SUMMARY
The quest for bacterial survival is exemplified by spores formed by some Firmicutes members. They turn up everywhere one looks, and their ubiquity reflects adaptations to the stresses bacteria face. Spores are impactful in public health, food safety, and biowarfare. Heat resistance is the hallmark of spores and is countered principally by a mineralized gel-like protoplast, termed the spore core, with reduced water which minimizes macromolecular movement/denaturation/aggregation. Dry heat, however, introduces mutations into spore DNA. Spores have countermeasures to extreme conditions that are multifactorial, but the fact that spore DNA is in a crystalline-like nucleoid in the spore core, likely due to DNA saturation with small acid-soluble spore proteins (SASPs), suggests that reduced macromolecular motion is also critical in spore dry heat resistance. SASPs are also central in the radiation resistance characteristic of spores, where the contributions of four spore features—SASP; Ca2+, with pyridine-2,6-dicarboxylic acid (CaDPA); photoproduct lyase; and low water content—minimize DNA damage. Notably, the spore environment steers UV photochemistry toward a product that germinated spores can repair without significant mutagenesis. This resistance extends to chemicals and macromolecules that could damage spores. Macromolecules are excluded by the spore coat which impedes the passage of moieties of ≥10 kDa. Additionally, damaging chemicals may be degraded or neutralized by coat enzymes/proteins. However, the principal protective mechanism here is the inner membrane, a compressed structure lacking lipid fluidity and presenting a barrier to the diffusion of chemicals into the spore core; SASP saturation of DNA also protects against genotoxic chemicals. Spores are also resistant to other stresses, including high pressure and abrasion. Regardless, overarching mechanisms associated with resistance seem to revolve around reduced molecular motion, a fine balance between rigidity and flexibility, and perhaps efficient repair.
KEYWORDS: bacterial spores, spore resistance
INTRODUCTION
Many species of Firmicutes, including those in both aerobic Bacillales and anaerobic Clostridiales groups, can cease growth and initiate the process of sporulation, forming endospores from growing cells when environmental conditions have changed so that continued growth is unlikely (1–3). Indeed, a common impetus for initiating sporulation is the depletion of environmental nutrients. However, the decision to form a spore is risky for several reasons. One reason is that the transformation from a growing cell to a dormant spore is metabolically expensive, i.e., many vegetative cell proteins initially in the developing spore will be degraded, many spore-specific proteins are made, and the mother cell dies, and its degradation products are then free to anyone else in the environment (1), all with no increase in cell numbers. In addition, while there is certainly a possible gain in acquiring the ability to form spores, as it can mean the survival of the population during “bad” times, there are trade-offs in (i) continually maintaining the many genes needed only for sporulation and (ii) ceasing growth, such that the decision to form spores can allow other organisms free rein to grow without competition from spore formers. The upside in this trade-off for the spore formers is, of course, that sporulation can lead to survival in bad times due to the metabolic dormancy and resistance properties of spores. Then, when the spore environment is again conducive to growth, the spores can return to life via germination and then outgrowth (4). That the developmental option of transforming a growing cell into a dormant, resistant spore is indeed advantageous is indicated by the fact that this option evolved more than 2 billion years ago in some anaerobic Firmicutes members prior to the great oxidation event that allowed the flourishing of the aerobic bacteria, and it is still a pathway chosen by many Firmicutes species (5, 6). Notably, most aspects of sporulation, spore structure, and spore germination are similar in spores of Bacillales and Clostridiales, and many key spore proteins of these different species are close homologs (5, 6). Note, however, that when sporulation is no longer a useful developmental option, such as when environmental nutrients are constantly replenished, sporulation can be selected against (7, 8), perhaps (i) due to the energetic cost of maintaining the many genes needed only for sporulation and (ii) in the absence of selection for the function of these genes.
Despite the advantages that spore formation has given to some Firmicutes species, what is good for spore formers may not be beneficial for other organisms, including humans, as growing or stationary-phase cells of many spore formers are major agents of food spoilage and food poisoning, while some others cause severe human diseases or intoxications, including anthrax, botulism, and tetanus, among others (1). Since spores are essentially everywhere in the environment (9) and are most often the vectors of the deleterious effects noted above, there is constant interest in spore eradication, which is made difficult by the high resistance of spores to just about everything (10–13). Indeed, standards for many food-sterilization regimens are based on the ability to inactivate spores of particular species of interest, such as toxigenic Clostridium botulinum (14, 15). Standards for autoclave function, whether steam or chemical, are often the ability to kill the most resistant spores, such as the very wet heat resistant spores of Geobacillus stearothermophilus (16). The mechanism(s) of spore resistance have also been a fertile field for research. A contemporary PubMed search for “bacterial spore resistance” identifies >650 papers over the past 5 years. Notably, advances still appear in this area, such as the relatively recent isolation of spores of the Bacillus subtilis group with greatly increased wet heat resistance, which appears to involve a novel resistance mechanism (17–19). This review article will reexamine what we do and do not know about spore resistance, focusing on the mechanisms of this resistance as commonly determined in work with spores of the model spore former Bacillus subtilis. Hopefully, resistance mechanisms elucidated in B. subtilis spores will be similar to those in other spore formers, and this similarity has generally been the case, even with spores of Clostridiales members (20–22). However, it would be of significant interest and potentially practical value to have more comparative analyses of the mechanisms of the resistance of spores of other species and genera.
As noted above, one advantage of spore formation is that because of their dormancy and extreme resistance properties, spores can survive for long periods in the absence of nutrients and under conditions that might preclude cell survival. There have been several controversial reports that claimed or suggested possible spore survival for tens of millions of years, either entombed in amber or in a liquid inclusion in a crystal in a salt mine (23, 24), but it is certainly not clear if these claims are valid. Indeed, very recent results on the projected long-term survival of spores of B. subtilis suggest that even with high concentrations of these spores, one individual could survive at most only ~10 million years when dry and exposed to natural background gamma radiation (25). However, there is one well documented report indicating that dry spores of several Bacillus species can survive for ≥100 years and then joyously return to life (26). There is also a long-term experiment in progress to monitor spore survival over 500 years (27). For humans, with whom survival for 100 years is generally a stretch, this sounds like spores are doing quite well. Given that there have been billions of years during which spores have been exposed to all manner of harsh environmental conditions, with the continuous selection for spores that can survive, spores present today are those whose ancestors have truly stood the test of time. This survival requires that cells of such spore formers undergo the following two major transitions: (i) from an actively metabolizing cell to a metabolically dormant spore that can survive for many years in the absence of exogenous nutrients or even the metabolism of endogenous nutrients and (ii) from a growing cell sensitive to all manner of severe environmental changes to a spore resistant to environmental changes, such as large fluctuations in temperature, radiation exposure, water availability up to desiccation, and toxic chemicals or enzymes found in the environment or produced by other organisms. Since spore-forming organisms that were inefficient in these major transitions were at risk of being selected out, the ancestors of the spores we see formed today have truly been through the fires of natural selection. Equally, it is worth bearing in mind the distinction between resistance properties selected for over evolutionary timescales and which pertain to physiological and or ecological processes versus those that are more likely to be fortuitous and are merely incidental to the original stress factor. The former properties include resilience in the face of predation, desiccation, and UV radiation, whereas the latter properties are generally resistance properties against anthropologic-associated stressors, such as extremes of heat (at least for spores of mesophiles), mechanical disruption, and exposure to organic solvents and various other toxic (often manufactured) chemicals.
There are two major reasons that spores can survive for so long, namely, their dormancy and their resistance. Dormant spores in water exhibit minimal, if any, metabolic activity, even of endogenous metabolites, and spores of several species accumulate minimal if any ATP even when incubated for long periods at physiological temperatures (28). Note that it was imperative that the spores used in reference 28 could not complete germination, as soon after this event, spores rapidly accumulated ATP even if from only endogenous resources. As a consequence of their dormancy, spores (i) are not at risk from starving to death and (ii) will produce minimal, if any, toxic by-products of oxidative metabolism that can damage DNA and proteins. The spore features that generate dormancy appear to be primarily the low water content in the spore core, which can be as low as 25% of wet weight; notably, at least one soluble protein in the spore core, the reporter fluorophore green fluorescent protein (GFP), is immobile in the spore core and yet is fully mobile when spores complete germination and their core water content rises to 80% of wet weight (29). The precise state of core water has been the subject of significant research, with different groups suggesting the spore core is in either a glass-like amorphous solid state (30) or a gel-like state with significant mobile water but with spore macromolecules rotationally immobilized (31, 32). The latter model is most consistent with recent detailed studies of spore core water properties by nuclear magnetic resonance techniques (33). The pH in the core, generally ~6.5 (34), is >1 unit lower than that in growing cells, which plays a crucial role in precluding the activity of a very pH-sensitive spore core enzyme that can initiate the catabolism of a major spore energy reserve, 3-phosphoglyceric acid (3PGA), of which its catabolism generates ATP soon after the completion of spore germination (35). Indeed, the ATP generated from 3PGA is essential for maintaining the viability of spores germinating in a poor medium (36). However, 3PGA is not utilized in mutant Bacillus spores that cannot complete germination because of their lack of both cortex lytic enzymes (CLEs) essential for the completion of spore germination, even upon incubation with germinants for long times at physiological temperatures (28, 37).
The second major reason for the extreme survival of spores is their resistance to all manner of agents (10, 13), including enzymes, wet and dry heat, radiation of multiple types and energy levels, desiccation, high pressures, and a host of chemicals and degradative enzymes. Perhaps unsurprisingly, these resistance properties are largely dependent on the novel components and structural features of spores.
SPORE STRUCTURAL FEATURES AND SPORE RESISTANCE
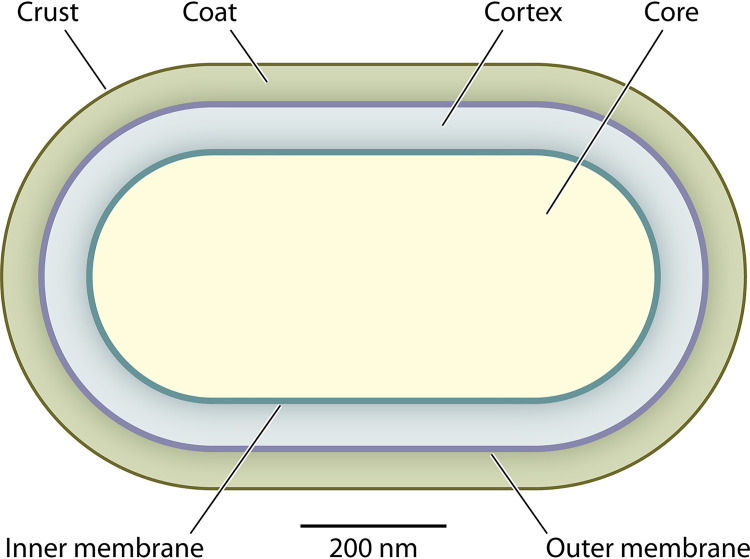
A major feature responsible for the extreme resistance of spores is their novel structure which is very different from that of a growing cell (Fig. 1). The innermost region of the spore is termed the core and is where spore DNA, RNA, and most soluble proteins are located. Given the importance of protecting spore DNA from damage for spores to survive, it is not surprising that some novel spore features help protect spore DNA (Fig. 2). Moreover, spores also have strategies to protect core proteins essential in macromolecular synthesis and metabolism. These protective spore features include the low-molecular-weight small acid-soluble spore proteins (SASPs) of the α/β type that saturate spore DNA (38), the low core water content noted above, and the 1:1 chelate of divalent cations, generally Ca2+, with pyridine-2,6-dicarboxylic acid (CaDPA) (Fig. 2A) that comprises ~25% of core dry weight (39). Surrounding the core is the inner membrane (IM), which has a phospholipid and fatty acid composition similar to that of the plasma membrane in growing cells (40). However, the permeability of the IM to small uncharged molecules, including even water, is quite low (32), and lipid probes in the IM are largely immobile as determined by fluorescence redistribution after photobleaching (41). Notably, occurring late in sporulation, ~30% of the IM is extruded into the spore core as the core volume contracts due to its reduced water content (42). However, upon germination when the core volume and the IM surface area expand, the IM extruded into the core is assimilated back into the IM.
FIG 1.
Schematic of a longitudinal cross-sectioned Bacillus subtilis spore showing the major morphological features visible by transmission electron microscopy. The germ cell wall is not distinguished but is located between the cortex and inner membrane. Spores of some species have an additional thin layer of coat-like material, the exosporium, which unlike the B. subtilis crust, is usually located distally with respect to the coat.
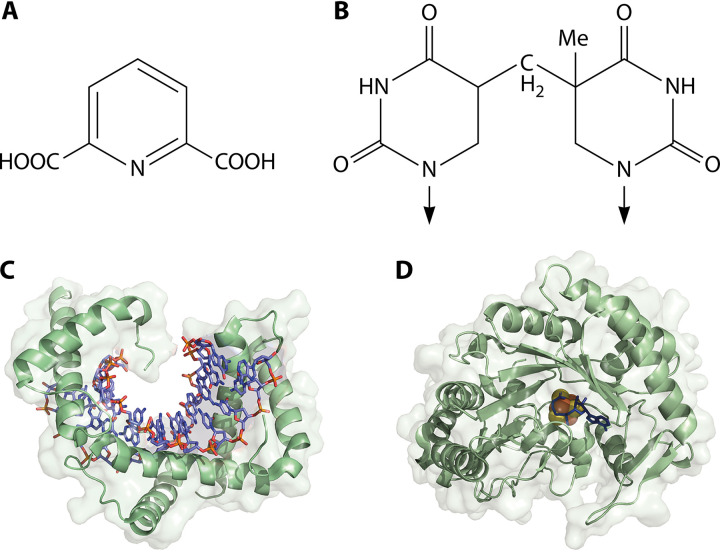
FIG 2.
