ABSTRACT
There are no licensed vaccines for enterotoxigenic Escherichia coli (ETEC), a common cause of children’s diarrhea and travelers’ diarrhea. ETEC strains producing enterotoxins (heat-labile toxin, LT; heat-stable toxin, STa) and adhesins CFA/I, CFA/II (CS1-CS3) or CFA/IV (CS4-CS6) attributed to a majority of ETEC-associated diarrheal cases, thus the two toxins (STa, LT) and the seven adhesins (CFA/I, CS1 to CS6) are historically the primary targets in ETEC vaccine development. Recent studies, however, revealed that ETEC strains with adhesins CS14, CS21, CS7, CS17, and CS12 are also prevalent and cause moderate-to-severe diarrhea; these adhesins are now considered antigen targets as well for ETEC vaccines. In this study, we applied the epitope- and structure-based multiepitope-fusion-antigen (MEFA) vaccinology platform and constructed a polyvalent protein to present immuno-dominant continuous B-cell epitopes of these five adhesins (also an STa toxoid); we then characterized this protein antigen’s (termed as adhesin MEFA-II) broad immunogenicity and evaluated antibody functions against each targeted adhesin and STa toxin. Data showed that mice intramuscularly immunized with adhesin MEFA-II protein developed robust IgG to the targeted adhesins and toxin STa. Importantly, the antigen-derived antibodies significantly inhibited adherence of ETEC bacteria expressing adhesin CS7, CS12, CS14, CS17, or CS21 and reduced STa enterotoxicity. These results indicated that adhesin MEFA-II protein is broadly immunogenic and induces cross-functional antibodies, suggesting adhesin MEFA-II can be an effective ETEC vaccine antigen; if included in an ETEC vaccine candidate, adhesin MEFA-II can expand vaccine coverage and increase efficacy against ETEC-associated children’s diarrhea and travelers’ diarrhea.
IMPORTANCE An effective vaccine is lacking against ETEC, a primary cause of children’s diarrhea and traveler’s diarrhea and a threat to global health. The key challenge in ETEC vaccine development is that ETEC bacteria express heterogeneous virulence determinants (>25 adhesins and two toxins). While the current strategy to target the seven most prevalent ETEC adhesins (CFA/I, CS1 to CS6) potentially lead to a vaccine against many clinical cases, the prevalence of ETEC strains shifts chronically and geographically, and ETEC expressing other adhesins, mainly CS7, CS12, CS14, CS17, and CS21, also cause moderate-to-severe diarrhea. However, it is impossible to develop an ETEC vaccine to target as many as 12 adhesins under conventional approaches. This study used a unique vaccinology platform to create a polyvalent antigen and demonstrated the antigen's broad immunogenicity and functions against the targeted ETEC adhesins, enabling the development of a broadly protective vaccine essentially against all of the important ETEC strains.
KEYWORDS: adhesin MEFA-II, MEFA (multiepitope fusion antigen), adhesin, ETEC (enterotoxigenic Escherichia coli), vaccine, diarrhea
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is a top-four cause of diarrhea in children aged less than 5 years in developing countries (children’s diarrhea) and the most common cause of diarrhea in children and adults, including military and civilian personnel traveling from the developed world to ETEC countries or regions of endemicity (travelers’ diarrhea) (1–3). Vaccines are considered a more practical and cost-effective countermeasure against ETEC-associated diarrhea and a better tool in combating antimicrobial resistance (AMR) (4, 5), especially as ETEC and other enteric bacteria are gaining AMR at an alarmingly high and increasing rate. Unfortunately, there are no vaccines licensed for ETEC diarrhea and many other infectious diseases.
There are a few ETEC vaccine candidates currently under preclinical or clinical investigation (6–8). These vaccine candidates primarily target the strains producing a few important ETEC adhesins and one or both ETEC enterotoxins. Adhesins and enterotoxins are the virulence determinants in causing ETEC-associated diarrhea. However, since an ETEC strain expressing one adhesin (out of >25 colonization factor antigen [CFA] and coli surface antigen [CS] adhesins) and either enterotoxin (heat-stable toxin [STa], heat-labile toxin [LT]) can cause diarrhea, it becomes very challenging to develop an effective ETEC vaccine to protect against the diverse ETEC strains or pathovars that produce heterogeneous adhesins and toxins (9–11). Epidemiological and clinical studies revealed that ETEC strains producing seven adhesins, CFA/I, CFA/II (CS1, CS2, CS3), and CFA/IV (CS4, SC5, CS6) [together with either or both enterotoxins (STa, LT)], are the dominant source to cause ETEC-associated diarrhea (12–15) and tend to be more significant in causing moderate to severe clinical cases (16). Some (or all) of these seven adhesins (CFA/I, CS1 to CS6), therefore, have been historically targeted as the main antigens of ETEC vaccine candidates (6, 8, 10).
A recent study estimated that ETEC strains expressing these seven adhesins (CFA/I, CS1 to CS6), however, are responsible for about 66% of ETEC diarrhea clinical cases and the strains producing other adhesins also play a significant role in causing children’s diarrhea and travelers’ diarrhea (16). Systematic surveys and cohort case studies uncovered that adhesins CS21 and CS14 are highly prevalent among ETEC strains isolated from diarrheal patients on a global scale (13, 15). Moreover, ETEC strains producing CS14 adhesin and STa toxin, as well as the strains producing CS21 adhesin and LT, are found to be significantly associated with moderate-to-severe diarrhea in children living in South Asia or sub-Saharan Africa (16). Indeed, ETEC strains that produce CS21 adhesin and STa toxin or STa and LT toxins are identified as the most prevalent cause of clinical diarrhea in US children as well as US adults traveling to Guatemala, Mexico, or India (17). It is also noticed that an ETEC isolate that produces CS7 adhesin and LT toxin was found to cause diarrhea in adult volunteers (18), and based on a longitudinal study of hospital patient samples, ETEC strains that express CS7, CS14, or CS21 adhesin (alone or together with CFA/I or CFA/II), as well as CS12 or CS17 adhesin, emerged as a dominant cause of clinical diarrhea in Bangladesh (19). Therefore, these adhesins are recommended to be also targeted for ETEC vaccine development. Indeed, it was estimated that the inclusion of CS7, CS12, CS14, CS17, and CS21 adhesin antigens to a vaccine that covers the seven major adhesins (CFA/I, CS1-CS6) (without ETEC toxin antigens) would improve vaccine protection significantly, from 64.3% to 86.2% against STa only ETEC infection, from 66% to 80.6% against STa+ ETEC (STa alone or together with LT) strains, and from 25% to 47.3% against LT only ETEC (16).
