlation could be established between these compounds and the antimicrobial activity of the EOs of S. macrostema. Regarding the most abundant compounds, it should be mentioned that piperitone (C10H16O, molecular weight: 152.23 g/mol) is a biologically active monocyclic monoterpene ketone with insecticidal activity. It is soluble in alcohol and ether with aroma of mint and camphor characteristic of eucalyptus and the genera Piper, Cymbopogon, Andropogon, and Mentha. These can be used in the production and synthesis of menthol and thymol [26,27]. In addition, piperitenone (C10H14O, molecular weight 150.22 g/mol) is a menthane monoterpenoid with antioxidant activity which is very hydrophobic and contains the strong-tasting menthol common in spearmint and rosemary [28], which generally occurs in the flowering stage and becomes pulegone or piperitone in the adult stage of the plant [29]. Likewise, piperitenone oxide (C10H14O2, molecular weight: 166 g/mol) is a monoterpenoid ketone that is soluble in ethanol, methanol, benzene, and petroleum [30], with cardiovascular, antimicrobial, insecticidal, and antifungal activities [31]. The biosynthetic pathway for the biogenesis of piperitone derivatives starts with the production of limonene. Then, a piperitenol compound is formed from which pulegone, menthane, and menthol are produced by one route, while piperitone, piperitone oxide, and menthol are produced by another route [17,29,32].
The biological activity of S. macrotema has been demonstrated in various tests and has been attributed to the high content of various terpenes and their synergistic action, the composition of which presents compositional variability. In general, the protection of a substrate against oxidative damage is attributed to phenolic compounds (e.g., eugenol, thymol) which are found in very low amounts in essential oils. However, since the major compounds in essential oils are monoterpenes and sesquiterpenes these compounds also contribute significantly to the antioxidant and antibacterial activity of these oils. [33]. Likewise, due to the ethnobotanical and medicinal usefulness of these plants, it is important to mention the toxicity of pulegone, since is a potent toxic compound (hepatotoxic and abortifacient) [34]; plants that contain it in high concentrations should be avoided. Piperitenone should also be investigated toxicologically because of the possible formation of the toxin p-cresol in plants with high contents of these compounds, as it is traditionally used in medicine [35]. Pulegone is abundant in S. macrostema in the early stages of development, so its use in this stage of development would not be recommended, but when it reaches a stage of greater maturity in which its concentration decreases, it can be utilized [17].
4. Materials and Methods
4.1. Plant Material
Maturing and wild plants (sample size n = 20) of S. macrostema were collected in 2020 in mountainous areas of the municipality of Angahuan (19°32′50″ N 102°13′34″ W, 2340 m above sea level), Uruapan Michoacán, western Mexico. The collection was carried out during the period of the most abundant plant development in this area, which corresponds to the period between September and January. The sampling area was located on the Purepecha plateau, with a height of 1700–3200 masl and cold weather almost all year round, with a temperature of 12–18 °C. The predominant vegetation consists of deciduous tropical forests, mainly including species of pines, oaks, and firs with dark or reddish soils formed from volcanic ash [36]. The leaves were detached from the plants manually and dried in a shady environment to avoid any alterations caused by solar radiation. The plant material was pulverized using a Wiley mill and sieved; the sample retained in the sieve (No. 60) was collected and stored in a refrigerator for analysis. The plants were identified at the Institute of Botany of the University of Guadalajara (IBUG), with the record number 185703.
4.2. Essential Oils Isolation
The essential oils were obtained by hydrodistillation based on commonly used methods [37], for which a glass system consisting of a balloon flask, condenser, heating mantle, and a continuous Clevenger trap with a 10 mL collector was necessary. Ground and washed plant material were weighed (300 g), and then 3000 mL of water were added. Heat was applied until the water was boiling, with an extraction time of 4 h. The essential oil isolated and retained in the trap, with a volume of approximately 1 mL, was separated by decantation. Residual moisture was removed from the essential oil with anhydrous sodium sulfate, stored in an amber bottle, and refrigerated at 4 °C. The necessary repetitions were made to obtain the quantity of essential oil which would be sufficient to carry out the tests.