A quartet of spore resistance-associated molecules. (A) Pyridine-2,6-dicarboxylic acid (DPA), which would be charged and chelated with Ca2+ ions in the spore core; (B) thyminyl-thymine adduct, often referred to as spore photoproduct (SP); (C) SASP-DNA complex (DNA, blue and orange) (Protein Data Bank [PDB] 2z3x); (D) SP lyase with S-adenosyl-l-methionine cofactor (blue) bound at the iron-sulfur cluster (yellow and orange) (PDB 4fhc).
Just outside the IM there is the first of two layers of peptidoglycan (PG), the germ cell wall which becomes the cell wall of the spore when it completes germination, and the PG structure in this layer appears identical to that in growing cell PG (1). The second and larger PG layer is the cortex, which has several differences in structure from that of germ cell wall PG. Specifically, many muramic acid residues are present as muramic acid-δ-lactam and others are attached to only a single alanine residue, instead of a muramyl-peptide with or without a cross-link (43, 44). The novel cortex PG features are recognized by specific hydrolases that initiate cortex-specific PG hydrolysis in spore germination (4, 45). It seems possible that the cortex undergoes expansion late in sporulation and by pressing against rigid spore coat elements (see below) exerts pressure on the spore core and IM leading to extrusion of core water, forcing some IM material into the core, and decreasing IM permeability (42). Notably, in the absence of most of the spore coat, IM permeability to water increases significantly (46). Outside of the cortex is the outer membrane, which while very important in spore formation, as yet has no known role in spore resistance (10).
The next layer is the spore coat, which itself may have several layers (47). The coat is composed primarily of spore-specific proteins, of which many are cross-linked to give a relatively rigid structure (48, 49), although at least outer layers can flex somewhat (50), and the coat plays some important roles in spore resistance (46, 51–54). The coat is also important in IM permeability as noted above and seems likely to be the rigid layer which ensures that any cortex expansion has effects inward on the IM and core (42). Equally, a recent atomic force microscope (AFM) led study indicated that the cortex is actually more stiff than the coat in B. subtilis spores (55). In addition, the coat layer has relatively low permeability to molecules (>10 kDa), which is something that is important for spore resistance to enzymes that hydrolyze PG (see below) (56). Spores of some species, including B. subtilis, have an additional relatively thin layer termed the crust just outside the coat made up of spore-specific proteins and glycoproteins (57), but it is not clear if the crust has a specific role in spore resistance (58). Instead of the crust, spores of some other species, for example Bacillus anthracis, have a seemingly analogous structure, the exosporium, which is similarly made up of spore-specific proteins and glycoproteins (59) but is much more distally located from the coat. While the exosporium may play a role in spore adhesion to abiotic surfaces (60–62), it is not clear if it plays any major roles in spore resistance, although it may prevent large molecules, such as antibodies, from accessing the more inner layers of the spores (56, 63, 64). In addition, the C. difficile exosporium has been shown to bind to host factors and help become internalized by epithelial cells (65).
WHAT ARE SPORES RESISTANT TO AND HOW RESISTANT ARE THEY?
It is often said that spores are extremely resistant, and as noted above, the list of what they are resistant to is long (10), and includes (i) UV and visible light and ionizing radiation; (ii) wet heat, probably the most familiar of spores’ resistance properties; (iii) dry heat, desiccation, and high vacuum; (iv) a host of chemicals, including those that alkylate, oxidize, or hydrolyze DNA and proteins, including strong oxidizing agents, such as peroxides or hypochlorite, as well as aldehydes, acid, and bases; (v) enzymes that degrade cell wall PG (also known as “eat resistance”); (vi) high hydrostatic pressure (HHP), which is used by the food industry; and (vii) a number of less well studied agents, including high pressure or supercritical gasses, and mechanical disruption.
As is perhaps not surprising, with spores of a particular strain or species, different sporulation media or conditions can give rise to spores with different resistance properties. The resistance property most often studied is to wet heat, but some studies have examined effects of sporulation conditions on spore resistance to dry heat, radiation, or chemicals, often hydrogen peroxide (66). Given that spores of several species, notably Bacillus atrophaeus and G. stearothermophilus are commonly used as biological indicators for autoclave-based sterilization procedures, there have been many studies on the effects of sporulation conditions on these spores’ wet heat resistance (16, 67, 68). However, the effects of sporulation conditions on spores of many other species, including B. subtilis, have also been studied (14, 69–73).
Variables in sporulation conditions examined for their effects on spore resistance include (i) temperature, (ii) water activity, (iii) pH, (iv) divalent cation type and amount, (v) addition of agents that increase the reducing power of sporulation media, and 6) relative medium richness. Much of the effect of these variables on sporulation output are summarized in a relatively recent review on this topic (3). However, although many of these variables can have effects on spore resistance, the reasons for most effects on spore resistance are not known. One exception is sporulation temperature, as higher temperatures generally give more wet heat resistant spores than lower temperatures. This effect is generally paralleled by lower core water content in spores made at higher temperatures, consistent with the major role for the low core water content in spore wet heat resistance. Another effect of sporulation temperature on spores of B. subtilis is some drastic changes in coat assembly in spores made at higher temperatures (73), which might be expected to alter spore wet heat resistance as well as resistance to predation and perhaps some chemicals, but this topic has not yet been studied in detail.
The addition or removal of specific divalent cations also often has significant effects on spore wet heat resistance (15), and these effects could certainly alter the divalent cations chelated with Pyridine-2,6-dicarboxylic acid (DPA), which is something that can also alter spore wet heat resistance (see below), although the precise explanation for the latter effect is not known. Unfortunately, this type of modulation of sporulation medium is generally not followed up by quantitation of the levels of divalent cations in the spores produced, and whether they are in the spore core or spore outer layers, so this topic is another area where further research is needed.
In addition, the reason(s) for effects of other modifications to sporulation conditions on spore resistance, such as changes in sporulation pH, water activity, reducing power, or medium richness, are not known, and generally, detailed analyses of the composition of the spores produced in the different conditions have not been carried out. Again, this topic is an area where further research is needed, in particular to determine the causes of effects of variations in sporulation conditions on spore resistance properties.
Most importantly, examination of the literature indicates that spore resistance to different agents discussed in this article compared with that of growing cells is >10-fold higher and most often much, much higher (Table 1). In the sections below, spore resistance to these potential killing agents will be discussed in detail.
TABLE 1.
Resistance of growing wild-type Bacillus subtilis cells, wild-type spores, and wild-type spores carrying the spoVA2mob transposable element to various treatments
| Treatment | Data for: |
Key resistance factors | ||
|---|---|---|---|---|
| Growing cells (wild type)a | Dormant spores (wild type)b | Dormant spores (spoVA2mob)b | ||
| Wet heat (93°C)c | <0.05 | 35 | >120 | Low core water, SASPs, transposon gene(s), coat |
| Dry heat (120°C)c | <5 | 170 | 200 | SASPs |
| UV at 254 nmc | <0.1 | 1.2 | 1.3 | SASPs, DNA repair, coat |
| NaOCl (2.5% available chlorine)c | <0.1 | 4.5 | 6.5 | Coat |
| H2O2 (11%)c | <0.5 | 205 | 280 | SASPs, coat, IM |
| HCHO (2.5%)c | <0.1 | 20 | >60 | SASPs, coat, IM |
| HNO2 (200 mM)c | <0.1 | 15 | >40 | SASPs, IM |
| C12H27N (1 mM)c | ND | 20 | >60 | IM |
| Desiccationd,e | <1 | >20 | NDf | SASPs |
| γ-Radiation (100 krads)e | 1 | 28 | NDf | SASPs, DNA repair |
UV and Visible Light and Ionizing Radiation
Spores of Firmicutes are generally 10- to 30-fold more resistant to 254-nm UV radiation, which is the optimum UV wavelength for damaging DNA, than growing cells. The major cause of spore UV resistance is the saturation of spore DNA with the α/β-type SASP mentioned above (12). All spore-forming Firmicutes genomes have 2 to 7 genes encoding these very homologous 60- to 80-residue proteins that in total make up ~5% of total spore core protein, and these genes are considered members of the set of genes essential in a spore former (5, 6). The α/β-type SASPs are synthesized only in the developing spore core very late in sporulation and are stable in the dormant spore. However, these proteins are rapidly degraded when spore germination is complete, via a process initiated by a SASP-specific endoprotease (74, 75). The free amino acids generated in this degradation provide amino acids for the synthesis of most new proteins soon after germination is complete. The α/β-type SASPs collectively saturate DNA in spores, and the core’s low water content is essential for the saturation, as when spores lacking the SASP-specific protease complete germination and core water content rises to 80% of wet weight, much of the α/β-type SASPs dissociate from the spore chromosome (76). Notably, the three regions of the α/β-type SASPs that are the most highly conserved throughout evolution are two that directly bind DNA and the region with the SASP-specific protease recognition sequence (12).
Saturation of DNA with α/β-type SASPs, either in spores or in vitro with purified proteins from a number of species, transitions DNA structure from a B to an A-like conformation (Fig. 2C) (77–79). This transition drastically alters the 254-nm UV photochemistry of the DNA from the generation of cyclobutane-type pyrimidine dimers (CPDs) and 6-4-bipyrimidine photoproducts (6-4PPs) in growing cells or DNA in vitro to a thyminyl-thymine adduct termed the spore photoproduct (SP) (Fig. 2B) in spores or DNA saturated in vitro with α/β-type SASPs from either Bacillus or Clostridium species (80, 81). Notably, wild-type (wt) spores of several species are much more UV resistant than spores lacking ~85% of α/β-type SASPs, termed α−β− spores, and UV irradiation of α−β− spores generates CPDs and most likely 6-4PPs. The large CaDPA depot in the spore core also is involved in spore UV resistance but sensitizes spore DNA to damage (82).
CPDs and 6-4PPs, as well as the SP, can be repaired when spores come back to life and enter outgrowth, and spores contain proteins involved in DNA repair, including RecA and SP lyase (Fig. 2D) as well as enzymes of nucleotide excision repair (NER) and base excision repair (BER) (83–85). In addition, a number of genes encoding DNA repair proteins, including RecA, are induced when UV-irradiated spores complete germination but not in germinated unirradiated spores, such that germinated spores with damaged DNA have higher levels of DNA repair proteins (85). Note that UV-treated spores, even if some are killed, can still germinate, which is also seen with spores killed by other agents. The CPDs and 6-4PPs are repaired primarily by a cut/patch process involving DNA backbone incision and replication by a low-fidelity DNA polymerase; this repair process is error prone and generates mutations. In contrast, SP repair does not break the DNA backbone as the SP lyase enzyme present in spores monomerizes SP to two thymine residues in an error-free process requiring S-adenosylmethionine once spores have completed germination (86, 87). Since 254-nm UV forms SP and CPD with relatively similar efficiency, it is the error-free nature of SP repair compared with that of CPDs and 6-4PPs which likely explains spore resistance to UV at 254 nm. As expected, spores which lack SP lyase are more sensitive to UV than wild-type spores, and the concomitant absence of other DNA repair proteins leads to even lower UV resistance (88). Thus, both α/β-type SASPs and DNA repair are crucial for full spore resistance to UV at 254 nm. The need for both α/β-type SASPS and DNA repair is also the case for resistance to UV at 222nm, as well as blue light at 400 nm, and SP is again the major photoproduct generated in wild-type spores at these two wavelengths, with minimal CPDs (89, 90).
Coats of spores of some species also contain various pigments, including carotenoids, and there is evidence that such pigments can provide some protection to spores against UV radiation (91, 92) and even more against 400 nm blue light (90). However, these pigments are not the major source of spore UV resistance, and spores of many species have no such pigments and are still very UV resistant. Spores can also be inactivated by pulsed white light from 200 to 1,100 nm (93, 94), and α/β-type SASPs and DNA repair are again important in resistance to this treatment (95). The spore coat is also important in resistance to pulsed white light. Spores with severe coat mutations are killed more rapidly than wild-type spores, and this treatment also removes some coat proteins from spores (96). Intriguingly, spores exposed to pulsed near-infrared light (700 to 1,000 nm) showed only 2-fold enhanced resistance to its bactericidal effects compared with vegetative cells, which is a surprisingly small deviation in sensitivity and one that warrants further investigation (97).