A vaccine, if it targets all of these 12 important adhesins (CFA/I, CS1 to CS7, CS12, CS14, CS17, CS21), would significantly improve protection against ETEC diarrhea. The challenge is that it becomes practically impossible to compose 12 or even seven important ETEC adhesin antigens into a vaccine product with conventional vaccine technologies. The current cocktail vaccine strategy to mix a few live attenuated or killed strains expressing several ETEC adhesins encounters challenges, including the complexity of product formulation, the need for a high oral dose that is often linked to reactogenicity, especially in young children, and the excessive amounts of somatic antigens, which results in poor host immune responses specific to the targeted virulence factors and unsatisfied efficacy against diarrhea (10). In contrast to the conventional approach, the novel vaccinology platform multiepitope-fusion-antigen (MEFA), which applies computational biology and protein modeling techniques to construct polyvalent antigens with broad immunogenicity (11, 20–22), can solve the antigen heterogeneity problem. Combined with the epitope vaccinology concept and structure vaccinology concept, the MEFA vaccinology platform enables the integration of multiple functional epitopes from heterogeneous virulence factors or strains into a backbone immunogen to produce a polyvalent immunogen with cross-protective immunity.
By applying this MEFA vaccinology platform, we have developed an ETEC vaccine candidate, named MecVax (multivalent enterotoxigenic E. coli vaccine) which is composed of two polyvalent protein antigens to target the seven ETEC adhesins (CFA/I and CS1 to CS6) and both ETEC toxins (8, 23–25). With the addition of another polyvalent adhesin immunogen to target the other important ETEC adhesins, such an ETEC vaccine candidate would expand protection coverage and amplify efficacy against ETEC diarrhea. In this study, we applied the MEFA vaccinology platform and constructed an ETEC adhesin MEFA protein to target CS7, CS12, CS14, CS17, and CS21 adhesins. We then examined this polyvalent adhesin MEFA protein for broad immunogenicity and subsequently evaluated MEFA-induced antibodies for cross-protection against the targeted five ETEC adhesins.
RESULTS
A polyvalent adhesin MEFA protein carrying epitopes of CS7, CS12, CS14, CS17, and CS21 adhesins as well as an STa toxoid was constructed and expressed.
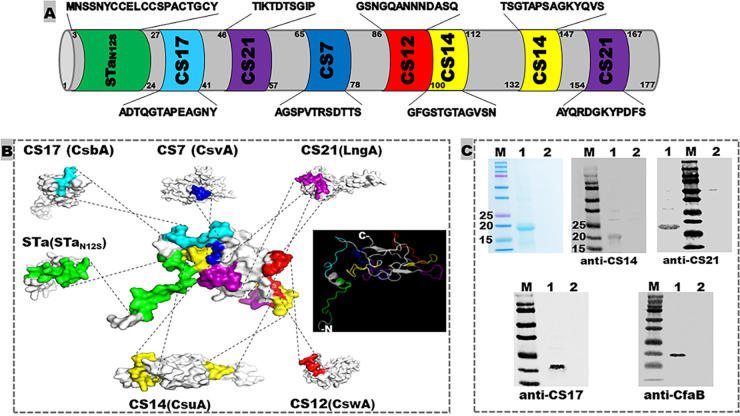
Immunodominant continuous B-cell epitopes ‘AGSPVTRSDTTS’ of adhesin CS7 major subunit CsvA (GenBank accession number AY009095), ‘GSNGQANNNDASQ’ of CS12 (CswA; AY009096), ‘TTSGTAPSAGKYQ’ and ‘GFGSTGTAGVSN’ of CS14 (CsuA; AY283611), ‘ADTQGTAPEAGNY’ of CS17 (CsbA; AY515609), and ‘AYQRDGKYPDFV’ and ‘TIKTDTSGIP’ of CS21 (LngA; NG036490) were in silico identified (based on a Random Forest algorithm which is trained on epitopes and non-epitopes determined from crystal structures to predict conformational epitopes). These epitopes were predicted antigenic (based on antigenicity propensity) and surface-exposed (based on hydrophilicity and protein modeling). With assistance from the MEFA vaccinology platform (21), these seven ETEC adhesin epitopes and a full-length STaN12S toxoid (MNSSNYCCELCCSPACTGCY; the 12th asparagine is substituted with serine) were integrated into the backbone protein CFA/I major subunit CfaB by substituting counterpart backbone epitopes (Fig. 1). After in silico optimization, this polyvalent adhesin MEFA protein termed adhesin MEFA-II (to separate from adhesin CFA/I/II/IIV MEFA which targets CFA/I, CS1 to CS6) had a molecule size of 18.1 kDa and an instability index of 22.93 (<40 is considered stable), with each of the adhesin epitopes of interest on MEFA-II confirmed in silico to be immunodominant, surface-exposed, and antigenic. The 19-amino acid STaN12S peptide itself is not immunogenic, and its immunogenicity was enhanced after being integrated into backbone CfaB, particularly for the 16th (from an antigenicity score of 0.434 in native peptide to 0.504 in MEFA-II), 17th (from 0.379 to 0.504), and 19th (from 0.255 to 0.503) residues, with an overall average residue score improved from 0.4112 to 0.452, based on Bepipred-2.0 predictor (Data Not Shown). A gene coding adhesin MEFA-II was synthesized after codon optimization and subsequently cloned into expression vector pET28a; the resultant plasmid was hosted by E. coli BL21 CodonPlus (DE3) strain. The MEFA protein was extracted at a yield of 40 mg per L of culture broth, at a purity over 95% estimated with SDS-PAGE Coomassie blue staining. The targeted protein was recognized by mouse serum polyclonal antibodies specific to CS14 major subunit CsuA, CS21 major subunit LngA, CS17 major subunit CsbA, and backbone CfaB in Western blot (Fig. 1).
FIG 1.
Illustration of ETEC adhesin MEFA-II structure and characterization of the recombinant protein. (A) Amino acid sequences and positions of the epitopes from the major subunit of CS7, CS12, CS14, CS17, and CS21, as well as STa toxoid STaN12S, on adhesin MEFA-II. (B) Adhesin MEFA-II protein modeling to show epitope structural location and surface presentation, as well as adhesin MEFA-II protein 3D structure. (C) Adhesin MEFA-II protein detection in SDS-PAGE Coomassie blue staining and Western blot with anti-CsuA (CS14 major subunit), anti-LngA (CS21 major subunit), anti-CsbA (CS17), or anti-CfaB (backbone) mouse sera (1:5,000). M = molecular marker, in kDa; 1 = adhesin MEFA-II protein; 2 = BL21 host cell inclusion body proteins (solubilized and refolded).
Adhesin MEFA-II protein induced antibody responses to five adhesins (CS7, CS12, CS14, CS17, and CS21) and toxin STa.