4.3. DPPH and TEAC Free Radical Scavenging Assay
The following reagents were used for this test: 0.2 mM DPPH (7.89 mg DPPH in 100 mL absolute ethanol), S. macrostema essential oil (1 to 100 mg/mL concentrations), limonene (1 to 100 mg/mL concentrations) as reference, and Trolox (0 at 0.1 mg/mL). In addition, 0.1 M Tris-HCl (pH 7.4) was added. The measurement of activity of S. macrostema essential oil against DPPH radicals was carried out according to Shimamura et al. (2014) [38], with modifications. In test tubes, 200 µL of sample solution to be analyzed, 800 µL of 0.1 M Tris-HCl buffer solution (pH 7.4), and 1000 µL of 0.2 mM DPPH solution were added in consecutive order. Each preparation was mixed for 10 s. The mixtures were left to rest for 30 min in the dark. The absorbance was measured at 517 nm (Jenway 6405 Uv/Vis Spectrophotometer, Dunmow, UK), in triplicate. A mixture of 200 µL of concentrated ethanol (99.5% or 99.7%), 800 µL of 0.1 M Tris-HCl buffer solution, and 1 mL of 0.2 mM DPPH was used as a blank. The percentage of inhibition was obtained by means of the following formula:
DPPH value was expressed in Trolox equivalents with the formula TEAC = IC50 Trolox (mg/mL)/IC50 sample (mg/mL). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) is a vitamin E-derived antioxidant with free radical activity. In many studies, the antioxidant activity of the samples against free radicals (DPPH, ABTC) is compared against Trolox as a standard antioxidant by means of the corresponding ratio (Trolox inhibition/compound inhibition), known as TEAC [39].
4.4. Antimicrobial Activity In Vitro
Antimicrobial tests were performed using the broth microdilution assay [40]. Essential oil serial dilutions of 1/10, 1/100, 1/500, and 1/1000 (v/v) were made. In addition, bacterial suspensions of Escherichia coli (ATCC® 8739) and Staphylococcus aureus (ATCC® 6538p), previously cultured overnight at 30 °C in Mueller–Hinton broth (MHB), were adjusted to densities of 0.5 according to the turbidity scale of McFarland (1.5 × 108 CFU/mL). Then, 100 µL of the extracts and 100 µL of the prepared bacterial suspensions were deposited in a 96-well microtiter plate (Costar TM, made of polystyrene). The inoculated plates were incubated at 37 ± 0.1 °C at 24 h with continuous agitation. The samples with bacteria and without extract were considered as controls. The antimicrobial activity (inhibition of growth and multiplication bacterial) was determined by measuring the absorbance at 620 nm using a spectrophotometer (Jenway 6405 Uv/Vis Spectrophotometer, UK). These measurements were performed in triplicate.
4.5. Thin Layer Chromatography–Direct Bioautography (TLC-DB)
This test was developed according to the method described by Jesionek et al. (2015) [41], with modifications. A suspension of microorganisms of Escherichia coli (ATCC® 8739) and Staphylococcus aureus (ATCC® 6538p) was prepared in Mueller–Hinton agar (MHA). Subsequently, they were inoculated in 100 mL of nutrient broth (0.5% agar) at 37 °C for 48 h. The concentration was adjusted by dilution with nutrient broth and absorption from 0.4 to 600 nm, corresponding to 4 × 107 UFC/mL. TLC plates were prepared with fluorescence factor (60 Merck®, Darmstadt, Germany, 10 × 10 cm), conditioning them at 45 °C for 12 h. For the development of the plates, approximately 10 µL of a methanol solution of the essential oils (30 µL/mL) of S. macrostema was deposited at the base of the plates, and solutions of M. Pugana and T. vulgaris were used for comparison. Elution was performed with toluene/ethyl acetate/petroleum ether (93:7:20) v/v/v. The bands eluted by fluorescence were observed at 254 nm. The test was carried out in triplicate. The plates were placed in a chamber at 25 °C for 90 min. They were immersed for 10 s in 50 mL of the bacterial suspension, removed, and dried by air flow, then placed into a steam chamber at 37 °C for 24 h in an incubator (Binder BD115, Camarillo, CA, USA). Subsequently, the plates were immersed for 60 s in aqueous tetrazolium salt MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) solution (Sigma Aldrich, Burlington, MA, USA, 0.6 mg/mL) with 0.1% Triton X-100, then removed and incubated for 1 h in a steam chamber at 37 °C. They were then immersed in 50 mL of ethanol (70% v/v) for 10 s and dried with air. The occurrence of light bands or spots indicated antibacterial activity in contrast to the bluish background. The distances were measured to calculate the Rf. The strongest light points indicated the fractions with the highest antimicrobial intensity levels. The fractions with coincident Rf were located, the silica was carefully scraped off with a spatula, and the contents were stored in an amber vial. Subsequently, the silica with the active fraction was diluted in 2 mL of dichloromethane (Sigma-Aldrich Merck subsidiary Mexico, Naucalpan de Juárez, Mexico), then filtered on PDVF membranes with 0.45 µm porosity and injected into the GC-MS equipment (Bruker Daltonics 436 SCION®, Bremen, Germany). The same parameters as those indicated in the chemical characterization of the essential oils were used.