As is true for spore resistance to UV and visible radiation, wild-type spore resistance to various types of ionizing radiation is also much greater than that of α−β− spores (38, 98, 99), indicating that α/β-type SASP binding also protects spore DNA against ionizing radiation. It also seems possible that spores with lower core water contents might exhibit higher ionizing radiation resistance due to less generation of free radicals from water splitting. While there is some evidence consistent with this possibility (98), this topic needs a thorough investigation. As seen with UV radiation, ionizing radiation was found to generate DNA damage in spores, as evidenced by increased mutation levels in survivors (100). Consequently, it is not surprising that spore resistance to ionizing radiation resistance is increased by the presence of a number of DNA repair enzymes in spores. They act once germination is complete and include enzymes involved in recombination repair, BER repair, abasic site repair, and homologous and nonhomologous end joining (38, 101, 102). Notably, spore radiation resistance parallels the incorporation of cysteine into developing spores (103), whereas spores produced in cysteine-enriched media show increased resistance to nonionizing UV between 280 and 400 nm (70). However, the underlying reasons for apparent cysteine-associated resistance to ionizing and nonionizing radiations have not been established.
Wet Heat
Resistance to wet heat is the signature resistance property of bacterial spores, and given that wet heat treatment is the most commonly used method for spore eradication from foods, guidelines for food sterilization are generally determined by the wet heat temperature and treatment time needed to eradicate the most dangerous spores (e.g., those of neurotoxin generating C. botulinum). Given the widespread use of wet heat for spore killing, it is perhaps not surprising that there has been an enormous amount of research on spore killing by and resistance to wet heat. This work has established that wet heat, including in an autoclave, does not kill spores of several Bacillus species by (i) DNA damage, (ii) inactivation of one or more essential germination components, or (iii) breaking down the spores’ IM permeability barrier so that CaDPA is released (16, 104–106); notably, CaDPA release from wet heat-treated spores of several species is well after spore death (104). Importantly, wet heat-treated spore populations, even if ~95% is killed, can still exhibit percentages of germination of >50% (104, 106). However, only a few percentages of these germinated spores enter outgrowth and almost all make minimal amounts of ATP or other high-energy intermediates (106). This work and other data (107, 108) have led to the suggestion that spore killing by wet heat is due to the inactivation of one or more crucial enzymes in the central metabolism in germinated spores—more on this later.
Pioneering work on spore wet heat resistance by Phil Gerhardt and Bob Marquis (109) established a number of additional features of spores that are important in their wet heat resistance, as follows: (i) the optimum growth temperature of the organism, with spores from organisms with higher optimum growth temperatures and having more intrinsically heat-resistant proteins, being more wet heat resistant (e.g., spores of the thermophile Geobacillus stearothermophilus); (ii) the degree of mineralization of the spore core and the specific cation(s) associated with DPA, with Ca2+ being the cation giving most wet heat-resistant spores and with higher CaDPA levels in the core also generally giving more wet heat-resistant spores (note this effect can be difficult to separate from effects of elevated core CaDPA on core water content, as noted below); (iii) the sporulation temperature, with higher sporulation temperatures that still allow sporulation giving more wet heat-resistant spores; and most importantly, (iv) the water content in the spore core which ranges from 25% to 55% wet weight, in contrast to the usual 80% of wet weight in growing cells, with lower core water contents found in most wet heat resistant spores. Indeed, spores made at higher temperatures generally give spores with lower core water content.
One major question about spore core water is how the low spore water content is achieved late during sporulation. A partial answer to this question is that it is due to the developing forespores taking up CaDPA made in the mother cell very late in sporulation. Indeed, CaDPA uptake is paralleled by extrusion of some core water. In B. subtilis spores, this process reduces core water from 45% of wet weight to ~35% of wet weight (110). However, well prior to CaDPA uptake by the developing spore, (i) the volume of the spore core has already decreased significantly as has spore core water content (111) and (ii) soon thereafter, as much as 30% of the IM of developing forespores is extruded into the spore core and the spore core only fuses with the IM upon completion of spore germination (42). A major question then is how core volume is decreased in forespore development prior to CaDPA uptake, and the only known candidate for this event is a mechanical effect of the spore cortex, presumably its expansion against the restraint exerted by the rigid spore coat layer exerting compression inward on the spore core. The importance of the coat in spore wet heat resistance is reflected in the lower wet heat resistance of genetically coatless B. subtilis spores (52). In addition, it is now well appreciated that spores undergo what has been termed “maturation” after spores are released from the sporangium (112). This process can lead to large increases in resistance to wet heat and hypochlorite and is most likely due to increased cross-linking of coat proteins by enzymes in the coat (48, 49), again providing strong evidence for the importance of the coat in spore resistance to wet heat. While the spore cortex expansion against the rigid coat seems a reasonable hypothesis for how much core water is extruded, more work is needed to completely understand this crucial event in sporulation. There is, however, no question that a low core water content can protect proteins against wet heat (110, 113), and many studies indicate that soluble core proteins are stable in wet spores to ~40°C higher temperatures than when heated in solution (107).
In addition to the mechanisms noted above, another very important mechanism protecting spores from wet heat is the saturation of spore DNA with the α/β-type SASPs described in the section above. At elevated temperatures, both pyrimidine and purine bases in DNA can undergo cleavage of the glycosidic bond generating an abasic site in vitro or in growing cells (114), with depurination more rapid than depyrimidination, and both processes are faster at lower pH values (115). Notably, the repair of abasic sites is significantly error prone and generates mutations, as noted above (115, 116). Since spores of multiple species in water are quite resistant to temperatures of >75°C for significant amounts of time, and with a spore core with a pH of ~6.5, one would expect there to be much depurination in DNA from heat-treated spores. However, this depurination is not observed, as there are minimal abasic sites in wild-type spores killed 99.9% by wet heat (114). In contrast, wet heat-treated α−β− spores killed to the same level as the wild-type spores had >20-fold higher levels of abasic sites, which also correlated with the frequencies of DNA strand breaks in these wet heat- α−β− treated spores (114). This prevention of depurination of DNA in wild-type spores is almost certainly due to the saturation of spore DNA by α/β-type SASPs, as (i) these proteins block DNA depurination in vitro and (ii) an α/β-type SASP variant that binds DNA poorly does not protect DNA from depurination in vitro and does not protect spores well against wet heat (117). DNA binding by α/β-type SASPs also greatly inhibits deamination of cytosine residues in DNA (115), another potential mutagenic event. Indeed, the α/β-type SASPs protect spore DNA from damage even at elevated temperatures, including at up to >100°C in a steam autoclave (16). Consequently, spore preparations killed by 90 to 95% by wet heat at various temperatures exhibit no increase in mutation frequency, in contrast to α−β− spores which (i) are significantly more sensitive to wet heat than wild-type spores (Table 1), (ii) accumulate high levels of mutants in spore populations killed by 90 to 95%, and (iii) become even less wet heat resistant if a crucial DNA repair protein such as RecA is also absent (85). In contrast, the loss of RecA has no effect on the resistance of wild-type spores to wet heat (85). Presumably, the protection of DNA by α/β-type SASP binding is so great that spores die from other effects of wet heat well before there is any DNA damage. This other lethal wet heat damage is most likely to spore proteins, and a small amount of protein denaturation takes place during spore killing by wet heat, and prior to CaDPA release, which takes place in only dead, wet heat-treated spores. Following all CaDPA release, there is rapid denaturation of large amounts of spore protein, presumably because of the increased core water content once CaDPA has been released (104). As noted above, there is good evidence that the spore coat also is important in spore resistance, as spores with severely defective coats have significantly lower wet heat resistance. Why the coat is important in wet heat resistance is not completely clear, but it may be multifactorial, i.e., in addition to its rigidity influencing cortex-mediated core volume, it may be because it, along with CaDPA, is important for maintaining a relatively immobile IM environment, as is discussed below.
A more subtle effect on spore wet heat resistance is seen when wild-type spores are treated with wet heat applied either conductively or by ohmic (i.e., electrical resistance) heating (118). Notably, ohmic heating kills wild-type spores more rapidly than the same temperature applied to these spores by way of conductive heating. A possible clue to the mechanism of this effect came from demonstrating that α−β− spores were killed equally well by wet heat applied either conductively or ohmically. These data suggest that spores’ high resistance to wet heat due to α/β-type SASP-binding can be subverted by ohmic heat, as the ohmic treatment may weaken interactions between α/β-type SASPs and DNA which are not extremely strong. Conceivably, dissociation of the proteins from DNA could lead to heat-induced damage on any naked DNA prior to α/β-type SASP rebinding to DNA when spores are cooled. However, recent work has shown that ohmic heat killing of wt B. subtilis spores is not associated with increased mutagenesis (S. Singh, M. Ali, J. H. Mok, G. Korza, P. Setlow, and S. K. Sastry, unpublished results), so further work is needed to determine exactly how ohmic heat accelerates wild-type spore killing.
As a reminder that “nature always finds a way,” a new mechanism of spore resistance to wet heat was identified a few years ago in spores of some extremely wet heat-resistant spores of B. subtilis and its close relatives that had survived in a plant processing foodstuffs at very high temperature (17, 18). The organisms giving these very heat-resistant spores had all acquired a transposon with an operon, termed spoVA2mob, that was likely expressed only in the developing spore. Notably, the extreme resistance of these spores was largely due to a gene on this operon called 2duf, denoting that it has two domains of unknown function, one being DUF421 (51). These heat-resistant spores had neither an elevated CaDPA content nor a decreased core water content (19), indicating that something new was giving rise to the extremely high spore wet heat resistance, and this was likely 2Duf alone or together with some other gene product expressed on the spoVA2mob operon. From its primary structure, 2Duf seems most likely to be an IM protein. Indeed, while not found in a proteomic analysis of spores carrying spoVA2mob (119), a 2Duf orthologue was found in the Bacillus cereus spore IM proteome but not in growing cells (120, 121). Notably, spores with 2Duf are highly resistant not only to wet heat but also to several agents that either modify the IM or must cross it to damage molecules in the core, either proteins damaged by H2O2 or DNA damaged by nitrite or formaldehyde (51). It has been known for a number of years that the spore IM has a much lower permeability than the plasma membrane of a growing cell, even to molecules like methylamine or even water (41). This low spore IM permeability is due in part to the spore coat, the removal of which may release cortex pressure on the core such that the IM can expand very slightly, and to the high level of CaDPA of the spore core (51). Note that a lipid probe in the spore IM is largely immobile (41), and the lipid mobility and permeability of the IM are both increased significantly in spores that lack coats and CaDPA (122); perhaps free coordination sites of Ca2+ in CaDPA bind to phospholipid headgroups on the inner IM leaflet and make the IM more rigid and less permeable. While the precise effect of 2Duf on IM structure is not well understood, recent results indicate that the presence of the spoVA2mob operon makes lipids in the IM more immobile and less permeable to agents that must enter the core to damage core components (51). In addition, the presence of 2Duf stabilizes IM germination proteins to wet heat (119) and makes IM germinant receptors more refractory to the heat activation that can potentiate spore germination (108).
The decreased IM permeability of spores containing 2Duf noted above seems likely to be the reason that spores with 2Duf have increased resistance to chemicals that must cross the IM to cause spore killing (51). While how this effect of 2Duf on IM properties could increase spore wet heat resistance is less obvious, a possible mechanism is suggested when the mode of spore killing by wet heat and hydrogen peroxide is considered, as this mode appears to be by the inactivation of one or more key enzymes needed for ATP generation in spore outgrowth (104–106, 123). Some of these proteins could be soluble enzymes for glycolysis, as spores have little if any of some enzymes of the tricarboxylic acid cycle (124, 125). However, most spore recovery media often have only low glucose levels and therefore most ATP must be generated in outgrowing aerobic spore formers by oxidative phosphorylation (125), and many of the enzymes for the latter are integral IM proteins (120). Thus, the increased resistance of these enzymes to wet heat and hydrogen peroxide in an IM with 2Duf may be due to a more rigid IM, with 2Duf providing increased resistance to key IM ATP-generating proteins. While this idea is only a hypothesis, it is consistent with several other facts, including that (i) 2Duf does not provide spores with increased resistance to dry heat or UV radiation, of which both kill spores by DNA damage, and (ii) the viability of wet heat-treated spores of several species can be significantly increased by the addition of 10 mM glucose to recovery media but not by addition of substrates, such as amino acids, that require oxidative phosphorylation to generate energy, which is also the case for B. subtilis spores treated with hydrogen peroxide (105, 126). Clearly, further work is needed to fully understand in detail all the effects of 2Duf on the IM of spores and how these effects modulate spore wet heat resistance.
In addition to the effects on spore wet heat resistance noted above, recent work has identified homologs of 2duf in the B. subtilis genome, specifically the yetF and ydfS genes, as also important in wet heat resistance in B. subtilis spores, with a yetF deletion reducing wet heat resistance the most (121). Mutations in these two genes also reduced spore resistance to agents that must cross the IM to damage core components, including H2O2, nitrite, and formaldehyde. The loss of YetF or YdfS also had slightly decreased rates of l-valine germination and had stronger effects on AGFK germination. The effects of the yetF mutation were complemented by reintroduction of the yetF gene at the amyE locus, and introduction of yetF at amyE in a wild-type strain increased spore wet heat resistance (121). Importantly, YetF and YdfS are predicted to be membrane proteins and have been found in the IM of spores, as was a YetF-GFP fusion (127, 128). While the CaDPA and core water content of yetF B. subtilis spores are identical to those of wild-type spores, the phospholipid composition of spores with and without yetF or ydfS was also examined, as work 13 years ago showed that changes in specific IM phospholipid levels could decrease B. subtilis spore resistance to wet heat and hydrogen peroxide (40). However, an analysis of IM phospholipid levels in spores with or without 2Duf, YetF, or YdfS found that there were no significant differences between the IM phospholipids in spores of these three strains (121, 123) While these latter results rule out a significant role for IM phospholipids in modulating effects of YetF and its homologs on spore resistance, the initial findings (40) of large effects of changes in IM phospholipid levels on spore wet heat and H2O2 resistance also provide further support for the major role of the IM in determining rates of spore killing by these two agents.