The adhesin MEFA protein was shown to be broadly immunogenic. Mice intramuscularly immunized with this MEFA protein, with or without adjuvant dmLT (double mutant heat-labile toxin, LTR192G/L211A), developed robust IgG responses to the five targeted adhesins (CS7, CS12, CS14, CS17, and CS21) and STa toxins (Fig. 2). The IgG titers (log10) to CS7, CS12, CS14, CS17, and CS21 (each subunit protein as the ELISA coating antigen) detected from the serum samples of the mice immunized with the MEFA protein (no dmLT adjuvant) were 4.3 ± 0.25, 4.7 ± 0.22, 5.1 ± 0.27, 4.0 ± 0.56, and 5.7 ± 0.12 (log10), respectively; anti-STa IgG titers were detected at 3.9 ± 0.76. When dmLT adjuvant was included, anti-CS7, -CS12, -CS14, -CS17 and anti-CS21 IgG titers were 4.2 ± 0.30, 4.4 ± 0.25, 5.3 ± 0.29, 4.3 ± 0.45, and 5.6 ± 0.24 (log10). Anti-STa IgG (4.3 ± 0.48) and anti-LT IgG (5.3 ± 0.19; derived from dmLT adjuvant) were detected in the sera of the group of mice immunized with adhesin MEFA-II and dmLT adjuvant. There were no significant differences in anti-adhesin and anti-STa IgG titers between the groups immunized with adhesin MEFA-II with or without dmLT. Additionally, anti-CFA/I IgG titers, which were derived from MEFA backbone CfaB, were detected at 4.3 ± 0.21 and 3.8 ± 0.50, respectively, from the two groups immunized with adhesin MEFA-II, with or without dmLT adjuvant. No antigen-specific antibodies were detected from the serum samples of the control mice or sera collected before the primary immunization. No antigen-specific IgA responses were detected from the mouse serum samples.
FIG 2.
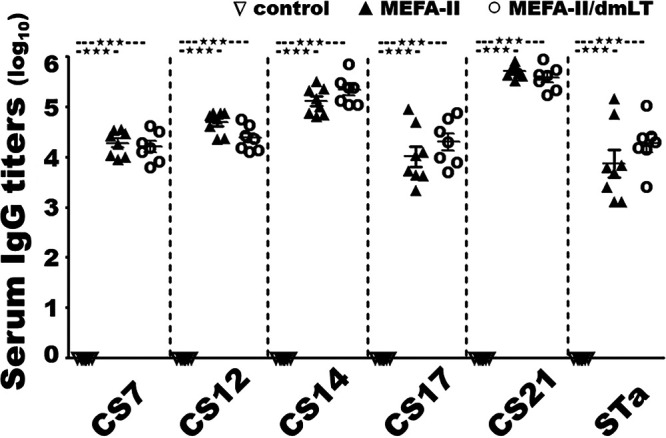
Mouse serum IgG titers (log10) from the group intramuscularly immunized with 25 μg adhesin MEFA-II (▴), 25 μg adhesin MEFA-II with 0.1 μg adjuvant dmLT (○), or 25 μL PBS (▽). ELISAs using major subunit protein CsvA, CswA, CsvA, CsbA, or LngA as the coating antigen were to titrate mouse serum anti-CS7, -CS12, -CS14, -CS17, and anti-CS21 IgG response, and STa-ovalbumin conjugates to titrate anti-STa IgG titers. Bars represented antibody titer means and standard deviations. *** indicated a P-value of less than 0.0001.
Mouse serum antibodies induced by adhesin MEFA-II broadly inhibited adherence of ETEC strains producing CS7, CS12, CS14, CS17, or CS21 adhesin.
ETEC bacteria expressing adhesin CS7, CS12, CS14, CS17, or CS21, after being incubated with heat-inactivated serum samples from the mice immunized with the adhesin MEFA-II protein, with or without adjuvant dmLT, showed a significant reduction in adherence to Caco-2 cells, compared to the bacteria incubated with the serum samples from the control group (Fig. 3). After incubation with the sera of the mice immunized with adhesin MEFA-II, in vitro adherence from CS7, CS12, CS14, CS17, and CS21 ETEC bacteria was reduced by 82%, 55%, 70%, 49%, and 62%, respectively. When the sera from the group immunized with adhesin MEFA-II and dmLT adjuvant were used, the adherence was reduced by 84%, 55%, 71%, 52%, and 62%. There were no significant differences in inhibiting adherence against these five adhesins between the two immunized groups (adhesin MEFA-II with or without adjuvant dmLT). Additionally, adherence to Caco-2 cells from H10407 bacteria (CFA/I, STa, LT) was reduced by 73% after incubation with the sera from the group immunized with adhesin MEFA-II without dmLT, and 74% incubated with sera of the group immunized with adhesin MEFA-II and dmLT adjuvant.
FIG 3.
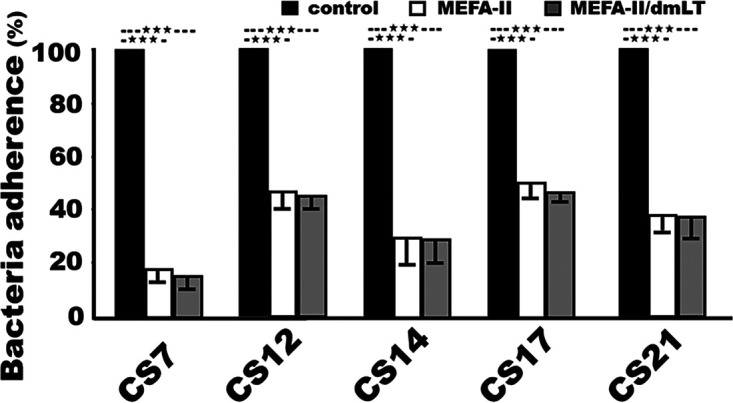
Mouse serum antibody adherence inhibition against ETEC field isolates expressing adhesin CS7 (JF2327), CS12 (JF3276), CS14 (JF2125), CS17 (JF2350), or CS21 (JF2101). Bacteria adherence to Caco-2 cells (%) from each ETEC isolate incubation with mouse sera from the group immunized with adhesin MEFA-II (white box), adhesin MEFA-II and adjuvant dmLT (gray box), or PBS (black box). Adherent bacteria were overnight cultured on agar plates and CFU were counted, with the CFU from the bacteria treated with the control mouse serum referred to as 100% (of adherence). Boxes and bars represented the means and standard deviations of the adherent bacteria (CFU in %). *** indicated a P-value of less than 0.0001.
Mouse serum antibodies induced by adhesin MEFA-II exhibited neutralization activity against STa toxin enterotoxicity.
After incubation with the serum samples of the mice immunized with adhesin MEFA-II protein, STa toxin was shown a significant reduction in stimulating the elevation of intracellular cGMP levels in T-84 cells, measured with a cyclic GMP EIA kit (Fig. 4). In contrast, the serum sample from the control mice was unable to prevent STa toxin from stimulating intracellular cGMP in T-84 cells. The intracellular cGMP levels were 40.7 ± 5.7 and 8.6 ± 1.5 (nM), respectively, in the T-84 cells treated with STa and sera of the mice immunized with adhesin MEFA-II or adhesin MEFA-II and dmLT adjuvant, significantly lower than the intracellular cGMP in the cells treated with STa and the control mouse sera were 87.1 ± 3.2 nM (P < 0.001). Sera from the group immunized with adhesin MEFA-II adjuvanted with dmLT showed significantly better in preventing STa from stimulation of cGMP (P = 0.01). The baseline cGMP in T-84 cells (without STa toxin or mouse sera) was 7.1 ± 0.64 nM (Fig. 4).
FIG 4.