4.6. Gas Chromatography-Mass Spectrometry
The S. macrostema EOs were analyzed by GC-MS according to the method used by Scalvenzi et al. 2017 [42]. A gas chromatograph (Bruker Daltonics 436 SCION®, Bremen, Germany) coupled to a triple quadrupole mass detector (model TQ-EVOQ) was used. A Bruker BR-5MS GC column (30 × 0.25 mm × 0.25 µm, Bremen, Germany) was also used. The maximum analysis temperature was 350 °C. The temperature program was as follows: 50 °C to 100 °C at 1 °C/min; 100 °C to 250 °C at 5 °C/min, and maintenance of this temperature for 10 min for a total analysis time of 90 min. The mass spectrometer conditions were ionization energy, 70 eV; emission current, 10 mAmp; scan rate, 1 scan/s; mass range, m/z 35–400 Da; trap temperature, 220 °C; and transfer line temperature, 260 °C. The carrier gas was helium (99.999%), with a flow 1 mL/min and a split radius of 1:50. The LIR values were calculated according to the formula proposed by Van Den Dool and Kratz, 1963 [43]. These were applied to programs with thermal gradients, using C8 to C20 alkanes with their respective retention times as standards, then compared against the retention rates reported in the literature [13]. The components were identified based on IR and by comparison with the mass spectra essential oil component libraries [12,13]. Bruker’s MS-Workstation software integrated with NIST-MS-Search Ver. 8.2.1 was used for this analysis.
5. Conclusions
The main biological properties of S. macrostema were evaluated due to its ethnomedicinal use to combat various diseases. A high percentage of volatile terpenes was found, most of them being oxygenated monoterpenes, with a high proportion of piperitone oxides and epoxides as well as linalool, pulegone, menthone, and limonene, among other common components in essential oils of aromatic plants with medicinal properties. The chemical composition of S. macrostema EOs showed variation in the type and quantity of compounds due to edaphoclimatic and phenological factors of the growing area. Likewise, the biological activity of these compounds was attributed more to synergistic action than to the individualized activity of the compounds in in vitro investigations. The evaluation of their biological properties is important for the characterization and knowledge of these biotic resources and may even guide the direct measurement of their effects on various diseases, including chronic degenerative diseases (e.g., diabetes, cancer, hypertension), either in cell lines or in controlled clinical studies.
Acknowledgments
The authors express their gratitude to the Department of Wood, Pulp and Paper of the University of Guadalajara, in whose laboratories the study was carried out.
Author Contributions
Investigation: E.A.O.M., L.B.R. and J.C.S.; original manuscript writing: J.J.V.R.; manuscript review J.A.S.G., C.A.M., M.R.S. and A.F.C.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in the laboratory of DMCyP and approved by the Institutional Review Board; 2023.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not Applicable.