The work on the effects of YetF and YdfS on spore resistance to date has focused on B. subtilis spores in which YetF is most likely present in higher levels in spores than that in vegetative cells, and it is perhaps higher than YdfS levels in spores. These two proteins are members of the DUF421 superfamily of proteins that are found in an enormous number of Firmicutes spore formers and which exhibit similar structures when AlphaFold models are compared (121). Even some non-spore-forming bacteria contain one or more members of this gene family, but to date, no function has been ascribed to these proteins in growing cells. Further research into the specific mechanisms whereby these DUF421 proteins modulate spore resistance seem likely to lead to further surprises.
While most work on spore wet heat treatment has used conductive heating, there are some treatments that generate wet heat by methods other than conductive means, with one such being microwave heating. However, while some work suggested that microwave irradiation of spores of several species had effects greater that a thermal effect alone (129), others have found no such additive effect (130–133), and there has been no significant study of the mechanisms of spore resistance to microwave heating. There are also treatments that combine a thermal effect with another treatment; one treatment such is carrying out sonication of spores at an elevated temperature, a process termed thermosonication (TS) (134–138). TS kills spores of many species. With B. subtilis spores, the factors important in resistance to this treatment are most importantly the α/β-type SASPs (137), although evidently TS treatment results in damage to the inner membrane and spore coat (135, 137, 138).
Dry Heat, Desiccation, and High Vacuum
Spores are generally resistant to ~20°C higher temperatures when dry than when heated in water but can be killed by dry heat, and this treatment is used in some situations requiring sterilization, including in spacecraft assembly (139). As with wet heat, the α/β-type SASPs are also an important factor in spore dry heat resistance, as DNA damage by dry heat, including depurination, is greatly slowed by DNA’s saturation with α/β-type SASPs, and α−β− spores are more sensitive to dry heat that wild-type spores (116). However, unlike with wet heat, wild-type spore populations killed 90 to 95% by dry heat accumulate high levels of mutations (116, 140, 141). Consequently, DNA repair when spores begin outgrowth is also important because their dry heat resistance and mutations in major DNA repair proteins, such as RecA, greatly decrease spore dry heat resistance (85). The DNA damage generated by dry heat treatment of spores includes both transitions and transversions (142).
Wild-type spores are generally very resistant to desiccation alone and can survive multiple cycles of hydration and desiccation at moderate vacuum (~10 Pa) (143). The α/β-type SASPs are a major factor in spore desiccation resistance at moderate vacuum, as α−β− spores are killed 30 to 75% by even one cycle of desiccation under moderate vacuum and then rehydration, with multiple cycles giving more and more killing; it is the processes of either desiccation or rehydration or both that give the killing of the α−β− spores, not the time in the desiccated state (143). Again, DNA damage seems to be the mechanism of the killing of the α−β− spores by desiccation, as there is increased mutagenesis of the survivors and the presence of DNA repair enzymes in spores is important in desiccation resistance of α−β− spores. While wild-type spores survive desiccation at moderate vacuum, desiccation at high vacuum (≤0.1 Pa) results in spore killing by DNA damage (27, 144). Again, the α/β-type SASPs as well as DNA repair during spore outgrowth are most important in resisting spore killing by high vacuum (101, 142, 145, 146).
Chemicals
Spores can be killed by a wide range of chemicals, including a variety of DNA-damaging agents; some amines and alcohols; and oxidizing agents, aldehydes, acids, and bases (11, 147). For potential DNA-damaging chemicals that can alkylate, deaminate, or oxidize DNA, spore resistance to these agents is due to (i) the low IM permeability, as increases or decreases in IM permeability correlate with more rapid or slower spore killing, respectively (123, 126, 148); and (ii) DNA protection by α/β-type SASPs, which is so effective that several peroxides, including hydrogen peroxide, do not kill wild-type spores by DNA damage but most likely kill them by oxidative damage to amino acid residues, in particular methionines, in one or more core enzymes (149–151). For example, even α/β-type SASPs bound to DNA in spores can undergo oxidation of methionine residues by multiple peroxides, as can methionines in other core proteins, and these agents can also oxidize lysine γ-amino groups in core proteins to aldehydes (152). Presumably, it is by protein damage that wild-type spores are killed by these peroxides. However, the protein(s) damaged by oxidizing agents which leads to spore death is not known, although killed peroxide-treated spores can germinate, albeit slowly (153). Indeed, germinated hydrogen peroxide-killed spores do not accumulate ATP, as is the case with germinated wet heat-killed spores, and perhaps both agents target similar IM enzymes involved in oxidative phosphorylation (104, 123, 154). In contrast to the behavior of wild-type spores, at least hydrogen peroxide kills α−β− spores by DNA damage, and DNA repair in peroxide-treated outgrowing α−β−spores is important in the resistance of these spores (85).
Larger, more hydrophilic potential DNA-damaging agents, especially if charged at neutral pH, do not kill spores by DNA damage. This group of chemicals includes strong oxidizing agents, such as hypochlorite, chlorine dioxide, or ozone, which do not kill spores by DNA damage as they presumably do not cross the IM, they do not kill α−β− spores more rapidly than wild-type spores, and the loss of DNA repair capacity has no effect on spore resistance to such agents (155, 156). In addition to the prevention of damage to spore core components by low IM permeability, major protection against these oxidizing agents as well as aldehydes (e.g., glutaraldehyde) is provided by the spore coat. Severe coat defects result in more rapid spore killing by such agents. However, decoated wild-type spores treated with such agents still are not killed by DNA damage, and even decoated α−β− spores are not killed by DNA damage (155–158). Thus, the major protection against such agents is likely by their detoxification in reactions with spore coat protein, so there is less damage to more inner spore layers. Indeed, the lethal damage whereby such agents kill spores appears to be in the IM, as while the killed spores can germinate, the IM often ruptures when the core swells during germination. However, the specific IM damage that causes this effect is not known but is not the oxidation of unsaturated fatty acids (126).
Another potential protective feature of the coat of spores of some species is that potential detoxification enzymes (e.g., catalase and superoxide dismutase) are present not only in the spore core but also in the coat (159, 160). The core enzymes play no role in spore resistance (161), even if their levels are greatly increased (162), presumably because these enzymes are inactive in the core’s low water content, but they can increase the resistance of the fully germinated spore, in which they become active (163). There is also evidence that at least superoxide dismutase in the coat of Bacillus anthracis spores can increase spore resistance to agents that generate superoxide (164).
Spores are also resistant to low and high pH values, such as with 1 M acid or base, but pH extremes do not kill spores by DNA damage, and the loss of DNA repair, α/β-type SASPs, or spore coats do not affect spore resistance to these pH extremes, which is not really understood. However, findings with spore resistance to a strong alkali has exemplified some of the potential for misleading results that can arise in studies of spore killing by chemicals. While B. subtilis spores treated with 1 M NaOH may give no colonies on nutrient plates, colony formation is fully restored on plates with low levels of lysozyme (165). Notably, given germinants, these alkali-treated spores rapidly release CaDPA in the initial step in spore germination, but they cannot degrade the cortex PG and complete the germination process (153). Presumably, the cortex-lytic enzymes are inactivated by strong alkali and lysozyme was a surrogate for these enzymes, as the treated spores were not truly dead. Another more insidious problem in assessing spore killing by chemical agents is that unless these are removed or fully inactivated, when spores are plated on nutrients to allow spore germination, toxic chemicals that have been carried over can rapidly kill the much more sensitive germinated spores. Sadly, this problem has led to more than one error in classifying a chemical agent as being able to kill dormant spores (166, 167).
Eat Resistance
Sporulating cells spend a large amount of time and energy in making all the specific proteins that comprise the spore coat, for example, ≥80 proteins in B. subtilis, which together make up >50% of total spore protein. Given this large energy expenditure, one would presume that the coat provides some major benefit to spores, and indeed it does, as it deals with a major problem spores face when in soil or water which have many other types of organisms, including lower eukaryotes and predatory bacteria, for all of whom bacteria are their favorite “meal.” The eukaryotic bacterivores ingest meals of soil or water, and enzymes in their digestive tract, most importantly PG hydrolases that lyse and kill any ingested bacteria, together with other hydrolases digest the meal to metabolites, allowing the predator to flourish. However, in this predator-rich environment, the spore can neither flee from its predators nor release some chemical defense but must stand its ground. The spore coat comes in here, as several studies have shown that intact spores of the model organism B. subtilis, a soil organism, are taken up readily either by the protozoan Tetrahymena thermophilus or the nematode Caenorhabditis elegans. However, the ingested spores are neither killed nor digested but rather are excreted no worse the wear from their brief sojourn in the digestive tract of these predators (53, 54). Notably, the spores’ resistance to these predators is dependent on their coat, as severely coatless spores are readily eaten, with increased sensitivity to digestion correlating with more severe coat defects. There are also multiple reports of spores of many different species surviving transit through the human gut (168, 169), although there have been no studies on the fate of coatless spores in the human gut.
There are also at least two species of bacteria, namely, Myxococcus xanthus and Cupriavides necator, that prey on other bacteria, including growing B. subtilis cells, but they cannot kill their spores (170). However, the mechanism of the resistance of B. subtilis spores to these predatory microbes is either not known (M. xanthus) or does not involve spore coats (C. necator) and may be due to dormancy and low IM permeability of the spores (170, 171). Note that spore dormancy and low IM permeability also preclude spore killing by antibiotics.
High Hydrostatic Pressure (HHP)
Spores are markedly resistant to HHP, perhaps because of their relatively rigid layers, but HHP resistance does not involve an intact spore coat (172). However, HHP treatment can potentiate spore killing by triggering their germination at either moderate pressures (150 to 330 MPa) or much higher pressures (up to 900 MPa) (173, 174). While germinated spores can be killed by HHP, generally this method is not efficient, and in the food industry, HHP treatment is carried out at high temperatures to kill the partially or completely germinated spores, which are much less wet heat resistant than dormant spores (138, 175–178).
Mechanical Disruption
Spores are much more resistant to mechanical disruption than growing cells, whether by sound waves (sonication) (134–138), by shaking with glass beads (179), or by piercing by silicon nanopillars (180). However, while studies of this type of spore killing have ruled out specific protective components, such as α/β-type SASPs and most spore core proteins, the specific mechanisms involved in spore resistance to mechanical disruption have not been identified, and even the removal of much of the spore coat does not sensitize spores to mechanical disruption or piercing (179, 180).
Miscellaneous
The past decade or so has seen the development and introduction of a fairly wide range of technologies aimed at inactivating spores in environments where traditional thermal and chemical approaches may not be appropriate (181). Various nonthermal gas plasma-based approaches have received considerable attention (182, 183), although knowledge of spore components damaged by nonthermal and low-pressure plasma samples and resistance counter measures is incomplete. However, the exposure of spores to cold atmospheric plasma was reported to damage the spore inner membrane and germination proteins, with the damage being caused by charged particles and reactive oxygen species as opposed to UV radiation (184). The configuration of plasma devices seems likely to modulate spore targets since a low-temperature nitrogen-oxygen plasma system was found to damage spore DNA via emission of UV-C and that SASPs and DNA repair enzymes conferred resistance in this regard (185). Another emerging approach concerns the application of supercritical gasses, usually CO2 coupled with chemical modifiers for sterilizing heat-sensitive surfaces in the health care and food sectors (186, 187). Few studies have pursued the physiological basis for spore damage in this regard; however, spores exposed to supercritical CO2 with peracetic acid were killed principally by damage to the inner membrane (188), with the coat and SASPs conferring various degrees of resistance to wet and dry spores, respectively. Finally, at the opposite end of the thermal spectrum, the exposure of spores to blast environment conditions, that is ~1,000°C for tens of milliseconds, resulted in efficient spore killing via damage to unknown spore proteins and perhaps the inner membrane (189). Evidently, there are limits to resistance, even for these most recalcitrant of microorganisms.
CONCLUDING REMARKS
After more than 7 decades of intensive research driven in no small part by the quest for eradication, spores continue to confound and surprise. Just when progress in understanding the basis of resistance might have been in danger of stalling, new protein players in spore resistance (17–19, 121) are suddenly discovered, adding to the mosaic of features that collectively underpin spore recalcitrance. How were these players missed for so long in a busy field of research? If anything, recent events in the field demonstrate once again that spores do not easily give up their secrets. Indeed, an assessment of the literature, such as in this review, suggests that the current knowledge of spore resistance to a number of important stress factors frequently lacks granularity. Hence, as we move forward, the objective should be adding detail to “the damaged inner membrane,” or the “undefined core protein,” or more precisely defining what is meant by “coat disruption.” Which proteins or lipids are involved? What is the effect at the nano and ultrastructural levels? What new and emerging techniques can we apply to probe the spore in such detail, and can we then use this knowledge to kill spores more efficiently? Clearly there is much left to be learned about spores, their resistance, and their ever-elusive Achilles’ heels.