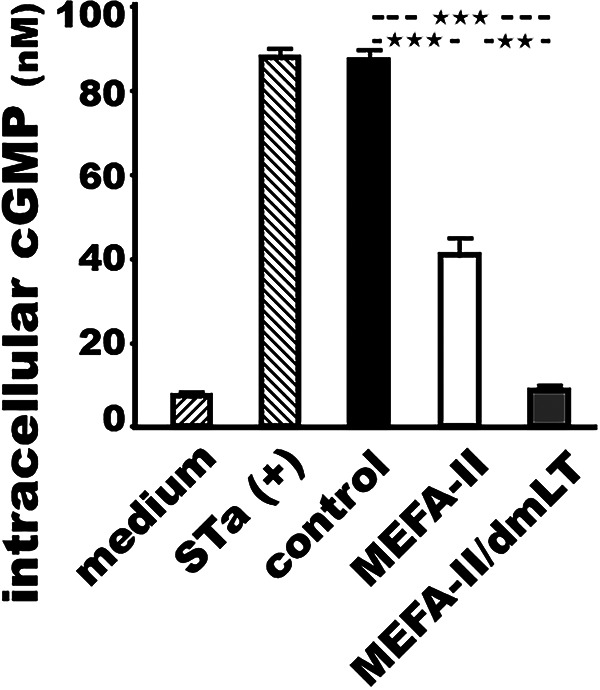
Mouse serum antibody neutralization against STa enterotoxicity. Intracellular cyclic GMP (nM; picomole per mL) in the T-84 cells after incubation with STa toxin (2 ng) which was treated with mouse sera from the group immunized with adhesin MEFA-II (white box), adhesin MEFA-II and adjuvant dmLT (gray box), or PBS (black box), measured with a cGMP EIA kit (Enzo Life Sciences) by following the manufacturer’s protocol. T-84 cells cultured with STa toxin alone served as a positive control to show STa enterotoxicity in the stimulation of cGMP, whereas T-84 cells with culture medium indicate cGMP baseline levels. Boxes and bars represented the means and standard deviations of the cGMP levels (nM). *** indicated a P-value of less than 0.0001 and ** of less than 0.001.
DISCUSSION
Results from the current study support the application of the epitope- and structure-based MEFA vaccinology platform in the construction of broadly immunogenic and cross-functional polyvalent immunogens. This MEFA platform has been applied to generate protein antigens with broad immunogenicity, including ETEC adhesin CFA/I/II/IV or tip MEFA, toxoid fusion, fimbria-toxoid MEFA, as well as a cholera MEFA (20, 22, 26–29). Data showed this polyvalent adhesin MEFA-II protein is broadly immunogenic and induces antibodies to inhibit adherence of CS7, CS12, CS14, CS17, and CS21 adhesins, the adhesins expressed by ETEC strains that are prevalent and cause moderate-to-severe diarrhea. Additionally, adhesin MEFA-II carries STa toxoid STaN12S and induces antibodies that neutralize STa enterotoxicity. Since STa toxin plays a more important role in causing ETEC-associated children’s diarrhea and travelers’ diarrhea (1, 2, 30), functional anti-STa antibodies are essential for an effective ETEC vaccine.
It needs to be pointed out that adhesin MEFA-II is not intended to be the sole antigen for an ETEC vaccine candidate. Unlike CFA/I and CS1 to CS6 adhesins, these five adhesins (CS7, CS12, CS14, CS14, CS21) targeted by adhesin MEFA-II are regarded as the second tier of ETEC adhesins in causing diarrhea. Instead, it is rather a potentially additive (not an alternate) adhesin antigen to ETEC vaccine candidate MecVax. MecVax is composed of two polyvalent proteins, adhesin CFA/I/II/IV MEFA and toxoid fusion 3xSTaN12S-mnLTR192G/L211A; this vaccine candidate not only induces functional antibodies against adherence from the seven first-tier or major ETEC adhesins (CFA/I, CS1 to CS6) and enterotoxicity of both enterotoxins (STa, LT) (23), it also protects against clinical diarrhea in pigs when challenged with a STa+ or an LT+ ETEC strain (8) and bacteria intestinal colonization in rabbits when inoculated with an ETEC expressing adhesin CFA/I (25) or CS1 to CS6 (data unpublished). With the addition of adhesin MEFA-II, MecVax would be expanded to protect against 12 ETEC adhesins (all of the important ETEC adhesins) and both ETEC enterotoxins, becoming an ETEC vaccine with an unprecedentedly broad spectrum to protect against ETEC diarrhea. Additionally, since STa toxin plays a more important role in causing children’s diarrhea and travelers’ diarrhea, additive protection against STa toxin from adhesin MEFA-II will enhance MecVax efficacy against STa-related ETEC diarrhea (after this MEFA-II protein be combined with MecVax). Surely, future studies on antigen compatibility among three polyvalent proteins and preclinical efficacy are needed to validate the candidacy of such an ETEC vaccine candidate for even broader coverage.
The current study is limited to antigen immunogenicity characterization and in vitro function assessment of MEFA-II-induced antibodies against bacterial adherence and STa enterotoxicity. Future studies with a rabbit colonization model and a pig passive protection model will be needed to evaluate adhesin MEFA-II in vivo protection against intestinal colonization from ETEC strains producing CS7, CS12, CS14, CS17, or CS21 adhesin or clinical diarrhea from a STa+ ETEC infection. It was observed that results from in vitro assays especially the antibody adherence inhibition assay tend to underestimate antibody in vivo protection against ETEC or Vibrio cholerae bacteria intestinal colonization (22, 25). For example, while rabbit serum antibodies (immunized with CFA/I/II/IV MEFA or MecVax) inhibited 60–70% of the adherence (in vitro) from ETEC H10407 (CFA/I, STa, LT) to Caco-2 cells, these rabbits showed over two logs (>99%) reduction (in vivo) of H10407 bacteria colonization in small intestines after challenge (25). It is expected that, therefore, antibodies derived from adhesin MEFA-II, which reduced 50% to 80% in vitro adherence from ETEC expressing any of the five targeted adhesins, will protect against bacteria intestinal colonization in future in vivo ETEC challenge studies.
We included two epitopes from the major subunit of CS14 or CS21 into adhesin MEFA-II because these two adhesins were reported as more prevalent or more significant in association with moderate-to-severe diarrhea than adhesins CS7, CS12, and CS17 (13, 15, 16). Additionally, dmLT (LTR192G/L211A) was used as the adjuvant in one immunized group. This dmLT is reported to stimulate an immunogen for antigen-specific antibody responses and potentially mucosal responses (31). While dmLT elicited anti-LT IgG responses in the immunized mice, it did not enhance adhesin MEFA-II protein in eliciting IgG to the five targeted adhesins or STa, nor did it stimulate antigen-specific IgA responses in the current study. Adjuvant dmLT or mLT (single mutant LT, LTR192G) is linked to stimulation of low levels of antigen-specific IgA responses in the mice parenterally immunized with a protein immunogen (32, 33). Those two studies that used 0.5 μg or 1.5 μg adjuvant, however, differed from the current study in which only 0.1 μg dmLT was included. Whether a high dose of dmLT adjuvant elevates adhesin MEFA-II antigen-specific IgG or IgA responses is to be examined in future studies.