Funding Statement
This research was support on internal funding of the Department of Cellulose Wood and Paper (DMCyP) of the University Center for Exact Sciences and Engineering (CUCEI) of the University of Guadalajara.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cantino P.D., Harley R.M., Wagstaff S.J. Genera of Labiatae Status and Classification. In: Harley R.M., Reynolds T., editors. Advances In Labiatae Science. Royal Botanic Gardens Press; Kew, Richmond: Surrey, UK: 1992. pp. 511–522. [Google Scholar]
- 2.Rzedowski G.C., Rzedowski J. Flora Fanerogámica del Valle de México. 2nd ed. Instituto de Ecología, A.C. y Comisión Nacional para el Conocimiento y Uso de la Biodivervisidad; Pátzcuaro, Michoacán, México: 2005. [(accessed on 1 June 2022)]. 1406p. Available online: https://www.biodiversidad.gob.mx/publicaciones/librosDig/pdf/Flora_del_Valle_de_Mx1.pdf. [Google Scholar]
- 3.Esquivel-García R., Pérez-Calix E., Ochoa-Zarzosa A., García-Pérez M.E. Ethnomedicinal plants used for the treatment of dermatological affections on the Purépecha Plateau, Michoacán, Mexico. Acta Bot. Mex. 2018;125:1339. doi: 10.21829/abm125.2018.1339. [DOI] [Google Scholar]
- 4.Martínez-Gordillo M., Fragoso-Martínez I., García-Peña M.R., Montiel O. Géneros de Lamiaceae de México, diversidad y endemismo. [(accessed on 15 June 2022)];Rev. Mex. Biodivers. 2013 84:30–86. doi: 10.7550/rmb.30158. Available online: https://www.redalyc.org/articulo.oa?id=42526150034. [DOI] [Google Scholar]
- 5.Bello González M.Á., Garciglia R.S., Carmona Fernández J. Propagación y crecimiento de Satureja macrostema Briq.(Lamiaceae) bajo condiciones controladas en Uruapan, Michoacán. [(accessed on 20 May 2022)];Cienc. Nicolaita. 2013 58:105–115. Available online: https://www.cic.cn.umich.mx/cn/issue/view/6. [Google Scholar]
- 6.Sefidkon F., Askari F., Sadeghzadeh F., Oulia P. Antimicrobial effects of the essential oils of Satureja mutica, S. edmondi and S. bachtiarica against salmonella paratifi a and b. [(accessed on 20 September 2022)];Iran. J. Biol. 2009 22:249–258. Available online: https://www.sid.ir/en/journal/ViewPaper.aspx?id=159158. [Google Scholar]
- 7.Miguel M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour. Fragr. J. 2010;25:291–312. doi: 10.1002/ffj.1961. [DOI] [Google Scholar]
- 8.Slavkovkovsaka V., Jancic J., Bojovic S., Milosavljevic S., Djovovic D. Variability of essential oils of Satureja montana L. and Satureja kitaibelii Wierzb. from the central part of the Balkan península. Phytochemistry. 2001;57:71–76. doi: 10.1016/S0031-9422(00)00458-1. [DOI] [PubMed] [Google Scholar]
- 9.Eminagaoglu O., Tepe B., Yumrutas O., Askin A., Daferera D., Polissiou M., Sokmen A. The in vitro antioxidative properties of the essential oils and methanol extracts of Satureja spicigera (K.Koch) Boiss. and Satureja cunifolia ten. Food Chem. 2007;100:339–343. doi: 10.1016/j.foodchem.2005.09.054. [DOI] [Google Scholar]
- 10.Güllüce M., Sokmen M., Daferera D., Agar G., Ozkan H., Kartal N. In vitro antibacterial, antifungal and antioxidant activities of the essential oil, methanol extracts of herbal parts and callus culture of Satureja hortensis L. J. Agric. Food Chem. 2003;51:3958–3965. doi: 10.1021/jf0340308. [DOI] [PubMed] [Google Scholar]
- 11.Ayub M.A., Goksen G., Fatima A., Zubair M., Abid M.A., Starowicz M. Comparison of Conventional Extraction Techniques with Superheated Steam Distillation on Chemical Characterization and Biological Activities of Syzygium aromaticum L. Essential Oil. Separations. 2023;10:27. doi: 10.3390/separations10010027. [DOI] [Google Scholar]
- 12.NIST Standard Reference Database . NIST/EPA/NIH Mass Spectral Library with Search Program. NIST; Gaithersburg, MD, USA: 2008. Data Version NIST 05:2008. [Google Scholar]
- 13.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. p. 804. [Google Scholar]