Biographies

Peter Setlow is Board of Trustees Distinguished Professor of Molecular Biology and Biophysics at UConn Health in Farmington, CT. He graduated from Swarthmore College in 1964 with a B.A. in Chemistry and did his Ph.D. in Biochemistry at Brandeis University with Nathan Kaplan. From 1968 to 1971, he did postdoctoral work with Arthur Kornberg at Stanford University School of Medicine, working on both Bacillus spore germination and Escherichia coli DNA polymerase. He then became an Assistant Professor of Biochemistry at UConn Health in 1971. His research on the formation, biochemistry, resistance, killing, and germination of spores of bacteria of Bacillus and Clostridium species has resulted in more than 540 publications on these topics. Dr. Setlow is a Fellow of the American Academy of Microbiology, an Honorary Member of the Society for Applied Microbiology, and is on the Editorial Boards of Applied and Environmental Microbiology and the Journal of Bacteriology.

Graham Christie is an Associate Professor at the Department of Chemical Engineering & Biotechnology, University of Cambridge. He graduated with a B.S. in Microbiology from the University of Strathclyde, Glasgow, in 1994, and then conducted his Ph.D. at the University of Warwick under the supervision of Crawford Dow. He joined the Institute of Biotechnology at the University of Cambridge in 2002, to work on the development of rapid diagnostics for bacterial spores, before turning his attention to more fundamental aspects of spore biology, principally germination and coat assembly. He was elected to the Fellowship at Peterhouse, Cambridge, in 2016.
REFERENCES
- 1.Setlow P, Johnson EA. 2012. Spores and their significance, p 45–79. In Doyle MP, Buchanan R (ed), Food Microbiology, Fundamentals and Frontiers, 4th ed. ASM Press, Washington, DC. [Google Scholar]
- 2.Shen A. 2020. Clostridioides difficile spore formation and germination: new insights and opportunities for intervention. Annu Rev Microbiol 74:545–566. 10.1146/annurev-micro-011320-011321. [DOI] [PubMed] [Google Scholar]
- 3.Bressuire-Isoard C, Broussolle V, Carlin F. 2018. Sporulation environment influences spore properties in Bacillus: evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol Rev 42:614–626. 10.1093/femsre/fuy021. [DOI] [PubMed] [Google Scholar]
- 4.Christie G, Setlow P. 2020. Bacillus spore germination: knowns, unknowns and what we need to learn. Cell Signal 74:109729. 10.1016/j.cellsig.2020.109729. [DOI] [PubMed] [Google Scholar]
- 5.Galperin MY, Mekhedov SL, Puigbo P, Smirnov S, Wolf YI, Rigden DJ. 2012. Genomic determinants of sporulation in Bacilli and Clostridia: towards the minimal set of sporulation-specific genes. Environ Microbiol 14:2870–2890. 10.1111/j.1462-2920.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galperin MY, Yutin N, Wolf YI, Vera Alvarez R, Koonin EV. 2022. Conservation and evolution of the sporulation gene set in diverse members of the Firmicutes. J Bacteriol 204:e0007922. 10.1128/jb.00079-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CT, Fishwick LK, Chokshi BM, Cuff MA, Jackson J, Oglesby T, Rioux AT, Rodriguez E, Stupp GS, Trupp AH, Woollcombe-Clarke JS, Wright TN, Zaragoza WJ, Drew JC, Triplett EW, Nicholson WL. 2011. Whole-genome sequencing and phenotypic analysis of Bacillus subtilis mutants following evolution under conditions of relaxed selection for sporulation. Appl Environ Microbiol 77:6867–6877. 10.1128/AEM.05272-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maughan H, Masel J, Birky CW, Jr, Nicholson WL. 2007. The roles of mutation accumulation and selection in loss of sporulation in experimental populations of Bacillus subtilis. Genetics 177:937–948. 10.1534/genetics.107.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64:548–572. 10.1128/MMBR.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setlow P. 2016. Spore resistance properties, p 201–215. In Eichenberger P, Driks A (ed), The Bacterial Spore: From Molecules to Systems. ASM Press, Washington, DC. [Google Scholar]
- 11.Setlow P. 2013. Resistance of bacterial spores to chemical agents, p 121–130. In Fraise AP, Maillard JY, Sattar SA (ed), Principles and practice of disinfection, preservation and sterilization, 5th ed. Wiley-Blackwell, Oxford, United Kingdom. [Google Scholar]
- 12.Setlow P. 2007. I will survive: DNA protection in bacterial spores. Trends Microbiol 15:172–180. 10.1016/j.tim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 14.Marshall KM, Nowaczyk L, II, Morrissey TR, Loeza V, Halik LA, Skinner GE, Reddy NR, Fleischman GJ, Larkin JW. 2015. Effect of sporulation temperature on the resistance of Clostridium botulinum type A spores to thermal and high pressure processing. J Food Prot 78:146–150. 10.4315/0362-028X.JFP-14-186. [DOI] [PubMed] [Google Scholar]
- 15.Lenz CA, Vogel RF. 2014. Effect of sporulation medium and its divalent cation content on the heat and high pressure resistance of Clostridium botulinum type E spores. Food Microbiol 44:156–167. 10.1016/j.fm.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Huesca-Espitia LC, Suvira M, Rosenbeck K, Korza G, Setlow B, Li W, Wang S, Li YQ, Setlow P. 2016. Effects of steam autoclave treatment on Geobacillus stearothermophilus spores. J Appl Microbiol 121:1300–1311. 10.1111/jam.13257. [DOI] [PubMed] [Google Scholar]
- 17.Berendsen EM, Boekhorst J, Kuipers OP, Wells-Bennik MH. 2016. A mobile genetic element profoundly increases heat resistance of bacterial spores. ISME J 10:2633–2642. 10.1038/ismej.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berendsen EM, Koning RA, Boekhorst J, de Jong A, Kuipers OP, Wells-Bennik MH. 2016. High-level heat resistance of spores of Bacillus amyloliquefaciens and Bacillus licheniformis results from the presence of a spoVA operon in a Tn1546 transposon. Front Microbiol 7:1912. 10.3389/fmicb.2016.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, Korza G, DeMarco AM, Kuipers OP, Li YQ, Setlow P. 2021. Properties of spores of Bacillus subtilis with or without a transposon that decreases spore germination and increases spore wet heat resistance. J Appl Microbiol 131:2918–2928. 10.1111/jam.15163. [DOI] [PubMed] [Google Scholar]
- 20.Meaney CA, Cartman ST, McClure PJ, Minton NP. 2016. The role of small acid-soluble proteins (SASPs) in protection of spores of Clostridium botulinum against nitrous acid. Int J Food Microbiol 216:25–30. 10.1016/j.ijfoodmicro.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Raju D, Setlow P, Sarker MR. 2007. Antisense-RNA-mediated decreased synthesis of small, acid-soluble spore proteins leads to decreased resistance of Clostridium perfringens spores to moist heat and UV radiation. Appl Environ Microbiol 73:2048–2053. 10.1128/AEM.02500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nerber HN, Sorg JA. 2021. The small acid-soluble proteins of Clostridioides difficile are important for UV resistance and serve as a check point for sporulation. PLoS Pathog 17:e1009516. 10.1371/journal.ppat.1009516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cano RJ, Borucki MK. 1995. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science 268:1060–1064. 10.1126/science.7538699. [DOI] [PubMed] [Google Scholar]
- 24.Vreeland RH, Rosenzweig WD, Powers DW. 2000. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 407:897–900. 10.1038/35038060. [DOI] [PubMed] [Google Scholar]
- 25.Horne WH, Volpe RP, Korza G, DePratti S, Conze IH, Shuryak I, Grebenc T, Matrosova VY, Gaidamakova EK, Tkavc R, Sharma A, Gostinčar C, Gunde-Cimerman N, Hoffman BM, Setlow P, Daly MJ. 2022. Effects of desiccation and freezing on microbial ionizing radiation survivability: considerations for Mars sample return. Astrobiology 22:1337–1350. 10.1089/ast.2022.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy MJ, Reader SL, Swierczynski LM. 1994. Preservation records of micro-organisms: evidence of the tenacity of life. Microbiology 140:2513–2529. 10.1099/00221287-140-10-2513. [DOI] [PubMed] [Google Scholar]
- 27.Ulrich N, Nagler K, Laue M, Cockell CS, Setlow P, Moeller R. 2018. Experimental studies addressing the longevity of Bacillus subtilis spores—the first data from a 500-year experiment. PLoS One 13:e0208425. 10.1371/journal.pone.0208425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh S, Korza G, Maciejewski M, Setlow P. 2015. Analysis of metabolism in dormant spores of Bacillus species by 31P nuclear magnetic resonance analysis of low-molecular-weight compounds. J Bacteriol 197:992–1001. 10.1128/JB.02520-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan AE, Koppel DE, Setlow B, Setlow P. 2003. A soluble protein is immobile in dormant spores of Bacillus subtilis but is mobile in germinated spores: implications for spore dormancy. Proc Natl Acad Sci USA 100:4209–4214. 10.1073/pnas.0636762100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedline AW, Zachariah MM, Johnson K, Thomas KJ, III, Middaugh AN, Garimella R, Powell DR, Vaishampayan PA, Rice CV. 2014. Water behavior in bacterial spores by deuterium NMR spectroscopy. J Phys Chem B 118:8945–8955. 10.1021/jp5025119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colas de la Noue A, Natali F, Fekraoui F, Gervais P, Martinez N, Perrier-Cornet JM, Peters J. 2020. The molecular dynamics of bacterial spore and the role of calcium dipicolinate in core properties at the sub-nanosecond time-scale. Sci Rep 10:8265. 10.1038/s41598-020-65093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sunde EP, Setlow P, Hederstedt L, Halle B. 2009. The physical state of water in bacterial spores. Proc Natl Acad Sci USA 106:19334–19339. 10.1073/pnas.0908712106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaieda S, Setlow B, Setlow P, Halle B. 2013. Mobility of core water in Bacillus subtilis spores by 2H NMR. Biophys J 105:2016–2023. 10.1016/j.bpj.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Setlow B, Setlow P. 1980. Measurements of the pH within dormant and germinated bacterial spores. Proc Natl Acad Sci USA 77:2474–2476. 10.1073/pnas.77.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setlow P, Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem 245:3637–3644. 10.1016/S0021-9258(18)62974-6. [DOI] [PubMed] [Google Scholar]
- 36.Korza G, Goulet M, DeMarco A, Wicander J, Setlow P. 2023. Role of Bacillus subtilis spore core water content and pH in the accumulation and utilization of spores’ large 3-phosphoglyceric acid depot, and the crucial role of this depot in generating ATP early during spore germination. Microorganisms 11:195. 10.3390/microorganisms11010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setlow B, Melly E, Setlow P. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage in spore germination. J Bacteriol 183:4894–4899. 10.1128/JB.183.16.4894-4899.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moeller R, Setlow P, Horneck G, Berger T, Reitz G, Rettberg P, Doherty AJ, Okayasu R, Nicholson WL. 2008. Roles of the major, small, acid-soluble spore proteins and spore-specific and universal DNA repair mechanisms in resistance of Bacillus subtilis spores to ionizing radiation from X rays and high-energy charged-particle bombardment. J Bacteriol 190:1134–1140. 10.1128/JB.01644-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Setlow B, Atluri S, Kitchel R, Koziol-Dube K, Setlow P. 2006. Role of dipicolinic acid in resistance and stability of spores of Bacillus subtilis with or without DNA-protective alpha/beta-type small acid-soluble proteins. J Bacteriol 188:3740–3747. 10.1128/JB.00212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffiths KK, Setlow P. 2009. Effects of modification of membrane lipid composition on Bacillus subtilis sporulation and spore properties. J Appl Microbiol 106:2064–2078. 10.1111/j.1365-2672.2009.04176.x. [DOI] [PubMed] [Google Scholar]
- 41.Cowan AE, Olivastro EM, Koppel DE, Loshon CA, Setlow B, Setlow P. 2004. Lipids in the inner membrane of dormant spores of Bacillus species are largely immobile. Proc Natl Acad Sci USA 101:7733–7738. 10.1073/pnas.0306859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laue M, Han HM, Dittmann C, Setlow P. 2018. Intracellular membranes of bacterial endospores are reservoirs for spore core membrane expansion during spore germination. Sci Rep 8:11388. 10.1038/s41598-018-29879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imae Y, Strominger JL. 1976. Conditional spore cortex-less mutants of Bacillus sphaericus 9602. J Biol Chem 251:1493–1499. 10.1016/S0021-9258(17)33767-5. [DOI] [PubMed] [Google Scholar]
- 44.Daniel RA, Drake S, Buchanan CE, Scholle R, Errington J. 1994. The Bacillus subtilis spoVD gene encodes a mother-cell-specific penicillin-binding protein required for spore morphogenesis. J Mol Biol 235:209–220. 10.1016/s0022-2836(05)80027-0. [DOI] [PubMed] [Google Scholar]