It was noted that serum antibodies from the mice immunized with adhesin MEFA-II and adjuvant dmLT were significantly better in neutralizing STa enterotoxicity compared to the serum antibodies from the group immunized with the adhesin MEFA-II alone, despite mouse serum anti-STa antibody titers were not significantly different between these two groups. The mechanism of dmLT adjuvant in enhancing anti-STa antibody neutralizing activity is unknown momently. Additionally, we did not test the neutralizing activity of dmLT-induced anti-LT antibodies against LT enterotoxicity in this study. However, the dmLT adjuvant, at a dose of 0.1 to 1 μg, was shown to induce antibodies that neutralized LT enterotoxicity (34). Because of that, we designed adhesin MEFA-II to include STa toxoid STaN12S (but not LT epitopes) and expected this protein antigen to induce functional antibodies against STa enterotoxicity (in addition to five adhesins), thus another ETEC vaccine candidate composed of two polyvalent adhesin proteins, CFA/I/II/IV MEFA and this adhesin MEFA-II, adjuvanted with dmLT, would be able to induce functional antibodies against 12 ETEC adhesins and both ETEC toxins. Ideally, by adding adhesin MEFA-II to MecVax (which consists of CFA/I/II/IV MEFA protein and toxoid fusion protein), a new vaccine candidate can induce functional antibodies against all of the 12 important adhesins and two toxins, with enhanced elicitation of neutralizing anti-STa antibodies. Future studies will reveal if the incorporation of another copy of STaN12S toxoid to adhesin MEFA-II would improve anti-STa antibody neutralizing activity (without dmLT adjuvant), and if combined with MecVax would lead to a vaccine candidate of broad protection against ETEC-associated children’s diarrhea and travelers’ diarrhea.
In conclusion, polyvalent adhesin MEFA-II protein constructed with the assistance of the epitope- and structure-based MEFA vaccinology platform exhibited broad immunogenicity and induced cross-functional antibodies against five ETEC adhesins (CS7, CS12, CS14, CS17, and CS21) and STa toxin. If adhesin MEFA-II is demonstrated in future studies to be broadly protective against ETEC intestinal colonization and antigenically compatible with the polyvalent CFA/I/II/IV adhesin or the two MecVax antigens (CFA/I/II/IV MEFA and toxoid fusion), a more broadly protective ETEC vaccine against all the important ETEC strains causing children’s diarrhea and travelers’ diarrhea can be developed.
MATERIALS AND METHODS
Ethics statement.
The care and handling of the mice used in this study complied with the Animal Welfare Act (1996 National Research Council Guidelines) and Policy on Humane Care and Use of Laboratory Animals (Public Health Service). The mouse immunization protocol (UIUC Protocol number 22054) was approved by the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee; animal use and care were supervised by the institutional attending veterinarians and staff.
Bacteria strains and plasmids.
Recombinant E. coli strain 9916 expressing the tag-less adhesin MEFA-II protein was constructed by transforming E. coli BL21 CodonPlus (DE3) (Agilent Technologies; Santa Clara, CA) with plasmid pET28α that had the synthetic adhesin MEFA-II gene cloned at the NcoI and EagI sites. Recombinant E. coli strains to express CS7, CS12, CS14, CS17, and CS21 major subunit proteins were constructed to express recombinant major subunit proteins CsvA, CswA, CsuA, CsbA, and LngA (to be used as ELISA coating antigens to titrate antigen-specific antibody responses). ETEC field isolates expressing adhesin CS7, CS12, CS14, CS17, or CS21 together with STa and/or LT toxin (35) were used in antibody adherence inhibition assays (Table 1).
TABLE 1.
The recombinant Escherichia coli strains expressing ETEC adhesin MEFA-II protein or the major subunits of CS7, CS12, CS14, CS17, or CS21 adhesin, as well as the ETEC field isolates expressing CS7, CS12, CS14, CS17, or CS21 adhesin used in antibody adherence inhibition assay were listed
| Strains | Relevant properties | Sources |
|---|---|---|
| BL21 CodenPlus (DE3) | B F– ompT hsdS(rB – mB –) dcm+ Tetr gal l (DE3) endA Hte [argU proL Camr | Agilent Technologies |
| 9916 | ‘Adhesin MEFA-II + pET28α’ (NcoI/EagI) in BL21 E. coli | This study |
| 9611 | ‘CS7 major subunit CsvA + pET28α’ (NcoI/EagI) in BL21 E. coli | This study |
| 9604 | ‘CS12 major subunit CswA + pET28α’ (NcoI/EagI) in BL21 E. coli | This study |
| 9579 | ‘CS14 major subunit CsuA + pET28α’ (NcoI/EagI) in BL21 E. coli | This study |
| 9580 | ‘CS17 major subunit CsbA + pET28α’ (NcoI/EagI) in BL21 E. coli | This study |
| 9582 | ‘CS21 major subunit LngA + pET28α’ (NcoI/EagI) in BL21 E. coli | This study |
| JE2327 | CS7, LT | Washington University at St Louis (35) |
| JF3276 | CS12/CS20, LT, STa | Washington University at St Louis (35) |
| JF2125 | CS14, LT | Washington University at St Louis (35) |
| JF2350 | CS17, LT | Washington University at St Louis (35) |
| JF2101 | CS21, STa | Washington University at St Louis (35) |
PCR primers to amplify adhesin subunit genes csvA, cswA, csuA, csbA, and lngA were listed in Table 2. Each amplified subunit gene was digested with restriction enzymes NcoI and EagI, ligated into vector pET28α, and expressed by E. coli BL21 CodonPlus (DE3) for each adhesin major subunit protein.
TABLE 2.
The PCR primers used in this study to amplify the CS7, CS12, CS14, CS17, and CS21 major subunit genes, with restriction site NcoI underlined in each forward primer and EagI in the reverse primer
| Primer | Sequences (5′ → 3′) |
|---|---|
| CS7(CsvA)-F | CGCCATGGAAATGGCAACCGTATCAG |
| CS7(CsvA)-R | TCGGCCGCTTTACGCCACATCCGAAGCACTT |
| CS12(CswA)-F | CATGCCATGGAAATGGCTGTTACTCTAATGAATAGCTCA |
| CS12(CswA)-R | GAGTCGGCCGCTTTATGAACACCAACAGTATTAAGTGAA |
| CS14(CsuA)-F | CATGCCATGGAA ATGGCTCTGAGCACAATATTTGTAG |
| CS14(CsuA)-R | GAGTCGGCCGCTTTAGTTTGTCGATTGAGTCATTACAATGGA |
| CS17(CsbA)-F | CTAGCTAGCTAGATGGCTATGGCGACTCTGTTTGCCA |
| CS17(CsbA)-R | GAGTCGGCCGCTTTATAATACCTTGGTAATTGCCTGCCTCA |
| CS21(LngA)-F | CATGCCATGGGCATGAGCCTGCTGGAAGTTATCATTGTT |
| CS21(LngA)-R | GAGTCGGCCGCTTTAACGGCTACCTAAAGTAATTGAG |
ETEC adhesin MEFA-II gene construction and protein expression.