- 14.Torres-Martínez R., García-Rodríguez M.Y., Ríos-Chávez P., Saavedra-Molina A., López-Meza J.E., Ochoa-Zarzosa A., Salgado Garciglia R. Antioxidant Activity of the Essential Oil and its Major Terpenes of Satureja macrostema (Moc. And Sessé ex Benth.) Briq. Pharmacogn. Mag. 2008;13:5875–5880. doi: 10.4103/pm.pm_316_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed A.H., Hussein K., Masaoud M., Al-Sham F. Radical Scavenging, Antibacterial Activities and Chemical Composition of Volatile Oil of Edible Mentha longifolia (L.) Hudson, Subs. Schimperi (Briq.) Briq, from Yemen. AJMAP. 2017;4:19–35. [Google Scholar]
- 16.Sharopov F.S., Sulaimonova V.A., Setzer W.N. Essential oil composition of Mentha longifolia from wild populations growing in Tajikistan. J. Med. Act. Plants. 2012;1:76–84. doi: 10.7275/R5736NTN. [DOI] [Google Scholar]
- 17.Espino Garibay F. Master’s Thesis. Michoacana University of San Nicolás de Hidalgo; Morelia, Mexico: 2010. Clasificación Molecular de Satureja macrostema (Benth) Briq y Erngium carlinae Delar F. y Evaluación de Actividad Antimicrobiana de sus Terpenoides. [Google Scholar]
- 18.Rojas-Olivos A., Solano-Gómez R., Granados-Echegoyen C., Santiago-Santiago L.A., García-Dávila J., Pérez-Pacheco R., Lagunez-Rivera L. Larvicidal effect of Clinopodium macrostemum essential oil extracted by microwave-assisted hydrodistillation against Culex quinquefasciatus (Diptera: Culicidae) Rev. Soc. Bras. Med. Trop. 2018;51:291–296. doi: 10.1590/0037-8682-0284-2017. [DOI] [PubMed] [Google Scholar]
- 19.Viturro C.I., Molina A.C., Heit C., Elechosa M., Molina A.M., Juares M. Evaluation of the composition of the essential oils of Satureja boliviana, S. odora and S. parvifolia obtained from samples collected in Tucumán, Argentina. Bol. Latinoam. Caribe Plantas Med. Aromát. 2007;6:288–289. [Google Scholar]
- 20.Jennan S., Fouad R., Nordine A., Farah A., Bennani B., Moja S., Greche H., Mahjoubi F. Chemical Composition and Antibacterial Screening of Aerial Parts of Essential Oils of Three Satureja species (Satureja briquetti, Satureja atlantica and Satureja alpina) Growing Wild in the Middle Atlas Mountains of Morocco. J. Essent. Oil Bear. Plants. 2018;21:741–748. doi: 10.1080/0972060X.2018.1486230. [DOI] [Google Scholar]
- 21.Viturro C.I., Molina A., Guy I., Charles B., Guinaudeau H., Fournet A. Essential oils of Satureja boliviana and S. parvifolia growing in the region of Jujuy, Argentina. Flavour. Fragr. J. 2000;15:377–382. doi: 10.1002/1099-1026(200011/12)15:6<377::AID-FFJ926>3.0.CO;2-P. [DOI] [Google Scholar]
- 22.Odumosu P., Ojerinde S., Egbuchiem M. Polyphenolic contents of some instant tea brands and their antioxidant activities. J. Appl. Pharm. Sci. 2015;5:100–105. doi: 10.7324/JAPS.2015.50918. [DOI] [Google Scholar]
- 23.Mihajilov-Krstev T., Radnovic D., Kitic D., Stojanovic-Radic Z., Zlatkovic B. Antimicrobial Activity of Satureja Hortensis L. Essential Oil Against Pathogenic Microbial Strains. Biotechnol. Biotechnol. Equip. 2009;23:1492–1496. doi: 10.2478/V10133-009-0018-2. [DOI] [Google Scholar]
- 24.Eftekhara F., Raeia F., Yousefzadib S., Ebrahimic N., Hadiand J. Antibacterial Activity and Essential Oil Composition of Satureja spicigera from Iran. Z. Nat. 2009;64:20–24. doi: 10.1515/znc-2009-1-204. [DOI] [PubMed] [Google Scholar]
- 25.Sefidkon F., Salehyar S., Mirza M., Dabiri M. The essential oil of Tagetes erecta L. occurring in Iran. Flavour. Fragr. J. 2004;19:579–581. doi: 10.1002/ffj.1360. [DOI] [Google Scholar]
- 26.O’Neil M.J. In: The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 15th ed. RSC Publishing, editor. Royal Society of Chemistry; Cambridge, UK: 2013. [(accessed on 10 July 2021)]. p. 2708. Available online: www.rsc.org/merck-index. [Google Scholar]
- 27.Picard M., Lytra G., Tempere S., Barbe J.-C., de Revel G., Marchand S. Identification of Piperitone as an Aroma Compound Contributing to the Positive Mint Nuances Perceived in Aged Red Bordeaux Wines. J. Agric. Food Chem. 2016;64:451–460. doi: 10.1021/acs.jafc.5b04869. [DOI] [PubMed] [Google Scholar]
- 28.TMIC (The Metabolomics Innovation Centre); INNOVATION.CA (Canada Foundation For Innovation) [(accessed on 2 March 2023)]. Available online: https://foodb.ca/compounds/FDB015974.