- 45.Popham DL, Helin J, Costello CE, Setlow P. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA 93:15405–15410. 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knudsen SM, Cermak N, Delgado FF, Setlow B, Setlow P, Manalis SR. 2016. Water and small-molecule permeation of dormant Bacillus subtilis spores. J Bacteriol 198:168–177. 10.1128/JB.00435-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Driks A, Eichenberger P. 2016. The spore coat. Microbiol Spectr 4:4.2.03. 10.1128/microbiolspec.TBS-0023-2016. [DOI] [PubMed] [Google Scholar]
- 48.Abhyankar W, Pandey R, Ter Beek A, Brul S, de Koning LJ, de Koster CG. 2015. Reinforcement of Bacillus subtilis spores by cross-linking of outer coat proteins during maturation. Food Microbiol 45:54–62. 10.1016/j.fm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Ursem R, Swarge B, Abhyankar WR, Buncherd H, de Koning LJ, Setlow P, Brul S, Kramer G. 2021. Identification of native cross-links in Bacillus subtilis spore coat proteins. J Proteome Res 20:1809–1816. 10.1021/acs.jproteome.1c00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chada VG, Sanstad EA, Wang R, Driks A. 2003. Morphogenesis of Bacillus spore surfaces. J Bacteriol 185:6255–6261. 10.1128/JB.185.21.6255-6261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanaan J, Murray J, Higgins R, Nana M, DeMarco AM, Korza G, Setlow P. 2022. Resistance properties and the role of the inner membrane and coat of Bacillus subtilis spores with extreme wet heat resistance. J Appl Microbiol 132:2157–2166. 10.1111/jam.15345. [DOI] [PubMed] [Google Scholar]
- 52.Ghosh S, Setlow B, Wahome PG, Cowan AE, Plomp M, Malkin AJ, Setlow P. 2008. Characterization of spores of Bacillus subtilis that lack most coat layers. J Bacteriol 190:6741–6748. 10.1128/JB.00896-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klobutcher LA, Ragkousi K, Setlow P. 2006. The Bacillus subtilis spore coat provides “eat resistance” during phagocytic predation by the protozoan Tetrahymena thermophila. Proc Natl Acad Sci USA 103:165–170. 10.1073/pnas.0507121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laaberki MH, Dworkin J. 2008. Role of spore coat proteins in the resistance of Bacillus subtilis spores to Caenorhabditis elegans predation. J Bacteriol 190:6197–6203. 10.1128/JB.00623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morisaku T, Kido Y, Asai K, Yui H. 2019. Mechanical properties of the coat protein layer and cortex in single Bacillus subtilis spores studied with an atomic force microscope and laser-induced surface deformation microscope. Anal Sci 35:45–48. 10.2116/analsci.18SDP02. [DOI] [PubMed] [Google Scholar]
- 56.Gerhardt P, Black SH. 1961. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol 82:750–760. 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKenney PT, Driks A, Eskandarian HA, Grabowski P, Guberman J, Wang KH, Gitai Z, Eichenberger P. 2010. A distance-weighted interaction map reveals a previously uncharacterized layer of the Bacillus subtilis spore coat. Curr Biol 20:934–938. 10.1016/j.cub.2010.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dubois T, Krzewinski F, Yamakawa N, Lemy C, Hamiot A, Brunet L, Lacoste AS, Knirel Y, Guerardel Y, Faille C. 2020. The sps genes encode an original Legionaminic acid pathway required for crust assembly in Bacillus subtilis. mBio 11:e01153-20. 10.1128/mBio.01153-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bressuire-Isoard C, Bornard I, Henriques AO, Carlin F, Broussolle V. 2016. Sporulation temperature reveals a requirement for CotE in the assembly of both the coat and exosporium layers of Bacillus cereus spores. Appl Environ Microbiol 82:232–243. 10.1128/AEM.02626-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Zhou K, Li N, Christie G, Wilson DI. 2017. Assessing the impact of germination and sporulation conditions on the adhesion of Bacillus spores to glass and stainless steel by fluid dynamic gauging. J Food Sci 82:2614–2625. 10.1111/1750-3841.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lequette Y, Garénaux E, Tauveron G, Dumez S, Perchat S, Slomianny C, Lereclus D, Guérardel Y, Faille C. 2011. Role played by exosporium glycoproteins in the surface properties of Bacillus cereus spores and in their adhesion to stainless steel. Appl Environ Microbiol 77:4905–4911. 10.1128/AEM.02872-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faille C, Lequette Y, Ronse A, Slomianny C, Garénaux E, Guerardel Y. 2010. Morphology and physico-chemical properties of Bacillus spores surrounded or not with an exosporium: consequences on their ability to adhere to stainless steel. Int J Food Microbiol 143:125–135. 10.1016/j.ijfoodmicro.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 63.Nishihara T, Takubo Y, Kawamata E, Koshikawa T, Ogaki J, Kondo M. 1989. Role of outer coat in resistance of Bacillus megaterium spore. J Biochem 106:270–273. 10.1093/oxfordjournals.jbchem.a122843. [DOI] [PubMed] [Google Scholar]
- 64.Koshikawa T, Beaman TC, Pankratz HS, Nakashio S, Corner TR, Gerhardt P. 1984. Resistance, germination, and permeability correlates of Bacillus megaterium spores successively divested of integument layers. J Bacteriol 159:624–632. 10.1128/jb.159.2.624-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro-Córdova P, Mora-Uribe P, Reyes-Ramírez R, Cofré-Araneda G, Orozco-Aguilar J, Brito-Silva C, Mendoza-León MJ, Kuehne SA, Minton NP, Pizarro-Guajardo M, Paredes-Sabja D. 2021. Entry of spores into intestinal epithelial cells contributes to recurrence of Clostridioides difficile infection. Nat Commun 12:1140. 10.1038/s41467-021-21355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stier P, Kulozik U. 2020. Effect of sporulation conditions following submerged cultivation on the resistance of Bacillus atrophaeus spores against inactivation by H2O2. Molecules 25:2985. 10.3390/molecules25132985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells-Bennik MHJ, Janssen PWM, Klaus V, Yang C, Zwietering MH, Den Besten HMW. 2019. Heat resistance of spores of 18 strains of Geobacillus stearothermophilus and impact of culturing conditions. Int J Food Microbiol 291:161–172. 10.1016/j.ijfoodmicro.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Mtimet N, Trunet C, Mathot AG, Venaille L, Leguérinel I, Coroller L, Couvert O. 2016. Die another day: fate of heat-treated Geobacillus stearothermophilus ATCC 12980 spores during storage under growth-preventing conditions. Food Microbiol 56:87–95. 10.1016/j.fm.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Malleck T, Daufouy G, André S, Broussolle V, Planchon S. 2018. Temperature impacts the sporulation capacities and spore resistance of Moorella thermoacetica. Food Microbiol 73:334–341. 10.1016/j.fm.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 70.Moeller R, Wassmann M, Reitz G, Setlow P. 2011. Effect of radioprotective agents in sporulation medium on Bacillus subtilis spore resistance to hydrogen peroxide, wet heat and germicidal and environmentally relevant UV radiation. J Appl Microbiol 110:1485–1494. 10.1111/j.1365-2672.2011.05004.x. [DOI] [PubMed] [Google Scholar]
- 71.Mtimet N, Guégan S, Durand L, Mathot AG, Venaille L, Leguérinel I, Coroller L, Couvert O. 2016. Effect of pH on Thermoanaerobacterium thermosaccharolyticum DSM 571 growth, spore heat resistance and recovery. Food Microbiol 55:64–72. 10.1016/j.fm.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 72.Moeller R, Vlašić I, Reitz G, Nicholson WL. 2012. Role of altered rpoB alleles in Bacillus subtilis sporulation and spore resistance to heat, hydrogen peroxide, formaldehyde, and glutaraldehyde. Arch Microbiol 194:759–767. 10.1007/s00203-012-0811-4. [DOI] [PubMed] [Google Scholar]
- 73.Isticato R, Lanzilli M, Petrillo C, Donadio G, Baccigalupi L, Ricca E. 2020. Bacillus subtilis builds structurally and functionally different spores in response to the temperature of growth. Environ Microbiol 22:170–182. 10.1111/1462-2920.14835. [DOI] [PubMed] [Google Scholar]
- 74.Carroll TM, Setlow P. 2005. Site-directed mutagenesis and structural studies suggest that the germination protease, GPR, in spores of Bacillus species is an atypical aspartic acid protease. J Bacteriol 187:7119–7125. 10.1128/JB.187.20.7119-7125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Traag BA, Pugliese A, Setlow B, Setlow P, Losick R. 2013. A conserved ClpP-like protease involved in spore outgrowth in Bacillus subtilis. Mol Microbiol 90:160–166. 10.1111/mmi.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanchez-Salas JL, Santiago-Lara ML, Setlow B, Sussman MD, Setlow P. 1992. Properties of Bacillus megaterium and Bacillus subtilis mutants which lack the protease that degrades small, acid-soluble proteins during spore germination. J Bacteriol 174:807–814. 10.1128/jb.174.3.807-814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Setlow P. 1992. DNA in dormant spores of Bacillus species is in an A-like conformation. Mol Microbiol 6:563–567. 10.1111/j.1365-2958.1992.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 78.Lee KS, Bumbaca D, Kosman J, Setlow P, Jedrzejas MJ. 2008. Structure of a protein-DNA complex essential for DNA protection in spores of Bacillus species. Proc Natl Acad Sci USA 105:2806–2811. 10.1073/pnas.0708244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohr SC, Sokolov NV, He CM, Setlow P. 1991. Binding of small acid-soluble spore proteins from Bacillus subtilis changes the conformation of DNA from B to A. Proc Natl Acad Sci USA 88:77–81. 10.1073/pnas.88.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicholson WL, Setlow B, Setlow P. 1991. Ultraviolet irradiation of DNA complexed with alpha/beta-type small, acid-soluble proteins from spores of Bacillus or Clostridium species makes spore photoproduct but not thymine dimers. Proc Natl Acad Sci USA 88:8288–8292. 10.1073/pnas.88.19.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fairhead H, Setlow P. 1992. Binding of DNA to alpha/beta-type small, acid-soluble proteins from spores of Bacillus or Clostridium species prevents formation of cytosine dimers, cytosine-thymine dimers, and bipyrimidine photoadducts after UV irradiation. J Bacteriol 174:2874–2880. 10.1128/jb.174.9.2874-2880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Douki T, Setlow B, Setlow P. 2005. Photosensitization of DNA by dipicolinic acid, a major component of spores of Bacillus species. Photochem Photobiol Sci 4:591–597. 10.1039/b503771a. [DOI] [PubMed] [Google Scholar]
- 83.Xue Y, Nicholson WL. 1996. The two major spore DNA repair pathways, nucleotide excision repair and spore photoproduct lyase, are sufficient for the resistance of Bacillus subtilis spores to artificial UV-C and UV-B but not to solar radiation. Appl Environ Microbiol 62:2221–2227. 10.1128/aem.62.7.2221-2227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moeller R, Douki T, Cadet J, Stackebrandt E, Nicholson WL, Rettberg P, Reitz G, Horneck G. 2007. UV-radiation-induced formation of DNA bipyrimidine photoproducts in Bacillus subtilis endospores and their repair during germination. Int Microbiol 10:39–46. [PubMed] [Google Scholar]
- 85.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. 10.1128/jb.178.12.3486-3495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rebeil R, Sun Y, Chooback L, Pedraza-Reyes M, Kinsland C, Begley TP, Nicholson WL. 1998. Spore photoproduct lyase from Bacillus subtilis spores is a novel iron-sulfur DNA repair enzyme which shares features with proteins such as class III anaerobic ribonucleotide reductases and pyruvate-formate lyases. J Bacteriol 180:4879–4885. 10.1128/JB.180.18.4879-4885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Setlow P, Li L. 2015. Photochemistry and photobiology of the spore photoproduct: a 50-year journey. Photochem Photobiol 91:1263–1290. 10.1111/php.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Setlow B, Setlow P. 1988. Decreased UV light resistance of spores of Bacillus subtilis strains deficient in pyrimidine dimer repair and small, acid-soluble spore proteins. Appl Environ Microbiol 54:1275–1276. 10.1128/aem.54.5.1275-1276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taylor W, Camilleri E, Craft DL, Korza G, Granados MR, Peterson J, Szczpaniak R, Weller SK, Moeller R, Douki T, Mok WWK, Setlow P. 2020. DNA damage kills bacterial spores and cells exposed to 222-nanometer UV radiation. Appl Environ Microbiol 86:e03039-19. 10.1128/AEM.03039-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Djouiai B, Thwaite JE, Laws TR, Commichau FM, Setlow B, Setlow P, Moeller R. 2018. Role of DNA repair and protective components in Bacillus subtilis spore resistance to inactivation by 400-nm-wavelength blue light. Appl Environ Microbiol 84:e01604-18. 10.1128/AEM.01604-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moeller R, Horneck G, Facius R, Stackebrandt E. 2005. Role of pigmentation in protecting Bacillus sp. endospores against environmental UV radiation. FEMS Microbiol Ecol 51:231–236. 10.1016/j.femsec.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 92.Khaneja R, Perez-Fons L, Fakhry S, Baccigalupi L, Steiger S, To E, Sandmann G, Dong TC, Ricca E, Fraser PD, Cutting SM. 2010. Carotenoids found in Bacillus. J Appl Microbiol 108:1889–1902. 10.1111/j.1365-2672.2009.04590.x. [DOI] [PubMed] [Google Scholar]