MEFA vaccinology platform was used for in silico identification of immunodominant continuous B-cell epitopes and the selection of a backbone for adhesin MEFA-II as described previously (21). Briefly, online software IEDB (www.iedb.org) and BepiPred-2.0 (http://tools.iedb.org/bcell/help/#Bepipred-2.0) were applied to identify the most immunogenic and continuous B-cell epitopes (based on antigenicity scores) from major subunits CS7 major subunit CsvA, CS12 major subunit CswA, CS14 major subunit CsuA, CS17 major subunit CsbA, and CS21 major subunit LngA, respectively. Epitope surface exposure was assessed with hydrophilicity, and epitope location and presentation were visualized with PyMol (www.pymol.org) and Phyre2 (www.sbg.bio.ic.ac.uk/~phyre2). CFA/I major subunit CfaB, which served as the backbone of CFA/I/II/IV MEFA (20), was selected as the backbone because of its strong immunogenicity, structural stability, and the presence of multiple continuous B-cell epitopes. By substituting the CfaB backbone epitopes with the most immunogenic epitope(s) predicted from each subunit, one epitope from CS7, CS12, or CS17 and two epitopes from CS14 or CS21, as well as a full-length STa toxoid STaN12S, adhesin MEFA-II was constructed initially in silico. The antigenicity, location, and presentation of each inserted epitope on the MEFA-II protein were assessed and confirmed with IEDB BepiPred-2.0, PyMol, and Phyre2. The MEFA-II protein was examined for stability with ExPASy (www.expasy.org). This adhesin MEFA-II gene was then codon optimized and synthesized by GenScript Biotech (Piscataway, NJ), cloned into pET28α (at the cloning sites of NcoI and EagI), and the plasmid was used to transform E. coli BL21CodonPlus (DE3) strain.
The expressed adhesin MEFA-II protein was extracted with bacteria protein extraction reagent (B-PER; Thermo Fisher Scientific; Waltham, MA) as described previously (20, 22, 36). Briefly, adhesin MEFA-II protein was expressed as an inclusion body protein (to increase protein yield), then solubilized with solubilization buffer (50 mM CAPs supplemented with 0.3% N-lauroylsarcosine and 1 mM dithiothreitol, pH 11.0), and refolded with 1 M Tris-HCl (pH 8.5) supplemented with 0.1 mM dithiothreitol in the first two buffer changes and then Tris-HCl buffer without dithiothreitol. After examination of the protein concentration, the refolded adhesin MEFA-II protein was aliquoted (1 mg per mL), characterized in SDS-PAGE with Coomassie blue staining and Western blot with mouse polyclonal antibodies to CS14 major subunit CsuA, CS21 major subunit LngA, CS17 major subunit CsbA, or backbone CfaB (1:5,000), and stored at −80°C.
Mouse intramuscular immunization with adhesin MEFA-II protein.
Eight-week-old female BALB/c mice (Charles River Laboratories; Wilmington, MA), 8 per group (23 out the 24 mice ordered were delivered, thus 7 mice in one group and 8 in the other groups), were used for intramuscular immunization. The first group was immunized with 25 μg adhesin MEFA-II protein (in 25 μL); the second group (7 mice) was immunized with 25 μg adhesin MEFA-II protein (in 25 μL) and 0.1 μg (in 1 μL) dmLT adjuvant (double mutant holotoxin-structured heat-labile toxin, LTR192G/L211A; provided by PATH); the third group was injected with 25 μL PBS as the control. Two boosters were followed with the same dose of the primary at the interval of 2 weeks. 2 weeks after the final booster, all mice were sacrificed. Blood samples were collected from each mouse before the primary and 2 weeks after the final booster. Serum samples were stored at −80°C until use.
Mouse serum antigen-specific antibody titration.
Serum samples collected from each mouse before the primary and 2 weeks after the final booster were titrated for IgG and IgA responses to the major subunit of CS7, CS12, CS14, CS17, or CS21 in ELISAs. As described previously (8, 20, 34), each adhesin major subunit recombinant protein (CsvA, CswA, CsuA, CsbA, LngA), 100 ng per well, was coated to 2HB 96-well microtiter plates (Thermo Fisher Scientific). To titrate anti-STa antibodies, STa-ovalbumin conjugates (10 ng per well) were used to coat Costar 96-well plates (Corning Inc.; Corning, NY); to titrate anti-LT (from dmLT adjuvant), cholera toxin (CT; Sigma, St. Louis, MO), 100 ng per well, was used to coat 2HB plates. Mouse serum samples, in 2-fold serial dilutions (1:200 to 1:25,600), were added to the coated wells accordingly. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG or IgA (1:5,000; Bethyl Laboratories; Montgomery, TX) was used as the secondary antibody, and 3,3’5,5′-tetramethylbenzidine (TMB) peroxidase substrate (KPL, Gaithersburg, MD) was used to visualize optical density. OD values were measured at the wavelength of 650 nm, and antibody titers were converted into log10.
Mouse serum antibody bacterial adherence inhibition assay.
Mouse serum samples pooled from each group were examined for antibody adherence inhibition activity against ETEC field isolates expressing CS7, CS12, CS14, CS17, or CS21 adhesin with Caco-2 cells. As described previously (8, 20, 24, 34), 3.0 to 3.5× 106 CFU of bacteria from each ETEC strain were first treated with 10% mannose, then mixed with 15 μL heat-inactivated mouse sera. After 1 h of incubation at room temperature (50 rpm), the serum/bacteria mixture was added to 95–100% confluent monolayered Caco-2 cells in wells of a 24-well tissue culture plate (6 to 7× 105 Caco-2 cells per well). Incubated in a 37°C and 5% CO2 incubator for 1 h, cells were rinsed to remove the nonadherent ETEC bacteria. Cells (with adherent ETEC bacteria) were then dislodged with 0.5% Triton (Sigma), collected, serially diluted, and plated on LB agar plates. Bacteria (CFU) were counted after overnight growth at 37°C. Bacteria CFU from each strain were converted to percentages, with the CFU number from the bacteria treated with the control mouse sera referred to as 100% adherence.
Mouse serum antibody neutralization against STa enterotoxicity.
Mouse sera pooled from each immunized group or the control group were examined for antibody neutralization activity against STa toxin enterotoxicity, by using T-84 cells (ATCC, CCL-248) and a cyclic GMP EIA kit (Enzo Life Sciences, Farmingdale, NY) as described previously (8, 24, 37). Briefly, 30 μL mouse serum sample was mixed with 2 ng STa toxin, in duplicates, and incubated at room temperature for 30 min. Each toxin/serum mixture was added to 95–100% confluent monolayered T-84 cells and incubated for 1 h in a 37°C and 5% CO2 incubator. After gentle washes with PBS to discard extracellular cGMP, T-84 cells were lysed with 0.1 M HCl supplemented with 0.5% Triton X-100 (Sigma) to release intracellular cGMP. The lysates were collected and intracellular cGMP levels (nanomolar, picomole per mL) were measured by using the cGMP EIA kit by following the manufacturer’s protocol (Enzo Life). T-84 cells incubated with STa toxin without sera served as a positive control to show the stimulation of intracellular cGMP by STa enterotoxicity, and T-84 cells cultured in Dulbecco’s Modified Eagle Medium/F12 culture medium were a negative control to show cGMP baseline levels.