- 29.Tavakkoli-Khaledi S., Asgarpanah J. Essential Oil Chemical Composition of Mentha mozaffarianii Jamzad Seeds. J. Mex. Chem. Soc. 2016;60:19–22. doi: 10.29356/jmcs.v60i1.66. [DOI] [Google Scholar]
- 30.Hendriks H., van Os F.H.L. Essential oil of two chemotypes of Mentha suaveolens during ontogenesis. Phytochemistry. 1976;15:1127–1130. doi: 10.1016/0031-9422(76)85115-1. [DOI] [Google Scholar]
- 31.Riani L.R., Macedo A.L., Chedier L.M., Pimenta D.S. Chemical analysis of essential oil and hydrolates of leaves, inflorescences and stems of Piper chimonanthifolium Kunth. Rev. Virtual Quim. 2017;9:1560–1569. doi: 10.21577/1984-6835.20170091. [DOI] [Google Scholar]
- 32.Sohell S., Rodney B. Menthofuran regulates essential oil biosynthesis in peppermint b controlling a downstream monoterpene reductactase. Proc. Natl. Acad. Sci. USA. 2003;100:14481–14486. doi: 10.1073/pnas.2436325100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaramillo B.E., Stashenko E., Martínez J.R. Volatile chemical composition of the Colombian Satureja brownie (Sw.) Briq. and determination of its antioxidant activity. Rev. Cuba. Plantas Med. 2010;15:52–63. [Google Scholar]
- 34.Petrakis A.E., Kimbaris C.A., Polissious G.M. Quantitative Determination of Pulegone in Pennyroyal oil by FT-IR Spectroscopy. J. Agric. Food Chem. 2009;57:10044–10048. doi: 10.1021/jf9026052. [DOI] [PubMed] [Google Scholar]
- 35.Madyastha K.M., Gaikwad N.W. Metabolic disposition of a monoterpene ketone, piperitenone, in rats: Evidence for the formation of a known toxin, p-cresol. Drug Metab. Dispos. 1999;27:74–80. [PubMed] [Google Scholar]
- 36.Orduña Trejo C., Castro Campillo A., Ramírez Pulido J. Mammals from the Tarascan Plateau, Michoacán, México. Rev. Mex. Mastozool. 1999;4:53–68. doi: 10.22201/ie.20074484e.1999.4.1.81. [DOI] [Google Scholar]
- 37.Guerra-Boone L., Alvarez-Román R., Salazar-Aranda R., Torres-Cirio A., Rivas-Galindo V.M., Waksman de Torres N., González González G.M., Pérez-López L.A. Chemical compositions and antimicrobial and antioxidant activities of the essential oils from Magnolia grandiflora, Chrysactinia mexicana, and Schinus molle found in northeast Mexico. Nat. Prod. Commun. 2013;8:135–138. doi: 10.1177/1934578X1300800133. [DOI] [PubMed] [Google Scholar]
- 38.Shimamura T., Sumikura Y., Yamazaki T., Tada A., Kashiwagi T., Ishikawa H., Matsui T., Sugimoto N., Akiyama H., Ukeda H. Applicability of the DPPH assay for evaluating the antioxidant capacity of food additives—Inter-laboratory evaluation study. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2014;30:717–721. doi: 10.2116/analsci.30.717. [DOI] [PubMed] [Google Scholar]
- 39.Olszowy Tomczyk M. How to express the antioxidant properties of substances properly? Chem. Pap. 2021;75:6157–6167. doi: 10.1007/s11696-021-01799-1. [DOI] [Google Scholar]
- 40.Borges C., Ferreira M., Saavedra J., Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013;19:256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 41.Jesionek W., Móricz Á.M., Ott P.G., Kocsis B., Horváth G., Choma I.M. TLC-Direct Bioautography and LC/MS as Complementary Methods in Identification of Antibacterial Agents in Plant Tinctures from the Asteraceae Family. J. AOAC Int. 2015;98:857–861. doi: 10.5740/jaoacint.SGE2-Choma. [DOI] [PubMed] [Google Scholar]
- 42.Scalvenzi L., Grandini A., Spagnoletti A., Tacchini M., Neill D., Ballesteros J.L., Sacchetti G., Guerrini A. Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile. Molecules. 2017;22:1163. doi: 10.3390/molecules22071163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Den Dool H., Kratz P. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this study are available upon request from the corresponding author.