- 93.Jo HL, Hwang HJ, Chung MS. 2019. Inactivation of Bacillus subtilis spores at various germination and outgrowth stages using intense pulsed light. Food Microbiol 82:409–415. 10.1016/j.fm.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 94.Trunet C, Mtimet N, Mathot AG, Postollec F, Leguérinel I, Couvert O, Carlin F, Coroller L. 2018. Effect of incubation temperature and pH on the recovery of Bacillus weihenstephanensis spores after exposure to a peracetic acid-based disinfectant or to pulsed light. Int J Food Microbiol 278:81–87. 10.1016/j.ijfoodmicro.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 95.Esbelin J, Malléa S, Clair G, Carlin F. 2016. Inactivation by pulsed light of Bacillus subtilis spores with impaired protection factors. Photochem Photobiol 92:301–307. 10.1111/php.12568. [DOI] [PubMed] [Google Scholar]
- 96.Clair G, Esbelin J, Malléa S, Bornard I, Carlin F. 2020. The spore coat is essential for Bacillus subtilis spore resistance to pulsed light, and pulsed light treatment eliminates some spore coat proteins. Int J Food Microbiol 323:108592. 10.1016/j.ijfoodmicro.2020.108592. [DOI] [PubMed] [Google Scholar]
- 97.Dikec J, Bechoua N, Winckler P, Perrier-Cornet JM. 2022. Effects of pulsed near infrared light (NIR) on Bacillus subtilis spores. J Photochem Photobiol B 234:112530. 10.1016/j.jphotobiol.2022.112530. [DOI] [PubMed] [Google Scholar]
- 98.Moeller R, Raguse M, Reitz G, Okayasu R, Li Z, Klein S, Setlow P, Nicholson WL. 2014. Resistance of Bacillus subtilis spore DNA to lethal ionizing radiation damage relies primarily on spore core components and DNA repair, with minor effects of oxygen radical detoxification. Appl Environ Microbiol 80:104–109. 10.1128/AEM.03136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y, Moeller R, Tran S, Dubovcova B, Akepsimaidis G, Meneses N, Drissner D, Mathys A. 2018. Geobacillus and Bacillus spore inactivation by low energy electron beam technology: resistance and influencing factors. Front Microbiol 9:2720. 10.3389/fmicb.2018.02720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takahashi N, Hieda K, Morohoshi F, Munakata N. 1999. Base substitution spectra of nalidixylate resistant mutations induced by monochromatic soft X and 60Co gamma-rays in Bacillus subtilis spores. J Radiat Res 40:115–124. 10.1269/jrr.40.115. [DOI] [PubMed] [Google Scholar]
- 101.Vlašić I, Mertens R, Seco EM, Carrasco B, Ayora S, Reitz G, Commichau FM, Alonso JC, Moeller R. 2014. Bacillus subtilis RecA and its accessory factors, RecF, RecO, RecR and RecX, are required for spore resistance to DNA double-strand break. Nucleic Acids Res 42:2295–2307. 10.1093/nar/gkt1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moeller R, Reitz G, Li Z, Klein S, Nicholson WL. 2012. Multifactorial resistance of Bacillus subtilis spores to high-energy proton radiation: role of spore structural components and the homologous recombination and non-homologous end joining DNA repair pathways. Astrobiology 12:1069–1077. 10.1089/ast.2012.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vinter V. 1961. Changes in radioresistance of sporulating cells of Bacillus cereus. Nature 189:589–590. 10.1038/189589a0. [DOI] [PubMed] [Google Scholar]
- 104.Coleman WH, Chen D, Li YQ, Cowan AE, Setlow P. 2007. How moist heat kills spores of Bacillus subtilis. J Bacteriol 189:8458–8466. 10.1128/JB.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coleman WH, Setlow P. 2009. Analysis of damage due to moist heat treatment of spores of Bacillus subtilis. J Appl Microbiol 106:1600–1607. 10.1111/j.1365-2672.2008.04127.x. [DOI] [PubMed] [Google Scholar]
- 106.Coleman WH, Zhang P, Li YQ, Setlow P. 2010. Mechanism of killing of spores of Bacillus cereus and Bacillus megaterium by wet heat. Lett Appl Microbiol 50:507–514. 10.1111/j.1472-765X.2010.02827.x. [DOI] [PubMed] [Google Scholar]
- 107.Warth AD. 1980. Heat stability of Bacillus cereus enzymes within spores and in extracts. J Bacteriol 143:27–34. 10.1128/jb.143.1.27-34.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wen J, Smelt J, Vischer NOE, de Vos AL, Setlow P, Brul S. 2022. Heat activation and inactivation of bacterial spores: is there an overlap? Appl Environ Microbiol 88:e0232421. 10.1128/aem.02324-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gerhardt P, Marquis RE. 1989. Spore thermoresistance mechanisms, p 43–63. In Smith I, Slepecky RA, Setlow P (ed), Regulation of prokaryotic development. American Society of Microbiology, Washington, DC. [Google Scholar]
- 110.Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182:5505–5512. 10.1128/JB.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tovar-Rojo F, Chander M, Setlow B, Setlow P. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 184:584–587. 10.1128/JB.184.2.584-587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez-Salas JL, Setlow B, Zhang P, Li YQ, Setlow P. 2011. Maturation of released spores is necessary for acquisition of full spore heat resistance during Bacillus subtilis sporulation. Appl Environ Microbiol 77:6746–6754. 10.1128/AEM.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Popham DL, Sengupta S, Setlow P. 1995. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl Environ Microbiol 61:3633–3638. 10.1128/aem.61.10.3633-3638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Setlow B, Setlow P. 1994. Heat inactivation of Bacillus subtilis spores lacking small, acid-soluble spore proteins is accompanied by generation of abasic sites in spore DNA. J Bacteriol 176:2111–2113. 10.1128/jb.176.7.2111-2113.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sohail A, Hayes CS, Divvela P, Setlow P, Bhagwat AS. 2002. Protection of DNA by alpha/beta-type small, acid-soluble proteins from Bacillus subtilis spores against cytosine deamination. Biochemistry 41:11325–11330. 10.1021/bi026332t. [DOI] [PubMed] [Google Scholar]
- 116.Setlow B, Setlow P. 1995. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by dry heat. Appl Environ Microbiol 61:2787–2790. 10.1128/aem.61.7.2787-2790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tovar-Rojo F, Setlow P. 1991. Effects of mutant small, acid-soluble spore proteins from Bacillus subtilis on DNA in vivo and in vitro. J Bacteriol 173:4827–4835. 10.1128/jb.173.15.4827-4835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schottroff F, Pyatkovskyy T, Reineke K, Setlow P, Sastry SK, Jaeger H. 2019. Mechanisms of enhanced bacterial endospore inactivation during sterilization by ohmic heating. Bioelectrochemistry 130:107338. 10.1016/j.bioelechem.2019.107338. [DOI] [PubMed] [Google Scholar]
- 119.Tu Z, Setlow P, Brul S, Kramer G. 2021. Molecular physiological characterization of a high heat resistant spore forming Bacillus subtilis food isolate. Microorganisms 9:667. 10.3390/microorganisms9030667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao X, Swarge BN, Dekker HL, Roseboom W, Brul S, Kramer G. 2021. The membrane proteome of spores and vegetative cells of the food-borne pathogen Bacillus cereus. Int J Mol Sci 22:12475. 10.3390/ijms222212475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu B, Kanaan J, Shames H, Wicander J, Aryal M, Li Y, Korza G, Brul S, Kramer G, Li YQ, Nichols FC, Hao B, Setlow P. 2023. Identification and characterization of new proteins crucial for bacterial spore resistance and germination. In revision. [DOI] [PMC free article] [PubMed]
- 122.Mokashi S, Kanaan J, Craft DL, Byrd B, Zenick B, Laue M, Korza G, Mok WWK, Setlow P. 2020. Killing of bacterial spores by dodecylamine and its effects on spore inner membrane properties. J Appl Microbiol 129:1511–1522. 10.1111/jam.14732. [DOI] [PubMed] [Google Scholar]
- 123.Korza G, DePratti S, Fairchild D, Wicander J, Kanaan J, Shames H, Nichols FC, Cowan AE, Setlow P. 2023. Expression of the 2Duf protein in wild-type Bacillus subtilis spores stabilizes inner membrane proteins and increases spore resistance to heat and hydrogen peroxide. J Appl Microbiol 10.1093/jambio/lxad040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kornberg A, Spudich JA, Nelson DL, Deutscher MP. 1968. Origin of proteins in sporulation. Annu Rev Biochem 37:51–78. 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- 125.Setlow B, Shay LK, Vary JC, Setlow P. 1977. Production of large amounts of acetate during germination of Bacillus megaterium spores in the absence of exogenous carbon sources. J Bacteriol 132:744–746. 10.1128/jb.132.2.744-746.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cortezzo DE, Koziol-Dube K, Setlow B, Setlow P. 2004. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes spores to subsequent stress. J Appl Microbiol 97:838–852. 10.1111/j.1365-2672.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- 127.Arrieta-Ortiz ML, Hafemeister C, Bate AR, Chu T, Greenfield A, Shuster B, Barry SN, Gallitto M, Liu B, Kacmarczyk T, Santoriello F, Chen J, Rodrigues CD, Sato T, Rudner DZ, Driks A, Bonneau R, Eichenberger P. 2015. An experimentally supported model of the Bacillus subtilis global transcriptional regulatory network. Mol Syst Biol 11:839. 10.15252/msb.20156236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng L, Abhyankar W, Ouwerling N, Dekker HL, van Veen H, van der Wel NN, Roseboom W, de Koning LJ, Brul S, de Koster CG. 2016. Bacillus subtilis spore inner membrane proteome. J Proteome Res 15:585–594. 10.1021/acs.jproteome.5b00976. [DOI] [PubMed] [Google Scholar]
- 129.Celandroni F, Longo I, Tosoratti N, Giannessi F, Ghelardi E, Salvetti S, Baggiani A, Senesi S. 2004. Effect of microwave radiation on Bacillus subtilis spores. J Appl Microbiol 97:1220–1227. 10.1111/j.1365-2672.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- 130.Park HS, Yang J, Choi HJ, Kim KH. 2017. Effective thermal inactivation of the spores of Bacillus cereus biofilms using microwave. J Microbiol Biotechnol 27:1209–1215. 10.4014/jmb.1702.02009. [DOI] [PubMed] [Google Scholar]
- 131.Jeng DK, Kaczmarek KA, Woodworth AG, Balasky G. 1987. Mechanism of microwave sterilization in the dry state. Appl Environ Microbiol 53:2133–2137. 10.1128/aem.53.9.2133-2137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Welt BA, Tong CH, Rossen JL, Lund DB. 1994. Effect of microwave radiation on inactivation of Clostridium sporogenes (PA 3679) spores. Appl Environ Microbiol 60:482–488. 10.1128/aem.60.2.482-488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ojha SC, Chankhamhaengdecha S, Singhakaew S, Ounjai P, Janvilisri T. 2016. Inactivation of Clostridium difficile spores by microwave irradiation. Anaerobe 38:14–20. 10.1016/j.anaerobe.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 134.Evelyn, Silva FVM. 2015. Use of power ultrasound to enhance the thermal inactivation of Clostridium perfringens spores in beef slurry. Int J Food Microbiol 206:17–23. 10.1016/j.ijfoodmicro.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 135.Fan L, Hou F, Muhammad AI, Ruiling LV, Watharkar RB, Guo M, Ding T, Liu D. 2019. Synergistic inactivation and mechanism of thermal and ultrasound treatments against Bacillus subtilis spores. Food Res Int 116:1094–1102. 10.1016/j.foodres.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 136.Fan L, Hou F, Idris Muhammad A, Bilyaminu Ismail B, Lv R, Ding T, Liu D. 2020. Proteomic responses of spores of Bacillus subtilis to thermosonication involve large-scale alterations in metabolic pathways. Ultrason Sonochem 64:104992. 10.1016/j.ultsonch.2020.104992. [DOI] [PubMed] [Google Scholar]
- 137.Fan L, Ismail BB, Hou F, Muhammad AI, Zou M, Ding T, Liu D. 2019. Thermosonication damages the inner membrane of Bacillus subtilis spores and impels their inactivation. Food Res Int 125:108514. 10.1016/j.foodres.2019.108514. [DOI] [PubMed] [Google Scholar]
- 138.Lv R, Zou M, Chantapakul T, Chen W, Muhammad AI, Zhou J, Ding T, Ye X, Liu D. 2019. Effect of ultrasonication and thermal and pressure treatments, individually and combined, on inactivation of Bacillus cereus spores. Appl Microbiol Biotechnol 103:2329–2338. 10.1007/s00253-018-9559-3. [DOI] [PubMed] [Google Scholar]