Statistical analyses.
Data were presented as means and standard deviations. Two-way ANOVA with the Bonferroni post hoc test, from GraphPad Prism 7 (San Diego, CA), was used to analyze mouse serum antibody titers (expressed in log10), antibody adherence inhibition activities (in %), and cGMP levels (nM). A calculated P-value less than 0.05 was considered significant. Samples were tested in triplicate (in antibody titration and antibody adherence inhibition assay) or duplicate (in cGMP EIA), and each assay was repeated.
ACKNOWLEDGMENTS
The ETEC field isolates expressing CS7, CS12, CS14, CS17, or CS21 adhesin were kindly provided by James Fleckenstein (Washington University at St. Louis), and adjuvant dmLT was supplied from PATH. Financial support for this study was provided by NIH R01AI121067.
Contributor Information
Weiping Zhang, Email: wpzhang@illinois.edu.
Charles M. Dozois, INRS Armand-Frappier Sante Biotechnologie Research Centre
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque ASG, Zaidi AKM, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, McMurry TL, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, Rajendiran R, Benny B, McGrath M, McCormick BJJ, Seidman JC, Lang D, Gottlieb M, Guerrant RL, Lima AAM, Leite JP, Samie A, Bessong PO, Page N, Bodhidatta L, Mason C, Shrestha S, Kiwelu I, Mduma ER, Iqbal NT, Bhutta ZA, Ahmed T, Haque R, Kang G, Kosek MN, MAL-ED Network Investigators ., et al. 2018. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 6:E1309–E1318. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang ZD, DuPont HL. 2017. Etiology of travellers' diarrhea. J Travel Med 24:S13–S16. doi: 10.1093/jtm/tax003. [DOI] [PubMed] [Google Scholar]
- 4.Walker RI, Wierzba TF, Mani S, Bourgeois AL. 2017. Vaccines against Shigella and enterotoxigenic Escherichia coli: a summary of the 2016 VASE Conference. Vaccine 35:6775–6782. doi: 10.1016/j.vaccine.2017.09.045. [DOI] [PubMed] [Google Scholar]
- 5.Frost I, Sati H, Garcia-Vello P, Hasso-Agopsowicz M, Lienhardt C, Gigante V, Beyer P. 2023. The role of bacterial vaccines in the fight against antimicrobial resistance: an analysis of the preclinical and clinical development pipeline. Lancet Microbe 4:e113–125. doi: 10.1016/S2666-5247(22)00303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Lundgren A, Wiklund G, Kaim J, Lofstrand M, Carlin N, Bourgeois AL, Maier N, Fix A, Wierzba T, Walker RI, Svennerholm AM. 2020. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis 20:208–219. doi: 10.1016/S1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riddle MS, Maciel M, Jr, Porter CK, Poole ST, Gutierrez RL, Gormley R, Laird RM, Sebeny PJ, Dori KE, Greenleaf ME, Hoq F, Turiansky GW, Jarell A, Hawk D, Tribble D, Savarino SJ. 2020. A first in human clinical trial assessing the safety and immunogenicity of transcutaneously delivered enterotoxigenic Escherichia coli fimbrial tip adhesin with heat-labile enterotoxin with mutation R192G. Vaccine 38:7040–7048. doi: 10.1016/j.vaccine.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Seo H, Garcia C, Ruan X, Duan Q, Sack DA, Zhang W. 2021. Preclinical characterization of immunogenicity and efficacy against diarrhea from mecVax, a multivalent enterotoxigenic E. coli vaccine candidate. Infect Immun 89:e0010621. doi: 10.1128/IAI.00106-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svennerholm A-M. 2011. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. The Indian J Medical Res 133:188–196. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Sack DA. 2012. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev Vaccines 11:677–694. doi: 10.1586/erv.12.37. [DOI] [PubMed] [Google Scholar]
- 11.Seo H, Duan Q, Zhang W. 2020. Vaccines against gastroenteritis, current progress and challenges. Gut Microbes 11:1486–1517. doi: 10.1080/19490976.2020.1770666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaastra W, Svennerholm AM. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol 4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 13.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 14.von Mentzer A, Connor TR, Wieler LH, Semmler T, Iguchi A, Thomson NR, Rasko DA, Joffre E, Corander J, Pickard D, Wiklund G, Svennerholm AM, Sjoling A, Dougan G. 2014. Identification of enterotoxigenic Escherichia coli (ETEC) clades with long-term global distribution. Nat Genet 46:1321–1326. doi: 10.1038/ng.3145. [DOI] [PubMed] [Google Scholar]
- 15.Kuhlmann FM, Martin J, Hazen TH, Vickers TJ, Pashos M, Okhuysen PC, Gomez-Duarte OG, Cebelinski E, Boxrud D, Del Canto F, Vidal R, Qadri F, Mitreva M, Rasko DA, Fleckenstein JM. 2019. Conservation and global distribution of non-canonical antigens in Enterotoxigenic Escherichia coli. PLoS Negl Trop Dis 13:e0007825. doi: 10.1371/journal.pntd.0007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidal RM, Muhsen K, Tennant SM, Svennerholm AM, Sow SO, Sur D, Zaidi AKM, Faruque ASG, Saha D, Adegbola R, Hossain MJ, Alonso PL, Breiman RF, Bassat Q, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ahmed S, Qureshi S, Quadri F, Hossain A, Das SK, Antonio M, Mandomando I, Nhampossa T, Acacio S, Omore R, Ochieng JB, Oundo JO, Mintz ED, O'Reilly CE, Berkeley LY, Livio S, Panchalingam S, Nasrin D, Farag TH, Wu Y, Sommerfelt H, Robins-Browne RM, Del Canto F, Hazen TH, Rasko DA, Kotloff KL, Nataro JP, Levine MM. 2019. Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 13:e0007037. doi: 10.1371/journal.pntd.0007037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kharat VB, Ahmed M, Jiang ZD, Riddle MS, DuPont HL. 2017. Colonization Factors in enterotoxigenic Escherichia coli strains in travelers to Mexico, Guatemala, and India compared with children in Houston, Texas. Am J Trop Med Hyg 96:83–87. doi: 10.4269/ajtmh.16-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenzie R, Porter CK, Cantrell JA, Denearing B, O'Dowd A, Grahek SL, Sincock SA, Woods C, Sebeny P, Sack DA, Tribble DR, Bourgeois AL, Savarino SJ. 2011. Volunteer challenge with enterotoxigenic Escherichia coli that express intestinal colonization factor fimbriae CS17 and CS19. J Infect Dis 204:60–64. doi: 10.1093/infdis/jir220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begum YA, Baby NI, Faruque AS, Jahan N, Cravioto A, Svennerholm AM, Qadri F. 2014. Shift in phenotypic characteristics of enterotoxigenic Escherichia coli (ETEC) isolated from diarrheal patients in Bangladesh. PLoS Negl Trop Dis 8:e3031. doi: 10.1371/journal.pntd.0003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruan X, Knudsen DE, Wollenberg KM, Sack DA, Zhang W. 2014. Multiepitope fusion antigen induces broadly protective antibodies that prevent adherence of Escherichia coli strains expressing colonization factor antigen I (CFA/I), CFA/II, and CFA/IV. Clin Vaccine Immunol 21:. doi: 10.1128/CVI.00652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li SKL, Zhang W. 2022. Multiepitope fusion antigen: MEFA, an epitope- and structure-based vaccinology platform for multivalent vaccine development. Methods Mol Biol 2414:151–169. doi: 10.1007/978-1-0716-1900-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upadhyay I, Li S, Ptacek G, Seo H, Sack DA, Zhang W. 2022. A polyvalent multiepitope protein cross-protects against Vibrio cholerae infection in rabbit colonization and passive protection models. Proc Natl Acad Sci USA 119:e2202938119. doi: 10.1073/pnas.2202938119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan Q, Lu T, Garcia C, Yanez C, Nandre RM, Sack DA, Zhang W. 2018. Co-administered tag-less toxoid fusion 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA (multiepitope fusion antigen) induce neutralizing antibodies to 7 adhesins (CFA/I, CS1-CS6) and both enterotoxins (LT, STa) of enterotoxigenic Escherichia coli (ETEC). Front Microbiol 9:e1198. doi: 10.3389/fmicb.2018.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia CY, Seo H, Sack DA, Zhang W. 2022. Intradermally administered enterotoxigenic Escherichia coli vaccine candidate MecVax induces functional serum immunoglobulin G antibodies against seven adhesins (CFA/I and CS1 through CS6) and both toxins (STa and LT). Appl Environ Microbiol 88:e0213921. doi: 10.1128/AEM.02139-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upadhyay I, Lauder KL, Li S, Ptacek G, Zhang W. 2022. Intramuscularly administered enterotoxigenic Escherichia coli (ETEC) vaccine candidate MecVax prevented H10407 intestinal colonization in an adult rabbit colonization model. Microbiol Spectr 10: e0147322. doi: 10.1128/spectrum.01473-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nandre RM, Ruan X, Duan Q, Sack DA, Zhang W. 2016. Antibodies derived from an enterotoxigenic Escherichia coli (ETEC) adhesin tip MEFA (multiepitope fusion antigen) against adherence of nine ETEC adhesins: CFA/I, CS1, CS2, CS3, CS4, CS5, CS6, CS21 and EtpA. Vaccine 34:3620–3625. doi: 10.1016/j.vaccine.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan X, Robertson DC, Nataro JP, Clements JD, Zhang W, Group. tSTVC . 2014. Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infect Immun 82:1823–1832. doi: 10.1128/IAI.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu T, Moxley RA, Zhang W. 2020. Application of a novel epitope and structure vaccinology-assisted fimbria-toxin multiepitope fusion antigen of enterotoxigenic Escherichia coli for multivalent vaccine development against porcine post-weaning diarrhea. Appl Environ Microbiol 86:e00274-20. doi: 10.1128/AEM.00274-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nandre R, Ruan X, Lu T, Duan Q, Sack D, Zhang W. 2018. Enterotoxigenic Escherichia coli adhesin-toxoid multiepitope fusion antigen CFA/I/II/IV-3xSTaN12S-mnLTR192G/L211A-derived antibodies inhibit adherence of seven adhesins, neutralize enterotoxicity of LT and STa toxins, and protect piglets against diarrhea. Infect Immun 86:e00550-17. doi: 10.1128/IAI.00550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turunen K, Antikainen J, Laaveri T, Kirveskari J, Svennerholm AM, Kantele A. 2020. Clinical aspects of heat-labile and heat-stable toxin-producing enterotoxigenic Escherichia coli: a prospective study among Finnish travellers. Travel Med Infect Dis 38:101855. doi: 10.1016/j.tmaid.2020.101855. [DOI] [PubMed] [Google Scholar]
- 31.Norton EB, Bauer DL, Weldon WC, Oberste MS, Lawson LB, Clements JD. 2015. The novel adjuvant dmLT promotes dose sparing, mucosal immunity and longevity of antibody responses to the inactivated polio vaccine in a murine model. Vaccine 33:1909–1915. doi: 10.1016/j.vaccine.2015.02.069. [DOI] [PubMed] [Google Scholar]
- 32.Lee T, Gutierrez RL, Maciel M, Poole S, Testa KJ, Trop S, Duplessis C, Lane A, Riddle MS, Hamer M, Alcala A, Prouty M, Maier N, Erdem R, Louis Bourgeois A, Porter CK. 2021. Safety and immunogenicity of intramuscularly administered CS6 subunit vaccine with a modified heat-labile enterotoxin from enterotoxigenic Escherichia coli. Vaccine 39:5548–5556. doi: 10.1016/j.vaccine.2021.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rollenhagen JE, Woods CM, O'Dowd A, Poole ST, Tian JH, Guebre-Xabier M, Ellingsworth L, Prouty MG, Glenn G, Savarino SJ. 2019. Evaluation of transcutaneous immunization as a delivery route for an enterotoxigenic E. coli adhesin-based vaccine with CfaE, the colonization factor antigen 1 (CFA/I) tip adhesin. Vaccine 37:6134–6138. doi: 10.1016/j.vaccine.2019.08.057. [DOI] [PubMed] [Google Scholar]
- 34.Seo H, Lu T, Mani S, Bourgeois AL, Walker R, Sack DA, Zhang W. 2020. Adjuvant effect of enterotoxigenic Escherichia coli (ETEC) double-mutant heat-labile toxin (dmLT) on systemic immunogenicity induced by the CFA/I/II/IV MEFA ETEC vaccine: dose-related enhancement of antibody responses to seven ETEC adhesins (CFA/I, CS1-CS6). Hum Vaccin Immunother 16:419–425. doi: 10.1080/21645515.2019.1649555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Q, Qadri F, Kansal R, Rasko DA, Sheikh A, Fleckenstein JM. 2015. Conservation and immunogenicity of novel antigens in diverse isolates of enterotoxigenic Escherichia coli. PLoS Negl Trop Dis 9:e0003446. doi: 10.1371/journal.pntd.0003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Han X, Upadhyay I, Zhang W. 2022. Characterization of functional B-cell epitopes at the amino terminus of Shigella invasion plasmid antigen B (IpaB). Appl Environ Microbiol 88:e0038422. doi: 10.1128/aem.00384-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo H, Duan Q, Upadhyay I, Zhang W. 2022. Evaluation of Multivalent enterotoxigenic Escherichia coli vaccine candidate MecVax antigen dose-dependent effect in a murine model. Appl Environ Microbiol 88:e0095922. doi: 10.1128/aem.00959-22. [DOI] [PMC free article] [PubMed] [Google Scholar]