- 139.Zammuto V, Fuchs FM, Fiebrandt M, Stapelmann K, Ulrich NJ, Maugeri TL, Pukall R, Gugliandolo C, Moeller R. 2018. Comparing spore resistance of Bacillus strains isolated from hydrothermal vents and spacecraft assembly facilities to environmental stressors and decontamination treatments. Astrobiology 18:1425–1434. 10.1089/ast.2017.1715. [DOI] [PubMed] [Google Scholar]
- 140.Zamenhof S. 1960. Effects of heating dry bacteria and spores on their phenotype and genotype. Proc Natl Acad Sci USA 46:101–105. 10.1073/pnas.46.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Northrop J, Slepecky RA. 1967. Sporulation mutations induced by heat in Bacillus subtilis. Science 155:838–839. 10.1126/science.155.3764.838. [DOI] [PubMed] [Google Scholar]
- 142.del Carmen Huesca Espitia L, Caley C, Bagyan I, Setlow P. 2002. Base-change mutations induced by various treatments of Bacillus subtilis spores with and without DNA protective small, acid-soluble spore proteins. Mutat Res 503:77–84. 10.1016/s0027-5107(02)00093-3. [DOI] [PubMed] [Google Scholar]
- 143.Fairhead H, Setlow B, Waites WM, Setlow P. 1994. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from being killed by freeze-drying. Appl Environ Microbiol 60:2647–2649. 10.1128/aem.60.7.2647-2649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Munakata N, Saitou M, Takahashi N, Hieda K, Morohoshi F. 1997. Induction of unique tandem-base change mutations in bacterial spores exposed to extreme dryness. Mutat Res 390:189–195. 10.1016/s0165-1218(97)00020-7. [DOI] [PubMed] [Google Scholar]
- 145.He L, Wang S, Cortesão M, Wu M, Moeller R, Setlow P, Li YQ. 2018. Single-cell analysis reveals individual spore responses to simulated space vacuum. NPJ Microgravity 4:26. 10.1038/s41526-018-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Horneck G, Moeller R, Cadet J, Douki T, Mancinelli RL, Nicholson WL, Panitz C, Rabbow E, Rettberg P, Spry A, Stackebrandt E, Vaishampayan P, Venkateswaran KJ. 2012. Resistance of bacterial endospores to outer space for planetary protection purposes—experiment PROTECT of the EXPOSE-E mission. Astrobiology 12:445–456. 10.1089/ast.2011.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Setlow P, Christie G. 2021. What’s new and notable in bacterial spore killing! World J Microbiol Biotechnol 37:144. 10.1007/s11274-021-03108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cortezzo DE, Setlow P. 2005. Analysis of factors that influence the sensitivity of spores of Bacillus subtilis to DNA damaging chemicals. J Appl Microbiol 98:606–617. 10.1111/j.1365-2672.2004.02495.x. [DOI] [PubMed] [Google Scholar]
- 149.Setlow B, Setlow P. 1993. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl Environ Microbiol 59:3418–3423. 10.1128/aem.59.10.3418-3423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Setlow B, Setlow CA, Setlow P. 1997. Killing bacterial spores by organic hydroperoxides. J Ind Microbiol Biotechnol 18:384–388. 10.1038/sj.jim.2900399. [DOI] [Google Scholar]
- 151.Palop A, Rutherford GC, Marquis RE. 1998. Inactivation of enzymes within spores of Bacillus megaterium ATCC 19213 by hydroperoxides. Can J Microbiol 44:465–470. 10.1139/w98-026. [DOI] [PubMed] [Google Scholar]
- 152.Hayes CS, Illades-Aguiar B, Casillas-Martinez L, Setlow P. 1998. In vitro and in vivo oxidation of methionine residues in small, acid-soluble spore proteins from Bacillus species. J Bacteriol 180:2694–2700. 10.1128/JB.180.10.2694-2700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Setlow B, Yu J, Li YQ, Setlow P. 2013. Analysis of the germination kinetics of individual Bacillus subtilis spores treated with hydrogen peroxide or sodium hypochlorite. Lett Appl Microbiol 57:259–265. 10.1111/lam.12113. [DOI] [PubMed] [Google Scholar]
- 154.Melly E, Cowan AE, Setlow P. 2002. Studies on the mechanism of killing of Bacillus subtilis spores by hydrogen peroxide. J Appl Microbiol 93:316–325. 10.1046/j.1365-2672.2002.01687.x. [DOI] [PubMed] [Google Scholar]
- 155.Young SB, Setlow P. 2003. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J Appl Microbiol 95:54–67. 10.1046/j.1365-2672.2003.01960.x. [DOI] [PubMed] [Google Scholar]
- 156.Young SB, Setlow P. 2004. Mechanisms of Bacillus subtilis spore resistance to and killing by aqueous ozone. J Appl Microbiol 96:1133–1142. 10.1111/j.1365-2672.2004.02236.x. [DOI] [PubMed] [Google Scholar]
- 157.Tennen R, Setlow B, Davis KL, Loshon CA, Setlow P. 2000. Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J Appl Microbiol 89:330–338. 10.1046/j.1365-2672.2000.01114.x. [DOI] [PubMed] [Google Scholar]
- 158.Riesenman PJ, Nicholson WL. 2000. Role of the spore coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl Environ Microbiol 66:620–626. 10.1128/AEM.66.2.620-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Checinska A, Burbank M, Paszczynski AJ. 2012. Protection of Bacillus pumilus spores by catalases. Appl Environ Microbiol 78:6413–6422. 10.1128/AEM.01211-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Henriques AO, Moran CP, Jr. 2007. Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol 61:555–588. 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- 161.Casillas-Martinez L, Setlow P. 1997. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents. J Bacteriol 179:7420–7425. 10.1128/jb.179.23.7420-7425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Casillas-Martinez L, Driks A, Setlow B, Setlow P. 2000. Lack of a significant role for the PerR regulator in Bacillus subtilis spore resistance. FEMS Microbiol Lett 188:203–208. 10.1111/j.1574-6968.2000.tb09194.x. [DOI] [PubMed] [Google Scholar]
- 163.Bagyan I, Casillas-Martinez L, Setlow P. 1998. The katX gene, which codes for the catalase in spores of Bacillus subtilis, is a forespore-specific gene controlled by sigmaF, and KatX is essential for hydrogen peroxide resistance of the germinating spore. J Bacteriol 180:2057–2062. 10.1128/JB.180.8.2057-2062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cybulski RJ, Jr, Sanz P, Alem F, Stibitz S, Bull RL, O'Brien AD. 2009. Four superoxide dismutases contribute to Bacillus anthracis virulence and provide spores with redundant protection from oxidative stress. Infect Immun 77:274–285. 10.1128/IAI.00515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Setlow B, Loshon CA, Genest PC, Cowan AE, Setlow C, Setlow P. 2002. Mechanisms of killing spores of Bacillus subtilis by acid, alkali and ethanol. J Appl Microbiol 92:362–375. 10.1046/j.1365-2672.2002.01540.x. [DOI] [PubMed] [Google Scholar]
- 166.Green J, Korza G, Granados MR, Zenick B, Schlievert PM, Mok WMK, Setlow P. 2020. Lack of efficient killing of purified dormant spores of Bacillales and Clostridiales species by glycerol monolaurate in a non-aqueous gel. Lett Appl Microbiol 70:407–412. 10.1111/lam.13290. [DOI] [PubMed] [Google Scholar]
- 167.Setlow P. 2019. Observations on research with spores of Bacillales and Clostridiales species. J Appl Microbiol 126:348–358. 10.1111/jam.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cutting SM. 2011. Bacillus probiotics. Food Microbiol 28:214–220. 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 169.Ghelardi E, Celandroni F, Salvetti S, Gueye SA, Lupetti A, Senesi S. 2015. Survival and persistence of Bacillus clausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J Appl Microbiol 119:552–559. 10.1111/jam.12848. [DOI] [PubMed] [Google Scholar]
- 170.Seccareccia I, Kovács ÁT, Gallegos-Monterrosa R, Nett M. 2016. Unraveling the predator-prey relationship of Cupriavidus necator and Bacillus subtilis. Microbiol Res 192:231–238. 10.1016/j.micres.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 171.Müller S, Strack SN, Ryan SE, Kearns DB, Kirby JR. 2015. Predation by Myxococcus xanthus induces Bacillus subtilis to form spore-filled megastructures. Appl Environ Microbiol 81:203–210. 10.1128/AEM.02448-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Paidhungat M, Setlow B, Daniels WB, Hoover D, Papafragkou E, Setlow P. 2002. Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Appl Environ Microbiol 68:3172–3175. 10.1128/AEM.68.6.3172-3175.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gould GW, Sale AJ. 1970. Initiation of germination of bacterial spores by hydrostatic pressure. J Gen Microbiol 60:335–346. 10.1099/00221287-60-3-335. [DOI] [PubMed] [Google Scholar]
- 174.Delbrück AI, Zhang Y, Heydenreich R, Mathys A. 2021. Bacillus spore germination at moderate high pressure: a review on underlying mechanisms, influencing factors, and its comparison with nutrient germination. Compr Rev Food Sci Food Saf 20:4159–4181. 10.1111/1541-4337.12789. [DOI] [PubMed] [Google Scholar]
- 175.Reineke K, Schlumbach K, Baier D, Mathys A, Knorr D. 2013. The release of dipicolinic acid–the rate-limiting step of Bacillus endospore inactivation during the high pressure thermal sterilization process. Int J Food Microbiol 162:55–63. 10.1016/j.ijfoodmicro.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 176.Modugno C, Peltier C, Simonin H, Dujourdy L, Capitani F, Sandt C, Perrier-Cornet JM. 2020. Understanding the effects of high pressure on bacterial spores using synchrotron infrared spectroscopy. Front Microbiol 10:3122. 10.3389/fmicb.2019.03122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Modugno C, Kmiha S, Simonin H, Aouadhi C, Diosdado Cañizares E, Lang E, André S, Mejri S, Maaroufi A, Perrier-Cornet JM. 2019. High pressure sensitization of heat-resistant and pathogenic foodborne spores to nisin. Food Microbiol 84:103244. 10.1016/j.fm.2019.103244. [DOI] [PubMed] [Google Scholar]
- 178.Lenz CA, Reineke K, Knorr D, Vogel RF. 2015. High pressure thermal inactivation of Clostridium botulinum type E endospores—kinetic modeling and mechanistic insights. Front Microbiol 6:652. 10.3389/fmicb.2015.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jones CA, Padula NL, Setlow P. 2005. Effect of mechanical abrasion on the viability, disruption and germination of spores of Bacillus subtilis. J Appl Microbiol 99:1484–1494. 10.1111/j.1365-2672.2005.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ghosh S, Niu S, Yankova M, Mecklenburg M, King SM, Ravichandran J, Kalia RK, Nakano A, Vashishta P, Setlow P. 2017. Analysis of killing of growing cells and dormant and germinated spores of Bacillus species by black silicon nanopillars. Sci Rep 7:17768. 10.1038/s41598-017-18125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reineke K, Mathys A. 2020. Endospore inactivation by emerging technologies: a review of target structures and inactivation mechanisms. Annu Rev Food Sci Technol 11:255–274. 10.1146/annurev-food-032519-051632. [DOI] [PubMed] [Google Scholar]
- 182.Liao X, Muhammad AI, Chen S, Hu Y, Ye X, Liu D, Ding T. 2019. Bacterial spore inactivation induced by cold plasma. Crit Rev Food Sci Nutr 59:2562–2572. 10.1080/10408398.2018.1460797. [DOI] [PubMed] [Google Scholar]
- 183.Puligundla P, Mok C. 2018. Inactivation of spores by nonthermal plasmas. World J Microbiol Biotechnol 34:143. 10.1007/s11274-018-2527-3. [DOI] [PubMed] [Google Scholar]
- 184.Wang S, Doona CJ, Setlow P, Li YQ. 2016. Use of raman spectroscopy and phase-contrast microscopy to characterize cold atmospheric plasma inactivation of individual bacterial spores. Appl Environ Microbiol 82:5775–5784. 10.1128/AEM.01669-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Roth S, Feichtinger J, Hertel C. 2010. Characterization of Bacillus subtilis spore inactivation in low-pressure, low-temperature gas plasma sterilization processes. J Appl Microbiol 108:521–531. 10.1111/j.1365-2672.2009.04453.x. [DOI] [PubMed] [Google Scholar]
- 186.Hart A, Anumudu C, Onyeaka H, Miri T. 2022. Application of supercritical fluid carbon dioxide in improving food shelf-life and safety by inactivating spores: a review. J Food Sci Technol 59:417–428. 10.1007/s13197-021-05022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park HS, Choi HJ, Kim MD, Kim KH. 2013. Addition of ethanol to supercritical carbon dioxide enhances the inactivation of bacterial spores in the biofilm of Bacillus cereus. Int J Food Microbiol 166:207–212. 10.1016/j.ijfoodmicro.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 188.Setlow B, Korza G, Blatt KM, Fey JP, Setlow P. 2016. Mechanism of Bacillus subtilis spore inactivation by and resistance to supercritical CO2 plus peracetic acid. J Appl Microbiol 120:57–69. 10.1111/jam.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Camilleri E, Korza G, Huesca-Espita LDC, Setlow B, Stamatis D, Setlow P. 2020. Mechanisms of killing of Bacillus thuringiensis Al Hakam spores in a blast environment with and without iodic acid. J Appl Microbiol 128:1378–1389. 10.1111/jam.14573. [DOI] [PubMed] [Google Scholar]